Ultrastructural Characteristics of the Juvenile Chum Salmon (Oncorhynchus keta) Cerebellum: Interneuron Composition, Neuro–Glial Interactions, Homeostatic Neurogenesis, and Synaptic Plasticity
Abstract
1. Introduction
2. Results
2.1. Morphological Organization of the Cerebellum of Juvenile Chum Salmon Oncorhynchus keta
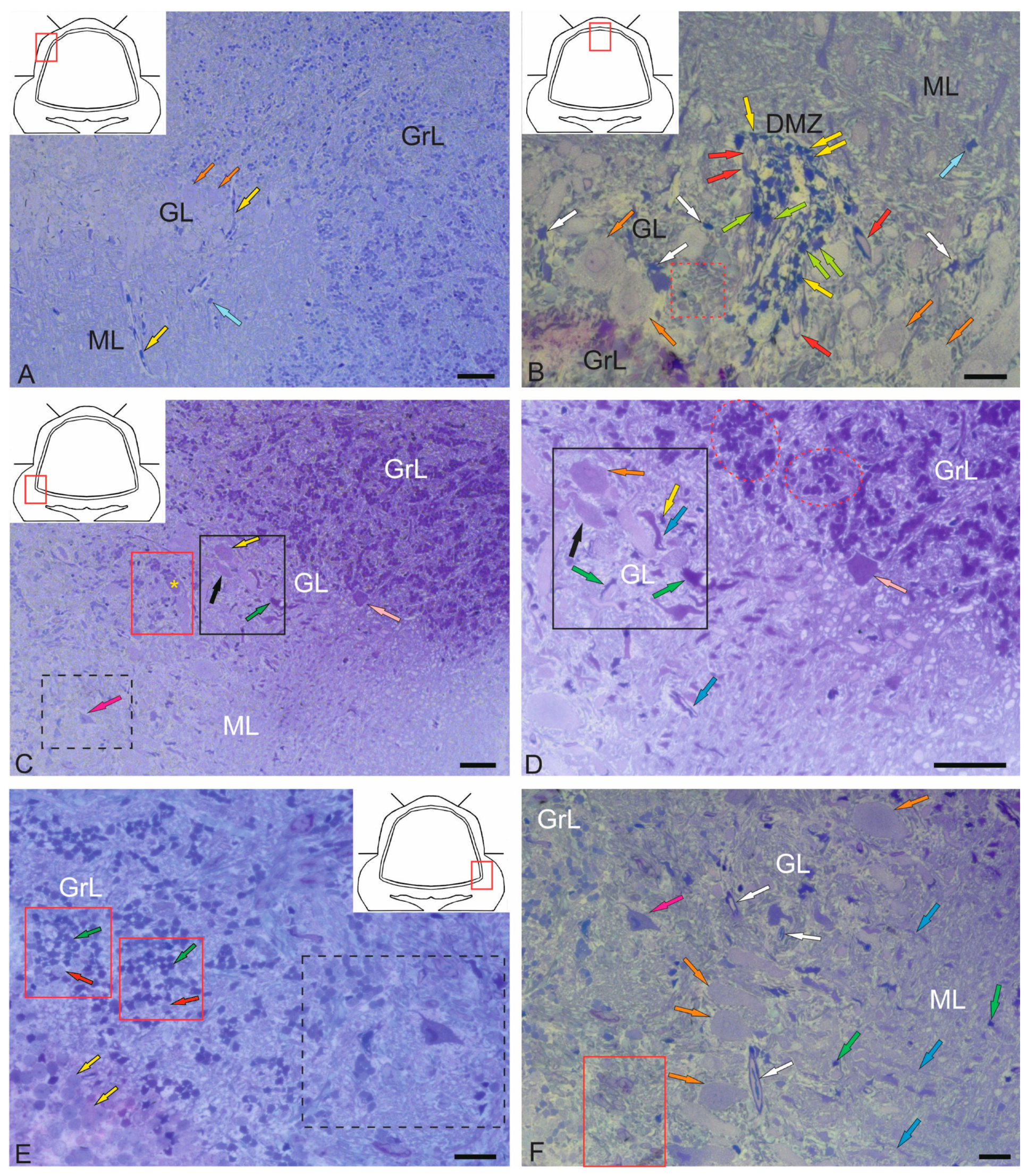
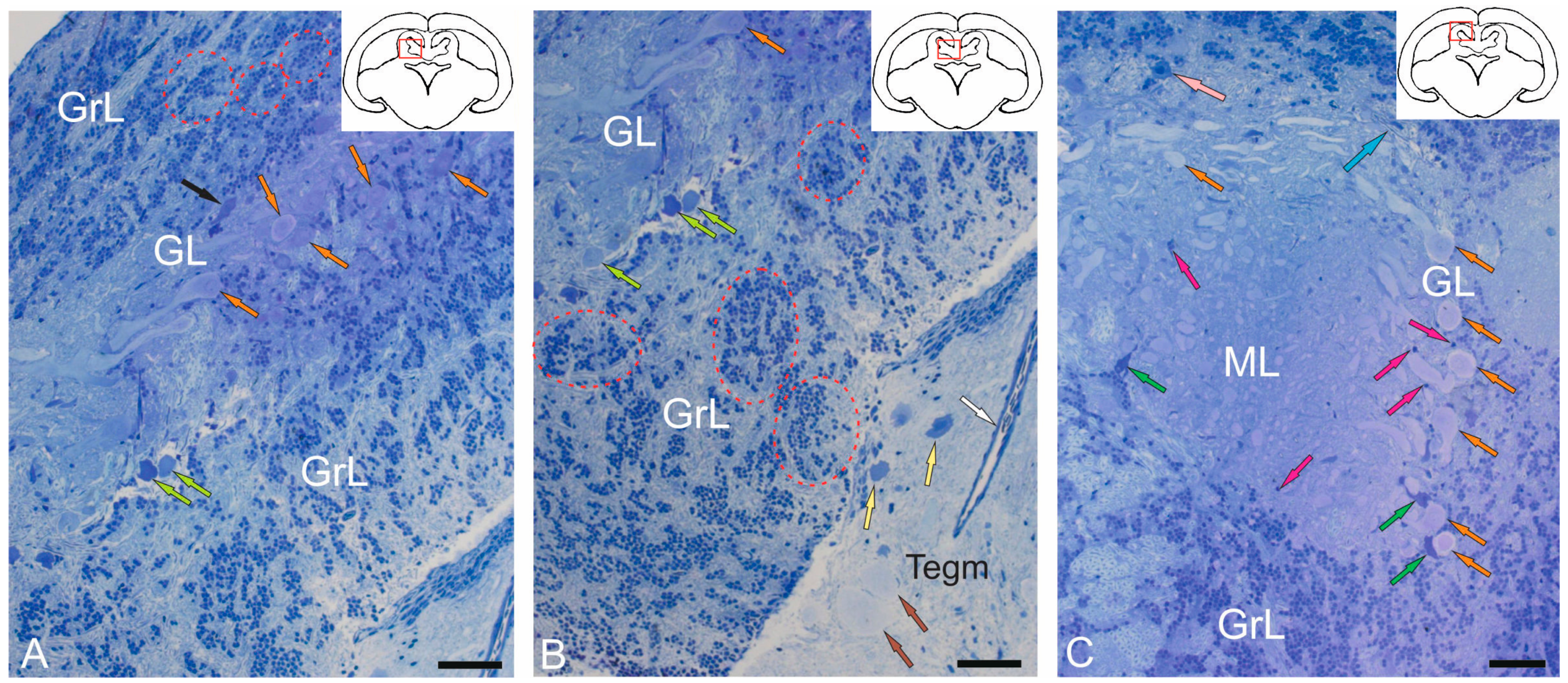
2.1.1. Organization of the Corpus Cerebellum
2.1.2. Organization of the Valvula Cerebelli
2.1.3. Organization of the Granular Eminences
2.2. Ultrastructural Organization of the Cerebellum of Juvenile Chum Salmon
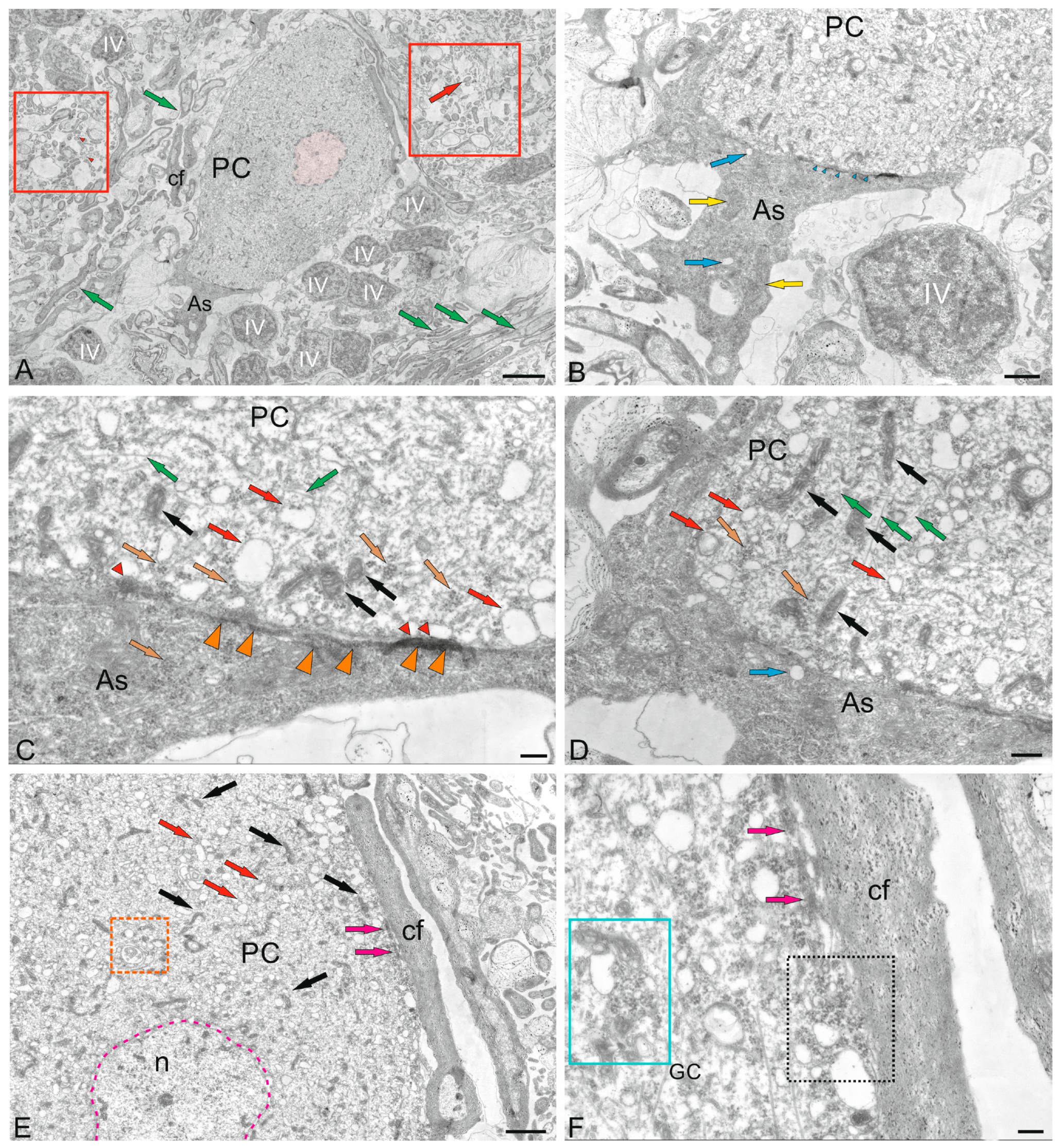
2.2.1. Molecular Layer Cells
2.2.2. Morphological Heterogeneity of Synaptic Structures
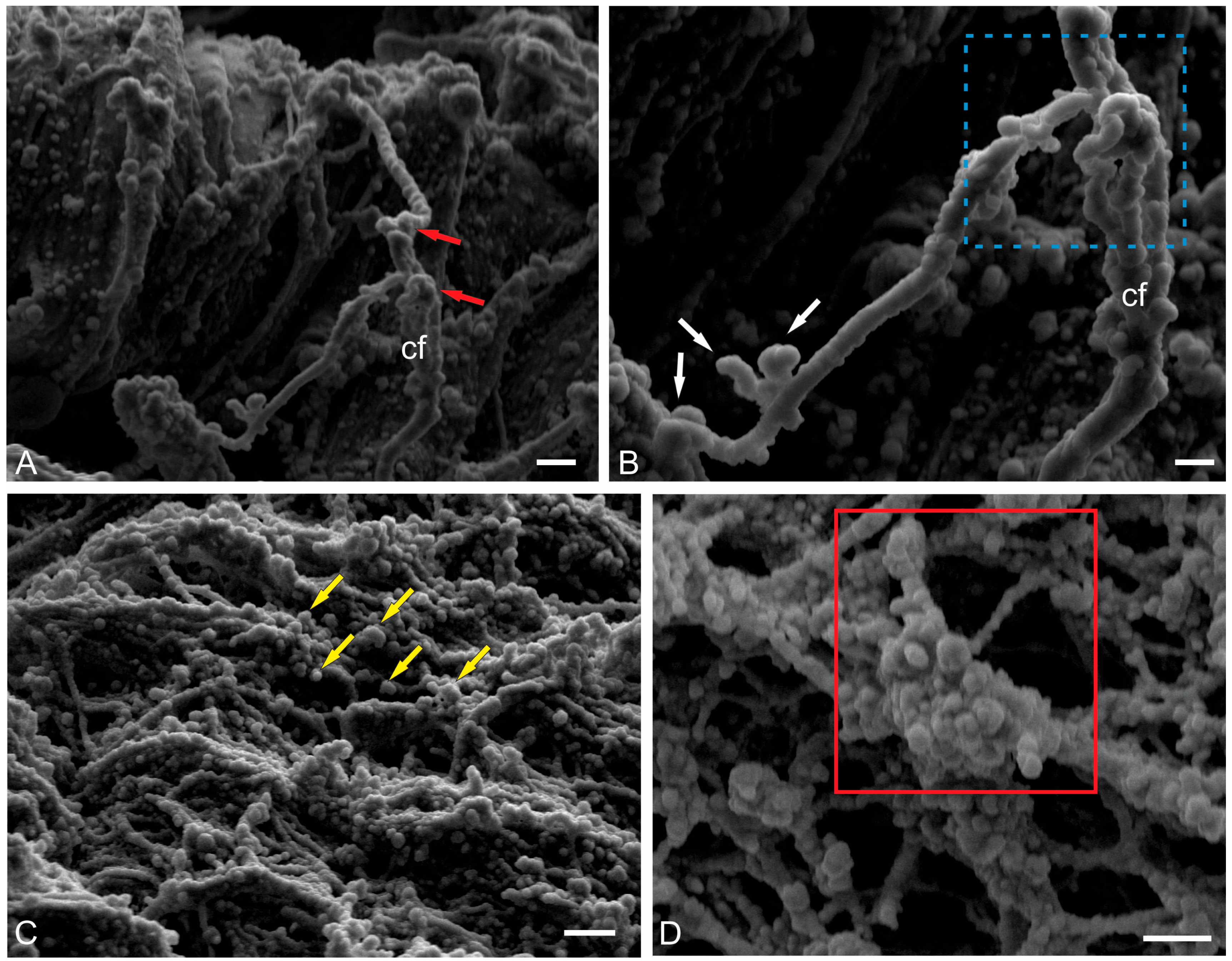
2.2.3. Ultrastructural Organization of Climbing Fibers and Neural Stem Progenitor Cells
2.2.4. Ultrastructural Organization of the Dorsal Matrix Zone
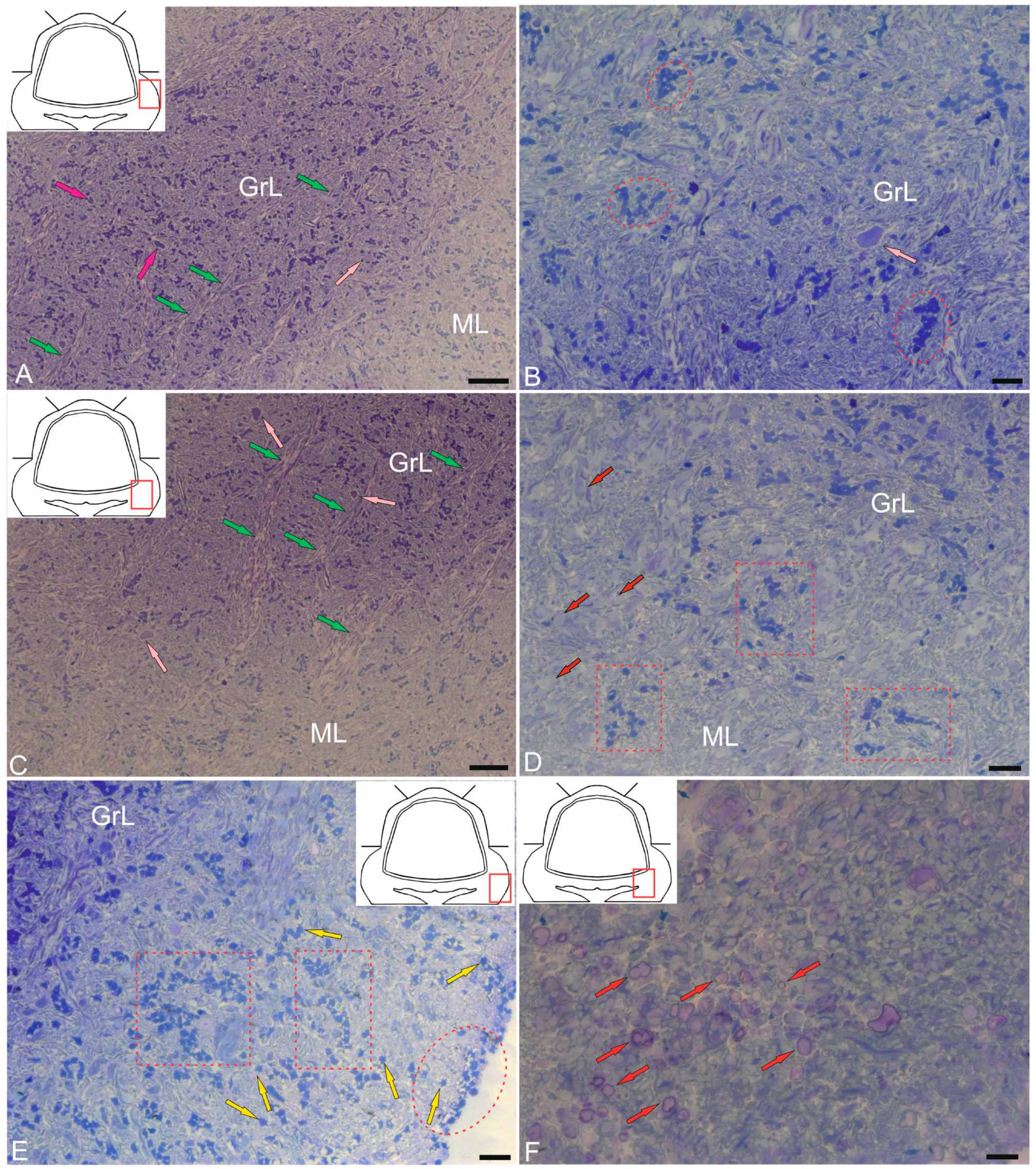
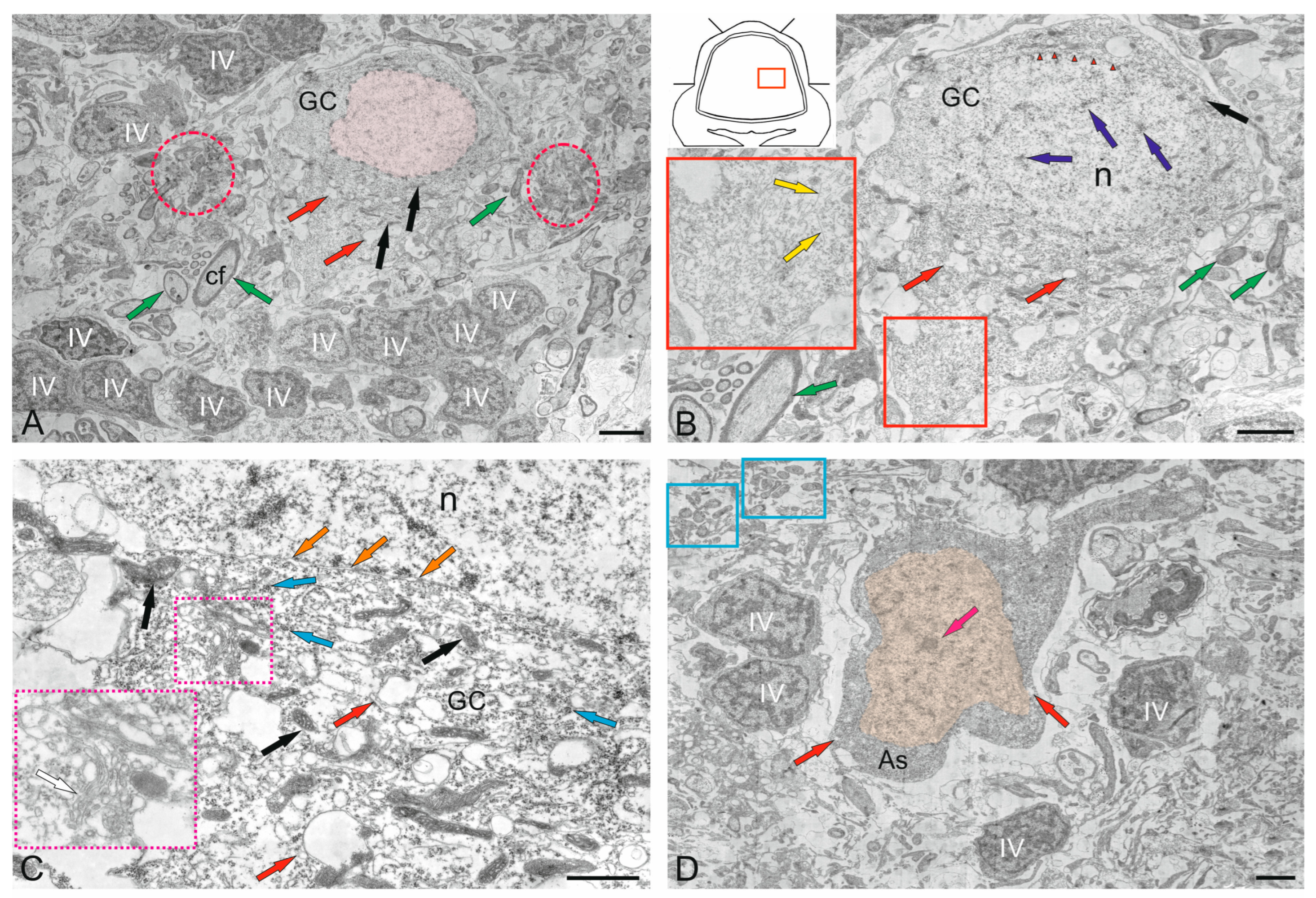
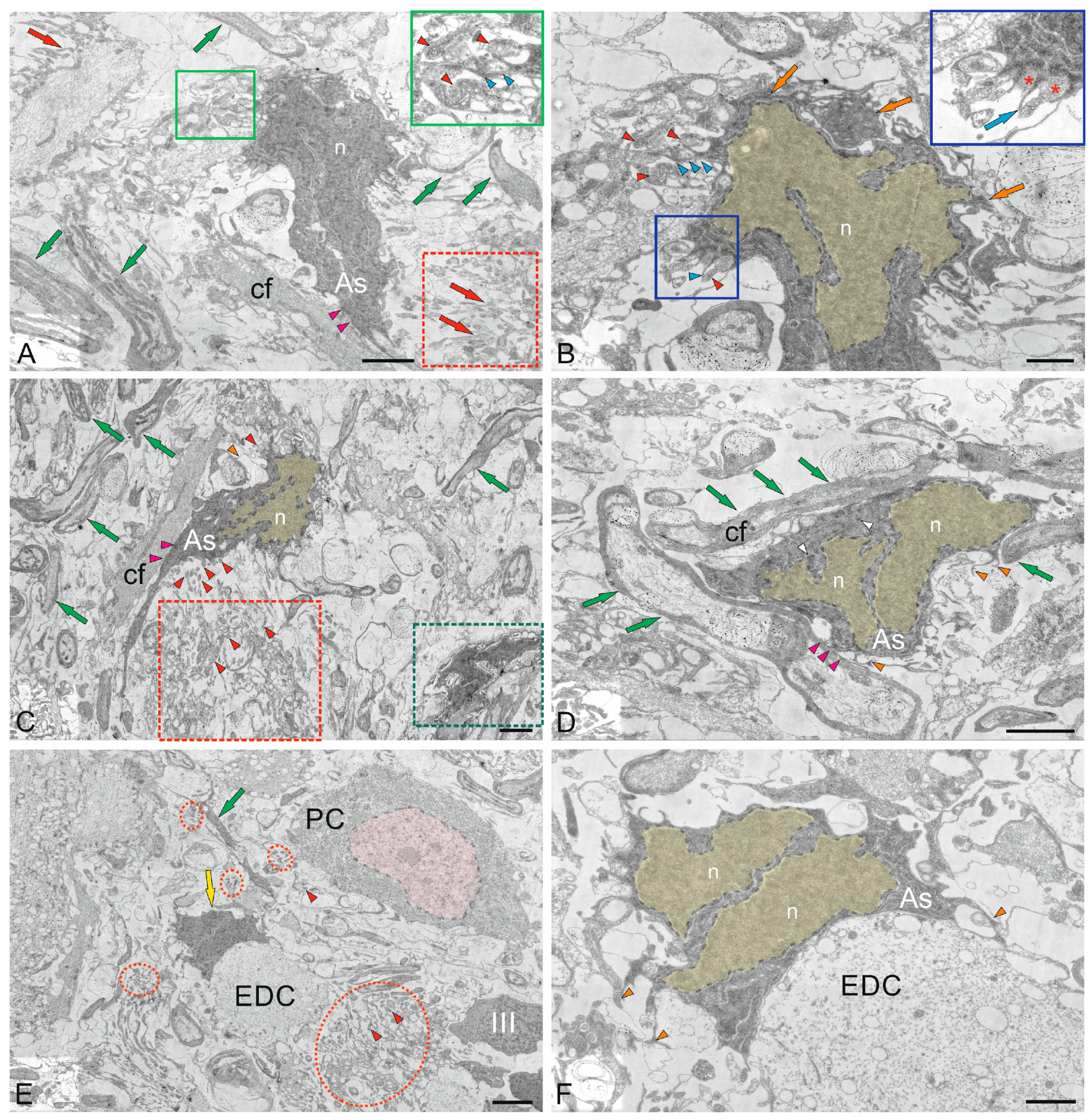
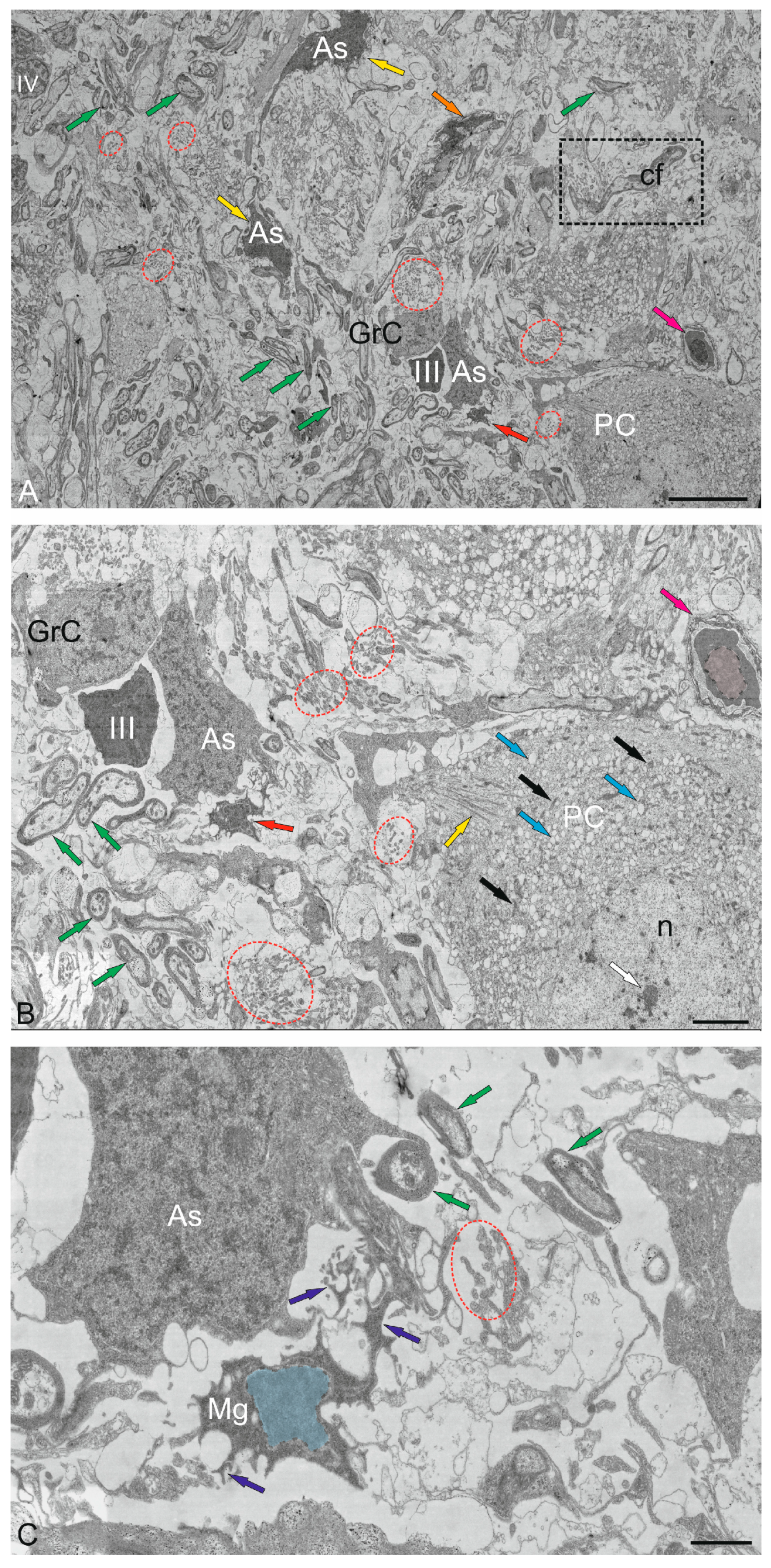
2.3. Ganglion Layer
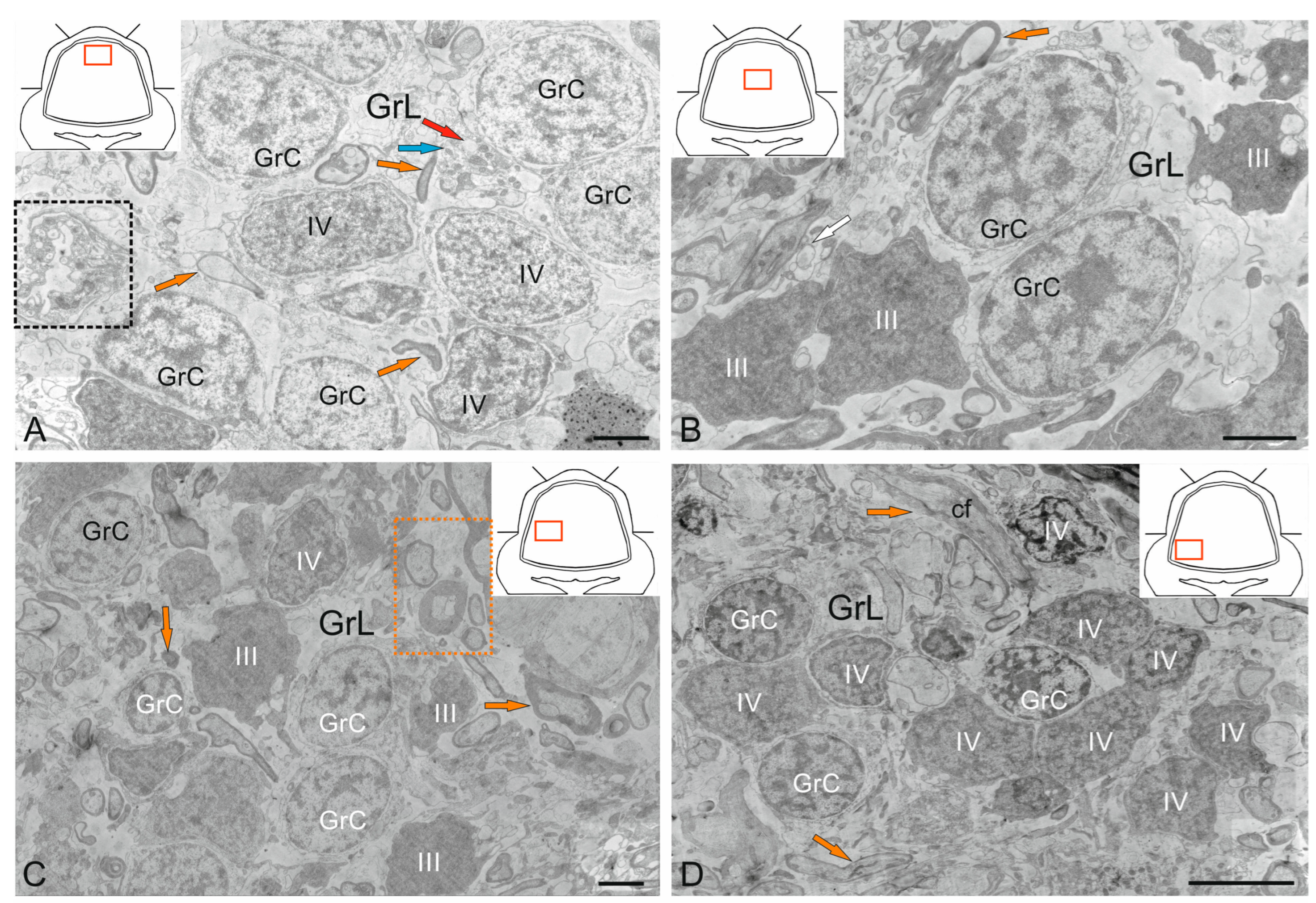
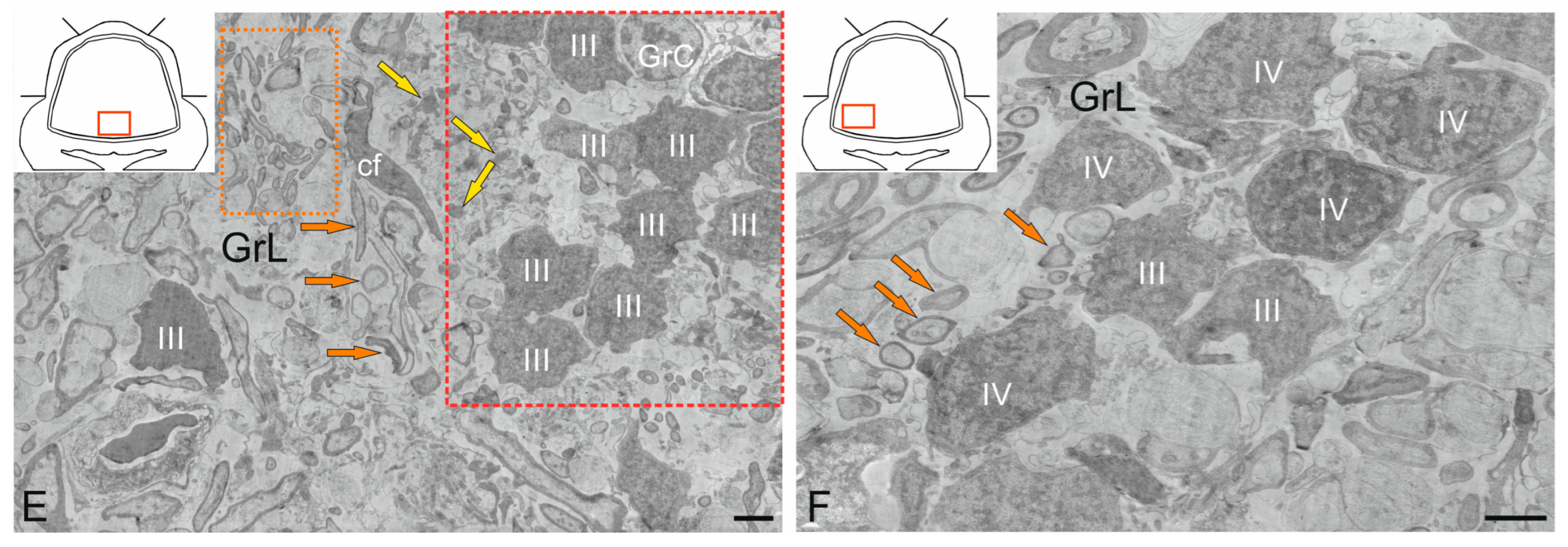
2.4. Granular Layer
2.4.1. Granular Cells
2.4.2. Golgi Cells
2.5. Cerebellar Glial Cells
2.5.1. Macroglia
2.5.2. Microglia
3. Discussion
3.1. Features of the Histological Structure and Immunohistochemical Labeling of the DMZ in the Cerebellum of Juvenile Chum Salmon O. keta
3.2. Molecular Layer
3.2.1. Features of the Synaptic Structure of the SCs
3.2.2. Adult-Type Neural Stem Progenitor Cells (aNSPCs) in the Molecular (ML) and Granular Layers (GrL) of the Cerebellum
3.2.3. Non-Glial Progenitors in the DMZ and GrL and Neoteny
3.3. Ganglion Layer
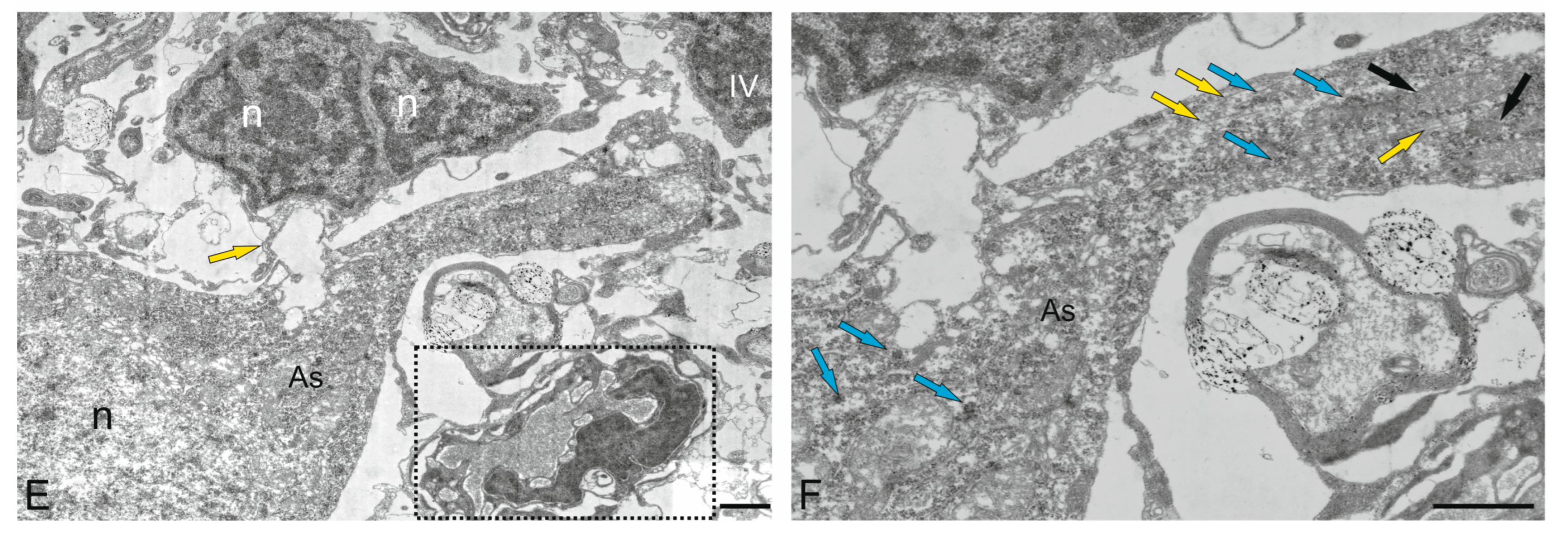
3.3.1. Neuro–Glial Relationships in the Ganglion Layer of the Juvenile Chum Salmon Cerebellum
3.3.2. Astrocytes
3.3.3. Microglia
4. Materials and Methods
4.1. Experimental Animals
4.2. Transmission Electron Microscopy
4.3. Cell Counting and Visualization
4.4. Scanning Electron Microscopy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meek, J.; Hafmans, T.G.; Maler, L.; Hawkes, R. Distribution of zebrin II in the gigantocerebellum of the mormyrid fish Gnathonemus petersii compared with other teleosts. J. Comp. Neurol. 1992, 316, 17–31. [Google Scholar] [CrossRef]
- Folgueira, M.; Anadon, R.; Yanez, J. Afferent and efferent connections of the cerebellum of a salmonid, the rainbow trout (Oncorhynchus mykiss): A tract-tracing study. J. Comp. Neurol. 2006, 497, 542–565. [Google Scholar] [CrossRef]
- Nieuwenuys, R. Comparative anatomy of the cerebellum. In Progress in Brain Research; Fox, C., Snider, R., Eds.; Elsevier: Amsterdam, The Netherlands, 1967; Volume 25, pp. 1–93. [Google Scholar]
- Pushchina, E.V.; Stukaneva, M.E.; Varaksin, A.A. Neural stem cells in the cerebellum of juvenile masu salmon (Oncorhynchus masu) arter mechanical inhury. In Teleost: Physiology, Evolution and Classification; Herleif, M., Ed.; Nava Science Publisher, Inc.: New York, NY, USA, 2018; pp. 73–95. [Google Scholar]
- Finger, T.E. Organization of the teleost cerebellum. In Fish Neurobiology; Northcutt, R.G., Davis, R.E., Eds.; University of Michigan Press: Ann Arbor, MI, USA, 1983; pp. 262–284. [Google Scholar]
- Meek, J.; Yang, J.Y.; Han, V.Z.; Bell, C.C. Morphological analysis of the mormyrid cerebellum using immunohistochemistry with emphasis on the unusual neuronal organization of the valvula. J. Comp. Neurol. 2008, 519, 396–421. [Google Scholar] [CrossRef]
- Nieuwenhuys, R.; Nicholson, C. A survey of the general morphology, the fiber connections, and the possible significance of gigantocerebellum of mormyrid fishes. In Neurobiology of Cerebellar Evolution and Development; Llinás, R., Ed.; American Medical Association: Chicago, IL, USA, 1969; pp. 107–134. [Google Scholar]
- Pushchina, E.; Bykova, M.E.; Vekhova, E.E.; Pimenova, E.A. Immunohistochemical and ultrastructural analysis of adult neurogenesis involving glial and non-glial progenitors in the cerebellum of juvenile chum salmon Oncorhynchus keta. Int. J. Mol. Sci. 2025, 26, 9267. [Google Scholar] [CrossRef]
- Murakami, T.; Morita, Y. Morphology and distribution of the projection neurons in the cerebellum in a teleost, Sebastiscus marmoratus. J. Comp. Neurol. 1987, 256, 607–623. [Google Scholar] [CrossRef]
- Pouwels, E. On the development of the cerebellum of the trout Salmo gairdneri. III. Development of neuronal elements. Anat. Embryol. 1978, 153, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Pouwels, E. On the development of the cerebellum of the trout Salmo gairdneri. IV. Development of the pattern of connectivity. Anat. Embryol. 1978, 153, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Kaiserman-Abramof, I.R.; Palay, S.L. Fine structural studies of the cerebellar cortex in a mormyrid fish. In Neurobiology of Cerebellar Evolution and Development; Llinás, R., Ed.; American Medical Association: Chicago, IL, USA, 1969; pp. 171–205. [Google Scholar]
- Pushchina, E.V.; Varaksin, A.A.; Shukla, S.; Obukhov, D.K. The Neurochemical Organization and Adult Neurogenesis in the Masu Salmon Brain; Nova Science Pub Inc.: Hauppauge, NY, USA, 2017; 267p. [Google Scholar]
- Pushchina, E.V.; Varaksin, A.A. Constitutive neurogenesis and neuronal plasticity in the adult cerebellum and brainstem of rainbow trout, Oncorhynchus mykiss. Int. J. Mol. Sci. 2024, 25, 5595. [Google Scholar] [CrossRef]
- Stukaneva, M.E.; Pushchina, E.V.; Varaksin, A.A. GFAP and PCNA marking in the cerebellum of masu salmon᾿s (Oncorhynchus masou) juvenile after mechanical injury. Rus. J. Dev. Biol. 2017, 48, 321–329. [Google Scholar] [CrossRef]
- Pushchina, E.V.; Stukaneva, M.E.; Varaksin, A.A. Hydrogen sulfide modulates adult and reparative neurogenesis in the cerebellum of juvenile masu salmon, Oncorhynchus masou. Int. J. Mol. Sci. 2020, 21, 9638. [Google Scholar] [CrossRef]
- Pushchina, E.V.; Bykova, M.E.; Varaksin, A.A. Post-traumatic expressions of aromatase B, glutamine synthetase, and cystathionine-beta-synthase in the cerebellum of juvenile chum salmon, Oncorhynchus keta. Int. J. Mol. Sci. 2024, 25, 3299. [Google Scholar] [CrossRef]
- Pushchina, E.V.; Varaksin, A.A. Morphological organization of large Golgi neurons in the cerebellum of the opisthocentrid Pholidapus dybowskii. Neurosci. Behav. Physiol. 2002, 32, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Pushchina, E.V.; Varaksin, A.A. Argyrophilic and nitroxydergic bipolar neurons (Lugaro cells) in the cerebellum of Pholidapus dybowskii. Zh. Evol. Biokhim. Fiziol. 2001, 37, 437–441. [Google Scholar] [PubMed]
- Meek, J.; Nieuwenhuys, R. Palisade pattern of mormyrid Purkinje cells. A correlated light and electron microscopic study. J. Comp. Neurol. 1991, 306, 156–192. [Google Scholar] [CrossRef]
- Torres, B.; Pastor, A.M.; Cabrera, B.; Salas, C.; Delgado-García, J.M. Afferents to the oculomotor nucleus in the goldfish (Carassius auratus) as revealed by retrograde labeling with horseradish peroxidase. J. Comp. Neurol. 1992, 324, 449–461. [Google Scholar] [CrossRef]
- Ikenaga, T.; Yoshida, M.; Uematsu, K. Morphology and immunohistochemistry of efferent neurons of the goldfish corpus cerebelli. J. Comp. Neurol. 2005, 487, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuys, R.; Nicholson, C. Aspects of the histology of the cerebellum of mormyrid fishes. In Neurobiology of Cerebellar Evolution and Development; Llinás, R., Ed.; American Medical Association: Chicago, IL, USA, 1969; pp. 135–169. [Google Scholar]
- Nieuwenhuys, R.; Pouwels, E.; Smulders-Kersten, E. The neuronal organization of cerebellar lobe C1 in the mormyrid fish Gnathonemus petersii (Teleostei). Z. Anat-Entwickl. Gesch. 1974, 144, 315–336. [Google Scholar] [CrossRef] [PubMed]
- Pushchina, E.V.; Karpenko, A.A. Distribution of cholineacetyltransferase histochemistry in isthmus and medulla of Onchorynchus masou. Tract-tracing observation on the ascending meso-pontine cholinergic system. Tsitologiia 2007, 49, 581–593. [Google Scholar]
- Porteros, A.; Arévalo, R.; Briñón, J.G.; Crespo, C.; Aijón, J.; Alonso, J.R. Parvalbumin immunoreactivity during the development of the cerebellum of the rainbow trout. Dev. Brain Res. 1998, 109, 221–227. [Google Scholar] [CrossRef]
- Pérez, S.E.; Yáñez, J.; Marín, O.; Anadón, R.; González, A.; Rodríguez-Moldes, I. Distribution of choline acetyltransferase (ChAT) immunoreactivity in the brain of the adult trout, and tract-tracing observations on the connections of the nuclei of the isthmus. J. Comp. Neurol. 2000, 428, 450–474. [Google Scholar] [CrossRef]
- Kalinichenko, S.G.; Motavkin, P.A. Cerebellar Cortex; Kupriyanov, V.V., Ed.; Nauka: Moscow, Russia, 2005; 319p. [Google Scholar]
- Bae, Y.K.; Kani, S.; Shimizu, T.; Tanabe, K.; Nojima, H.; Kimura, Y.; Higashijima, S.; Hibi, M. Anatomy of zebrafish cerebellum and screen for mutations affecting its development. Dev. Biol. 2009, 330, 406–426. [Google Scholar] [CrossRef] [PubMed]
- Kaslin, J.; Brand, M. Cerebellar development and neurogenesis in zebrafish. In Handbook of the Cerebellum and Cerebellar Disorders; Manto, M., Gruol, D., Schmahmann, J., Koibuchi, N., Sillitoe, R., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Obukhov, D.K.; Andreeva, N.G. Evolutionary Morphology of the Nervous System in Vertebrates, 3rd ed.; Textbook; Revised and Expanded; Higher Education: Moscow, Russia, 2024. [Google Scholar]
- Castejón, O.J.; Castejón, H.V.; Alvarado, M.V. Further observations on cerebellar climbing fibers. A study by means of light microscopy, confocal laser scanning microscopy and scanning and transmission electron microscopy. Biocell 2000, 24, 197–212. [Google Scholar]
- Matsui, H.; Namikawa, K.; Babaryka, A.; Köster, R.W. Functional regionalization of the teleost cerebellum analyzed in vivo. Proc. Natl. Acad. Sci. USA 2014, 111, 11846–11851. [Google Scholar] [CrossRef]
- Lindsey, B.W.; Darabie, A.; Tropepe, V. The cellular composition of neurogenic periventricular zones in the adult zebrafish forebrain. J. Comp. Neurol. 2012, 520, 2275–2316. [Google Scholar] [CrossRef]
- Stukaneva, M.E.; Pushchina, E.V. Constitutive neurogenesis in the brain of different vertebrate groups. Neurophysiology 2020, 52, 456–470. [Google Scholar] [CrossRef]
- Lindsey, B.W. A Comparative view of cerebellar morphology and diversity in fishes. In Development of the Cerebellum from Molecular Aspects to Diseases; Springer: Berlin/Heidelberg, Germany, 2023; pp. 155–171. Available online: https://link.springer.com/chapter/10.1007/978-3-031-23104-9_8 (accessed on 7 November 2025).
- Pushchina, E.V.; Pimenova, E.A.; Kapustyanov, I.A.; Bykova, M.E. Ultrastructural study and immunohistochemical characteristics of mesencephalic tegmentum in juvenile chum salmon (Oncorhynchus keta) brain after acute traumatic injury. Int. J. Mol. Sci. 2025, 26, 644. [Google Scholar] [CrossRef] [PubMed]
- York, R.A.; Byrne, A.L.; Abdilleh, K.; Patil, C.; Steelman, T.; Finger, T.E.; Russell, D.F. Behavior evolution contributes to hindbrain diversification among lake malawi cichlid fish. Sci. Rep. 2019, 9, 19994. [Google Scholar] [CrossRef] [PubMed]
- Ikenaga, T. Teleost fish. In Handbook of the Cerebellum and Cerebellar Disorders; Manto, M., Gruol, D.L., Schmahmann, J., Koibuchi, N., Rossi, F., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 1463–1480. [Google Scholar]
- Han, V.Z.; Meek, J.; Campbell, H.R.; Bell, C.C. Cell morphology and circuitry in the central lobes of the mormyrid cerebellum. J. Comp. Neurol. 2006, 497, 309–325. [Google Scholar] [CrossRef]
- Nieuwenhuys, R.; Ten Donkelaar, H.J.; Nicholson, C. (Eds.) The Central Nervous System of Vertebrates; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Caron, A.; Trzuskot, L.; Lindsey, B.W. Uncovering the spectrum of adult zebrafish neural stem cell cycle regulators. Front Cell Dev. Biol. 2022, 10, 941893. [Google Scholar] [CrossRef]
- Tong, Z.; Yin, Z. Distribution, contribution and regulation of nestin+ cells. J. Adv. Res. 2024, 61, 47–63. [Google Scholar] [CrossRef]
- Pose-Méndez, S.; Schramm, P.; Valishetti, K.; Köster, R.W. Development, circuitry, and function of the zebrafish cerebellum. Cell Mol. Life Sci. 2023, 80, 227. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Matsuda, K.; Yamaguchi, S.; Asakawa, K.; Miyasaka, N.; Lal, P.; Yoshihara, Y.; Koga, A.; Kawakami, K.; Shimizu, T.; et al. Establishment of Gal4 transgenic zebrafish lines for analysis of development of cerebellar neural circuitry. Dev. Biol. 2015, 397, 1–17. [Google Scholar] [CrossRef]
- Ito, M. Historical review of the significance of the cerebellum and the role of Purkinje cells in motor learning. Ann. N. Y. Acad. Sci. 2002, 978, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Ito, M. The molecular organization of cerebellar long-term depression. Nat. Rev. Neurosci. 2002, 3, 896–902. [Google Scholar] [CrossRef]
- Ito, M. Cerebellar circuitry as a neuronal machine. Prog. Neurobiol. 2006, 78, 272–303. [Google Scholar] [CrossRef]
- Alonso, J.R.; Arevalo, R.; Brinon, J.G.; Lara, J.; Weruaga, E.; Aijon, J. Parvalbumin.immunoreactive neurons and fibres in the teleost cerebellum. Anat. Embryol. 1992, 185, 355–361. [Google Scholar] [CrossRef]
- Chang, W.; Pedroni, A.; Hohendorf, V.; Giacomello, S.; Hibi, M.; Köster, R.W.; Ampatzis, K. Functionally distinct Purkinje cell types show temporal precision in encoding locomotion. Proc. Natl. Acad. Sci. USA 2020, 117, 17330–17337. [Google Scholar] [CrossRef]
- Favre-Bulle, I.A.; Vanwalleghem, G.; Taylor, M.A.; Rubinsztein-Dunlop, H.; Scott, E.K. Cellular-resolution imaging of vestibular processing across the larval zebrafish brain. Curr. Biol. 2018, 28, 3711–3722.e3. [Google Scholar] [CrossRef]
- Zupanc, G.K.H.; Hinsch, K.; Gage, F.H. Proliferation, migration, neuronal differentiation, and long-term survival of new cells in the adult zebrafish brain. J. Comp. Neurol. 2005, 488, 290–319. [Google Scholar] [CrossRef]
- Delgado, L.M.; Schmachtenberg, O. Neurogenesis in the adult goldfish cerebellum. Anat. Rec. 2011, 294, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Maruska, K.P.; Carpenter, R.E.; Fernald, R.D. Characterization of cell proliferation throughout the brain of the African cichlid fish Astatotilapia burtoni and its regulation by social status. J. Comp. Neurol. 2012, 520, 3471–3491. [Google Scholar] [CrossRef] [PubMed]
- Tozzini, E.T.; Baumgart, M.; Battistoni, G.; Cellerino, A. Adult neurogenesis in the short-lived teleost Nothobranchius furzeri: Localization of neurogenic niches, molecular characterizing and effects of aging. Aging Cell 2012, 11, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Kuroyanagi, Y.; Okuyama, T.; Suehiro, Y.; Imada, H.; Shimado, A.; Naruse, K.; Takeda, H.; Kubo, T.; Tareuchi, H. Proliferation zones in adult medaka (Oryzias latipes) brain. Brain Res. 2010, 1323, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Zupanc, G.K.H.; Horschke, I. Proliferation zones in the brain of adult gymnotiform fish: A quantitative mapping study. J. Comp. Neurol. 1995, 353, 213–233. [Google Scholar] [CrossRef]
- Feliciano, D.M.; Bordey, A.; Bonfanti, L. Noncanonical sites of adult neurogenesis in the mammalian brain. Cold Spring Harb. Perspect. Biol. 2015, 7, a018846. [Google Scholar] [CrossRef]
- Pushchina, E.V.; Kapustyanov, I.A.; Kluka, G.G. Adult neurogenesis of teleost fish determines high neuronal plasticity and regeneration. Int. J. Mol. Sci. 2024, 25, 3658. [Google Scholar] [CrossRef]
- Pushchina, E.V.; Varaksin, A.A.; Obukhov, D.K. Molecular markers of adult neurogenesis in the telencephalon and tectum of rainbow trout. Int. J. Mol. Sci. 2022, 23, 1234. [Google Scholar] [CrossRef]
- Mommsen, T.P. Growth and Metabolism; CRC: Boca Raton, FL, USA, 1998. [Google Scholar]
- Biga, P.R.; Goetz, F.W. Zebrafish and giant danio models for muscle growth: Determinate vs. indeterminate growth as determined by morphometric analysis. Am. J. Phys. Regul. Integr. Comp. Phys. 2006, 291, R1327–R1337. [Google Scholar] [CrossRef]
- Kaslin, J.; Ganz, J.; Geffarth, M.; Grandel, H.; Hans, S.; Brand, M. Stem cells in the adult zebrafish cerebellum: Initiation and maintenance of a novel stem cell niche. J. Neurosci. 2009, 29, 6142–6153. [Google Scholar] [CrossRef]
- Grandel, H.; Kaslin, J.; Ganz, J.; Wenzel, I.; Brand, M. Neural stem cells and neurogenesis in the adult zebrafish brain: Origin, proliferation dynamics, migration and cell fate. Dev. Biol. 2006, 295, 263–277. [Google Scholar] [CrossRef]
- Lindsey, B.W.; Di Donato, S.; Kaslin, J.; Tropepe, V. Sensory-specific modulation of adult neurogenesis in sensory structures is associated with the type of stem cell present in the neurogenic niche of the zebrafish brain. Eur. J. Neurosci. 2014, 40, 3591–3607. [Google Scholar] [CrossRef]
- Zupanc, G.K.H. Adult neurogenesis in the central nervous system of teleost fish: From stem cells to function and evolution. J. Exp. Biol. 2021, 224, jeb226357. [Google Scholar] [CrossRef] [PubMed]
- Ming, G.L.; Song, H. Adult neurogenesis in the mammalian brain: Significant answers and significant questions. Neuron 2011, 70, 687–702. [Google Scholar] [CrossRef]
- Lindsey, B.W.; Tropepe, V. Changes in the social environment induce neurogenic plasticity predominantly in niches residing in sensory structures of the zebrafish brain independently of cortisol levels. Dev. Neurobiol. 2014, 74, 1053–1077. [Google Scholar] [CrossRef]
- Sergio, R.; Panopoulos, A.D.; Herrerías, A.; Bissig, K.-D.; Lutz, M.; Travis Berggren, W.; Verma, I.M.; Belmonte, J.C.I. A High proliferation rate is required for cell reprogramming and maintenance of human embryonic stem cell identity. Curr. Biol. 2011, 21, 45–52. [Google Scholar] [CrossRef]
- Bonett, R.M.; Ledbetter, N.M.; Hess, A.J.; Herrboldt, M.A.; Denoël, M. Repeated ecological and life cycle transitions make salamanders an ideal model for evolution and development. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2022, 251, 957–972. [Google Scholar] [CrossRef]
- Bruce, R.C. Size-mediated tradeoffs in life-history traits in dusky salamanders. Copeia 2013, 262–267. [Google Scholar] [CrossRef]
- Phung, T.X.; Nascimento, J.C.S.; Novarro, A.J.; Wiens, J.J. Correlated and decoupled evolution of adult and larval body size in frogs. Proc. Roy. Soc. 2020, 287, 20201474. [Google Scholar] [CrossRef] [PubMed]
- Chi-Kuo, H.; Brunet, A. The African turquoise killifish: A research organism to study vertebrate aging and diapause. Aging Cell 2018, 17, e12757. [Google Scholar] [CrossRef]
- Rodríguez-Moldes, I. Brain and nervous system functional morphology of the brains of cartilaginous fishes. Encycl. Fish Physiol. 2011, pp. 26–36. Available online: https://www.sciencedirect.com/science/article/abs/pii/B9780123745538000046?via%3Dihub (accessed on 7 November 2025).
- Pose-Méndez, S.; Rodríguez-Moldes, I.; Candal, E.; Mazan, S.; Anadón, R. A developmental study of the cerebellar nucleus in the catshark, a basal gnathostome. Brain Behav. Evol. 2017, 89, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Huesa, G.; Anadón, R.; Yáñez, J. Afferent and efferent connections of the cerebellum of the chondrostean Acipenser baeri: A carbocyanine dye (DiI) tracing study. J. Comp. Neurol. 2003, 460, 327–344. [Google Scholar] [CrossRef]
- Ikenaga, T.; Shimomai, R.; Hagio, H.; Kimura, S.; Matsumoto, K.; Kato, D.I.; Uesugi, K.; Takeuchi, A.; Yamamoto, N.; Hibi, M. Morphological analysis of the cerebellum and its efferent system in a basal actinopterygian fish, Polypterus senegalus. J. Comp. Neurol. 2022, 530, 1231–1246. [Google Scholar] [CrossRef]
- Puschina, E.V.; Varaksin, A.A. Hydrogen sulfide-, parvalbumin-, and GABA-producing systems in the masu salmon brain. Neurophysiology 2011, 43, 90–102. [Google Scholar] [CrossRef]
- Lannoo, M.J.; Ross, L.; Maler, L.; Hawkes, R. Development of the cerebellum and its extracerebellar Purkinje cell projection in teleost fishes as determined by zebrin II immunocytochemistry. Prog. Neurobiol. 1991, 37, 329–363. [Google Scholar] [CrossRef] [PubMed]
- Puzdrowski, R.L. Anti-zebrin II immunopositivity in the cerebellum and octavolateral nuclei in two species of stingrays. Brain Behav. Evol. 1997, 50, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Pose-Méndez, S.; Candal, E.; Mazan, S.; Rodríguez-Moldes, I. Morphogenesis of the cerebellum and cerebellum-related structures in the shark Scyliorhinus canicula: Insights on the ground pattern of the cerebellar ontogeny. Brain Struct. Funct. 2016, 221, 1691–1717. [Google Scholar] [CrossRef]
- Chang, W.; Pedroni, A.; Köster, R.W.; Giacomello, S.; Ampatzis, K. Purkinje cells located in the adult zebrafish valvula cerebelli exhibit variable functional responses. Sci. Rep. 2021, 11, 18408. [Google Scholar] [CrossRef] [PubMed]
- Kapustyanov, I.A.; Pushchina, E.V.; Varaksin, A.A.; Obukhov, D.K. Induction of apoptotic activity in case of mechanical damage to the mecencephalic tegmentum of juvenile chum salmon Oncorhynchus keta. Genes Cells 2017, 12, 111. [Google Scholar]
- Anvari Far, H.; Adineh, H.; Khamisabadi, S.; Pahlavan, M. Apoptosis in fish: Environmental factors and programmed cell death. Cell Tissue Res. 2017, 368, 425–439. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Zupanc, G.K.H.; Zupanc, M.M.; Kucera, J.; Zupanc, G.; Watanabe, K. Spatio-temporal distribution of microglia/macrophages during regeneration in the cerebellum of adult teleost fish, Apteronotus leptorhynchus: A quantitative analysis. Brain Behav. Evol. 2003, 62, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, R.S.; Wellbrock, U.M.; Zupanc, G.K. Apoptotic cell death, long-term persistence, and neuronal differentiation of aneuploid cells generated in the adult brain of teleost fish. Dev. Neurobiol. 2008, 68, 1257–1268. [Google Scholar] [CrossRef] [PubMed]
- Pushchina, E.V.; Varaksin, A.A.; Obukhov, D.K.; Prudnikov, I.M. GFAP expression in the optic nerve and increased H2S generation in the integration centers of the rainbow trout (Oncorhynchus mykiss) brain after unilateral eye injury. Neural Regen. Res. 2020, 15, 1867–1886. [Google Scholar] [CrossRef]
- Ghali, R.P.; Leclerc, L.; Levine, R.L. Mononuclear cell proliferation and hyperplasia during Wallerian degeneration in the visual system of the goldfish in the presence or absence of regenerating optic axons. Brain Res. 2000, 854, 178–188. [Google Scholar] [CrossRef]
- Becker, T.; Becker, C.G. Regenerating descending axons preferentially reroute to the gray matter in the presence of a general macrophage/microglial reaction caudal to a spinal transection in adult zebrafish. J. Comp. Neurol. 2001, 433, 131–147. [Google Scholar] [CrossRef]
- Crotti, A.; Ransohoff, R.M. Microglial physiology and pathophysiology: Insights from genome-wide transcriptional profiling. Immunity 2016, 44, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Garland, E.F.; Hartnell, I.J.; Boche, D. Microglia and astrocyte function and communication: What do we know in humans? Front Neurosci. 2022, 16, 824888. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Astrogliosis. Cold Spring Harb. Perspect. Biol. 2014, 7, a020420. [Google Scholar] [CrossRef] [PubMed]
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Verkhratsky, A. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef]
- Zamanian, J.L.; Xu, L.; Foo, L.C.; Nouri, N.; Zhou, L.; Giffard, R.G.; Barres, B.A. Genomic analysis of reactive astrogliosis. J. Neurosci. 2012, 32, 6391–6410. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Kono, T.; Ishihara, Y.; Itoh, K. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Pekny, M.; Pekna, M. Astrocyte reactivity and reactive astrogliosis: Costs and benefits. Physiol. Rev. 2014, 94, 1077–1098. [Google Scholar] [CrossRef]
- Perez-Nievas, B.G.; Serrano-Pozo, A. Deciphering the astrocyte reaction in Alzheimer’s disease. Front. Aging Neurosci. 2018, 10, 114. [Google Scholar] [CrossRef]
- Marlatt, M.W.; Bauer, J.; Aronica, E.; Van Haastert, E.S.; Hoozemans, J.J.; Joels, M.; Lucassen, P.J. Proliferation in the alzheimer hippocampus is due to microglia, not astroglia, and occurs at sites of amyloid deposition. Neural. Plast. 2014, 2014, 693851. [Google Scholar] [CrossRef]
- Pelvig, D.P.; Pakkenberg, H.; Regeur, L.; Oster, S.; Pakkenberg, B. Neocortical glial cell numbers in Alzheimer’s disease. A stereological study. Dement. Geriatr. Cogn. Disord. 2003, 16, 212–219. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Gómez-Isla, T.; Growdon, J.H.; Frosch, M.P.; Hyman, B.T. A phenotypic change but not proliferation underlies glial responses in Alzheimer disease. Am. J. Pathol. 2013, 182, 2332–2344. [Google Scholar] [CrossRef]
- Oberheim, N.A.; Wang, X.; Goldman, S.; Nedergaard, M. Astrocytic complexity distinguishes the human brain. Trends Neurosci. 2006, 29, 547–553. [Google Scholar] [CrossRef]
- Sosunov, A.A.; Wu, X.; Tsankova, N.M.; Guilfoyle, E.; Mckhann, G.M.; Goldman, J.E. Phenotypic heterogeneity and plasticity of isocortical and hippocampal astrocytes in the human brain. J. Neurosci. 2014, 34, 2285–2298. [Google Scholar] [CrossRef]
- Chapouton, P.; Jagasia, R.; Bally-Cuif, L. Adult neurogenesis in non-mammalian vertebrates. Bioessays 2007, 29, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Alunni, A.; Bally-Cuif, L. A comparative view of regenerative neurogenesis in vertebrates. Development 2016, 143, 741–753. [Google Scholar] [CrossRef]
- Zupanc, G.K.; Sîrbulescu, R.F. Teleost fish as a model system to study successful regeneration of the central nervous system. Curr. Top. Microbiol. Immunol. 2013, 367, 193–233. [Google Scholar] [PubMed]
- Shigetomi, E.; Jackson, W.T.; Ransom, C.B.; Amara, S.G.; Kato, S. Two forms of astrocyte calcium excitability have distinct effects on NMDA receptor-mediated slow inward currents in pyramidal neurons. J. Neurosci. 2008, 28, 6659–6663. [Google Scholar] [CrossRef]
- Kim, H.B.; Kim, Y.; Choi, J.H.; Park, S.S.; Lee, H.K.; Kim, J.H. Astrocytes promote ethanol-induced enhancement of intracellular Ca2+ signals through intercellular communication with neurons. Science 2021, 24, 102436. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, S.R.; Kim, A.E.; Packer, J.G.; Kim, D.H.; Ryu, J.H.; Wu, Y.; Kwon, H. Astrocyte-derived estrogen regulates reactive astrogliosis and is neuroprotective following ischemic brain injury. J. Neurosci. 2020, 40, 9751–9771. [Google Scholar] [CrossRef]
- Fuente-Martin, E.; Lopez, M.; Mendez, P.; Mallo, F.; Gallego, R.; Dieguez, C.; Nogueiras, R. Estrogen, astrocytes and the neuroendocrine control of metabolism. Rev. Endocr. Metab. Disord. 2013, 14, 331–338. [Google Scholar] [CrossRef]
- Porter, J.T.; McCarthy, K.D. Astrocytic neurotransmitter receptors in situ and in vivo. Prog. Neurobiol. 1997, 51, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Var, S.R.; Byrd-Jacobs, C.A. Microglial response patterns following damage to the zebrafish olfactory bulb. IBRO Rep. 2019, 7, 70–79. [Google Scholar] [CrossRef]
- Bhattarai, P.; Thomas, A.K.; Cosacak, M.I.; Papadimitriou, C.; Mashkaryan, V.; Zhang, Y.; Kizil, C. Modeling amyloid-β42 toxicity and neurodegeneration in adult zebrafish brain. J. Vis. Exp. 2017, 128, 56014. [Google Scholar]
- Kyritsis, N.; Kizil, C.; Zocher, S.; Kroehne, V.; Kaslin, J.; Freudenreich, D.; Iltzsche, A.; Brand, M. Acute inflammation initiates the regenerative response in the adult zebrafish brain. Science 2012, 338, 1353–1356. [Google Scholar] [CrossRef]
- Cosacak, M.I.; Papadimitriou, C.; Kizil, C. Regeneration, plasticity, and induced molecular programs in adult zebrafish brain. BioMed Res. Int. 2015, 2015, 769763. [Google Scholar] [CrossRef]
- Sharma, C.; Kang, S.C. Garcinol pacifies acrylamide induced cognitive impairments, neuroinflammation and neuronal apoptosis by modulating GSK signaling and activation of pCREB by regulating cathepsin B in the brain of zebrafish larvae. Food Chem. Toxicol. 2020, 138, 111246. [Google Scholar] [CrossRef]
- Var, S.R.; Byrd-Jacobs, C.A. Role of macrophages and microglia in zebrafish regeneration. Int. J. Mol. Sci. 2020, 21, 4768. [Google Scholar] [CrossRef] [PubMed]
- Boche, D.; Perry, V.H.; Nicoll, V.A.R. Review: Activation patterns of microglia and their identification in the human brain. Neuropathol. Appl. Neurobiol. 2013, 39, 3–18. [Google Scholar] [CrossRef]
- Cserép, C.; Pósfai, B.; Dénes, Á. Shaping neuronal fate: Functional heterogeneity of direct microglia-neuron interactions. Neuron 2021, 109, 222–240. [Google Scholar] [CrossRef]
- Battisti, W.P.; Cuoghi, B.; Turrini, M.; Fornari, R.; Mancini, D.; Spagnoli, M.; Cacciabue, D.; Passamonti, M. Macrophages, microglia, and astrocytes are rapidly activated after crush injury of the goldfish optic nerve: A light and electron microscopic analysis. J. Comp. Neurol. 1995, 354, 306–320. [Google Scholar] [CrossRef]
- Oosterhof, N.; Holtman, I.R.; Kuil, L.E.; Van Der Linde, H.C.; Boddeke, E.W.; Eggen, B.J.; Van Ham, T.J. Identification of a conserved and acute neurodegeneration-specific microglial transcriptome in the zebrafish. Glia 2017, 65, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.; Du, M.; Li, R.; Li, K.; Huang, X.; Duan, D.; Ai, X.; Yao, F.; Zhang, L.; Hu, Z.; et al. Glia maturation factor beta is required for reactive gliosis after traumatic brain injury in zebrafish. Exp. Neurol. 2018, 305, 129–138. [Google Scholar] [CrossRef]
- Acarin, L.; Castro, M.C.; Vela, J.M.; González, B. Demonstration of poly-N-acetyl lactosamine residues in ameboid and ramified microglial cells in rat brain by tomato lectin binding. J. Histochem. Cytochem. 1994, 42, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Velasco, A.; Aijón, J.; Lara, J.M.; Muñoz, A.; Leal, E.; Gutiérrez, J.C.; Varela, C.; González, J. Enzyme histochemical identification of microglial cells in the retina of a fish (Tinca tinca). Neurosci. Lett. 1999, 263, 101–104. [Google Scholar] [CrossRef]
- Cuoghi, B.; Marini, M. Ultrastructural and cytochemical features of the supramedullary neurons of the pufferfish Diodon holacanthus (L.) (Osteichthyes). Tissue Cell 2001, 33, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Cuoghi, B.; Mola, L. Microglia of teleosts: Facing a challenge in neurobiology. Eur. J. Histochem. 2007, 51, 231–240. [Google Scholar]
- Nakanishi, M.; Aoyama, Y.; Shimizu, H.; Matsuda, T.; Saito, Y.; Yamamoto, Y.; Watanabe, K. Microglia-derived interleukin-6 and leukemia inhibitory factor promote astrocytic differentiation of neural stem/progenitor cells. Eur. J. Neurosci. 2007, 25, 649–658. [Google Scholar] [CrossRef]
- Peri, F.; Nusslein-Volhard, C. Live imaging of neuronal degradation by microglia reveals a role for v0-ATPase a1 in phagosomal fusion in vivo. Cell 2008, 133, 916–927. [Google Scholar] [CrossRef]
- Svahn, A.; Graeber, M.B.; Ellett, F.E.; Lieschke, G.J.; Rinkwitz, S.; Bennett, M.R.; Becker, T.S. Development of ramified microglia from early macrophages in the zebrafish optic tectum. Dev. Neurobiol. 2013, 73, 60–71. [Google Scholar] [CrossRef]
- Neumann, H.; Kotter, M.R.N.; Franklin, R. Debris clearance by microglia: An essential link between degeneration and regeneration. Brain J. Neurol. 2009, 132, 288–295. [Google Scholar] [CrossRef]
- Najafi, A.R.; Crapser, J.; Jiang, S.; Ng, W.; Mortazavi, A.; West, B.L.; Green, K. A limited capacity for microglial repopulation in the adult brain. Glia 2018, 66, 2385–2396. [Google Scholar] [CrossRef]
- Cherry, J.D.; Tripodis, Y.; Alvarez, V.E.; Huber, B.; Kiernan, P.T.; Daneshvar, D.; Mez, J.; Montenigro, P.; Solomon, T.M.; Alosco, M.L.; et al. Microglial neuroinflammation contributes to tau accumulation in chronic traumatic encephalopathy. Acta Neuropathol. Commun. 2016, 4, 112. [Google Scholar] [CrossRef] [PubMed]
- Moritz, C.; Berardi, F.; Abate, C.; Peri, F. Live imaging reveals a new role for the sigma-1 (σ1) receptor in allowing microglia to leave brain injuries. Neurosci. Lett. 2015, 591, 13–18. [Google Scholar] [CrossRef]
- Oosterhof, N.; Boddeke, E.; Van Ham, T.J. Immune cell dynamics in the CNS: Learning from the zebrafish. Glia 2015, 63, 719–735. [Google Scholar] [CrossRef] [PubMed]
- Pope, H.M.; Voigt, M.M. Peripheral glia have a pivotal role in the initial response to axon degeneration of peripheral sensory neurons in zebrafish. PLoS ONE 2014, 9, e103283. [Google Scholar] [CrossRef] [PubMed]
- Mironov, A.A.; Komissarchik, Y.Y.; Mironov, V.A. Metody Electronnoi Microscopii v biologii i Meditsine: Metodicheskoe Rukovodstvo (Methods of Electron Microscopy in Biology and Medicine: A Methodological Guide); Nauka: St. Petersburg, Russia, 1994. [Google Scholar]


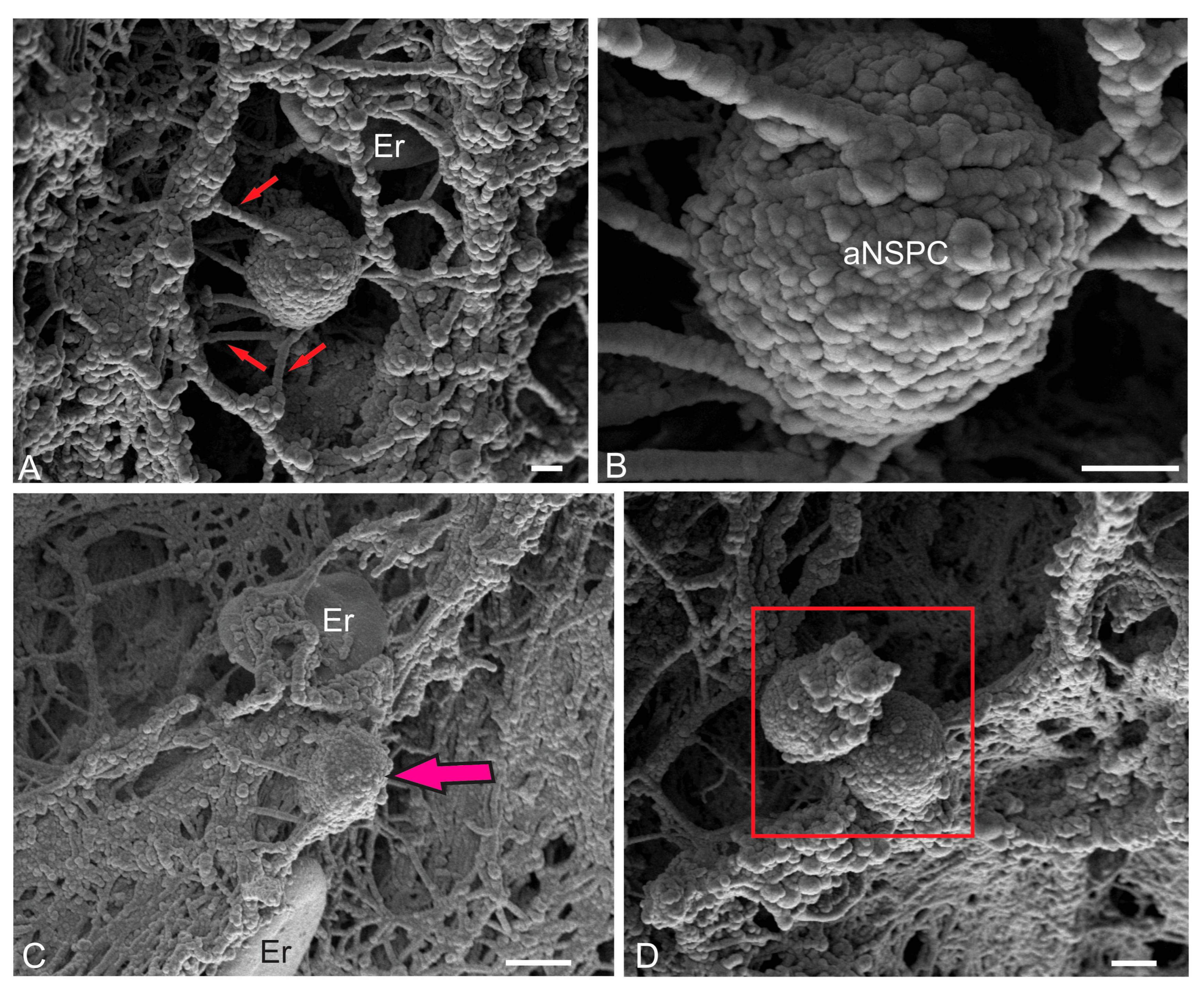
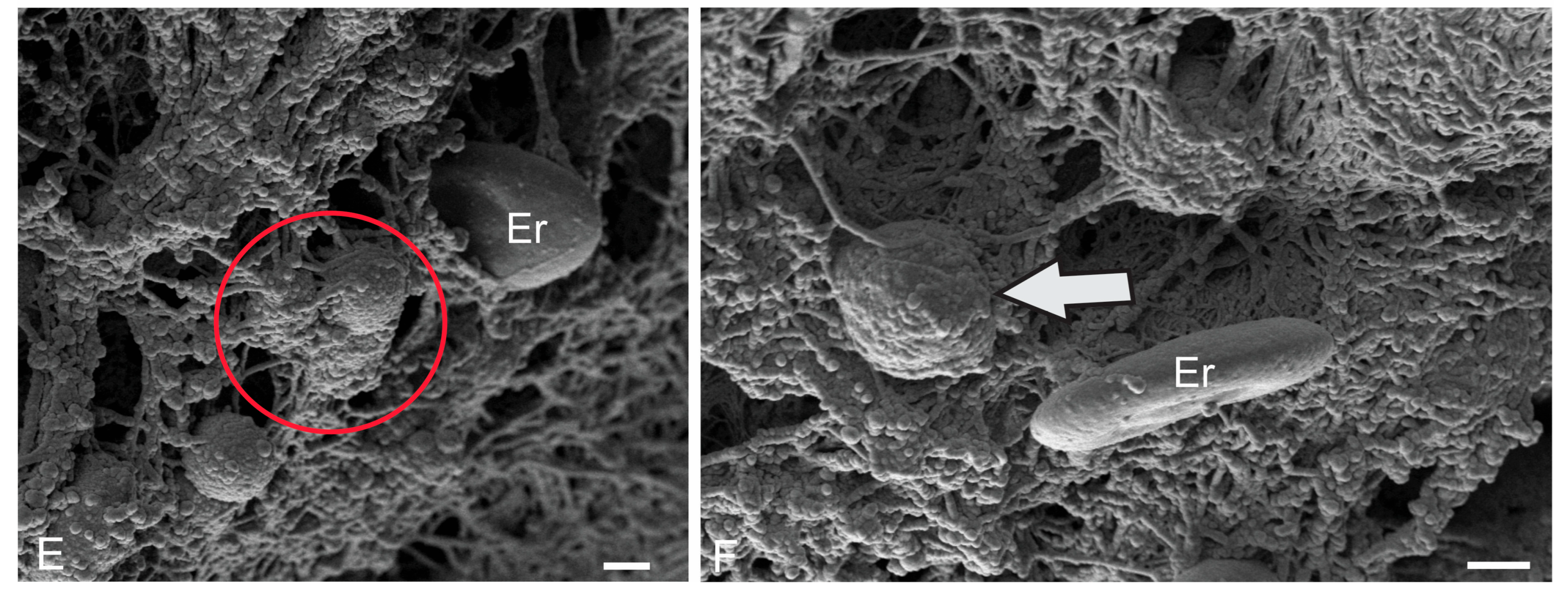
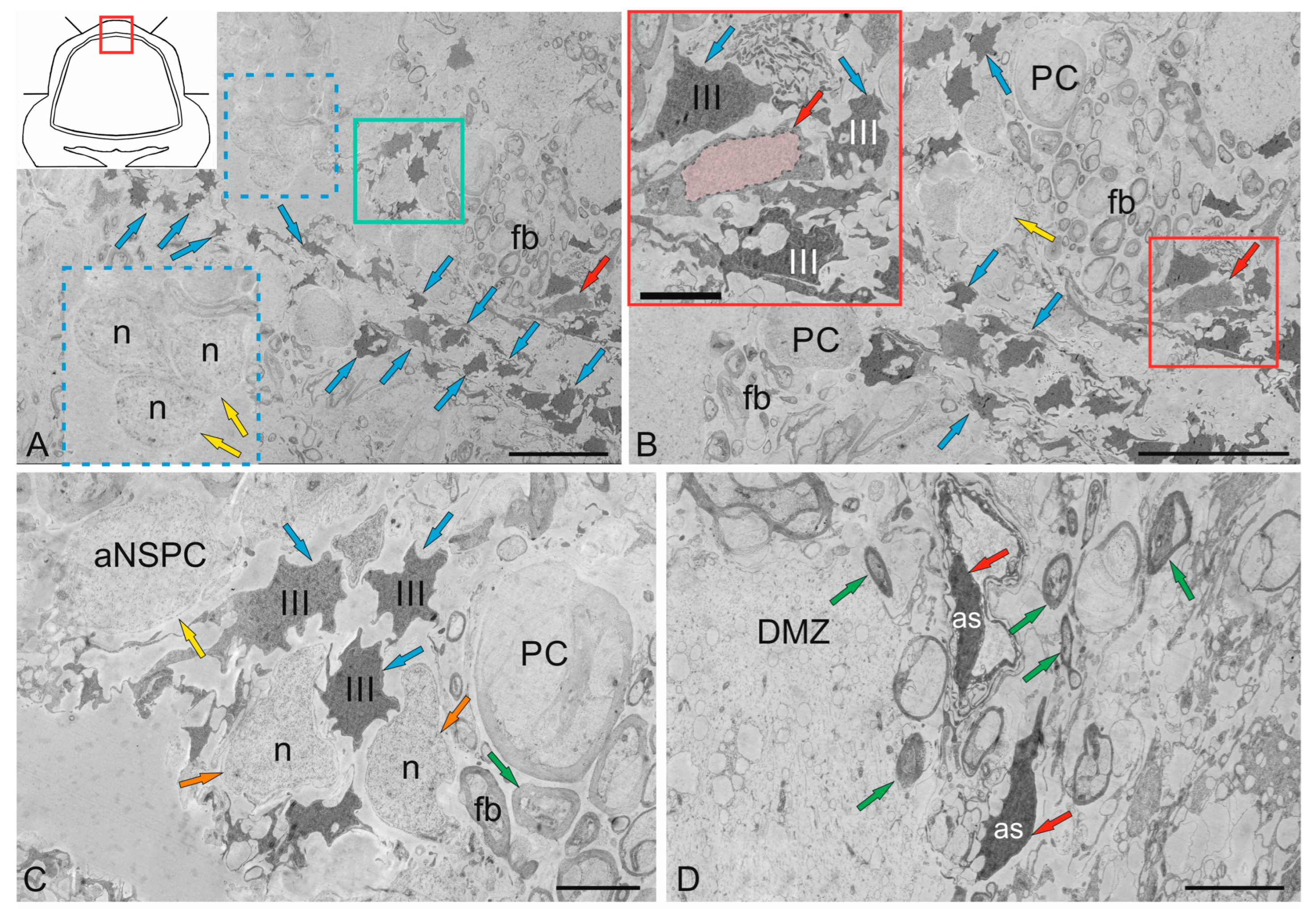
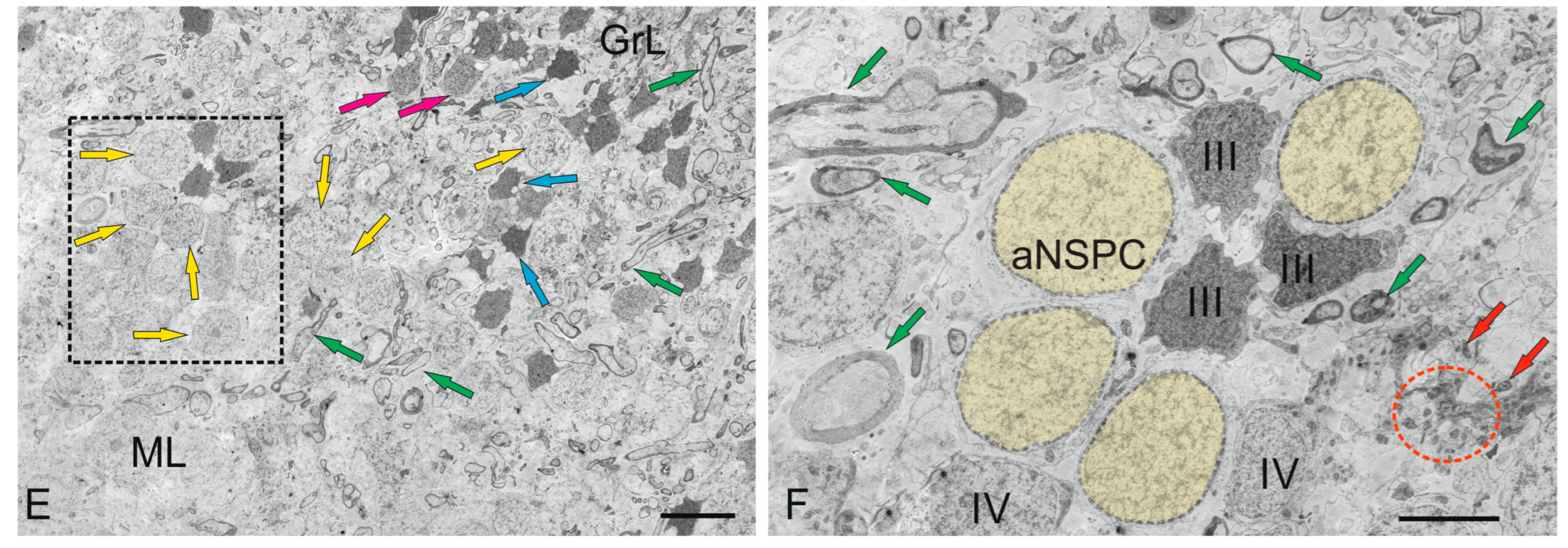

| Molecular layer | |||||
| Type of Cells | Stellate cells | Non-glial aNSPCs (DMZ) | |||
| Long axis of cell soma (µm) | 8.47 ± 0.66 | 7.71 ± 0.45 | |||
| Short axis of cell soma (µm) | 7.48 ± 0.78 | 6.06 ± 0.53 | |||
| Sample size | n = 20 | n = 15 | |||
| Nucleus | |||||
| Contour | Round or oval | Round, smooth | |||
| Long axis (µm) | 6.79 ± 0.76 | 6.65 ± 0.36 | |||
| Short axis (µm) | 5.02 ± 0.21 | 5.46 ± 0.54 | |||
| Chromatin | Denser euchromatin and heterochromatin | reticular euchromatin | |||
| Color | Medium | Light | |||
| Nucleoli | 1–2 are rarely found | No or 1 | |||
| Cytoplasm | |||||
| Percentage/color | Medium | Light | |||
| Mitochondria | Many | Few | |||
| Vacuoles | Some | No | |||
| Lipid droplets | Yes | No | |||
| Dense bodies | No | No | |||
| Cell contacts | No | Non-glial aNSPCs, glial aNSPCs III | |||
| Glial aNSPCs (GrL, ML) | |||||
| Type of Cells | III | IV | DMZ | ||
| Long axis of cell soma (µm) | 5.55 ± 0.6 | 5.67 ± 1.13 | 5.32 ± 0.92 | ||
| Short axis of cell soma (µm) | 3.87 ± 0.67 | 3.81 ± 0.42 | 3.07 ± 0.66 | ||
| Sample size | n = 15 | n = 15 | n = 20 | ||
| Nucleus | |||||
| Contour | elongated; irregular ± invaginations | ovoid, irregular ± invaginations | irregular ± invaginations | ||
| Long axis (µm) | 4.81 ± 0.48 | 4.87 ± 1.21 | 4.73 ± 0.9 | ||
| Short axis (µm) | 3.39 ± 0.63 | 3.39 ± 0.35 | 2.62 ± 0.67 | ||
| Chromatin | evenly distributed; non-clumped | reticulated; clumped hetero | reticulated; clumped hetero | ||
| Color | Medium | dark | Dark | ||
| Nucleoli | 1–2 | 1 or 2, rarely visible | No visible | ||
| Cytoplasm | |||||
| Percentage/color | scanty, dark | scanty, medium | scanty, medium | ||
| Mitochondria | Few | few | single | ||
| Vacuoles | No | several, rarely encountered | no | ||
| Lipid droplets | 0-1 | no | no | ||
| Dense bodies | Yes | no | no | ||
| Localization | ML, GrL | ML, GL, GrL | ML | ||
| Cell contacts | III, IV | III, IV | III, NEC | ||
| Granular layer | |||||
| Type of Cells | Granular cells (GrC) | Golgi cells (GC) | Astrocytes (As) | ||
| Long axis of cell soma (µm) | 6.19 ± 0.71 | 18.3 ± 0.81 | 13.9 ± 1.4 | ||
| Short axis of cell soma (µm) | 3.96 ± 0.53 | 13.02 ± 0.95 | 9.6 ± 0.9 | ||
| Sample size | n = 15 | n = 10 | n = 10 | ||
| Nucleus | |||||
| Contour | ovoid; elongated, smooth | ovoid; elongated, ± invaginations | ovoid; irregular ± invaginations | ||
| Long axis (µm) | 5.55 ± 0.63 | 12.54 ± 0.98 | 11.3 ± 1.2 | ||
| Short axis (µm) | 3.52 ± 0.59 | 7.6 ± 0.95 | 8.4 ± 0.8 | ||
| Chromatin | clumped heterochromatin | reticulated euchromatin, clumped heterochromatin | clumped heterochromatin | ||
| Color | Light | light | Light | ||
| Nucleoli | No | no | 1 | ||
| Cytoplasm | |||||
| Percentage/color | scanty, light, foamy | abundant/light | abundant, dark | ||
| Mitochondria | Few | many | Few | ||
| Vacuoles | Few | many | Few | ||
| Lipid droplets | No | no | No | ||
| Dense bodies | No | No | No | ||
| Cell contacts | III, IV aNSPC | Protoplasmic astrocytes | aNSPC IV | ||
| Ganglion layer | |||||
| Type of Cells | Purkinje cells | Eurydendroid cells | |||
| Long axis of cell soma (µm) | 23.28 ± 2.99 | 22.79 ± 0.74 | |||
| Short axis of cell soma (µm) | 18.55 ± 3.22 | 16.91 ± 0.73 | |||
| Sample size | n = 10 | n = 10 | |||
| Nucleus | |||||
| Contour | elongate, oval, smooth ± invaginations | oval, elongate, smooth | |||
| Long axis (µm) | 11.22 ±1.65 | 13.56 ± 1.83 | |||
| Short axis (µm) | 8.53 ± 1.45 | 10.17 ± 0.69 | |||
| Chromatin | euchromatin, heterochromatin | clumped heterochromatin | |||
| Color | Light | Light | |||
| Nucleoli | 1 or 2, rarely visible | 1 or 2, rarely visible | |||
| Cytoplasm | |||||
| Percentage/color | Medium | Medium | |||
| Mitochondria | Many | Many | |||
| Vacuoles | Few | Present | |||
| Lipid droplets | Yes | Many | |||
| Dense bodies | No | No | |||
| Cell contacts | Protoplasmic astrocytes, pfs | Protoplasmic astrocytes | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pushchina, E.V.; Vekhova, E.E.; Bykova, M.E. Ultrastructural Characteristics of the Juvenile Chum Salmon (Oncorhynchus keta) Cerebellum: Interneuron Composition, Neuro–Glial Interactions, Homeostatic Neurogenesis, and Synaptic Plasticity. Int. J. Mol. Sci. 2025, 26, 11123. https://doi.org/10.3390/ijms262211123
Pushchina EV, Vekhova EE, Bykova ME. Ultrastructural Characteristics of the Juvenile Chum Salmon (Oncorhynchus keta) Cerebellum: Interneuron Composition, Neuro–Glial Interactions, Homeostatic Neurogenesis, and Synaptic Plasticity. International Journal of Molecular Sciences. 2025; 26(22):11123. https://doi.org/10.3390/ijms262211123
Chicago/Turabian StylePushchina, Evgeniya V., Evgeniya E. Vekhova, and Mariya E. Bykova. 2025. "Ultrastructural Characteristics of the Juvenile Chum Salmon (Oncorhynchus keta) Cerebellum: Interneuron Composition, Neuro–Glial Interactions, Homeostatic Neurogenesis, and Synaptic Plasticity" International Journal of Molecular Sciences 26, no. 22: 11123. https://doi.org/10.3390/ijms262211123
APA StylePushchina, E. V., Vekhova, E. E., & Bykova, M. E. (2025). Ultrastructural Characteristics of the Juvenile Chum Salmon (Oncorhynchus keta) Cerebellum: Interneuron Composition, Neuro–Glial Interactions, Homeostatic Neurogenesis, and Synaptic Plasticity. International Journal of Molecular Sciences, 26(22), 11123. https://doi.org/10.3390/ijms262211123






