Response of Plants to Touch Stress at Morphological, Physiological and Molecular Levels
Abstract
1. Introduction
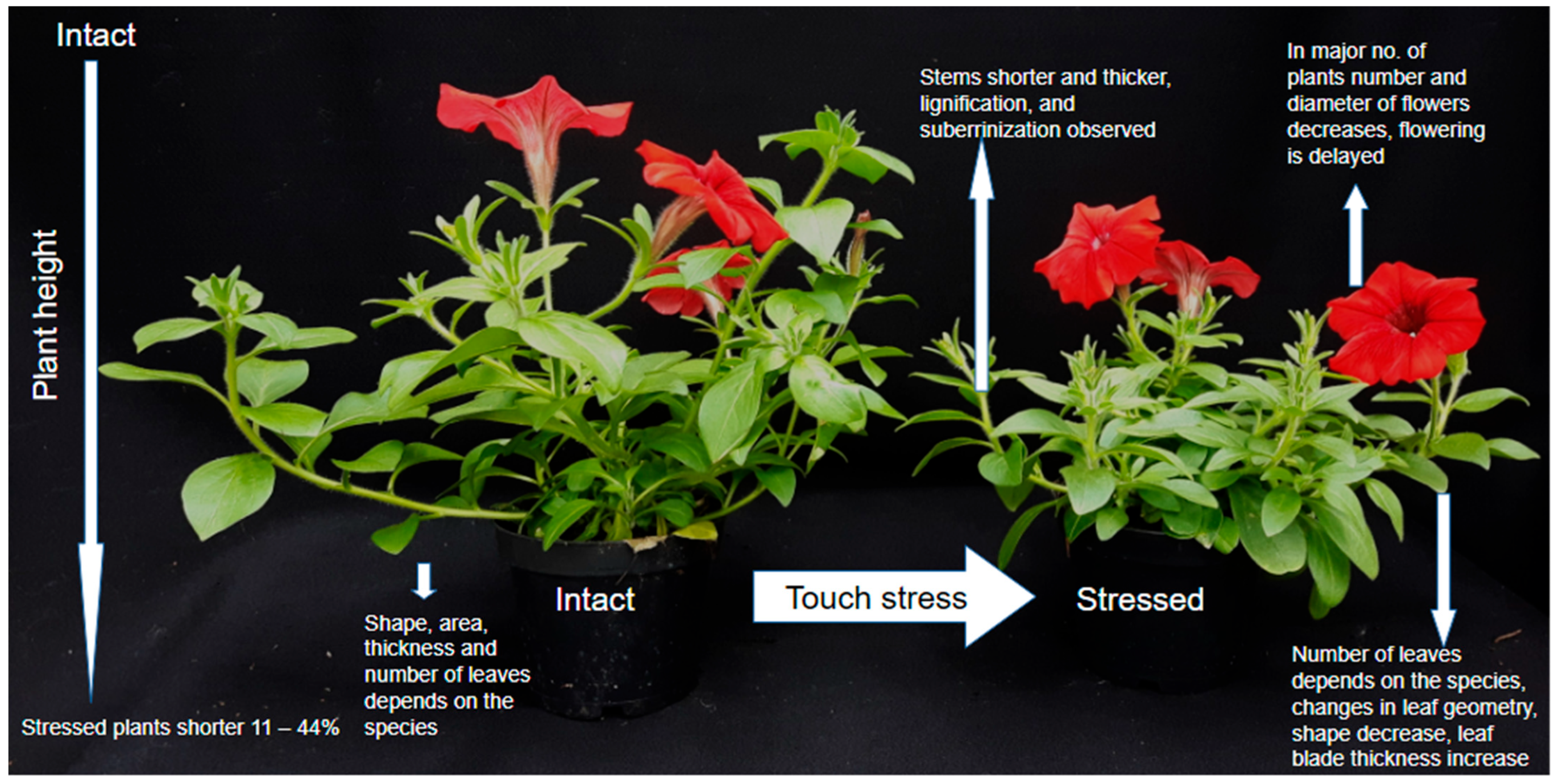
2. Plant Response on the Morphological and Anatomical Level
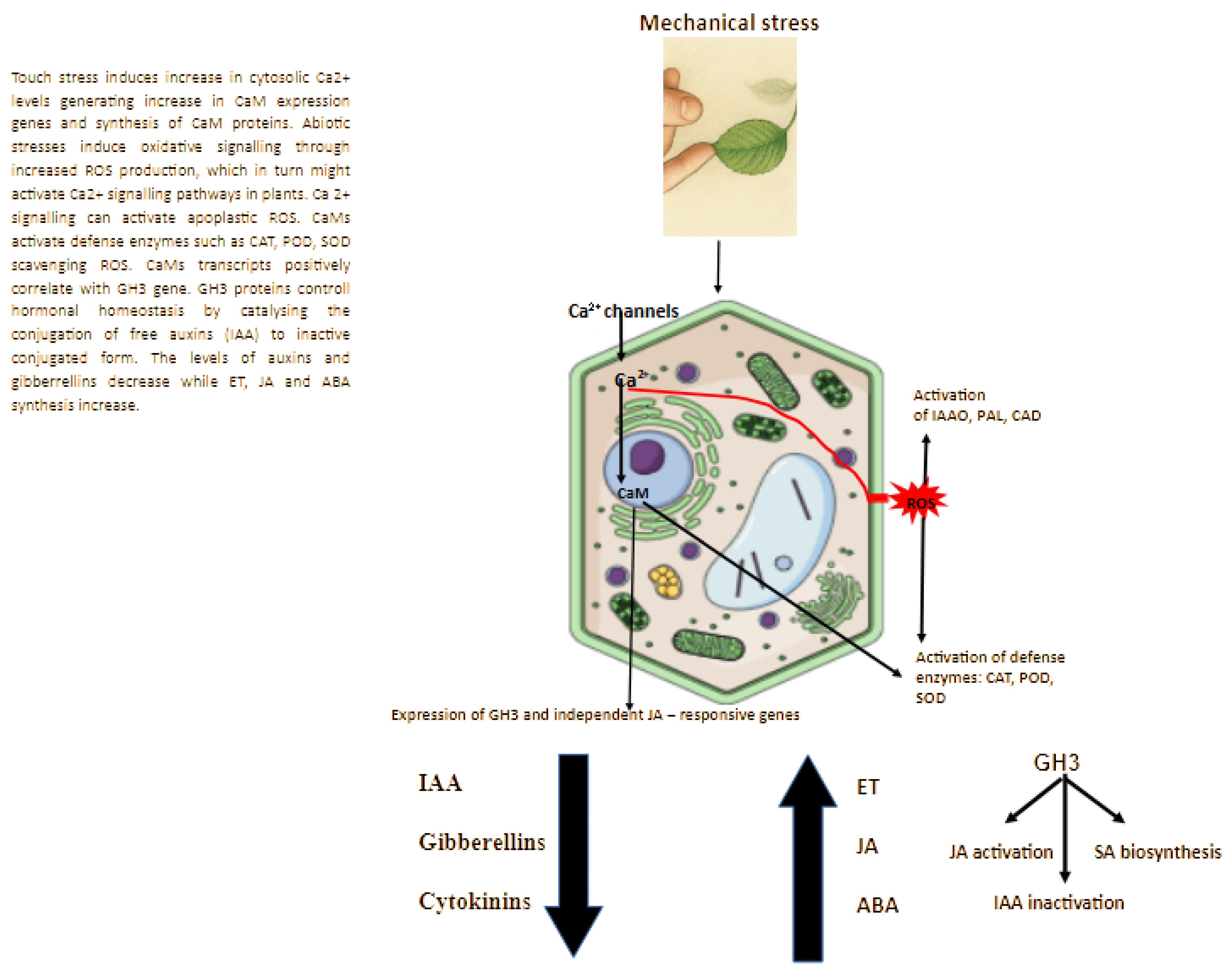
3. Plant Response at the Hormonal and Enzymatic Level
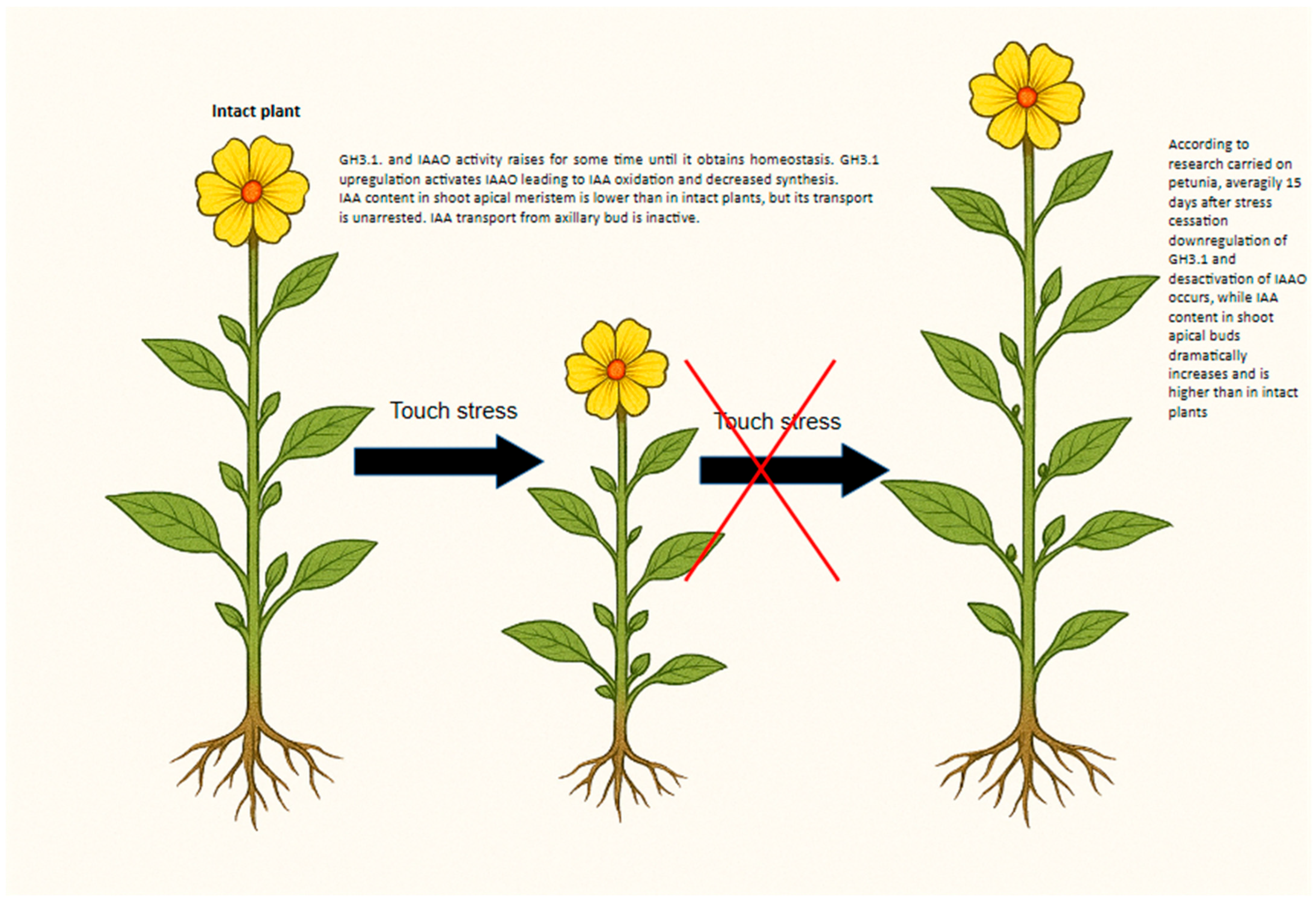
3.1. Phytohormones
3.2. Enzymes Induced to Thigmomorphogenesis
4. Plant Response at the Molecular Level—TouCH Gene Expression
5. Thigmomorphogenesis Mechanism in Monocots
5.1. Stem Reaction to Mechanical Stress in Monocots
5.2. Differences in Secondary Cell Wall Structure Between Eudicots and Monocotyledons
5.3. Differences in Specific Gene Expression Between Eudicots and Monocotyledons
6. Conclusions
Funding
Conflicts of Interest
References
- Jaffe, M.J. Thigmomorphogenesis: The response of plant growth and development to mechanical stimulation: With special reference to Bryonia dioica. Planta 1973, 114, 143–157. [Google Scholar] [CrossRef]
- Hindhaugh, R.; Bosch, M.; Donnison, I.S. Mechanical stimulation in wheat triggers age- and dose-dependent alterations in growth, development and grain characteristics. Ann. Bot. 2021, 128, 589–603. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jaffe, M.J. Thigmomorphogenesis: A detailed characterization of the response of beans (Phaseolus vulgaris L.) to mechanical stimulation. Z. Pflanzenphysiol. 1976, 77, 437–453. [Google Scholar] [CrossRef]
- Telewski, F.W. Mechanosensing and plant growth regulators elicited during the thigmomorphogenetic response. Front. For. Glob. Chang. 2021, 3, 574096. [Google Scholar] [CrossRef]
- Ahmad, P.; Bhardwaj, R.; Tuteja, N. Plant Signaling Under Abiotic Stress Environment. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Ahmad, P., Prasad, M., Eds.; Springer: New York, NY, USA, 2012. [Google Scholar] [CrossRef]
- Miransari, M. Role of Phytohormone Signaling During Stress. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Ahmad, P., Prasad, M., Eds.; Springer: New York, NY, USA, 2012. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Li, R.; Ge, Y.; Li, Y.; Li, R. Plants’ Response to Abiotic Stress: Mechanisms and Strategies. Int. J. Mol. Sci. 2023, 24, 10915. [Google Scholar] [CrossRef]
- Kohli, A.; Sreenivasulu, N.; Lakshmanan, P.; Kumar, P.P. The phytohormone crosstalk paradigm takes center stage in understanding how plants respond to abiotic stresses. Plant Cell Rep. 2013, 32, 945–957. [Google Scholar] [CrossRef]
- Chang, Y.; Zhu, C.; Jiang, J.; Zhang, H.; Zhu, J.; Duan, C. Epigenetic regulation in plant abiotic stress responses. J. Integr. Plant Biol. 2020, 62, 563–580. [Google Scholar] [CrossRef]
- Chehab, E.W.; Eich, E.; Braam, J. Thigmomorphogenesis: A complex plant response to mechano-stimulation. J. Exp. Bot. 2009, 60, 43–56. [Google Scholar] [CrossRef]
- Ghosh, R.; Barbacci, A.; Leblanc-Fournier, N. Mechanostimulation: A promising alternative for sustainable agriculture practices. J. Exp. Bot. 2021, 72, 2877–2888. [Google Scholar] [CrossRef]
- Mishra, R.C.; Grover, A. Intergenic sequence between Arabidopsis caseinolytic protease B-cytoplasmic/heat shock protein100 and choline kinase genes functions as a heat-inducible bidirectional promoter. Plant Physiol. 2014, 166, 1646–1658. [Google Scholar] [CrossRef]
- Jaffe, M.J.; Forbes, S. Thigmomorphogenesis: The effect of mechanical perturbation on plants. Plant Growth Regul. 1993, 12, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Braam, J. In touch: Plant responses to mechanical stimuli. New Phytol. 2005, 165, 373–389. [Google Scholar] [CrossRef]
- Telewski, F.W.; Jaffe, M.J. Thigmomorphogenesis: The role of ethylene in the response of Pinus taeda and Abies fraseri to mechanical perturbation. Physiol. Plant. 1986, 66, 227–233. [Google Scholar] [CrossRef]
- Biddington, N.L. The effects of mechanically-induced stress in plants—A review. Plant Growth Regul. 1986, 4, 103–123. [Google Scholar] [CrossRef]
- Jaffe, M.J.; Leopold, A.C.; Staples, R.C. Thigmo responses in plants and fungi. Am. J. Bot. 2002, 89, 375–382. [Google Scholar] [CrossRef]
- Niklas, K.J. Effects of vibration on mechanical properties and biomass allocation pattern of Capsella bursa-pastoris (Cruciferae). Ann. Bot. 1998, 82, 147–156. [Google Scholar] [CrossRef]
- Anten, N.P.; Casado-Garcia, R.; Nagashima, H. Effects of mechanical stress and plant density on mechanical characteristics, growth, and lifetime reproduction of tobacco plants. Am. Nat. 2005, 166, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, A.; Yin, H.; Zhang, J. Influence of water stress on endogenous hormone contents and cell damage of maize seedlings. J. Integr. Plant Biol. 2008, 50, 427–434. [Google Scholar] [CrossRef]
- Smith, V.C.; Ennos, A.R. The effects of air flow and stem flexure on the mechanical and hydraulic properties of the stems of sunflowers Helianthus annuus L. J. Exp. Bot. 2003, 54, 845–849. [Google Scholar] [CrossRef]
- Telewski, F.W.; Aloni, R.; Sauter, J.J. Physiology of secondary tissues of populus. In Biology of Populus and Its Implications for Management and Conservation; NRC Research Press: Ottawa, ON, Canada, 1996; pp. 301–329. [Google Scholar]
- Badel, E.; Ewers, F.W.; Cochard, H.; Telewski, F.W. Acclimation of mechanical and hydraulic functions in trees: Impact of the thigmomorphogenetic process. Front. Plant Sci. 2015, 6, 266. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gardiner, B.; Berry, P.; Moulia, B. Wind impacts on plant growth, mechanics, and damage. Plant Sci. 2016, 245, 94–118. [Google Scholar] [CrossRef]
- Böhm, J.; Scherzer, S.; Krol, E.; Kreuzer, I.; von Meyer, K.; Lorey, C.; Mueller, T.D.; Shabala, L.; Monte, I.; Solano, R.; et al. The Venus Flytrap Dionaea muscipula Counts Prey-Induced Action Potentials to Induce Sodium Uptake. Curr. Biol. 2016, 26, 286–295. [Google Scholar] [CrossRef]
- Jędrzejuk, A.; Kuźma, N.; Nawrot, K.; Budzyński, R.; Orłowski, A. Mechanical stimulation affects growth dynamics, IAA content and activity of POD and IAA oxidase in Petunia × atkinsiana. Sci. Hortic. 2020, 274, 109661. [Google Scholar] [CrossRef]
- Johjima, T.; Latimer, J.G.; Wakita, H. Brushing influences transplant growth and subsequent yield of four cultivars of tomato and their hybrid lines. J. Am. Soc. Hortic. Sci. 1992, 117, 384–388. [Google Scholar] [CrossRef]
- Urbancsok, J.; Donev, E.N.; Sivan, P.; van Zalen, E.; Barbut, F.R.; Derba-Maceluch, M.; Šimura, J.; Yassin, Z.; Gandla, M.L.; Karady, M.; et al. Flexure wood formation via growth reprogramming in hybrid aspen involves jasmonates and polyamines and transcriptional changes resembling tension wood development. New Phytol. 2023, 240, 2312–2334. [Google Scholar] [CrossRef]
- Groover, A. Gravitropisms and reaction woods of forest trees—Evolution, functions and mechanisms. New Phytol. 2016, 211, 790–802. [Google Scholar] [CrossRef]
- Hamant, O. Widespread mechanosensing controls the structure behind the architecture in plants. Curr. Opin. Plant Biol. 2013, 16, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Latimer, J.G.; Johjima, T.; Harada, K. The effect of mechanical stress on transplant growth and subsequent yield of four cultivars of cucumber. Sci. Hortic. 1991, 47, 221–230. [Google Scholar] [CrossRef]
- Coutand, C.; Julien, J.L.; Moulia, B.; Mauget, J.C.; Guitard, D. Biomechanical study of the effect of a controlled bending on tomato stem elongation: Global mechanical analysis. J. Exp. Bot. 2000, 51, 1813–1824. [Google Scholar] [CrossRef] [PubMed]
- Autio, J.; Voipio, I.; Koivunen, T. Responses of Aster, Dusty Miller, and Petunia Seedlings to Daily Exposure to Mechanical Stress. HortScience 1994, 29, 1449–1452. [Google Scholar] [CrossRef]
- Jędrzejuk, A.; Kuźma, N.; Orłowski, A.; Budzyński, R.; Gehl, C.; Serek, M. Mechanical stimulation decreases auxin and gibberellic acid synthesis but does not affect auxin transport in axillary buds; it also stimulates peroxidase activity in Petunia × atkinsiana. Molecules 2023, 28, 2714. [Google Scholar] [CrossRef]
- Watt, N.T.; Taylor, D.R.; Gillott, A.; Thomas, D.A.; Perera, W.S.; Hooper, N.M. Reactive oxygen species-mediated beta-cleavage of the prion protein in the cellular response to oxidative stress. J. Biol. Chem. 2005, 280, 35914–35921. [Google Scholar] [CrossRef]
- Braam, J.; Davis, R.W. Rain, wind-, and touch-induced expression of calmodulin and calmodulin-related genes in Arabidopsis. Cell 1990, 60, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Börnke, F.; Rocksch, T. Thigmomorphogenesis—Control of plant growth by mechanical stimulation. Sci. Hortic. 2020, 234, 344–353. [Google Scholar] [CrossRef]
- Marler, T.E. Thigmomorphogenesis and biomechanical responses of shade-grown Serianthes nelsonii plants to stem flexure. Plant Signal. Behav. 2019, 14, 1601953. [Google Scholar] [CrossRef]
- Monshausen, G.B.; Messerli, M.A.; Gilroy, S. Imaging of the yellow cameleon 3.6 indicator reveals that elevations in cytosolic Ca2+ follow oscillating increases in growth in root hairs of Arabidopsis. Plant Physiol. 2008, 147, 1690–1698. [Google Scholar] [CrossRef] [PubMed]
- Sablowski, R. Coordination of plant cell growth and division: Collective control or mutual agreement? Curr. Opin. Plant Biol. 2016, 34, 54–60. [Google Scholar] [CrossRef]
- Nick, P. Microtubules, signalling and abiotic stress. Plant J. 2013, 75, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Zajączkowska, U.; Dmitruk, D.; Sekulska-Nalewajko, J.; Gocławski, J.; Dołkin-Lewko, A.; Łotocka, B. The impact of mechanical stress on anatomy, morphology, and gene expression in Urtica dioica L. Planta 2024, 260, 46. [Google Scholar] [CrossRef]
- Anten, N.P.; Alcalá-Herrera, R.; Schieving, F.; Onoda, Y. Wind and mechanical stimuli differentially affect leaf traits in Plantago major. New Phytol. 2010, 188, 554–564. [Google Scholar] [CrossRef]
- Cipollini, D.F., Jr. Wind-induced mechanical stimulation increases pest resistance in common bean. Oecologia 1997, 111, 84–90. [Google Scholar] [CrossRef]
- Wada, K.C.; Takeno, K. Stress-induced flowering. Plant Signal. Behav. 2010, 5, 944–947. [Google Scholar] [CrossRef]
- Blanvillain, R.; Wei, S.; Wei, P.; Kim, J.H.; Ow, D.W. Stress tolerance to stress escape in plants: Role of the OXS2 zinc-finger transcription factor family. EMBO J. 2011, 30, 3812–3822. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C. Hormonal modulation of plant immunity. Annu. Rev. Cell. Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [PubMed]
- Riboni, M.; Robustelli Test, A.; Galbiati, M.; Tonelli, C.; Conti, L. Environmental stress and flowering time: The photoperiodic connection. Plant Signal. Behav. 2014, 9, e29036. [Google Scholar] [CrossRef] [PubMed]
- Rasool, S.; Latef, A.A.H.A.; Ahmad, P. Chickpea: Role and responses under abiotic and biotic stress. In Legumes Under Environmental Stress: Yield, Improvement and Adaptations; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 67–79. [Google Scholar]
- Kazan, K.; Lyons, R. The link between flowering time and stress tolerance. J. Exp. Bot. 2016, 67, 47–60. [Google Scholar] [CrossRef]
- Takeno, K. Stress-induced flowering: The third category of flowering response. J. Exp. Bot. 2016, 67, 4925–4934. [Google Scholar] [CrossRef]
- Morel, P.; Crespel, L.; Galopin, G.; Moulia, B. Effect of mechanical stimulation on the growth and branching of garden rose. Sci. Hortic. 2012, 135, 59–64. [Google Scholar] [CrossRef]
- Graham, T.; Wheeler, R. Mechanical stimulation modifies canopy architecture and improves volume utilization efficiency in bell pepper: Implications for bioregenerative life-support and vertical farming. Open Agric. 2017, 2, 42–51. [Google Scholar] [CrossRef]
- Sparke, M.A.; Wünsche, J.N. Mechanosensing of plants. Hortic. Rev. 2020, 47, 43–83. [Google Scholar]
- Gartner, B.L. Root biomechanics and whole-plant allocation patterns: Responses of tomato plants to stem flexure. J. Exp. Bot. 1994, 45, 1647–1654. [Google Scholar] [CrossRef]
- Darwin, C. The Effects of Cross and Self Fertilisation in the Vegetable Kingdom; Appleton, D., Ed.; John Murray: London, UK, 1877. [Google Scholar]
- Ennos, A.R. The scaling of root anchorage. J. Theor. Biol. 1993, 161, 61–75. [Google Scholar] [CrossRef]
- Ennos, A.R.; Crook, M.J.; Grimshaw, C. The anchorage mechanics of maize, Zea mays. J. Exp. Bot. 1993, 44, 147–153. [Google Scholar] [CrossRef]
- Reubens, B.; Pannemans, B.; Danjon, F.; De Proft, M.; De Baets, S.; De Baerdemaeker, J.; Poesen, J.; Muys, B. The effect of mechanical stimulation on root and shoot development of young containerised Quercus robur and Robinia pseudoacacia trees. Trees-Struct. Funct. 2009, 23, 1213–1228. [Google Scholar] [CrossRef]
- Goodman, A.M.; Ennos, A.R. Responses of the root systems of sunflower and maize to unidirectional stem flexure. Ann. Bot. 1998, 82, 347–357. [Google Scholar] [CrossRef]
- Richter, G.L.; Monshausen, G.B.; Krol, A.; Gilroy, S. Mechanical stimuli modulate lateral root organogenesis. Plant Physiol. 2009, 151, 1855–1866. [Google Scholar] [CrossRef] [PubMed]
- Coutand, C. Mechanosensing and thigmomorphogenesis, a physiological and biomechanical point of view. Plant Sci. 2010, 179, 168–182. [Google Scholar] [CrossRef]
- Jaffe, M.J.; Wakefield, A.H.; Telewski, F.; Gulley, E.; Biro, R. Computer-assisted image analysis of plant growth, thigmomorphogenesis and gravitropism. Plant Physiol. 1985, 77, 722–730. [Google Scholar] [CrossRef]
- Peacock, K.; Berg, A.R. Effect of mechanical stress on sunflower (Helianthus annuus L.) Hypocotyl growth. Ann. Bot. 1994, 74, 661–666. [Google Scholar] [CrossRef]
- Young, I.M.; Montagu, K.; Conroy, J.; Bengough, A.G. Mechanical impedance of root growth directly reduces leaf elongation rates of cereals. New Phytol. 1997, 135, 613–619. [Google Scholar] [CrossRef]
- Garner, L.C.; BjörKman, T. Mechanical Conditioning for Controlling Excessive Elongation in Tomato Transplants: Sensitivity to Dose, Frequency, and Timing of Brushing. J. Am. Soc. Hortic. Sci. 1996, 121, 894–900. [Google Scholar] [CrossRef]
- Garner, L.C.; BjörKman, T. Using Impedance for Mechanical Conditioning of Tomato Transplants to Control Excessive Stem Elongation. HortScience 1997, 32, 227–229. [Google Scholar] [CrossRef]
- Garner, L.C.; Langton, A.; BjörKman, T. Commercial Adaptations of Mechanical Stimulation for the Control of Transplant Growth. Acta Hortic. 1996, 435, 219–230. [Google Scholar] [CrossRef]
- Beyl, C.A.; Mitchell, C.A. Alteration of Growth, Exudation Rate, and Endogenous Hormone Profiles in Mechanically Dwarfed Sunflower1. J. Am. Soc. Hortic. Sci. 1983, 108, 257–262. [Google Scholar] [CrossRef]
- Ley-Ngardigal, B.; Roman, H.; Brouard, N.; Huché-Thélier, L.; Guérin, V.; Leduc, N. Recurrent symmetrical bendings cause dwarfing in Hydrangea through spatial molecular regulation of xylem cell walls. Front. Plant Sci. 2024, 14, 1268272. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, P.; Liu, W.C.; Han, C.; Wang, S.; Bai, M.Y.; Song, C.P. Reactive oxygen species: Multidimensional regulators of plant adaptation to abiotic stress and development. J. Integr. Plant Biol. 2024, 66, 330–367. [Google Scholar] [CrossRef]
- Hartmann, F.P.; Tinturier, E.; Julien, J.L.; Leblanc-Fournier, N. Between Stress and Response: Function and Localization of Mechanosensitive Ca2+ Channels in Herbaceous and Perennial Plants. Int. J. Mol. Sci. 2021, 22, 11043. [Google Scholar] [CrossRef]
- Wang, L.; Ma, C.; Wang, S.; Yang, F.; Sun, Y.; Tang, J.; Luo, J.; Wu, J. Ethylene and jasmonate signaling converge on gibberellin catabolism during thigmomorphogenesis in Arabidopsis. Plant Physiol. 2024, 194, 758–773. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.A. Influence of mechanical stress on auxin-stimulated growth of excised pea stem sections. Physiol. Plant. 1977, 41, 129–134. [Google Scholar] [CrossRef]
- Erner, Y.; Jaffe, M.J. Thigmomorphogenesis: The involvement of auxin and abscisic acid in growth retardation due to mechanical perturbation. Plant Cell Physiol. 1982, 23, 935–941. [Google Scholar] [CrossRef]
- Hofinger, M.; Chapelle, B.; Boyer, N.; Gaspar, T. GCMS identification and titration of IAA in mechanically perturbed Bryonia dioica. Plant Physiol. 1979, 63, 52. [Google Scholar]
- Saidi, Y.; Peter, M.; Finka, A.; Cicekli, C.; Vigh, L.; Goloubinoff, P. Membrane lipid composition affects plant heat sensing and modulates Ca2+-dependent heat shock response. Plant Signal. Behav. 2010, 5, 1530–1533. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Onoda, Y.A.; Niels, P. Challenges to understand plant responses to wind. Plant Signal. Behav. 2011, 6, 1057–1059. [Google Scholar] [CrossRef]
- Castro-Estrada, J.; Salazar, S.M.; Mariotti-Martínez, J.A.; Cabello, J.V.; Chan, R.L.; Welchen, E. Mechanical stress induces anatomical changes, tomato early flowering, and increased yield involving ethylene and auxins. J. Exp. Bot. 2025, eraf252. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.; Gladala-Kostarz, A.; Hindhaugh, R.; Doonan, J.H.; Bosch, M. Mechanical stimulation in plants: Molecular insights, morphological adaptations, and agricultural applications in monocots. BMC Biol. 2025, 23, 58. [Google Scholar] [CrossRef]
- Tretner, C.; Huth, U.; Hause, B. Mechanostimulation of Medicago truncatula leads to enhanced levels of jasmonic acid. J. Exp. Bot. 2008, 59, 2847–2856. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Ota, Y. A relationship between growth inhibition and abscisic acid content by mechanical stimulation in rice plant. Jpn. J. Crop Sci. 1980, 49, 615–616. [Google Scholar] [CrossRef]
- Giraudat, J. Abscisic acid signaling. Curr. Opin. Cell Biol. 1995, 7, 232–238. [Google Scholar] [CrossRef]
- Whitehead, F.H. Experimental studies of the effect of wind on plant growth and anatomy. II. Helianthus annuus. New Phytol. 1962, 61, 59–62. [Google Scholar] [CrossRef]
- Weyers, J.D.B.; Hillman, J.R. Sensitivity of commelina stomata to abscisic acid. Planta 1979, 146, 623–628. [Google Scholar] [CrossRef]
- Singh, A.; Roychoudhury, A. Abscisic acid in plants under abiotic stress: Crosstalk with major phytohormones. Plant Cell Rep. 2023, 42, 961–974. [Google Scholar] [CrossRef] [PubMed]
- Shu, K.; Liu, X.D.; Xie, Q.; He, Z.H. Two Faces of One Seed: Hormonal Regulation of Dormancy and Germination. Mol. Plant 2016, 9, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, J.; Poree, F.; Schaeufele, R.; Helmke, H.; Frackenpohl, J.; Lehr, S.; von Koskull-Döring, P.; Christmann, A.; Schnyder, H.; et al. Abscisic Acid Receptors and Coreceptors Modulate Plant Water Use Efficiency and Water Productivity. Plant Physiol. 2019, 180, 1066–1080. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shu, K.; Zhou, W.; Chen, F.; Luo, X.; Yang, W. Abscisic Acid and Gibberellins Antagonistically Mediate Plant Development and Abiotic Stress Responses. Front. Plant Sci. 2018, 9, 416. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Emenecker, R.J.; Strader, L.C. Auxin-Abscisic Acid Interactions in Plant Growth and Development. Biomolecules 2020, 10, 281. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, K.; Wang, S.; Wu, H.; Wang, H. Protein Levels of Several Arabidopsis Auxin Response Factors Are Regulated by Multiple Factors and ABA Promotes ARF6 Protein Ubiquitination. Int. J. Mol. Sci. 2020, 21, 9437. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, L.R.; Wang, Y.B.; He, S.B.; Hao, F.S. Mechanisms for abscisic acid inhibition of primary root growth. Plant Signal. Behav. 2018, 13, e1500069. [Google Scholar] [CrossRef]
- Sun, S.; Li, X.; Gao, S.; Nie, N.; Zhang, H.; Yang, Y.; He, S.; Liu, Q.; Zhai, H. A Novel WRKY Transcription Factor from, ItfWRKY70, confers drought tolerance in sweet potato. Int. J. Mol. Sci. 2022, 23, 686. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, X.; Wang, M.; Xie, L.; Wu, Z.; Yu, J.; Wang, Y.; Zhang, Z.; Jia, Y.; Liu, Q. The miR528-D3 module regulates plant height in rice by modulating the gibberellin and abscisic acid metabolisms. Rice 2022, 15, 27. [Google Scholar] [CrossRef]
- Wu, H.; He, Q.; He, B.; He, S.; Zeng, L.; Yang, L.; Zhang, H.; Wei, Z.; Hu, X.; Hu, J.; et al. Gibberellin signaling regulates lignin biosynthesis to modulate rice seed shattering. Plant Cell 2023, 35, 4383–4404. [Google Scholar] [CrossRef]
- Davière, J.M.; Achard, P. Gibberellin signaling in plants. Development 2013, 140, 1147–1151. [Google Scholar] [CrossRef]
- Zhao, Q. Lignification: Flexibility, biosynthesis and regulation. Trends Plant Sci. 2016, 21, 713–721. [Google Scholar] [CrossRef]
- Lange, M.J.P.; Lange, T. Touch-induced changes in Arabidopsis morphology dependent on gibberellin breakdown. Nat. Plants 2015, 1, 14025. [Google Scholar] [CrossRef]
- Suge, H. Dehydration and Drought Resistance in Phaseiolus vulgaris as Affected by Mechanical Stress; Reports of the Institute for Agricultural Research; Tohoku University: Sendai, Japan, 1980; Volume 31, pp. 1–10. [Google Scholar]
- Brenya, E.; Pervin, M.; Chen, Z.H.; Tissue, D.T.; Johnson, S.; Braam, J.; Cazzonelli, C.I. Mechanical stress acclimation in plants: Linking hormones and somatic memory to thigmomorphogenesis. Plant Cell Environ. 2022, 45, 989–1010. [Google Scholar] [CrossRef]
- Wang, M.; Fan, X.; Ding, F. Jasmonate: A Hormone of Primary Importance for Temperature Stress Response in Plants. Plants 2023, 12, 4080. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Milmanda, G.L. Touch me not! Jasmonic acid and ethylene converge on gibberellins breakdown to regulate touch-induced morphogenesis. Plant Physiol. 2024, 194, 601–603. [Google Scholar] [CrossRef] [PubMed]
- Kuźma, N.; Klimek-Chodacka, M.; Budzyński, R.; Barański, R.; Jędrzejuk, A. The response of Petunia × atkinsiana ‘Pegasus Special Burgundy Bicolor’ to mechanical stress encompassing morphological changes as well as physiological and molecular factors. Sci. Rep. 2025, 15, 1583. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, D.; Bangerth, F. Stress induced ethylene evolution and its possible relationship to auxin-transport, cytokinin levels, and flower bud induction in shoots of apple seedlings and bearing apple trees. Plant Growth Regul. 1998, 24, 127–134. [Google Scholar] [CrossRef]
- Morita, M.T.; Tasaka, M. Gravity sensing and signaling. Curr. Opin. Plant Biol. 2004, 7, 712–718. [Google Scholar] [CrossRef]
- Esmon, C.A.; Tinsley, A.G.; Ljung, K.; Sandberg, G.; Hearne, L.B.; Liscum, E. A gradient of auxin and auxin-dependent transcription precedes tropic growth responses. Proc. Natl. Acad. Sci. USA 2006, 103, 236–241. [Google Scholar] [CrossRef]
- Xu, J.; Chen, L.; Sun, H.; Wusiman, N.; Sun, W.; Li, B.; Gao, Y.; Kong, J.; Zhang, D.; Zhang, X.; et al. Crosstalk between cytokinin and ethylene signaling pathways regulates leaf abscission in cotton in response to chemical defoliants. J. Exp. Bot. 2019, 70, 1525–1538. [Google Scholar] [CrossRef] [PubMed]
- Iida, H. Mugifumi, a beneficial farm work of adding mechanical stress by treading to wheat and barley seedlings. Front. Plant Sci. 2014, 5, 453. [Google Scholar] [CrossRef]
- Mitchell, C.A. Recent Advances in Plant Response to Mechanical Stress: Theory and Application. HortScience 1996, 31, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Telewski Frank, W. Flexure Wood: Mechanical stress induced secondary xylem formation. In Secondary Xylem Biology; Academic Press: Cambridge, MA, USA, 2016; pp. 73–91. [Google Scholar]
- Onguso, J.M.; Mizutani, F.; Hossain, A.B.M.S. The effect of trunk electric vibration on the growth, yield and fruit quality of peach trees (Prunus persica [L.] Batsch). Sci. Hortic. 2006, 108, 359–363. [Google Scholar] [CrossRef]
- Penninckx, I.A.M.A.; Thomma, B.P.H.J.; Buchala, A.; Metraux, J.-P.; Broekaert, W.F. Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin. Plant Cell 1998, 10, 2103–2113. [Google Scholar] [CrossRef]
- Norman-Setterblad, C.; Vidal, S.; Palva, E.T. Interacting signal pathways control defense gene expression in Arabidopsis in response to cell wall-degrading enzymes from Erwinia carotovora. Mol. Plant-Microbe Interact. 2000, 13, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Gan, L.; Shen, Z.; Xia, K. Interactions between jasmonates and ethylene in the regulation of root hair development in Arabidopsis. J. Exp. Bot. 2006, 57, 1299–1308. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, M.; Meng, Z.; Wang, B.; Chen, M. Research Progress on the Roles of Cytokinin in Plant Response to Stress. Int. J. Mol. Sci. 2020, 21, 6574. [Google Scholar] [CrossRef]
- Pappachan, E. Developmental and tissue-specific expression of a tomato anionic peroxidase (Tap1) gene by a minimal promoter, with wound and pathogen induction by an additional 5′-Flanking Region. Plant Mol. Biol. 1993, 22, 475–490. [Google Scholar]
- Quiroga, M.; Guerrero, C.; Botella, M.A.; Barceló, A.; Amaya, I.; Medina, M.I.; Alonso, F.J.; de Forchetti, S.M.; Tigier, H.; Valpuesta, V. A tomato peroxidase involved in the synthesis of lignin and suberin. Plant Physiol. 2000, 122, 1119–1127. [Google Scholar] [CrossRef]
- Potters, G.; Pasternak, T.P.; Guisez, Y.; Jansen, M.A. Different stresses, similar morphogenic responses: Integrating a plethora of pathways. Plant Cell Environ. 2009, 32, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Van Moerkercke, A.; Duncan, O.; Zander, M.; Šimura, J.; Broda, M.; Vanden Bossche, R.; Lewsey, M.G.; Lama, S.; Singh, K.B.; Ljung, K.; et al. A MYC2/MYC3/MYC4-dependent transcription factor network regulates water spray-responsive gene expression and jasmonate levels. Proc. Natl. Acad. Sci. USA 2019, 116, 23345–23356. [Google Scholar] [CrossRef]
- De Jaegher, G.; Boyer, N.; Bon, M.-C.; Gaspar, T. Thigmomorphogenesis in bryonia dioica: Early events in ethylene biosynthesis pathway. Biochem. Physiol. Pflanz. 1987, 182, 49–56. [Google Scholar] [CrossRef]
- Ekinci, M.; Yildirim, E.; Ağar, G.; Yüksel, E.A. Determination of cadmium and/or drought stress effects on some plant phytohormone contents and hormone gene expressions in bean (Phaseolus vulgaris L). Turk. J. Agric. For. 2023, 47, 402–411. [Google Scholar] [CrossRef]
- Li, S.-W.; Leng, Y.; Feng, L.; Zeng, X.-Y. Involvement of abscisic acid in regulating antioxidative defense systems and iaa-oxidase activity and improving adventitious rooting in mung bean [Vigna radiata (L.) Wilczek] seedlings under cadmium stress. Environ. Sci. Pollut. Res. 2014, 21, 525–537. [Google Scholar] [CrossRef]
- De Jaegher, G.; Boyer, N.; Gaspar, T. Thigmomorphogenesis in Bryonia dioica: Change in soluble and wall peroxidase, phenylalanine ammonialyase activity, cellulose, lignin content and monomeric constituents. Plant Growth Regul. 1985, 3, 133–148. [Google Scholar] [CrossRef]
- Anten, N.P.R.; Casado-Garcia, R.; Pierik, R.; Pons, T.L. Ethylene sensitivity affects changes in growth patterns, but not stem properties, in response to mechanical stress in tobacco. Physiol. Plant. 2006, 128, 274–282. [Google Scholar] [CrossRef]
- Liu, C.Y.; Zhang, F.; Zhang, D.J.; Srivastava, A.K.; Wu, Q.S.; Zou, Y.N. Mycorrhiza stimulates root-hair growth and IAA synthesis and transport in trifoliate orange under drought stress. Sci. Rep. 2018, 8, 1978. [Google Scholar] [CrossRef]
- Stelmach, B.A.; Müller, A.; Hennig, P.; Laudert, D.; Andert, L.; Weiler, E.W. Quantitation of the octadecanoid 12-oxo-phytodienoic acid, a signalling compound in plant mechanotransduction. Phytochemistry 1998, 47, 539–546. [Google Scholar] [CrossRef]
- Kavi Kishor Polavarapu, B.; Sreenivasulu, N. Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant Cell Environ. 2014, 37, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Nazir, F.; Fariduddin, Q.; Khan, T.A. Hydrogen peroxide as a signalling molecule in plants and its crosstalk with other plant growth regulators under heavy metal stress. Chemosphere 2020, 252, 126486. [Google Scholar] [CrossRef] [PubMed]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Normanly, J.; Grisafi, P.; Fink, G.R.; Bartel, B. Arabidopsis mutants resistant to the auxin effects of indole-3-acetonitrile are defective in the nitrilase encoded by the NIT1 gene. Plant Cell 1997, 9, 1781–1790. [Google Scholar] [CrossRef]
- Ali, M.; Shi, L.; Khan, M.A.; Ali, A.; Hu, S.; Shen, J. Auxin biodynamics and its integral role in enhancing plant resilience to environmental cues. Physiol. Plant. 2025, 177, e70165. [Google Scholar] [CrossRef]
- Ostin, A.; Kowalyczk, M.; Bhalerao, R.P.; Sandberg, G. Metabolism of indole-3-acetic acid in Arabidopsis. Plant Physiol. 1998, 118, 285–296. [Google Scholar] [CrossRef]
- Gladala-Kostarz, A. The Impact of Wind and Mechanical Stress on the Growth and Development of Brachypodium distachyon Stems. Ph.D. Thesis, Aberystwyth University, Aberystwyth, UK, 2019. [Google Scholar]
- Potocka, I.; Szymanowska-Pułka, J. Morphological responses of plant roots to mechanical stress. Ann. Bot. 2018, 122, 711–723. [Google Scholar] [CrossRef]
- Baluska, F.; Wojtaszek, P.; Volkmann, D.; Barlow, P. The architecture of polarized cell growth: The unique status of elongating plant cells. Bioessays 2003, 25, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.R.; Johnson, K.A.; Braam, J.; James, M.N. Comparative modeling of the three-dimensional structure of the calmodulin-related TCH2 protein from Arabidopsis. Proteins 1997, 27, 144–153. [Google Scholar] [CrossRef]
- Mccormack, E.; Braam, J. Calmodulins and related potential calcium sensors of Arabidopsis. New Phytol. 2003, 159, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Delk, N.A.; Johnson, K.A.; Chowdhury, N.I.; Braam, J. CML24, regulated in expression by diverse stimuli, encodes a potential Ca2+ sensor that functions in responses to abscisic acid, daylength, and ion stress. Plant Physiol. 2005, 139, 240–253. [Google Scholar] [CrossRef]
- Purugganan, M.M. Functions of the Calmodulin-Related TCH3 and TCH4-Xyloglucan Endotransglycosylase in Arabidopsis Plants. Ph.D. Thesis, Rice University, Houston, TX, USA, 1998. [Google Scholar]
- Campbell, P.; Braam, J. Co-And/Or Post-translational modifications are critical for tch4 xet activity. Plant J. 1998, 15, 553–561. [Google Scholar] [CrossRef]
- Rose, A.B. Requirements for intron-mediated enhancement of gene expression in Arabidopsis. RNA 2002, 8, 1444–1453. [Google Scholar] [CrossRef] [PubMed]
- Arteca, J.M.; Arteca, R.N. A multi-responsive gene encoding 1-aminocyclopropane-1-carboxylate synthase (Acs6) in mature Arabidopsis leaves. Plant Mol. Biol. 1999, 39, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Tatsuki, M.; Mori, H. Rapid and transient expression of 1-aminocyclopropane-1-carboxylate synthase isogenes by touch and wound stimuli in tomato. Plant Cell Physiol. 1999, 40, 709–715. [Google Scholar] [CrossRef]
- Botella, M.A.; Xu, Y.; Prabha, T.N.; Zhao, Y.; Narasimhan, M.L.; Wilson, K.A.; Nielsen, S.S.; Bressan, R.A.; Hasegawa, P.M. Differential expression of soybean cysteine proteinase inhibitor genes during development and in response to wounding and methyl jasmonate. Plant Physiol. 1996, 112, 1201–1210. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.; Choi, K.Y.; Hepp, R.; Lee, J.Y.; Lim, M.K.; Chatani-Hinze, M.; Roche, P.A.; Kim, D.G.; Ahn, Y.S.; et al. Calmodulin dynamically regulates the trafficking of the metabotropic glutamate receptor mGluR5. Proc. Natl. Acad. Sci. USA 2008, 105, 12575–12580. [Google Scholar] [CrossRef]
- Mizoguchi, T.; Irie, K.; Hirayama, T.; Hayashida, N.; Yamaguchi-Shinozaki, K.; Matsumoto, K.; Shinozaki, K. A gene encoding a mitogen-activated protein kinase kinase kinase is induced simultaneously with genes for a mitogen-activated protein kinase and an S6 ribosomal protein kinase by touch, cold, and water stress in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 1996, 93, 765–769. [Google Scholar] [CrossRef]
- Braam, J. Regulated expression of the calmodulin-related tch genes in cultured Arabidopsis cells: Induction by calcium and heat shock. Proc. Natl. Acad. Sci. USA 1992, 89, 3213–3216. [Google Scholar] [CrossRef]
- Braam, J.; Sistrunk, M.L.; Polisensky, D.H.; Xu, W.; Purugganan, M.M.; Antosiewicz, D.M.; Campbell, P.; Johnson, K.A. Life in a changing world: TCH gene regulation of expression and responses to environmental signals. Physiol. Plant. 1996, 98, 909–916. [Google Scholar] [CrossRef]
- Antosiewicz, J.; Damiani, E.; Jassem, W.; Wozniak, M.; Orena, M.; Greci, L. Influence of structure on the antioxidant activity of indolinic nitroxide radicals. Free Radic. Biol. Med. 1997, 22, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Botella, J.R.; Arteca, R.N.; Frangos, J.A. A mechanical strain-induced 1-Aminocyclopropane-1-Carboxylic acid synthase gene. Proc. Natl. Acad. Sci. USA 1995, 92, 1595–1598. [Google Scholar] [CrossRef]
- Kimbrough, J.M.; Salinas-Mondragon, R.; Boss, W.F.; Brown, C.S.; Sederoff, H.W. The fast and transient transcriptional network of gravity and mechanical stimulation in the Arabidopsis root apex. Plant Physiol. 2004, 136, 2790–2805. [Google Scholar] [CrossRef]
- Brenya, E.; Chen, Z.-H.; Tissue, D.; Papanicolaou, A.; Cazzonelli, C.I. Prior exposure of Arabidopsis seedlings to mechanical stress heightens jasmonic acid-mediated defense against necrotrophic pathogens. BMC Plant Biol. 2020, 20, 548. [Google Scholar] [CrossRef]
- Mauch, F.; Kmecl, A.; Schaffrath, U.; Volrath, S.; Görlach, J.; Ward, E.; Ryals, J.; Dudler, R. Mechanosensitive expression of a lipoxygenase gene in wheat. Plant Physiol. 1997, 114, 1561–1566. [Google Scholar] [CrossRef]
- Suza Walter, P.; Staswick Paul, E. The role of Jar1 in jasmonoyl-l-isoleucine production during Arabidopsis wound response. Planta 2008, 227, 1221–1232. [Google Scholar] [CrossRef] [PubMed]
- Plasencia, F.A.; Estrada, Y.; Flores, F.B.; Ortíz-Atienza, A.; Lozano, R.; Egea, I. The Ca2+ Sensor Calcineurin B-Like Protein 10 in Plants: Emerging New Crucial Roles for Plant Abiotic Stress Tolerance. Front. Plant Sci. 2021, 11, 599944. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.; Hettenhausen, C.; Lange, T.; Wünsche, H.; Fang, J.; Baldwin, I.T.; Wu, J. High levels of jasmonic acid antagonize the biosynthesis of gibberellins and inhibit the growth of Nicotiana attenuata stems. Plant J. 2013, 73, 591–606. [Google Scholar] [CrossRef]
- Lee, D.; Polisensky, D.H.; Braam, J. Genome-wide identification of touch- and darkness-regulated Arabidopsis genes: A focus on calmodulin-like and XTH genes. New Phytol. 2005, 165, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Baena, M.S.; Hernandes-Lopes, J.; Van Sluys, M.A. Reaching the top through a tortuous path: Helical growth in climbing plants. Curr. Opin. Plant Biol. 2021, 59, 101982. [Google Scholar] [CrossRef]
- Pomiès, L.; Decourteix, M.; Franchel, J.; Moulia, B.; Leblanc-Fournier, N. Poplar stem transcriptome is massively remodelled in response to single or repeated mechanical stimuli. BMC Genom. 2017, 18, 300. [Google Scholar] [CrossRef]
- Bashford, M.T.; Hickey, S.E.; Curry, C.J.; Toriello, H.V. American College of Medical Genetics and Genomics (ACMG) Professional Practice and Guidelines Committee. Addendum: ACMG Practice Guideline: Lack of evidence for MTHFR polymorphism testing. Genet. Med. 2020, 22, 2125. [Google Scholar] [CrossRef]
- Niklas, K.J.; Spatz, H.C. Mechanical properties of wood disproportionately increase with increasing density. Am. J. Bot. 2012, 99, 169–170. [Google Scholar] [CrossRef]
- Robertson, D.J.; Smith, S.L.; Cook, D.D. On measuring the bending strength of septate grass stems. Am. J. Bot. 2015, 102, 5–11. [Google Scholar] [CrossRef]
- Ookawa, T.; Hobo, T.; Yano, M.; Murata, K.; Ando, T.; Miura, H.; Asano, K.; Ochiai, Y.; Ikeda, M.; Nishitani, R. New approach for rice improvement using a pleiotropic QTL gene for lodging resistance and yield. Nat. Commun. 2010, 1, 132. [Google Scholar] [CrossRef]
- Samad, S.; Kurokura, T.; Koskela, E.; Toivainen, T.; Patel, V.; Mouhu, K.; Sargent, D.J.; Hytönen, T. Additive QTLs on three chromosomes control flowering time in woodland strawberry (Fragaria vesca L.). Hortic. Res. 2017, 4, 17020. [Google Scholar] [CrossRef]
- Zargar, O.; Li, Q.; Nwaobi, C.; Pharr, M.; Finlayson, S.A.; Muliana, A. Thigmostimulation alters anatomical and biomechanical properties of bioenergy sorghum stems. J. Mech. Behav. Biomed. Mater. 2022, 127, 105090. [Google Scholar] [CrossRef] [PubMed]
- Erndwein, L.; Cook, D.D.; Robertson, D.J.; Sparks, E.E. Field-based mechanical phenotyping of cereal crops to assess lodging resistance. Appl. Plant Sci. 2020, 8, e11382. [Google Scholar] [CrossRef] [PubMed]
- McCahill, I.W.; Abushal, L.T.; Khahani, B.; Probert, C.F.; Flockhart, E.L.; Gregory, G.A.; Li, E.Z.; Zhang, Y.; Baumgart, L.A.; O’Malley, R.C.; et al. Shoring up the Base: The Development and Regulation of Cortical Sclerenchyma in Grass Nodal Roots. Plant Physiol. 2025, 199, kiaf215. [Google Scholar] [CrossRef]
- Lemloh, M.-L.; Pohl, A.; Weber, E.; Zeiger, M.; Bauer, P.; Weiss, I.M.; Schneider, A.S. Structure-property relationships in mechanically stimulated Sorghum bicolor stalks. Bioinspired Mater. 2014, 1, 1–11. [Google Scholar] [CrossRef]
- Coomey, J.H.; MacKinnon, K.J.; McCahill, I.W.; Khahani, B.; Handakumbura, P.P.; Trabucco, G.M.; Mazzola, J.; Leblanc, N.A.; Kheam, R.; Hernandez-Romero, M.; et al. Mechanically induced localisation of SECONDARY WALL INTERACTING bZIP is associated with thigmomorphogenic and secondary cell wall gene expression. Quant Plant Biol. 2024, 5, e5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gladala-Kostarz, A.; Doonan, J.H.; Bosch, M. Mechanical stimulation in Brachypodium distachyon: Implications for fitness, productivity, and cell wall properties. Plant Cell Environ. 2020, 43, 1314–1330. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Finlayson, S.A. Sorghum tiller bud growth is repressed by contact with the overlying leaf. Plant Cell Environ. 2019, 42, 2120–2132. [Google Scholar] [CrossRef] [PubMed]
- Darwish, E.; Ghosh, R.; Bentzer, J.; Tsardakas, R.N.; Proux-Wera, E.; Kamal, N.; Spannagl, M.; Hause, B.; Sirijovski, N.; Van Aken, O. The dynamics of touch-responsive gene expression in cereals. Plant J. 2023, 116, 282–302. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jędrzejuk, A.; Kuźma, N. Response of Plants to Touch Stress at Morphological, Physiological and Molecular Levels. Int. J. Mol. Sci. 2025, 26, 11120. https://doi.org/10.3390/ijms262211120
Jędrzejuk A, Kuźma N. Response of Plants to Touch Stress at Morphological, Physiological and Molecular Levels. International Journal of Molecular Sciences. 2025; 26(22):11120. https://doi.org/10.3390/ijms262211120
Chicago/Turabian StyleJędrzejuk, Agata, and Natalia Kuźma. 2025. "Response of Plants to Touch Stress at Morphological, Physiological and Molecular Levels" International Journal of Molecular Sciences 26, no. 22: 11120. https://doi.org/10.3390/ijms262211120
APA StyleJędrzejuk, A., & Kuźma, N. (2025). Response of Plants to Touch Stress at Morphological, Physiological and Molecular Levels. International Journal of Molecular Sciences, 26(22), 11120. https://doi.org/10.3390/ijms262211120





