Synthesis of Biomass-Derived Graphene Nanomaterials by Chemical Activation with KOH
Abstract
1. Introduction
2. Results
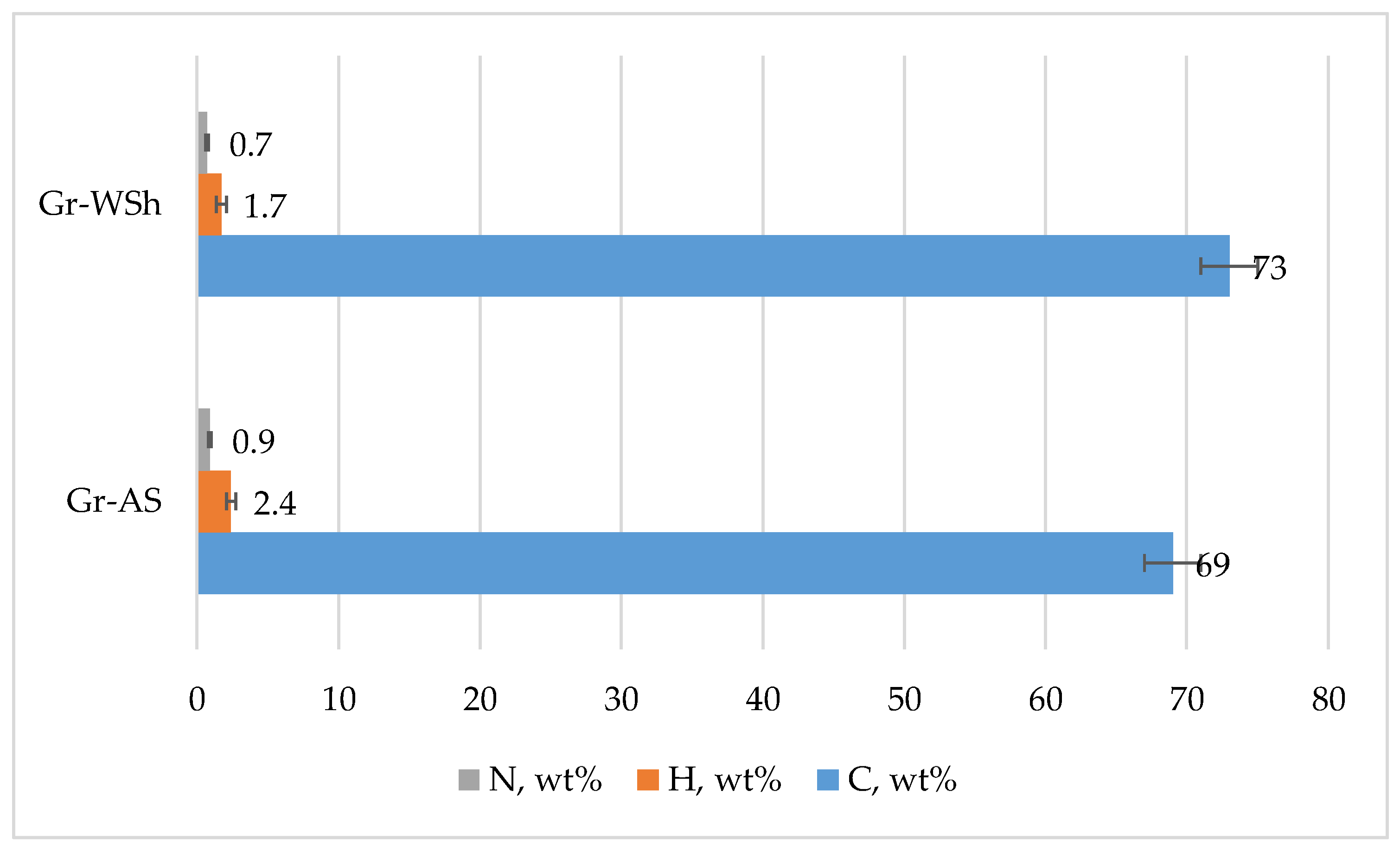
2.1. Material Composition
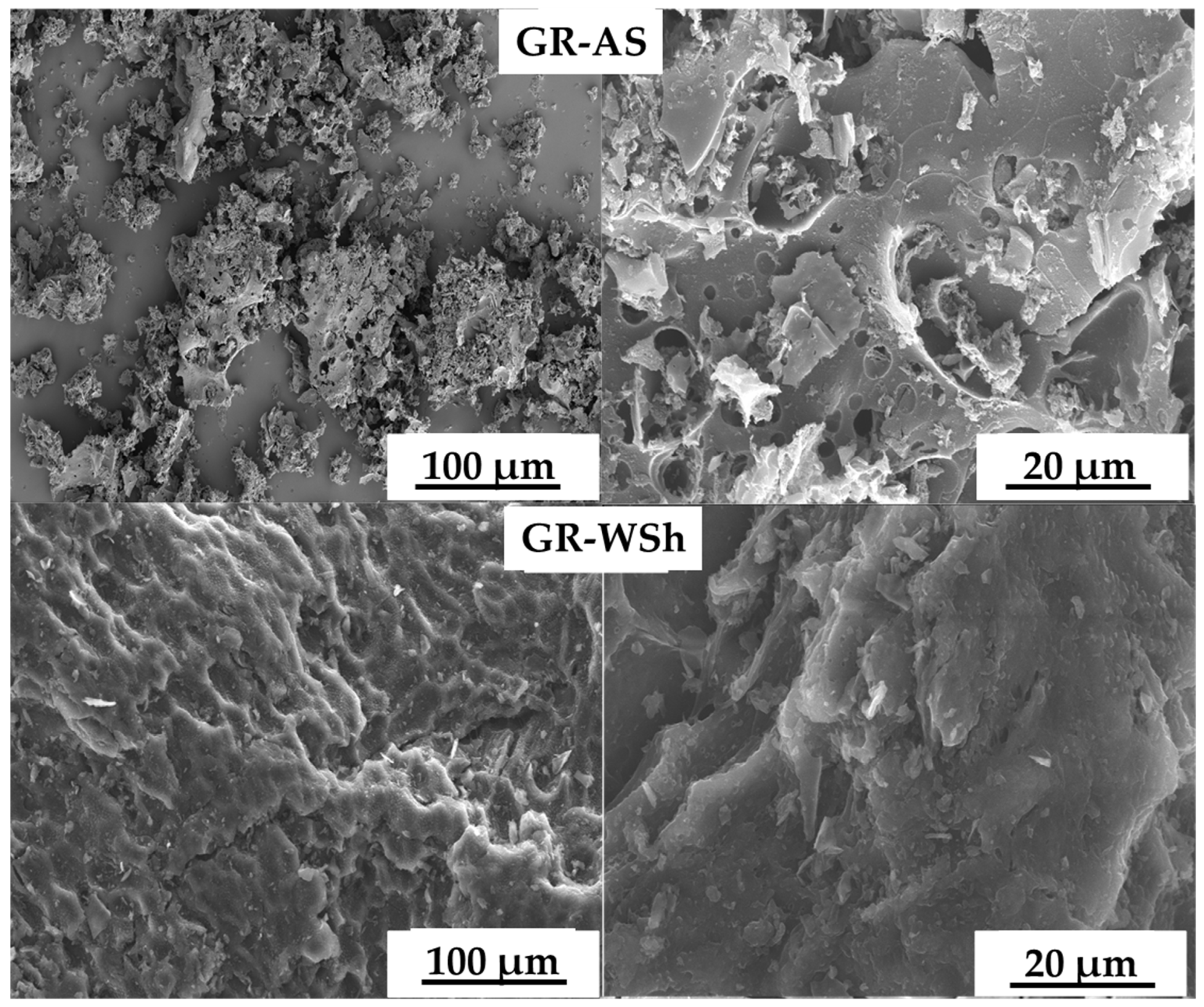
2.2. Morphology of Biomass-Derived Graphene
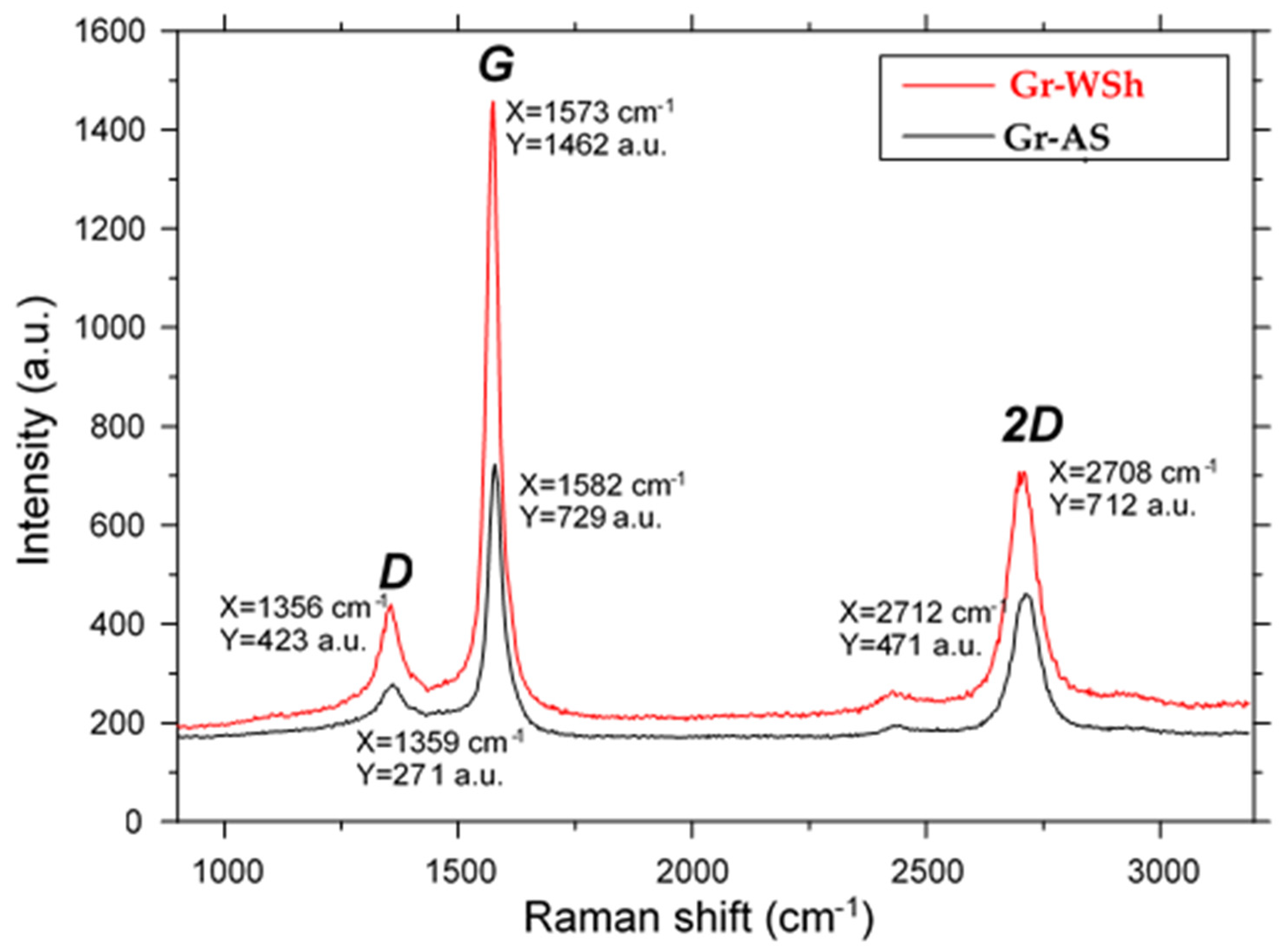
2.3. Raman Spectroscopy
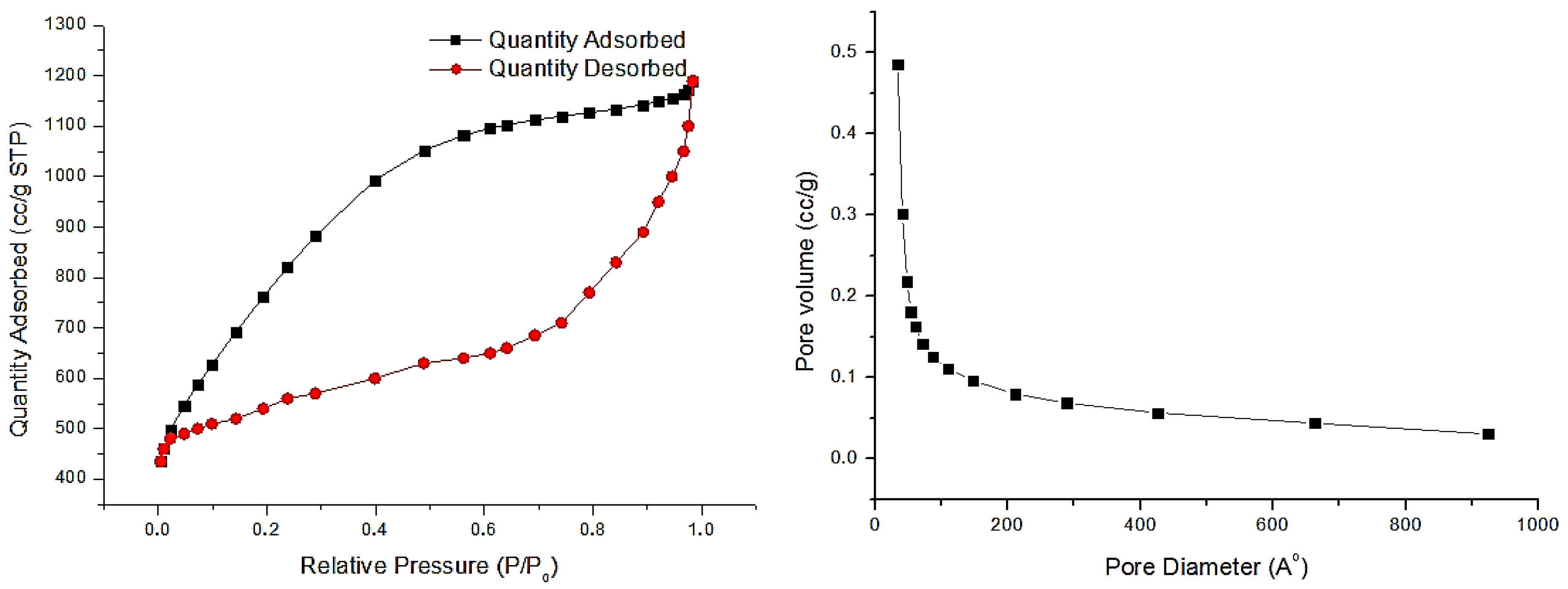
2.4. Specific Surface by BET Method
2.5. Transmission Electron Microscopy
3. Discussion
4. Methods and Materials
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RH | Rise husk |
| AS | Apricot stones |
| WSh | Walnut shells |
| SEM | Scanning electron microscope |
| BET | Brunauer–Emmett–Teller method |
| CVD | Chemical vapor deposition |
References
- Murugadoss, V.; Reddy, S.N.; Subramania, A.K.; Dhandapani, P.; Rajendra, S.P.; AlSalhi, M.S.; Angaiah, S. Influence of high-shear exfoliation and the stabilizer on the formation of exfoliated graphene nanosheets and its supercapacitive performances. J. Iran. Chem. Soc. 2025, 22, 733–742. [Google Scholar] [CrossRef]
- Bonaventura, E.; Martella, C.; Macis, S.; Dhungana, D.S.; Krotkus, S.; Heuken, M.; Lupi, S.; Molle, A.; Grazianetti, C. Optical properties of two-dimensional tin nanosheets epitaxially grown on graphene. Nanotechnology 2024, 35, 23LT01. [Google Scholar] [CrossRef]
- Li, S.; Wang, J.; Huang, M. Direct CVD Growth of Twisted Graphene: Unveiling Mechanisms and Prospects for Future Applications. Small 2025, 21, 2500628. [Google Scholar] [CrossRef]
- Fayed, M.I. Determination of some apricot seed and kernel physical and mechanical properties. Agric. Eng. Int. CIGR E-J. 2020, 22, 229–237. [Google Scholar]
- Hu, Z.; Zhao, H.; Wang, B.; Zhang, C.; Lu, H. Study on the performance of biochar prepared from walnut shell and traditional graphene electrode plate in the treatment of domestic sewage in microbial fuel cells. Water Sci. Technol. 2024, 89, 2880–2893. [Google Scholar] [CrossRef] [PubMed]
- Onovo, H.O.; Agbeleye, A.A.; Akano, T.T.; Oloyede, O.R.; Oyegbami, S.O.; Amoo, E.O. Characterization of Rice Husk for Graphene Extraction and Metallization. ABUAD J. Eng. Res. Dev. 2024, 7, 521–531. [Google Scholar] [CrossRef]
- Shaikhiev, I.; Shaykhieva, K.; Sverguzova, S.; Fomina, E.; Vinogradenko, Y.; Fediuk, R.; Amran, M.; Svintsov, A.P.; de Azevedo, A.R.G.; Gunasekaran, M. Removing Pollutants from Sewage Waters with Ground Apricot Kernel Shell Material. Materials 2022, 15, 3428. [Google Scholar] [CrossRef]
- Gedik, B.K.; Önal, Y.; Başar, C.A.; Akbulut, Y. Chemical Vapor Deposition Synthesis of Carbon Nanotube Using Pyrolysis Gas Products of Apricot Kernel Shell. Adv. Sci. Eng. Med. 2020, 12, 556–563. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, P.K.; Sharma, K. Synergistic Effects of Heated Walnut Shell Powder and Reduced Graphene Oxide in Paraffin Wax Composites for Marine Thermal Applications. Int. J. Marit. Eng. 2024, 1, 277–286. [Google Scholar] [CrossRef]
- Duisenbek, A.; Beisenova, Y.; Beissenov, R.; Askaruly, K.; Yeleuov, M.; Abdisattar, A. Onion husk-derived high surface area graphene-like carbon for supercapacitor electrode material application. Heliyon 2024, 10, e32915. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raghavan, N.; Thangavel, S.; Venugopal, G. A short review on preparation of graphene from waste and bioprecursors. Appl. Mater. Today 2017, 7, 246–254. [Google Scholar] [CrossRef]
- Jasim, S.A.; Rachchh, N.; Pallathadka, H.; Sanjeevi, R.; Bokov, D.O.; Bobonazarovna, S.F.; Jabbar, H.S.; Mahajan, S.; Mustafa, Y.F.; Alhadrawi, M. Recent advances in carbon-based materials derived from diverse green biowaste for sensing applications: A comprehensive overview from the perspective of synthesis method and application. RSC Adv. 2024, 14, 39787–39803. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abbas, S.; Abbas, A.; Zahra, T.; Kazmi, J.; Ahmad, W.; Ahmed, N.; Lim, T.M.; Cong, H. Green and gram-scale synthesis of uniform graphene quantum dots from biomass waste: A highly selective probe for nanomolar Hg2+ sensing. Mater. Today Chem. 2025, 47, 102830. [Google Scholar] [CrossRef]
- ASTM E870-82 Standard Test; Methods for Analysis of Wood Fuels. ASTM International: West Conshohocken, PA, USA, 2019. [CrossRef]
- Jandosov, J.M.; Shikina, N.V.; Bijsenbayev, M.A.; Shamalov, M.E.; Ismagilov, Z.R.; Mansurov, Z.A. Evaluation of Synthetic Conditions for H3PO4 Chemically Activated Rice Husk and Preparation of Honeycomb Monoliths. Eurasian Chem. Tech. J. 2009, 11, 245–252. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.; Li, G.; Guo, S. Emission reduction effect and carbon market efficiency of carbon emissions trading policy in China. Energy 2020, 196, 117117. [Google Scholar] [CrossRef]
- Ogidi, S.; Yusuf, M.; Sanni, K.; Andrea, D.; Abdulsalam, A. Removal of Heavy Metals from Produced Water Using Activated Carbon from Coconut Husk Enhanced with Graphene Oxide. J. Eng. Res. Rep. 2024, 26, 42–52. [Google Scholar] [CrossRef]
- Chailuecha, C.; Klinbumrung, A.; Chaopanich, P.; Sirirak, R. Graphene-like porous carbon nanostructure from corn husk: Synthesis and characterization. Mater. Today Proc. 2021, 47, 512. [Google Scholar] [CrossRef]
- Yang, P.; Hu, J.; Zhou, X.; Xia, J.; Shi, J.; He, J. Synthesis of graphene nanosheets modified with the Fe3O4@phenol formaldehyde resin or PFR nanoparticles for their application in bio-imagine and thermal treatment. J. Appl. Polym. Sci. 2017, 134, 45007. [Google Scholar] [CrossRef]
- YYang, X.; Li, S.; Zhang, S.; Chen, X.; Peng, S. Facile assembly of cellulose-derived 3D-graphene/lithium hydroxide monohydrate nanocomposite for low-temperature chemical heat storage. Cellulose 2019, 26, 4815–4825. [Google Scholar] [CrossRef]
- Xabela, S.; Moutloali, R.M. 2-(N-3-Sulfopropyl-N,N-dimethyl ammonium)ethyl methacrylate modified graphene oxide embedded into cellulose acetate ultrafiltration membranes for improved performance. J. Appl. Polym. Sci. 2022, 139, 52336. [Google Scholar] [CrossRef]
- Seitzhanova, M.; Azat, S.; Yeleuov, M.; Taurbekov, A.; Mansurov, Z.; Doszhanov, E.; Berndtsson, R. Production of Graphene Membranes from Rice Husk Biomass Waste for Improved Desalination. Nanomaterials 2024, 14, 224. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seitzhanova, M.A.; Mansurov, Z.A.; Yeleuov, M.; Roviello, V.; Di Capua, R. The Characteristics of Graphene Obtained from Rice Husk and Graphite. Eurasian Chem.-Technol. J. 2019, 21, 149–156. [Google Scholar] [CrossRef]
- Saha, J.K.; Dutta, A. A Review of Graphene: Material Synthesis from Biomass Sources. Waste Biomass Valorization 2022, 13, 1385–1429. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suwaki, R.; Mori, M.; Ishii, T.; Ito, H.; Yoshida, W.; Nakayama, M.; Ueda, T.; Myochin, H.; Noguera, M.G.; Zhang, J.; et al. Generation of Graphene-like Carbon Materials Derived from Biomass Using Iron Species. ACS Appl. Nano Mater. 2025, 8, 17643–17652. [Google Scholar] [CrossRef]
- Watcharamaisakul, S.; Janphuang, N.; Chueangam, W.; Srisom, K.; Rueangwittayanon, A.; Rittihong, U.; Tunmee, S.; Chanlek, N.; Pornsetmetakul, P.; Wirojsirasak, W.; et al. Synthesis of Turbostratic Graphene Derived from Biomass Waste Using Long Pulse Joule Heating Technique. Nanomaterials 2025, 15, 468. [Google Scholar] [CrossRef]
- Othman, C.; Ermala, F.; Ismail, M.S.; Yusof, N.; Samitsu, S.; Yusop, M.Z.; Arifin, T.; Fatihah, N.; Alias, N.H.; Jaafar, J.; et al. Methane adsorption by porous graphene derived from rice husk ashes under various stabilization temperatures. Carbon Lett. 2020, 30, 535–543. [Google Scholar] [CrossRef]
- Ismail, M.S.; Yusof, N.; Yusop, M.M.; Ismail, A.F.; Jaafar, J.; Aziz, F.; Karim, Z.A. Synthesis and characterization of graphene derived from rice husks. Malays. J. Fundam. Appl. Sci. 2019, 15, 516–521. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Lou, F.; Buan, M.E.M.; Sheridan, E.; Chen, D. Enhancing capacitance of supercapacitor with both organic electrolyte and ionic liquid electrolyte on a biomass-derived carbon. RSC Adv. 2017, 7, 23859–23865. [Google Scholar] [CrossRef]
- Rani, M.U.; Nanaji, K.; Rao, T.N.; Deshpande, A.S. Corn husk derived activated carbon with enhanced electrochemical performance for high-voltage supercapacitors. J. Power Sources 2020, 471, 228387. [Google Scholar] [CrossRef]
- Chiu, Y.-H.; Lin, L.-Y. Effect of activating agents for producing activated carbon using a facile one-step synthesis with waste coffee grounds for symmetric supercapacitors. J. Taiwan Inst. Chem. Eng. 2019, 101, 177–185. [Google Scholar] [CrossRef]
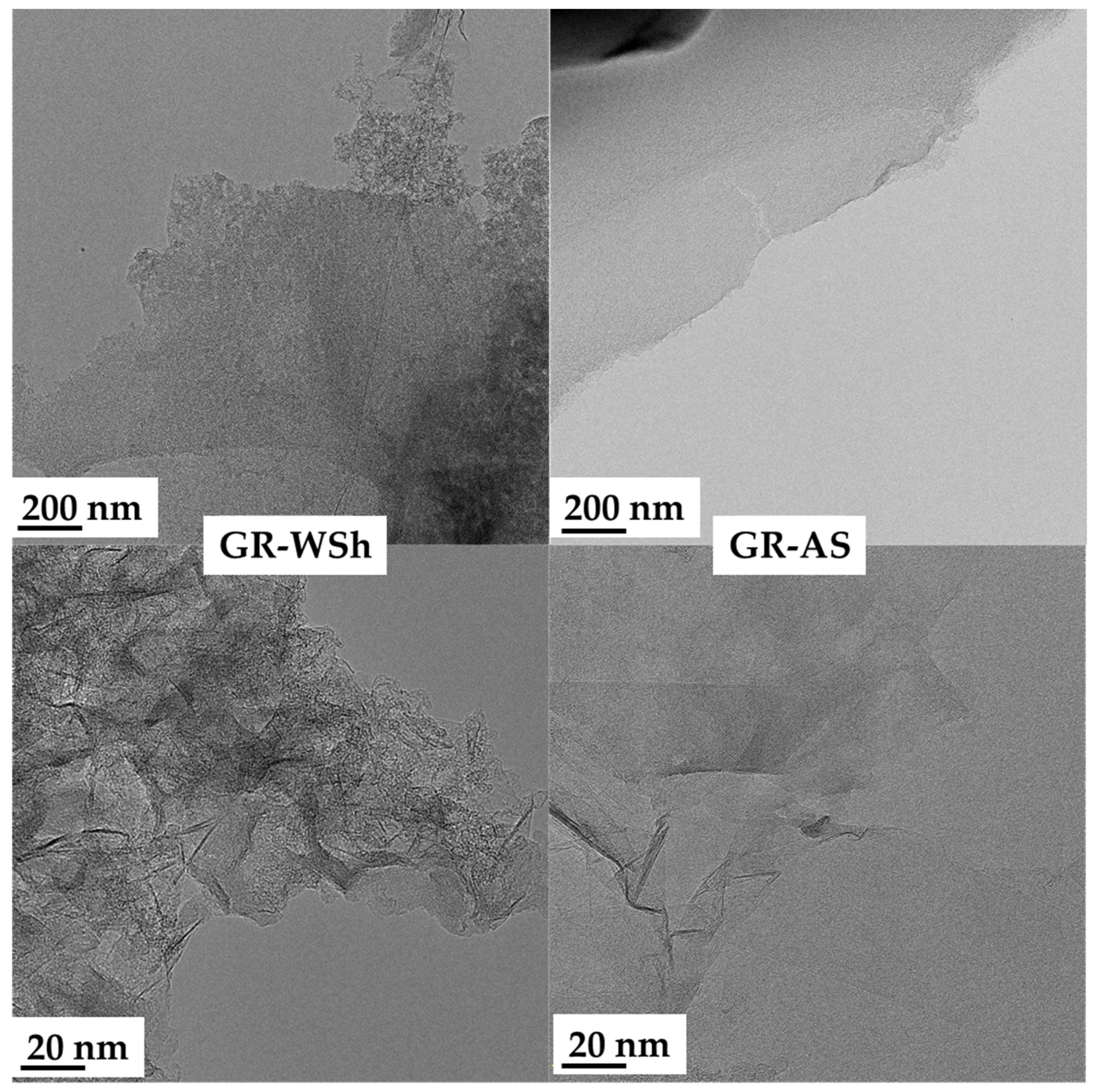

| Raw Material | Label of Biomass-derived Graphene | Mechanical Characteristics | Particle Size (SEM) | Yield, % |
|---|---|---|---|---|
| Apricot stones (AS) | Gr-AS | Aggregates and fragile fragments | ~600–90 nm | 3.1 |
| Walnut shells (WSh) | Gr-WSh | Aggregates and fragile fragments | ~700–85 nm | 2.9 |
| ID Sample | Ratio of Biomass/KOH, g/g | IG | IG | I2D | IG/I2D | ID/IG | Number of Layers |
|---|---|---|---|---|---|---|---|
| Gr-AS | 1/5 | 271 | 729 | 471 | 1.54 | 0.28 | 4–5 |
| Gr-WSh | 1/5 | 423 | 1462 | 712 | 2.05 | 0.37 | 4–5 |
| Biomass-Derived Graphene | Specific Surface Area, m2/g | Specific Pore Volume, cm3/g | Average Pore Size, nm |
|---|---|---|---|
| Gr-AS | 1358.680 | 0.058 | 25.321 |
| Gr-WSh | 1235.491 | 0.086 | 25.723 |
| Material Type | Carbonation Conditions | Specific Surface Area, m2/g | Ref |
|---|---|---|---|
| Biomass-derived graphene | 850 °C, 45 min, Ar | 350–450 | The present study |
| Coconut husk | 700 °C, 1.5 h, N2 | 250–300 | [17] |
| Corn biomass | 650 °C, 1 h, Ar | 220–310 | [18] |
| Phenol formaldehyde resin | 800 °C, 2 h, N2 | 350–400 | [19] |
| Modified cellulose precursor | 700 °C, 1 h, CO2 | 280–360 | [20,21] |
| Sample | Specific Surface Area, m2/g | IG/I2D Ratio | Carbon Content | Ref |
|---|---|---|---|---|
| Biomass-derived graphene | ~1300 | 1.61 ± 0.10 | 75 wt.% | The present study |
| Rice husk-derived graphene | 1001–1556 | 1.34 ± 0.10 | - | [27] |
| - | 0.9024 | - | [28] | |
| Onion husk-derived graphene | 1924 | 1.4 ± 0.10 | 98 wt.% | [10] |
| Maximum Heating Temperature | From 40 to 1200 °C |
|---|---|
| Power consumption | 3 × 2 kW = 6 kW (power of one wire: 2 kW, 220 V, R = 24.2 Ohm) |
| Maximum heating temperature | 1200 °C (<1 h) |
| Camera material | Aluminosilicate ceramics |
| Dimensions of the working chamber | OD 220 × 1D120 × 720 mm, |
| Chamber volume | 8138 cm3 |
| Resistance wire | HRE, diameter 1.5 mm, length 29.48 m. |
| Temperature controller block | automatic switch, is a short circuit protection; electromagnetic starter—switches heating elements on and off; double START-STOP button—manual heating control; programmable temperature controller—sets and maintains the required (can be specified, defined) temperature; power section on thyristors provides smooth regulation of heating power. |
| Temperature sensor | Thermocouple type K (chromel-alumel) |
| Stage | Conditions and Parameters | Description of the Process |
|---|---|---|
| Washing and drying | Repeated washing with distilled water; drying at 383 K, 1 h | Removal of impurities |
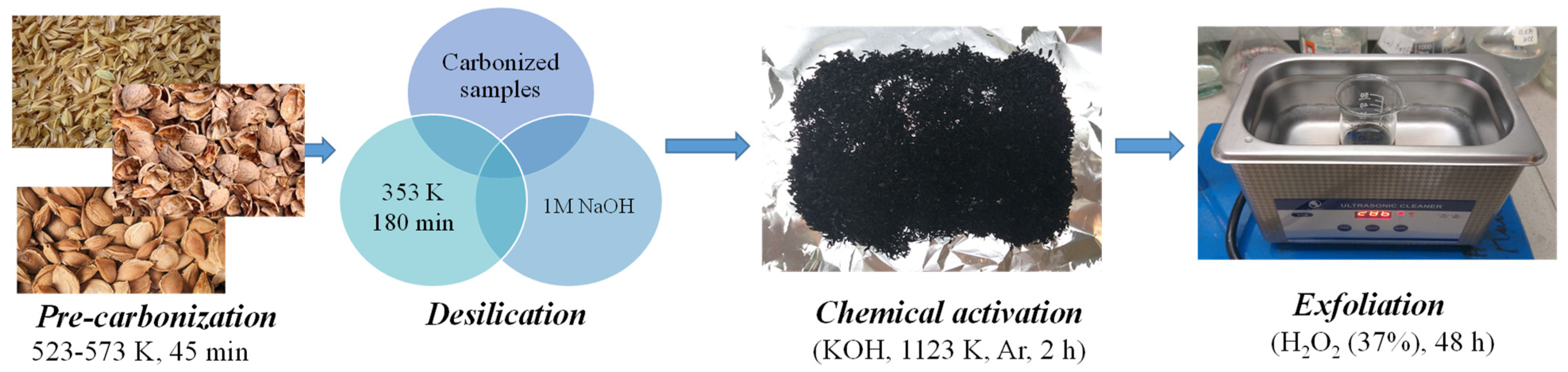
| Pre-carbonation | 523–573 K, 45 min, Ar, 5 cm3/min, rotating reactor | Initial stage of heat treatment |
| Desilication | 1 M NaOH (60 g in 3 L), 353 K, 3 h | Removal of silicon compounds |
| Washing and drying after desilication | Wash to neutral pH, dry at 383 K, 2 h | Preparing for activation |
| Chemical activation | Mixture with KOH (1:5), pressing, annealing in Ar at 1123 K, 2 h | Formation of a porous structure |
| Washing and drying of activated material | Wash to neutrality, dry at 373 K, 24 h | Removal of reagent residues |
| Exfoliation | Treatment 37% H2O2, 60 °C, continuous magnetic stirring at 400 rpm, 48 h, ratio of 1:10 (solid to liquid, w/v), wash-dry | Removal of amorphous carbon |
| Product yield | ~3% by weight | Final products: Gr-RH, Gr-WSh, Gr-AS |
| Method of Analysis | Device | Main Parameters | Location of the Event |
|---|---|---|---|
| SEM | Nova NanoSem 450 FEI/Termofisher (Waltham, MA, USA) | at 3.00 kV in high vacuum mode, using an Everhart Thornley Detector (ETD) and Through the Lens Detector (TLD) for details micrographs and elemental microanalysis (EDX) at 15.00 kV | “ACE” laboratory of University of Naples Federico II (Naples, Italy) |
| Elemental analysis | PRIMACS100 analyzer (Skalar Analytical B.V., Breda, The Netherlands) and CHN 628 LECO elemental analyzer (LECO Corporation, St. Joseph, MI, USA) | ASTM E870 procedure using EDTA as standard | “ACE” laboratory of University of Naples Federico II (Naples, Italy) |
| Raman spectroscopy | Jasco NRS-3100 (Jasco, Tokyo, Japan) | Laser 514 nm, spectral resolution 4 cm−1, accumulation time 30 s | National Open Nanotechnology Laboratory (Almaty, Kazakhstan) |
| Specific surface area analysis (BET method) | Sorbtometer-M (Thermo Fisher Scientific, Waltham, MA, USA) | Low temperature nitrogen adsorption, surface area range: 1–3000 m2/g | Institute of Combustion Problems (Almaty, Kazakhstan) |
| Transmission electron microscopy | HRTEM, FEI Talos F200X G2, USA (Thermo Fisher Scientific, Waltham, MA, USA) | at accelerating voltages between 200 kV and 300 kV, with the powder specimens dispersed onto copper grids coated with ultrathin carbon films | State Key Laboratory of Precision Blasting, Jianghan University (Wuhan, China) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seitzhanova, M.; Kudyarova, Z.; Rakhimova, B.; Tugelbayeva, L.; Tauanov, Z. Synthesis of Biomass-Derived Graphene Nanomaterials by Chemical Activation with KOH. Int. J. Mol. Sci. 2025, 26, 11255. https://doi.org/10.3390/ijms262311255
Seitzhanova M, Kudyarova Z, Rakhimova B, Tugelbayeva L, Tauanov Z. Synthesis of Biomass-Derived Graphene Nanomaterials by Chemical Activation with KOH. International Journal of Molecular Sciences. 2025; 26(23):11255. https://doi.org/10.3390/ijms262311255
Chicago/Turabian StyleSeitzhanova, Makpal, Zhanar Kudyarova, Bibigul Rakhimova, Lyaila Tugelbayeva, and Zhandos Tauanov. 2025. "Synthesis of Biomass-Derived Graphene Nanomaterials by Chemical Activation with KOH" International Journal of Molecular Sciences 26, no. 23: 11255. https://doi.org/10.3390/ijms262311255
APA StyleSeitzhanova, M., Kudyarova, Z., Rakhimova, B., Tugelbayeva, L., & Tauanov, Z. (2025). Synthesis of Biomass-Derived Graphene Nanomaterials by Chemical Activation with KOH. International Journal of Molecular Sciences, 26(23), 11255. https://doi.org/10.3390/ijms262311255






