Semaphorin3A Rewires CD4+ T-Cell Metabolism via AKT/mTORC1 Inhibition in Health and Rheumatoid Arthritis
Abstract
1. Introduction
2. Results
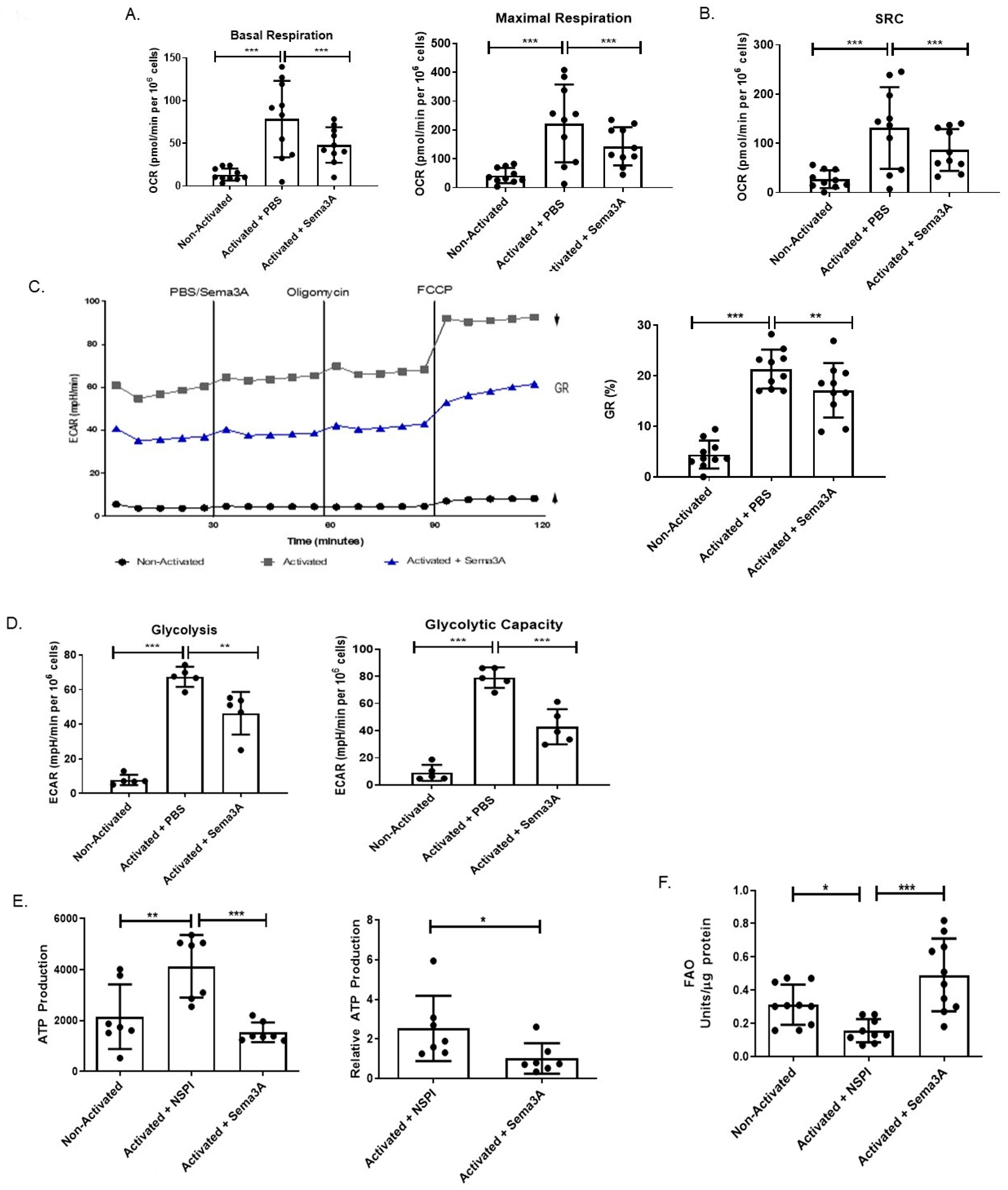
2.1. Sema3A Downregulates OXPHOS and Mitochondrial Membrane Potential in Activated T Cells
2.2. Sema3A Downregulates Glycolysis in Activated T Cells
2.3. Immmunometabolic Reprogramming: Sema3A Increases FAO
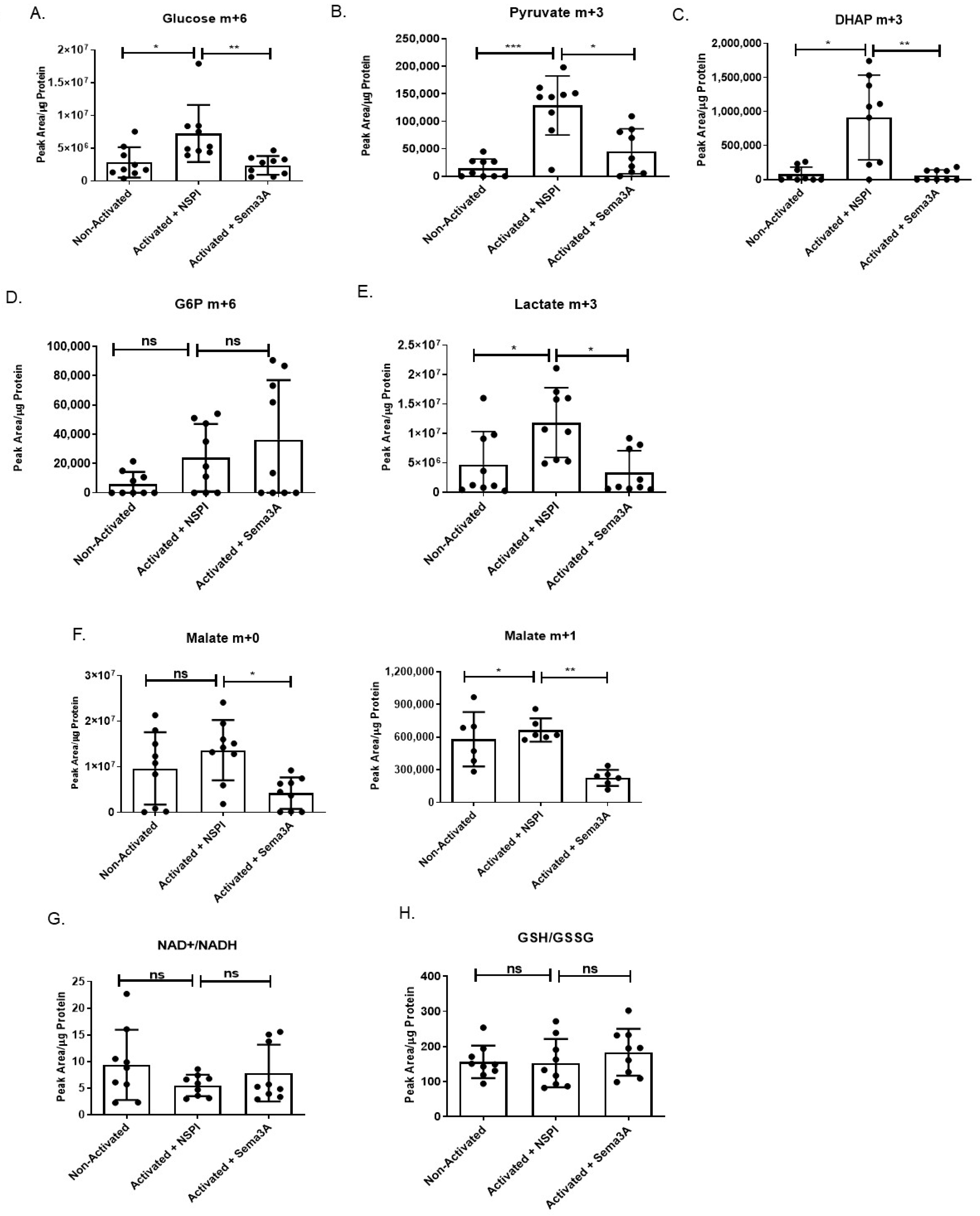
2.4. Sema3A Downregulates Key Metabolites During Glycolysis
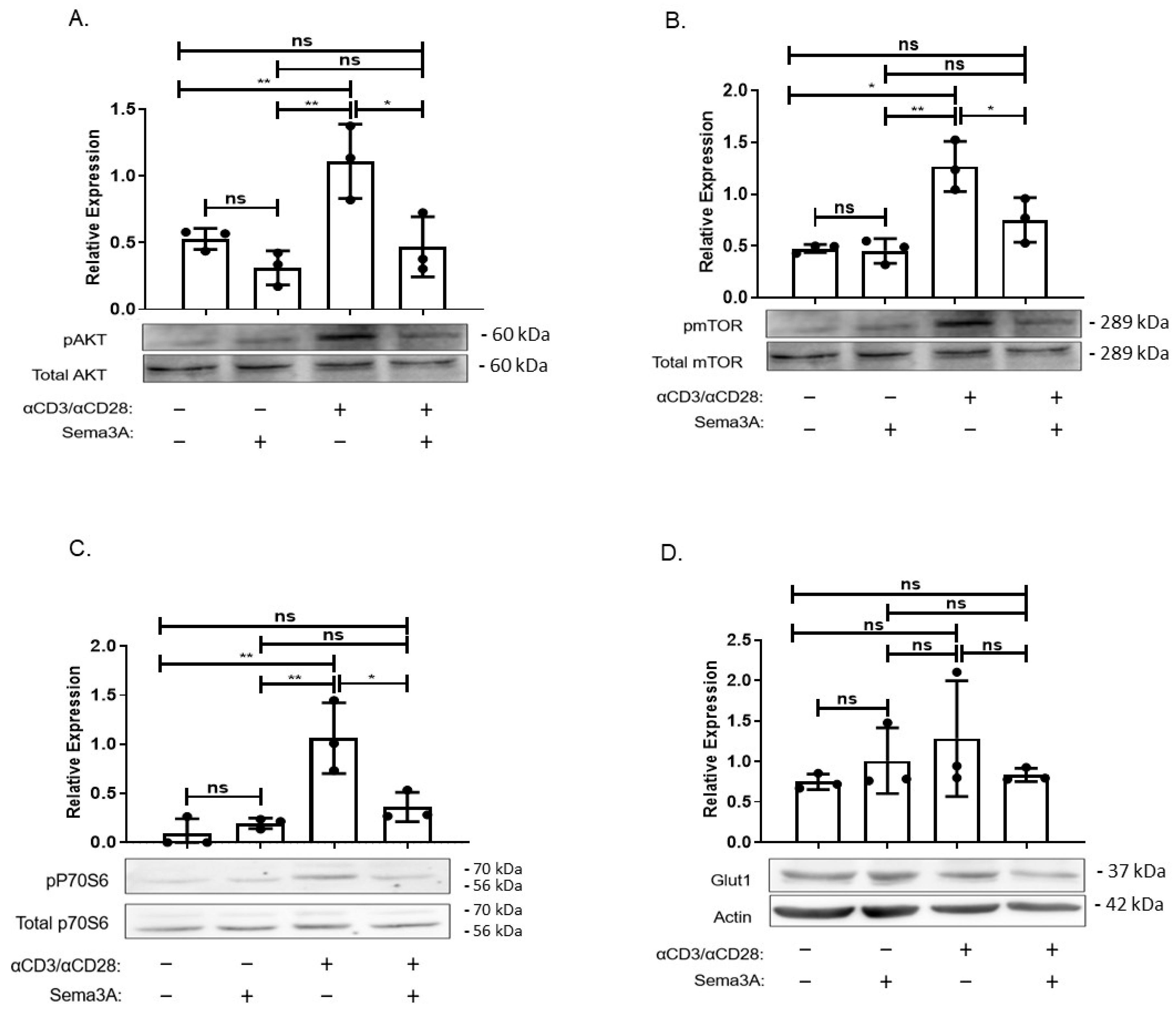
2.5. Sema3A’s Effect on the PI3K/AKT/mTORC1 Signaling Pathway
2.6. Sema3A Inhibits OXPHOS in RA Patients
2.7. Sema3A Downregulates Glycolysis in RA Patients
2.8. Sema3A Decreases ATP Production in RA
2.9. Sema3A Increases FAO in RA
3. Discussion
4. Materials and Methods
4.1. Preparation and Purification of Sema3A
4.2. CD4+ T Cell Isolation
4.3. Extracellular Flux Analysis
4.4. TMRE Staining
4.5. Mass Spectrometry Analysis
4.6. Western Blot Analysis
4.7. ATP Determination Assay
4.8. Fatty Acid Oxidation (FAO)
4.9. Study Population
4.10. Statistical Analysis
4.11. Antibodies
4.12. Supplemental Methods
4.12.1. Cell Proliferation Assay
4.12.2. Glucose Quantification Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McKinney, E.F.; Smith, K.G.C. Metabolic exhaustion in infection, cancer and autoimmunity review-article. Nat. Immunol. 2018, 19, 213–221. [Google Scholar] [CrossRef]
- Of, F.; To, U.; Trends, C.E.; Countries, I.N.D.; Geographic, T.H.E.; Of, D.; Diseases, A. S Usceptibility To a Utoimmune and a Llergic D Iseases. N. Engl. J. Med. 2002, 347, 911–920. [Google Scholar]
- Freitag, J.; Berod, L.; Kamradt, T.; Sparwasser, T. Immunometabolism and autoimmunity. Immunol. Cell Biol. 2016, 94, 925–934. [Google Scholar] [CrossRef]
- Pearce, E.L.; Pearce, E.J. Metabolic pathways in immune cell activation and quiescence. Immunity 2013, 38, 633–643. [Google Scholar] [CrossRef]
- Chavakis, T. Immunometabolism: Where Immunology and Metabolism Meet. J. Innate Immun. 2022, 14, 1–3. [Google Scholar] [CrossRef]
- Chi, H. Immunometabolism at the intersection of metabolic signaling, cell fate, and systems immunology. Cell. Mol. Immunol. 2022, 19, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Okano, T.; Saegusa, J.; Takahashi, S.; Ueda, Y.; Morinobu, A. Immunometabolism in rheumatoid arthritis. Immunol. Med. 2018, 41, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Buck, M.D.; O’Sullivan, D.; Pearce, E.L. T cell metabolism drives immunity. J. Exp. Med. 2015, 212, 1345–1360. [Google Scholar] [CrossRef] [PubMed]
- Piranavan, P.; Bhamra, M.; Perl, A. Metabolic Targets for Treatment of Autoimmune Diseases. Immunometabolism 2020, 2, e200012. [Google Scholar] [CrossRef]
- Gerriets, V.A.; Kishton, R.J.; Nichols, A.G.; Macintyre, A.N.; Inoue, M.; Ilkayeva, O.; Winter, P.S.; Liu, X.; Priyadharshini, B.; Slawinska, M.E.; et al. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J. Clin. Investig. 2015, 125, 194–207. [Google Scholar] [CrossRef]
- Mangal, J.L.; Basu, N.; Wu, H.-J.J.; Acharya, A.P. Immunometabolism: An Emerging Target for Immunotherapies to Treat Rheumatoid Arthritis. Immunometabolism 2021, 3, e210032. [Google Scholar] [CrossRef]
- Weyand, C.M.; Goronzy, J.J. Immunometabolism in early and late stages of rheumatoid arthritis. Nat. Rev. Rheumatol. 2017, 13, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Weyand, C.M.; Goronzy, J.J. Immunometabolism in the development of rheumatoid arthritis. Immunol. Rev. 2020, 294, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Vadasz, Z.; Toubi, E. Semaphorin 3A-a marker for disease activity and a potential putative disease-modifying treatment in systemic lupus erythematosus. Lupus 2012, 21, 1266–1270. [Google Scholar] [CrossRef] [PubMed]
- Bejar, J.; Kessler, O.; Sabag, A.D.; Sabo, E.; Itzhak, O.B.; Neufeld, G.; Vadasz, Z. Semaphorin3A: A potential therapeutic tool for lupus nephritis. Front. Immunol. 2018, 9, 1–6. [Google Scholar] [CrossRef]
- Rimar, D.; Nov, Y.; Rosner, I.; Slobodin, G.; Rozenbaum, M.; Halasz, K.; Haj, T.; Jiries, N.; Kaly, L.; Boulman, N.; et al. Semaphorin 3A: An immunoregulator in systemic sclerosis. Rheumatol. Int. 2015, 35, 1625–1630. [Google Scholar] [CrossRef]
- Cozacov, R.; Halasz, K.; Haj, T.; Vadasz, Z. Semaphorin 3A: Is a key player in the pathogenesis of asthma. Clin. Immunol. 2017, 184, 70–72. [Google Scholar] [CrossRef]
- Catalano, A. The Neuroimmune Semaphorin-3A Reduces Inflammation and Progression of Experimental Autoimmune Arthritis. J. Immunol. 2010, 185, 6373–6383. [Google Scholar] [CrossRef]
- Eiza, N.; Kessler, O.; Sabag, A.; Neufeld, G.; Jones, E.Y.; Vadasz, Z. Truncated-semaphorin3A is a potential regulatory molecule to restore immune homeostasis in immune-mediated diseases. Front. Pharmacol. 2023, 13, 1085892. [Google Scholar] [CrossRef]
- Xiang, R.; Xu, Y.; Zhang, W.; Kong, Y.G.; Tan, L.; Chen, S.M.; Deng, Y.Q.; Tao, Z.Z. Semaphorin 3A inhibits allergic inflammation by regulating immune responses in a mouse model of allergic rhinitis. Int. Forum Allergy Rhinol. 2019, 9, 528–537. [Google Scholar] [CrossRef]
- Toubi, E.; Vadasz, Z. Semaphorin3A is a promising therapeutic tool for bronchial asthma. Allergy Eur. J. Allergy Clin. Immunol. 2020, 75, 481–483. [Google Scholar] [CrossRef]
- Liu, L.N.; Li, X.M.; Ye, D.Q.; Pan, H.F. Emerging role of semaphorin-3A in autoimmune diseases. Inflammopharmacology 2018, 26, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Lepelletier, Y.; Moura, I.C.; Hadj-Slimane, R.; Renand, A.; Fiorentino, S.; Baude, C.; Shirvan, A.; Barzilai, A.; Hermine, O. Immunosuppressive role of semaphorin-3A on T cell proliferation is mediated by inhibition of actin cytoskeleton reorganization. Eur. J. Immunol. 2006, 36, 1782–1793. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Yan, W. T-cell immunometabolism against cancer. Cancer Lett. 2016, 382, 255–258. [Google Scholar] [CrossRef]
- Poznanski, S.M.; Barra, N.G.; Ashkar, A.A.; Schertzer, J.D. Immunometabolism of T cells and NK cells: Metabolic control of effector and regulatory function. Inflamm. Res. 2018, 67, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Rezaeepoor, M.; Ganjalikhani-hakemi, M.; Shapoori, S.; Eskandari, N.; Sharifi, M.; Etemadifar, M.; Mansuorian, M. Semaphorin-3A as An Immune Modulator Is Suppressed by MicroRNA-145-5p. Cell J. 2018, 20, 113–119. [Google Scholar] [CrossRef]
- Natarajan, D.; Plakkot, B.; Tiwari, K.; Ekambaram, S.; Wang, W.; Rudolph, M.; Mohammad, M.A.; Chacko, S.K.; Subramanian, M.; Tarantini, S.; et al. Chronic β3-AR stimulation activates distinct thermogenic mechanisms in brown and white adipose tissue and improves systemic metabolism in aged mice. Aging Cell 2024, 23, e14321. [Google Scholar] [CrossRef]
- Wu, J.; Jin, Z.; Zheng, H.; Yan, L.J. Sources and implications of NADH/NAD+ redox imbalance in diabetes and its complications. Diabetes, Metab. Syndr. Obes. 2016, 9, 145–153. [Google Scholar] [CrossRef]
- Flohé, L. The fairytale of the GSSG/GSH redox potential. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 3139–3142. [Google Scholar] [CrossRef]
- Orozco, J.M.; Krawczyk, P.A.; Scaria, S.M.; Cangelosi, A.L.; Chan, S.H.; Kunchok, T.; Lewis, C.A.; Sabatini, D.M. Dihydroxyacetone phosphate signals glucose availability to mTORC1. Nat. Metab. 2020, 2, 893–901. [Google Scholar] [CrossRef]
- Dufner, A.; Thomas, G. Ribosomal S6 kinase signaling and the control of translation. Exp. Cell Res. 1999, 253, 100–109. [Google Scholar] [CrossRef]
- Stöckl, S.; Reichart, J.; Zborilova, M.; Johnstone, B.; Grässel, S. Semaphorin 3A-Neuropilin-1 Signaling Modulates MMP13 Expression in Human Osteoarthritic Chondrocytes. Int. J. Mol. Sci. 2022, 23, 14180. [Google Scholar] [CrossRef] [PubMed]
- Sumi, C.; Hirose, N.; Yanoshita, M.; Takano, M.; Nishiyama, S.; Okamoto, Y.; Asakawa, Y.; Tanimoto, K. Semaphorin 3A Inhibits Inflammation in Chondrocytes under Excessive Mechanical Stress. Mediat. Inflamm. 2018, 2018, 5703651. [Google Scholar] [CrossRef]
- Sang, Y.; Tsuji, K.; Inoue-torii, A.; Fukushima, K.; Kitamura, S. Semaphorin3A-Inhibitor Ameliorates Doxorubicin-Induced Podocyte Injury. Int. J. Mol. Sci. 2020, 21, 4099. [Google Scholar] [CrossRef]
- Analyzer, M. Bioenergetics in T. cells using a SEF, G Measuring bioenergetics in T cells using a Seahorse. Physiol. Behav. 2017, 176, 139–148. [Google Scholar] [CrossRef]
- Marchetti, P.; Fovez, Q.; Germain, N.; Khamari, R.; Kluza, J. Mitochondrial spare respiratory capacity: Mechanisms, regulation, and significance in non-transformed and cancer cells. FASEB J. 2020, 34, 13106–13124. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J.; Lum, J.J.; Hatzivassiliou, G.; Thompson, C.B. The Biology of Cancer: Metabolic Reprogramming Fuels Cell Growth and Proliferation. Cell Metab. 2008, 7, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, A.; Fontanesi, F.; Díaz, F. Evaluation of the mitochondrial respiratory Chain and oxidative phosphorylation system using polarography and spectrophotometric enzyme assays. Curr. Protoc. Hum. Genet. 2009, 19, 1–13. [Google Scholar] [CrossRef]
- Crowley, L.C.; Christensen, M.E.; Waterhouse, N.J. Measuring mitochondrial transmembrane potential by TMRE staining. Cold Spring Harb. Protoc. 2016, 2016, 1092–1096. [Google Scholar] [CrossRef]
- Chapter 2 Biochemical targets for anthelmintic activity. Pharmacochem. Libr. 1997, 25, 46–70. [CrossRef]
- Harada, H.; Andersen, J.S.; Mann, M.; Terada, N.; Korsmeyer, S.J. p70S6 kinase signals cell survival as well as growth, inactivating the pro-apoptotic molecule BAD. Proc. Natl. Acad. Sci. USA 2001, 98, 9666–9670. [Google Scholar] [CrossRef] [PubMed]


| Patients Characteristics | n = 32 |
|---|---|
| Age (yr.) | 26–78 (59) |
| Sex | |
| Males | 20/32 (62.5%) |
| Females | 12/32 (37.5%) |
| Disease Duration (yr.) | 1–23 (8.6) |
| CDAI Range | 19–84 (33.2) |
| Comorbidities | |
| Dyslipidemia | 1/32 (3.1%) |
| Asthma | 1/32 (3.1%) |
| Hypertension | 5/32 (15.6%) |
| Hypothyroidism | 2/32 (6.2%) |
| Ischemic Heart Disease | 1/32 (3.1%) |
| HBV | 1/32 (3.1%) |
| Treatments | |
| Tocilizumab | 2/32 (6.2%) |
| Methotrexate | 2/32 (6.2%) |
| Rituximab | 1/32 (3.1%) |
| Leflunomide | 1/32 (3.1%) |
| Prednisone | 3/32 (9.4%) |
| Hydroxychloroquine | 1/32 (3.1%) |
| NSAIDs | 1/32 (3.1%) |
| Infliximab | 1/32 (3.1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mubariki, R.; Eiza, N.; Sabag, A.D.; Keret, S.; Rimar, D.; Slobodin, G.; Zisman, D.; Toubi, E.; Vadasz, Z. Semaphorin3A Rewires CD4+ T-Cell Metabolism via AKT/mTORC1 Inhibition in Health and Rheumatoid Arthritis. Int. J. Mol. Sci. 2025, 26, 11160. https://doi.org/10.3390/ijms262211160
Mubariki R, Eiza N, Sabag AD, Keret S, Rimar D, Slobodin G, Zisman D, Toubi E, Vadasz Z. Semaphorin3A Rewires CD4+ T-Cell Metabolism via AKT/mTORC1 Inhibition in Health and Rheumatoid Arthritis. International Journal of Molecular Sciences. 2025; 26(22):11160. https://doi.org/10.3390/ijms262211160
Chicago/Turabian StyleMubariki, Raeda, Nasren Eiza, Adi D. Sabag, Shiri Keret, Doron Rimar, Gleb Slobodin, Devy Zisman, Elias Toubi, and Zahava Vadasz. 2025. "Semaphorin3A Rewires CD4+ T-Cell Metabolism via AKT/mTORC1 Inhibition in Health and Rheumatoid Arthritis" International Journal of Molecular Sciences 26, no. 22: 11160. https://doi.org/10.3390/ijms262211160
APA StyleMubariki, R., Eiza, N., Sabag, A. D., Keret, S., Rimar, D., Slobodin, G., Zisman, D., Toubi, E., & Vadasz, Z. (2025). Semaphorin3A Rewires CD4+ T-Cell Metabolism via AKT/mTORC1 Inhibition in Health and Rheumatoid Arthritis. International Journal of Molecular Sciences, 26(22), 11160. https://doi.org/10.3390/ijms262211160







