Transcriptomics and Plant Hormone Analysis Reveal the Mechanism of Exogenous GA3 Promoting Reflowering of Phalaenopsis ‘Hatuyuki’
Abstract
1. Introduction
2. Results
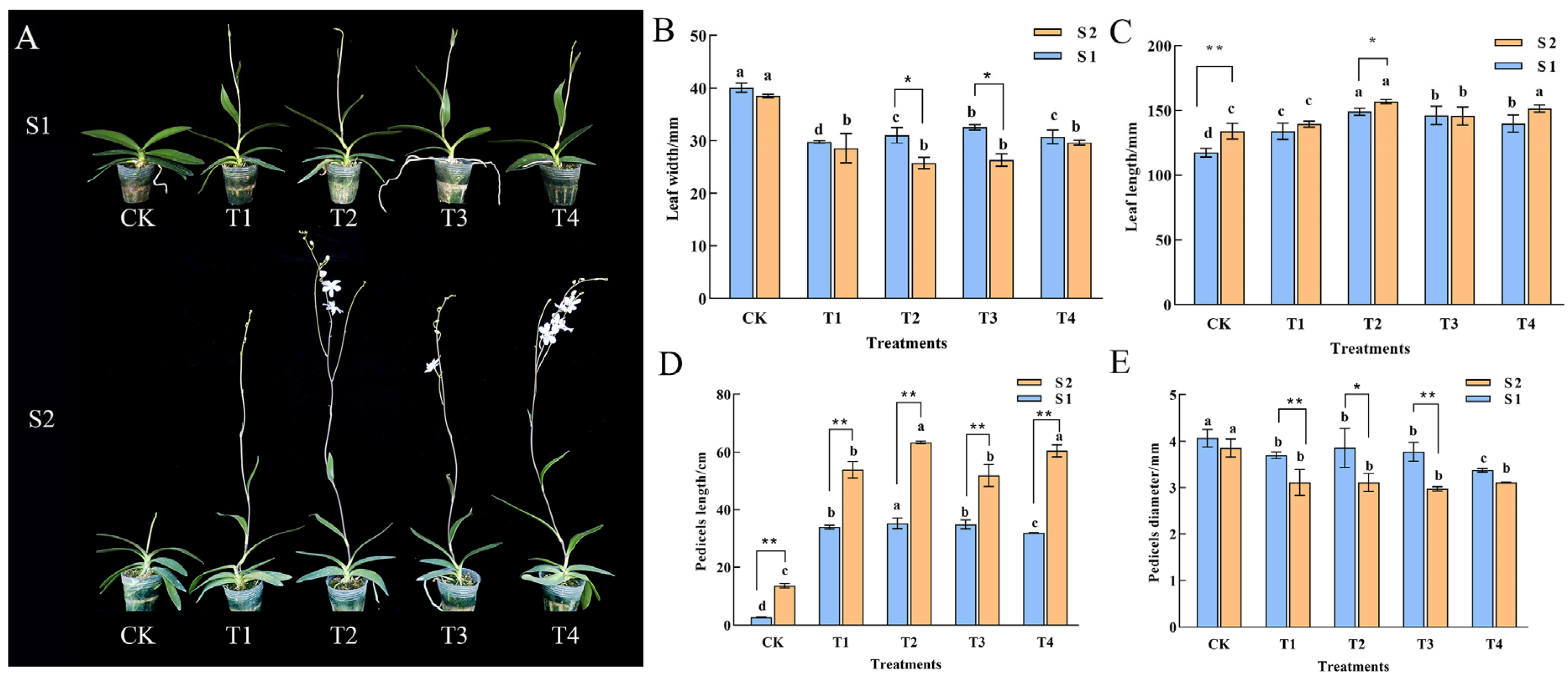
2.1. Plant Growth
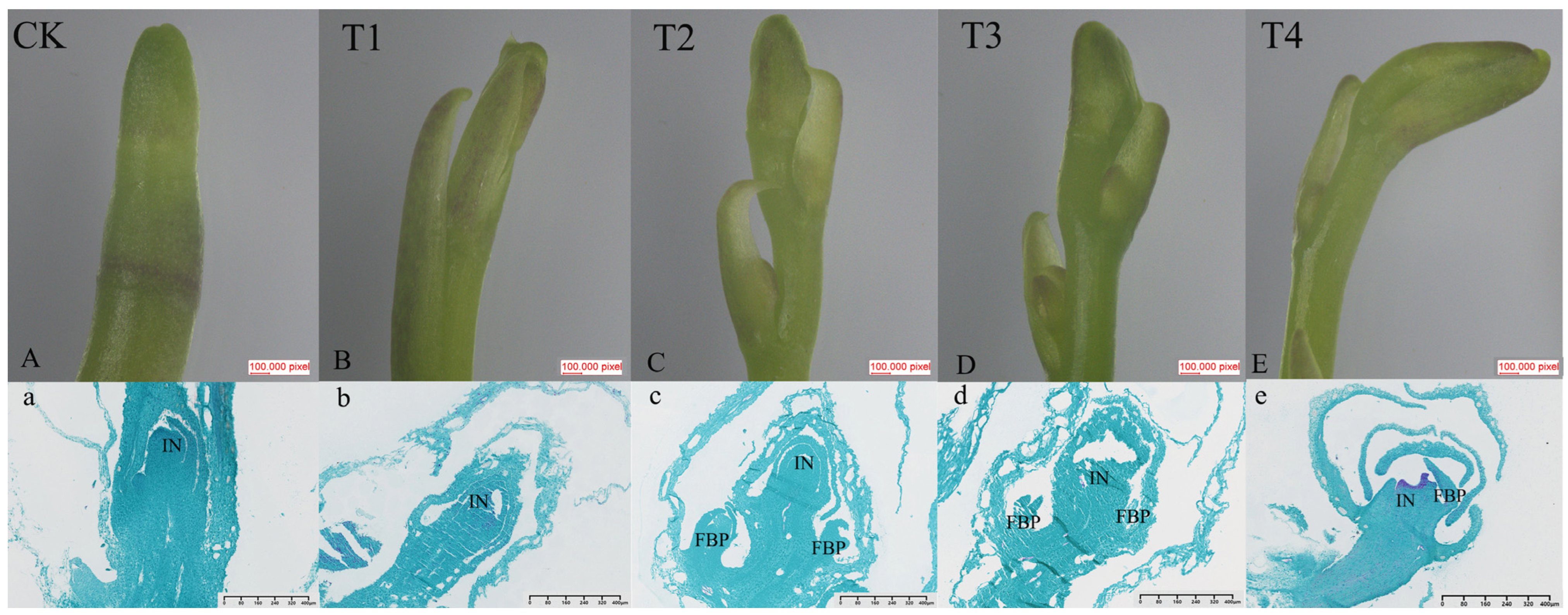
2.2. Morphological and Cytological Characterization of Spike
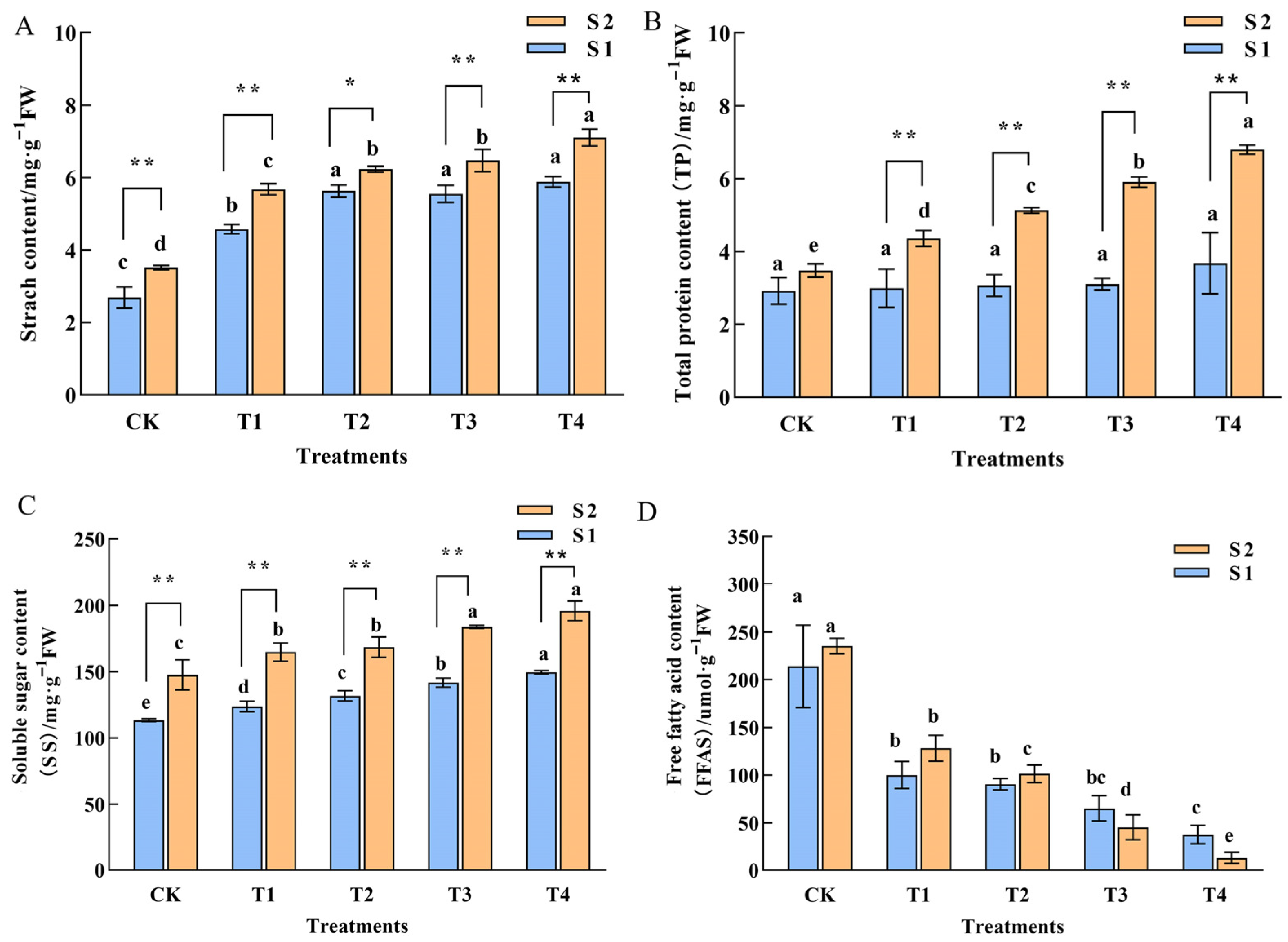
2.3. Physiological Characterization of Phalaenopsis ‘Hatuyuki’ Under GA3 Treatment
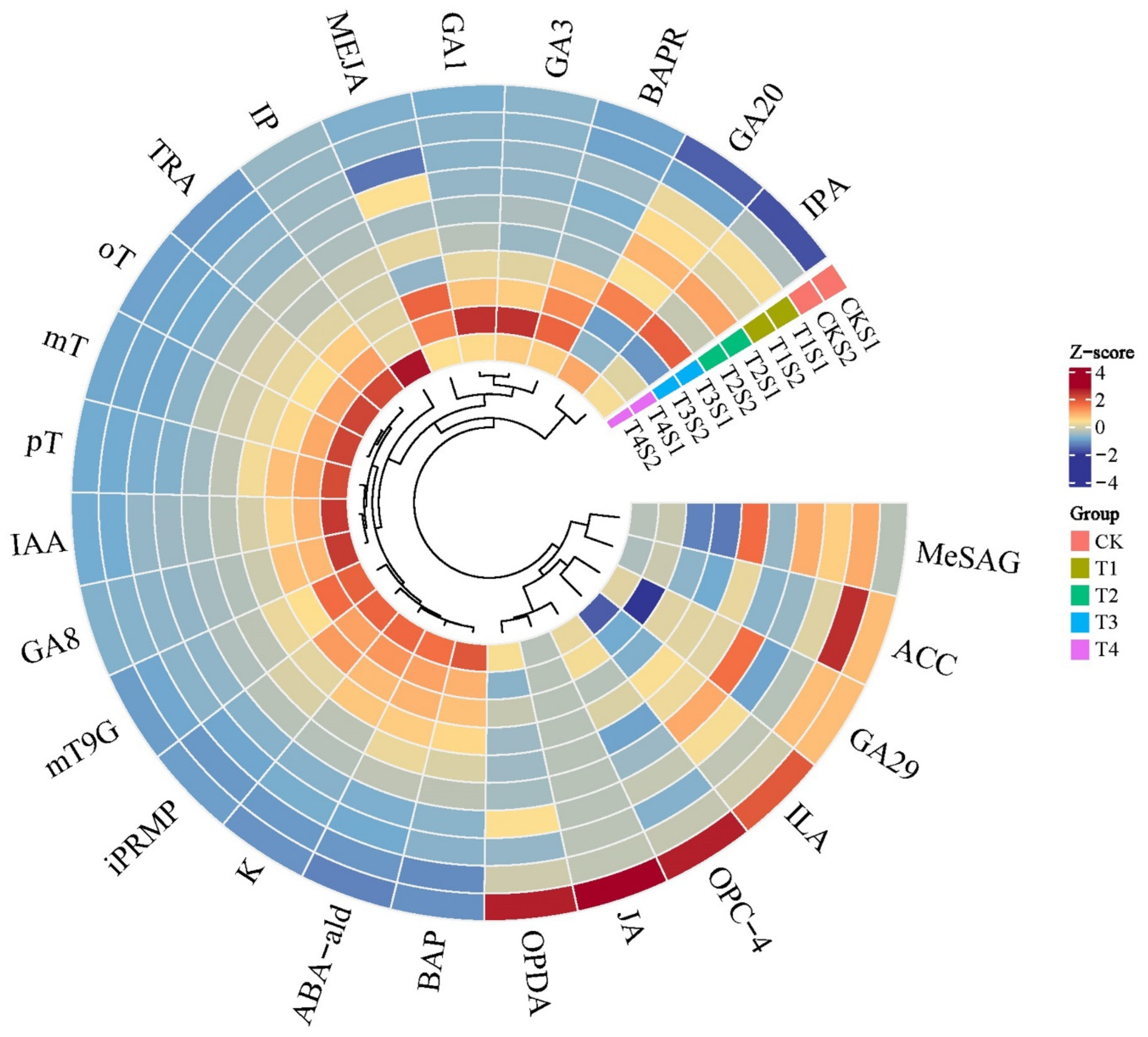
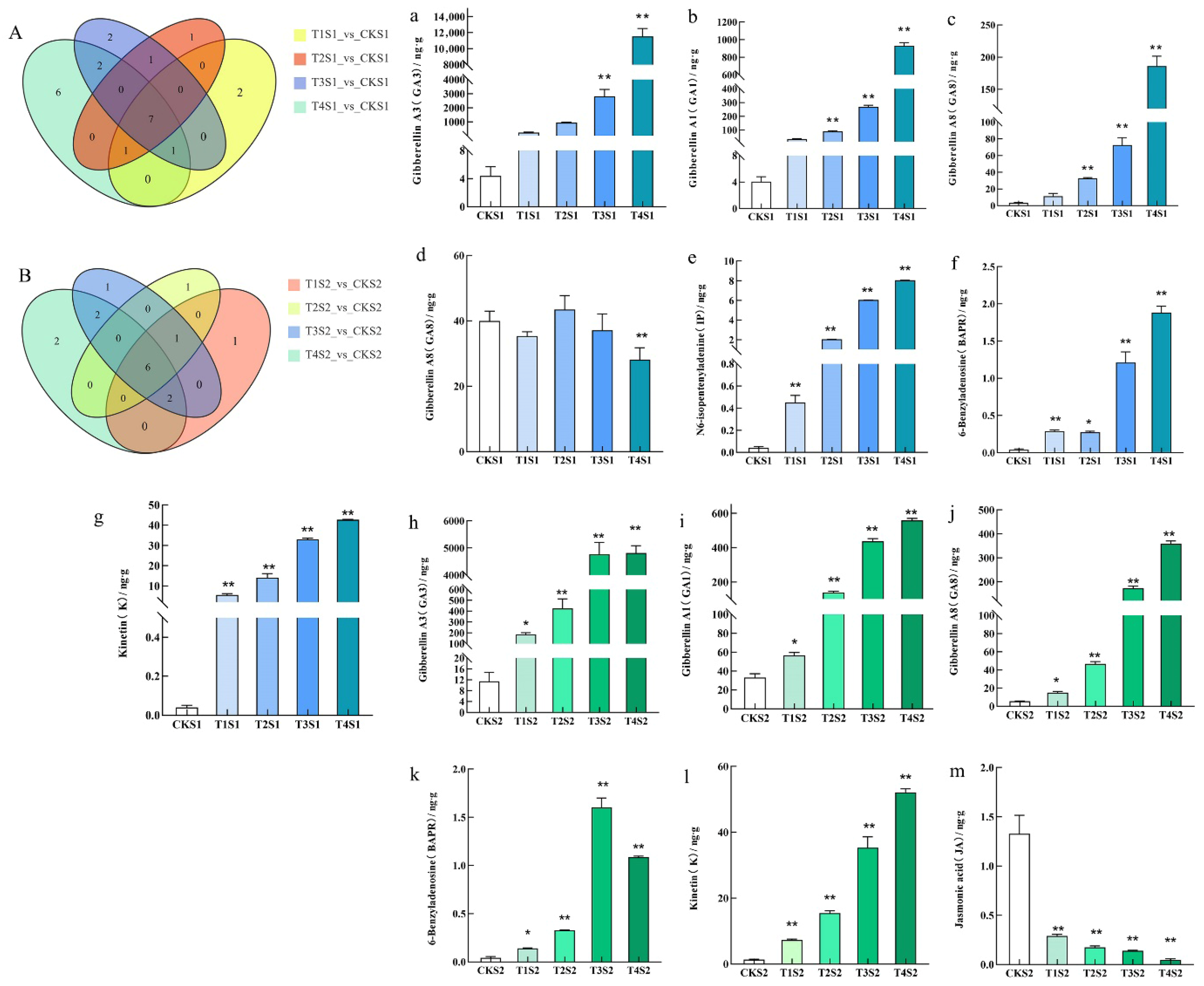
2.4. Changes in Endogenous Hormone Contents
2.5. Quality Analysis of Transcriptome Sequencing Data
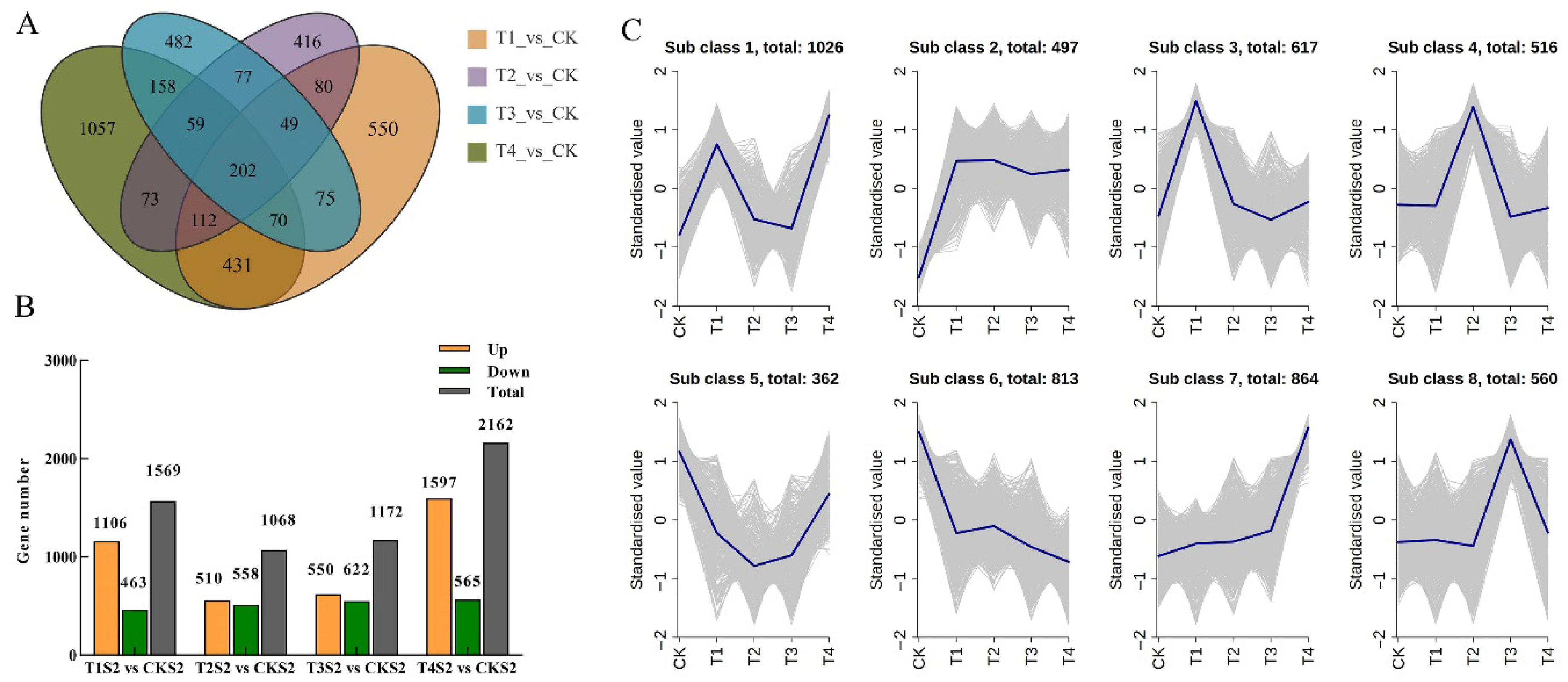
2.6. Differentially Expressed Genes Screening and Analysis
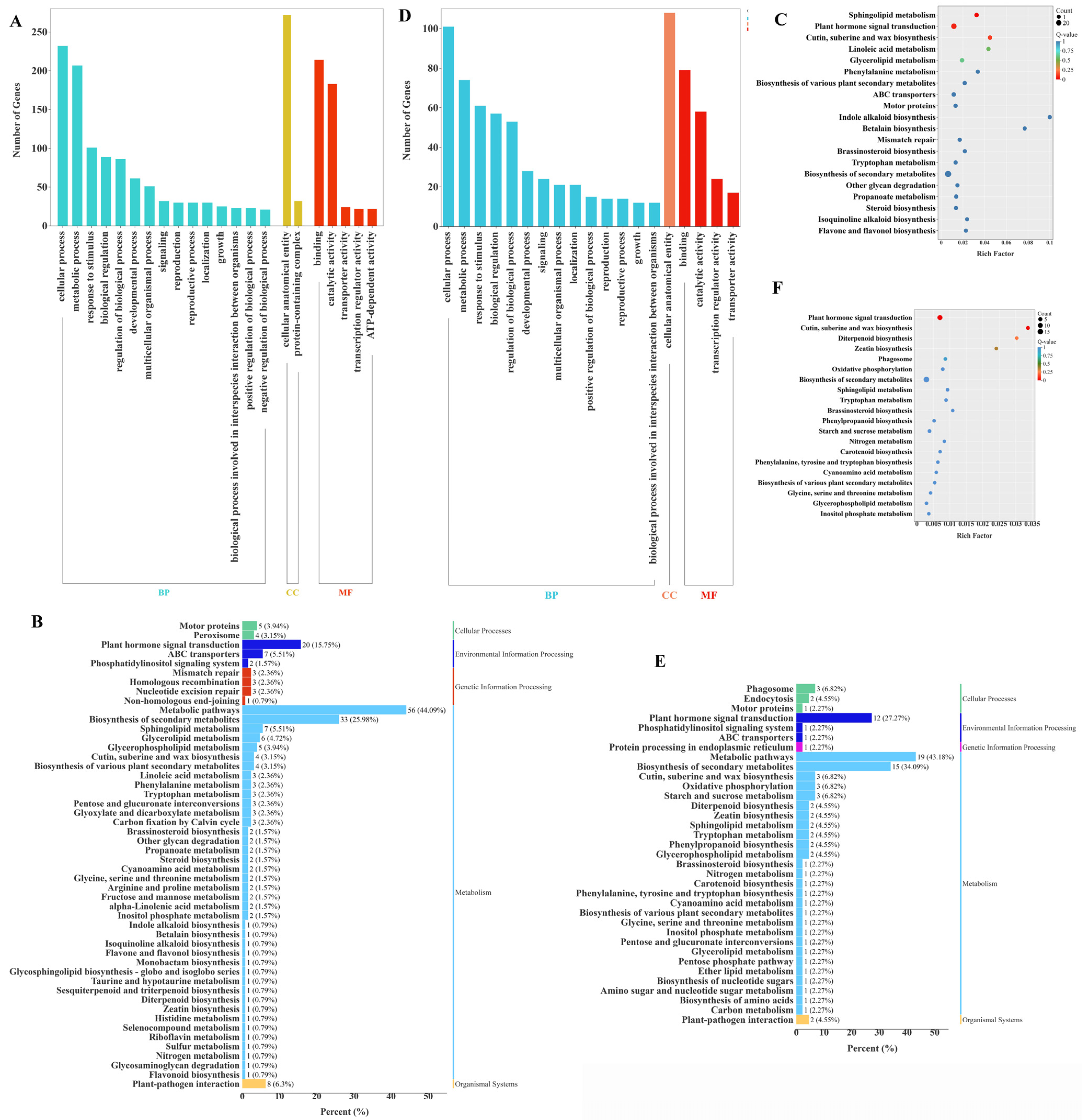
2.7. GO and KEGG Enrichment Analyses of DEGs
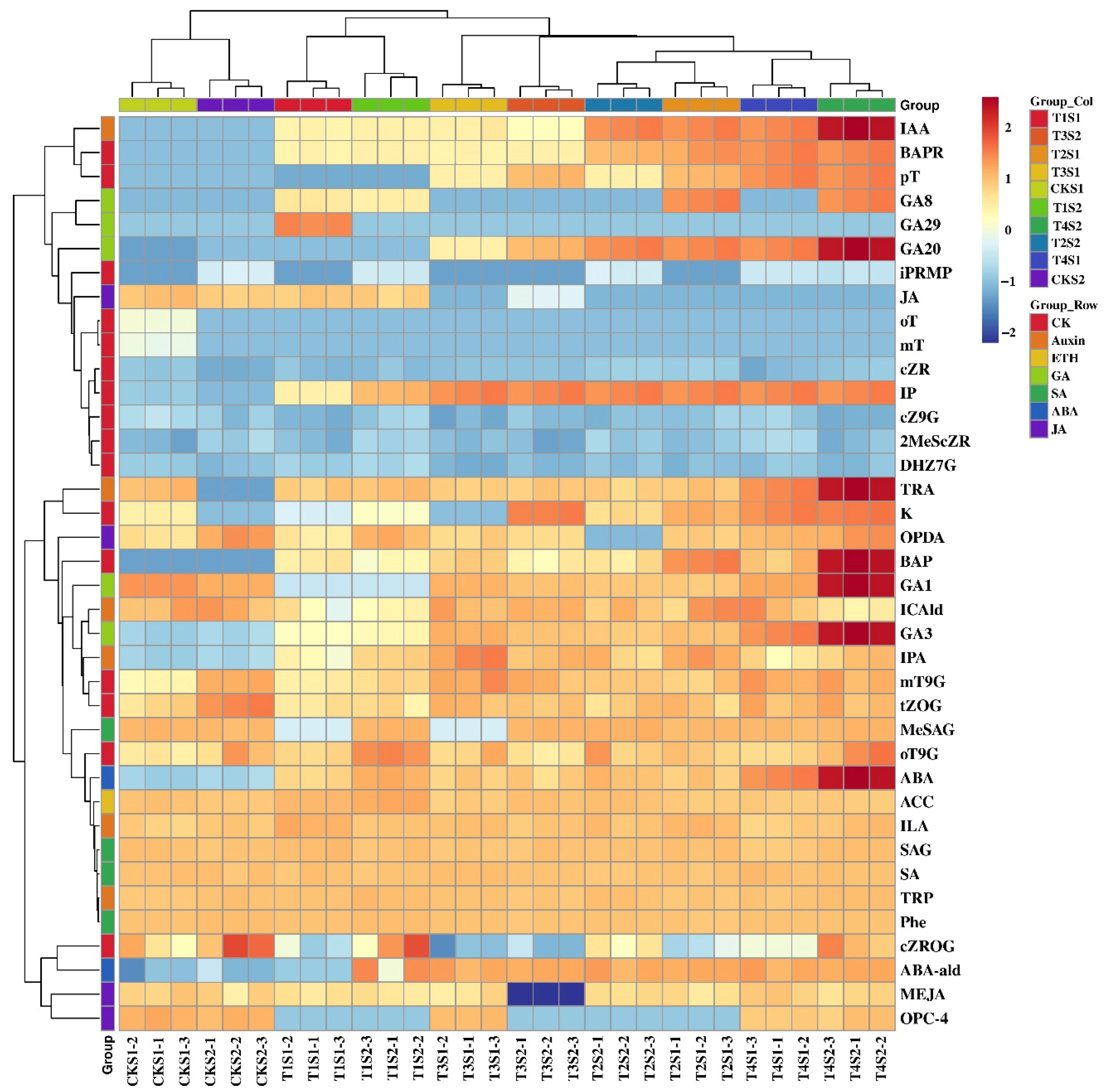
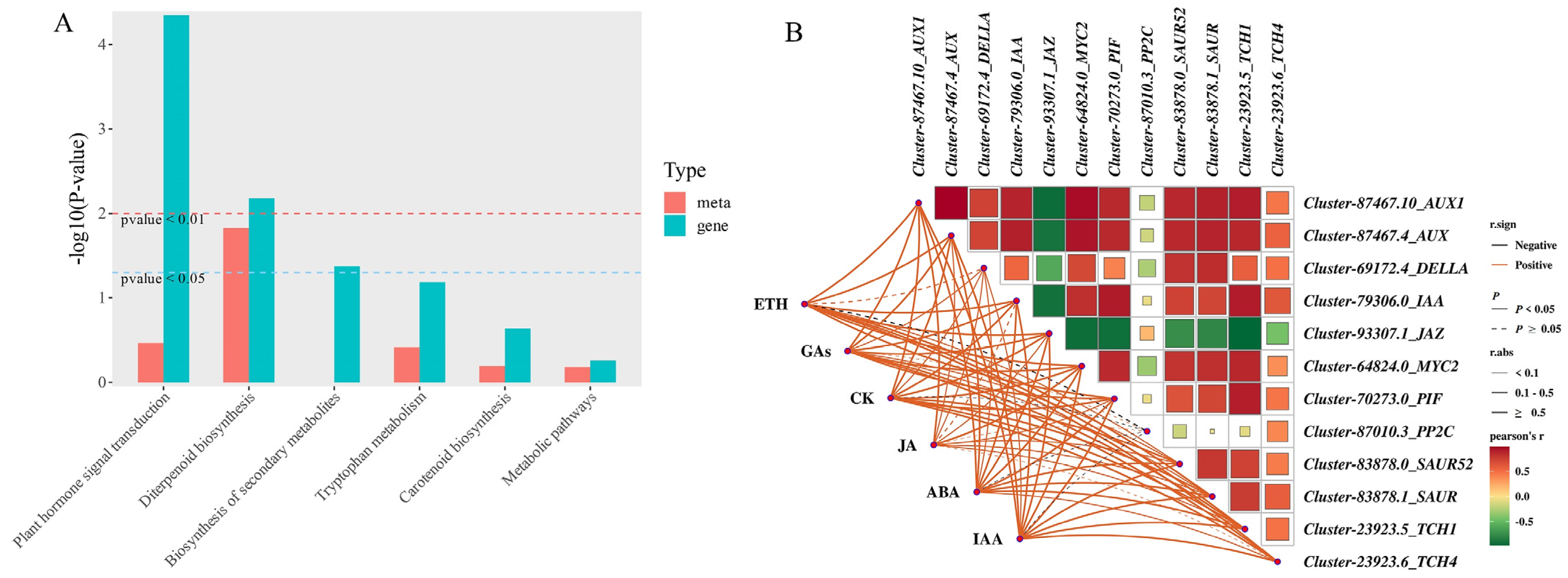
2.8. Combined Analysis of Endogenous Hormones and Transcriptome
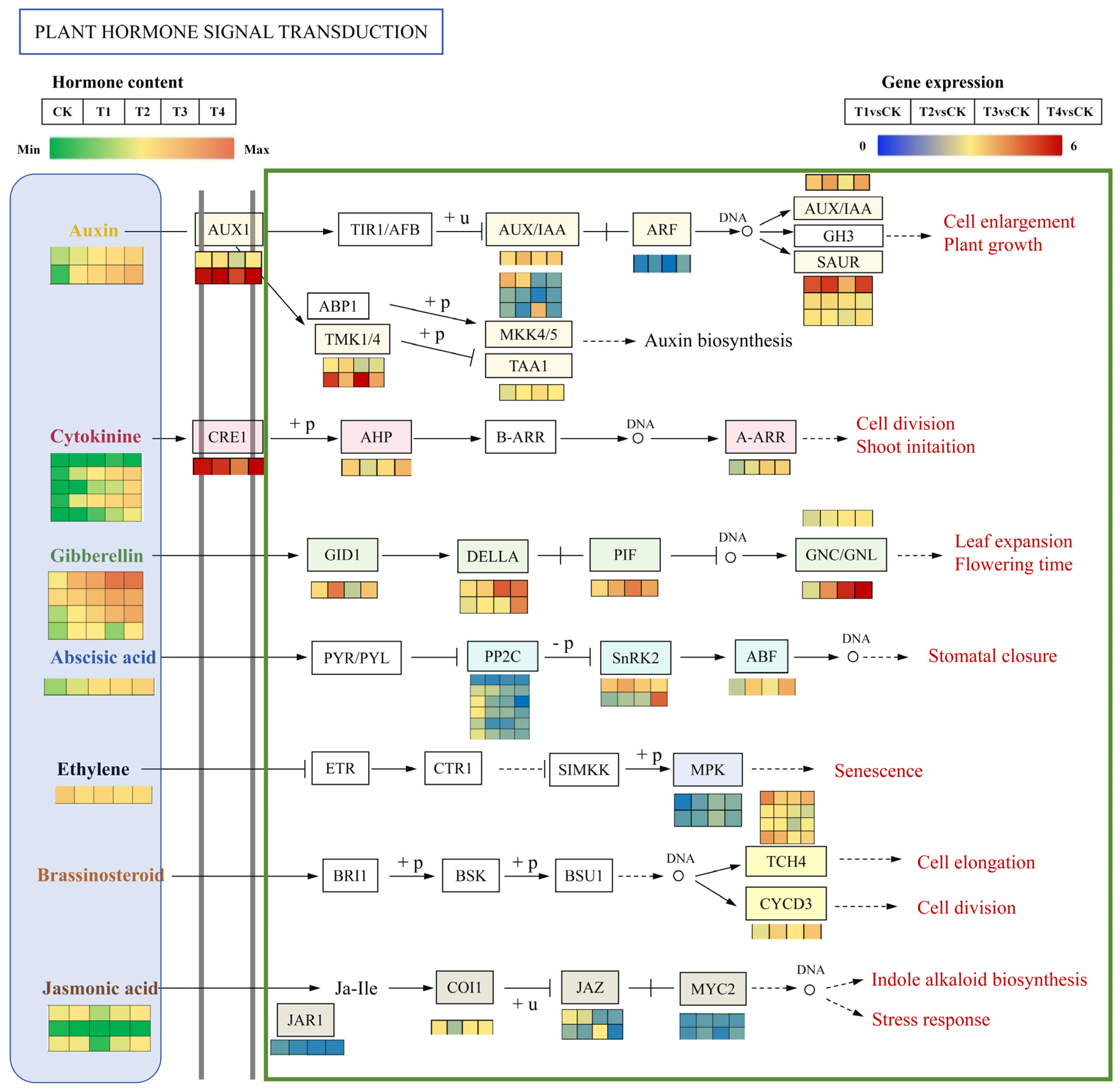
2.9. DEGs Related to Endogenous Hormone Signal Transduction
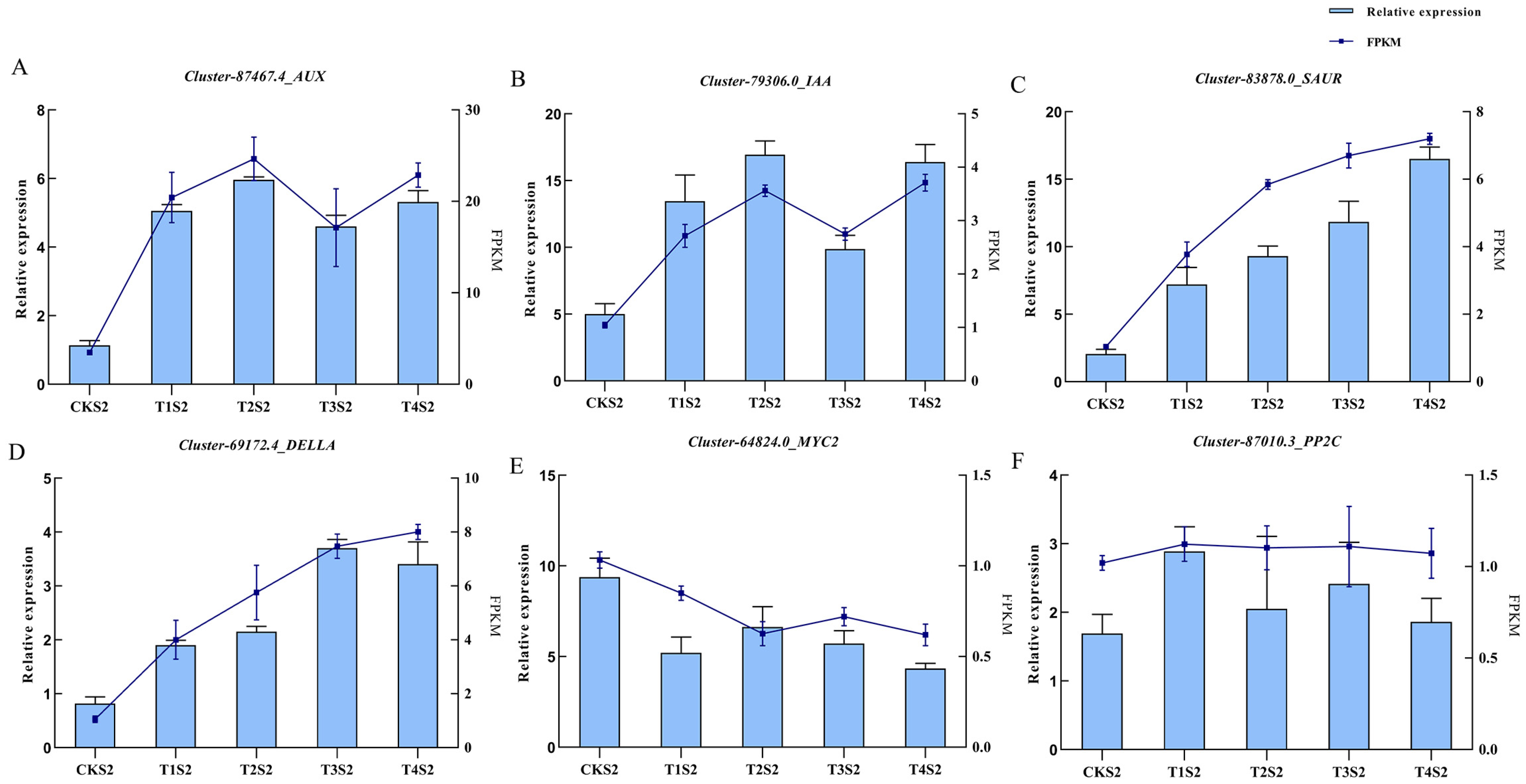
2.10. qRT-PCR Verification
3. Discussion
3.1. The Effects of GA3 Treatment on the Growth and Physiology of Phalaenopsis ‘Hatuyuki’
3.2. The Effect of GA3 Treatment on the Content of Endogenous Hormones in Phalaenopsis ‘Hatuyuki’
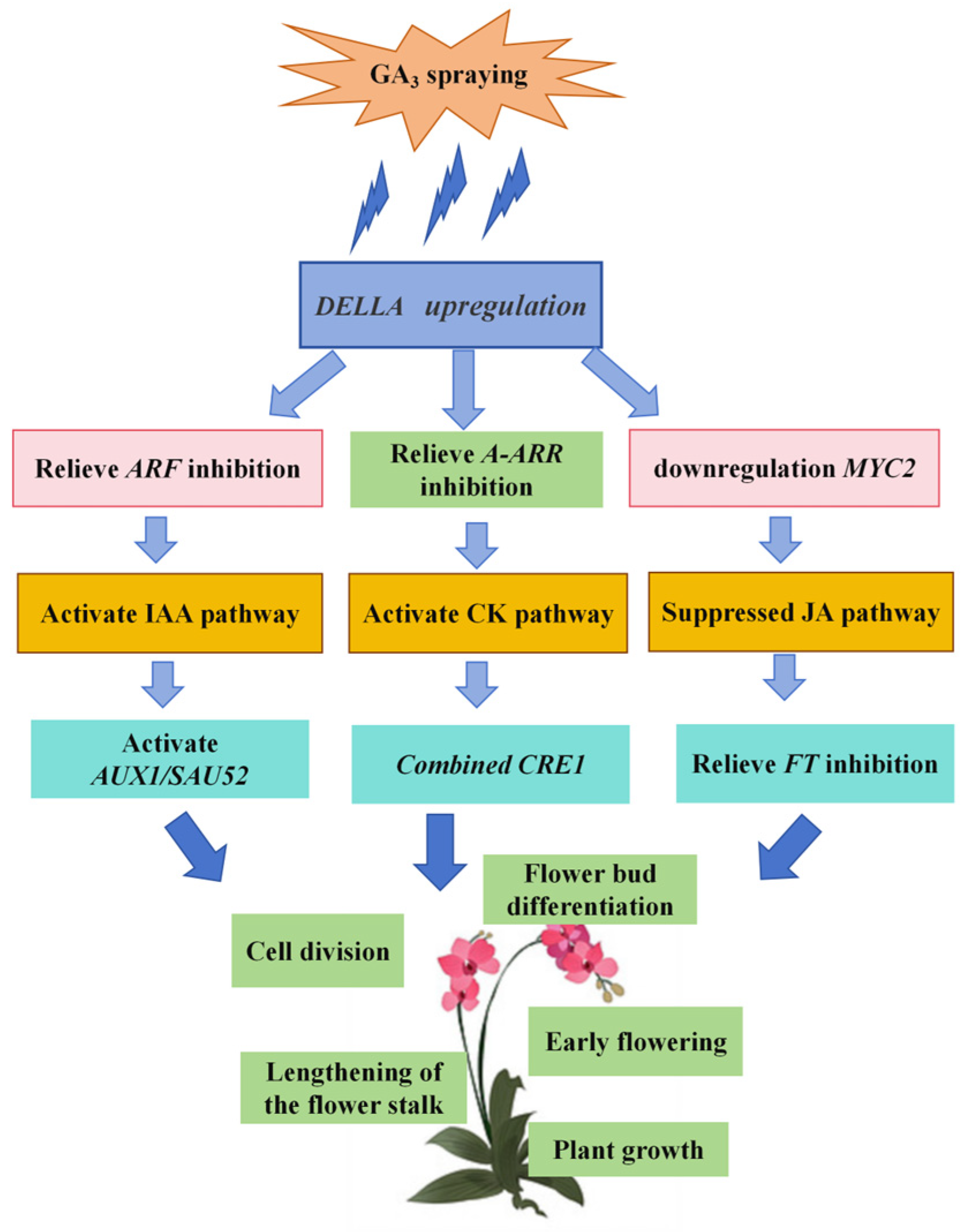
3.3. The Plant Hormone Signaling Pathway Regulates the Flower Bud Differentiation and Flower Spike Growth of Phalaenopsis ‘Hatuyuki’
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Growth Measurement
4.3. Paraffin Section Preparation
4.4. Measurement of Leaf Physiological Indicators
4.5. Measurement of Endogenous Hormone Content
4.6. RNA Extraction, Transcriptome Sequencing, and Data Analysis
4.7. qRT-PCR Validation
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hsu, C.-C.; Chen, H.-H.; Chen, W.-H. Phalaenopsis. In Ornamental Crops; Springer: Berlin/Heidelberg, Germany, 2018; pp. 567–625. [Google Scholar]
- Proietti, S.; Scariot, V.; De Pascale, S.; Paradiso, R. Flowering mechanisms and environmental stimuli for flower transition: Bases for production scheduling in greenhouse floriculture. Plants 2022, 11, 432. [Google Scholar] [CrossRef]
- Han, C.; Dong, F.; Qi, Y.; Wang, Y.; Zhu, J.; Li, B.; Zhang, L.; Lv, X.; Wang, J. The Breeding, Cultivation, and Potential Applications of Ornamental Orchids with a Focus on Phalaenopsis—A Brief Review. Plants 2025, 14, 1689. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Lee, A.K. Comparative Analysis of the Quality of Domestically Distributed Cut Phalaenopsis Flowers Based on the Season and Place of Origin. Horticulturae 2021, 7, 382. [Google Scholar] [CrossRef]
- Fan, Z.; Gao, Y.; Diao, X.; Wang, Y.; Zhang, Q. Inheritance of reblooming bearded Iris hybrids phenotypic traits. J. China Agric. Univ. 2018, 23, 29–37. [Google Scholar]
- Fan, Z.; Gao, Y.; Ren, Y.; Guan, C.; Liu, R.; Zhang, Q. To bloom once or more times: The reblooming mechanisms of Iris germanica revealed by transcriptome profiling. BMC Genom. 2020, 21, 553. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Cheng, F.-Y.; Wang, R.; Zhong, Y.; He, C. Transcriptome comparison reveals key candidate genes responsible for the unusual reblooming trait in tree peonies. PLoS ONE 2013, 8, e79996. [Google Scholar] [CrossRef]
- Pérez-Rojas, M.; Díaz-Ramírez, D.; Ortíz-Ramírez, C.I.; Galaz-Ávalos, R.M.; Loyola-Vargas, V.M.; Ferrándiz, C.; Abraham-Juárez, M.d.R.; Marsch-Martínez, N. The role of cytokinins during the development of strawberry flowers and receptacles. Plants 2023, 12, 3672. [Google Scholar] [CrossRef]
- Ramirez-Villacis, D.X.; Erazo-Garcia, P.; Quijia-Pillajo, J.; Llerena-Llerena, S.; Barriga-Medina, N.; Jones, C.D.; Leon-Reyes, A. Influence of Grafting on Rootstock Rhizosphere Microbiome Assembly in Rosa sp.‘Natal Brier’. Biology 2023, 12, 663. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Xue, Y.; Ren, X.; Xue, J.; Zhang, X. Defoliation, not gibberellin, induces tree peony autumn reflowering regulated by carbon allocation and metabolism in buds and leaves. Plant Physiol. Biochem. 2020, 151, 545–555. [Google Scholar] [CrossRef]
- Li, X.-K.; Qin, X.-M.; Cui, J.-L. Transcriptional regulatory mechanisms of flavonoid metabolism from flower bud to flower in Hemerocallis citrina. Sci. Hortic. 2024, 326, 112766. [Google Scholar] [CrossRef]
- Dong, F.; Qi, Y.; Wang, Y.; Wang, C.; Zhu, J.; Wang, C.; Ma, L.; Zhang, J.; Lv, X. Screening of flower bud differentiation conditions and changes in metabolite content of Phalaenopsis pulcherrima. S. Afr. J. Bot. 2024, 171, 529–535. [Google Scholar] [CrossRef]
- Gao, X.; Hao, Y.; Yang, X.; Shi, Y.; Zhang, L.; Yan, R. Transcriptomics and Metabolomics Analysis Reveal the Mechanism of Hormones Regulation of the Flower Bud Formation and Development in Phalaenopsis Orchid. J. Plant Growth Regul. 2025, 44, 4073–4089. [Google Scholar] [CrossRef]
- Runkle, E. Environmental and hormonal regulation of flowering in Phalaenopsis orchids: A mini review. I. Int. Orchid. Symp. 2010, 878, 263–267. [Google Scholar] [CrossRef]
- Bao, S.; Hua, C.; Shen, L.; Yu, H. New insights into gibberellin signaling in regulating flowering in Arabidopsis. J. Integr. Plant Biol. 2020, 62, 118–131. [Google Scholar] [CrossRef]
- Shah, S.H.; Islam, S.; Mohammad, F.; Siddiqui, M.H. Gibberellic acid: A versatile regulator of plant growth, development and stress responses. J. Plant Growth Regul. 2023, 42, 7352–7373. [Google Scholar] [CrossRef]
- Sun, T.-P. Gibberellin metabolism, perception and signaling pathways in Arabidopsis. Arab. Book/Am. Soc. Plant Biol. 2008, 6, e0103. [Google Scholar] [CrossRef]
- Sun, L.; Jiang, Z.; Ju, Y.; Zou, X.; Wan, X.; Chen, Y.; Yin, Z. A potential endogenous gibberellin-mediated signaling cascade regulated floral transition in Magnolia× soulangeana ‘Changchun’. Mol. Genet. Genom. 2021, 296, 207–222. [Google Scholar] [CrossRef]
- Li, B.; Wang, Q.; Qiu, Z.; Lu, Z.; Zhang, J.; He, Q.; Yang, J.; Zhang, H.; Zhu, X.; Chen, X. Transcriptomic Analysis of Gibberellin-Mediated Flower Opening Process in Tree Peony (Paeonia suffruticosa). Plants 2025, 14, 1002. [Google Scholar] [CrossRef]
- Xing, L.-B.; Zhang, D.; Li, Y.-M.; Shen, Y.-W.; Zhao, C.-P.; Ma, J.-J.; An, N.; Han, M.-Y. Transcription profiles reveal sugar and hormone signaling pathways mediating flower induction in apple (Malus domestica Borkh.). Plant Cell Physiol. 2015, 56, 2052–2068. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, J.C.; Ono, E.O.; Rodrigues, J.D. Gibberellic acid in vegetative and reproductive development of Phalaenopsis orchid hybrid genus. Hortic. Bras. 2012, 30, 71–74. [Google Scholar] [CrossRef]
- Lee, H.B.; Lee, J.-H.; Jeong, S.J.; An, S.K.; Kang, B.-C.; Kim, K.S. Intermittent high temperature reduces leaf sugar content and inhibits inflorescence initiation in Phalaenopsis hybrid. Environ. Exp. Bot. 2021, 189, 104562. [Google Scholar] [CrossRef]
- Muñoz-Fambuena, N.; Mesejo, C.; González-Mas, M.C.; Iglesias, D.J.; Primo-Millo, E.; Agustí, M. Gibberellic acid reduces flowering intensity in sweet orange [Citrus sinensis (L.) Osbeck] by repressing CiFT gene expression. J. Plant Growth Regul. 2012, 31, 529–536. [Google Scholar] [CrossRef]
- Li, Z.; Xiao, W.; Chen, H.; Zhu, G.; Lv, F. Transcriptome analysis reveals endogenous hormone changes during spike development in Phalaenopsis. Int. J. Mol. Sci. 2022, 23, 10461. [Google Scholar] [CrossRef]
- Farman, S.; Mushtaq, A.; Azeem, M.W. Plant growth regulators (PGRs) and their applications: A review. Int. J. Chem. Biochem. Sci. 2019, 15, 94–103. [Google Scholar]
- Siddiqui, M.W.; Zavala, J.F.A.; Hwang, C.-A.A. Postharvest Management Approaches for Maintaining Quality of Fresh Produce; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Van Tongerlo, E.; van Ieperen, W.; Dieleman, J.A.; Marcelis, L.F.M. Vegetative traits can predict flowering quality in Phalaenopsis orchids despite large genotypic variation in response to light and temperature. PLoS ONE 2021, 16, e0251405. [Google Scholar] [CrossRef]
- Li, J.; Wen, J.; Wu, K.; Li, L.; Fang, L.; Zeng, S. Integrating Physiology, Cytology, and Transcriptome to Reveal the Leaf Variegation Mechanism in Phalaenopsis Chia E Yenlin Variegata Leaves. Biomolecules 2024, 14, 963. [Google Scholar] [CrossRef]
- Lopez, R.G.; Runkle, E.S. Environmental physiology of growth and flowering of orchids. HortScience 2005, 40, 1969–1973. [Google Scholar] [CrossRef]
- Mehrpouyan, S.; Minow, M.A.; Patterson, J.A.; Tetlow, I.J.; Emes, M.J. Starch as a source, starch as a sink: The bifunctional role of starch in carbon allocation. J. Exp. Bot. 2017, 68, 4433–4453. [Google Scholar] [CrossRef]
- Wang, L.; Li, H.; Sun, P.; Suo, Y.; Han, W.; Diao, S.; Mai, Y.; Li, F.; Fu, J. Effects of plant growth regulators, soil moisture contents, and carbon/nitrogen ratios on sex differentiation in persimmon (Diospyros kaki Thunb.) flowers. J. Plant Growth Regul. 2021, 40, 1121–1138. [Google Scholar] [CrossRef]
- Boriboonkaset, T.; Theerawitaya, C.; Yamada, N.; Pichakum, A.; Supaibulwatana, K.; Cha-Um, S.; Takabe, T.; Kirdmanee, C. Regulation of some carbohydrate metabolism-related genes, starch and soluble sugar contents, photosynthetic activities and yield attributes of two contrasting rice genotypes subjected to salt stress. Protoplasma 2013, 250, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Goicoechea, N.; Aguirreolea, J.; Cenoz, S.; Garcia-Mina, J. Verticillium dahliae modifies the concentrations of proline, soluble sugars, starch, soluble protein and abscisic acid in pepper plants. Eur. J. Plant Pathol. 2000, 106, 19–25. [Google Scholar] [CrossRef]
- Chen, J.; Yan, B.; Tang, Y.; Xing, Y.; Li, Y.; Zhou, D.; Guo, S. Symbiotic and asymbiotic germination of Dendrobium officinale (Orchidaceae) respond differently to exogenous gibberellins. Int. J. Mol. Sci. 2020, 21, 6104. [Google Scholar] [CrossRef]
- Yin, Y.; Li, J.; Guo, B.; Li, L.; Ma, G.; Wu, K.; Yang, F.; Zhu, G.; Fang, L.; Zeng, S. Exogenous GA3 promotes flowering in Paphiopedilum callosum (Orchidaceae) through bolting and lateral flower development regulation. Hortic. Res. 2022, 9, uhac091. [Google Scholar] [CrossRef]
- El-Desoky, N.I.; Hashem, N.M.; Elkomy, A.G.; Abo-Elezz, Z.R. Improving rabbit doe metabolism and whole reproductive cycle outcomes via fatty acid-rich moringa oleifera leaf extract supplementation in free and nano-encapsulated forms. Animals 2022, 12, 764. [Google Scholar] [CrossRef] [PubMed]
- Shakirova, F.M.; Avalbaev, A.M.; Bezrukova, M.V.; Kudoyarova, G.R. Role of endogenous hormonal system in the realization of the antistress action of plant growth regulators on plants. Plant Stress 2010, 4, 32–38. [Google Scholar]
- Gao, X. Regulation of Flower Bud Differentiation by Exogenous Hormones in Fragrant Phalaenopsis and Key Gene Regulating. 2024. Available online: https://link.cnki.net/doi/10.27257/d.cnki.gnxhc.2024.002323 (accessed on 1 June 2025).
- Huang, X.; Liu, L.; Qiang, X.; Meng, Y.; Li, Z.; Huang, F. Integrative metabolomic and transcriptomic analysis elucidates that the mechanism of phytohormones regulates floral bud development in Alfalfa. Plants 2024, 13, 1078. [Google Scholar] [CrossRef] [PubMed]
- Kolachevskaya, O.O.; Myakushina, Y.A.; Getman, I.A.; Lomin, S.N.; Deyneko, I.V.; Deigraf, S.V.; Romanov, G.A. Hormonal regulation and crosstalk of auxin/cytokinin signaling pathways in potatoes in vitro and in relation to vegetation or tuberization stages. Int. J. Mol. Sci. 2021, 22, 8207. [Google Scholar] [CrossRef]
- Kushwah, S.; Laxmi, A. The interaction between glucose and cytokinin signal transduction pathway in Arabidopsis thaliana. Plant Cell Environ. 2014, 37, 235–253. [Google Scholar] [CrossRef]
- Lin, Q.; Zhang, Z.; Wu, F.; Feng, M.; Sun, Y.; Chen, W.; Cheng, Z.; Zhang, X.; Ren, Y.; Lei, C. The APC/CTE E3 ubiquitin ligase complex mediates the antagonistic regulation of root growth and tillering by ABA and GA. Plant Cell 2020, 32, 1973–1987. [Google Scholar] [CrossRef]
- Sang, D.; Chen, D.; Liu, G.; Liang, Y.; Huang, L.; Meng, X.; Chu, J.; Sun, X.; Dong, G.; Yuan, Y. Strigolactones regulate rice tiller angle by attenuating shoot gravitropism through inhibiting auxin biosynthesis. Proc. Natl. Acad. Sci. USA 2014, 111, 11199–11204. [Google Scholar] [CrossRef]
- Zhu, J.; Li, X.; Huang, J.; Wang, L.; Zheng, Q.; Li, H.; Chen, Y.; Tang, J.; Hao, X.; Wang, X. Transcriptomics and plant hormone analysis reveal the mechanism of branching angle formation in tea plants (Camellia sinensis). Int. J. Mol. Sci. 2025, 26, 604. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, Y.; Niu, Q.; He, L.; Teng, Y.; Bai, S. Abscisic acid (ABA) promotes the induction and maintenance of pear (Pyrus pyrifolia white pear group) flower bud endodormancy. Int. J. Mol. Sci. 2018, 19, 310. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Y.; Pan, J.; Lou, D.; Hu, Y.; Yu, D. The bHLH transcription factors MYC2, MYC3, and MYC4 are required for jasmonate-mediated inhibition of flowering in Arabidopsis. Mol. Plant 2017, 10, 1461–1464. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Nakamura, S.; Katsura, J.; Maruyama, Y.; Ohtsubo, K. Relationship between fatty acid composition and starch properties of 30 japonica rice cultivars. Cereal Chem. 2019, 96, 228–242. [Google Scholar] [CrossRef]
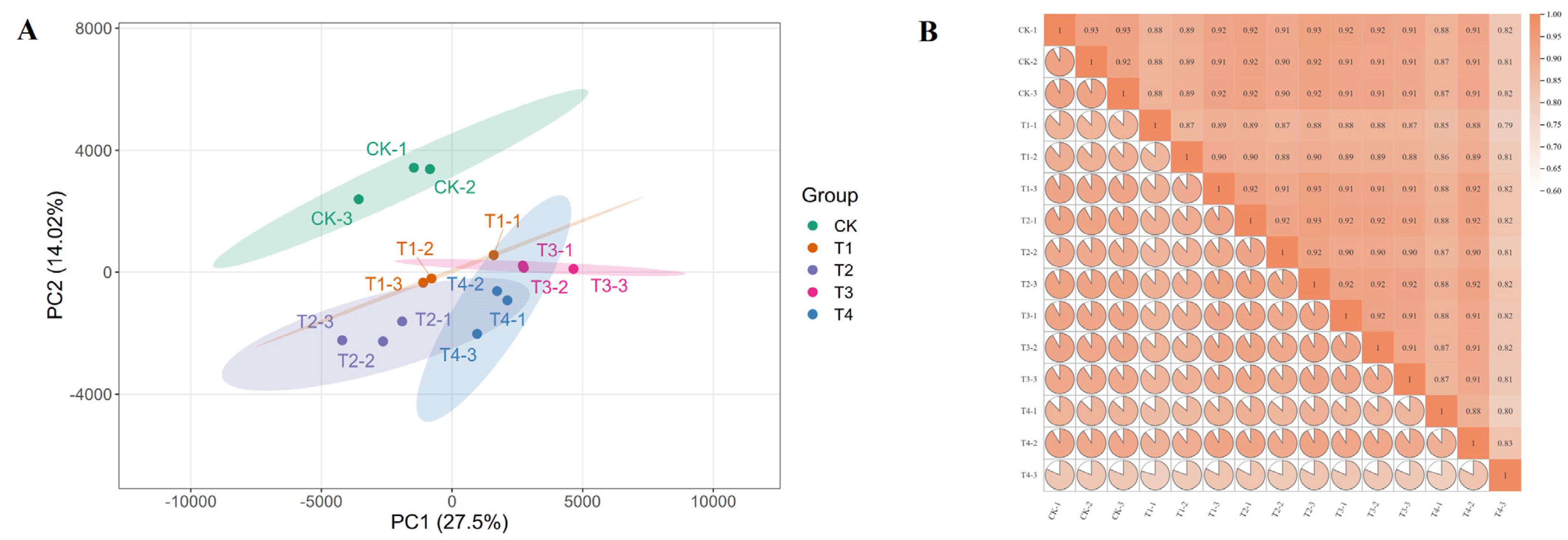
| Sample | Raw Reads | Clean Reads | Clean Base (G) | Q20 (%) | Q30 (%) | GC Content (%) | Total Mapped Reads (%) | Uniquely Mapped Reads (%) | Multiple Mapped Reads (%) | Known Genes (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| CK-1 | 54,841,928 | 52,869,856 | 7.93 | 99.04 | 96.59 | 47.32 | 15.68 | 12.31 | 0.12 | 83.26 |
| CK-2 | 54,802,054 | 52,852,302 | 7.93 | 99.04 | 96.61 | 47.41 | 19.86 | 13.65 | 0.08 | 77.25 |
| CK-3 | 52,919,290 | 50,998,418 | 7.65 | 99.11 | 96.79 | 47.05 | 18.87 | 10.56 | 0.23 | 78.56 |
| T1-1 | 57,579,628 | 55,103,474 | 8.27 | 98.99 | 96.42 | 47.07 | 20.23 | 15.32 | 0.06 | 81.23 |
| T1-2 | 55,800,648 | 53,275,878 | 7.99 | 99.04 | 96.56 | 47.05 | 18.63 | 12.33 | 0.09 | 74.56 |
| T1-3 | 62,108,194 | 59,591,940 | 8.94 | 99.14 | 96.87 | 47.19 | 16.48 | 8.89 | 0.07 | 77.52 |
| T2-1 | 72,932,666 | 69,883,844 | 10.48 | 99.07 | 96.67 | 47.03 | 19.58 | 10.69 | 0.06 | 78.59 |
| T2-2 | 64,222,592 | 61,359,334 | 9.2 | 99.11 | 96.77 | 46.92 | 20.15 | 11.25 | 0.11 | 80.23 |
| T2-3 | 71,058,798 | 68,631,230 | 10.29 | 99.22 | 97.04 | 47.05 | 20.36 | 10.36 | 0.06 | 81.06 |
| T3-1 | 57,826,344 | 55,470,314 | 8.32 | 99.02 | 96.53 | 47.66 | 19.68 | 10.25 | 0.09 | 77.56 |
| T3-2 | 54,212,068 | 52,227,802 | 7.83 | 99.05 | 96.65 | 47.56 | 18.59 | 8.56 | 0.05 | 81.56 |
| T3-3 | 63,936,974 | 61,490,882 | 9.22 | 99.06 | 96.66 | 47.69 | 19.65 | 7.65 | 0.05 | 82.21 |
| T4-1 | 53,961,002 | 51,805,616 | 7.77 | 99.06 | 96.61 | 47.03 | 14.36 | 4.38 | 0.08 | 79.56 |
| T4-2 | 57,041,832 | 54,870,944 | 8.23 | 99.07 | 96.65 | 47.48 | 13.65 | 7.66 | 0.06 | 78.58 |
| T4-3 | 65,232,004 | 62,537,240 | 9.38 | 99.15 | 96.91 | 46.25 | 12.24 | 6.25 | 0.07 | 76.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Yang, M.; Feng, L.; Hu, Q.; Hu, Y.; Zhang, X.; Zheng, J. Transcriptomics and Plant Hormone Analysis Reveal the Mechanism of Exogenous GA3 Promoting Reflowering of Phalaenopsis ‘Hatuyuki’. Int. J. Mol. Sci. 2025, 26, 11069. https://doi.org/10.3390/ijms262211069
Ma X, Yang M, Feng L, Hu Q, Hu Y, Zhang X, Zheng J. Transcriptomics and Plant Hormone Analysis Reveal the Mechanism of Exogenous GA3 Promoting Reflowering of Phalaenopsis ‘Hatuyuki’. International Journal of Molecular Sciences. 2025; 26(22):11069. https://doi.org/10.3390/ijms262211069
Chicago/Turabian StyleMa, Xiaohua, Min Yang, Lei Feng, Qingdi Hu, Yaping Hu, Xule Zhang, and Jian Zheng. 2025. "Transcriptomics and Plant Hormone Analysis Reveal the Mechanism of Exogenous GA3 Promoting Reflowering of Phalaenopsis ‘Hatuyuki’" International Journal of Molecular Sciences 26, no. 22: 11069. https://doi.org/10.3390/ijms262211069
APA StyleMa, X., Yang, M., Feng, L., Hu, Q., Hu, Y., Zhang, X., & Zheng, J. (2025). Transcriptomics and Plant Hormone Analysis Reveal the Mechanism of Exogenous GA3 Promoting Reflowering of Phalaenopsis ‘Hatuyuki’. International Journal of Molecular Sciences, 26(22), 11069. https://doi.org/10.3390/ijms262211069






