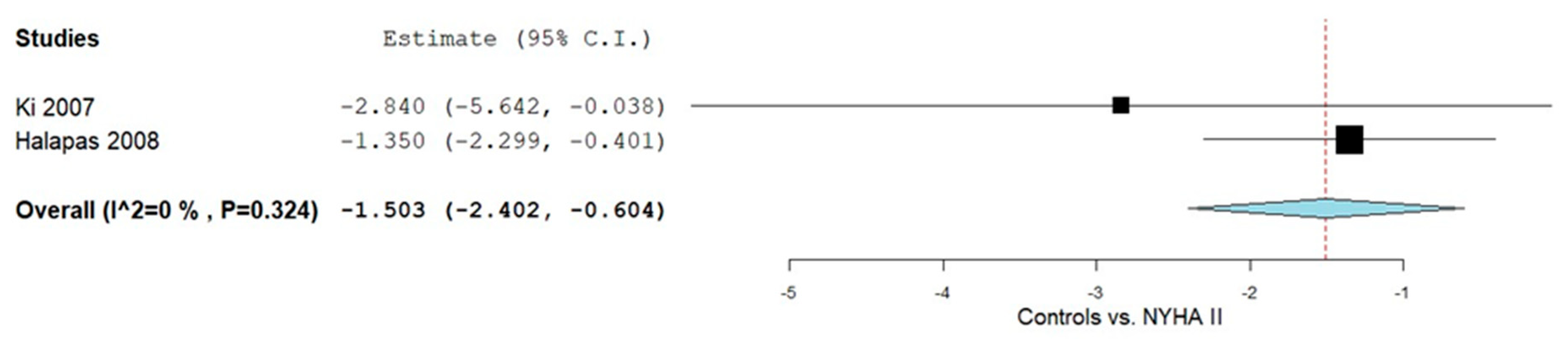Exploring the Clinical Utility of Osteoprotegerin in Heart Failure—A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Strategy
2.2. Eligibility Criteria
2.3. Risk of Bias Assessment in Individual Studies
2.4. Summary Measures and Synthesis of Results
3. Results
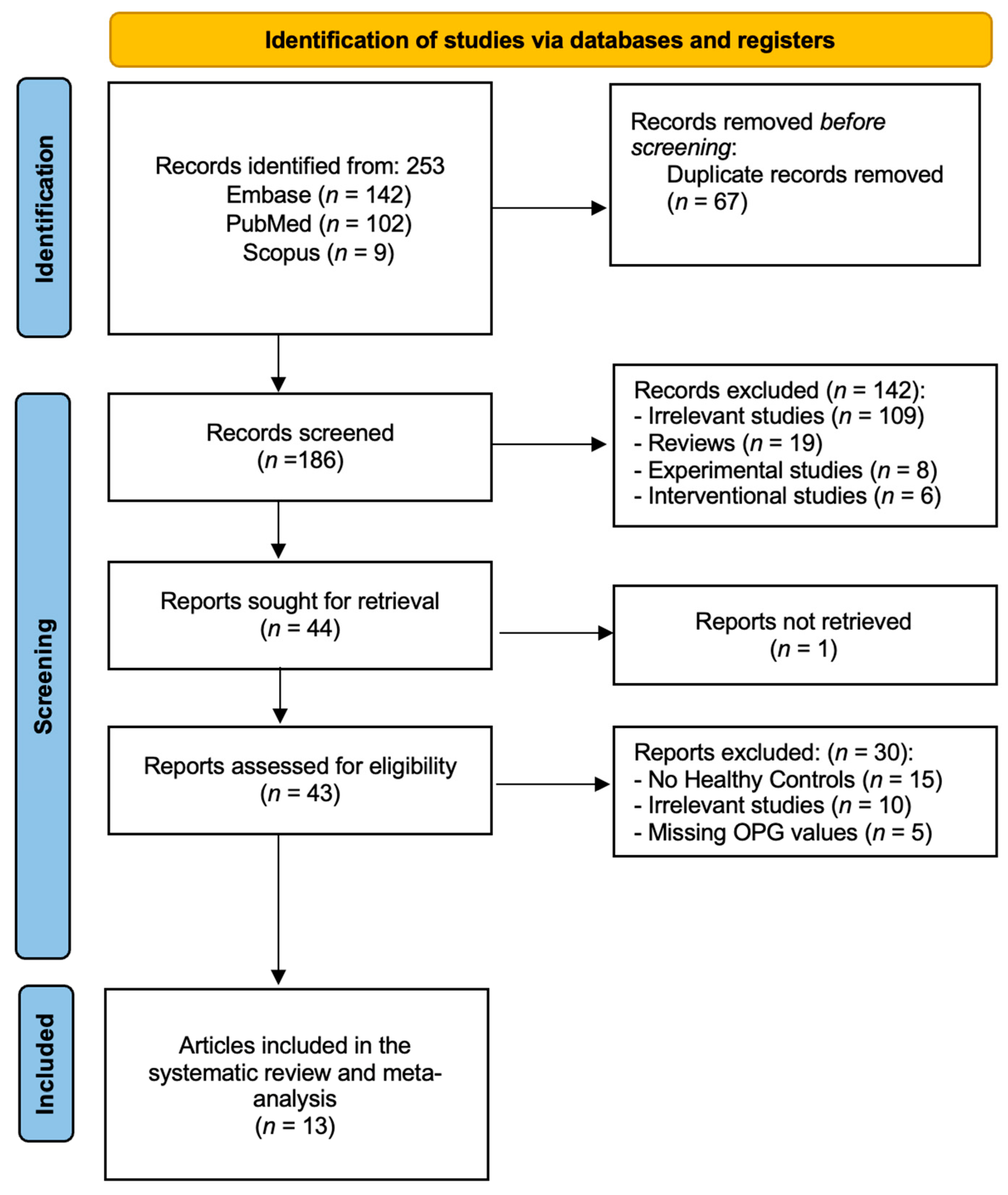
3.1. General Results
3.2. Study Characteristics
3.3. Definition of HF
3.4. OPG Levels in HF
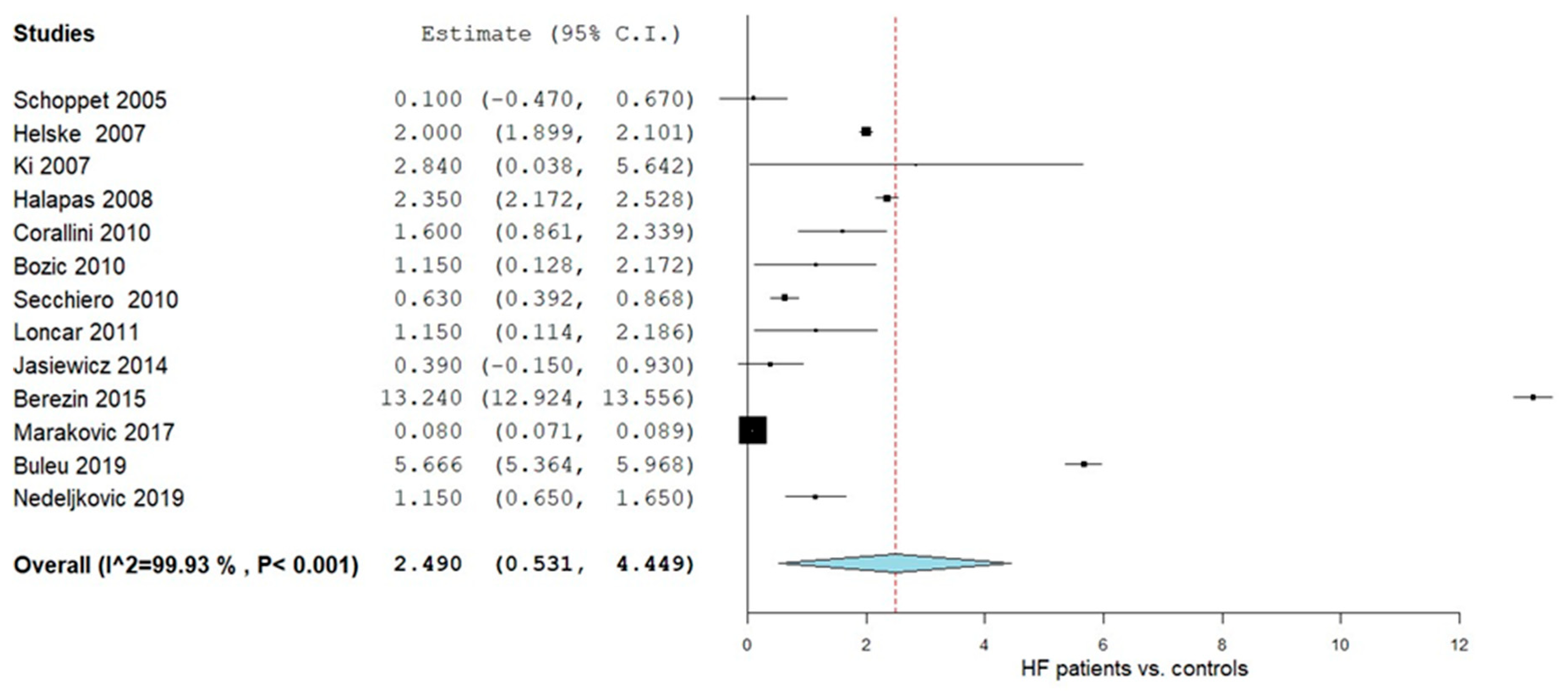
3.4.1. OPG Levels in HF Patients vs. Controls
3.4.2. Controls vs. NYHA Class II
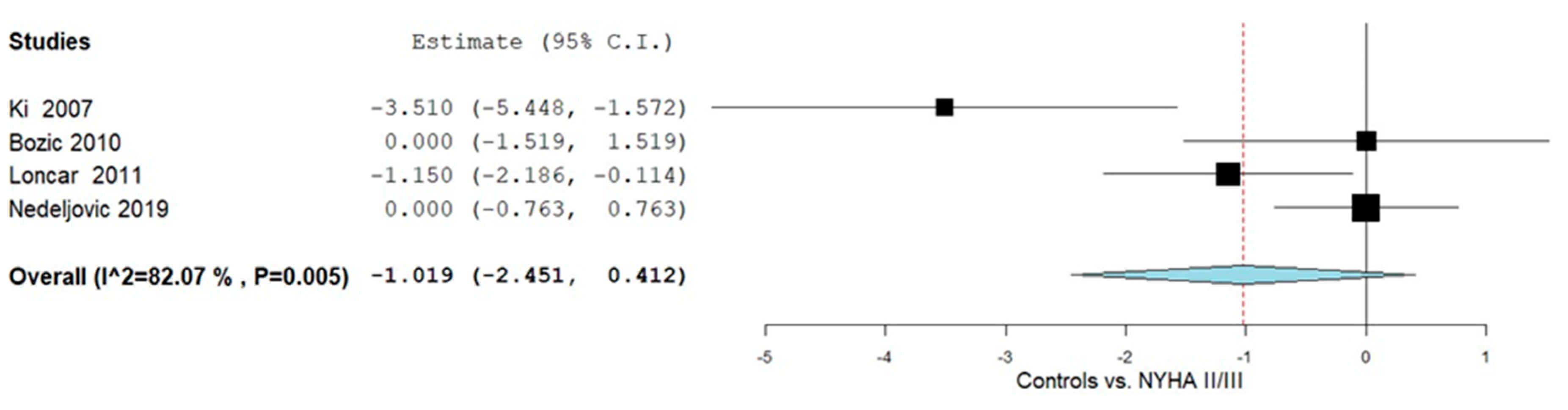
3.4.3. Controls vs. NYHA Class II and III
3.5. Quality Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2022, 118, 3272–3287, Erratum in Cardiovasc. Res. 2023, 119, 1453. [Google Scholar] [CrossRef]
- Shahim, B.; Kapelios, C.J.; Savarese, G.; Lund, L.H. Global Public Health Burden of Heart Failure: An Updated Review. Card. Fail. Rev. 2023, 9, e11. [Google Scholar] [CrossRef]
- Berezin, A.E.; Berezin, A.A. Biomarkers in Heart Failure: From Research to Clinical Practice. Ann. Lab. Med. 2023, 43, 225–236. [Google Scholar] [CrossRef]
- Akhtar, S.M.M.; Ali, A.; Fareed, A.; Hasan, J. Osteoprotegerin (OPG): A potential biomarker for adverse cardiovascular events in stable coronary artery disease. Health Sci. Rep. 2024, 7, e2253. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.H.; Oh, T.R.; Choi, H.S.; Kim, C.S.; Bae, E.H.; Ma, S.K.; Oh, K.-H.; Jung, J.Y.; Hyun, Y.Y.; Kim, S.W.; et al. Circulating osteoprotegerin as a cardiac biomarker for left ventricular diastolic dysfunction in patients with pre-dialysis chronic kidney disease: The KNOW-CKD study. Clin. Res. Cardiol. 2024, 113, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Gori, M.; Senni, M.; Metra, M. High-Sensitive Cardiac Troponin for Prediction of Clinical Heart Failure. Circulation 2017, 135, 1506–1508. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.E.; Liu, C.; Lyass, A.; Courchesne, P.; Pencina, M.J.; Vasan, R.S.; Larson, M.G.; Levy, D. Galectin-3, a Marker of Cardiac Fibrosis, Predicts Incident Heart Failure in the Community. J. Am. Coll. Cardiol. 2012, 60, 1249–1256. [Google Scholar] [CrossRef]
- Parikh, R.H.; Seliger, S.L.; Christenson, R.; Gottdiener, J.S.; Psaty, B.M.; deFilippi, C.R. Soluble ST2 for Prediction of Heart Failure and Cardiovascular Death in an Elderly, Community-Dwelling Population. J. Am. Heart Assoc. 2016, 5, e003188. [Google Scholar] [CrossRef]
- Cruciat, R.C.; Gazi, G.; Ismaiel, A.; Leucuta, D.-C.; Al Srouji, N.; Popa, S.-L.; Ismaiel, M.; Ensar, D.; Dumitrascu, D.L. Unlocking the potential of biomarkers: The promise of adrenomedullin and its precursors in diagnosing and assessing heart failure. Int. J. Cardiol. 2025, 418, 132659. [Google Scholar] [CrossRef]
- Kuku, K.O.; Oyetoro, R.; Hashemian, M.; Livinski, A.A.; Shearer, J.J.; Joo, J.; Psaty, B.M.; Levy, D.; Ganz, P.; Roger, V.L. Proteomics for heart failure risk stratification: A systematic review. BMC Med. 2024, 22, 34. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ (Clin. Res. ed.) 2021, 372, n71. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. 2000. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 25 November 2024).
- Lo, C.K.; Mertz, D.; Loeb, M. Newcastle-Ottawa Scale: Comparing reviewers’ to authors’ assessments. BMC Med. Res. Methodol. 2014, 14, 45. [Google Scholar] [CrossRef]
- Wallace, B.C.; Dahabreh, I.J.; Trikalinos, T.A.; Lau, J.; Trow, P.; Schmid, C.H. Closing the Gap between Methodologists and End-Users: R as a Computational Back-End. J. Stat. Softw. 2012, 49, 1–15. [Google Scholar] [CrossRef]
- Viechtbauer, W. Conducting Meta-Analyses in R with the metafor Package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Akl, E.; Altman, D.; Aluko, P.; Beaton, D.; Berlin, J.; Bhaumik, B.; Bingham, C.; Boers, M.; Booth, A.; Boutron, I.; et al. Cochrane Handbook for Systematic Reviews of Interventions; Wiley-Blackwell: Hoboken, NJ, USA, 2019. [Google Scholar]
- Chen, Y.-H.; Wu, Y.-W.; Yang, W.-S.; Wang, S.-S.; Lee, C.-M.; Chou, N.-K.; Hsu, R.-B.; Lin, Y.-H.; Lin, M.-S.; Ho, Y.-L.; et al. Relationship between Bone Mineral Density and Serum Osteoprotegerin in Patients with Chronic Heart Failure. PLoS ONE 2012, 7, e44242. [Google Scholar] [CrossRef] [PubMed]
- Erkol, A.; Oduncu, V.; Pala, S.; Kızılırmak, F.; Kılıcgedik, A.; Yılmaz, F.; Güler, A.; Karabay, C.Y.; Kırma, C. Plasma osteoprotegerin level on admission is associated with no-reflow phenomenon after primary angioplasty and subsequent left ventricular remodeling in patients with acute ST-segment elevation myocardial infarction. Atherosclerosis 2012, 221, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Erkol, A.; Pala, S.; Kırma, C.; Oduncu, V.; Dündar, C.; Izgi, A.; Tigen, K.; Gibson, C.M. Relation of circulating osteoprotegerin levels on admission to microvascular obstruction after primary percutaneous coronary intervention. Am. J. Cardiol. 2011, 107, 857–862. [Google Scholar] [CrossRef]
- Fuernau, G.; Zaehringer, S.; Eitel, I.; de Waha, S.; Droppa, M.; Desch, S.; Schuler, G.; Adams, V.; Thiele, H. Osteoprotegerin in ST-elevation myocardial infarction: Prognostic impact and association with markers of myocardial damage by magnetic resonance imaging. Int. J. Cardiol. 2013, 167, 2134–2139. [Google Scholar] [CrossRef]
- Gullestad, L.; Ueland, T.; Vinge, L.E.; Finsen, A.; Yndestad, A.; Aukrust, P. Inflammatory cytokines in heart failure: Mediators and markers. Cardiology 2012, 122, 23–35. [Google Scholar] [CrossRef]
- Jansson, A.M.; Hartford, M.; Omland, T.; Karlsson, T.; Lindmarker, P.; Herlitz, J.; Ueland, T.; Aukrust, P.; Caidahl, K. Multimarker risk assessment including osteoprotegerin and CXCL16 in acute coronary syndromes. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 3041–3049. [Google Scholar] [CrossRef]
- Leistner, D.M.; Seeger, F.H.; Fischer, A.; Röxe, T.; Klotsche, J.; Iekushi, K.; Seeger, T.; Assmus, B.; Honold, J.; Karakas, M.; et al. Elevated levels of the mediator of catabolic bone remodeling RANKL in the bone marrow environment link chronic heart failure with osteoporosis. Circ. Heart Fail. 2012, 5, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Omland, T.; Drazner, M.H.; Ueland, T.; Abedin, M.; Murphy, S.A.; Aukrust, P.; de Lemos, J.A. Plasma osteoprotegerin levels in the general population: Relation to indices of left ventricular structure and function. Hypertension 2007, 49, 1392–1398. [Google Scholar] [CrossRef] [PubMed]
- Omland, T.; Ueland, T.; Jansson, A.M.; Persson, A.; Karlsson, T.; Smith, C.; Herlitz, J.; Aukrust, P.; Hartford, M.; Caidahl, K. Circulating osteoprotegerin levels and long-term prognosis in patients with acute coronary syndromes. J. Am. Coll. Cardiol. 2008, 51, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, E.R.; Ueland, T.; Seifert, R.; Aukrust, P.; Schartum-Hansen, H.; Ebbing, M.; Bleie, Ø.; Igland, J.; Svingen, G.; Nordrehaug, J.E.; et al. Serum osteoprotegerin levels and long-term prognosis in patients with stable angina pectoris. Atherosclerosis 2010, 212, 644–649. [Google Scholar] [CrossRef]
- Røysland, R.; Bonaca, M.P.; Omland, T.; Sabatine, M.; Murphy, S.A.; Scirica, B.M.; Bjerre, M.; Flyvbjerg, A.; Braunwald, E.; Morrow, D.A. Osteoprotegerin and cardiovascular mortality in patients with non-ST elevation acute coronary syndromes. Heart 2012, 98, 786–791. [Google Scholar] [CrossRef]
- Røysland, R.; Masson, S.; Omland, T.; Milani, V.; Bjerre, M.; Flyvbjerg, A.; Di Tano, G.; Misuraca, G.; Maggioni, A.P.; Tognoni, G.; et al. Prognostic value of osteoprotegerin in chronic heart failure: The GISSI-HF trial. Am. Heart J. 2010, 160, 286–293. [Google Scholar] [CrossRef]
- Schnabel, R.B.; Larson, M.G.; Yamamoto, J.F.; Kathiresan, S.; Rong, J.; Levy, D.; Keaney, J.F., Jr.; Wang, T.J.; Vasan, R.S.; Benjamin, E.J. Relation of multiple inflammatory biomarkers to incident atrial fibrillation. Am. J. Cardiol. 2009, 104, 92–96. [Google Scholar] [CrossRef]
- Ueland, T.; Aukrust, P.; Dahl, C.P.; Husebye, T.; Solberg, O.G.; Tønnessen, T.; Aakhus, S.; Gullestad, L. Osteoprotegerin levels predict mortality in patients with symptomatic aortic stenosis. J. Intern. Med. 2011, 270, 452–460. [Google Scholar] [CrossRef]
- Ueland, T.; Dahl, C.P.; Kjekshus, J.; Hulthe, J.; Böhm, M.; Mach, F.; Goudev, A.; Lindberg, M.; Wikstrand, J.; Aukrust, P.; et al. Osteoprotegerin predicts progression of chronic heart failure: Results from CORONA. Circ. Heart Fail. 2011, 4, 145–152. [Google Scholar] [CrossRef]
- Balion, C.M.; Santaguida, P.; McKelvie, R.; Hill, S.A.; McQueen, M.J.; Worster, A.; Raina, P.S. Physiological, pathological, pharmacological, biochemical and hematological factors affecting BNP and NT-proBNP. Clin. Biochem. 2008, 41, 231–239. [Google Scholar] [CrossRef]
- Candido, R.; Toffoli, B.; Corallini, F.; Bernardi, S.; Zella, D.; Voltan, R.; Grill, V.; Celeghini, C.; Fabris, B. Human full-length osteoprotegerin induces the proliferation of rodent vascular smooth muscle cells both in vitro and in vivo. J. Vasc. Res. 2010, 47, 252–261. [Google Scholar] [CrossRef]
- Demyanets, S.; Huber, K.; Wojta, J. Inflammation and the cardiovascular system. Eur. Surg. 2011, 43, 78–89. [Google Scholar] [CrossRef]
- Feng, W.; Li, W.; Liu, W.; Wang, F.; Li, Y.; Yan, W. IL-17 induces myocardial fibrosis and enhances RANKL/OPG and MMP/TIMP signaling in isoproterenol-induced heart failure. Exp. Mol. Pathol. 2009, 87, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Gruson, D.; Ahn, S.A.; Rousseau, M.F. Biomarkers of inflammation and cardiac remodeling: The quest of relevant companions for the risk stratification of heart failure patients is still ongoing. Biochem. Med. (Zagreb) 2011, 21, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Henkens, M.; van Ommen, A.M.; Remmelzwaal, S.; Valstar, G.B.; Wang, P.; Verdonschot, J.A.J.; Hazebroek, M.R.; Hofstra, L.; van Empel, V.P.M.; Beulens, J.W.J.; et al. The HFA-PEFF score identifies ‘early-HFpEF’ phenogroups associated with distinct biomarker profiles. ESC Heart Fail. 2022, 9, 2032–2036. [Google Scholar] [CrossRef]
- Koo, H.M.; Do, H.M.; Kim, E.J.; Lee, M.J.; Shin, D.H.; Kim, S.J.; Oh, H.J.; Yoo, D.E.; Kim, J.K.; Park, J.T.; et al. Elevated osteoprotegerin is associated with inflammation, malnutrition and new onset cardiovascular events in peritoneal dialysis patients. Atherosclerosis 2011, 219, 925–930. [Google Scholar] [CrossRef]
- Pincott, E.S.; Burch, M. New biomarkers in heart failure. Prog. Pediatr. Cardiol. 2011, 31, 49–52. [Google Scholar] [CrossRef]
- Schlieper, G.; Westenfeld, R.; Brandenburg, V.; Ketteler, M. Inhibitors of calcification in blood and urine. Semin. Dial. 2007, 20, 113–121. [Google Scholar] [CrossRef]
- Vik, A.; Brodin, E.; Børvik, T.; Sveinbjørnsson, B.; Hansen, J.B. Serum osteoprotegerin in young survivors of myocardial infarction. Thromb. Haemost. 2006, 95, 881–885. [Google Scholar] [CrossRef]
- Gupta, S.; Drazner, M.H.; de Lemos, J.A. Newer biomarkers in heart failure. Heart Fail. Clin. 2009, 5, 579–588. [Google Scholar] [CrossRef]
- Manhenke, C.; Ørn, S.; von Haehling, S.; Wollert, K.C.; Ueland, T.; Aukrust, P.; Voors, A.A.; Squire, I.; Zannad, F.; Anker, S.D.; et al. Clustering of 37 circulating biomarkers by exploratory factor analysis in patients following complicated acute myocardial infarction. Int. J. Cardiol. 2013, 166, 729–735. [Google Scholar] [CrossRef]
- Siasos, G.; Oikonomou, E.; Maniatis, K.; Georgiopoulos, G.; Kokkou, E.; Tsigkou, V.; Zaromitidou, M.; Antonopoulos, A.; Vavuranakis, M.; Stefanadis, C.; et al. Prognostic significance of arterial stiffness and osteoprotegerin in patients with stable coronary artery disease. Eur. J. Clin. Investig. 2018, 48, e12890. [Google Scholar] [CrossRef]
- Ueland, T.; Yndestad, A.; Dahl, C.P.; Gullestad, L.; Aukrust, P. TNF revisited: Osteoprotegerin and TNF-related molecules in heart failure. Curr. Heart Fail. Rep. 2012, 9, 92–100. [Google Scholar] [CrossRef]
- Yue, H.; Li, W.; Desnoyer, R.; Karnik, S.S. Role of nuclear unphosphorylated STAT3 in angiotensin II type 1 receptor-induced cardiac hypertrophy. Cardiovasc. Res. 2010, 85, 90–99. [Google Scholar] [CrossRef]
- Schoppet, M.; Ruppert, V.; Hofbauer, L.C.; Henser, S.; Al-Fakhri, N.; Christ, M.; Pankuweit, S.; Maisch, B. TNF-related apoptosis-inducing ligand and its decoy receptor osteoprotegerin in nonischemic dilated cardiomyopathy. Biochem. Biophys. Res. Commun. 2005, 338, 1745–1750. [Google Scholar] [CrossRef] [PubMed]
- Helske, S.; Kovanen, P.T.; Lindstedt, K.A.; Salmela, K.; Lommi, J.; Turto, H.; Werkkala, K.; Kupari, M. Increased circulating concentrations and augmented myocardial extraction of osteoprotegerin in heart failure due to left ventricular pressure overload. Eur. J. Heart Fail. 2007, 9, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.H.; Oh, T.R.; Choi, H.S.; Kim, C.S.; Oh, K.H.; Lee, J.; Oh, Y.K.; Jung, J.Y.; Choi, K.H.; Ma, S.K.; et al. Association of Circulating Osteoprotegerin Level with Blood Pressure Variability in Patients with Chronic Kidney Disease. J. Clin. Med. 2021, 11, 178. [Google Scholar] [CrossRef] [PubMed]
- Halapas, A.; Zacharoulis, A.; Theocharis, S.; Karavidas, A.; Korres, D.; Papadopoulos, K.; Katopodis, H.; Stavropoulou, A.; Lembessis, P.; Xiromeritis, C.; et al. Serum levels of the osteoprotegerin, receptor activator of nuclear factor kappa-B ligand, metalloproteinase-1 (MMP-1) and tissue inhibitors of MMP-1 levels are increased in men 6 months after acute myocardial infarction. Clin. Chem. Lab. Med. 2008, 46, 510–516. [Google Scholar] [CrossRef]
- Bozic, B.; Loncar, G.; Prodanovic, N.; Radojicic, Z.; Cvorovic, V.; Dimkovic, S.; Popovic-Brkic, V. Relationship between high circulating adiponectin with bone mineral density and bone metabolism in elderly males with chronic heart failure. J. Card. Fail. 2010, 16, 301–307. [Google Scholar] [CrossRef]
- Corallini, F.; Secchiero, P.; Beltrami, A.P.; Cesselli, D.; Puppato, E.; Ferrari, R.; Beltrami, C.A.; Zauli, G. TNF-alpha modulates the migratory response of mesenchymal stem cells to TRAIL. Cell. Mol. Life Sci. CMLS 2010, 67, 1307–1314. [Google Scholar] [CrossRef]
- Secchiero, P.; Corallini, F.; Beltrami, A.P.; Ceconi, C.; Bonasia, V.; Di Chiara, A.; Ferrari, R.; Zauli, G. An imbalanced OPG/TRAIL ratio is associated to severe acute myocardial infarction. Atherosclerosis 2010, 210, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Loncar, G.; Bozic, B.; Dimkovic, S.; Prodanovic, N.; Radojicic, Z.; Cvorovic, V.; Putnikovic, B.; Popovic, V. Association of increased parathyroid hormone with neuroendocrine activation and endothelial dysfunction in elderly men with heart failure. J. Endocrinol. Investig. 2011, 34, e78–e85. [Google Scholar] [CrossRef] [PubMed]
- Jasiewicz, M.; Knapp, M.; Waszkiewicz, E.; Musiał, W.J.; Kamiński, K.A. Potential pathogenic role of soluble receptor activator of nuclear factor-ĸB ligand and osteoprotegerin in patients with pulmonary arterial hypertension. Pol. Arch. Med. Wewn. 2014, 124, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Berezin, A.E.; Kremzer, A.A.; Berezina, T.A.; Martovitskaya, Y.V. Pattern of circulating microparticles in chronic heart failure patients with metabolic syndrome: Relevance to neurohumoral and inflammatory activation. BBA Clin. 2015, 4, 69–75. [Google Scholar] [CrossRef][Green Version]
- Makarović, S.; Makarović, Z.; Bilić-Ćurčić, I.; Milas-Ahić, J.; Mihaljević, I.; Franceschi, M.; Jukić, T. Serum Osteoprotegerin in Patients with Calcified Aortic Valve Stenosis in Relation to Heart Failure. Acta Clin. Croat. 2017, 56, 733–741. [Google Scholar] [CrossRef]
- Buleu, F.N.; Luca, C.T.; Tudor, A.; Badalica-Petrescu, M.; Caraba, A.; Pah, A.; Georgescu, D.; Christodorescu, R.; Dragan, S. Correlations between Vascular Stiffness Indicators, OPG, and 25-OH Vitamin D3 Status in Heart Failure Patients. Medicina 2019, 55, 309. [Google Scholar] [CrossRef]
- Nedeljkovic, B.B.; Loncar, G.; Vizin, T.; Radojicic, Z.; Brkic, V.P.; Kos, J. Relationship of High Circulating Cystatin C to Biochemical Markers of Bone Turnover and Bone Mineral Density in Elderly Males with a Chronic Heart Failure. J. Med. Biochem. 2019, 38, 53–62. [Google Scholar] [CrossRef]
- Montagnana, M.; Lippi, G.; Danese, E.; Guidi, G.C. The role of osteoprotegerin in cardiovascular disease. Ann. Med. 2013, 45, 254–264. [Google Scholar] [CrossRef]
- Dutka, M.; Bobiński, R.; Wojakowski, W.; Francuz, T.; Pająk, C.; Zimmer, K. Osteoprotegerin and RANKL-RANK-OPG-TRAIL signalling axis in heart failure and other cardiovascular diseases. Heart Fail. Rev. 2022, 27, 1395–1411. [Google Scholar] [CrossRef]
- Kamimura, D.; Buckley, L.; Claggett, B.; Yu, B.; Coresh, J.; Matsushita, K.; Chang, P.; Hoogeveen, R.; Ballantyne, C.; Hall, M.; et al. Abstract 10769: Osteoprotegerin and Incident Heart Failure: The Atherosclerosis Risk in Communities Study. Circulation 2021, 144. [Google Scholar] [CrossRef]
- Ueland, T.; Yndestad, A.; Øie, E.; Florholmen, G.; Halvorsen, B.; Frøland, S.S.; Simonsen, S.; Christensen, G.; Gullestad, L.; Aukrust, P. Dysregulated osteoprotegerin/RANK ligand/RANK axis in clinical and experimental heart failure. Circulation 2005, 111, 2461–2468. [Google Scholar] [CrossRef]
- Ma, T.; Zhao, J.; Yan, Y.; Liu, J.; Zang, J.; Zhang, Y.; Ruan, K.; Xu, H.; He, W. Plasma osteoprotegerin predicts adverse cardiovascular events in stable coronary artery disease: The PEACE trial. Front. Cardiovasc. Med. 2023, 10, 1178153. [Google Scholar] [CrossRef]
- Friões, F.; Laszczynska, O.; Almeida, P.B.; Silva, N.; Guimarães, J.T.; Omland, T.; Azevedo, A.; Bettencourt, P. Prognostic Value of Osteoprotegerin in Acute Heart Failure. Can. J. Cardiol. 2015, 31, 1266–1271. [Google Scholar] [CrossRef][Green Version]
- Giannitsi, S.; Bougiakli, M.; Bechlioulis, A.; Naka, K. Endothelial dysfunction and heart failure: A review of the existing bibliography with emphasis on flow mediated dilation. JRSM Cardiovasc. Dis. 2019, 8, 2048004019843047. [Google Scholar] [CrossRef]
- Yin, Z.; Zhang, J.; Shen, Z.; Qin, J.J.; Wan, J.; Wang, M. Regulated vascular smooth muscle cell death in vascular diseases. Cell Prolif. 2024, 57, e13688. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gazi, G.; Gazi, G.; Cruciat, R.C.; Leucuta, D.-C.; Popa, S.-L.; Ismaiel, A. Exploring the Clinical Utility of Osteoprotegerin in Heart Failure—A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2025, 26, 11053. https://doi.org/10.3390/ijms262211053
Gazi G, Gazi G, Cruciat RC, Leucuta D-C, Popa S-L, Ismaiel A. Exploring the Clinical Utility of Osteoprotegerin in Heart Failure—A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2025; 26(22):11053. https://doi.org/10.3390/ijms262211053
Chicago/Turabian StyleGazi, Gifar, Gabi Gazi, Robert Cristian Cruciat, Daniel-Corneliu Leucuta, Stefan-Lucian Popa, and Abdulrahman Ismaiel. 2025. "Exploring the Clinical Utility of Osteoprotegerin in Heart Failure—A Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 26, no. 22: 11053. https://doi.org/10.3390/ijms262211053
APA StyleGazi, G., Gazi, G., Cruciat, R. C., Leucuta, D.-C., Popa, S.-L., & Ismaiel, A. (2025). Exploring the Clinical Utility of Osteoprotegerin in Heart Failure—A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences, 26(22), 11053. https://doi.org/10.3390/ijms262211053