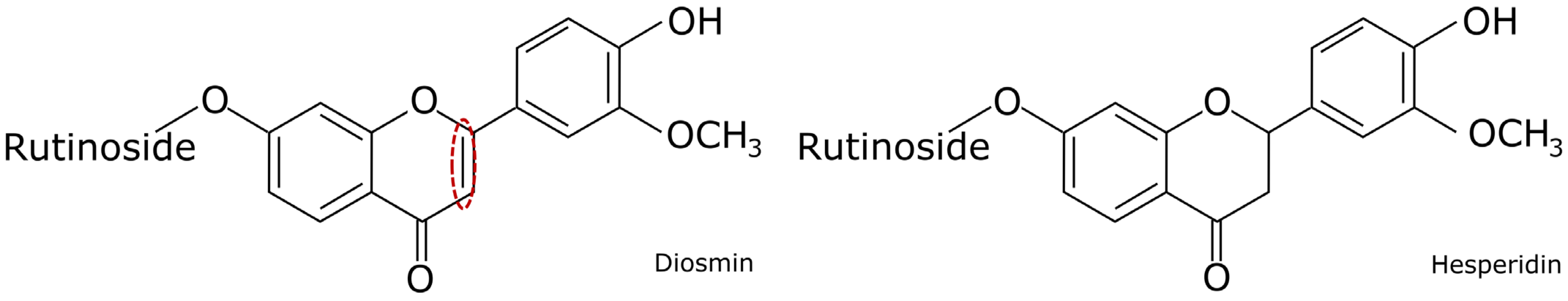
Diosmin or Hesperidin? Comparison of Antioxidative Action of Two Venoactive Flavonoids in Type 1 Diabetic Rats
Abstract
1. Introduction
2. Results
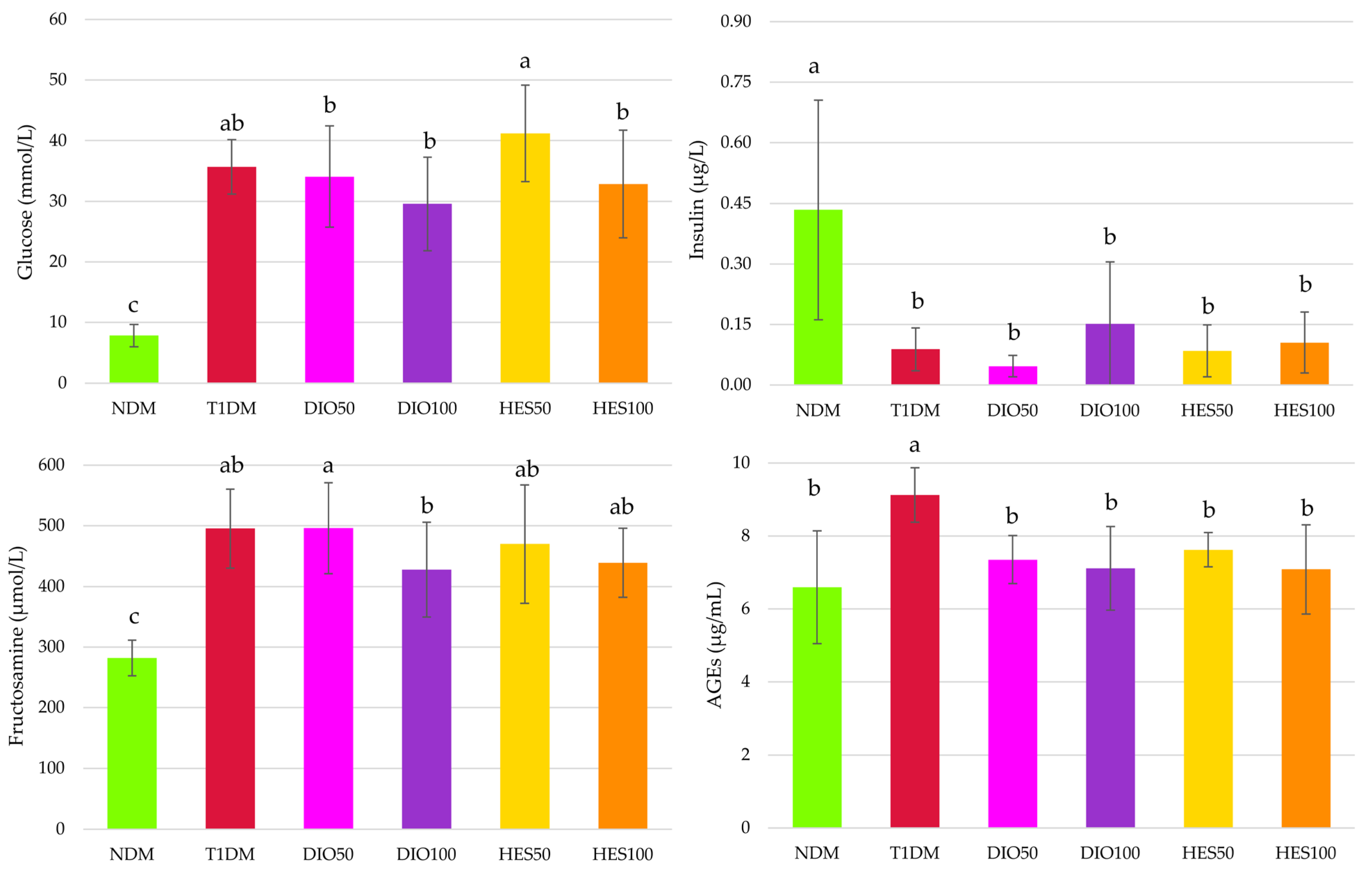
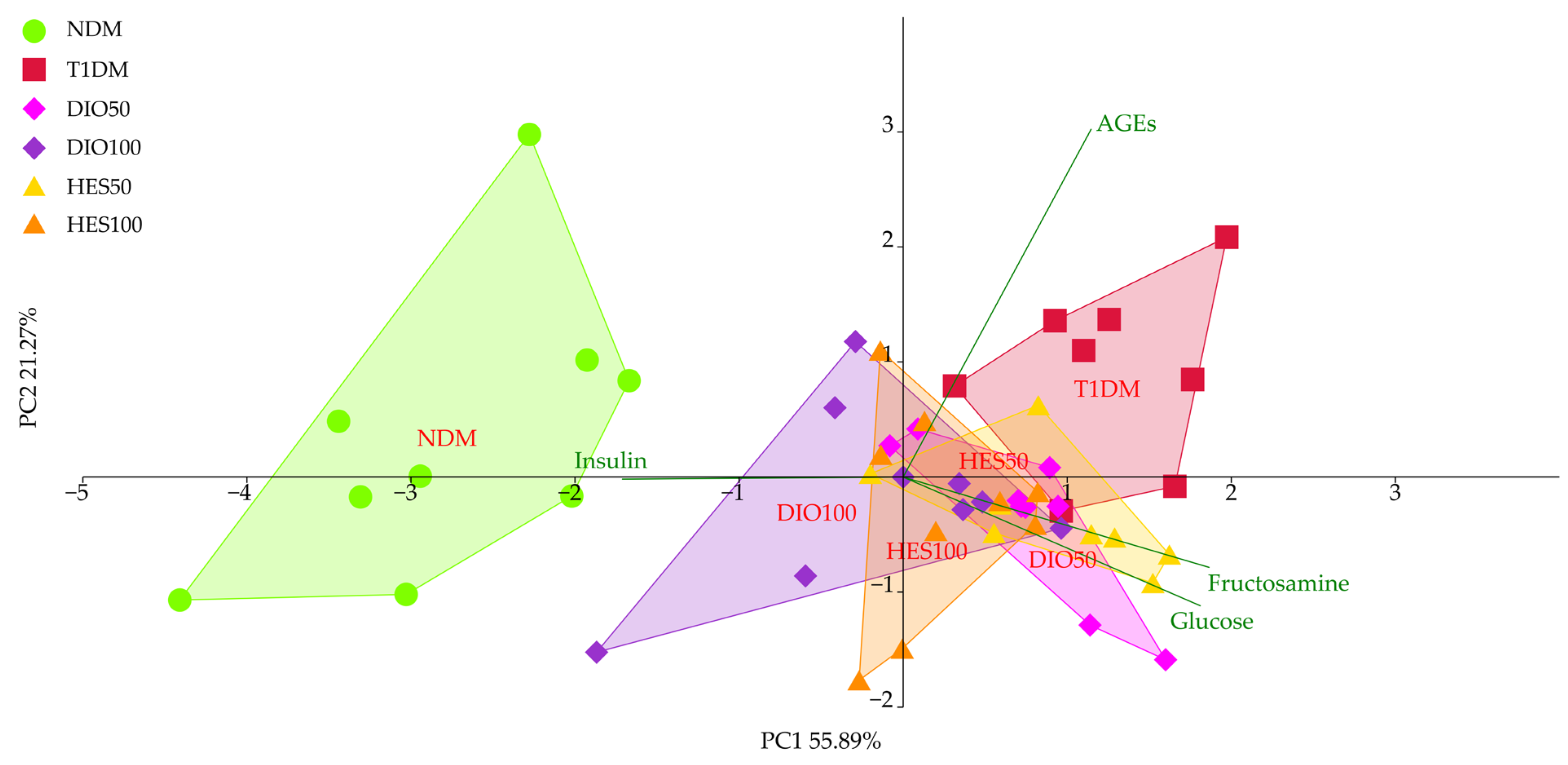
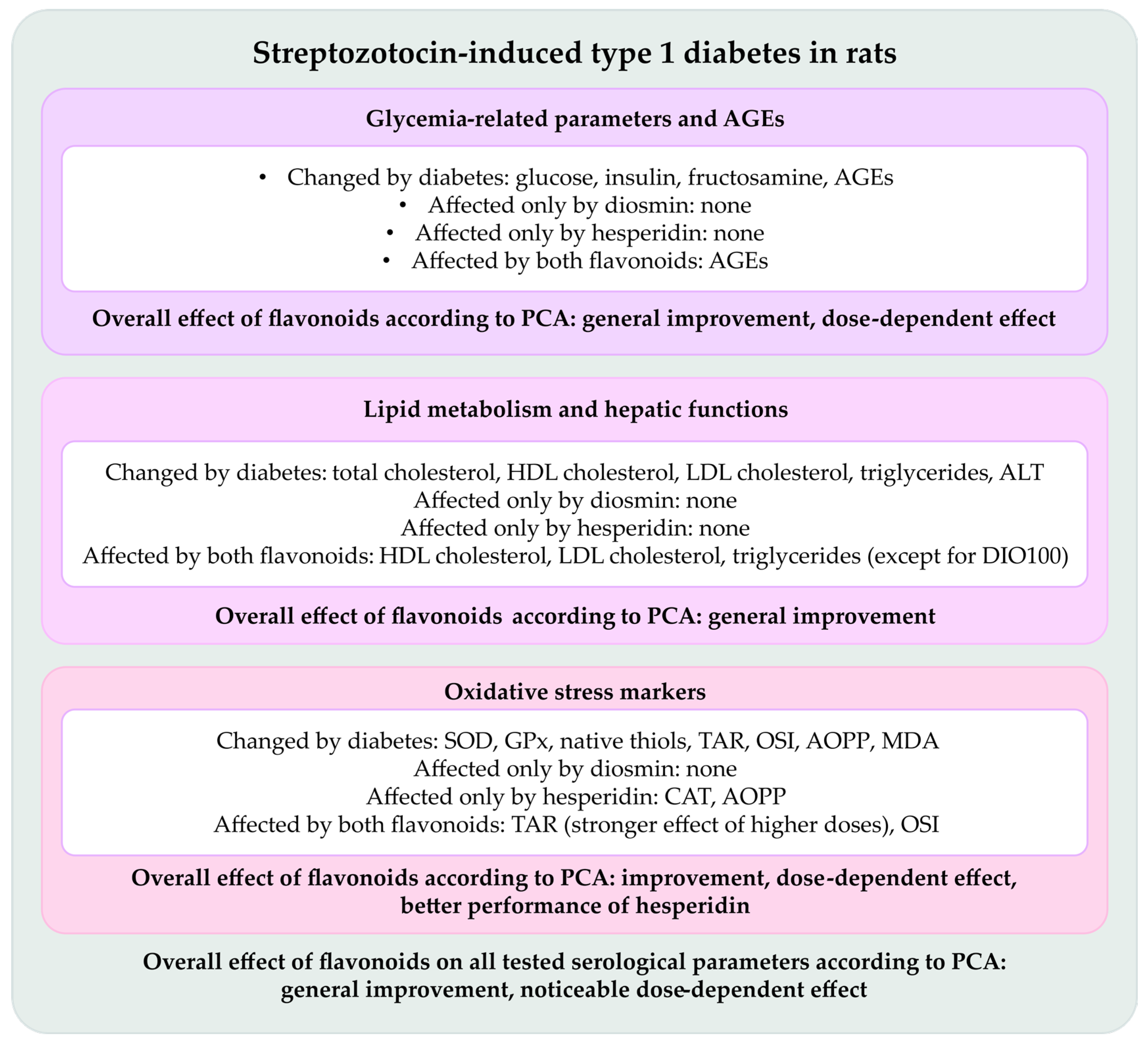
2.1. Effect of Tested Flavonoids on Carbohydrate Metabolism and AGEs Levels
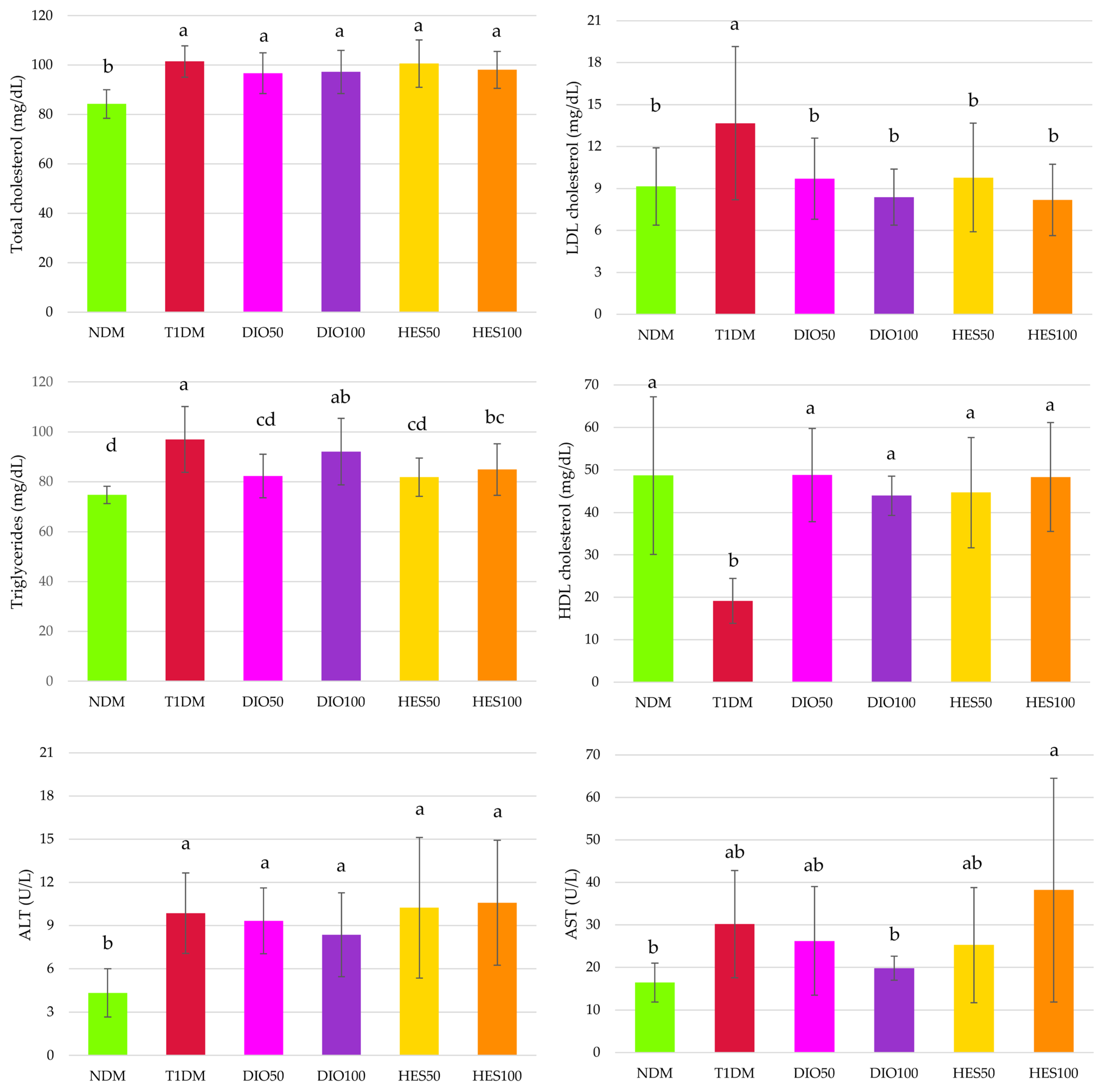
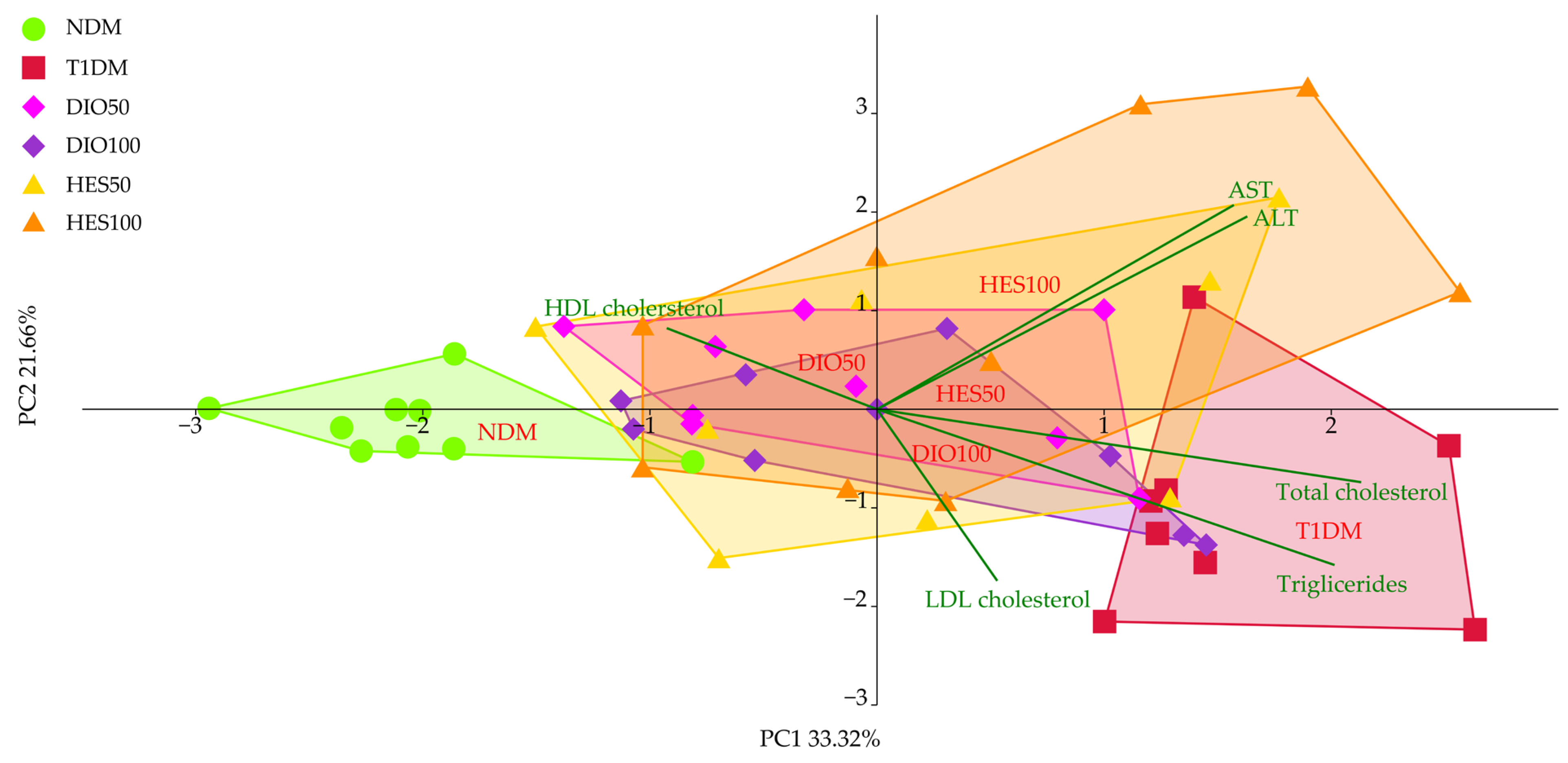
2.2. Effect of Tested Flavonoids on Lipid Metabolism and Hepatic Functions
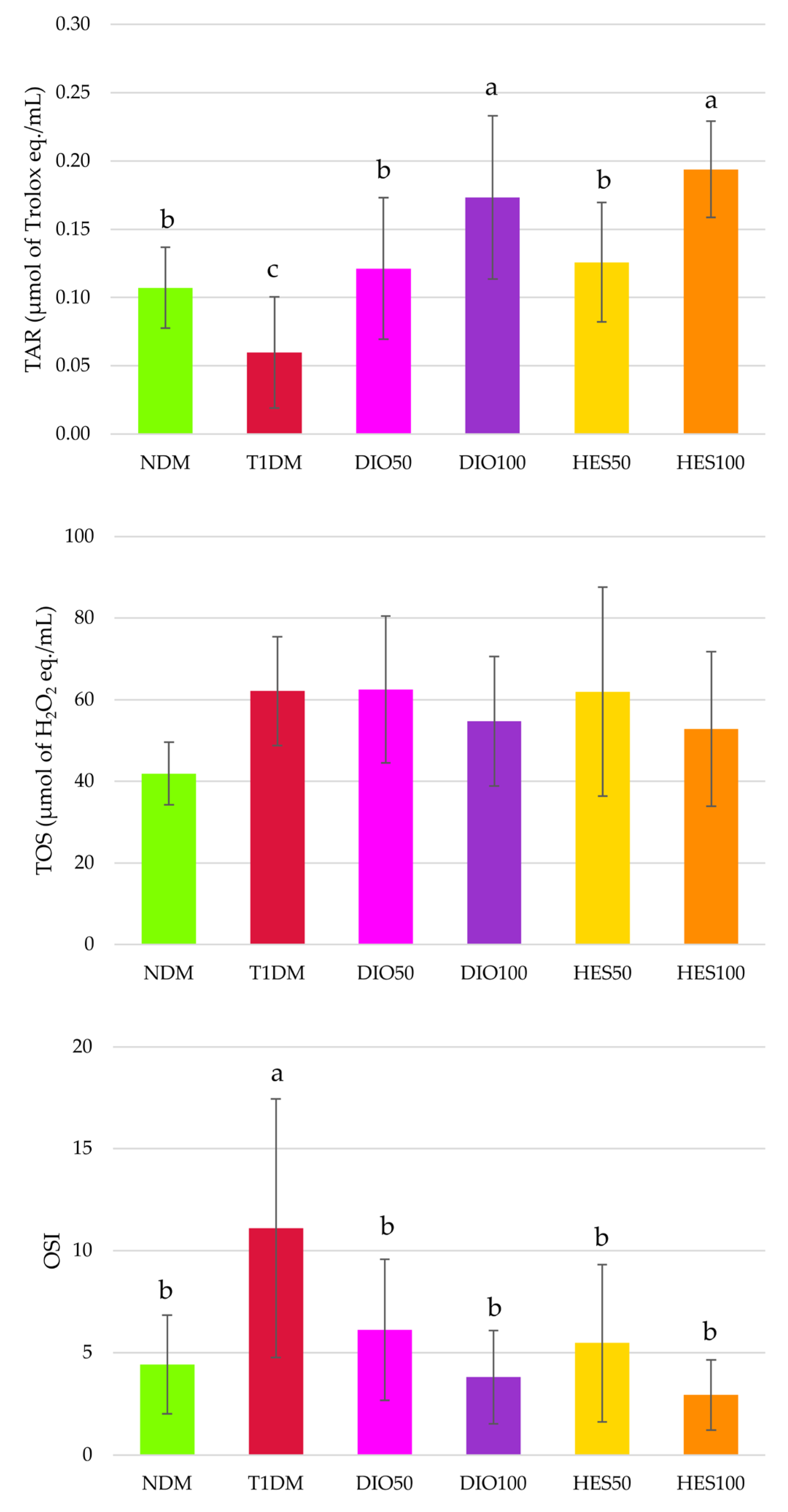
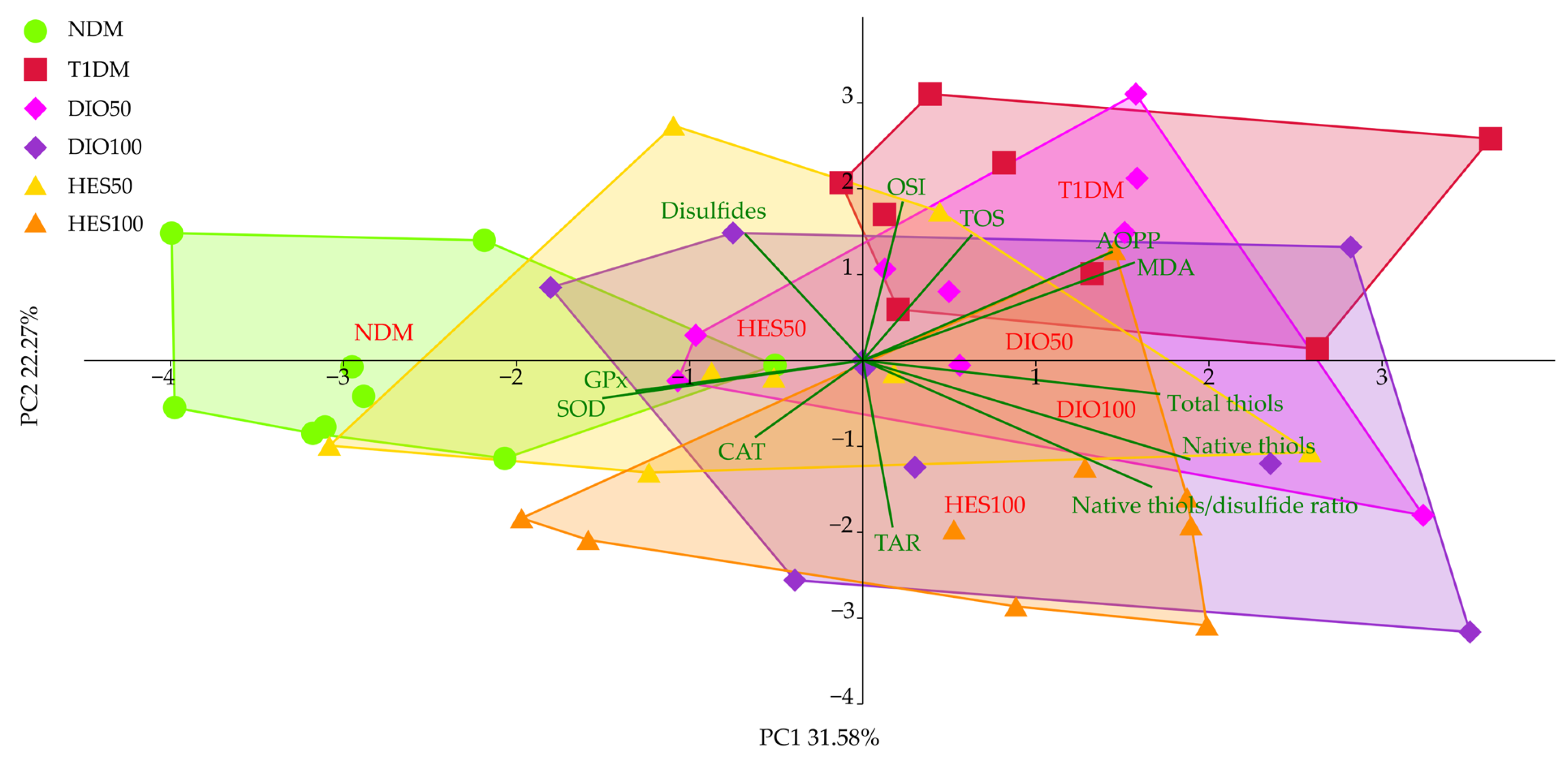
2.3. Effect of Tested Flavonoids on Oxidative Stress Markers
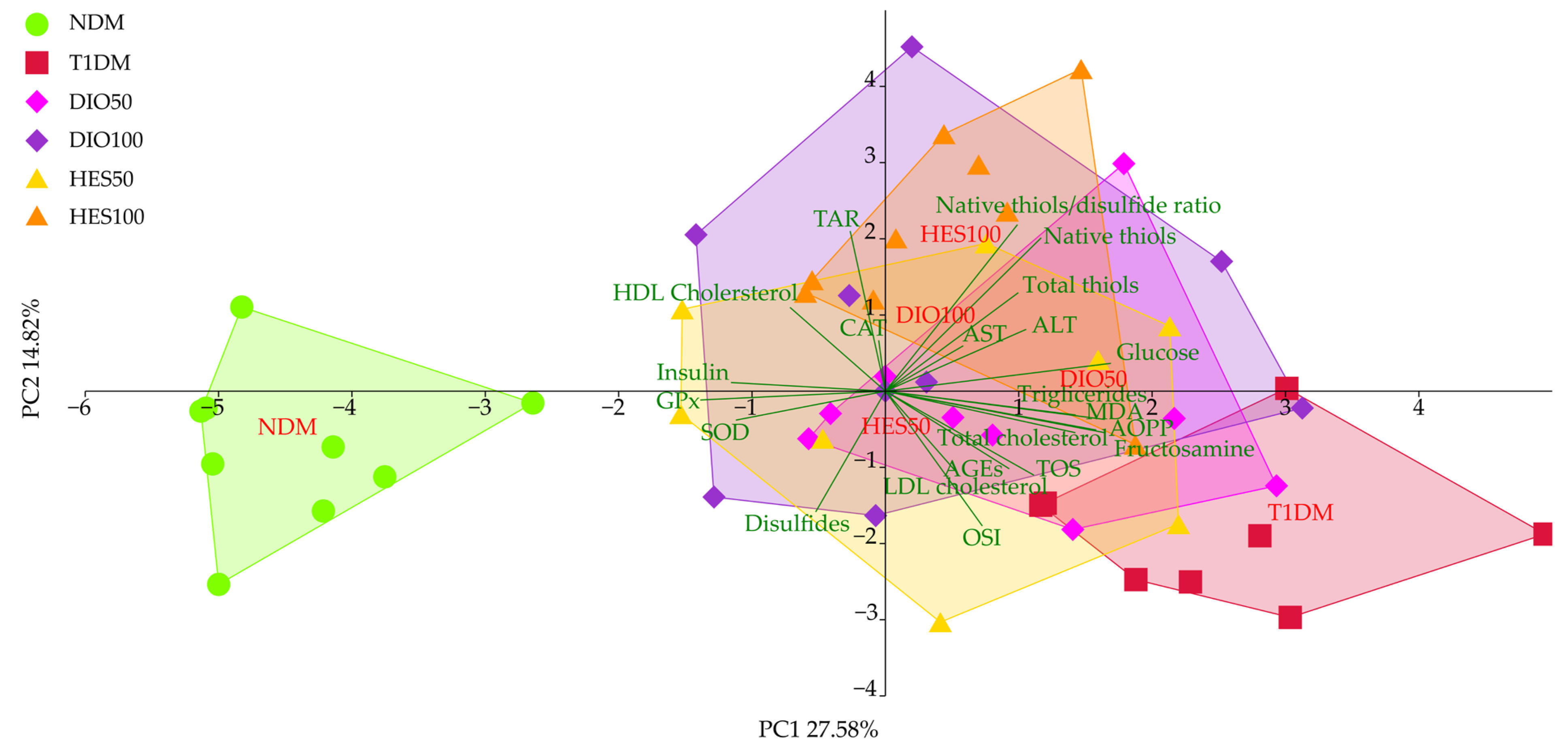
3. Discussion
4. Materials and Methods
4.1. Chemicals and Kits
4.2. Animals and Experimental Design
- NDM—control rats in which diabetes was not induced (nondiabetic rats)
- T1DM—control rats in which type 1 diabetes was induced
- DIO50—type 1 diabetic rats administered with diosmin at a dose of 50 mg/kg
- DIO100—type 1 diabetic rats administered with diosmin at a dose of 100 mg/kg
- HES50—type 1 diabetic rats administered with hesperidin at a dose of 50 mg/kg
- HES100—type 1 diabetic rats administered with hesperidin at a dose of 100 mg/kg
4.3. Carbohydrate Metabolism Markers and Advanced Glycation End-Product Analysis
4.4. Lipid Metabolism Markers Analysis
4.5. Antioxidative Enzymes Activity Analysis
4.6. Oxidative Damage Markers Analysis
4.7. Oxidative Stress Status Analysis
4.8. Thiols Content Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AGEs | Advanced glycation end products |
| ALT | Alanine transaminase |
| AOPP | Advanced oxidation protein products |
| AST | Asparagine transaminase |
| CAT | Catalase |
| CVD | Chronic venous disease |
| CVI | Chronic venous insufficiency |
| DIO50 | Type 1 diabetic rats treated with diosmin at a dose of 50 mg/kg |
| DIO100 | Type 1 diabetic rats treated with diosmin at a dose of 100 mg/kg |
| DM | Diabetes mellitus |
| GPx | Glutathione peroxidase |
| GSH | Glutathione |
| HDL | High-density lipoprotein |
| HES50 | Type 1 diabetic rats treated with hesperidin at a dose of 50 mg/kg |
| HES100 | Type 1 diabetic rats treated with hesperidin at a dose of 100 mg/kg |
| LDL | Low-density lipoprotein |
| MDA | Malondialdehyde |
| NDM | Nondiabetic control rats |
| OSI | Oxidative stress index |
| PC1 | Principal component 1 |
| PC2 | Principal component 2 |
| PCA | Principal component analysis |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| T1DM | Type 1 diabetic control rats |
| TAR | Total antioxidative response |
| TOS | Total oxidative status |
References
- Domingueti, C.P.; Dusse, L.M.S.; Carvalho, M.D.G.; De Sousa, L.P.; Gomes, K.B.; Fernandes, A.P. Diabetes mellitus: The linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J. Diabetes Complicat. 2016, 30, 738–745. [Google Scholar] [CrossRef]
- Guo, H.; Wu, H.; Li, Z. The pathogenesis of diabetes. Int. J. Mol. Sci. 2023, 24, 6978. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Sharma, N.; Arora, S.; Verma, S. Diabetes: A review of its pathophysiology, and advanced methods of mitigation. Curr. Med. Res. Opin. 2024, 40, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Templer, S.; Abdo, S.; Wong, T. Preventing diabetes complications. Intern. Med. J. 2024, 54, 1264–1274. [Google Scholar] [CrossRef] [PubMed]
- Gastaldi, G.; Pannier, F.; Roztočil, K.; Lugli, M.; Mansilha, A.; Haller, H.; Rabe, E.; Van Rijn, M.J. Chronic venous disease and diabetic microangiopathy: Pathophysiology and commonalities. Int. Angiol. 2021, 40, 457–469. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martínez, O.; García-Montero, C.; Álvarez-Mon, M.A.; Chaowen, C.; Ruiz-Grande, F.; Pekarek, L.; Monserrat, J.; Asúnsolo, A.; García-Honduvilla, N.; et al. Understanding chronic venous disease: A critical overview of its pathophysiology and medical management. J. Clin. Med. 2021, 10, 3239. [Google Scholar] [CrossRef]
- Castro-Ferreira, R.; Cardoso, R.; Leite-Moreira, A.; Mansilha, A. The role of endothelial dysfunction and inflammation in chronic venous disease. Ann. Vasc. Surg. 2018, 46, 380–393. [Google Scholar] [CrossRef]
- González, P.; Lozano, P.; Ros, G.; Solano, F. Hyperglycemia and oxidative stress: An integral, updated and critical overview of their metabolic interconnections. Int. J. Mol. Sci. 2023, 24, 9352. [Google Scholar] [CrossRef]
- Black, H.S. A synopsis of the associations of oxidative stress, ROS, and antioxidants with diabetes mellitus. Antioxidants 2022, 11, 2003. [Google Scholar] [CrossRef]
- Jarošíková, R.; Roztočil, K.; Husáková, J.; Dubský, M.; Bém, R.; Wosková, V.; Fejfarová, V. Chronic venous disease and its intersections with diabetes mellitus. Physiol. Res. 2023, 72, 280–286. [Google Scholar] [CrossRef]
- Okley, D.V. Systemic phlebotropic drugs in pharmacotherapy of chronic venous insufficiency of the lower extremities. News Farm. 2015, 4, 74–77. [Google Scholar] [CrossRef]
- Chaitidis, N.; Kokkinidis, D.G.; Papadopoulou, Z.; Kyriazopoulou, M.; Schizas, D.; Bakoyiannis, C. Treatment of chronic venous disorder: A comprehensive review. Dermatol. Ther. 2022, 35, e15238. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, R.E.; Ali, D.E.; El-Shiekh, R.A.; El-Alfy, A.N.; Abd El Hafeez, M.S.; Reda, A.M.; Fayek, N.M. Therapeutic applications of natural products in the management of venous diseases: A comprehensive review. Inflammopharmacology 2025, 33, 1673–1712. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Zapata, M.J.; Vernooij, R.W.; Simancas-Racines, D.; Uriona Tuma, S.M.; Stein, A.T.; Moreno Carriles, R.M.M.; Vargas, E.; Bonfill Cosp, X. Phlebotonics for venous insufficiency. Cochrane Database Syst. Rev. 2020, 3, CD003229. [Google Scholar] [CrossRef] [PubMed]
- Gama, C.R.B.; Nunes, C.P.; Steinbruch, M.; Suchmacher, M.; Gama, G.F.; Kaufman, R.; Mezitis, S.; Sitnoveter, A.L.; Ezri, T.G.B.; Daher, J.P.L.; et al. Comparative efficacy and safety of a horse chestnut formulation vs. diosmin-hesperidin in chronic venous insufficiency: Randomized double-blind trial. Int. J. Clin. Med. 2025, 16, 279–292. [Google Scholar] [CrossRef]
- Roy, J.; Azamthulla, M.; Mukkerjee, D. Hesperidin and diosmin—A novel drugs. Int. J. Pharm. Res. Technol. 2020, 10, 25–33. [Google Scholar]
- Szymański, M.; Młynarek, D.; Szymański, A.; Matławska, I. Simultaneous determination of diosmin and hesperidin in pharmaceuticals by RPLC using ionic liquids as mobile phase modifiers. Iran. J. Pharm. Res. 2016, 15, 141–148. [Google Scholar]
- Borko, Y.; Kovalevska, I. Studies of physico-chemical and pharmaco-technological parameters of bioflavonoids diosmin and hesperidin. Sci. J. ScienceRise Pharm. Sci. 2019, 5, 42–46. [Google Scholar] [CrossRef]
- Hassan, M.A.; Elmageed, G.M.A.; El-Qazaz, I.G.; El-Sayed, D.S.; El-Samad, L.M.; Abdou, H.M. The synergistic influence of polyflavonoids from Citrus aurantifolia on diabetes treatment and their modulation of the PI3K/AKT/FOXO1 signaling pathways: Molecular docking analyses and in vivo investigations. Pharmaceutics 2023, 15, 2306. [Google Scholar] [CrossRef]
- Cova, D.; De Angelis, L.; Giavarini, F.; Palladini, G.; Perego, R. Pharmacokinetics and metabolism of oral diosmin in healthy volunteers. Int. J. Clin. Parmacol. Toxicol. 1992, 30, 29–33. [Google Scholar]
- Kanaze, F.I.; Bounartzi, M.I.; Georgarakis, M.; Niopas, I. Pharmacokinetics of the citrus flavanone aglycones hesperetin and naringenin after single oral administration in human subjects. Eur. J. Clin. Nutr. 2007, 61, 472–477. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Mustafa, S.; Akbar, M.; Khan, M.A.; Sunita, K.; Parveen, S.; Pawar, J.S.; Massey, S.; Agarwal, N.R.; Husain, S.A. Plant metabolite diosmin as the therapeutic agent in human diseases. Curr. Res. Pharmacol. Drug Discov. 2022, 3, 100122. [Google Scholar] [CrossRef] [PubMed]
- Huwait, E.; Mobashir, M. Potential and therapeutic roles of diosmin in human diseases. Biomedicines 2022, 10, 1076. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, A.; Mirzaei, A.; Khalilabad, S.N.; Askari, V.R.; Rahimi, V.B. Promising influences of hesperidin and hesperetin against diabetes and its complications: A systematic review of molecular, cellular, and metabolic effects. EXCLI J. 2023, 22, 1235–1263. [Google Scholar] [PubMed]
- Rajasekar, M.; Baskaran, P.; Mary, J.; Sivakumar, M.; Selvam, M. Revisiting diosmin for their potential biological properties and applications. Carbohydr. Polym. Technol. Appl. 2024, 7, 100419. [Google Scholar] [CrossRef]
- Li, C.; Schluesener, H. Health-promoting effects of the citrus flavanone hesperidin. Crit. Rev. Food Sci. Nutr. 2017, 57, 613–631. [Google Scholar] [CrossRef]
- Vinayagam, R.; Xu, B. Antidiabetic properties of dietary flavonoids: A cellular mechanism review. Nutr. Metab. 2015, 12, 60. [Google Scholar] [CrossRef]
- Gerges, S.H.; Wahdan, S.A.; Elsherbiny, D.A.; El-Demerdash, E. Pharmacology of diosmin, a citrus flavone glycoside: An updated review. Eur. J. Drug Metab. Pharmacokinet. 2022, 47, 1–18. [Google Scholar] [CrossRef]
- Wdowiak, K.; Walkowiak, J.; Pietrzak, R.; Bazan-Woźniak, A.; Cielecka-Piontek, J. Bioavailability of hesperidin and its aglycone hesperetin—Compounds found in citrus fruits as a parameter conditioning the pro-health potential (neuroprotective and antidiabetic activity)—Mini-review. Nutrients 2022, 14, 2647. [Google Scholar] [CrossRef]
- Otto-Buczkowska, E.; Jainta, N. Pharmacological treatment in diabetes mellitus type 1—Insulin and what else ? Int. J. Endocrinol. Metab. 2018, 16, e13008. [Google Scholar] [CrossRef]
- Lefever, E.; Vliebergh, J.; Mathieu, C. Improving the treatment of patients with diabetes using insulin analogues: Current findings and future directions. Expert Opin. Drug Saf. 2021, 20, 155–170. [Google Scholar] [CrossRef]
- Dahiru, M.M.; Nadro, M.S. A review of the mechanisms of action and side effects of anti-diabetic agents. Trends Pharmacol. Sci. 2022, 8, 195–210. [Google Scholar]
- Dilworth, L.; Stennett, D.; Facey, A.; Omoruyi, F.; Mohansingh, S.; Omoruyi, F.O. Diabetes and the associated complications: The role of antioxidants in diabetes therapy and care. Biomed. Pharmacother. 2024, 181, 117641. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, X.; Cao, S.; Duan, Y.; Xu, C.; Gan, D.; He, W. Dietary antioxidant indices in relation to all-cause and cause-specific mortality among adults with diabetes: A prospective cohort study. Front. Nutr. 2022, 9, 849727. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.C.; Lin, M.H.; Cheng, J.T.; Wu, M.C. Diosmin, a citrus nutrient, activates imidazoline receptors to alleviate blood glucose and lipids in type 1-like diabetic rats. Nutrients 2017, 9, 684. [Google Scholar] [CrossRef]
- Hsu, C.C.; Lin, M.H.; Cheng, J.T.; Wu, M.C. Antihyperglycaemic action of diosmin, a citrus flavonoid, is induced through endogenous β-endorphin in type I-like diabetic rats. Clin. Exp. Pharmacol. Physiol. 2017, 44, 549–555. [Google Scholar]
- Akiyama, S.; Katsumata, S.; Suzuki, K.; Ishimi, Y.; Wu, J.; Uehara, M. Hypoglycemic and hypolipidemic effects dietary hesperidin exerts in streptozotocin-induced marginal type 1 diabetic rats. J. Clin. Biochem. Nutr. 2010, 46, 87–92. [Google Scholar] [CrossRef]
- Shehata, A.S.; Mohamed, D.A.; Hagras, S.M.; El-Beah, S.M.; Elnegris, H.M. The role of hesperidin in ameliorating retinal changes in rats with experimentally induced type 1 diabetes mellitus and the active role of vascular endothelial growth factor and glial fibrillary acidic protein. Anat. Cell Biol. 2021, 54, 456–478. [Google Scholar] [CrossRef]
- Shehata, A.S.; Amer, M.G.; Abd El-Haleem, M.R.; Karam, R.A. The ability of hesperidin compared to that of insulin for preventing osteoporosis induced by type I diabetes in young male albino rats: A histological and biochemical study. Exp. Toxicol. Pathol. 2017, 69, 203–212. [Google Scholar] [CrossRef]
- Kandemir, F.M.; Ozkaraca, M.; Küçükler, S.; Caglayan, C.; Hanedan, B. Preventive effects of hesperidin on diabetic nephropathy induced by streptozotocin via modulating TGF-β1 and oxidative DNA damage. Toxin Rev. 2018, 37, 287–293. [Google Scholar]
- Keenoy, B.M.; Vetrmmen, J.; de Leeuw, I. The effect of flavonoid treatment on the glycation and antioxidant status in type 1 diabetic patients. Diabetes Nutr. Metab. 1999, 12, 256–263. [Google Scholar]
- Carballo-Villalobos, A.I.; González-Trujano, M.-E.; Pellicer, F.; López-Muñoz, F.J. Antihyperalgesic effect of hesperidin improves with diosmin in experimental neuropathic pain. BioMed Res. Int. 2016, 2016, 8263463. [Google Scholar] [CrossRef]
- Yang, M.; Tanaka, T.; Hirose, Y.; Deguchi, T.; Mori, H.; Kawada, Y. Chemopreventive effects of diosmin and hesperidin on n-butyl-n-(4-hydroxybutyl) nitrosamine-induced urinary-bladder carcinogenesis in male ICR mice. Int. J. Cancer 1997, 73, 719–724. [Google Scholar] [CrossRef]
- Tanaka, T.; Makita, H.; Kawabata, K.; Mori, H.; Kakumoto, M.; Satoh, K.; Hara, A.; Sumida, T.; Tanaka, T.; Ogawa, H. Chemoprevention of azoxymethane-induced rat colon carcinogenesis by the naturally occurring flavonoids, diosmin and hesperidin. Carcinogenesis 1997, 18, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Makita, H.; Kawabata, K.; Mori, H.; Kakumoto, M.; Satoh, K.; Hara, A.; Sumida, T.; Fukutani, K.; Tanaka, T.; et al. Modulation of N-methyl-N-amylnitrosamine-induced rat oesophageal tumourigenesis by dietary feeding of diosmin and hesperidin, both alone and in combination. Carcinogenesis 1997, 18, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Makita, H.; Ohnishi, M.; Mori, H.; Satoh, K.; Hara, A.; Sumida, T.; Fukutani, K.; Tanaka, T.; Ogawa, H. Chemoprevention of 4-nitroquinoline 1-oxide-induced oral carcinogenesis in rats by flavonoids diosmin and hesperidin, each alone and in combination. Cancer Res. 1997, 57, 246–252. [Google Scholar] [PubMed]
- Elhelaly, A.E.; AlBasher, G.; Alfarraj, S.; Almeer, R.; Bahbah, E.I.; Fouda, M.M.A.; Bungău, S.G.; Aleya, L.; Abdel-Daim, M.M. Protective effects of hesperidin and diosmin against acrylamide-induced liver, kidney, and brain oxidative damage in rats. Environ. Sci. Pollut. Res. 2019, 26, 35151–35162. [Google Scholar] [CrossRef]
- Bednarska, K.; Fecka, I. Potential of vasoprotectives to inhibit non-enzymatic protein glycation, and reactive carbonyl and oxygen species uptake. Int. J. Mol. Sci. 2021, 22, 10026. [Google Scholar] [CrossRef]
- Pinna, C.; Sala, A. Sex-specific activity of hesperidin, diosmin and genistein on human umbilical vein. Biomed. Res. Clin. Pract. Res. 2019, 4, 1–5. [Google Scholar] [CrossRef]
- Osama, H.; Hamed, E.O.; Mahmoud, M.A.; Abdelrahim, M.E.A. The effect of hesperidin and diosmin individually or in combination on metabolic profile and neuropathy among diabetic patients with metabolic syndrome: A randomized controlled trial. J. Diet. Suppl. 2023, 20, 749–762. [Google Scholar] [CrossRef]
- Borymska, W.; Borymski, S.; Zych, M.; Kaczmarczyk-Żebrowska, I. Porównanie potencjału antyoksydacyjnego dwóch flawonoidów flebotropowych—Diosminy i hesperydyny—W przebiegu cukrzycy typu 1. In Proceedings of the 7 Śląskie Farmaceutyczne Spotkanie Naukowe “Od Nauki do Pacjenta”, Sosnowiec, Poland, 21–28 November 2024; p. 91. [Google Scholar]
- Issaq, H.J.; Xiao, Z.; Veenstra, T.D. Serum and plasma proteomics. Chem. Rev. 2007, 107, 3601–3620. [Google Scholar] [CrossRef]
- Liu, L.; Aa, J.; Wang, G.; Yan, B.; Zhang, Y.; Wang, X.; Zhao, C.; Cao, B.; Shi, J.; Li, M.; et al. Differences in metabolite profile between blood plasma and serum. Anal. Biochem. 2010, 406, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Szkudelski, T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol. Res. 2001, 50, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Lenzen, S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 2008, 51, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Klasik-Ciszewska, S.; Londzin, P.; Grzywnowicz, K.; Borymska, W.; Zych, M.; Kaczmarczyk-Żebrowska, I.; Folwarczna, J. Effect of chrysin, a flavonoid present in food, on the skeletal system in rats with experimental type 1 diabetes. Nutrients 2025, 17, 316. [Google Scholar] [CrossRef]
- Wojnar, W.; Kaczmarczyk-Sedlak, I.; Zych, M. Diosmin ameliorates the effects of oxidative stress in lenses of streptozotocin-induced type 1 diabetic rats. Pharmacol. Rep. 2017, 69, 995–1000. [Google Scholar] [CrossRef]
- Sundaram, R.; Nandhakumar, E.; Haseena Banu, H. Hesperidin, a citrus flavonoid ameliorates hyperglycemia by regulating key enzymes of carbohydrate metabolism in streptozotocin-induced diabetic rats. Toxicol. Mech. Methods 2019, 29, 644–653. [Google Scholar] [CrossRef]
- Pari, L.; Srinivasan, S. Antihyperglycemic effect of diosmin on hepatic key enzymes of carbohydrate metabolism in streptozotocin-nicotinamide-induced diabetic rats. Biomed. Pharmacother. 2010, 64, 477–481. [Google Scholar] [CrossRef]
- Malmström, H.; Walldius, G.; Grill, V.; Jungner, I.; Gudbjörnsdottir, S.; Hammar, N. Fructosamine is a useful indicator of hyperglycaemia and glucose control in clinical and epidemiological studies—Cross-sectional and longitudinal experience from the AMORIS cohort. PLoS ONE 2014, 9, e111463. [Google Scholar] [CrossRef]
- Shams-Rad, S.; Mohammadi, M.; Ramezani-Jolfaie, N.; Zarei, S.; Mohsenpour, M.; Salehi-Abargouei, A. Hesperidin supplementation has no effect on blood glucose control: A systematic review and meta-analysis of randomized controlled clinical trials. Br. J. Clin. Pharmacol. 2020, 86, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Sakasai-Sakai, A.; Takata, T.; Takino, J.; Koriyama, Y. Effects of toxic AGEs (TAGE) on human health. Cells 2022, 11, 2178. [Google Scholar] [CrossRef] [PubMed]
- Kuzan, A. Toxicity of advanced glycation end products (Review). Biomed. Rep. 2021, 14, 46. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Petroianu, G.; Adem, A. Advanced glycation end products and diabetes mellitus: Mechanisms and perspectives. Biomolecules 2022, 12, 542. [Google Scholar] [CrossRef]
- Hafizur, R.M.; Momin, S.; Fatima, N. Prevention of advanced glycation end-products formation in diabetic rats through beta-cell modulation by Aegle marmelos. BMC Complement. Altern. Med. 2017, 17, 227. [Google Scholar] [CrossRef]
- Li, L.; Song, Q.; Zhang, X.; Yan, Y.; Wang, X. Allicin alleviates diabetes mellitus by inhibiting the formation of advanced glycation end products. Molecules 2022, 27, 8793. [Google Scholar] [CrossRef]
- El-Fawal, R.; El Fayoumi, H.M.; Mahmoud, M.F. Effects of diosmin and crocin on metabolic syndrome-associated cardio-vascular complications in rats. Naunyn. Schmiedebergs. Arch. Pharmacol. 2019, 392, 1523–1536. [Google Scholar] [CrossRef]
- Patil, K.K.; Meshram, R.J.; Dhole, N.A.; Gacche, R.N. Role of dietary flavonoids in amelioration of sugar induced cataractogenesis. Arch. Biochem. Biophys. 2016, 593, 1–11. [Google Scholar] [CrossRef]
- Khan, M.S.; Rehman, M.T.; Ismael, M.A.; AlAjmi, M.F.; Alruwaished, G.I.; Alokail, M.S.; Khan, M.R. Bioflavonoid (hesperidin) restrains protein oxidation and advanced glycation end product formation by targeting AGEs and glycolytic enzymes. Cell Biochem. Biophys. 2021, 79, 833–844. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Xu, G.; Wei, D. Hesperidin exerts the gestational diabetes mellitus via AGEs-RAGE signalling pathway. Int. J. Pharmacol. 2019, 15, 604–615. [Google Scholar] [CrossRef]
- Shi, X.; Liao, S.; Mi, H.; Guo, C.; Qi, D.; Li, F.; Zhang, C.; Yang, Z. Hesperidin prevents retinal and plasma abnormalities in streptozotocin-induced diabetic rats. Molecules 2012, 17, 12868–12881. [Google Scholar] [CrossRef]
- Lee, J.; Yun, J.S.; Ko, S.H. Advanced glycation end products and their effect on vascular complications in type 2 diabetes mellitus. Nutrients 2022, 14, 3086. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.Y.; Kim, Y.S. The role of advanced glycation end products in diabetic vascular complications. Diabetes Metab. J. 2018, 42, 188–195. [Google Scholar] [CrossRef]
- Bierhansl, L.; Conradi, L.-C.; Treps, L.; Dewerchin, M.; Carmeliet, P. Central role of metabolism in endothelial cell function and vascular disease. Physiology 2017, 32, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.; Nakamura, N.; Suematsu, M.; Kaseda, K.; Matsui, T. Advanced glycation end products: A molecular target for vascular complications in diabetes. Mol. Med. 2015, 21, S32–S40. [Google Scholar] [CrossRef] [PubMed]
- Vergès, B. Dyslipidemia in type 1 diabetes: A masked danger. Trends Endocrinol. Metab. 2020, 31, 422–434. [Google Scholar] [CrossRef]
- Athyros, V.G.; Doumas, M.; Imprialos, K.P.; Stavropoulos, K.; Georgianou, E.; Katsimardou, A.; Karagiannis, A. Diabetes and lipid metabolism. Hormones 2018, 17, 61–67. [Google Scholar] [CrossRef]
- Haffar, S.; Izzy, M.; Habib, H.; Sugihara, T.; Li, D.K.; Sharma, A.; Wang, Z.; Murad, M.H.; Watt, K.D.; Bazerbachi, F. Liver chemistries in glycogenic hepatopathy associated with type 1 diabetes mellitus: A systematic review and pooled analysis. Liver Int. 2021, 41, 1545–1555. [Google Scholar] [CrossRef]
- Ozkol, H.; Tuluce, Y.; Dilsiz, N.; Koyuncu, I. Therapeutic potential of some plant extracts used in turkish traditional medicine on streptozocin-induced type 1 diabetes mellitus in rats. J. Membr. Biol. 2013, 246, 47–55. [Google Scholar] [CrossRef]
- Komeili, G.; Hashemi, M.; Bameri-Niafar, M. Evaluation of antidiabetic and antihyperlipidemic effects of peganum harmala seeds in diabetic rats. Cholesterol 2016, 2016, 7389864. [Google Scholar] [CrossRef]
- Ahmadvand, H.; Ghasemi-Dehnoo, M. Antiatherogenic, hepatoprotective, and hypolipidemic effects of coenzyme Q10 in alloxan-induced type 1 diabetic rats. ARYA Atheroscler. 2014, 10, 192–198. [Google Scholar] [PubMed]
- Srinivasan, S.; Pari, L. Antihyperlipidemic effect of diosmin: A citrus flavonoid on lipid metabolism in experimental diabetic rats. J. Funct. Foods 2013, 5, 484–492. [Google Scholar] [CrossRef]
- Liu, C.; Hao, S.; Zhang, M.; Wang, X.; Chu, B.; Wen, T.; Dang, R.; Sun, H. Natural diosmin alleviating obesity and nonalcoholic fatty liver disease by regulating the activating the AMP-activated protein kinase (AMPK) pathway. Chin. J. Nat. Med. 2025, 23, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Yıldızhan, K.; Bayir, M.H.; Huyut, Z.; Altındağ, F. Effect of hesperidin on lipid profile, inflammation and apoptosis in experimental diabetes. Dokl. Biochem. Biophys. 2025, 521, 198–205. [Google Scholar] [CrossRef]
- Ahmed, O.M.; Mahmoud, A.M.; Abdel-Moneim, A.; Ashour, M.B. Antidiabetic effects of hesperidin and naringin in type 2 diabetic rats. Diabetol. Croat. 2012, 41, 53–67. [Google Scholar]
- Wu, C.-H.; Lin, J.-A.; Hsieh, W.-C.; Yen, G.-C. Low-Density-Lipoprotein (LDL)-bound flavonoids increase the resistance of LDL to oxidation and glycation under pathophysiological concentrations of glucose in vitro. J. Agric. Food Chem. 2009, 57, 5058–5064. [Google Scholar]
- Doğduş, M.; Koç, A. Higher serum Endocan levels are involved in the pathophysiology of chronic venous insufficiency. Ege Tıp Derg. 2020, 59, 310–315. [Google Scholar] [CrossRef]
- Papachristoforou, E.; Lambadiari, V.; Maratou, E.; Makrilakis, K. Association of glycemic indices (hyperglycemia, glucose variability, and hypoglycemia) with oxidative stress and diabetic complications. J. Diabetes Res. 2020, 2020, 7489795. [Google Scholar] [CrossRef]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stresses and their classifications. Chem. Biol. Interact. 2016, 224, 164–175. [Google Scholar] [CrossRef]
- Ali, M.M.; Agha, F.G. Amelioration of streptozotocin-induced diabetes mellitus, oxidative stress and dyslipidemia in rats by tomato extract lycopene. Scand. J. Clin. Lab. Investig. 2009, 69, 371–379. [Google Scholar] [CrossRef]
- Kabay, S.C.; Ozden, H.; Guven, G.; Ustuner, M.C.; Degirmecni, I.; Olgun, E.G.; Unal, N. Protective effects of vitamin E on central nervous system in streptozotocin-induced diabetic rats. Clin. Investig. Med. 2009, 32, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Grabia, M.; Socha, K.; Soroczyńska, J.; Bossowski, A.; Markiewicz-Żukowska, R. Determinants related to oxidative stress parameters in pediatric patients with type 1 diabetes mellitus. Nutrients 2023, 15, 2048. [Google Scholar] [CrossRef] [PubMed]
- Alghazeer, R.; Alghazir, N.; Awayn, N.; Ahtiwesh, O.; Elgahmasi, S. Biomarkers of oxidative stress and antioxidant defense in patients with type 1 diabetes mellitus. Ibnosina J. Med. Biomed. Sci. 2018, 10, 198–204. [Google Scholar] [CrossRef]
- Yildirim, Z.; Yildirim, F.; Ucgun, N.I.; Kilic, N. The evaluation of the oxidative stress parameters in nondiabetic and diabetic senile cataract patients. Biol. Trace Elem. Res. 2009, 128, 135–143. [Google Scholar] [CrossRef]
- Piwowar, A.; Knapik-Kordecka, M.; Warwas, M. AOPP and its relations with selected markers of oxidative/antioxidative system in type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2007, 77, 188–192. [Google Scholar] [CrossRef]
- Agarwal, S.; Tripathi, R.; Mohammed, A.; Rizvi, S.I.; Mishra, N. Effects of thymol supplementation against type 2 diabetes in streptozotocin-induced rat model. Plant Arch. 2020, 20 (Suppl. S1), 863–869. [Google Scholar]
- Astari, L.F.; Cahyono, H.A.; Widjajanto, E. Correlation of interleukin-10, superoxide dismutase (SOD), and malondialdehyde (MDA) levels with HbA1c in pediatric type 1 diabetes mellitus. J. Trop. Life Sci. 2017, 7, 286–292. [Google Scholar] [CrossRef]
- Chandran, K.; Lee, S.M.; Shen, L.; Tng, E.L. Fructosamine and HbA1c: A correlational study in a southeast asian population. J. ASEAN Fed. Endocr. Soc. 2024, 39, 26–30. [Google Scholar] [CrossRef]
- Hasan, H.F.; Abdel-Rafei, M.K.; Galal, S.M. Diosmin attenuates radiation-induced hepatic fibrosis by boosting PPAR-γ expression and hampering miR-17-5p-activated canonical Wnt-β-catenin signaling. Biochem. Cell Biol. 2017, 95, 400–414. [Google Scholar] [CrossRef]
- Barreca, D.; Laganà, G.; Bruno, G.; Magazù, S.; Bellocco, E. Diosmin binding to human serum albumin and its preventive action against degradation due to oxidative injuries. Biochimie 2013, 95, 2042–2049. [Google Scholar] [CrossRef]
- Kumar, R.; Akhtar, F.; Rizvi, S.I. Hesperidin attenuates altered redox homeostasis in an experimental hyperlipidaemic model of rat. Clin. Exp. Pharmacol. Physiol. 2020, 47, 571–582. [Google Scholar]
- Shakour, N.; Mahdinezhad, M.R.; Hadjzadeh, M.A.R.; Sahebkar, A.; Hadizadeh, F. Serum biochemical evaluation following administration of imidazolyl thiazolidinedione in streptozotocin-induced diabetic rats. J. Mol. Histol. 2024, 55, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Van Laer, K.; Hamilton, C.J.; Messens, J. Low-molecular-weight thiols in thiol-disulfide exchange. Antioxid. Redox Signal. 2013, 18, 1642–1653. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.M.; Meki, A.R.M.A. Oxidative stress in streptozotocin-induced diabetic rats: Effects of garlic oil and melatonin. Comp. Biochem. Physiol.—Part A 2003, 135, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Al-Matubsi, H.; Rashan, L.; Aburayyan, W.; Al Hanbali, O.; Abuarqoub, D.; Efferth, T. Antidiabetic and antioxidant properties of Boswellia sacra oleo-gum in streptozotocin-induced diabetic rats. J. Ayurveda Integr. Med. 2024, 15, 101014. [Google Scholar] [CrossRef]
- Salama, A.; Asaad, G.F.; Shaheen, A. Chrysin ameliorates STZ-induced diabetes in rats: Possible impact of modulation of TLR4/NF-κβ pathway. Res. Pharm. Sci. 2022, 17, 1–11. [Google Scholar] [CrossRef]
- Shiming, Z.; Mak, K.K.; Balijepalli, M.K.; Chakravarthi, S.; Pichika, M.R. Swietenine potentiates the antihyperglycemic and antioxidant activity of metformin in streptozotocin induced diabetic rats. Biomed. Pharmacother. 2021, 139, 111576. [Google Scholar] [CrossRef]
- Hamamcioglu, A.C.; Severcan, C.; Bayraktaroglu, T. Relationship of thiol/disulphide homeostasis with oxidative stress parameters in non-diabetic, prediabetic and type 2 diabetic Turkish women. Istanbul J. Pharm. 2022, 52, 121–128. [Google Scholar] [CrossRef]
- Dickinson, D.A.; Forman, H.J. Glutathione in defense and signaling: Lessons from a small thiol. Ann. N. Y. Acad. Sci. 2002, 973, 488–504. [Google Scholar] [CrossRef]
- Rațiu, S.; Mariș, M.I.; Furdui-Lința, A.V.; Sima, L.V.; Bratu, T.I.; Sturza, A.; Muntean, D.M.; Crețu, O.M. Oxidative stress in the pathophysiology of chronic venous disease: An overview. Antioxidants 2025, 14, 989. [Google Scholar] [CrossRef]
- Geravandi, S.; Emamgholipour, S.; Pakdaman, M.; Sari, A.A.; Esmaeili, A. Principal components of type 2 diabetes risk: An exploratory factor analysis in an Iranian cohort. BMC Public Health 2025, 25, 256. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, L.; Wang, S.; Huang, R.; Zheng, L.; Liang, S.; Zhang, L.; Xu, J. An integrated metabonomic approach to studying metabolic profiles in rat models with insulin resistance induced by high fructose. Mol. Biosyst. 2014, 10, 1803–1811. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Jiang, Q.; Ji, H.; Ning, J.; Li, C.; Zheng, H. Type 1 diabetes induces cognitive dysfunction in rats associated with alterations of the gut microbiome and metabolomes in serum and hippocampus. BBA—Mol. Basis Dis. 2019, 1865, 165541. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, V.; Ahmed, S.S.S.J.; Ebenezar, K.K. Multivariate analysis and molecular interaction of curcumin with PPARγ in high fructose diet induced insulin resistance in rats. Springerplus 2016, 5, 1732. [Google Scholar] [CrossRef]
- Abdou, H.M.; Elmageed, G.M.A.; Hussein, H.K.; Yamari, I.; Chtita, S.; El-Samad, L.M.; Hassan, M.A. Antidiabetic effects of quercetin and silk sericin in attenuating dysregulation of hepatic gluconeogenesis in diabetic rats through potential modulation of PI3K/Akt/FOXO1 signaling: In vivo and in silico studies. Xenobiotics 2025, 15, 16. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, Z.; Shu, Z.; Jia, Z. A plasma metabolomic analysis revealed the metabolic regulatory mechanism of the water extract of Dendrobium huoshanense in improving streptozotocin—Induced type 1 diabetes model rats. J. Nat. Med. 2025, 79, 750–772. [Google Scholar] [CrossRef]
- Ma, L.; Liu, J.; Deng, M.; Zhou, L.; Zhang, Q.; Xiao, X. Metabolomics analysis of serum and urine in type 1 diabetes patients with different time in range derived from continuous glucose monitoring. Diabetol. Metab. Syndr. 2024, 16, 21. [Google Scholar] [CrossRef]
- Jain, D.; Bansal, M.K.; Dalvi, R.; Upganlawar, A.; Somani, R. Protective effect of diosmin against diabetic neuropathy in experimental rats. J. Integr. Med. 2014, 12, 35–41. [Google Scholar] [CrossRef]
- El-Marasy, S.A.; Abdallah, H.M.I.; El-Shenawy, S.M.; El-Khatib, A.S.; El-Shabrawy, O.A.; Kenawy, S.A. Anti-depressant effect of hesperidin in diabetic rats. Can. J. Physiol. Pharmacol. 2014, 952, 945–952. [Google Scholar] [CrossRef]
- Visnagri, A.; Kandhare, A.D.; Chakravarty, S.; Ghosh, P.; Bodhankar, S.L. Hesperidin, a flavanoglycone attenuates experimental diabetic neuropathy via modulation of cellular and biochemical marker to improve nerve functions. Pharm. Biol. 2014, 52, 814–828. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Friedlander, M.; Capeillère-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Erel, O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin. Biochem. 2004, 37, 112–119. [Google Scholar] [CrossRef]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef]
- Kosecik, M.; Erel, O.; Sevinc, E.; Selek, S. Increased oxidative stress in children exposed to passive smoking. Int. J. Cardiol. 2005, 100, 61–64. [Google Scholar] [CrossRef]
- Erel, O.; Neselioglu, S. A novel and automated assay for thiol/disulphide homeostasis. Clin. Biochem. 2014, 47, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]

| NDM | T1DM | DIO50 | DIO100 | HES50 | HES100 | |
|---|---|---|---|---|---|---|
| PC1 | −2.78 ± 0.88 d | 1.24 ± 0.54 a | 0.75 ± 0.51 ab | −0.19 ± 0.85 c | 0.78 ± 0.55 ab | 0.31 ± 0.60 bc |
| PC2 | 0.32 ± 1.23 ab | 0.90 ± 0.78 a | −0.34 ± 0.67 b | −0.17 ± 0.83 b | −0.28 ± 0.46 b | −0.36 ± 0.90 b |
| NDM | T1DM | DIO50 | DIO100 | HES50 | HES100 | |
|---|---|---|---|---|---|---|
| PC1 | −2.03 ± 0.56 c | 1.59 ± 0.62 a | −0.13 ± 0.91 b | 0.17 ± 1.04 b | −0.03 ± 1.10 b | 0.50 ± 1.25 b |
| PC2 | −0.15 ± 0.34 abc | −1.02 ± 1.08 c | 0.26 ± 0.66 ab | −0.26 ± 0.76 bc | −0.02 ± 1.26 ab | 0.71 ± 1.35 a |
| NDM | T1DM | DIO50 | DIO100 | HES50 | HES100 | |
|---|---|---|---|---|---|---|
| SOD [U/mg of protein] | 0.24 ± 0.03 a | 0.19 ± 0.02 b | 0.19 ± 0.03 b | 0.20 ± 0.03 b | 0.20 ± 0.04 b | 0.20 ± 0.04 b |
| CAT [nmol/min/mg of protein] | 0.48 ± 0.13 cb | 0.30 ± 0.11 c | 0.32 ± 0.12 c | 0.42 ± 0.15 c | 0.90 ± 0.59 a | 0.81 ± 0.60 ab |
| GPx [nmol/min/mg of protein] | 39.68 ± 9.19 a | 20.18 ± 3.08 b | 21.42 ± 3.33 b | 23.40 ± 4.05 b | 25.98 ± 5.37 b | 26.29 ± 5.79 b |
| Total thiols [nmol/L] | 99.50 ± 23.13 | 117.18 ± 17.97 | 113.78 ± 19.70 | 114.84 ± 25.84 | 107.75 ± 27.30 | 118.47 ± 19.25 |
| Native thiols [nmol/L] | 37.17 ± 14.90 b | 63.46 ± 20.86 a | 61.75 ± 19.42 a | 66.56 ± 33.25 a | 54.18 ± 27.33 ab | 76.50 ± 18.61 a |
| Disulfides [nmol/L] | 31.17 ± 9.63 a | 26.86 ± 5.73 ab | 26.01 ± 3.24 ab | 24.14 ± 5.53 b | 26.79 ± 8.95 ab | 20.99 ± 2.49 b |
| Native thiols/ disulfides ratio | 1.28 ± 0.57 c | 2.51 ± 1.02 abc | 2.41 ± 0.85 bc | 3.19 ± 2.32 ab | 2.19 ± 1.18 bc | 3.68 ± 1.01 a |
| NDM | T1DM | DIO50 | DIO100 | HES50 | HES100 | |
|---|---|---|---|---|---|---|
| PC1 | −2.76 ± 1.07 c | 1.12 ± 1.34 a | 0.78 ± 1.36 ab | 0.85 ± 1.83 ab | −0.48 ± 1.63 b | 0.87 ± 1.22 ab |
| PC2 | −0.11 ± 0.95 bc | 1.69 ± 1.03 a | 0.75 ± 1.43 ab | −0.76 ± 1.58 cd | 0.29 ± 1.51 bc | −1.52 ± 1.35 d |
| NDM | T1DM | DIO50 | DIO100 | HES50 | HES100 | |
|---|---|---|---|---|---|---|
| PC1 | −4.43 ± 0.83 c | 2.54 ± 1.23 a | 0.96 ± 1.21 b | 0.40 ± 1.64 b | 0.24 ± 1.44 b | 0.71 ± 0.83 b |
| PC2 | −0.72 ± 1.02 cd | −1.83 ± 0.92 d | −0.23 ± 1.34 bc | 1.00 ± 1.81 ab | −0.43 ± 1.67 cd | 1.92 ± 1.53 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borymska, W.; Borymski, S.; Zych, M.; Dudek, S.; Kaczmarczyk-Żebrowska, I. Diosmin or Hesperidin? Comparison of Antioxidative Action of Two Venoactive Flavonoids in Type 1 Diabetic Rats. Int. J. Mol. Sci. 2025, 26, 11252. https://doi.org/10.3390/ijms262311252
Borymska W, Borymski S, Zych M, Dudek S, Kaczmarczyk-Żebrowska I. Diosmin or Hesperidin? Comparison of Antioxidative Action of Two Venoactive Flavonoids in Type 1 Diabetic Rats. International Journal of Molecular Sciences. 2025; 26(23):11252. https://doi.org/10.3390/ijms262311252
Chicago/Turabian StyleBorymska, Weronika, Sławomir Borymski, Maria Zych, Sławomir Dudek, and Ilona Kaczmarczyk-Żebrowska. 2025. "Diosmin or Hesperidin? Comparison of Antioxidative Action of Two Venoactive Flavonoids in Type 1 Diabetic Rats" International Journal of Molecular Sciences 26, no. 23: 11252. https://doi.org/10.3390/ijms262311252
APA StyleBorymska, W., Borymski, S., Zych, M., Dudek, S., & Kaczmarczyk-Żebrowska, I. (2025). Diosmin or Hesperidin? Comparison of Antioxidative Action of Two Venoactive Flavonoids in Type 1 Diabetic Rats. International Journal of Molecular Sciences, 26(23), 11252. https://doi.org/10.3390/ijms262311252






