Genetic Diversity of Selected High-Risk HPV Types Prevalent in Africa and Not Covered by Current Vaccines: A Pooled Sequence Data Analysis
Abstract
1. Introduction
2. Results
2.1. Sequence Dataset Composition
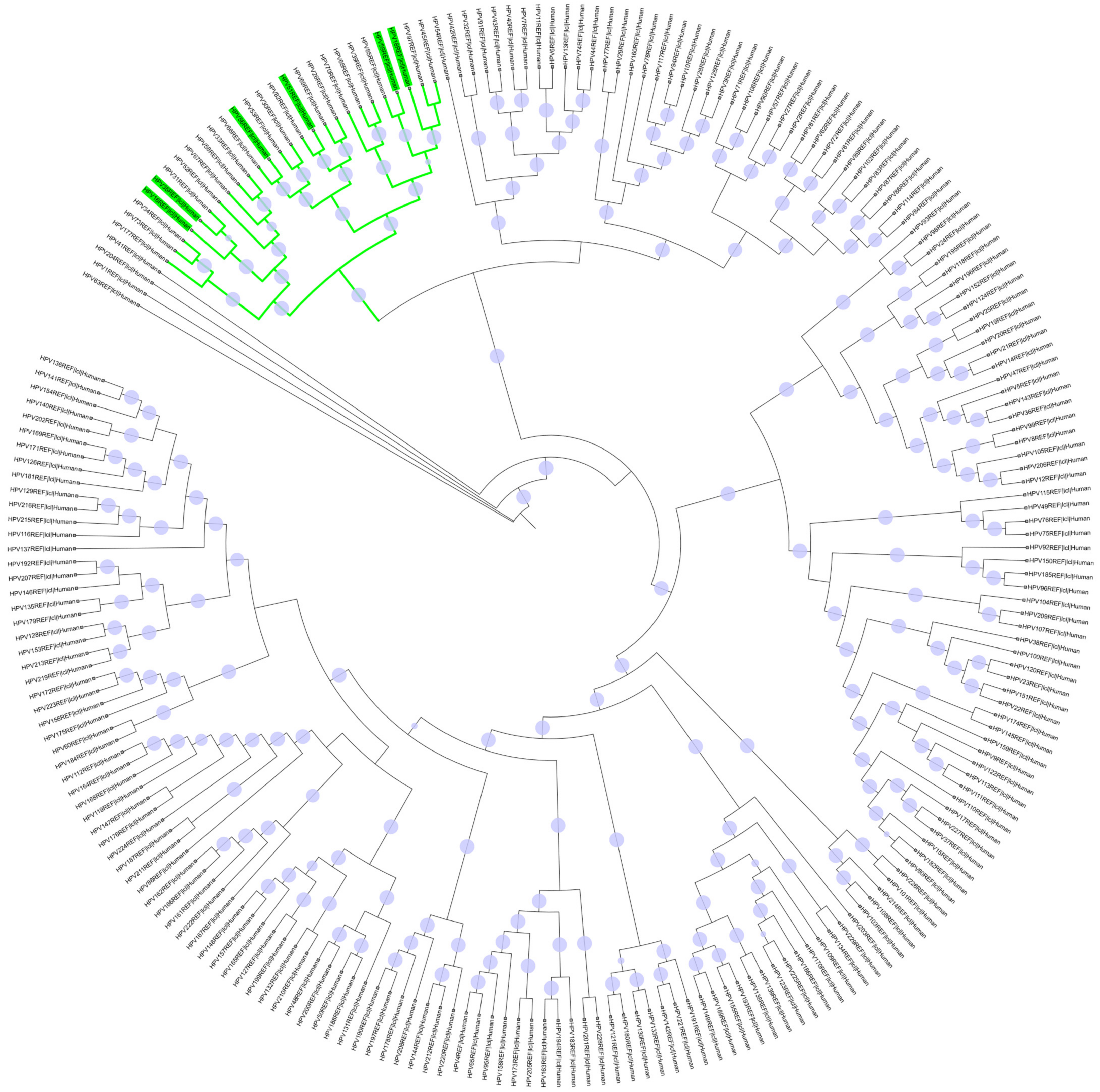
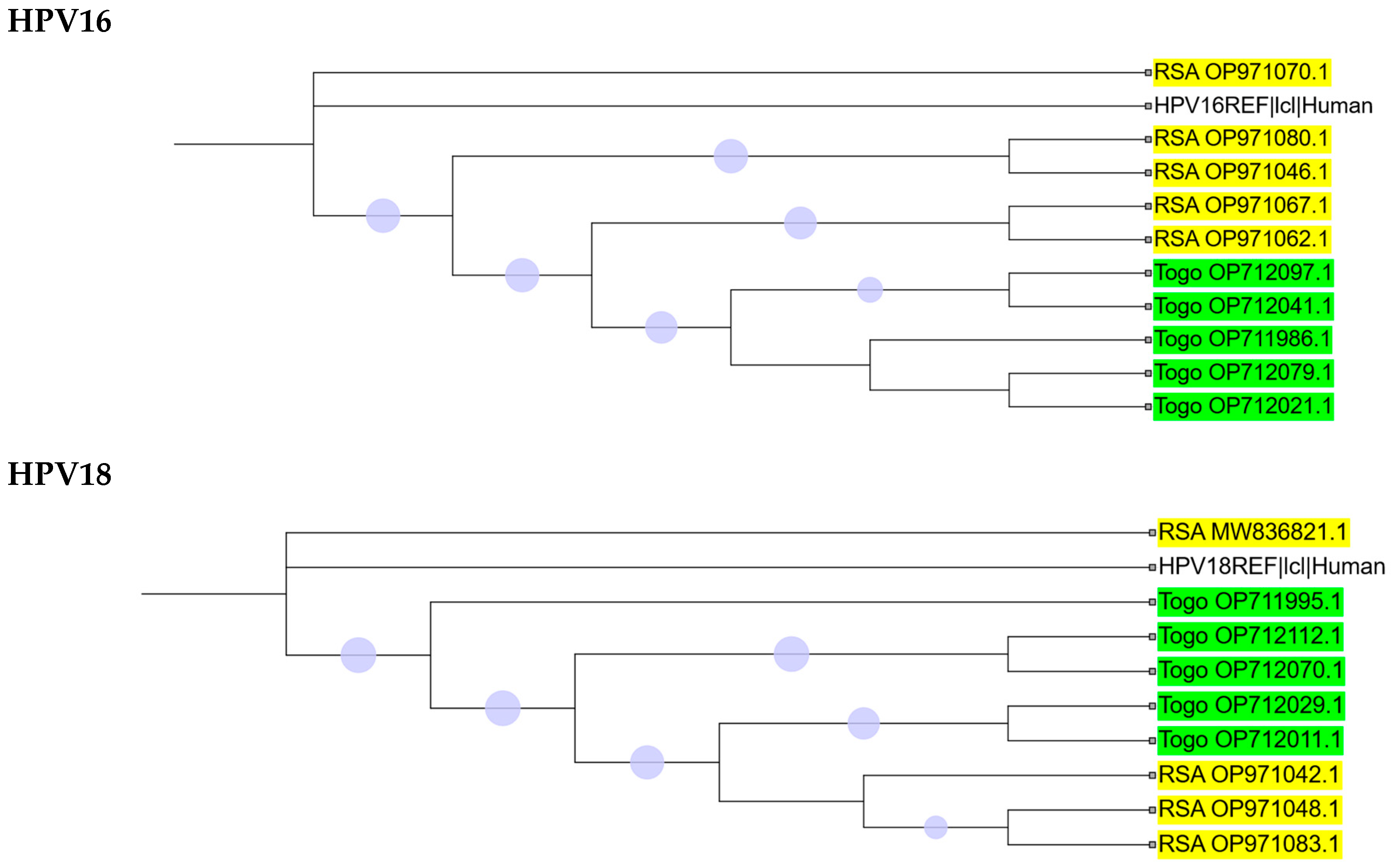
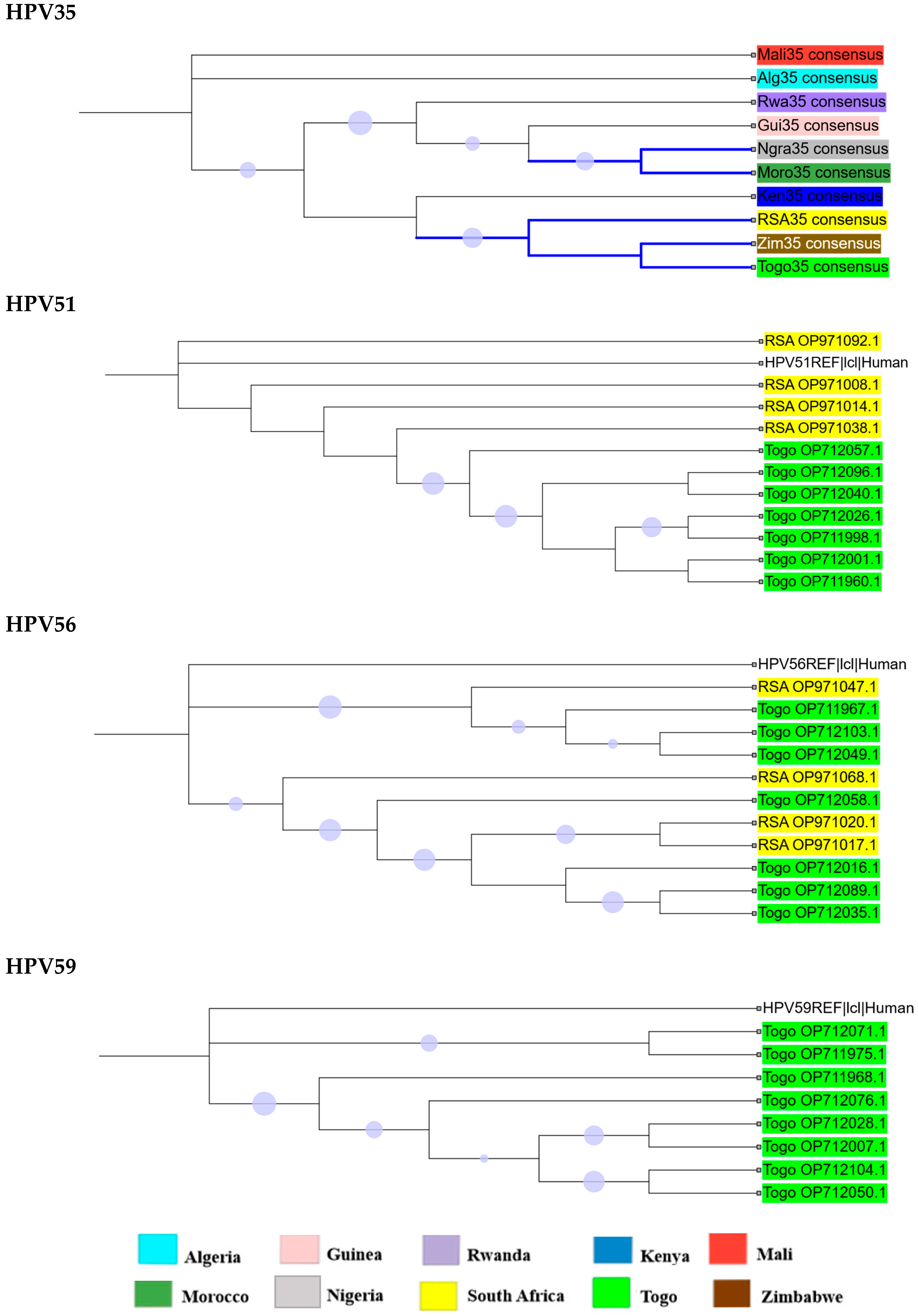
2.2. Phylogenetic Analysis
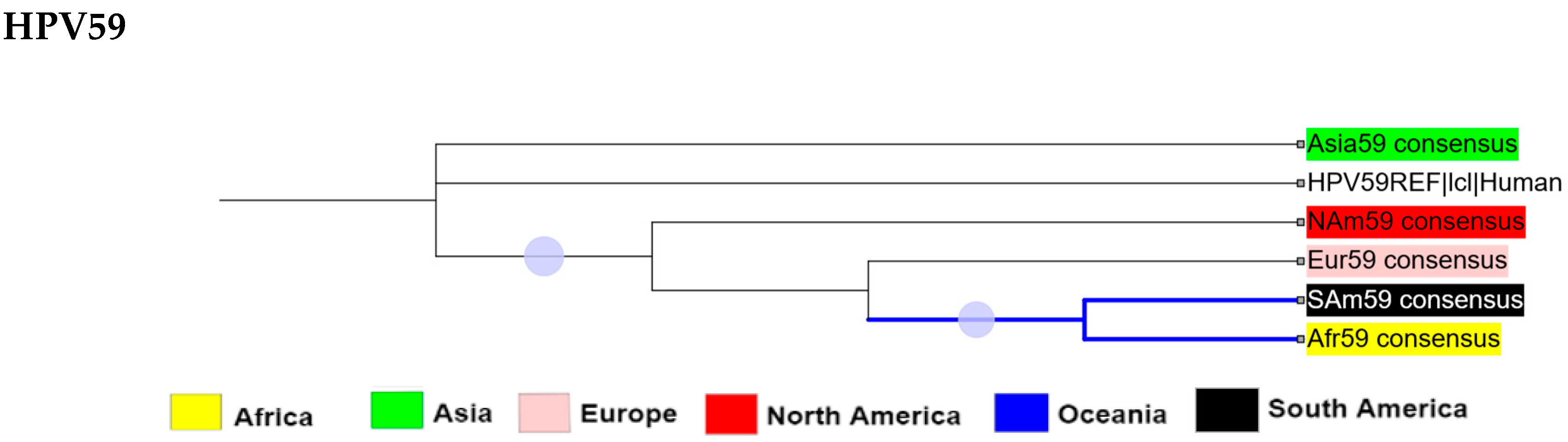
2.3. Intra-Africa Diversity
2.4. Oncoprotein/L1 Variant Analysis
3. Discussion
4. Methods and Materials
4.1. Database Mining and Nucleotide Sequence Retrieval
4.2. Sequence Data Sorting and Annotation
4.3. Nucleotide Sequence Alignments and Phylogenetic Analysis
4.4. Variation Calling and Amino Acid Translation
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HR-HPV | high-risk human papillomavirus |
| NCBI | national centre for biotechnology information |
| ORF | open reading frame |
| SNP | single nucleotide polymorphism |
| VIMS | vaccine innovation and manufacturing strategy |
References
- WHO. Global Cancer Observatory; WHO: Geneva, Switzerland, 2025; Available online: https://gco.iarc.fr/tomorrow/en/dataviz/isotype?cancers=23&single_unit=50000&types=0 (accessed on 16 July 2025).
- Yuan, Y.; Cai, X.; Shen, F.; Ma, F. HPV post-infection microenvironment and cervical cancer. Cancer Lett. 2021, 497, 243–254. [Google Scholar] [CrossRef]
- Jary, A.; Teguete, I.; Sidibé, Y.; Kodio, A.; Dolo, O.; Burrel, S.; Boutolleau, D.; Beauvais-Remigereau, L.; Sayon, S.; Kampo, M. Prevalence of cervical HPV infection, sexually transmitted infections and associated antimicrobial resistance in women attending cervical cancer screening in Mali. Int. J. Infect. Dis. 2021, 108, 610–616. [Google Scholar] [CrossRef]
- de Sanjose, S.; Brotons, M.; Pavon, M.A. The natural history of human papillomavirus infection. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 47, 2–13. [Google Scholar] [CrossRef]
- Laganà, A.S.; Chiantera, V.; Gerli, S.; Proietti, S.; Lepore, E.; Unfer, V.; Carugno, J.; Favilli, A. Preventing persistence of HPV infection with natural molecules. Pathogens 2023, 12, 416. [Google Scholar] [CrossRef]
- Szymonowicz, K.A.; Chen, J. Biological and clinical aspects of HPV-related cancers. Cancer Biol. Med. 2020, 17, 864. [Google Scholar] [CrossRef]
- Ribeiro, D.V.; Steffens, S.M.; Fedrizzi, E.N. The impact of the HPV vaccine on the world: Initial outcomes and challenges. Braz. J. Sex. Transm. Dis. 2020, 32, e203204. [Google Scholar] [CrossRef]
- Huber, J.; Mueller, A.; Sailer, M.; Regidor, P.-A. Human papillomavirus persistence or clearance after infection in reproductive age. What is the status? Review of the literature and new data of a vaginal gel containing silicate dioxide, citric acid, and selenite. Women’s Health 2021, 17, 17455065211020702. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- WHO. Accelerating the Elimination of Cervical Cancer as a Public Health Problem: Towards Achieving 90–70–90 Targets by 2030; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Doorbar, J.; Quint, W.; Banks, L.; Bravo, I.G.; Stoler, M.; Broker, T.R.; Stanley, M.A. The biology and life-cycle of human papillomaviruses. Vaccine 2012, 30, F55–F70. [Google Scholar] [CrossRef]
- Bruni, L.; Albero, G.; Serrano, B.; Mena, M.; Collado, J.J.; Gómez, D.; Muñoz, J.; Bosch, F.; de Sanjosé, S. Human Papillomavirus and Related Diseases in Africa: Summary Report 10 March 2023; IARC Information Centre on HPV and Cancer (HPV Information Centre): Barcelona, Spain, 2023. [Google Scholar]
- Bruni, L.; Albero, G.; Rowley, J.; Alemany, L.; Arbyn, M.; Giuliano, A.R.; Markowitz, L.E.; Broutet, N.; Taylor, M. Global and regional estimates of genital human papillomavirus prevalence among men: A systematic review and meta-analysis. Lancet Glob. Health 2023, 11, e1345–e1362. [Google Scholar] [CrossRef]
- Bosch, F.X.; Burchell, A.N.; Schiffman, M.; Giuliano, A.R.; de Sanjose, S.; Bruni, L.; Tortolero-Luna, G.; Kjaer, S.K.; Munoz, N. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine 2008, 26, K1–K16. [Google Scholar] [CrossRef]
- Bruni, L.; Albero, G.; Serrano, B.; Mena, M.; Collado, J.; Gómez, D.; Muñoz, J.; Bosch, F.; de Sanjosé, S. Human Papillomavirus and Related Diseases in the World: Summary Report 10 March 2023; IARC Information Centre on HPV and Cancer (HPV Information Centre): Barcelona, Spain, 2023. [Google Scholar]
- Ghosh, A.; Chatterjee, S.; Dawn, A.; Das, A. HPV Vaccines–An Overview. Indian J. Dermatol. 2025, 70, 188–200. [Google Scholar] [CrossRef]
- Wang, R.; Huang, H.; Yu, C.; Li, X.; Wang, Y.; Xie, L. Current status and future directions for the development of human papillomavirus vaccines. Front. Immunol. 2024, 15, 1362770. [Google Scholar] [CrossRef]
- Oncology, T.L. HPV vaccination in south Asia: New progress, old challenges. Oncology 2022, 23, 1233. [Google Scholar]
- WHO. WHO Adds an HPV Vaccine for Single-Dose Use; WHO: Geneva, Switzerland, 2024; Available online: https://www.who.int/news/item/04-10-2024-who-adds-an-hpv-vaccine-for-single-dose-use (accessed on 16 September 2025).
- Gomez, F.; Hirbo, J.; Tishkoff, S.A. Genetic variation and adaptation in Africa: Implications for human evolution and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a008524. [Google Scholar] [CrossRef]
- Ghattas, M.; Dwivedi, G.; Lavertu, M.; Alameh, M.-G. Vaccine technologies and platforms for infectious diseases: Current progress, challenges, and opportunities. Vaccines 2021, 9, 1490. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiu, K.; Ren, J.; Zhao, Y.; Cheng, P. Roles of human papillomavirus in cancers: Oncogenic mechanisms and clinical use. Signal Transduct. Target. Ther. 2025, 10, 44. [Google Scholar] [CrossRef]
- DiMaio, D.; Mattoon, D. Mechanisms of cell transformation by papillomavirus E5 proteins. Oncogene 2001, 20, 7866–7873. [Google Scholar] [CrossRef]
- Peng, Q.; Wang, L.; Zuo, L.; Gao, S.; Jiang, X.; Han, Y.; Lin, J.; Peng, M.; Wu, N.; Tang, Y. HPV E6/E7: Insights into their regulatory role and mechanism in signaling pathways in HPV-associated tumor. Cancer Gene Ther. 2024, 31, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Prado, J.C.; Calleja-Macias, I.E.; Bernard, H.-U.; Kalantari, M.; Macay, S.A.; Allan, B.; Williamson, A.-L.; Chung, L.-P.; Collins, R.J.; Zuna, R.E. Worldwide genomic diversity of the human papillomaviruses-53, 56, and 66, a group of high-risk HPVs unrelated to HPV-16 and HPV-18. Virology 2005, 340, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Calleja-Macias, I.E.; Kalantari, M.; Huh, J.; Ortiz-Lopez, R.; Rojas-Martinez, A.; Gonzalez-Guerrero, J.F.; Williamson, A.-L.; Hagmar, B.; Wiley, D.J.; Villarreal, L. Genomic diversity of human papillomavirus-16, 18, 31, and 35 isolates in a Mexican population and relationship to European, African, and Native American variants. Virology 2004, 319, 315–323. [Google Scholar] [CrossRef]
- Calleja-Macias, I.E.; Villa, L.L.; Prado, J.C.; Kalantari, M.; Allan, B.; Williamson, A.-L.; Chung, L.-P.; Collins, R.J.; Zuna, R.E.; Dunn, S.T. Worldwide genomic diversity of the high-risk human papillomavirus types 31, 35, 52, and 58, four close relatives of human papillomavirus type 16. J. Virol. 2005, 79, 13630–13640. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, S.; Hankins, C.; Money, D.; Pourreaux, K.; Franco, E.; Coutlée, F.; Canadian Women’s HIV Study Group. Polymorphism of the L1 capsid gene and persistence of human papillomavirus type 52 infection in women at high risk or infected by HIV. JAIDS J. Acquir. Immune Defic. Syndr. 2007, 44, 61–65. [Google Scholar] [CrossRef]
- Gagnon, S.; Hankins, C.; Tremblay, C.; Forest, P.; Pourreaux, K.; Coutlée, F.; Canadian Women’s HIV Study Group. Viral polymorphism in human papillomavirus types 33 and 35 and persistent and transient infection in the genital tract of women. J. Infect. Dis. 2004, 190, 1575–1585. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, S.; Hankins, C.; Tremblay, C.; Pourreaux, K.; Forest, P.; Rouah, F.; Coutlée, F. Polymorphism of human papillomavirus type 31 isolates infecting the genital tract of HIV—seropositive and HIV—seronegative women at risk for HIV infection. J. Med. Virol. 2005, 75, 213–221. [Google Scholar] [CrossRef]
- Raiol, T.; Wyant, P.S.; de Amorim, R.M.S.; Cerqueira, D.M.; Milanezi Nv, G.; Brigido Md, M.; Sichero, L.; Martins, C.R.F. Genetic variability and phylogeny of the high-risk HPV-31, -33, -35, -52, and -58 in central Brazil. J. Med. Virol. 2009, 81, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Schiffman, M.; Herrero, R.; DeSalle, R.; Anastos, K.; Segondy, M.; Sahasrabuddhe, V.V.; Gravitt, P.E.; Hsing, A.W.; Burk, R.D. Evolution and taxonomic classification of human papillomavirus 16 (HPV16)-related variant genomes: HPV31, HPV33, HPV35, HPV52, HPV58 and HPV67. PLoS ONE 2011, 6, e20183. [Google Scholar] [CrossRef]
- Harper, D.M.; DeMars, L.R. HPV vaccines–a review of the first decade. Gynecol. Oncol. 2017, 146, 196–204. [Google Scholar] [CrossRef]
- Dehghani, B.; Hasanshahi, Z.; Hashempour, T.; Motamedifar, M. The possible regions to design Human Papilloma Viruses vaccine in Iranian L1 protein. Biologia 2020, 75, 749–759. [Google Scholar] [CrossRef]
- Oumeslakht, L.; Ababou, M.; Badaoui, B.; Qmichou, Z. Worldwide genetic variations in high-risk human papillomaviruses capsid L1 gene and their impact on vaccine efficiency. Gene 2021, 782, 145533. [Google Scholar] [CrossRef]
- Tsakogiannis, D.; Nikolaidis, M.; Zagouri, F.; Zografos, E.; Kottaridi, C.; Kyriakopoulou, Z.; Tzioga, L.; Markoulatos, P.; Amoutzias, G.D.; Bletsa, G. Mutation profile of HPV16 L1 and L2 genes in different geographic areas. Viruses 2022, 15, 141. [Google Scholar] [CrossRef]
- Alsanea, M.; Alsaleh, A.; Obeid, D.; Alhadeq, F.; Alahideb, B.; Alhamlan, F. Genetic variability in the E6, E7, and L1 genes of human papillomavirus types 16 and 18 among women in Saudi Arabia. Viruses 2022, 15, 109. [Google Scholar] [CrossRef]
- Ahmed, A.I.; Bissett, S.L.; Beddows, S. Amino acid sequence diversity of the major human papillomavirus capsid protein: Implications for current and next generation vaccines. Infect. Genet. Evol. 2013, 18, 151–159. [Google Scholar] [CrossRef]
- Jing, Y.; Wang, T.; Chen, Z.; Ding, X.; Xu, J.; Mu, X.; Cao, M.; Chen, H. Phylogeny and polymorphism in the long control regions E6, E7, and L1 of HPV Type 56 in women from southwest China. Mol. Med. Rep. 2018, 17, 7131–7141. [Google Scholar] [CrossRef]
- Basukala, O.; Banks, L. The not-so-good, the bad and the ugly: HPV E5, E6 and E7 oncoproteins in the orchestration of carcinogenesis. Viruses 2021, 13, 1892. [Google Scholar] [CrossRef]
- Venuti, A.; Paolini, F.; Nasir, L.; Corteggio, A.; Roperto, S.; Campo, M.S.; Borzacchiello, G. Papillomavirus E5: The smallest oncoprotein with many functions. Mol. Cancer 2011, 10, 140. [Google Scholar] [CrossRef]
- Li, T.; Yang, Z.; Zhang, C.; Wang, S.; Mei, B. Genetic variation of E6 and E7 genes of human papillomavirus type 16 from central China. Virol. J. 2023, 20, 217. [Google Scholar] [CrossRef]
- Bletsa, G.; Zagouri, F.; Amoutzias, G.; Nikolaidis, M.; Zografos, E.; Markoulatos, P.; Tsakogiannis, D. Genetic variability of the HPV16 early genes and LCR. Present and future perspectives. Expert Rev. Mol. Med. 2021, 23, e19. [Google Scholar] [CrossRef] [PubMed]
- Boumba, L.M.A.; Assoumou, S.Z.; Hilali, L.; Mambou, J.V.; Moukassa, D.; Ennaji, M.M. Genetic variability in E6 and E7 oncogenes of human papillomavirus Type 16 from Congolese cervical cancer isolates. Infect. Agents Cancer 2015, 10, 15. [Google Scholar] [CrossRef]
- He, J.; Li, T.; Cheng, C.; Li, N.; Gao, P.; Lei, D.; Liang, R.; Ding, X. The polymorphism analysis and therapy vaccine target epitopes screening of HPV-35 E6 E7 among the threaten α-9 HPV in Sichuan area. Virol. J. 2024, 21, 213. [Google Scholar] [CrossRef]
- Hiller, T.; Stubenrauch, F.; Iftner, T. Isolation and functional analysis of five HPVE6 variants with respect to p53 degradation. J. Med. Virol. 2008, 80, 478–483. [Google Scholar] [CrossRef]
- Yuan, H.-B.; Yu, J.-H.; Gan, J.; Qiu, Y.; Yan, Z.-Y.; Xu, H.-H. Genetic variations and carcinogenicity analysis of E6/E7 oncogenes in HPV31 and HPV35 in Taizhou, China. Virol. J. 2025, 22, 35. [Google Scholar] [CrossRef] [PubMed]
- Mboumba Bouassa, R.-S.; Avala Ntsigouaye, J.; Lemba Tsimba, P.C.; Nodjikouambaye, Z.A.; Sadjoli, D.; Mbeko Simaleko, M.; Camengo, S.P.; Longo, J.D.D.; Grésenguet, G.; Veyer, D. Genetic diversity of HPV35 in Chad and the Central African Republic, two landlocked countries of Central Africa: A cross-sectional study. PLoS ONE 2024, 19, e0297054. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.; Boland, J.F.; Li, H.; Burk, R.; Yeager, M.; Anderson, S.K.; Wentzensen, N.; Schiffman, M.; Mirabello, L.; Dean, M. HPV16 E7 nucleotide variants found in cancer-free subjects affect E7 protein expression and transformation. Cancers 2022, 14, 4895. [Google Scholar] [CrossRef] [PubMed]
- Maueia, C.; Murahwa, A.T.; Carulei, O.; Taku, O.; Mbulawa, Z.; Manjate, A.; Valey, Z.O.; Mussá, T.; Williamson, A.-L. Genetic variability in HPV 33 and 35 E6 and E7 genes from South African and Mozambican women with different cervical cytology status. Virol. J. 2025, 22, 234. [Google Scholar] [CrossRef]
- Soheili, M.; Keyvani, H.; Soheili, M.; Nasseri, S. Human papilloma virus: A review study of epidemiology, carcinogenesis, diagnostic methods, and treatment of all HPV-related cancers. Med. J. Islam. Repub. Iran 2021, 35, 65. [Google Scholar] [CrossRef]
- Schiffman, M.; Castle, P.E.; Jeronimo, J.; Rodriguez, A.C.; Wacholder, S. Human papillomavirus and cervical cancer. Lancet 2007, 370, 890–907. [Google Scholar] [CrossRef]
- Murenzi, G.; Mungo, C. Impact of the human papillomavirus vaccine in low-resource settings. Lancet Glob. Health 2023, 11, e997–e998. [Google Scholar] [CrossRef]
- Sayers, E.W.; Beck, J.; Bolton, E.E.; Brister, J.R.; Chan, J.; Connor, R.; Feldgarden, M.; Fine, A.M.; Funk, K.; Hoffman, J. Database resources of the National Center for Biotechnology Information in 2025. Nucleic Acids Res. 2025, 53, D20–D29. [Google Scholar] [CrossRef]
- Van Doorslaer, K.; Li, Z.; Xirasagar, S.; Maes, P.; Kaminsky, D.; Liou, D.; Sun, Q.; Kaur, R.; Huyen, Y.; McBride, A.A. The Papillomavirus Episteme: A major update to the papillomavirus sequence database. Nucleic Acids Res. 2017, 45, D499–D506. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, Y.; Gao, W.; Ke, Y.; Lu, Z. Whole-genome analysis of human papillomavirus types 16, 18, and 58 isolated from cervical Precancer and Cancer samples in Chinese women. Sci. Rep. 2017, 7, 263. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; Von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v6: Recent updates to the phylogenetic tree display and annotation tool. Nucleic Acids Res. 2024, 52, W78–W82. [Google Scholar] [CrossRef] [PubMed]
- DSTI. Building a Local Vaccine Capability Critical in the Era of Health Challenges; DSTI: Pretoria, South Africa, 2024. Available online: https://www.dsti.gov.za/index.php/media-room/latest-news/4363-building-a-local-vaccine-capability-critical-in-the-era-of-health-challenges (accessed on 5 February 2025).
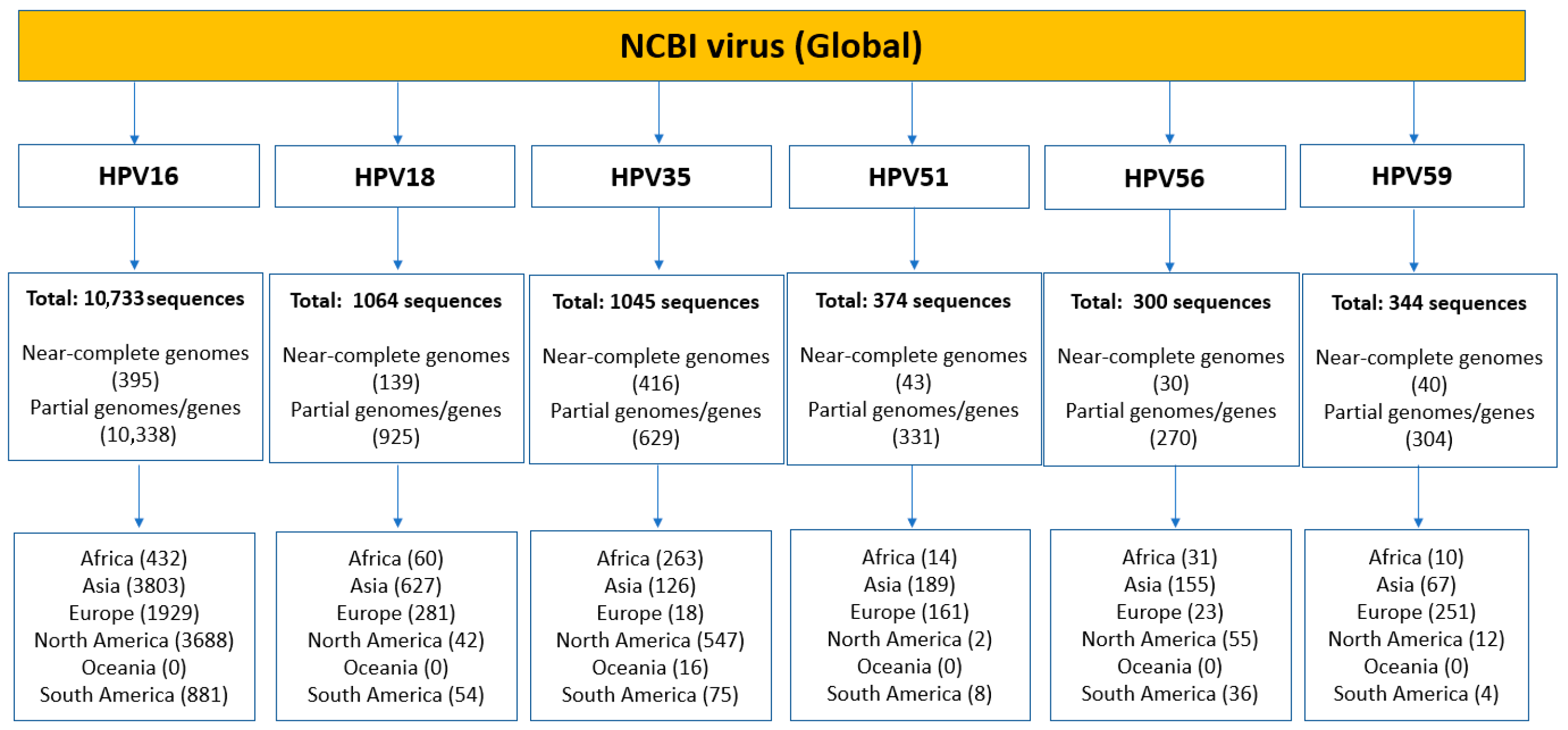
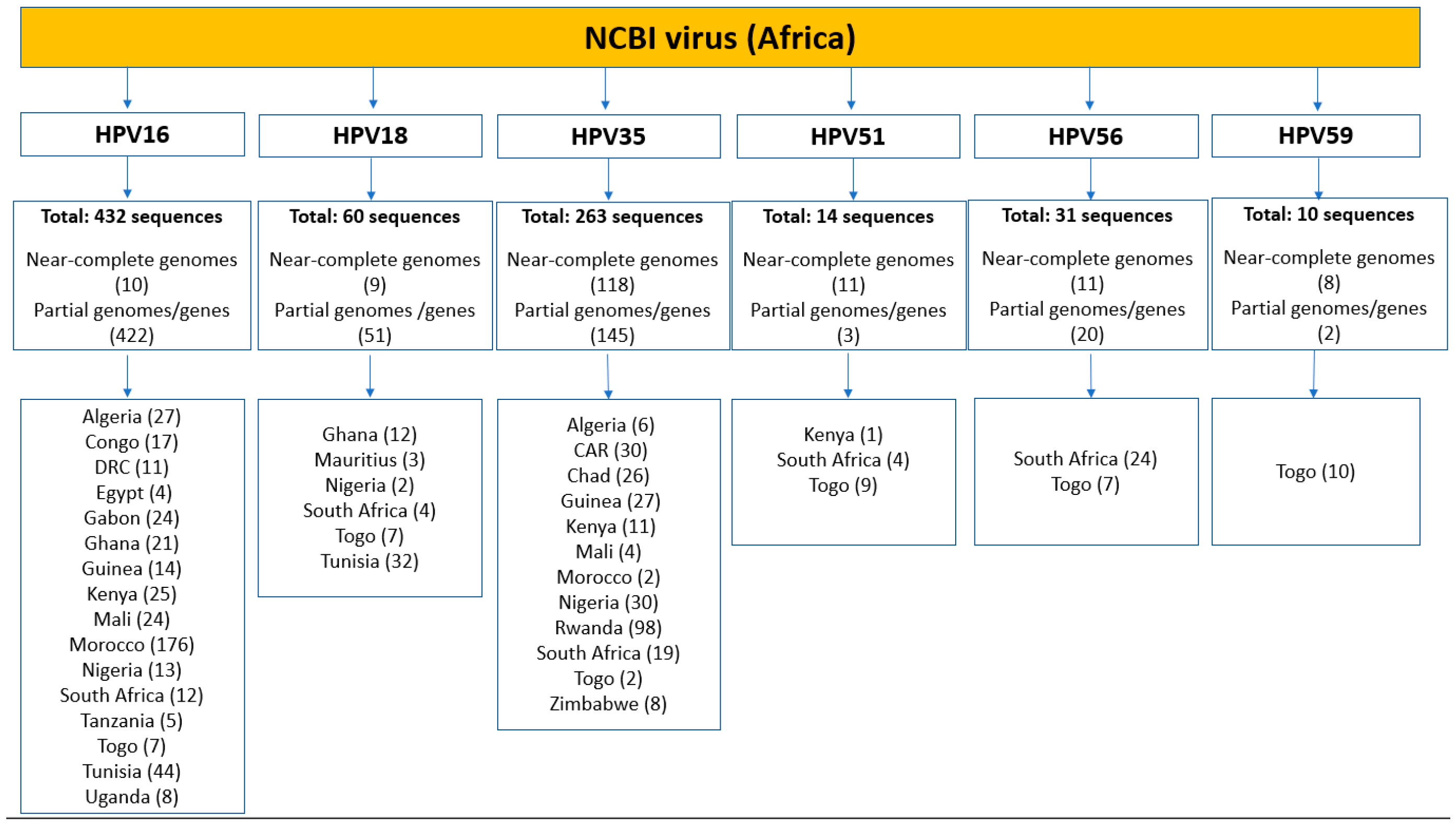


| HPV | Gene Size (Bases) a | Protein Length (Amino Acids) | Geographical Distribution | Number of Sequences (N) |
|---|---|---|---|---|
| E5 | ||||
| 16 | 252 | 83 | South Africa (5), Togo (6) | 11 |
| 18 | 222 | 73 | South Africa (4), Togo (6) | 10 |
| 35 | 252 | 83 | Algeria (5), Guinea (27), Kenya (8), Mali (4), Morocco (2), Nigeria (28), Rwanda (92), South Africa (14), Togo (2), Zimbabwe (7) | 189 |
| 51 | NA | NA | NA | NA |
| 56 | NA | NA | NA | NA |
| 59 | 222 | 73 | Togo (10) | 10 |
| TOTAL | 220 | |||
| E6 | ||||
| 16 | 456 | 151 | South Africa (5), Togo (6) | 11 |
| 18 | 477 | 158 | Nigeria (2), South Africa (4), Togo (5) | 11 |
| 35 | 450 | 149 | Algeria (5), Central Africa Republic (4), Chad (4), Guinea (24), Kenya (9), Mali (4), Morocco (2), Nigeria (25), Rwanda (82), South Africa (18), Togo (2), Zimbabwe (8) | 187 |
| 51 | 456 | 151 | South Africa (4), Togo (8) | 12 |
| 56 | 468 | 155 | South Africa (4), Togo (7) | 11 |
| 59 | 483 | 160 | Togo (10) | 10 |
| TOTAL | 242 | |||
| E7 | ||||
| 16 | 297 | 98 | Republic of the Congo (2), South Africa (5), Togo (6) | 13 |
| 18 | 318 | 105 | South Africa (4), Togo (5) | 9 |
| 35 | 300 | 99 | Algeria (5), Guinea (26), Kenya (10), Mali (4), Morocco (2), Nigeria (27), Rwanda (94), South Africa (18), Togo (2), Zimbabwe (8) | 196 |
| 51 | 306 | 101 | South Africa (4), Togo (8) | 12 |
| 56 | 318 | 105 | South Africa (4), Togo (7) | 11 |
| 59 | 324 | 107 | Togo (10) | 10 |
| TOTAL | 251 | |||
| L1 | ||||
| 16 | 1518 | 505 | South Africa (6), Togo (7) | 13 |
| 18 | 1524 | 507 | South Africa (4), Togo (7) | 11 |
| 35 | 1509 | 502 | Algeria (6), Guinea (27), Kenya (10), Mali (4), Morocco (2), Nigeria (28), Rwanda (86), South Africa (18), Togo (2), Zimbabwe (8) | 191 |
| 51 | 1515 | 504 | South Africa (4), Togo (9) | 13 |
| 56 | 1500 | 499 | South Africa (4), Togo (7) | 11 |
| 59 | 1527 | 508 | Togo (10) | 10 |
| TOTAL | 249 | |||
| HPV | Number of Variants | |
|---|---|---|
| Synonymous | Non-Synonymous | |
| 16 | 6 | 5 |
| 18 | 2 | 4 |
| 35 | 6 | 9 |
| 51 | N/A | N/A |
| 56 | N/A | N/A |
| 59 | 2 | 5 |
| Total | 16 | 23 |
| HPV | Number of Variants | |
|---|---|---|
| Synonymous | Non-Synonymous | |
| 16 | 4 | 6 |
| 18 | 7 | 1 |
| 35 | 9 | 10 |
| 51 | 3 | 2 |
| 56 | 4 | 3 |
| 59 | 4 | 1 |
| Total | 31 | 23 |
| HPV | Number of Variants | |
|---|---|---|
| Synonymous | Non-Synonymous | |
| 16 | 3 | 2 |
| 18 | 2 | 3 |
| 35 | 3 | 4 |
| 51 | 1 | 3 |
| 56 | 0 | 5 |
| 59 | 1 | 4 |
| Total | 10 | 21 |
| HPV | Number of Variants | |
|---|---|---|
| Synonymous | Non-Synonymous | |
| 16 | 17 | 9 |
| 18 | 15 | 5 |
| 35 | 44 | 10 |
| 51 | 25 | 7 |
| 56 | 13 | 4 |
| 59 | 15 | 19 |
| Total | 129 | 54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nyide, B.; Thomas, M.; Banks, L.; Mkhize, P.P.; Matume, N.D. Genetic Diversity of Selected High-Risk HPV Types Prevalent in Africa and Not Covered by Current Vaccines: A Pooled Sequence Data Analysis. Int. J. Mol. Sci. 2025, 26, 11056. https://doi.org/10.3390/ijms262211056
Nyide B, Thomas M, Banks L, Mkhize PP, Matume ND. Genetic Diversity of Selected High-Risk HPV Types Prevalent in Africa and Not Covered by Current Vaccines: A Pooled Sequence Data Analysis. International Journal of Molecular Sciences. 2025; 26(22):11056. https://doi.org/10.3390/ijms262211056
Chicago/Turabian StyleNyide, Babalwa, Miranda Thomas, Lawrence Banks, Pamela P. Mkhize, and Nontokozo D. Matume. 2025. "Genetic Diversity of Selected High-Risk HPV Types Prevalent in Africa and Not Covered by Current Vaccines: A Pooled Sequence Data Analysis" International Journal of Molecular Sciences 26, no. 22: 11056. https://doi.org/10.3390/ijms262211056
APA StyleNyide, B., Thomas, M., Banks, L., Mkhize, P. P., & Matume, N. D. (2025). Genetic Diversity of Selected High-Risk HPV Types Prevalent in Africa and Not Covered by Current Vaccines: A Pooled Sequence Data Analysis. International Journal of Molecular Sciences, 26(22), 11056. https://doi.org/10.3390/ijms262211056






