Effect of Central Injection of Anandamide on LPS-Dependent Suppression of GnRH/LH Secretion in Ewes During the Follicular Phase of the Estrous Cycle
Abstract
1. Introduction
2. Results
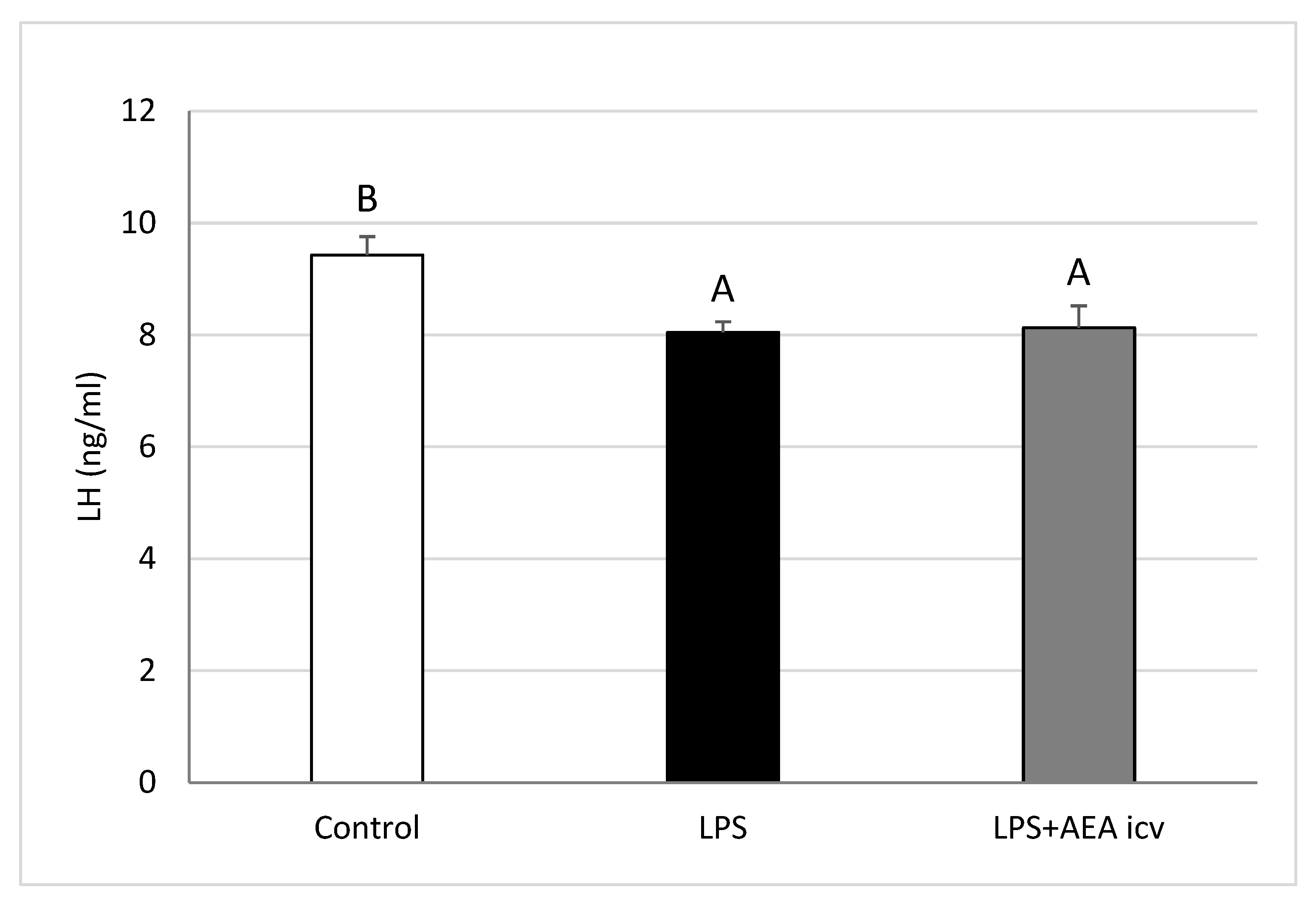
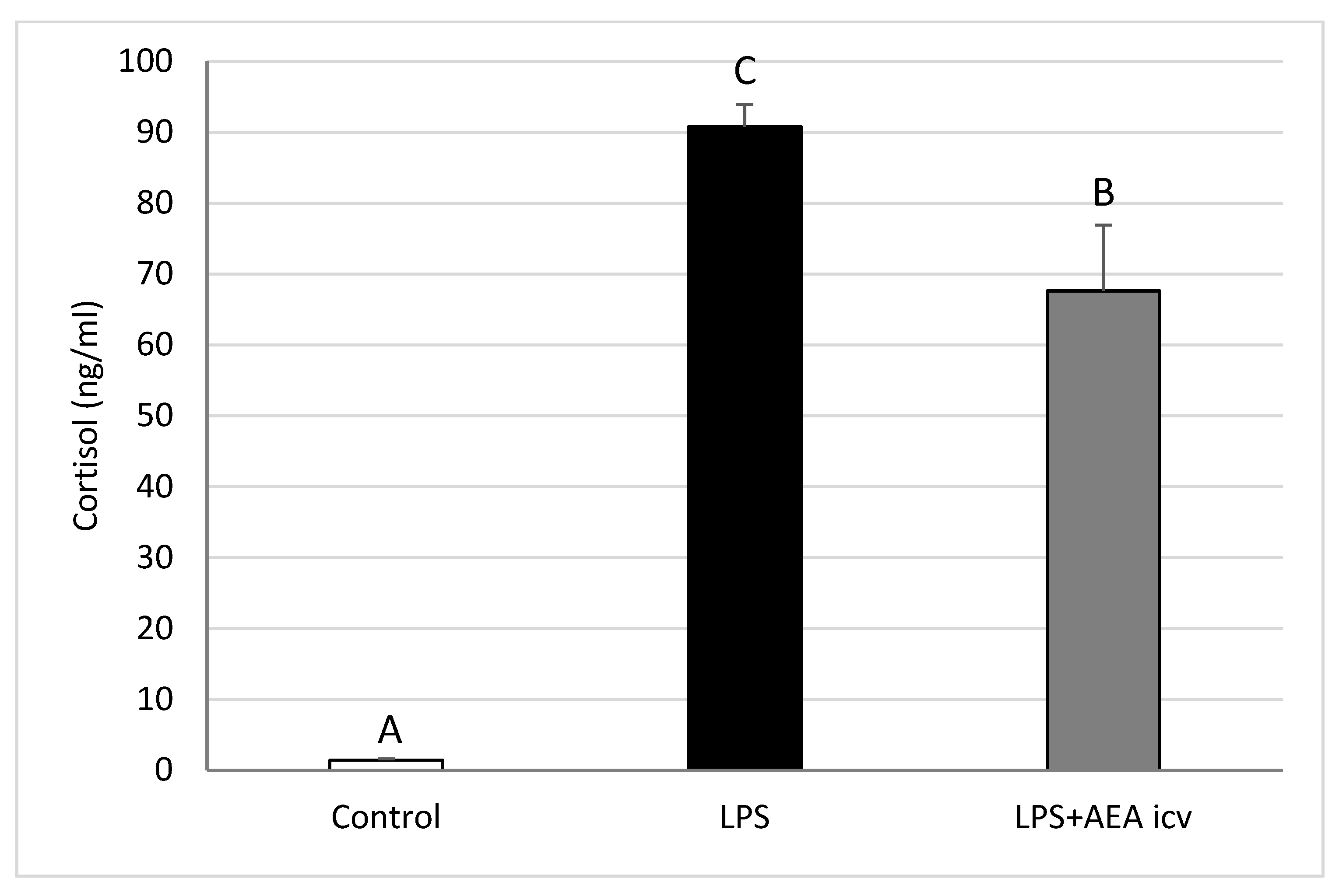
2.1. Blood LH and Cortisol Concentrations
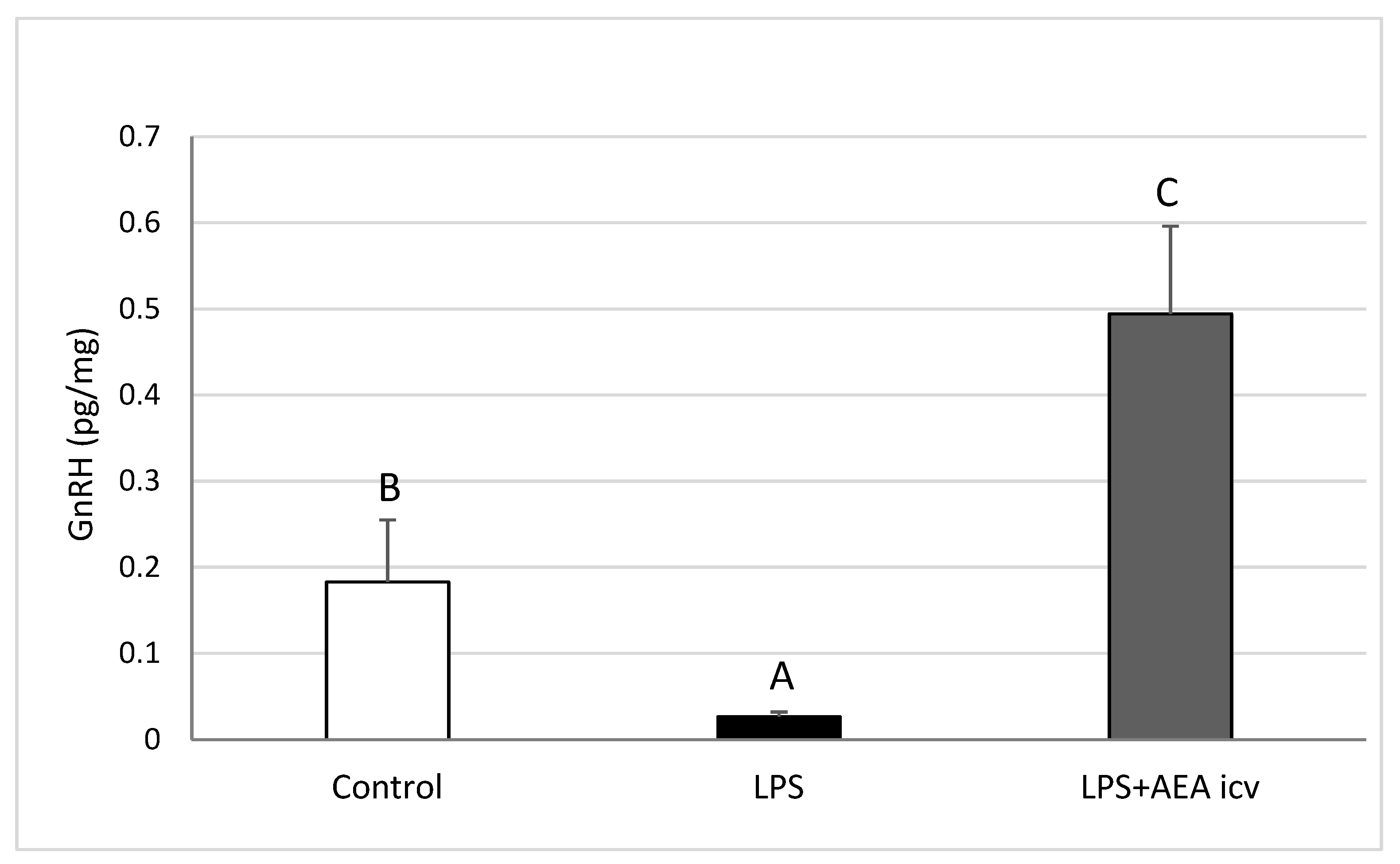
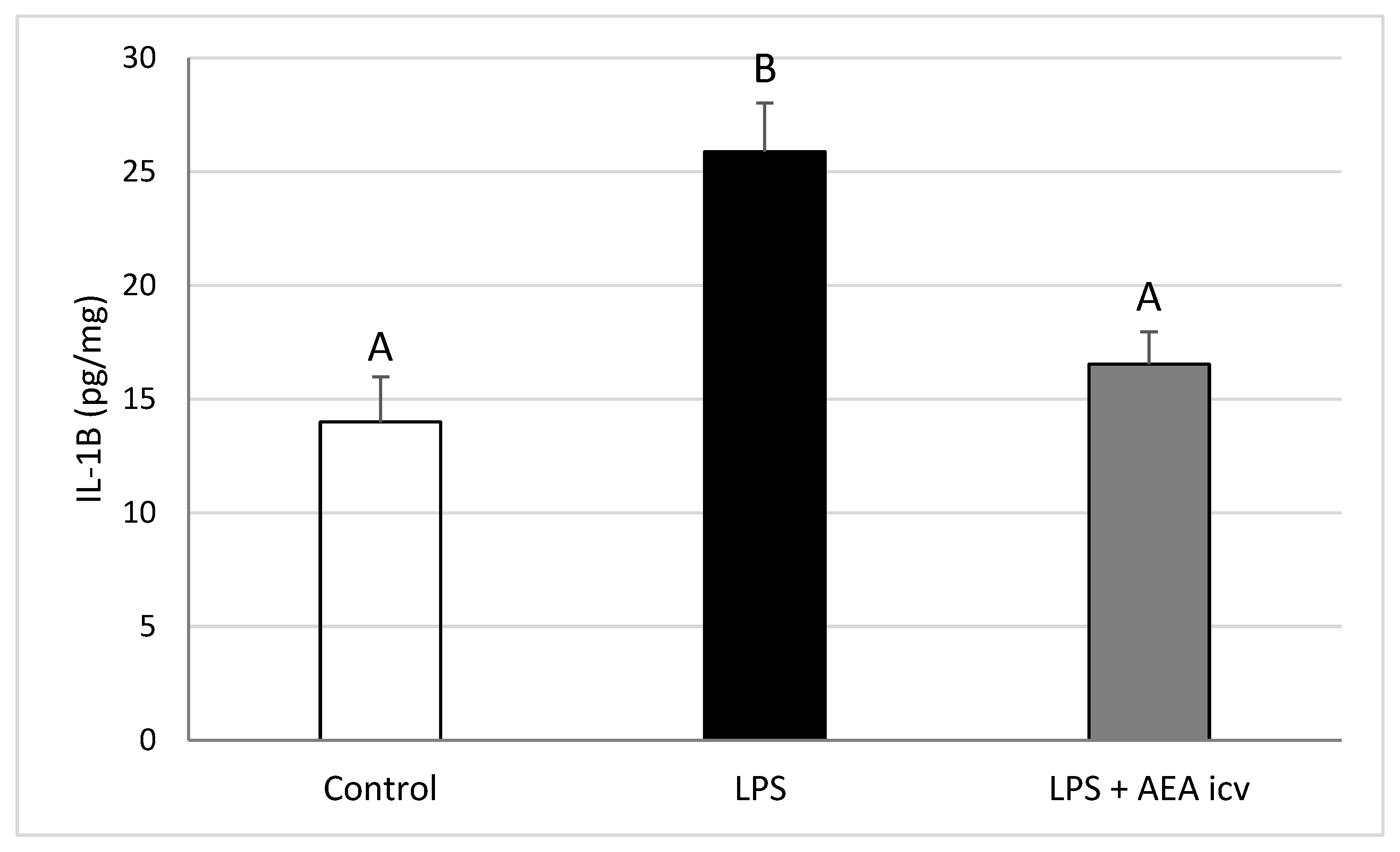
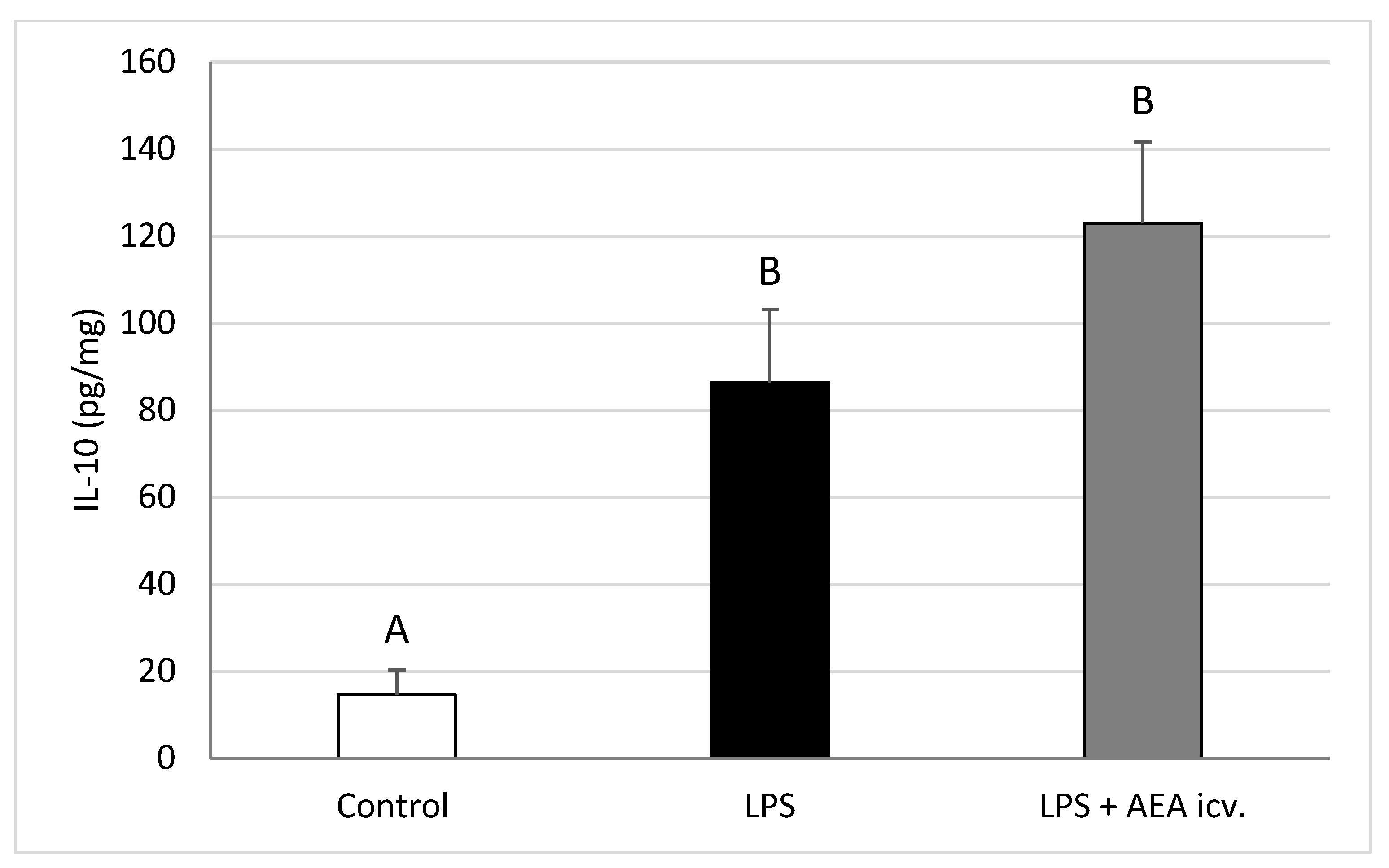
2.2. GnRH and Cytokine Levels in the POA
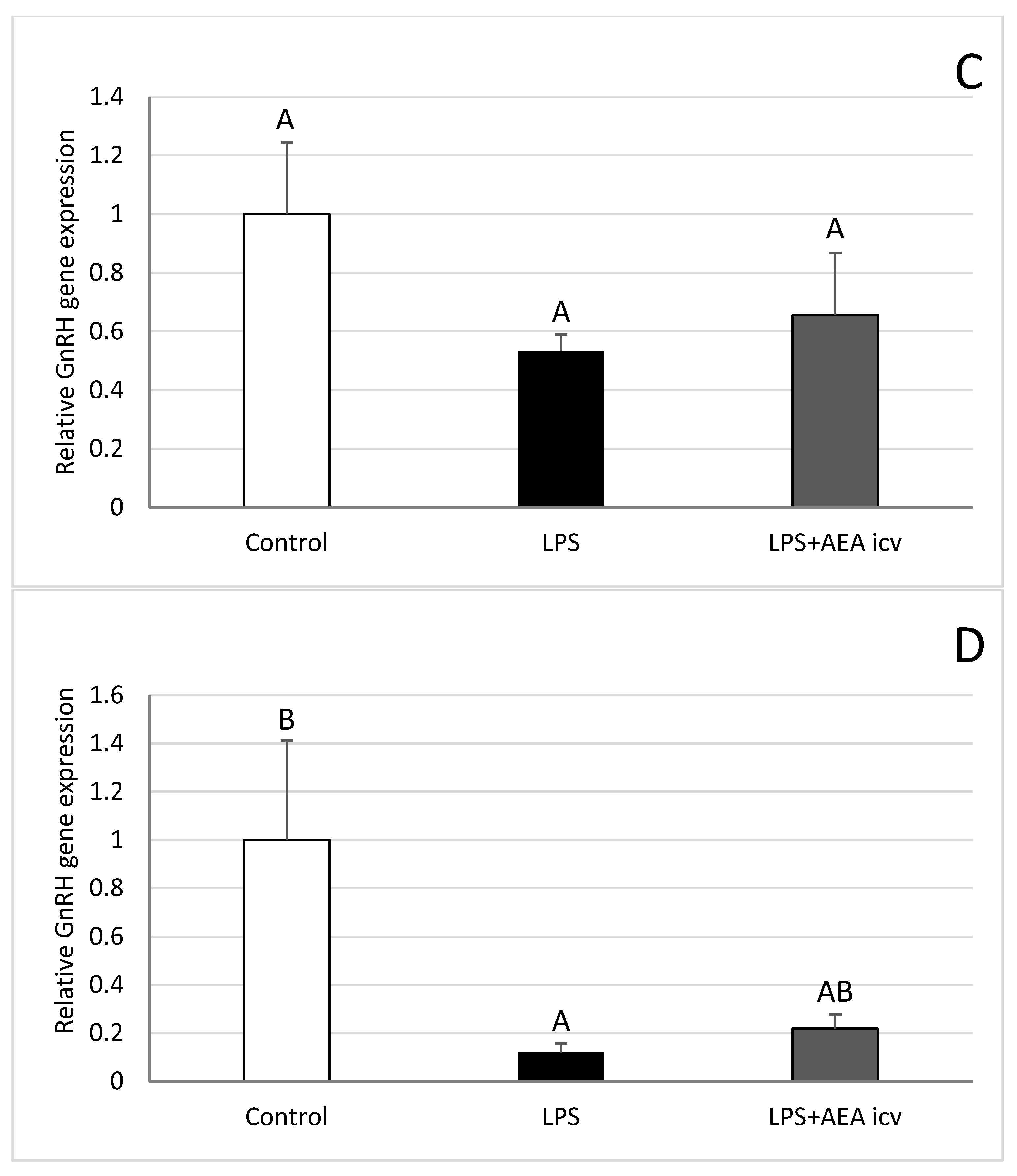
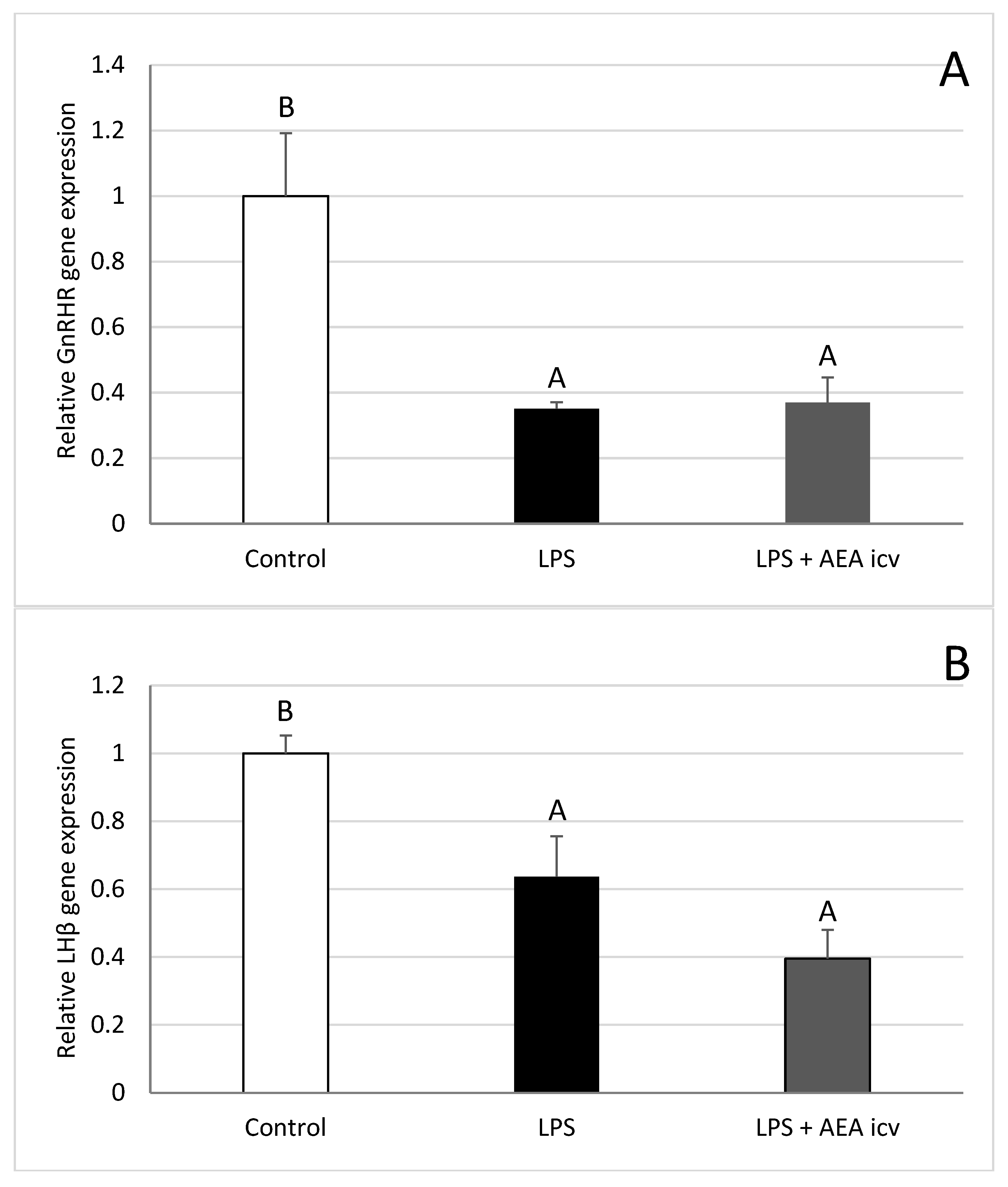
2.3. GnRH and GnRHR Gene Expression in the Hypothalamus
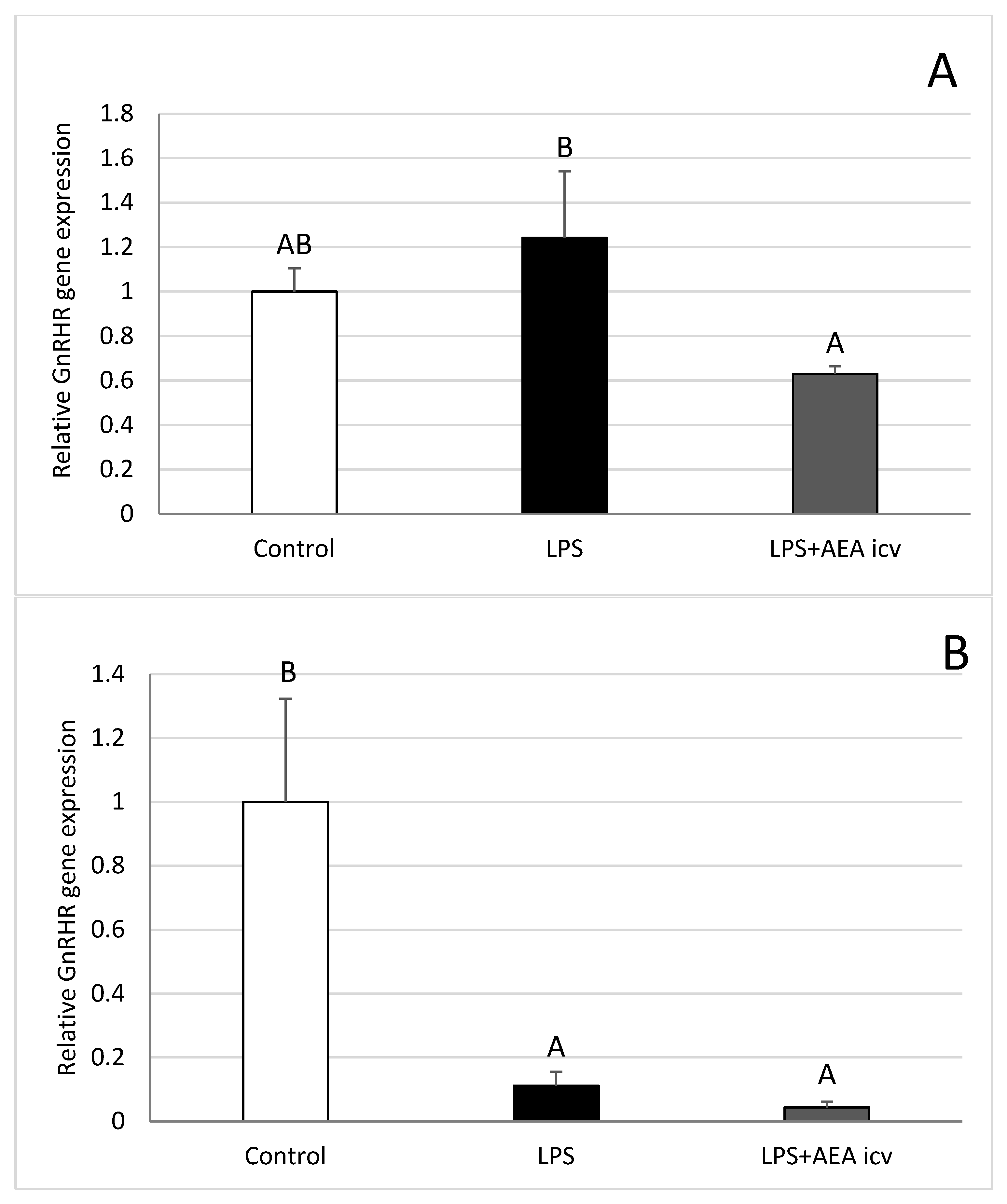
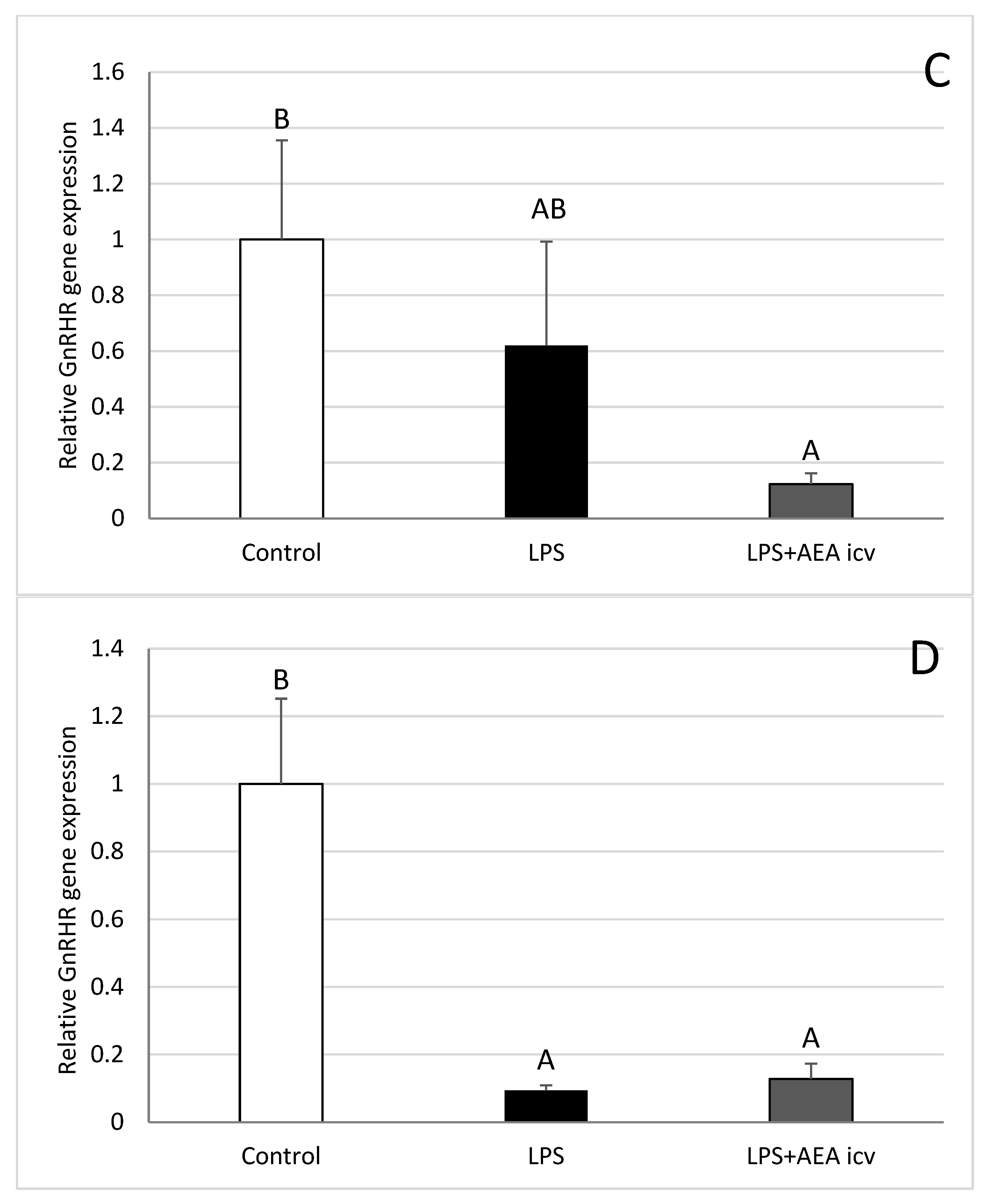
2.4. GnRHR and LH Gene Expression in AP
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Procedures
4.2. Assays
4.2.1. Radioimmunoassay for LH, FSH, and Cortisol
4.2.2. ELISA for GnRH Concentration in the POA
4.2.3. Relative Gene Expression
4.2.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Herman, A.P.; Tomczyk, M.; Wójcik, M.; Bochenek, J.; Antushevich, H.; Herman, A.; Wiechetek, W.; Szczepkowska, A.; Marciniak, E.; Tomaszewska-Zaremba, D. Effect of Caffeine on the Inflammatory-Dependent Changes in the GnRH/LH Secretion in a Female Sheep Model. Int. J. Mol. Sci. 2024, 25, 2663. [Google Scholar] [CrossRef]
- Weiss, G.; Goldsmith, L.T.; Taylor, R.N.; Bellet, D.; Taylor, H.S. Inflammation in Reproductive Disorders. Reprod. Sci. 2009, 16, 216–229. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Cronin, J.G.; Healey, G.D.; Gabler, C.; Heuwieser, W.; Streyl, D.; Bromfield, J.J.; Miyamoto, A.; Fergani, C.; Dobson, H. Innate Immunity and Inflammation of the Bovine Female Reproductive Tract in Health and Disease. Reproduction 2014, 148, R41–R51. [Google Scholar] [CrossRef]
- Daniel, J.A.; Abrams, M.S.; deSouza, L.; Wagner, C.G.; Whitlock, B.K.; Sartin, J.L. Endotoxin Inhibition of Luteinizing Hormone in Sheep. Domest. Anim. Endocrinol. 2003, 25, 13–19. [Google Scholar] [CrossRef]
- Refojo, D.; Arias, P.; Moguilevsky, J.A.; Feleder, C. Effect of Bacterial Endotoxin on in Vivo Pulsatile Gonadotropin Secretion in Adult Male Rats. Neuroendocrinology 1998, 67, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.Y.; Harris, T.G.; Battaglia, D.F.; Viguie, C.; Karsch, F.J. Endotoxin Inhibits Pituitary Responsiveness to Gonadotropin-Releasing Hormone. Endocrinology 2001, 142, 8. [Google Scholar] [CrossRef]
- Haziak, K.; Herman, A.P.; Wojtulewicz, K.; Pawlina, B.; Paczesna, K.; Bochenek, J.; Tomaszewska-Zaremba, D. Effect of CD14/TLR4 Antagonist on GnRH/LH Secretion in Ewe during Central Inflammation Induced by Intracerebroventricular Administration of LPS. J. Anim. Sci. Biotechnol. 2018, 9, 52. [Google Scholar] [CrossRef]
- Herman, A.P.; Krawczyńska, A.; Bochenek, J.; Dobek, E.; Herman, A.; Tomaszewska-Zaremba, D. LPS-Induced Inflammation Potentiates the IL-1-Mediated Reduction of LH Secretion from the Anterior Pituitary Explants. Clin. Dev. Immunol. 2013, 2013, 926937. [Google Scholar] [CrossRef] [PubMed]
- Spangelo, B.L.; Judd, A.M.; Isakson, P.C.; Macleod, R.M. Interleukin-6 Stimulates Anterior Pituitary Hormone Release in Vitro. Endocrinology 1989, 125, 575–577. [Google Scholar] [CrossRef] [PubMed]
- Wojtulewicz, K.; Krawczyńska, A.; Tomaszewska-Zaremba, D.; Wójcik, M.; Herman, A.P. Effect of Acute and Prolonged Inflammation on the Gene Expression of Proinflammatory Cytokines and Their Receptors in the Anterior Pituitary Gland of Ewes. Int. J. Mol. Sci. 2020, 21, 6939. [Google Scholar] [CrossRef]
- Zakharova, L.; Sharova, V.; Izvolskaia, M. Mechanisms of Reciprocal Regulation of Gonadotropin-Releasing Hormone (GnRH)-Producing and Immune Systems: The Role of GnRH, Cytokines and Their Receptors in Early Ontogenesis in Normal and Pathological Conditions. Int. J. Mol. Sci. 2021, 22, 114. [Google Scholar] [CrossRef]
- Bridgeman, M.B.; Abazia, D.T. Medicinal Cannabis: History, Pharmacology, And Implications for the Acute Care Setting. Pharm. Ther. 2017, 42, 180–188. [Google Scholar]
- Imtiaz, S.; Nigatu, Y.T.; Ali, F.; Douglas, L.; Hamilton, H.A.; Rehm, J.; Rueda, S.; Schwartz, R.M.; Wells, S.; Elton-Marshall, T. Cannabis Legalization and Cannabis Use, Daily Cannabis Use and Cannabis-Related Problems among Adults in Ontario, Canada (2001–2019). Drug Alcohol. Depend. 2023, 244, 109765. [Google Scholar] [CrossRef] [PubMed]
- Morano, A.; Fanella, M.; Albini, M.; Cifelli, P.; Palma, E.; Giallonardo, A.T.; Di Bonaventura, C. Cannabinoids in the Treatment of Epilepsy: Current Status and Future Prospects. Neuropsychiatr. Dis. Treat. 2020, 16, 381–396. [Google Scholar] [CrossRef] [PubMed]
- Blebea, N.M.; Pricopie, A.I.; Vlad, R.-A.; Hancu, G. Phytocannabinoids: Exploring Pharmacological Profiles and Their Impact on Therapeutical Use. Int. J. Mol. Sci. 2024, 25, 4204. [Google Scholar] [CrossRef]
- Laaboudi, F.-Z.; Rejdali, M.; Amhamdi, H.; Salhi, A.; Elyoussfi, A.; Ahari, M. In the Weeds: A Comprehensive Review of Cannabis; Its Chemical Complexity, Biosynthesis, and Healing Abilities. Toxicol. Rep. 2024, 13, 101685. [Google Scholar] [CrossRef]
- Devane, W.A.; Hanuš, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and Structure of a Brain Constituent That Binds to the Cannabinoid Receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef]
- Artmann, A.; Petersen, G.; Hellgren, L.I.; Boberg, J.; Skonberg, C.; Nellemann, C.; Hansen, S.H.; Hansen, H.S. Influence of Dietary Fatty Acids on Endocannabinoid and N-Acylethanolamine Levels in Rat Brain, Liver and Small Intestine. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2008, 1781, 200–212. [Google Scholar] [CrossRef]
- Di Marzo, V.; Bisogno, T.; De Petrocellis, L. The Biosynthesis, Fate and Pharmacological Properties of Endocannabinoids. In Cannabinoids; Pertwee, R.G., Ed.; Handbook of Experimental Pharmacology; Springer: Heidelberg/Berlin, Germany, 2005; Volume 168, pp. 147–185. ISBN 978-3-540-22565-2. [Google Scholar]
- Pertwee, R.G. Pharmacology of Cannabinoid CB1 and CB2 Receptors. Pharmacol. Ther. 1997, 74, 129–180. [Google Scholar] [CrossRef]
- El-Talatini, M.R.; Taylor, A.H.; Elson, J.C.; Brown, L.; Davidson, A.C.; Konje, J.C. Localisation and Function of the Endocannabinoid System in the Human Ovary. PLoS ONE 2009, 4, e4579. [Google Scholar] [CrossRef]
- Capozzi, A.; Caissutti, D.; Mattei, V.; Gado, F.; Martellucci, S.; Longo, A.; Recalchi, S.; Manganelli, V.; Riitano, G.; Garofalo, T.; et al. Anti-Inflammatory Activity of a CB2 Selective Cannabinoid Receptor Agonist: Signaling and Cytokines Release in Blood Mononuclear Cells. Molecules 2021, 27, 64. [Google Scholar] [CrossRef]
- Gammon, C.M.; Freeman, G.M.; Xie, W.; Petersen, S.L.; Wetsel, W.C. Regulation of Gonadotropin-Releasing Hormone Secretion by Cannabinoids. Endocrinology 2005, 146, 4491–4499. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M. Endocannabinoids: Friends and Foes of Reproduction. Prog. Lipid Res. 2009, 48, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Dey, S.K. Endocannabinoid Signaling in Female Reproduction. ACS Chem. Neurosci. 2012, 3, 349–355. [Google Scholar] [CrossRef]
- Tomaszewska-Zaremba, D.; Tomczyk, M.; Wojtulewicz, K.; Bochenek, J.; Pałatyńska, K.; Herman, A.P. Effect of Central Administration of Indomethacin on Anandamide-Induced GnRH/LH Secretion in the Hypothalamus of Anoestrous Ewes. J. Vet. Res. 2024, 68, 451–459. [Google Scholar] [CrossRef]
- Tomaszewska-Zaremba, D.; Gajewska, A.; Misztal, T. Anti-Inflammatory Effects of Cannabinoids in Therapy of Neurodegenerative Disorders and Inflammatory Diseases of the CNS. Int. J. Mol. Sci. 2025, 26, 6570. [Google Scholar] [CrossRef] [PubMed]
- Leonard, B.E.; Aricioglu, F. Cannabinoids and neuroinflammation: Therapeutic implications. J. Affect. Disord. Rep. 2023, 12, 100463. [Google Scholar] [CrossRef]
- Morcuende, A.; García-Gutiérrez, M.S.; Tambaro, S.; Nieto, E.; Manzanares, J.; Femenia, T. Immunomodulatory Role of CB2 Receptors in Emotional and Cognitive Disorders. Front. Psychiatry 2022, 13, 866052. [Google Scholar] [CrossRef]
- Herman, A.; Skipor, J.; Krawczyńska, A.; Bochenek, J.; Wojtulewicz, K.; Pawlina, B.; Antushevich, H.; Herman, A.; Tomaszewska-Zaremba, D. Effect of Central Injection of Neostigmine on the Bacterial Endotoxin Induced Suppression of GnRH/LH Secretion in Ewes during the Follicular Phase of the Estrous Cycle. Int. J. Mol. Sci. 2019, 20, 4598. [Google Scholar] [CrossRef]
- Kalra, P.S.; Edwards, T.G.; Xu, B.; Jain, M.; Kalra, S.P. The Anti-Gonadotropic Effects of Cytokines: The Role of Neuropeptides. Domest. Anim. Endocrinol. 1998, 15, 321–332. [Google Scholar] [CrossRef]
- Wu, S.; Wolfe, A. Signaling of Cytokines Is Important in Regulation of GnRH Neurons. Mol. Neurobiol. 2012, 45, 119–125. [Google Scholar] [CrossRef]
- Wagner, S.; Auffermann, W.; Buser, P.; Lim, T.H.; Kircher, B.; Pflugfelder, P.; Higgins, C.B. Diagnostic Accuracy and Estimation of the Severity of Valvular Regurgitation from the Signal Void on Cine Magnetic Resonance Images. Am. Heart J. 1989, 118, 760–767. [Google Scholar] [CrossRef]
- Banks, W.A.; Ortiz, L.; Plotkin, S.R.; Kastin, A.J. Human Interleukin (IL) 1 Alpha, Murine IL-1 Alpha and Murine IL-1 Beta Are Transported from Blood to Brain in the Mouse by a Shared Saturable Mechanism. J. Pharmacol. Exp. Ther. 1991, 259, 988–996. [Google Scholar] [CrossRef]
- Herman, A.P.; Krawczyńska, A.; Bochenek, J.; Antushevich, H.; Herman, A.; Tomaszewska-Zaremba, D. Peripheral Injection of SB203580 Inhibits the Inflammatory-Dependent Synthesis of Proinflammatory Cytokines in the Hypothalamus. BioMed Res. Int. 2014, 2014, 475152. [Google Scholar] [CrossRef]
- Smith, J.A.; Das, A.; Ray, S.K.; Banik, N.L. Role of Pro-Inflammatory Cytokines Released from Microglia in Neurodegenerative Diseases. Brain Res. Bull. 2012, 87, 10–20. [Google Scholar] [CrossRef]
- Chen, G.; McCuskey, R.S.; Reichlin, S. Blood Interleukin-6 and Tumor Necrosis Factor-α Elevation after Intracerebroventricular Injection of Escherichia coli Endotoxin in the Rat Is Determined by Two Opposing Factors: Peripheral Induction by LPS Transferred from Brain to Blood and Inhibition of Peripheral Response by a Brain-Mediated Mechanism. Neuroimmunomodulation 2000, 8, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Robinson, S.M. Minimal Penetration of Lipopolysaccharide across the Murine Blood–Brain Barrier. Brain Behav. Immun. 2010, 24, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Scorticati, C.; Fernández-Solari, J.; De Laurentiis, A.; Mohn, C.; Prestifilippo, J.P.; Lasaga, M.; Seilicovich, A.; Billi, S.; Franchi, A.; McCann, S.M.; et al. The Inhibitory Effect of Anandamide on Luteinizing Hormone-Releasing Hormone Secretion Is Reversed by Estrogen. Proc. Natl. Acad. Sci. USA 2004, 101, 11891–11896. [Google Scholar] [CrossRef]
- Fernández-Solari, J.; Scorticati, C.; Mohn, C.; De Laurentiis, A.; Billi, S.; Franchi, A.; McCann, S.M.; Rettori, V. Alcohol Inhibits Luteinizing Hormone-Releasing Hormone Release by Activating the Endocannabinoid System. Proc. Natl. Acad. Sci. USA 2004, 101, 3264–3268. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska-Zaremba, D.; Mateusiak, K.; Przekop, F. The Role of GabaA Receptors in the Neural Systems of the Ventromedial Hypothalamus-Nucleus Infundibular Region in the Control of GnRH Release in Ewes during Follicular Phase. Exp. Clin. Endocrinol. Diabetes 2003, 111, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Avraham, Y.; Mechoulam, R.; Berry, E.M. Low Dose Anandamide Affects Food Intake, Cognitive Function, Neurotransmitter and Corticosterone Levels in Diet-Restricted Mice. Eur. J. Pharmacol. 2000, 392, 147–156. [Google Scholar] [CrossRef]
- Ciechanowska, M.; Łapot, M.; Malewski, T.; Mateusiak, K.; Misztal, T.; Przekop, F. Effects of GABAA Receptor Modulation on the Expression of GnRH Gene and GnRH Receptor (GnRH-R) Gene in the Hypothalamus and GnRH-R Gene in the Anterior Pituitary Gland of Follicular-Phase Ewes. Anim. Reprod. Sci. 2009, 111, 235–248. [Google Scholar] [CrossRef]
- Scorticati, C.; Mohn, C.; De Laurentiis, A.; Vissio, P.; Fernández Solari, J.; Seilicovich, A.; McCann, S.M.; Rettori, V. The Effect of Anandamide on Prolactin Secretion Is Modulated by Estrogen. Proc. Natl. Acad. Sci. USA 2003, 100, 2134–2139. [Google Scholar] [CrossRef]
- Herman, A.P.; Tomaszewska-Zaremba, D. Effect of Endotoxin on the Expression of GnRH and GnRHR Genes in the Hypothalamus and Anterior Pituitary Gland of Anestrous Ewes. Anim. Reprod. Sci. 2010, 120, 105–111. [Google Scholar] [CrossRef]
- Haziak, K.; Herman, A.; Tomaszewska-Zaremba, D. The Effect of LPS on LH Release and Gene Expression of LH-β, GnRH-R and TLR4 in the Anterior Pituitary of Follicular Phase Ewes—An in Vitro Study. J. Anim. Feed. Sci. 2013, 22, 97–105. [Google Scholar] [CrossRef]
- Lohrer, P.; Gloddek, J.; Nagashima, A.C.; Korali, Z.; Hopfner, U.; Pereda, M.P.; Arzt, E.; Stalla, G.K.; Renner, U. Lipopolysaccharide directly stimulates the intrapituitary interleukin-6 production by folliculostellate cells via specific receptors and the p38alpha mitogen-activated protein kinase/nuclear factor-kappaB pathway. Endocrinology 2000, 144, 4457–4465. [Google Scholar] [CrossRef]
- Wenger, T.; Croix, D.; Tramu, G. The Effect of Chronic Prepubertal Administration of Marihuana (Delta-9-Tetrahydrocannabinol) on the Onset of Puberty and the Postpubertal Reproductive Functions in Female Rats. Biol. Reprod. 1988, 39, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Steger, R.W.; Silverman, A.Y.; Siler-Khodr, T.M.; Asch, R.H. The Effect of Λ9-Tetrahydrocannabinol on the Positive and Negative Feedback Control of Luteinizing Hormone Release. Life Sci. 1980, 27, 1911–1916. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska-Zaremba, D.; Wojtulewicz, K.; Paczesna, K.; Tomczyk, M.; Biernacka, K.; Bochenek, J.; Herman, A.P. The Influence of Anandamide on the Anterior Pituitary Hormone Secretion in Ewes—Ex Vivo Study. Animals 2020, 10, 706. [Google Scholar] [CrossRef]
- Dokladny, K.; Lobb, R.; Wharton, W.; Ma, T.Y.; Moseley, P.L. LPS-induced cytokine levels are repressed by elevated expression of HSP70 in rats: Possible role of NF-kappaB. Cell Stress Chaperon. 2010, 5, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Dorresteijn, M.J.; Draisma, A.; van der Hoeven, J.G.; Pickkers, P. Lipopolysaccharide-stimulated whole blood cytokine production does not predict the inflammatory response in human endotoxemia. Innate Immun. 2010, 16, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Marquette, C.; Van Dam, A.M.; Ban, E.; Lanièce, P.; Crumeyrolle-Arias, M.; Fillion, G.; Berkenbosch, F.; Haour, F. Rat interleukin-1 beta binding sites in rat hypothalamus and pituitary gland. Neuroendocrinology 1995, 62, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Jindal, K.C.; Chaudhary, R.S.; Singla, A.K.; Gangwal, S.S.; Khanna, S. Dissolution Test Method for Rifampicin-Isoniazid Fixed Dose Formulations. J. Pharm. Biomed. Anal. 1994, 12, 493–497. [Google Scholar] [CrossRef]
- Warzecha, Z.; Dembinski, A.; Ceranowicz, P.; Dembinski, M.; Cieszkowski, J.; Kownacki, P.; Konturek, P.C. Role of Sensory Nerves in Gastroprotective Effect of Anandamide in Rats. J. Physiol. Pharmacol. 2011, 62, 207–217. [Google Scholar]
- Mecha, M.; Feliú, A.; Iñigo, P.M.; Mestre, L.; Carrillo-Salinas, F.J.; Guaza, C. Cannabidiol Provides Long-Lasting Protection against the Deleterious Effects of Inflammation in a Viral Model of Multiple Sclerosis: A Role for A2A Receptors. Neurobiol. Dis. 2013, 59, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Croxford, J.L.; Miller, S.D. Immunoregulation of a Viral Model of Multiple Sclerosis Using the Synthetic Cannabinoid R(+)WIN55,212. J. Clin. Investig. 2003, 111, 1231–1240. [Google Scholar] [CrossRef]
- Correa, F.; Hernangómez, M.; Mestre, L.; Loría, F.; Spagnolo, A.; Docagne, F.; Di Marzo, V.; Guaza, C. Anandamide Enhances IL-10 Production in Activated Microglia by Targeting CB2 Receptors: Roles of ERK1/2, JNK, and NF-κB. Glia 2010, 58, 135–147. [Google Scholar] [CrossRef]
- Battista, N.; Meccariello, R.; Cobellis, G.; Fasano, S.; Di Tommaso, M.; Pirazzi, V.; Konje, J.C.; Pierantoni, R.; Maccarrone, M. The Role of Endocannabinoids in Gonadal Function and Fertility along the Evolutionary Axis. Mol. Cell. Endocrinol. 2012, 355, 1–14. [Google Scholar] [CrossRef]
- Turcotte, C.; Chouinard, F.; Lefebvre, J.S.; Flamand, N. Regulation of Inflammation by Cannabinoids, the Endocannabinoids 2-Arachidonoyl-Glycerol and Arachidonoyl-Ethanolamide, and Their Metabolites. J. Leukoc. Biol. 2015, 97, 1049–1070. [Google Scholar] [CrossRef]
- Stella, N. Endocannabinoid Signaling in Microglial Cells. Neuropharmacology 2009, 56, 244–253. [Google Scholar] [CrossRef]
- De Meij, J.; Alfanek, Z.; Morel, L.; Decoeur, F.; Leyrolle, Q.; Picard, K.; Carrier, M.; Aubert, A.; Séré, A.; Lucas, C.; et al. Microglial cannabinoid type 1 receptor regulates brain inflammation in a sex-specific manner. Cannabis Cannabinoid Res. 2021, 6, 488–507. [Google Scholar] [CrossRef]
- Tanasescu, R.; Constantinescu, C.S. Cannabinoids and the Immune System: An Overview. Immunobiology 2010, 215, 588–597. [Google Scholar] [CrossRef]
- Aguilera, G.; Abou-Samra, A.B.; Harwood, J.P.; Catt, K.J. Corticotropin-releasing factor receptors: Characterization and actions in the anterior pituitary. Adv. Exp. Med. Biol. 1988, 245, 83–105. [Google Scholar] [CrossRef]
- Aydin, C.; Yalcin, M. Peripheral mechanisms involved in the pressor and bradycardic effects of centrally administered arachidonic acid. Prostag. Leukotr. Ess. Fat. Acids 2008, 78, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, M.; Savci, V. Restoration of blood pressure by centrally injected U-46619, a thromboxane A2 analog, in haemorhaged hypotensive rats: Investigation of different brain areas. Pharmacology 2004, 70, 177–187. [Google Scholar] [CrossRef]
- Rettori, V.; McCann, S.M. The Mechanism of Action of Alcohol to Suppress Gonadotropin Secretion. Mol. Psychiatry 1997, 2, 350–354. [Google Scholar] [CrossRef]
- Erkan, L.G.; Altinbas, B.; Guvenc, G.; Alcay, S.; Toker, M.B.; Ustuner, B.; Kucuksen, D.U.; Yalcin, M. Brain thromboxane A2 via arachidonic acid cascade induces the hypothalamic-pituitary-gonadal axis activation in rats. Auton. Neurosci. 2015, 189, 50–55. [Google Scholar] [CrossRef]
- Tomczyk, M.; Tomaszewska-Zaremba, D.; Bochenek, J.; Herman, A.; Herman, A.P. Anandamide Influences Interleukin-1β Synthesis and IL-1 System Gene Expressions in the Ovine Hypothalamus during Endo-Toxin-Induced Inflammation. Animals 2021, 11, 484. [Google Scholar] [CrossRef] [PubMed]
- Oakley, A.E.; Breen, K.M.; Clarke, I.J.; Karsch, F.J.; Wagenmaker, E.R.; Tilbrook, A.J. Cortisol reduces gonadotropin-releasing hormone pulse frequency in follicular phase ewes: Influence of ovarian steroids. Endocrinology 2009, 150, 341–349. [Google Scholar] [CrossRef]
- Rodovalho-Callegari, F.V.; Rodrigues-Santos, I.; Lucion, A.B.; Rodovalho, G.V.; Leite, C.M.; De Paula, B.B.; Pestana-Oliveira, N.; Anselmo-Franci, J.A. Acute stress anticipates and amplifies the luteinizing hormone pre-ovulatory surge in rats: Role of noradrenergic neurons. Brain Res. 2022, 1781, 147805. [Google Scholar] [CrossRef] [PubMed]
- Riebe, C.J.; Wotjak, C.T. Endocannabinoids and stress. Stress 2011, 14, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Strzetelski, J. Standards for Ruminant Feeding; Instytut Zootechniki PIB: Kraków, Poland, 2009; ISBN 978-83-7607-072-8. [Google Scholar]
- Przybył, B.; Wójcik-Gładysz, A.; Gajewska, A.; Szlis, M. Brain-Derived Neurotrophic Factor (BDNF) Affects Somatotrophic Axis Activity in Sheep. J. Anim. Feed. Sci. 2021, 30, 329–339. [Google Scholar] [CrossRef]
- Naylor, D.; Sharma, A.; Li, Z.; Monteith, G.; Sullivan, T.; Canovas, A.; Karrow, N.A. Short communication: Characterizing ovine serum stress biomarkers during endotoxemia. J. Dairy. Sci. 2020, 103, 5501–5508. [Google Scholar] [CrossRef] [PubMed]
- Welento, J.; Szteyn, S.; Milart, Z. Observations on the Stereotaxic Configuration of the Hypothalamus Nuclei in the Sheep. Anat. Anz. 1969, 124, 1–27. [Google Scholar] [PubMed]
- Rasmussen, R. Quantification on the LightCycler. In Rapid Cycle Real-Time PCR; Meuer, S., Wittwer, C., Nakagawara, K.-I., Eds.; Springer: Heidelberg/Berlin, Germany, 2001; pp. 21–34. ISBN 978-3-540-66736-0. [Google Scholar]

| Graph. | No. of Animal | Experimental Treatment I (IV) | Dose [ng/kg] | Experimental Treatment II (IV/ICV) | Dose [µM/Animal] |
|---|---|---|---|---|---|
| Control | 6 | NaCl | 0 | NaCl | 0 |
| LPS IV | 6 | LPS | 400 | NaCl (iv.) | 0 |
| LPS IV and AEA ICV | 6 | LPS | 400 | AEA (ICV) | 100 |
| Total | 18 | ||||
| GenBank Acc. No. | Gene | Amplicon Size [bp] | Forward/Reverse | Sequence 5′ → 3′ | Ref. |
|---|---|---|---|---|---|
| NM_001034034 | GAPDH glyceraldehyde-3-phosphate dehydrogenase | 134 | forward | AGAAGGCTGGGGCTCACT | [45] |
| reverse | GGCATTGCTGACAATCTTGA | ||||
| U39357 | ACTB beta actin | 168 | forward | CTTCCTTCCTGGGCATGG | [45] |
| reverse | GGGCAGTGATCTCTTTCTGC | ||||
| BC108088.1 | HDAC1 histone deacetylase1 | 115 | forward | CTGGGGACCTACGGGATATT | [35] |
| reverse | GACATGACCGGCTTGAAAAT | ||||
| NM_001009397 | GnRHR gonadotropin-releasing hormone receptor | 150 | forward | TCTTTGCTGGACCACAGTTAT | [45] |
| reverse | GGCAGCTGAAGGTGAAAAAG | ||||
| U02517 | GnRH gonadotropin-releasing hormone | 123 | forward | GCCCTGGAGGAAAGAGAAAT | [45] |
| reverse | GAGGAGAATGGGACTGGTGA | ||||
| X52488 | LHB luteinizing hormone beta-subunit | 184 | forward | AGATGCTCCAGGGACTGCT | [45] |
| reverse | TGCTTCATGCTGAGGCAGTA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojtulewicz, K.; Tomaszewska-Zaremba, D.; Tomczyk, M.; Bochenek, J.; Herman, A.P. Effect of Central Injection of Anandamide on LPS-Dependent Suppression of GnRH/LH Secretion in Ewes During the Follicular Phase of the Estrous Cycle. Int. J. Mol. Sci. 2025, 26, 11246. https://doi.org/10.3390/ijms262311246
Wojtulewicz K, Tomaszewska-Zaremba D, Tomczyk M, Bochenek J, Herman AP. Effect of Central Injection of Anandamide on LPS-Dependent Suppression of GnRH/LH Secretion in Ewes During the Follicular Phase of the Estrous Cycle. International Journal of Molecular Sciences. 2025; 26(23):11246. https://doi.org/10.3390/ijms262311246
Chicago/Turabian StyleWojtulewicz, Karolina, Dorota Tomaszewska-Zaremba, Monika Tomczyk, Joanna Bochenek, and Andrzej P. Herman. 2025. "Effect of Central Injection of Anandamide on LPS-Dependent Suppression of GnRH/LH Secretion in Ewes During the Follicular Phase of the Estrous Cycle" International Journal of Molecular Sciences 26, no. 23: 11246. https://doi.org/10.3390/ijms262311246
APA StyleWojtulewicz, K., Tomaszewska-Zaremba, D., Tomczyk, M., Bochenek, J., & Herman, A. P. (2025). Effect of Central Injection of Anandamide on LPS-Dependent Suppression of GnRH/LH Secretion in Ewes During the Follicular Phase of the Estrous Cycle. International Journal of Molecular Sciences, 26(23), 11246. https://doi.org/10.3390/ijms262311246








