Xyloglucan Endotransglycosylase/Hydrolase Downregulation Increases Nicotiana benthamiana Tolerance to Tobacco Mosaic Virus Infection
Abstract
1. Introduction
2. Results
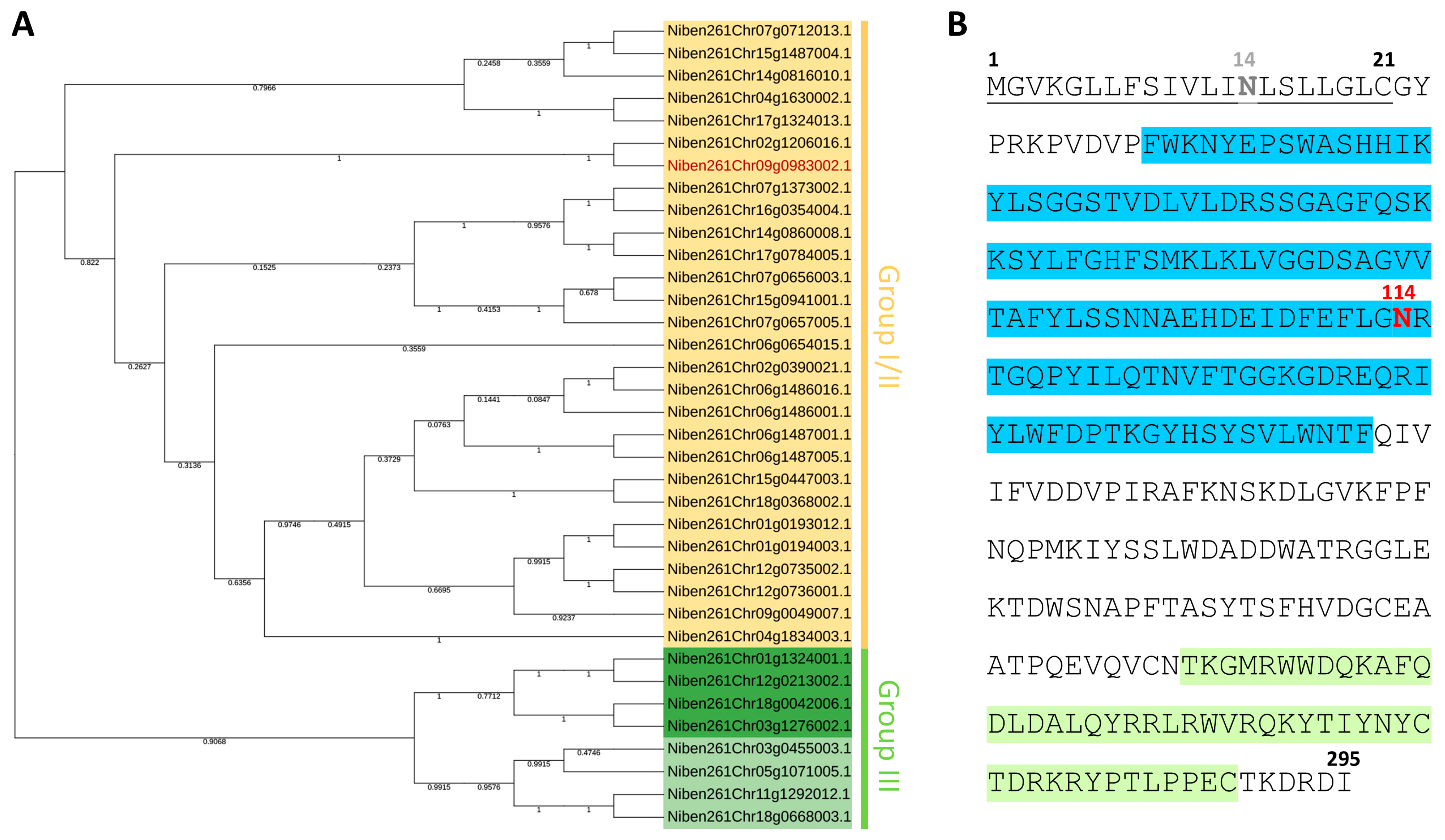
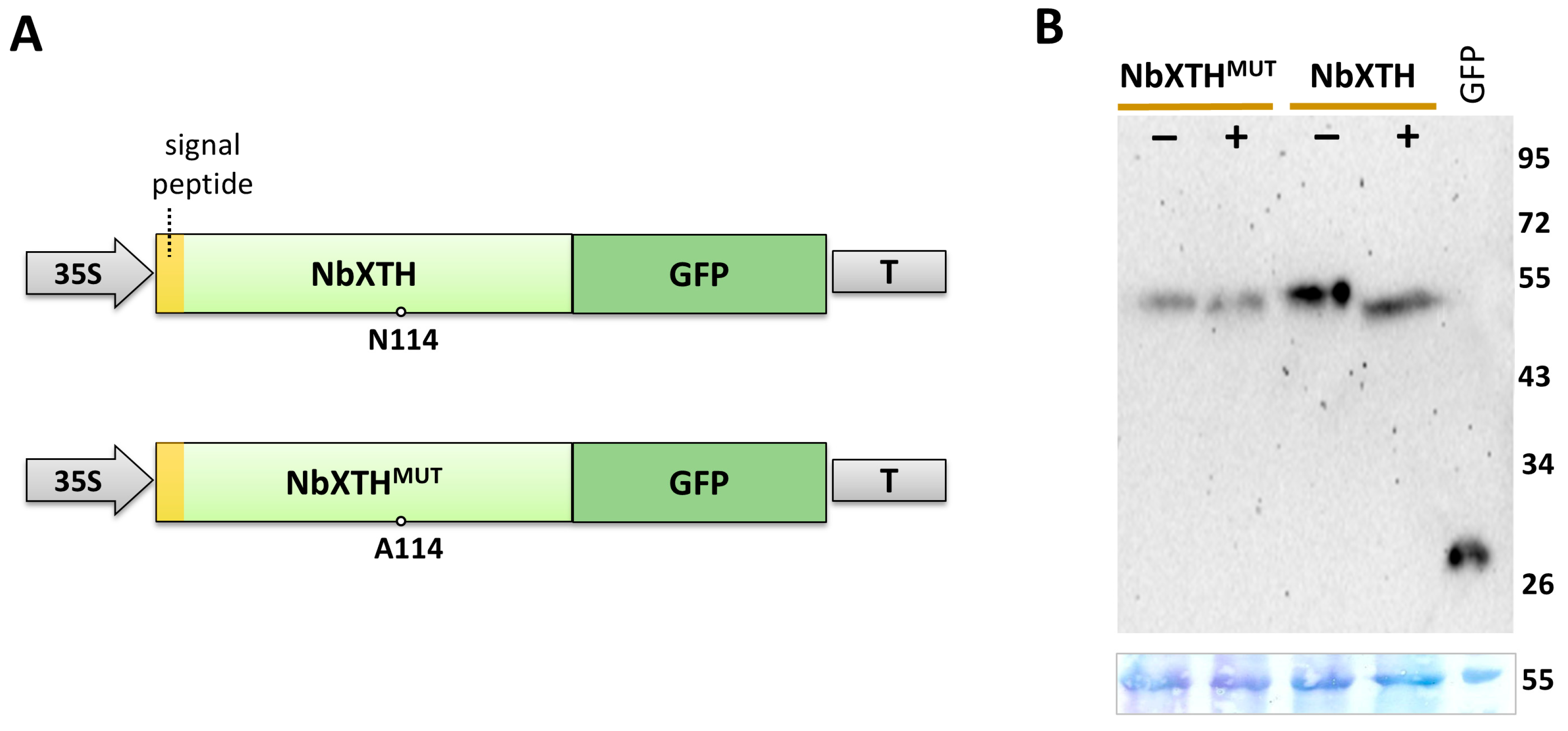
2.1. NbXTH Identification and Analysis
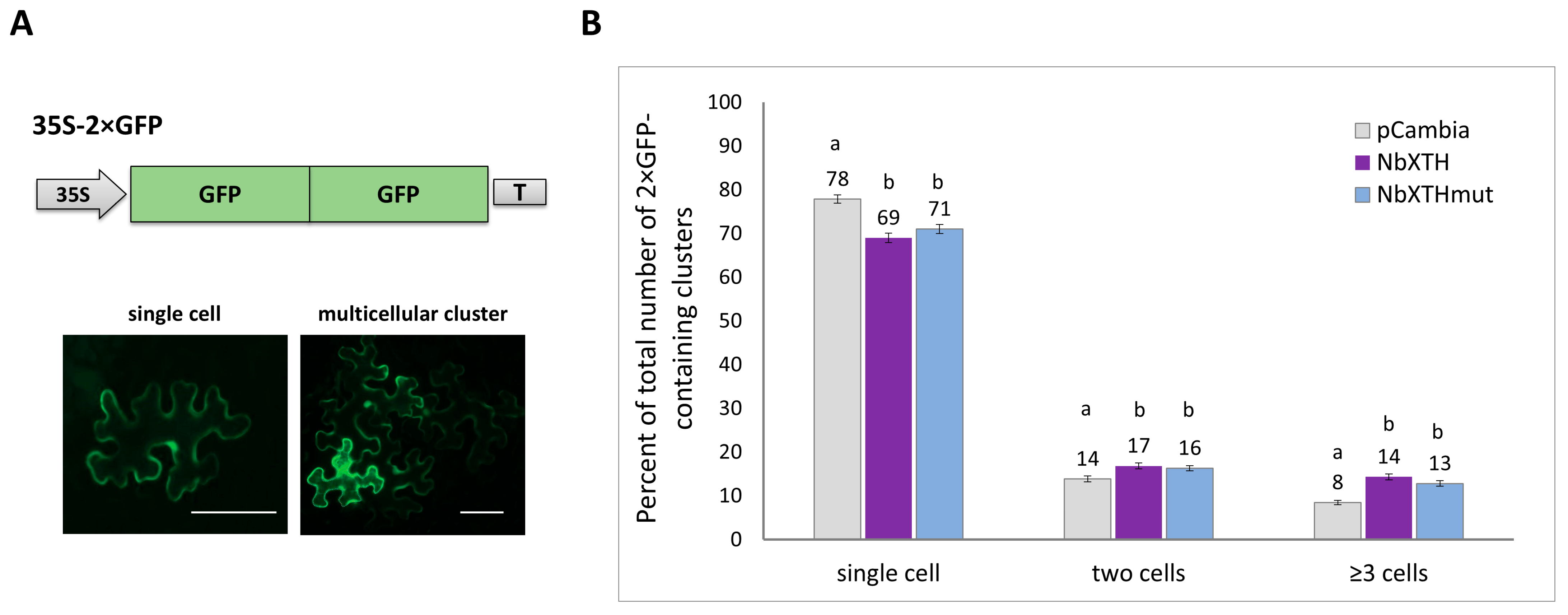
2.2. NbXTH Affects Intercellular Transport of Macromolecules
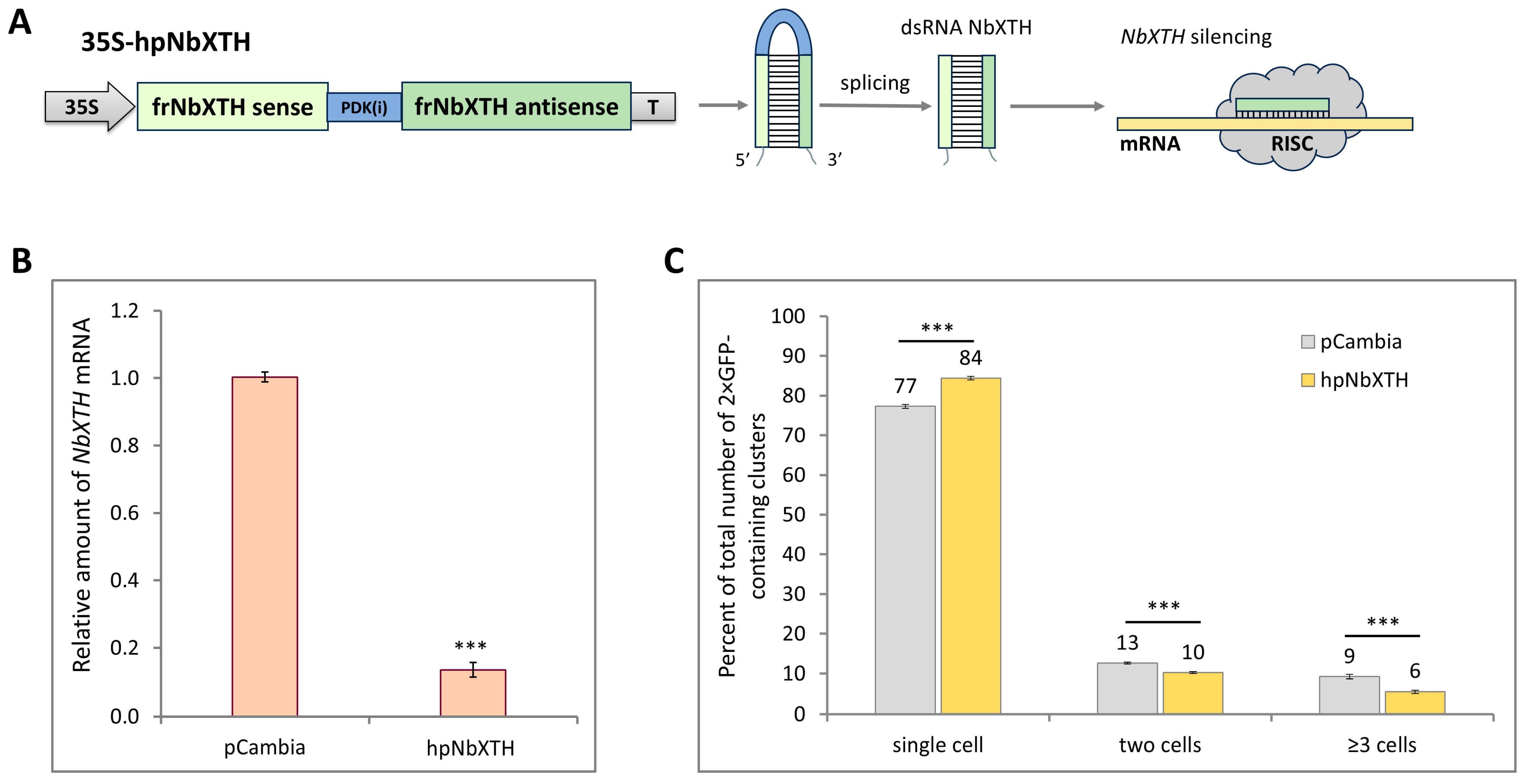
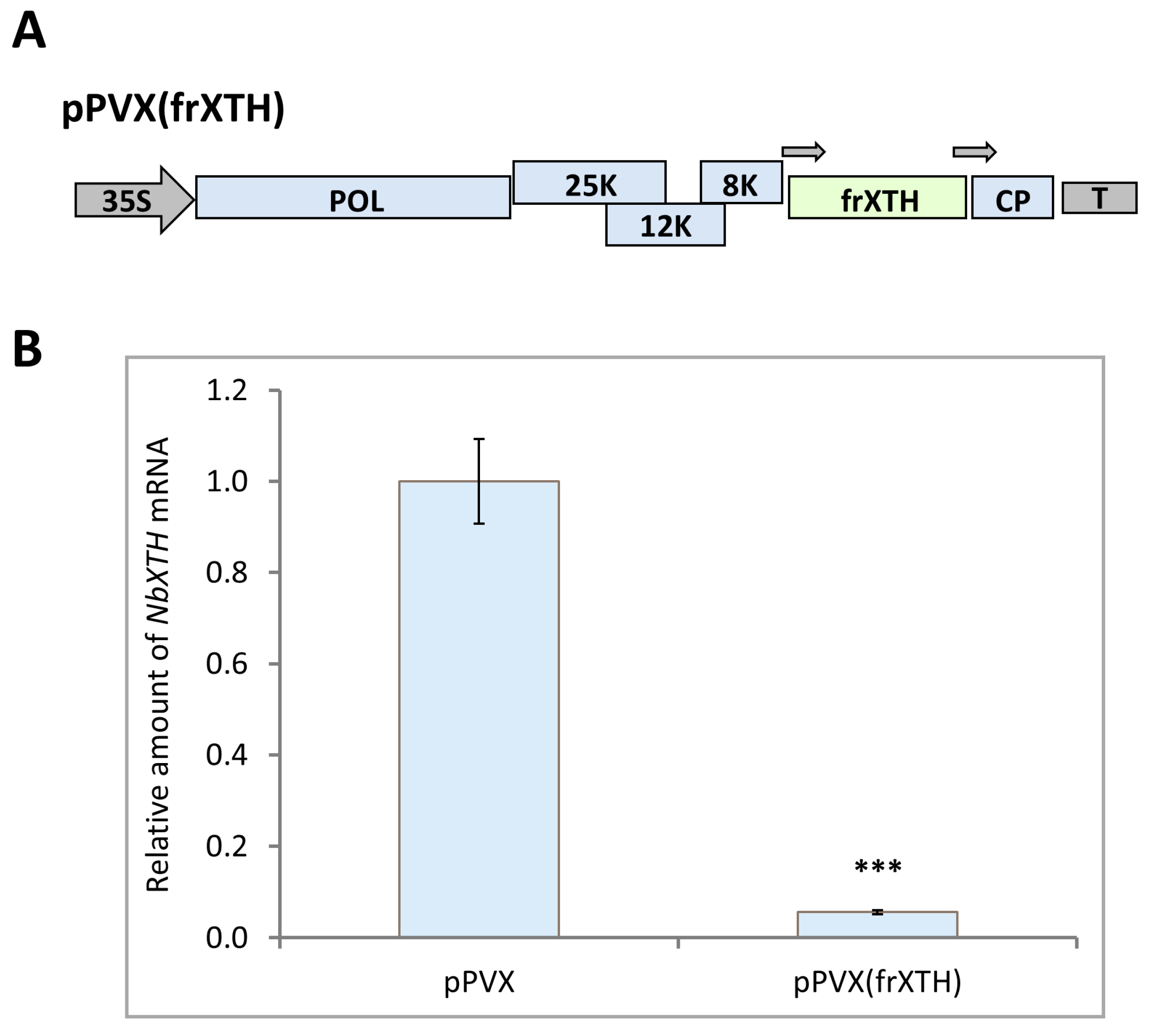
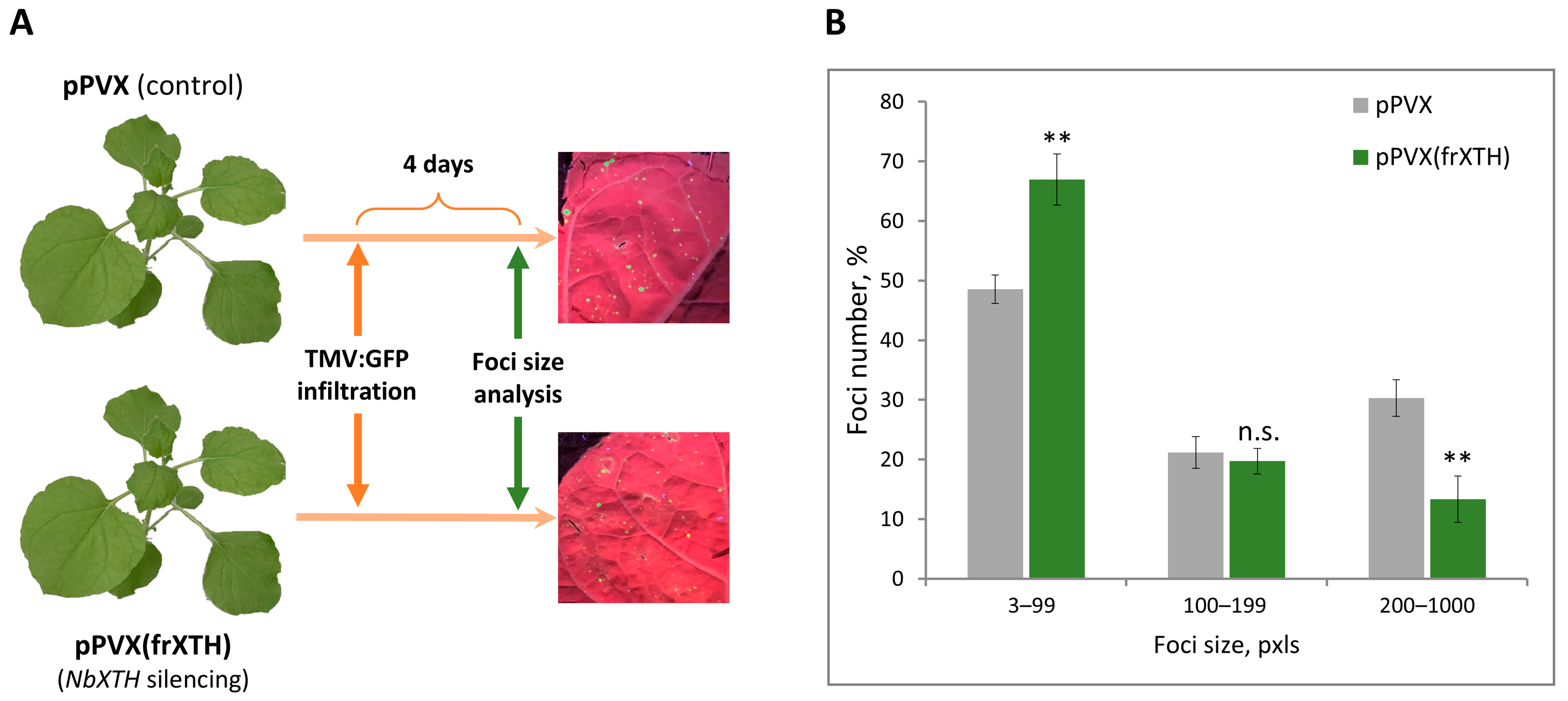
2.3. NbXTH Is Involved in the Development of Tobacco Mosaic Infection
3. Discussion
4. Materials and Methods
4.1. N. benthamiana XTHs Identification and Phylogenetic Analysis
4.2. Plant Growth Conditions
4.3. Plasmid Constructs
4.4. Agroinfiltration
4.5. Protein Extract Preparation and PNGase Treatment
4.6. Western Blot Analysis
4.7. Plant Inoculation for Systemic Infection
4.8. GFP Imaging and Intercellular Transport Analysis
4.9. TMV Foci Visualization and Quantification
4.10. Quantitative Real-Time PCR (qRT-PCR) Analysis of Transcript Concentrations
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Molina, A.; Jordá, L.; Torres, M.Á.; Martín-Dacal, M.; Berlanga, D.J.; Fernández-Calvo, P.; Gómez-Rubio, E.; Martín-Santamaría, S. Plant Cell Wall-Mediated Disease Resistance: Current Understanding and Future Perspectives. Mol. Plant 2024, 17, 699–724. [Google Scholar] [CrossRef]
- Munzert, K.S.; Engelsdorf, T. Plant Cell Wall Structure and Dynamics in Plant-Pathogen Interactions and Pathogen Defence. J. Exp. Bot. 2025, 76, 228–242. [Google Scholar] [CrossRef]
- Harholt, J.; Suttangkakul, A.; Vibe Scheller, H. Biosynthesis of Pectin. Plant Physiol. 2010, 153, 384–395. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Structure and Growth of Plant Cell Walls. Nat. Rev. Mol. Cell Biol. 2024, 25, 340–358. [Google Scholar] [CrossRef]
- Seifert, G.J.; Blaukopf, C. Irritable Walls: The Plant Extracellular Matrix and Signaling. Plant Physiol. 2010, 153, 467–478. [Google Scholar] [CrossRef]
- Kim, S.-J.; Brandizzi, F. The Plant Secretory Pathway for the Trafficking of Cell Wall Polysaccharides and Glycoproteins. Glycobiology 2016, 26, 940–949. [Google Scholar] [CrossRef]
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The Carbohydrate-Active EnZymes Database (CAZy): An Expert Resource for Glycogenomics. Nucleic Acids Res. 2009, 37, D233–D238. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.K.C.; Braam, J.; Fry, S.C.; Nishitani, K. The XTH Family of Enzymes Involved in Xyloglucan Endotransglucosylation and Endohydrolysis: Current Perspectives and a New Unifying Nomenclature. Plant Cell Physiol. 2002, 43, 1421–1435. [Google Scholar] [CrossRef] [PubMed]
- Eklöf, J.M.; Brumer, H. The XTH Gene Family: An Update on Enzyme Structure, Function, and Phylogeny in Xyloglucan Remodeling. Plant Physiol. 2010, 153, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.; Braam, J. Xyloglucan Endotransglycosylases: Diversity of Genes, Enzymes and Potential Wall-Modifying Functions. Trends Plant Sci. 1999, 4, 361–366. [Google Scholar] [CrossRef]
- Ishida, K.; Yokoyama, R. Reconsidering the Function of the Xyloglucan Endotransglucosylase/Hydrolase Family. J. Plant Res. 2022, 135, 145–156. [Google Scholar] [CrossRef]
- Le Gall, H.; Philippe, F.; Domon, J.-M.; Gillet, F.; Pelloux, J.; Rayon, C. Cell Wall Metabolism in Response to Abiotic Stress. Plants 2015, 4, 112–166. [Google Scholar] [CrossRef]
- Kuluev, B.; Mikhaylova, E.; Berezhneva, Z.; Nikonorov, Y.; Postrigan, B.; Kudoyarova, G.; Chemeris, A. Expression Profiles and Hormonal Regulation of Tobacco NtEXGT Gene and Its Involvement in Abiotic Stress Response. Plant Physiol. Biochem. 2017, 111, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Liu, Y.; Shen, Y.; Li, W. A Surprising Diversity of Xyloglucan Endotransglucosylase/Hydrolase in Wheat: New in Sight to the Roles in Drought Tolerance. Int. J. Mol. Sci. 2023, 24, 9886. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento-López, L.G.; López-Espinoza, M.Y.; Juárez-Verdayes, M.A.; López-Meyer, M. Genome-Wide Characterization of the Xyloglucan Endotransglucosylase/Hydrolase Gene Family in Solanum lycopersicum L. and Gene Expression Analysis in Response to Arbuscular Mycorrhizal Symbiosis. PeerJ 2023, 11, e15257. [Google Scholar] [CrossRef]
- Li, J.; Li, M.; Dai, Y.; Lai, S.; Zhang, S. The Role of XTHs in Cell Wall Immobilization of Metal Ions. Plant Physiol. Biochem. PPB 2025, 229, 110475. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Purugganan, M.M.; Polisensky, D.H.; Antosiewicz, D.M.; Fry, S.C.; Braam, J. Arabidopsis TCH4, Regulated by Hormones and the Environment, Encodes a Xyloglucan Endotransglycosylase. Plant Cell 1995, 7, 1555–1567. [Google Scholar] [CrossRef][Green Version]
- Xu, W.; Campbell, P.; Vargheese, A.K.; Braam, J. The Arabidopsis XET-Related Gene Family: Environmental and Hormonal Regulation of Expression. Plant J. Cell Mol. Biol. 1996, 9, 879–889. [Google Scholar] [CrossRef]
- Wu, Y.; Jeong, B.-R.; Fry, S.C.; Boyer, J.S. Change in XET Activities, Cell Wall Extensibility and Hypocotyl Elongation of Soybean Seedlings at Low Water Potential. Planta 2005, 220, 593–601. [Google Scholar] [CrossRef]
- Cho, S.K.; Kim, J.E.; Park, J.-A.; Eom, T.J.; Kim, W.T. Constitutive Expression of Abiotic Stress-Inducible Hot Pepper CaXTH3, Which Encodes a Xyloglucan Endotransglucosylase/Hydrolase Homolog, Improves Drought and Salt Tolerance in Transgenic Arabidopsis Plants. FEBS Lett. 2006, 580, 3136–3144. [Google Scholar] [CrossRef]
- Wan, J.; He, M.; Hou, Q.; Zou, L.; Yang, Y.; Wei, Y.; Chen, X. Cell Wall Associated Immunity in Plants. Stress Biol. 2021, 1, 3. [Google Scholar] [CrossRef]
- Kozieł, E.; Otulak-Kozieł, K.; Bujarski, J.J. Plant Cell Wall as a Key Player During Resistant and Susceptible Plant-Virus Interactions. Front. Microbiol. 2021, 12, 656809. [Google Scholar] [CrossRef] [PubMed]
- Zavaliev, R.; Ueki, S.; Epel, B.L.; Citovsky, V. Biology of Callose (β-1,3-Glucan) Turnover at Plasmodesmata. Protoplasma 2011, 248, 117–130. [Google Scholar] [CrossRef]
- Iglesias, V.A.; Meins, F. Movement of Plant Viruses Is Delayed in a Beta-1,3-Glucanase-Deficient Mutant Showing a Reduced Plasmodesmatal Size Exclusion Limit and Enhanced Callose Deposition. Plant J. Cell Mol. Biol. 2000, 21, 157–166. [Google Scholar] [CrossRef]
- Bucher, G.L.; Tarina, C.; Heinlein, M.; Di Serio, F.; Meins, F.; Iglesias, V.A. Local Expression of Enzymatically Active Class I Beta-1, 3-Glucanase Enhances Symptoms of TMV Infection in Tobacco. Plant J. Cell Mol. Biol. 2001, 28, 361–369. [Google Scholar] [CrossRef]
- Goyer, A.; Hamlin, L.; Crosslin, J.M.; Buchanan, A.; Chang, J.H. RNA-Seq Analysis of Resistant and Susceptible Potato Varieties during the Early Stages of Potato Virus Y Infection. BMC Genom. 2015, 16, 472. [Google Scholar] [CrossRef] [PubMed]
- Otulak-Kozieł, K.; Kozieł, E.; Lockhart, B.E.L. Plant Cell Wall Dynamics in Compatible and Incompatible Potato Response to Infection Caused by Potato Virus Y (PVYNTN). Int. J. Mol. Sci. 2018, 19, 862. [Google Scholar] [CrossRef] [PubMed]
- Lotan, T.; Fluhr, R. Xylanase, a Novel Elicitor of Pathogenesis-Related Proteins in Tobacco, Uses a Non-Ethylene Pathway for Induction. Plant Physiol. 1990, 93, 811–817. [Google Scholar] [CrossRef]
- Otulak-Kozieł, K.; Kozieł, E.; Bujarski, J.J. Spatiotemporal Changes in Xylan-1/Xyloglucan and Xyloglucan Xyloglucosyl Transferase (XTH-Xet5) as a Step-In of Ultrastructural Cell Wall Remodelling in Potato–Potato Virus Y (PVYNTN) Hypersensitive and Susceptible Reaction. Int. J. Mol. Sci. 2018, 19, 2287. [Google Scholar] [CrossRef]
- Ershova, N.; Sheshukova, E.; Kamarova, K.; Arifulin, E.; Tashlitsky, V.; Serebryakova, M.; Komarova, T. Nicotiana benthamiana Kunitz Peptidase Inhibitor-like Protein Involved in Chloroplast-to-Nucleus Regulatory Pathway in Plant-Virus Interaction. Front. Plant Sci. 2022, 13, 1041867. [Google Scholar] [CrossRef]
- Ershova, N.; Kamarova, K.; Sheshukova, E.; Antimonova, A.; Komarova, T. A Novel Cellular Factor of Nicotiana benthamiana Susceptibility to Tobamovirus Infection. Front. Plant Sci. 2023, 14, 1224958. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xu, Z.; Ding, A.; Kong, Y. Genome-Wide Identification and Expression Profiling Analysis of the Xyloglucan Endotransglucosylase/Hydrolase Gene Family in Tobacco (Nicotiana tabacum L.). Genes 2018, 9, 273. [Google Scholar] [CrossRef]
- Strasser, R.; Stadlmann, J.; Schähs, M.; Stiegler, G.; Quendler, H.; Mach, L.; Glössl, J.; Weterings, K.; Pabst, M.; Steinkellner, H. Generation of Glyco-Engineered Nicotiana benthamiana for the Production of Monoclonal Antibodies with a Homogeneous Human-like N-Glycan Structure. Plant Biotechnol. J. 2008, 6, 392–402. [Google Scholar] [CrossRef]
- Zanini, A.A.; Burch-Smith, T.M. New Insights into Plasmodesmata: Complex ‘Protoplasmic Connecting Threads’. J. Exp. Bot. 2024, 75, 5557. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, T.; Zhang, F.; Zhang, Y.; Liang, Y. RNA Interference: A Natural Immune System of Plants to Counteract Biotic Stressors. Cells 2019, 8, 38. [Google Scholar] [CrossRef]
- Rodrigues, S.P.; Ventura, J.A.; Aguilar, C.; Nakayasu, E.S.; Almeida, I.C.; Fernandes, P.M.B.; Zingali, R.B. Proteomic Analysis of Papaya (Carica papaya L.) Displaying Typical Sticky Disease Symptoms. Proteomics 2011, 11, 2592–2602. [Google Scholar] [CrossRef] [PubMed]
- DeBlasio, S.L.; Johnson, R.; Mahoney, J.; Karasev, A.; Gray, S.M.; MacCoss, M.J.; Cilia, M. Insights Into the Polerovirus–Plant Interactome Revealed by Coimmunoprecipitation and Mass Spectrometry. Mol. Plant-Microbe Interact. 2015, 28, 467–481. [Google Scholar] [CrossRef]
- Dorokhov, Y.L.; Sheshukova, E.V.; Byalik, T.E.; Komarova, T.V. Diversity of Plant Virus Movement Proteins: What Do They Have in Common? Processes 2020, 8, 1547. [Google Scholar] [CrossRef]
- Driouich, A.; Staehelin, L.A. The Plant Golgi Apparatus: Structural Organization and Functional Properties. In The Golgi Apparatus; Berger, E.G., Roth, J., Eds.; Birkhäuser: Basel, Switzerland, 1997; pp. 275–301. ISBN 978-3-0348-9810-2. [Google Scholar]
- Ito, Y.; Uemura, T.; Nakano, A. Formation and Maintenance of the Golgi Apparatus in Plant Cells. In International Review of Cell and Molecular Biology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 310, pp. 221–287. ISBN 978-0-12-800180-6. [Google Scholar]
- Hawes, C.; Satiat-Jeunemaitre, B. The Plant Golgi Apparatus—Going with the Flow. Biochim. Biophys. Acta BBA Mol. Cell Res. 2005, 1744, 93–107. [Google Scholar] [CrossRef]
- Zabotina, O.A.; Zhang, N.; Weerts, R. Polysaccharide Biosynthesis: Glycosyltransferases and Their Complexes. Front. Plant Sci. 2021, 12, 625307, Erratum in Front. Plant Sci. 2021, 12, 720709. [Google Scholar] [CrossRef]
- Matsui, T.; Takita, E.; Sato, T.; Kinjo, S.; Aizawa, M.; Sugiura, Y.; Hamabata, T.; Sawada, K.; Kato, K. N-Glycosylation at Noncanonical Asn-X-Cys Sequences in Plant Cells. Glycobiology 2011, 21, 994–999. [Google Scholar] [CrossRef]
- Saint-Jore-Dupas, C.; Nebenführ, A.; Boulaflous, A.; Follet-Gueye, M.-L.; Plasson, C.; Hawes, C.; Driouich, A.; Faye, L.; Gomord, V. Plant N -Glycan Processing Enzymes Employ Different Targeting Mechanisms for Their Spatial Arrangement along the Secretory Pathway. Plant Cell 2006, 18, 3182–3200. [Google Scholar] [CrossRef]
- Strasser, R. Biological Significance of Complex N-Glycans in Plants and Their Impact on Plant Physiology. Front. Plant Sci. 2014, 5, 363. [Google Scholar] [CrossRef]
- De Caroli, M.; Manno, E.; Piro, G.; Lenucci, M.S. Ride to Cell Wall: Arabidopsis XTH11, XTH29 and XTH33 Exhibit Different Secretion Pathways and Responses to Heat and Drought Stress. Plant J. 2021, 107, 448–466. [Google Scholar] [CrossRef]
- Johansson, P.; Brumer, H., III; Baumann, M.J.; Kallas, Å.M.; Henriksson, H.; Denman, S.E.; Teeri, T.T.; Jones, T.A. Crystal Structures of a Poplar Xyloglucan Endotransglycosylase Reveal Details of Transglycosylation Acceptor Binding. Plant Cell 2004, 16, 874–886. [Google Scholar] [CrossRef]
- Kallas, Å.M.; Piens, K.; Denman, S.E.; Henriksson, H.; Fäldt, J.; Johansson, P.; Brumer, H.; Teeri, T.T. Enzymatic Properties of Native and Deglycosylated Hybrid Aspen (Populus tremula × tremuloides) Xyloglucan Endotransglycosylase 16A Expressed in Pichia pastoris. Biochem. J. 2005, 390, 105–113. [Google Scholar] [CrossRef]
- Campbell, P.; Braam, J. In Vitro Activities of Four Xyloglucan Endotransglycosylases from Arabidopsis. Plant J. Cell Mol. Biol. 1999, 18, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Azim, M.F.; Burch-Smith, T.M. Organelles-Nucleus-Plasmodesmata Signaling (ONPS): An Update on Its Roles in Plant Physiology, Metabolism and Stress Responses. Curr. Opin. Plant Biol. 2020, 58, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Ganusova, E.E.; Reagan, B.C.; Fernandez, J.C.; Azim, M.F.; Sankoh, A.F.; Freeman, K.M.; McCray, T.N.; Patterson, K.; Kim, C.; Burch-Smith, T.M. Chloroplast-to-Nucleus Retrograde Signalling Controls Intercellular Trafficking via Plasmodesmata Formation. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190408. [Google Scholar] [CrossRef] [PubMed]
- Carlotto, N.; Wirth, S.; Furman, N.; Ferreyra Solari, N.; Ariel, F.; Crespi, M.; Kobayashi, K. The Chloroplastic DEVH-Box RNA Helicase INCREASED SIZE EXCLUSION LIMIT 2 Involved in Plasmodesmata Regulation Is Required for Group II Intron Splicing. Plant Cell Environ. 2016, 39, 165–173. [Google Scholar] [CrossRef]
- Germain, A.; Herlich, S.; Larom, S.; Kim, S.H.; Schuster, G.; Stern, D.B. Mutational Analysis of Arabidopsis Chloroplast Polynucleotide Phosphorylase Reveals Roles for Both RNase PH Core Domains in Polyadenylation, RNA 3’-End Maturation and Intron Degradation. Plant J. Cell Mol. Biol. 2011, 67, 381–394. [Google Scholar] [CrossRef]
- Burch-Smith, T.M.; Brunkard, J.O.; Choi, Y.G.; Zambryski, P.C. Organelle–Nucleus Cross-Talk Regulates Plant Intercellular Communication via Plasmodesmata. Proc. Natl. Acad. Sci. USA 2011, 108, E1451–E1460. [Google Scholar] [CrossRef]
- Herbers, K.; Lorences, E.P.; Barrachina, C.; Sonnewald, U. Functional Characterisation of Nicotiana tabacum Xyloglucan Endotransglycosylase (NtXET-1): Generation of Transgenic Tobacco Plants and Changes in Cell Wall Xyloglucan. Planta 2001, 212, 279–287. [Google Scholar] [CrossRef]
- Sheshukova, E.V.; Komarova, T.V.; Ershova, N.M.; Shindyapina, A.V.; Dorokhov, Y.L. An Alternative Nested Reading Frame May Participate in the Stress-Dependent Expression of a Plant Gene. Front. Plant Sci. 2017, 8, 2137. [Google Scholar] [CrossRef]
- Chapman, S.; Kavanagh, T.; Baulcombe, D. Potato Virus X as a Vector for Gene Expression in Plants. Plant J. Cell Mol. Biol. 1992, 2, 549–557. [Google Scholar] [CrossRef]
- Komarova, T.V.; Skulachev, M.V.; Zvereva, A.S.; Schwartz, A.M.; Dorokhov, Y.L.; Atabekov, J.G. New Viral Vector for Efficient Production of Target Proteins in Plants. Biochemistry 2006, 71, 846–850. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Zavaliev, R.; Epel, B.L. Imaging Callose at Plasmodesmata Using Aniline Blue: Quantitative Confocal Microscopy. In Plasmodesmata Methods and Protocols; Springer: New York, NY, USA, 2015; Volume 1217, pp. 105–119. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ershova, N.M.; Sheshukova, E.V.; Kamarova, K.A.; Alimova, A.R.; Savchenko, Y.Y.; Antimonova, A.A.; Komarova, T.V. Xyloglucan Endotransglycosylase/Hydrolase Downregulation Increases Nicotiana benthamiana Tolerance to Tobacco Mosaic Virus Infection. Int. J. Mol. Sci. 2025, 26, 11183. https://doi.org/10.3390/ijms262211183
Ershova NM, Sheshukova EV, Kamarova KA, Alimova AR, Savchenko YY, Antimonova AA, Komarova TV. Xyloglucan Endotransglycosylase/Hydrolase Downregulation Increases Nicotiana benthamiana Tolerance to Tobacco Mosaic Virus Infection. International Journal of Molecular Sciences. 2025; 26(22):11183. https://doi.org/10.3390/ijms262211183
Chicago/Turabian StyleErshova, Natalia M., Ekaterina V. Sheshukova, Kamila A. Kamarova, Alfiya R. Alimova, Yana Y. Savchenko, Alexandra A. Antimonova, and Tatiana V. Komarova. 2025. "Xyloglucan Endotransglycosylase/Hydrolase Downregulation Increases Nicotiana benthamiana Tolerance to Tobacco Mosaic Virus Infection" International Journal of Molecular Sciences 26, no. 22: 11183. https://doi.org/10.3390/ijms262211183
APA StyleErshova, N. M., Sheshukova, E. V., Kamarova, K. A., Alimova, A. R., Savchenko, Y. Y., Antimonova, A. A., & Komarova, T. V. (2025). Xyloglucan Endotransglycosylase/Hydrolase Downregulation Increases Nicotiana benthamiana Tolerance to Tobacco Mosaic Virus Infection. International Journal of Molecular Sciences, 26(22), 11183. https://doi.org/10.3390/ijms262211183





