Transcriptome and Biochemical Analysis of the Mechanism of Low-Temperature Germination in Acer truncatum Bunge Seeds
Abstract
1. Introduction
2. Results
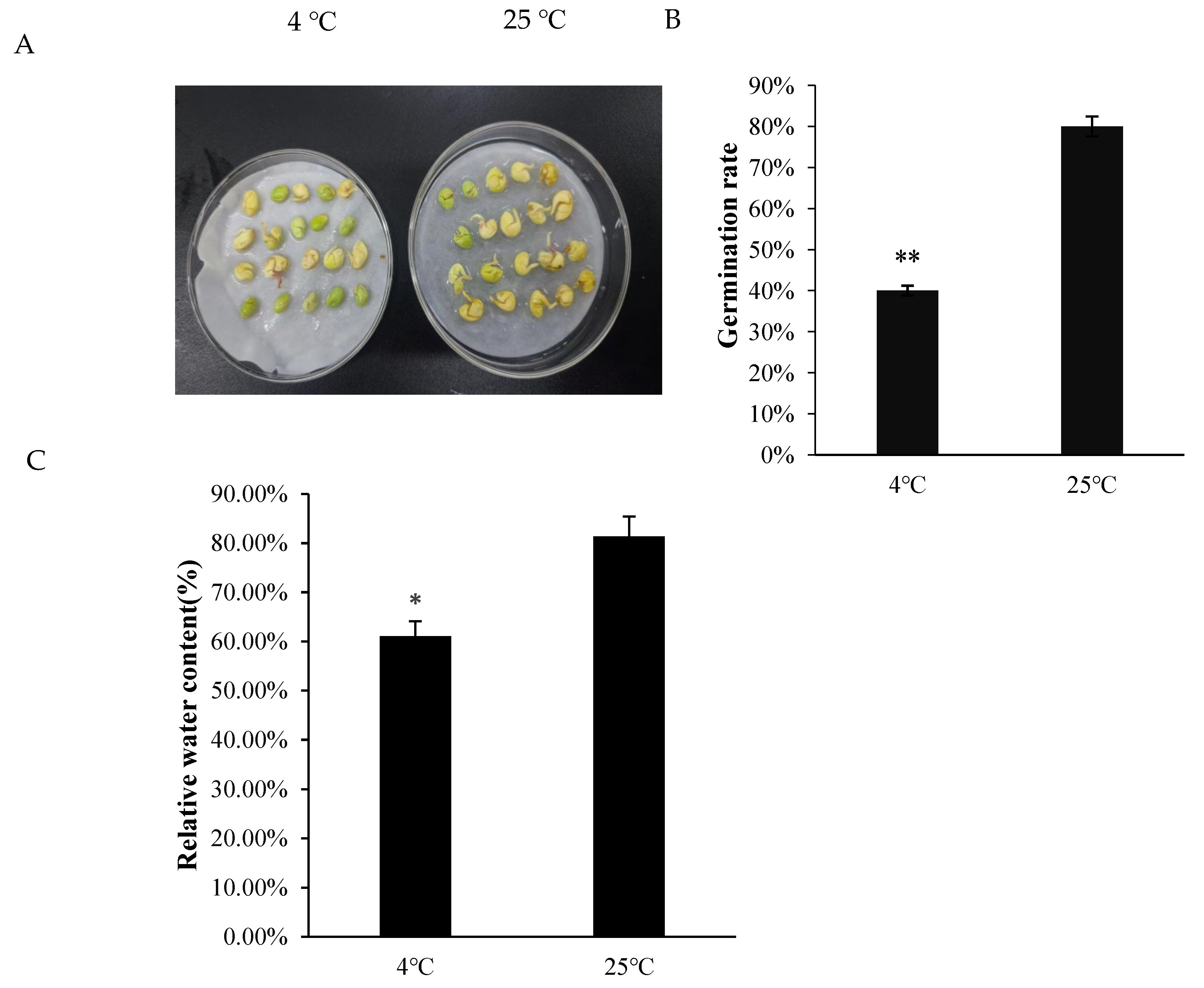
2.1. Physiological Changes of A. truncatum Seeds Under Low-Temperature Stress
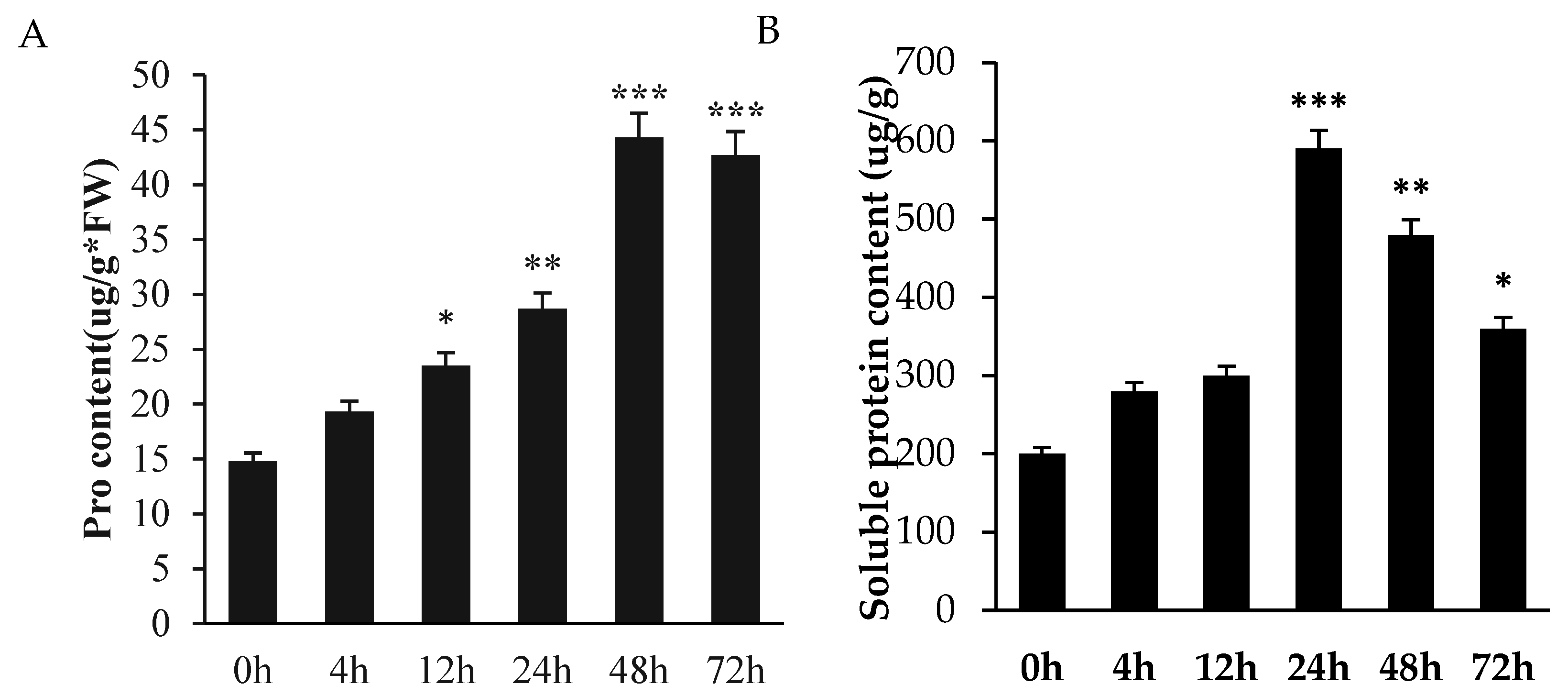
2.2. Temporal Patterns of Soluble Osmolytes in Germinating A. truncatum Seeds
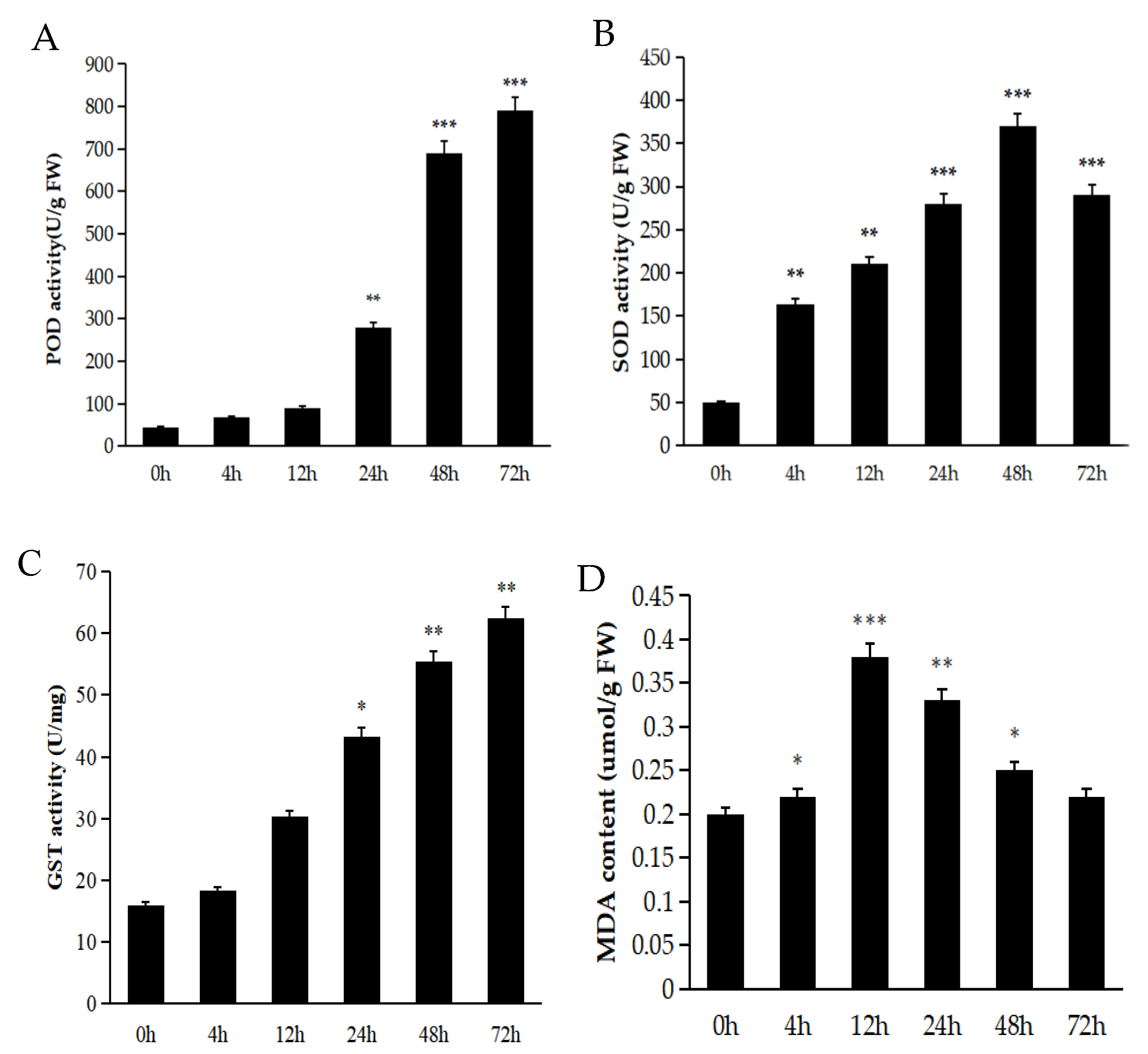
2.3. Antioxidant Enzymes Play a Crucial Role in Aiding Plants to Eliminate Superoxide Free Radicals
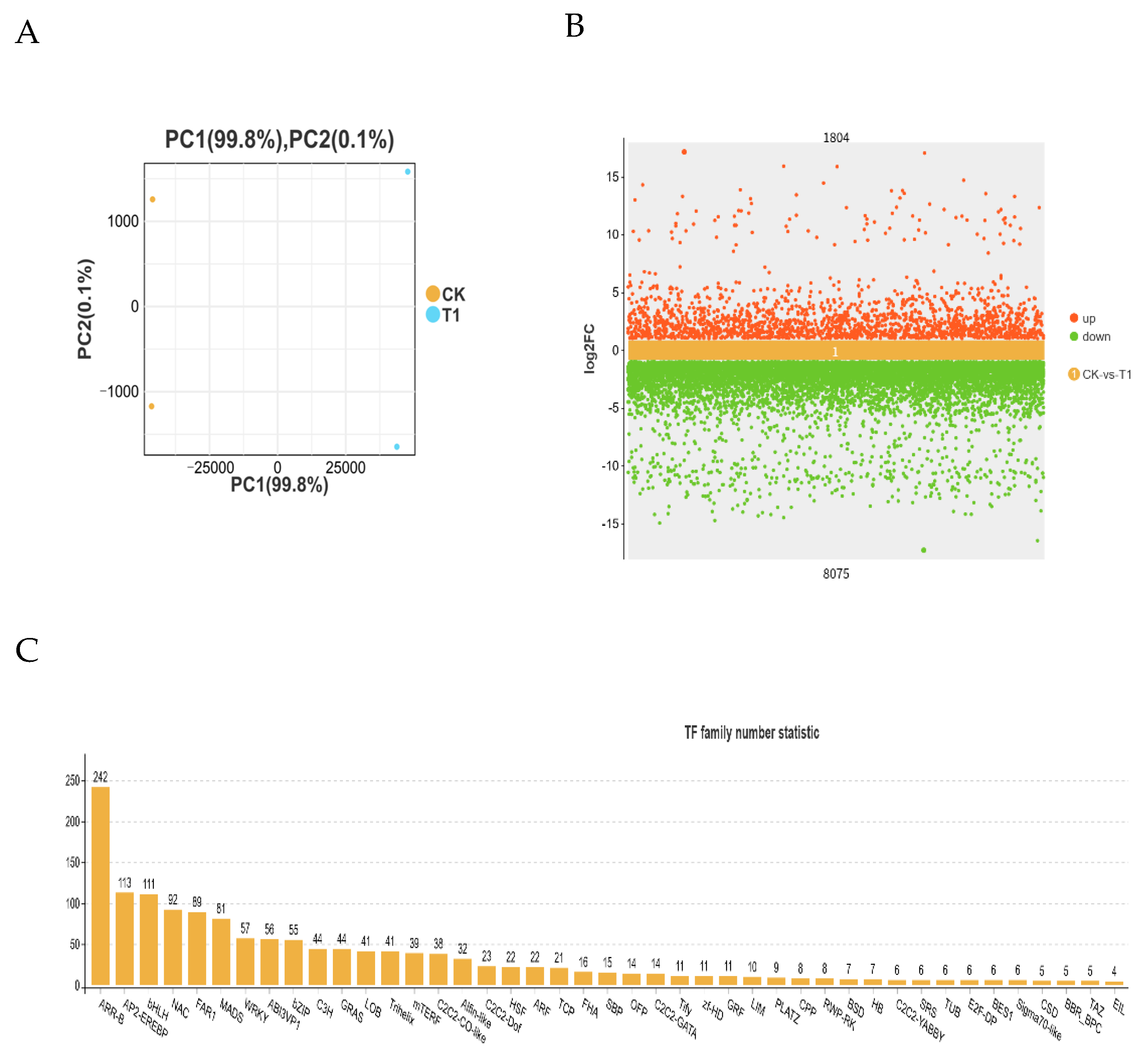
2.4. Transcriptome Sequencing Results and Transcription Factor Analysis
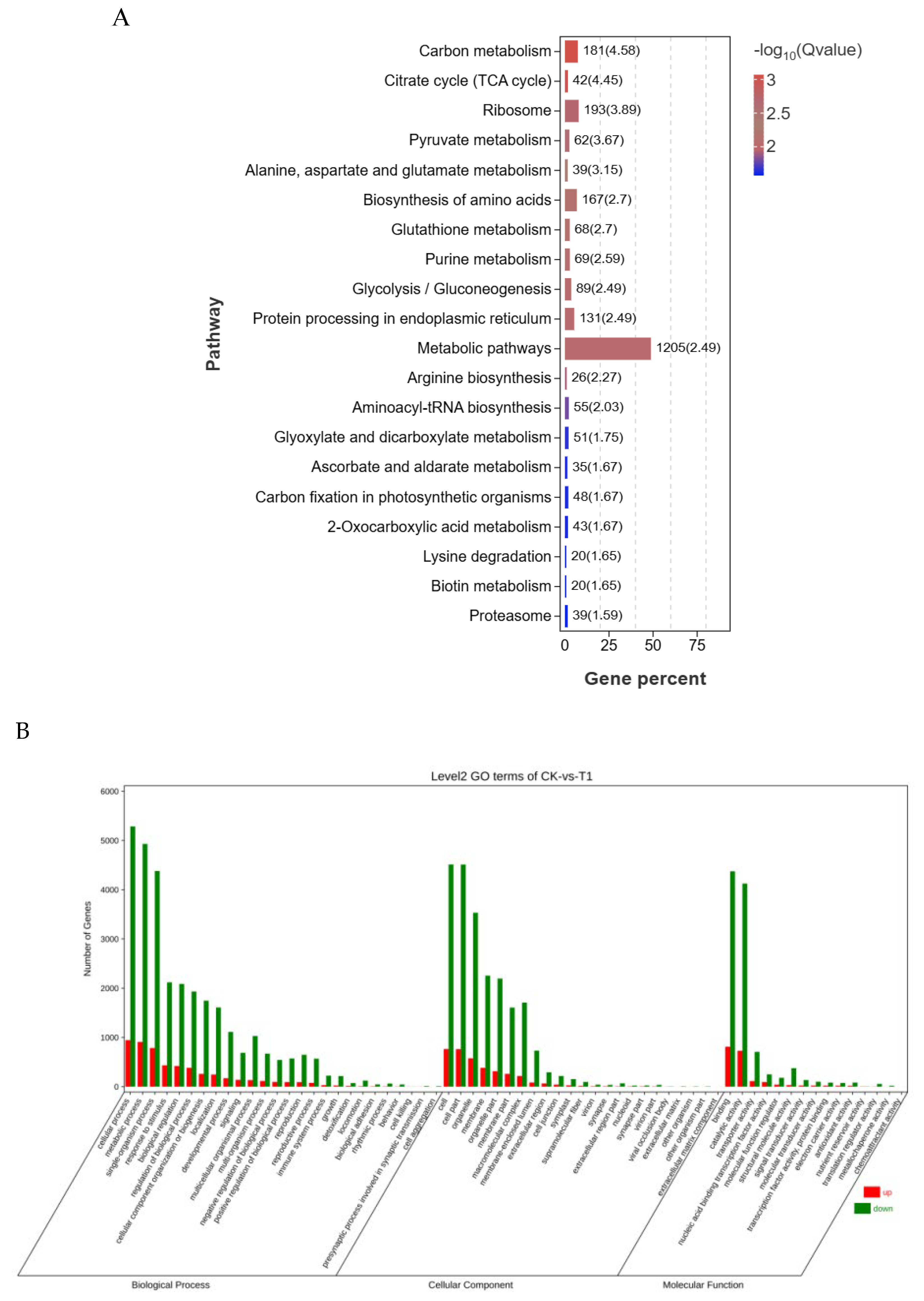
2.5. GO and KEGG Enrichment Analysis of the Differentially Expressed Genes (DEGs)
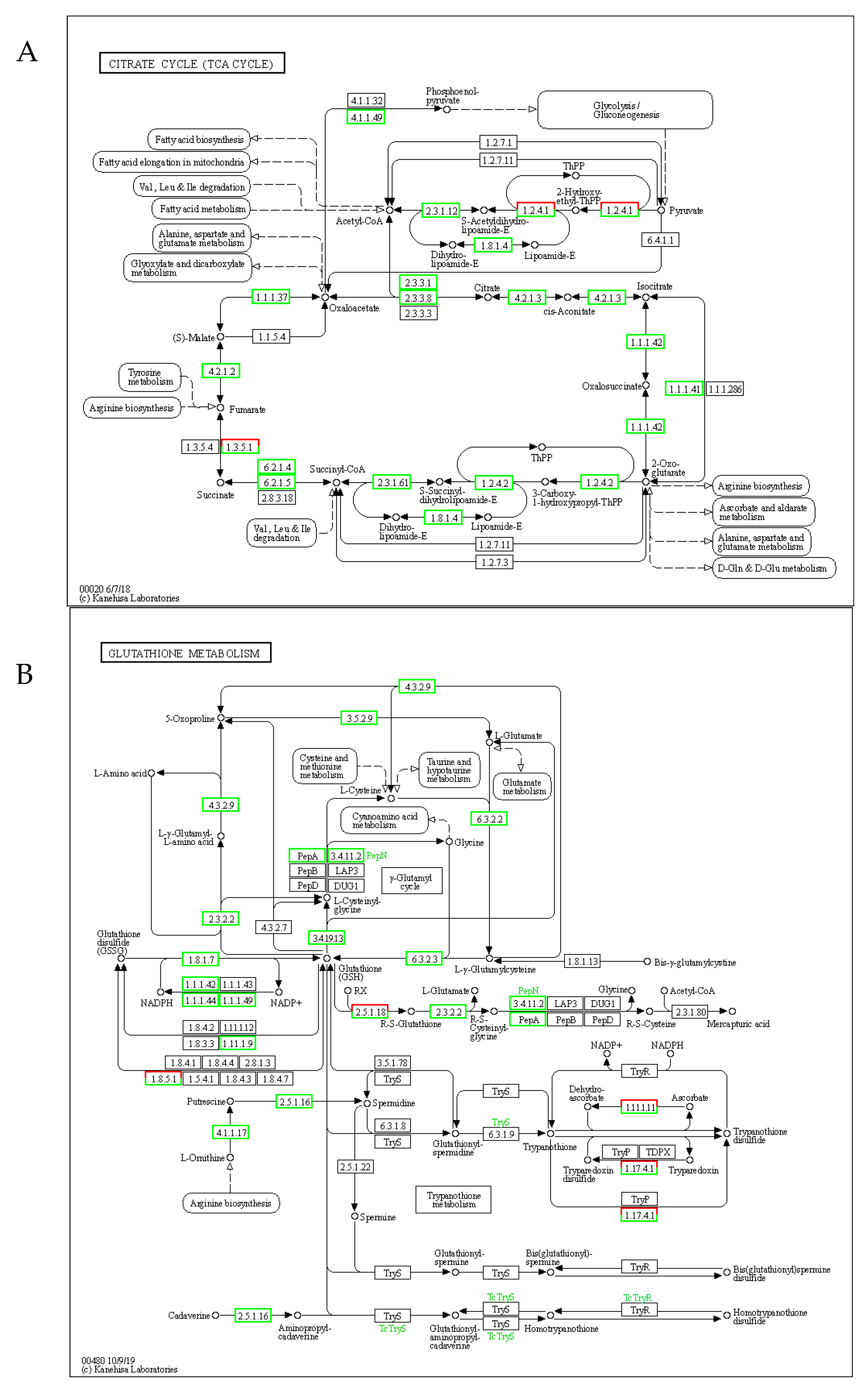
2.6. Interplay Between the TCA Cycle and Glutathione Redox System in Stress Responses
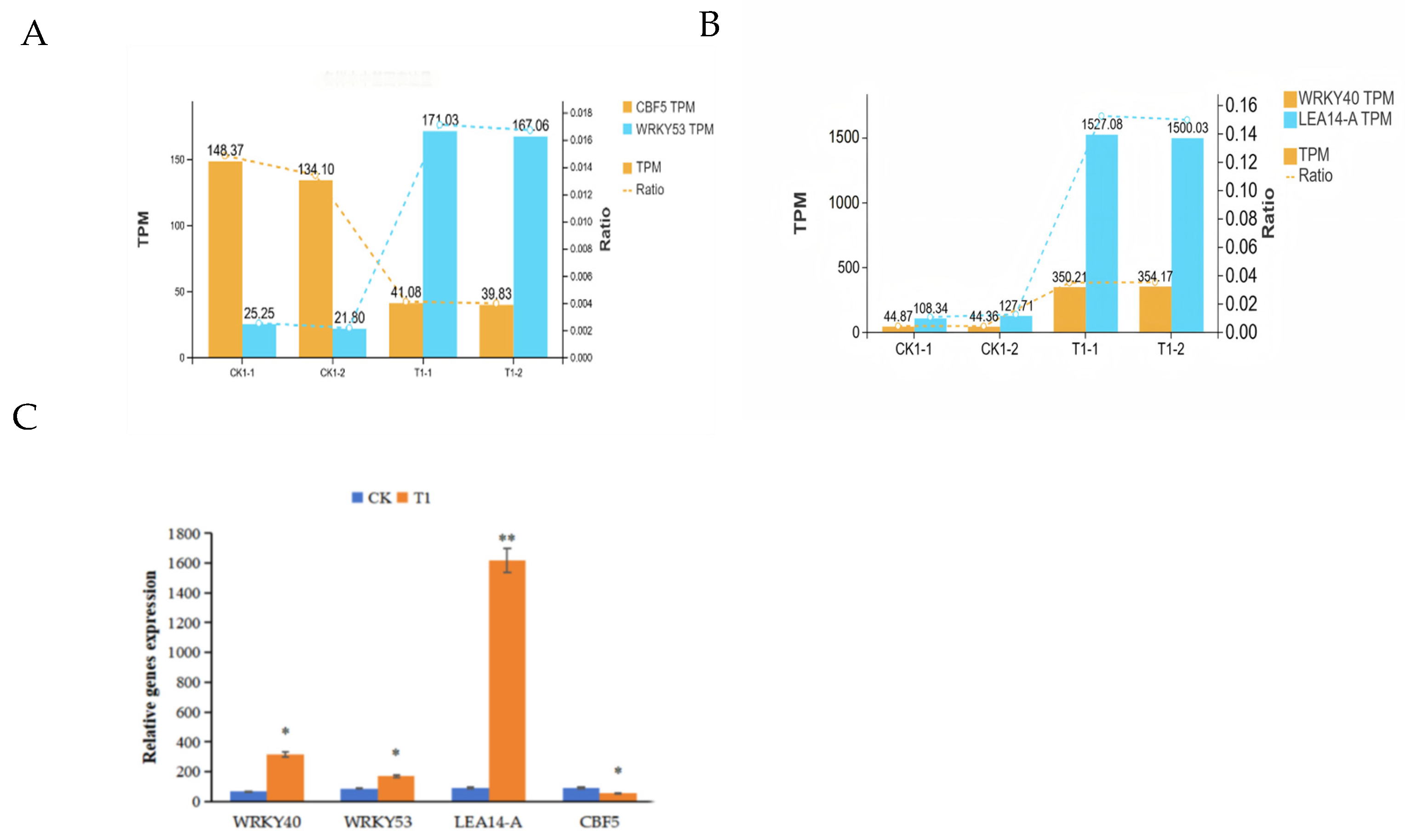
2.7. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis of Key Candidate Genes
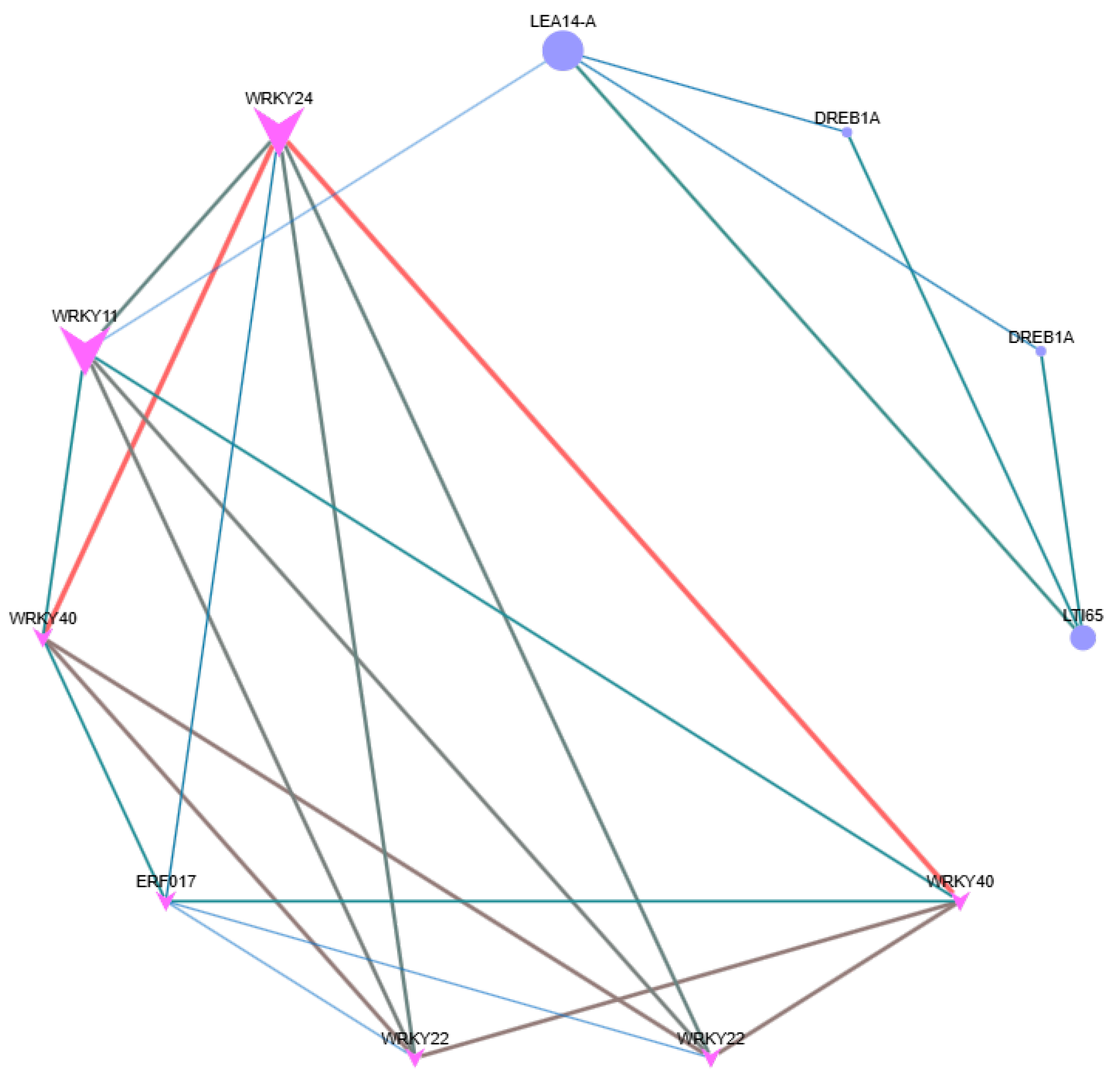
2.8. Network Relationships of Hub Genes in Four Modules with Significant Correlation
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Low-Temperature Treatment
4.2. Quantification of Seed Relative Water Content in A. truncatum
4.3. Determination of Soluble Protein Content
4.4. Determination Method of Physiological Indicators
4.5. RNA-Seq Preparation and Assembly
4.6. Identification and Enrichment Analysis of Differentially Expressed Genes (DEGs)
4.7. Validation of Key Candidate DEGs by qRT-PCR
4.8. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Allsup, C.M.; George, I.; Lankau, R.A. Shifting microbial communities can enhance tree tolerance to changing climates. Science 2023, 380, 835–840. [Google Scholar] [CrossRef]
- Larran, A.S.; Pajoro, A.; Qüesta, J.I. Is winter coming? Impact of the changing climate on plant responses to cold temperature. Plant Cell Environ. 2023, 46, 3175–3193. [Google Scholar] [CrossRef]
- Zhao, Q.; Han, R.; Cai, K.; Yan, H.; Li, Y.; Qu, G.; Liu, L.; Zhao, X. Identification and Analysis of the CBF Gene Family in Three Species of Acer under Cold Stress. Int. J. Mol. Sci. 2023, 24, 2088. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Wei, J.; Cai, K.; Zhang, H.; Ge, L.; Ren, Z.; Zhao, C.; Zhao, X. Genome-Wide Identification and Analysis of the WRKY Gene Family and Cold Stress Response in A. truncatum. Genes 2021, 12, 1867. [Google Scholar] [CrossRef]
- Han, Z.; Xu, X.; Zhang, S.; Zhao, Q.; Li, H.; Cui, Y.; Li, X.; Wang, L.; Chen, S.; Zhao, X. Transcriptomics Profiling of Acer pseudosieboldianum Molecular Mechanism against Freezing Stress. Int. J. Mol. Sci. 2022, 23, 14676. [Google Scholar] [CrossRef]
- Farooq, M.A.; Ma, W.; Shen, S.; Gu, A. Underlying Biochemical and Molecular Mechanisms for Seed Germination. Int. J. Mol. Sci. 2022, 23, 8502. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Wang, D.; Gong, Q.; You, J.; Ren, Q.; An, H.; Zhou, Y.; Jiang, H. Metabolomic analysis of rapeseed priming with H2O2 in response to germination under chilling stress. Plant Growth Regul. 2023, 99, 477–491. [Google Scholar] [CrossRef]
- Neta, I.C.S.; Pinho, É.V.d.R.V.; de Abreu, V.M.; Vilela, D.R.; Santos, M.C.; dos Santos, H.O.; Ferreira, R.A.D.C.; Von Pinho, R.G.; Vasconcellos, R.C.d.C. Gene expression and genetic control to cold tolerance during maize seed germination. BMC Plant Biol. 2020, 20, 188. [Google Scholar] [CrossRef]
- Fujino, K.; Sekiguchi, H.; Matsuda, Y.; Sugimoto, K.; Ono, K.; Yano, M. Molecular identification of a major quantitative trait locus, qLTG3-1, controlling low-temperature germinability in rice. Proc. Natl. Acad. Sci. USA 2008, 105, 12623–12628. [Google Scholar] [CrossRef]
- Tian, Z.; Ji, Y.; Sun, L.; Xu, X.; Fan, D.; Zhong, H.; Liang, Z.; Gunther, F. Changes in production potentials of rapeseed in the Yangtze River Basin of China under climate change: A multi-model ensemble approach. J. Geogr. Sci. 2018, 28, 1700–1714. [Google Scholar] [CrossRef]
- Trischuk, R.G.; Schilling, B.S.; Low, N.H.; Gray, G.R.; Gusta, L.V. Cold acclimation, de-acclimation and re-acclimation of spring canola, winter canola and winter wheat: The role of carbohydrates, cold-induced stress proteins and vernalization. Environ. Exp. Bot. 2014, 106, 156–163. [Google Scholar] [CrossRef]
- Cai, K. Physiological effects of jasmonic acid on rice seedlings under low temperature stress. Hubei Agric. Sci. 2014, 53, 3512–3515. [Google Scholar]
- Singh, S.; Singh, P.; Tomar, R.S.; Sharma, R.A.; Singh, S.K. Proline: A Key Player to Regulate Biotic and Abiotic Stress in Plants. In Towards Sustainable Natural Resources; Rani, M., Chaudhary, B.S., Jamal, S., Kumar, P., Eds.; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Mwenye, O.J.; van Rensburg, L.; van Biljon, A.; van der Merwe, R. The role of proline and root traits on selection for drought-stress tolerance in soybeans: A review. S. Afr. J. Plant Soil 2016, 33, 245–256. [Google Scholar] [CrossRef]
- Luo, T.; Zhang, Y.; Zhang, C.; Nelson, M.N.; Yuan, J.; Guo, L.; Xu, Z. Genome-Wide Association Mapping Unravels the Genetic Control of Seed Vigor under Low-Temperature Conditions in Rapeseed (Brassica napus L.). Plants 2021, 10, 426. [Google Scholar] [CrossRef] [PubMed]
- Trotel-Aziz, P.; Niogret, M.; Deleu, C.; Bouchereau, A.; Aziz, A.; Larher, F.R. The control of proline consumption by abscisic acid during osmotic stress recovery of canola leaf discs. Physiol. Plant. 2003, 117, 213–221. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Xu, Q.; Wang, D.; Di, H.; Huang, J.; Yang, X.; Wang, Z.; Zhang, L.; Dong, L.; et al. Identification of candidate tolerance genes to low-temperature during maize germination by GWAS and RNA-seq approaches. BMC Plant Biol. 2020, 20, 333. [Google Scholar] [CrossRef]
- Tang, J.; Tian, X.; Mei, E.; He, M.; Gao, J.; Yu, J.; Xu, M.; Liu, J.; Song, L.; Li, X.; et al. WRKY53 negatively regulates rice cold tolerance at the booting stage by fine-tuning anther gibberellin levels. Plant Cell. 2022, 34, 4495–4515. [Google Scholar] [CrossRef]
- Yang, L.; Liu, H.; Lei, L.; Wang, J.; Zheng, H.; Xin, W.; Zou, D. Combined QTL-sequencing, linkage map**, and RNA-sequencing identify candidate genes and KASP markers for low-temperature germination in Oryza sativa L. ssp. Japonica. Planta 2023, 257, 122. [Google Scholar] [CrossRef]
- Zhang, W.B.; Qiu, P.C.; Jiang, H.W.; Liu, C.Y.; Xin, D.W.; Li, C.D.; Hu, G.H.; Chen, Q.S. Dissection of genetic overlap of drought and low-temperature tolerance QTLs at the germination stage using backcross introgression lines in soybean. Mol. Biol. Rep. 2012, 39, 6087–6094. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, P.; Zou, C.; Chen, Z.; Yuan, G.; Gao, S.; Pan, G.; Shen, Y.; Ma, L. Comprehensive analysis of transcriptional data on seed germination of two maize inbred lines under low-temperature conditions. Plant Physiol. Biochem. 2023, 201, 107874. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, W.; Jiang, M.; Yang, L.; Zhou, X. QTL mapping for low temperature germination in rapeseed. Sci. Rep. 2021, 11, 23382. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Kazuoka, T.; Oeda, K. Heat-Stable COR (Cold-Regulated) Proteins Associated with Freezing Tolerance in Spinach. Plant Cell Physiol. 1992, 33, 1107–1114. [Google Scholar] [CrossRef]
- El-Siddig, K.; Ebert, G.; Lüdders, P. Tamarind (Tamarindus indica L.): A review on a multipurpose tree with promising future in the Sudan. Angew. Bot. 1999, 73, 202–205. [Google Scholar]
- Prasad, T.K. Role of Catalase in Inducing Chilling Tolerance in Pre-Emergent Maize Seedlings. Plant Physiol. 1997, 114, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ni, C.; Li, T.; Han, L.; Du, P.; Xiao, K. TaLBD1, a LOB transcription factor gene in T. aestivum, confers plant adaptation to low-N stress via modulating N acquisition-associated processes. Plant Cell Tissue Organ Cult. 2023, 153, 19–35. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, J.; Yang, Z.E.; Chen, E.Y.; Zhang, C.J.; Zhang, X.Y.; Li, F.G. Genome-wide analysis of GRAS transcription factor gene family in Gossypium hirsutum L. BMC Genom. 2018, 19, 348. [Google Scholar] [CrossRef]
- Dorjee, T.; Cui, Y.; Zhang, Y.; Liu, Q.; Li, X.; Sumbur, B.; Yan, H.; Bing, J.; Geng, Y.; Zhou, Y.; et al. Characterization of NAC Gene Family in Ammopiptanthus mongolicus and Functional Analysis of AmNAC24, an Osmotic and Cold-Stress-Induced NAC Gene. Biomolecules 2024, 14, 182. [Google Scholar] [CrossRef]
- Cao, Y.; Li, K.; Li, Y.; Zhao, X.; Wang, L. MYB Transcription Factors as Regulators of Secondary Metabolism in Plants. Biology 2020, 9, 61. [Google Scholar] [CrossRef]
- Ren, C.; Wang, H.; Zhou, Z.; Jia, J.; Zhang, Q.; Liang, C.; Li, W.; Zhang, Y.; Yu, G. Genome-wide identification of the B3 gene family in soybean and the response to melatonin under cold stress. Front. Plant Sci. 2023, 13, 1091907. [Google Scholar] [CrossRef]
- Wang, X.; Ding, Y.; Li, Z.; Shi, Y.; Wang, J.; Hua, J.; Gong, Z.; Zhou, J.-M.; Yang, S. PUB25 and PUB26 Promote Plant Freezing Tolerance by Degrading the Cold Signaling Negative Regulator MYB15. Dev. Cell 2019, 51, 222–235.e5. [Google Scholar] [CrossRef]
- Zhao, X.; Hui, B.; Hu, L.; Cheng, Q.; Via, B.K.; Nadel, R.; Starkey, T.; Enebak, S. Potential of near infrared spectroscopy to monitor variations in soluble sugars in Loblolly pine seedlings after cold acclimation. Agric. For. Meteorol. 2017, 232, 536–542. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Popov, V.N.; Naraikina, N.V. Change of antioxidant enzyme activity during low-temperature hardening of Nicotiana tabacum L. and Secale cereale L. Russian. J. Plant Physiol. 2020, 67, 898–905. [Google Scholar]
- Gao, J.; Guo, W.; Liu, Q.; Liu, M.; Shang, C.; Song, Y.; Hao, R.; Li, L.; Feng, X. The physiological response of Apricot flowers to low-temperature stress. Plants 2024, 13, 1002. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, C.; Chen, Y.; Zhang, Q.; Li, Q.; Qi, W. Changes in the leaf physiological characteristics and tissue-specific distribution of Ginsenosides in Panax ginseng during flowering stage under low-temperature stress. Front. Bioeng. Biotechnol. 2021, 9, 637324. [Google Scholar]
- Chen, M.; Zhu, X.; Hou, M.; Luo, W.; Jiang, Y.; Yu, Y.; Wang, J.; Yuan, H.; Huang, X.; Hua, J. Effects of low-temperature stress on cold resistance biochemical characteristics of Dali and Siqiu tea seedlings. Horticulturae 2024, 10, 823. [Google Scholar] [CrossRef]
- Yadav, S.K. Low-temperature stress tolerance mechanisms in plants. A review. Agron. Sustain. Dev. 2010, 30, 515–527. [Google Scholar] [CrossRef]
- Ritonga, F.N.; Chen, S. Physiological and Molecular Mechanism Involved in Cold Stress Tolerance in Plants. Plants 2020, 9, 560. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yao, L.; Hao, X.; Li, N.; Wang, Y.; Ding, C.; Lei, L.; Qian, W.; Zeng, J.; Yang, Y.; et al. Transcriptional and physiological analyses reveal the association of ROS metabolism with cold tolerance in tea plant. Environ. Exp. Bot. 2019, 160, 45–58. [Google Scholar] [CrossRef]
- Lee, J.H.; Yu, D.J.; Kim, S.J.; Choi, D.; Lee, H.J. Intraspecies differences in cold hardiness, carbohydrate content and β-amylase gene expression of Vaccinium corymbosum during cold acclimation and deacclimation. Tree Physiol. 2012, 32, 1533–1540. [Google Scholar] [CrossRef]
- Peng, T.; Jia, M.M.; Liu, J.H. RNAi-based functional elucidation of PtrPRP, a gene encoding a hybrid proline rich protein, in cold tolerance of Poncirus trifoliata. Front. Plant Sci. 2015, 6, 808. [Google Scholar] [CrossRef]
- Li, J.; Yang, Y.; Iqbal, A.; Qadri, R.; Shi, P.; Wang, Y.; Wu, Y.; Fan, H.; Wu, G. Correlation analysis of cold-related gene expression with physiological and biochemical indicators under cold stress in oil palm. PLoS ONE 2019, 14, e0225768. [Google Scholar] [CrossRef] [PubMed]
- Tardieu, F.; Simonneau, T.; Muller, B. The physiological basis of drought tolerance in crop plants: A scenario-dependent probabilistic approach. Ann. Rev. Plant Biol. 2018, 69, 733–759. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Tai, H.; Li, S.; Gao, W.; Zhao, M.; Xie, C.; Li, W. bHLH122 is important for drought and osmotic stress resistance in Arabidopsis and in the repression of ABA catabolism. New Phytol. 2014, 201, 1192–1204. [Google Scholar] [CrossRef]
- Rasheed, S.; Bashir, K.; Matsui, A.; Tanaka, M.; Seki, M. Transcriptomic analysis of soil-grown Arabidopsis thaliana roots and shoots in response to a drought stress. Front. Plant Sci. 2016, 7, 180–200. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, S.; Bashir, K.; Nakaminami, K.; Hanada, K.; Matsui, A.; Seki, M. Drought stress differentially regulates the expression of small open reading frames (sORFs) in Arabidopsis roots and shoots. Plant Signal. Behav. 2016, 11, e1215792. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, Y.; Li, Y.; Zhang, X.; Shi, N.; Zhao, J.; Yang, H. Dynamic changes in the transcriptome landscape of Arabidopsis thaliana in response to cold stress. Front. Plant Sci. 2022, 13, 983460. [Google Scholar] [CrossRef]
- Wang, Y.; Wan, C.; Li, L.; Xiang, Z.; Wang, J.; Li, Y.; Zhao, D. Transcriptomic Analysis of Yunwu Tribute Tea Leaves under Cold Stress. Curr. Issues Mol. Biol. 2023, 45, 699–720. [Google Scholar] [CrossRef]
- Li, W.; Yang, B.; Xu, J.; Peng, L.; Sun, S.; Huang, Z.; Jiang, X.; He, Y.; Wang, Z. A genome-wide association study reveals that the 2-oxoglutarate/malate translocator mediates seed vigor in rice. Plant J. 2021, 108, 478–491. [Google Scholar] [CrossRef]
- Hayashi, T.; Harada, A.; Sakai, T.; Takagi, S. Ca2+ transient induced by extracellular changes in osmotic pressure in Arabidopsis leaves: Differential involvement of cell wall-plasma membrane adhesion. Plant Cell Environ. 2006, 29, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, J. Experimental Principles and Techniques of Plant Physiology and Biochemistry; Higher Education Press: Beijing, China, 2015. [Google Scholar]
- Meng, H.; Zhao, J.; Yang, Y.; Diao, K.; Zheng, G.; Li, T.; Dai, X.; Li, J. PeGSTU58, a Glutathione S-Transferase from Populus euphratica, Enhances Salt and Drought Stress Tolerance in Transgenic Arabidopsis. Int. J. Mol. Sci. 2023, 24, 9354. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Cai, T.; Olyarchuk, J.G.; Wei, L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, H.; Zhou, L.; Qin, Y.; Ji, S.; Wang, P.; Liu, Y.; Liu, J.; Ma, J.; Sun, H.; Zhu, X.; et al. Transcriptome and Biochemical Analysis of the Mechanism of Low-Temperature Germination in Acer truncatum Bunge Seeds. Int. J. Mol. Sci. 2025, 26, 11193. https://doi.org/10.3390/ijms262211193
Meng H, Zhou L, Qin Y, Ji S, Wang P, Liu Y, Liu J, Ma J, Sun H, Zhu X, et al. Transcriptome and Biochemical Analysis of the Mechanism of Low-Temperature Germination in Acer truncatum Bunge Seeds. International Journal of Molecular Sciences. 2025; 26(22):11193. https://doi.org/10.3390/ijms262211193
Chicago/Turabian StyleMeng, Huijing, Linpo Zhou, Yiming Qin, Shuang Ji, Pengpeng Wang, Yufan Liu, Jiawen Liu, Jingyu Ma, Hexiang Sun, Xiuhong Zhu, and et al. 2025. "Transcriptome and Biochemical Analysis of the Mechanism of Low-Temperature Germination in Acer truncatum Bunge Seeds" International Journal of Molecular Sciences 26, no. 22: 11193. https://doi.org/10.3390/ijms262211193
APA StyleMeng, H., Zhou, L., Qin, Y., Ji, S., Wang, P., Liu, Y., Liu, J., Ma, J., Sun, H., Zhu, X., & Ru, G. (2025). Transcriptome and Biochemical Analysis of the Mechanism of Low-Temperature Germination in Acer truncatum Bunge Seeds. International Journal of Molecular Sciences, 26(22), 11193. https://doi.org/10.3390/ijms262211193



