The Adaptation of Cancer Cells to Serum Deprivation Is Mediated by mTOR-Dependent Cholesterol Synthesis
Abstract
1. Introduction
2. Results
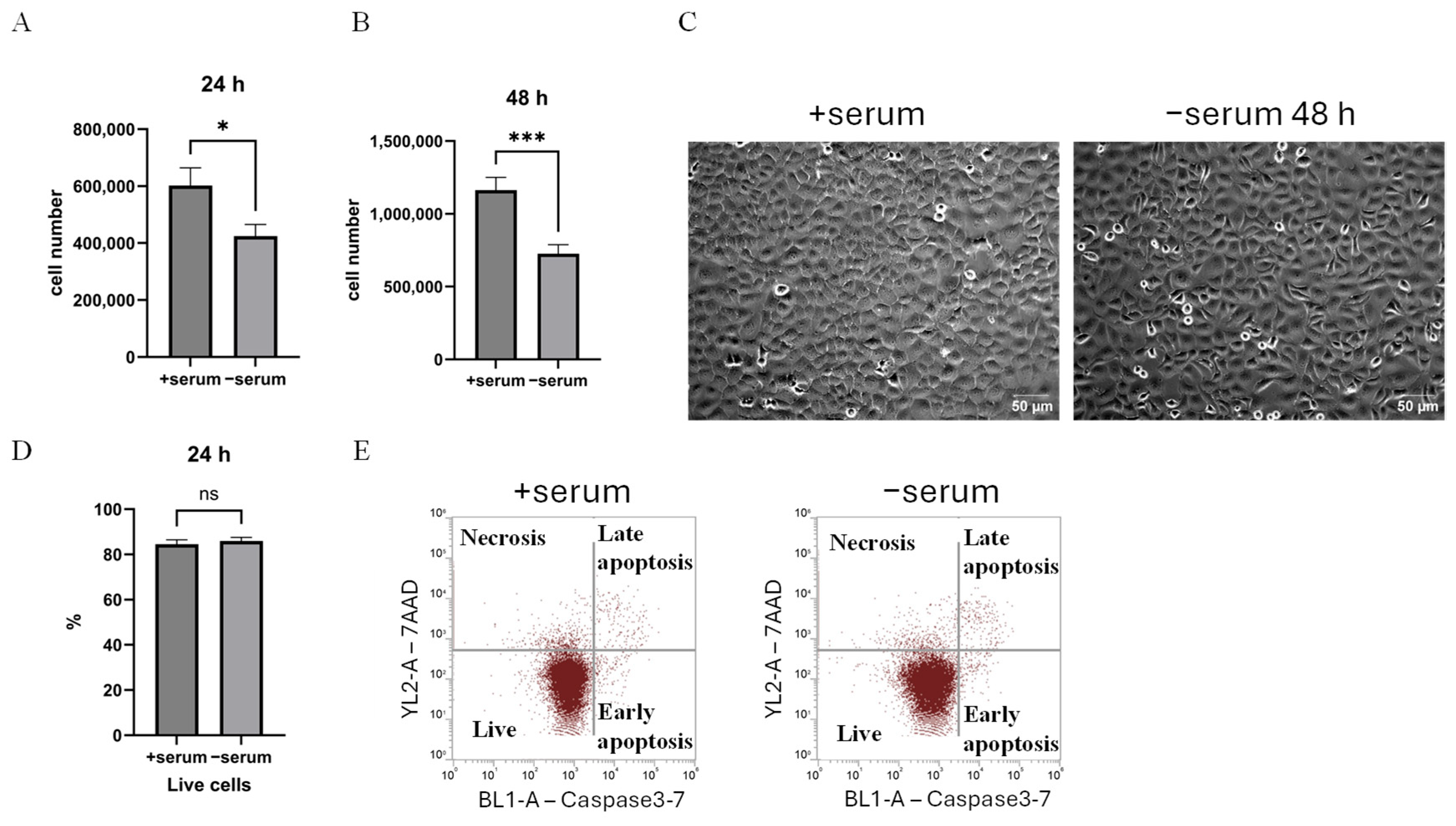
2.1. Serum Deprivation of Cancer Cells Reduces Proliferation but Does Not Induce Apoptosis
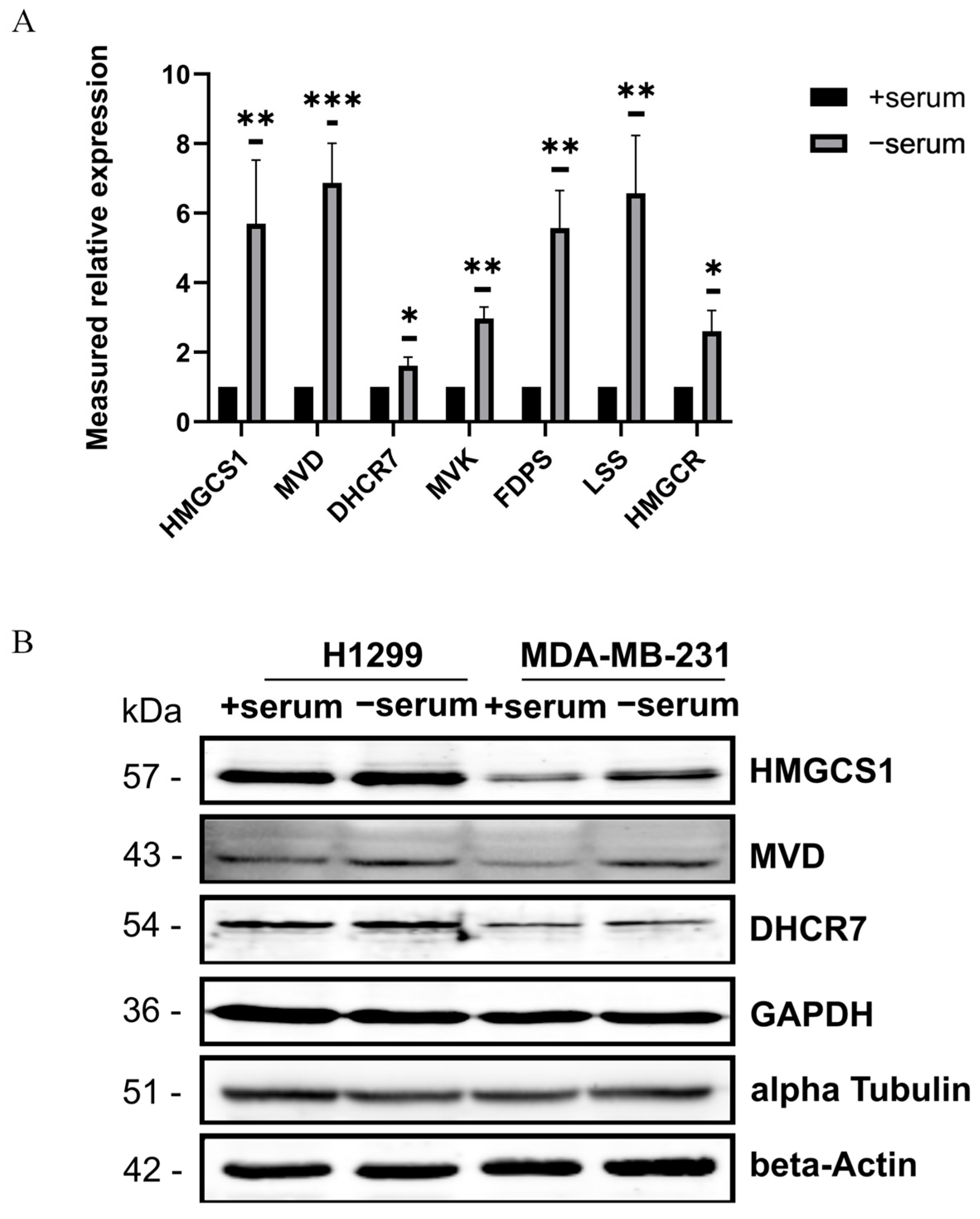
2.2. Transcriptomic Profiling Reveals Activation of Cholesterol Biosynthesis Pathway in Serum-Deprived Cancer Cells
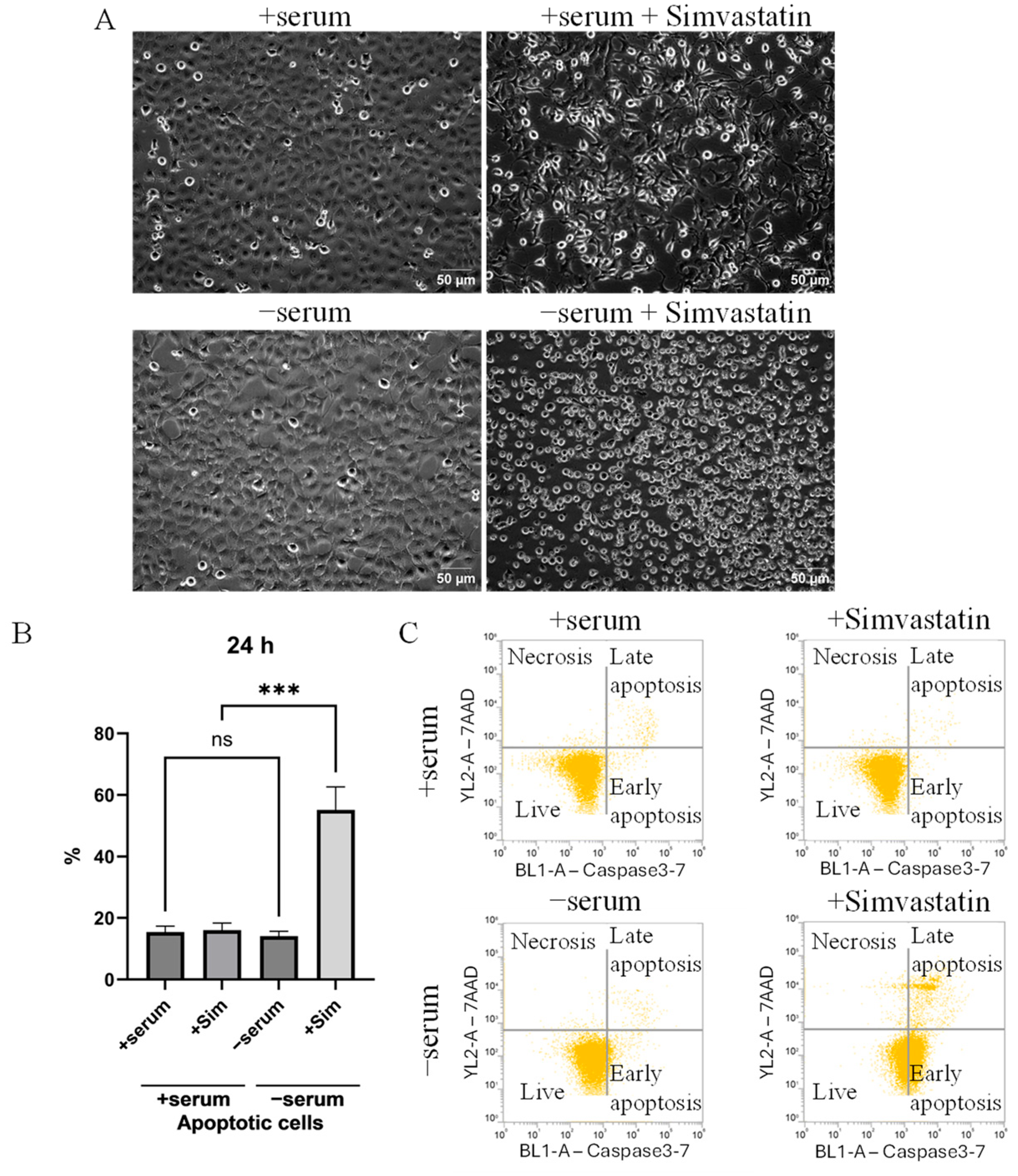
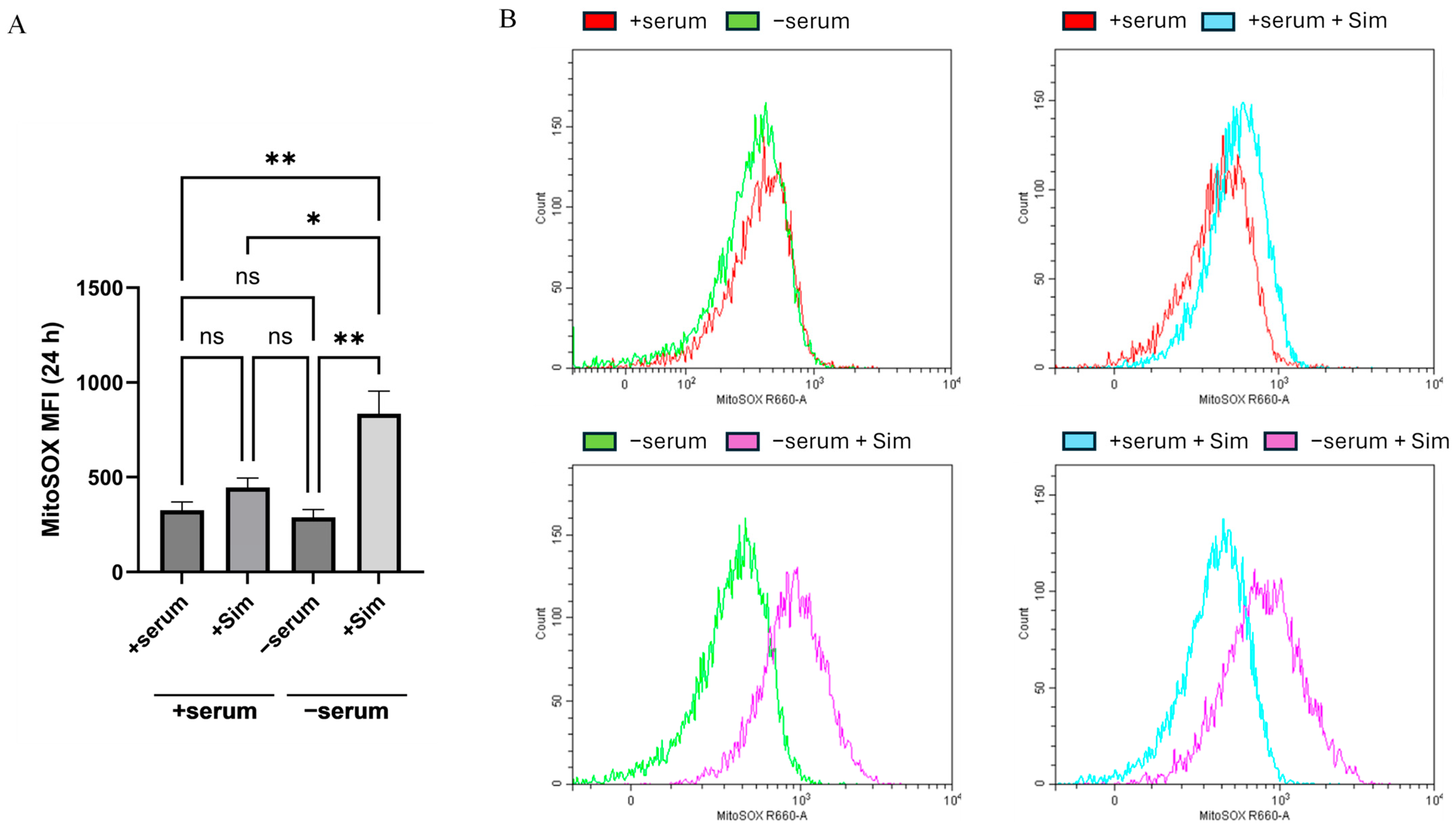
2.3. Simvastatin-Induced Apoptosis in Serum-Deprived Cancer Cells Mediated by a Mitochondrial ROS Generation
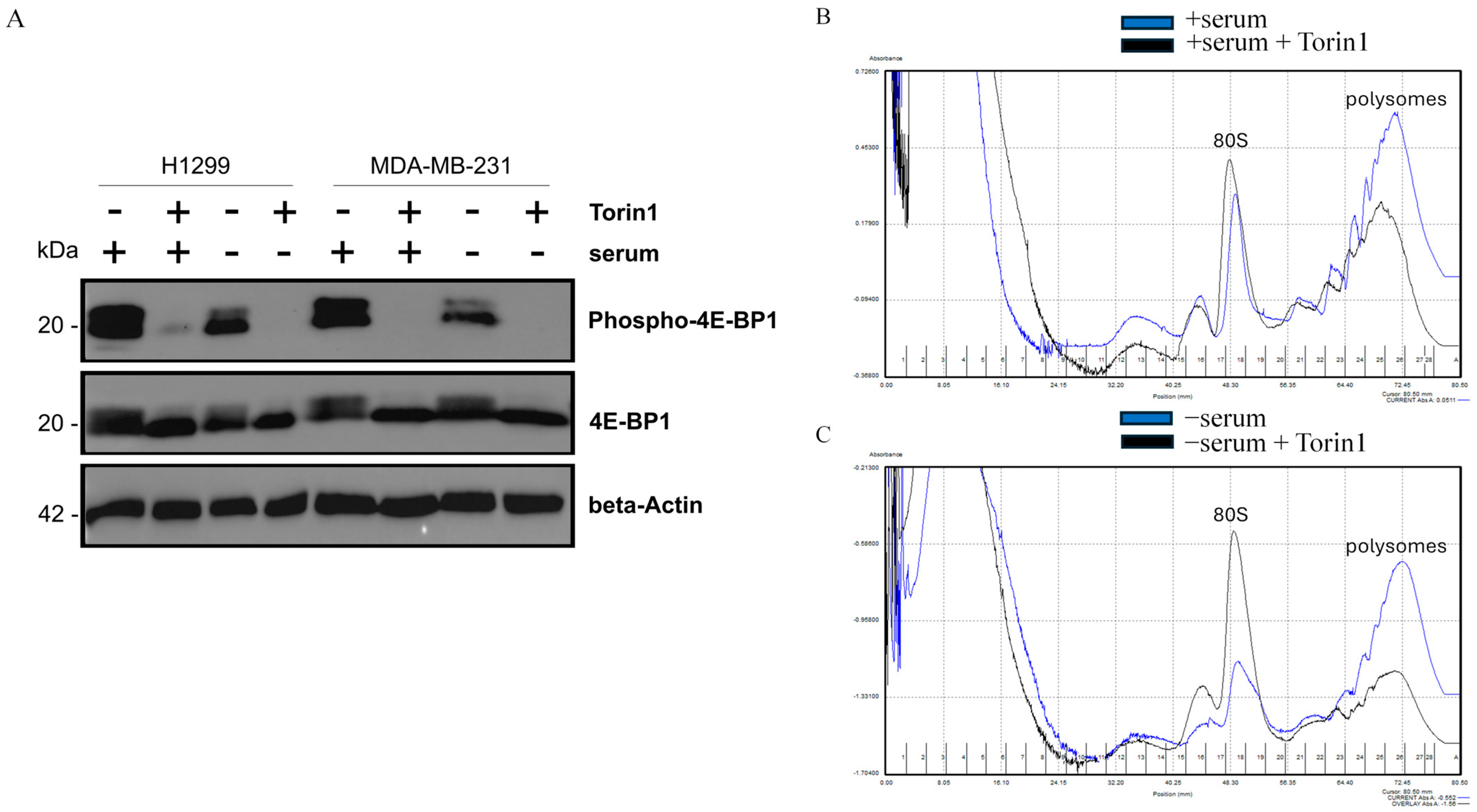
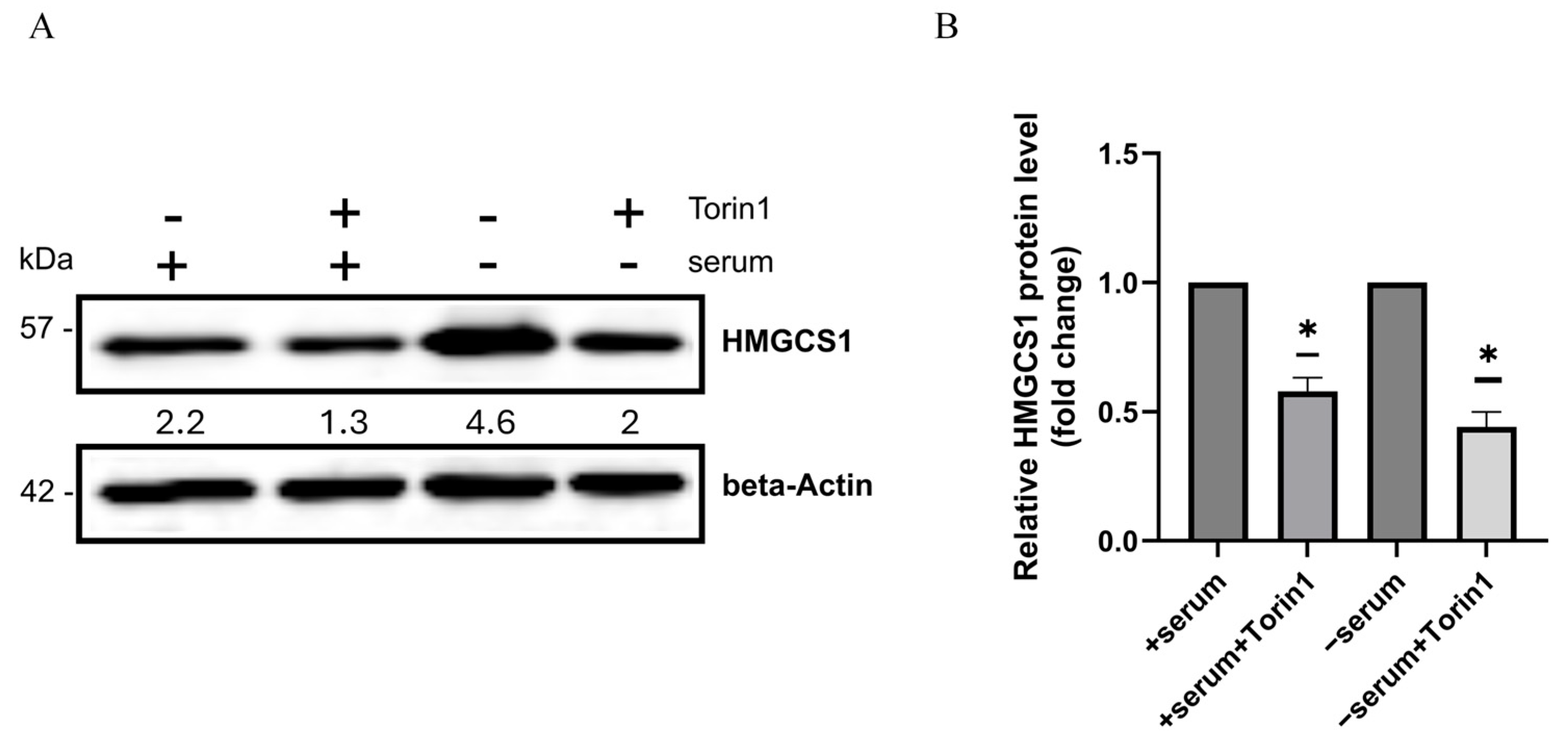
2.4. The Serum-Dependent Adaptive Upregulation of a Cholesterol Synthesis Pathway Is Mediated by mTOR Kinase Activity
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cell Counting and Size Analysis
4.3. RNA Isolation, Library Preparation, and Sequencing
4.4. Transcriptomic and Pathway Analysis
4.5. Flow Cytometry
4.6. Mitochondrial ROS Measurement
4.7. Quantitative Real-Time PCR
4.8. Immunoblotting
4.9. Subcellular Fractionation to Obtain the Soluble Nuclear Fractions and Cytoplasmic Fractions from Cancer Cells
4.10. Sucrose Gradient Fractionation
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| mTOR | mechanistic target of rapamycin |
| HMGCS1 | 3-hydroxy-3-methylglutaryl-CoA synthase 1 |
| MVD | mevalonate diphosphate decarboxylase |
| DHCR7 | 7-dehydrocholesterol reductase |
| MVK | mevalonate kinase |
| FDPS | farnesyl diphosphate synthase |
| LSS | lanosterol synthase |
| HMGCR | 3-hydroxy-3-methylglutaryl-CoA reductase |
References
- DeBerardinis, R.J.; Lum, J.J.; Hatzivassiliou, G.; Thompson, C.B. The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008, 7, 11–20. [Google Scholar] [CrossRef]
- Cairns, R.A.; Harris, I.S.; Mak, T.W. Regulation of cancer cell metabolism. Nat. Rev. Cancer 2011, 11, 85–95. [Google Scholar] [CrossRef]
- Viale, A.; Corti, D.; Draetta, G.F. Tumors and mitochondrial respiration: A neglected connection. Cancer Res. 2015, 75, 3685–3686. [Google Scholar] [CrossRef] [PubMed]
- Martin-Perez, M.; Urdiroz-Urricelqui, U.; Bigas, C.; Benitah, S.A. The role of lipids in cancer progression and metastasis. Cell Metab. 2022, 34, 1675–1699. [Google Scholar] [CrossRef] [PubMed]
- Winkelkotte, A.M.; Al-Shami, K.; Chaves-Filho, A.B.; Vogel, F.C.E.; Schulze, A. Interactions of Fatty Acid and Cholesterol Metabolism with Cellular Stress Response Pathways in Cancer. Cold Spring Harb. Perspect. Med. 2025, 15, a041548. [Google Scholar] [CrossRef]
- Lewis, C.A.; Brault, C.; Peck, B.; Bensaad, K.; Griffiths, B.; Mitter, R.; Chakravarty, P.; East, P.; Dankworth, B.; Alibhai, D.; et al. SREBP maintains lipid biosynthesis and viability of cancer cells under lipid- and oxygen-deprived conditions and defines a gene signature associated with poor survival in glioblastoma multiforme. Oncogene 2015, 34, 5128–5140. [Google Scholar] [CrossRef]
- Peck, B.; Schug, Z.T.; Zhang, Q.; Dankworth, B.; Jones, D.T.; Smethurst, E.; Patel, R.; Mason, S.; Jiang, M.; Saunders, R.; et al. Inhibition of fatty acid desaturation is detrimental to cancer cell survival in metabolically compromised environments. Cancer Metab. 2016, 4, 6. [Google Scholar] [CrossRef]
- Munir, R.; Lisec, J.; Swinnen, J.V.; Zaidi, N. Lipid metabolism in cancer cells under metabolic stress. Br. J. Cancer 2019, 120, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Sokol, K.H.; Lee, C.J.; Rogers, T.J.; Waldhart, A.; Ellis, A.E.; Madireddy, S.; Daniels, S.R.; House, R.R.J.; Ye, X.; Olesnavich, M.; et al. Lipid availability influences ferroptosis sensitivity in cancer cells by regulating polyunsaturated fatty acid trafficking. Cell Chem. Biol. 2025, 32, 408–422.e406. [Google Scholar] [CrossRef]
- Simigdala, N.; Gao, Q.; Pancholi, S.; Roberg-Larsen, H.; Zvelebil, M.; Ribas, R.; Folkerd, E.; Thompson, A.; Bhamra, A.; Dowsett, M.; et al. Cholesterol biosynthesis pathway as a novel mechanism of resistance to estrogen deprivation in estrogen receptor-positive breast cancer. Breast Cancer Res. 2016, 18, 58. [Google Scholar] [CrossRef]
- Kim, J.; Guan, K.L. mTOR as a central hub of nutrient signalling and cell growth. Nat. Cell Biol. 2019, 21, 63–71. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Cho, S.; Blenis, J. mTORC1, the maestro of cell metabolism and growth. Genes Dev. 2025, 39, 109–131. [Google Scholar] [CrossRef]
- Rabanal-Ruiz, Y.; Otten, E.G.; Korolchuk, V.I. mTORC1 as the main gateway to autophagy. Essays Biochem. 2017, 61, 565–584. [Google Scholar] [CrossRef]
- Wang, G.; Chen, L.; Qin, S.; Zhang, T.; Yao, J.; Yi, Y.; Deng, L. Mechanistic Target of Rapamycin Complex 1: From a Nutrient Sensor to a Key Regulator of Metabolism and Health. Adv. Nutr. 2022, 13, 1882–1900. [Google Scholar] [CrossRef]
- Bakan, I.; Laplante, M. Connecting mTORC1 signaling to SREBP-1 activation. Curr. Opin. Lipidol. 2012, 23, 226–234. [Google Scholar] [CrossRef]
- Han, J.; Wang, Y. mTORC1 signaling in hepatic lipid metabolism. Protein Cell 2018, 9, 145–151. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. Regulation of mTORC1 and its impact on gene expression at a glance. J. Cell Sci. 2013, 126 Pt 8, 1713–1719. [Google Scholar] [CrossRef]
- Düvel, K.; Yecies, J.L.; Menon, S.; Raman, P.; Lipovsky, A.I.; Souza, A.L.; Triantafellow, E.; Ma, Q.; Gorski, R.; Cleaver, S.; et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol. Cell 2010, 39, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- KEGG. KEGG Orthology: HMGCS (K01641). Kyoto Encyclopedia of Genes and Genomes. Available online: https://www.genome.jp/dbget-bin/www_bget?ko:K01641 (accessed on 30 September 2025).
- KEGG. KEGG Orthology: MVD K01597. Kyoto Encyclopedia of Genes and Genomes. Available online: https://www.genome.jp/dbget-bin/www_bget?ko:K01597 (accessed on 30 September 2025).
- KEGG. KEGG Orthology: DHCR7 (K00213)/Enzyme 1.3.1.21/Reaction R07487. Kyoto Encyclopedia of Genes and Genomes. Available online: https://www.genome.jp/dbget-bin/www_bget?K00213+1.3.1.21+R07487 (accessed on 30 September 2025).
- KEGG. KEGG Orthology: MVK (K00869). Kyoto Encyclopedia of Genes and Genomes. Available online: https://www.genome.jp/dbget-bin/www_bget?ko:K00869 (accessed on 30 September 2025).
- KEGG. KEGG Orthology: FDPS (K00787). Kyoto Encyclopedia of Genes and Genomes. Available online: https://www.genome.jp/dbget-bin/www_bget?ko:K00787 (accessed on 30 September 2025).
- KEGG. KEGG Orthology: LSS (K01852). Kyoto Encyclopedia of Genes and Genomes. Available online: https://www.genome.jp/dbget-bin/www_bget?ko:K01852 (accessed on 30 September 2025).
- KEGG. KEGG Enzyme: HMGCR (1.1.1.34). Kyoto Encyclopedia of Genes and Genomes. Available online: https://www.genome.jp/dbget-bin/www_bget?ec:1.1.1.34 (accessed on 30 September 2025).
- Technology, C.S. Phospho-4E-BP1 (Thr37/46) Rabbit mAb #2855. Cell Signaling Technology. Available online: https://www.cellsignal.com/products/primary-antibodies/phospho-4e-bp1-thr37-46-236b4-rabbit-mab/2855?srsltid=AfmBOopZmDm9kJnWsiBsct6aqBlMl4JGA5gzlKMtdArbeVEZ7DOVvCRN (accessed on 30 September 2025).
- Abetov, D.A.; Kiyan, V.S.; Zhylkibayev, A.A.; Sarbassova, D.A.; Alybayev, S.D.; Spooner, E.; Song, M.S.; Bersimbaev, R.I.; Sarbassov, D.D. Formation of mammalian preribosomes proceeds from intermediate to composed state during ribosome maturation. J. Biol. Chem. 2019, 294, 10746–10757. [Google Scholar] [CrossRef] [PubMed]
- Kazyken, D.; Kaz, Y.; Kiyan, V.; Zhylkibayev, A.A.; Chen, C.H.; Agarwal, N.K.; dos Sarbassov, D. The nuclear import of ribosomal proteins is regulated by mTOR. Oncotarget 2014, 5, 9577–9593. [Google Scholar] [CrossRef] [PubMed]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552 Pt 2, 335–344. [Google Scholar] [CrossRef]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef]
- Serrano, J.J.; Medina, M. Metabolic Reprogramming at the Edge of Redox: Connections Between Metabolic Reprogramming and Cancer Redox State. Int. J. Mol. Sci. 2025, 26, 498. [Google Scholar] [CrossRef]
- Jalmukhambetova, A.; Baltabekova, A.; Tolebay, A.; Rakhimgerey, N.; Molnár, F.; Thanh Pham, T.; Burska, A.N.; Sarbassov, D.D. Oxidative stress induces cortical stiffening and cytoskeletal remodelling in pre-apoptotic cancer cells. Cell Stress. 2025, 9, 182–193. [Google Scholar] [CrossRef]
- Goicoechea, L.; Conde de la Rosa, L.; Torres, S.; García-Ruiz, C.; Fernández-Checa, J.C. Mitochondrial cholesterol: Metabolism and impact on redox biology and disease. Redox Biol. 2023, 61, 102643. [Google Scholar] [CrossRef] [PubMed]
- Ribas, V.; García-Ruiz, C.; Fernández-Checa, J.C. Mitochondria, cholesterol and cancer cell metabolism. Clin. Transl. Med. 2016, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- ATCC. NCI-H1299 (CRL-5803). American Type Culture Collection. Available online: https://www.atcc.org/products/crl-5803 (accessed on 30 September 2025).
- ATCC. MDA-MB-231. American Type Culture Collection. Available online: https://www.atcc.org/products/htb-26 (accessed on 30 September 2025).
- Coulter, B. Multisizer 4e Coulter Counter. Beckman Coulter. Available online: https://www.beckman.com/cell-counters-and-analyzers/multisizer-4e (accessed on 30 September 2025).
- Illumina. NovaSeq 6000 Sequencing Platform. Illumina. Available online: https://sapac.illumina.com/systems/sequencing-platforms/novaseq.html (accessed on 30 September 2025).
- Coulter, B. CytoFLEX S (Flow Cytometer). Beckman Coulter. Available online: https://www.beckman.com/flow-cytometry/research-flow-cytometers/cytoflex-s (accessed on 30 September 2025).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilyassova, B.; Rakhimgerey, N.; Rakhimova, S.; Satvaldina, N.; Daniyarov, A.; Akilzhanova, A.; Kairov, U.; Begimbetova, D.; Sarbassov, D.D. The Adaptation of Cancer Cells to Serum Deprivation Is Mediated by mTOR-Dependent Cholesterol Synthesis. Int. J. Mol. Sci. 2025, 26, 10932. https://doi.org/10.3390/ijms262210932
Ilyassova B, Rakhimgerey N, Rakhimova S, Satvaldina N, Daniyarov A, Akilzhanova A, Kairov U, Begimbetova D, Sarbassov DD. The Adaptation of Cancer Cells to Serum Deprivation Is Mediated by mTOR-Dependent Cholesterol Synthesis. International Journal of Molecular Sciences. 2025; 26(22):10932. https://doi.org/10.3390/ijms262210932
Chicago/Turabian StyleIlyassova, Bayansulu, Nargiz Rakhimgerey, Saule Rakhimova, Nazerke Satvaldina, Asset Daniyarov, Ainur Akilzhanova, Ulykbek Kairov, Dinara Begimbetova, and Dos D. Sarbassov. 2025. "The Adaptation of Cancer Cells to Serum Deprivation Is Mediated by mTOR-Dependent Cholesterol Synthesis" International Journal of Molecular Sciences 26, no. 22: 10932. https://doi.org/10.3390/ijms262210932
APA StyleIlyassova, B., Rakhimgerey, N., Rakhimova, S., Satvaldina, N., Daniyarov, A., Akilzhanova, A., Kairov, U., Begimbetova, D., & Sarbassov, D. D. (2025). The Adaptation of Cancer Cells to Serum Deprivation Is Mediated by mTOR-Dependent Cholesterol Synthesis. International Journal of Molecular Sciences, 26(22), 10932. https://doi.org/10.3390/ijms262210932





