Integrated Transcriptomic and Metabolomic Analysis Reveals Biochar-Induced Enhancement of Growth and Secondary Metabolism in the Medicinal Plant Echinacea purpurea
Abstract
1. Introduction
2. Results
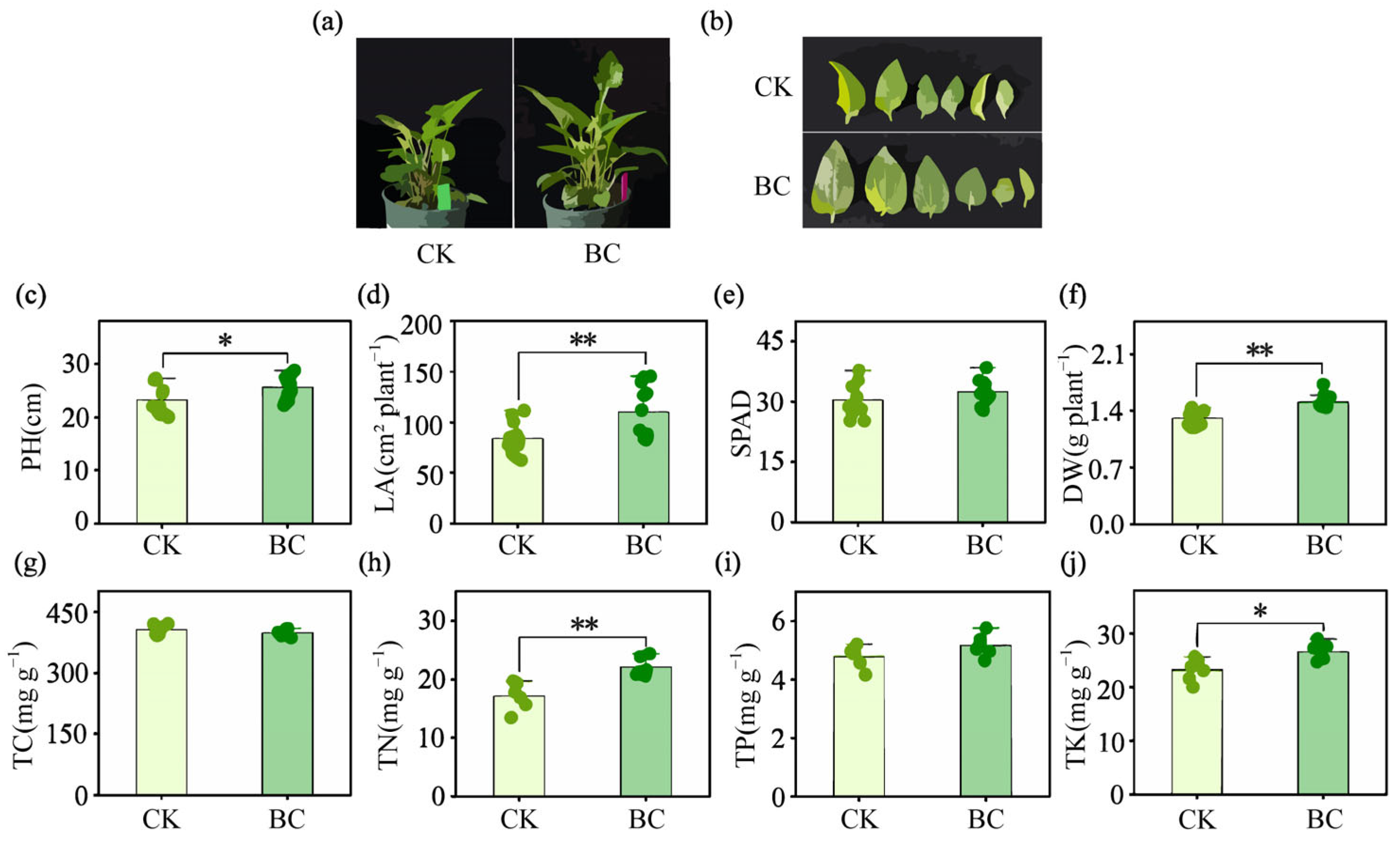
2.1. Plant Growth and Nutrient Accumulation
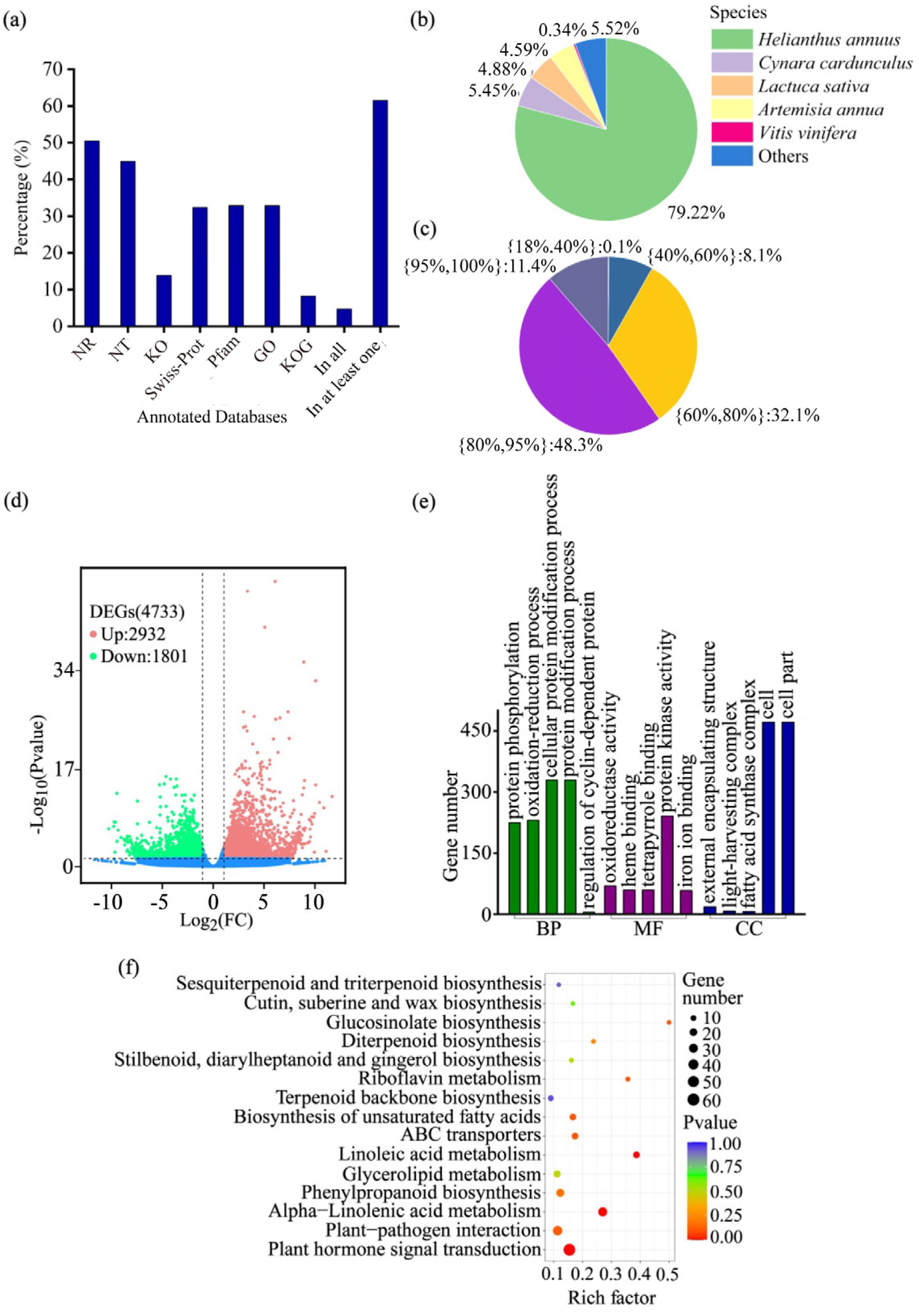
2.2. Overview of RNA Sequencing, Assembly, and Functional Annotation
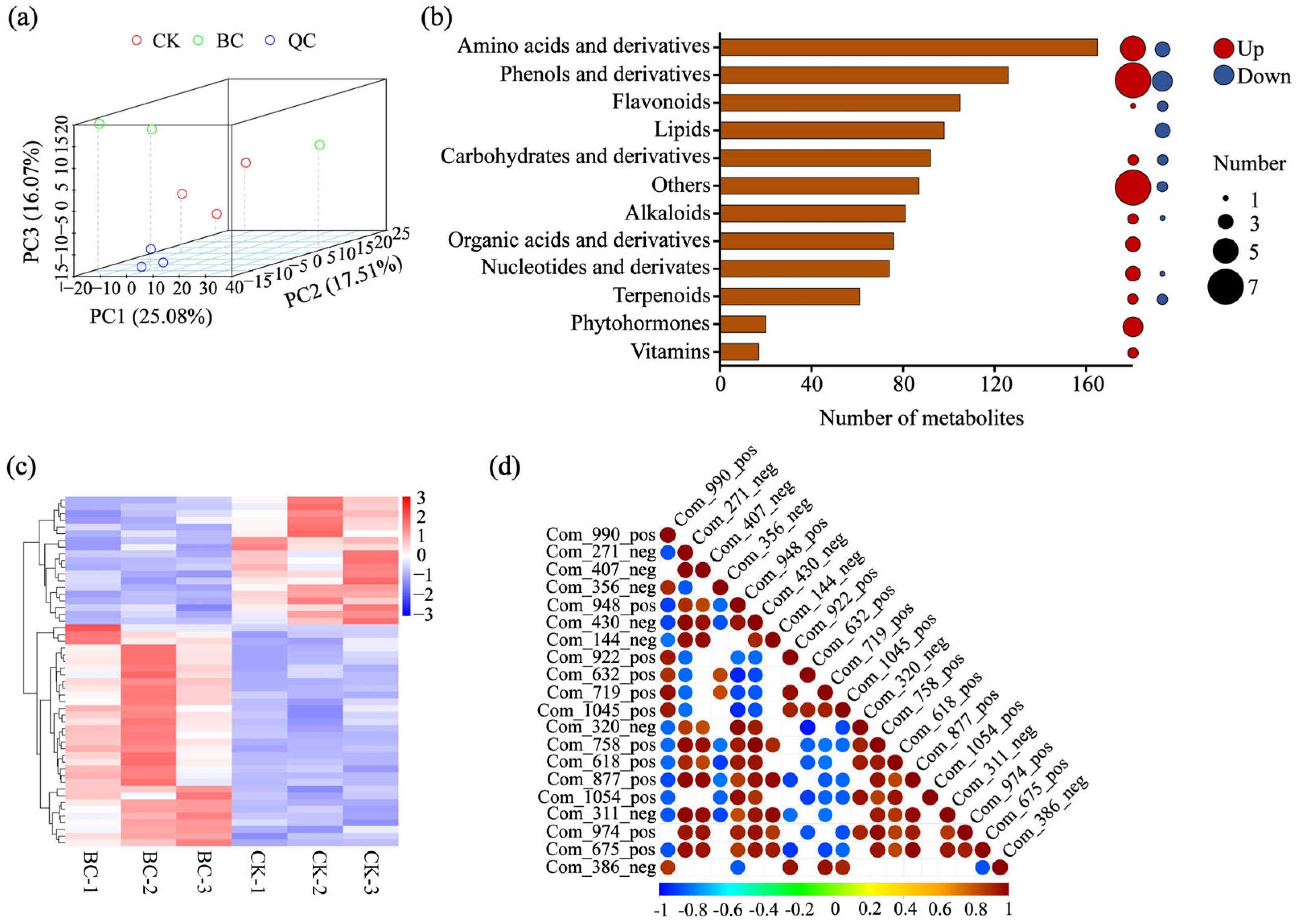
2.3. Overview of Metabolomic Profiling and Differential Accumulation Metabolites
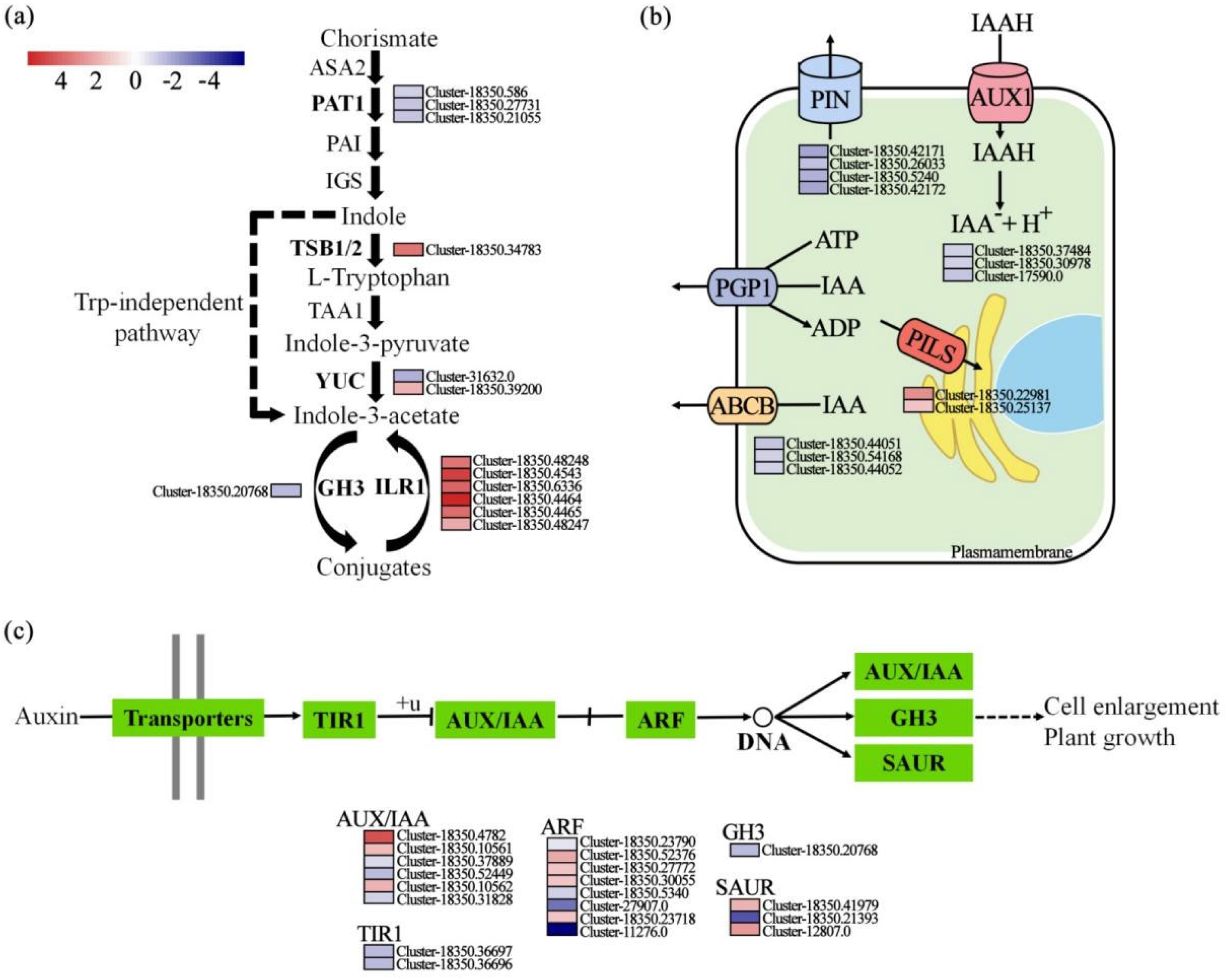
2.4. Association Analysis of Metabolome and Transcriptome
3. Discussion
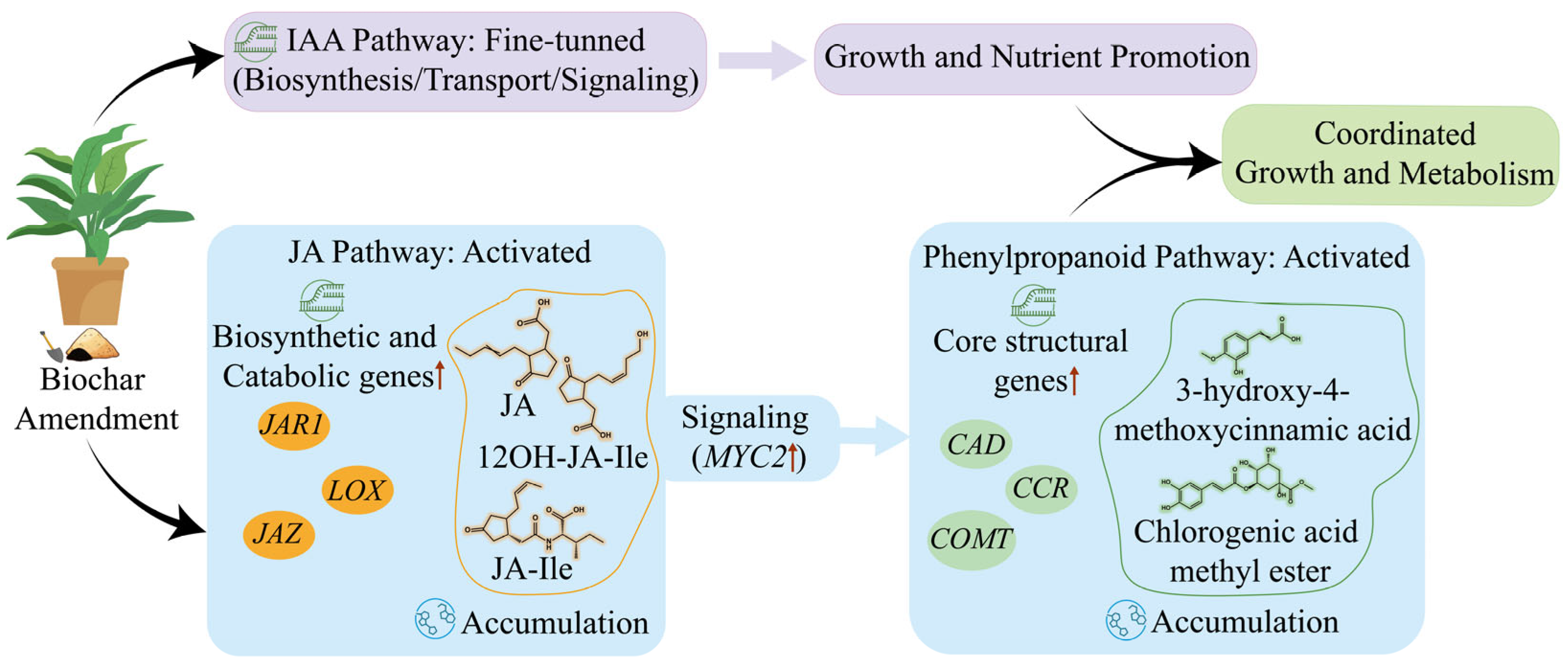
3.1. Growth Enhancement and Transcriptomic Reprogramming in E. purpurea Mediated by Biochar
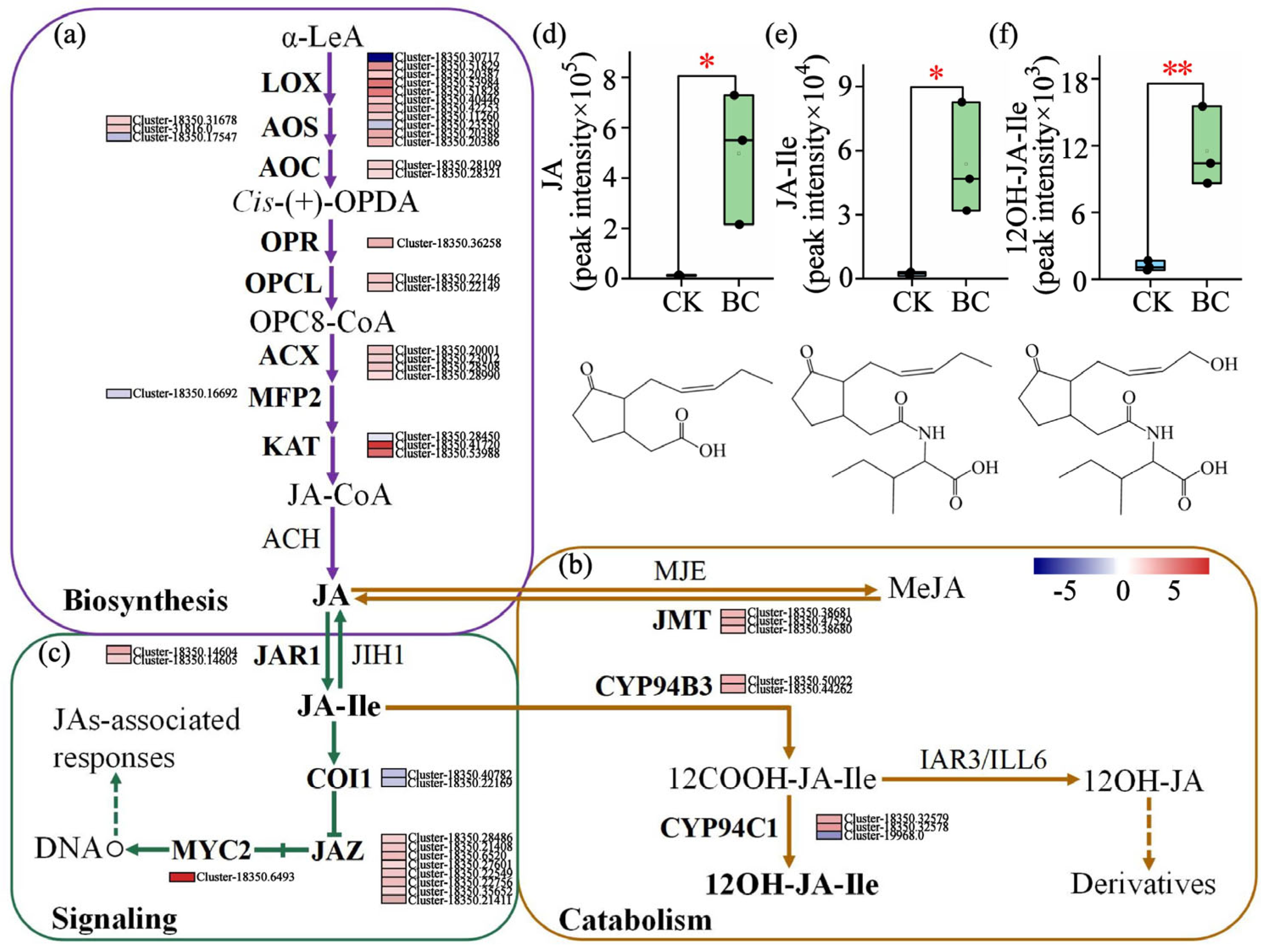
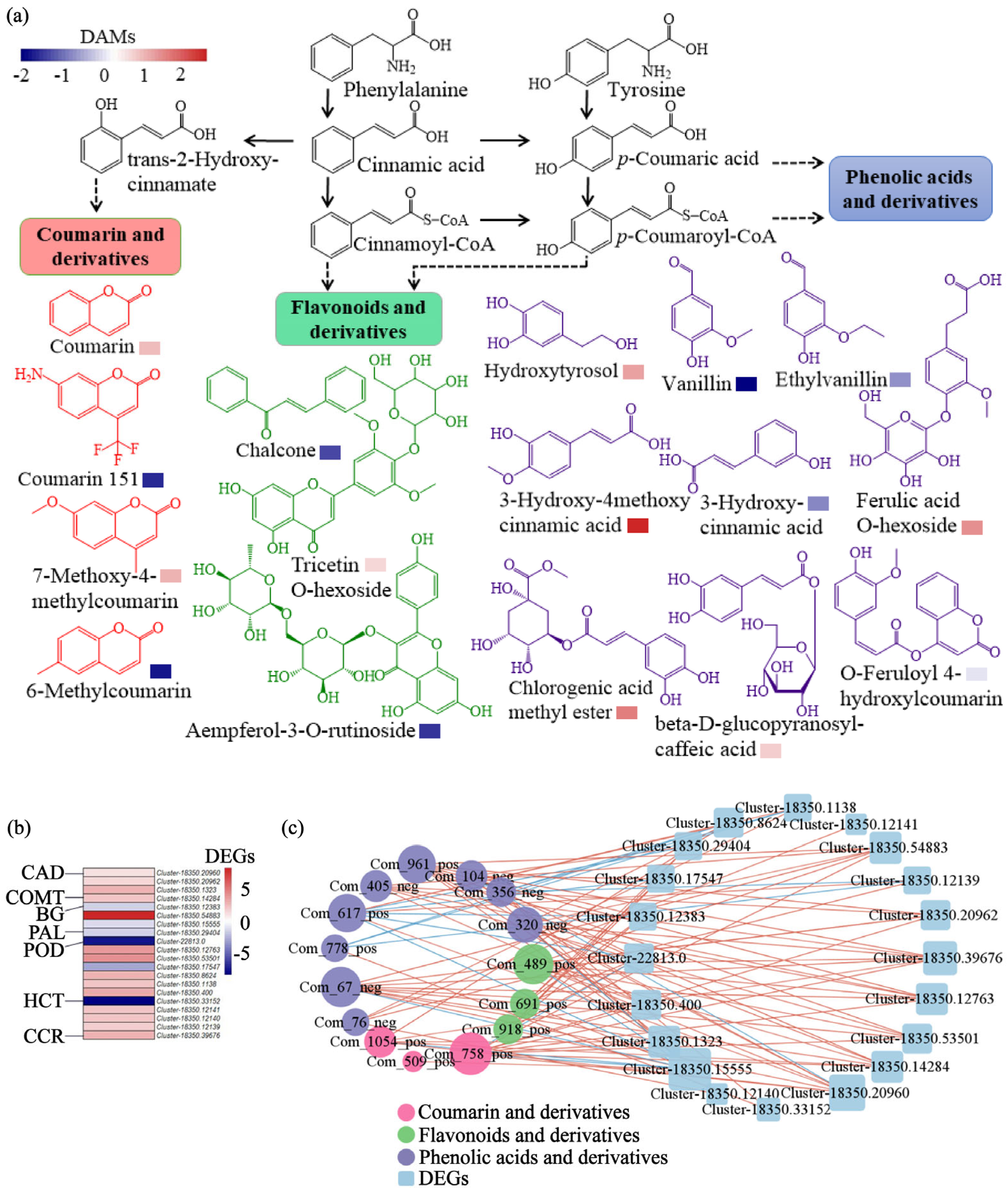
3.2. Enrichment of Secondary Metabolites and Activation of Jasmonic Acid Signaling and Phenylpropanoid Biosynthesis in E. purpurea Mediated by Biochar
4. Materials and Methods
4.1. Soil Preparation, Experimental Design, and Plant Cultivation
4.2. Growth and Elemental Composition Measurement
4.3. Transcriptomic Sequencing and qRT-PCR
4.4. Metabolomic Analysis
4.5. Data and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barnes, J.; Anderson, L.A.; Gibbons, S.; Phillipson, J.D. Echinacea Species (Echinacea Angustifolia (DC.) Hell., Echinacea Pallida (Nutt.) Nutt., Echinacea Purpurea (L.) Moench): A Review of Their Chemistry, Pharmacology and Clinical Properties. J. Pharm. Pharmacol. 2005, 57, 929–954. [Google Scholar] [CrossRef]
- Burlou-Nagy, C.; Bănică, F.; Jurca, T.; Vicaș, L.G.; Marian, E.; Muresan, M.E.; Bácskay, I.; Kiss, R.; Fehér, P.; Pallag, A. Echinacea Purpurea (L.) Moench: Biological and Pharmacological Properties. A Review. Plants 2022, 11, 1244. [Google Scholar] [CrossRef] [PubMed]
- Saeidnia, S.; Manayi, A.; Vazirian, M. Echinacea Purpurea: Pharmacology, Phytochemistry and Analysis Methods. Pharmacogn. Rev. 2015, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Xu, T.; Li, Q.; Yang, F.; Wang, C.; Huang, T.; Hao, Z. Polysaccharide from Echinacea Purpurea Reduce the Oxidant Stress in Vitro and in Vivo. Int. J. Biol. Macromol. 2020, 149, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Tahmasebi, A.; Ebrahimie, E.; Pakniyat, H.; Ebrahimi, M.; Mohammadi-Dehcheshmeh, M. Insights from the Echinacea Purpurea (L.) Moench Transcriptome: Global Reprogramming of Gene Expression Patterns towards Activation of Secondary Metabolism Pathways. Ind. Crops Prod. 2019, 132, 365–376. [Google Scholar] [CrossRef]
- Iakab, M.; Domokos, E.; Benedek, K.; Molnár, K.; Kentelky, E.; Buta, E.; Dulf, F.V. The Importance of Mycorrhizal Fungi in the Development and Secondary Metabolite Production of Echinacea Purpurea and Relatives (Asteraceae): Current Research Status and Perspectives. Horticulturae 2022, 8, 1106. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Zhang, W.; Zeng, Y.; Jiao, M.; Ye, C.; Li, Y.; Liu, C.; Wang, J. Integration of High-Throughput Omics Technologies in Medicinal Plant Research: The New Era of Natural Drug Discovery. Front. Plant Sci. 2023, 14, 1073848. [Google Scholar] [CrossRef]
- Rai, A.; Saito, K.; Yamazaki, M. Integrated Omics Analysis of Specialized Metabolism in Medicinal Plants. Plant J. 2017, 90, 764–787. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, S.; Li, R.; Zhao, Q. Unraveling the Specialized Metabolic Pathways in Medicinal Plant Genomes: A Review. Front. Plant Sci. 2024, 15, 1459533. [Google Scholar] [CrossRef]
- He, D.; Rao, X.; Deng, J.; Damaris, R.N.; Yang, P. Integration of Metabolomics and Transcriptomics Analyses Investigates the Accumulation of Secondary Metabolites in Maturing Seed Plumule of Sacred Lotus (Nelumbo Nucifera). Food Res. Int. 2023, 163, 112172. [Google Scholar] [CrossRef]
- Ren, C.; Chen, C.; Dong, S.; Wang, R.; Xian, B.; Liu, T.; Xi, Z.; Pei, J.; Chen, J. Integrated Metabolomics and Transcriptome Analysis on Flavonoid Biosynthesis in Flowers of Safflower (Carthamus Tinctorius L.) during Colour-Transition. PeerJ 2022, 10, e13591. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Ahmad, N.; Meng, W.; Zhao, S.; Chang, Y.; Wang, N.; Zhang, M.; Yao, N.; Liu, X.; Zhang, J. Integrated Metabolomics and Transcriptomics Provide Key Molecular Insights into Floral Stage-Driven Flavonoid Pathway in Safflower. Int. J. Mol. Sci. 2024, 25, 11903. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zou, Y.; Jiang, Z.; Tu, L.; Wu, X.; Li, D.; Wang, J.; Huang, L.; Xu, C.; Gao, W. Multiomics Driven Identification of Glycosyltransferases in Flavonoid Glycoside Biosynthesis in Safflower. Hortic. Plant J. 2024, in press. [Google Scholar] [CrossRef]
- Guo, E.; Yuan, M.; Xu, L.; Ren, Q.; Wang, Z.; Li, Z.; Wu, Z.; Liu, W.; Zhao, Y.; Feng, F.; et al. Identification of Three Key Enzymes Involved in the Biosynthesis of Tetracyclic Oxindole Alkaloids in Uncaria Rhynchophylla. Bioorganic Chem. 2023, 136, 106545. [Google Scholar] [CrossRef]
- Xu, W.; Cheng, Y.; Guo, Y.; Yao, W.; Qian, H. Effects of Geographical Location and Environmental Factors on Metabolite Content and Immune Activity of Echinacea Purpurea in China Based on Metabolomics Analysis. Ind. Crops Prod. 2022, 189, 115782. [Google Scholar] [CrossRef]
- Moghadam, A.; Taghizadeh, M.S.; Haghi, R.; Tahmasebi, A.; Niazi, A.; Ebrahimie, E. Exploring Novel Insights: Methyl Jasmonate Treatment Reveals Novel lncRNA-Mediated Regulation of Secondary Metabolite Biosynthesis Pathways in Echinacea Purpurea. Food Biosci. 2024, 57, 103457. [Google Scholar] [CrossRef]
- Lin, J.; Yang, Q.; Su, J.; Xing, H.; Pan, H.; Yu, J.-B.; Bai, M.; Li, Y.; Huang, H.-Y.; Liang, X.; et al. Transcriptomics and Metabolomics Analyses of the Effects of Plant Age and Organ on Caffeic Acid Derivative Accumulation in Echinacea Purpurea. Plant Physiol. Biochem. 2025, 226, 110051. [Google Scholar] [CrossRef]
- Singh, B.; Macdonald, L.M.; Kookana, R.S.; Van Zwieten, L.; Butler, G.; Joseph, S.; Weatherley, A.; Kaudal, B.B.; Regan, A.; Cattle, J.; et al. Opportunities and Constraints for Biochar Technology in Australian Agriculture: Looking beyond Carbon Sequestration. Soil Res. 2014, 52, 739–750. [Google Scholar] [CrossRef]
- Xiang, Y.; Liu, Y.; Niazi, N.K.; Bolan, N.; Zhao, L.; Zhang, S.; Xue, J.; Yao, B.; Li, Y. Biochar Addition Increased Soil Bacterial Diversity and Richness: Large-Scale Evidence of Field Experiments. Sci. Total Environ. 2023, 893, 164961. [Google Scholar] [CrossRef]
- Yu, H.; Zou, W.; Chen, J.; Chen, H.; Yu, Z.; Huang, J.; Tang, H.; Wei, X.; Gao, B. Biochar Amendment Improves Crop Production in Problem Soils: A Review. J. Environ. Manag. 2019, 232, 8–21. [Google Scholar] [CrossRef]
- Zhang, R.; Tan, S.; Zhang, B.; Hu, P.; Li, L. Cerium-Promoted Ginsenosides Accumulation by Regulating Endogenous Methyl Jasmonate Biosynthesis in Hairy Roots of Panax Ginseng. Molecules 2021, 26, 5623. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Basak, B.B.; Gajbhiye, N.A.; Kalariya, K.A.; Manivel, P. Sustainable Fertilization through Co-Application of Biochar and Chemical Fertilizers Improves Yield, Quality of Andrographis Paniculata and Soil Health. Ind. Crops Prod. 2019, 140, 111607. [Google Scholar] [CrossRef]
- Nigam, N.; Khare, P.; Ahsan, M.; Yadav, V.; Shanker, K.; Singh, R.P.; Panday, V.; Das, P.; Singh, A.; Yadav, R.; et al. Biochar Amendment Reduced the Risk Associated with Metal Uptake and Improved Metabolite Content in Medicinal Herbs. Physiol. Plant. 2021, 173, 321–339. [Google Scholar] [CrossRef] [PubMed]
- Moradi, R.; Pourghasemian, N.; Naghizadeh, M. Effect of Beeswax Waste Biochar on Growth, Physiology and Cadmium Uptake in Saffron. J. Clean. Prod. 2019, 229, 1251–1261. [Google Scholar] [CrossRef]
- Huang, H.; Liu, B.; Liu, L.; Song, S. Jasmonate Action in Plant Growth and Development. J. Exp. Bot. 2017, 68, 1349–1359. [Google Scholar] [CrossRef]
- Delgado, C.; Mora-Poblete, F.; Ahmar, S.; Chen, J.-T.; Figueroa, C.R. Jasmonates and Plant Salt Stress: Molecular Players, Physiological Effects, and Improving Tolerance by Using Genome-Associated Tools. Int. J. Mol. Sci. 2021, 22, 3082. [Google Scholar] [CrossRef]
- Biederman, L.A.; Harpole, W.S. Biochar and Its Effects on Plant Productivity and Nutrient Cycling: A Meta-analysis. GCB Bioenergy 2013, 5, 202–214. [Google Scholar] [CrossRef]
- Dai, Y.; Zheng, H.; Jiang, Z.; Xing, B. Combined Effects of Biochar Properties and Soil Conditions on Plant Growth: A Meta-Analysis. Sci. Total Environ. 2020, 713, 136635. [Google Scholar] [CrossRef]
- He, Y.; Yao, Y.; Ji, Y.; Deng, J.; Zhou, G.; Liu, R.; Shao, J.; Zhou, L.; Li, N.; Zhou, X.; et al. Biochar Amendment Boosts Photosynthesis and Biomass in C3 but Not C4 Plants: A Global Synthesis. GCB Bioenergy 2020, 12, 605–617. [Google Scholar] [CrossRef]
- O’Brien, J.A.; Vega, A.; Bouguyon, E.; Krouk, G.; Gojon, A.; Coruzzi, G.; Gutiérrez, R.A. Nitrate Transport, Sensing, and Responses in Plants. Mol. Plant 2016, 9, 837–856. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Peng, Y.; Kong, Y.; Li, J.; Sun, C. Combined Effects of Biochar Addition and Nitrogen Fertilizer Reduction on the Rhizosphere Metabolomics of Maize (Zea Mays L.) Seedlings. Plant Soil 2018, 433, 19–35. [Google Scholar] [CrossRef]
- Bao, Z.; Dai, W.; Su, X.; Liu, Z.; An, Z.; Sun, Q.; Jing, H.; Lin, L.; Chen, Y.; Meng, J. Long-term Biochar Application Promoted Soil Aggregate-associated Potassium Availability and Maize Potassium Uptake. GCB Bioenergy 2024, 16, e13134. [Google Scholar] [CrossRef]
- Martinsen, V.; Mulder, J.; Shitumbanuma, V.; Sparrevik, M.; Børresen, T.; Cornelissen, G. Farmer-led Maize Biochar Trials: Effect on Crop Yield and Soil Nutrients under Conservation Farming. J. Plant Nutr. Soil Sci. 2014, 177, 681–695. [Google Scholar] [CrossRef]
- Jeffery, S.; Verheijen, F.G.A.; Van Der Velde, M.; Bastos, A.C. A Quantitative Review of the Effects of Biochar Application to Soils on Crop Productivity Using Meta-Analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Olmo, M.; Villar, R.; Salazar, P.; Alburquerque, J.A. Changes in Soil Nutrient Availability Explain Biochar’s Impact on Wheat Root Development. Plant Soil 2016, 399, 333–343. [Google Scholar] [CrossRef]
- Mandal, S.; Thangarajan, R.; Bolan, N.S.; Sarkar, B.; Khan, N.; Ok, Y.S.; Naidu, R. Biochar-Induced Concomitant Decrease in Ammonia Volatilization and Increase in Nitrogen Use Efficiency by Wheat. Chemosphere 2016, 142, 120–127. [Google Scholar] [CrossRef]
- Viger, M.; Hancock, R.D.; Miglietta, F.; Taylor, G. More Plant Growth but Less Plant Defence? First Global Gene Expression Data for Plants Grown in Soil Amended with Biochar. GCB Bioenergy 2015, 7, 658–672. [Google Scholar] [CrossRef]
- Sun, C.; Wang, D.; Shen, X.; Li, C.; Liu, J.; Lan, T.; Wang, W.; Xie, H.; Zhang, Y. Effects of Biochar, Compost and Straw Input on Root Exudation of Maize (Zea Mays L.): From Function to Morphology. Agric. Ecosyst. Environ. 2020, 297, 106952, Erratum in Agric. Ecosyst. Environ. 2021, 308, 107216. [Google Scholar] [CrossRef]
- Wang, D.; Sun, C.; Cui, M.; Shen, X.; Zhang, Y.; Xiao, J.; Liu, P.; Zhang, Y.; Xie, H. An Integrated Analysis of Transcriptome and Metabolome Provides Insights into the Responses of Maize (Zea Mays L.) Roots to Different Straw and Fertilizer Conditions. Environ. Exp. Bot. 2022, 194, 104732. [Google Scholar] [CrossRef]
- Ke, M.; Gao, Z.; Chen, J.; Qiu, Y.; Zhang, L.; Chen, X. Auxin Controls Circadian Flower Opening and Closure in the Waterlily. BMC Plant Biol. 2018, 18, 143. [Google Scholar] [CrossRef]
- Hardoim, P.R.; De Carvalho, T.L.G.; Ballesteros, H.G.F.; Bellieny-Rabelo, D.; Rojas, C.A.; Venancio, T.M.; Ferreira, P.C.G.; Hemerly, A.S. Genome-Wide Transcriptome Profiling Provides Insights into the Responses of Maize (Zea Mays L.) to Diazotrophic Bacteria. Plant Soil 2020, 451, 121–143. [Google Scholar] [CrossRef]
- Barbez, E.; Kubeš, M.; Rolčík, J.; Béziat, C.; Pěnčík, A.; Wang, B.; Rosquete, M.R.; Zhu, J.; Dobrev, P.I.; Lee, Y.; et al. A Novel Putative Auxin Carrier Family Regulates Intracellular Auxin Homeostasis in Plants. Nature 2012, 485, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Barbez, E.; Laňková, M.; Pařezová, M.; Maizel, A.; Zažímalová, E.; Petrášek, J.; Friml, J.; Kleine-Vehn, J. Single-Cell-Based System to Monitor Carrier Driven Cellular Auxin Homeostasis. BMC Plant Biol. 2013, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The Effect of Developmental and Environmental Factors on Secondary Metabolites in Medicinal Plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, F.; Chen, J.; Younis, A.; Abideen, Z.; Naveed, M.; Koyro, H.-W.; Siddique, K.H.M. Biochar, Compost, and Biochar–Compost Blend Applications Modulate Growth, Photosynthesis, Osmolytes, and Antioxidant System of Medicinal Plant Alpinia Zerumbet. Front. Plant Sci. 2021, 12, 707061. [Google Scholar] [CrossRef]
- Zhang, F.; Yao, J.; Ke, J.; Zhang, L.; Lam, V.Q.; Xin, X.-F.; Zhou, X.E.; Chen, J.; Brunzelle, J.; Griffin, P.R.; et al. Structural Basis of JAZ Repression of MYC Transcription Factors in Jasmonate Signalling. Nature 2015, 525, 269–273. [Google Scholar] [CrossRef]
- Sheard, L.B.; Tan, X.; Mao, H.; Withers, J.; Ben-Nissan, G.; Hinds, T.R.; Kobayashi, Y.; Hsu, F.-F.; Sharon, M.; Browse, J.; et al. Jasmonate Perception by Inositol-Phosphate-Potentiated COI1–JAZ Co-Receptor. Nature 2010, 468, 400–405. [Google Scholar] [CrossRef]
- Wasternack, C.; Strnad, M. Jasmonates Are Signals in the Biosynthesis of Secondary Metabolites—Pathways, Transcription Factors and Applied Aspects—A Brief Review. New Biotechnol. 2019, 48, 1–11. [Google Scholar] [CrossRef]
- Xu, P.; Zhao, P.-X.; Cai, X.-T.; Mao, J.-L.; Miao, Z.-Q.; Xiang, C.-B. Integration of Jasmonic Acid and Ethylene Into Auxin Signaling in Root Development. Front. Plant Sci. 2020, 11, 271. [Google Scholar] [CrossRef]
- Zheng, H.; Fu, X.; Shao, J.; Tang, Y.; Yu, M.; Li, L.; Huang, L.; Tang, K. Transcriptional Regulatory Network of High-Value Active Ingredients in Medicinal Plants. Trends Plant Sci. 2023, 28, 429–446. [Google Scholar] [CrossRef]
- Zhang, R.; Li, C.; Guo, R.; Li, Z.; Zhang, B. Harnessing Jasmonate Pathways: PgJAR1’s Impact on Ginsenoside Accumulation in Ginseng. Plants 2025, 14, 847. [Google Scholar] [CrossRef]
- Guo, X.-X.; Yang, X.-Q.; Yang, R.-Y.; Zeng, Q.-P. Salicylic Acid and Methyl Jasmonate but Not Rose Bengal Enhance Artemisinin Production through Invoking Burst of Endogenous Singlet Oxygen. Plant Sci. 2010, 178, 390–397. [Google Scholar] [CrossRef]
- Li, S.; Zhang, P.; Zhang, M.; Fu, C.; Zhao, C.; Dong, Y.; Guo, A.; Yu, L. Transcriptional Profile of Taxus Chinensis Cells in Response to Methyl Jasmonate. BMC Genom. 2012, 13, 295. [Google Scholar] [CrossRef]
- Yang, T.; Deng, L.; Wang, Q.; Sun, C.; Ali, M.; Wu, F.; Zhai, H.; Xu, Q.; Xin, P.; Cheng, S.; et al. Tomato CYP94C1 Inactivates Bioactive JA-Ile to Attenuate Jasmonate-Mediated Defense during Fruit Ripening. Mol. Plant 2024, 17, 509–512. [Google Scholar] [CrossRef]
- Fu, X.; Peng, B.; Hassani, D.; Xie, L.; Liu, H.; Li, Y.; Chen, T.; Liu, P.; Tang, Y.; Li, L.; et al. AaWRKY9 Contributes to Light- and Jasmonate-mediated to Regulate the Biosynthesis of Artemisinin in Artemisia Annua. New Phytol. 2021, 231, 1858–1874. [Google Scholar] [CrossRef]
- Raziq, A.; Zhang, K.; Sun, W.; Ahmad, N.; Zhao, H.; Raza, M.A.; Ahmed, S.; Din, A.M.U.; Zhao, S.; Pan, J.; et al. Transcriptome Profiling of MYB-Overexpressed Transgenic Lines Provides Crucial Molecular Insights into Anthocyanin and Remodel the Biosynthesis Regulatory Network in Nicotiana Tabacum. Ind. Crops Prod. 2024, 213, 118374. [Google Scholar] [CrossRef]
- Du, T.; Niu, J.; Su, J.; Li, S.; Guo, X.; Li, L.; Cao, X.; Kang, J. SmbHLH37 Functions Antagonistically With SmMYC2 in Regulating Jasmonate-Mediated Biosynthesis of Phenolic Acids in Salvia Miltiorrhiza. Front. Plant Sci. 2018, 9, 1720. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Zhou, W.; Su, J.; Wang, X.; Li, L.; Wang, L.; Cao, X.; Wang, Z. Overexpression of SmMYC2 Increases the Production of Phenolic Acids in Salvia Miltiorrhiza. Front. Plant Sci. 2017, 8, 1804. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhu, X.; Yu, Z.; Hu, B.; Liu, P.; Zhang, F.; Chen, M.; Wang, J.; Huang, Y.; Yuan, H.; et al. Agronomic Characteristics, Objective Quantitative, Metabolome and Transcriptome Analysis Reveal the Influence of Fertilization Treatments on Fresh Leaf Characteristics and Finished Tea Quality. Food Chem. 2025, 482, 144183. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Li, L.; Qin, L.; Huang, Y.; Ye, Y.; Wang, M.; Wang, Y.; Xu, Y.; Luo, F.; Mei, H. Targeted Metabolomics Reveals Impact of N Application on Accumulation of Amino Acids, Flavonoids and Phytohormones in Tea Shoots under Soil Nutrition Deficiency Stress. Forests 2022, 13, 1629. [Google Scholar] [CrossRef]
- You, Y.; Zhao, M.; Wang, L.; Gu, W.; Cheng, Y.; Zhang, Y.; Song, W.; Jiang, R.; Zhao, X. Different Fertilizers Regulated the Microbiome and Metabolism of Pinus Koraiensis to Promote Nutrient Cycling. Ind. Crops Prod. 2025, 232, 121334. [Google Scholar] [CrossRef]
- Gao, Z.; Zhu, N.; Yang, M.; Cui, X.; Han, C.; Guo, X.; Leng, F.; Cheng, Z.; Luo, W.; Wang, Y. Integrating Transcriptome, Metabolome and Microbiome to Explore the Interaction Mechanism between Secondary Metabolites and Root-Associated Bacteria of Codonopsis Pilosula. Ind. Crops Prod. 2025, 227, 120790. [Google Scholar] [CrossRef]
- Li, Q.; Xu, M.; Wu, F.; Guo, Z.; Yang, N.; Li, L.; Wen, W.; Xu, D. Integrated Transcriptomics and Metabolomics Provide Insights into the Biosynthesis of Militarine in the Cell Suspension Culture System of Bletilla Striata. Adv. Biotechnol. 2024, 2, 25. [Google Scholar] [CrossRef]
- Sun, C.; Shen, X.; Zhang, Y.; Song, T.; Xu, L.; Xiao, J. Molecular Defensive Mechanism of Echinacea Purpurea (L.) Moench against PAH Contaminations. Int. J. Mol. Sci. 2023, 24, 11020. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, L.; Liu, L.; Liu, J.; Fu, Y.; Zhang, Y.; Sun, C.; Zhang, Y. Integrated Transcriptomic and Metabolomic Analysis Reveals Biochar-Induced Enhancement of Growth and Secondary Metabolism in the Medicinal Plant Echinacea purpurea. Int. J. Mol. Sci. 2025, 26, 11249. https://doi.org/10.3390/ijms262311249
Tan L, Liu L, Liu J, Fu Y, Zhang Y, Sun C, Zhang Y. Integrated Transcriptomic and Metabolomic Analysis Reveals Biochar-Induced Enhancement of Growth and Secondary Metabolism in the Medicinal Plant Echinacea purpurea. International Journal of Molecular Sciences. 2025; 26(23):11249. https://doi.org/10.3390/ijms262311249
Chicago/Turabian StyleTan, Liru, Ling Liu, Jun Liu, Yawen Fu, Ying Zhang, Caixia Sun, and Yulan Zhang. 2025. "Integrated Transcriptomic and Metabolomic Analysis Reveals Biochar-Induced Enhancement of Growth and Secondary Metabolism in the Medicinal Plant Echinacea purpurea" International Journal of Molecular Sciences 26, no. 23: 11249. https://doi.org/10.3390/ijms262311249
APA StyleTan, L., Liu, L., Liu, J., Fu, Y., Zhang, Y., Sun, C., & Zhang, Y. (2025). Integrated Transcriptomic and Metabolomic Analysis Reveals Biochar-Induced Enhancement of Growth and Secondary Metabolism in the Medicinal Plant Echinacea purpurea. International Journal of Molecular Sciences, 26(23), 11249. https://doi.org/10.3390/ijms262311249





