TDP-43 Regulates Rab4 Levels to Support Synaptic Vesicle Recycling and Neuromuscular Connectivity in Drosophila and Human ALS Models
Abstract
1. Introduction
2. Results
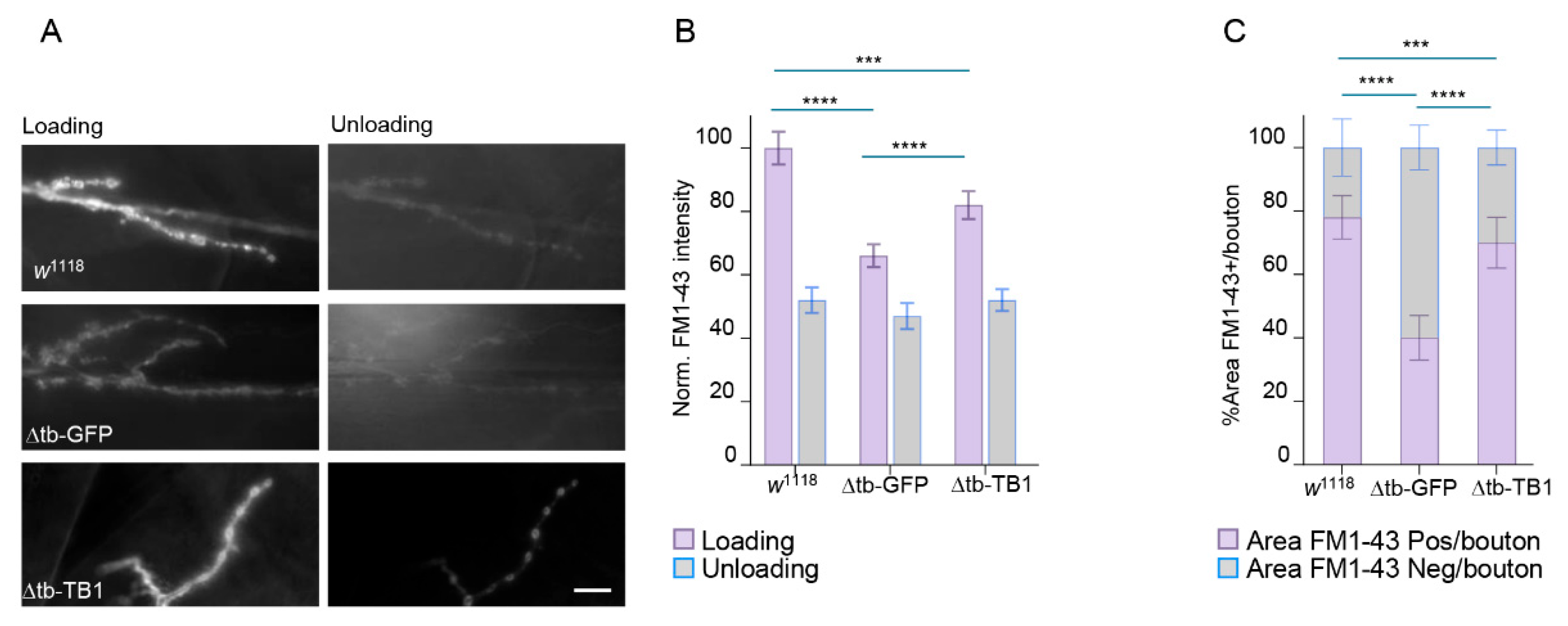
2.1. TDP-43 Controls Synaptic Vesicle Recycling at NMJs
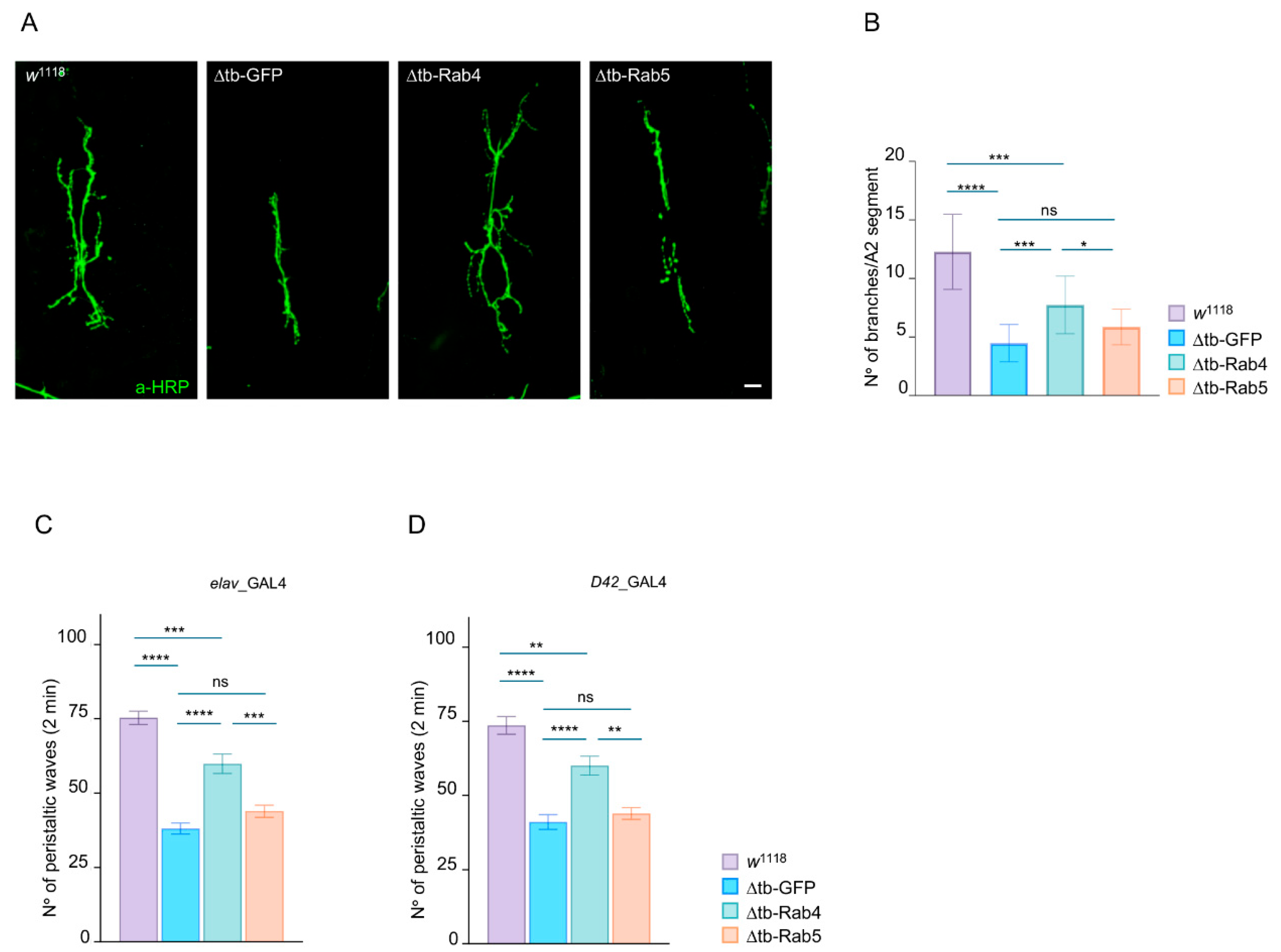
2.2. Rab4 Restores Locomotory Behaviors and Synaptic Growth in TDP-43-Null Flies
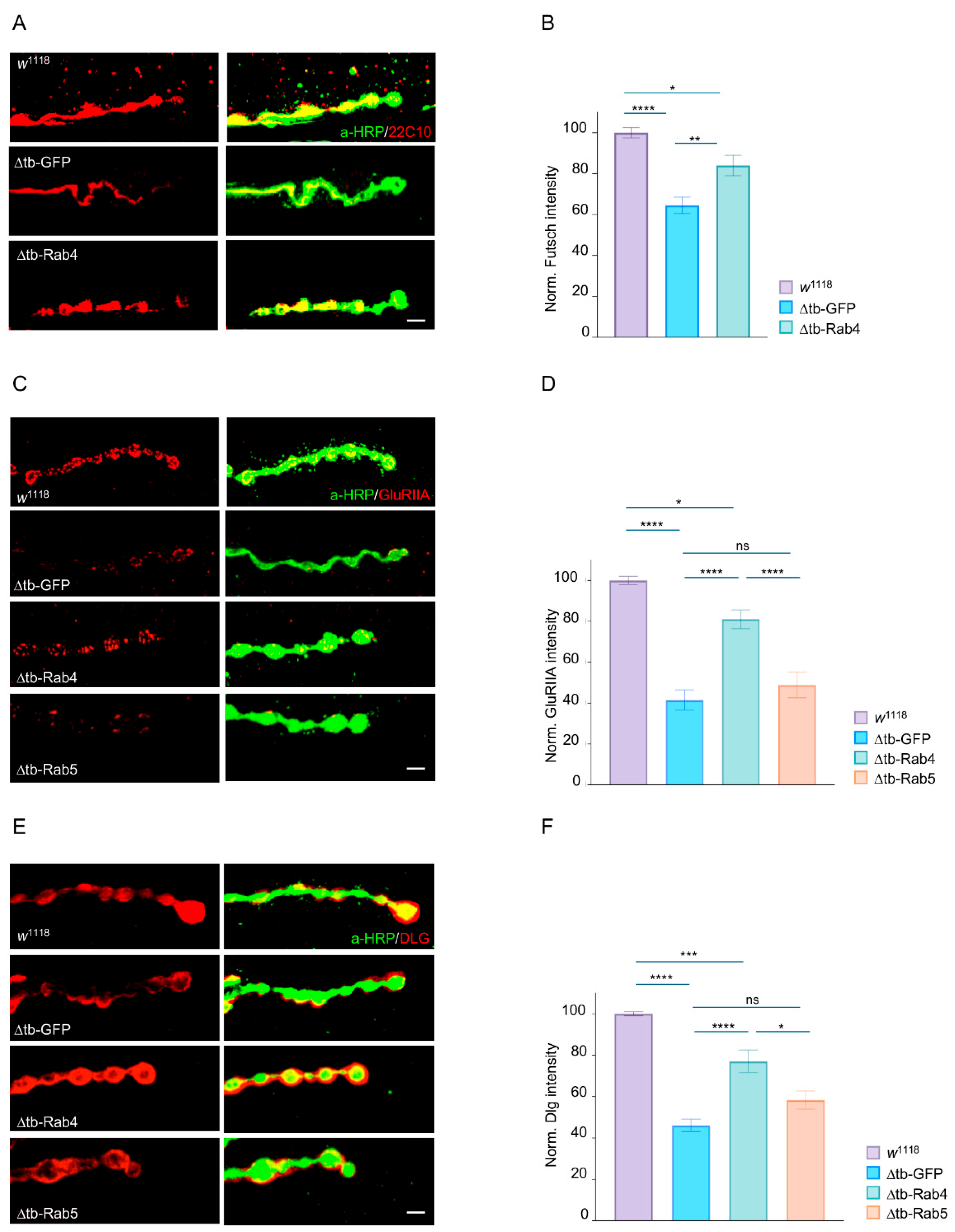
2.3. Rab4 Promotes the Maturation of Presynaptic and Postsynaptic Terminals
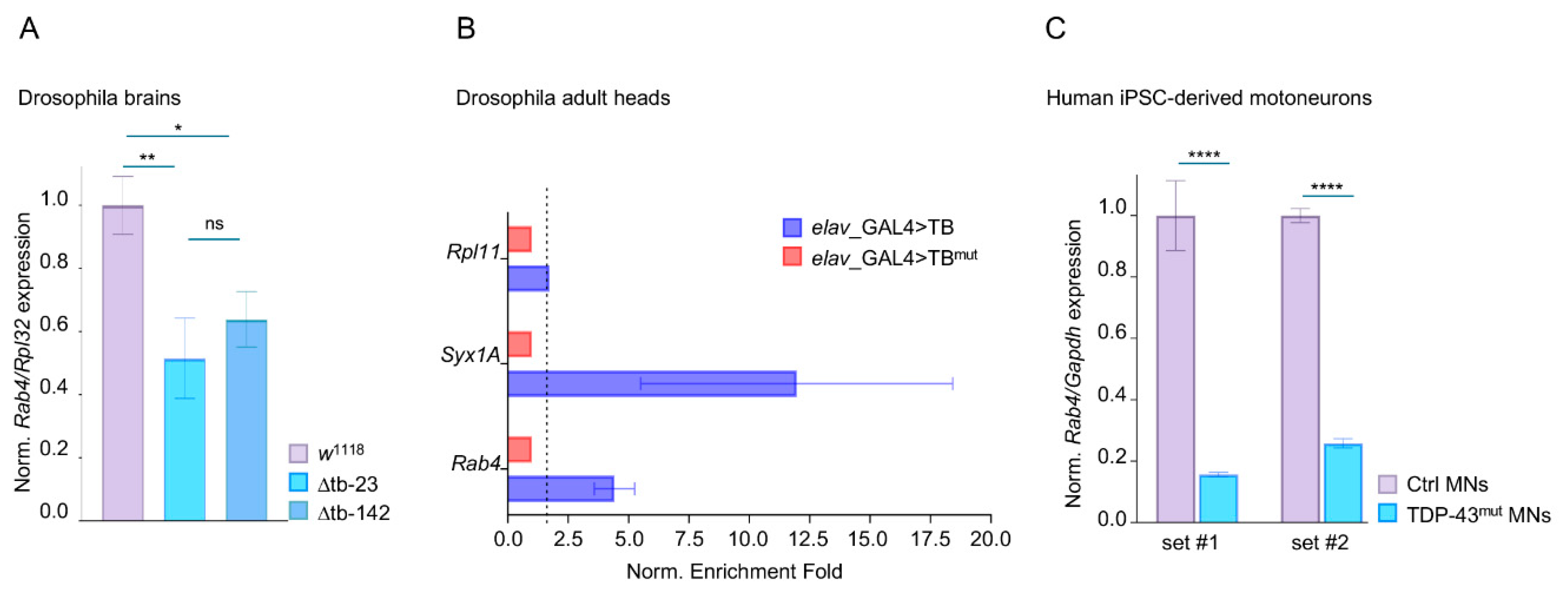
2.4. TDP-43 Binds Rab4 mRNA and Regulates Its Expression Levels in Drosophila and Human Motoneurons
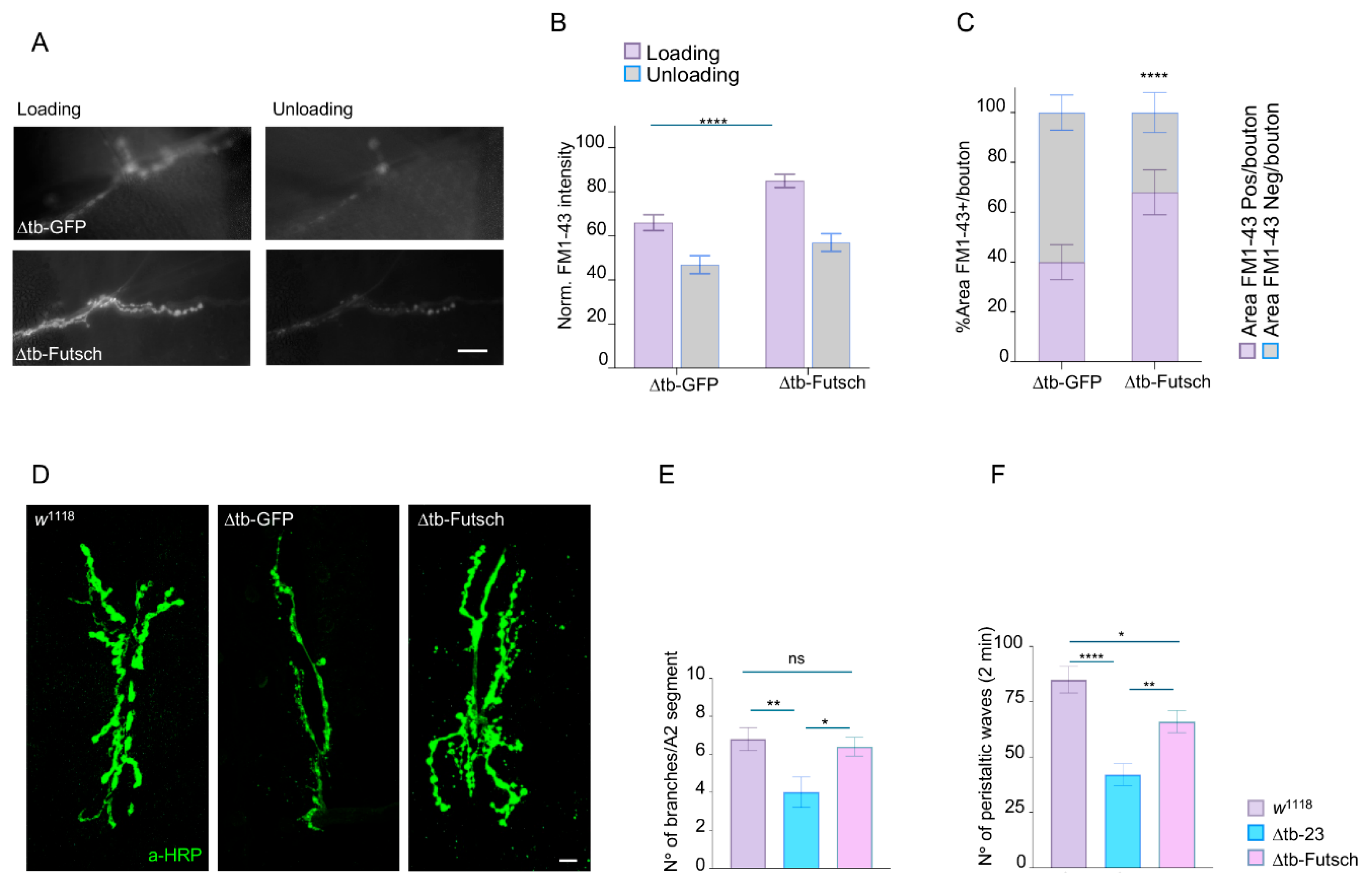
2.5. Futsch/MAP1B Expression Recovers Presynaptic Vesicle Recycling, Synaptic Growth, and Locomotor Behavior in TDP-43 Mutant Flies
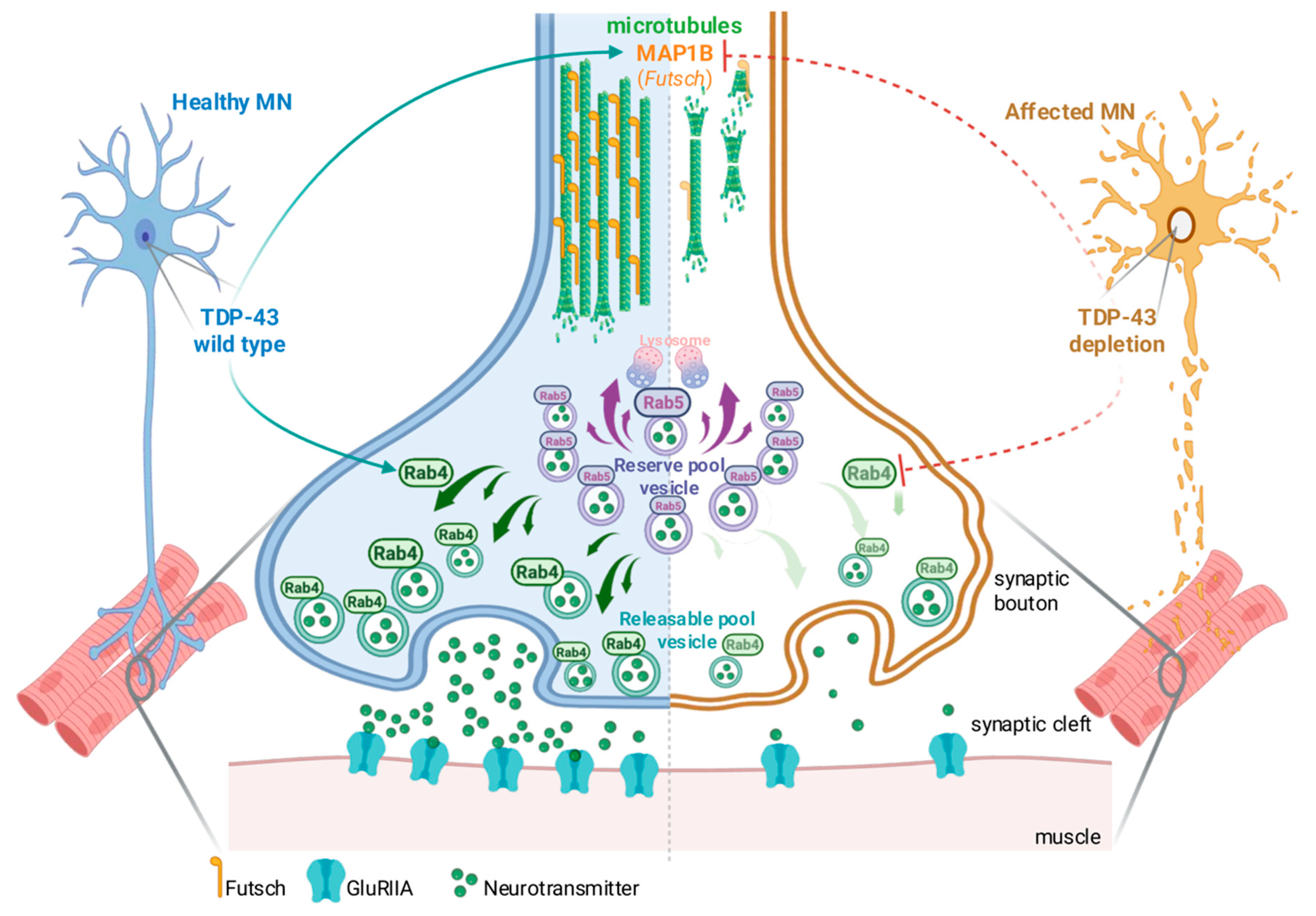
3. Discussion
3.1. TDP-43 Dysfunction and the Challenge of Transcriptomic Complexity in ALS
3.2. Rab4-Dependent Vesicle Recycling Arises as a Functional Bottleneck Regulated by TDP-43
3.3. MAP1B/futsch Links Vesicle Dynamics and Cytoskeletal Assembly Through a Self-Reinforcing Module
3.4. A Modular Response Capable of Bypassing TDP-43 Loss of Function
3.5. Implications for ALS and Neurodegenerative Disease
4. Material and Methods
4.1. Fly Strains
4.2. Fly Maintenance
4.3. Larval Movement
4.4. Climbing Assay
4.5. NMJs Characterization
4.6. FM 1-43 In Vivo Assay and Quantification
4.7. Immunohistochemistry
4.8. Quantification of Confocal Images
4.9. RNA Extraction
4.10. qRT-PCR
4.11. Immunoprecipitation for RNA Enrichment
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masrori, P.; Van Damme, P. Amyotrophic Lateral Sclerosis: A Clinical Review. Eur. J. Neurol. 2020, 27, 1918–1929. [Google Scholar] [CrossRef]
- Mead, R.J.; Shan, N.; Reiser, H.J.; Marshall, F.; Shaw, P.J. Amyotrophic Lateral Sclerosis: A Neurodegenerative Disorder Poised for Successful Therapeutic Translation. Nat. Rev. Drug Discov. 2023, 22, 185–212. [Google Scholar] [CrossRef]
- Van Damme, P.; Robberecht, W.; Van Den Bosch, L. Modelling Amyotrophic Lateral Sclerosis: Progress and Possibilities. Dis. Models Mech. 2017, 10, 537–549. [Google Scholar] [CrossRef]
- Taylor, J.P.; Brown, R.H.; Cleveland, D.W. Decoding ALS: From Genes to Mechanism. Nature 2016, 539, 197–206. [Google Scholar] [CrossRef]
- Su, W.-M.; Cheng, Y.-F.; Jiang, Z.; Duan, Q.-Q.; Yang, T.-M.; Shang, H.-F.; Chen, Y.-P. Predictors of Survival in Patients with Amyotrophic Lateral Sclerosis: A Large Meta-Analysis. EBioMedicine 2021, 74, 103732. [Google Scholar] [CrossRef] [PubMed]
- Vasta, R.; De Mattei, F.; Tafaro, S.; Canosa, A.; Manera, U.; Grassano, M.; Palumbo, F.; Cabras, S.; Matteoni, E.; Di Pede, F.; et al. Changes to Average Survival of Patients With Amyotrophic Lateral Sclerosis (1995–2018): Results From the Piemonte and Valle d’Aosta Registry. Neurology 2025, 104, e213467. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.-C.; Polymenidou, M.; Cleveland, D.W. Converging Mechanisms in ALS and FTD: Disrupted RNA and Protein Homeostasis. Neuron 2013, 79, 416–438. [Google Scholar] [CrossRef]
- Kim, G.; Gautier, O.; Tassoni-Tsuchida, E.; Ma, X.R.; Gitler, A.D. ALS Genetics: Gains, Losses, and Implications for Future Therapies. Neuron 2020, 108, 822–842. [Google Scholar] [CrossRef]
- Baughn, M.W.; Melamed, Z.; López-Erauskin, J.; Beccari, M.S.; Ling, K.; Zuberi, A.; Presa, M.; Gonzalo-Gil, E.; Maimon, R.; Vazquez-Sanchez, S.; et al. Mechanism of STMN2 Cryptic Splice-Polyadenylation and Its Correction for TDP-43 Proteinopathies. Science 2023, 379, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Baralle, M.; Buratti, E.; Baralle, F.E. The Role of TDP-43 in the Pathogenesis of ALS and FTLD. Biochem. Soc. Trans. 2013, 41, 1536–1540. [Google Scholar] [CrossRef]
- Mehta, P.R.; Brown, A.-L.; Ward, M.E.; Fratta, P. The Era of Cryptic Exons: Implications for ALS-FTD. Mol. Neurodegener. 2023, 18, 16. [Google Scholar] [CrossRef]
- Igaz, L.M.; Kwong, L.K.; Xu, Y.; Truax, A.C.; Uryu, K.; Neumann, M.; Clark, C.M.; Elman, L.B.; Miller, B.L.; Grossman, M.; et al. Enrichment of C-Terminal Fragments in TAR DNA-Binding Protein-43 Cytoplasmic Inclusions in Brain but Not in Spinal Cord of Frontotemporal Lobar Degeneration and Amyotrophic Lateral Sclerosis. Am. J. Pathol. 2008, 173, 182–194. [Google Scholar] [CrossRef]
- Neumann, M.; Sampathu, D.M.; Kwong, L.K.; Truax, A.C.; Micsenyi, M.C.; Chou, T.T.; Bruce, J.; Schuck, T.; Grossman, M.; Clark, C.M.; et al. Ubiquitinated TDP-43 in Frontotemporal Lobar Degeneration and Amyotrophic Lateral Sclerosis. Science 2006, 314, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, O.G.; Chien, M.Z.Y.J.; Wlaschin, J.J.; Barattucci, S.; Harley, P.; Mattedi, F.; Mehta, P.R.; Pisliakova, M.; Ryadnov, E.; Keuss, M.J.; et al. Creation of de Novo Cryptic Splicing for ALS and FTD Precision Medicine. Science 2024, 386, 61–69. [Google Scholar] [CrossRef]
- Scialò, C.; Zhong, W.; Jagannath, S.; Wilkins, O.; Caredio, D.; Hruska-Plochan, M.; Lurati, F.; Peter, M.; De Cecco, E.; Celauro, L.; et al. Seeded Aggregation of TDP-43 Induces Its Loss of Function and Reveals Early Pathological Signatures. Neuron 2025, 113, 1614–1628.e11. [Google Scholar] [CrossRef]
- Seddighi, S.; Qi, Y.A.; Brown, A.-L.; Wilkins, O.G.; Bereda, C.; Belair, C.; Zhang, Y.-J.; Prudencio, M.; Keuss, M.J.; Khandeshi, A.; et al. Mis-Spliced Transcripts Generate de Novo Proteins in TDP-43-Related ALS/FTD. Sci. Transl. Med. 2024, 16, eadg7162. [Google Scholar] [CrossRef]
- Polymenidou, M.; Lagier-Tourenne, C.; Hutt, K.R.; Huelga, S.C.; Moran, J.; Liang, T.Y.; Ling, S.-C.; Sun, E.; Wancewicz, E.; Mazur, C.; et al. Long Pre-mRNA Depletion and RNA Missplicing Contribute to Neuronal Vulnerability from Loss of TDP-43. Nat. Neurosci. 2011, 14, 459–468. [Google Scholar] [CrossRef]
- Tollervey, J.R.; Curk, T.; Rogelj, B.; Briese, M.; Cereda, M.; Kayikci, M.; König, J.; Hortobágyi, T.; Nishimura, A.L.; Zupunski, V.; et al. Characterizing the RNA Targets and Position-Dependent Splicing Regulation by TDP-43. Nat. Neurosci. 2011, 14, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Sanelli, T.; Dib, S.; Sheps, D.; Findlater, J.; Bilbao, J.; Keith, J.; Zinman, L.; Rogaeva, E.; Robertson, J. RNA Targets of TDP-43 Identified by UV-CLIP Are Deregulated in ALS. Mol. Cell Neurosci. 2011, 47, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Hallegger, M.; Chakrabarti, A.M.; Lee, F.C.Y.; Lee, B.L.; Amalietti, A.G.; Odeh, H.M.; Copley, K.E.; Rubien, J.D.; Portz, B.; Kuret, K.; et al. TDP-43 Condensation Properties Specify Its RNA-Binding and Regulatory Repertoire. Cell 2021, 184, 4680–4696.e22. [Google Scholar] [CrossRef]
- Saloner, R.; Staffaroni, A.M.; Dammer, E.B.; Johnson, E.C.B.; Paolillo, E.W.; Wise, A.; Heuer, H.W.; Forsberg, L.K.; Lario-Lago, A.; Webb, J.D.; et al. Large-Scale Network Analysis of the Cerebrospinal Fluid Proteome Identifies Molecular Signatures of Frontotemporal Lobar Degeneration. Nat. Aging 2025, 5, 1143–1158, Correction in Nat. Aging 2025, 5, 1372. [Google Scholar] [CrossRef] [PubMed]
- Faura, J.; Heeman, B.; Pottier, C.; Baker, M.C.; DeJesus-Hernandez, M.; Küçükali, F.; Heiß, L.; Wynants, S.; Van den Broeck, M.; De Rijk, P.; et al. Analysis of the Splicing Landscape of the Frontal Cortex in FTLD-TDP Reveals Subtype Specific Patterns and Cryptic Splicing. Acta Neuropathol. 2025, 149, 59. [Google Scholar] [CrossRef]
- Romano, G.; Klima, R.; Buratti, E.; Verstreken, P.; Baralle, F.E.; Feiguin, F. Chronological Requirements of TDP-43 Function in Synaptic Organization and Locomotive Control. Neurobiol. Dis. 2014, 71, 95–109. [Google Scholar] [CrossRef]
- Godena, V.K.; Romano, G.; Romano, M.; Appocher, C.; Klima, R.; Buratti, E.; Baralle, F.E.; Feiguin, F. TDP-43 Regulates Drosophila Neuromuscular Junctions Growth by Modulating Futsch/MAP1B Levels and Synaptic Microtubules Organization. PLoS ONE 2011, 6, e17808. [Google Scholar] [CrossRef]
- Romano, G.; Holodkov, N.; Klima, R.; Feiguin, F. TDP-43 Regulates GAD1 mRNA Splicing and GABA Signaling in Drosophila CNS. Sci. Rep. 2021, 11, 18761. [Google Scholar] [CrossRef]
- Romano, G.; Holodkov, N.; Klima, R.; Grilli, F.; Guarnaccia, C.; Nizzardo, M.; Rizzo, F.; Garcia, R.; Feiguin, F. Downregulation of Glutamic Acid Decarboxylase in Drosophila TDP-43-Null Brains Provokes Paralysis by Affecting the Organization of the Neuromuscular Synapses. Sci. Rep. 2018, 8, 1809. [Google Scholar] [CrossRef]
- Strah, N.; Romano, G.; Introna, C.; Klima, R.; Marzullo, M.; Ciapponi, L.; Megighian, A.; Nizzardo, M.; Feiguin, F. TDP-43 Promotes the Formation of Neuromuscular Synapses through the Regulation of Disc-Large Expression in Drosophila Skeletal Muscles. BMC Biol. 2020, 18, 34. [Google Scholar] [CrossRef] [PubMed]
- Verstreken, P.; Ohyama, T.; Bellen, H.J. FM 1–43 Labeling of Synaptic Vesicle Pools at the Drosophila Neuromuscular Junction. Methods Mol. Biol. 2008, 440, 349–369. [Google Scholar] [CrossRef]
- Chua, C.E.L.; Tang, B.L. Role of Rab GTPases and Their Interacting Proteins in Mediating Metabolic Signalling and Regulation. Cell. Mol. Life Sci. 2015, 72, 2289–2304. [Google Scholar] [CrossRef]
- Dey, S.; Banker, G.; Ray, K. Anterograde Transport of Rab4-Associated Vesicles Regulates Synapse Organization in Drosophila. Cell Rep. 2017, 18, 2452–2463. [Google Scholar] [CrossRef] [PubMed]
- Falk, J.; Konopacki, F.A.; Zivraj, K.H.; Holt, C.E. Rab5 and Rab4 Regulate Axon Elongation in the Xenopus Visual System. J. Neurosci. 2014, 34, 373–391. [Google Scholar] [CrossRef]
- Hoogenraad, C.C.; Popa, I.; Futai, K.; Martinez-Sanchez, E.; Wulf, P.S.; van Vlijmen, T.; Dortland, B.R.; Oorschot, V.; Govers, R.; Monti, M.; et al. Neuron Specific Rab4 Effector GRASP-1 Coordinates Membrane Specialization and Maturation of Recycling Endosomes. PLoS Biol. 2010, 8, e1000283, Correction in PLoS Biol. 2010, 8, 10.1371. [Google Scholar] [CrossRef]
- Martinez-Arroyo, O.; Selma-Soriano, E.; Ortega, A.; Cortes, R.; Redon, J. Small Rab GTPases in Intracellular Vesicle Trafficking: The Case of Rab3A/Raphillin-3A Complex in the Kidney. Int. J. Mol. Sci. 2021, 22, 7679. [Google Scholar] [CrossRef]
- Sönnichsen, B.; De Renzis, S.; Nielsen, E.; Rietdorf, J.; Zerial, M. Distinct Membrane Domains on Endosomes in the Recycling Pathway Visualized by Multicolor Imaging of Rab4, Rab5, and Rab11. J. Cell Biol. 2000, 149, 901–914. [Google Scholar] [CrossRef] [PubMed]
- Stenmark, H. Rab GTPases as Coordinators of Vesicle Traffic. Nat. Rev. Mol. Cell Biol. 2009, 10, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Villarroel-Campos, D.; Henríquez, D.R.; Bodaleo, F.J.; Oguchi, M.E.; Bronfman, F.C.; Fukuda, M.; Gonzalez-Billault, C. Rab35 Functions in Axon Elongation Are Regulated by P53-Related Protein Kinase in a Mechanism That Involves Rab35 Protein Degradation and the Microtubule-Associated Protein 1B. J. Neurosci. 2016, 36, 7298–7313. [Google Scholar] [CrossRef]
- White, J.A.; Krzystek, T.J.; Hoffmar-Glennon, H.; Thant, C.; Zimmerman, K.; Iacobucci, G.; Vail, J.; Thurston, L.; Rahman, S.; Gunawardena, S. Excess Rab4 Rescues Synaptic and Behavioral Dysfunction Caused by Defective HTT-Rab4 Axonal Transport in Huntington’s Disease. Acta Neuropathol. Commun. 2020, 8, 97. [Google Scholar] [CrossRef]
- Wucherpfennig, T.; Wilsch-Bräuninger, M.; González-Gaitán, M. Role of Drosophila Rab5 during Endosomal Trafficking at the Synapse and Evoked Neurotransmitter Release. J. Cell Biol. 2003, 161, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Ayala, Y.M.; Zago, P.; D’Ambrogio, A.; Xu, Y.-F.; Petrucelli, L.; Buratti, E.; Baralle, F.E. Structural Determinants of the Cellular Localization and Shuttling of TDP-43. J. Cell Sci. 2008, 121, 3778–3785. [Google Scholar] [CrossRef]
- Lagier-Tourenne, C.; Polymenidou, M.; Cleveland, D.W. TDP-43 and FUS/TLS: Emerging Roles in RNA Processing and Neurodegeneration. Hum. Mol. Genet. 2010, 19, R46–R64. [Google Scholar] [CrossRef]
- Buratti, E. Chapter One—Functional Significance of TDP-43 Mutations in Disease. In Advances in Genetics; Friedmann, T., Dunlap, J.C., Goodwin, S.F., Eds.; Academic Press: Cambridge, MA, USA, 2015; Volume 91, pp. 1–53. [Google Scholar]
- Chia, R.; Chiò, A.; Traynor, B.J. Novel Genes Associated with Amyotrophic Lateral Sclerosis: Diagnostic and Clinical Implications. Lancet Neurol. 2018, 17, 94–102. [Google Scholar] [CrossRef]
- Todd, T.W.; Shao, W.; Yong-jie, Z.; Petrucelli, L. The Endo-Lysosomal Pathway and ALS/FTD. Trends Neurosci. 2023, 46, 1025–1041. [Google Scholar] [CrossRef]
- Schwenk, B.M.; Hartmann, H.; Serdaroglu, A.; Schludi, M.H.; Hornburg, D.; Meissner, F.; Orozco, D.; Colombo, A.; Tahirovic, S.; Michaelsen, M.; et al. TDP-43 Loss of Function Inhibits Endosomal Trafficking and Alters Trophic Signaling in Neurons. EMBO J. 2016, 35, 2350–2370. [Google Scholar] [CrossRef]
- Roos, J.; Hummel, T.; Ng, N.; Klämbt, C.; Davis, G.W. Drosophila Futsch Regulates Synaptic Microtubule Organization and Is Necessary for Synaptic Growth. Neuron 2000, 26, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Mosca, T.J.; Hong, W.; Dani, V.S.; Favaloro, V.; Luo, L. Trans-Synaptic Teneurin Signalling in Neuromuscular Synapse Organization and Target Choice. Nature 2012, 484, 237–241, Correction in Nature 2016, 637, E29. [Google Scholar] [CrossRef] [PubMed]
- Ball, R.W.; Peled, E.S.; Guerrero, G.; Isacoff, E.Y. BMP Signaling and Microtubule Organization Regulate Synaptic Strength. Neuroscience 2015, 291, 155–166. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shi, Q.; Lin, Y.Q.; Saliba, A.; Xie, J.; Neely, G.G.; Banerjee, S. Tubulin Polymerization Promoting Protein, Ringmaker, and MAP1B Homolog Futsch Coordinate Microtubule Organization and Synaptic Growth. Front. Cell. Neurosci. 2019, 13, 192. [Google Scholar] [CrossRef]
- Hirokawa, N. Microtubule Organization and Dynamics Dependent on Microtubule-Associated Proteins. Curr. Opin. Cell Biol. 1994, 6, 74–81. [Google Scholar] [CrossRef]
- Chen, L.; Stone, M.C.; Tao, J.; Rolls, M.M. Axon Injury and Stress Trigger a Microtubule-Based Neuroprotective Pathway. Proc. Natl. Acad. Sci. USA 2012, 109, 11842–11847. [Google Scholar] [CrossRef]
- DiTella, M.C.; Feiguin, F.; Carri, N.; Kosik, K.S.; Cáceres, A. MAP-1B/TAU Functional Redundancy during Laminin-Enhanced Axonal Growth. J. Cell Sci. 1996, 109, 467–477. [Google Scholar] [CrossRef]
- Mann, J.R.; Donnelly, C.J. RNA Modulates Physiological and Neuropathological Protein Phase Transitions. Neuron 2021, 109, 2663–2681. [Google Scholar] [CrossRef]
- Feiguin, F.; Godena, V.K.; Romano, G.; D’Ambrogio, A.; Klima, R.; Baralle, F.E. Depletion of TDP-43 Affects Drosophila Motoneurons Terminal Synapsis and Locomotive Behavior. FEBS Lett. 2009, 583, 1586–1592. [Google Scholar] [CrossRef]
- Jan, L.Y.; Jan, Y.N. Antibodies to Horseradish Peroxidase as Specific Neuronal Markers in Drosophila and in Grasshopper Embryos. Proc. Natl. Acad. Sci. USA 1982, 79, 2700–2704. [Google Scholar] [CrossRef]
- Romano, G.; Appocher, C.; Scorzeto, M.; Klima, R.; Baralle, F.E.; Megighian, A.; Feiguin, F. Glial TDP-43 Regulates Axon Wrapping, GluRIIA Clustering and Fly Motility by Autonomous and Non-Autonomous Mechanisms. Hum. Mol. Genet. 2015, 24, 6134–6145. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Verstreken, P.; Ohyama, T.; Haueter, C.; Habets, R.L.P.; Lin, Y.Q.; Swan, L.E.; Ly, C.V.; Venken, K.J.T.; De Camilli, P.; Bellen, H.J. Tweek, an Evolutionarily Conserved Protein, Is Required for Synaptic Vesicle Recycling. Neuron 2009, 63, 203–215. [Google Scholar] [CrossRef]
- Guichet, A.; Wucherpfennig, T.; Dudu, V.; Etter, S.; Wilsch-Bräuniger, M.; Hellwig, A.; González-Gaitán, M.; Huttner, W.B.; Schmidt, A.A. Essential Role of Endophilin A in Synaptic Vesicle Budding at the Drosophila Neuromuscular Junction. EMBO J. 2002, 21, 1661–1672. [Google Scholar] [CrossRef]
- Klima, R.; Romano, G.; Gbadamosi, M.; Megighian, A.; Feiguin, F. Immuno-Electrophysiology on Neuromuscular Junctions of Drosophila Third Instar Larva. Bio Protoc. 2021, 11, e3913. [Google Scholar] [CrossRef] [PubMed]
- Thomas, U.; Kim, E.; Kuhlendahl, S.; Koh, Y.H.; Gundelfinger, E.D.; Sheng, M.; Garner, C.C.; Budnik, V. Synaptic Clustering of the Cell Adhesion Molecule Fasciclin II by Discs-Large and Its Role in the Regulation of Presynaptic Structure. Neuron 1997, 19, 787–799. [Google Scholar] [CrossRef]
- Romano, G.; Klima, R.; Feiguin, F. Immunoprecipitation for Protein-Protein Interactions and for RNA Enrichment in Drosophila Melanogaster. BIO-Protoc. 2021, 11, e4250. [Google Scholar] [CrossRef] [PubMed]
| Target | Species | Forward | Reverse |
|---|---|---|---|
| Rab4 (Figure 4A) | D. mel | 5′-CTGAATGATGCTCGCACTCT-3′ | 5′-AAGGTGCTTGCTTCCAGAAA-3′ |
| Rab4 (Figure 4B) | D. mel | 5′-GGCAGCGGTAAGAGTTGTCT-3′ | 5′-GAATCTCTCCTGACCGGCTG-3′ |
| Syx1A | D. mel | 5′-TGTTCACGCAGGGCATCATC-3′ | 5′-GCCGTCTGCACATAGTCCATAG-3′ |
| Rpl11 | D. mel | 5′-CCATCGGTATCTATGGTCTGGA-3′ | 5′-CATCGTATTTCTGCTGGAACCA-3′ |
| Rpl32 | D. mel | 5′-AAGCGGCGACGCACTCTGTT-3′ | 5′-GCCCAGCATACAGGCCCAAG-3′ |
| Rab4 | human | 5′-CAGAAAGAATGGGCTCAGGT-3′ | 5′- TGCTCTCCTAACAACCACACT-3′ |
| Gapdh | human | 5′-GTCCACTGGCGTCTTCAC-3′ | 5′-AGGCATTGCTGATGATCTTGA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gbadamosi, M.; Romano, G.; Simbula, M.; Canarutto, G.; Ottoboni, L.; Corti, S.; Feiguin, F. TDP-43 Regulates Rab4 Levels to Support Synaptic Vesicle Recycling and Neuromuscular Connectivity in Drosophila and Human ALS Models. Int. J. Mol. Sci. 2025, 26, 11030. https://doi.org/10.3390/ijms262211030
Gbadamosi M, Romano G, Simbula M, Canarutto G, Ottoboni L, Corti S, Feiguin F. TDP-43 Regulates Rab4 Levels to Support Synaptic Vesicle Recycling and Neuromuscular Connectivity in Drosophila and Human ALS Models. International Journal of Molecular Sciences. 2025; 26(22):11030. https://doi.org/10.3390/ijms262211030
Chicago/Turabian StyleGbadamosi, Monsurat, Giulia Romano, Michela Simbula, Giulia Canarutto, Linda Ottoboni, Stefania Corti, and Fabian Feiguin. 2025. "TDP-43 Regulates Rab4 Levels to Support Synaptic Vesicle Recycling and Neuromuscular Connectivity in Drosophila and Human ALS Models" International Journal of Molecular Sciences 26, no. 22: 11030. https://doi.org/10.3390/ijms262211030
APA StyleGbadamosi, M., Romano, G., Simbula, M., Canarutto, G., Ottoboni, L., Corti, S., & Feiguin, F. (2025). TDP-43 Regulates Rab4 Levels to Support Synaptic Vesicle Recycling and Neuromuscular Connectivity in Drosophila and Human ALS Models. International Journal of Molecular Sciences, 26(22), 11030. https://doi.org/10.3390/ijms262211030







