1. Introduction
Plant–pathogen interactions play a critical role in determining plant health, crop productivity and ecosystem stability. A comprehensive understanding of these interactions is essential for developing effective disease control strategies and ensuring global food security [
1].
Xylella fastidosa is a Gram-negative bacterium that colonizes the xylem, the tissue responsible for water transport in plants, and causes devasting diseases in several crops. In grapevines (
Vitis vinifera), it is the etiological agent of Pierce’s disease (PD), characterized by leaf scorch, wilting, and progressive decline, often leading to plant death [
2]. PD disrupts water transport and induces progressive physiological deterioration in
V. vinifera. The pathosystem involves bacterial colonization of xylem vessels, biofilm formation, and vessel occlusion, collectively causing water stress, leaf scorch, and tissue necrosis [
3]. Infection triggers the plant to form tyloses and other xylem blockages that interrupt water flow and impose severe physiological stress. PD is a major concern for grape production worldwide, such as in Majorca (Spain) and Taiwan and possibly spreading to the northern (Europe and China) and southern hemisphere (Chile, Argentina, South Africa, Australia, and New Zealand) [
4], particularly in California, where it causes substantial economic losses estimated at over USD 100 million [
5]. The pathogen is transmitted by xylem-feeding insects, and biofilm formation further restricts water movement. Although some wild grape species exhibit natural resistance, cultivated
V. vinifera varieties remain highly susceptible. Upon infection,
V. vinifera undergoes extensive transcriptional reprogramming, characterized by the activation of defense-related genes and downregulation of many metabolic pathways. These alterations highlight the complex molecular responses that enable the plant to cope up with
X. fastidiosa infection [
6].
Despite extensive research on the pathophysiological effects of PD, the dynamic transcriptomic responses of
V. vinifera to
X. fastidiosa remain poorly understood. Addressing this knowledge gap is crucial for elucidating the molecular mechanisms underlying susceptibility and resistance. PD progression in
V. vinifera can be broadly divided into two phases based on symptom development and physiological changes. Phase I (early infection) marks the onset of bacterial colonization, characterized by limited xylem blockage, mild leaf scorch, and activation of defense and oxidative stress pathways. Phase II (advanced infection) involves extensive bacterial proliferation, systemic vessel occlusion, chlorosis, and dehydration, leading to severe wilting and necrosis. This transition represents a shift from active defense to metabolic decline and transcriptional suppression as the infection progresses [
4,
7].
Ingel et al. (2021) integrated microscopy, micro-CT, and RNA-seq to associate
X. fastidiosa infection with tylose formation and starch depletion in
V. vinifera, revealing broad transcriptional shifts related to ethylene signaling, cell wall biogenesis, and photosynthetic decline [
6]. However, their analysis primarily describes overall expression trends without resolving the underlying regulatory network or distinguishing infection-induced changes from normal temporal variation [
6].
Therefore, this study used a computational, systems-biology approach to examine transcriptional regulation and interaction networks [
8], re-analyzing the GSE152164 RNA-seq dataset using a multi-contrast DESeq2 framework that compares infected and control samples across disease phases and time points, which enabled the separation of infection-specific from temporal variation [
6,
9]. We further constructed STRING-based protein–protein interaction (PPI) networks of differentially expressed genes (DEGs) using the Cytoscape STRING app to identify key regulatory hubs and functional modules involved in the grapevine response to
X. fastidiosa infection.
2. Results
2.1. Identification of Differentially Expressed Genes
To investigate the transcriptional changes associated with
X. fastidiosa infection in
V. vinifera, we analyzed RNA-seq data from the Gene Expression Omnibus (GEO) dataset GSE152164, comprising 12 samples across Phases I and II under both control and infected conditions [
6]. Differential expression analysis was performed using DESeq2, with the following pairwise contrasts: Phase I control vs. Phase I infected, Phase II control vs. Phase II infected, Phase I infected vs. Phase II infected, and Phase I control vs. Phase II control. Genes with a threshold of FDR < 0.05 and |log
2 FC| ≥ 1 were considered significantly differentially expressed. Genes with log
2 FC > 1 were classified as upregulated, and those with log
2 FC ≤ −1 were considered as downregulated.
Table 1 summarizes the DEG counts for all contrasts, and
Figure 1 shows the corresponding volcano plots.
In the first pairwise comparison (Phase I control vs. Phase I onfected), 1093 DEGs were identified, comprising 986 upregulated and 107 downregulated genes, indicating a strong host response during the early phase of infection (
Supplementary Material I-i). In contrast, the Phase II control vs. Phase II infected comparison yielded only 136 DEGs (133 were upregulated, and 3 were downregulated), suggesting a markedly reduced transcriptional response at the later phase of infection (
Table 1) (
Supplementary Material I-ii). Beyond infection-specific comparisons, we examined temporal variation within the control and infected groups. The Phase I control vs. Phase II control contrast produced the largest number of DEGs (1270), predominantly downregulated (1063) genes, reflecting developmental or age-related transcriptional shifts independent of infection (
Supplementary Material I-iii). Moreover, the Phase I infected vs. Phase II infected comparison produced 336 DEGs (43 upregulated and 293 downregulated), indicating progressive reprogramming of disease-related pathways as infection advances over various stages (
Table 1 and
Supplementary Material I-iv).
2.2. Phase-Specific and Temporal Dynamics of Differentially Expressed Genes in Response to Xylella fastidiosa Infection
Table 2 provides a detailed summary of common and phase-specific DEGs identified in
V. vinifera during
X. fastidiosa infection. This comparative framework highlights both infection-induced and time-dependent transcriptional reprogramming, providing insights into how the grapevine defense network evolves between early and late infection stages. Among the infection–phase comparisons, the most extensive transcriptional reprogramming occurred in the “early-phase-specific response (Phase I only)” category, which encompassed 991 DEGs (884 upregulated and 107 downregulated). This strong early activation reflects rapid induction of defense-related signaling, stress-responsive transcription factors, and primary metabolic reprogramming upon pathogen perception.
The “core infection response (intersection)” category comprised 102 DEGs, all upregulated and shared between Phases I and II infected samples. These genes likely represent a sustained defense module characterized by persistent immune activation, enhanced secondary metabolism, and strengthened stress tolerance throughout infection progression.
In contrast, the “late-phase-specific response (Phase II only)” subset contained only 34 DEGs (31 upregulated and 3 downregulated), suggesting that once the primary defense program is established during Phase I, Phase II involves minimal new transcriptional activation. These late-phase genes may reflect long-term defense adaptation, secondary signaling cascades, or compensatory processes that follow the initial immune response (
Table 2,
Figure 2).
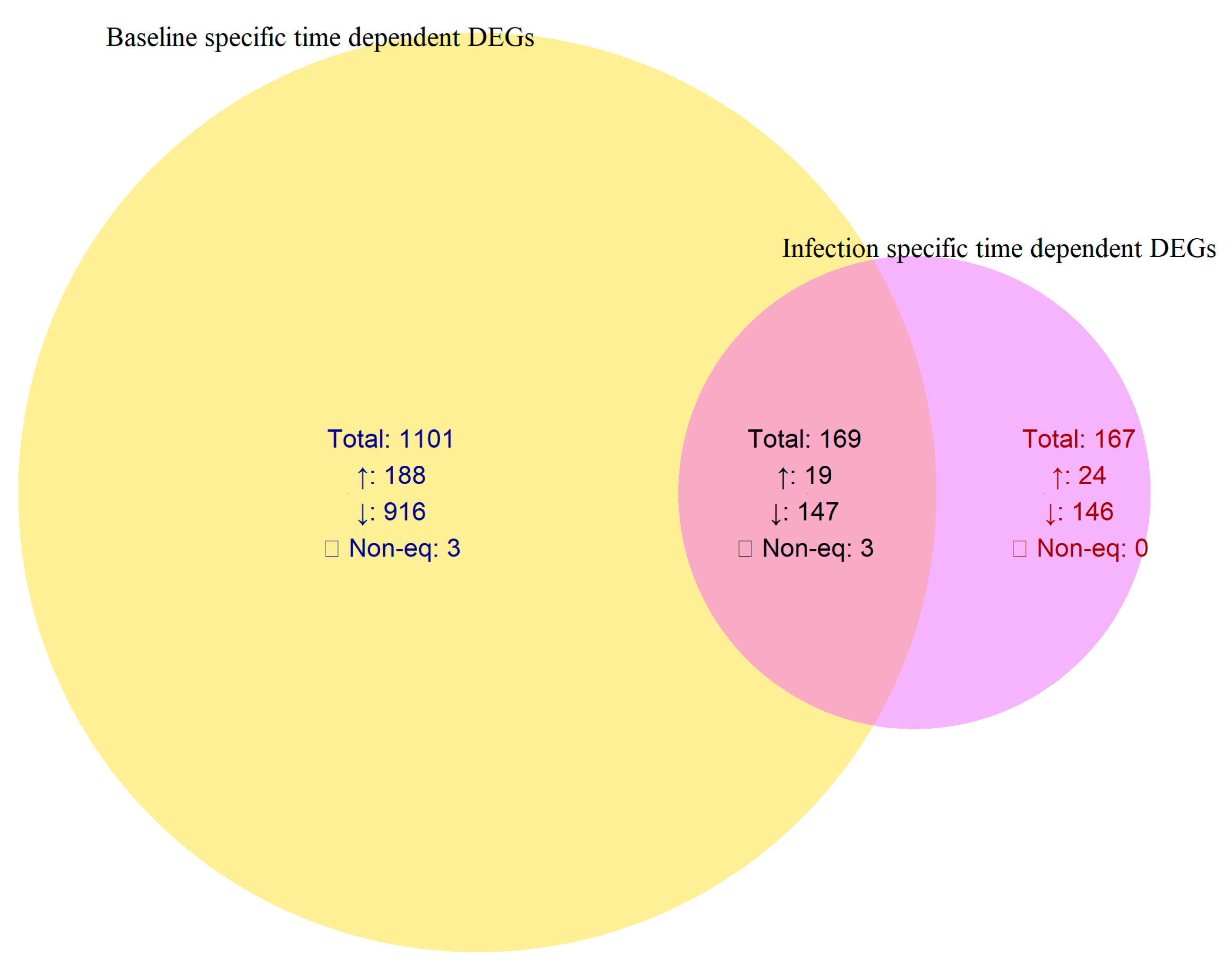
In comparison, the control samples exhibited a broader set of temporally regulated genes (1101 DEGs; 188 upregulated and 916 downregulated), while infection induced a smaller but distinct subset (167 DEGs; 24 upregulated and 146 downregulated). These infection-specific temporal DEGs represent genes modulated exclusively by pathogen rather than normal developmental transitions (
Table 2,
Figure 3).
Overall, these findings underscore a phase-resolved transcriptional landscape in which V. vinifera mounts a strong early transcriptional response upon infection, maintains a subset of core responses into later phases, and undergoes a distinct temporal shift, especially widespread downregulation, unique to infected plants. This pattern reflects a tightly regulated balance between growth, energy use, and immune preparedness throughout disease progression.
2.3. Gene Ontology (GO) Enrichment Analysis
Gene Ontology (GO) enrichment analysis was performed using DAVID Bioinformatics Resources v6.8 [
10] to identify enriched molecular functions (MFs), biological process (BPs), and cellular component (CC) terms among the DEGs (
Supplementary Material I-v). GO term annotation and classification were based on the GO database, and only terms with a
p-value < 0.05 were considered significantly enriched [
11,
12].
In MF enrichment, upregulated DEGs were primarily associated with ATP binding, suggesting that many DEGs are involved in energy-dependent biochemical processes. The enrichment of protein kinase activity indicates active involvement of phosphorylation-mediated signaling pathways in cellular responses, while ATPase-coupled transmembrane transporter activity highlights the role of ATP-driven transporters in maintaining ion and metabolite homeostasis across membranes. Collectively, these findings denote that energy metabolism, signal transduction, and transport-associated molecular functions are key mechanisms modulated during
X. fastidiosa infection. Among downregulated DEGs, enriched MF terms included serine-type endopeptidase activity and transmembrane transporter activity (
Table 3), reflecting reduced proteolytic and transport functions during disease progression (
Supplementary Material I-v).
In BP enrichment, upregulated DEGs were predominantly involved in immune response, hormone-mediated pathways, signal transduction, defense response, lipid metabolism, and regulation of systemic acquired resistance (
Table 4). No significantly enriched BP terms were detected among downregulated DEGs (
Supplementary Material I-v).
For CC enrichment, upregulated DEGs were mainly localized to the plasma membrane and other membrane-associated compartments, underscoring the role of membrane-bound proteins in stress signaling and pathogen perception (
Table 5). No significantly enriched CC terms were detected among downregulated DEGs (
Supplementary Material I-v).
2.4. STRING Protein–Protein Interaction (PPI) Network and Hub-Gene Identification
To examine the functional relationships among DEGs in
V. vinifera during
X. fastidiosa infection, a PPI network was constructed using the STRING database [
13,
14] via the stringApp plugin in Cytoscape [
14]. A combined score threshold of 0.40 was selected to balance interaction coverage and reliability, given the limited experimentally validated data available for
V. vinifera in STRING. Networks generated with higher confidence thresholds (>0.70) became excessively fragmented. After construction, isolated nodes (degree = 0) and small disconnected subnetworks (<3 nodes) were removed to retain the main connected network. The resulting network exhibited sufficient density for robust topological and clustering analyses using CytoHubba (version 0.1) and MCODE (version 2.2.0).
The initial STRING analysis using DEGs from Phases I and II infected samples identified approximately 1126 nodes and 981 edges (
Supplementary Material II-i). After removing disconnected and isolated nodes, the final core network comprised 413 nodes and 921 edges. Topological analysis revealed a clustering coefficient of 0.295, network diameter of 16, characteristic path length of 5.76, network density of 0.011, and an average of 4.46 neighbors per node (
Figure 4). These parameters suggest a moderately dense network characterized by a highly interconnected central core and several loosely associated peripheral modules. The core region exhibited strong interconnectivity among genes involved in ribosomal function, defense signaling, oxidative stress response, and secondary metabolism—key biological processes activated during pathogen invasion—whereas the peripheral clusters represented stress-specific or phase-dependent responses to
X. fastidiosa infection.
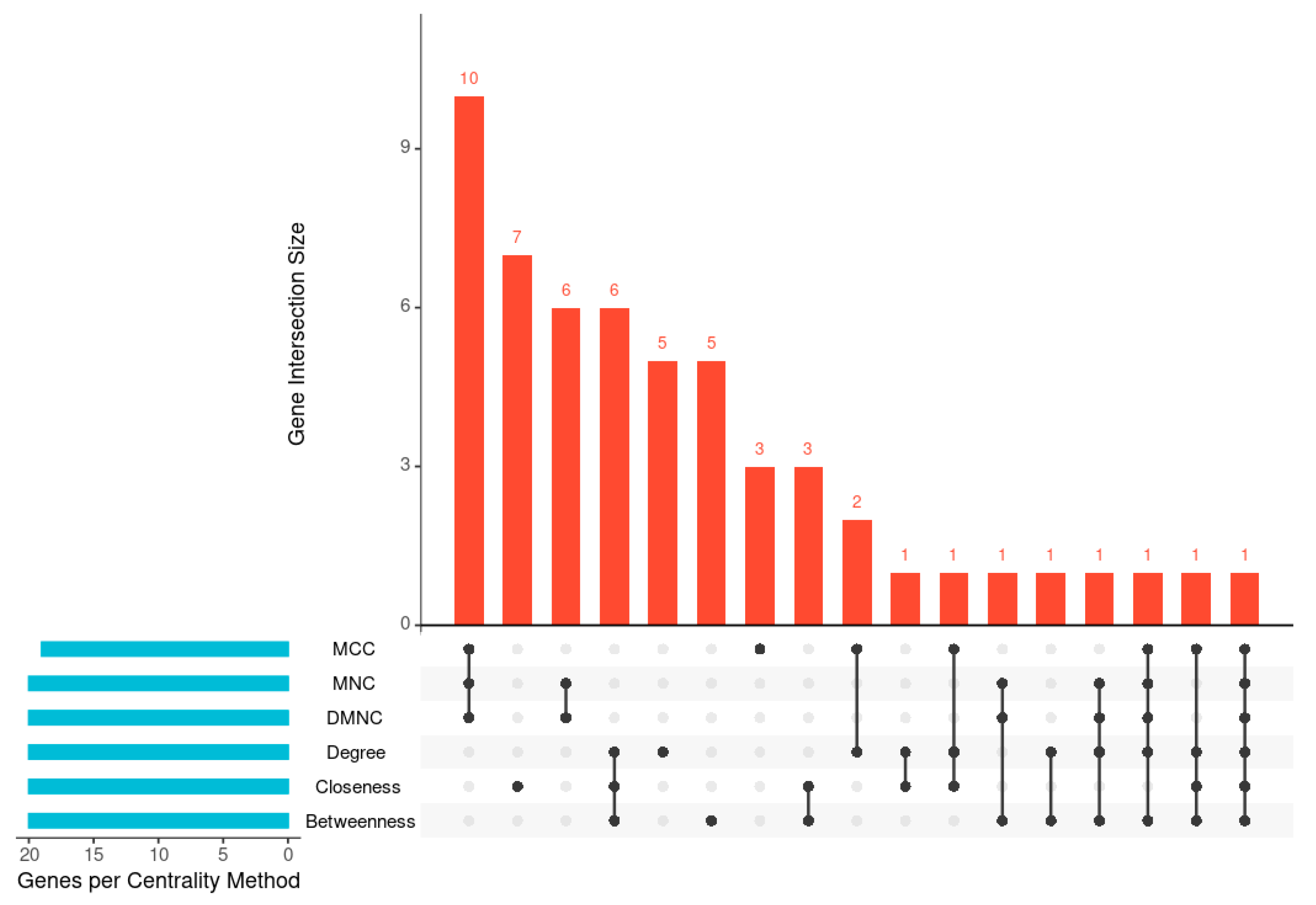
To identify key nodes within the PPI network (
Figure 4), six CytoHubba centrality algorithms (degree, betweenness, closeness, MCC, MNC, and DMNC) were applied (
Table 6). The UpSet plot (
Figure 5,
Supplementary Material II-ii) illustrates the overlap among the top 20 hub genes identified by these algorithms. One gene was shared across all six methods, while the largest intersection comprised ten genes common to three algorithms (MCC, MNC, and DMNC). To enhance analytical robustness, only genes considered by at least three independent algorithms were designated as high-confidence hub genes. This refinement yielded 21 consistently identified hubs representing biologically significant nodes within the
V. vinifera defense network (
Table 6 and
Table 7).
The top 21 identified hub genes include VIT_16s0050g02220 (chitinase), VIT_19s0014g03660 (chlorophyll a-b binding protein, chloroplastic), VIT_18s0001g14500 (endoplasmin homolog), VIT_02s0025g04250 (thaumatin-like protein), VIT_17s0000g07560 (enhanced disease susceptibility 1), VIT_07s0031g00470 (DNA polymerase), VIT_14s0066g01240 (L-aspartate oxidase), VIT_18s0001g03910 (nitrate reductase), VIT_03s0088g00810 (SCP domain-containing protein), VIT_10s0116g01650 (adenylyl-sulphate reductase (glutathione)), VIT_16s0098g01580 (luminal-binding protein 5), VIT_02s0025g04310 (thaumatin-like protein), VIT_03s0091g00160 (basic secretory protease), VIT_07s0129g00360 (peroxidase), VIT_10s0003g02890, VIT_10s0003g02900, VIT_12s0028g00320, VIT_12s0055g01110, VIT_17s0000g06350, VIT_18s0089g01170, and VIT_19s0014g00160 (all encoding chlorophyll a-b binding protein, chloroplastic).
Among the 21 identified hub genes, three genes, viz. VIT_18s0001g14500 (endoplasmin homolog), VIT_02s0025g04250 (thaumatin-like protein), and VIT_17s0000g06350 (chlorophyll a/b-binding protein, chloroplastic) were also present among the 167 genes exhibiting infection-specific temporal expression patterns common to both phases. These hub genes are functionally associated with plant defense and environmental stress responses [
15,
16], highlighting their potential roles in the adaptive response of grapevine’s to
X. fastidiosa infection.
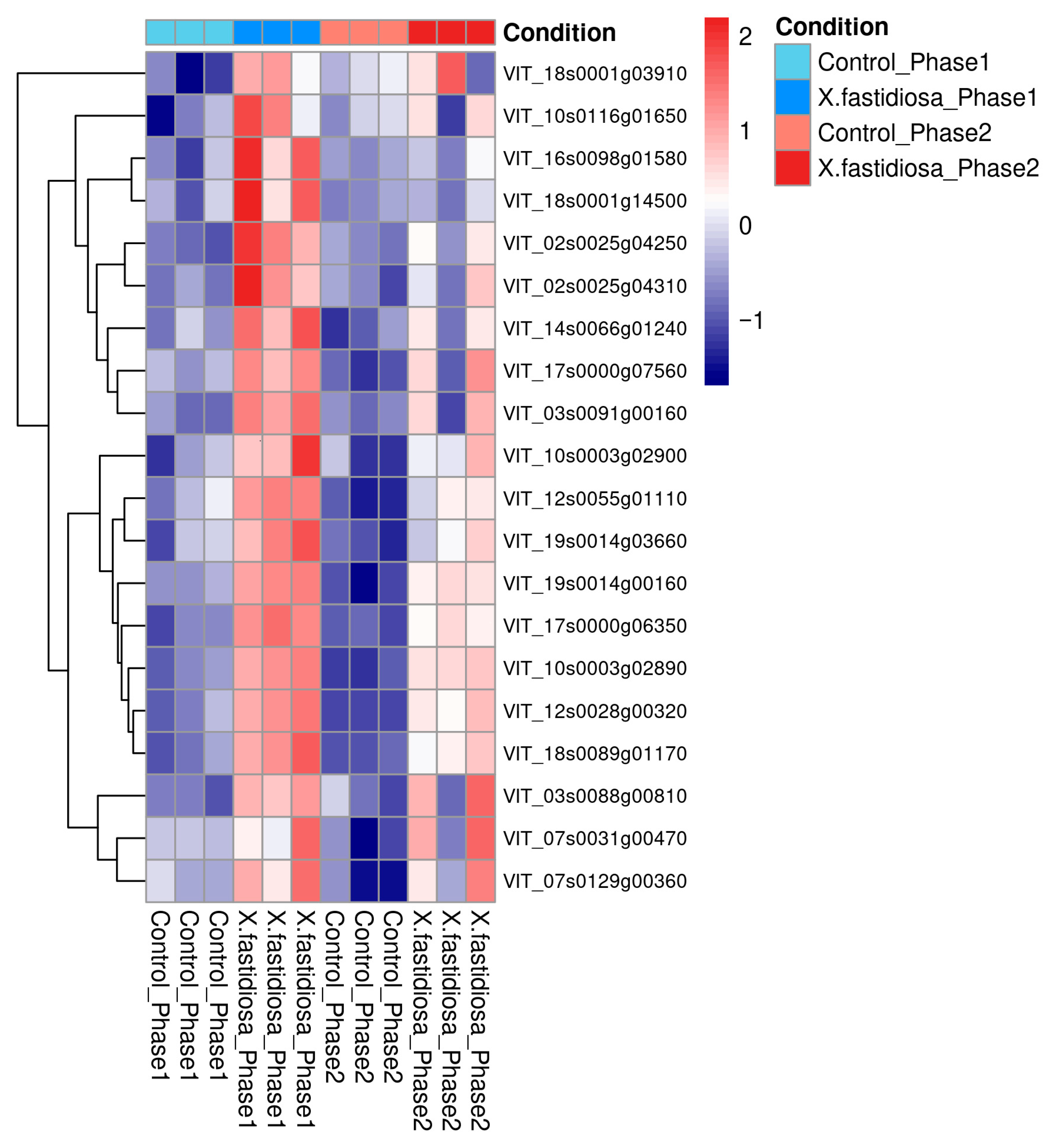
A heatmap of the 21 hub genes revealed that most were upregulated in Phases I and II relative to healthy controls, indicating their sustained involvement in the infection response (
Figure 6). Photosynthesis-related chlorophyll a/b binding proteins (CABs) appeared as hubs owing to network co-expression; however, they likely represent metabolic consequence nodes reflecting secondary effects of infection rather than direct defense regulators. Conversely, defense-related genes such as EDS1, thaumatin-like protein, chitinase, and endoplasmin constitute active components of the grapevine immune system, mediating signaling and stress adaptation. These functionally relevant genes represent promising molecular candidate genes and potential targets for understanding and managing PD in
V. vinifera (
Figure 6).
2.5. Cluster Modules Within the PPI Network
The PPI network was clustered using the MCODE algorithm with the following parameters: node score cutoff = 0.2, K-core = 2, degree cutoff = 2, and maximum depth = 100. The three most highly interconnected clusters were selected for downstream analysis (
Supplementary Material I-iv) [
17]. Within each cluster,
seed genes represent highly connected nodes automatically selected by MCODE as the initial points for cluster expansion. We also examined whether these clusters contained any of the 21 previously identified hub genes, as their co-occurrence within modules would indicate significant biological importance.
Cluster 1 exhibited a cluster score of 6.000 and contained 63 nodes with a density of 22. Within this cluster, two seed genes (VIT-18s0042g00740 and VIT-14s0068g00930) and one hub gene (VIT-03s0088g00810) were identified (
Supplementary Material II-iii). Cluster 2 showed the highest complexity, with a cluster score of 5.804, encompassing 148 nodes, and a density of 52. This cluster contained three seed genes (VIT-16s0050g01160, VIT-16s0098g01580, and VIT-04s0044g00860) and eight hub genes (VIT-16s0050g02220, VIT-18s001g14500, VIT-02s0025g04250, VIT-18s0001g03910, VIT-10s0115g01650, VIT-02s0025g04310, VIT-03s0091g00160, and VIT-07s0129g00360), among which one gene, VIT-16s0098g01580, functioned as a hub and seed node (
Supplementary Material II-iii). Cluster 3 had a cluster score of 5.120 and comprised 64 nodes with a density of 26. This cluster contained two seed genes (VIT-01s0127g00260 and VIT-13s0019g04140) and eight hub genes (VIT-19s0014h03660, VIT-10s0003g02890, VIT-10s003g02900, VIT-12s0028g00320, VIT-12s00055g01110, VIT-17s0000g06350, VIT-18s0089g01170, and VIT-19s0014g00160) (
Supplementary Material II-iii).
Functional annotation of these top-ranked clusters revealed significant enrichment in defense response, cell wall modification, and signal transduction processes, underscoring that the clustered hub genes act cooperatively to regulate multiple layers of the V. vinifera defense network in response to X. fastidiosa infection.
3. Discussion
Differential expression genes (DEGs) analysis remains a widely adopted approach for elucidating transcriptional changes across biological conditions, particularly when applied to high-throughput RNA sequencing data [
8,
18]. The study by Ingel et al. (2021) provides a foundational understanding of
Xylella fastidiosa infection in
Vitis vinifera, demonstrating that tylosis formation and starch depletion are associated with extensive transcriptional reprogramming across disease phases [
6]. Our re-analysis advances this study by introducing a four-way contrast design that distinguishes developmental from infection-specific effects and resolves phase-dependent transcriptional dynamics. This analytical framework not only confirms the early activation of ethylene-mediated defense signaling and cell-wall remodeling but also identifies key regulatory hub genes associated with late-phase metabolic decline. Integrating PPI network topology with DEG analysis advances the dataset from a descriptive transcriptomic survey to a systems-level representation of the grapevine defense network against
X. fastidiosa.
Using the GSE152164 dataset comprising 12 samples across Phases I and II (infected and control), DEG analysis was performed in DESeq2 [
19] to evaluate four pairwise contrasts: Phase I control vs. infected, Phase II control vs. infected, Phase I control vs. Phase II control, and Phase I infected vs. Phase II infected. The results are consistent with those of Ingel et al. (2021), who report 5651 DEGs, with higher induction during the early infection stage (4636 DEGs in Phase I) and fewer in the later stage (2662 DEGs in Phase II) [
6]. In our re-analysis, 1093 DEGs (986 upregulated) were detected in Phase I infected vines, indicating strong transcriptional reprogramming. In contrast, only 136 DEGs were detected in Phase II, indicating a marked decline in transcriptional activity as the disease progresses. These results remain broadly consistent with the expression patterns reported by Ingel et al. (2021) [
6]. The smaller number of DEGs identified in our analysis reflects the application of stricter statistical thresholds; while Ingel et al. [
6] employed an unadjusted
p < 0.05 without a fold-change cut off, we applied a Benjamini–Hochberg adjusted
p-value (FDR) < 0.05 and |log
2 FC| ≥ 1, producing a more conservative and biologically reliable DEG set.
Developmental variation also exerts a strong influence in transcriptomic differences. Overall, 1270 DEGs were identified between Phases I and II controls, underscoring substantial temporal reprogramming unrelated to infection [
20] (
Table 2). This observation emphasizes the importance of comparing infected and control samples within each phase to minimize developmental confounding. The transition from Phase I to II in infected vines yielded 336 DEGs (43 upregulated and 293 downregulated), signifying repression of early defense genes and broad metabolic downshift (
Table 2).
Phase-wise comparison of DEGs provides deeper insights into the temporal progression of
X. fastidiosa infection in
V. vinifera. A core set of 102 genes remained upregulated across both phases in the infected vine plants, indicating persistent defense-related activation throughout disease progression. In contrast, 991 genes were exclusively induced in Phase I, reflecting a strong but transient early response, whereas only 34 DEGs were uniquely detected in Phase II, suggesting minimal new transcriptional activation during advanced infection (
Table 2;
Figure 2). To distinguish infection-driven transcriptional changes from normal developmental variation, temporal DEGs in infected and control vine plants were directly compared. Among the 336 DEGs identified across the infected time course (Phase I → Phase II), 167 overlapped with DEGs from the control time course, indicating expression changes associated with normal vine maturation. The remaining 167 DEGs (24 upregulated and 146 downregulated) were unique to the infected vine plants and represent infection-specific temporal regulators. These genes likely mediate the transcriptional reprogramming underlying disease progression and symptom manifestation (
Table 2;
Figure 3).
Functional interpretation of these DEGs began with GO enrichment analysis, which revealed significant enrichment in defense-related molecular functions, including ATP binding, kinase activity, and transmembrane transport regulation. These results showed strong activation of signaling and energy-driven mechanisms during infection. To elucidate the broader functional context, a PPI network analysis was subsequently performed to examine how these genes integrate within grapevine defense networks.
The PPI network analysis further reinforced the centrality of early defense mechanisms in
V. vinifera during
X. fastidiosa infection. The STRING-based network, constructed using the StringApp in Cytoscape, exhibited a moderately dense topology with a highly connected core, indicating coordinated regulation among defense-associated genes. Application of six CytoHubba centrality algorithms (degree, betweenness, closeness, MCC, MNC, and DMNC) identified 21 high-confidence hub genes, including VIT_16s0050g02220 (chitinase), VIT_19s0014g03660 (chlorophyll a-b binding protein), VIT_18s0001g14500 (endoplasmin homolog), and VIT_02s0025g04250 (thaumatin-like protein). Furthermore, 3 of the 21 hub genes, VIT_18s0001g14500, VIT_02s0025g04250, and VIT_17s0000g06350, also appeared as infection-specific temporal DEGs, each previously implicated in defense responses [
20].
Some of the identified hub genes function as key regulators of plant immunity and stress adaptation. Chitinase (VIT_16s0050g02220), a classical PR-3 protein, mediates antifungal defense and signaling and has previously been associated with grapevine responses to
X. fastidiosa [
21,
22,
23]. VIT_ 02s0025g04250 (thaumatin-like protein; PR-5 family) contributes to pathogen restriction via antifungal activity and cell wall modification [
20,
24]. VIT_17s0000g07560 (EDS1) functions as a pivotal node in salicylic acid (SA)-mediated and effector-triggered immunity [
25,
26]. VIT_18s0001g14500 (endoplasmin), an ER-localized HSP90 chaperone, regulates the unfolded protein response (UPR), relieving ER stress and ensuring proper folding and secretion of defense-related glycoproteins [
27,
28]. Additional hub genes, including VIT_18s0001g03910 (nitrate reductase) and VIT_10s0116g01650 (adenylyl-sulfate reductase), participate in nitrogen and sulfur metabolism—pathways often reprogrammed under oxidative or pathogen-induced stress [
29,
30]. VIT_08s0040g02590 (glutathione S-transferase) further indicates the activation of antioxidant and detoxification mechanisms that maintain cellular redox balance during infection [
31].
A subset of chlorophyll a/b binding (CAB) protein isoforms, including (
VIT_10s0003g02890,
VIT_12s0028g00320, and related genes) also emerged among the identified hub genes. While these genes are generally downregulated during infection [
32], their prominence within the interaction network likely reflects co-expression with stress-responsive modules rather than a direct involvement in defense signaling. The CAB genes displayed a biphasic expression pattern—transient induction in Phase I followed by repression in Phase II—suggesting an early photoprotective or compensatory response that diminishes as photosynthetic inhibition becomes established [
15,
29,
33]. Thus, while CABs may act as responsive metabolic indicators, defense-related genes such as chitinase, thaumatin-like protein, and EDS1 constitute genuine functional regulators of grapevine immunity [
26,
34].
Overall, our systems-biology-based re-analysis of RNA-seq data reveals that Vitis vinifera mounts a coordinated, multi-layered defense against Xylella fastidiosa. This defense involves early activation of pathogenesis-related proteins, molecular chaperones, and antioxidant enzymes, followed by a progressive suppression of photosynthetic and primary metabolic processes in the late phase of infection. These results underscore the close interdependence between defense signaling and metabolic reprogramming, defining a transcriptional signature that characterizes PD progression in grapevine.
Limitations
Although this re-analysis provides valuable systems-level insight into the transcriptional response of
Vitis vinifera’s to
Xylella fastidiosa, certain limitations should be acknowledged. The study lacks an independent validation dataset and experimental confirmation to substantiate the identified DEGs and hub genes. Consequently, our findings are based solely on computational inference from the RNA-seq data. In addition, the dataset includes only three biological replicates per condition (Phase I control, Phase I infected, Phase II control, and Phase II infected), which limits statistical power and reduces sensitivity for detecting subtle transcriptional changes. However, the original study by Ingel et al. (2021) [
6] provides complementary experimental evidence through microscopy and physiological assays, which validates the disease-related changes in xylem structure and starch metabolism. Future studies incorporating quantitative biological replication and qualitative molecular validation of functional assays will be essential to strengthen and extend these findings.
5. Conclusions
This study provides a comprehensive systems-level perspective on the molecular response of V. vinifera to X. fastidiosa infection, emphasizing the dynamic and phase-dependent nature of its defense regulation. Transcriptomic profiling reveals a strong and coordinated early-phase activation of defense-associated pathways involved in immune signaling, oxidative stress tolerance, and pathogen recognition, reflecting the rapid attempt of the grapevine to restrict pathogen proliferation before symptom onset. As infection progresses, the marked reduction in transcriptional activity observed in the late phase likely reflects both active suppression of host gene expression and functional deterioration of xylem tissues resulting from bacterial colonization and vascular occlusion. This temporal transition indicates that V. vinifera progressively redirects metabolic resources from growth to sustained defense maintenance under continuous pathogen pressure, an adaptive but metabolically expensive trade-off that manifests as hallmark symptoms of Pierce’s disease, including chlorosis, vascular blockage, and physiological decline. These identified hub genes likely function as key regulatory nodes coordinating immune activation and stress adaptation in grapevine during X. fastidiosa infection.
Overall, this study delineates a temporally structured defense framework in V. vinifera characterized by an early surge of transcriptional activation, limited late-phase induction, and a stable core of persistently upregulated defense genes. The identification of 167 infection-specific temporal DEGs highlights promising molecular candidates for early disease detection, functional validation, and genetic improvement strategies aimed at enhancing PD resistance in grapevine. Future multi-omics investigations and cultivar-level analyses are crucial to translate these molecular insights into practical tools for disease management and grapevine breeding.