Ethanol–Withanolides Interactions: Compound-Specific Effects on Zebrafish Larvae Locomotor Behavior and GABAA Receptor Subunit Expression
Abstract
1. Introduction
2. Results
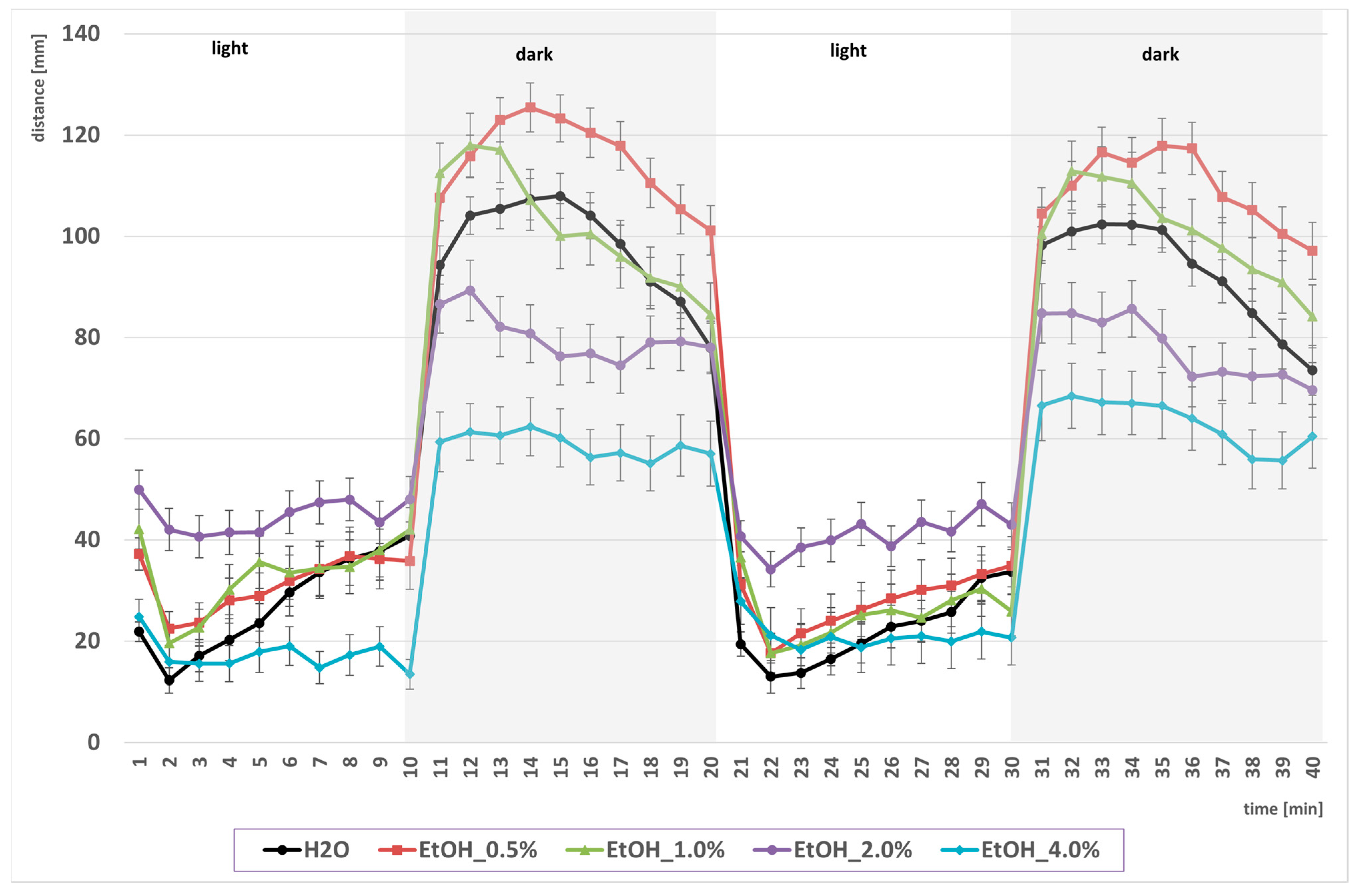
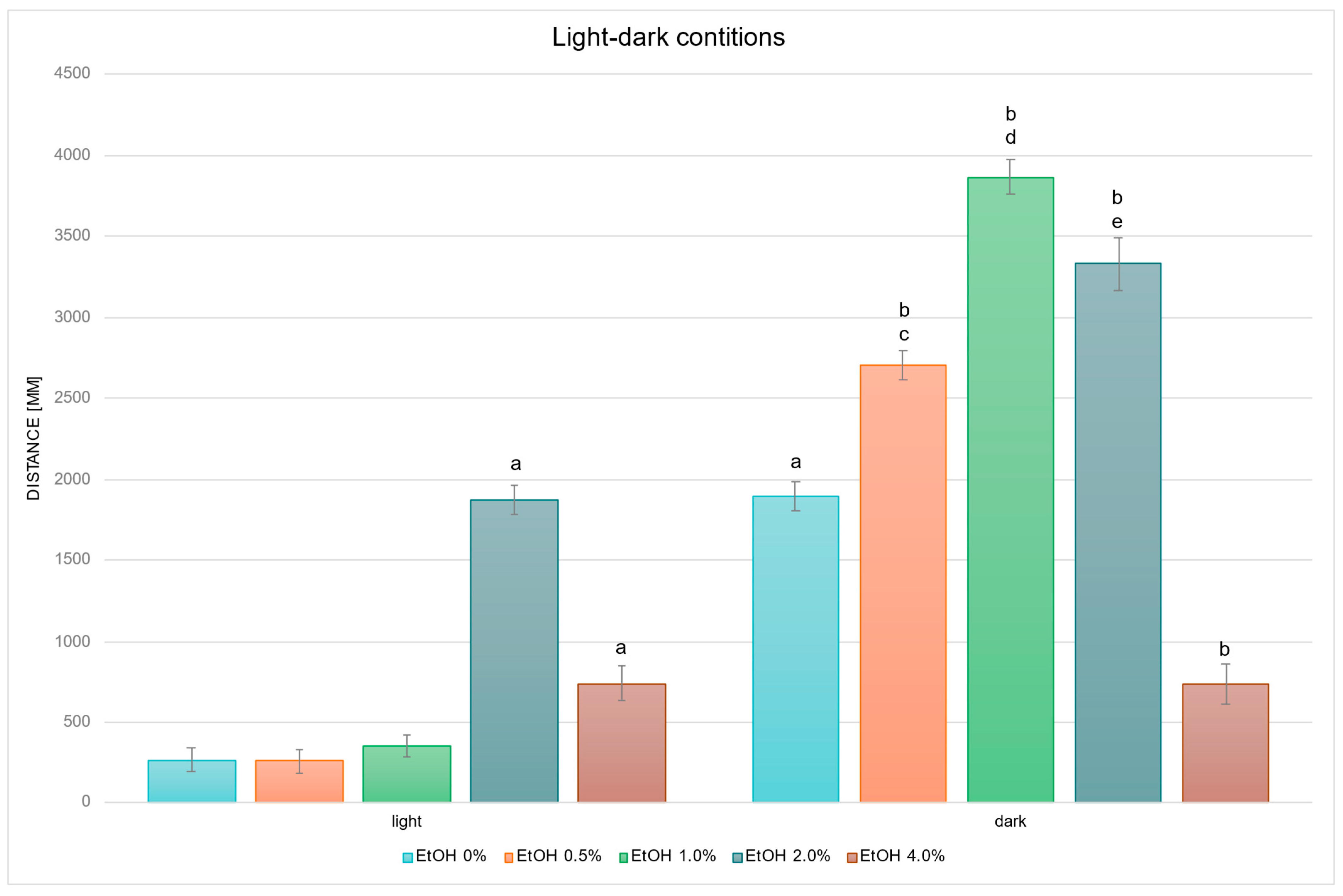
2.1. Biphasic Locomotor Response to EtOH Exposure
2.2. Withanolide Modulation of EtOH-Driven Locomotor Activity
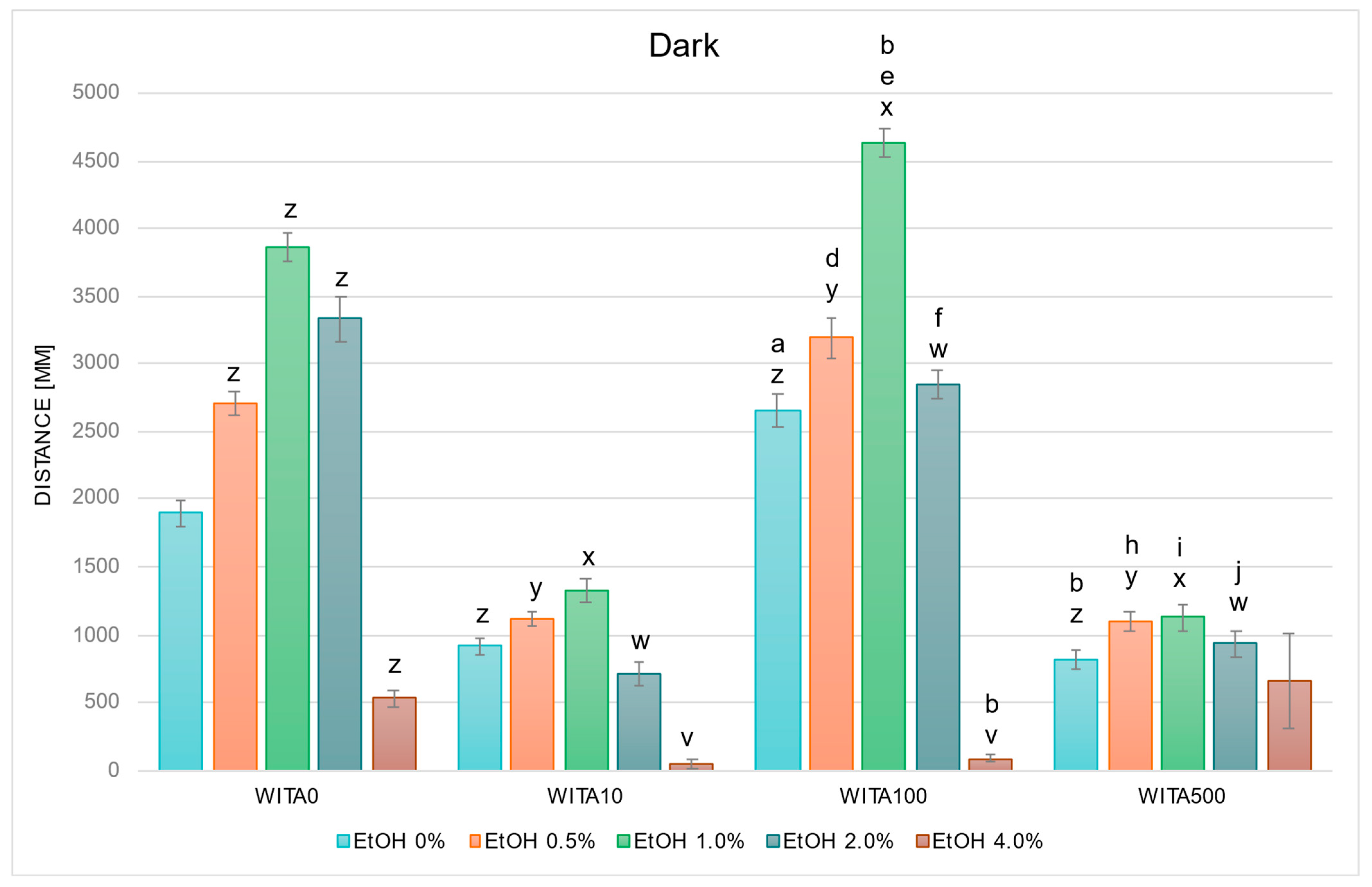
2.2.1. WITA Alters EtOH-Induced Behavior
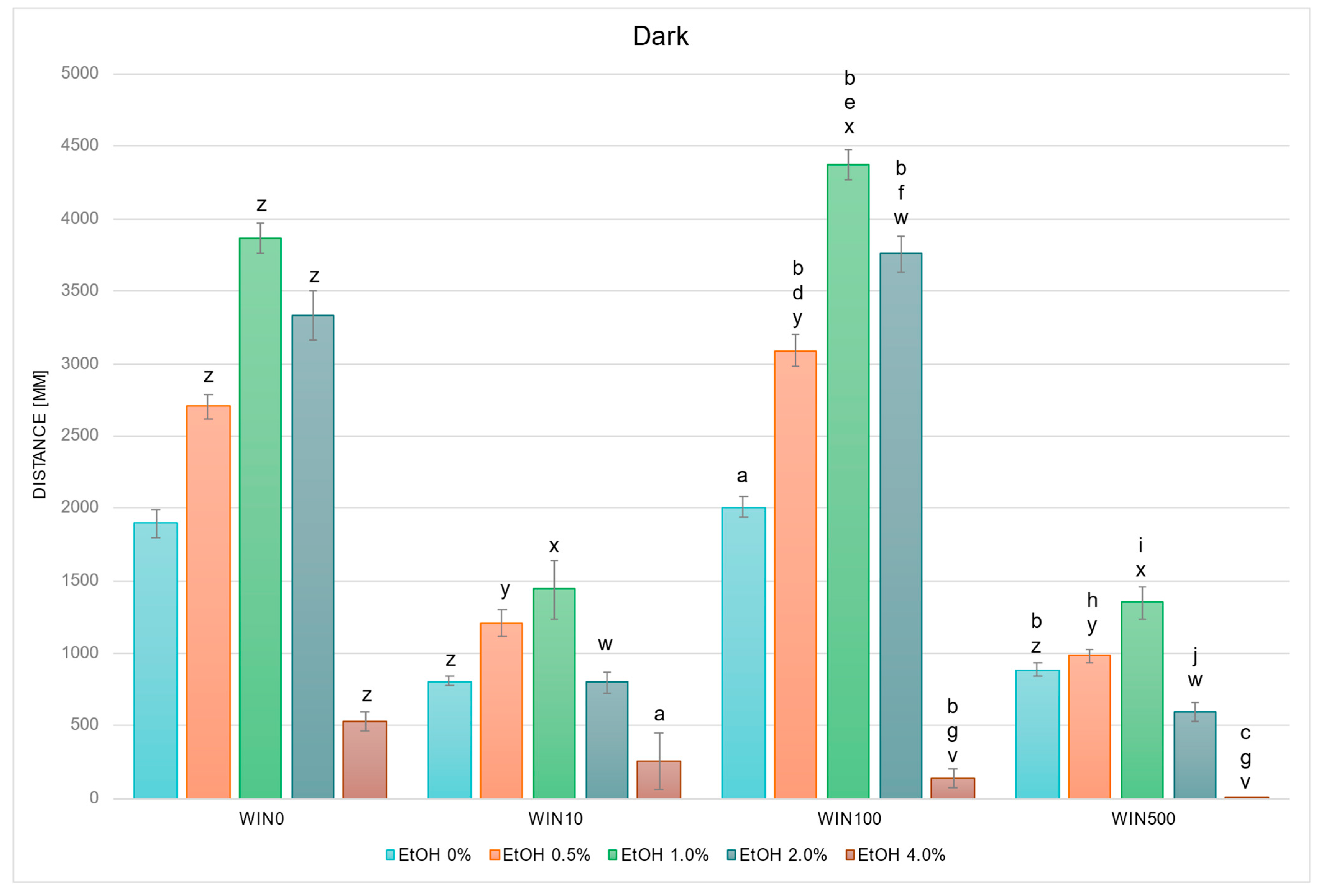
2.2.2. WIN Modifies Locomotor Response Under EtOH Exposure
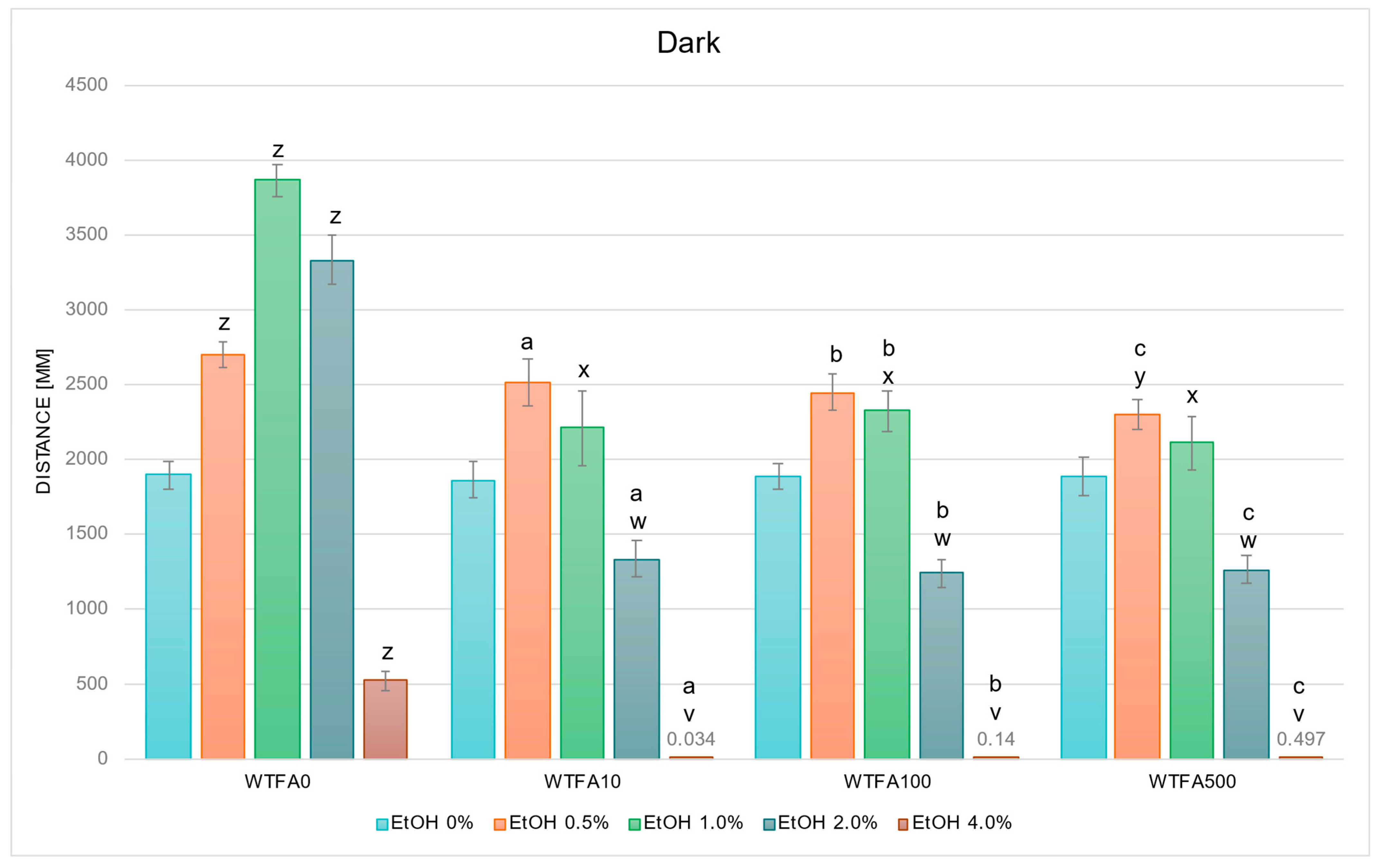
2.2.3. WTFA Alters EtOH-Induced Behavior
2.3. Effects on GABAA Receptor Subunit Gene Expression
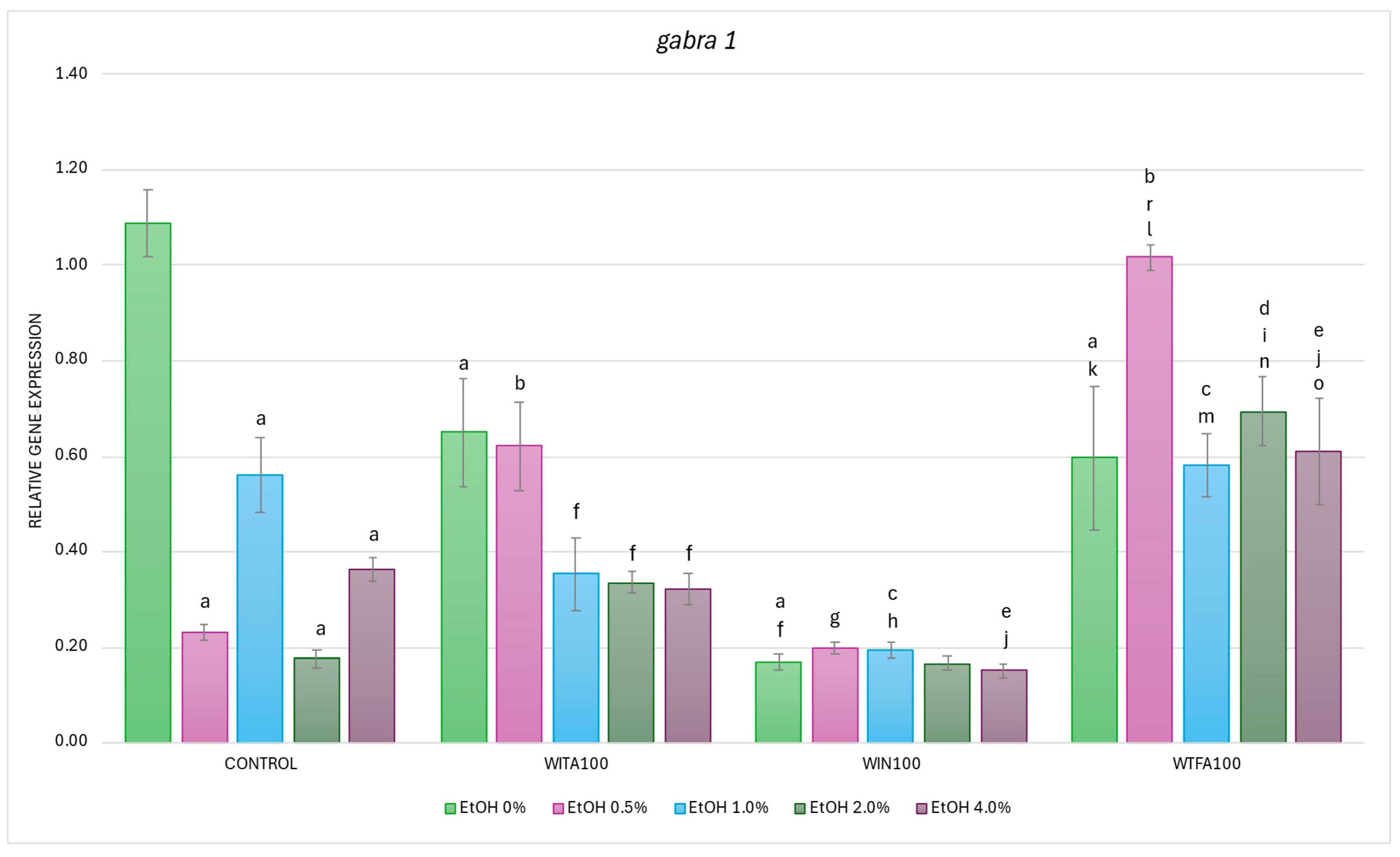
2.3.1. gabra1 Expression Affected by EtOH and Withanolides
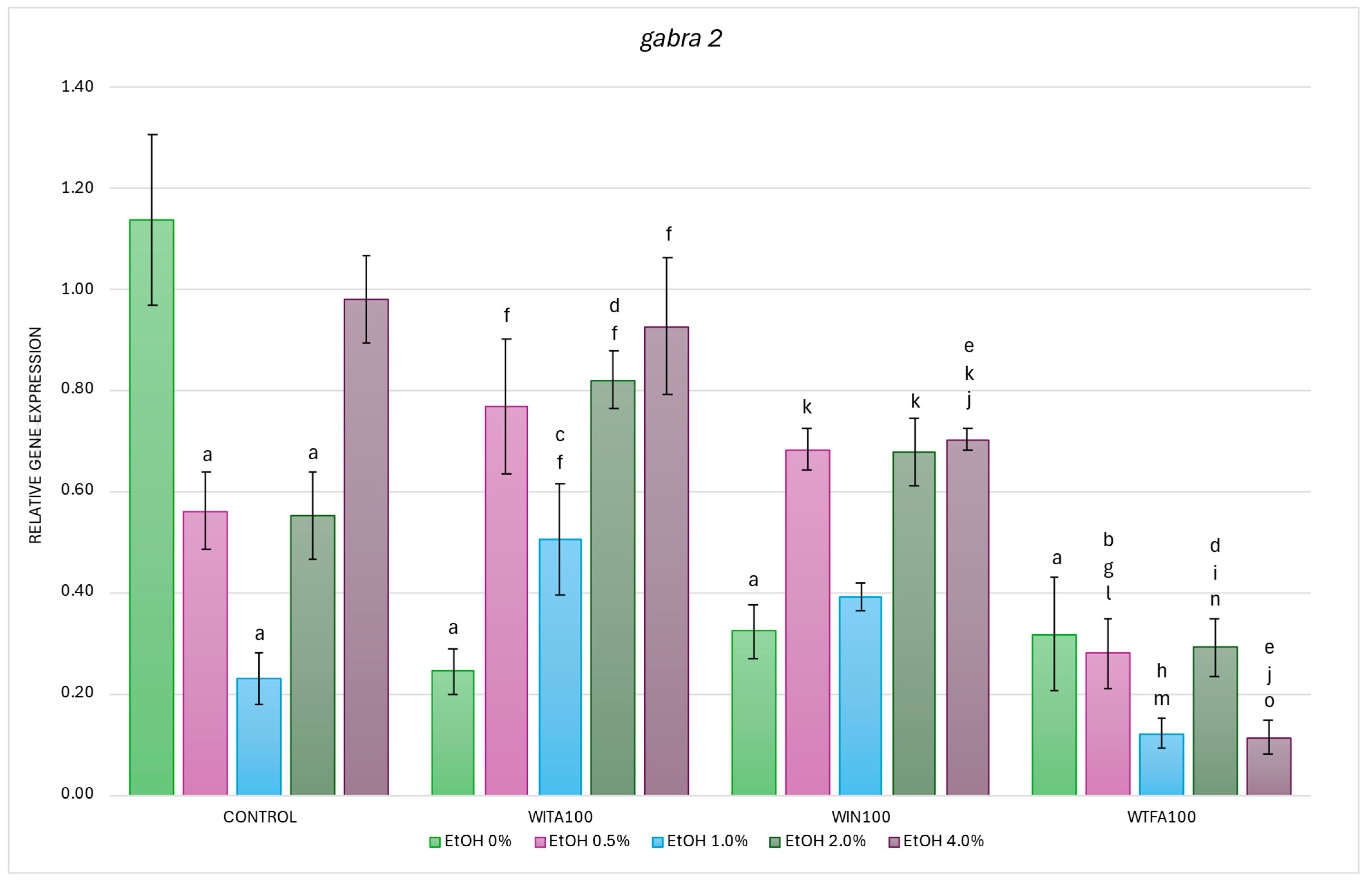
2.3.2. gabra2 Expression Affected by EtOH and Withanolides
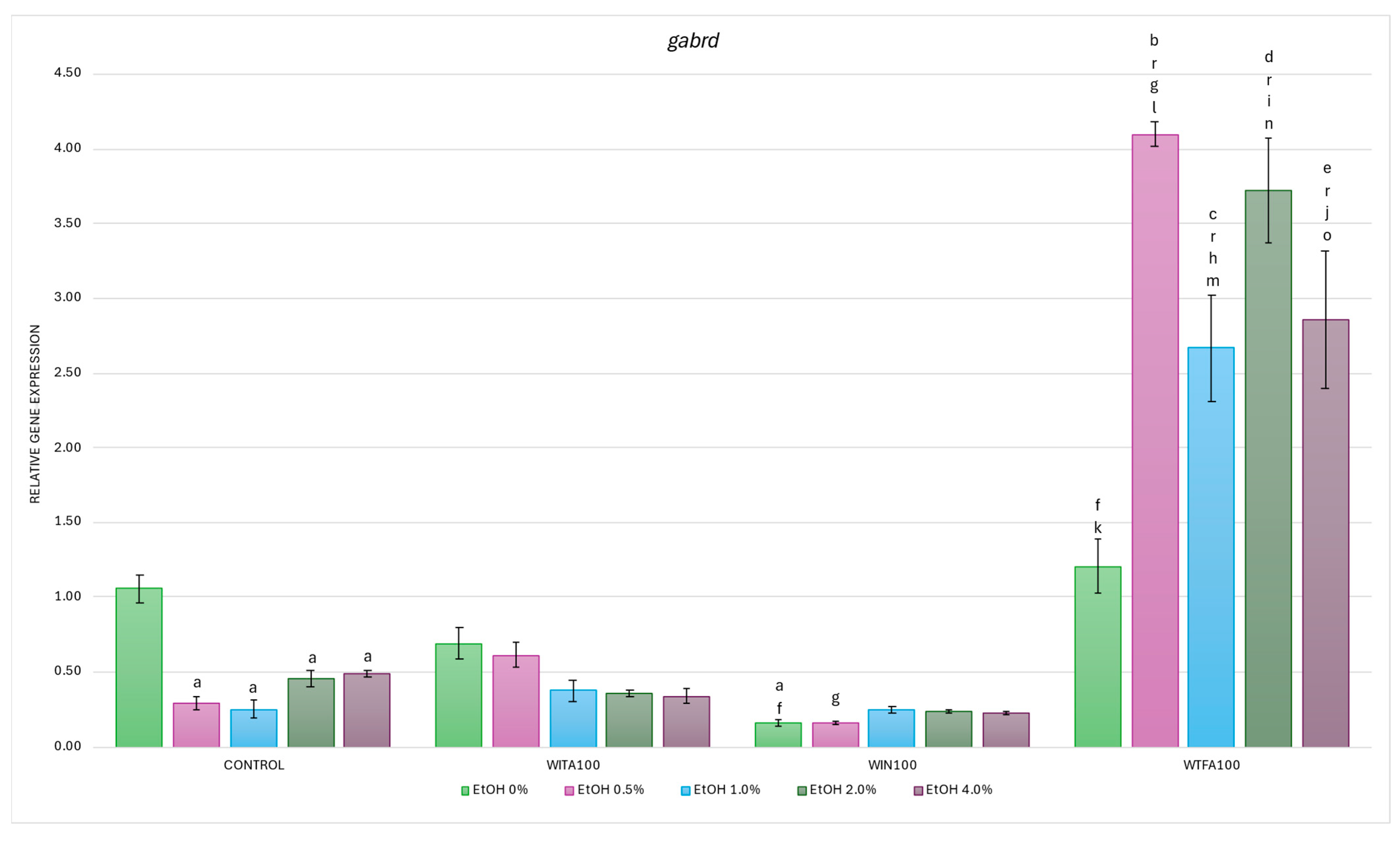
2.3.3. gabrd Expression Affected by EtOH and Withanolides
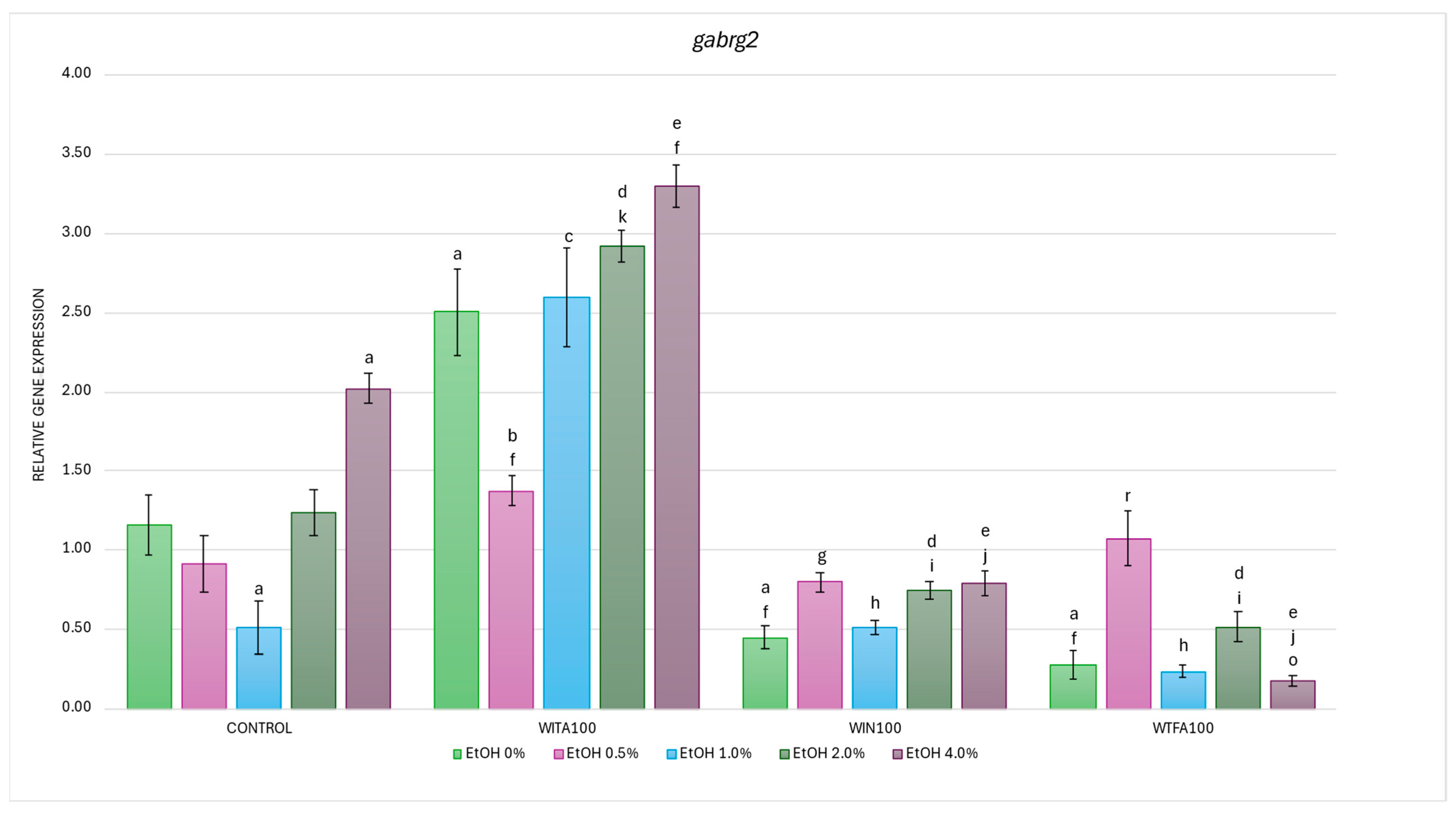
2.3.4. gabrg2 Expression Affected by EtOH and Withanolides
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Behavioral Assays
4.3. Molecular Analyses
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | analysis of variance |
| CNS | central nervous system |
| DPF | days post-fertilization |
| EtOH | ethanol |
| GABA | gamma-aminobutyric acid |
| GABAA | gamma-aminobutyric acid type A |
| IL-6 | interleukin 6 |
| KCC2 | potassium–chloride cotransporter 2 |
| NK | natural killer (cell) |
| NKCC1 | sodium–potassium–chloride cotransporter 1 |
| NMDA | N-methyl-D-aspartate |
| RT-qPCR | reverse transcription quantitative PCR |
| SEM | standard error of the mean |
| TNF-α | tumor necrosis factor alpha |
| WIN | withanone |
| WITA | withanolide A |
| WS | Withania somnifera |
| WTFA | withaferin A |
References
- Song, F.; Walker, M.P. Sleep, alcohol, and caffeine in financial traders. PLoS ONE 2023, 18, e0291675. [Google Scholar] [CrossRef]
- Fernández-Solà, J. The effects of ethanol on the heart: Alcoholic cardiomyopathy. Nutrients 2020, 12, 572. [Google Scholar] [CrossRef]
- Matsumoto, C.; Miedema, M.D.; Ofman, P.; Gaziano, J.M.; Sesso, H.D. An expanding knowledge of the mechanisms and effects of alcohol consumption on cardiovascular disease. J. Cardiopulm. Rehabil. Prev. 2014, 34, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Kamal, H.; Tan, G.C.; Ibrahim, S.F.; Shaikh, M.F.; Mohamed, I.N.; Mohamed, R.M.P.; Hamid, A.A.; Ugusman, A.; Kumar, J. Alcohol use disorder, neurodegeneration, Alzheimer’s and Parkinson’s disease: Interplay between oxidative stress, neuroimmune response and excitotoxicity. Front. Cell. Neurosci. 2020, 14, 282. [Google Scholar] [CrossRef] [PubMed]
- Pervin, Z.; Stephen, J.M. Effect of alcohol on the central nervous system to develop neurological disorder: Pathophysiological and lifestyle modulation can be potential therapeutic options for alcohol-induced neurotoxication. AIMS Neurosci. 2021, 8, 390–413. [Google Scholar] [CrossRef] [PubMed]
- de Menezes, R.F.; Bergmann, A.; Thuler, L.C.S. Alcohol Consumption and Risk of Cancer: A Systematic Literature Review. Asian Pac. J. Cancer Prev. 2013, 14, 4965–4972. [Google Scholar] [CrossRef]
- Begun, A.L. Introduction to psychoactive substances. In The Routledge Handbook of Social Work and Addictive Behaviors; Begun, A.L., Murray, M.M., Eds.; Routledge: New York, NY, USA, 2020; pp. 18–38. ISBN 9780429203121. [Google Scholar]
- World Health Organization. Global Status Report on Alcohol and Health and Treatment of Substance Use Disorders; WHO: Geneva, Switzerland, 2024. [Google Scholar]
- Lopes, G.M.; Nóbrega, B.A.; Del Prette, G.; Scivoletto, S. Use of psychoactive substances by adolescents: Current panorama. Rev. Bras. Psychiatry 2013, 35 (Suppl. S1), S51–S61. [Google Scholar] [CrossRef]
- Manthey, J.; Kilian, C.; Carr, S.; Bartak, M.; Bloomfield, K.; Braddick, F.; Gual, A.; Neufeld, M.; O’Donnell, A.; Petruzelka, B.; et al. Use of alcohol, tobacco, cannabis, and other substances during the first wave of the SARS-CoV-2 pandemic in Europe: A survey on 36,000 European substance users. Subst. Abuse Treat. Prev. Policy 2021, 16, 36. [Google Scholar] [CrossRef]
- Mounteney, J.; Griffiths, P.; Sedefov, R.; Noor, A.; Vicente, J.; Simon, R. The drug situation in Europe: An overview of data available on illicit drugs and new psychoactive substances from European monitoring in 2015. Addiction 2016, 111, 34–48. [Google Scholar] [CrossRef]
- European Monitoring Centre for Drugs and Drug Addiction. Health Responses to New Psychoactive Substances. Available online: https://www.emcdda.europa.eu/publications/pods/health-responses-for-new-psychoactive-substances_en (accessed on 31 March 2025).
- Pohorecky, L.A. Biphasic action of ethanol. Biobehav. Rev. 1977, 1, 231–240. [Google Scholar] [CrossRef]
- Gilpin, N.W.; Koob, G.F. Neurobiology of alcohol dependence: Focus on motivational mechanisms. Alcohol Res. Health 2008, 31, 185–195. [Google Scholar]
- Eckardt, M.J.; File, S.E.; Gessa, G.L.; Grant, K.A.; Guerri, C.; Hoffman, P.L.; Kalant, H.; Koob, G.F.; Li, T.K.; Tabakoff, B. Effects of moderate alcohol consumption on the central nervous system. Alcohol. Clin. Exp. Res. 1998, 22, 998–1040. [Google Scholar] [CrossRef]
- Mirijello, A.; Sestito, L.; Antonelli, M.; Gasbarrini, A.; Addolorato, G. Identification and management of acute alcohol intoxication. Eur. J. Intern. Med. 2023, 108, 1–8. [Google Scholar] [CrossRef]
- Koob, G.F. A role for GABA mechanisms in the motivational effects of alcohol. Biochem. Pharmacol. 2004, 68, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Roberto, M.; Madamba, S.G.; Moore, S.D.; Tallent, M.K.; Siggins, G.R. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc. Natl. Acad. Sci. USA 2003, 100, 2053–2058. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F.; Volkow, N.D. Neurocircuitry of addiction. Neuropsychopharmacology 2010, 35, 217–238, Erratum in Neuropsychopharmacology 2010, 35, 1051. [Google Scholar] [CrossRef] [PubMed]
- de Boer, S.F.; Olivier, B.; Veening, J.; Koolhaas, J.M. The neurobiology of offensive aggression: Revealing a modular view. Physiol. Behav. 2015, 146, 111–127. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.-J.; Fowler, J.S.; Tomasi, D. Addiction circuitry in the human brain. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 321–336. [Google Scholar] [CrossRef]
- Benke, D.; Fritschy, J.M.; Trzeciak, A.; Bannwarth, W.; Mohler, H. Distribution, prevalence, and drug binding profile of gamma-aminobutyric acid type A receptor subtypes differing in the beta-subunit variant. J. Biol. Chem. 1994, 269, 27100–27107. [Google Scholar] [CrossRef]
- Olsen, R.W.; Sieghart, W. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: Classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol. Rev. 2008, 60, 243–260. [Google Scholar] [CrossRef]
- Minier, F.; Sigel, E. Positioning of the alpha-subunit isoforms confers a functional signature to gamma-aminobutyric acid type A receptors. Proc. Natl. Acad. Sci. USA 2004, 101, 7769–7774. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Suryanarayanan, A.; Abriam, A.; Snyder, B.; Olsen, R.W.; Spigelman, I. Mechanisms of reversible GABAA receptor plasticity after ethanol intoxication. J. Neurosci. 2007, 27, 12367–12377. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Faria, L.C.; Mody, I. Low ethanol concentrations selectively augment the tonic inhibition mediated by delta subunit-containing GABAA receptors in hippocampal neurons. J. Neurosci. 2004, 24, 8379–8382. [Google Scholar] [CrossRef] [PubMed]
- Wallner, M.; Hanchar, H.J.; Olsen, R.W. Ethanol enhances α4β3δ and α6β3δ γ-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc. Natl. Acad. Sci. USA 2003, 100, 15218–15223. [Google Scholar] [CrossRef]
- Kumar, S.; Porcu, P.; Werner, D.F.; Matthews, D.B.; Diaz-Granados, J.L.; Helfand, R.S.; Morrow, A.L. The role of GABA(A) receptors in the acute and chronic effects of ethanol: A decade of progress. Psychopharmacology 2009, 205, 529–564. [Google Scholar] [CrossRef]
- Devaud, L.L.; Fritschy, J.M.; Sieghart, W.; Morrow, A.L. Bidirectional alterations of GABA(A) receptor subunit peptide levels in rat cortex during chronic ethanol consumption and withdrawal. J. Neurochem. 1997, 69, 126–130. [Google Scholar] [CrossRef]
- Hansen, A.W.; Almeida, F.B.; Bandiera, S.; Pulcinelli, R.R.; Caletti, G.; Agnes, G.; Fernandes de Paula, L.; Nietiedt, N.A.; Nin, M.S.; Tannhauser Barros, H.M.; et al. Correlations between subunits of GABAA and NMDA receptors after chronic alcohol treatment or withdrawal, and the effect of taurine in the hippocampus of rats. Alcohol 2020, 82, 63–70. [Google Scholar] [CrossRef]
- Enoch, M.-A. The role of GABA(A) receptors in the development of alcoholism. Pharmacol. Biochem. Behav. 2008, 90, 95–104. [Google Scholar] [CrossRef]
- Enoch, M.-A.; Hodgkinson, C.A.; Yuan, Q.; Albaugh, B.; Virkkunen, M.; Goldman, D. GABRG1 and GABRA2 as independent predictors for alcoholism in two populations. Neuropsychopharmacology 2009, 34, 1245–1254. [Google Scholar] [CrossRef]
- Newman, E.L.; Gunner, G.; Huynh, P.; Gachette, D.; Moss, S.J.; Smart, T.G.; Rudolph, U.; DeBold, J.F.; Miczek, K.A. Effects of Gabra2 Point Mutations on Alcohol Intake: Increased Binge-Like and Blunted Chronic Drinking by Mice. Alcohol. Clin. Exp. Res. 2016, 40, 2445–2455. [Google Scholar] [CrossRef]
- Chandrasekar, R. Alcohol and NMDA receptor: Current research and future direction. Front. Mol. Neurosci. 2013, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Allgaier, C. Ethanol sensitivity of NMDA receptors. Neurochem. Int. 2002, 41, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Bhattacharjee, B.; Sandhanam, K.; Ghose, S.; Barman, D.; Sahu, R.K. Market overview of herbal medicines for lifestyle diseases. In Role of Herbal Medicines: Management of Lifestyle Diseases; Dhara, A.K., Mandal, S.C., Eds.; Springer Nature: Singapore, 2023; pp. 597–614. ISBN 978-981-99-7702-4. [Google Scholar]
- Lippert, A.; Renner, B. Herb-Drug Interaction in Inflammatory Diseases: Review of Phytomedicine and Herbal Supplements. J. Clin. Med. 2022, 11, 1567. [Google Scholar] [CrossRef]
- Sivertsen, K.; Lukic, M.; Kristoffersen, A.E. Gender specific association between the use of complementary and alternative medicine (CAM) and alcohol consumption and injuries caused by drinking in the sixth Tromsø study. BMC Complement. Altern. Med. 2018, 18, 239. [Google Scholar] [CrossRef]
- Karakaya, R.E. Assessment of the use of herbal supplements and attitudes of adults taking medication. J. Contemp. Med. 2025, 15, 45–50. [Google Scholar] [CrossRef]
- Bashir, A.; Nabi, M.; Tabassum, N.; Afzal, S.; Ayoub, M. An updated review on phytochemistry and molecular targets of Withania somnifera (L.) Dunal (Ashwagandha). Front. Pharmacol. 2023, 14, 1049334. [Google Scholar] [CrossRef]
- Zahiruddin, S.; Basist, P.; Parveen, A.; Parveen, R.; Khan, W.; Ahmad, S. Ashwagandha in brain disorders: A review of recent developments. J. Ethnopharmacol. 2020, 257, 112876. [Google Scholar] [CrossRef]
- Mikulska, P.; Malinowska, M.; Ignacyk, M.; Szustowski, P.; Nowak, J.; Pesta, K.; Szeląg, M.; Szklanny, D.; Judasz, E.; Kaczmarek, G.; et al. Ashwagandha (Withania somnifera)—Current Research on the Health-Promoting Activities: A Narrative Review. Pharmaceutics 2023, 15, 1057. [Google Scholar] [CrossRef]
- D’Cruz, M.; Andrade, C. Potential clinical applications of Ashwagandha (Withania somnifera) in medicine and neuropsychiatry. Expert Rev. Clin. Pharmacol. 2022, 15, 1067–1080. [Google Scholar] [CrossRef]
- Weathermon, R.; Crabb, D.W. Alcohol and medication interactions. Alcohol Res. Health 1999, 23, 40–54. [Google Scholar]
- Witkiewitz, K.; Vowles, K.E. Alcohol and Opioid Use, Co-Use, and Chronic Pain in the Context of the Opioid Epidemic: A Critical Review. Alcohol. Clin. Exp. Res. 2018, 42, 478–488. [Google Scholar] [CrossRef]
- MacKillop, J.; Agabio, R.; Feldstein Ewing, S.W.; Heilig, M.; Kelly, J.F.; Leggio, L.; Lingford-Hughes, A.; Palmer, A.A.; Parry, C.D.; Ray, L.; et al. Hazardous drinking and alcohol use disorders. Nat. Rev. Dis. Primers 2022, 8, 80, Erratum in Nat. Rev. Dis. Primers 2024, 10, 69. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J.; Moylan, S.; Camfield, D.A.; Pase, M.P.; Mischoulon, D.; Berk, M.; Jacka, F.N.; Schweitzer, I. Complementary medicine, exercise, meditation, diet, and lifestyle modification for anxiety disorders: A review of current evidence. Evid. Based Complement. Altern. Med. 2012, 2012, 809653. [Google Scholar] [CrossRef] [PubMed]
- Jang, G.R.; Harris, R.Z. Drug interactions involving ethanol and alcoholic beverages. Expert Opin. Drug Metab. Toxicol. 2007, 3, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Na, S.; Li, J.; Zhang, H.; Li, Y.; Yang, Z.; Zhong, Y.; Dong, G.; Yang, J.; Yue, J. The induction of cytochrome P450 2E1 by ethanol leads to the loss of synaptic proteins via PPARα down-regulation. Toxicology 2017, 385, 18–27. [Google Scholar] [CrossRef]
- Chan, L.-N.; Anderson, G.D. Pharmacokinetic and pharmacodynamic drug interactions with ethanol (alcohol). Clin. Pharmacokinet. 2014, 53, 1115–1136. [Google Scholar] [CrossRef]
- Horizon Databook: The World’s Largest Portal for Market Reports & Statistics Global Ashwagandha Supplements Market Size & Outlook. Available online: https://www.grandviewresearch.com/horizon/outlook/ashwagandha-supplements-market-size/global (accessed on 1 April 2025).
- Pandit, S.; Srivastav, A.K.; Sur, T.K.; Chaudhuri, S.; Wang, Y.; Biswas, T.K. Effects of Withania somnifera Extract in Chronically Stressed Adults: A Randomized Controlled Trial. Nutrients 2024, 16, 1293. [Google Scholar] [CrossRef]
- Wiciński, M.; Fajkiel-Madajczyk, A.; Kurant, Z.; Kurant, D.; Gryczka, K.; Falkowski, M.; Wiśniewska, M.; Słupski, M.; Ohla, J.; Zabrzyński, J. Can Ashwagandha Benefit the Endocrine System?—A Review. Int. J. Mol. Sci. 2023, 24, 16513. [Google Scholar] [CrossRef]
- Yin, H.; Cho, D.H.; Park, S.J.; Han, S.K. GABA-mimetic actions of Withania somnifera on substantia gelatinosa neurons of the trigeminal subnucleus caudalis in mice. Am. J. Chin. Med. 2013, 41, 1043–1051. [Google Scholar] [CrossRef]
- Park, C.W.; Hong, K.-B.; Suh, H.J.; Ahn, Y. Sleep-promoting activity of amylase-treated Ashwagandha (Withania somnifera L. Dunal) root extract via GABA receptors. J. Food Drug Anal. 2023, 31, 278–288. [Google Scholar] [CrossRef]
- Sood, A.; Mehrotra, A.; Dhawan, D.K.; Sandhir, R. Neuroprotective effects of Withania somnifera on ischemic stroke are mediated via anti-inflammatory response and modulation of neurotransmitter levels. Neurochem. Int. 2024, 180, 105867. [Google Scholar] [CrossRef]
- Balkrishna, A.; Solleti, S.K.; Singh, H.; Sharma, N.; Varshney, A. Withanolides from Withania somnifera Ameliorate Neutrophil Infiltration in Endotoxin-Induced Peritonitis by Regulating Oxidative Stress and Inflammatory Cytokines. Planta Med. 2022, 88, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Dar, N.J.; Hamid, A.; Ahmad, M. Pharmacologic overview of Withania somnifera, the Indian Ginseng. Cell. Mol. Life Sci. 2015, 72, 4445–4460. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Choi, H.-S.; Han, K.; Sim, W.; Suh, H.J.; Ahn, Y. Ashwagandha (Withania somnifera (L.) dunal) root extract containing withanolide a alleviates depression-like behavior in mice by enhancing the brain-derived neurotrophic factor pathway under unexpected chronic mild stress. J. Ethnopharmacol. 2025, 340, 119224. [Google Scholar] [CrossRef] [PubMed]
- Orrù, A.; Marchese, G.; Casu, G.; Casu, M.A.; Kasture, S.; Cottiglia, F.; Acquas, E.; Mascia, M.P.; Anzani, N.; Ruiu, S. Withania somnifera root extract prolongs analgesia and suppresses hyperalgesia in mice treated with morphine. Phytomedicine 2014, 21, 745–752. [Google Scholar] [CrossRef]
- Soman, S.; Korah, P.K.; Jayanarayanan, S.; Mathew, J.; Paulose, C.S. Oxidative stress induced NMDA receptor alteration leads to spatial memory deficits in temporal lobe epilepsy: Ameliorative effects of Withania somnifera and Withanolide A. Neurochem. Res. 2012, 37, 1915–1927. [Google Scholar] [CrossRef]
- Paul, S.; Chakraborty, S.; Anand, U.; Dey, S.; Nandy, S.; Ghorai, M.; Saha, S.C.; Patil, M.T.; Kandimalla, R.; Proćków, J.; et al. Withania somnifera (L.) Dunal (Ashwagandha): A comprehensive review on ethnopharmacology, pharmacotherapeutics, biomedicinal and toxicological aspects. Biomed. Pharmacother. 2021, 143, 112175. [Google Scholar] [CrossRef]
- Saleem, S.; Muhammad, G.; Hussain, M.A.; Altaf, M.; Bukhari, S.N.A. Withania somnifera L.: Insights into the phytochemical profile, therapeutic potential, clinical trials, and future prospective. Iran. J. Basic Med. Sci. 2020, 23, 1501–1526. [Google Scholar] [CrossRef]
- Spina, L.; Longoni, R.; Rosas, M.; Collu, M.; Peana, A.T.; Espa, E.; Kasture, S.; Cotti, E.; Acquas, E. Withania somnifera Dunal (Indian ginseng) impairs acquisition and expression of ethanol-elicited conditioned place preference and conditioned place aversion. J. Psychopharmacol. 2015, 29, 1191–1199. [Google Scholar] [CrossRef]
- Haque, I.M.; Mishra, A.; Kalra, B.S.; Chawla, S. Role of Standardized Plant Extracts in Controlling Alcohol Withdrawal Syndrome—An Experimental Study. Brain Sci. 2021, 11, 919. [Google Scholar] [CrossRef]
- Bassareo, V.; Talani, G.; Frau, R.; Porru, S.; Rosas, M.; Kasture, S.B.; Peana, A.T.; Loi, E.; Sanna, E.; Acquas, E. Inhibition of Morphine- and Ethanol-Mediated Stimulation of Mesolimbic Dopamine Neurons by Withania somnifera. Front. Neurosci. 2019, 13, 545. [Google Scholar] [CrossRef] [PubMed]
- Maccioni, R.; Serra, M.; Marongiu, J.; Cottiglia, F.; Maccioni, E.; Bassareo, V.; Morelli, M.; Kasture, S.B.; Acquas, E. Effects of docosanyl ferulate, a constituent of Withania somnifera, on ethanol- and morphine-elicited conditioned place preference and ERK phosphorylation in the accumbens shell of CD1 mice. Psychopharmacology 2022, 239, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Candelario, M.; Cuellar, E.; Reyes-Ruiz, J.M.; Darabedian, N.; Feimeng, Z.; Miledi, R.; Russo-Neustadt, A.; Limon, A. Direct evidence for GABAergic activity of Withania somnifera on mammalian ionotropic GABAA and GABAρ receptors. J. Ethnopharmacol. 2015, 171, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Abbas, K.; Iram, F.; Raza, M.T.; Mustafa, M.; Zehra, Z. Molecular Docking and Dynamics Studies of Withania somnifera Derived Compounds as GABA-A Receptor Modulators for Insomnia. Chronobiol. Med. 2024, 6, 77–86. [Google Scholar] [CrossRef]
- Cocco, A.; Rönnberg, A.M.C.; Jin, Z.; André, G.I.; Vossen, L.E.; Bhandage, A.K.; Thörnqvist, P.-O.; Birnir, B.; Winberg, S. Characterization of the γ-aminobutyric acid signaling system in the zebrafish (Danio rerio Hamilton) central nervous system by reverse transcription-quantitative polymerase chain reaction. Neuroscience 2017, 343, 300–321. [Google Scholar] [CrossRef]
- Monesson-Olson, B.; McClain, J.J.; Case, A.E.; Dorman, H.E.; Turkewitz, D.R.; Steiner, A.B.; Downes, G.B. Expression of the eight GABAA receptor α subunits in the developing zebrafish central nervous system. PLoS ONE 2018, 13, e0196083. [Google Scholar] [CrossRef]
- Sadamitsu, K.; Shigemitsu, L.; Suzuki, M.; Ito, D.; Kashima, M.; Hirata, H. Characterization of zebrafish GABAA receptor subunits. Sci. Rep. 2021, 11, 6242. [Google Scholar] [CrossRef]
- Choi, T.-Y.; Choi, T.-I.; Lee, Y.-R.; Choe, S.-K.; Kim, C.-H. Zebrafish as an animal model for biomedical research. Exp. Mol. Med. 2021, 53, 310–317. [Google Scholar] [CrossRef]
- Tran, S.; Gerlai, R. Time-course of behavioural changes induced by ethanol in zebrafish (Danio rerio). Behav. Brain Res. 2013, 252, 204–213. [Google Scholar] [CrossRef]
- de Esch, C.; van der Linde, H.; Slieker, R.; Willemsen, R.; Wolterbeek, A.; Woutersen, R.; De Groot, D. Locomotor activity assay in zebrafish larvae: Influence of age, strain and ethanol. Neurotoxicol. Teratol. 2012, 34, 425–433. [Google Scholar] [CrossRef]
- Guo, N.; Lin, J.; Peng, X.; Chen, H.; Zhang, Y.; Liu, X.; Li, Q. Influences of acute ethanol exposure on locomotor activities of zebrafish larvae under different illumination. Alcohol 2015, 49, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Hillman, C.; Kearn, J.; Parker, M.O. A unified approach to investigating 4 dpf zebrafish larval behaviour through a standardised light/dark assay. Prog. Neuropsychopharmacol. Biol. Psychiatry 2024, 134, 111084. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, B.; Bjerke, S.; Kobayashi, K.; Guo, S. Acute effects of alcohol on larval zebrafish: A genetic system for large-scale screening. Pharmacol. Biochem. Behav. 2004, 77, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Irons, T.D.; MacPhail, R.C.; Hunter, D.L.; Padilla, S. Acute neuroactive drug exposures alter locomotor activity in larval zebrafish. Neurotoxicol. Teratol. 2010, 32, 84–90. [Google Scholar] [CrossRef]
- MacPhail, R.C.; Brooks, J.; Hunter, D.L.; Padnos, B.; Irons, T.D.; Padilla, S. Locomotion in larval zebrafish: Influence of time of day, lighting and ethanol. Neurotoxicology 2009, 30, 52–58. [Google Scholar] [CrossRef]
- Basnet, R.M.; Zizioli, D.; Taweedet, S.; Finazzi, D.; Memo, M. Zebrafish larvae as a behavioral model in neuropharmacology. Biomedicines 2019, 7, 23. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, N.; Cagetti, E.; Houser, C.R.; Olsen, R.W.; Spigelman, I. Chronic intermittent ethanol-induced switch of ethanol actions from extrasynaptic to synaptic hippocampal GABAA receptors. J. Neurosci. 2006, 26, 1749–1758. [Google Scholar] [CrossRef]
- Edenberg, H.J.; Dick, D.M.; Xuei, X.; Tian, H.; Almasy, L.; Bauer, L.O.; Crowe, R.R.; Goate, A.; Hesselbrock, V.; Jones, K.; et al. Variations in GABRA2, encoding the α2 subunit of the GABAA receptor, are associated with alcohol dependence and with brain oscillations. Am. J. Hum. Genet. 2004, 74, 705–714. [Google Scholar] [CrossRef]
- Mihalek, R.M.; Bowers, B.J.; Wehner, J.M.; Kralic, J.E.; VanDoren, M.J.; Morrow, A.L.; Homanics, G.E. GABAA-Receptor δ Subunit Knockout Mice Have Multiple Defects in Behavioral Responses to Ethanol. Alcohol Clin. Exp. Res. 2001, 25, 1708–1718. [Google Scholar] [CrossRef]
- Liao, M.; Kundap, U.; Rosch, R.E.; Burrows, D.R.W.; Meyer, M.P.; Ouled Amar Bencheikh, B.; Cossette, P.; Samarut, É. Targeted knockout of GABA-A receptor gamma 2 subunit provokes transient light-induced reflex seizures in zebrafish larvae. Dis. Model. Mech. 2019, 12, 040782. [Google Scholar] [CrossRef] [PubMed]
- Berro, L.F.; Rüedi-Bettschen, D.; Cook, J.E.; Golani, L.K.; Li, G.; Jahan, R.; Rashid, F.; Cook, J.M.; Rowlett, J.K.; Platt, D.M. GABAA Receptor Subtypes and the Abuse-Related Effects of Ethanol in Rhesus Monkeys: Experiments with Selective Positive Allosteric Modulators. Alcohol. Clin. Exp. Res. 2019, 43, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ari, Y. Excitatory actions of gaba during development: The nature of the nurture. Nat. Rev. Neurosci. 2002, 3, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.; Brustein, E.; Liao, M.; Mercado, A.; Babilonia, E.; Mount, D.B.; Drapeau, P. Neurogenic role of the depolarizing chloride gradient revealed by global overexpression of KCC2 from the onset of development. J. Neurosci. 2008, 28, 1588–1597. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, S.; Du, J. KCC2-dependent subcellular E(Cl) difference of ON-OFF retinal ganglion cells in larval zebrafish. Front. Neural Circuits 2013, 7, 103. [Google Scholar] [CrossRef]
- Jones, E.F.; Butler, M.G.; Trendafilova, D.; Mendez, M.S.; Jernigan, L.A.; Gahtan, E.; Steele, J. In vivo tracking of KCC2b expression during early brain development. J. Comp. Neurol. 2023, 531, 48–57. [Google Scholar] [CrossRef]
- Gerlai, R. Zebrafish antipredatory responses: A future for translational research? Behav. Brain Res. 2010, 207, 223–231. [Google Scholar] [CrossRef]
- Fontana, B.D.; Alnassar, N.; Parker, M.O. The zebrafish (Danio rerio) anxiety test battery: Comparison of behavioral responses in the novel tank diving and light-dark tasks following exposure to anxiogenic and anxiolytic compounds. Psychopharmacology 2022, 239, 287–296. [Google Scholar] [CrossRef]
- Belelli, D.; Harrison, N.L.; Maguire, J.; Macdonald, R.L.; Walker, M.C.; Cope, D.W. Extrasynaptic GABAA receptors: Form, pharmacology, and function. J. Neurosci. 2009, 29, 12757–12763. [Google Scholar] [CrossRef]
- Möykkynen, T.; Korpi, E.R. Acute effects of ethanol on glutamate receptors. Basic Clin. Pharmacol. Toxicol. 2012, 111, 4–13. [Google Scholar] [CrossRef]
- Modi, S.J.; Tiwari, A.; Ghule, C.; Pawar, S.; Saste, G.; Jagtap, S.; Singh, R.; Deshmukh, A.; Girme, A.; Hingorani, L. Pharmacokinetic Study of Withanosides and Withanolides from Withania somnifera Using Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry (UHPLC-MS/MS). Molecules 2022, 27, 1476. [Google Scholar] [CrossRef]
- Aleström, P.; D’Angelo, L.; Midtlyng, P.J.; Schorderet, D.F.; Schulte-Merker, S.; Sohm, F.; Warner, S. Zebrafish: Housing and husbandry recommendations. Lab. Anim. 2020, 54, 213–224. [Google Scholar] [CrossRef]
- Westerfield, M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio), 4th ed.; University of Oregon Press: Eugene, OR, USA, 2000. [Google Scholar]
- Christou, M.; Kavaliauskis, A.; Ropstad, E.; Fraser, T.W.K. DMSO effects larval zebrafish (Danio rerio) behavior, with additive and interaction effects when combined with positive controls. Sci. Total Environ. 2020, 709, 134490. [Google Scholar] [CrossRef] [PubMed]
- Ingebretson, J.J.; Masino, M.A. Quantification of locomotor activity in larval zebrafish: Considerations for the design of high-throughput behavioral studies. Front. Neural Circuits 2013, 7, 109. [Google Scholar] [CrossRef] [PubMed]
- Jarema, K.A.; Hunter, D.L.; Hill, B.N.; Olin, J.K.; Britton, K.N.; Waalkes, M.R.; Padilla, S. Developmental Neurotoxicity and Behavioral Screening in Larval Zebrafish with a Comparison to Other Published Results. Toxics 2022, 10, 256. [Google Scholar] [CrossRef] [PubMed]
- Burgess, H.A.; Granato, M. Modulation of locomotor activity in larval zebrafish during light adaptation. J. Exp. Biol. 2007, 210, 2526–2539. [Google Scholar] [CrossRef]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Houseright, R.A.; Rosowski, E.E.; Lam, P.-Y.; Tauzin, S.J.M.; Mulvaney, O.; Dewey, C.N.; Huttenlocher, A. Cell type specific gene expression profiling reveals a role for complement component C3 in neutrophil responses to tissue damage. Sci. Rep. 2020, 10, 15716. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Nolan, T.; Pfaffl, M.W. Quantitative real-time RT-PCR—A perspective. J. Mol. Endocrinol. 2005, 34, 597–601. [Google Scholar] [CrossRef]
- Bustin, S.A.; Wittwer, C.T. MIQE: A step toward more robust and reproducible quantitative PCR. Clin. Chem. 2017, 63, 1537–1538. [Google Scholar] [CrossRef]
- Tang, R.; Dodd, A.; Lai, D.; McNabb, W.C.; Love, D.R. Validation of zebrafish (Danio rerio) reference genes for quantitative real-time RT-PCR normalization. Acta Biochim. Biophys. Sin. 2007, 39, 384–390. [Google Scholar] [CrossRef]
| Gene | Accession Number | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|---|
| ef1a | FJ915061.1 | CTGGAGGCCAGCTCAAACAT | ATCAAGAAGAGTAGTACCGCTAGCATTAC |
| gabra1 | NM_001077326.1 | TGAGTCAGAGACAAGAGTGTTC | CTTCCACCCCACATCATTCTC |
| gabra2 | LC596832.1 | CAGACACTTTCTTTCATAACGG | TCCTCAAGATGCATTGGG |
| gabrd | XM_695007.8 | AACTTTCGTCCAGGGATCGG | TGGTGTATTCCATGTTGGCTTC |
| gabrg2 | NM_001256250.1 | ACGGCTATGGACCTCTTCGT | TTTGAGGAAAAGAGCCGCAGG |
| Primer | Initial Denaturation | Denaturation (45 Cycles) | Annealing | Melting Curve Analysis |
|---|---|---|---|---|
| ef1a | 95 °C, 120 s | 95 °C, 15 s | 59 °C, 60 s | 95 °C to 60 °C |
| gabra1, gabra2, gabrd, gabrg2 | 60 °C, 60 s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czora-Poczwardowska, K.; Kujawski, R.; Jarczak, W.; Cicha, E.; Mikołajczak, P.; Szulc, M. Ethanol–Withanolides Interactions: Compound-Specific Effects on Zebrafish Larvae Locomotor Behavior and GABAA Receptor Subunit Expression. Int. J. Mol. Sci. 2025, 26, 10991. https://doi.org/10.3390/ijms262210991
Czora-Poczwardowska K, Kujawski R, Jarczak W, Cicha E, Mikołajczak P, Szulc M. Ethanol–Withanolides Interactions: Compound-Specific Effects on Zebrafish Larvae Locomotor Behavior and GABAA Receptor Subunit Expression. International Journal of Molecular Sciences. 2025; 26(22):10991. https://doi.org/10.3390/ijms262210991
Chicago/Turabian StyleCzora-Poczwardowska, Kamila, Radosław Kujawski, Weronika Jarczak, Emilia Cicha, Przemysław Mikołajczak, and Michał Szulc. 2025. "Ethanol–Withanolides Interactions: Compound-Specific Effects on Zebrafish Larvae Locomotor Behavior and GABAA Receptor Subunit Expression" International Journal of Molecular Sciences 26, no. 22: 10991. https://doi.org/10.3390/ijms262210991
APA StyleCzora-Poczwardowska, K., Kujawski, R., Jarczak, W., Cicha, E., Mikołajczak, P., & Szulc, M. (2025). Ethanol–Withanolides Interactions: Compound-Specific Effects on Zebrafish Larvae Locomotor Behavior and GABAA Receptor Subunit Expression. International Journal of Molecular Sciences, 26(22), 10991. https://doi.org/10.3390/ijms262210991









