The Anti-Inflammatory and Skin Barrier Function Recovery Effects of Carica papaya Peel in Mice with Contact Dermatitis
Abstract
1. Introduction
2. Results
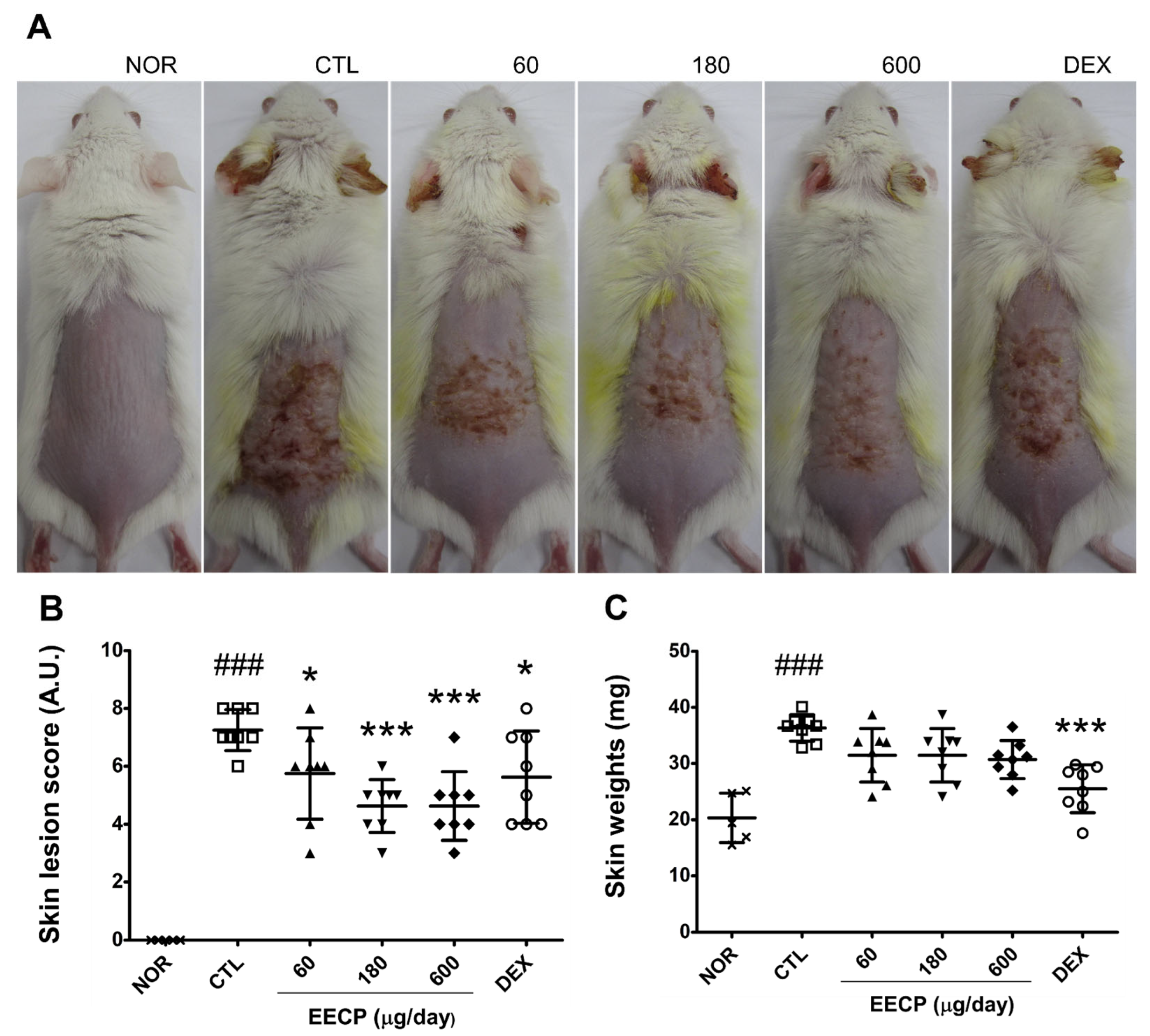
2.1. EECP Improved CD Symptoms and Reduced Skin Lesion Scores
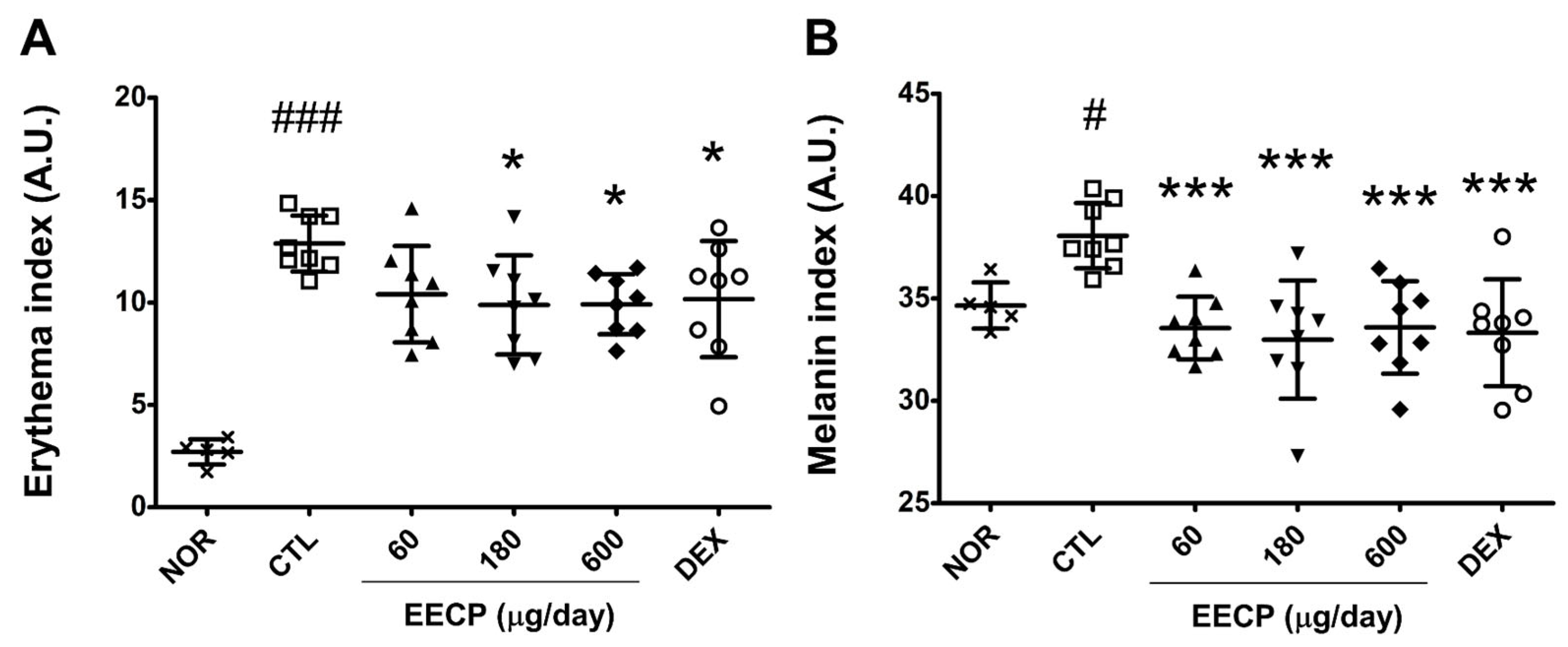
2.2. EECP Alleviated Erythema and Melanin Indices
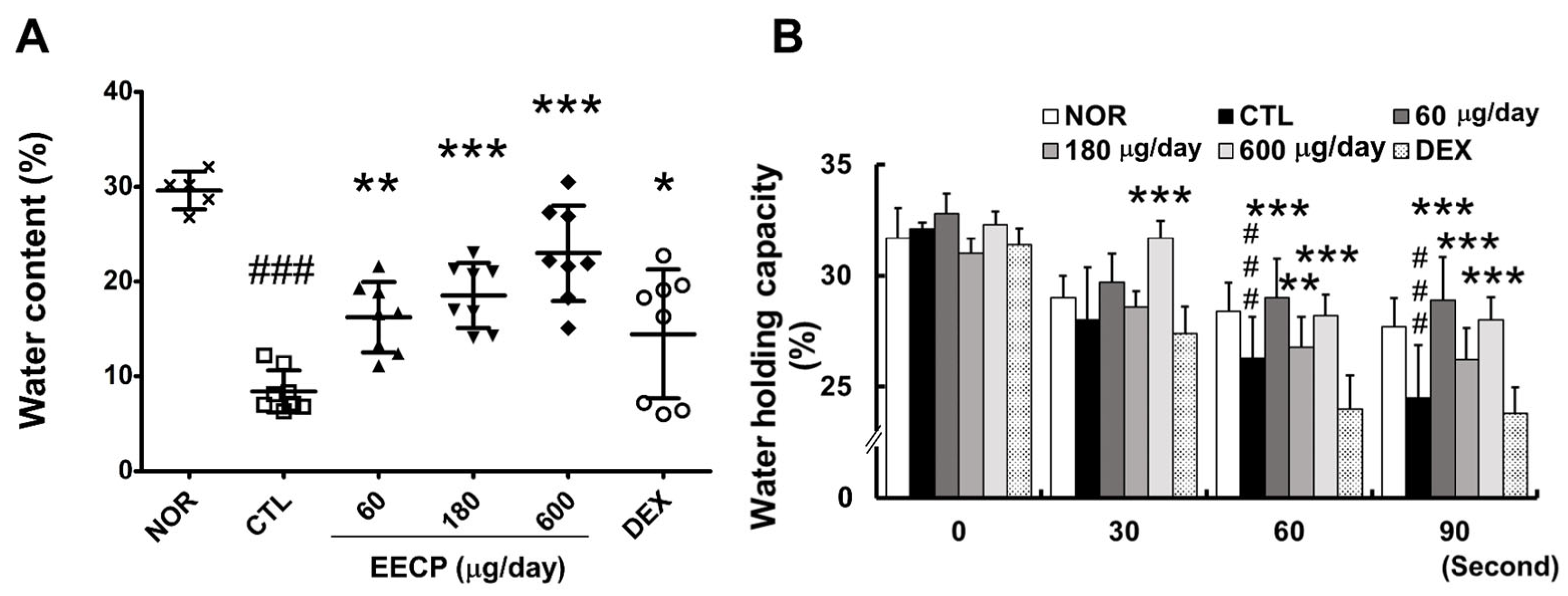
2.3. EECP Increased Skin Water Content and Water-Holding Capacity
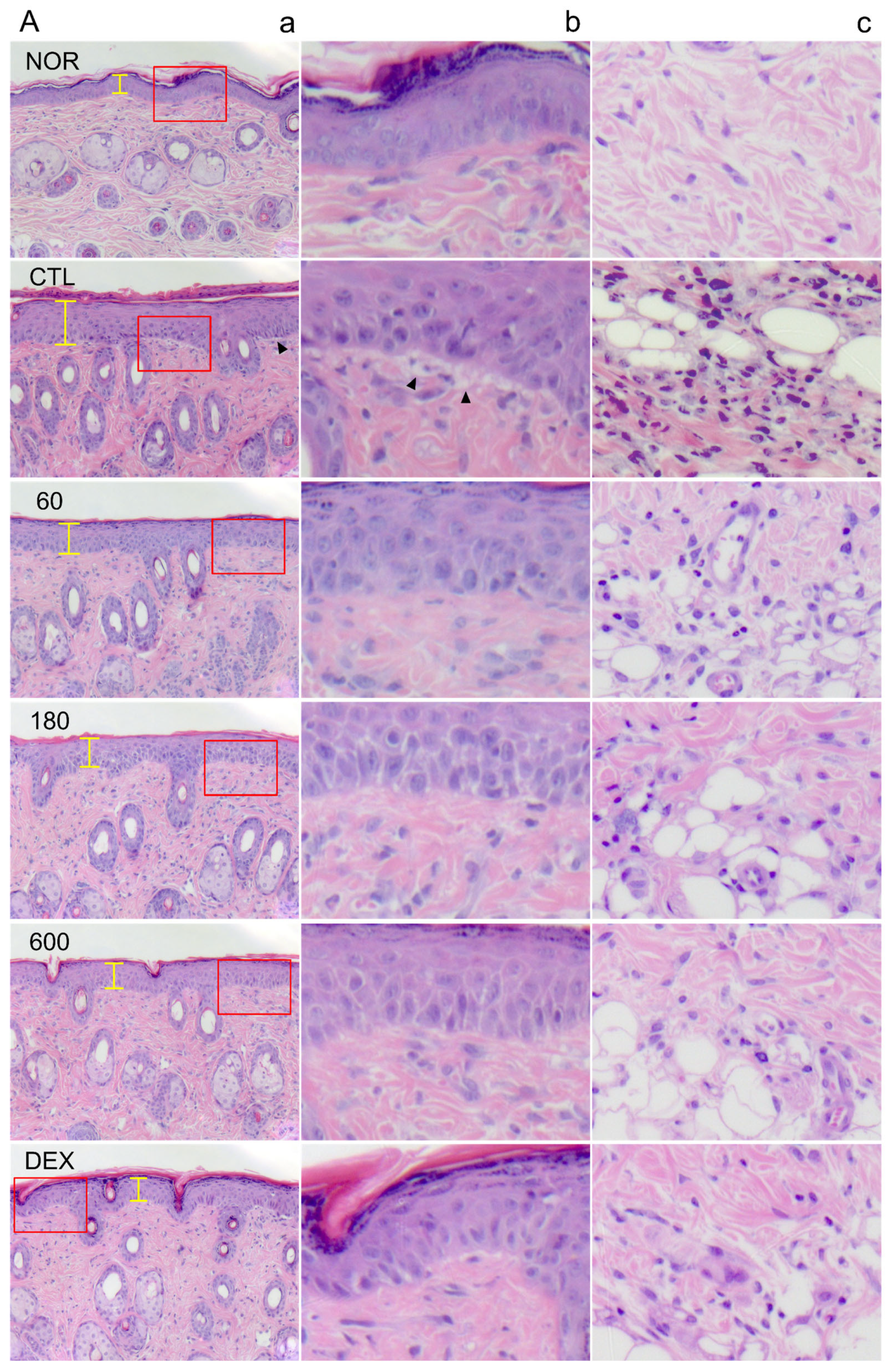
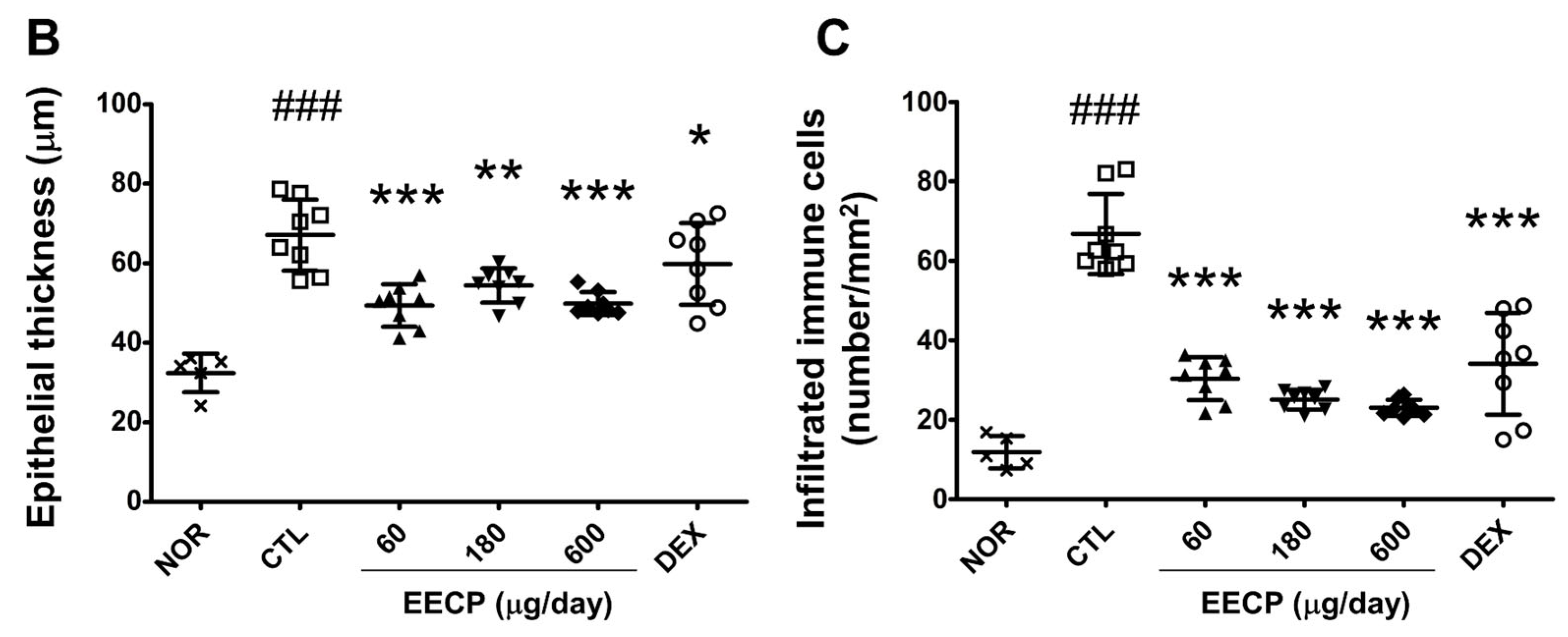
2.4. EECP Effectively Inhibited Epidermal Hyperplasia and Immune Cell Infiltration of Inflamed Tissues
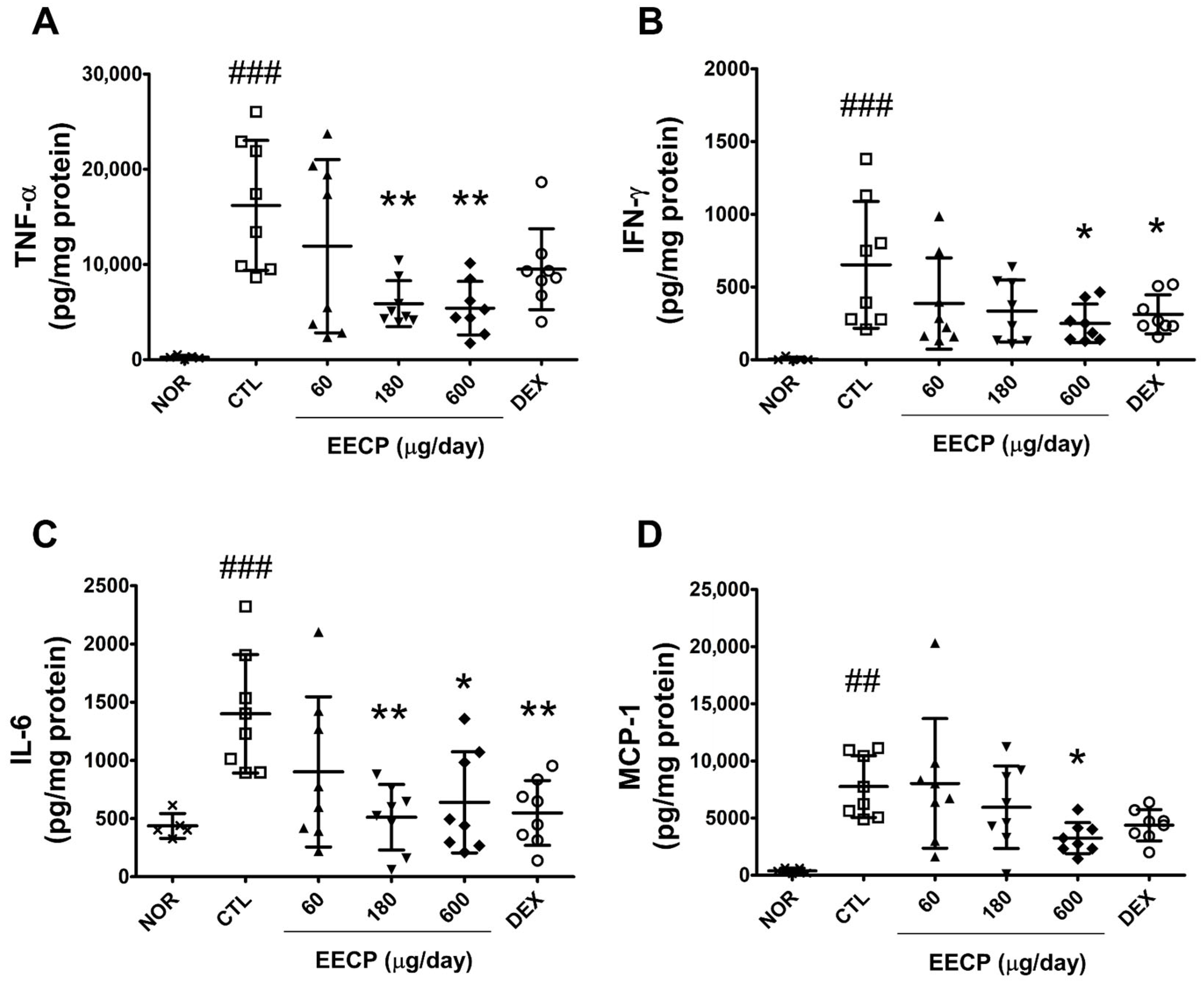
2.5. EECP Reduced the Production of TNF-α, IFN-γ, IL-6 and MCP-1 in Inflamed Tissues
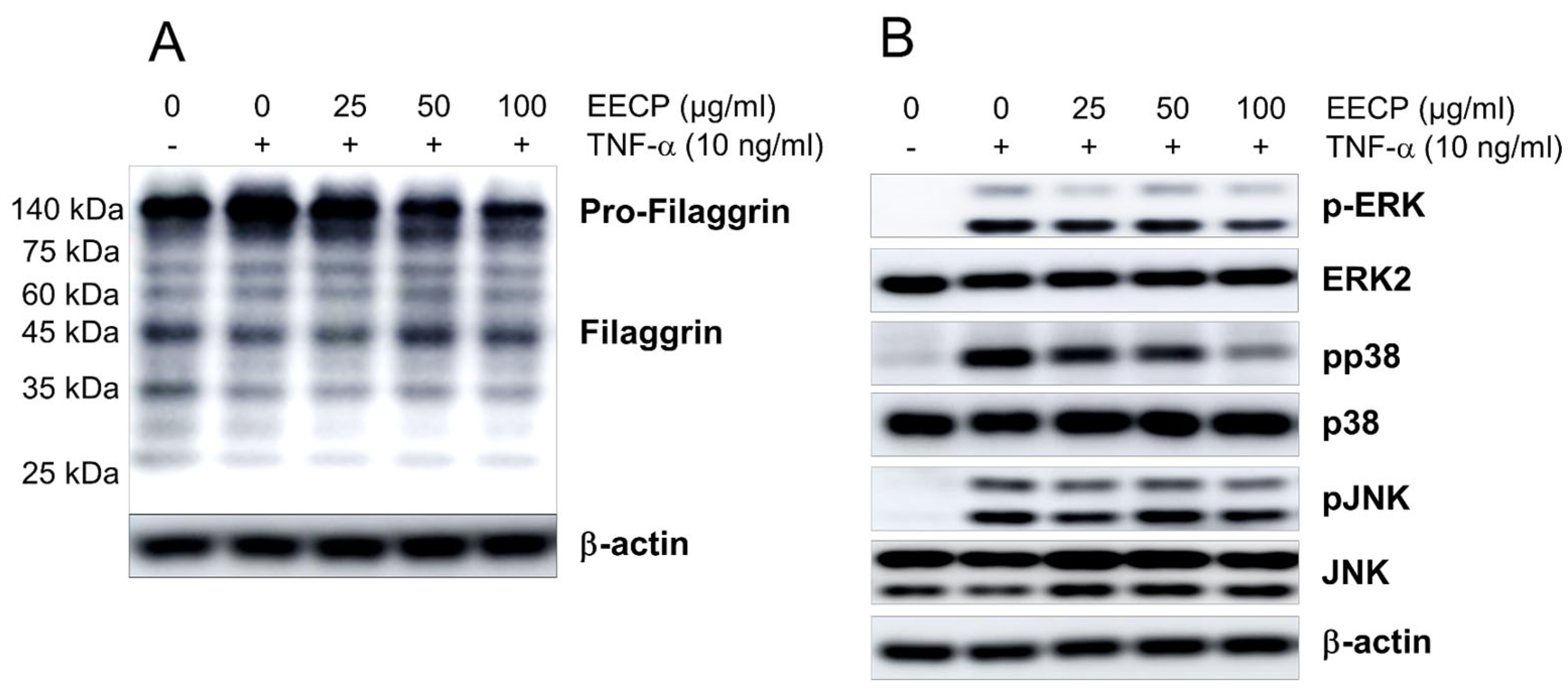
2.6. EECP Inhibited TNF-Induced Pro-Filaggrin Accumulation and Phosphorylations of ERK and P38, and Restored Filaggrin Expression in HaCaT Cells
2.7. EECP Inhibited TNF-α-Induced ICAM-1 and Cytokine Expression in HaCaT Cells
3. Discussion
4. Materials and Methods
4.1. Preparation of the Papaya Peel Extract
4.2. Animals
4.3. Induction of CD and the Experimental Design
4.4. Skin Observation and Assessment of Skin Lesions and Weight
4.5. Assessment of Skin Color
4.6. Skin Water Content and Water-Holding Capacity
4.7. Histopathological Examination
4.8. Quantitative Analysis of Epidermal Thickness and Immune Cell Infiltration
4.9. Cytokine Levels
4.10. Cell Culture
4.11. Protein Extraction and Western Blot Analysis
4.12. Total RNA Isolation and Quantitative Real-Time PCR
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yew, Y.W.; Kuan, A.H.Y.; Ge, L.; Yap, C.W.; Heng, B.H. Psychosocial impact of skin diseases: A population-based study. PLoS ONE 2020, 15, e0244765. [Google Scholar] [CrossRef] [PubMed]
- Dini, I.; Laneri, S. The new challenge of green cosmetics: Natural food ingredients for cosmetic formulations. Molecules 2021, 26, 3921. [Google Scholar] [CrossRef] [PubMed]
- Akanda, M.K.M.; Hasan, A.H.M.N.; Mehjabin, S.; Parvez, G.M.M.; Yasmin, S.; Akhtar, M.S.; Anjum, S.; Ashique, S.; Sulatana, R.; Ansari, M.Y. Carica papaya in health and disease: A review of its bioactive compounds for treating various disease conditions, including anti-inflammatory and anti-arthritic activities. Inflammopharmacology 2025, 33, 3051–3084. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, A.B.; Amos, I.A.; Adebisi, A.O.; Gboyega, E.A.; Oluwabukunmi, B.; Chekwube, N. Therapeutic benefits of Carica papaya: A review on its pharmacological activities and characterization of papain. Arab. J. Chem. 2023, 17, 105369. [Google Scholar]
- Kong, Y.R.; Jong, Y.X.; Balakrishnan, M.; Bok, Z.K.; Weng, J.K.K.; Tay, K.C.; Goh, B.H.; Ong, Y.S.; Chan, K.G.; Lee, L.H.; et al. Beneficial Role of Carica papaya Extracts and Phytochemicals on Oxidative Stress and Related Diseases: A Mini Review. Biology 2021, 10, 287. [Google Scholar] [CrossRef]
- Mukta, M.M.; Hossain, M.F.; Arefin, P.; Ahmed, S. Investigation of Nutritional Properties and Antioxidant Potentials of Papaya (Carica papaya L. var BARI Papaya-1) Peels Available in Bangladesh. SSRN. 2025. Available online: https://ssrn.com/abstract=5111428 (accessed on 26 January 2025).
- Chaiwut, P.; Nitsawang, S.; Shank, L.; Kanasawud, P. A comparative study on properties and proteolytic components of Papaya peel and latex proteases. Chiang Mai J. Sci. 2007, 34, 109–118. [Google Scholar]
- Nieto Calvache, J.; Cueto, M.; Farroni, A.; de Escalada Pla, M.; Gerschenson, L.N. Antioxidant characterization of new dietary fiber concentrates from Papaya pulp and peel (Carica papaya L.). J. Funct. Foods 2016, 27, 319–328. [Google Scholar] [CrossRef]
- Vyumvuhore, R.; Tfayli, A.; Baillet-Guffroy, A.; Biniek, K.; Dauskardt, R.; Duplan, H.; Delalleau, A.; Manfait, M. The relationship between water loss, mechanical stress, and molecular structure of human stratum corneum ex vivo. J. Biophotonics 2015, 8, 217–225. [Google Scholar] [CrossRef]
- Yosipovitch, G.; Misery, L.; Proksch, E.; Metz, M.; Ständer, S.; Schmelz, M. Skin barrier damage and itch: Review of mechanisms, topical management and future directions. Acta Derm. Venereol. 2019, 99, 1201–1209. [Google Scholar] [CrossRef]
- Doi, T.; Mizukawa, Y.; Shimoda, Y.; Yamazaki, Y.; Shiohara, T. Importance of water content of the stratum corneum in mouse models for contact hypersensitivity. J. Investig. Dermatol. 2017, 137, 151–158. [Google Scholar] [CrossRef]
- Denda, M.; Sato, J.; Tsuchiya, T.; Elias, P.M.; Feingold, K.R. Low humidity stimulates epidermal DNA synthesis and amplifies the hyperproliferative response to barrier disruption: Implication for seasonal exacerbations of inflammatory dermatoses. J. Investig. Dermatol. 1998, 111, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Diepgen, T.L.; Ofenloch, R.F.; Bruze, M.; Bertuccio, P.; Cazzaniga, S.; Coenraads, P.J.; Elsner, P.; Goncalo, M.; Svensson, Å.; Naldi, L. Prevalence of contact allergy in the general population in different European regions. Br. J. Dermatol. 2016, 174, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Trautmann, A.; Akdis, M.; Bröcker, E.B.; Blaser, K.; Akdis, C.A. New insights into the role of T cells in atopic dermatitis and allergic contact dermatitis. Trends Immunol. 2001, 22, 530–532. [Google Scholar] [CrossRef]
- Dickel, H. Management of contact dermatitis. Allergo J. Int. 2023, 32, 57–76. [Google Scholar] [CrossRef]
- Kim, K.; Lee, D.; Kim, H.Y.; Kim, S.; Lyu, J.H.; Park, S.; Park, Y.C.; Kim, H. Anti-Inflammatory Effects of Spirodela polyrhiza (L.) SCHLEID. Extract on Contact Dermatitis in Mice-Its Active Compounds and Molecular Targets. Int. J. Mol. Sci. 2023, 24, 13271. [Google Scholar] [CrossRef]
- Koorneef, L.L.; van den Berg, S.A.; Havekes, L.M.; Rensen, P.C.N.; van Dijk, K.W. Dexamethasone-associated metabolic effects in male mice are partially caused by depletion of endogenous corticosterone. Front. Endocrinol. 2022, 13, 960279. [Google Scholar] [CrossRef]
- Gisondi, P.; Ellis, C.N.; Girolomoni, G. Pimecrolimus in dermatology: Atopic dermatitis and beyond. Int. J. Clin. Pract. 2005, 59, 969–974. [Google Scholar] [CrossRef]
- Martín-Santiago, A.; Puig, S.; Arumi, D.; Rebollo Laserna, F.J. Safety Profile and Tolerability of Topical Phosphodiesterase 4 Inhibitors for the Treatment of Atopic Dermatitis: A Systematic Review and Meta-Analysis. Curr. Ther. Res. 2022, 96, 100679. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Muguet, V.; Christen-Zaech, S. Restoring skin hydration and barrier function: Mechanistic insights into basic emollients for xerosis cutis. Int. J. Dermatol. 2025, 64, 5–12. [Google Scholar] [CrossRef]
- Del Rosso, J.Q.; Kircik, L. Skin 101: Understanding the fundamentals of skin barrier physiology—Why is this important for clinicians? J. Clin. Aesthet. Dermatol. 2021, 18, 7–15. [Google Scholar]
- Eberlein-König, B.; Schäfer, T.; Huss-Marp, J.; Darsow, U.; Möhrenschlager, M.; Herbert, O.; Abeck, D.; Krämer, U.; Behrendt, H.; Ring, J. Skin surface pH, stratum corneum hydration, trans-epidermal water loss and skin roughness related to atopic eczema and skin dryness in a population of primary school children. Acta Derm. Venereol. 2000, 80, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Kang, Y.M.; Lee, M.; An, H.J. Papain Suppresses Atopic Skin Inflammation through Anti-Inflammatory Activities Using In Vitro and In Vivo Models. Antioxidants 2024, 13, 928. [Google Scholar] [CrossRef] [PubMed]
- Gurung, S.; Skalko-Basnet, N. Wound healing properties of Carica papaya latex: In vitro evaluation in mice burn model. J. Ethnopharmacol. 2009, 121, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Falduto, M.T.; Young, A.P.; Hickson, R.C. Exercise inhibits glucocorticoid-induced glutamine synthetase expression in red skeletal muscles. Am. J. Physiol. Cell Physiol. 1992, 262, C214–C220. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Kirsner, R. Proinflammatory cytokines regulate epidermal stem cells in wound epithelialization. Stem Cell Res. Ther. 2020, 11, 232. [Google Scholar] [CrossRef]
- Kaur, S.; Zilmer, K.; Leping, V.; Zilmer, M. Allergic contact dermatitis is associated with significant oxidative stress. Dermatol. Res. Pract. 2014, 2014, 415638. [Google Scholar] [CrossRef]
- Nakamura, K.; Williams, I.R.; Kupper, T.S. Keratinocyte-derived monocyte chemoattractant protein 1 (MCP-1): Analysis in a transgenic model demonstrates MCP-1 can recruit dendritic and Langerhans cells to skin. J. Investig. Dermatol. 1995, 105, 635–643. [Google Scholar] [CrossRef]
- Dmochowski, M.; Sokołowska-Wojdyło, M. Spongiotic reaction patterns in autoimmune bullous dermatoses (Review). Exp. Ther. Med. 2021, 21, 351. [Google Scholar]
- Cao, Y.; Lai, K.M.; Fu, K.C.; Kuo, C.L.; Tan, Y.J.; Yu, L.L.; Huang, D. Dual Functionality of Papaya Leaf Extracts: Anti-Coronavirus Activity and Anti-Inflammation Mechanism. Foods 2024, 13, 3274. [Google Scholar] [CrossRef]
- Ferreira, P.M.; Ferreira, A.M.; Lopes, G.R.; Freitas, A.C. Carotenoids and their interaction with the immune system. Antioxidants 2023, 12, 829. [Google Scholar]
- Flieger, J.; Raszewska-Famielec, M.; Radzikowska-Büchner, E.; Flieger, W. Skin Protection by Carotenoid Pigments. Int. J. Mol. Sci. 2024, 25, 1431. [Google Scholar] [CrossRef]
- Leyva-López, N.; Gutierrez-Grijalva, E.P.; Ambriz-Perez, D.L.; Heredia, J.B. Flavonoids as cytokine modulators: A possible therapy for inflammation-related diseases. Int. J. Mol. Sci. 2016, 17, 921. [Google Scholar] [CrossRef]
- Lee, S.G.; Karadeniz, F.; Seo, Y.; Kong, C.S. Anti-Melanogenic Effects of Flavonoid Glycosides from Limonium tetragonum (Thunb.) Bullock via Inhibition of Tyrosinase and Tyrosinase-Related Proteins. Molecules 2017, 22, 1480. [Google Scholar] [CrossRef]
- Kim, B.E.; Howell, M.D.; Guttman-Yassky, E.; Gilleaudeau, P.M.; Cardinale, I.R.; Boguniewicz, M.; Krueger, J.G.; Leung, D.Y. TNF-α downregulates filaggrin and loricrin through c-Jun N-terminal kinase: Role for TNF-α antagonists to improve skin barrier. J. Investig. Dermatol. 2011, 131, 1272–1279. [Google Scholar] [CrossRef]
- Furue, M. Regulation of Filaggrin, Loricrin, and Involucrin by IL-4, IL-13, IL-17A, IL-22, AHR, and NRF2: Pathogenic Implications in Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 5382. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, H.; Li, W.; Qiang, M.; Dong, T.; Li, H. Role of vitamin C in skin diseases. Front. Physiol. 2018, 9, 819. [Google Scholar] [CrossRef]
- Zeze, N.; Kido-Nakahara, M.; Tsuji, G.; Maehara, E.; Sato, Y.; Sakai, S.; Fujishima, K.; Hashimoto-Hachiya, A.; Furue, M.; Nakahara, T. Role of ERK Pathway in the Pathogenesis of Atopic Dermatitis and Its Potential as a Therapeutic Target. Int. J. Mol. Sci. 2022, 23, 3467. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Yang, H.; Liu, E.; Wang, H. P38/ERK MAPK signaling pathways are involved in the regulation of filaggrin and involucrin by IL-17. Mol. Med. Rep. 2017, 16, 8863–8867. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, T.; Katayama, I.; Yokozeki, H.; Nishioka, K. ICAM-1 expression on keratinocytes in mechanically-injured skin of a patient with atopic dermatitis. J. Dermatol. Sci. 1996, 12, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Lehtola, T.; Nummenmaa, E.; Nieminen, R.; Hämäläinen, M.; Vuolteenaho, K.; Moilanen, E. The glucocorticoid dexamethasone alleviates allergic inflammation through a mitogen-activated protein kinase phosphatase-1-dependent mechanism in mice. Basic Clin. Pharmacol. Toxicol. 2024, 134, 686–694. [Google Scholar] [CrossRef]
- Oakley, R.H.; Cidlowski, J.A. The Biology of the Glucocorticoid Receptor: New Signaling Mechanisms in Health and Disease. J. Allergy Clin. Immunol. 2013, 132, 1033–1044. [Google Scholar] [CrossRef]
- Voskas, D.; Babichev, Y.; Ling, L.S.; Alami, J.; Shaked, Y.; Kerbel, R.S.; Ciruna, B.; Dumont, D.J. An eosinophil immune response characterizes the inflammatory skin disease observed in Tie-2 transgenic mice. J. Leukoc. Biol. 2008, 84, 59–67. [Google Scholar] [CrossRef]
- Orzechowski, A.; Jank, M.; Gajewska, M.; Balasinska, B.; Ostaszewski, P.; Wilczak, J.; Waręski, P.; Fuller, J., Jr. Rats with a glucocorticoid-induced catabolic state show symptoms of oxidative stress and spleen atrophy: The effects of age and recovery. J. Vet. Med. Ser. A 2002, 49, 256–263. [Google Scholar] [CrossRef]
- Schoepe, S.; Schäcke, H.; May, E.; Asadullah, K. Glucocorticoid therapy-induced skin atrophy. Exp. Dermatol. 2006, 15, 406–420. [Google Scholar] [CrossRef]
- Ferguson, P.; Akinsulie, A.; Bergfeld, W.F.; Belsito, D.V.; Cohen, D.E.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Peterson, L.A.; Shank, R.C.; et al. Safety Assessment of Carica papaya (Papaya)-Derived Ingredients as Used in Cosmetics. Int. J. Toxicol. 2025, 44 (Suppl. S3), 36S–56S. [Google Scholar] [CrossRef]
- Pallavi, A.G.; Vaibhav, A.J.; Vikas, B.W. Biological Study of Carica papaya and Formulation and Evaluation of Herbal Papaya Soap. Int. J. Sci. Dev. Res. 2022, 7, 880–894. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Sun, K.; Kim, Y.; Son, H.; Lee, J.; Kim, S.; Kim, H. The Anti-Inflammatory and Skin Barrier Function Recovery Effects of Carica papaya Peel in Mice with Contact Dermatitis. Int. J. Mol. Sci. 2025, 26, 11122. https://doi.org/10.3390/ijms262211122
Park S, Sun K, Kim Y, Son H, Lee J, Kim S, Kim H. The Anti-Inflammatory and Skin Barrier Function Recovery Effects of Carica papaya Peel in Mice with Contact Dermatitis. International Journal of Molecular Sciences. 2025; 26(22):11122. https://doi.org/10.3390/ijms262211122
Chicago/Turabian StylePark, Seonah, Kyoungmin Sun, Yeojin Kim, Hyorhan Son, Jimi Lee, Soyeon Kim, and Hyungwoo Kim. 2025. "The Anti-Inflammatory and Skin Barrier Function Recovery Effects of Carica papaya Peel in Mice with Contact Dermatitis" International Journal of Molecular Sciences 26, no. 22: 11122. https://doi.org/10.3390/ijms262211122
APA StylePark, S., Sun, K., Kim, Y., Son, H., Lee, J., Kim, S., & Kim, H. (2025). The Anti-Inflammatory and Skin Barrier Function Recovery Effects of Carica papaya Peel in Mice with Contact Dermatitis. International Journal of Molecular Sciences, 26(22), 11122. https://doi.org/10.3390/ijms262211122




