Raman Spectroscopy of Cell-Free Cervicovaginal Lavage for HPV Lesion Diagnosis: A Pilot Study
Abstract
1. Introduction
2. Results
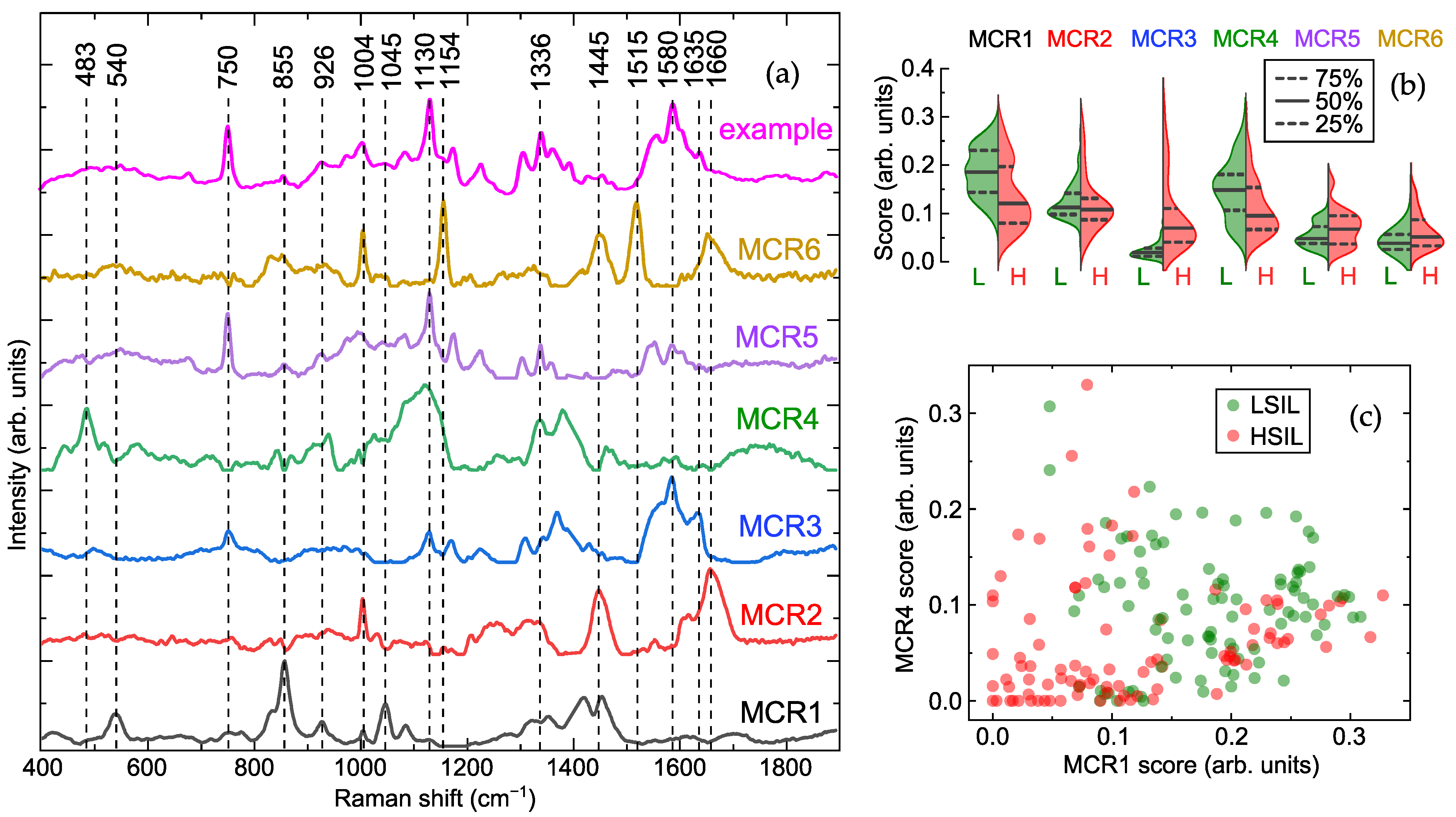
2.1. Cell-Free Cervicovaginal Lavage Composition Revealed by Spectral Analysis
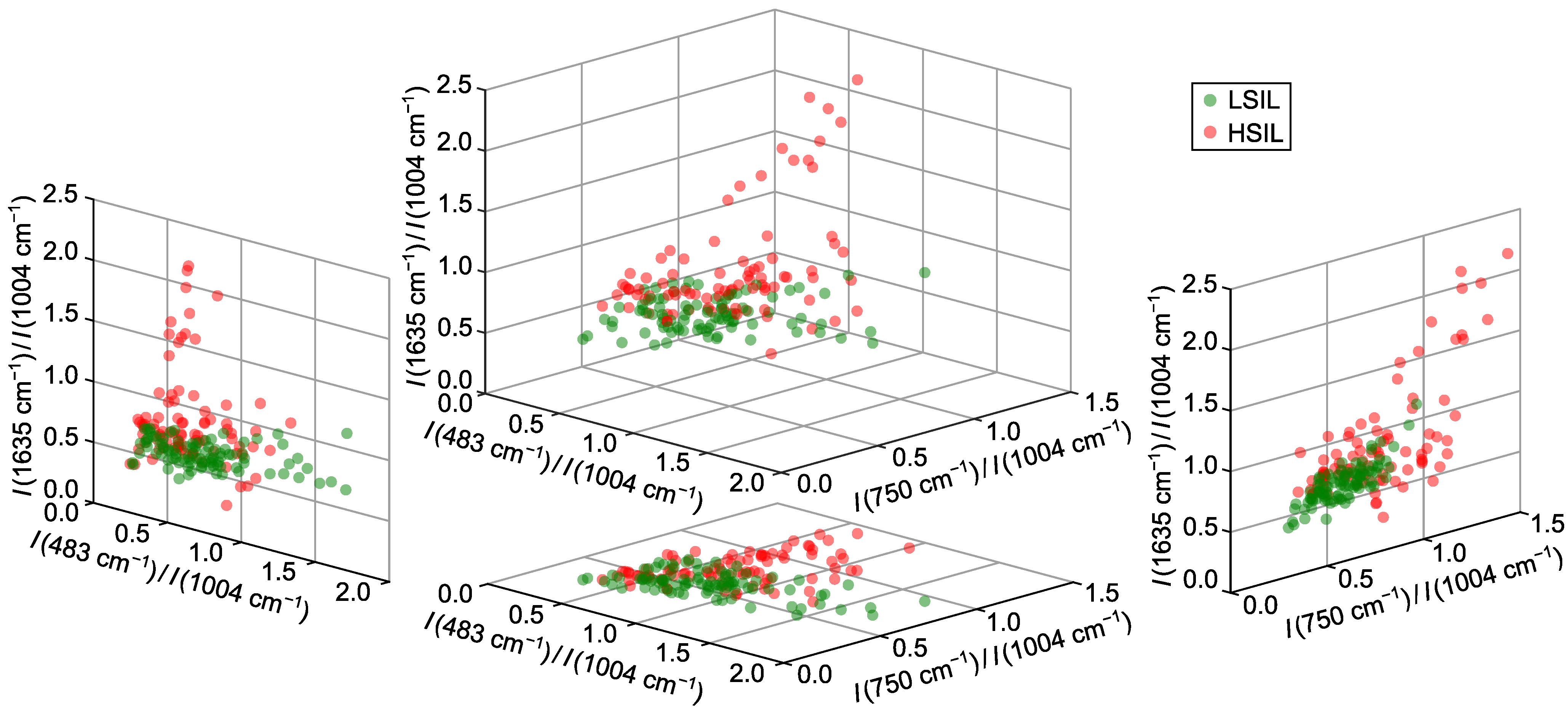
2.2. Optimal Spectral Ratios for Differentiating Cervical Lesions via Raman Spectroscopy
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Raman Spectroscopy
4.3. Raman Data Processing and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, Y.H.; Chang, B.; Choi, J.H.; Park, H.K.; Choi, S. Biochemical fingerprints of human papillomavirus infection and cervical dysplasia using cervical fluids: Spectral pattern investigation. Microsc. Res. Tech. 2016, 79, 966–972. [Google Scholar] [CrossRef]
- Shaikh, R.; Daniel, A.; Lyng, F.M. Raman Spectroscopy for Early Detection of Cervical Cancer, a Global Women’s Health Issue—A Review. Molecules 2023, 28, 2502. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guideline for Screening and Treatment of Cervical Pre-Cancer Lesions for Cervical Cancer Prevention: Use of Dual-Stain Cytology to Triage Women After a Positive Test for Human Papillomavirus (HPV); World Health Organization: Geneva, Switzerland, 2024.
- Espinoza, H.; Ha, K.T.; Pham, T.T.; Espinoza, J.L. Genetic Predisposition to Persistent Human Papillomavirus-Infection and Virus-Induced Cancers. Microorganisms 2021, 9, 92. [Google Scholar] [CrossRef]
- Davies, K.R.; Cantor, S.B.; Cox, D.D.; Follen, M. An Alternative Approach for Estimating the Accuracy of Colposcopy in Detecting Cervical Precancer. PLoS ONE 2015, 10, e0126573. [Google Scholar] [CrossRef]
- Kaur, G.; Shivani, u.; Zutshi, V.; Yadav, A.K. Role of Multiple Cervical Biopsies on Colposcopy for the Detection of Premalignant and Malignant Lesions of the Cervix. J. Colposc. Low. Genit. Tract Pathol. 2024, 2, 97–103. [Google Scholar] [CrossRef]
- Hariprasad, R.; Mittal, S.; Basu, P. Role of colposcopy in the management of women with abnormal cytology. Cytojournal 2021, 19, 40. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guideline for Screening and Treatment of Cervical Pre-Cancer Lesions for Cervical Cancer Prevention, 2nd ed.; World Health Organization: Geneva, Switzerland, 2021; pp. xvi, 97.
- Liu, M.; Lu, J.; Zhi, Y.; Ruan, Y.; Cao, G.; Xu, X.; An, X.; Gao, J.; Li, F. Microendoscopy in vivo for the pathological diagnosis of cervical precancerous lesions and early cervical cancer. Infect. Agents Cancer 2023, 18, 26. [Google Scholar] [CrossRef]
- Cui, X.; Yang, D.; Zhang, J.; Zhao, Y.; Cui, Z.; Wang, C.; Qiao, Y. Clinical value of optical coherence tomography in the early diagnosis of cervical cancer and precancerous lesions: A cross-sectional study. Front. Oncol. 2024, 14, 1423128. [Google Scholar] [CrossRef] [PubMed]
- Starodubtseva, N.L.; Brzhozovskiy, A.G.; Bugrova, A.E.; Kononikhin, A.S.; Indeykina, M.I.; Gusakov, K.I.; Chagovets, V.V.; Nazarova, N.M.; Frankevich, V.E.; Sukhikh, G.T.; et al. Label-free cervicovaginal fluid proteome profiling reflects the cervix neoplastic transformation. J. Mass Spectrom. 2019, 54, 693–703. [Google Scholar] [CrossRef] [PubMed]
- de Souza Leão, L.Q.; de Andrade, J.C.; Marques, G.M.; Guimarães, C.C.; de Fátima Vieira de Albuquerque, R.; e Silva, A.S.; de Araujo, K.P.; de Oliveira, M.P.; Gonçalves, A.F.; Figueiredo, H.F.; et al. Rapid prediction of cervical cancer and high-grade precursor lesions: An integrated approach using low-field 1H NMR and chemometric analysis. Clin. Chim. Acta 2025, 574, 120346. [Google Scholar] [CrossRef] [PubMed]
- Traynor, D.; Martin, C.M.; White, C.; Reynolds, S.; D’Arcy, T.; O’Leary, J.J.; Lyng, F.M. Raman Spectroscopy of Liquid-Based Cervical Smear Samples as a Triage to Stratify Women Who Are HPV-Positive on Screening. Cancers 2021, 13, 2008. [Google Scholar] [CrossRef]
- Sitarz, K.; Czamara, K.; Bialecka, J.; Klimek, M.; Zawilinska, B.; Szostek, S.; Kaczor, A. HPV Infection Significantly Accelerates Glycogen Metabolism in Cervical Cells with Large Nuclei: Raman Microscopic Study with Subcellular Resolution. Int. J. Mol. Sci. 2020, 21, 2667. [Google Scholar] [CrossRef]
- Vargis, E.; Tang, Y.W.; Khabele, D.; Mahadevan-Jansen, A. Near-infrared Raman Microspectroscopy Detects High-risk Human Papillomaviruses. Transl. Oncol. 2012, 5, 172–179. [Google Scholar] [CrossRef]
- Duraipandian, S.; Zheng, W.; Ng, J.; Low, J.J.; Ilancheran, A.; Huang, Z. Near-infrared-excited confocal Raman spectroscopy advances in vivo diagnosis of cervical precancer. J. Biomed. Opt. 2013, 18, 067007. [Google Scholar] [CrossRef]
- Barik, A.K.; M, S.P.; N, M.; Pai, M.V.; Upadhya, R.; Pai, A.K.; Lukose, J.; Chidangil, S. A micro-Raman spectroscopy study of inflammatory condition of human cervix: Probing of tissues and blood plasma samples. Photodiagnosis Photodyn. Ther. 2022, 39, 102948. [Google Scholar] [CrossRef]
- Rubina, S.; Amita, M.; Kedar K., D.; Bharat, R.; Krishna, C.M. Raman spectroscopic study on classification of cervical cell specimens. Vib. Spectrosc. 2013, 68, 115–121. [Google Scholar] [CrossRef]
- Sikirzhytskaya, A.; Sikirzhytski, V.; Lednev, I.K. Raman spectroscopic signature of vaginal fluid and its potential application in forensic body fluid identification. Forensic Sci. Int. 2012, 216, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Sikirzhytskaya, A.; Sikirzhytski, V.; Pérez-Almodóvar, L.; Lednev, I.K. Raman spectroscopy for the identification of body fluid traces: Semen and vaginal fluid mixture. Forensic Chem. 2023, 32, 100468. [Google Scholar] [CrossRef]
- George, N.; Singh, H.; Jotaniya, R.; Pandya, S.R. Raman spectroscopy for the determination of forensically important bio-fluids. Forensic Sci. Int. 2022, 340, 111441. [Google Scholar] [CrossRef]
- Choi, S.; Park, H.K.; Min, G.E.; Kim, Y.H. Biochemical investigations of human papillomavirus-infected cervical fluids. Microsc. Res. Tech. 2015, 78, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Matveeva, I.; Bratchenko, I.; Khristoforova, Y.; Bratchenko, L.; Moryatov, A.; Kozlov, S.; Kaganov, O.; Zakharov, V. Multivariate Curve Resolution Alternating Least Squares Analysis of In Vivo Skin Raman Spectra. Sensors 2022, 22, 9588. [Google Scholar] [CrossRef] [PubMed]
- Yakimov, B.P.; Venets, A.V.; Schleusener, J.; Fadeev, V.V.; Lademann, J.; Shirshin, E.A.; Darvin, M.E. Blind source separation of molecular components of the human skin in vivo: Non-negative matrix factorization of Raman microspectroscopy data. Analyst 2021, 146, 3185–3196. [Google Scholar] [CrossRef]
- Rimskaya, E.; Gorevoy, A.; Yakimova, A.; Makarova, N.; Starodubtseva, N.; Kudryashov, S.; Nazarenko, R.; Kalinina, E.; Frankevich, V.; Sukhikh, G. Enhancing male fertility diagnostics with seminal plasma Raman spectroscopy. Spectrochim. Acta Part Mol. Biomol. Spectrosc. 2025, 340, 126237. [Google Scholar] [CrossRef]
- Angle, K.J.; Nowak, C.M.; Grassian, V.H. Organic acid evaporation kinetics from aqueous aerosols: Implications for aerosol buffering capacity in the atmosphere. Environ. Sci. Atmos. 2023, 3, 316–327. [Google Scholar] [CrossRef]
- Rygula, A.; Majzner, K.; Marzec, K.M.; Kaczor, A.; Pilarczyk, M.; Baranska, M. Raman spectroscopy of proteins: a review. J. Raman Spectrosc. 2013, 44, 1061–1076. [Google Scholar] [CrossRef]
- Krafft, C. Raman Spectroscopy of Proteins and Nucleic Acids: From Amino Acids and Nucleotides to Large Assemblies. In Encyclopedia of Analytical Chemistry, 1st ed.; Meyers, R.A., Ed.; Wiley: Hoboken, NJ, USA, 2018; pp. 1–15. [Google Scholar] [CrossRef]
- Darvin, M.E.; Sterry, W.; Lademann, J.; Vergou, T. The Role of Carotenoids in Human Skin. Molecules 2011, 16, 10491–10506. [Google Scholar] [CrossRef]
- Parker, S.F.; Tavender, S.M.; Dixon, N.M.; Herman, H.; Williams, K.P.J.; Maddams, W.F. Raman Spectrum of beta-Carotene Using Laser Lines from Green (514.5 nm) to Near-Infrared (1064 nm): Implications for the Characterization of Conjugated Polyenes. Appl. Spectrosc. 1999, 53, 86–91. [Google Scholar] [CrossRef]
- Rimskaya, E.; Gorevoy, A.; Shelygina, S.; Perevedentseva, E.; Timurzieva, A.; Saraeva, I.; Melnik, N.; Kudryashov, S.; Kuchmizhak, A. Multi-Wavelength Raman Differentiation of Malignant Skin Neoplasms. Int. J. Mol. Sci. 2024, 25, 7422. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, A.; Piuri, G.; Porta, M.D.; Mazzucchelli, S.; Bonizzi, A.; Truffi, M.; Sevieri, M.; Allevi, R.; Corsi, F.; Cazzola, R.; et al. Raman spectroscopy characterization of the major classes of plasma lipoproteins. Vib. Spectrosc. 2020, 109, 103073. [Google Scholar] [CrossRef]
- Ahlawat, S.; Kumar, N.; Uppal, A.; Kumar Gupta, P. Visible Raman excitation laser induced power and exposure dependent effects in red blood cells. J. Biophotonics 2017, 10, 415–422. [Google Scholar] [CrossRef]
- Grytsyk, N.; Boubegtiten-Fezoua, Z.; Javahiraly, N.; Omeis, F.; Devaux, E.; Hellwig, P. Surface-enhanced resonance Raman spectroscopy of heme proteins on a gold grid electrode. Spectrochim. Acta Part Mol. Biomol. Spectrosc. 2020, 230, 118081. [Google Scholar] [CrossRef]
- Wiercigroch, E.; Szafraniec, E.; Czamara, K.; Pacia, M.Z.; Majzner, K.; Kochan, K.; Kaczor, A.; Baranska, M.; Malek, K. Raman and infrared spectroscopy of carbohydrates: A review. Spectrochim. Acta Part Mol. Biomol. Spectrosc. 2017, 185, 317–335. [Google Scholar] [CrossRef]
- Terán, M.; Ruiz, J.J.; Loza-Alvarez, P.; Masip, D.; Merino, D. Open Raman spectral library for biomolecule identification. Chemom. Intell. Lab. Syst. 2025, 264, 105476. [Google Scholar] [CrossRef]
- Lee, D.; Du, J.; Yu, R.; Su, Y.; Heath, J.R.; Wei, L. Visualizing Subcellular Enrichment of Glycogen in Live Cancer Cells by Stimulated Raman Scattering. Anal. Chem. 2020, 92, 13182–13191. [Google Scholar] [CrossRef] [PubMed]
- Kopec, M.; Imiela, A.; Abramczyk, H. Monitoring glycosylation metabolism in brain and breast cancer by Raman imaging. Sci. Rep. 2019, 9, 166. [Google Scholar] [CrossRef]
- Rao, S.; Bálint, Š.; Cossins, B.; Guallar, V.; Petrov, D. Raman Study of Mechanically Induced Oxygenation State Transition of Red Blood Cells Using Optical Tweezers. Biophys. J. 2009, 96, 209–216. [Google Scholar] [CrossRef]
- Talari, A.C.S.; Movasaghi, Z.; Rehman, S.; Rehman, I.U. Raman Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2015, 50, 46–111. [Google Scholar] [CrossRef]
- Mirmonsef, P.; Hotton, A.L.; Gilbert, D.; Gioia, C.J.; Maric, D.; Hope, T.J.; Landay, A.L.; Spear, G.T. Glycogen Levels in Undiluted Genital Fluid and Their Relationship to Vaginal pH, Estrogen, and Progesterone. PLoS ONE 2016, 11, e0153553. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Sullivan, M.A.; Gunter, J.H.; Kryza, T.; Lyons, N.; He, Y.; Hooper, J.D. Revisiting Glycogen in Cancer: A Conspicuous and Targetable Enabler of Malignant Transformation. Front. Oncol. 2020, 10, 592455. [Google Scholar] [CrossRef]
- Arizmendi-Izazaga, A.; Navarro-Tito, N.; Jiménez-Wences, H.; Mendoza-Catalán, M.A.; Martínez-Carrillo, D.N.; Zacapala-Gómez, A.E.; Olea-Flores, M.; Dircio-Maldonado, R.; Torres-Rojas, F.I.; Soto-Flores, D.G.; et al. Metabolic Reprogramming in Cancer: Role of HPV 16 Variants. Pathogens 2021, 10, 347. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, F.; Zhou, K.; Huang, K.; Zhu, Q.; Luo, X.; Yu, J.; Shi, Z. Oncogenic role of ABHD5 in endometrial cancer. Cancer Manag. Res. 2019, 11, 2139–2150. [Google Scholar] [CrossRef]
- Ma, J.; Yao, Z.; Ma, L.; Zhu, Q.; Zhang, J.; Li, L.; Liu, C. Glucose metabolism reprogramming in gynecologic malignant tumors. J. Cancer 2024, 15, 2627–2645. [Google Scholar] [CrossRef]
- Pucino, V.; Bombardieri, M.; Pitzalis, C.; Mauro, C. Lactate at the crossroads of metabolism, inflammation, and autoimmunity. Eur. J. Immunol. 2017, 47, 14–21. [Google Scholar] [CrossRef]
- Walenta, S.; Wetterling, M.; Lehrke, M.; Schwickert, G.; Sundfør, K.; Rofstad, E.; Mueller-Klieser, W. High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res. 2000, 60, 916–921. [Google Scholar]
- Pavlides, S.; Whitaker-Menezes, D.; Castello-Cros, R.; Flomenberg, N.; Witkiewicz, A.; Frank, P.; Casimiro, M.; Wang, C.; Fortina, P.; Addya, S.; et al. The reverse Warburg effect: Aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle 2009, 8, 3984–4001. [Google Scholar] [CrossRef] [PubMed]
- Halestrap, A.P. Monocarboxylic Acid Transport. Compr. Physiol. 2013, 3, 1611–1643. [Google Scholar] [CrossRef]
- Montal, E.D.; Bhalla, K.; Dewi, R.E.; Ruiz, C.F.; Haley, J.A.; Ropell, A.E.; Gordon, C.; Haley, J.D.; Girnun, G.D. Inhibition of phosphoenolpyruvate carboxykinase blocks lactate utilization and impairs tumor growth in colorectal cancer. Cancer Metab. 2019, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Hui, S.; Ghergurovich, J.M.; Morscher, R.J.; Jang, C.; Teng, X.; Lu, W.; Esparza, L.A.; Reya, T.; Zhan, L.; Yanxiang Guo, J.; et al. Glucose feeds the TCA cycle via circulating lactate. Nature 2017, 551, 115–118. [Google Scholar] [CrossRef]
- Sajnani, K.; Islam, F.; Smith, R.A.; Gopalan, V.; Lam, A.K.Y. Genetic alterations in Krebs cycle and its impact on cancer pathogenesis. Biochimie 2017, 135, 164–172. [Google Scholar] [CrossRef]
- Li, T.; Copeland, C.; Le, A. Glutamine Metabolism in Cancer. In The Heterogeneity of Cancer Metabolism; Le, A., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 17–38. [Google Scholar] [CrossRef]
- Mullen, A.R.; Wheaton, W.W.; Jin, E.S.; Chen, P.H.; Sullivan, L.B.; Cheng, T.; Yang, Y.; Linehan, W.M.; Chandel, N.S.; DeBerardinis, R.J. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature 2012, 481, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Eskander, R.N.; Tewari, K.S. Targeting angiogenesis in advanced cervical cancer. Ther. Adv. Med. Oncol. 2014, 6, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Minion, L.E.; Tewari, K.S. Cervical cancer – State of the science: From angiogenesis blockade to checkpoint inhibition. Gynecol. Oncol. 2018, 148, 609–621. [Google Scholar] [CrossRef]
- Tomao, F.; Papa, A.; Rossi, L.; Zaccarelli, E.; Caruso, D.; Zoratto, F.; Panici, P.B.; Tomao, S. Angiogenesis and antiangiogenic agents in cervical cancer. Oncotargets Ther. 2014, 7, 2237–2248. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, S.; Zhang, X.; Han, Y.; Tan, M.; Fan, J.; Du, J.; Fan, Y.; Zhao, X. The biomechanical signature of tumor invasion. Genes Dis. 2025, 13, 101771. [Google Scholar] [CrossRef]
- Betsou, F.; Lehmann, S.; Ashton, G.; Barnes, M.; Benson, E.E.; Coppola, D.; DeSouza, Y.; Eliason, J.; Glazer, B.; Guadagni, F.; et al. Standard Preanalytical Coding for Biospecimens: Defining the Sample PREanalytical Code. Cancer Epidemiol. Biomarkers Prev. 2010, 19, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Hernandes, V.V.; Barbas, C.; Dudzik, D. A review of blood sample handling and pre-processing for metabolomics studies. Electrophoresis 2017, 38, 2232–2241. [Google Scholar] [CrossRef]
- Ellervik, C.; Vaught, J. Preanalytical Variables Affecting the Integrity of Human Biospecimens in Biobanking. Clin. Chem. 2015, 61, 914–934. [Google Scholar] [CrossRef]
- Bai, G.; Gajer, P.; Nandy, M.; Ma, B.; Yang, H.; Sakamoto, J.; Blanchard, M.; Ravel, J.; Brotman, R. Comparison of Storage Conditions for Human Vaginal Microbiome Studies. PLoS ONE 2012, 7, e36934. [Google Scholar] [CrossRef]
- Petry, K.U.; Nieminen, P.J.; Leeson, S.C.; Bergeron, C.O.; Redman, C.W. 2017 update of the European Federation for Colposcopy (EFC) performance standards for the practice of colposcopy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 224, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Borisov, A.V.; Snegerev, M.S.; Colón-Rodríguez, S.; Fikiet, M.A.; Lednev, I.K.; Kistenev, Y.V. Identification of semen traces at a crime scene through Raman spectroscopy and machine learning. Sci. Rep. 2024, 14, 23070. [Google Scholar] [CrossRef]
- Zhao, J.; Lui, H.; McLean, D.I.; Zeng, H. Automated autofluorescence background subtraction algorithm for biomedical Raman spectroscopy. Appl. Spectrosc. 2007, 61, 1225–1232. [Google Scholar] [CrossRef]
- Zhao, J.; Lui, H.; McLean, D.I.; Zeng, H. Real-Time Raman Spectroscopy for Noninvasive in vivo Skin Analysis and Diagnosis. In New Developments in Biomedical Engineering; Campolo, D., Ed.; IntechOpen: Rijeka, Croatia, 2010; Chapter 24. [Google Scholar] [CrossRef]
- Bratchenko, L.; Bratchenko, I. Avoiding Overestimation and the ‘Black Box’ Problem in Biofluids Multivariate Analysis by Raman Spectroscopy: Interpretation and Transparency With the SP-LIME Algorithm. J. Raman Spectrosc. 2025, 56, 353–364. [Google Scholar] [CrossRef]
- Bratchenko, L.A.; Khristoforova, Y.A.; Pimenova, I.A.; Tupikova, E.N.; Skuratova, M.A.; Dvoynikov-Sechnoy, G.A.; Wang, S.; Lebedev, P.A.; Bratchenko, I.A. SERS-based technique for accessible and rapid diagnosis of multiple myeloma in blood serum analysis. Light. Adv. Manuf. 2025, 6, 284. [Google Scholar] [CrossRef]
- Adamczyk, A.; Tipping, W.; Mazuryk, O.; Graham, D.; Baranska, M.; Majzner, K. Sensitive Detection and Identification Method of Erythrocyte-like Cells upon Doxorubicin Induced Differentiation with Vibrational Techniques. Anal. Chem. 2025, 97, 16966–16974. [Google Scholar] [CrossRef] [PubMed]
- Lyng, F.; Faoláin, E.; Conroy, J.; Meade, A.; Knief, P.; Duffy, B.; Hunter, M.; Byrne, J.; Kelehan, P.; Byrne, H. Vibrational spectroscopy for cervical cancer pathology, from biochemical analysis to diagnostic tool. Exp. Mol. Pathol. 2007, 82, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Movasaghi, Z.; Rehman, S.; Rehman, I.U. Raman Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2007, 42, 493–541. [Google Scholar] [CrossRef]
- Konorov, S.O.; Schulze, H.G.; Piret, J.M.; Turner, R.F.B.; Blades, M.W. Evidence of marked glycogen variations in the characteristic Raman signatures of human embryonic stem cells. J. Raman Spectrosc. 2011, 42, 1135–1141. [Google Scholar] [CrossRef]
- Olaetxea, I.; Valero, A.; Lopez, E.; Lafuente, H.; Izeta, A.; Jaunarena, I.; Seifert, A. Machine Learning-Assisted Raman Spectroscopy for pH and Lactate Sensing in Body Fluids. Anal. Chem. 2020, 92, 13888–13895. [Google Scholar] [CrossRef] [PubMed]
- Golparvar, A.; Boukhayma, A.; Loayza, T.; Caizzone, A.; Enz, C.; Carrara, S. Very Selective Detection of Low Physiopathological Glucose Levels by Spontaneous Raman Spectroscopy with Univariate Data Analysis. BioNanoScience 2021, 11, 871–877. [Google Scholar] [CrossRef]
- De Gelder, J.; De Gussem, K.; Vandenabeele, P.; Moens, L. Reference database of Raman spectra of biological molecules. J. Raman Spectrosc. 2007, 38, 1133–1147. [Google Scholar] [CrossRef]
- Golparvar, A.; Kim, J.; Boukhayma, A.; Briand, D.; Carrara, S. Highly accurate multimodal monitoring of lactate and urea in sweat by soft epidermal optofluidics with single-band Raman scattering. Sens. Actuators Chem. 2023, 387, 133814. [Google Scholar] [CrossRef]
- Vlasov, A.V.; Maliar, N.L.; Bazhenov, S.V.; Nikelshparg, E.I.; Brazhe, N.A.; Vlasova, A.D.; Osipov, S.D.; Sudarev, V.V.; Ryzhykau, Y.L.; Bogorodskiy, A.O.; et al. Raman Scattering: From Structural Biology to Medical Applications. Crystals 2020, 10, 38. [Google Scholar] [CrossRef]
- Zhu, G.; Zhu, X.; Fan, Q.; Wan, X. Raman spectra of amino acids and their aqueous solutions. Spectrochim. Acta Part Mol. Biomol. Spectrosc. 2011, 78, 1187–1195. [Google Scholar] [CrossRef]
- Rimskaya, E.; Shelygina, S.; Timurzieva, A.; Saraeva, I.; Perevedentseva, E.; Melnik, N.; Kudrin, K.; Reshetov, D.; Kudryashov, S. Multispectral Raman Differentiation of Malignant Skin Neoplasms In Vitro: Search for Specific Biomarkers and Optimal Wavelengths. Int. J. Mol. Sci. 2023, 24, 14748. [Google Scholar] [CrossRef]
- Synytsya, A.; Judexova, M.; Hoskovec, D.; Miskovicova, M.; Petruzelka, L. Raman spectroscopy at different excitation wavelengths (1064, 785 and 532 nm) as a tool for diagnosis of colon cancer. J. Raman Spectrosc. 2014, 45, 903–911. [Google Scholar] [CrossRef]
- Bergholt, M.S.; Zheng, W.; Lin, K.; Huang, Z.; Ho, K.Y.; Yeoh, K.G.; Teh, M.; So, J.B.Y. Characterizing variability in in vivo Raman spectra of different anatomical locations in the upper gastrointestinal tract toward cancer detection. J. Biomed. Opt. 2011, 16, 037003. [Google Scholar] [CrossRef]
- Shang, L.; Tang, J.; Wu, J.; Shang, H.; Huang, X.; Bao, Y.; Xu, Z.; Wang, H.; Yin, J. Polarized Micro-Raman Spectroscopy and 2D Convolutional Neural Network Applied to Structural Analysis and Discrimination of Breast Cancer. Biosensors 2023, 13, 65. [Google Scholar] [CrossRef]
- Udensi, J.; Loughman, J.; Loskutova, E.; Byrne, H.J. Raman Spectroscopy of Carotenoid Compounds for Clinical Applications—A Review. Molecules 2022, 27, 9017. [Google Scholar] [CrossRef]
- Tfaili, S.; Gobinet, C.; Josse, G.; Angiboust, J.F.; Manfait, M.; Piot, O. Confocal Raman microspectroscopy for skin characterization: a comparative study between human skin and pig skin. Analyst 2012, 137, 3673–3682, Correction in Analyst 2020, 145, 4699–4700. https://doi.org/10.1039/D0AN90060E. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Lin, J.; Wu, Y.; Feng, S.; Li, Y.; Yu, Y.; Xi, G.; Zeng, H.; Chen, R. Investigation on the interactions of lymphoma cells with paclitaxel by Raman spectroscopy. Spectroscopy 2011, 25, 23–32. [Google Scholar] [CrossRef]
- Abramczyk, H.; Brozek-Pluska, B.; Kopec, M.; Surmacki, J.; Błaszczyk, M.; Radek, M. Redox Imbalance and Biochemical Changes in Cancer by Probing Redox-Sensitive Mitochondrial Cytochromes in Label-Free Visible Resonance Raman Imaging. Cancers 2021, 13, 960. [Google Scholar] [CrossRef]
- Amaral, S.; Da Costa, R.; Wübbeling, F.; Redmann, K.; Schlatt, S. Raman micro-spectroscopy analysis of different sperm regions: a species comparison. Mol. Hum. Reprod. 2017, 24, 185–202. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Moy, A.J.; Nguyen, H.T.M.; Zhang, J.; Fox, M.C.; Sebastian, K.R.; Reichenberg, J.S.; Markey, M.K.; Tunnell, J.W. Raman active components of skin cancer. Biomed. Opt. Express 2017, 8, 2835–2850. [Google Scholar] [CrossRef] [PubMed]
- Czamara, K.; Majzner, K.; Pacia, M.Z.; Kochan, K.; Kaczor, A.; Baranska, M. Raman spectroscopy of lipids: a review. J. Raman Spectrosc. 2015, 46, 4–20. [Google Scholar] [CrossRef]
- Silveira, L.; Sathaiah, S.; Zângaro, R.A.; Pacheco, M.T.T.; Chavantes, M.C.; Pasqualucci, C.A.G. Correlation between near-infrared Raman spectroscopy and the histopathological analysis of atherosclerosis in human coronary arteries. Lasers Surg. Med. 2002, 30, 290–297. [Google Scholar] [CrossRef]
- Huang, Z.; McWilliams, A.; Lui, H.; McLean, D.I.; Lam, S.; Zeng, H. Near-infrared Raman spectroscopy for optical diagnosis of lung cancer. Int. J. Cancer 2003, 107, 1047–1052. [Google Scholar] [CrossRef]
- Gniadecka, M.; Wulf, H.C.; Nymark Mortensen, N.; Faurskov Nielsen, O.; Christensen, D.H. Diagnosis of Basal Cell Carcinoma by Raman Spectroscopy. J. Raman Spectrosc. 1997, 28, 125–129. [Google Scholar] [CrossRef]
- Mahadevan-Jansen, A.; Richards-Kortum, R.R. Raman spectroscopy for the detection of cancers and precancers. J. Biomed. Opt. 1996, 1, 31–70. [Google Scholar] [CrossRef]
- ChemicalBook. Sodium Lactate (312-85-6). Available online: https://www.chemicalbook.com/SpectrumEN_312-85-6_Raman.htm (accessed on 6 November 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rimskaya, E.; Gorevoy, A.; Devyatkina, A.; Nazarova, N.; Starodubtseva, N.; Abakarova, P.; Mgeryan, A.; Kudryashov, S.; Prilepskaya, V.; Sukhikh, G. Raman Spectroscopy of Cell-Free Cervicovaginal Lavage for HPV Lesion Diagnosis: A Pilot Study. Int. J. Mol. Sci. 2025, 26, 11064. https://doi.org/10.3390/ijms262211064
Rimskaya E, Gorevoy A, Devyatkina A, Nazarova N, Starodubtseva N, Abakarova P, Mgeryan A, Kudryashov S, Prilepskaya V, Sukhikh G. Raman Spectroscopy of Cell-Free Cervicovaginal Lavage for HPV Lesion Diagnosis: A Pilot Study. International Journal of Molecular Sciences. 2025; 26(22):11064. https://doi.org/10.3390/ijms262211064
Chicago/Turabian StyleRimskaya, Elena, Alexey Gorevoy, Anastasia Devyatkina, Niso Nazarova, Natalia Starodubtseva, Patimat Abakarova, Anna Mgeryan, Sergey Kudryashov, Vera Prilepskaya, and Gennady Sukhikh. 2025. "Raman Spectroscopy of Cell-Free Cervicovaginal Lavage for HPV Lesion Diagnosis: A Pilot Study" International Journal of Molecular Sciences 26, no. 22: 11064. https://doi.org/10.3390/ijms262211064
APA StyleRimskaya, E., Gorevoy, A., Devyatkina, A., Nazarova, N., Starodubtseva, N., Abakarova, P., Mgeryan, A., Kudryashov, S., Prilepskaya, V., & Sukhikh, G. (2025). Raman Spectroscopy of Cell-Free Cervicovaginal Lavage for HPV Lesion Diagnosis: A Pilot Study. International Journal of Molecular Sciences, 26(22), 11064. https://doi.org/10.3390/ijms262211064




