ACE2: Friend or Foe in Post-COVID-19 Neurodegeneration?
Abstract
1. Introduction
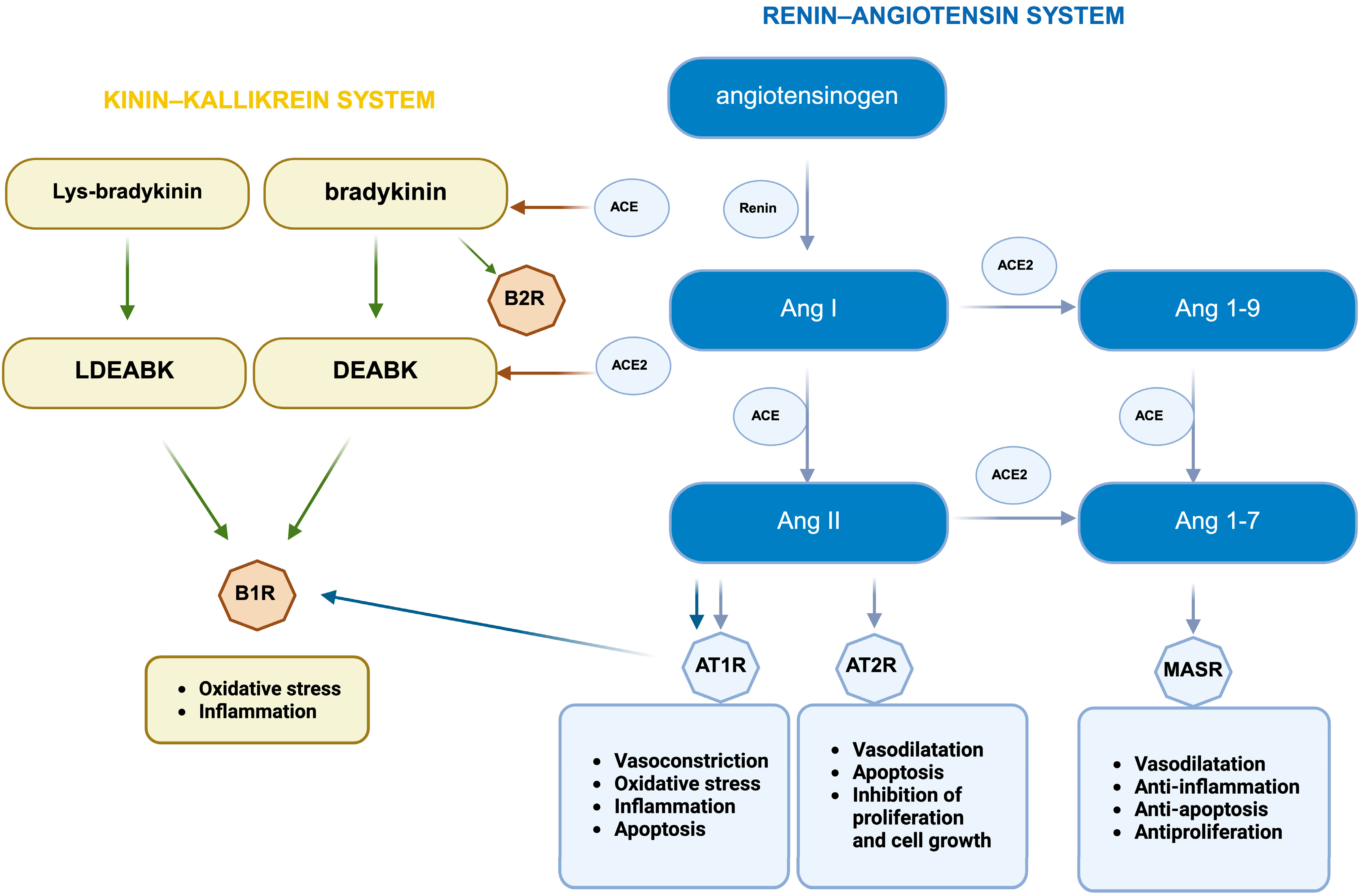
2. Brain RAS and ACE2
3. ACE2 Expression
4. ACE2
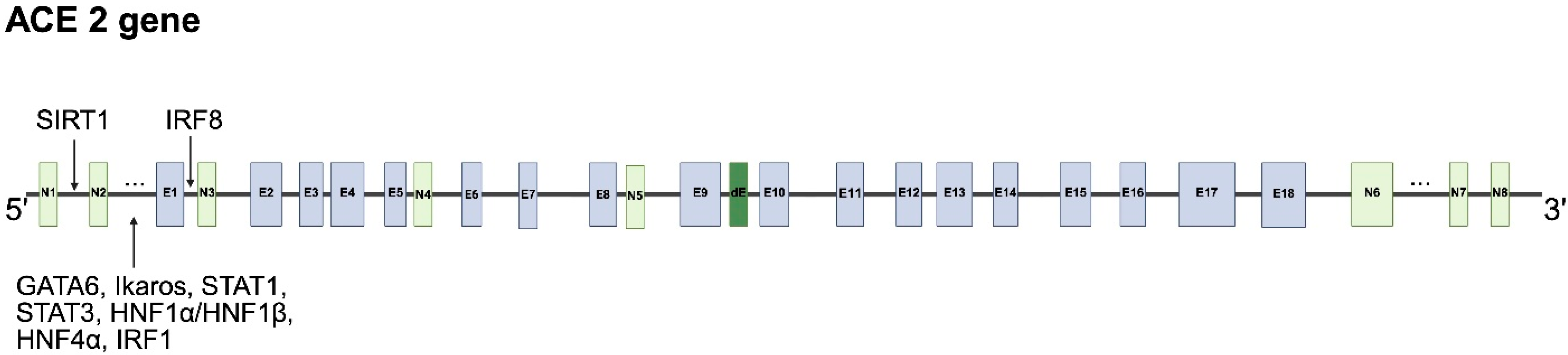
4.1. Human ACE2 Gene, Alternative Splicing and mRNA Transcripts
4.2. Full-Length ACE2
4.3. Regulation at the Transcriptional Level
4.4. Regulation of ACE2 mRNA Expression by Non-Coding RNAs
4.5. Alternative Regulation of ACE2 mRNA Expression
4.6. Post-Translational Regulation of ACE2 Expression
4.7. ACE2 Processing by Proteases
4.8. ACE2: A Chaperone for the Membrane Trafficking of Amino Acid Transporters
4.9. Role of the ACE2 Endodomain
4.10. Exosomal Full-Length ACE2
4.11. The ACE2 Isoform 4
5. The Impact of ACE2 on Selected Neurotransmitter Systems in the Brain
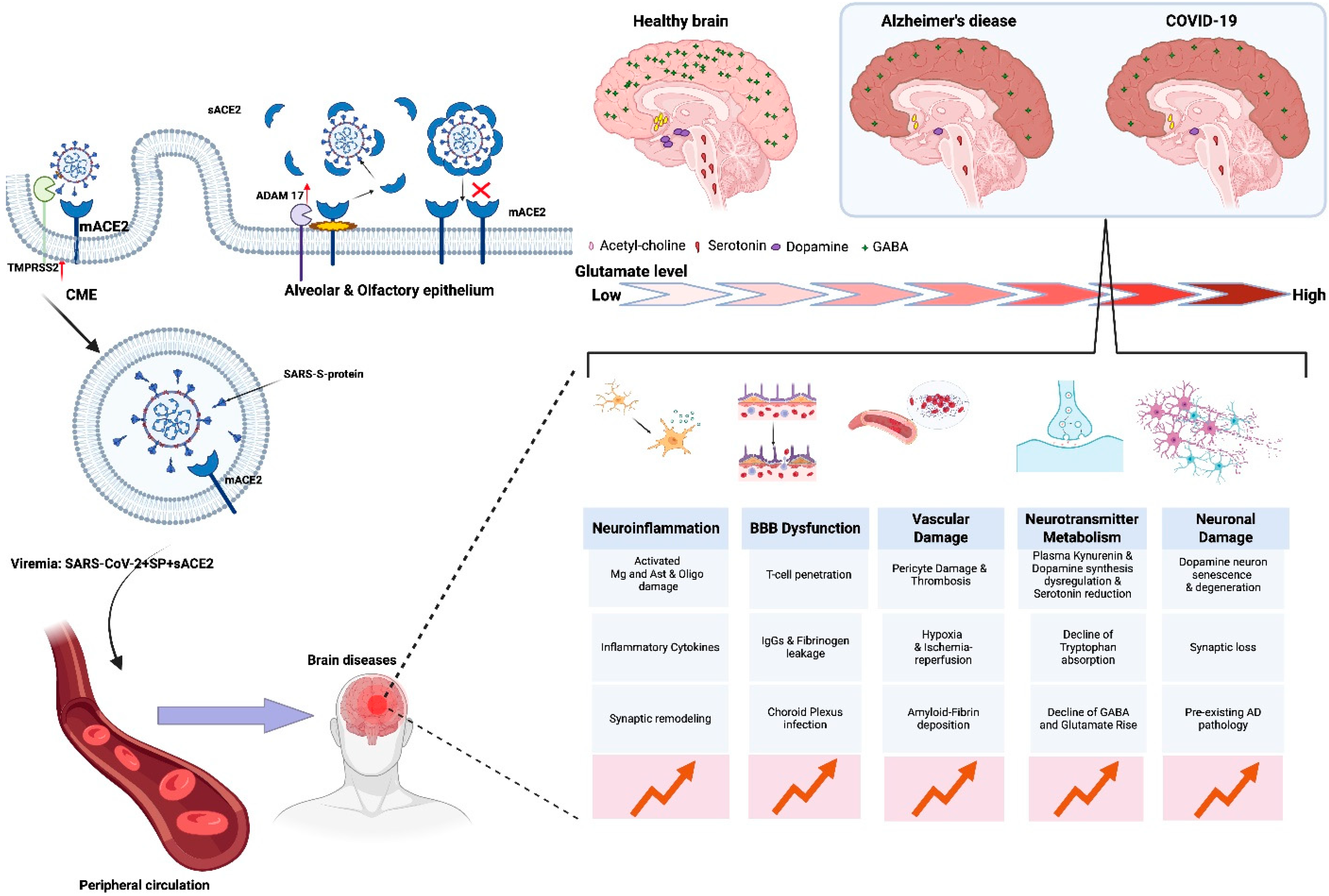
6. ACE2 as a Cell Receptor for SARS-CoV-2 Entry
7. Is Alzheimer’s Disease a Comorbidity or a Consequence of COVID-19-Induced Neuropathology?
8. Concluding Remarks and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kanugula, A.K.; Kaur, J.; Batra, J.; Ankireddypalli, A.R.; Velagapudi, R. Renin-Angiotensin System: Updated Understanding and Role in Physiological and Pathophysiological States. Cureus 2023, 15, e40725. [Google Scholar] [CrossRef]
- Alenina, N.; Bader, M. ACE2 in Brain Physiology and Pathophysiology: Evidence from Transgenic Animal Models. Neurochem. Res. 2019, 44, 1323–1329. [Google Scholar] [CrossRef]
- Eriksson, U.; Danilczyk, U.; Penninger, J.M. Just the beginning: Novel functions for angiotensin-converting enzymes. Curr. Biol. 2002, 12, R745–R752. [Google Scholar] [CrossRef]
- Santos, R.A.S.; Oudit, G.Y.; Verano-Braga, T.; Canta, G.; Steckelings, U.M.; Bader, M. The renin-angiotensin system: Going beyond the classical paradigms. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H958–H970. [Google Scholar] [CrossRef]
- Gilbert, A.; Liu, J.; Cheng, G.; An, C.; Deo, K.; Gorret, A.M.; Qin, X. A review of urinary angiotensin converting enzyme 2 in diabetes and diabetic nephropathy. Biochem. Med. 2019, 29, 010501. [Google Scholar] [CrossRef]
- Sharma, N.; Anders, H.J.; Gaikwad, A.B. Fiend and friend in the renin angiotensin system: An insight on acute kidney injury. Biomed. Pharmacother. 2019, 110, 764–774. [Google Scholar] [CrossRef]
- Reveret, L.; Leclerc, M.; Emond, V.; Tremblay, C.; Loiselle, A.; Bourassa, P.; Bennett, D.A.; Hébert, S.S.; Calon, F. Higher angiotensin-converting enzyme 2 (ACE2) levels in the brain of individuals with Alzheimer’s disease. Acta Neuropathol. Commun. 2023, 11, 159, Erratum in Acta Neuropathol. Commun. 2023, 11, 173. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Alquisiras-Burgos, I.; Peralta-Arrieta, I.; Alonso-Palomares, L.A.; Zacapala-Gómez, A.E.; Salmerón-Bárcenas, E.G.; Aguilera, P. Neurological Complications Associated with the Blood-Brain Barrier Damage Induced by the Inflammatory Response During SARS-CoV-2 Infection. Mol. Neurobiol. 2021, 58, 520–535. [Google Scholar] [CrossRef]
- Ashraf, U.M.; Abokor, A.A.; Edwards, J.M.; Waigi, E.W.; Royfman, R.S.; Hasan, S.A.; Smedlund, K.B.; Hardy, A.M.G.; Chakravarti, R.; Koch, L.G. SARS-CoV-2, ACE2 expression, and systemic organ invasion. Physiol. Genomics 2021, 53, 51–60. [Google Scholar] [CrossRef]
- Beyerstedt, S.; Casaro, E.B.; Rangel, É.B. COVID-19: Angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 905–919. [Google Scholar] [CrossRef]
- Scialo, F.; Daniele, A.; Amato, F.; Pastore, L.; Matera, M.G.; Cazzola, M.; Castaldo, G.; Bianco, A. ACE2: The Major Cell Entry Receptor for SARS-CoV-2. Lung 2020, 198, 867–877. [Google Scholar] [CrossRef]
- Jami, G.; Ataee, M.; Esmaeili, V.; Chamani, S.; Rezaei, A.; Naghizadeh, A. Characterization of the angiotensin-converting enzyme 2 (ACE2), the main receptor for the SARS-CoV-2 virus. Am. J. Clin. Exp. Immunol. 2023, 12, 24–44. [Google Scholar]
- Coelho, S.V.A.; Souza, G.L.E.; Bezerra, B.B.; Lima, L.R.; Correa, I.A.; de Almeida, D.V.; Silva-Aguiar, R.P.D.; Pinheiro, A.A.S.; Sirois, P.; Caruso-Neves, C.; et al. SARS-CoV-2 Replication in a Blood-Brain Barrier Model Established with Human Brain Microvascular Endothelial Cells Induces Permeability and Disables ACE2-Dependent Regulation of Bradykinin B1 Receptor. Int. J. Mol. Sci. 2025, 26, 5540. [Google Scholar] [CrossRef]
- Rasmi, Y.; Shokati, A.; Hatamkhani, S.; Farnamian, Y.; Naderi, R.; Jalali, L. Assessment of the relationship between the dopaminergic pathway and severe acute respiratory syndrome coronavirus 2 infection, with related neuropathological features, and potential therapeutic approaches in COVID-19 infection. Rev. Med. Virol. 2024, 34, e2506. [Google Scholar] [CrossRef]
- Eid, H.M.A.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Fawzy, M.N.; Papadakis, M.; Almutawif, Y.A.; Alexiou, A.; Batiha, G.E. GABA and GABAergic dysfunction in COVID-19: Piecing the puzzle with targeting immunity and several inflammatory pathways. Cytokine 2025, 193, 156976. [Google Scholar] [CrossRef]
- Amadoro, G.; Latina, V.; Stigliano, E.; Micera, A. COVID-19 and Alzheimer’s Disease Share Common Neurological and Ophthalmological Manifestations: A Bidirectional Risk in the Post-Pandemic Future. Cells 2023, 12, 2601. [Google Scholar] [CrossRef]
- Granholm, A.C. Long-Term Effects of SARS-CoV-2 in the Brain: Clinical Consequences and Molecular Mechanisms. J. Clin. Med. 2023, 12, 3190. [Google Scholar] [CrossRef]
- Cai, M.; Xie, Y.; Topol, E.J.; Al-Aly, Z. Three-year outcomes of post-acute sequelae of COVID-19. Nat. Med. 2024, 30, 1564–1573. [Google Scholar] [CrossRef]
- Ding, Q.; Zhao, H. Long-term effects of SARS-CoV-2 infection on human brain and memory. Cell Death Discov. 2023, 9, 196. [Google Scholar] [CrossRef]
- Rukavina Mikusic, N.L.; Pineda, A.M.; Gironacci, M.M. Angiotensin-(1–7) and Mas receptor in the brain. Explor. Med. 2021, 2, 268–293. [Google Scholar] [CrossRef]
- Gheblawi, M.; Wang, K.; Viveiros, A.; Nguyen, Q.; Zhong, J.C.; Turner, A.J.; Raizada, M.K.; Grant, M.B.; Oudit, G.Y. Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System: Celebrating the 20th Anniversary of the Discovery of ACE2. Circ. Res. 2020, 126, 1456–1474. [Google Scholar] [CrossRef]
- Gross, L.Z.F.; Sacerdoti, M.; Piiper, A.; Zeuzem, S.; Leroux, A.E.; Biondi, R.M. ACE2, the Receptor that Enables Infection by SARS-CoV-2: Biochemistry, Structure, Allostery and Evaluation of the Potential Development of ACE2 Modulators. ChemMedChem 2020, 15, 1682–1690. [Google Scholar] [CrossRef]
- García-Escobar, A.; Vera-Vera, S.; Jurado-Román, A.; Jiménez-Valero, S.; Galeote, G.; Moreno, R. Calcium Signaling Pathway Is Involved in the Shedding of ACE2 Catalytic Ectodomain: New Insights for Clinical and Therapeutic Applications of ACE2 for COVID-19. Biomolecules 2022, 12, 76. [Google Scholar] [CrossRef]
- Gonzalez, S.M.; Siddik, A.B.; Su, R.C. Regulated Intramembrane Proteolysis of ACE2: A Potential Mechanism Contributing to COVID-19 Pathogenesis? Front. Immunol. 2021, 12, 612807. [Google Scholar] [CrossRef]
- Al-Qahtani, Z.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Albuhadily, A.K.; Ali, N.H.; Alexiou, A.; Papadakis, M.; Saad, H.M.; Batiha, G.E. The potential role of brain renin-angiotensin system in the neuropathology of Parkinson disease: Friend, foe or turncoat? J. Cell. Mol. Med. 2024, 28, e18495. [Google Scholar] [CrossRef]
- Morgun, A.V.; Salmin, V.V.; Boytsova, E.B.; Lopatina, O.L.; Salmina, A.B. Molecular Mechanisms of Proteins—Targets for SARS-CoV-2 (Review). Sovrem. Tekhnologii Med. 2021, 12, 98–108. [Google Scholar] [CrossRef]
- Lindskog, C.; Méar, L.; Virhammar, J.; Fällmar, D.; Kumlien, E.; Hesselager, G.; Casar-Borota, O.; Rostami, E. Protein Expression Profile of ACE2 in the Normal and COVID-19-Affected Human Brain. J. Proteome Res. 2022, 21, 2137–2145. [Google Scholar] [CrossRef]
- Chen, R.; Wang, K.; Yu, J.; Howard, D.; French, L.; Chen, Z.; Wen, C.; Xu, Z. The Spatial and Cell-Type Distribution of SARS-CoV-2 Receptor ACE2 in the Human and Mouse Brains. Front. Neurol. 2021, 11, 573095. [Google Scholar] [CrossRef]
- Tayler, H.M.; MacLachlan, R.; Güzel, Ö.; Fisher, R.A.; Skrobot, O.A.; Abulfadl, M.A.; Kehoe, P.G.; Miners, J.S. Altered Gene Expression Within the Renin-Angiotensin System in Normal Aging and Dementia. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, glad241. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, W.; Lukiw, W. Ubiquity of the SARS-CoV-2 receptor ACE2 and upregulation in limbic regions of Alzheimer’s disease brain. Folia Neuropathol. 2021, 59, 232–238. [Google Scholar] [CrossRef]
- Pistollato, F.; Petrillo, M.; Clerbaux, L.A.; Leoni, G.; Ponti, J.; Bogni, A.; Brogna, C.; Cristoni, S.; Sanges, R.; Mendoza-de Gyves, E.; et al. Effects of Spike protein and toxin-like peptides found in COVID-19 patients on human 3D neuronal/glial model undergoing differentiation: Possible implications for SARS-CoV-2 impact on brain development. Reprod. Toxicol. 2022, 111, 34–48. [Google Scholar] [CrossRef]
- Mesmoudi, S.; Lapina, C.; Rodic, M.; Peschanski, D. Multi-Data Integration Towards a Global Understanding of the Neurological Impact of Human Brain Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Front. Integr. Neurosci. 2022, 16, 756604. [Google Scholar] [CrossRef]
- Bu, F.; Guan, R.; Wang, W.; Liu, Z.; Yin, S.; Zhao, Y.; Chai, J. Bioinformatics and systems biology approaches to identify the effects of COVID-19 on neurodegenerative diseases: A review. Medicine 2022, 101, e32100. [Google Scholar] [CrossRef]
- Zhang, Y.; Archie, S.R.; Ghanwatkar, Y.; Sharma, S.; Nozohouri, S.; Burks, E.; Mdzinarishvili, A.; Liu, Z.; Abbruscato, T.J. Potential role of astrocyte angiotensin converting enzyme 2 in the neural transmission of COVID-19 and a neuroinflammatory state induced by smoking and vaping. Fluids Barriers CNS 2022, 19, 46, Erratum in Fluids Barriers CNS 2022, 19, 91. [Google Scholar] [CrossRef]
- Lima, R.S.; Rocha, L.P.C.; Moreira, P.R. Genetic and epigenetic control of ACE2 expression and its possible role in COVID-19. Cell Biochem. Funct. 2021, 39, 713–726. [Google Scholar] [CrossRef]
- Tipnis, S.R.; Hooper, N.M.; Hyde, R.; Karran, E.; Christie, G.; Turner, A.J. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J. Biol. Chem. 2000, 275, 33238–33243. [Google Scholar] [CrossRef]
- Rehman, S.U.; Tabish, M. Alternative splicing of ACE2 possibly generates variants that may limit the entry of SARS-CoV-2: A potential therapeutic approach using SSOs. Clin. Sci. 2020, 134, 1143–1150. [Google Scholar] [CrossRef]
- Stocker, N.; Radzikowska, U.; Wawrzyniak, P.; Tan, G.; Huang, M.; Ding, M.; Akdis, C.A.; Sokolowska, M. Regulation of angiotensin-converting enzyme 2 isoforms by type 2 inflammation and viral infection in human airway epithelium. Mucosal Immunol. 2023, 16, 5–16. [Google Scholar] [CrossRef]
- Blume, C.; Jackson, C.L.; Spalluto, C.M.; Legebeke, J.; Nazlamova, L.; Conforti, F.; Perotin, J.M.; Frank, M.; Butler, J.; Crispin, M.; et al. A novel ACE2 isoform is expressed in human respiratory epithelia and is upregulated in response to interferons and RNA respiratory virus infection. Nat. Genet. 2021, 53, 205–214. [Google Scholar] [CrossRef]
- Onabajo, O.O.; Banday, A.R.; Stanifer, M.L.; Yan, W.; Obajemu, A.; Santer, D.M.; Florez-Vargas, O.; Piontkivska, H.; Vargas, J.M.; Ring, T.J.; et al. Interferons and viruses induce a novel truncated ACE2 isoform and not the full-length SARS-CoV-2 receptor. Nat. Genet. 2020, 52, 1283–1293. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.W.; Chuang, H.C.; Tan, T.H. ACE2 in chronic disease and COVID-19: Gene regulation and post-translational modification. J. Biomed. Sci. 2023, 30, 71. [Google Scholar] [CrossRef]
- Zipeto, D.; Palmeira, J.D.F.; Argañaraz, G.A.; Argañaraz, E.R. ACE2/ADAM17/TMPRSS2 Interplay May Be the Main Risk Factor for COVID-19. Front. Immunol. 2020, 11, 576745. [Google Scholar] [CrossRef]
- Badawi, S.; Ali, B.R. ACE2 Nascence, trafficking, and SARS-CoV-2 pathogenesis: The saga continues. Hum. Genom. 2021, 15, 8. [Google Scholar] [CrossRef]
- Clarke, N.E.; Fisher, M.J.; Porter, K.E.; Lambert, D.W.; Turner, A.J. Angiotensin converting enzyme (ACE) and ACE2 bind integrins and ACE2 regulates integrin signalling. PLoS ONE 2012, 7, e34747. [Google Scholar] [CrossRef] [PubMed]
- Niehues, R.V.; Wozniak, J.; Wiersch, F.; Lilienthal, E.; Tacken, N.; Schumertl, T.; Garbers, C.; Ludwig, A.; Düsterhöft, S. The collectrin-like part of the SARS-CoV-1 and -2 receptor ACE2 is shed by the metalloproteinases ADAM10 and ADAM17. FASEB J. 2022, 36, e22234. [Google Scholar] [CrossRef]
- Descamps, G.; Verset, L.; Trelcat, A.; Hopkins, C.; Lechien, J.R.; Journe, F.; Saussez, S. ACE2 Protein Landscape in the Head and Neck Region: The Conundrum of SARS-CoV-2 Infection. Biology 2020, 9, 235. [Google Scholar] [CrossRef]
- Rushworth, C.A.; Guy, J.L.; Turner, A.J. Residues affecting the chloride regulation and substrate selectivity of the angiotensin-converting enzymes (ACE and ACE2) identified by site-directed mutagenesis. FEBS J. 2008, 275, 6033–6042. [Google Scholar] [CrossRef]
- Towler, P.; Staker, B.; Prasad, S.G.; Menon, S.; Tang, J.; Parsons, T.; Ryan, D.; Fisher, M.; Williams, D.; Dales, N.A.; et al. ACE2 X-ray structures reveal a large hinge-bending motion important for inhibitor binding and catalysis. J. Biol. Chem. 2004, 279, 17996–18007. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yan, R.; Zhou, Q. ACE2, B0AT1, and SARS-CoV-2 Spike protein: Structural and functional implications. Curr. Opin. Struct. Biol. 2022, 74, 102388. [Google Scholar] [CrossRef]
- Zhang, Y.; Niu, G.; Flisikowska, T.; Schnieke, A.; Flisikowski, K. A tissue- and gender-specific regulation of the SARS-CoV-2 receptor ACE2 by p53 in pigs. Biochem. Biophys. Res. Commun. 2021, 553, 25–29. [Google Scholar] [CrossRef]
- Zhao, S.; Ghosh, A.; Lo, C.S.; Chenier, I.; Scholey, J.W.; Filep, J.G.; Ingelfinger, J.R.; Zhang, S.L.; Chan, J.S.D. Nrf2 Deficiency Upregulates Intrarenal Angiotensin-Converting Enzyme-2 and Angiotensin 1-7 Receptor Expression and Attenuates Hypertension and Nephropathy in Diabetic Mice. Endocrinology 2018, 159, 836–852. [Google Scholar] [CrossRef] [PubMed]
- Elibol, B.; Kilic, U. High Levels of SIRT1 Expression as a Protective Mechanism Against Disease-Related Conditions. Front. Endocrinol. 2018, 9, 614. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Zhou, L. EZH2-mediated H3K27me3 inhibits ACE2 expression. Biochem. Biophys. Res. Commun. 2020, 526, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Chen, Y.H.; Liu, P.J.; Hu, W.C.; Lu, K.C.; Tsai, K.W. The emerging role of miRNAs in the pathogenesis of COVID-19: Protective effects of nutraceutical polyphenolic compounds against SARS-CoV-2 infection. Int. J. Med. Sci. 2022, 19, 1340–1356. [Google Scholar] [CrossRef] [PubMed]
- Holohan, K.N.; Lahiri, D.K.; Schneider, B.P.; Foroud, T.; Saykin, A.J. Functional microRNAs in Alzheimer’s disease and cancer: Differential regulation of common mechanisms and pathways. Front. Genet. 2013, 3, 323. [Google Scholar] [CrossRef] [PubMed]
- Hardin, L.T.; Xiao, N. miRNAs: The Key Regulator of COVID-19 Disease. Int. J. Cell. Biol. 2022, 2022, 1645366. [Google Scholar] [CrossRef]
- Zabłocka, A.; Kazana, W.; Sochocka, M.; Stańczykiewicz, B.; Janusz, M.; Leszek, J.; Orzechowska, B. Inverse Correlation Between Alzheimer’s Disease and Cancer: Short Overview. Mol. Neurobiol. 2021, 58, 6335–6349. [Google Scholar] [CrossRef]
- Hu, Y.K.; Wang, X.; Li, L.; Du, Y.H.; Ye, H.T.; Li, C.Y. MicroRNA-98 induces an Alzheimer’s disease-like disturbance by targeting insulin-like growth factor 1. Neurosci. Bull. 2013, 29, 745–751. [Google Scholar] [CrossRef]
- Jia, L.H.; Liu, Y.N. Downregulated serum miR-223 servers as biomarker in Alzheimer’s disease. Cell Biochem. Funct. 2016, 34, 233–237. [Google Scholar] [CrossRef]
- Pimenta, R.; Viana, N.I.; Dos Santos, G.A.; Candido, P.; Guimarães, V.R.; Romão, P.; Silva, I.A.; de Camargo, J.A.; Hatanaka, D.M.; Queiroz, P.G.S.; et al. MiR-200c-3p expression may be associated with worsening of the clinical course of patients with COVID-19. Mol. Biol. Res. Commun. 2021, 10, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Amakiri, N.; Kubosumi, A.; Tran, J.; Reddy, P.H. Amyloid Beta and MicroRNAs in Alzheimer’s Disease. Front. Neurosci. 2019, 13, 430. [Google Scholar] [CrossRef]
- Abdolahi, S.; Hosseini, M.; Rezaei, R.; Mohebbi, S.R.; Rostami-Nejad, M.; Mojarad, E.N.; Mirjalali, H.; Yadegar, A.; Asadzadeh Aghdaei, H.; Zali, M.R.; et al. Evaluation of miR-200c-3p and miR-421-5p levels during immune responses in the admitted and recovered COVID-19 subjects. Infect. Genet. Evol. 2022, 98, 105207. [Google Scholar] [CrossRef]
- Sun, N.N.; Yu, C.H.; Pan, M.X.; Zhang, Y.; Zheng, B.J.; Yang, Q.J.; Zheng, Z.M.; Meng, Y. Mir-21 Mediates the Inhibitory Effect of Ang (1-7) on AngII-induced NLRP3 Inflammasome Activation by Targeting Spry1 in lung fibroblasts. Sci. Rep. 2017, 7, 14369, Erratum in Sci. Rep. 2020, 10, 21896. [Google Scholar] [CrossRef]
- Dong, Y.; Xiong, J.; Ji, L.; Xue, X. MiR-421 Aggravates Neurotoxicity and Promotes Cell Death in Parkinson’s Disease Models by Directly Targeting MEF2D. Neurochem. Res. 2021, 46, 299–308. [Google Scholar] [CrossRef]
- Zhang, R.; Wu, Y.; Zhao, M.; Liu, C.; Zhou, L.; Shen, S.; Liao, S.; Yang, K.; Li, Q.; Wan, H. Role of HIF-1alpha in the regulation ACE and ACE2 expression in hypoxic human pulmonary artery smooth muscle cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2009, 297, L631–L640. [Google Scholar] [CrossRef]
- Li, W.; Wang, R.; Ma, J.Y.; Wang, M.; Cui, J.; Wu, W.B.; Liu, R.M.; Zhang, C.X.; Li, W.; Wang, S.M. A Human Long Non-Coding RNA ALT1 Controls the Cell Cycle of Vascular Endothelial Cells via ACE2 and Cyclin D1 Pathway. Cell. Physiol. Biochem. 2017, 43, 1152–1167. [Google Scholar] [CrossRef]
- Diallo, I.; Jacob, R.A.; Vion, E.; Kozak, R.A.; Mossman, K.; Provost, P. Altered microRNA Transcriptome in Cultured Human Airway Cells upon Infection with SARS-CoV-2. Viruses 2023, 15, 496. [Google Scholar] [CrossRef]
- Wei, C.; Henderson, H.; Spradley, C.; Li, L.; Kim, I.K.; Kumar, S.; Hong, N.; Arroliga, A.C.; Gupta, S. Circulating miRNAs as potential marker for pulmonary hypertension. PLoS ONE 2013, 8, e64396. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Gao, F.; Hao, J.; Liu, Z. microRNA-1246 mediates lipopolysaccharide-induced pulmonary endothelial cell apoptosis and acute lung injury by targeting angiotensin-converting enzyme 2. Am. J. Transl. Res. 2017, 9, 1287–1296. [Google Scholar] [PubMed]
- Guo, R.; Fan, G.; Zhang, J.; Wu, C.; Du, Y.; Ye, H.; Li, Z.; Wang, L.; Zhang, Z.; Zhang, L.; et al. A 9-microRNA Signature in Serum Serves as a Noninvasive Biomarker in Early Diagnosis of Alzheimer’s Disease. J. Alzheimers Dis. 2017, 60, 1365–1377. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.L.; Strohm, L.; Zhu, Y.; Chia, R.S.L.; Chong, J.R.; Suresh, D.D.; Zhou, L.H.; Too, H.P.; Hilal, S.; Radivoyevitch, T.; et al. Extracellular Vesicle-Enriched miRNA-Biomarkers Show Improved Utility for Detecting Alzheimer’s Disease Dementia and Medial Temporal Atrophy. J. Alzheimers Dis. 2024, 99, 1317–1331. [Google Scholar] [CrossRef]
- Wiese, O.J.; Allwood, B.W.; Zemlin, A.E. COVID-19 and the renin-angiotensin system (RAS): A spark that sets the forest alight? Med. Hypotheses 2020, 144, 110231. [Google Scholar] [CrossRef]
- Sato, T.; Suzuki, T.; Watanabe, H.; Kadowaki, A.; Fukamizu, A.; Liu, P.P.; Kimura, A.; Ito, H.; Penninger, J.M.; Imai, Y.; et al. Apelin is a positive regulator of ACE2 in failing hearts. J. Clin. Investig. 2013, 123, 5203–5211. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Xiang, Y.; Qu, X.; Liu, H.; Liu, C.; Li, G.; Han, L.; Qin, X. Apelin-13 Suppresses Neuroinflammation Against Cognitive Deficit in a Streptozotocin-Induced Rat Model of Alzheimer’s Disease Through Activation of BDNF-TrkB Signaling Pathway. Front. Pharmacol. 2019, 10, 395. [Google Scholar] [CrossRef]
- Connolly, K.; Lehoux, M.; O’Rourke, R.; Assetta, B.; Erdemir, G.A.; Elias, J.A.; Lee, C.G.; Huang, Y.A. Potential role of chitinase-3-like protein 1 (CHI3L1/YKL-40) in neurodegeneration and Alzheimer’s disease. Alzheimers Dement. 2023, 19, 9–24. [Google Scholar] [CrossRef]
- Kamle, S.; Ma, B.; He, C.H.; Akosman, B.; Zhou, Y.; Lee, C.M.; El-Deiry, W.S.; Huntington, K.; Liang, O.; Machan, J.T.; et al. Chitinase 3-like-1 is a therapeutic target that mediates the effects of aging in COVID-19. JCI Insight 2021, 6, e148749. [Google Scholar] [CrossRef]
- Koka, V.; Huang, X.R.; Chung, A.C.; Wang, W.; Truong, L.D.; Lan, H.Y. Angiotensin II up-regulates angiotensin I-converting enzyme (ACE), but down-regulates ACE2 via the AT1-ERK/p38 MAP kinase pathway. Am. J. Pathol. 2008, 172, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Elshafei, A.; Khidr, E.G.; El-Husseiny, A.A.; Gomaa, M.H. RAAS, ACE2 and COVID-19; a mechanistic review. Saudi J. Biol. Sci. 2021, 28, 6465–6470. [Google Scholar] [CrossRef]
- Song, B.; Jin, H.; Yu, X.; Zhang, Z.; Yu, H.; Ye, J.; Xu, Y.; Zhou, T.; Oudit, G.Y.; Ye, J.Y.; et al. Angiotensin-converting enzyme 2 attenuates oxidative stress and VSMC proliferation via the JAK2/STAT3/SOCS3 and profilin-1/MAPK signaling pathways. Regul. Pept. 2013, 185, 44–51. [Google Scholar] [CrossRef]
- Gong, J.; Lu, Z.; Li, R.; Xu, C.; Jin, X. ACE2 inhibits proliferation of smooth muscle cell through AT1R and its downstream signaling pathway. J. Biosci. 2023, 48, 25. [Google Scholar] [CrossRef]
- Yang, H.; Khalil, R.A. ADAM and ADAMTS disintegrin and metalloproteinases as major factors and molecular targets in vascular malfunction and disease. Adv. Pharmacol. 2022, 94, 255–363. [Google Scholar] [CrossRef]
- Town, T.; Laouar, Y.; Pittenger, C.; Mori, T.; Szekely, C.A.; Tan, J.; Duman, R.S.; Flavell, R.A. Blocking TGF-beta-Smad2/3 innate immune signaling mitigates Alzheimer-like pathology. Nat. Med. 2008, 14, 681–687. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, J.; Yan, Y.; Ruan, Z.; Su, Y.; Wang, J.; Huang, H.; Zhang, Y.; Wang, W.; Gao, J.; et al. Angiotensin-converting enzyme 2 regulates autophagy in acute lung injury through AMPK/mTOR signaling. Arch. Biochem. Biophys. 2019, 672, 108061. [Google Scholar] [CrossRef]
- Pang, L.; Liu, Z.; Zhou, K.; Chen, P.; Pan, E.; Che, Y.; Qi, X. ACE2 Rescues Impaired Autophagic Flux Through the PI3K/AKT Pathway After Subarachnoid Hemorrhage. Neurochem. Res. 2022, 47, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Zhang, J.; Wang, C.; Jain, P.P.; Xiong, M.; Shi, X.; Lei, Y.; Chen, S.; Yin, Q.; Thistlethwaite, P.A.; et al. MDM2-Mediated Ubiquitination of Angiotensin-Converting Enzyme 2 Contributes to the Development of Pulmonary Arterial Hypertension. Circulation 2020, 142, 1190–1204. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Gu, X.H.; Li, M.; Cheng, Z.J.; Tian, S.; Liao, Y.; Liu, X. MicroRNA-155-5p Targets SKP2, Activates IKKβ, Increases Aβ Aggregation, and Aggravates a Mouse Alzheimer Disease Model. J. Neuropathol. Exp. Neurol. 2022, 81, 16–26. [Google Scholar] [CrossRef]
- Porchietto, E.; Morello, G.; Cicilese, G.; Rainero, I.; Rubino, E.; Tamagno, E.; Boschi, S.; Guglielmotto, M. UCH-L1 in Alzheimer’s Disease: A Crucial Player in Dementia-Associated Mechanisms. Int. J. Mol. Sci. 2025, 26, 9012. [Google Scholar] [CrossRef] [PubMed]
- Chuang, H.C.; Hsueh, C.H.; Hsu, P.M.; Huang, R.H.; Tsai, C.Y.; Chung, N.H.; Chow, Y.H.; Tan, T.H. SARS-CoV-2 Spike protein enhances MAP4K3/GLK-induced ACE2 stability in COVID-19. EMBO Mol. Med. 2022, 14, e15904. [Google Scholar] [CrossRef]
- Sahin, U.; de Thé, H.; Lallemand-Breitenbach, V. Sumoylation in Physiology, Pathology and Therapy. Cells 2022, 11, 814. [Google Scholar] [CrossRef]
- Conz, A.; Musi, C.A.; Russo, L.; Borsello, T.; Colnaghi, L. Super-resolution study of PIAS SUMO E3-ligases in hippocampal and cortical neurons. Eur. J. Histochem. 2021, 65, 3241. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yong, H.; Wang, W.; Gao, Y.; Wang, P.; Chen, X.; Lu, J.; Zheng, J.; Bai, J. GSK3326595 is a promising drug to prevent SARS-CoV-2 Omicron and other variants infection by inhibiting ACE2-R671 di-methylation. J. Med. Virol. 2023, 95, e28158. [Google Scholar] [CrossRef]
- Shajahan, A.; Archer-Hartmann, S.; Supekar, N.T.; Gleinich, A.S.; Heiss, C.; Azadi, P. Comprehensive characterization of N- and O- glycosylation of SARS-CoV-2 human receptor angiotensin converting enzyme 2. Glycobiology 2021, 31, 410–424. [Google Scholar] [CrossRef]
- Jia, H.P.; Look, D.C.; Tan, P.; Shi, L.; Hickey, M.; Gakhar, L.; Chappell, M.C.; Wohlford-Lenane, C.; McCray, P.B., Jr. Ectodomain shedding of angiotensin converting enzyme 2 in human airway epithelia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 297, L84–L96. [Google Scholar] [CrossRef] [PubMed]
- Healy, E.F. How tetraspanin-mediated cell entry of SARS-CoV-2 can dysregulate the shedding of the ACE2 receptor by ADAM17. Biochem. Biophys. Res. Commun. 2022, 593, 52–56. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.C.; Dos Santos, V.M.; da Silva, M.V.B.; Prazeres, T.C.M.M.; Cartágenes, M.D.S.S.; Calzerra, N.T.M.; de Queiroz, T.M. Involvement of shedding induced by ADAM17 on the nitric oxide pathway in hypertension. Front. Mol. Biosci. 2022, 9, 1032177. [Google Scholar] [CrossRef]
- Bobkova, N.V. The Balance between Two Branches of RAS Can Protect from Severe COVID-19 Course. Biochem. Suppl. Ser. A Membr. Cell. Biol. 2021, 15, 36–51. [Google Scholar] [CrossRef]
- Jing, W.; Procko, E. ACE2-based decoy receptors for SARS coronavirus 2. Proteins 2021, 89, 1065–1078. [Google Scholar] [CrossRef]
- Yeung, M.L.; Teng, J.L.L.; Jia, L.; Zhang, C.; Huang, C.; Cai, J.P.; Zhou, R.; Chan, K.H.; Zhao, H.; Zhu, L.; et al. Soluble ACE2-mediated cell entry of SARS-CoV-2 via interaction with proteins related to the renin-angiotensin system. Cell 2021, 184, 2212–2228.e12, Erratum in Cell 2023, 186, 5428–5432. [Google Scholar] [CrossRef]
- Lin, M.S.; Chao, T.L.; Chou, Y.C.; Yi, Y.; Chen, C.L.; Huang, K.Y.; Chang, S.Y.; Yang, P.C. The ACE2 decoy receptor can overcome immune escape by rapid mutating SARS-CoV-2 variants and reduce cytokine induction and clot formation. J. Biomed. Sci. 2025, 32, 59. [Google Scholar] [CrossRef]
- Deshotels, M.R.; Xia, H.; Sriramula, S.; Lazartigues, E.; Filipeanu, C.M. Angiotensin II mediates angiotensin converting enzyme type 2 internalization and degradation through an angiotensin II type I receptor-dependent mechanism. Hypertension 2014, 64, 1368–1375, Erratum in Hypertension 2014, 64, e8. [Google Scholar] [CrossRef]
- Dong, F.; Li, H.; Liu, L.; Yao, L.L.; Wang, J.; Xiang, D.; Ma, J.; Zhang, G.; Zhang, S.; Li, J.; et al. ACE2 negatively regulates the Warburg effect and suppresses hepatocellular carcinoma progression via reducing ROS-HIF1α activity. Int. J. Biol. Sci. 2023, 19, 2613–2629. [Google Scholar] [CrossRef]
- Engler, M.; Albers, D.; Von Maltitz, P.; Groß, R.; Münch, J.; Cirstea, I.C. ACE2-EGFR-MAPK signaling contributes to SARS-CoV-2 infection. Life Sci. Alliance 2023, 6, e202201880. [Google Scholar] [CrossRef] [PubMed]
- Gekle, M.; Dubourg, V.; Schwerdt, G.; Benndorf, R.A.; Schreier, B. The role of EGFR in vascular AT1R signaling: From cellular mechanisms to systemic relevance. Biochem. Pharmacol. 2023, 217, 115837. [Google Scholar] [CrossRef] [PubMed]
- Heurich, A.; Hofmann-Winkler, H.; Gierer, S.; Liepold, T.; Jahn, O.; Pöhlmann, S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus Spike protein. J. Virol. 2014, 88, 1293–1307. [Google Scholar] [CrossRef] [PubMed]
- Muhanna, D.; Arnipalli, S.R.; Kumar, S.B.; Ziouzenkova, O. Osmotic Adaptation by Na+-Dependent Transporters and ACE2: Correlation with Hemostatic Crisis in COVID-19. Biomedicines 2020, 8, 460. [Google Scholar] [CrossRef]
- Singer, D.; Camargo, S.M. Collectrin and ACE2 in renal and intestinal amino acid transport. Channels 2011, 5, 410–423. [Google Scholar] [CrossRef] [PubMed]
- Bröer, A.; Hu, Z.; Kukułowicz, J.; Yadav, A.; Zhang, T.; Dai, L.; Bajda, M.; Yan, R.; Bröer, S. Cryo-EM structure of ACE2-SIT1 in complex with tiagabine. J. Biol. Chem. 2024, 300, 107687. [Google Scholar] [CrossRef]
- Severe COVID-19 GWAS Group; Ellinghaus, D.; Degenhardt, F.; Bujanda, L.; Buti, M.; Albillos, A.; Invernizzi, P.; Fernández, J.; Prati, D.; Baselli, G.; et al. Genomewide Association Study of Severe COVID-19 with Respiratory Failure. N. Engl. J. Med. 2020, 383, 1522–1534. [Google Scholar] [CrossRef]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Y.; Leung, E.L.; Yao, X. In silico study of intrinsic dynamics of full-length apo-ACE2 and RBD-ACE2 complex. Comput. Struct. Biotechnol. J. 2021, 19, 5455–5465. [Google Scholar] [CrossRef]
- El-Baba, T.J.; Lutomski, C.A.; Burnap, S.A.; Bolla, J.R.; Baker, L.A.; Baldwin, A.J.; Struwe, W.B.; Robinson, C.V. Uncovering the Role of N-Glycan Occupancy on the Cooperative Assembly of Spike and Angiotensin Converting Enzyme 2 Complexes: Insights from Glycoengineering and Native Mass Spectrometry. J. Am. Chem. Soc. 2023, 145, 8021–8032. [Google Scholar] [CrossRef]
- Li, H.Z.; Pike, A.C.W.; Lotsaris, I.; Chi, G.; Hansen, J.S.; Lee, S.C.; Rödström, K.E.J.; Bushell, S.R.; Speedman, D.; Evans, A.; et al. Structure and function of the SIT1 proline transporter in complex with the COVID-19 receptor ACE2. Nat. Commun. 2024, 15, 5503. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, A.; Pawnikar, S.; Miao, Y. Mechanism of Ligand Recognition by Human ACE2 Receptor. J. Phys. Chem. Lett. 2021, 12, 4814–4822. [Google Scholar] [CrossRef]
- O’Mara, M.; Oakley, A.; Bröer, S. Mechanism and putative structure of B(0)-like neutral amino acid transporters. J. Membr. Biol. 2006, 213, 111–118. [Google Scholar] [CrossRef]
- Chu, P.L.; Le, T.H. Role of collectrin, an ACE2 homologue, in blood pressure homeostasis. Curr. Hypertens. Rep. 2014, 16, 490. [Google Scholar] [CrossRef]
- Chen, Q.; Gao, F.; Gao, Y.; Yang, S.; Cao, J.; Deng, H.; Yang, F.; Wang, Y.; Yuan, L. Intestinal ACE2 regulates glucose metabolism in diet-induced obese mice through a novel gut-islet axis mediated by tryptophan. Obesity 2023, 31, 1311–1325. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Perlot, T.; Rehman, A.; Trichereau, J.; Ishiguro, H.; Paolino, M.; Sigl, V.; Hanada, T.; Hanada, R.; Lipinski, S.; et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 2012, 487, 477–481. [Google Scholar] [CrossRef]
- Camargo, S.M.; Singer, D.; Makrides, V.; Huggel, K.; Pos, K.M.; Wagner, C.A.; Kuba, K.; Danilczyk, U.; Skovby, F.; Kleta, R.; et al. Tissue-specific amino acid transporter partners ACE2 and collectrin differentially interact with hartnup mutations. Gastroenterology 2009, 136, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Kang, Z.; Gong, H.; Xu, D.; Wang, J.; Li, Z.; Li, Z.; Cui, X.; Xiao, J.; Zhan, J.; et al. Digestive system is a potential route of COVID-19: An analysis of single-cell coexpression pattern of key proteins in viral entry process. Gut 2020, 69, 1010–1018. [Google Scholar] [CrossRef]
- Chen, Y.; Dinges, M.M.; Green, A.; Cramer, S.E.; Larive, C.K.; Lytle, C. Absorptive transport of amino acids by the rat colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G189–G202. [Google Scholar] [CrossRef] [PubMed]
- Kravetz, Z.; Schmidt-Kastner, R. New aspects for the brain in Hartnup disease based on mining of high-resolution cellular mRNA expression data for SLC6A19. IBRO Neurosci. Rep. 2023, 14, 393–397. [Google Scholar] [CrossRef]
- Kowalczuk, S.; Bröer, A.; Munzinger, M.; Tietze, N.; Klingel, K.; Bröer, S. Molecular cloning of the mouse IMINO system: An Na+-and Cl--dependent proline transporter. Biochem. J. 2005, 386, 417–422. [Google Scholar] [CrossRef]
- Singer, D.; Camargo, S.M.; Huggel, K.; Romeo, E.; Danilczyk, U.; Kuba, K.; Chesnov, S.; Caron, M.G.; Penninger, J.M.; Verrey, F. Orphan transporter SLC6A18 is renal neutral amino acid transporter B0AT3. J. Biol. Chem. 2009, 284, 19953–19960. [Google Scholar] [CrossRef] [PubMed]
- Vuille-dit-Bille, R.N.; Camargo, S.M.; Emmenegger, L.; Sasse, T.; Kummer, E.; Jando, J.; Hamie, Q.M.; Meier, C.F.; Hunziker, S.; Forras-Kaufmann, Z.; et al. Human intestine luminal ACE2 and amino acid transporter expression increased by ACE-inhibitors. Amino Acids 2015, 47, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Y.; Zhang, Y.; Shen, Y.; Xu, K.; Liu, Y.; Wang, Z.; Yan, R. Structural insight into the substrate recognition and transport mechanism of amino acid transporter complex ACE2-B0AT1 and ACE2-SIT1. Cell Discov. 2023, 9, 93. [Google Scholar] [CrossRef]
- Yang, G.; Chu, P.L.; Rump, L.C.; Le, T.H.; Stegbauer, J. ACE2 and the Homolog Collectrin in the Modulation of Nitric Oxide and Oxidative Stress in Blood Pressure Homeostasis and Vascular Injury. Antioxid. Redox Signal. 2017, 26, 645–659. [Google Scholar] [CrossRef]
- Kliche, J.; Kuss, H.; Ali, M.; Ivarsson, Y. Cytoplasmic short linear motifs in ACE2 and integrin β3 link SARS-CoV-2 host cell receptors to mediators of endocytosis and autophagy. Sci. Signal 2021, 14, eabf1117. [Google Scholar] [CrossRef]
- Zhang, Q.; Gefter, J.; Sneddon, W.B.; Mamonova, T.; Friedman, P.A. ACE2 interaction with cytoplasmic PDZ protein enhances SARS-CoV-2 invasion. iScience 2021, 24, 102770. [Google Scholar] [CrossRef]
- Fanning, A.S.; Anderson, J.M. PDZ domains: Fundamental building blocks in the organization of protein complexes at the plasma membrane. J. Clin. Investig. 1999, 103, 767–772. [Google Scholar] [CrossRef]
- Caillet-Saguy, C.; Wolff, N. PDZ-Containing Proteins Targeted by the ACE2 Receptor. Viruses 2021, 13, 2281. [Google Scholar] [CrossRef]
- Shenoy, S.K.; Lefkowitz, R.J. β-Arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol. Sci. 2011, 32, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Gahmberg, C.G.; Grönholm, M. How integrin phosphorylations regulate cell adhesion and signaling. Trends Biochem. Sci. 2022, 47, 265–278. [Google Scholar] [CrossRef]
- Mia, M.S.; Hossain, D.; Woodbury, E.; Kelleher, S.; Palamuttam, R.J.; Rao, R.; Steen, P.; Jarajapu, Y.P.; Mathew, S. Integrin β1 is a key determinant of the expression of angiotensin-converting enzyme 2 (ACE2) in the kidney epithelial cells. Eur. J. Cell. Biol. 2023, 102, 151316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Z.; Nguyen, H.T.T.; Watson, A.J.; Lao, Q.; Li, A.; Zhu, J. Integrin α5β1 contributes to cell fusion and inflammation mediated by SARS-CoV-2 Spike via RGD-independent interaction. Proc. Natl. Acad. Sci. USA 2023, 120, e2311913120. [Google Scholar] [CrossRef]
- Han, H.Y.; Zhang, J.P.; Ji, S.Q.; Liang, Q.M.; Kang, H.C.; Tang, R.H.; Zhu, S.Q.; Xue, Z. αν and β1 Integrins mediate Aβ-induced neurotoxicity in hippocampal neurons via the FAK signaling pathway. PLoS ONE 2013, 8, e64839. [Google Scholar] [CrossRef]
- Wang, J.; Chen, S.; Bihl, J. Exosome-Mediated Transfer of ACE2 (Angiotensin-Converting Enzyme 2) from Endothelial Progenitor Cells Promotes Survival and Function of Endothelial Cell. Oxid. Med. Cell. Longev. 2020, 2020, 4213541. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Cheng, C.; Liu, S. Angiotensin-converting enzyme 2 augments the effects of endothelial progenitor cells-exosomes on vascular smooth muscle cell phenotype transition. Cell Tissue Res. 2020, 382, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Wang, Y.; Liu, J.; Jin, X.; Xiang, Z.; Li, S.; Shi, Y.; Chen, Y.; Zhong, W.; Ma, X. MiR-17-5p Mediates the Effects of ACE2-Enriched Endothelial Progenitor Cell-Derived Exosomes on Ameliorating Cerebral Ischemic Injury in Aged Mice. Mol. Neurobiol. 2023, 60, 3534–3552. [Google Scholar] [CrossRef]
- Ng, K.W.; Attig, J.; Bolland, W.; Young, G.R.; Major, J.; Wrobel, A.G.; Gamblin, S.; Wack, A.; Kassiotis, G. Tissue-specific and interferon-inducible expression of nonfunctional ACE2 through endogenous retroelement co-option. Nat. Genet. 2020, 52, 1294–1302. [Google Scholar] [CrossRef]
- Samuel, C.E. Interferon at the crossroads of SARS-CoV-2 infection and COVID-19 disease. J. Biol. Chem. 2023, 299, 104960. [Google Scholar] [CrossRef]
- Busnadiego, I.; Fernbach, S.; Pohl, M.O.; Karakus, U.; Huber, M.; Trkola, A.; Stertz, S.; Hale, B.G. Antiviral Activity of Type I.; II.; and III Interferons Counterbalances ACE2 Inducibility and Restricts SARS-CoV-2. mBio 2020, 11, e01928-20. [Google Scholar] [CrossRef]
- de Souza, A.S.; de Freitas Amorim, V.M.; Guardia, G.D.A.; Dos Santos, F.R.C.; Dos Santos, F.F.; de Souza, R.F.; de Araujo Juvenal, G.; Huang, Y.; Ge, P.; Jiang, Y.; et al. Molecular Dynamics Analysis of Fast-Spreading Severe Acute Respiratory Syndrome Coronavirus 2 Variants and Their Effects on the Interaction with Human Angiotensin-Converting Enzyme 2. ACS Omega. 2022, 7, 30700–30709. [Google Scholar] [CrossRef]
- Mpekoulis, G.; Frakolaki, E.; Taka, S.; Ioannidis, A.; Vassiliou, A.G.; Kalliampakou, K.I.; Patas, K.; Karakasiliotis, I.; Aidinis, V.; Chatzipanagiotou, S.; et al. Alteration of L-Dopa decarboxylase expression in SARS-CoV-2 infection and its association with the interferon-inducible ACE2 isoform. PLoS ONE 2021, 16, e0253458. [Google Scholar] [CrossRef]
- Chai, Y.L.; Lee, J.H.; Chong, J.R.; Ballard, C.; Francis, P.T.; Kennedy, B.K.; Arumugam, T.V.; Chen, C.P.; Aarsland, D.; Lai, M.K.P. Inflammatory panel cytokines are elevated in the neocortex of late-stage Alzheimer’s disease but not Lewy body dementias. J. Neuroinflammation. 2023, 20, 111. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.Y.; Chung, Y.C.; Jin, B.K. Interleukin-4 and Interleukin-13 Exacerbate Neurotoxicity of Prothrombin Kringle-2 in Cortex In Vivo via Oxidative Stress. Int. J. Mol. Sci. 2019, 20, 1927. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Sriramula, S.; Lazartigues, E. Excessive Glutamate Stimulation Impairs ACE2 Activity Through ADAM17-Mediated Shedding in Cultured Cortical Neurons. Cell. Mol. Neurobiol. 2018, 38, 1235–1243. [Google Scholar] [CrossRef]
- Parekh, R.U.; Sriramula, S. Activation of Kinin B1R Upregulates ADAM17 and Results in ACE2 Shedding in Neurons. Int. J. Mol. Sci. 2020, 22, 145. [Google Scholar] [CrossRef]
- Silva, C.C.; Correa, A.M.B.; Kushmerick, C.; Sharma, N.M.; Patel, K.P.; de Almeida, J.F.Q.; Moreira, F.A.; Ferreira, A.J.; Fontes, M.A.P. Angiotensin-converting enzyme 2 activator, DIZE in the basolateral amygdala attenuates the tachycardic response to acute stress by modulating glutamatergic tone. Neuropeptides 2020, 83, 102076. [Google Scholar] [CrossRef] [PubMed]
- Durante, W. Glutamine Deficiency Promotes Immune and Endothelial Cell Dysfunction in COVID-19. Int. J. Mol. Sci. 2023, 24, 7593. [Google Scholar] [CrossRef]
- Jia, X.; Zhu, J.; Bian, X.; Liu, S.; Yu, S.; Liang, W.; Jiang, L.; Mao, R.; Zhang, W.; Rao, Y. Importance of glutamine in synaptic vesicles revealed by functional studies of SLC6A17 and its mutations pathogenic for intellectual disability. Elife 2023, 12, RP86972. [Google Scholar] [CrossRef]
- Hägglund, M.G.; Hellsten, S.V.; Bagchi, S.; Ljungdahl, A.; Nilsson, V.C.; Winnergren, S.; Stephansson, O.; Rumaks, J.; Svirskis, S.; Klusa, V.; et al. Characterization of the transporter B0AT3 (Slc6a17) in the rodent central nervous system. BMC Neurosci. 2013, 14, 54. [Google Scholar] [CrossRef]
- Bhat, S.; El-Kasaby, A.; Freissmuth, M.; Sucic, S. Functional and Biochemical Consequences of Disease Variants in Neurotransmitter Transporters: A Special Emphasis on Folding and Trafficking Deficits. Pharmacol. Ther. 2021, 222, 107785, Erratum in Pharmacol. Ther. 2021, 225, 107816. [Google Scholar] [CrossRef] [PubMed]
- Burnyasheva, A.O.; Stefanova, N.A.; Kolosova, N.G.; Telegina, D.V. Changes in the Glutamate/GABA System in the Hippocampus of Rats with Age and during Alzheimer’s Disease Signs Development. Biochemistry 2023, 88, 1972–1986. [Google Scholar] [CrossRef] [PubMed]
- Pozdnyakova, N.; Krisanova, N.; Pastukhov, A.; Tarasenko, A.; Dudarenko, M.; Chernykh, A.; Pashenko, A.; Ryabukhin, S.; Tolstanova, G.; Volochnyuk, D.; et al. Neuromodulation by selective angiotensin-converting enzyme 2 inhibitors. Neuroscience 2022, 498, 155–173. [Google Scholar] [CrossRef]
- Grone, B.P.; Maruska, K.P. Three Distinct Glutamate Decarboxylase Genes in Vertebrates. Sci. Rep. 2016, 6, 30507. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, F.; Yuan, L. ACE2 Regulates Glycolipid Metabolism in Multiple Tissues. Front. Biosci. 2024, 29, 17. [Google Scholar] [CrossRef]
- Chen, Q.; Gao, Y.; Yang, F.; Deng, H.; Wang, Y.; Yuan, L. Angiotensin-converting enzyme 2 improves hepatic insulin resistance by regulating GABAergic signaling in the liver. J. Biol. Chem. 2022, 298, 102603. [Google Scholar] [CrossRef]
- Sánchez-Huertas, C.; Rico, B. CREB-Dependent Regulation of GAD65 Transcription by BDNF/TrkB in Cortical Interneurons. Cereb. Cortex 2011, 21, 777–788. [Google Scholar] [CrossRef]
- Mao, R.; Hu, M.; Liu, X.; Ye, L.; Xu, B.; Sun, M.; Xu, S.; Shao, W.; Tan, Y.; Xu, Y.; et al. Impairments of GABAergic transmission in hippocampus mediate increased susceptibility of epilepsy in the early stage of Alzheimer’s disease. Cell Commun. Signal. 2024, 22, 147. [Google Scholar] [CrossRef]
- Parekh, R.U.; Robidoux, J.; Sriramula, S. Kinin B1 Receptor Blockade Prevents Angiotensin II-induced Neuroinflammation and Oxidative Stress in Primary Hypothalamic Neurons. Cell. Mol. Neurobiol. 2020, 40, 845–857. [Google Scholar] [CrossRef]
- Dominguez-Meijide, A.; Rodriguez-Perez, A.I.; Diaz-Ruiz, C.; Guerra, M.J.; Labandeira-Garcia, J.L. Dopamine modulates astroglial and microglial activity via glial renin-angiotensin system in cultures. Brain Behav. Immun. 2017, 62, 277–290. [Google Scholar] [CrossRef]
- Rodriguez-Pallares, J.; Rey, P.; Parga, J.A.; Muñoz, A.; Guerra, M.J.; Labandeira-Garcia, J.L. Brain angiotensin enhances dopaminergic cell death via microglial activation and NADPH-derived ROS. Neurobiol. Dis. 2008, 31, 58–73. [Google Scholar] [CrossRef]
- Joglar, B.; Rodriguez-Pallares, J.; Rodriguez-Perez, A.I.; Rey, P.; Guerra, M.J.; Labandeira-Garcia, J.L. The inflammatory response in the MPTP model of Parkinson’s disease is mediated by brain angiotensin: Relevance to progression of the disease. J. Neurochem. 2009, 109, 656–669. [Google Scholar] [CrossRef]
- Garrido-Gil, P.; Valenzuela, R.; Villar-Cheda, B.; Lanciego, J.L.; Labandeira-Garcia, J.L. Expression of angiotensinogen and receptors for angiotensin and prorenin in the monkey and human substantia nigra: An intracellular renin-angiotensin system in the nigra. Brain Struct. Funct. 2013, 218, 373–388. [Google Scholar] [CrossRef] [PubMed]
- Quijano, A.; Diaz-Ruiz, C.; Lopez-Lopez, A.; Villar-Cheda, B.; Muñoz, A.; Rodriguez-Perez, A.I.; Labandeira-Garcia, J.L. Angiotensin Type-1 Receptor Inhibition Reduces NLRP3 Inflammasome Upregulation Induced by Aging and Neurodegeneration in the Substantia Nigra of Male Rodents and Primary Mesencephalic Cultures. Antioxidants 2022, 11, 329. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pinilla, E.; Rodríguez-Pérez, A.I.; Navarro, G.; Aguinaga, D.; Moreno, E.; Lanciego, J.L.; Labandeira-García, J.L.; Franco, R. Dopamine D2 and angiotensin II type 1 receptors form functional heteromers in rat striatum. Biochem. Pharmacol. 2015, 96, 131–142. [Google Scholar] [CrossRef]
- Aschrafi, A.; Berndt, A.; Kowalak, J.A.; Gale, J.R.; Gioio, A.E.; Kaplan, B.B. Angiotensin II mediates the axonal trafficking of tyrosine hydroxylase and dopamine β-hydroxylase mRNAs and enhances norepinephrine synthesis in primary sympathetic neurons. J. Neurochem. 2019, 150, 666–677. [Google Scholar] [CrossRef]
- Labandeira-Garcia, J.L.; Labandeira, C.M.; Guerra, M.J.; Rodriguez-Perez, A.I. The role of the brain renin-angiotensin system in Parkinson’s disease. Transl. Neurodegener. 2024, 13, 22. [Google Scholar] [CrossRef] [PubMed]
- Shebl, N.; Salama, M. Exploring dopa decarboxylase as an ideal biomarker in Parkinson’s disease with focus on regulatory mechanisms, cofactor influences, and metabolic implications. NPJ Biomed. Innov. 2025, 2, 2. [Google Scholar] [CrossRef]
- Blum, K.; Cadet, J.L.; Baron, D.; Badgaiyan, R.D.; Brewer, R.; Modestino, E.J.; Gold, M.S. Putative COVID-19 Induction of Reward Deficiency Syndrome (RDS) and Associated Behavioral Addictions with Potential Concomitant Dopamine Depletion: Is COVID-19 Social Distancing a Double Edged Sword? Subst. Use Misuse 2020, 55, 2438–2442. [Google Scholar] [CrossRef]
- Labandeira, C.M.; Pedrosa, M.A.; Quijano, A.; Valenzuela, R.; Garrido-Gil, P.; Sanchez-Andrade, M.; Suarez-Quintanilla, J.A.; Rodriguez-Perez, A.I.; Labandeira-Garcia, J.L. Angiotensin type-1 receptor and ACE2 autoantibodies in Parkinson’s disease. NPJ Parkinsons Dis. 2022, 8, 76. [Google Scholar] [CrossRef]
- Villar-Cheda, B.; Dominguez-Meijide, A.; Valenzuela, R.; Granado, N.; Moratalla, R.; Labandeira-Garcia, J.L. Aging-related dysregulation of dopamine and angiotensin receptor interaction. Neurobiol. Aging 2014, 35, 1726–1738. [Google Scholar] [CrossRef]
- Villar-Cheda, B.; Rodríguez-Pallares, J.; Valenzuela, R.; Muñoz, A.; Guerra, M.J.; Baltatu, O.C.; Labandeira-Garcia, J.L. Nigral and striatal regulation of angiotensin receptor expression by dopamine and angiotensin in rodents: Implications for progression of Parkinson’s disease. Eur. J. Neurosci. 2010, 32, 1695–1706. [Google Scholar] [CrossRef]
- Singer, D.; Camargo, S.M.; Ramadan, T.; Schäfer, M.; Mariotta, L.; Herzog, B.; Huggel, K.; Wolfer, D.; Werner, S.; Penninger, J.M.; et al. Defective intestinal amino acid absorption in Ace2 null mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G686–G695. [Google Scholar] [CrossRef] [PubMed]
- Klempin, F.; Mosienko, V.; Matthes, S.; Villela, D.C.; Todiras, M.; Penninger, J.M.; Bader, M.; Santos, R.A.S.; Alenina, N. Depletion of angiotensin-converting enzyme 2 reduces brain serotonin and impairs the running-induced neurogenic response. Cell. Mol. Life Sci. 2018, 75, 3625–3634. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.C.; Devason, A.S.; Umana, I.C.; Cox, T.O.; Dohnalová, L.; Litichevskiy, L.; Perla, J.; Lundgren, P.; Etwebi, Z.; Izzo, L.T.; et al. Serotonin reduction in post-acute sequelae of viral infection. Cell 2023, 186, 4851–4867.e20. [Google Scholar] [CrossRef]
- Leitzke, M. Is the post-COVID-19 syndrome a severe impairment of acetylcholine-orchestrated neuromodulation that responds to nicotine administration? Bioelectron. Med. 2023, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Kalashnyk, O.; Lykhmus, O.; Sullivan, R.; Komisarenko, S.; Skok, M. Agonists or positive allosteric modulators of α7 nicotinic acetylcholine receptor prevent interaction of SARS-CoV-2 receptor-binding domain with astrocytoma cells. Biochem. Biophys. Res. Commun. 2024, 709, 149825. [Google Scholar] [CrossRef]
- Chen, F.; Chen, Y.; Ke, Q.; Wang, Y.; Gong, Z.; Chen, X.; Cai, Y.; Li, S.; Sun, Y.; Peng, X.; et al. ApoE4 associated with severe COVID-19 outcomes via downregulation of ACE2 and imbalanced RAS pathway. J. Transl. Med. 2023, 21, 103. [Google Scholar] [CrossRef]
- Piccarducci, R.; Giacomelli, C.; Bertilacchi, M.S.; Benito-Martinez, A.; Di Giorgi, N.; Daniele, S.; Signore, G.; Rocchiccioli, S.; Vilar, M.; Marchetti, L.; et al. Apolipoprotein E ε4 triggers neurotoxicity via cholesterol accumulation.; acetylcholine dyshomeostasis.; and PKCε mislocalization in cholinergic neuronal cells. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166793. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Wang, Q.; Xue, Q.; Li, W.; Li, X.; Wu, Y. The Dual Role of Kinin/Kinin Receptors System in Alzheimer’s Disease. Front. Mol. Neurosci. 2019, 12, 234. [Google Scholar] [CrossRef]
- Mavrikaki, M.; Lee, J.D.; Solomon, I.H.; Slack, F.J. Severe COVID-19 is associated with molecular signatures of aging in the human brain. Nat. Aging 2022, 2, 1130–1137. [Google Scholar] [CrossRef]
- Zlacká, J.; Stebelová, K.; Zeman, M.; Herichová, I. Interactions of renin-angiotensin system and COVID-19: The importance of daily rhythms in ACE2, ADAM17 and TMPRSS2 expression. Physiol. Res. 2021, 70, S177–S194. [Google Scholar] [CrossRef]
- Miller, N.L.; Clark, T.; Raman, R.; Sasisekharan, R. Glycans in Virus-Host Interactions: A Structural Perspective. Front. Mol. Biosci. 2021, 8, 666756. [Google Scholar] [CrossRef]
- Bejoy, J.; Williams, C.I.; Cole, H.J.; Manzoor, S.; Davoodi, P.; Battaile, J.I.; Kaushik, A.; Nikolaienko, S.I.; Brelidze, T.I.; Gychka, S.G.; et al. Effects of Spike proteins on angiotensin converting enzyme 2 (ACE2). Arch. Biochem. Biophys. 2023, 748, 109769. [Google Scholar] [CrossRef]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef]
- Lim, S.; Zhang, M.; Chang, T.L. ACE2-Independent Alternative Receptors for SARS-CoV-2. Viruses 2022, 14, 2535. [Google Scholar] [CrossRef]
- Kettunen, P.; Lesnikova, A.; Räsänen, N.; Ojha, R.; Palmunen, L.; Laakso, M.; Lehtonen, Š.; Kuusisto, J.; Pietiläinen, O.; Saber, S.H.; et al. SARS-CoV-2 Infection of Human Neurons Is TMPRSS2 Independent, Requires Endosomal Cell Entry, and Can Be Blocked by Inhibitors of Host Phosphoinositol-5 Kinase. J. Virol. 2023, 97, e0014423. [Google Scholar] [CrossRef] [PubMed]
- Essalmani, R.; Jain, J.; Susan-Resiga, D.; Andréo, U.; Evagelidis, A.; Derbali, R.M.; Huynh, D.N.; Dallaire, F.; Laporte, M.; Delpal, A.; et al. Distinctive Roles of Furin and TMPRSS2 in SARS-CoV-2 Infectivity. J. Virol. 2022, 96, e0012822, Erratum in J. Virol. 2022, 96, e0074522. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhu, Q.; Fox, D.M.; Gao, C.; Stanley, S.A.; Luo, K. SARS-CoV-2 down-regulates ACE2 through lysosomal degradation. Mol. Biol. Cell 2022, 33, ar147. [Google Scholar] [CrossRef]
- Liu, M.; Lu, B.; Li, Y.; Yuan, S.; Zhuang, Z.; Li, G.; Wang, D.; Ma, L.; Zhu, J.; Zhao, J.; et al. P21-activated kinase 1 (PAK1)-mediated cytoskeleton rearrangement promotes SARS-CoV-2 entry and ACE2 autophagic degradation. Signal Transduct. Target. Ther. 2023, 8, 385. [Google Scholar] [CrossRef]
- Chatterjee, B.; Thakur, S.S. SARS-CoV-2 Infection Triggers Phosphorylation: Potential Target for Anti-COVID-19 Therapeutics. Front. Immunol. 2022, 13, 829474. [Google Scholar] [CrossRef]
- Shajahan, S.R.; Kumar, S.; Ramli, M.D.C. Unravelling the connection between COVID-19 and Alzheimer’s disease: A comprehensive review. Front. Aging Neurosci. 2024, 15, 1274452. [Google Scholar] [CrossRef]
- Li, S.; Sun, J.; Li, H.; Han, Z.; Wang, T.; Gao, S.; Zhu, P.; Chen, Y.; Yan, P.; Wang, M.; et al. Expression of SARS-CoV-2 entry receptor ACE2 in human brain and its association with Alzheimer’s disease and COVID-19. Mol. Psychiatry. 2025, 30, 3257–3268. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Shults, N.V.; Gychka, S.G.; Harris, B.T.; Suzuki, Y.J. Protein Expression of Angiotensin-Converting Enzyme 2 (ACE2) is Upregulated in Brains with Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 1687. [Google Scholar] [CrossRef]
- Lim, K.H.; Yang, S.; Kim, S.H.; Joo, J.Y. Elevation of ACE2 as a SARS-CoV-2 entry receptor gene expression in Alzheimer’s disease. J. Infect. 2020, 81, e33–e34. [Google Scholar] [CrossRef] [PubMed]
- Lennol, M.P.; García-Ayllón, M.S.; Avilés-Granados, C.; Trasciatti, C.; Tolassi, C.; Quaresima, V.; Arici, D.; Cristillo, V.; Volonghi, I.; Caprioli, F.; et al. Increased Cerebrospinal Fluid Angiotensin-Converting Enzyme 2 Fragments as a Read-Out of Brain Infection in Patients With COVID-19 Encephalopathy. J. Infect. Dis. 2025, 231, e929–e940. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Zhang, L.; Qin, C. Overexpression of ACE2 ameliorates Aβ-induced blood-brain barrier damage and angiogenesis by inhibiting NF-κB/VEGF/VEGFR2 pathway. Animal Model Exp. Med. 2023, 6, 237–244, Erratum in Animal Model Exp. Med. 2024, 7, 966–967.. [Google Scholar] [CrossRef] [PubMed]
- Neff, R.A.; Wang, M.; Vatansever, S.; Guo, L.; Ming, C.; Wang, Q.; Wang, E.; Horgusluoglu-Moloch, E.; Song, W.M.; Li, A.; et al. Molecular subtyping of Alzheimer’s disease using RNA sequencing data reveals novel mechanisms and targets. Sci. Adv. 2021, 7, eabb5398. [Google Scholar] [CrossRef]
- Kehoe, P.G.; Wong, S.; Al Mulhim, N.; Palmer, L.E.; Miners, J.S. Angiotensin-converting enzyme 2 is reduced in Alzheimer’s disease in association with increasing amyloid-β and tau pathology. Alzheimers Res. Ther. 2016, 8, 50. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; Miura, Y.; Tanabe, C.; Maeda, T.; Terayama, Y.; Turner, A.J.; Zou, K.; Komano, H. Conversion of Aβ43 to Aβ40 by the successive action of angiotensin-converting enzyme 2 and angiotensin-converting enzyme. J. Neurosci. Res. 2014, 92, 1178–1186. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Kempuraj, D. Role of SARS-CoV-2 Spike-Protein-Induced Activation of Microglia and Mast Cells in the Pathogenesis of Neuro-COVID. Cells 2023, 12, 688. [Google Scholar] [CrossRef]
- Lambert, D.W.; Yarski, M.; Warner, F.J.; Thornhill, P.; Parkin, E.T.; Smith, A.I.; Hooper, N.M.; Turner, A.J. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2). J. Biol. Chem. 2005, 280, 30113–30119. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Edsfeldt, A.; Svensson, J.; Ruge, T.; Goncalves, I.; Swärd, P. ADAM-17 Activity and Its Relation to ACE2: Implications for Severe COVID-19. Int. J. Mol. Sci. 2024, 25, 5911. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, F.; Wang, T.; Han, Z.; Shang, H.; Han, K.; Zhu, P.; Gao, S.; Wang, X.; Xue, Y.; et al. Shared genetics and causal association between plasma levels of SARS-CoV-2 entry receptor ACE2 and Alzheimer’s disease. CNS Neurosci. Ther. 2024, 30, e14873. [Google Scholar] [CrossRef]
- Najm, R.; Jones, E.A.; Huang, Y. Apolipoprotein E4, inhibitory network dysfunction, and Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Gkouskou, K.; Vasilogiannakopoulou, T.; Andreakos, E.; Davanos, N.; Gazouli, M.; Sanoudou, D.; Eliopoulos, A.G. COVID-19 enters the expanding network of apolipoprotein E4-related pathologies. Redox Biol. 2021, 41, 101938. [Google Scholar] [CrossRef]
- Zhang, H.; Shao, L.; Lin, Z.; Long, Q.X.; Yuan, H.; Cai, L.; Jiang, G.; Guo, X.; Yang, R.; Zhang, Z.; et al. APOE interacts with ACE2 inhibiting SARS-CoV-2 cellular entry and inflammation in COVID-19 patients. Signal Transduct. Target. Ther. 2022, 7, 261. [Google Scholar] [CrossRef]
- Konings, S.C.; Nyberg, E.; Martinsson, I.; Torres-Garcia, L.; Klementieva, O.; Guimas Almeida, C.; Gouras, G.K. Apolipoprotein E intersects with amyloid-β within neurons. Life Sci. Alliance 2023, 6, e202201887, Erratum in Life Sci. Alliance 2024, 7, e202402875.. [Google Scholar] [CrossRef]
- Ali, A.B.; Islam, A.; Constanti, A. The fate of interneurons, GABAA receptor sub-types and perineuronal nets in Alzheimer’s disease. Brain Pathol. 2023, 33, e13129. [Google Scholar] [CrossRef]
- Pszczołowska, M.; Walczak, K.; Misków, W.; Antosz, K.; Batko, J.; Karska, J.; Leszek, J. Molecular cross-talk between long COVID-19 and Alzheimer’s disease. Geroscience 2024, 46, 2885–2899, Erratum in Geroscience 2024, 46, 5393.. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, D.F.; Zou, Q.C.; Xie, X.; Xu, L.; Feng, X.L.; Li, X.; Han, J.B.; Yu, D.; Deng, Z.H.; et al. SARS-CoV-2 Spike protein S2 subunit modulates γ-secretase and enhances amyloid-β production in COVID-19 neuropathy. Cell Discov. 2022, 8, 99. [Google Scholar] [CrossRef]
- Shu, S.; Xu, S.Y.; Ye, L.; Liu, Y.; Cao, X.; Jia, J.Q.; Bian, H.J.; Liu, Y.; Zhu, X.L.; Xu, Y. Prefrontal parvalbumin interneurons deficits mediate early emotional dysfunction in Alzheimer’s disease. Neuropsychopharmacology 2023, 48, 391–401. [Google Scholar] [CrossRef]
- Motaghinejad, M.; Gholami, M. Possible Neurological and Mental Outcomes of COVID-19 Infection: A Hypothetical Role of ACE-2\Mas\BDNF Signaling Pathway. Int. J. Prev. Med. 2020, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Bu, J.; Bruckner, S.R.; Sengoku, T.; Geddes, J.W.; Estus, S. Glutamate regulates caveolin expression in rat hippocampal neurons. J. Neurosci. Res. 2003, 72, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Gaudreault, S.B.; Dea, D.; Poirier, J. Increased caveolin-1 expression in Alzheimer’s disease brain. Neurobiol. Aging 2004, 25, 753–759. [Google Scholar] [CrossRef]
- Chen, F.; Chen, Y.; Wang, Y.; Ke, Q.; Cui, L. The COVID-19 pandemic and Alzheimer’s disease: Mutual risks and mechanisms. Transl. Neurodegener. 2022, 11, 40. [Google Scholar] [CrossRef]
- Xiao, H.; Wei, J.; Yuan, L.; Li, J.; Zhang, C.; Liu, G.; Liu, X. Sex hormones in COVID-19 severity: The quest for evidence and influence mechanisms. J. Cell. Mol. Med. 2024, 28, e18490. [Google Scholar] [CrossRef]
- Gorenshtein, A.; Leibovitch, L.; Liba, T.; Stern, S.; Stern, Y. Gender Disparities in Neurological Symptoms of Long COVID: A Systematic Review and Meta-Analysis. Neuroepidemiology 2025, 59, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Serrano Del Pueblo, V.M.; Serrano-Heras, G.; Romero Sánchez, C.M.; Landete, P.P.; Rojas-Bartolome, L.; Feria, I.; Morris, R.G.M.; Strange, B.; Mansilla, F.; Zhang, L.; et al. Brain and cognitive changes in patients with long COVID compared with infection-recovered control subjects. Brain. 2024, 147, 3611–3623. [Google Scholar] [CrossRef]
- Ren, W.; Zhu, Y.; Lan, J.; Chen, H.; Wang, Y.; Shi, H.; Feng, F.; Chen, D.Y.; Close, B.; Zhao, X.; et al. Susceptibilities of Human ACE2 Genetic Variants in Coronavirus Infection. J. Virol. 2022, 96, e0149221. [Google Scholar] [CrossRef]
- Tukiainen, T.; Villani, A.C.; Yen, A.; Rivas, M.A.; Marshall, J.L.; Satija, R.; Aguirre, M.; Gauthier, L.; Fleharty, M.; Kirby, A.; et al. Landscape of X chromosome inactivation across human tissues. Nature 2017, 550, 244–248, Erratum in Nature 2018, 555, 274. [Google Scholar] [CrossRef]
- Konwar, C.; Asiimwe, R.; Inkster, A.M.; Merrill, S.M.; Negri, G.L.; Aristizabal, M.J.; Rider, C.F.; MacIsaac, J.L.; Carlsten, C.; Kobor, M.S. Risk-focused differences in molecular processes implicated in SARS-CoV-2 infection: Corollaries in DNA methylation and gene expression. Epigenetics Chromatin. 2021, 14, 54. [Google Scholar] [CrossRef]
- Jacobsen, H.; Klein, S.L. Sex Differences in Immunity to Viral Infections. Front. Immunol. 2021, 12, 720952. [Google Scholar] [CrossRef]
- Barber, A.J.; Del Genio, C.L.; Swain, A.B.; Pizzi, E.M.; Watson, S.C.; Tapiavala, V.N.; Zanazzi, G.J.; Gaur, A.B. Age, sex and Alzheimer’s disease: A longitudinal study of 3xTg-AD mice reveals sex-specific disease trajectories and inflammatory responses mirrored in postmortem brains from Alzheimer’s patients. Alzheimers Res. Ther. 2024, 16, 134. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, N.T.; Mielke, M.M. Sex Differences in Alzheimer’s Disease. Neurol. Clin. 2023, 41, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Liu, X. ACE2, a therapeutic target of COVID-19, needs to be treated with caution. Arch. Virol. 2025, 170, 143. [Google Scholar] [CrossRef]
- Chen, Y.H.; Jan, J.S.; Yang, C.H.; Yen, T.L.; Linh, T.T.D.; Annavajjula, S.; Satapathy, M.K.; Tsao, S.Y.; Hsieh, C.Y. Cognitive Sequelae of COVID-19: Mechanistic Insights and Therapeutic Approaches. CNS Neurosci. Ther. 2025, 31, e70348. [Google Scholar] [CrossRef] [PubMed]
- Scarano, N.; Musumeci, F.; Casini, B.; Brullo, C.; D’Ursi, P.; Fossa, P.; Schenone, S.; Cichero, E. Alzheimer’s Disease Etiology Hypotheses and Therapeutic Strategies: A Perspective. Int. J. Mol. Sci. 2025, 26, 6980. [Google Scholar] [CrossRef]
- Ramachandran, A.K.; Das, S.; Shenoy, G.G.; Mudgal, J.; Joseph, A. Relation between Apolipoprotein E in Alzheimer’s Disease and SARS-CoV-2 and their Treatment Strategy: A Review. CNS Neurol. Disord. Drug Targets. 2024, 23, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, S.; Cho, M.; Stumpff, J.C.; Bice, P.J.; İş, Ö.; Ertekin-Taner, N.; Saykin, A.J.; Nho, K. Cell-specific transcriptional signatures of vascular cells in Alzheimer’s disease: Perspectives, pathways, and therapeutic directions. Mol. Neurodegener. 2025, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.D.; Liang, K.; Shetty, A.K. Complications of COVID-19 on the Central Nervous System: Mechanisms and Potential Treatment for Easing Long COVID. Aging Dis. 2023, 14, 1492–1510. [Google Scholar] [CrossRef]
- Tiwari, V.; Singh, J.; Tiwari, P.; Chaturvedi, S.; Gupta, S.; Mishra, A.; Singh, S.; Wahajuddin, M.; Hanif, K.; Shukla, S. ACE2/ANG-(1–7)/Mas receptor axis activation prevents inflammation and improves cognitive functions in streptozotocin induced rat model of Alzheimer’s disease-like phenotypes. Eur. J. Pharmacol. 2023, 946, 175623. [Google Scholar] [CrossRef]
- Kuber, B.; Fadnavis, M.; Chatterjee, B. Role of angiotensin receptor blockers in the context of Alzheimer’s disease. Fundam. Clin. Pharmacol. 2023, 37, 429–445. [Google Scholar] [CrossRef]
- Lee, H.W.; Kim, S.; Jo, Y.; Kim, Y.; Ye, B.S.; Yu, Y.M. Neuroprotective effect of angiotensin II receptor blockers on the risk of incident Alzheimer’s disease: A nationwide population-based cohort study. Front. Aging Neurosci. 2023, 15, 1137197. [Google Scholar] [CrossRef]
- De Lorenzo, R.; Sciorati, C.; Lorè, N.I.; Capobianco, A.; Tresoldi, C.; Cirillo, D.M.; Ciceri, F.; Rovere-Querini, P.; Manfredi, A.A. Chitinase-3-like protein-1 at hospital admission predicts COVID-19 outcome: A prospective cohort study. Sci. Rep. 2022, 12, 7606. [Google Scholar] [CrossRef]
- Villareal, J.A.B.; Bathe, T.; Hery, G.P.; Phillips, J.L.; Tsering, W.; Prokop, S. Deterioration of neuroimmune homeostasis in Alzheimer’s Disease patients who survive a COVID-19 infection. J. Neuroinflamm. 2024, 21, 202. [Google Scholar] [CrossRef]
- Mwale, P.F.; Hsieh, C.T.; Yen, T.L.; Jan, J.S.; Taliyan, R.; Yang, C.H.; Yang, W.B. Chitinase-3-like-1: A multifaceted player in neuroinflammation and degenerative pathologies with therapeutic implications. Mol. Neurodegener. 2025, 20, 7. [Google Scholar] [CrossRef]
- Kamle, S.; Ma, B.; Lee, C.M.; Schor, G.; Zhou, Y.; Lee, C.G.; Elias, J.A. Host chitinase 3-like-1 is a universal therapeutic target for SARS-CoV-2 viral variants in COVID-19. Elife 2022, 11, e78273. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Jiang, W.; Li, Y.; Lee, C.G.; Elias, J.A.; Tang, C.; Huang, Y.A. CHI3L1/YKL-40 signaling inhibits neurogenesis in models of Alzheimer’s disease. Sci. Adv. 2025, 11, eadv1492. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kononova, S.V.; Bobkova, N.V.; Poltavtseva, R.A.; Leonov, S.; Sukhikh, G.T. ACE2: Friend or Foe in Post-COVID-19 Neurodegeneration? Int. J. Mol. Sci. 2025, 26, 11104. https://doi.org/10.3390/ijms262211104
Kononova SV, Bobkova NV, Poltavtseva RA, Leonov S, Sukhikh GT. ACE2: Friend or Foe in Post-COVID-19 Neurodegeneration? International Journal of Molecular Sciences. 2025; 26(22):11104. https://doi.org/10.3390/ijms262211104
Chicago/Turabian StyleKononova, Svetlana V., Natalia V. Bobkova, Rimma A. Poltavtseva, Sergey Leonov, and Gennadiy T. Sukhikh. 2025. "ACE2: Friend or Foe in Post-COVID-19 Neurodegeneration?" International Journal of Molecular Sciences 26, no. 22: 11104. https://doi.org/10.3390/ijms262211104
APA StyleKononova, S. V., Bobkova, N. V., Poltavtseva, R. A., Leonov, S., & Sukhikh, G. T. (2025). ACE2: Friend or Foe in Post-COVID-19 Neurodegeneration? International Journal of Molecular Sciences, 26(22), 11104. https://doi.org/10.3390/ijms262211104





