Cardiac Involvement in Myotonic Dystrophy Type 1: Mechanisms, Clinical Perspectives, and Emerging Therapeutic Strategies
Abstract
1. Introduction
2. Cardiac Clinical Phenotype
2.1. Cardiac Involvement in the Congenital Form of DM1
2.2. Cardiac Involvement in the Classical Form of DM1
2.3. Cardiac Involvement in Mild or Late-Onset DM1
2.4. Structural Impairments
2.5. Cardiac Autonomic Nervous System Imbalance
3. Pathophysiology and Role of Ion Channels in DM1 Cardiac Abnormalities
3.1. Sodium Channel Dysfunction
3.2. Calcium Channel Dysfunction
3.3. Potassium Channel Dysfunction
4. Indirect Effects on Cardiac Dysfunction
4.1. Type 2 Diabetes Mellitus
4.2. Respiratory Dysfunction
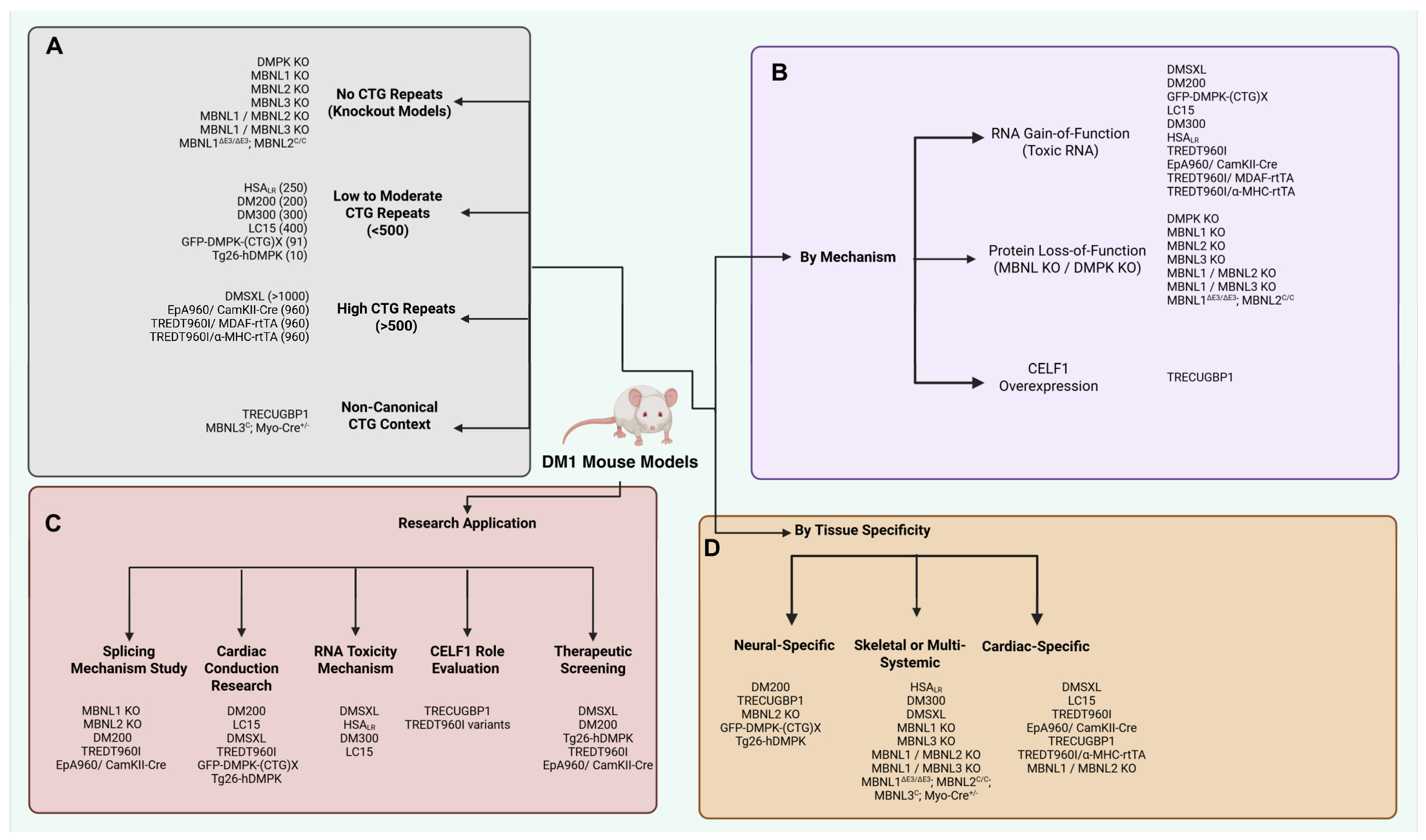
5. Animal Models and In Vitro Studies of DM1
5.1. The DMSXL Mouse Model
5.2. LC15 Mouse Model
5.3. EpA960 Mouse Model
5.4. Tetracycline-Inducible CUG960
5.5. DMPK Knockout Mouse Model
5.6. In Vitro Studies
6. Therapeutic Pipeline
6.1. siRNA and anti-miRNA Molecules
6.2. Antisense Oligonucleotides
6.3. CRISPR-Cas9 Gene-Editing
6.4. Small-Molecule Therapies
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brook, J.D.; McCurrach, M.E.; Harley, H.G.; Buckler, A.J.; Church, D.; Aburatani, H.; Hunter, K.; Stanton, V.P.; Thirion, J.-P.; Hudson, T.; et al. Molecular basis of myotonic dystrophy: Expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell 1992, 68, 799–808, Erratum in Cell, 1992, 69, 385. https://doi.org/10.1016/0092-8674(92)90418-c. [Google Scholar] [CrossRef]
- De Antonio, M.; Dogan, C.; Hamroun, D.; Mati, M.; Zerrouki, S.; Eymard, B.; Katsahian, S.; Bassez, G. Unravelling the myotonic dystrophy type 1 clinical spectrum: A systematic registry-based study with implications for disease classification. Rev. Neurol. 2016, 172, 572–580. [Google Scholar] [CrossRef]
- Wenninger, S.; Montagnese, F.; Schoser, B. Core Clinical Phenotypes in Myotonic Dystrophies. Front. Neurol. 2018, 9, 303. [Google Scholar] [CrossRef]
- Meola, G.; Cardani, R. Myotonic dystrophies: An update on clinical aspects, genetic, pathology, and molecular pathomechanisms. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2015, 1852, 594–606. [Google Scholar] [CrossRef] [PubMed]
- Ashizawa, T.; Epstein, H.F. Ethnic distribution of myotonic dystrophy gene. Lancet 1991, 338, 642–643. [Google Scholar] [CrossRef]
- Liao, Q.; Zhang, Y.; He, J.; Huang, K. Global Prevalence of Myotonic Dystrophy: An Updated Systematic Review and Meta-Analysis. Neuroepidemiology 2022, 56, 163–173. [Google Scholar] [CrossRef]
- Yotova, V.; Labuda, D.; Zietkiewicz, E.; Gehl, D.; Lovell, A.; Lefebvre, J.-F.; Bourgeois, S.; Lemieux-Blanchard, É.; Labuda, M.; Vézina, H.; et al. Anatomy of a founder effect: Myotonic dystrophy in Northeastern Quebec. Hum. Genet. 2005, 117, 177–187. [Google Scholar] [CrossRef]
- Han, J.Y.; Jang, W.; Park, J. Intergenerational Influence of Gender and the DM1 Phenotype of the Transmitting Parent in Korean Myotonic Dystrophy Type 1. Genes 2022, 13, 1465. [Google Scholar] [CrossRef] [PubMed]
- Zeesman, S.; Carson, N.; Whelan, D.T. Paternal transmission of the congenital form of myotonic dystrophy type 1: A new case and review of the literature. Am. J. Med. Genet. 2002, 107, 222–226. [Google Scholar] [CrossRef]
- Hiromasa, S.; Ikeda, T.; Kubota, K.; Hattori, N.; Coto, H.; Maldonado, C.; Kupersmith, J. Ventricular tachycardia and sudden death in myotonic dystrophy. Am. Heart J. 1988, 115, 914–915. [Google Scholar] [CrossRef] [PubMed]
- Mankodi, A.; Thornton, C.A. Myotonic syndromes. Curr. Opin. Neurol. 2002, 15, 545–552. [Google Scholar] [CrossRef]
- Itoh, H.; Tamura, T. Cardiac Involvements in Myotonic Dystrophy. In Myotonic Dystrophy: Disease Mechanism, Current Management and Therapeutic Development; Takahashi, M.P., Matsumura, T., Eds.; Springer: Singapore, 2018; pp. 63–76. ISBN 978-981-13-0508-5. [Google Scholar]
- Bienias, P.; Łusakowska, A.; Ciurzyński, M.; Rymarczyk, Z.; Irzyk, K.; Kurnicka, K.; Kamińska, A.; Pruszczyk, P. Supraventricular and Ventricular Arrhythmias Are Related to the Type of Myotonic Dystrophy but Not to Disease Duration or Neurological Status. Pacing Clin. Electrophysiol. 2016, 39, 959–968. [Google Scholar] [CrossRef]
- McBride, D.; Deshmukh, A.; Shore, S.; Elafros, M.A.; Liang, J.J. Cardiac Involvement and Arrhythmias Associated with Myotonic Dystrophy. RCM 2022, 23, 126. [Google Scholar] [CrossRef]
- Groh, W.J.; Groh, M.R.; Saha, C.; Kincaid, J.C.; Simmons, Z.; Ciafaloni, E.; Pourmand, R.; Otten, R.F.; Bhakta, D.; Nair, G.V.; et al. Electrocardiographic abnormalities and sudden death in myotonic dystrophy type 1. N. Engl. J. Med. 2008, 358, 2688–2697. [Google Scholar] [CrossRef]
- Christensen, A.H.; Bundgaard, H.; Schwartz, M.; Hansen, S.H.; Svendsen, J.H. Cardiac myotonic dystrophy mimicking arrhythmogenic right ventricular cardiomyopathy in a young sudden cardiac death victim. Circ. Arrhythm. Electrophysiol. 2008, 1, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Petri, H.; Mohammad, B.J.Y.; Kristensen, A.T.; Thune, J.J.; Vissing, J.; Køber, L.; Witting, N.; Bundgaard, H.; Christensen, A.H. Natural history of cardiac involvement in myotonic dystrophy type 1—Emphasis on the need for lifelong follow-up. Int. J. Cardiol. 2024, 406, 132070. [Google Scholar] [CrossRef]
- Bhakta, D.; Shen, C.; Kron, J.; Epstein, A.E.; Pascuzzi, R.M.; Groh, W.J. Pacemaker and implantable cardioverter-defibrillator use in a US myotonic dystrophy type 1 population. J. Cardiovasc. Electrophysiol. 2011, 22, 1369–1375. [Google Scholar] [CrossRef]
- Nigro, G.; Papa, A.A.; Politano, L. The heart and cardiac pacing in Steinert disease. Acta Myol. 2012, 31, 110–116. [Google Scholar] [PubMed]
- Hawkins, A.M.; Hawkins, C.L.; Abdul Razak, K.; Khoo, T.K.; Tran, K.; Jackson, R.V. Respiratory dysfunction in myotonic dystrophy type 1: A systematic review. Neuromuscul. Disord. 2019, 29, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Zhao, L.; Jin, P. A case of early onset diabetes with myotonic dystrophy type 1. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2023, 48, 930–934. [Google Scholar] [CrossRef]
- Gutiérrez Gutiérrez, G.; Díaz-Manera, J.; Almendrote, M.; Azriel, S.; Eulalio Bárcena, J.; Cabezudo García, P.; Camacho Salas, A.; Casanova Rodríguez, C.; Cobo, A.M.; Díaz Guardiola, P.; et al. Clinical guide for the diagnosis and follow-up of myotonic dystrophy type 1, MD1 or Steinert’s disease. Neurol. (Engl. Ed.) 2020, 35, 185–206. [Google Scholar] [CrossRef]
- Kaminsky, P.; Brembilla-Perrot, B.; Pruna, L.; Poussel, M.; Chenuel, B. Age, conduction defects and restrictive lung disease independently predict cardiac events and death in myotonic dystrophy. Int. J. Cardiol. 2013, 162, 172–178. [Google Scholar] [CrossRef]
- Chong-Nguyen, C.; Wahbi, K.; Algalarrondo, V.; Bécane, H.M.; Radvanyi-Hoffman, H.; Arnaud, P.; Furling, D.; Lazarus, A.; Bassez, G.; Béhin, A.; et al. Association Between Mutation Size and Cardiac Involvement in Myotonic Dystrophy Type 1. Circ. Cardiovasc. Genet. 2017, 10, e001526. [Google Scholar] [CrossRef]
- Gossios, T.D.; Providencia, R.; Creta, A.; Segal, O.R.; Nikolenko, N.; Turner, C.; Lopes, L.R.; Wahbi, K.; Savvatis, K. An overview of heart rhythm disorders and management in myotonic dystrophy type 1. Heart Rhythm. 2022, 19, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Hawley, R.J.; Milner, M.R.; Gottdiener, J.S.; Cohen, A. Myotonic heart disease: A clinical follow-up. Neurology 1991, 41, 259–262. [Google Scholar] [CrossRef] [PubMed]
- McNally, E.M.; Mann, D.L.; Pinto, Y.; Bhakta, D.; Tomaselli, G.; Nazarian, S.; Groh, W.J.; Tamura, T.; Duboc, D.; Itoh, H.; et al. Clinical Care Recommendations for Cardiologists Treating Adults With Myotonic Dystrophy. J. Am. Heart Assoc. 2020, 9, e014006. [Google Scholar] [CrossRef]
- Wahbi, K.; Sebag, F.A.; Lellouche, N.; Lazarus, A.; Bécane, H.-M.; Bassez, G.; Stojkovic, T.; Fayssoil, A.; Laforêt, P.; Béhin, A.; et al. Atrial flutter in myotonic dystrophy type 1: Patient characteristics and clinical outcome. Neuromuscul. Disord. 2016, 26, 227–233. [Google Scholar] [CrossRef]
- Brembilla-Perrot, B.; Schwartz, J.; Huttin, O.; Frikha, Z.; Sellal, J.M.; Sadoul, N.; Blangy, H.; Olivier, A.; Louis, S.; Kaminsky, P. Atrial flutter or fibrillation is the most frequent and life-threatening arrhythmia in myotonic dystrophy. Pacing Clin. Electrophysiol. 2014, 37, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Stoyanov, N.; Winterfield, J.; Varma, N.; Gollob, M.H. Atrial arrhythmias in the young: Early onset atrial arrhythmias preceding a diagnosis of a primary muscular dystrophy. Europace 2014, 16, 1814–1820. [Google Scholar] [CrossRef]
- Russo, V.; Sperlongano, S.; Gallinoro, E.; Rago, A.; Papa, A.A.; Golino, P.; Politano, L.; Nazarian, S.; Nigro, G. Prevalence of Left Ventricular Systolic Dysfunction in Myotonic Dystrophy Type 1: A Systematic Review. J. Card. Fail. 2020, 26, 849–856. [Google Scholar] [CrossRef]
- Petri, H.; Vissing, J.; Witting, N.; Bundgaard, H.; Køber, L. Cardiac manifestations of myotonic dystrophy type 1. Int. J. Cardiol. 2012, 160, 82–88. [Google Scholar] [CrossRef]
- Brunet Garcia, L.; Hajra, A.; Field, E.; Wacher, J.; Walsh, H.; Norrish, G.; Manzur, A.; Muntoni, F.; Munot, P.; Robb, S.; et al. Cardiac Manifestations of Myotonic Dystrophy in a Pediatric Cohort. Front. Pediatr. 2022, 10, 910660. [Google Scholar] [CrossRef]
- Wahbi, K.; Babuty, D.; Probst, V.; Wissocque, L.; Labombarda, F.; Porcher, R.; Bécane, H.M.; Lazarus, A.; Béhin, A.; Laforêt, P.; et al. Incidence and predictors of sudden death, major conduction defects and sustained ventricular tachyarrhythmias in 1388 patients with myotonic dystrophy type 1. Eur. Heart J. 2017, 38, 751–758. [Google Scholar] [CrossRef]
- Clementy, N.; Labombarda, F.; Grolleau, F.; Algalarrondo, V.; Bassez, G.; Bécane, H.-M.; Béhin, A.; Chapon, F.; El Hachmi, M.; Fayssoil, A.; et al. Electrocardiogram vs Electrophysiological Study and Major Conduction Delays in Myotonic Dystrophy Type 1. JAMA Cardiol. 2025, e253055. [Google Scholar] [CrossRef]
- Echenne, B.; Bassez, G. Chapter 144—Congenital and infantile myotonic dystrophy. In Handbook of Clinical Neurology; Dulac, O., Lassonde, M., Sarnat, H.B., Eds.; Pediatric Neurology Part III; Elsevier: Amsterdam, The Netherlands, 2013; Volume 113, pp. 1387–1393. [Google Scholar]
- Ho, G.; Carey, K.A.; Cardamone, M.; Farrar, M.A. Myotonic dystrophy type 1: Clinical manifestations in children and adolescents. Arch. Dis. Child. 2019, 104, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Zagorda, B.; Camdessanché, J.-P.; Féasson, L. Pregnancy and myopathies: Reciprocal impacts between pregnancy, delivery, and myopathies and their treatments. A clinical review. Rev. Neurol. 2021, 177, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Pregnancy Course and Outcome in Women with Hereditary Neuromuscular Disorders: Comparison of Obstetric Risks in 178 Patients—ClinicalKey. Available online: https://www.clinicalkey.com/#!/content/playContent/1-s2.0-S0301211512000917?returnurl=https:%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS0301211512000917%3Fshowall%3Dtrue&referrer= (accessed on 27 November 2024).
- Bird, T.D. Myotonic Dystrophy Type 1. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Thornton, C.A. Myotonic Dystrophy. Neurol. Clin. 2014, 32, 705–719. [Google Scholar] [CrossRef]
- Seifert, B.A.; Reddi, H.V.; Kang, B.E.; Bean, L.J.H.; Shealy, A.; Rose, N.C.; ACMG Laboratory Quality Assurance Committee. Electronic address: Documents@acmg.net Myotonic dystrophy type 1 testing, 2024 revision: A technical standard of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 2024, 26, 101145. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.; Hilton-Jones, D. Myotonic dystrophy: Diagnosis, management and new therapies. Curr. Opin. Neurol. 2014, 27, 599–606. [Google Scholar] [CrossRef]
- Mahadevan, M.S.; Yadava, R.S.; Mandal, M. Cardiac Pathology in Myotonic Dystrophy Type 1. Int. J. Mol. Sci. 2021, 22, 11874. [Google Scholar] [CrossRef]
- Clarke, N.R.; Kelion, A.D.; Nixon, J.; Hilton-Jones, D.; Forfar, J.C. Does cytosine-thymine-guanine (CTG) expansion size predict cardiac events and electrocardiographic progression in myotonic dystrophy? Heart 2001, 86, 411–416. [Google Scholar] [CrossRef]
- Phillips, M.F.; Harper, P.S. Cardiac disease in myotonic dystrophy. Cardiovasc. Res. 1997, 33, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Pelargonio, G.; Russo, A.D.; Sanna, T.; De Martino, G.; Bellocci, F. Myotonic Dystrophy and the Heart. Heart 2002, 88, 665–670. [Google Scholar] [CrossRef]
- McNally, E.M.; Sparano, D. Mechanisms and management of the heart in Myotonic Dystrophy. Heart 2011, 97, 1094–1100. [Google Scholar] [CrossRef]
- Bucci, E.; Testa, M.; Licchelli, L.; Frattari, A.; El Halabieh, N.A.; Gabriele, E.; Pignatelli, G.; De Santis, T.; Fionda, L.; Vanoli, F.; et al. A 34-year longitudinal study on long-term cardiac outcomes in DM1 patients with normal ECG at baseline at an Italian clinical centre. J. Neurol. 2018, 265, 885–895. [Google Scholar] [CrossRef]
- Ho, G.; Cardamone, M.; Farrar, M. Congenital and childhood myotonic dystrophy: Current aspects of disease and future directions. World J. Clin. Pediatr. 2015, 4, 66–80. [Google Scholar] [CrossRef]
- Hermans, M.C.; Faber, C.G.; Bekkers, S.C.; de Die-Smulders, C.E.; Gerrits, M.M.; Merkies, I.S.; Snoep, G.; Pinto, Y.M.; Schalla, S. Structural and functional cardiac changes in myotonic dystrophy type 1: A cardiovascular magnetic resonance study. J. Cardiovasc. Magn. Reson. 2012, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Bhakta, D.; Lowe, M.R.; Groh, W.J. Prevalence of structural cardiac abnormalities in patients with myotonic dystrophy type I. Am. Heart J. 2004, 147, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Goldberger, J.J.; Arora, R.; Buckley, U.; Shivkumar, K. Autonomic Nervous System Dysfunction: JACC Focus Seminar. J. Am. Coll. Cardiol. 2019, 73, 1189–1206. [Google Scholar] [CrossRef]
- Hardin, B.A.; Lowe, M.R.; Bhakta, D.; Groh, W.J. Heart rate variability declines with increasing age and CTG repeat length in patients with myotonic dystrophy type 1. Ann. Noninvasive Electrocardiol. 2003, 8, 227–232. [Google Scholar] [CrossRef]
- Di Leo, R.; Rodolico, C.; De Gregorio, C.; Recupero, A.; Coglitore, S.; Annesi, G.; Toscano, A.; Messina, C.; Vita, G. Cardiovascular autonomic control in myotonic dystrophy type 1: A correlative study with clinical and genetic data. Neuromuscul. Disord. 2004, 14, 136–141. [Google Scholar] [CrossRef]
- Fregonezi, G.; Araújo, T.; Dourado Junior, M.E.; Ferezini, J.; Silva, E.; Resqueti, V. Heart rate variability in myotonic dystrophy type 1 patients. Arq. Bras. Cardiol. 2012, 98, 353–360. [Google Scholar] [CrossRef]
- Konieczny, P.; Stepniak-Konieczna, E.; Sobczak, K. MBNL proteins and their target RNAs, interaction and splicing regulation. Nucleic Acids Res. 2014, 42, 10873–10887. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.M.; McCurrach, M.E.; Taneja, K.L.; Singer, R.H.; Housman, D.E. Expansion of a CUG trinucleotide repeat in the 3′ untranslated region of myotonic dystrophy protein kinase transcripts results in nuclear retention of transcripts. Proc. Natl. Acad. Sci. USA 1997, 94, 7388–7393. [Google Scholar] [CrossRef] [PubMed]
- Sicot, G.; Gomes-Pereira, M. RNA toxicity in human disease and animal models: From the uncovering of a new mechanism to the development of promising therapies. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2013, 1832, 1390–1409. [Google Scholar] [CrossRef]
- Liu, J.; Guo, Z.-N.; Yan, X.-L.; Yang, Y.; Huang, S. Brain Pathogenesis and Potential Therapeutic Strategies in Myotonic Dystrophy Type 1. Front. Aging Neurosci. 2021, 13, 755392. [Google Scholar] [CrossRef]
- Sznajder, Ł.J.; Swanson, M.S. Short Tandem Repeat Expansions and RNA-Mediated Pathogenesis in Myotonic Dystrophy. Int. J. Mol. Sci. 2019, 20, 3365. [Google Scholar] [CrossRef]
- Ho, T.H.; Savkur, R.S.; Poulos, M.G.; Mancini, M.A.; Swanson, M.S.; Cooper, T.A. Colocalization of muscleblind with RNA foci is separable from mis-regulation of alternative splicing in myotonic dystrophy. J. Cell Sci. 2005, 118, 2923–2933. [Google Scholar] [CrossRef]
- Suenaga, K.; Lee, K.-Y.; Nakamori, M.; Tatsumi, Y.; Takahashi, M.P.; Fujimura, H.; Jinnai, K.; Yoshikawa, H.; Du, H.; Ares, M.; et al. Muscleblind-like 1 knockout mice reveal novel splicing defects in the myotonic dystrophy brain. PLoS ONE 2012, 7, e33218. [Google Scholar] [CrossRef]
- Lin, X.; Miller, J.W.; Mankodi, A.; Kanadia, R.N.; Yuan, Y.; Moxley, R.T.; Swanson, M.S.; Thornton, C.A. Failure of MBNL1-dependent post-natal splicing transitions in myotonic dystrophy. Hum. Mol. Genet. 2006, 15, 2087–2097. [Google Scholar] [CrossRef] [PubMed]
- Berger, D.S.; Ladd, A.N. Repression of nuclear CELF activity can rescue CELF-regulated alternative splicing defects in skeletal muscle models of myotonic dystrophy. PLoS Curr. 2012, 4, RRN1305. [Google Scholar] [CrossRef]
- Kuyumcu-Martinez, N.M.; Wang, G.-S.; Cooper, T.A. Increased steady-state levels of CUGBP1 in myotonic dystrophy 1 are due to PKC-mediated hyperphosphorylation. Mol. Cell 2007, 28, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Philips, A.V.; Timchenko, L.T.; Cooper, T.A. Disruption of splicing regulated by a CUG-binding protein in myotonic dystrophy. Science 1998, 280, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Shi, J.-J.; Chen, R.-Y.; Li, C.-Y.; Liu, Y.-J.; Lu, J.-F.; Yang, G.-J.; Cao, J.-F.; Chen, J. Curriculum vitae of CUG binding protein 1 (CELF1) in homeostasis and diseases: A systematic review. Cell. Mol. Biol. Lett. 2024, 29, 32. [Google Scholar] [CrossRef]
- Blech-Hermoni, Y.; Dasgupta, T.; Coram, R.J.; Ladd, A.N. Identification of Targets of CUG-BP, Elav-Like Family Member 1 (CELF1) Regulation in Embryonic Heart Muscle. PLoS ONE 2016, 11, e0149061. [Google Scholar] [CrossRef]
- Timchenko, L. Correction of RNA-Binding Protein CUGBP1 and GSK3β Signaling as Therapeutic Approach for Congenital and Adult Myotonic Dystrophy Type 1. Int. J. Mol. Sci. 2019, 21, 94. [Google Scholar] [CrossRef] [PubMed]
- Charlet-B, N.; Savkur, R.S.; Singh, G.; Philips, A.V.; Grice, E.A.; Cooper, T.A. Loss of the muscle-specific chloride channel in type 1 myotonic dystrophy due to misregulated alternative splicing. Mol. Cell 2002, 10, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Savkur, R.S.; Philips, A.V.; Cooper, T.A. Aberrant regulation of insulin receptor alternative splicing is associated with insulin resistance in myotonic dystrophy. Nat. Genet. 2001, 29, 40–47. [Google Scholar] [CrossRef]
- Pierre, M.; Djemai, M.; Poulin, H.; Chahine, M. NaV1.5 knockout in iPSCs: A novel approach to study NaV1.5 variants in a human cardiomyocyte environment. Sci. Rep. 2021, 11, 17168. [Google Scholar] [CrossRef]
- Freyermuth, F.; Rau, F.; Kokunai, Y.; Linke, T.; Sellier, C.; Nakamori, M.; Kino, Y.; Arandel, L.; Jollet, A.; Thibault, C.; et al. Splicing misregulation of SCN5A contributes to cardiac-conduction delay and heart arrhythmia in myotonic dystrophy. Nat. Commun. 2016, 7, 11067. [Google Scholar] [CrossRef]
- Walzik, S.; Schroeter, A.; Benndorf, K.; Zimmer, T. Alternative Splicing of the Cardiac Sodium Channel Creates Multiple Variants of Mutant T1620K Channels. PLoS ONE 2011, 6, e19188. [Google Scholar] [CrossRef] [PubMed]
- Murphy, L.L.; Moon-Grady, A.J.; Cuneo, B.F.; Wakai, R.T.; Yu, S.; Kunic, J.D.; Benson, D.W.; George, A.L. Developmentally regulated SCN5A splice variant potentiates dysfunction of a novel mutation associated with severe fetal arrhythmia. Heart Rhythm. 2012, 9, 590–597. [Google Scholar] [CrossRef]
- Onkal, R.; Mattis, J.H.; Fraser, S.P.; Diss, J.K.J.; Shao, D.; Okuse, K.; Djamgoz, M.B.A. Alternative splicing of Nav1.5: An electrophysiological comparison of ‘neonatal’ and ‘adult’ isoforms and critical involvement of a lysine residue. J. Cell. Physiol. 2008, 216, 716–726. [Google Scholar] [CrossRef]
- Li, W.; Yin, L.; Shen, C.; Hu, K.; Ge, J.; Sun, A. SCN5A Variants: Association With Cardiac Disorders. Front. Physiol. 2018, 9, 1372. [Google Scholar] [CrossRef]
- Pang, P.D.; Alsina, K.M.; Cao, S.; Koushik, A.B.; Wehrens, X.H.T.; Cooper, T.A. CRISPR-Mediated Expression of the Fetal Scn5a Isoform in Adult Mice Causes Conduction Defects and Arrhythmias. J. Am. Heart Assoc. 2018, 7, e010393. [Google Scholar] [CrossRef]
- Poulin, H.; Mercier, A.; Djemai, M.; Pouliot, V.; Deschenes, I.; Boutjdir, M.; Puymirat, J.; Chahine, M. iPSC-derived cardiomyocytes from patients with myotonic dystrophy type 1 have abnormal ion channel functions and slower conduction velocities. Sci. Rep. 2021, 11, 2500. [Google Scholar] [CrossRef]
- Algalarrondo, V.; Wahbi, K.; Sebag, F.; Gourdon, G.; Beldjord, C.; Azibi, K.; Balse, E.; Coulombe, A.; Fischmeister, R.; Eymard, B.; et al. Abnormal sodium current properties contribute to cardiac electrical and contractile dysfunction in a mouse model of myotonic dystrophy type 1. Neuromuscul. Disord. 2015, 25, 308–320. [Google Scholar] [CrossRef]
- Veerman, C.C.; Wilde, A.A.M.; Lodder, E.M. The cardiac sodium channel gene SCN5A and its gene product NaV1.5: Role in physiology and pathophysiology. Gene 2015, 573, 177–187. [Google Scholar] [CrossRef]
- Pierre, M.; Djemai, M.; Chapotte-Baldacci, C.-A.; Pouliot, V.; Puymirat, J.; Boutjdir, M.; Chahine, M. Cardiac involvement in patient-specific induced pluripotent stem cells of myotonic dystrophy type 1: Unveiling the impact of voltage-gated sodium channels. Front. Physiol. 2023, 14, 1258318. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.D.; Hell, J.W. CaV1.2 Signaling Complexes in the Heart. J. Mol. Cell. Cardiol. 2013, 58, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Ginjupalli, V.K.M.; Cupelli, M.; Reisqs, J.-B.; Sleiman, Y.; El-Sherif, N.; Gourdon, G.; Puymirat, J.; Chahine, M.; Boutjdir, M. Electrophysiological basis of cardiac arrhythmia in a mouse model of myotonic dystrophy type 1. Front. Physiol. 2023, 14, 1257682. [Google Scholar] [CrossRef]
- Cupelli, M.; Ginjupalli, V.K.M.; Reisqs, J.-B.; Sleiman, Y.; El-Sherif, N.; Gourdon, G.; Puymirat, J.; Chahine, M.; Boutjdir, M. Calcium handling abnormalities increase arrhythmia susceptibility in DMSXL myotonic dystrophy type 1 mice. Biomed. Pharmacother. 2024, 180, 117562. [Google Scholar] [CrossRef]
- Kaliman, P.; Catalucci, D.; Lam, J.T.; Kondo, R.; Gutiérrez, J.C.P.; Reddy, S.; Palacín, M.; Zorzano, A.; Chien, K.R.; Ruiz-Lozano, P. Myotonic Dystrophy Protein Kinase Phosphorylates Phospholamban and Regulates Calcium Uptake in Cardiomyocyte Sarcoplasmic Reticulum*. J. Biol. Chem. 2005, 280, 8016–8021. [Google Scholar] [CrossRef] [PubMed]
- Kaliman, P.; Llagostera, E. Myotonic dystrophy protein kinase (DMPK) and its role in the pathogenesis of myotonic dystrophy 1. Cell. Signal. 2008, 20, 1935–1941. [Google Scholar] [CrossRef]
- Costa, A.; Cruz, A.C.; Martins, F.; Rebelo, S. Protein Phosphorylation Alterations in Myotonic Dystrophy Type 1: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 3091. [Google Scholar] [CrossRef]
- Tylock, K.M.; Auerbach, D.S.; Tang, Z.Z.; Thornton, C.A.; Dirksen, R.T. Biophysical mechanisms for QRS- and QTc-interval prolongation in mice with cardiac expression of expanded CUG-repeat RNA. J. Gen. Physiol. 2020, 152, e201912450. [Google Scholar] [CrossRef]
- Rawshani, A.; McGuire, D.K.; Omerovic, E.; Sattar, N.; McMurray, J.J.V.; Smith, U.; Redfors, B.; Bergfeldt, L.; Eliasson, B.; Borén, J.; et al. Cardiac arrhythmias and conduction abnormalities in patients with type 2 diabetes. Sci. Rep. 2023, 13, 1192. [Google Scholar] [CrossRef] [PubMed]
- Takeshima, K.; Ariyasu, H.; Ishibashi, T.; Kawai, S.; Uraki, S.; Koh, J.; Ito, H.; Akamizu, T. Myotonic dystrophy type 1 with diabetes mellitus, mixed hypogonadism and adrenal insufficiency. Endocrinol. Diabetes Metab. Case Rep. 2018, 2018, 17–0143. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xian, H.; Xu, Y.; Li, W.; Guo, J.; Wan, K.; Wang, J.; Xu, Z.; Zhang, Q.; Han, Y.; et al. The impact of type 2 diabetes mellitus on the clinical profile, myocardial fibrosis, and prognosis in non-ischemic dilated cardiomyopathy: A prospective cohort study. Cardiovasc. Diabetol. 2024, 23, 48. [Google Scholar] [CrossRef]
- Arrieta, F.; Iglesias, P.; Pedro-Botet, J.; Becerra, A.; Ortega, E.; Obaya, J.C.; Nubiola, A.; Maldonado, G.F.; Campos, M.d.M.; Petrecca, R.; et al. Diabetes mellitus and cardiovascular risk: Update of the recommendations of the Diabetes and Cardiovascular Disease working group of the Spanish Diabetes Society (SED, 2018). Clín. Investig. Arterioscler. (Engl. Ed.) 2018, 30, 137–153. [Google Scholar] [CrossRef]
- Nieuwenhuis, S.; Okkersen, K.; Widomska, J.; Blom, P.; ’t Hoen, P.A.C.; van Engelen, B.; Glennon, J.C. Insulin Signaling as a Key Moderator in Myotonic Dystrophy Type 1. Front. Neurol. 2019, 10, 1229. [Google Scholar] [CrossRef]
- Andreadi, A.; Bellia, A.; Di Daniele, N.; Meloni, M.; Lauro, R.; Della-Morte, D.; Lauro, D. The molecular link between oxidative stress, insulin resistance, and type 2 diabetes: A target for new therapies against cardiovascular diseases. Curr. Opin. Pharmacol. 2022, 62, 85–96. [Google Scholar] [CrossRef]
- Seki, Y.; Yamada, T.; Kiyosue, A.; Kimura, K.; Uehara, M.; Hatano, M.; Sasako, T.; Shirota, Y.; Sudo, A.; Ishiura, H.; et al. Asymptomatic myocardial infarction in a patient with myotonic dystrophy type 1. J. Cardiol. Cases 2022, 26, 248–251. [Google Scholar] [CrossRef]
- Pepe, M.; Addabbo, F.; Cecere, A.; Tritto, R.; Napoli, G.; Nestola, P.L.; Cirillo, P.; Biondi-Zoccai, G.; Giordano, S.; Ciccone, M.M. Acute Hyperglycemia-Induced Injury in Myocardial Infarction. Int. J. Mol. Sci. 2024, 25, 8504. [Google Scholar] [CrossRef]
- Giuliani, L.; Di Toro, A.; Urtis, M.; Smirnova, A.; Concardi, M.; Favalli, V.; Serio, A.; Grasso, M.; Arbustini, E. Hereditary muscle diseases and the heart: The cardiologist’s perspective. Eur. Heart J. Suppl. 2020, 22, E13–E19. [Google Scholar] [CrossRef]
- Singh, R.B.; Fedacko, J.; Pella, D.; Fatima, G.; Elkilany, G.; Moshiri, M.; Hristova, K.; Jakabcin, P.; Vaňova, N. High Exogenous Antioxidant, Restorative Treatment (Heart) for Prevention of the Six Stages of Heart Failure: The Heart Diet. Antioxidants 2022, 11, 1464. [Google Scholar] [CrossRef]
- Oktay, A.A.; Paul, T.K.; Koch, C.A.; Lavie, C.J. Diabetes, Cardiomyopathy, and Heart Failure. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Wahbi, K.; Porcher, R.; Laforêt, P.; Fayssoil, A.; Bécane, H.M.; Lazarus, A.; Sochala, M.; Stojkovic, T.; Béhin, A.; Leonard-Louis, S.; et al. Development and Validation of a New Scoring System to Predict Survival in Patients With Myotonic Dystrophy Type 1. JAMA Neurol. 2018, 75, 573–581. [Google Scholar] [CrossRef]
- Russo, V.; Antonini, G.; Massa, R.; Casali, C.; Mauriello, A.; Martino, A.M.; Marconi, R.; Garibaldi, M.; Franciosa, P.; Zecchin, M.; et al. Comprehensive Cardiovascular Management of Myotonic Dystrophy Type 1 Patients: A Report from the Italian Neuro-Cardiology Network. J. Cardiovasc. Dev. Dis. 2024, 11, 63. [Google Scholar] [CrossRef]
- Rossi, S.; Della Marca, G.; Ricci, M.; Perna, A.; Nicoletti, T.F.; Brunetti, V.; Meleo, E.; Calvello, M.; Petrucci, A.; Antonini, G.; et al. Prevalence and predictor factors of respiratory impairment in a large cohort of patients with Myotonic Dystrophy type 1 (DM1): A retrospective, cross sectional study. J. Neurol. Sci. 2019, 399, 118–124. [Google Scholar] [CrossRef]
- Hartog, L.; Zhao, J.; Reynolds, J.; Brokamp, G.; Vilson, F.; Arnold, W.D.; LoRusso, S. Factors Influencing the Severity and Progression of Respiratory Muscle Dysfunction in Myotonic Dystrophy Type 1. Front. Neurol. 2021, 12, 658532. [Google Scholar] [CrossRef]
- Nguyen, T.-H.; Conotte, S.; Belayew, A.; Declèves, A.-E.; Legrand, A.; Tassin, A. Hypoxia and Hypoxia-Inducible Factor Signaling in Muscular Dystrophies: Cause and Consequences. Int. J. Mol. Sci. 2021, 22, 7220. [Google Scholar] [CrossRef]
- Carrell, S.T.; Carrell, E.M.; Auerbach, D.; Pandey, S.K.; Bennett, C.F.; Dirksen, R.T.; Thornton, C.A. Dmpk gene deletion or antisense knockdown does not compromise cardiac or skeletal muscle function in mice. Hum. Mol. Genet. 2016, 25, 4328–4338. [Google Scholar] [CrossRef]
- Porquet, F.; Weidong, L.; Jehasse, K.; Gazon, H.; Kondili, M.; Blacher, S.; Massotte, L.; Di Valentin, E.; Furling, D.; Gillet, N.A.; et al. Specific DMPK-promoter targeting by CRISPRi reverses myotonic dystrophy type 1-associated defects in patient muscle cells. Mol. Ther. Nucleic Acids 2023, 32, 857–871. [Google Scholar] [CrossRef]
- Huang, K.; Wang, D.-D.; Hu, W.-B.; Zeng, W.-Q.; Xu, X.; Li, Q.-X.; Bi, F.-F.; Yang, H.; Qiu, J. Calcitriol increases MBNL1 expression and alleviates myotonic dystrophy phenotypes in HSALR mouse models. J. Transl. Med. 2022, 20, 588. [Google Scholar] [CrossRef]
- Mankodi, A.; Logigian, E.; Callahan, L.; McClain, C.; White, R.; Henderson, D.; Krym, M.; Thornton, C.A. Myotonic dystrophy in transgenic mice expressing an expanded CUG repeat. Science 2000, 289, 1769–1773. [Google Scholar] [CrossRef]
- Seznec, H.; Agbulut, O.; Sergeant, N.; Savouret, C.; Ghestem, A.; Tabti, N.; Willer, J.C.; Ourth, L.; Duros, C.; Brisson, E.; et al. Mice transgenic for the human myotonic dystrophy region with expanded CTG repeats display muscular and brain abnormalities. Hum. Mol. Genet. 2001, 10, 2717–2726. [Google Scholar] [CrossRef]
- Huguet, A.; Medja, F.; Nicole, A.; Vignaud, A.; Guiraud-Dogan, C.; Ferry, A.; Decostre, V.; Hogrel, J.-Y.; Metzger, F.; Hoeflich, A.; et al. Molecular, physiological, and motor performance defects in DMSXL mice carrying >1,000 CTG repeats from the human DM1 locus. PLoS Genet. 2012, 8, e1003043. [Google Scholar] [CrossRef]
- Morriss, G.R.; Rajapakshe, K.; Huang, S.; Coarfa, C.; Cooper, T.A. Mechanisms of skeletal muscle wasting in a mouse model for myotonic dystrophy type 1. Hum. Mol. Genet. 2018, 27, 2789–2804. [Google Scholar] [CrossRef]
- Wang, P.-Y.; Lin, Y.-M.; Wang, L.-H.; Kuo, T.-Y.; Cheng, S.-J.; Wang, G.-S. Reduced cytoplasmic MBNL1 is an early event in a brain-specific mouse model of myotonic dystrophy. Hum. Mol. Genet. 2017, 26, 2247–2257. [Google Scholar] [CrossRef]
- Yadava, R.S.; Yu, Q.; Mandal, M.; Rigo, F.; Bennett, C.F.; Mahadevan, M.S. Systemic therapy in an RNA toxicity mouse model with an antisense oligonucleotide therapy targeting a non-CUG sequence within the DMPK 3’UTR RNA. Hum. Mol. Genet. 2020, 29, 1440–1453. [Google Scholar] [CrossRef]
- Matynia, A.; Ng, C.H.; Dansithong, W.; Chiang, A.; Silva, A.J.; Reddy, S. Muscleblind1, but not Dmpk or Six5, contributes to a complex phenotype of muscular and motivational deficits in mouse models of myotonic dystrophy. PLoS ONE 2010, 5, e9857. [Google Scholar] [CrossRef]
- Kanadia, R.N.; Johnstone, K.A.; Mankodi, A.; Lungu, C.; Thornton, C.A.; Esson, D.; Timmers, A.M.; Hauswirth, W.W.; Swanson, M.S. A muscleblind knockout model for myotonic dystrophy. Science 2003, 302, 1978–1980. [Google Scholar] [CrossRef]
- Charizanis, K.; Lee, K.-Y.; Batra, R.; Goodwin, M.; Zhang, C.; Yuan, Y.; Shiue, L.; Cline, M.; Scotti, M.M.; Xia, G.; et al. Muscleblind-like 2-mediated alternative splicing in the developing brain and dysregulation in myotonic dystrophy. Neuron 2012, 75, 437–450. [Google Scholar] [CrossRef]
- Poulos, M.G.; Batra, R.; Li, M.; Yuan, Y.; Zhang, C.; Darnell, R.B.; Swanson, M.S. Progressive impairment of muscle regeneration in muscleblind-like 3 isoform knockout mice. Hum. Mol. Genet. 2013, 22, 3547–3558. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Li, M.; Manchanda, M.; Batra, R.; Charizanis, K.; Mohan, A.; Warren, S.A.; Chamberlain, C.M.; Finn, D.; Hong, H.; et al. Compound loss of muscleblind-like function in myotonic dystrophy. EMBO Mol. Med. 2013, 5, 1887–1900. [Google Scholar] [CrossRef]
- Choi, J.; Personius, K.E.; DiFranco, M.; Dansithong, W.; Yu, C.; Srivastava, S.; Dixon, D.M.; Bhatt, D.B.; Comai, L.; Vergara, J.L.; et al. Muscleblind-Like 1 and Muscleblind-Like 3 Depletion Synergistically Enhances Myotonia by Altering Clc-1 RNA Translation. eBioMedicine 2015, 2, 1034–1047. [Google Scholar] [CrossRef]
- Thomas, J.D.; Sznajder, Ł.J.; Bardhi, O.; Aslam, F.N.; Anastasiadis, Z.P.; Scotti, M.M.; Nishino, I.; Nakamori, M.; Wang, E.T.; Swanson, M.S. Disrupted prenatal RNA processing and myogenesis in congenital myotonic dystrophy. Genes. Dev. 2017, 31, 1122–1133. [Google Scholar] [CrossRef]
- Ward, A.J.; Rimer, M.; Killian, J.M.; Dowling, J.J.; Cooper, T.A. CUGBP1 overexpression in mouse skeletal muscle reproduces features of myotonic dystrophy type 1. Hum. Mol. Genet. 2010, 19, 3614–3622. [Google Scholar] [CrossRef]
- Koshelev, M.; Sarma, S.; Price, R.E.; Wehrens, X.H.T.; Cooper, T.A. Heart-specific overexpression of CUGBP1 reproduces functional and molecular abnormalities of myotonic dystrophy type 1. Hum. Mol. Genet. 2010, 19, 1066–1075. [Google Scholar] [CrossRef]
- Mahadevan, M.S.; Yadava, R.S.; Yu, Q.; Balijepalli, S.; Frenzel-McCardell, C.D.; Bourne, T.D.; Phillips, L.H. Reversible model of RNA toxicity and cardiac conduction defects in myotonic dystrophy. Nat. Genet. 2006, 38, 1066–1070. [Google Scholar] [CrossRef]
- O’Cochlain, D.F.; Perez-Terzic, C.; Reyes, S.; Kane, G.C.; Behfar, A.; Hodgson, D.M.; Strommen, J.A.; Liu, X.-K.; van den Broek, W.; Wansink, D.G.; et al. Transgenic overexpression of human DMPK accumulates into hypertrophic cardiomyopathy, myotonic myopathy and hypotension traits of myotonic dystrophy. Hum. Mol. Genet. 2004, 13, 2505–2518. [Google Scholar] [CrossRef]
- Rao, A.N.; Campbell, H.M.; Guan, X.; Word, T.A.; Wehrens, X.H.T.; Xia, Z.; Cooper, T.A. Reversible cardiac disease features in an inducible CUG repeat RNA–expressing mouse model of myotonic dystrophy. JCI Insight 2021, 6, e143465. [Google Scholar] [CrossRef]
- Dincã, D.M.; Lallemant, L.; González-Barriga, A.; Cresto, N.; Braz, S.O.; Sicot, G.; Pillet, L.-E.; Polvèche, H.; Magneron, P.; Huguet-Lachon, A.; et al. Myotonic dystrophy RNA toxicity alters morphology, adhesion and migration of mouse and human astrocytes. Nat. Commun. 2022, 13, 3841, Erratum in Nat. Commun., 2022, 13, 4091. https://doi.org/10.1038/s41467-022-31774-7. [Google Scholar] [CrossRef]
- Gomes-Pereira, M.; Foiry, L.; Nicole, A.; Huguet, A.; Junien, C.; Munnich, A.; Gourdon, G. CTG trinucleotide repeat “big jumps”: Large expansions, small mice. PLoS Genet. 2007, 3, e52. [Google Scholar] [CrossRef]
- Panaite, P.-A.; Kuntzer, T.; Gourdon, G.; Lobrinus, J.A.; Barakat-Walter, I. Functional and histopathological identification of the respiratory failure in a DMSXL transgenic mouse model of myotonic dystrophy. Dis. Models Mech. 2013, 6, 622–631. [Google Scholar] [CrossRef]
- Golini, E.; Rigamonti, M.; Raspa, M.; Scavizzi, F.; Falcone, G.; Gourdon, G.; Mandillo, S. Excessive rest time during active phase is reliably detected in a mouse model of myotonic dystrophy type 1 using home cage monitoring. Front. Behav. Neurosci. 2023, 17, 1130055. [Google Scholar] [CrossRef]
- Wang, G.-S.; Kearney, D.L.; De Biasi, M.; Taffet, G.; Cooper, T.A. Elevation of RNA-binding protein CUGBP1 is an early event in an inducible heart-specific mouse model of myotonic dystrophy. J. Clin. Investig. 2007, 117, 2802–2811. [Google Scholar] [CrossRef]
- Braz, S.O.; Acquaire, J.; Gourdon, G.; Gomes-Pereira, M. Of Mice and Men: Advances in the Understanding of Neuromuscular Aspects of Myotonic Dystrophy. Front. Neurol. 2018, 9, 519. [Google Scholar] [CrossRef]
- Gomes-Pereira, M.; Cooper, T.A.; Gourdon, G. Myotonic dystrophy mouse models: Towards rational therapy development. Trends Mol. Med. 2011, 17, 506–517. [Google Scholar] [CrossRef]
- Berul, C.I.; Maguire, C.T.; Aronovitz, M.J.; Greenwood, J.; Miller, C.; Gehrmann, J.; Housman, D.; Mendelsohn, M.E.; Reddy, S. DMPK dosage alterations result in atrioventricular conduction abnormalities in a mouse myotonic dystrophy model. J. Clin. Investig. 1999, 103, R1–R7. [Google Scholar] [CrossRef]
- Carrell, S.; Carrell, E.; Auerbach, D.; Pandey, S.; Bennett, F.; Dirksen, R.; Thornton, C. Silencing of Myotonic Dystrophy Protein Kinase (DMPK) Does Not Affect Cardiac or Muscle Function in Mice (I4.008). Neurology 2016, 86, I4.008. [Google Scholar] [CrossRef]
- Spitalieri, P.; Talarico, R.V.; Caioli, S.; Murdocca, M.; Serafino, A.; Girasole, M.; Dinarelli, S.; Longo, G.; Pucci, S.; Botta, A.; et al. Modelling the pathogenesis of Myotonic Dystrophy type 1 cardiac phenotype through human iPSC-derived cardiomyocytes. J. Mol. Cell. Cardiol. 2018, 118, 95–109. [Google Scholar] [CrossRef]
- Weeden, T.; Picariello, T.; Quinn, B.; Spring, S.; Shen, P.-Y.; Qiu, Q.; Vieira, B.F.; Schlaefke, L.; Russo, R.J.; Chang, Y.-A.; et al. FORCE platform overcomes barriers of oligonucleotide delivery to muscle and corrects myotonic dystrophy features in preclinical models. Commun. Med. 2025, 5, 22. [Google Scholar] [CrossRef]
- Kim, E.Y.; Barefield, D.Y.; Vo, A.H.; Gacita, A.M.; Schuster, E.J.; Wyatt, E.J.; Davis, J.L.; Dong, B.; Sun, C.; Page, P.; et al. Distinct pathological signatures in human cellular models of myotonic dystrophy subtypes. JCI Insight 2019, 4, e122686. [Google Scholar] [CrossRef]
- Izzo, M.; Battistini, J.; Provenzano, C.; Martelli, F.; Cardinali, B.; Falcone, G. Molecular Therapies for Myotonic Dystrophy Type 1: From Small Drugs to Gene Editing. Int. J. Mol. Sci. 2022, 23, 4622. [Google Scholar] [CrossRef]
- Klein, A.F.; Dastidar, S.; Furling, D.; Chuah, M.K. Therapeutic Approaches for Dominant Muscle Diseases: Highlight on Myotonic Dystrophy. Curr. Gene Ther. 2015, 15, 329–337. [Google Scholar] [CrossRef]
- Bisset, D.R.; Stepniak-Konieczna, E.A.; Zavaljevski, M.; Wei, J.; Carter, G.T.; Weiss, M.D.; Chamberlain, J.R. Therapeutic impact of systemic AAV-mediated RNA interference in a mouse model of myotonic dystrophy. Hum. Mol. Genet. 2015, 24, 4971–4983. [Google Scholar] [CrossRef]
- Study of AOC 1001 in Adult Myotonic Dystrophy Type 1 (DM1) Patients (MARINA). Available online: https://clinicaltrials.gov/ct2/show/NCT05027269 (accessed on 2 December 2024).
- Pandey, S.K.; Wheeler, T.M.; Justice, S.L.; Kim, A.; Younis, H.S.; Gattis, D.; Jauvin, D.; Puymirat, J.; Swayze, E.E.; Freier, S.M.; et al. Identification and Characterization of Modified Antisense Oligonucleotides Targeting DMPK in Mice and Nonhuman Primates for the Treatment of Myotonic Dystrophy Type 1. J. Pharmacol. Exp. Ther. 2015, 355, 329–340. [Google Scholar] [CrossRef]
- A Phase 1/2a Dose-Escalating Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of ARO-DM1 in Subjects with Type 1 Myotonic Dystrophy Who Are ≥18 to ≤ 65 Years (NCT06138743). Available online: https://clinicaltrials.gov/study/NCT06138743 (accessed on 2 September 2025).
- ARTHEx Biotech S.L. A Phase 1/2a Double-Blind, Placebo-Controlled, Single- and Multiple Ascending Dose Study to Assess the Safety, Tolerability, PK, PD and Efficacy of IV Administration of ATX-01 In Male and Female Participants Aged 18 to 64 with Classic DM1. Available online: https://clinicaltrials.gov/study/NCT06300307 (accessed on 2 September 2025).
- Schwartz, J.L.; Jones, K.L.; Yeo, G.W. Repeat RNA expansion disorders of the nervous system: Post-transcriptional mechanisms and therapeutic strategies. Crit. Rev. Biochem. Mol. Biol. 2021, 56, 31–53. [Google Scholar] [CrossRef]
- Jauvin, D.; Chrétien, J.; Pandey, S.K.; Martineau, L.; Revillod, L.; Bassez, G.; Lachon, A.; MacLeod, A.R.; Gourdon, G.; Wheeler, T.M.; et al. Targeting DMPK with Antisense Oligonucleotide Improves Muscle Strength in Myotonic Dystrophy Type 1 Mice. Mol. Ther. Nucleic Acids 2017, 7, 465–474. [Google Scholar] [CrossRef]
- Thornton, C.A.; Moxley, R.T.; Eichinger, K.; Heatwole, C.; Mignon, L.; Arnold, W.D.; Ashizawa, T.; Day, J.W.; Dent, G.; Tanner, M.K.; et al. Antisense oligonucleotide targeting DMPK in patients with myotonic dystrophy type 1: A multicentre, randomised, dose-escalation, placebo-controlled, phase 1/2a trial. Lancet Neurol. 2023, 22, 218–228. [Google Scholar] [CrossRef]
- Ait Benichou, S.; Jauvin, D.; De Serres-Bérard, T.; Bennett, F.; Rigo, F.; Gourdon, G.; Boutjdir, M.; Chahine, M.; Puymirat, J. Enhanced Delivery of Ligand-Conjugated Antisense Oligonucleotides (C16-HA-ASO) Targeting Dystrophia Myotonica Protein Kinase Transcripts for the Treatment of Myotonic Dystrophy Type 1. Hum. Gene Ther. 2022, 33, 810–820. [Google Scholar] [CrossRef]
- Pascual-Gilabert, M.; Artero, R.; López-Castel, A. The myotonic dystrophy type 1 drug development pipeline: 2022 edition. Drug Discov. Today 2023, 28, 103489. [Google Scholar] [CrossRef]
- Llagostera, E.; López, M.J.Á.; Scimia, C.; Catalucci, D.; Párrizas, M.; Ruiz-Lozano, P.; Kaliman, P. Altered β-adrenergic response in mice lacking myotonic dystrophy protein kinase (DMPK). Muscle Nerve 2012, 45, 128–130. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.F.; Varela, M.A.; Arandel, L.; Holland, A.; Naouar, N.; Arzumanov, A.; Seoane, D.; Revillod, L.; Bassez, G.; Ferry, A.; et al. Peptide-conjugated oligonucleotides evoke long-lasting myotonic dystrophy correction in patient-derived cells and mice. J. Clin. Investig. 2019, 129, 4739–4744. [Google Scholar] [CrossRef]
- Vertex Pharmaceuticals Incorporated. A Phase 1/2, Randomized, Double-Blind, Placebo-Controlled Single- and Multiple-Dose Escalation Study Evaluating the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of VX-670 in Adult Subjects with Myotonic Dystrophy Type 1. Available online: https://www.clinicaltrials.gov/study/NCT06185764 (accessed on 2 September 2025).
- Wang, Y.; Hao, L.; Wang, H.; Santostefano, K.; Thapa, A.; Cleary, J.; Li, H.; Guo, X.; Terada, N.; Ashizawa, T.; et al. Therapeutic Genome Editing for Myotonic Dystrophy Type 1 Using CRISPR/Cas9. Mol. Ther. 2018, 26, 2617–2630. [Google Scholar] [CrossRef]
- Dastidar, S.; Ardui, S.; Singh, K.; Majumdar, D.; Nair, N.; Fu, Y.; Reyon, D.; Samara, E.; Gerli, M.F.M.; Klein, A.F.; et al. Efficient CRISPR/Cas9-mediated editing of trinucleotide repeat expansion in myotonic dystrophy patient-derived iPS and myogenic cells. Nucleic Acids Res. 2018, 46, 8275–8298. [Google Scholar] [CrossRef]
- Bérenger-Currias, N.; Martinat, C.; Baghdoyan, S. Pluripotent Stem Cells in Disease Modeling and Drug Discovery for Myotonic Dystrophy Type 1. Cells 2023, 12, 571. [Google Scholar] [CrossRef]
- Ikeda, M.; Taniguchi-Ikeda, M.; Kato, T.; Shinkai, Y.; Tanaka, S.; Hagiwara, H.; Sasaki, N.; Masaki, T.; Matsumura, K.; Sonoo, M.; et al. Unexpected Mutations by CRISPR-Cas9 CTG Repeat Excision in Myotonic Dystrophy and Use of CRISPR Interference as an Alternative Approach. Mol. Ther. Methods Clin. Dev. 2020, 18, 131–144. [Google Scholar] [CrossRef]
- Sandonà, M.; Cavioli, G.; Renzini, A.; Cedola, A.; Gigli, G.; Coletti, D.; McKinsey, T.A.; Moresi, V.; Saccone, V. Histone Deacetylases: Molecular Mechanisms and Therapeutic Implications for Muscular Dystrophies. Int. J. Mol. Sci. 2023, 24, 4306. [Google Scholar] [CrossRef]
- Neault, N.; Ravel-Chapuis, A.; Baird, S.D.; Lunde, J.A.; Poirier, M.; Staykov, E.; Plaza-Diaz, J.; Medina, G.; Abadía-Molina, F.; Jasmin, B.J.; et al. Vorinostat Improves Myotonic Dystrophy Type 1 Splicing Abnormalities in DM1 Muscle Cell Lines and Skeletal Muscle from a DM1 Mouse Model. Int. J. Mol. Sci. 2023, 24, 3794. [Google Scholar] [CrossRef]
- Zhang, F.; Bodycombe, N.E.; Haskell, K.M.; Sun, Y.L.; Wang, E.T.; Morris, C.A.; Jones, L.H.; Wood, L.D.; Pletcher, M.T. A flow cytometry-based screen identifies MBNL1 modulators that rescue splicing defects in myotonic dystrophy type I. Hum. Mol. Genet. 2017, 26, 3056–3068. [Google Scholar] [CrossRef]
- Ravel-Chapuis, A.; Duchesne, E.; Jasmin, B.J. Pharmacological and exercise-induced activation of AMPK as emerging therapies for myotonic dystrophy type 1 patients. J. Physiol. 2022, 600, 3249–3264. [Google Scholar] [CrossRef]
- Bassez, G.; Audureau, E.; Hogrel, J.-Y.; Arrouasse, R.; Baghdoyan, S.; Bhugaloo, H.; Gourlay-Chu, M.-L.; Le Corvoisier, P.; Peschanski, M. Improved mobility with metformin in patients with myotonic dystrophy type 1: A randomized controlled trial. Brain 2018, 141, 2855–2865. [Google Scholar] [CrossRef]
- Horrigan, J.; Gomes, T.B.; Snape, M.; Nikolenko, N.; McMorn, A.; Evans, S.; Yaroshinsky, A.; Della Pasqua, O.; Oosterholt, S.; Lochmüller, H. A Phase 2 Study of AMO-02 (Tideglusib) in Congenital and Childhood-Onset Myotonic Dystrophy Type 1 (DM1). Pediatr. Neurol. 2020, 112, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, N.; Cavus, O.; Wallace, M.J.; Bobik, J.T.; You, K.; Takenaka, S.S.; Abdallah, D.; Mohler, E.J.; Antwi-Boasiako, S.; Murphy, N.P.; et al. Evaluation of Tideglusib as a Disease Modifying Therapy in Murine Models of Arrhythmogenic Cardiomyopathy. JACC Basic Transl. Sci. 2025, 10, 101281. [Google Scholar] [CrossRef]
- AMO-02—AMO-Pharma.com. Available online: https://www.amo-pharma.com/amo_02.htm (accessed on 12 February 2025).
- Clinical Trials Register. Available online: https://www.clinicaltrialsregister.eu/ctr-search/trial/2018-000692-32/IT (accessed on 12 February 2025).
- Harmony Biosciences Presents Positive Data for Pitolisant in the Treatment of Excessive Daytime Sleepiness and Fatigue in Myotonic Dystrophy Type 1|Harmony Biosciences. Available online: https://ir.harmonybiosciences.com/news-releases/news-release-details/harmony-biosciences-presents-positive-data-pitolisant-treatment/ (accessed on 12 February 2025).
- Logigian, E.L.; Martens, W.B.; Moxley, R.T.; McDermott, M.P.; Dilek, N.; Wiegner, A.W.; Pearson, A.T.; Barbieri, C.A.; Annis, C.L.; Thornton, C.A.; et al. Mexiletine is an effective antimyotonia treatment in myotonic dystrophy type 1(LOE Classification). Neurology 2010, 74, 1441–1448. [Google Scholar] [CrossRef]
- Lupin Ltd. A Randomized, Double-Blind, Placebo-Controlled, Multi-Center Study to Investigate the Efficacy and Safety of Once Daily Mexiletine PR During 26 Weeks of Treatment in Patients with Myotonic Dystrophy Type 1 and Type 2 (Phase 3). Available online: https://www.clinicaltrials.gov/study/NCT04700046 (accessed on 2 September 2025).
- Lawless, M.; Arnold, W.; Agriesti, J.; Moravec, T.; Moravec, T.; Moravec, T. Investigation of Ranolazine as an Anti-myotonia Treatment in Myotonic Dystrophy Type 1 (P5.443). Neurology 2018, 90, P5.443. [Google Scholar] [CrossRef]
- Arnold, W. Open Label Trial of Ranolazine in Myotonia Congenita, Paramyotonia Congenita, & Myotonic Dystrophy Type 1. Available online: https://www.clinicaltrials.gov/study/NCT02251457 (accessed on 2 September 2025).
- Edokpolor, K.S.; Banerjee, A.; McEachin, Z.T.; Gu, J.; Kosti, A.; Arboleda, J.D.; García, P.S.; Wang, E.T.; Bassell, G.J. Altered Behavioral Responses Show GABA Sensitivity in Muscleblind-Like 2-Deficient Mice: Implications for CNS Symptoms in Myotonic Dystrophy. eNeuro 2022, 9, ENEURO.0218-22.2022. [Google Scholar] [CrossRef]
- Sampson, J.; Wang, E.; Day, J.; Gutmann, L.; Mezerhane, E.; Seto, A.; Ehrich, E. Results of Double-blind, Placebo-controlled, Dose Range Finding, Crossover Study of Single Day Administration of ERX-963 (IV Flumazenil) in Adults with Myotonic Dystrophy Type 1 (2834). Neurology 2021, 96, 2834. [Google Scholar] [CrossRef]
- Mishra, S.K.; Hicks, S.M.; Frias, J.A.; Vangaveti, S.; Nakamori, M.; Cleary, J.D.; Reddy, K.; Berglund, J.A. Quercetin selectively reduces expanded repeat RNA levels in models of myotonic dystrophy. bioRxiv 2023. [Google Scholar] [CrossRef]
- Nakamori, M.; Nakatani, D.; Sato, T.; Hasuike, Y.; Kon, S.; Saito, T.; Nakamura, H.; Takahashi, M.P.; Hida, E.; Komaki, H.; et al. Erythromycin for myotonic dystrophy type 1: A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. eClinicalMedicine 2024, 67, 102390. [Google Scholar] [CrossRef]
- Berthomier, A. Results of Phase II Trial Testing Erythromycin in DM1; Institut de Myologie: Paris, France, 2024. [Google Scholar]
- DM1. Available online: https://www.aviditybiosciences.com/pipeline/dm1/ (accessed on 12 February 2025).
- Dyne Therapeutics Announces Initiation of Phase 1/2 ACHIEVE Clinical Trial of DYNE-101 for the Treatment of Myotonic Dystrophy Type 1|Dyne Therapeutics, Inc. Available online: https://investors.dyne-tx.com/news-releases/news-release-details/dyne-therapeutics-announces-initiation-phase-12-achieve-clinical/ (accessed on 12 February 2025).
- Dyne Therapeutics. A Randomized, Placebo-Controlled, Multiple Ascending Dose Study Assessing Safety, Tolerability, Pharmacodynamics, Efficacy, and Pharmacokinetics of DYNE-101 Administered to Participants with Myotonic Dystrophy Type 1. Available online: https://clinicaltrials.gov/study/NCT05481879 (accessed on 2 September 2025).
- De Serres-Bérard, T.; Ait Benichou, S.; Jauvin, D.; Boutjdir, M.; Puymirat, J.; Chahine, M. Recent Progress and Challenges in the Development of Antisense Therapies for Myotonic Dystrophy Type 1. Int. J. Mol. Sci. 2022, 23, 13359. [Google Scholar] [CrossRef]
- Ait Benichou, S.; Jauvin, D.; De Serres-Bérard, T.; Pierre, M.; Ling, K.K.; Bennett, C.F.; Rigo, F.; Gourdon, G.; Chahine, M.; Puymirat, J. Antisense oligonucleotides as a potential treatment for brain deficits observed in myotonic dystrophy type 1. Gene Ther. 2022, 29, 698–709. [Google Scholar] [CrossRef]
- Vertex and Entrada Therapeutics Establish Collaboration to Discover and Develop Endosomal Escape Vehicle (EEV) Therapeutics for Myotonic Dystrophy Type 1 (DM1)|Vertex Pharmaceuticals. Available online: https://investors.vrtx.com/news-releases/news-release-details/vertex-and-entrada-therapeutics-establish-collaboration-discover (accessed on 12 February 2025).
- Cardinali, B.; Provenzano, C.; Izzo, M.; Voellenkle, C.; Battistini, J.; Strimpakos, G.; Golini, E.; Mandillo, S.; Scavizzi, F.; Raspa, M.; et al. Time-controlled and muscle-specific CRISPR/Cas9-mediated deletion of CTG-repeat expansion in the DMPK gene. Mol. Ther. Nucleic Acids 2021, 27, 184–199. [Google Scholar] [CrossRef] [PubMed]
- Izzo, M.; Battistini, J.; Golini, E.; Voellenkle, C.; Provenzano, C.; Orsini, T.; Strimpakos, G.; Scavizzi, F.; Raspa, M.; Baci, D.; et al. Muscle-specific gene editing improves molecular and phenotypic defects in a mouse model of myotonic dystrophy type 1. Clin. Transl. Med. 2025, 15, e70227. [Google Scholar] [CrossRef]
- Hanifi, M.; Ates-Kalkan, P.S.; Wen, S.; Fischer, M.; Kroesen, A.; Yu, Z.; Wood, M.; Thongjuea, S.; Mead, A.; Fulga, T.A.; et al. Robust CRISPR/dCas13 RNA blockers specifically perturb miRNA-target interactions and rescue type 1 myotonic dystrophy pathology. bioRxiv 2024. [Google Scholar] [CrossRef]
- Patient Dosing Commenced for Phase 1/2 ArthemiR Trial of ATX-01 in Myotonic Dystrophy Type 1. Available online: https://www.neurologylive.com/view/patient-dosing-commenced-phase-1-2-arthemir-trial-atx-01-myotonic-dystrophy-type-1 (accessed on 12 February 2025).
- Our Approach & Focus. Available online: https://www.arthexbiotech.com/our-approach-focus (accessed on 12 February 2025).

| Form of DM1 | CTG Repeat Range | Onset * | Key Cardiac Features | Skeletal Muscle and Other Systemic Features | Severity | Primary Challenges | References |
|---|---|---|---|---|---|---|---|
| Mild (Late-Onset) Form | 50–150 | Middle to late adulthood | Mild conduction abnormalities; occasional first-degree AV block; low incidence of structural abnormalities. | Cataracts, mild myotonia, minimal muscle weakness. | Least severe | Often undiagnosed due to subtle symptoms; may miss early intervention for cardiac monitoring. | [24,27,41,42,43,44,45] |
| Classical (Adult-Onset) Form | 250–500 | Late teens to early adulthood | Progressive conduction defects, including PR prolongation and bundle branch block; QTc (corrected QT) prolongation; moderate arrhythmia risk. | Progressive muscle weakness, severe myotonia, cataracts, insulin resistance. | Moderate to severe | Requires ongoing cardiac monitoring due to arrhythmia risk; symptomatic management of muscle issues. | [24,43,44,45,46,47,48,49] |
| Congenital Form | >1000 | Birth or early infancy | Severe conduction delays; AV block, high risk of ventricular arrhythmias; QTc prolongation; structural abnormalities including fibrosis. | Severe hypotonia, respiratory distress, developmental delay, cognitive impairments, dysphagia. | Most severe | Immediate cardiac and respiratory support; early intervention needed for developmental support. | [24,33,45,49,50] |
| Juvenile Form | 500–1000 | Childhood to early adolescence | PR and QRS prolongation; moderate QTc prolongation; risk of atrial and ventricular arrhythmias. | Cognitive deficits, myotonia, gastrointestinal issues, mild developmental delay. | Severe | Progressive arrhythmia risk; requires multidisciplinary care to address systemic complications. | [24,45,49] |
| Model | Generation Strategy | Phenotype Manifestations | Limitations | Research Application | References |
|---|---|---|---|---|---|
| DMPK KO | DMPK gene deletion | Mild skeletal myopathy and cardiac defects | Does not fully replicate DM1 pathology, particularly RNA gain-of-function effects; potential compensatory mechanisms may mask phenotypes. | Studying the role of DMPK in muscle and cardiac function; assessing the impact of DMPK loss. | [107,108] |
| HSALR | Overexpression of human skeletal actin with 250 CTG repeats | Myotonia, muscle weakness, RNA foci formation, splicing defects | Limited to skeletal muscles; does not model for cardiac aspect of DM1 | Investigation of RNA toxicity in skeletal muscle. | [109,110] |
| DM300 | Insertion of a 45 kb human genomic fragment containing DMPK with 300 CTG repeats | Myotonia, impaired glucose metabolism, muscle atrophy, and RNA foci. | CTG repeat instability in subsequent generations; high mortality; limited splicing alterations | Studying DMPK transcription toxicities in tissues. | [111] |
| DMSXL | Insertion of a 45 kb human genomic fragment containing DMPK with >1000 CTG repeats. (Over the generations of DM300) | Motor deficits, RNA foci, MBNL1 sequestration, cerebellar dysfunction, splicing alterations, behavioral abnormalities, cardiac conduction, electrophysiological abnormalities | Decline in transgene expression with age; severe weight loss; high mortality rates | Studying congenital and adult-onset DM1, therapeutic testing | [81,85,86,112] |
| TREDT960I/MDAF-rtTA | Insertion of a tetracycline-responsive expanded transgene with DMPK exon 11–15 transgenes, heart-specific rtTA expression. | RNA foci, MBNL1 sequestration, CELF1 protein upregulation, alternative Splicing alterations, myopathy, and muscle wasting | There is no reproduction of CTG repeat continuity. | Studying and understanding the various mechanisms of CUG-induced muscle wasting. | [113] |
| EpA960/ CamKII-Cre | Inducible expression of DMPK exon 15 with 960 CTG repeats; brain-specific Cre expression | RNA foci, MBNL1 sequestration, CELF1 upregulation, splicing alterations, learning deficits, brain atrophy, neurotransmission dysfunction | Does not reproduce CTG repeat instability; limited to neural tissues | Identifying neural degeneration related to CTG repeat expansions | [114] |
| DM200 | Inducible expression of DMPK 3′ UTR with 200 CTG repeats replacing coding sequence with GFP | Cardiac conduction abnormalities, MBNL1 sequestration, RNA foci, and myotonia | Splicing changes in the heart not fully characterized | Investigation of splicing defects and their progression. | [115] |
| MBNL1 KO | Deletion of MBNL1 exon 3 | Splicing abnormalities, myotonia, cataracts | Mild brain alterations; limited spliceopathy compared to DM1 | Investigating MBNL1 function, splicing defect studies | [116,117] |
| MBNL2 KO | Deletion of MBNL2 exon 2 | Spatial memory deficits, reduced synaptic plasticity, and splicing alterations | Does not replicate DM1 muscle phenotype | Evaluating MBNL2’s role in splicing regulation and DM1 phenotype | [118] |
| MBNL3 KO | Deletion of MBNL3 exon 2 | Delayed muscle regeneration, neonatal hypotonia | MBNL3 truncation; limited impact on adult muscle function | Assessing functional redundancy among MBNL proteins | [119] |
| MBNL1/MBNL2 KO | Double knockout of MBNL1 and MBNL2 | Myopathy, motor deficits, brain tissue alterations, and skeletal abnormalities. | Reduced lifespan and high mortality before birth. | Evaluating combined loss of MBNL1 and MBNL2 in DM1 muscle phenotype | [120] |
| MBNL1/MBNL3 KO | Double knockout of MBNL2 and MBNL3 by deleting MBLN1 exon 3 and MBNL exon 2 | Impaired chloride conductance, reduced muscle strength, myopathy, and myotonia | Minor brain alterations and limited spliceopathy. | Evaluating combined loss of MBNL1 and MBNL3 in DM1 muscle phenotype | [121] |
| Mbnl1ΔE3/ΔE3; Mbnl2C//C; Mbnl3C; Myo-Cre+/− | Mbnl1 knockout: muscle-specific Cre-mediated MBNL2 and MBNL3 knockout | Spliceopathy, myopathy, muscle wasting, and respiratory difficulties. | Reduced lifespan and high mortality before birth. | Evaluating loss of all the MBNL proteins and their role in DM1 muscular phenotype | [122] |
| TRECUGBP1 | Insertion of a tetracycline-responsive human expressing CELF1 transgene; heart-specific reverse tet trans activator (rtTA) | Splicing alterations and systolic dysfunction. | Limited to cardiac pathology. | Evaluating contribution of CELF 1 expression to DM1 cardiac phenotype. | [123,124] |
| GFP-DMPK-(CTG)X | Overexpression of DMPK 3′ UTR with either the wild-type, 11, or expanded, 91, CTG repeats. | RNA foci formation, myotonia, cardiac conduction defects, splicing abnormalities. | Potential for permanent overexpression of human DMPK; does not fully replicate multisystemic aspects of DM1. | Understanding the role of DMPK expression and RNA toxicity in DM1 pathogenesis; evaluating therapeutic interventions targeting RNA toxicity. | [125] |
| Tg26-hDMPK | Overexpression of human DMPK gene in transgenic mice | Myocardial hypertrophy, fibrosis, cardiomyopathy, intracellular calcium overload, reduced blood pressure, and myopathy. | Deficits in chloride channels necessitating use of hyper excitability regulators. Over-expression of hDMPK and increased risks of hypotension. Reduced blood pressure. | Understanding the role of proper expression of hDMPK in ion homeostasis, viability control in muscle cell types, and cytoarchitectural infrastructure. | [126] |
| TREDT960I/α-MHC-rtTA | Insertion of a tetracycline-responsive transgene containing DMPK exons 11–15 with 960 interrupted CTG repeats; heart-specific rtTA expression under the α-myosin heavy chain promoter. | RNA foci, MBNL1 sequestration, CELF1 protein upregulation, alternative splicing alterations, arrhythmias. | Does not reproduce CTG repeat continuity; limited to cardiac tissue. | Studying changes in ionic transport in cardio myocytes with CUG toxicities. | [127] |
| LC15 | Insertion of the expanded CTG repeat from the DMPK 3′ UTR downstream of a luciferase reporter gene under the control of the CMV-βA promoter. | Prolonged QRS and corrected QT (QTc) intervals, increased susceptibility to ventricular arrhythmias upon flecainide administration, RNA foci formation. | Limited to cardiac defects; does not model multisystemic aspects of DM1. | Evaluating cardiac conduction abnormalities and arrhythmogenic susceptibility in DM1. | [90] |
| DM1 iPSC-CM study | Key Findings | Affected Channels/Genes | Clinical Relevance | References |
|---|---|---|---|---|
| Spitalieri et al., 2018 | Accumulation of RNA foci and MBNL1 sequestration Mis-splicing of SCN5A leading to fetal isoform expression Reduced Na+ and Ca2+ current densities Prolonged action potentials and decreased conduction velocities Impaired calcium transient propagation Observation of arrhythmogenic events | MBNL1 MBNL2 TNNT2 SCN5A CACNA1C KCNH2 KCNQ1 KCND3 | Recapitulates molecular markers of DM1 Demonstrates altered electrophysiological parameters and biomechanical behavior consistent with unstable cardiac function | [137] |
| Poulin et al., 2021 | Abnormal ion channel functions Slower conduction velocities | SCN5A CACNA1C KCNH2 | Highlights the arrhythmogenic potential due to ion channel dysfunction in DM1 cardiomyocytes | [80] |
| Kim et al., 2019 | Presence of MBNL1-positive intranuclear foci Aberrant splicing of target genes Distinct Ca2+ transient abnormalities | MBNL1 TNNT2 SCN5A | Differentiates pathological signatures between DM1 and DM2 Emphasizes the role of MBNL1 sequestration in DM1 cardiac pathology | [139] |
| Pierre et al., 2023 | Accumulation of RNA foci and MBNL1 sequestration Mis-splicing of SCN5A leading to fetal isoform expression Reduced Na+ and Ca2+ current densities Prolonged action potentials and decreased conduction velocities Impaired calcium transient propagation Observation of arrhythmogenic events | SCN5A DMPK MBNL1 | Provides insights into molecular and electrophysiological mechanisms underlying DM1 cardiac involvement Highlights the critical role of voltage-gated sodium channels in DM1-related cardiac dysfunctions | [83] |
| Therapeutic Class | Drug Candidate | Mechanism | Preclinical/Clinical Model | Current Status | Key Findings | Limitations | References |
|---|---|---|---|---|---|---|---|
| Small Molecules | Tideglusib | GSK3β inhibitor; reduces RNA foci and normalizes CELF1 | HSALR, DMSXL mice; muscle biopsies from patients | Phase III | Improves myotonia, muscle strength, and cognitive symptoms | Limited long-term data and unproven in adults. | [166] |
| Metformin | Activates AMPK pathway; modulates glucose metabolism | iPSC-CMs, DM1 patient trials | Phase III | Enhances muscle function and motility | Insufficient multisystem benefit and lack of robust long-term data. | [163,167] | |
| Pitolisant | Histamine H3 antagonist; targets daytime sleepiness | Clinical trials | Phase II | Reduces excessive daytime sleepiness in DM1 patients | Limited to non-muscular symptoms (EDS, fatigue) and no effect on multisystemic. | [168] | |
| Mexiletine | Sodium channel blocker; reduces myotonia | Clinical trials | Phase III | Decreases muscle stiffness, improves handgrip strength | GI intolerance, unproven benefit for fatigue and multisystem features. | [169,170] | |
| Ranolazine | Sodium channel blocker; targets arrhythmias | Clinical trials | Completed Phase I | Limited impact on muscle function, improves heart rhythm | Not recommended to those with existing long QTc and limited long-term safety. | [171,172] | |
| Flumazenil | GABA receptor modulator; treats cognitive symptoms | Clinical trials | Phase 1 | Reduces hypersomnia, improves cognitive function | Short duration of action, primarily targets CNS symptoms, limited availability and access. | [173,174] | |
| Quercetin | Reduces toxic mRNA levels; exhibits senolytic activity | Cellular and animal models of DM1 | Preclinical | Selectively reduces expanded repeat RNA levels and reverses accelerated aging phenotypes in DM1 models | Reversion of benefit and possible cell toxicity at higher doses and Long-term safety unstudied in DM1. | [175] | |
| Vorinostat | Targets DMPK and inhibits mutant DMPK levels | HSALR models | Preclinical | Reduces DMPK, rescued MBNL1 sequestration and spliceopathy. | Potential off-target effects, toxicity at higher concentrations, and unknown long-term safety. | [160] | |
| Erythromycin | Antibiotic; reduces RNA foci accumulation | Cell and mouse models | Phase II | Improves splicing, decreases foci | Modest efficacy and GI side effects for long-term usage. | [176,177] | |
| siRNA molecules | AOC 1001 | siRNA targeting DMPK; reduces DMPK mRNA via TfR1-mediated delivery | Clinical trials | Phase I/II | Reduces DMPK mRNA in muscle tissues, corrects splicing | Off-target risks and possible immune response to antibody-oligonucleotide conjugate. | [178] |
| ARO-DM1 | siRNA is a ligand conjugated via TRiM to target DMPK | Clinical trails | Phase I/IIa | Reduces DMPK RNA in skeletal muscle. | Off-target risks, unknown safety, and immune response. | [145] | |
| Antisense Oligonucleotides | DYNE-101 | ASO conjugated with monoclonal antibody for hTfR1 targeting | Clinical trials | Phase I/II | Reduces DMPK RNA in skeletal and cardiac muscle, splicing correction | Unknow long-term safety and moderate side effects. | [179,180] |
| IONIS-DMPKRx | ASO; targets DMPK mRNA for degradation | DMSXL mouse models | Preclinical | Reduces RNA foci, restores MBNL protein levels | Primarily impacts muscle, not multisystemic and insufficient concentration in muscle. | [150,181,182] | |
| Pip6a-PMO-CAG7 | Peptide-PMO conjugate; targets CUG repeats | HSALR model | Preclinical | Decreases RNA foci and rescues splicing | Benefit and delivery efficiency in cardiac, CNS tissues unproven. | [153] | |
| ENTR-701 | Peptide-conjugated PMO; blocks CUG repeats | HSALR model, patient-derived cells | Preclinical/sold to Vertex Therapeutics | Reduces RNA foci, corrects splicing defects | Delivery efficiency unproven, potential immune or off-target. | [183] | |
| VX-670 | Peptide-conjugated PMO; blocks CUG repeats | Clinical trails | Phase I/II | Reduces RNA foci, corrects splicing defects | Efficacy unproven, mechanism and tissue distribution still under study. | [154] | |
| Gene Editing | AAV-CRISPR-SaCas9 | CRISPR/Cas9; excises CTG repeats | DMSXL model | Preclinical | Reduces RNA foci, rescues muscle weakness | Very early-stage preclinical trails, delivery efficiency, potential off-target, immune response. | [184,185] |
| AAV-PIN-dCas9 | dCas9-PIN fusion; degrades toxic RNA | Adult and neonatal HSALR models | Lead selection | Reduces RNA foci, rescues muscle weakness | Immunogenicity. Delivery efficiency, packaging constraint, off-target effects. | [186] | |
| Anti-miRNA | ATX-01 | Inhibit MBNL regulator microRNA-23b (over expresses MBNL) | Clinical trails | Phase I/II | Improves splicing, rescues muscle phenotypes | Immunogenicity and off-target. | [146,187,188] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ginjupalli, V.K.M.; Reisqs, J.-B.; Cupelli, M.; Chahine, M.; Boutjdir, M. Cardiac Involvement in Myotonic Dystrophy Type 1: Mechanisms, Clinical Perspectives, and Emerging Therapeutic Strategies. Int. J. Mol. Sci. 2025, 26, 10992. https://doi.org/10.3390/ijms262210992
Ginjupalli VKM, Reisqs J-B, Cupelli M, Chahine M, Boutjdir M. Cardiac Involvement in Myotonic Dystrophy Type 1: Mechanisms, Clinical Perspectives, and Emerging Therapeutic Strategies. International Journal of Molecular Sciences. 2025; 26(22):10992. https://doi.org/10.3390/ijms262210992
Chicago/Turabian StyleGinjupalli, Vamsi Krishna Murthy, Jean-Baptiste Reisqs, Michael Cupelli, Mohamed Chahine, and Mohamed Boutjdir. 2025. "Cardiac Involvement in Myotonic Dystrophy Type 1: Mechanisms, Clinical Perspectives, and Emerging Therapeutic Strategies" International Journal of Molecular Sciences 26, no. 22: 10992. https://doi.org/10.3390/ijms262210992
APA StyleGinjupalli, V. K. M., Reisqs, J.-B., Cupelli, M., Chahine, M., & Boutjdir, M. (2025). Cardiac Involvement in Myotonic Dystrophy Type 1: Mechanisms, Clinical Perspectives, and Emerging Therapeutic Strategies. International Journal of Molecular Sciences, 26(22), 10992. https://doi.org/10.3390/ijms262210992






