Integrative Transcriptomic and Metabolomic Analysis Reveals That Acanthopanax senticosus Fruit Ameliorates Cisplatin-Induced Acute Kidney Injury by Suppressing the NF-κB/PI3K-AKT Pathway via UGT1A1 Regulation
Abstract
1. Introduction
2. Results
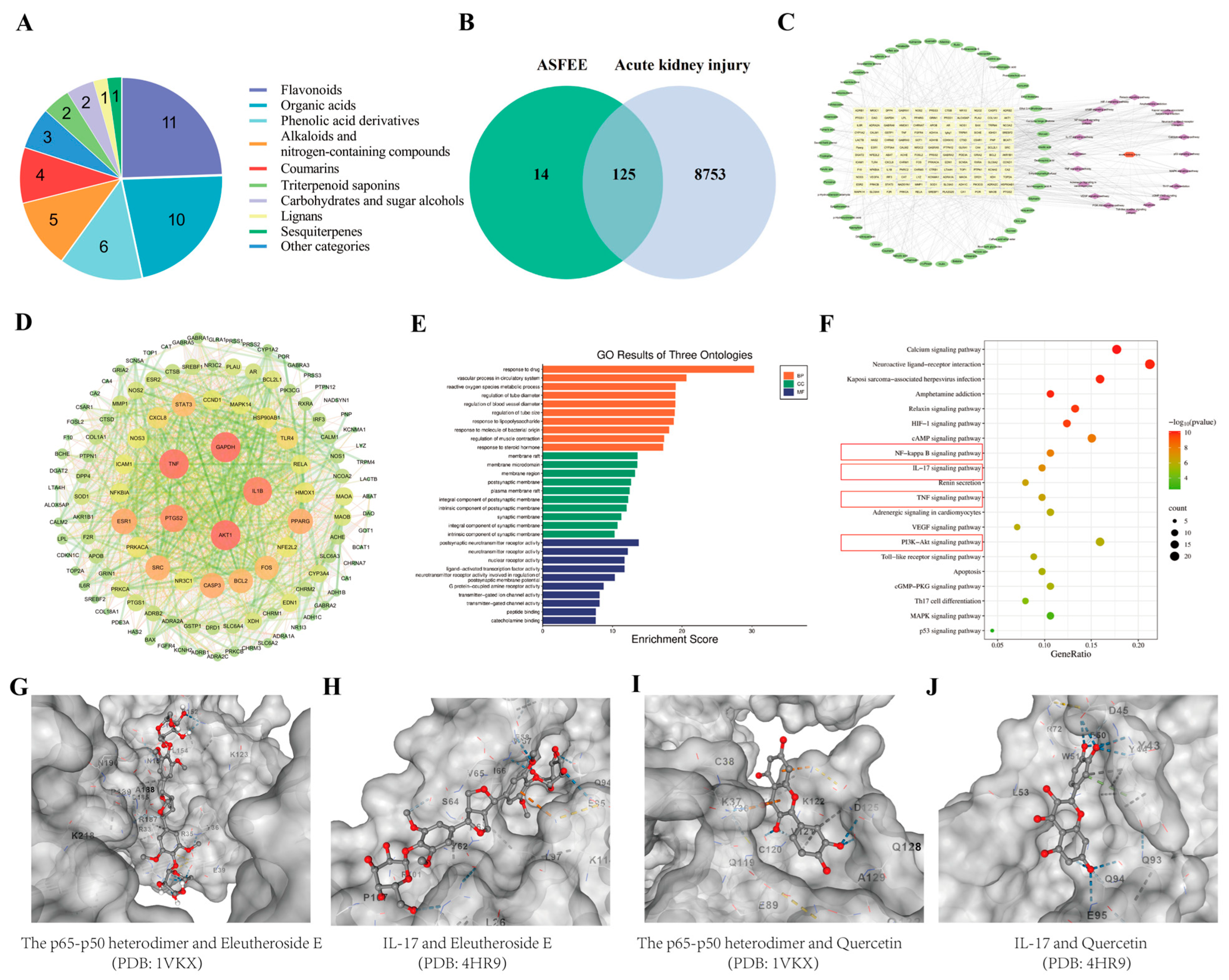
2.1. Analysis of Chemical Constituents in ASFEE by UPLC-MS/MS
2.2. Analysis of Network Pharmacology
2.2.1. Drug Targets of ASFEE and Disease Targets of AKI
2.2.2. GO Enrichment and KEGG Pathway Analysis
2.2.3. Molecular Docking Analysis
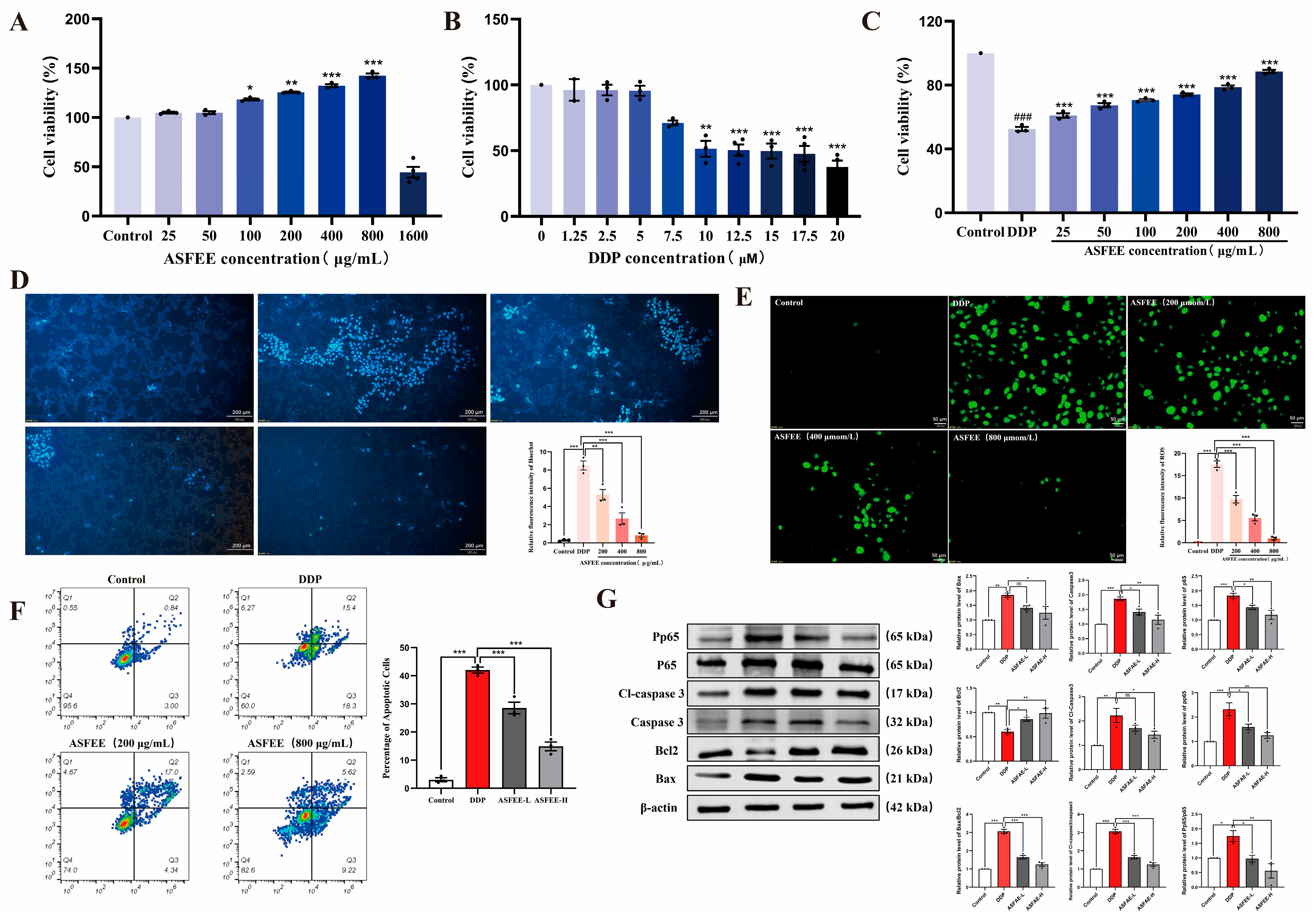
2.3. The Effect of ASFEE on DDP-Induced HK-2 Cell Injury
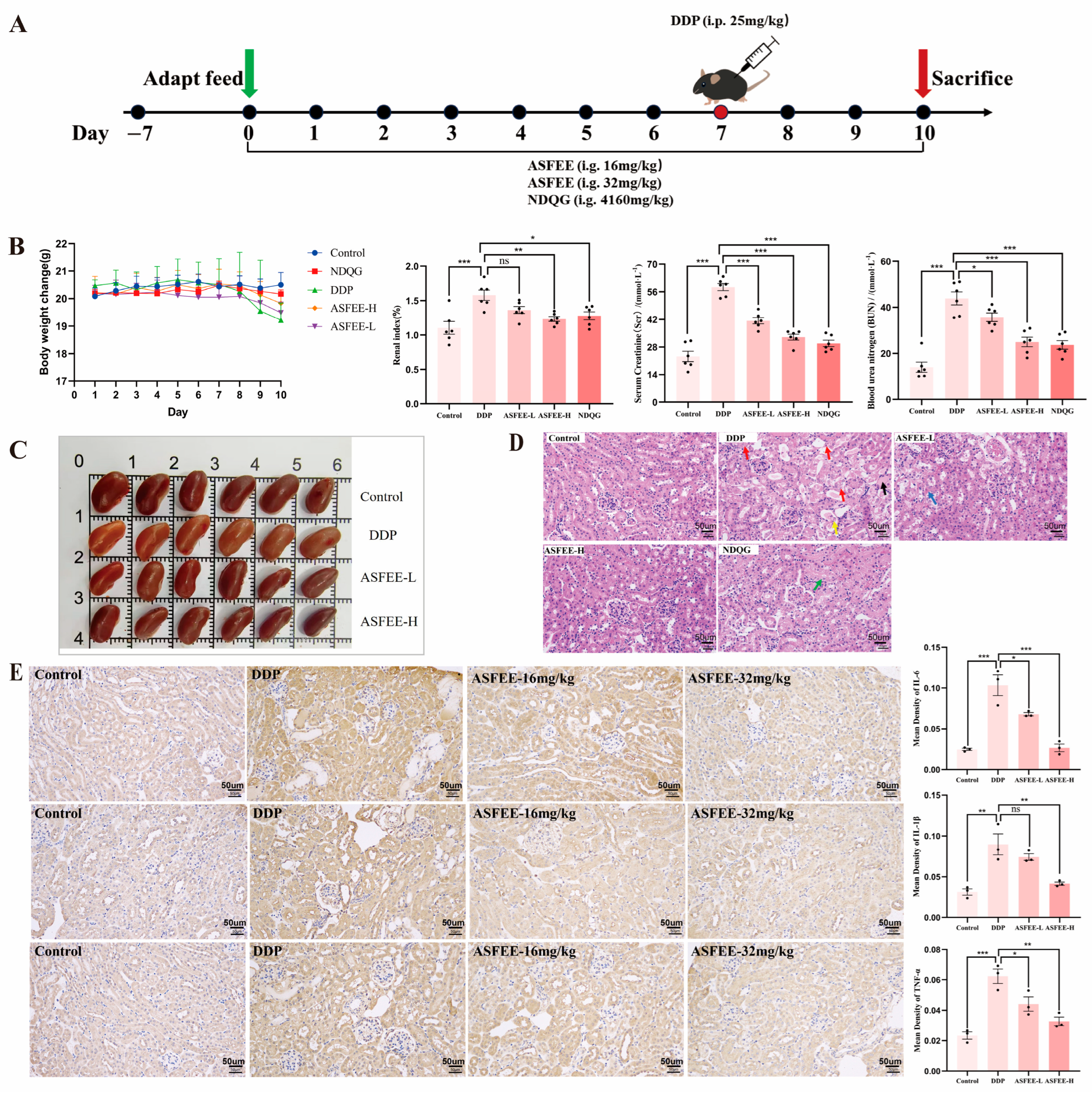
2.4. Analysis of Serum Biochemical Indicators and Kidney Histopathology Features
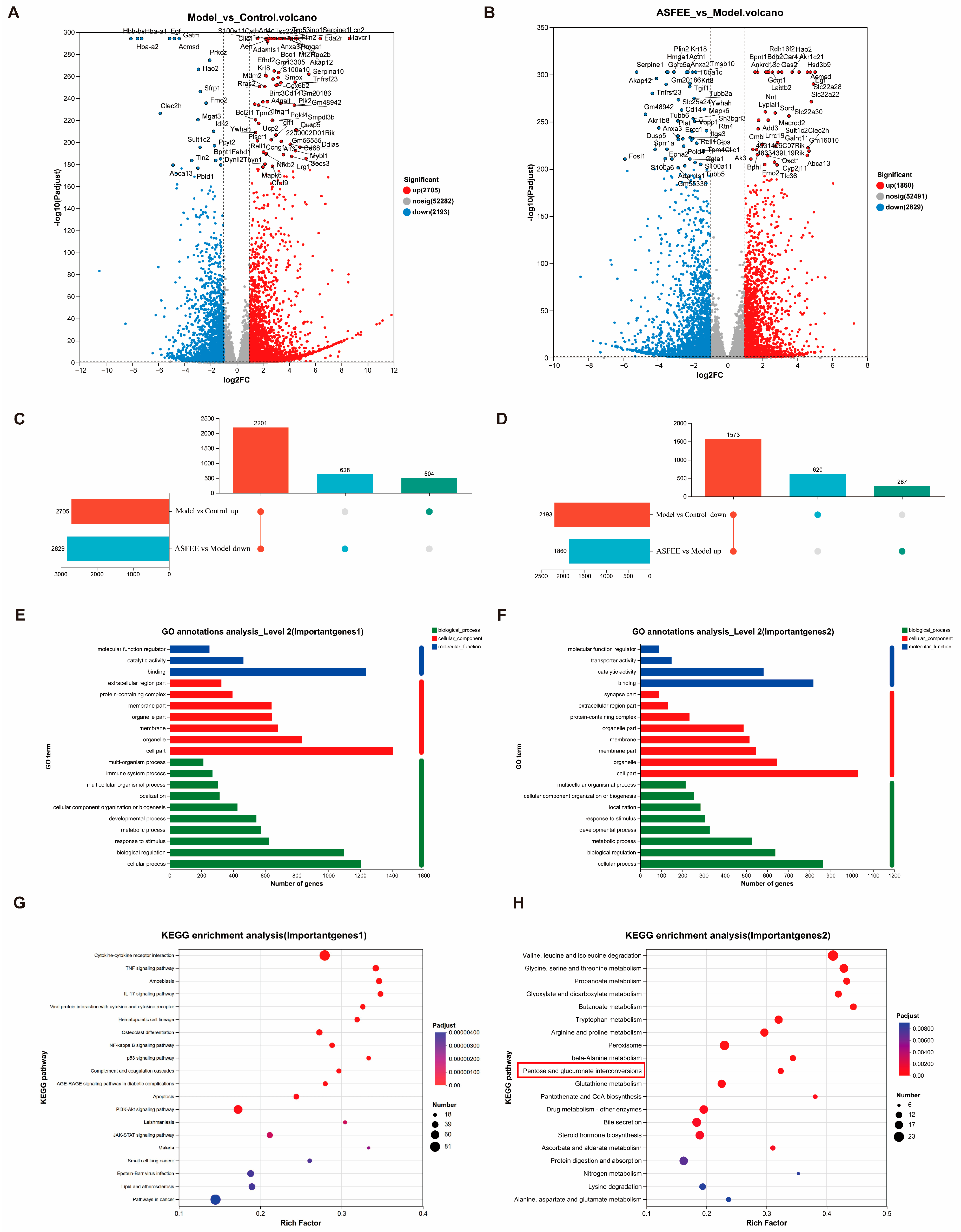
2.5. Transcriptome Analysis in Kidney Tissue
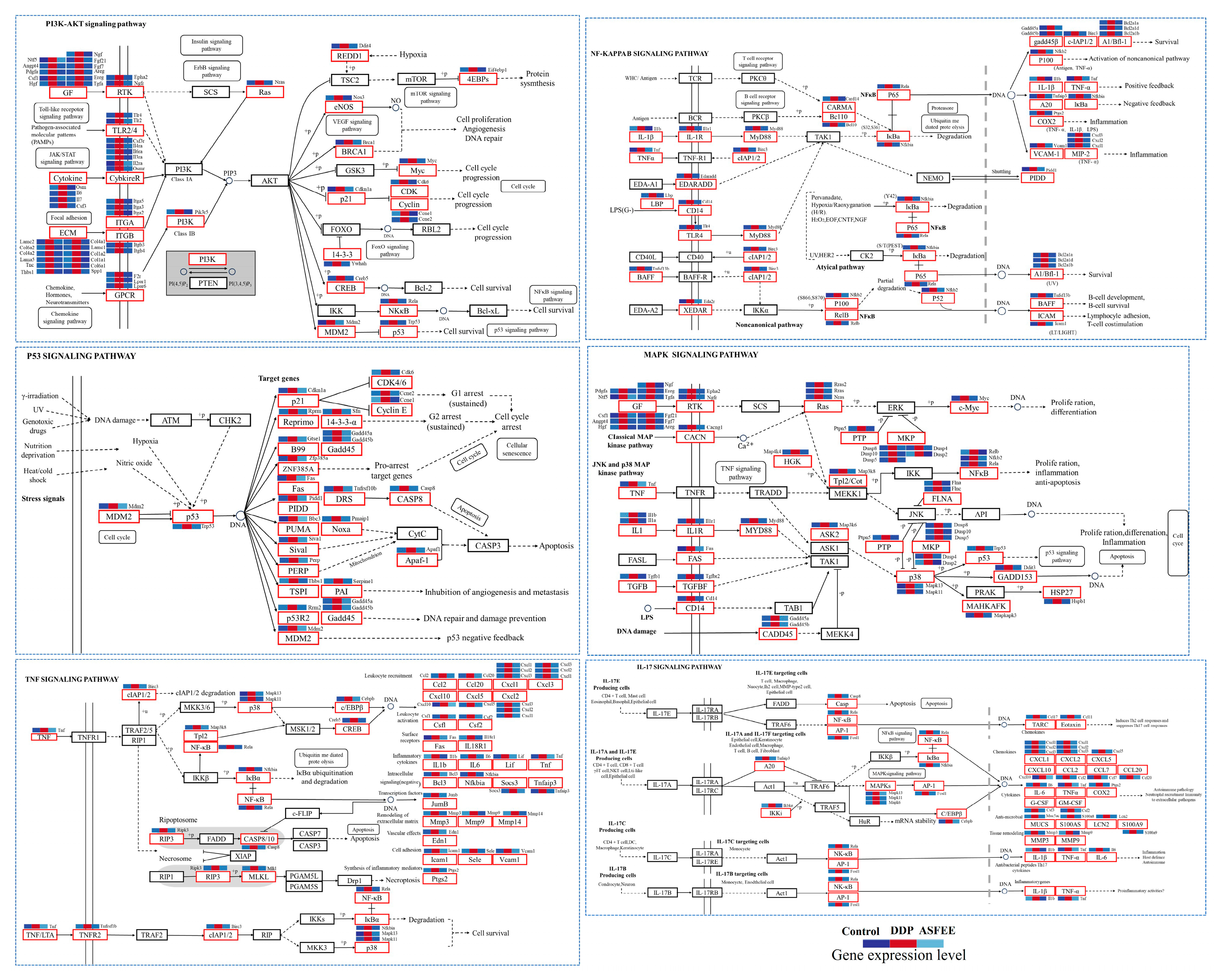
2.6. Effect of ASFEE on Kidney Inflammation-Related Pathways
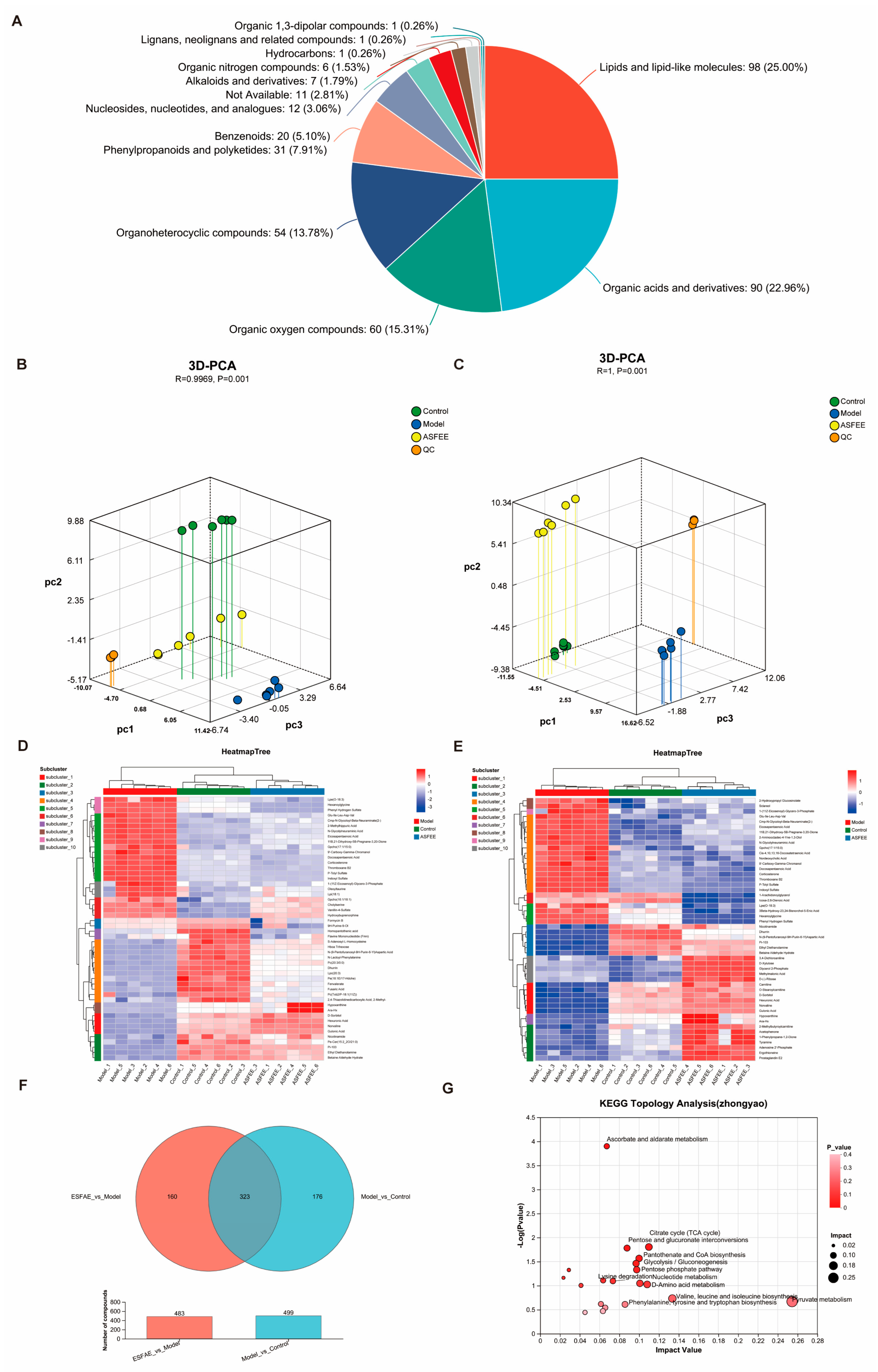
2.7. Metabolomic Analysis in Kidney Tissue
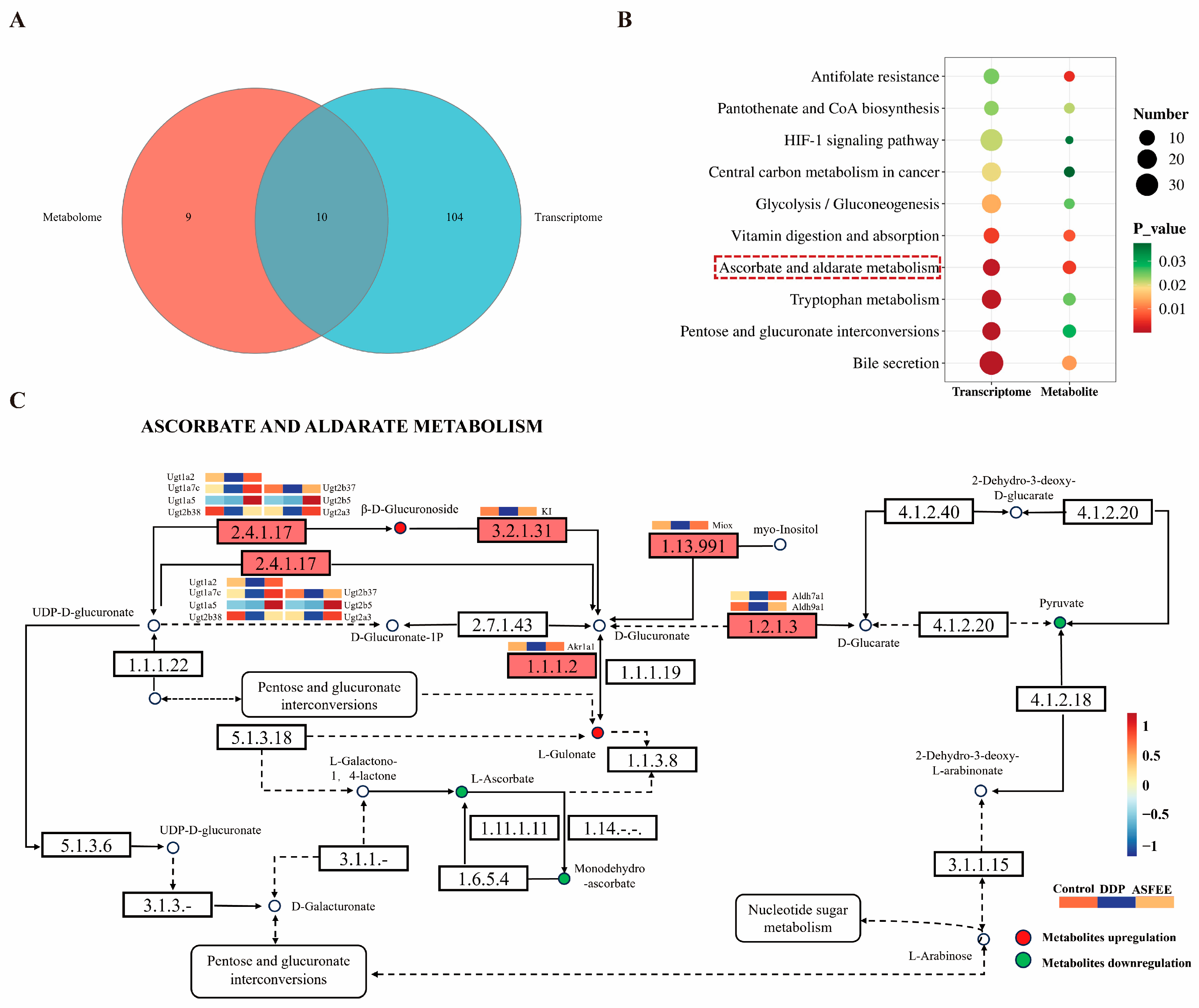
2.8. Integrated Analysis of ASFEE Protection Against DDP-Induced AKI
2.9. Validation of Key Genes and Proteins
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. UPLC-MS/MS Analysis
4.3. Network Pharmacology Construction
4.4. Molecular Docking
4.5. Cell Culture and Treatment
4.6. Hoechst 33258 Dyeing
4.7. Assessment of ROS Levels
4.8. Annexin V-FITC/PI Apoptosis Detection
4.9. Experimental Animals
4.10. SCr and BUN
4.11. H&E Staining and ROS Detection
4.12. Immunohistochemistry
4.13. Total RNA Isolation and Transcriptome Analysis
4.14. Untargeted LC-MS Metabolomics Analysis
4.15. RT-qPCR
4.16. Western Blot
4.17. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASFEE | Acanthopanax senticosus fruit ethanol extract |
| AKI | acute kidney injury |
| DDP | cisplatin |
| UHPLC-Q-TOF/MS | Ultra-High Performance Liquid Chromatography-Quadrupole-Time of Flight/Mass Spectrometry |
| BUN | Blood Urea Nitrogen |
| Scr | Serum Creatinine |
| DEGs | Differentially Expressed Genes |
| DAMs | Differentially Accumulated Metabolites |
| ESI | Electrospray Ionization |
| VIP | Variable Importance in Projection |
References
- Czopek, A.; Moorhouse, R.; Gallacher, P.J.; Pugh, D.; Ivy, J.R.; Farrah, T.E.; Godden, E.; Hunter, R.W.; Webb, D.J.; Tharaux, P.L.; et al. Endothelin blockade prevents the long-term cardiovascular and kidney sequelae of acute kidney injury in mice. Sci. Transl. Med. 2022, 14, eabf5074. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Nie, S.; Zhao, X.Y.; Xu, X.; Xu, H.; Liu, B.C.; Weng, J.P.; Chen, C.B.; Liu, H.F.; Yang, Q.Q.; et al. Incidence, risk factors and outcome of postoperative acute kidney injury in China. Nephrol. Dial. Transplant. 2024, 39, 967–977. [Google Scholar] [CrossRef]
- Tan, H.L.; Yap, J.Q.; Qian, Q. Acute Kidney Injury: Tubular Markers and Risk for Chronic Kidney Disease and End-Stage Kidney Failure. Blood Purif. 2016, 41, 144–150. [Google Scholar] [CrossRef]
- Gao, J.Z.; Deng, Q.X.; Yu, J.; Wang, C.; Wei, W. Role of kidney tubular epithelial cells and macrophages in cisplatin-induced acute kidney injury. Life Sci. 2024, 339, 122450. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.K.; Xie, Y.C.; Xiao, B.N.; He, X.L.; Ying, G.H.; Zha, H.Y.; Yang, C.; Jin, X.J.; Li, G.L.; Ping, L.; et al. Isorhamnetin alleviates cisplatin-induced acute kidney injury via enhancing fatty acid oxidation. Free Radic. Biol. Med. 2024, 212, 22–33. [Google Scholar] [CrossRef]
- Li, P.P.; Li, D.P.; Lu, Y.F.; Pan, S.K.; Cheng, F.; Li, S.; Zhang, X.N.; Huo, J.L.; Liu, D.W.; Liu, Z.S. GSTT1/GSTM1 deficiency aggravated cisplatin-induced acute kidney injury via ROS-triggered ferroptosis. Front. Immunol. 2024, 15, 1457230. [Google Scholar] [CrossRef]
- Tang, C.Y.; Livingston, M.J.; Safirstein, R.; Dong, Z. Cisplatin nephrotoxicity: New insights and therapeutic im-plications. Nat. Rev. Nephrol. 2023, 19, 53–72. [Google Scholar] [CrossRef]
- Li, Q.; Jin, S.B.; Chen, J.; Wang, J.; Huo, S.J.; Zhang, R.G. Effect of aeanthopanax senticosus on antioxidants and serum TNF-α during acute kidney ischemia-reperfusioninjury in rats. Hainan Med. J. 2015, 7, 941–943. [Google Scholar]
- Li, Q.; Jin, S.B.; She, Z.X.; Zhao, Z.Y.; Chen, Y.; Li, F.; Huo, S.J. Effect of Acanthopanax senticosus Pretreatment on Acute kidney Ischemic Reperfusion Injury in Rats. Chin. J. Cell Biol. 2016, 38, 1505–1511. [Google Scholar]
- Han, Y.; Zhang, A.H.; Sun, H.; Zhang, Y.Z.; Meng, X.C.; Yan, G.L.; Liu, L.; Wang, X.J. High-throughput ultra high performance liquid chromatography combined with mass spectrometry approach for the rapid analysis and charac-terization of multiple constituents of the fruit of Acanthopanax senticosus (Rupr. et Maxim.) Harms. J. Sep. Sci. 2017, 40, 2178–2187. [Google Scholar] [CrossRef]
- Wang, X.Y.; Lin, H.; Diao, C.Y.; An, R.; Wang, H.Z.; Che, C.L. Biological Activity and Application Progress of Ac-anthopanax senticosus. Fruit. Agric. Technol. 2023, 43, 10–13. [Google Scholar]
- Bian, X.B.; Chen, L.Z.; Bian, X.F.; Li, L.L.; Liu, D.; Liu, S.Y.; Xu, L.; Huo, X.Y.; Yang, X.H. Protective effect of Tibetan medicine Qiwei Tiexie pills on liver injury induced by acetaminophen overdose: An integrated strategy of network pharmacology, metabolomics and transcriptomics. Phytomedicine 2024, 123, 155221. [Google Scholar] [CrossRef]
- Tan, S.; Zou, Z.; Luan, X.; Chen, C.; Li, S.; Zhang, Z.; Quan, M.; Li, X.; Zhu, W.; Yang, G. Synthesis, Anti-Inflammatory Activities, and Molecular Docking Study of Novel Pyxinol Derivatives as Inhibitors of NF-κB Activation. Molecules 2024, 29, 1711. [Google Scholar] [CrossRef]
- Xia, X.; Zeng, H.; Wang, H.; Li, X.; Zhang, S.; Yang, L.; He, J. Revealing the Active Constituents and Mechanisms of Semiliquidambar cathayensis Chang Roots against Rheumatoid Arthritis through Network Pharmacology, Molecular Docking, and in Vivo Experiment. Chem. Biodivers. 2023, 20, e202200916. [Google Scholar] [CrossRef]
- Li, Y.Z.; Shi, L.; Zhao, F.; Luo, Y.W.; Zhang, M.J.; Wu, X.F.; Zhu, J.F. PIM1 attenuates cisplatin-induced AKI by inhibiting Drp1 activation. Cell Signal. 2024, 113, 110969. [Google Scholar] [CrossRef]
- Tlemsani, C.; Huillard, O.; Arrondeau, J.; Boudou-Rouquette, P.; Cessot, A.; Blanchet, B.; Thomas-Schoemann, A.; Coriat, R.; Durand, J.P.; Giroux, J.; et al. Effect of glucuronidation on transport and tissue accumulation of tyrosine kinase inhibitors: Consequences for the clinical management of sorafenib and regorafenib. Expert Opin. Drug Metab. Toxicol. 2015, 11, 785–794. [Google Scholar] [CrossRef]
- Karbownik, A.; Miedziaszczyk, M.; Grabowski, T.; Stanisławiak-Rudowicz, J.; Jaźwiec, R.; Wolc, A.; Grześkowiak, E.; Szałek, E. In vivo assessment of potential for UGT-inhibition-based drug-drug interaction between sorafenib and tapentadol. Biomed. Pharmacother. 2020, 130, 110530. [Google Scholar] [CrossRef] [PubMed]
- Holford, P.; Carr, A.C.; Jovic, T.H.; Ali, S.R.; Whitaker, I.S.; Marik, P.E.; Smith, A.D. Vitamin C-An Adjunctive Therapy for Respiratory Infection, Sepsis and COVID-19. Nutrients 2020, 12, 3760. [Google Scholar] [CrossRef] [PubMed]
- Hemilä, H.; de Man, A.M.E. Vitamin C and COVID-19. Front. Med. 2020, 7, 559811. [Google Scholar] [CrossRef] [PubMed]
- Azzam, S.M.; Anwar, H.M.; Abd El-Slam, A.H.; Diab, M.S.M.; Ibrahim, H.M.; Yousef, A.M.; Sabry, F.M.; Darwish, I.A.; Kaliyamoorthy, K.; Salem, G.E.M.; et al. The protective role of vitamin C against linezolid-induced hepato-kidney toxicity in a rat model. Front. Pharmacol. 2025, 16, 1551062. [Google Scholar] [CrossRef]
- Liang, H.; Song, K. Elucidating ascorbate and aldarate metabolism pathway characteristics via integration of untargeted metabolomics and transcriptomics of the kidney of high-fat diet-fed obese mice. PLoS ONE 2024, 19, e0300705. [Google Scholar] [CrossRef]
- Kinsey, G.R.; Li, L.; Okusa, M.D. Inflammation in Acute Kidney Injury. Nephron Exp. Nephrol. 2008, 109, e102–e107. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Zhang, Y.Z.; Wang, Q.; Zang, Y.W.; Li, Z.Y.; Duan, Z.C.; Ren, J.C.; Xu, Z.H. A polysaccharide from Huaier ameliorates cisplatin nephrotoxicity by decreasing oxidative stress and apoptosis via PI3K/AKT signaling. Int. J. Biol. Macromol. 2019, 139, 932–943. [Google Scholar] [CrossRef]
- Ma, Z.N.; Liu, Z.; Wang, Z.; Ren, S.; Tang, S.; Wang, Y.P.; Xiao, S.Y.; Chen, C.; Li, W. Supplementation of American ginseng berry extract mitigated cisplatin-evoked nephrotoxicity by suppressing ROS-mediated activation of MAPK and NF-κB signaling pathways. Food Chem. Toxicol. 2017, 110, 62–73. [Google Scholar] [CrossRef]
- Park, E.J.; Dusabimana, T.; Je, J.; Jeong, K.; Yun, S.P.; Kim, H.J.; Kim, H.; Park, S.W. Honokiol Protects the Kidney from Kidney Ischemia and Reperfusion Injury by Upregulating the Glutathione Biosynthetic Enzymes. Biomedicines 2020, 8, 352. [Google Scholar] [CrossRef]
- Ullah, M.; Liu, D.D.; Rai, S.; Dadhania, A.; Jonnakuti, S.; Concepcion, W.; Thakor, A.S. Reversing Acute Kidney Injury Using Pulsed Focused Ultrasound and MSC Therapy: A Role for HSP-Mediated PI3K/AKT Signaling. Mol. Ther. Methods Clin. Dev. 2020, 17, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Yuan, Y.T.; Gao, Y.T.; Li, X.; Tian, F.; Liu, F.; Du, R.C.; Li, P.F.; Wang, F.; Xu, S.M.; et al. MicroRNA-31 regulating apoptosis by mediating the phosphatidylinositol-3 kinase/protein kinase B signaling pathway in treatment of spinal cord injury. Brain Dev. 2019, 41, 649–661. [Google Scholar] [CrossRef]
- Zhao, W.; Li, J.; Li, Y.; Chen, Y.; Jin, H.X. Preventive Effect of Collagen Peptides from Acaudina molpadioides on Acute Kidney Injury through Attenuation of Oxidative Stress and Inflammation. Oxidative Med. Cell. Longev. 2022, 2022, 8186838. [Google Scholar] [CrossRef]
- Li, X.X.; Bechara, R.; Zhao, J.J.; McGeachy, M.J.; Gaffen, S.L. IL-17 receptor-based signaling and implications for disease. Nat. Immunol. 2019, 20, 1594–1602. [Google Scholar] [CrossRef]
- Zhang, Z.R.; Tang, Z.; Ma, X.W.; Sun, K.; Fan, L.P.; Fang, J.; Pan, J.P.; Wang, X.J.; An, H.Z.; Zhou, J. TAOK1 negatively regulates IL-17-mediated signaling and inflammation. Cell. Mol. Immunol. 2018, 15, 794–802, Erratum in Cell. Mol. Immunol. 2018, 15, 940. [Google Scholar] [CrossRef]
- Kim, K.H.; Hong, G.L.; Jung, D.Y.; Karunasagara, S.; Jeong, W.I.; Jung, J.Y. IL-17 deficiency aggravates the streptozotocin-induced diabetic nephropathy through the reduction of autophagosome formation in mice. Mol. Med. 2021, 27, 25. [Google Scholar]
- Song, Z.Y.; Li, Z.Y.; Pan, T.; Liu, T.; Gong, B.F.; Wang, Z.X.; Liu, K.; Fan, H.Y. Protopanaxadiol prevents cispla-tin-induced acute kidney injury by regulating ferroptosis. J. Pharm. Pharmacol. 2024, 76, 884–896. [Google Scholar] [CrossRef]
- Guo, S.S.; Zhou, L.; Liu, X.Q.; Gao, L.; Li, Y.Y.; Wu, Y.G. Baicalein alleviates cisplatin-induced acute kidney in-jury by inhibiting ALOX12-dependent ferroptosis. Phytomedicine 2024, 130, 155757. [Google Scholar] [CrossRef]
- Tatsumi, N.; Tokumitsu, S.; Nakano, M.; Fukami, T.; Nakajima, M. miR-141-3p commonly regulates human UGT1A isoforms via different mechanisms. Drug Metab. Pharmacokinet. 2018, 33, 203–210. [Google Scholar] [CrossRef]
- Li, W.; Chen, Y.N.; Chen, Y.Y.; Wang, Z.; Wang, Z.; Jiang, L.L.; Shi, H.C.; Liu, Y. Inhibition of UGT1A1*1 and UGT1A1*6 catalyzed glucuronidation of SN-38 by silybins. Chem. Biol. Interact. 2022, 368, 110248. [Google Scholar] [CrossRef]
- Ptaszyńska, A.A.; Załuski, D. Extracts from Eleutherococcus senticosus (Rupr. et Maxim.) Maxim. Roots: A New Hope Against Honeybee Death Caused by Nosemosis. Molecules 2020, 25, 4452. [Google Scholar] [CrossRef]
- Huang, X.Y.; Zhang, M.X.; Song, Y.; Sun, B.W.; Lin, L.; Song, X.L.; Li, C. Integrated network pharmacology to investigate the mechanism of Salvia miltiorrhiza Bunge in the treatment of myocardial infarction. J. Cell. Mol. Med. 2023, 27, 3514–3525. [Google Scholar] [CrossRef]
- Zhou, Q.Y.; Quirk, J.D.; Hu, Y.; Yan, H.M.; Gaut, J.P.; Pham, C.T.N.; Wickline, S.A.; Pan, H. Rapamycin Perfluorocarbon Nanoparticle Mitigates Cisplatin-Induced Acute Kidney Injury. Int. J. Mol. Sci. 2023, 24, 6086. [Google Scholar] [CrossRef]
- Wang, P.P.; Ouyang, J.; Zhou, K.Q.; Hu, D.D.; Zhang, S.N.; Zhang, A.H.; Yang, Y.W. Olesoxime protects against cisplatin-induced acute kidney injury by attenuating mitochondrial dysfunction. Biomed. J. 2025, 48, 100730. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, L.; Tang, Z.; Ma, X.; Zhang, Q.; Han, Y.; Wang, Q.; Liu, J.; Bian, X.; Gao, L.; Xu, M.; et al. Integrative Transcriptomic and Metabolomic Analysis Reveals That Acanthopanax senticosus Fruit Ameliorates Cisplatin-Induced Acute Kidney Injury by Suppressing the NF-κB/PI3K-AKT Pathway via UGT1A1 Regulation. Int. J. Mol. Sci. 2025, 26, 11131. https://doi.org/10.3390/ijms262211131
Han L, Tang Z, Ma X, Zhang Q, Han Y, Wang Q, Liu J, Bian X, Gao L, Xu M, et al. Integrative Transcriptomic and Metabolomic Analysis Reveals That Acanthopanax senticosus Fruit Ameliorates Cisplatin-Induced Acute Kidney Injury by Suppressing the NF-κB/PI3K-AKT Pathway via UGT1A1 Regulation. International Journal of Molecular Sciences. 2025; 26(22):11131. https://doi.org/10.3390/ijms262211131
Chicago/Turabian StyleHan, Liu, Zebo Tang, Xiangyu Ma, Qiuyue Zhang, Yu Han, Qi Wang, Jinlong Liu, Xuefeng Bian, Liancong Gao, Mengran Xu, and et al. 2025. "Integrative Transcriptomic and Metabolomic Analysis Reveals That Acanthopanax senticosus Fruit Ameliorates Cisplatin-Induced Acute Kidney Injury by Suppressing the NF-κB/PI3K-AKT Pathway via UGT1A1 Regulation" International Journal of Molecular Sciences 26, no. 22: 11131. https://doi.org/10.3390/ijms262211131
APA StyleHan, L., Tang, Z., Ma, X., Zhang, Q., Han, Y., Wang, Q., Liu, J., Bian, X., Gao, L., Xu, M., & Sun, X. (2025). Integrative Transcriptomic and Metabolomic Analysis Reveals That Acanthopanax senticosus Fruit Ameliorates Cisplatin-Induced Acute Kidney Injury by Suppressing the NF-κB/PI3K-AKT Pathway via UGT1A1 Regulation. International Journal of Molecular Sciences, 26(22), 11131. https://doi.org/10.3390/ijms262211131







