Surface Acoustic Waves-Based Molecular Recognition of a Collagen Receptor on Human Erythrocytes
Abstract
1. Introduction
2. Results
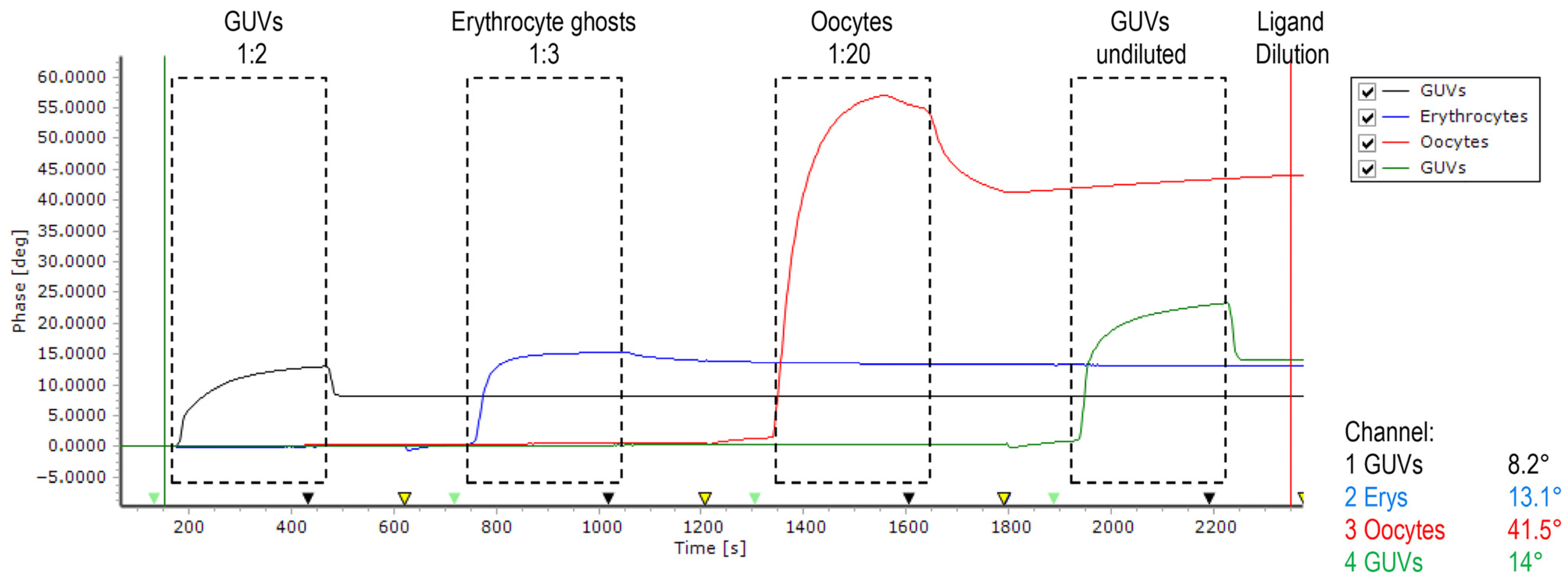
2.1. Biosensor-Based Investigation of Disintegrins Binding
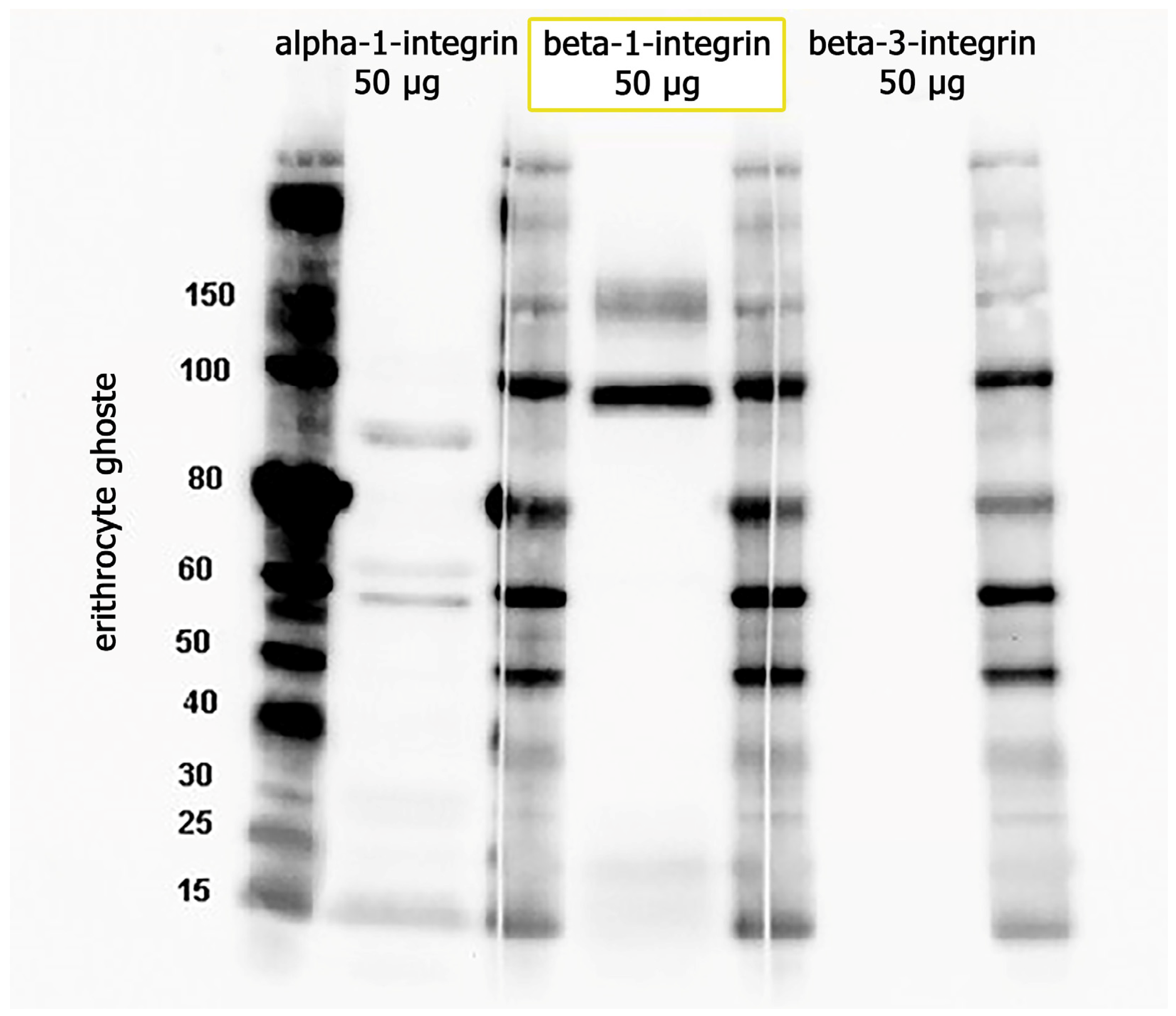
2.2. Immunoblotting
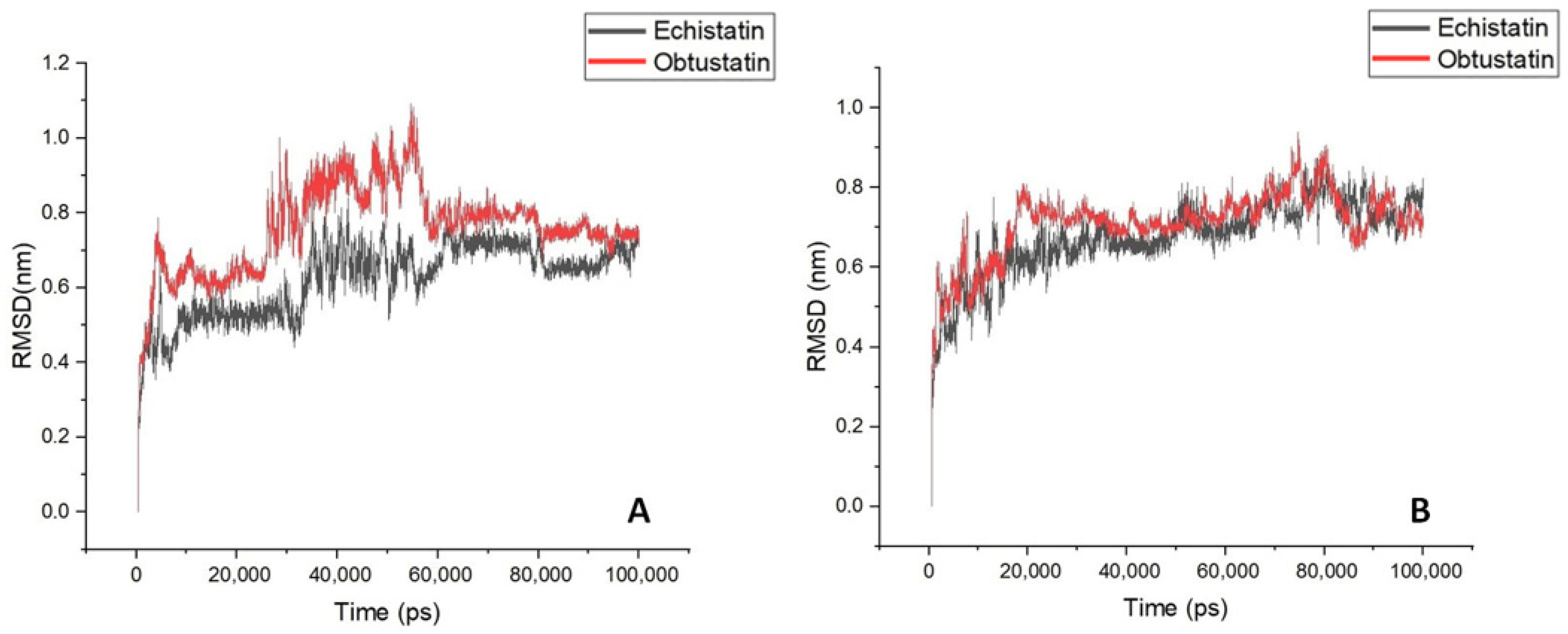
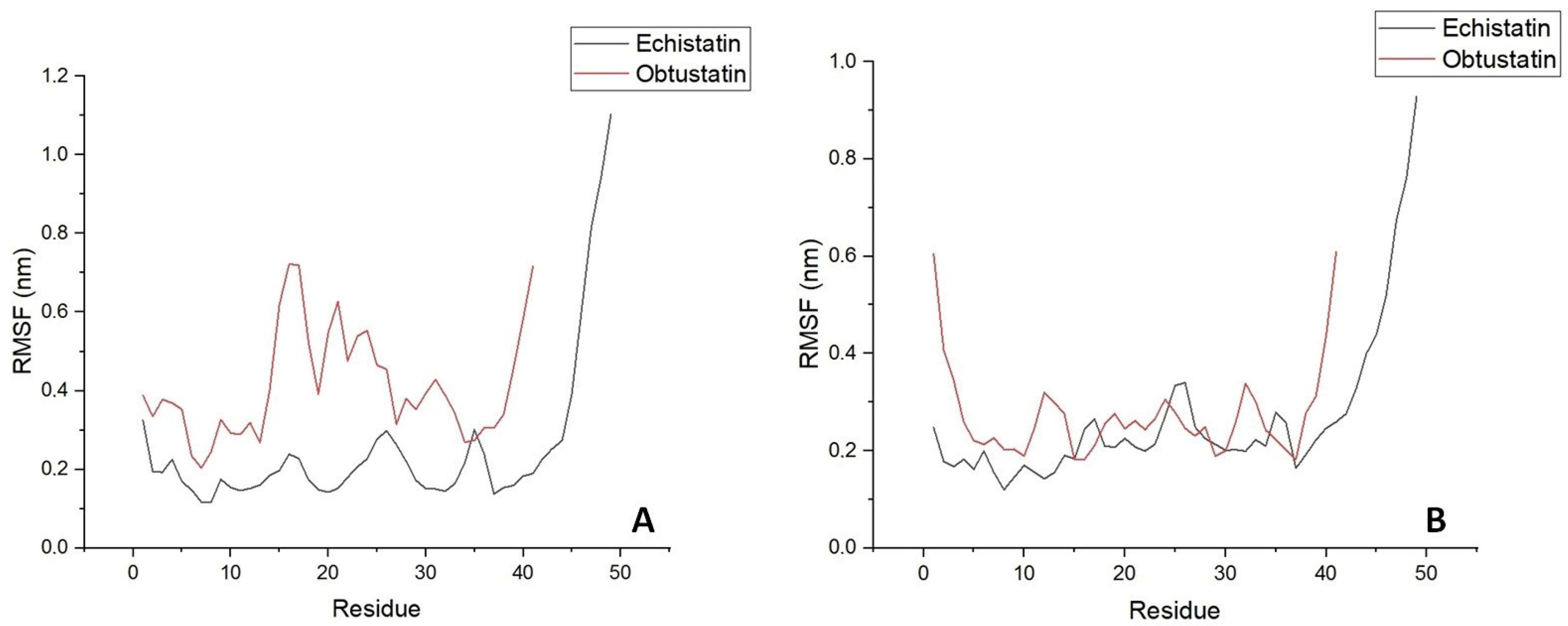
2.3. Bioinformatics Processing and Molecular Dynamic Simulation
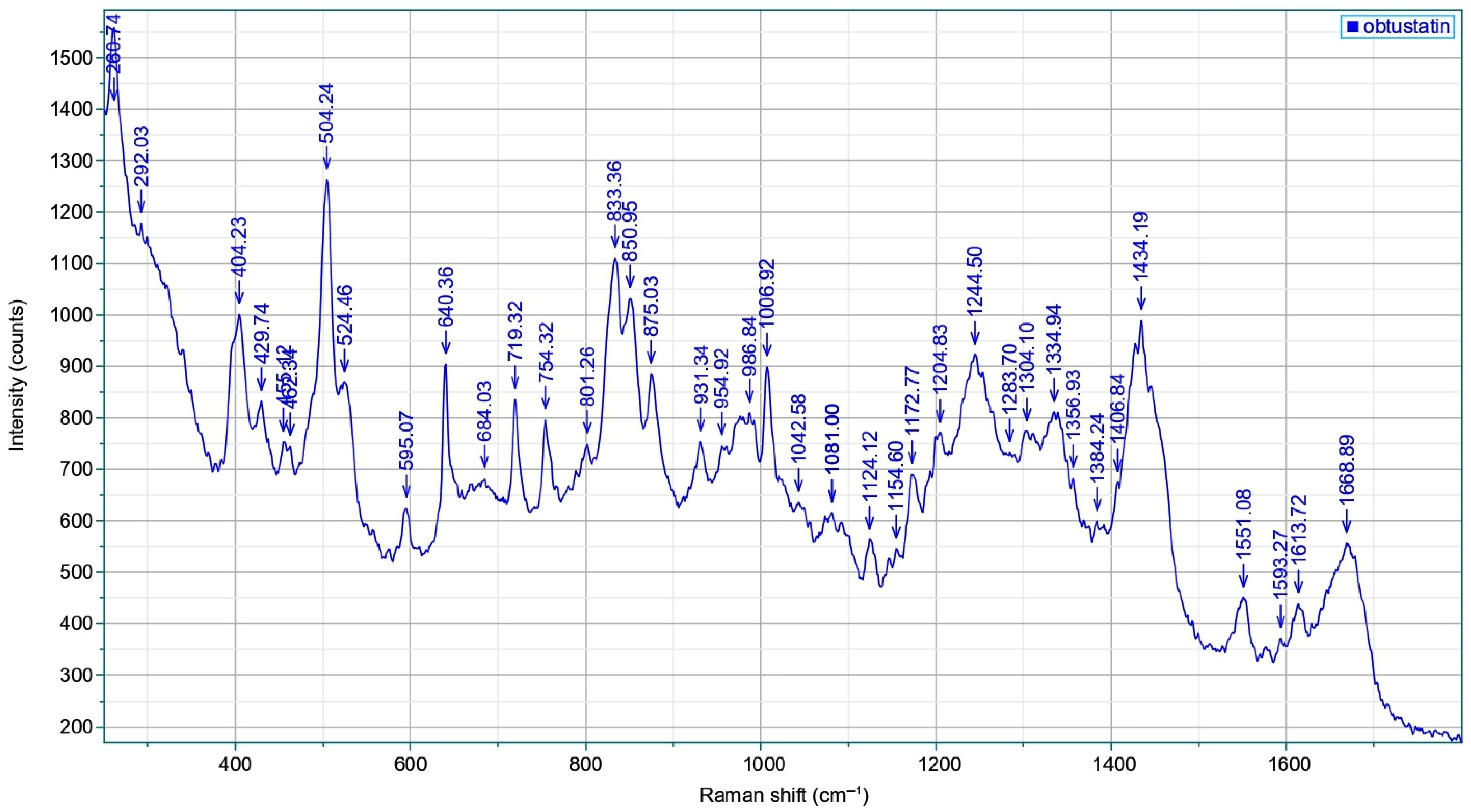
2.4. Raman Spectroscopy
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Phospholipid Processing and GUV Formation
4.3. Erythrocyte Ghosts
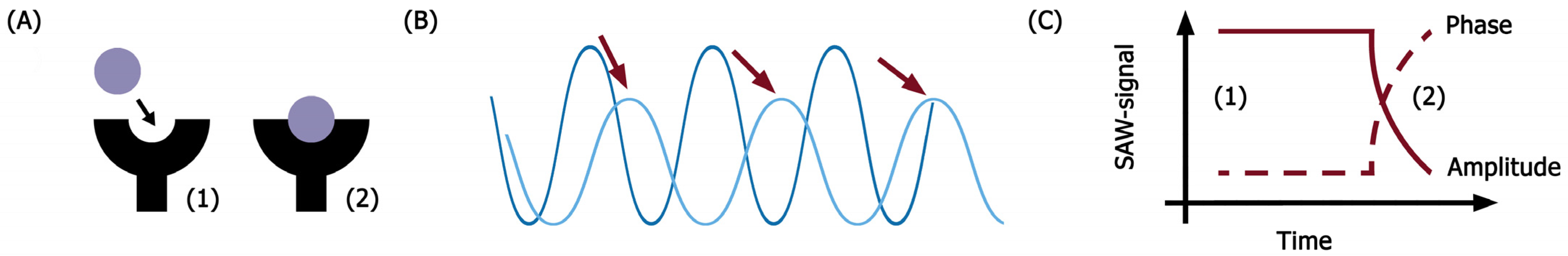
4.4. SAW Biosensor Measurements
4.5. Protein Extraction and SDS-PAGE
4.6. Immunoblotting
4.7. Raman Spectroscopy
4.8. MD Simulation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GUV | giant unilamellar vesicles |
| SAW | surface acoustic wave |
| RBC | red blood cells |
| AFM | atomic force microscopy |
| KTS/RGD | lisine-threonine-serine/arginine-glycine-aspartic acid |
| SDS-PAGE | sodium dodecyl sulphate polyacrylamide gel electrophoresis |
| RMSD | root mean square deviation |
| RMSF | root mean square fluctuation |
| LIBS | ligand-induced binding site |
| vWFA | von Willebrand factor A |
| MIDAS | metal ion-dependent adhesion site |
| VTE | venous thromboembolism |
References
- Ławkowska, K.; Bonowicz, K.; Jerka, D.; Bai, Y.; Gagat, M. Integrins in Cardiovascular Health and Disease: Molecular Mechanisms and Therapeutic Opportunities. Biomolecules 2025, 15, 233. [Google Scholar] [CrossRef]
- Savitri, C.; Ha, S.S.; Kwon, J.W.; Kim, S.H.; Kim, Y.M.; Park, H.M.; Kwon, H.; Ji, M.J.; Park, K. Human Fibroblast-Derived Matrix Hydrogel Accelerates Regenerative Wound Remodeling Through the Interactions with Macrophages. Adv. Sci. 2024, 11, e2305852. [Google Scholar] [CrossRef]
- Vasconcelos, A.A.; Estrada, J.C.; David, V.; Wermelinger, L.S.; Almeida, F.C.L.; Zingali, R.B. Structure-Function Relationship of the Disintegrin Family: Sequence Signature and Integrin Interaction. Front. Mol. Biosci. 2021, 8, 783301. [Google Scholar] [CrossRef] [PubMed]
- Clissa, P.B.; Della-Casa, M.S.; Zychar, B.C.; Sanabani, S.S. The Role of Snake Venom Disintegrins in Angiogenesis. Toxins 2024, 16, 127. [Google Scholar] [CrossRef] [PubMed]
- Janus-Bell, E.; Mangin, P.H. The relative importance of platelet integrins in hemostasis, thrombosis and beyond. Haematologica 2023, 108, 1734–1747. [Google Scholar] [CrossRef]
- Brown, M.C.; Staniszewska, I.; Del Valle, L.; Tuszynski, G.P.; Marcinkiewicz, C. Angiostatic activity of obtustatin as alpha1beta1 integrin inhibitor in experimental melanoma growth. Int. J. Cancer 2008, 123, 2195–2203. [Google Scholar] [CrossRef]
- Pasini, E.M.; Lutz, H.U.; Mann, M.; Thomas, A.W. Red blood cell (RBC) membrane proteomics--Part I: Proteomics and RBC physiology. J. Proteom. 2010, 73, 403–420. [Google Scholar] [CrossRef]
- Wilson, M.C.; Trakarnsanga, K.; Heesom, K.J.; Cogan, N.; Green, C.; Toye, A.M.; Parsons, S.F.; Anstee, D.J.; Frayne, J. Comparison of the Proteome of Adult and Cord Erythroid Cells, and Changes in the Proteome Following Reticulocyte Maturation. Mol. Cell. Proteom. MCP 2016, 15, 1938–1946. [Google Scholar] [CrossRef] [PubMed]
- Bryk, A.H.; Wiśniewski, J.R. Quantitative Analysis of Human Red Blood Cell Proteome. J. Proteome Res. 2017, 16, 2752–2761. [Google Scholar] [CrossRef]
- Carvalho, F.A.; Connell, S.; Miltenberger-Miltenyi, G.; Pereira, S.V.; Tavares, A.; Ariëns, R.A.; Santos, N.C. Atomic force microscopy-based molecular recognition of a fibrinogen receptor on human erythrocytes. ACS Nano 2010, 4, 4609–4620. [Google Scholar] [CrossRef]
- De Oliveira, S.; Vitorino de Almeida, V.; Calado, A.; Rosário, H.S.; Saldanha, C. Integrin-associated protein (CD47) is a putative mediator for soluble fibrinogen interaction with human red blood cells membrane. Biochim. Biophys. Acta 2012, 1818, 481–490. [Google Scholar] [CrossRef]
- Sokolova, I.A.; Muravyov, A.V.; Khokhlova, M.D.; Rikova, S.Y.; Lyubin, E.V.; Gafarova, M.A.; Skryabina, M.N.; Fedyanin, A.A.; Kryukova, D.V.; Shahnazarov, A.A. An effect of glycoprotein IIb/IIIa inhibitors on the kinetics of red blood cells aggregation. Clin. Hemorheol. Microcirc. 2014, 57, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Thomson-Luque, R.; Wang, C.; Ntumngia, F.B.; Xu, S.; Szekeres, K.; Conway, A.; Adapa, S.R.; Barnes, S.J.; Adams, J.H.; Jiang, R.H.Y. In-depth phenotypic characterization of reticulocyte maturation using mass cytometry. Blood Cells Mol. Dis. 2018, 72, 22–33. [Google Scholar] [CrossRef]
- Atkinson, R.A.; Saudek, V.; Pelton, J.T. Echistatin: The refined structure of a disintegrin in solution by 1H NMR and restrained molecular dynamics. Int. J. Pept. Protein Res. 1994, 43, 563–572. [Google Scholar] [CrossRef]
- Paz Moreno-Murciano, M.; Monleón, D.; Marcinkiewicz, C.; Calvete, J.J.; Celda, B. NMR solution structure of the non-RGD disintegrin obtustatin. J. Mol. Biol. 2003, 329, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Sanz, L.; Ayvazyan, N.; Calvete, J.J. Snake venomics of the Armenian mountain vipers Macrovipera lebetina obtusa and Vipera raddei. J. Proteom. 2008, 71, 198–209. [Google Scholar] [CrossRef]
- Marcinkiewicz, C.; Weinreb, P.H.; Calvete, J.J.; Kisiel, D.G.; Mousa, S.A.; Tuszynski, G.P.; Lobb, R.R. Obtustatin: A potent selective inhibitor of alpha1beta1 integrin in vitro and angiogenesis in vivo. Cancer Res. 2003, 63, 2020–2023. [Google Scholar]
- Hunter, E.J.; Hamaia, S.W.; Gullberg, D.; Malcor, J.D.; Farndale, R.W. Selectivity of the collagen-binding integrin inhibitors, TC-I-15 and obtustatin. Toxicol. Appl. Pharmacol. 2021, 428, 115669. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J.; Marcinkiewicz, C.; Sanz, L. KTS and RTS-disintegrins: Anti-angiogenic viper venom peptides specifically targeting the alpha 1 beta 1 integrin. Curr. Pharm. Des. 2007, 13, 2853–2859. [Google Scholar] [CrossRef]
- Oursler, M.J.; Spelsberg, T.C. Echistatin, a potential new drug for osteoporosis. Endocrinology 1993, 132, 939–940. [Google Scholar] [CrossRef]
- Lin, Y.; Rao, J.; Zha, X.L.; Xu, H. Angiopoietin-like 3 induces podocyte F-actin rearrangement through integrin α(V)β3/FAK/PI3K pathway-mediated Rac1 activation. BioMed Res. Int. 2013, 2013, 135608. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Huang, C.H.; Chang, Y.T.; Chen, C.Y.; Shiu, J.H.; Cheng, C.H.; Su, Y.F.; Chuang, W.J. Structural and Functional Differences of Rhodostomin and Echistatin in Integrin Recognition and Biological Implications. Proteins 2025, 93, 1627–1644. [Google Scholar] [CrossRef] [PubMed]
- Perpeet, M.; Glass, S.; Gronewold, T.M.; Kiwitz, A.; Malavé, A.; Stoyanov, I.; Tewes, M.; Quandt, E. SAW sensor system for marker-free molecular interaction analysis. Anal. Lett. 2006, 39, 1747–1757. [Google Scholar] [CrossRef]
- Mandal, D.; Banerjee, S. Surface Acoustic Wave (SAW) Sensors: Physics, Materials, and Applications. Sensors 2022, 22, 820. [Google Scholar] [CrossRef] [PubMed]
- Gronewold, T.M.; Baumgartner, A.; Quandt, E.; Famulok, M. Discrimination of single mutations in cancer-related gene fragments with a surface acoustic wave sensor. Anal. Chem. 2006, 78, 4865–4871. [Google Scholar] [CrossRef]
- Andrä, J.; Böhling, A.; Gronewold, T.M.; Schlecht, U.; Perpeet, M.; Gutsmann, T. Surface acoustic wave biosensor as a tool to study the interaction of antimicrobial peptides with phospholipid and lipopolysaccharide model membranes. Langmuir ACS J. Surf. Colloids 2008, 24, 9148–9153. [Google Scholar] [CrossRef]
- Grabka, M.; Jasek, K.; Witkiewicz, Z. Surface Acoustic Wave Immunosensor for Detection of Botulinum Neurotoxin. Sensors 2023, 23, 7688. [Google Scholar] [CrossRef]
- Casalinuovo, I.A.; Pierro, D.; Bruno, E.; Francesco, P.; Coletta, M. Experimental use of a new surface acoustic wave sensor for the rapid identification of bacteria and yeasts. Lett. Appl. Microbiol. 2006, 42, 24–29. [Google Scholar] [CrossRef]
- Tamarin, O.; Comeau, S.; Déjous, C.; Moynet, D.; Rebière, D.; Bezian, J.; Pistré, J. Real time device for biosensing: Design of a bacteriophage model using love acoustic waves. Biosens. Bioelectron. 2003, 18, 755–763. [Google Scholar] [CrossRef]
- Al-Kaddah, S.; Reder-Christ, K.; Klocek, G.; Wiedemann, I.; Brunschweiger, M.; Bendas, G. Analysis of membrane interactions of antibiotic peptides using ITC and biosensor measurements. Biophys. Chem. 2010, 152, 145–152. [Google Scholar] [CrossRef]
- Schlesinger, M.; Schmitz, P.; Zeisig, R.; Naggi, A.; Torri, G.; Casu, B.; Bendas, G. The inhibition of the integrin VLA-4 in MV3 melanoma cell binding by non-anticoagulant heparin derivatives. Thromb. Res. 2012, 129, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Slamnoiu, S.; Vlad, C.; Stumbaum, M.; Moise, A.; Lindner, K.; Engel, N.; Vilanova, M.; Diaz, M.; Karreman, C.; Leist, M.; et al. Identification and affinity-quantification of ß-amyloid and α-synuclein polypeptides using on-line SAW-biosensor-mass spectrometry. J. Am. Soc. Mass Spectrom. 2014, 25, 1472–1481. [Google Scholar] [CrossRef] [PubMed]
- Ghazaryan, N.A.; Ghulikyan, L.A.; Kishmiryan, A.V.; Kirakosyan, G.R.; Nazaryan, O.H.; Ghevondyan, T.H.; Zakaryan, N.A.; Ayvazyan, N.M. Anti-tumor effect investigation of obtustatin and crude Macrovipera lebetina obtusa venom in S-180 sarcoma bearing mice. Eur. J. Pharmacol. 2015, 764, 340–345. [Google Scholar] [CrossRef]
- Ghazaryan, N.; Movsisyan, N.; Macedo, J.C.; Vaz, S.; Ayvazyan, N.; Pardo, L.; Logarinho, E. The antitumor efficacy of monomeric disintegrin obtustatin in S-180 sarcoma mouse model. Investig. New Drugs 2019, 37, 1044–1051. [Google Scholar] [CrossRef]
- Pocheć, E.; Lityńska, A.; Amoresano, A.; Casbarra, A. Glycosylation profile of integrin alpha 3 beta 1 changes with melanoma progression. Biochim. Et Biophys. Acta 2003, 1643, 113–123. [Google Scholar] [CrossRef]
- Zhao, X.; Lasell, T.K.; Melançon, P. Localization of large ADP-ribosylation factor-guanine nucleotide exchange factors to different Golgi compartments: Evidence for distinct functions in protein traffic. Mol. Biol. Cell 2002, 13, 119–133. [Google Scholar] [CrossRef]
- Moloney, D.J.; Panin, V.M.; Johnston, S.H.; Chen, J.; Shao, L.; Wilson, R.; Wang, Y.; Stanley, P.; Irvine, K.D.; Haltiwanger, R.S.; et al. Fringe is a glycosyltransferase that modifies Notch. Nature 2000, 406, 369–375. [Google Scholar] [CrossRef]
- Daidone, I.; Aschi, M.; Patamia, M.; Bozzi, A.; Petruzzelli, R. Structural and dynamical properties of KTS-disintegrins: A comparison between Obtustatin and Lebestatin. Biopolymers 2013, 99, 47–54. [Google Scholar] [CrossRef]
- Moreno-Murciano, M.P.; Monleón, D.; Calvete, J.J.; Celda, B.; Marcinkiewicz, C. Amino acid sequence and homology modeling of obtustatin, a novel non-RGD-containing short disintegrin isolated from the venom of Vipera lebetina obtuse. Protein Sci. A Publ. Protein Soc. 2003, 12, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Mozhaeva, V.; Kudryavtsev, D.; Prokhorov, K.; Utkin, Y.; Gudkov, S.; Garnov, S.; Kasheverov, I.; Tsetlin, V. Toxins’ classification through Raman spectroscopy with principal component analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 278, 121276. [Google Scholar] [CrossRef] [PubMed]
- Saudek, V.; Atkinson, R.A.; Lepage, P.; Pelton, J.T. The secondary structure of echistatin from 1H-NMR, circular-dichroism and Raman spectroscopy. Eur. J. Biochem. 1991, 202, 329–338. [Google Scholar] [CrossRef]
- Sugeta, H.; Go, A.; Miyazawa, T. Vibrational Spectra and Molecular Conformations of Dialkyl Disulfides. Bull. Chem. Soc. Jpn. 1973, 46, 3407–3411. [Google Scholar] [CrossRef]
- Xiong, J.P.; Stehle, T.; Zhang, R.; Joachimiak, A.; Frech, M.; Goodman, S.L.; Arnaout, M.A. Crystal structure of the extracellular segment of integrin alpha Vbeta3 in complex with an Arg-Gly-Asp ligand. Science 2002, 296, 151–155. [Google Scholar] [CrossRef]
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef] [PubMed]
- Jaudon, F.; Cingolani, L.A. Unlocking mechanosensitivity: Integrins in neural adaptation. Trends Cell Biol. 2024, 34, 1029–1043. [Google Scholar] [CrossRef]
- Hughes, P.E.; Diaz-Gonzalez, F.; Leong, L.; Wu, C.; McDonald, J.A.; Shattil, S.J.; Ginsberg, M.H. Breaking the integrin hinge, A defined structural constraint regulates integrin signaling. J. Biol. Chem. 1996, 271, 6571–6574. [Google Scholar] [CrossRef]
- Johnson, M.S.; Chouhan, B.S. Evolution of Integrin I Domains; Advances in Experimental Medicine and Biology; Springer: Dordrecht, The Netherlands, 2014; Volume 819, pp. 1–19. [Google Scholar] [CrossRef]
- Barczyk, M.; Carracedo, S.; Gullberg, D. Integrins. Cell Tissue Res. 2010, 339, 269–280. [Google Scholar] [CrossRef]
- Cui, Y.; Rolova, T.; Fagerholm, S.C. The role of integrins in brain health and neurodegenerative diseases. Eur. J. Cell Biol. 2024, 103, 151441. [Google Scholar] [CrossRef] [PubMed]
- Kondo, N.; Ueda, Y.; Kinashi, T. LFA1 Activation: Insights from a Single-Molecule Approach. Cells 2022, 11, 1751. [Google Scholar] [CrossRef] [PubMed]
- Moser, M.; Legate, K.R.; Zent, R.; Fässler, R. The tail of integrins, talin, and kindlins. Science 2009, 324, 895–899. [Google Scholar] [CrossRef]
- Zhu, J.; Luo, B.H.; Xiao, T.; Zhang, C.; Nishida, N.; Springer, T.A. Structure of a complete integrin ectodomain in a physiologic resting state and activation and deactivation by applied forces. Mol. Cell 2008, 32, 849–861. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, B.S.; Kessler, H.; Kossatz, S.; Reuning, U. RGD-Binding Integrins Revisited: How Recently Discovered Functions and Novel Synthetic Ligands (Re-)Shape an Ever-Evolving Field. Cancers 2021, 13, 1711. [Google Scholar] [CrossRef]
- Khamessi, O.; Ben Mabrouk, H.; Hkimi, C.; Rtima, R.; Kamoun, S.; Kharrat, R.; Ghedira, K. DisintegrinDB: The first integrated database resource of disintegrins from snake venoms. Biochem. Biophys. Res. Commun. 2022, 597, 77–82. [Google Scholar] [CrossRef]
- Fens, M.H.; van Wijk, R.; Andringa, G.; van Rooijen, K.L.; Dijstelbloem, H.M.; Rasmussen, J.T.; de Vooght, K.M.; Schiffelers, R.M.; Gaillard, C.A.; van Solinge, W.W. A role for activated endothelial cells in red blood cell clearance: Implications for vasopathology. Haematologica 2012, 97, 500–508. [Google Scholar] [CrossRef]
- Chaar, V.; Picot, J.; Renaud, O.; Bartolucci, P.; Nzouakou, R.; Bachir, D.; Galactéros, F.; Colin, Y.; Le Van Kim, C.; El Nemer, W. Aggregation of mononuclear and red blood cells through an {alpha}4{beta}1-Lu/basal cell adhesion molecule interaction in sickle cell disease. Haematologica 2010, 95, 1841–1848. [Google Scholar] [CrossRef]
- Himbert, S.; Alsop, R.J.; Rose, M.; Hertz, L.; Dhaliwal, A.; Moran-Mirabal, J.M.; Verschoor, C.P.; Bowdish, D.M.; Kaestner, L.; Wagner, C.; et al. The Molecular Structure of Human Red Blood Cell Membranes from Highly Oriented, Solid Supported Multi-Lamellar Membranes. Sci. Rep. 2017, 7, 39661. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, J.R.; Wolberg, A.S. Red blood cells in thrombosis. Blood 2017, 130, 1795–1799. [Google Scholar] [CrossRef]
- Khamessi, O.; Ben Mabrouk, H.; Kamoun, S.; Hkimi, C.; Ghedira, K.; Kharrat, R. The First Snake Venom KTS/Disintegrins-Integrin Interactions Using Bioinformatics Approaches. Molecules 2022, 28, 325. [Google Scholar] [CrossRef]
- Kates, M. Techniques of Lipidology: Isolation, Analysis and Identification of Lipids; North-Holland Pub. Co.: Amsterdam, The Netherlands, 1972. [Google Scholar]
- Angelova, M.I.; Soleau, S.; Meleard, P.; Faucon, J.F.; Bothorel, P. Preparation of Giant Vesicles by External Fields, Kinetics and Application; Progress in Colloid & Polymer Science; Steinkopff-Verlag: Darmstadt, Germany, 1992; Volume 89, pp. 127–131. [Google Scholar]
- Ghazaryan, N.A.; Ghulikyan, L.A.; Ayvazyan, N.M. Morphological changes of proteolipid giant unilamellar vesicles affected by Macrovipera lebetina obtusa venom visualized with fluorescence microscope. J. Membr. Biol. 2013, 246, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Dodge, J.T.; Mitchell, C.; Hanahan, D.J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch. Biochem. Biophys. 1963, 100, 119–130. [Google Scholar] [CrossRef]
- Kirakosyan, G.; Mohamadvarzi, M.; Ghulikyan, L.; Zaqaryan, N.; Kishmiryan, A.; Ayvazyan, N. Morphological and functional alteration of erythrocyte ghosts and giant unilamellar vesicles caused by Vipera latifi venom. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2016, 190, 48–53. [Google Scholar] [CrossRef]
- Schlesinger, M.; Simonis, D.; Schmitz, P.; Fritzsche, J.; Bendas, G. Binding between heparin and the integrin VLA-4. Thromb. Haemost. 2009, 102, 816–822. [Google Scholar] [CrossRef]
- Gronewold, T.M.; Schlecht, U.; Quandt, E. Analysis of proteolytic degradation of a crude protein mixture using a surface acoustic wave sensor. Biosens. Bioelectron. 2007, 22, 2360–2365. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Jung, J.; Kobayashi, C.; Torre, D.U.; Takada, S.; Sugita, Y. Implementation of residue-level coarse-grained models in GENESIS for large-scale molecular dynamics simulations. PLoS Comput. Biol. 2022, 18, e1009578. [Google Scholar] [CrossRef]
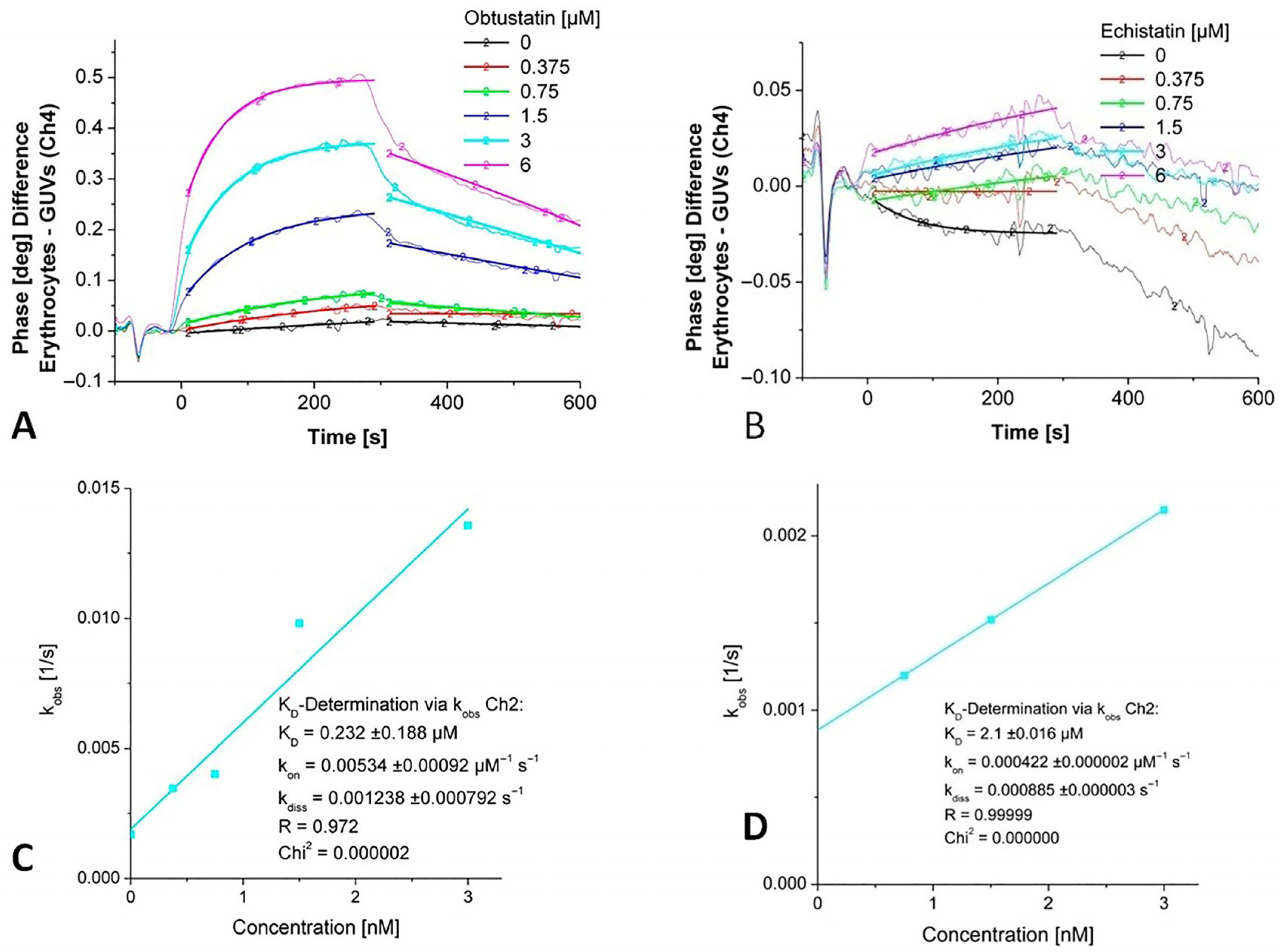

| Obtustatin | Echistatin | |
|---|---|---|
| kon | (5.34 ± 0.9) × 103 M−1 s−1 | (4.22 ± 0.002) × 102 M−1 s−1 |
| koff | (1.238 ± 0.8) × 10−3 s−1 | (8.85 ± 0.03) × 10−4 s−1 |
| KD | (2.32 ± 1.88)× 10−7 M | (2.1 ± 0.016) × 10−6 M |
| Echistatin (P17347)----ECESGPCCRNCKFLKEGTICKR—ARGD-DMDDYCNGKTCDCPRNPHKGPAT Obtustatin (P83469)---CTTGPCCRQCKLKPAGTTCW-----KTS--LTSHYCTGKSCDCPLYPG-------- | ||
| Name | Echistatin | Obtustatin |
| Glutamic acid | 3 | 0 |
| Cysteine | 8 | 8 |
| Serine | 1 | 3 |
| Glycine | 5 | 4 |
| Proline | 4 | 4 |
| Arginine | 4 | 1 |
| Asparagine | 3 | 0 |
| Lysine | 5 | 4 |
| Threonine | 3 | 7 |
| Leucine | 1 | 3 |
| Isoleucine | 1 | 0 |
| Alanine | 2 | 1 |
| Phenylalanine | 1 | 0 |
| Aspartic acid | 5 | 1 |
| Methionine | 1 | 0 |
| Tyrosine | 1 | 2 |
| Histidine | 1 | 1 |
| Glutamine | 0 | 1 |
| Tryptophan | 0 | 1 |
| Total residues | 49 | 41 |
| References for amino acids | [14] | [15] |
| Cat. Number | Antibody | Source | Dilution | Manufacturer |
|---|---|---|---|---|
| ab75872 | Anti-Integrin beta 3 antibody | Rabbit monoclonal | 1:1000 | Abcam |
| ab179471 | Anti-Integrin beta 1 antibody | Rabbit monoclonal | 1:1000 | Abcam |
| ab181434 | Anti-Integrin alpha 1 antibody—C-terminal | Rabbit polyclonal | 1:1000 | Abcam |
| NA934-100UL | ECL Anti-Rabbit IgG, HRP Linked | Donkey/polyclonal | 1:10,000 | GE Healthcare |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghukasyan, G.; Ghazaryan, N.; Torosyan, M.; Movsisyan, N.; Meltonyan, A.; Ayvazyan, N. Surface Acoustic Waves-Based Molecular Recognition of a Collagen Receptor on Human Erythrocytes. Int. J. Mol. Sci. 2025, 26, 11258. https://doi.org/10.3390/ijms262311258
Ghukasyan G, Ghazaryan N, Torosyan M, Movsisyan N, Meltonyan A, Ayvazyan N. Surface Acoustic Waves-Based Molecular Recognition of a Collagen Receptor on Human Erythrocytes. International Journal of Molecular Sciences. 2025; 26(23):11258. https://doi.org/10.3390/ijms262311258
Chicago/Turabian StyleGhukasyan, Gevorg, Narine Ghazaryan, Michael Torosyan, Naira Movsisyan, Ashot Meltonyan, and Naira Ayvazyan. 2025. "Surface Acoustic Waves-Based Molecular Recognition of a Collagen Receptor on Human Erythrocytes" International Journal of Molecular Sciences 26, no. 23: 11258. https://doi.org/10.3390/ijms262311258
APA StyleGhukasyan, G., Ghazaryan, N., Torosyan, M., Movsisyan, N., Meltonyan, A., & Ayvazyan, N. (2025). Surface Acoustic Waves-Based Molecular Recognition of a Collagen Receptor on Human Erythrocytes. International Journal of Molecular Sciences, 26(23), 11258. https://doi.org/10.3390/ijms262311258








