Nanomaterials in Drug Delivery: Leveraging Artificial Intelligence and Big Data for Predictive Design
Abstract
1. Introduction
2. Nanomaterials in Drug Delivery
2.1. Liposome
2.2. Polymeric Nanoparticle
2.3. Dendrimer
2.4. Metallic Nanoparticle
2.5. Silica-Based Nanoparticle
2.6. Carbon-Based Nanoparticle
3. Role of Artificial Intelligence in Nanomaterial Design
3.1. Machine Learning
3.2. Deep Learning
3.3. Generative Design Strategies
3.4. Critical Perspective on AI-Driven Modeling Approaches
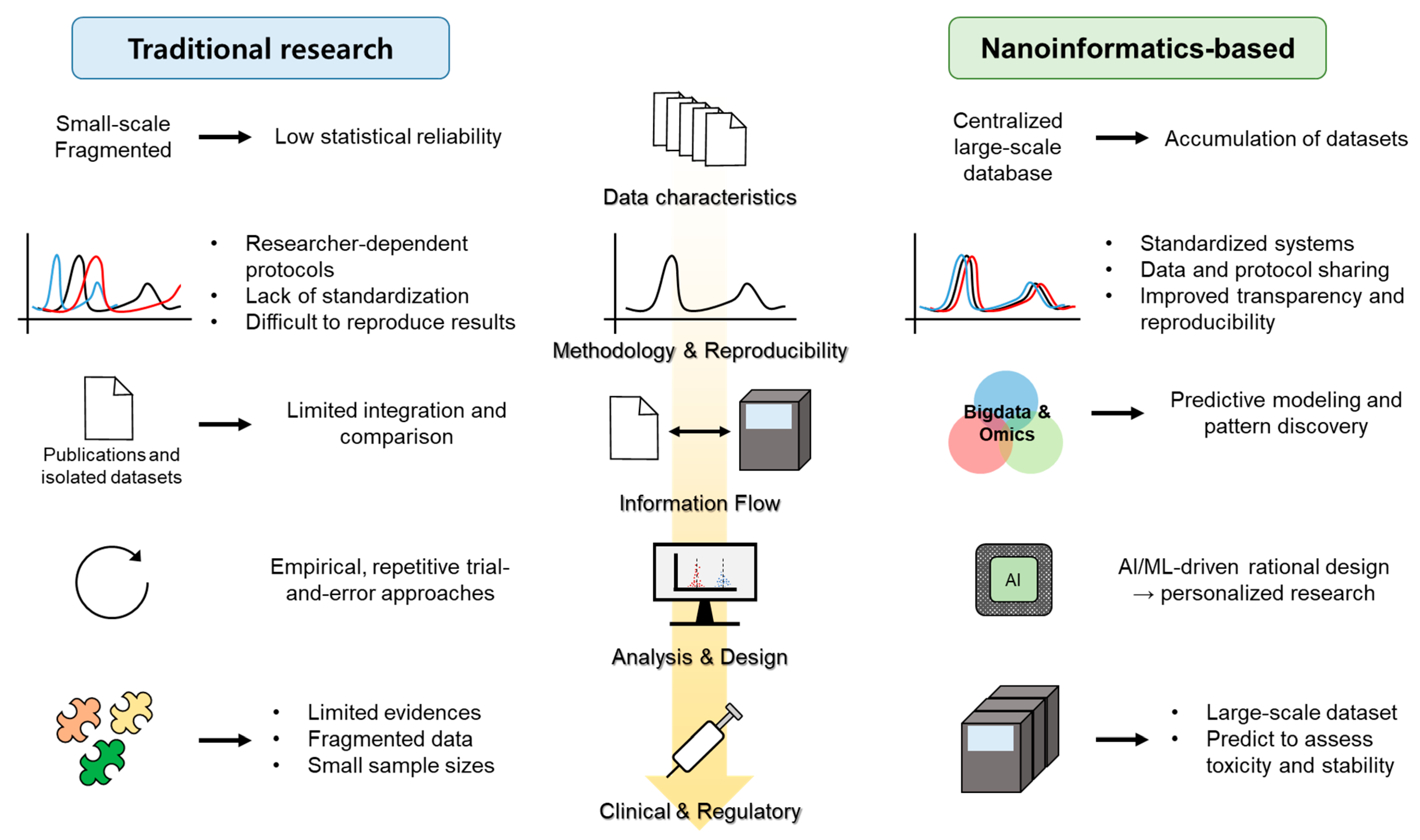
4. Big Data and Nanoinformatics
4.1. Emergence of Nanoinformatics
4.2. Major Databases and Repositories
4.3. Integration with Big Data Analytics
4.4. Regulatory Science and Translational Impact
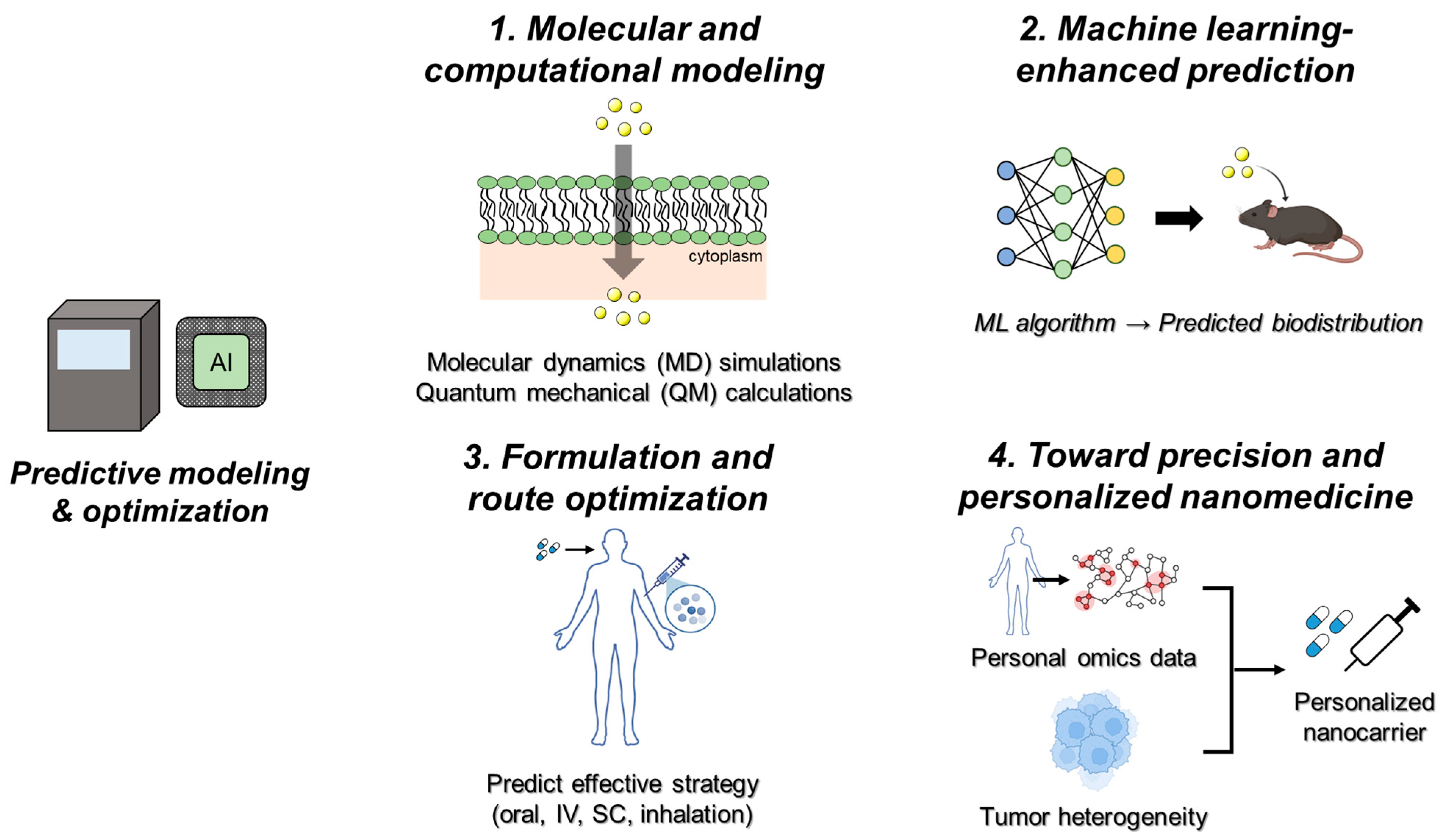
5. Predictive Modeling and Optimization
5.1. Molecular and Computational Modeling
5.2. Machine Learning-Enhanced Prediction
5.3. Formulation and Route Optimization
5.4. Toward Precision and Personalized Nanomedicine
5.5. Translational and Regulatory Perspectives
6. Opportunities and Challenges
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Q.; Liu, Y.; Li, C.; Xu, B.; Xu, S.; Liu, B. Machine Learning-Enhanced Nanoparticle Design for Precision Cancer Drug Delivery. Adv. Sci. 2025, 12, e03138. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, A.; Patne, A.Y.; Mohapatra, S.; Mohapatra, S.S. Convergence of Nanotechnology and Machine Learning: The State of the Art, Challenges, and Perspectives. Int. J. Mol. Sci. 2024, 25, 12368. [Google Scholar] [CrossRef] [PubMed]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-based medicines: A review of FDA-approved materials and clinical trials to date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Barenholz, Y.C. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Wang, A.Z.; Langer, R.; Farokhzad, O.C. Nanoparticle delivery of cancer drugs. Annu. Rev. Med. 2012, 63, 185–198. [Google Scholar] [CrossRef]
- Etheridge, M.L.; Campbell, S.A.; Erdman, A.G.; Haynes, C.L.; Wolf, S.M.; McCullough, J. The big picture on nanomedicine: The state of investigational and approved nanomedicine products. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 1–14. [Google Scholar] [CrossRef]
- Hare, J.I.; Lammers, T.; Ashford, M.B.; Puri, S.; Storm, G.; Barry, S.T. Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Adv. Drug Deliv. Rev. 2017, 108, 25–38. [Google Scholar] [CrossRef]
- Monopoli, M.P.; Aberg, C.; Salvati, A.; Dawson, K.A. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 2012, 7, 779–786. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, M.; Jirvankar, P.S. Harnessing artificial intelligence for enhanced nanoparticle design in precision oncology. AIMS Bioeng. 2024, 11, 574–597. [Google Scholar] [CrossRef]
- Dorsey, P.J.; Lau, C.L.; Chang, T.C.; Doerschuk, P.C.; D’Addio, S.M. Review of machine learning for lipid nanoparticle formulation and process development. J. Pharm. Sci. 2024, 113, 3413–3433. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Qin, S.; Li, Y.; Hasan, N.; Li, Y.V.; Liu, J. Machine learning integrated with in vitro experiments for study of drug release from PLGA nanoparticles. Sci. Rep. 2025, 15, 4218. [Google Scholar] [CrossRef]
- Ali, R.; Balamurali, M.; Varamini, P. Deep learning-based artificial intelligence to investigate targeted nanoparticles’ uptake in TNBC cells. Int. J. Mol. Sci. 2022, 23, 16070. [Google Scholar] [CrossRef]
- Wang, T.; Russo, D.P.; Demokritou, P.; Jia, X.; Huang, H.; Yang, X.; Zhu, H. An online nanoinformatics platform empowering computational modeling of nanomaterials by nanostructure annotations and machine learning toolkits. Nano Lett. 2024, 24, 10228–10236. [Google Scholar] [CrossRef]
- Saha, S. How nanoinformatics could pave the way to safer design of engineered nanomaterials? Front. Nanotechnol. 2025, 7, 1559053. [Google Scholar] [CrossRef]
- Shirzad, M.; Shaban, M.; Mohammadzadeh, V.; Rahdar, A.; Fathi-karkan, S.; Hoseini, Z.S.; Najafi, M.; Kharaba, Z.; Aboudzadeh, M.A. Artificial Intelligence-Assisted Design of Nanomedicines for Breast Cancer Diagnosis and Therapy: Advances, Challenges, and Future Directions. BioNanoScience 2025, 15, 354. [Google Scholar] [CrossRef]
- Nguyen, T.K.M.; Ki, M.R.; Lee, C.S.; Pack, S.P. Nanosized and tunable design of biosilica particles using novel silica-forming peptide-modified chimeric ferritin templates. J. Ind. Eng. Chem. 2019, 73, 198–204. [Google Scholar] [CrossRef]
- Abdelhamid, M.A.; Yeo, K.B.; Ki, M.-R.; Pack, S.P. Self-encapsulation and controlled release of recombinant proteins using novel silica-forming peptides as fusion linkers. Int. J. Biol. Macromol. 2019, 125, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Min, K.H.; Shin, J.W.; Ki, M.-R.; Pack, S.P. Green synthesis of silver nanoparticles on biosilica diatomite: Well-dispersed particle formation and reusability. Process Biochem. 2023, 125, 232–238. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, X.; Wu, Y.; Chen, X.; Feng, L.; Xie, N.; Shen, G. Nanotechnology’s frontier in combatting infectious and inflammatory diseases: Prevention and treatment. Signal Transduct. Target. Ther. 2024, 9, 34. [Google Scholar] [CrossRef]
- Gao, C.; Xiong, R.; Zhang, Z.-y.; Peng, H.; Gu, Y.-k.; Xu, W.; Yang, W.-t.; Liu, Y.; Gao, J.; Yin, Y. Hybrid nanostructures for neurodegenerative disease theranostics: The art in the combination of biomembrane and non-biomembrane nanostructures. Transl. Neurodegener. 2024, 13, 43. [Google Scholar] [CrossRef]
- Zuccari, G.; Villa, C.; Iurilli, V.; Barabino, P.; Zorzoli, A.; Marimpietri, D.; Caviglia, D.; Russo, E. AmBisome® formulations for pediatrics: Stability, cytotoxicity, and cost-effectiveness studies. Pharmaceutics 2024, 16, 466. [Google Scholar] [CrossRef]
- Miguel, R.D.A.; Hirata, A.S.; Jimenez, P.C.; Lopes, L.B.; Costa-Lotufo, L.V. Beyond Formulation: Contributions of Nanotechnology for Translation of Anticancer Natural Products into New Drugs. Pharmaceutics 2022, 14, 1722. [Google Scholar] [CrossRef]
- Lancet, J.E.; Uy, G.L.; Cortes, J.E.; Newell, L.F.; Lin, T.L.; Ritchie, E.K.; Stuart, R.K.; Strickland, S.A.; Hogge, D.; Solomon, S.R.; et al. CPX-351 (cytarabine and daunorubicin) Liposome for Injection Versus Conventional Cytarabine Plus Daunorubicin in Older Patients With Newly Diagnosed Secondary Acute Myeloid Leukemia. J. Clin. Oncol. 2018, 36, 2684–2692. [Google Scholar] [CrossRef]
- Kim, T.Y.; Kim, D.W.; Chung, J.Y.; Shin, S.G.; Kim, S.C.; Heo, D.S.; Kim, N.K.; Bang, Y.J. Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clin. Cancer Res. 2004, 10, 3708–3716. [Google Scholar] [CrossRef]
- Carballo-Diéguez, A.; Giguere, R.; Dolezal, C.; Chen, B.A.; Kahn, J.; Zimet, G.; Mabragaña, M.; Leu, C.S.; McGowan, I. “Tell Juliana”: Acceptability of the candidate microbicide VivaGel® and two placebo gels among ethnically diverse, sexually active young women participating in a phase 1 microbicide study. AIDS Behav. 2012, 16, 1761–1774. [Google Scholar] [CrossRef]
- Frank, J.A.; Zywicke, H.; Jordan, E.; Mitchell, J.; Lewis, B.K.; Miller, B.; Bryant, L.H.; Bulte, J.W. Magnetic intracellular labeling of mammalian cells by combining (FDA-approved) superparamagnetic iron oxide MR contrast agents and commonly used transfection agents. Acad. Radiol. 2002, 9, S484–S487. [Google Scholar] [CrossRef]
- Gangopadhyay, S.; Nikam, R.R.; Gore, K.R. Folate receptor-mediated siRNA delivery: Recent developments and future directions for RNAi therapeutics. Nucleic Acid Ther. 2021, 31, 245–270. [Google Scholar] [CrossRef] [PubMed]
- Rastinehad, A.R.; Anastos, H.; Wajswol, E.; Winoker, J.S.; Sfakianos, J.P.; Doppalapudi, S.K.; Carrick, M.R.; Knauer, C.J.; Taouli, B.; Lewis, S.C. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc. Natl. Acad. Sci. USA 2019, 116, 18590–18596. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Kim, J.S.; Kim, W.S. Photothermal therapy using gold nanoparticles and a long-pulsed 755-nm alexandrite laser to treat facial photoaging in Asian skin: A prospective clinical trial. Lasers Surg. Med. 2022, 54, 1060–1070. [Google Scholar] [CrossRef]
- Ki, M.-R.; Kim, J.K.; Kim, S.H.; Nguyen, T.K.M.; Kim, K.H.; Pack, S.P. Compartment-restricted and rate-controlled dual drug delivery system using a biosilica-enveloped ferritin cage. J. Ind. Eng. Chem. 2020, 81, 367–374. [Google Scholar] [CrossRef]
- Ki, M.-R.; Nguyen, T.K.M.; Jun, H.S.; Pack, S.P. Biosilica-enveloped ferritin cage for more efficient drug deliveries. Process Biochem. 2018, 68, 182–189. [Google Scholar] [CrossRef]
- Xu, H.; Chang, J.; Wu, H.; Wang, H.; Xie, W.; Li, Y.; Li, X.; Zhang, Y.; Fan, L. Carbon Dots with Guanidinium and Amino Acid Functional Groups for Targeted Small Interfering RNA Delivery toward Tumor Gene Therapy. Small 2023, 19, 2207204. [Google Scholar] [CrossRef]
- Lee, D.K.; Kee, T.; Liang, Z.; Hsiou, D.; Miya, D.; Wu, B.; Osawa, E.; Chow, E.K.; Sung, E.C.; Kang, M.K.; et al. Clinical validation of a nanodiamond-embedded thermoplastic biomaterial. Proc. Natl. Acad. Sci. USA 2017, 114, E9445–E9454. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Mohamed, M.; Abu Lila, A.S.; Shimizu, T.; Alaaeldin, E.; Hussein, A.; Sarhan, H.A.; Szebeni, J.; Ishida, T. PEGylated liposomes: Immunological responses. Sci. Technol. Adv. Mater. 2019, 20, 710–724. [Google Scholar] [CrossRef]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef]
- Thi, T.T.H.; Suys, E.J.A.; Lee, J.S.; Nguyen, D.H.; Park, K.D.; Truong, N.P. Lipid-Based Nanoparticles in the Clinic and Clinical Trials: From Cancer Nanomedicine to COVID-19 Vaccines. Vaccines 2021, 9, 359. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.S.; Baliga, V.; Londhe, V.Y. Liposomal Formulations: A Recent Update. Pharmaceutics 2025, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Alsaab, H.O.; Alharbi, F.D.; Alhibs, A.S.; Alanazi, N.B.; Alshehri, B.Y.; Saleh, M.A.; Alshehri, F.S.; Algarni, M.A.; Almugaiteeb, T.; Uddin, M.N.; et al. PLGA-Based Nanomedicine: History of Advancement and Development in Clinical Applications of Multiple Diseases. Pharmaceutics 2022, 14, 2728. [Google Scholar] [CrossRef] [PubMed]
- Omidian, H.; Wilson, R.L.; Castejon, A.M. Recent Advances in Peptide-Loaded PLGA Nanocarriers for Drug Delivery and Regenerative Medicine. Pharmaceuticals 2025, 18, 127. [Google Scholar] [CrossRef]
- Kanasty, R.; Dorkin, J.R.; Vegas, A.; Anderson, D. Delivery materials for siRNA therapeutics. Nat. Mater. 2013, 12, 967–977. [Google Scholar] [CrossRef]
- Sristi; Almalki, W.H.; Karwasra, R.; Gupta, G.; Singh, S.; Sharma, A.; Sahebkar, A.; Kesharwani, P. Chapter Nine—Advances in the polymeric nanoparticulate delivery systems for RNA therapeutics. In Progress in Molecular Biology and Translational Science; Chu, D.-T., Than, V.T., Eds.; Academic Press: Cambridge, MA, USA, 2024; Volume 204, pp. 219–248. [Google Scholar]
- Lee, K.S.; Chung, H.C.; Im, S.A.; Park, Y.H.; Kim, C.S.; Kim, S.-B.; Rha, S.Y.; Lee, M.Y.; Ro, J. Multicenter phase II trial of Genexol-PM, a Cremophor-free, polymeric micelle formulation of paclitaxel, in patients with metastatic breast cancer. Breast Cancer Res. Treat. 2008, 108, 241–250. [Google Scholar] [CrossRef]
- Hamaguchi, T.; Matsumura, Y.; Suzuki, M.; Shimizu, K.; Goda, R.; Nakamura, I.; Nakatomi, I.; Yokoyama, M.; Kataoka, K.; Kakizoe, T. NK105, a paclitaxel-incorporating micellar nanoparticle formulation, can extend in vivo antitumour activity and reduce the neurotoxicity of paclitaxel. Br. J. Cancer 2005, 92, 1240–1246. [Google Scholar] [CrossRef]
- Armstrong, A.; Brewer, J.; Newman, C.; Alakhov, V.; Pietrzynski, G.; Campbell, S.; Corrie, P.; Ranson, M.; Valle, J. SP1049C as first-line therapy in advanced (inoperable or metastatic) adenocarcinoma of the oesophagus: A phase II window study. J. Clin. Oncol. 2006, 24 (Suppl. 18), 4080. [Google Scholar] [CrossRef]
- Kokaz, S.F.; Deb, P.K.; Borah, P.; Bania, R.; Venugopala, K.N.; Nair, A.B.; Singh, V.; Al-Shar’i, N.A.; Hourani, W.; Gupta, G. Dendrimers: Properties and applications in biomedical field. In Nanoengineering of Biomaterials; Wiley: Hoboken, NJ, USA, 2021; pp. 215–243. [Google Scholar] [CrossRef]
- Svenson, S.; Tomalia, D.A. Dendrimers in biomedical applications—Reflections on the field. Adv. Drug Deliv. Rev. 2012, 64, 102–115. [Google Scholar] [CrossRef]
- Price, C.F.; Tyssen, D.; Sonza, S.; Davie, A.; Evans, S.; Lewis, G.R.; Xia, S.; Spelman, T.; Hodsman, P.; Moench, T.R. SPL7013 Gel (VivaGel®) retains potent HIV-1 and HSV-2 inhibitory activity following vaginal administration in humans. PLoS ONE 2011, 6, e24095. [Google Scholar] [CrossRef]
- Luong, D.; Kesharwani, P.; Deshmukh, R.; Amin, M.C.I.M.; Gupta, U.; Greish, K.; Iyer, A.K. PEGylated PAMAM dendrimers: Enhancing efficacy and mitigating toxicity for effective anticancer drug and gene delivery. Acta Biomater. 2016, 43, 14–29. [Google Scholar] [CrossRef]
- Dykman, L.; Khlebtsov, N. Gold nanoparticles in biomedical applications: Recent advances and perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282. [Google Scholar] [CrossRef]
- Khorasani, A.; Shahbazi-Gahrouei, D.; Safari, A. Recent metal nanotheranostics for cancer diagnosis and therapy: A review. Diagnostics 2023, 13, 833. [Google Scholar] [CrossRef]
- Jaque, D.; Maestro, L.M.; del Rosal, B.; Haro-Gonzalez, P.; Benayas, A.; Plaza, J.; Rodríguez, E.M.; Solé, J.G. Nanoparticles for photothermal therapies. Nanoscale 2014, 6, 9494–9530. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, A.; Kolosnjaj-Tabi, J.; Abou-Hassan, A.; Plan Sangnier, A.; Curcio, A.; Silva, A.K.; Di Corato, R.; Neveu, S.; Pellegrino, T.; Liz--Marzán, L.M. Magnetic (hyper) thermia or photothermia? Progressive comparison of iron oxide and gold nanoparticles heating in water, in cells, and in vivo. Adv. Funct. Mater. 2018, 28, 1803660. [Google Scholar] [CrossRef]
- Huang, Y.; Hsu, J.C.; Koo, H.; Cormode, D.P. Repurposing ferumoxytol: Diagnostic and therapeutic applications of an FDA-approved nanoparticle. Theranostics 2022, 12, 796. [Google Scholar] [CrossRef] [PubMed]
- Slowing, I.I.; Trewyn, B.G.; Giri, S.; Lin, V.Y. Mesoporous silica nanoparticles for drug delivery and biosensing applications. Adv. Funct. Mater. 2007, 17, 1225–1236. [Google Scholar] [CrossRef]
- Abdelhamid, M.A.; Khalifa, H.O.; Ki, M.-R.; Pack, S.P. Nanoengineered silica-based biomaterials for regenerative medicine. Int. J. Mol. Sci. 2024, 25, 6125. [Google Scholar] [CrossRef]
- Mamaeva, V.; Rosenholm, J.M.; Bate-Eya, L.T.; Bergman, L.; Peuhu, E.; Duchanoy, A.; Fortelius, L.E.; Landor, S.; Toivola, D.M.; Lindén, M. Mesoporous silica nanoparticles as drug delivery systems for targeted inhibition of Notch signaling in cancer. Mol. Ther. 2011, 19, 1538–1546. [Google Scholar] [CrossRef]
- Dutta Gupta, Y.; Mackeyev, Y.; Krishnan, S.; Bhandary, S. Mesoporous silica nanotechnology: Promising advances in augmenting cancer theranostics. Cancer Nanotechnol. 2024, 15, 9. [Google Scholar] [CrossRef]
- Abdelhamid, M.A.; Pack, S.P. Biomimetic and bioinspired silicifications: Recent advances for biomaterial design and applications. Acta Biomater. 2021, 120, 38–56. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Lu, M.-m.; Zhao, Y.-w.; Zhang, F.; Tan, Y.-f.; Zheng, X.; Pan, Y.; Xiao, X.-a.; Wang, Z.; Dong, W.-f. The shape effect of magnetic mesoporous silica nanoparticles on endocytosis, biocompatibility and biodistribution. Acta Biomater. 2017, 49, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S.K.; Srivastava, R. Drug Delivery with Carbon-Based Nanomaterials as Versatile Nanocarriers: Progress and Prospects. Front. Nanotechnol. 2021, 3, 644564. [Google Scholar] [CrossRef]
- Saleh, M.; Gul, A.; Nasir, A.; Moses, T.O.; Nural, Y.; Yabalak, E. Comprehensive review of Carbon-based nanostructures: Properties, synthesis, characterization, and cross-disciplinary applications. J. Ind. Eng. Chem. 2025, 146, 176–212. [Google Scholar] [CrossRef]
- Parveen, A.; Chatterjee, A.; Karak, P. Biomedical Applications of Carbon-Based Nanomaterials: Exploring Recent Advances in Therapeutics, Diagnostics, and Tissue Engineering. Adv. Pharm. Bull. 2025, 15, 232–247. [Google Scholar] [CrossRef]
- Topol, E.J. High-performance medicine: The convergence of human and artificial intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef]
- Vamathevan, J.; Clark, D.; Czodrowski, P.; Dunham, I.; Ferran, E.; Lee, G.; Li, B.; Madabhushi, A.; Shah, P.; Spitzer, M. Applications of machine learning in drug discovery and development. Nat. Rev. Drug Discov. 2019, 18, 463–477. [Google Scholar] [CrossRef]
- Gmeiner, W.H.; Ghosh, S. Nanotechnology for cancer treatment. Nanotechnol. Rev. 2014, 3, 111–122. [Google Scholar] [CrossRef]
- Cabral, H.; Kataoka, K. Progress of drug-loaded polymeric micelles into clinical studies. J. Control. Release 2014, 190, 465–476. [Google Scholar] [CrossRef]
- Chou, W.C.; Canchola, A.; Zhang, F.; Lin, Z. Machine Learning and Artificial Intelligence in Nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2025, 17, e70027. [Google Scholar] [CrossRef]
- Alqarni, S.; Huwaimel, B. Predicting PLGA nanoparticle size and zeta potential in synthesis for application of drug delivery via machine learning analysis. Sci. Rep. 2025, 15, 20765. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Tang, Y.; Wang, C.; Li, Y.; Zhang, J.; Luo, Y.; Xu, Z.; Wu, F.; Wang, S. Interpretable XGBoost-SHAP model predicts nanoparticles delivery efficiency based on tumor genomic mutations and nanoparticle properties. ACS Appl. Bio Mater. 2023, 6, 4326–4335. [Google Scholar] [CrossRef] [PubMed]
- Almansour, K.; Alqahtani, A.S. Utilization of machine learning approach for production of optimized PLGA nanoparticles for drug delivery applications. Sci. Rep. 2025, 15, 8840. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.E.; Ghandehari, H.; Facelli, J.C. A review of the applications of data mining and machine learning for the prediction of biomedical properties of nanoparticles. Comput. Methods Programs Biomed. 2016, 132, 93–103. [Google Scholar] [CrossRef]
- Bhosekar, S.; Singh, P.; Garg, D.; Ravi, V.; Diwakar, M. A Review of Deep Learning-based Multi-modal Medical Image Fusion. Open Bioinform. J. 2025, 18, e1875036237069. [Google Scholar] [CrossRef]
- Mishra, D.; Chaturvedi, B.; Soni, V.; Valecha, D.; Goel, M.; Ansari, J.R. Impact of bridging the gap between Artificial Intelligence and nanomedicine in healthcare. Next Nanotechnol. 2025, 8, 100203. [Google Scholar] [CrossRef]
- Chou, W.-C.; Chen, Q.; Yuan, L.; Cheng, Y.-H.; He, C.; Monteiro-Riviere, N.A.; Riviere, J.E.; Lin, Z. An artificial intelligence-assisted physiologically-based pharmacokinetic model to predict nanoparticle delivery to tumors in mice. J. Control. Release 2023, 361, 53–63. [Google Scholar] [CrossRef]
- Bals, J.; Epple, M. Deep learning for automated size and shape analysis of nanoparticles in scanning electron microscopy. RSC Adv. 2023, 13, 2795–2802. [Google Scholar] [CrossRef]
- Chandrasekar, V.; Panicker, A.J.; Singh, A.V.; Bhadra, J.; Sadasivuni, K.K.; Aboumarzouk, O.M.; Al Ansari, A.; Dakua, S.P. Artificial Intelligence Enabled Biomineralization for Eco-Friendly Nanomaterial Synthesis: Charting Future Trends. Nano Select. 2025, 6, e202400118. [Google Scholar] [CrossRef]
- Leong, Y.X.; Tan, E.X.; Leong, S.X.; Lin Koh, C.S.; Thanh Nguyen, L.B.; Ting Chen, J.R.; Xia, K.; Ling, X.Y. Where nanosensors meet machine learning: Prospects and challenges in detecting Disease X. ACS Nano 2022, 16, 13279–13293. [Google Scholar] [CrossRef] [PubMed]
- Elton, D.C.; Boukouvalas, Z.; Fuge, M.D.; Chung, P.W. Deep learning for molecular design—A review of the state of the art. Mol. Syst. Des. Eng. 2019, 4, 828–849. [Google Scholar] [CrossRef]
- Gómez-Bombarelli, R.; Wei, J.N.; Duvenaud, D.; Hernández-Lobato, J.M.; Sánchez-Lengeling, B.; Sheberla, D.; Aguilera-Iparraguirre, J.; Hirzel, T.D.; Adams, R.P.; Aspuru-Guzik, A. Automatic chemical design using a data-driven continuous representation of molecules. ACS Cent. Sci. 2018, 4, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Schleder, G.R.; Padilha, A.C.; Acosta, C.M.; Costa, M.; Fazzio, A. From DFT to machine learning: Recent approaches to materials science—A review. J. Phys. Mater. 2019, 2, 032001. [Google Scholar] [CrossRef]
- Zhou, Z.; Kearnes, S.; Li, L.; Zare, R.N.; Riley, P. Optimization of Molecules via Deep Reinforcement Learning. Sci. Rep. 2019, 9, 10752, Correction in Sci. Rep. 2020, 10, 10478. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Baek, M.; DiMaio, F.; Anishchenko, I.; Dauparas, J.; Ovchinnikov, S.; Lee, G.R.; Wang, J.; Cong, Q.; Kinch, L.N.; Schaeffer, R.D.; et al. Accurate prediction of protein structures and interactions using a three-track neural network. Science 2021, 373, 871–876. [Google Scholar] [CrossRef]
- Lutz, I.D.; Wang, S.; Norn, C.; Courbet, A.; Borst, A.J.; Zhao, Y.T.; Dosey, A.; Cao, L.; Xu, J.; Leaf, E.M.; et al. Top-down design of protein architectures with reinforcement learning. Science 2023, 380, 266–273. [Google Scholar] [CrossRef]
- Sanchez-Lengeling, B.; Aspuru-Guzik, A. Inverse molecular design using machine learning: Generative models for matter engineering. Science 2018, 361, 360–365. [Google Scholar] [CrossRef]
- Wang, Y.; Song, H.; Teng, Y.; Huang, G.; Qian, J.; Wang, H.; Dong, S.; Ha, J.; Ma, Y.; Chang, M.; et al. A Generative Artificial Intelligence Copilot for Biomedical Nanoengineering. ACS Nano 2025, 19, 19394–19407. [Google Scholar] [CrossRef]
- Singh, A.V.; Ansari, M.H.D.; Rosenkranz, D.; Maharjan, R.S.; Kriegel, F.L.; Gandhi, K.; Kanase, A.; Singh, R.; Laux, P.; Luch, A. Artificial intelligence and machine learning in computational nanotoxicology: Unlocking and empowering nanomedicine. Adv. Healthc. Mater. 2020, 9, 1901862. [Google Scholar] [CrossRef]
- Hickman, R.J.; Bannigan, P.; Bao, Z.; Aspuru-Guzik, A.; Allen, C. Self-driving laboratories: A paradigm shift in nanomedicine development. Matter 2023, 6, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Lodhi, A.; Jindal, A.; Tomar, D.; Kumar, A.; Sharma, K. Artificial Intelligence in Nanocarrier Design and Drug Delivery via Nanorobotics-Based Personalised Medicine for Cancer Diagnostics and Therapy. In Generative Intelligence in Healthcare; CRC Press: Boca Raton, FL, USA, 2025; pp. 91–124. [Google Scholar]
- Villa Nova, M.; Lin, T.P.; Shanehsazzadeh, S.; Jain, K.; Ng, S.C.Y.; Wacker, R.; Chichakly, K.; Wacker, M.G. Nanomedicine ex machina: Between model-informed development and artificial intelligence. Front. Digit. Health 2022, 4, 799341. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Agrahari, V. Emerging Trends and Translational Challenges in Drug and Vaccine Delivery. Pharmaceutics 2024, 16, 98. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Nehal, N.; Gull, A.; Parveen, R.; Khan, S.; Khan, S.; Ali, J. Explicating the transformative role of artificial intelligence in designing targeted nanomedicine. Expert. Opin. Drug Deliv. 2025, 22, 971–991. [Google Scholar] [CrossRef]
- Wei, Z.; Zhuo, S.; Zhang, Y.; Wu, L.; Gao, X.; He, S.; Bo, X.; Zhou, W. Machine learning reshapes the paradigm of nanomedicine research. Acta Pharm. Sin. B 2025, in press. [Google Scholar] [CrossRef]
- Faria, M.; Björnmalm, M.; Thurecht, K.J.; Kent, S.J.; Parton, R.G.; Kavallaris, M.; Johnston, A.P.R.; Gooding, J.J.; Corrie, S.R.; Boyd, B.J.; et al. Minimum information reporting in bio–nano experimental literature. Nat. Nanotechnol. 2018, 13, 777–785. [Google Scholar] [CrossRef]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Thomas, D.G.; Klaessig, F.; Harper, S.L.; Fritts, M.; Hoover, M.D.; Gaheen, S.; Stokes, T.H.; Reznik-Zellen, R.; Freund, E.T.; Klemm, J.D.; et al. Informatics and standards for nanomedicine technology. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2011, 3, 511–532. [Google Scholar] [CrossRef]
- Ostraat, M.L.; Mills, K.C.; Guzan, K.A.; Murry, D. The Nanomaterial Registry: Facilitating the sharing and analysis of data in the diverse nanomaterial community. Int. J. Nanomed. 2013, 8 (Suppl. S1), 7–13. [Google Scholar] [CrossRef]
- Ke, W.; He, R.; Jensen, M.A.; Dobrovolskaia, M.A. Transforming Cancer Nanotechnology Data Analysis and User Experience. Part I: Current Challenges and Solutions Provided by caNanoLab. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2025, 17, e70030. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 update. Nucleic Acids Res. 2022, 51, D1373–D1380. [Google Scholar] [CrossRef]
- Gernand, J.M.; Casman, E.A. Machine Learning for Nanomaterial Toxicity Risk Assessment. IEEE Intell. Syst. 2014, 29, 84–88. [Google Scholar] [CrossRef]
- Ahmad, F.; Mahmood, A.; Muhmood, T. Machine learning-integrated omics for the risk and safety assessment of nanomaterials. Biomater. Sci. 2021, 9, 1598–1608. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Xie, J.; Yang, F.; Luo, Y.; Du, J.; Xiang, H. Advances and prospects of precision nanomedicine in personalized tumor theranostics. Front. Cell Dev. Biol. 2024, 12, 1514399. [Google Scholar] [CrossRef] [PubMed]
- Fadeel, B.; Farcal, L.; Hardy, B.; Vázquez-Campos, S.; Hristozov, D.; Marcomini, A.; Lynch, I.; Valsami-Jones, E.; Alenius, H.; Savolainen, K. Advanced tools for the safety assessment of nanomaterials. Nat. Nanotechnol. 2018, 13, 537–543. [Google Scholar] [CrossRef]
- Robertson, A.S.; Reisin Miller, A.; Dolz, F. Supporting a data-driven approach to regulatory intelligence. Nat. Rev. Drug Discov. 2021, 20, 161–162. [Google Scholar] [CrossRef]
- Holzinger, A.; Langs, G.; Denk, H.; Zatloukal, K.; Müller, H. Causability and explainability of artificial intelligence in medicine. Wiley Interdiscip. Rev. Data Min. Knowl. Discov. 2019, 9, e1312. [Google Scholar] [CrossRef]
- Jahandoost, A.; Dashti, R.; Houshmand, M.; Hosseini, S.A. Utilizing machine learning and molecular dynamics for enhanced drug delivery in nanoparticle systems. Sci. Rep. 2024, 14, 26677. [Google Scholar] [CrossRef]
- Lee, H. Molecular Modeling of Protein Corona Formation and Its Interactions with Nanoparticles and Cell Membranes for Nanomedicine Applications. Pharmaceutics 2021, 13, 637. [Google Scholar] [CrossRef]
- Bunker, A.; Róg, T. Mechanistic Understanding from Molecular Dynamics Simulation in Pharmaceutical Research 1: Drug Delivery. Front. Mol. Biosci. 2020, 7, 604770. [Google Scholar] [CrossRef]
- Peng, S.; Wang, W.; Zhang, R.; Wu, C.; Pan, X.; Huang, Z. Nano-Formulations for Pulmonary Delivery: Past, Present, and Future Perspectives. Pharmaceutics 2024, 16, 161. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, Z.; Wang, Z.; Lee, D.; Ma, Y.; Wilhelm, S.; Wang, H.; Kim, B.Y.S.; Jiang, W. Multi-omics approaches to decipher the interactions of nanoparticles and biological systems. Nat. Rev. Bioeng. 2025, 3, 333–348. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles─From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef] [PubMed]
- Cheimarios, N.; Harrison, S.; Jensen, A.C.Ø.; Karatzas, P.; Tsoumanis, A.; Doganis, P.; Tsiros, P.; Winkler, D.A.; Lofts, S.; Jensen, K.A. NanoSolveIT integration of tools for assessment of human and environmental exposure to nanomaterials. In Handbook of Functionalized Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2021; pp. 81–120. [Google Scholar]
- Ahmad, A.; Imran, M.; Sharma, N. Precision Nanotoxicology in Drug Development: Current Trends and Challenges in Safety and Toxicity Implications of Customized Multifunctional Nanocarriers for Drug-Delivery Applications. Pharmaceutics 2022, 14, 2463. [Google Scholar] [CrossRef]
- Lee, D.; Huntoon, K.; Lux, J.; Kim, B.Y.S.; Jiang, W. Engineering nanomaterial physical characteristics for cancer immunotherapy. Nat. Rev. Bioeng. 2023, 1, 499–517. [Google Scholar] [CrossRef]
- Kim, B.Y.; Rutka, J.T.; Chan, W.C. Nanomedicine. N. Engl. J. Med. 2010, 363, 2434–2443. [Google Scholar] [CrossRef]
- Rodríguez-Gómez, F.D.; Monferrer, D.; Penon, O.; Rivera-Gil, P. Regulatory pathways and guidelines for nanotechnology-enabled health products: A comparative review of EU and US frameworks. Front. Med. 2025, 12, 1544393. [Google Scholar] [CrossRef]
- Shan, X.; Cai, Y.; Zhu, B.; Zhou, L.; Sun, X.; Xu, X.; Yin, Q.; Wang, D.; Li, Y. Rational strategies for improving the efficiency of design and discovery of nanomedicines. Nat. Commun. 2024, 15, 9990. [Google Scholar] [CrossRef]
- Char, D.S.; Shah, N.H.; Magnus, D. Implementing Machine Learning in Health Care—Addressing Ethical Challenges. N. Engl. J. Med. 2018, 378, 981–983. [Google Scholar] [CrossRef]
- Bhange, M.; Telange, D. Convergence of nanotechnology and artificial intelligence in the fight against liver cancer: A comprehensive review. Discov. Oncol. 2025, 16, 77. [Google Scholar] [CrossRef]
- Lendvai, G.F.; Gosztonyi, G. Algorithmic Bias as a Core Legal Dilemma in the Age of Artificial Intelligence: Conceptual Basis and the Current State of Regulation. Laws 2025, 14, 41. [Google Scholar] [CrossRef]
- Mirakhori, F.; Niazi, S.K. Harnessing the AI/ML in Drug and Biological Products Discovery and Development: The Regulatory Perspective. Pharmaceuticals 2025, 18, 47. [Google Scholar] [CrossRef]
- Oualikene-Gonin, W.; Jaulent, M.C.; Thierry, J.P.; Oliveira-Martins, S.; Belgodère, L.; Maison, P.; Ankri, J. Artificial intelligence integration in the drug lifecycle and in regulatory science: Policy implications, challenges and opportunities. Front. Pharmacol. 2024, 15, 1437167. [Google Scholar] [CrossRef]
- Correia Pinheiro, L.; Arlett, P.; Roes, K.; Musuamba Tshinanu, F.; Westman, G.; Frias, Z.; Hamann, H.; Berenguer Jornet, J.; Khan, I.; Larsen, J.; et al. Artificial Intelligence in European Medicines Regulation: From Vision to Action. Harnessing the Capabilities of Artificial Intelligence for the Benefit of Public and Animal Health. Clin. Pharmacol. Ther. 2025, 117, 335–336. [Google Scholar] [CrossRef]


| Nanomaterial Platform | Key Features | Therapeutic Advantages | Approved Products/Status |
|---|---|---|---|
| Liposome | Phospholipid bilayer vesicles; PEGylation to prolong circulation; size ~50–200 nm | Encapsulation of both hydrophilic & hydrophobic drugs; immune evasion; reduced systemic toxicity | Approved products: Doxil® (doxorubicin), LipoDox®, AmBisome® (amphotericin B) Myocet (doxorubicin-citrate) ®, Vyxeos(daunorubicin + Cytarabine)® [7,26,27,28] |
| Polymeric NP/Micelle | Biodegradable polymers (PLGA, PCL); tunable size & degradation rate; surface functionalization possible | Sustained/controlled drug release; delivery of siRNA, peptides; improved bioavailability of hydrophobic drugs | Approved/marketed: Genexol-PM (paclitaxel micelle) [29] Clinical trials: NK105 and SP1049C |
| Dendrimer | Highly branched, monodisperse macromolecules; multivalent surface groups for drug conjugation | High drug loading; multivalent targeting; imaging + therapy (theranostics) | Marketed: Vivagel® (SPL7013) [30] |
| Metallic NP | Gold, silver, iron oxide nanoparticles; unique optical/magnetic properties; possible surface modification | Theranostics; stimuli-responsive drug release (light, heat, magnetic field); antimicrobial effects | Approved: SPION-based MRI contrast agents (Feridex®, Ferumoxytol®) [31,32] Clinical trials: Photothermal therapy [33,34] |
| Silica NP (Mesoporous) | High surface area; tunable pore size (2–10 nm); easy surface functionalization | High drug loading; co-delivery of multiple agents; potential for imaging-guided therapy | Preclinical studies: Biosilica-enveloped ferritin cages [35,36] |
| Carbon-based NP | Diverse structural; high surface area; unique bonding properties of carbon atoms | High physical and chemical stability; tunable surface functionalization; suitable for drug/gene delivery, bioimaging, and photothermal & photodynamic therapies | Preclinical and translational studies: Amino acid-mimicking carbon dots for siRNA delivery [37]; nanodiamond-based treatment for apical lesions showing clinical potential [38] |
| Research Stage | AI Contribution | Representative Methods | Platform/Database | Outcome |
|---|---|---|---|---|
| Design & Screening | Predict nanocarrier physicochemical properties | ML, ensemble models | NanoCommons | Efficient candidate ranking |
| Mechanistic Understanding | Reveal nonlinear synthesis–performance links | DL, multimodal integration | caNanoLab | Mechanistic insight, hypothesis generation |
| Optimization & Discovery | Explore novel chemical space | GAN, VAE, RL | Nanomaterial Registry | Novel design proposals with improved profiles |
| Clinical Translation & Regulation | Improve reproducibility and transparency | XAI, data governance | NanoSolveIT/FDA Predictive Toxicology Roadmap | Higher regulatory confidence, cost/time reduction |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Kim, D.H.; Pack, S.P. Nanomaterials in Drug Delivery: Leveraging Artificial Intelligence and Big Data for Predictive Design. Int. J. Mol. Sci. 2025, 26, 11121. https://doi.org/10.3390/ijms262211121
Han Y, Kim DH, Pack SP. Nanomaterials in Drug Delivery: Leveraging Artificial Intelligence and Big Data for Predictive Design. International Journal of Molecular Sciences. 2025; 26(22):11121. https://doi.org/10.3390/ijms262211121
Chicago/Turabian StyleHan, Youngji, Dong Hyun Kim, and Seung Pil Pack. 2025. "Nanomaterials in Drug Delivery: Leveraging Artificial Intelligence and Big Data for Predictive Design" International Journal of Molecular Sciences 26, no. 22: 11121. https://doi.org/10.3390/ijms262211121
APA StyleHan, Y., Kim, D. H., & Pack, S. P. (2025). Nanomaterials in Drug Delivery: Leveraging Artificial Intelligence and Big Data for Predictive Design. International Journal of Molecular Sciences, 26(22), 11121. https://doi.org/10.3390/ijms262211121







