Self-Assembled Nanoparticles from Cationic Dipeptides and D-π-A Chromophores for Near-Infrared Photothermal Therapy
Abstract
1. Introduction
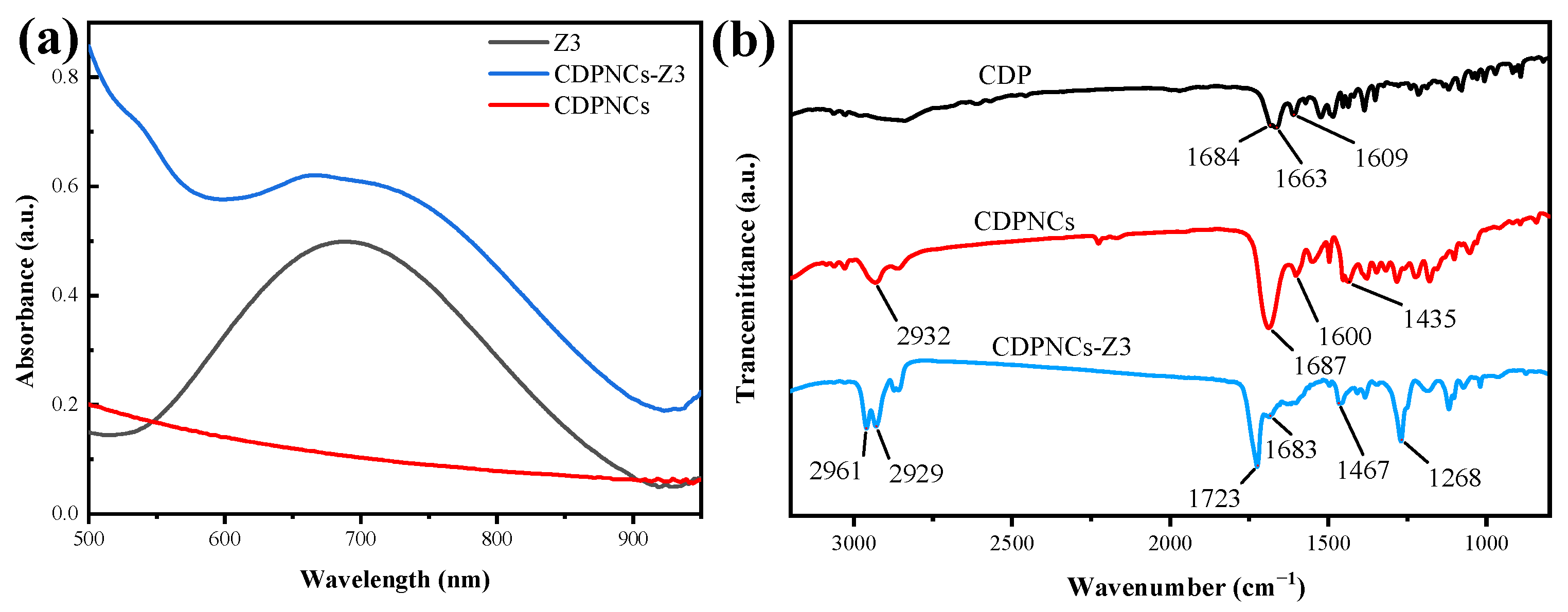
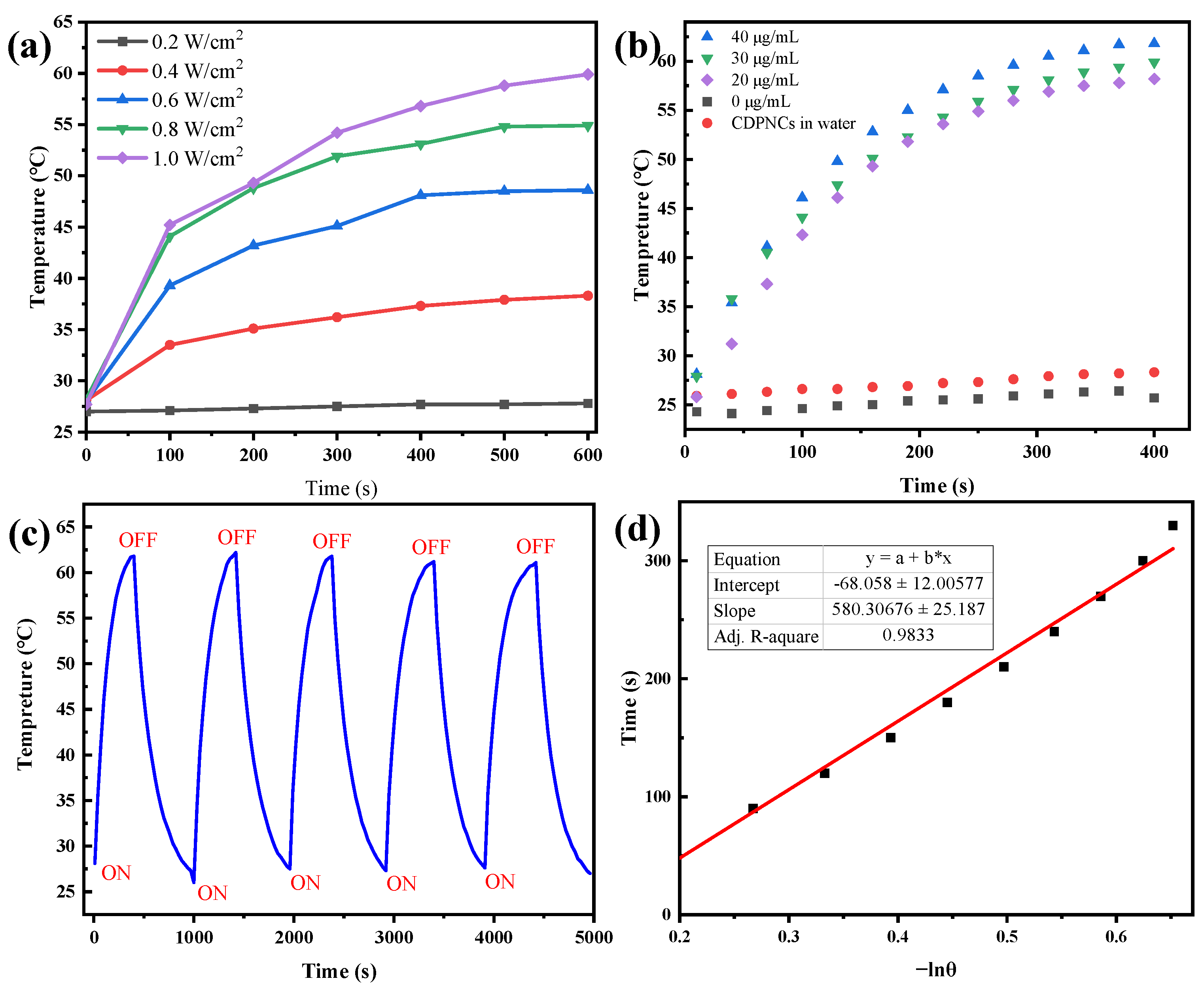
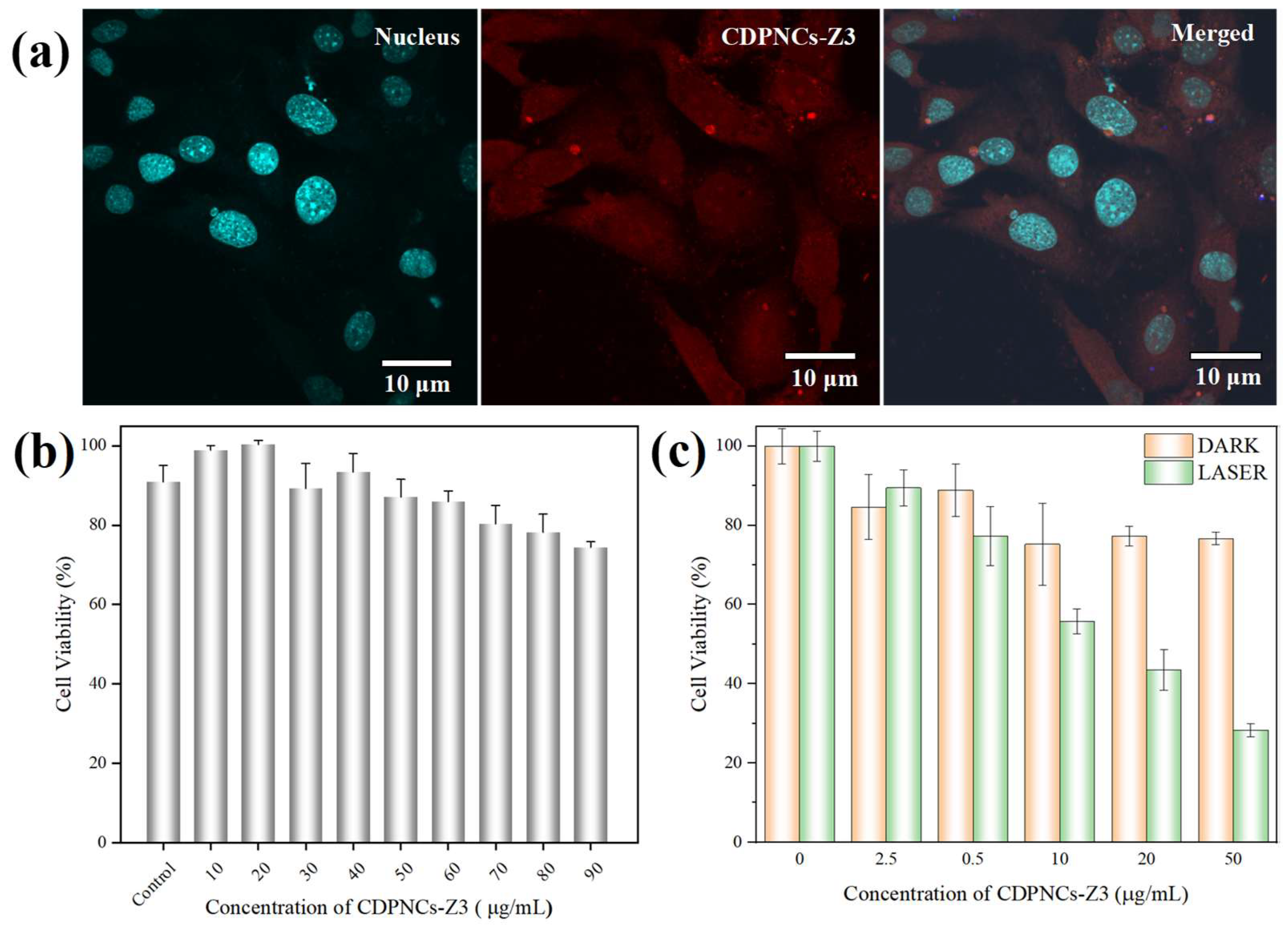
2. Results
3. Discussion
4. Materials and Methods
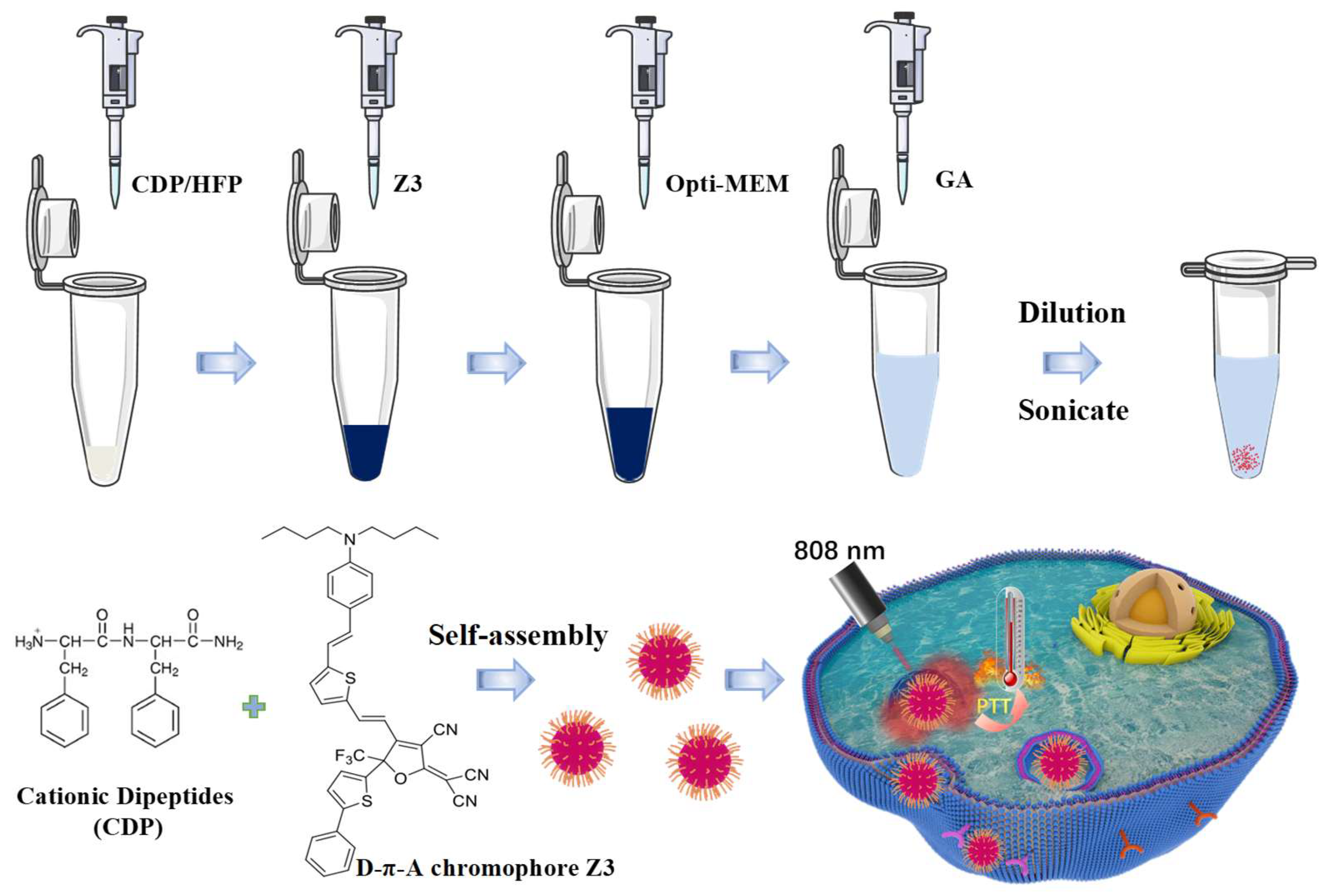
4.1. Preparation of CDPNCs-Z3 Assemblies
4.2. Morphological Characterization of CDPNCs-Z3
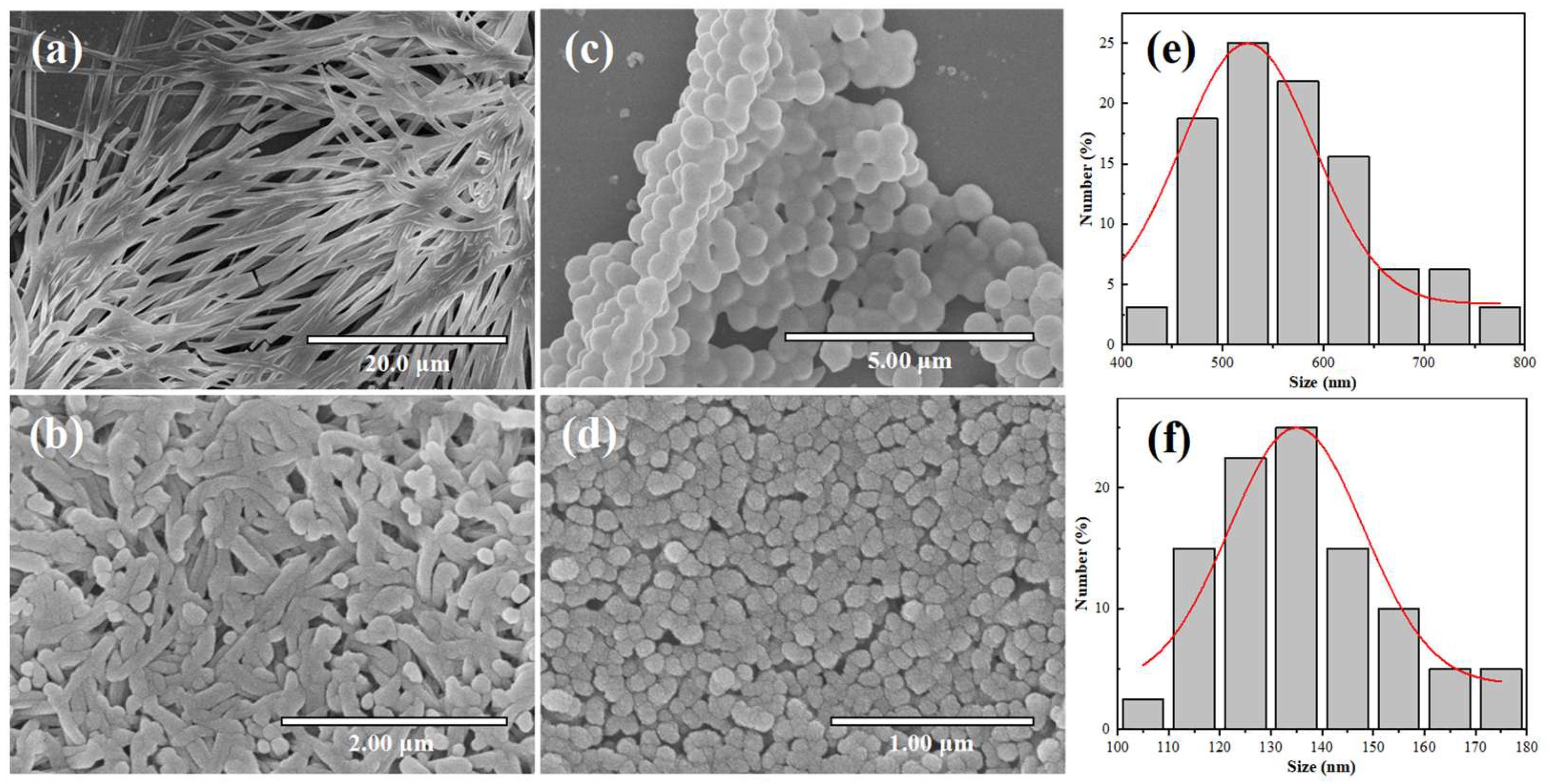
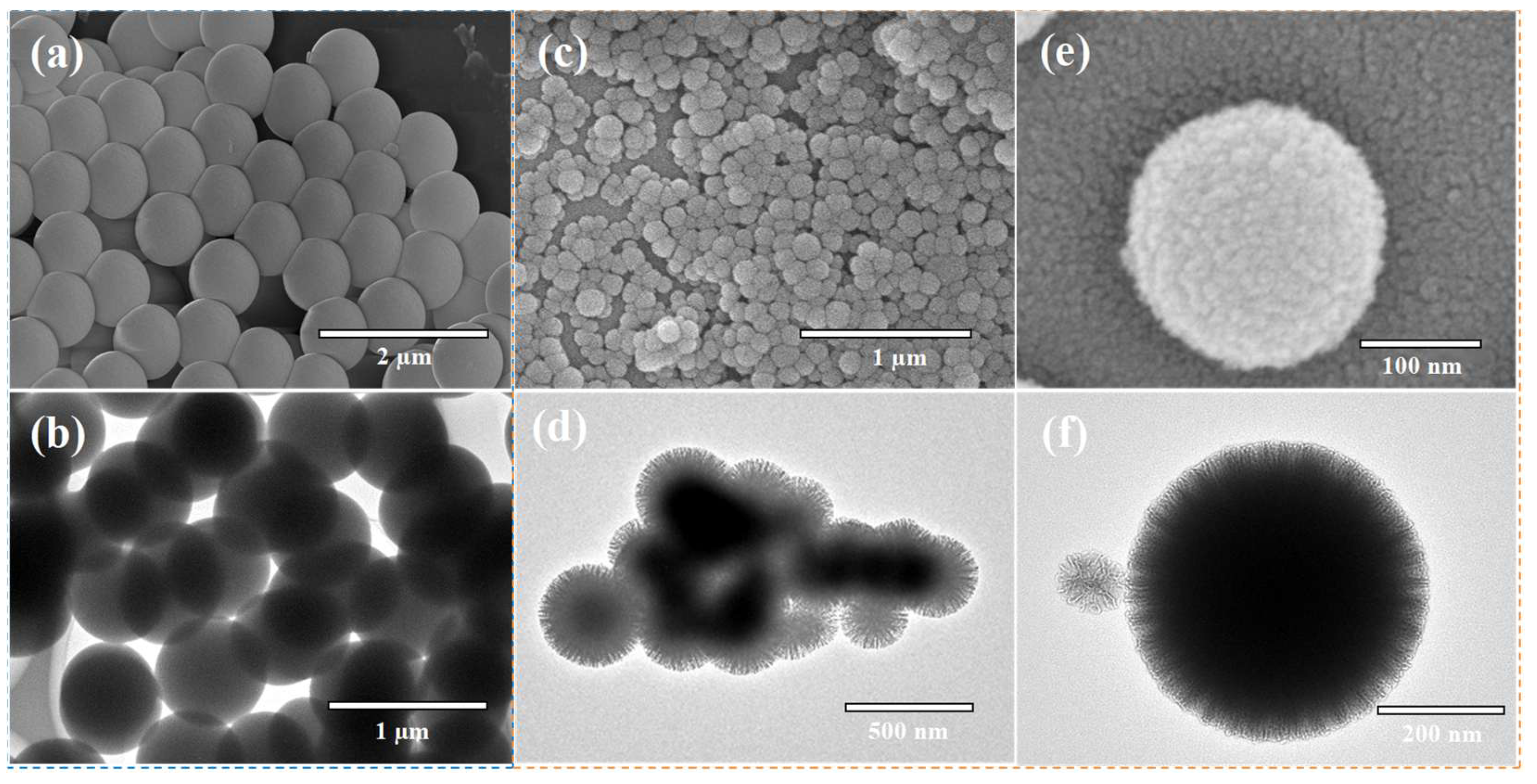
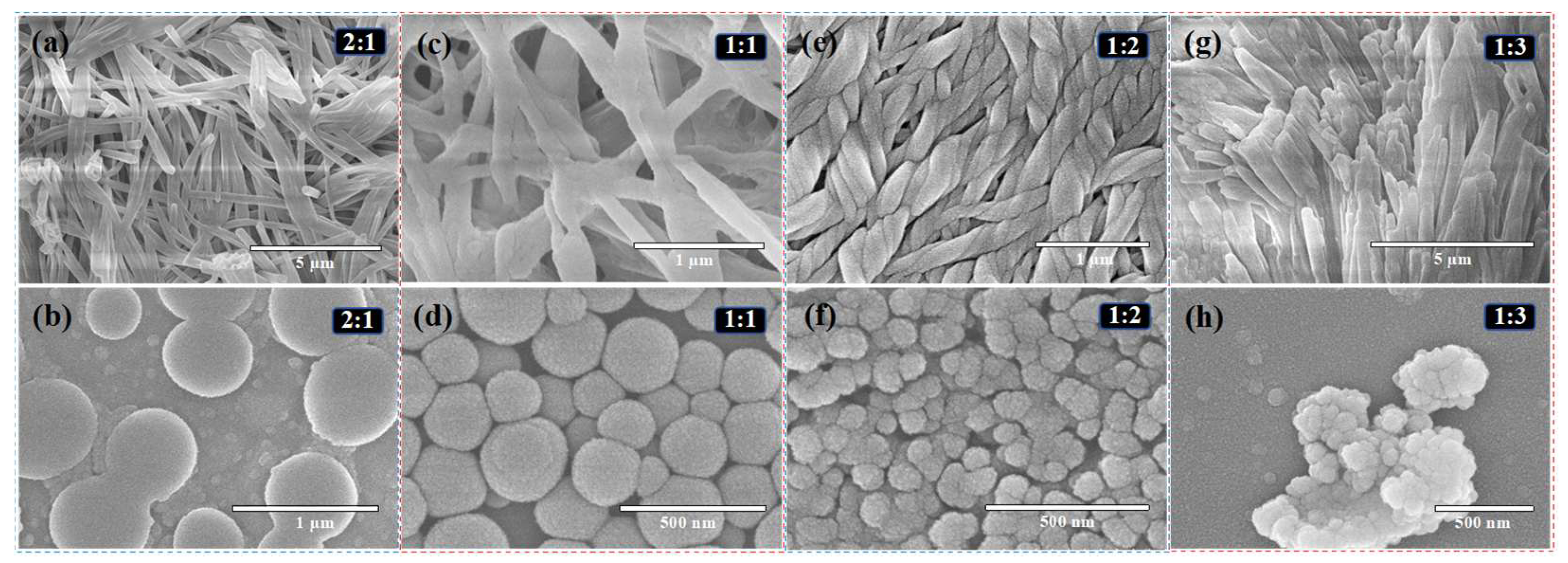
4.2.1. Scanning Electron Microscopy (SEM) Analysis
4.2.2. Transmission Electron Microscopy (TEM) Analysis
4.2.3. Particle Size Analysis
4.3. Spectroscopic Characterization
4.3.1. Ultraviolet-Visible (UV-Vis) Absorption Spectroscopy
4.3.2. Fourier-Transform Infrared (FTIR) Spectroscopy
4.4. Cytotoxicity Assay
4.5. Photothermal Performance Characterization of CDPNCs-Z3 Assemblies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, F.; Yang, S.; Zhao, C.; Liu, W.; Yao, X.; Yu, H.; Sun, X.; Liu, Y. γ-Glutamyl Transpeptidase-Activatable Near-Infrared Nanoassembly for Tumor Fluorescence Imaging-Guided Photothermal Therapy. Theranostics 2021, 11, 7045–7056. [Google Scholar] [CrossRef]
- Bian, W.Q.; Pan, Z.X.; Wang, Y.K.; Long, W.; Chen, Z.F.; Chen, N.P.; Zeng, Y.X.; Yuan, J.P.; Liu, X.J.; Lu, Y.-J.; et al. A Mitochondria-Targeted Thiazoleorange-Based Photothermal Agent for Enhanced Photothermal Therapy for Tumors. Bioorg. Chem. 2021, 113, 104954. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, X.; Hu, D.; Wang, P.; Liu, Q.; Zhang, X.; Jiang, J.; Liu, X.; Sheng, Z.; Liu, B.; et al. Phototheranostics: Active Targeting of Orthotopic Glioma Using Biomimetic Proteolipid Nanoparticles. ACS Nano 2019, 13, 386–398, Erratum in ACS Nano 2021, 15, 10733. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Verwilst, P.; Sharma, A.; Shin, J.; Sessler, J.L.; Kim, J.S. Organic Molecule-Based Photothermal Agents: An Expanding Photothermal Therapy Universe. Chem. Soc. Rev. 2018, 47, 2280–2297. [Google Scholar] [CrossRef] [PubMed]
- Carrese, B.; Cavallini, C.; Sanità, G.; Armanetti, P.; Silvestri, B.; Calì, G.; Pota, G.; Luciani, G.; Menichetti, L.; Lamberti, A. Controlled Release of Doxorubicin for Targeted Chemo-Photothermal Therapy in Breast Cancer HS578T Cells Using Albumin Modified Hybrid Nanocarriers. Int. J. Mol. Sci. 2021, 22, 11228. [Google Scholar] [CrossRef] [PubMed]
- Demina, P.A.; Khaydukov, K.V.; Babayeva, G.; Varaksa, P.O.; Atanova, A.V.; Stepanov, M.E.; Nikolaeva, M.E.; Krylov, I.V.; Evstratova, I.I.; Pokrovsky, V.S.; et al. Upconversion Nanoparticles Intercalated in Large Polymer Micelles for Tumor Imaging and Chemo/Photothermal Therapy. Int. J. Mol. Sci. 2023, 24, 10574. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Z.; Wei, Y.; Qi, W.; Li, J. Inorganic and Organic Hybrid Nanoarchitectonics for Biomedical Application. Adv. Colloid Interface Sci. 2025, 346, 103682. [Google Scholar] [CrossRef]
- Guo, S.; Gu, D.; Yang, Y.; Tian, J.; Chen, X. Near-Infrared Photodynamic and Photothermal Co-Therapy Based on Organic Small Molecular Dyes. J. Nanobiotechnol. 2023, 21, 348. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Y.; Tang, W.; Yue, S.; Wang, W.; Yao, H.; Xu, J.; Chen, Z.; Zhu, J. Self-Regenerating Photothermal Agents for Tandem Photothermal and Thermodynamic Tumor Therapy. Small Methods 2025, 9, 2400697. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; Chen, W.; Chen, H.; Yin, J. Near-Infrared Small Molecular Fluorescent Dyes for Photothermal Therapy. Chin. Chem. Lett. 2019, 30, 1353–1360. [Google Scholar] [CrossRef]
- Guo, B.; Huang, Z.M.; Shi, Q.; Middha, E.; Xu, S.D.; Li, L.X.; Wu, M.; Jiang, J.W.; Hu, Q.; Fu, Z.; et al. Organic Small Molecule Based Photothermal Agents with Molecular Rotors for Malignant Breast Cancer Therapy. Adv. Funct. Mater. 2020, 30, 1907093. [Google Scholar] [CrossRef]
- Lv, F.; Fan, X.; Liu, D.; Song, F. Photothermal Agents Based on Small Organic Fluorophores with Intramolecular Motion. Acta Biomater. 2022, 149, 16–29. [Google Scholar] [CrossRef]
- Du, B.; Liu, R.; Qu, C.; Qian, K.; Suo, Y.; Wu, F.; Chen, H.; Li, X.; Li, Y.; Liu, H.; et al. J-Aggregates Albumin-Based NIR-II Fluorescent Dye Nanoparticles for Cancer Phototheranostics. Mater. Today Bio. 2022, 16, 100366. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Zhang, G.-Q.; Ding, D. Design of Superior Phototheranostic Agents Guided by Jablonski Diagrams. Chem. Soc. Rev. 2020, 49, 8179–8234. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Zhao, X. Melanin-Like Nanomedicine in Photothermal Therapy Applications. Int. J. Mol. Sci. 2021, 22, 399. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Fang, Y.; Kwok, R.T.K.; Zhang, X.; Hu, X.; Lam, J.W.Y.; Ding, D.; Tang, B.Z. Highly Stable Organic Small Molecular Nanoparticles as an Advanced and Biocompatible Phototheranostic Agent of Tumor in Living Mice. ACS Nano 2017, 11, 7177–7188. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Zhang, Z.; Ji, L.; Zhang, J.; Wang, Q.; Guo, T.; Ni, S.; Cai, R.; Mu, X.; et al. Recent Progress on NIR-II Photothermal Therapy. Front. Chem. 2021, 9, 728066. [Google Scholar] [CrossRef]
- Lou, H.; Ji, A.; Qu, C.; Liu, H.; Jiang, L.; Chen, H.; Cheng, Z. A Small-Molecule Based Organic Nanoparticle for Photothermal Therapy and Near-Infrared-IIb Imaging. ACS Appl. Mater. Interfaces 2022, 14, 35454–35465. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, G. NIR Light-Responsive Nanocarriers for Controlled Release. J. Photochem. Photobiol. C 2021, 47, 100420. [Google Scholar] [CrossRef]
- Wang, Q.; Niu, X.; Yang, L.; Liu, J.; Wang, J.; Xu, X.; Tang, W.; Huang, W.; Fan, Q. Asymmetric Small Organic Molecule-Based NIR-II Fluorophores for High Performance Tumor Phototheranostics. Mater. Chem. Front. 2021, 5, 5689–5697. [Google Scholar] [CrossRef]
- Dai, Y.; Sun, J.; Fang, L.; Zhao, J.; Yu, F.; Wang, B. Recent Advancements of Porphyrin-Based Supramolecular Nanomaterials for Phototherapy. Coord. Chem. Rev. 2026, 547, 217121. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, J.; Chen, D.; Miao, S.; Wen, J.; Liu, C.; Xue, S.; Liu, Y.; Zhang, Q.; Shen, Y. “Cluster Bomb” Based Bismuth Nano-in-Micro Spheres Formed Dry Powder Inhalation for Thermo-Radio Sensitization Effects of Lung Metastatic Breast Cancer. Adv. Healthc. Mater. 2023, 12, 2202622. [Google Scholar] [CrossRef]
- Li, F.; Li, T.; Zhi, D.; Xu, P.; Wang, W.; Hu, Y.; Zhang, Y.; Wang, S.; Matula Thomas, J.; Beauchamp Norman, J.; et al. Novel Ultrasmall Multifunctional Nanodots for Dual-Modal MR/NIR-II Imaging-Guided Photothermal Therapy. Biomaterials 2020, 256, 120219. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Yang, Z.; Shen, H.; Chen, J.; Weitz, D.A.; Chen, D.; Sheng, J.; Liang, T. Interfacial Engineering of Biocompatible Nanocapsules for Near-Infrared-Triggered Drug Release and Photothermal Therapy. Adv. Sci. 2025, 12, 2410844. [Google Scholar] [CrossRef]
- Singh, M.; Kaur, G.; Singh, I. Molecular Self-Assembly of Peptides into Supramolecular Nanoarchitectures for Target-Specific Drug Delivery. ACS Appl. Bio Mater. 2025, 8, 4467–4488. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, S.; Guerin, T.; Toth, I.; Stephenson, R.J. Recent Advances in Self-Assembled Peptides: Implications for Targeted Drug Delivery and Vaccine Engineering. Adv. Drug Deliv. Rev. 2017, 110–111, 169–187. [Google Scholar] [CrossRef]
- Li, S.; Xing, R.; Chang, R.; Zou, Q.; Yan, X. Nanodrugs Based on Peptide-Modulated Self-Assembly: Design, Delivery and Tumor Therapy. Curr. Opin. Colloid Interface Sci. 2018, 35, 17–25. [Google Scholar] [CrossRef]
- Zhu, D.N.; Zhang, H.L.; Huang, Y.Z.; Lian, B.P.; Ma, C.; Han, L.Z.; Chen, Y.; Wu, S.M.; Li, N.; Zhang, W.J.; et al. A Self-Assembling Amphiphilic Peptide Dendrimer-Based Drug Delivery System for Cancer Therapy. Pharmaceutics 2021, 13, 1092. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, Y.; Xing, R.; Yan, X. Covalently Assembled Dipeptide Nanoparticles with Adjustable Fluorescence Emission for Multicolor Bioimaging. ChemBioChem 2019, 20, 555–560. [Google Scholar] [CrossRef]
- Ma, H.; Fei, J.; Li, Q.; Li, J. Photo-Induced Reversible Structural Transition of Cationic Diphenylalanine Peptide Self-Assembly. Small 2015, 11, 1787–1791. [Google Scholar] [CrossRef]
- Layek, B.; Singh, J. Editorial of Special Issue “Surface-Functionalized Nanoparticles as Drug Carriers”. Int. J. Mol. Sci. 2019, 20, 6352. [Google Scholar] [CrossRef]
- Kost, J.; Mathiowitz, E.; Azagury, A. Advances in Drug Delivery and Theranostics. Adv. Funct. Mater. 2021, 31, 2108838. [Google Scholar] [CrossRef]
- Yan, X.; He, Q.; Wang, K.; Duan, L.; Cui, Y.; Li, J. Transition of Cationic Dipeptide Nanotubes into Vesicles and Oligonucleotide Delivery. Angew. Chem. Int. Ed. 2007, 46, 2431–2434. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qing, Z.; Li, Y.; Zou, Z.; Yang, S.; Yang, R. Natural Peptide Probe Screened for High-Performance Fluorescent Sensing of Copper Ion: Especially Sensitivity, Rapidity, and Environment-Friendliness. ACS Omega 2019, 4, 793–800. [Google Scholar] [CrossRef]
- Liu, B.; Feng, W.; Ge, J.; Liu, Z.; Feng, S.; Chen, Z.; Bo, S. Organic Nanomedicine Containing Nonlinear Optical Chromophores for Ultrastable Photo-to-Heat Converting Theranostics in the Near-Infrared Window. Dye. Pigment. 2023, 210, 110962. [Google Scholar] [CrossRef]
- Li, Q.; Jia, Y.; Li, J.B. Controlled Assembly of Chiral Structure of Diphenylalanine Peptide. Acta Chim. Sin. 2019, 77, 1173. [Google Scholar] [CrossRef]
- Chen, W.; Sun, S.; Huang, G.; Ni, S.; Xu, L.; Dang, L.; Phillips, D.L.; Li, M.-D. Unprecedented Improvement of Near-Infrared Photothermal Conversion Efficiency to 87.2% by Ultrafast Non-Radiative Decay of Excited States of Self-Assembly Cocrystal. J. Phys. Chem. Lett. 2021, 12, 5796–5801. [Google Scholar] [CrossRef]
- Du, C.L.; Zhao, J.; Fei, J.B.; Cui, Y.; Li, J.B. Assembled Microcapsules by Doxorubicin and Polysaccharide as High Effective Anticancer Drug Carriers. Adv. Healthc. Mater. 2013, 2, 1246–1251. [Google Scholar] [CrossRef]
- Feng, W.; Zhao, W.; Chen, Z.; Zhang, C.; Ge, J.; Zheng, X.; Ren, H.; Bo, S. Organic Dye Nanoparticles with a Special D−π–A Structure for Photoacoustic Imaging and Photothermal Therapy. ACS Appl. Bio Mater. 2020, 3, 5722–5729. [Google Scholar] [CrossRef]
- Zhang, H.; Fei, J.B.; Yan, X.H.; Wang, A.H.; Li, J.B. Enzyme-Responsive Release of Doxorubicin from Monodisperse Dipeptide-Based Nanocarriers for Highly Efficient Cancer Treatment In Vitro. Adv. Funct. Mater. 2015, 25, 1193–1204. [Google Scholar] [CrossRef]
- Huang, Y.; Ye, D.; Liu, X.J.; Chen, H.; Luo, X.X.; Huang, B.; Zhou, N.Y.; Wang, H.X.; Zou, Q.C.; Fang, S.B.; et al. Spiky Tubular Nanoparticles with Low Protein Corona can Realize Efficient and Non-destructive Penetration through Endothelial Barrier. J. Control. Release 2024, 374, 1–14. [Google Scholar] [CrossRef]
- Huang, X.H.; Neretina, S.; El-Sayed, M. A Gold nanorods: From Synthesis and Properties to Biological and Biomedical Applications. Adv. Mater. 2009, 21, 4880–4910. [Google Scholar] [CrossRef] [PubMed]
- Decuzzi, P.; Ferrari, M. The Receptor-Mediated Endocytosis of Nonspherical Particles. Biophys. J. 2008, 94, 3790–3797. [Google Scholar] [CrossRef]
- Yang, D.J.; Gao, S.; Fang, Y.; Lin, X.J.; Jin, X.C.; Wang, X.Y.; Ke, L.Y.; Shi, K. The π-π Stacking-Guided Supramolecular Self-assembly of Nanomedicine for Effective Delivery of Antineoplastic Therapies. Nanomedicine 2018, 13, 3159–3177. [Google Scholar] [CrossRef]
- Dang, H.P.; Tian, Y.L.; Cheng, Q.; Teng, C.C.; Xie, K.; Yan, L.F. Galactose Conjugated BODIPY Dye and Hydrogen Bonding Promoted J-aggregates for Efficiently Targeted NIR-II Fluorescence Assistant Photothermal Therapy. J. Colloid Interface Sci. 2022, 612, 287–297. [Google Scholar] [CrossRef]
- Krenacs, T.; Meggyeshazi, N.; Forika, G.; Kiss, E.; Hamar, P.; Szekely, T.; Vancsik, T. Modulated Electro-Hyperthermia-Induced Tumor Damage Mechanisms Revealed in Cancer Models. Int. J. Mol. Sci. 2020, 21, 6270. [Google Scholar] [CrossRef]
- Hu, Q.; Wang, K.; Qiu, L. 6-Aminocaproic Acid as a Linker to Improve Near-Infrared Fluorescence Imaging and Phtothermal Cancer Therapy of PEGylated Indocyanine Green. Colloid Surf. B 2021, 197, 111372. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wan, H.; Jia, H.; Liu, L.; Wang, J. Porous Pt Nanoparticles with High Near-Infrared Photothermal Conversion Efficiencies for Photothermal Therapy. Adv. Healthc. Mater. 2016, 5, 3165–3172. [Google Scholar] [CrossRef]
- Santos, J.A.V.; Silva, D.; Marques, M.P.M.; Batista De Carvalho, L.A.E. Platinum-Based Chemotherapy: Trends in Organic Nanodelivery Systems. Nanoscale 2024, 16, 14640–14686. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.; Feng, L.; Zeng, Y.; Lin, J.; Bo, S.; Sun, N.; Zhang, X. Self-Assembled Nanoparticles from Cationic Dipeptides and D-π-A Chromophores for Near-Infrared Photothermal Therapy. Int. J. Mol. Sci. 2025, 26, 11235. https://doi.org/10.3390/ijms262211235
Zhou W, Feng L, Zeng Y, Lin J, Bo S, Sun N, Zhang X. Self-Assembled Nanoparticles from Cationic Dipeptides and D-π-A Chromophores for Near-Infrared Photothermal Therapy. International Journal of Molecular Sciences. 2025; 26(22):11235. https://doi.org/10.3390/ijms262211235
Chicago/Turabian StyleZhou, Wei, Liangxin Feng, Yanfei Zeng, Jiaxuan Lin, Shuhui Bo, Nan Sun, and Xiaoming Zhang. 2025. "Self-Assembled Nanoparticles from Cationic Dipeptides and D-π-A Chromophores for Near-Infrared Photothermal Therapy" International Journal of Molecular Sciences 26, no. 22: 11235. https://doi.org/10.3390/ijms262211235
APA StyleZhou, W., Feng, L., Zeng, Y., Lin, J., Bo, S., Sun, N., & Zhang, X. (2025). Self-Assembled Nanoparticles from Cationic Dipeptides and D-π-A Chromophores for Near-Infrared Photothermal Therapy. International Journal of Molecular Sciences, 26(22), 11235. https://doi.org/10.3390/ijms262211235





