Nicotine-Induced VEGF Levels in NSCLC Cells Are Modulated by PKA, Hyaluronan, and p53
Abstract
1. Introduction
2. Results and Discussion
2.1. PKA Regulates the Levels of VEGF in the Media of A549 and H1299 Cells Untreated or Treated with Nicotine
2.2. The Levels of VEGF Decreased upon Blocking PKA Activity and HA Synthesis in Both Cell Lines and Increased upon Blocking p53 Activity in A549 Cells
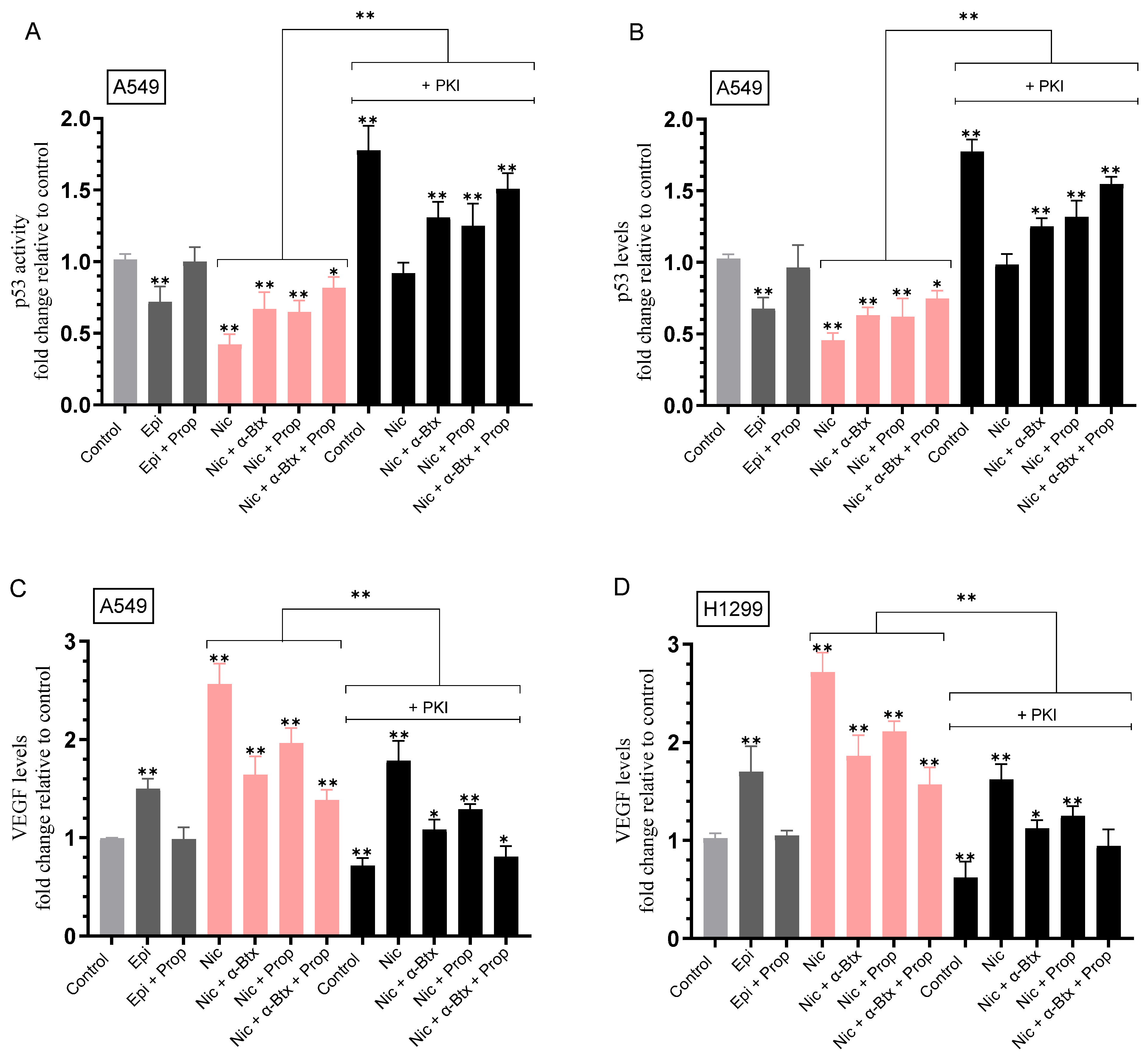
2.3. Nicotine-Induced PKA Activation Decreased by Co-Treatment with α-Btx and/or Prop, While HA Levels Correlated with the Activity of PKA
2.4. Opposite Effects Were Observed on the Activity of p53 in A549 Cells and the Levels of VEGF in the Media upon Treatment with Nicotine, an Effect Decreased by Co-Treatment with α-Btx and/or Prop, and upon PKA Inhibition
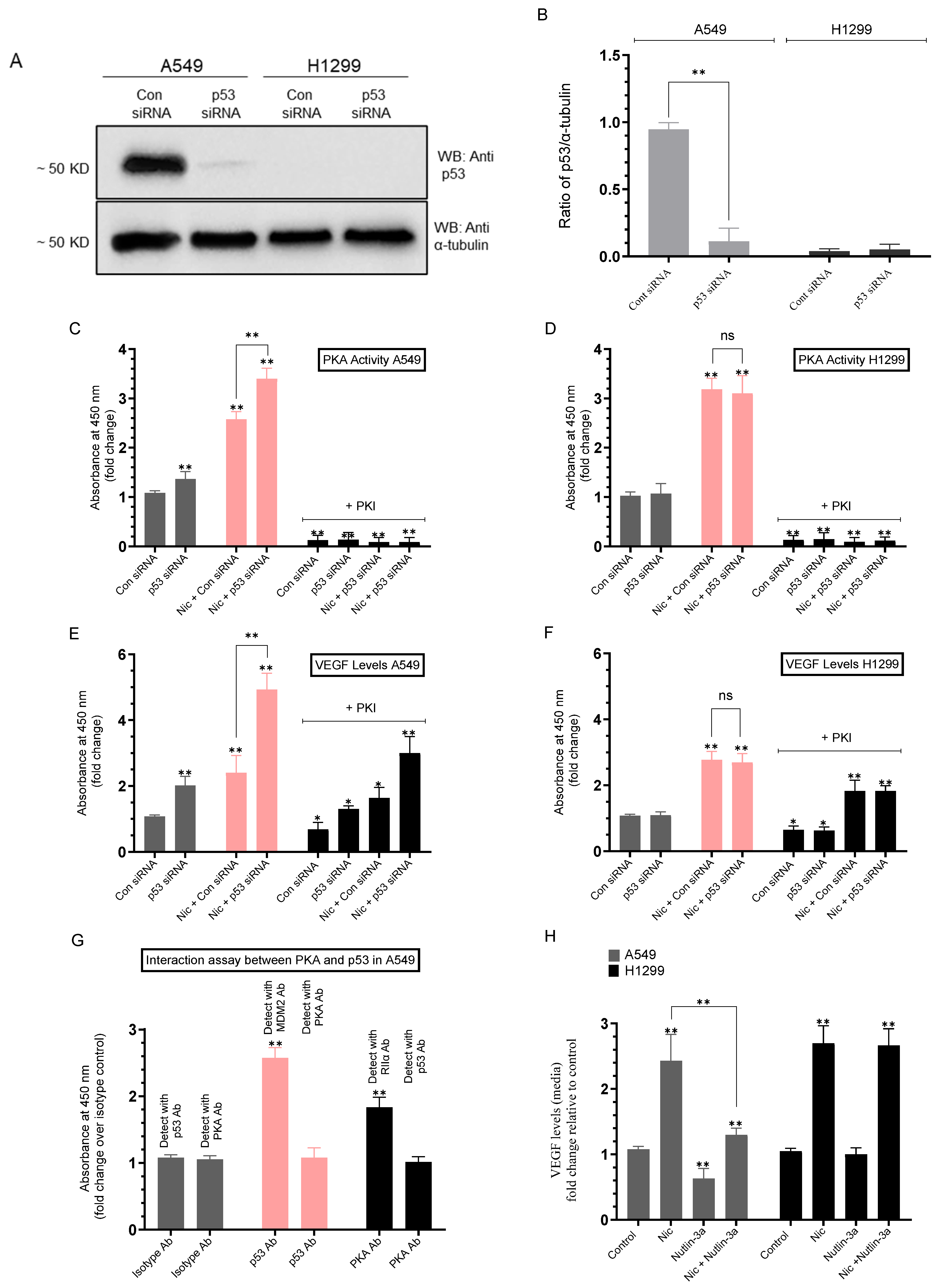
2.5. Knockdown of p53 Increased the PKA Activity and the Levels of VEGF in the Media of A549 Cells Untreated or Treated with Nicotine
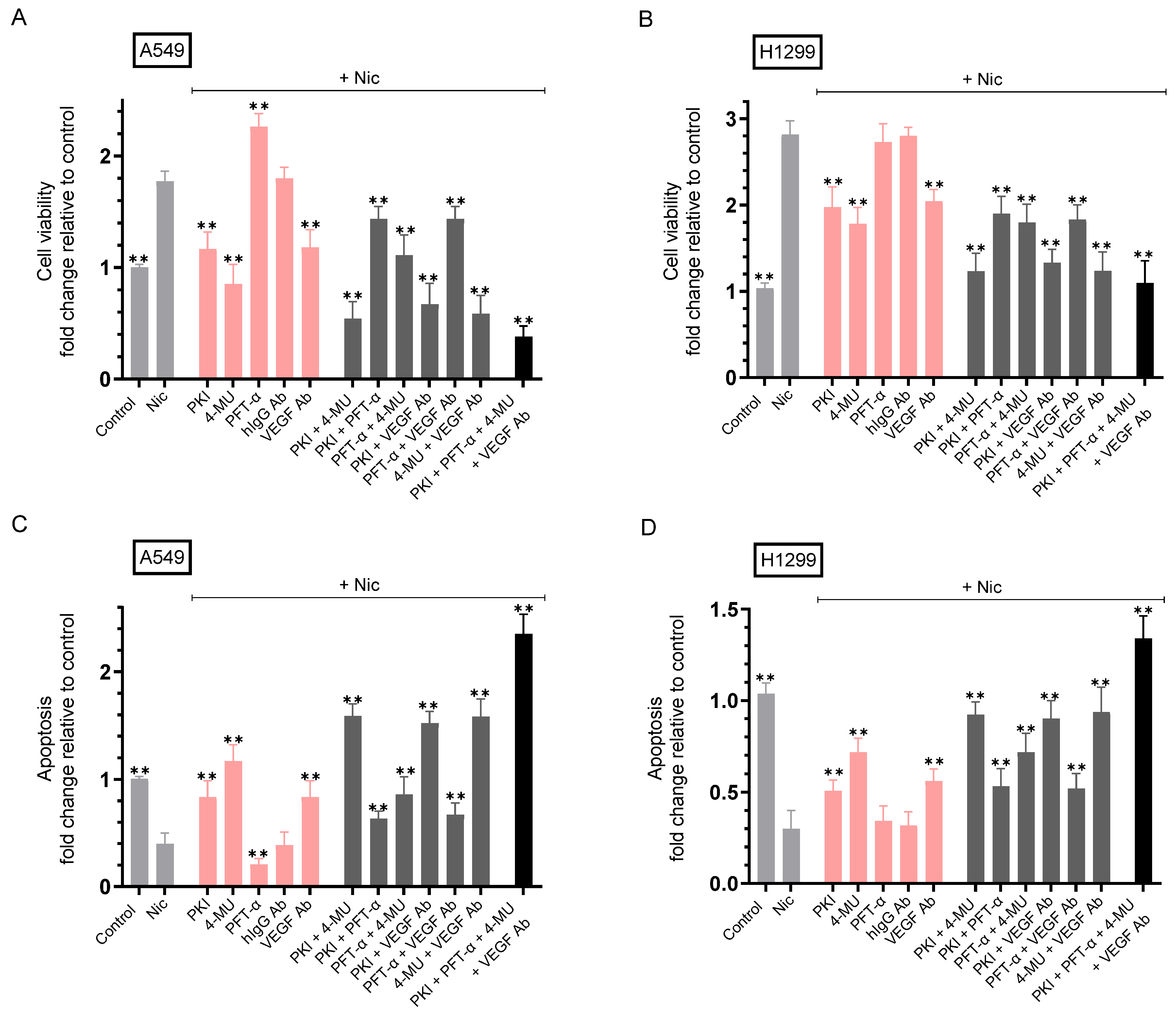
2.6. Co-Treatment with Nicotine and Either PKI, 4-MU, VEGF Antibodies, or in Combination Resulted in Decreased Cell Viability and Increased Apoptosis Compared to A549 and H1299 Cell Treatment Using Only Nicotine
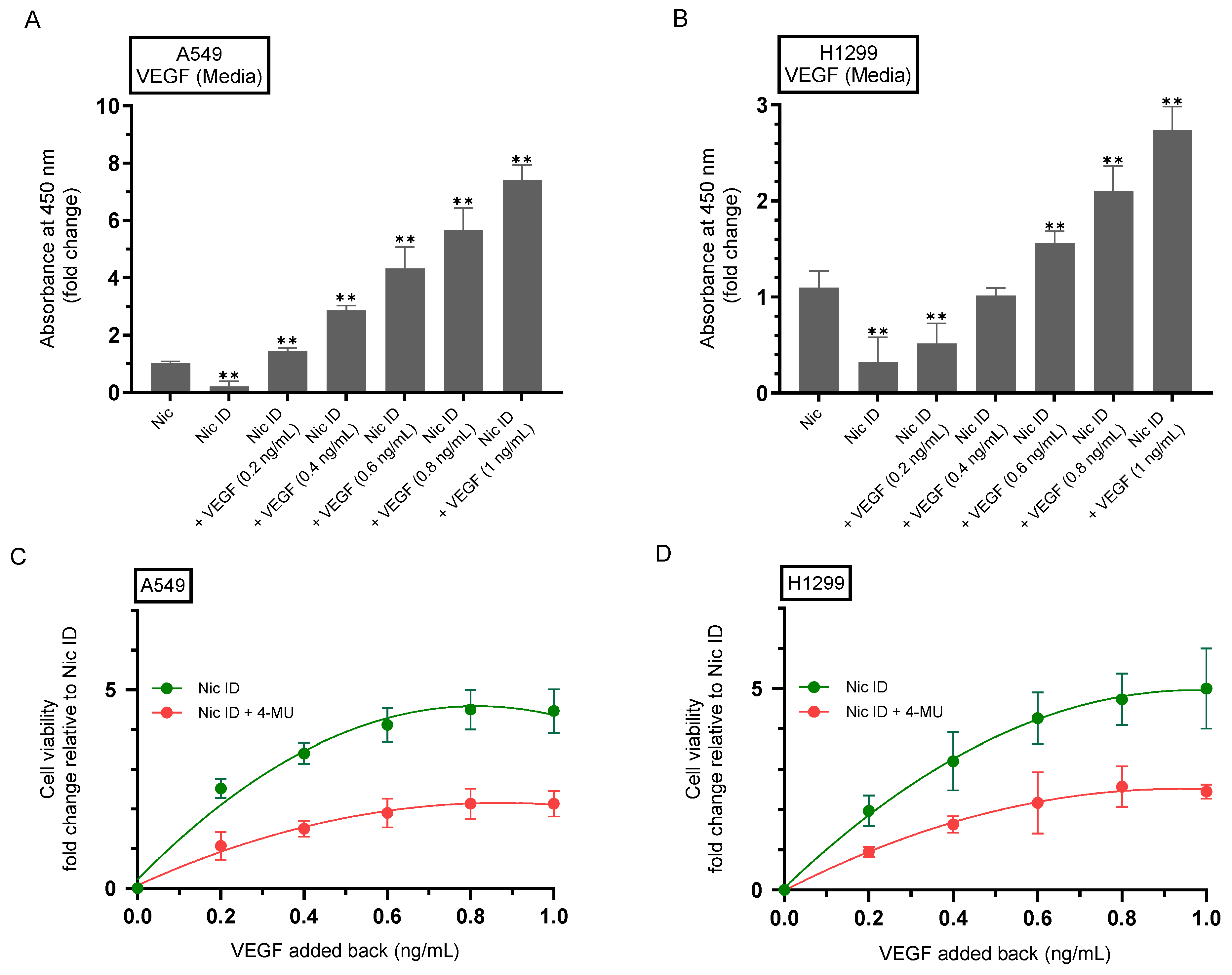
2.7. 4-MU Reduces the Rescue of Cell Viability Resulting from Addition of Purified VEGF to Nicotine-Treated Cell Media Immunodepleted of Secreted VEGF
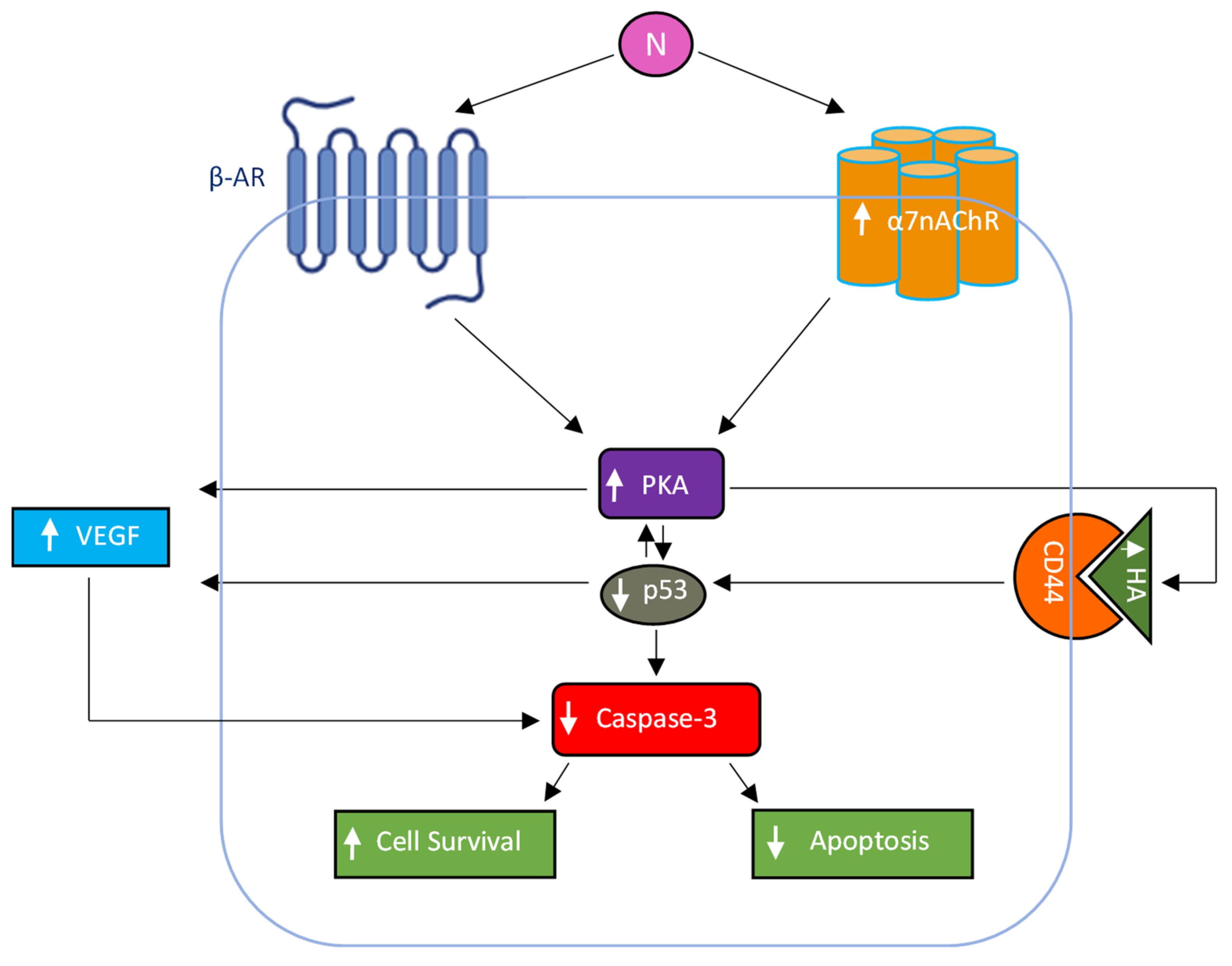
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. MTT Assay
4.4. Caspase-3 Assay
4.5. p53 Transcription Factor Activity Assay
4.6. Quantitation of Protein Levels and Normalization to α-Tubulin
4.7. VEGF Concentration Determination
4.8. PKA Assay
4.9. HA Quantitation
4.10. Western Blotting
4.11. siRNA Transfection
4.12. Immunodepletion
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Custodio, A.B.; González-Larriba, J.L.; Bobokova, J.; Calles, A.; Alvarez, R.; Cuadrado, E.; Manzano, A.; Díaz-Rubio, E. Prognostic and Predictive Markers of Benefit from Adjuvant Chemotherapy in Early-Stage Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2009, 4, 891–910. [Google Scholar] [CrossRef]
- Rotow, J.; Bivona, T.G. Understanding and Targeting Resistance Mechanisms in NSCLC. Nat. Rev. Cancer 2017, 17, 637–658. [Google Scholar] [CrossRef]
- Siddiqui, F.; Siddiqui, A.H. Lung Cancer. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Chen, R.-J.; Chang, L.W.; Lin, P.; Wang, Y.-J. Epigenetic Effects and Molecular Mechanisms of Tumorigenesis Induced by Cigarette Smoke: An Overview. J. Oncol. 2011, 2011, 654931. [Google Scholar] [CrossRef]
- Dasgupta, P.; Rizwani, W.; Pillai, S.; Kinkade, R.; Kovacs, M.; Rastogi, S.; Banerjee, S.; Carless, M.; Kim, E.; Coppola, D.; et al. Nicotine Induces Cell Proliferation, Invasion and Epithelial-Mesenchymal Transition in a Variety of Human Cancer Cell Lines. Int. J. Cancer 2009, 124, 36–45. [Google Scholar] [CrossRef]
- Schaal, C.; Chellappan, S.P. Nicotine-Mediated Cell Proliferation and Tumor Progression in Smoking-Related Cancers. Mol. Cancer Res. 2014, 12, 14–23. [Google Scholar] [CrossRef]
- Russo, P.; Cardinale, A.; Margaritora, S.; Cesario, A. Nicotinic Receptor and Tobacco-Related Cancer. Life Sci. 2012, 91, 1087–1092. [Google Scholar] [CrossRef]
- Egleton, R.D.; Brown, K.C.; Dasgupta, P. Nicotinic Acetylcholine Receptors in Cancer: Multiple Roles in Proliferation and Inhibition of Apoptosis. Trends Pharmacol. Sci. 2008, 29, 151–158. [Google Scholar] [CrossRef]
- Zhao, Y. The Oncogenic Functions of Nicotinic Acetylcholine Receptors. J. Oncol. 2016, 2016, 9650481. [Google Scholar] [CrossRef]
- Friedman, J.R.; Richbart, S.D.; Merritt, J.C.; Brown, K.C.; Nolan, N.A.; Akers, A.T.; Lau, J.K.; Robateau, Z.R.; Miles, S.L.; Dasgupta, P. Acetylcholine Signaling System in Progression of Lung Cancers. Pharmacol. Ther. 2019, 194, 222–254. [Google Scholar] [CrossRef]
- Xi, H.-J.; Wu, R.-P.; Liu, J.-J.; Zhang, L.-J.; Li, Z.-S. Role of Acetylcholinesterase in Lung Cancer. Thorac. Cancer 2015, 6, 390–398. [Google Scholar] [CrossRef]
- Gahring, L.C.; Myers, E.J.; Dunn, D.M.; Weiss, R.B.; Rogers, S.W. Nicotinic Alpha 7 Receptor Expression and Modulation of the Lung Epithelial Response to Lipopolysaccharide. PLoS ONE 2017, 12, e0175367. [Google Scholar] [CrossRef]
- Dom, A.M.; Buckley, A.W.; Brown, K.C.; Egleton, R.D.; Marcelo, A.J.; Proper, N.A.; Weller, D.E.; Shah, Y.H.; Lau, J.K.; Dasgupta, P. The A7-Nicotinic Acetylcholine Receptor and MMP-2/-9 Pathway Mediate the Proangiogenic Effect of Nicotine in Human Retinal Endothelial Cells. Investig. Opthalmology Vis. Sci. 2011, 52, 4428–4438. [Google Scholar] [CrossRef]
- Spindel, E.R. Cholinergic Targets in Lung Cancer. Curr. Pharm. Des. 2016, 22, 2152–2159. [Google Scholar] [CrossRef]
- Albuquerque, E.X.; Pereira, E.F.R.; Alkondon, M.; Rogers, S.W. Mammalian Nicotinic Acetylcholine Receptors: From Structure to Function. Physiol. Rev. 2009, 89, 73–120. [Google Scholar] [CrossRef]
- Pohanka, M. Alpha7 Nicotinic Acetylcholine Receptor Is a Target in Pharmacology and Toxicology. Int. J. Mol. Sci. 2012, 13, 2219–2238. [Google Scholar] [CrossRef]
- Niu, X.-M.; Lu, S. Acetylcholine Receptor Pathway in Lung Cancer: New Twists to an Old Story. World J. Clin. Oncol. 2014, 5, 667–676. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, P.; Zhu, L.; Zhao, Q.; Lu, X.; Bo, S. Blockade of A7 Nicotinic Acetylcholine Receptors Inhibit Nicotine-Induced Tumor Growth and Vimentin Expression in Non-Small Cell Lung Cancer through MEK/ERK Signaling Way. Oncol. Rep. 2017, 38, 3309–3318. [Google Scholar] [CrossRef]
- Bele, T.; Turk, T.; Križaj, I. Nicotinic Acetylcholine Receptors in Cancer: Limitations and Prospects. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 166875. [Google Scholar] [CrossRef]
- Shi, D.; Guo, W.; Chen, W.; Fu, L.; Wang, J.; Tian, Y.; Xiao, X.; Kang, T.; Huang, W.; Deng, W. Nicotine Promotes Proliferation of Human Nasopharyngeal Carcinoma Cells by Regulating α7AChR, ERK, HIF-1α and VEGF/PEDF Signaling. PLoS ONE 2012, 7, e43898. [Google Scholar] [CrossRef]
- Al Khashali, H.; Darweesh, B.; Ray, R.; Haddad, B.; Wozniak, C.; Ranzenberger, R.; Goel, S.; Khalil, J.; Guthrie, J.; Heyl, D.; et al. Regulation of Vascular Endothelial Growth Factor Signaling by Nicotine in a Manner Dependent on Acetylcholine-and/or β-Adrenergic-Receptors in Human Lung Cancer Cells. Cancers 2023, 15, 5500. [Google Scholar] [CrossRef]
- Thaker, P.H.; Han, L.Y.; Kamat, A.A.; Arevalo, J.M.; Takahashi, R.; Lu, C.; Jennings, N.B.; Armaiz-Pena, G.; Bankson, J.A.; Ravoori, M.; et al. Chronic Stress Promotes Tumor Growth and Angiogenesis in a Mouse Model of Ovarian Carcinoma. Nat. Med. 2006, 12, 939–944, Correction in Nat. Med. 2021, 12, 2246. [Google Scholar] [CrossRef]
- Niklińska, W.; Burzykowski, T.; Chyczewski, L.; Nikliński, J. Expression of Vascular Endothelial Growth Factor (VEGF) in Non-Small Cell Lung Cancer (NSCLC): Association with P53 Gene Mutation and Prognosis. Lung Cancer 2001, 34 (Suppl. S2), S59–S64. [Google Scholar] [CrossRef]
- Lutgendorf, S.K.; Cole, S.; Costanzo, E.; Bradley, S.; Coffin, J.; Jabbari, S.; Rainwater, K.; Ritchie, J.M.; Yang, M.; Sood, A.K. Stress-Related Mediators Stimulate Vascular Endothelial Growth Factor Secretion by Two Ovarian Cancer Cell Lines. Clin. Cancer Res. 2003, 9, 4514–4521. [Google Scholar]
- Nilsson, M.B.; Le, X.; Heymach, J.V. β-Adrenergic Signaling in Lung Cancer: A Potential Role for Beta-Blockers. J. Neuroimmune Pharmacol. 2020, 15, 27–36. [Google Scholar] [CrossRef]
- Wong, H.P.S.; Yu, L.; Lam, E.K.Y.; Tai, E.K.K.; Wu, W.K.K.; Cho, C.-H. Nicotine Promotes Colon Tumor Growth and Angiogenesis through Beta-Adrenergic Activation. Toxicol. Sci. 2007, 97, 279–287. [Google Scholar] [CrossRef]
- Wong, H.P.S.; Yu, L.; Lam, E.K.Y.; Tai, E.K.K.; Wu, W.K.K.; Cho, C.H. Nicotine Promotes Cell Proliferation via Alpha7-Nicotinic Acetylcholine Receptor and Catecholamine-Synthesizing Enzymes-Mediated Pathway in Human Colon Adenocarcinoma HT-29 Cells. Toxicol. Appl. Pharmacol. 2007, 221, 261–267. [Google Scholar] [CrossRef]
- Sastry, K.S.R.; Karpova, Y.; Prokopovich, S.; Smith, A.J.; Essau, B.; Gersappe, A.; Carson, J.P.; Weber, M.J.; Register, T.C.; Chen, Y.Q.; et al. Epinephrine Protects Cancer Cells from Apoptosis via Activation of cAMP-Dependent Protein Kinase and BAD Phosphorylation. J. Biol. Chem. 2007, 282, 14094–14100. [Google Scholar] [CrossRef]
- Liu, X.; Wu, W.K.K.; Yu, L.; Sung, J.J.Y.; Srivastava, G.; Zhang, S.T.; Cho, C.H. Epinephrine Stimulates Esophageal Squamous-Cell Carcinoma Cell Proliferation via Beta-Adrenoceptor-Dependent Transactivation of Extracellular Signal-Regulated Kinase/Cyclooxygenase-2 Pathway. J. Cell. Biochem. 2008, 105, 53–60. [Google Scholar] [CrossRef]
- Al-Wadei, H.A.N.; Al-Wadei, M.H.; Schuller, H.M. Cooperative Regulation of Non-Small Cell Lung Carcinoma by Nicotinic and Beta-Adrenergic Receptors: A Novel Target for Intervention. PLoS ONE 2012, 7, e29915. [Google Scholar] [CrossRef]
- Schuller, H.M.; Porter, B.; Riechert, A. Beta-Adrenergic Modulation of NNK-Induced Lung Carcinogenesis in Hamsters. J. Cancer Res. Clin. Oncol. 2000, 126, 624–630. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, C.; Chen, W.; Qiu, L.; Li, S.; Wang, T.; Xie, H.; Li, Y.; Li, C.; Li, L. The Stress Hormone Norepinephrine Promotes Tumor Progression through Β2-Adrenoreceptors in Oral Cancer. Arch. Oral. Biol. 2020, 113, 104712, Correction in Arch. Oral. Biol. 2022, 133, 105295. [Google Scholar] [CrossRef]
- Hu, P.; He, J.; Liu, S.; Wang, M.; Pan, B.; Zhang, W. Β2-Adrenergic Receptor Activation Promotes the Proliferation of A549 Lung Cancer Cells via the ERK1/2/CREB Pathway. Oncol. Rep. 2016, 36, 1757–1763. [Google Scholar] [CrossRef]
- Ray, R.; Al Khashali, H.; Haddad, B.; Wareham, J.; Coleman, K.-L.; Alomari, D.; Ranzenberger, R.; Guthrie, J.; Heyl, D.; Evans, H.G. Regulation of Cisplatin Resistance in Lung Cancer Cells by Nicotine, BDNF, and a β-Adrenergic Receptor Blocker. Int. J. Mol. Sci. 2022, 23, 12829. [Google Scholar] [CrossRef]
- Ray, R.; Goel, S.; Al Khashali, H.; Darweesh, B.; Haddad, B.; Wozniak, C.; Ranzenberger, R.; Khalil, J.; Guthrie, J.; Heyl, D.; et al. Regulation of Soluble E-Cadherin Signaling in Non-Small-Cell Lung Cancer Cells by Nicotine, BDNF, and β-Adrenergic Receptor Ligands. Biomedicines 2023, 11, 2555. [Google Scholar] [CrossRef]
- Goel, S.; Wozniak, C.; Sabri, A.; Haddad, B.; Lopo, B.; Cobos, A.; Sarofim, S.; Guthrie, J.; Heyl, D.; Evans, H.G. Lactoferrin Treatment Activates Acetylcholinesterase, Decreasing Acetylcholine Levels in Non-Small Cell Lung Cancer (NSCLC) Cell Culture Supernatants, Inhibiting Cell Survival. FEBS Open Bio 2025. epub ahead of print. [Google Scholar] [CrossRef]
- Raman, P.S.; Alves, C.S.; Wirtz, D.; Konstantopoulos, K. Distinct Kinetic and Molecular Requirements Govern CD44 Binding to Hyaluronan versus Fibrin(Ogen). Biophys. J. 2012, 103, 415–423. [Google Scholar] [CrossRef]
- Misra, S.; Heldin, P.; Hascall, V.C.; Karamanos, N.K.; Skandalis, S.S.; Markwald, R.R.; Ghatak, S. HA/CD44 Interactions as Potential Targets for Cancer Therapy. FEBS J. 2011, 278, 1429–1443. [Google Scholar] [CrossRef]
- Misra, S.; Hascall, V.C.; Markwald, R.R.; Ghatak, S. Interactions between Hyaluronan and Its Receptors (CD44, RHAMM) Regulate the Activities of Inflammation and Cancer. Front. Immunol. 2015, 6, 201. [Google Scholar] [CrossRef]
- Sherman, L.S.; Matsumoto, S.; Su, W.; Srivastava, T.; Back, S.A. Hyaluronan Synthesis, Catabolism, and Signaling in Neurodegenerative Diseases. Int. J. Cell Biol. 2015, 2015, e368584. [Google Scholar] [CrossRef]
- Liu, M.; Tolg, C.; Turley, E. Dissecting the Dual Nature of Hyaluronan in the Tumor Microenvironment. Front. Immunol. 2019, 10, 947. [Google Scholar] [CrossRef]
- Toole, B.P. Hyaluronan: From Extracellular Glue to Pericellular Cue. Nat. Rev. Cancer 2004, 4, 528–539. [Google Scholar] [CrossRef]
- Toole, B.P.; Slomiany, M.G. Hyaluronan, CD44 and Emmprin: Partners in Cancer Cell Chemoresistance. Drug Resist. Updat. 2008, 11, 110–121. [Google Scholar] [CrossRef]
- Chanmee, T.; Ontong, P.; Kimata, K.; Itano, N. Key Roles of Hyaluronan and Its CD44 Receptor in the Stemness and Survival of Cancer Stem Cells. Front. Oncol. 2015, 5, 180. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, S.; Karnad, A.; Freeman, J.W. The Biology and Role of CD44 in Cancer Progression: Therapeutic Implications. J. Hematol. Oncol. 2018, 11, 64. [Google Scholar] [CrossRef]
- Kultti, A.; Li, X.; Jiang, P.; Thompson, C.B.; Frost, G.I.; Shepard, H.M. Therapeutic Targeting of Hyaluronan in the Tumor Stroma. Cancers 2012, 4, 873–903. [Google Scholar] [CrossRef]
- Godar, S.; Ince, T.A.; Bell, G.W.; Feldser, D.; Donaher, J.L.; Bergh, J.; Liu, A.; Miu, K.; Watnick, R.S.; Reinhardt, F.; et al. Growth-Inhibitory and Tumor- Suppressive Functions of P53 Depend on Its Repression of CD44 Expression. Cell 2008, 134, 62–73. [Google Scholar] [CrossRef]
- Dhar, D.; Antonucci, L.; Nakagawa, H.; Kim, J.Y.; Glitzner, E.; Caruso, S.; Shalapour, S.; Yang, L.; Valasek, M.A.; Lee, S.; et al. Liver Cancer Initiation Requires P53 Inhibition by CD44-Enhanced Growth Factor Signaling. Cancer Cell 2018, 33, 1061–1077.e6. [Google Scholar] [CrossRef]
- Nagy, N.; Kuipers, H.F.; Frymoyer, A.R.; Ishak, H.D.; Bollyky, J.B.; Wight, T.N.; Bollyky, P.L. 4-Methylumbelliferone Treatment and Hyaluronan Inhibition as a Therapeutic Strategy in Inflammation, Autoimmunity, and Cancer. Front. Immunol. 2015, 6, 123. [Google Scholar] [CrossRef]
- Madden, K.S.; Szpunar, M.J.; Brown, E.B. β-Adrenergic Receptors (β-AR) Regulate VEGF and IL-6 Production by Divergent Pathways in High β-AR-Expressing Breast Cancer Cell Lines. Breast Cancer Res. Treat. 2011, 130, 747–758. [Google Scholar] [CrossRef]
- Tang, J.; Li, Z.; Lu, L.; Cho, C.H. β-Adrenergic System, a Backstage Manipulator Regulating Tumour Progression and Drug Target in Cancer Therapy. Semin. Cancer Biol. 2013, 23, 533–542. [Google Scholar] [CrossRef]
- Wang, H.; Li, M.; Lin, W.; Wang, W.; Zhang, Z.; Rayburn, E.R.; Lu, J.; Chen, D.; Yue, X.; Shen, F.; et al. Extracellular Activity of Cyclic AMP-Dependent Protein Kinase as a Biomarker for Human Cancer Detection: Distribution Characteristics in a Normal Population and Cancer Patients. Cancer Epidemiol. Biomarkers Prev. 2007, 16, 789–795. [Google Scholar] [CrossRef]
- Al-Wadei, H.A.N.; Ullah, M.F.; Al-Wadei, M.H. Intercepting Neoplastic Progression in Lung Malignancies via the Beta Adrenergic (β-AR) Pathway: Implications for Anti-Cancer Drug Targets. Pharmacol. Res. 2012, 66, 33–40. [Google Scholar] [CrossRef]
- Guo, R.; Liu, T.; Shasaltaneh, M.D.; Wang, X.; Imani, S.; Wen, Q. Targeting Adenylate Cyclase Family: New Concept of Targeted Cancer Therapy. Front. Oncol. 2022, 12, 829212. [Google Scholar] [CrossRef]
- Schuller, H.M. A New Twist to Neurotransmitter Receptors and Cancer. J. Cancer Metastasis Treat. 2017, 3, 71–77. [Google Scholar] [CrossRef]
- Lo, K.W.-H.; Kan, H.M.; Gagnon, K.A.; Laurencin, C.T. One-Day Treatment of Small Molecule 8-Bromo-Cyclic AMP Analogue Induces Cell-Based VEGF Production for In Vitro Angiogenesis and Osteoblastic Differentiation. J. Tissue Eng. Regen. Med. 2016, 10, 867–875. [Google Scholar] [CrossRef]
- Bradbury, D.; Clarke, D.; Seedhouse, C.; Corbett, L.; Stocks, J.; Knox, A. Vascular Endothelial Growth Factor Induction by Prostaglandin E2 in Human Airway Smooth Muscle Cells Is Mediated by E Prostanoid EP2/EP4 Receptors and SP-1 Transcription Factor Binding Sites. J. Biol. Chem. 2005, 280, 29993–30000. [Google Scholar] [CrossRef]
- Shi, J.; Liu, F.; Zhang, W.; Liu, X.; Lin, B.; Tang, X. Epigallocatechin-3-Gallate Inhibits Nicotine-Induced Migration and Invasion by the Suppression of Angiogenesis and Epithelial-Mesenchymal Transition in Non-Small Cell Lung Cancer Cells. Oncol. Rep. 2015, 33, 2972–2980. [Google Scholar] [CrossRef]
- Zhang, Q.; Tang, X.; Zhang, Z.-F.; Velikina, R.; Shi, S.; Le, A.D. Nicotine Induces Hypoxia-Inducible Factor-1alpha Expression in Human Lung Cancer Cells via Nicotinic Acetylcholine Receptor-Mediated Signaling Pathways. Clin. Cancer Res. 2007, 13, 4686–4694. [Google Scholar] [CrossRef]
- Jarzynka, M.J.; Guo, P.; Bar-Joseph, I.; Hu, B.; Cheng, S.-Y. Estradiol and Nicotine Exposure Enhances A549 Bronchioloalveolar Carcinoma Xenograft Growth in Mice through the Stimulation of Angiogenesis. Int. J. Oncol. 2006, 28, 337–344. [Google Scholar] [CrossRef]
- Daijo, H.; Hoshino, Y.; Kai, S.; Suzuki, K.; Nishi, K.; Matsuo, Y.; Harada, H.; Hirota, K. Cigarette Smoke Reversibly Activates Hypoxia-Inducible Factor 1 in a Reactive Oxygen Species-Dependent Manner. Sci. Rep. 2016, 6, 34424. [Google Scholar] [CrossRef]
- Shin, V.Y.; Wu, W.K.K.; Chu, K.-M.; Wong, H.P.S.; Lam, E.K.Y.; Tai, E.K.K.; Koo, M.W.L.; Cho, C.-H. Nicotine Induces Cyclooxygenase-2 and Vascular Endothelial Growth Factor Receptor-2 in Association with Tumor-Associated Invasion and Angiogenesis in Gastric Cancer. Mol. Cancer Res. 2005, 3, 607–615. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.; Xiao, Y.; Yang, M.; Chen, J.; Jian, Y.; Chen, X.; Shi, D.; Chen, X.; Ouyang, Y.; et al. Nicotine-Mediated OTUD3 Downregulation Inhibits VEGF-C mRNA Decay to Promote Lymphatic Metastasis of Human Esophageal Cancer. Nat. Commun. 2021, 12, 7006. [Google Scholar] [CrossRef]
- Bates, D.O. Vascular Endothelial Growth Factors and Vascular Permeability. Cardiovasc. Res. 2010, 87, 262–271. [Google Scholar] [CrossRef]
- Jung, J.-J.; Tiwari, A.; Inamdar, S.M.; Thomas, C.P.; Goel, A.; Choudhury, A. Secretion of Soluble Vascular Endothelial Growth Factor Receptor 1 (sVEGFR1/sFlt1) Requires Arf1, Arf6, and Rab11 GTPases. PLoS ONE 2012, 7, e44572, Correction in PLoS ONE 2012, 7, 10.1371/annotation/19ed28f9-9a01-4af9-857d-3ec4aaeceea1. [Google Scholar] [CrossRef]
- Piccioni, F.; Fiore, E.; Bayo, J.; Atorrasagasti, C.; Peixoto, E.; Rizzo, M.; Malvicini, M.; Tirado-González, I.; García, M.G.; Alaniz, L.; et al. 4-Methylumbelliferone Inhibits Hepatocellular Carcinoma Growth by Decreasing IL-6 Production and Angiogenesis. Glycobiology 2015, 25, 825–835. [Google Scholar] [CrossRef]
- Lokeshwar, V.B.; Lopez, L.E.; Munoz, D.; Chi, A.; Shirodkar, S.P.; Lokeshwar, S.D.; Escudero, D.O.; Dhir, N.; Altman, N. Antitumor Activity of Hyaluronic Acid Synthesis Inhibitor 4-Methylumbelliferone In Prostate Cancer Cells. Cancer Res. 2010, 70, 2613–2623. [Google Scholar] [CrossRef]
- Yates, T.J.; Lopez, L.E.; Lokeshwar, S.D.; Ortiz, N.; Kallifatidis, G.; Jordan, A.; Hoye, K.; Altman, N.; Lokeshwar, V.B. Dietary Supplement 4-Methylumbelliferone: An Effective Chemopreventive and Therapeutic Agent for Prostate Cancer. J. Natl. Cancer Inst. 2015, 107, djv085. [Google Scholar] [CrossRef]
- Ban, H.; Uchakina, O.; McKallip, R.J. Hyaluronic Acid Inhibitor 4-Methylumbelliferone Activates the Intrinsic Apoptosis Pathway in K562 Chronic Myelogenous Leukemia Cells. AntiCancer Res. 2015, 35, 5231–5240. [Google Scholar]
- Ludwig, N.; Szczepanski, M.J.; Gluszko, A.; Szafarowski, T.; Azambuja, J.H.; Dolg, L.; Gellrich, N.-C.; Kampmann, A.; Whiteside, T.L.; Zimmerer, R.M. CD44(+) Tumor Cells Promote Early Angiogenesis in Head and Neck Squamous Cell Carcinoma. Cancer Lett. 2019, 467, 85–95. [Google Scholar] [CrossRef]
- Murphy, J.F.; Lennon, F.; Steele, C.; Kelleher, D.; Fitzgerald, D.; Long, A.C. Engagement of CD44 Modulates Cyclooxygenase Induction, VEGF Generation, and Proliferation in Human Vascular Endothelial Cells. FASEB J. 2005, 19, 446–448. [Google Scholar] [CrossRef]
- Chaudhary, K.R.; Yan, S.X.; Heilbroner, S.P.; Sonett, J.R.; Stoopler, M.B.; Shu, C.; Halmos, B.; Wang, T.J.C.; Hei, T.K.; Cheng, S.K. Effects of β-Adrenergic Antagonists on Chemoradiation Therapy for Locally Advanced Non-Small Cell Lung Cancer. J. Clin. Med. 2019, 8, 575. [Google Scholar] [CrossRef]
- Huang, Q.; Tan, Q.; Mao, K.; Yang, G.; Ma, G.; Luo, P.; Wang, S.; Mei, P.; Wu, F.; Xu, J.; et al. The Role of Adrenergic Receptors in Lung Cancer. Am. J. Cancer Res. 2018, 8, 2227–2237. [Google Scholar]
- Kuroda, Y.; Higashi, H. Regulation of Hyaluronan Production by Β2 Adrenergic Receptor Signaling. Biochem. Biophys. Res. Commun. 2021, 575, 50–55. [Google Scholar] [CrossRef]
- Price, D.; Muterspaugh, R.; Clegg, B.; Williams, A.; Stephens, A.; Guthrie, J.; Heyl, D.; Guy Evans, H. IGFBP-3 Blocks Hyaluronan-CD44 Signaling, Leading to Increased Acetylcholinesterase Levels in A549 Cell Media and Apoptosis in a P53-Dependent Manner. Sci. Rep. 2020, 10, 5083–5099. [Google Scholar] [CrossRef]
- Hara, M.R.; Kovacs, J.J.; Whalen, E.J.; Rajagopal, S.; Strachan, R.T.; Grant, W.; Towers, A.J.; Williams, B.; Lam, C.M.; Xiao, K.; et al. A Stress Response Pathway Regulates DNA Damage through Β2-Adrenoreceptors and β-Arrestin-1. Nature 2011, 477, 349–353. [Google Scholar] [CrossRef]
- Walia, M.K.; Taylor, S.; Ho, P.W.M.; Martin, T.J.; Walkley, C.R. Tolerance to Sustained Activation of the cAMP/Creb Pathway Activity in Osteoblastic Cells Is Enabled by Loss of P53. Cell Death Dis. 2018, 9, 844. [Google Scholar] [CrossRef]
- Hu, W.; Feng, Z.; Levine, A.J. The Regulation of Multiple P53 Stress Responses Is Mediated through MDM2. Genes Cancer 2012, 3, 199–208. [Google Scholar] [CrossRef]
- Wang, S.; Hu, Y. A7 Nicotinic Acetylcholine Receptors in Lung Cancer. Oncol. Lett. 2018, 16, 1375–1382. [Google Scholar] [CrossRef]
- Malmlöf, M.; Roudier, E.; Högberg, J.; Stenius, U. MEK-ERK-Mediated Phosphorylation of Mdm2 at Ser-166 in Hepatocytes. Mdm2 Is Activated in Response to Inhibited Akt Signaling. J. Biol. Chem. 2007, 282, 2288–2296, Correction in J. Biol. Chem. 2016, 291, 26588. [Google Scholar] [CrossRef]
- Cheng, W.-L.; Chen, K.-Y.; Lee, K.-Y.; Feng, P.-H.; Wu, S.-M. Nicotinic-nAChR Signaling Mediates Drug Resistance in Lung Cancer. J. Cancer 2020, 11, 1125–1140. [Google Scholar] [CrossRef]
- Puliyappadamba, V.T.; Cheriyan, V.T.; Thulasidasan, A.K.T.; Bava, S.V.; Vinod, B.S.; Prabhu, P.R.; Varghese, R.; Bevin, A.; Venugopal, S.; Anto, R.J. Nicotine-Induced Survival Signaling in Lung Cancer Cells Is Dependent on Their P53 Status While Its down-Regulation by Curcumin Is Independent. Mol. Cancer 2010, 9, 220. [Google Scholar] [CrossRef]
- Zhang, C.; Ding, X.-P.; Zhao, Q.-N.; Yang, X.-J.; An, S.-M.; Wang, H.; Xu, L.; Zhu, L.; Chen, H.-Z. Role of A7-Nicotinic Acetylcholine Receptor in Nicotine-Induced Invasion and Epithelial-to-Mesenchymal Transition in Human Non-Small Cell Lung Cancer Cells. Oncotarget 2016, 7, 59199–59208. [Google Scholar] [CrossRef]
- Arunrungvichian, K.; Vajragupta, O.; Hayakawa, Y.; Pongrakhananon, V. Targeting Alpha7 Nicotinic Acetylcholine Receptors in Lung Cancer: Insights, Challenges, and Therapeutic Strategies. ACS Pharmacol. Transl. Sci. 2024, 7, 28–41. [Google Scholar] [CrossRef]
- Adler, V.; Pincus, M.R.; Minamoto, T.; Fuchs, S.Y.; Bluth, M.J.; Brandt-Rauf, P.W.; Friedman, F.K.; Robinson, R.C.; Chen, J.M.; Wang, X.W.; et al. Conformation-Dependent Phosphorylation of P53. Proc. Natl. Acad. Sci. USA 1997, 94, 1686–1691. [Google Scholar] [CrossRef]
- Naderi, E.H.; Jochemsen, A.G.; Blomhoff, H.K.; Naderi, S. Activation of cAMP Signaling Interferes with Stress-Induced P53 Accumulation in ALL-Derived Cells by Promoting the Interaction between P53 and HDM2. Neoplasia 2011, 13, 653–663. [Google Scholar] [CrossRef]
- Lee, Y.-M.; Lim, J.-H.; Chun, Y.-S.; Moon, H.-E.; Lee, M.K.; Huang, L.E.; Park, J.-W. Nutlin-3, an Hdm2 Antagonist, Inhibits Tumor Adaptation to Hypoxia by Stimulating the FIH-Mediated Inactivation of HIF-1alpha. Carcinogenesis 2009, 30, 1768–1775. [Google Scholar] [CrossRef]
- Shen, H.; Maki, C.G. Pharmacologic Activation of P53 by Small-Molecule MDM2 Antagonists. Curr. Pharm. Des. 2011, 17, 560–568. [Google Scholar] [CrossRef]
- Barr, M.P.; Gray, S.G.; Gately, K.; Hams, E.; Fallon, P.G.; Davies, A.M.; Richard, D.J.; Pidgeon, G.P.; O’Byrne, K.J. Vascular Endothelial Growth Factor Is an Autocrine Growth Factor, Signaling through Neuropilin-1 in Non-Small Cell Lung Cancer. Mol. Cancer 2015, 14, 45, Correction in Mol. Cancer 2020, 19, 16. [Google Scholar] [CrossRef]
- Li, H.; Ma, N.; Wang, J.; Wang, Y.; Yuan, C.; Wu, J.; Luo, M.; Yang, J.; Chen, J.; Shi, J.; et al. Nicotine Induces Progressive Properties of Lung Adenocarcinoma A549 Cells by Inhibiting Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Expression and Plasma Membrane Localization. Technol. Cancer Res. Treat. 2018, 17, 1533033818809984. [Google Scholar] [CrossRef]
- Sidorova, M.; Petrikaitė, V. The Effect of Beta Adrenoreceptor Blockers on Viability and Cell Colony Formation of Non-Small Cell Lung Cancer Cell Lines A549 and H1299. Molecules 2022, 27, 1938. [Google Scholar] [CrossRef]
- Duan, Y.; Li, J.; Wang, F.; Wei, J.; Yang, Z.; Sun, M.; Liu, J.; Wen, M.; Huang, W.; Chen, Z.; et al. Protein Modifications throughout the Lung Cancer Proteome Unravel the Cancer-Specific Regulation of Glycolysis. Cell Rep. 2021, 37, 110137. [Google Scholar] [CrossRef]
- Yu, M.; Liu, T.; Chen, Y.; Li, Y.; Li, W. Combination Therapy with Protein Kinase Inhibitor H89 and Tetrandrine Elicits Enhanced Synergistic Antitumor Efficacy. J. Exp. Clin. Cancer Res. 2018, 37, 114. [Google Scholar] [CrossRef]
- Wen, J.; Fu, J.-H.; Zhang, W.; Guo, M. Lung Carcinoma Signaling Pathways Activated by Smoking. Chin. J. Cancer 2011, 30, 551–558. [Google Scholar] [CrossRef]
- Shin, V.Y.; Wu, W.K.K.; Ye, Y.-N.; So, W.H.L.; Koo, M.W.L.; Liu, E.S.L.; Luo, J.-C.; Cho, C.-H. Nicotine Promotes Gastric Tumor Growth and Neovascularization by Activating Extracellular Signal-Regulated Kinase and Cyclooxygenase-2. Carcinogenesis 2004, 25, 2487–2495. [Google Scholar] [CrossRef]
- Espana-Serrano, L.; Chougule, M.B. Enhanced Anticancer Activity of PF-04691502, a Dual PI3K/mTOR Inhibitor, in Combination With VEGF siRNA Against Non–Small-Cell Lung Cancer. Mol. Ther. Nucleic Acids 2016, 5, e384. [Google Scholar] [CrossRef]
- Liu, B.; Peng, X.-C.; Zheng, X.-L.; Wang, J.; Qin, Y.-W. MiR-126 Restoration down-Regulate VEGF and Inhibit the Growth of Lung Cancer Cell Lines in Vitro and in Vivo. Lung Cancer 2009, 66, 169–175. [Google Scholar] [CrossRef]
- Riquelme, E.; Suraokar, M.; Behrens, C.; Lin, H.Y.; Girard, L.; Nilsson, M.B.; Simon, G.; Wang, J.; Coombes, K.R.; Lee, J.J.; et al. VEGF/VEGFR-2 Upregulates EZH2 Expression in Lung Adenocarcinoma Cells and EZH2 Depletion Enhances the Response to Platinum-Based and VEGFR-2-Targeted Therapy. Clin. Cancer Res. 2014, 20, 3849–3861. [Google Scholar] [CrossRef]
- Hu, B.; Ma, Y.; Yang, Y.; Zhang, L.; Han, H.; Chen, J. CD44 Promotes Cell Proliferation in Non-Small Cell Lung Cancer. Oncol. Lett. 2018, 15, 5627–5633. [Google Scholar] [CrossRef]
- Díaz, M.A.; Fusco, M.; Benítez, C.A.; Gayet, F.; García, L.; Victoria, L.; Jaramillo, S.; Bayo, J.; Zubieta, M.R.; Rizzo, M.M.; et al. Targeting Hyaluronan Metabolism-Related Molecules Associated with Resistant Tumor-Initiating Cells Potentiates Chemotherapy Efficacy in Lung Cancer. Sci. Rep. 2024, 14, 16803. [Google Scholar] [CrossRef]
- Karalis, T.; Shiau, A.K.; Gahman, T.C.; Skandalis, S.S.; Heldin, C.-H.; Heldin, P. Identification of a Small Molecule Inhibitor of Hyaluronan Synthesis, DDIT, Targeting Breast Cancer Cells. Cancers 2022, 14, 5800. [Google Scholar] [CrossRef]
- Nagase, H.; Kudo, D.; Suto, A.; Yoshida, E.; Suto, S.; Negishi, M.; Kakizaki, I.; Hakamada, K. 4-Methylumbelliferone Suppresses Hyaluronan Synthesis and Tumor Progression in SCID Mice Intra-Abdominally Inoculated With Pancreatic Cancer Cells. Pancreas 2017, 46, 190–197. [Google Scholar] [CrossRef]
- Moran, D.M.; Maki, C.G. Nutlin-3a Induces Cytoskeletal Rearrangement and Inhibits the Migration and Invasion Capacity of P53 Wild-Type Cancer Cells. Mol. Cancer Ther. 2010, 9, 895–905. [Google Scholar] [CrossRef]
- Zajkowicz, A.; Krześniak, M.; Matuszczyk, I.; Głowala-Kosińska, M.; Butkiewicz, D.; Rusin, M. Nutlin-3a, an MDM2 Antagonist and P53 Activator, Helps to Preserve the Replicative Potential of Cancer Cells Treated with a Genotoxic Dose of Resveratrol. Mol. Biol. Rep. 2013, 40, 5013–5026. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, W.; Jiang, X.; Bai, R.; Luo, Y.; Gao, Y.; Li, S.; Huang, Z.; Gong, Y.; Xie, C. Differential Effects of WRAP53 Transcript Variants on Non-Small Cell Lung Cancer Cell Behaviors. PLoS ONE 2023, 18, e0281132. [Google Scholar] [CrossRef]
- Gudikote, J.P.; Cascone, T.; Poteete, A.; Sitthideatphaiboon, P.; Wu, Q.; Morikawa, N.; Zhang, F.; Peng, S.; Tong, P.; Li, L.; et al. Inhibition of Nonsense-Mediated Decay Rescues P53β/γ Isoform Expression and Activates the P53 Pathway in MDM2-Overexpressing and Select P53-Mutant Cancers. J. Biol. Chem. 2021, 297, 101163. [Google Scholar] [CrossRef]
- Srivenugopal, K.S.; Shou, J.; Mullapudi, S.R.S.; Lang, F.F.; Rao, J.S.; Ali-Osman, F. Enforced Expression of Wild-Type P53 Curtails the Transcription of the O6-Methylguanine-DNA Methyltransferase Gene in Human Tumor Cells and Enhances Their Sensitivity to Alkylating Agents. Clin. Cancer Res. 2001, 7, 1398–1409. [Google Scholar]
- Khan, M.R.; Xiang, S.; Song, Z.; Wu, M. The P53-Inducible Long Noncoding RNA TRINGS Protects Cancer Cells from Necrosis under Glucose Starvation. EMBO J. 2017, 36, 3483–3500. [Google Scholar] [CrossRef]
- Vaughan, C.A.; Singh, S.; Subler, M.A.; Windle, J.J.; Inoue, K.; Fry, E.A.; Pillappa, R.; Grossman, S.R.; Windle, B.; Andrew Yeudall, W.; et al. The Oncogenicity of Tumor-Derived Mutant P53 Is Enhanced by the Recruitment of PLK3. Nat. Commun. 2021, 12, 704. [Google Scholar] [CrossRef]
- Kojima, K.; Konopleva, M.; Samudio, I.J.; Shikami, M.; Cabreira-Hansen, M.; McQueen, T.; Ruvolo, V.; Tsao, T.; Zeng, Z.; Vassilev, L.T.; et al. MDM2 Antagonists Induce P53-Dependent Apoptosis in AML: Implications for Leukemia Therapy. Blood 2005, 106, 3150–3159. [Google Scholar] [CrossRef]
- Song, J.M.; Im, J.; Nho, R.S.; Han, Y.H.; Upadhyaya, P.; Kassie, F. Hyaluronan-CD44/RHAMM Interaction-Dependent Cell Proliferation and Survival in Lung Cancer Cells. Mol. Carcinog. 2019, 58, 321–333. [Google Scholar] [CrossRef]
- Coleman, K.-L.; Chiaramonti, M.; Haddad, B.; Ranzenberger, R.; Henning, H.; Al Khashali, H.; Ray, R.; Darweesh, B.; Guthrie, J.; Heyl, D.; et al. Phosphorylation of IGFBP-3 by Casein Kinase 2 Blocks Its Interaction with Hyaluronan, Enabling HA-CD44 Signaling Leading to Increased NSCLC Cell Survival and Cisplatin Resistance. Cells 2023, 12, 405. [Google Scholar] [CrossRef]
- Benowitz, N.L. Pharmacology of Nicotine: Addiction, Smoking-Induced Disease, and Therapeutics. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 57–71. [Google Scholar] [CrossRef] [PubMed]
- St. Helen, G.; Havel, C.; Dempsey, D.; Jacob, P.; Benowitz, N.L. Nicotine Delivery, Retention, and Pharmacokinetics from Various Electronic Cigarettes. Addiction 2016, 111, 535–544. [Google Scholar] [CrossRef]
- Esther, C.R.; O’Neal, W.K.; Alexis, N.E.; Koch, A.L.; Cooper, C.B.; Barjaktarevic, I.; Raffield, L.M.; Bowler, R.P.; Comellas, A.P.; Peters, S.P.; et al. Prolonged, Physiologically Relevant Nicotine Concentrations in the Airways of Smokers. Am. J. Physiol. Cell. Mol. Physiol. 2023, 324, L32–L37. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.J.; Mehta, T.; Hinton, A.; Sloan, R.; Nshimiyimana, J.; Tackett, A.P.; Roberts, M.E.; Brinkman, M.C.; Wagener, T.L. E-Cigarette Nicotine Delivery Among Young Adults by Nicotine Form, Concentration, and Flavor: A Crossover Randomized Clinical Trial. JAMA Netw. Open 2024, 7, e2426702. [Google Scholar] [CrossRef]
- Hiler, M.; Breland, A.; Spindle, T.; Maloney, S.; Lipato, T.; Karaoghlanian, N.; Shihadeh, A.; Lopez, A.; Ramôa, C.; Eissenberg, T. Electronic Cigarette User Plasma Nicotine Concentration, Puff Topography, Heart Rate, and Subjective Effects: Influence of Liquid Nicotine Concentration and User Experience. Exp. Clin. Psychopharmacol. 2017, 25, 380–392. [Google Scholar] [CrossRef]
- Alsharairi, N.A. Scutellaria Baicalensis and Their Natural Flavone Compounds as Potential Medicinal Drugs for the Treatment of Nicotine-Induced Non-Small-Cell Lung Cancer and Asthma. Int. J. Environ. Res. Public Health 2021, 18, 5243. [Google Scholar] [CrossRef] [PubMed]
- Muterspaugh, R.; Price, D.; Esckilsen, D.; McEachern, S.; Guthrie, J.; Heyl, D.; Evans, H.G. Interaction of Insulin-Like Growth Factor-Binding Protein 3 With Hyaluronan and Its Regulation by Humanin and CD44. Biochemistry 2018, 57, 5726–5737. [Google Scholar] [CrossRef]
- Dorandish, S.; Devos, J.; Clegg, B.; Price, D.; Muterspaugh, R.; Guthrie, J.; Heyl, D.L.; Evans, H.G. Biochemical Determinants of the IGFBP-3-Hyaluronan Interaction. FEBS Open Bio 2020, 10, 1668–1684. [Google Scholar] [CrossRef]
- Price, D.; Dorandish, S.; Williams, A.; Iwaniec, B.; Stephens, A.; Marshall, K.; Guthrie, J.; Heyl, D.; Evans, H.G. Humanin Blocks the Aggregation of Amyloid-β Induced by Acetylcholinesterase, an Effect Abolished in the Presence of IGFBP-3. Biochemistry 2020, 59, 1981–2002. [Google Scholar] [CrossRef]
- Al Khashali, H.; Wareham, J.; Ray, R.; Haddad, B.; Coleman, K.-L.; Ranzenberger, R.; McCombs, P.; Guthrie, J.; Heyl, D.; Evans, H.G. Opposing Roles of IGFBP-3 and Heparanase in Regulating A549 Lung Cancer Cell Survival. Cells 2022, 11, 3533. [Google Scholar] [CrossRef] [PubMed]
- Atali, S.; Dorandish, S.; Devos, J.; Williams, A.; Price, D.; Taylor, J.; Guthrie, J.; Heyl, D.; Evans, H.G. Interaction of Amyloid Beta with Humanin and Acetylcholinesterase Is Modulated by ATP. FEBS Open Bio 2020, 10, 2805–2823. [Google Scholar] [CrossRef]
- Dorandish, S.; Williams, A.; Atali, S.; Sendo, S.; Price, D.; Thompson, C.; Guthrie, J.; Heyl, D.; Evans, H.G. Regulation of Amyloid-β Levels by Matrix Metalloproteinase-2/9 (MMP2/9) in the Media of Lung Cancer Cells. Sci. Rep. 2021, 11, 9708. [Google Scholar] [CrossRef]
- Al Khashali, H.; Ray, R.; Coleman, K.-L.; Atali, S.; Haddad, B.; Wareham, J.; Guthrie, J.; Heyl, D.; Evans, H.G. Regulation of the Soluble Amyloid Precursor Protein α (sAPPα) Levels by Acetylcholinesterase and Brain-Derived Neurotrophic Factor in Lung Cancer Cell Media. Int. J. Mol. Sci. 2022, 23, 10746. [Google Scholar] [CrossRef] [PubMed]
- Dorandish, S.; Atali, S.; Ray, R.; Al Khashali, H.; Coleman, K.-L.; Guthrie, J.; Heyl, D.; Evans, H.G. Differences in the Relative Abundance of ProBDNF and Mature BDNF in A549 and H1299 Human Lung Cancer Cell Media. Int. J. Mol. Sci. 2021, 22, 7059. [Google Scholar] [CrossRef] [PubMed]

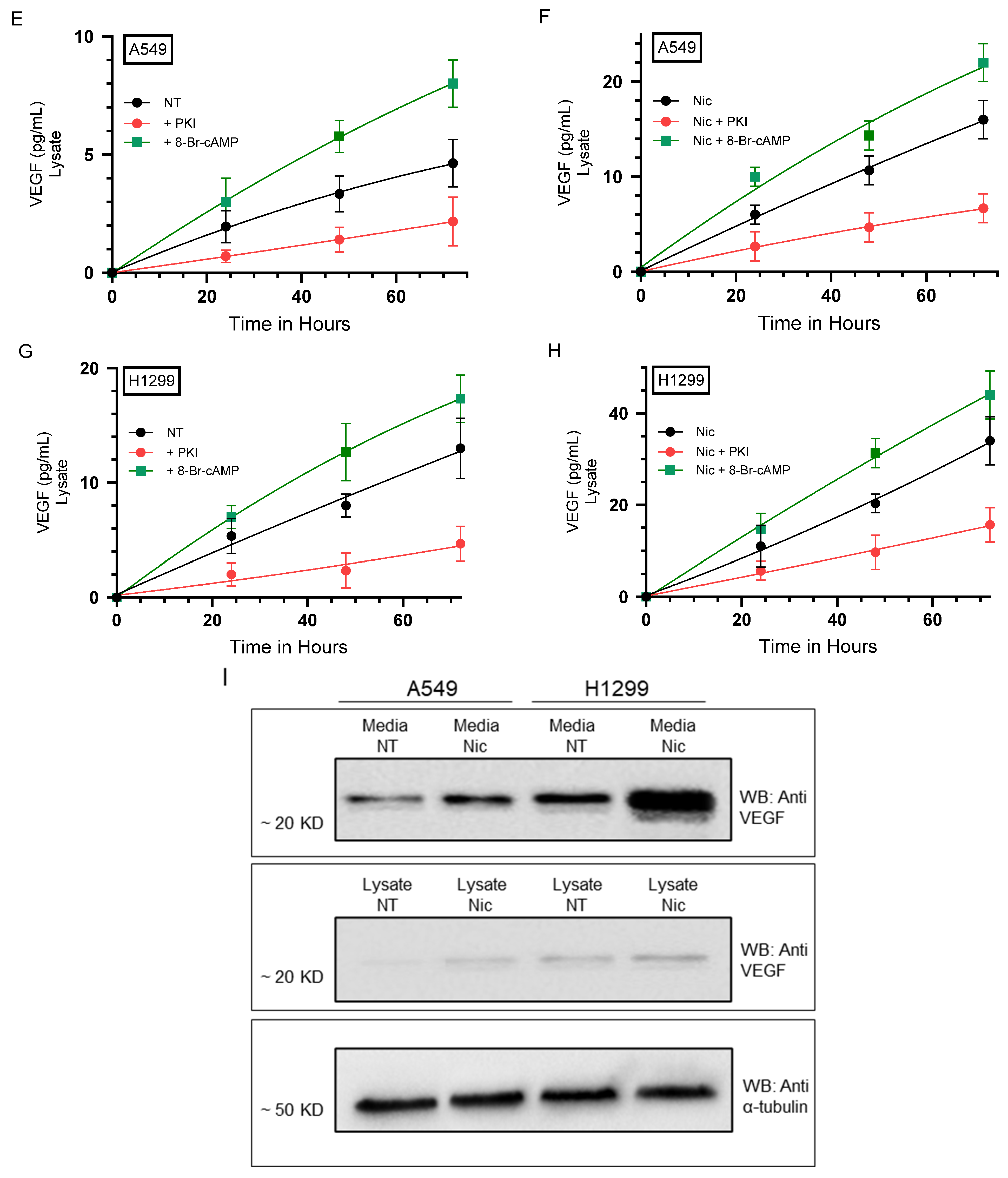


| A549 Cell Summary—VEGF Levels | ||
|---|---|---|
| Condition (72 h) | VEGF Level Fold Change ± SD | p-value |
| Control | VEGF levels increased over time | |
| 8-Br-cAMP | ~1.85-fold ± 0.22 ↑ vs. control | 0.017 |
| PKI | ~1.45-fold ± 0.17 ↓ vs. control | 0.026 |
| Nicotine | ~2.45-fold ± 0.28 ↑ vs. control | 0.022 |
| Nicotine + 8-Br-cAMP | ~1.25-fold ± 0.15 ↑ vs. nicotine | 0.018 |
| Nicotine + PKI | ~1.45-fold ± 0.16↓ vs. nicotine | 0.009 |
| H1299 Cell Summary–VEGF Levels | ||
| Condition (72 h) | VEGF Level Fold Change ± SD | p-value |
| Control | VEGF levels increased over time | |
| 8-Br-cAMP | ~1.40-fold ± 0.16 ↑ vs. control | 0.027 |
| PKI | ~1.60-fold ± 0.19 ↓ vs. control | 0.013 |
| Nicotine | ~2.65-fold ± 0.30 ↑ vs. control | 0.007 |
| Nicotine + 8-Br-cAMP | ~1.20-fold ± 0.14 ↑ vs. nicotine | 0.024 |
| Nicotine + PKI | ~1.60-fold ± 0.20 ↓ vs. nicotine | 0.004 |
| A549 Cell Summary | ||
|---|---|---|
| Condition | VEGF Fold Change ± SD | p-value |
| Nicotine + PKI | ~1.40-fold ± 0.16 ↓ vs. nicotine | 0.009 |
| Nicotine + 4-MU | ~1.40-fold ± 0.14 ↓ vs. nicotine | 0.008 |
| Nicotine + PKI + 4-MU | ~2.00-fold ± 0.23 ↓ vs. nicotine | 0.007 |
| Nicotine + PFT-α | ~1.50-fold ± 0.42 ↑ vs. nicotine | 0.009 |
| Nicotine + PKI + PFT-α | ~1.40-fold ± 0.16 ↓ vs. nicotine + PFT-α | 0.008 |
| Nicotine + 4-MU + PFT-α | ~1.40-fold ± 0.17 ↓ vs. nicotine + PFT-α | 0.008 |
| Nicotine + PKI + 4-MU + PFT-α | ~2.00-fold ± 0.24 ↓ vs. nicotine + PFT-α | 0.009 |
| H1299 Cell Summary | ||
| Condition | VEGF Fold Change ± SD | p-value |
| Nicotine + PKI | ~1.55-fold ± 0.18 ↓ vs. nicotine | 0.008 |
| Nicotine + 4-MU | ~1.55-fold ± 0.19 ↓ vs. nicotine | 0.006 |
| Nicotine + PKI + 4-MU | ~2.00-fold ± 0.23 ↓ vs. nicotine | 0.003 |
| Nicotine + PFT-α | No effect (p53-null) vs. nicotine | n.s. |
| Nicotine + PKI + PFT-α | No effect vs. nicotine + PKI | n.s. |
| Nicotine + 4-MU + PFT-α | No effect vs. nicotine + 4-MU | n.s. |
| Nicotine + PKI + 4-MU + PFT-α | No effect vs. nicotine + PKI + 4-MU | n.s. |
| A549 Cell Summary—PKA Activity | ||
|---|---|---|
| Condition | PKA Activity Fold Change ± SD | p-value |
| Epinephrine | ~2.85-fold ± 0.33 ↑ vs. control | 0.003 |
| Epinephrine + Propranolol | Blocked effect | n.s. |
| Nicotine | ~2.55-fold ± 0.29 ↑ vs. control | 0.002 |
| Nicotine + α-Btx | ~1.40-fold ± 0.16 ↓ vs. nicotine | 0.004 |
| Nicotine + Propranolol | ~1.25-fold ± 0.14 ↓ vs. nicotine | 0.003 |
| Nicotine + α-Btx + Propranolol | ~2.00-fold ± 0.22 ↓ vs. nicotine | 0.004 |
| PKI | Blocked activity | n.s. |
| H1299 Cell Summary—PKA Activity | ||
| Condition | PKA Activity Fold Change ± SD | p-value |
| Epinephrine | ~3.50-fold ± 0.40 ↑vs. control | 0.001 |
| Epinephrine + Propranolol | Blocked effect | n.s. |
| Nicotine | ~3.15-fold ± 0.35 ↑ vs. control | 0.005 |
| Nicotine + α-Btx | ~1.35-fold ± 0.15 ↓ vs. nicotine | 0.003 |
| Nicotine + Propranolol | ~1.20-fold ± 0.14 ↓ vs. nicotine | 0.002 |
| Nicotine + α-Btx + Propranolol | ~1.70-fold ± 0.19 ↓ vs. nicotine | 0.005 |
| PKI | Blocked activity | n.s. |
| A549 Cell Summary—HA Levels | ||
| Condition | HA Level Fold Change ± SD | p-value |
| Epinephrine | ~2.20-fold ± 0.25 ↑ vs. control | 0.004 |
| Epinephrine + Propranolol | Blocked effect | n.s. |
| Nicotine | ~2.00-fold ± 0.22 ↑ vs. control | 0.006 |
| Nicotine + α-Btx | ~1.50-fold ± 0.19 ↓ vs. nicotine | 0.003 |
| Nicotine + Propranolol | ~1.20-fold ± 0.15 ↓ vs. nicotine | 0.002 |
| Nicotine + α-Btx + Propranolol | ~1.85-fold ± 0.21 ↓ vs. nicotine | 0.004 |
| PKI (no nicotine) | ~2.30-fold ± 0.26 ↓ vs. control | 0.003 |
| Nicotine + PKI | ~1.50-fold ± 0.17 ↑ vs. control + PKI | 0.007 |
| Nicotine + PKI + α-Btx | ~1.28-fold ± 0.15 ↓ vs. nicotine + PKI | 0.008 |
| Nicotine + PKI + Propranolol | ~1.05-fold ± 0.12 ↓ vs. nicotine + PKI | 0.007 |
| Nicotine + PKI + α-Btx + Propranolol | ~1.34-fold ± 0.15 ↓ vs. nicotine + PKI | 0.006 |
| H1299 Cell Summary—HA Levels | ||
| Condition | HA Level Fold Change ± SD | p-value |
| Epinephrine | ~2.65-fold ± 0.30 ↑ vs. control | 0.005 |
| Epinephrine + Propranolol | Blocked effect | n.s. |
| Nicotine | ~2.30-fold ± 0.26 ↑ vs. control | 0.006 |
| Nicotine + α-Btx | ~1.48-fold ± 0.17 ↓ vs. nicotine | 0.003 |
| Nicotine + Propranolol | ~1.25-fold ± 0.14 ↓ vs. nicotine | 0.001 |
| Nicotine + α-Btx + Propranolol | ~1.75-fold ± 0.19 ↓ vs. nicotine | 0.004 |
| PKI (no nicotine) | ~1.95-fold ± 0.22 ↓ vs. control | 0.006 |
| Nicotine + PKI | ~1.45-fold ± 0.16 ↑ vs. control + PKI | 0.031 |
| Nicotine + PKI + α-Btx | ~1.30-fold ± 0.15 ↓ vs. nicotine + PKI | 0.003 |
| Nicotine + PKI + Propranolol | ~1.08-fold ± 0.13 ↓ vs. nicotine + PKI | 0.002 |
| Nicotine + PKI + α-Btx + Propranolol | ~1.40-fold ± 0.16 ↓ vs. nicotine + PKI | 0.006 |
| A549 Cell Summary—p53 Activity | ||
|---|---|---|
| Condition | p53 Activity Fold Change ± SD | p-value |
| Epinephrine | ~1.40-fold ± 0.16 ↓ vs. control | 0.004 |
| Epinephrine + Propranolol | Abolished effect | n.s. |
| Nicotine | ~2.40-fold ± 0.27 ↓ vs. control | 0.008 |
| Nicotine + α-Btx | ~1.60-fold ± 0.18 ↑ vs. nicotine | 0.007 |
| Nicotine + Propranolol | ~1.60-fold ± 0.19 ↑ vs. nicotine | 0.002 |
| Nicotine + α-Btx + Propranolol | ~1.95-fold ± 0.22 ↑ vs. nicotine | 0.003 |
| PKI (no nicotine) | ~1.75-fold ± 0.2 ↑ vs. control | 0.006 |
| Nicotine + PKI | ~2.20-fold ± 0.25 ↑ vs. nicotine | 0.004 |
| Nicotine + PKI + α-Btx | ~1.40-fold ± 0.15 ↑ vs. nicotine + PKI | 0.006 |
| Nicotine + PKI + Propranolol | ~1.35-fold ± 0.15 ↑ vs. nicotine + PKI | 0.003 |
| Nicotine + PKI + α-Btx + Propranolol | ~1.65-fold ± 0.19 ↑ vs. nicotine + PKI | 0.001 |
| A549 Cell Summary—VEGF Levels | ||
| Condition | VEGF Level Fold Change ± SD | p-value |
| Epinephrine | ~1.50-fold ± 0.170 ↑ vs. control | 0.004 |
| Epinephrine + Propranolol | Abolished effect | n.s. |
| Nicotine | ~2.55-fold ± 0.29 ↑ vs. control | 0.006 |
| Nicotine + α-Btx | ~1.55-fold ± 0.17 ↓ vs. nicotine | 0.004 |
| Nicotine + Propranolol | ~1.30-fold ± 0.15 ↓ vs. nicotine | 0.006 |
| Nicotine + α-Btx + Propranolol | ~1.85-fold ± 0.21 ↓ vs. nicotine | 0.007 |
| PKI (no nicotine) | ~1.40-fold ± 0.15 ↓ vs. control | 0.005 |
| Nicotine + PKI | ~1.45-fold ± 0.16 ↓ vs. nicotine | 0.004 |
| Nicotine + PKI + α-Btx | ~1.65-fold ± 0.19 ↓ vs. nicotine + PKI | 0.030 |
| Nicotine + PKI + Propranolol | ~1.35-fold ± 0.16 ↓ vs. nicotine + PKI | 0.040 |
| Nicotine + PKI + α-Btx + Propranolol | ~2.20-fold ± 0.26 ↓ vs. nicotine + PKI | 0.003 |
| H1299 Cell Summary—VEGF Levels | ||
| Condition | VEGF Level Fold Change ± SD | p-value |
| Epinephrine | ~1.70-fold ± 0.19 ↑ vs. control | 0.007 |
| Epinephrine + Propranolol | Blocked effect | n.s. |
| Nicotine | ~2.70-fold ± 0.32 ↑ vs. control | 0.002 |
| Nicotine + α-Btx | ~1.45-fold ± 0.16 ↓ vs. nicotine | 0.005 |
| Nicotine + Propranolol | ~1.30-fold ± 0.15 ↓ vs. nicotine | 0.003 |
| Nicotine + α-Btx + Propranolol | ~1.70-fold ± 0.20 ↓ vs. nicotine | 0.004 |
| PKI (no nicotine) | ~1.60-fold ± 0.19 ↓ vs. control | 0.003 |
| Nicotine + PKI | ~1.70-fold ± 0.21 ↓ vs. nicotine | 0.002 |
| Nicotine + PKI + α-Btx | ~1.40-fold ± 0.16 ↓ vs. nicotine + PKI | 0.030 |
| Nicotine + PKI + Propranolol | ~1.30-fold ± 0.15 ↓ vs. nicotine + PKI | 0.026 |
| Nicotine + PKI + α-Btx + Propranolol | ~1.70-fold ± 0.20 ↓ vs. nicotine + PKI | 0.003 |
| A549 Cell Summary—PKA Activity | ||
|---|---|---|
| Condition | PKA Activity Fold Change ± SD | p-value |
| p53 siRNA (untreated) | ~1.35-fold ± 0.15 ↑ vs. control siRNA | 0.004 |
| p53 siRNA + Nicotine | ~1.30-fold ± 0.15 ↑ vs. control siRNA + Nicotine | 0.002 |
| H1299 (any condition) | No effect (p53-null) | n.s. |
| A549 Cell Summary—VEGF Levels | ||
| Condition | VEGF Level Fold Change ± SD | p-value |
| p53 siRNA (untreated) | ~2.00-fold ± 0.22 ↑ vs. control siRNA | 0.006 |
| p53 siRNA + Nicotine | ~2.00-fold ± 0.23 ↑ vs. control siRNA + Nicotine | 0.002 |
| Control siRNA + PKI | ~1.45-fold ± 0.16 ↓ vs. control siRNA (no PKI) | 0.027 |
| p53 siRNA + PKI | ~1.55-fold ± 0.18 ↓ vs. p53 siRNA (no PKI) | 0.004 |
| Control siRNA + Nicotine + PKI | ~1.45-fold ± 0.17 ↓ vs. control siRNA + Nicotine (no PKI) | 0.031 |
| p53 siRNA + Nicotine + PKI | ~1.65-fold ± 0.19 ↓ vs. p53 siRNA + Nicotine (no PKI) | 0.005 |
| H1299 Cell Summary—VEGF Levels | ||
| Condition | VEGF Level Fold Change ± SD | p-value |
| Nicotine (control or p53 siRNA) | ~2.70-fold ± 0.31 ↑ vs. untreated | 0.006 |
| Control siRNA + PKI | ~1.55-fold ± 0.18 ↓ vs. control siRNA (no PKI) | 0.039 |
| p53 siRNA + PKI | ~1.55-fold ± 0.19 ↓ vs. p53 siRNA (no PKI) | 0.040 |
| Control siRNA + Nicotine + PKI | ~1.60-fold ± 0.18 ↓ vs. control siRNA + Nicotine (no PKI) | 0.004 |
| p53 siRNA + Nicotine + PKI | ~1.60-fold ± 0.19 ↓ vs. p53 siRNA + Nicotine (no PKI) | 0.005 |
| A549 Cell Summary—Cell Viability | ||
|---|---|---|
| Condition | Cell Viability Fold Change ± SD | p-value |
| Nicotine | ~1.75-fold ± 0.20 ↑ vs. control | 0.002 |
| Nicotine + PKI | ~1.53-fold ± 0.17 ↓ vs. nicotine | 0.006 |
| Nicotine + 4-MU | ~2.00-fold ± 0.24 ↓ vs. nicotine | 0.003 |
| Nicotine + PFT-α | ~1.30-fold ± 0.15 ↑ vs. nicotine | 0.005 |
| Nicotine + VEGF antibody | ~1.53-fold ± 0.18 ↓ vs. nicotine + hIgG | 0.004 |
| Nicotine + PKI + 4-MU | ~3.30-fold ± 0.38 ↓ vs. nicotine | 0.005 |
| Nicotine + PKI + PFT-α | ~1.23-fold ± 0.14 ↓ vs. nicotine | 0.008 |
| Nicotine + PFT-α + 4-MU | ~1.60-fold ± 0.19 ↓ vs. nicotine | 0.006 |
| Nicotine + PKI + VEGF antibody | ~2.65-fold ± 0.32 ↓ vs. nicotine | 0.002 |
| Nicotine + PFT-α + VEGF antibody | ~1.23-fold ± 0.15 ↓ vs. nicotine | 0.008 |
| Nicotine + 4-MU + VEGF antibody | ~3.05-fold ± 0.36 ↓ vs. nicotine | 0.003 |
| Nicotine + PKI + PFT-α + 4-MU + VEGF antibody | ~4.66-fold ± 0.52 ↓ vs. nicotine | 0.002 |
| H1299 Cell Summary—Cell Viability | ||
| Condition | Cell Viability Fold Change ± SD | p-value |
| Nicotine | ~2.80-fold ± 0.32 ↑ vs. control | 0.002 |
| Nicotine + PKI | ~1.43-fold ± 0.17 ↓ vs. nicotine | 0.008 |
| Nicotine + 4-MU | ~1.58-fold ± 0.19 ↓ vs. nicotine | 0.007 |
| Nicotine + PFT-α | No effect (p53-null) vs. nicotine | n.s. |
| Nicotine + hIgG | No effect vs. nicotine | n.s. |
| Nicotine + VEGF antibody | ~1.38-fold ± 0.16 ↓ vs. nicotine + hIgG | 0.004 |
| Nicotine + PKI + 4-MU | ~2.28-fold ± 0.27 ↓ vs. nicotine | 0.003 |
| Nicotine + PKI + PFT-α | No effect (p53-null) vs. nicotine + PKI | n.s. |
| Nicotine + PFT-α + 4-MU | No effect (p53-null) vs. nicotine + 4-MU | n.s. |
| Nicotine + PFT-α + VEGF antibody | No effect (p53-null) vs. nicotine + VEGF antibody | n.s. |
| Nicotine + PKI + VEGF antibody | ~2.10-fold ± 0.24 ↓ vs. nicotine | 0.004 |
| Nicotine + 4-MU + VEGF antibody | ~2.26-fold ± 0.27 ↓ vs. nicotine | 0.006 |
| Nicotine + PKI + PFT-α + 4-MU + VEGF antibody | ~2.58-fold ± 0.30 ↓ vs. nicotine | 0.004 |
| A549 Cell Summary—Apoptosis | ||
| Condition | Apoptosis Fold Change ± SD | p-value |
| Nicotine | ~2.50-fold ± 0.30 ↓ vs. control | 0.002 |
| Nicotine + PKI | ~2.10-fold ± 0.24 ↑ vs. nicotine | 0.006 |
| Nicotine + 4-MU | ~2.93-fold ± 0.33 ↑ vs. nicotine | 0.003 |
| Nicotine + PFT-α | ~2.00-fold ± 0.23 ↓ vs. nicotine | 0.004 |
| Nicotine + VEGF antibody | ~2.10-fold ± 0.24 ↑ vs. nicotine + hIgG | 0.005 |
| Nicotine + PKI + 4-MU | ~4.00-fold ± 0.46 ↑ vs. nicotine | 0.004 |
| Nicotine + PKI + PFT-α | ~1.58-fold ± 0.18 ↑ vs. nicotine | 0.006 |
| Nicotine + PFT-α + 4-MU | ~2.15-fold ± 0.25 ↑ vs. nicotine | 0.007 |
| Nicotine + PKI + VEGF antibody | ~3.80-fold ± 0.45 ↑ vs. nicotine | 0.002 |
| Nicotine + PFT-α + VEGF antibody | ~1.68-fold ± 0.20 ↑ vs. nicotine | 0.008 |
| Nicotine + 4-MU + VEGF antibody | ~3.95-fold ± 0.47 ↑ vs. nicotine | 0.002 |
| Nicotine + PKI + PFT-α + 4-MU + VEGF antibody | ~5.88-fold ± 0.70 ↑ vs. nicotine | 0.003 |
| H1299 Cell Summary—Apoptosis | ||
| Condition | Apoptosis Fold Change ± SD | p-value |
| Nicotine | ~3.30-fold ± 0.38 ↓ vs. control | 0.003 |
| Nicotine + PKI | ~1.70-fold ± 0.20 ↑ vs. nicotine | 0.005 |
| Nicotine + 4-MU | ~2.40-fold ± 0.27 ↑ vs. nicotine | 0.004 |
| Nicotine + PFT-α | No effect (p53-null) vs. nicotine | n.s. |
| Nicotine + hIgG | No effect vs. nicotine | n.s. |
| Nicotine + VEGF antibody | ~1.85-fold ± 0.22 ↑ vs. nicotine + hIgG | 0.007 |
| Nicotine + PKI + 4-MU | ~3.10-fold ± 0.36 ↑ vs. nicotine | 0.004 |
| Nicotine + PKI + PFT-α | No effect (p53-null) vs. nicotine + PKI | n.s. |
| Nicotine + PFT-α + 4-MU | No effect (p53-null) vs. nicotine + 4-MU | n.s. |
| Nicotine + PFT-α + VEGF antibody | No effect (p53-null) vs. nicotine + VEGF antibody | n.s. |
| Nicotine + PKI + VEGF antibody | ~3.00-fold ± 0.34 ↑ vs. nicotine | 0.002 |
| Nicotine + 4-MU + VEGF antibody | ~3.13-fold ± 0.37 ↑ vs. nicotine | 0.006 |
| Nicotine + PKI + PFT-α + 4-MU + VEGF antibody | ~4.45-fold ± 0.51 ↑ vs. nicotine | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wozniak, C.; Cobos, A.; Sabri, A.; Goel, S.; Lopo, B.; Sarofim, S.; Chutipassakul, C.; Guthrie, J.; Heyl, D.; Evans, H.G. Nicotine-Induced VEGF Levels in NSCLC Cells Are Modulated by PKA, Hyaluronan, and p53. Int. J. Mol. Sci. 2025, 26, 11103. https://doi.org/10.3390/ijms262211103
Wozniak C, Cobos A, Sabri A, Goel S, Lopo B, Sarofim S, Chutipassakul C, Guthrie J, Heyl D, Evans HG. Nicotine-Induced VEGF Levels in NSCLC Cells Are Modulated by PKA, Hyaluronan, and p53. International Journal of Molecular Sciences. 2025; 26(22):11103. https://doi.org/10.3390/ijms262211103
Chicago/Turabian StyleWozniak, Caroline, Alvaro Cobos, Aya Sabri, Stuti Goel, Brooke Lopo, Sarah Sarofim, Chanidapa Chutipassakul, Jeffrey Guthrie, Deborah Heyl, and Hedeel Guy Evans. 2025. "Nicotine-Induced VEGF Levels in NSCLC Cells Are Modulated by PKA, Hyaluronan, and p53" International Journal of Molecular Sciences 26, no. 22: 11103. https://doi.org/10.3390/ijms262211103
APA StyleWozniak, C., Cobos, A., Sabri, A., Goel, S., Lopo, B., Sarofim, S., Chutipassakul, C., Guthrie, J., Heyl, D., & Evans, H. G. (2025). Nicotine-Induced VEGF Levels in NSCLC Cells Are Modulated by PKA, Hyaluronan, and p53. International Journal of Molecular Sciences, 26(22), 11103. https://doi.org/10.3390/ijms262211103







