Juvenile and Osteoarthritic Human Chondrocytes Under Cyclic Tensile Strain: Transcriptional, Metabolic and Kinase Responses
Abstract
1. Introduction
2. Results
2.1. Gene Expression Analysis of Relevant Biomarkers in Unstimulated Chondrocytes
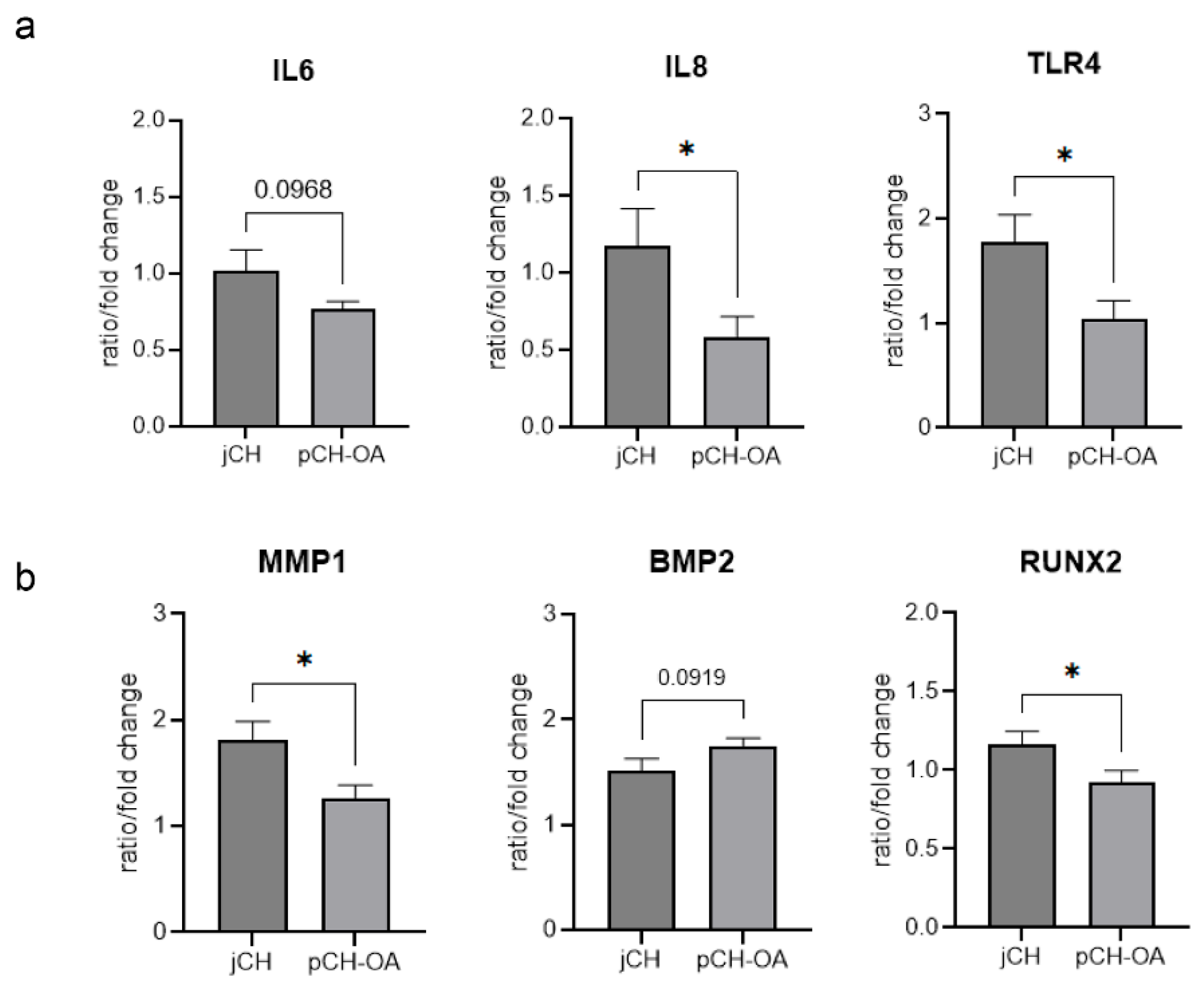
2.2. Effect of Mechanical Stimulation on the Relative Gene Expression of Relevant Chondrocytes Biomarkers
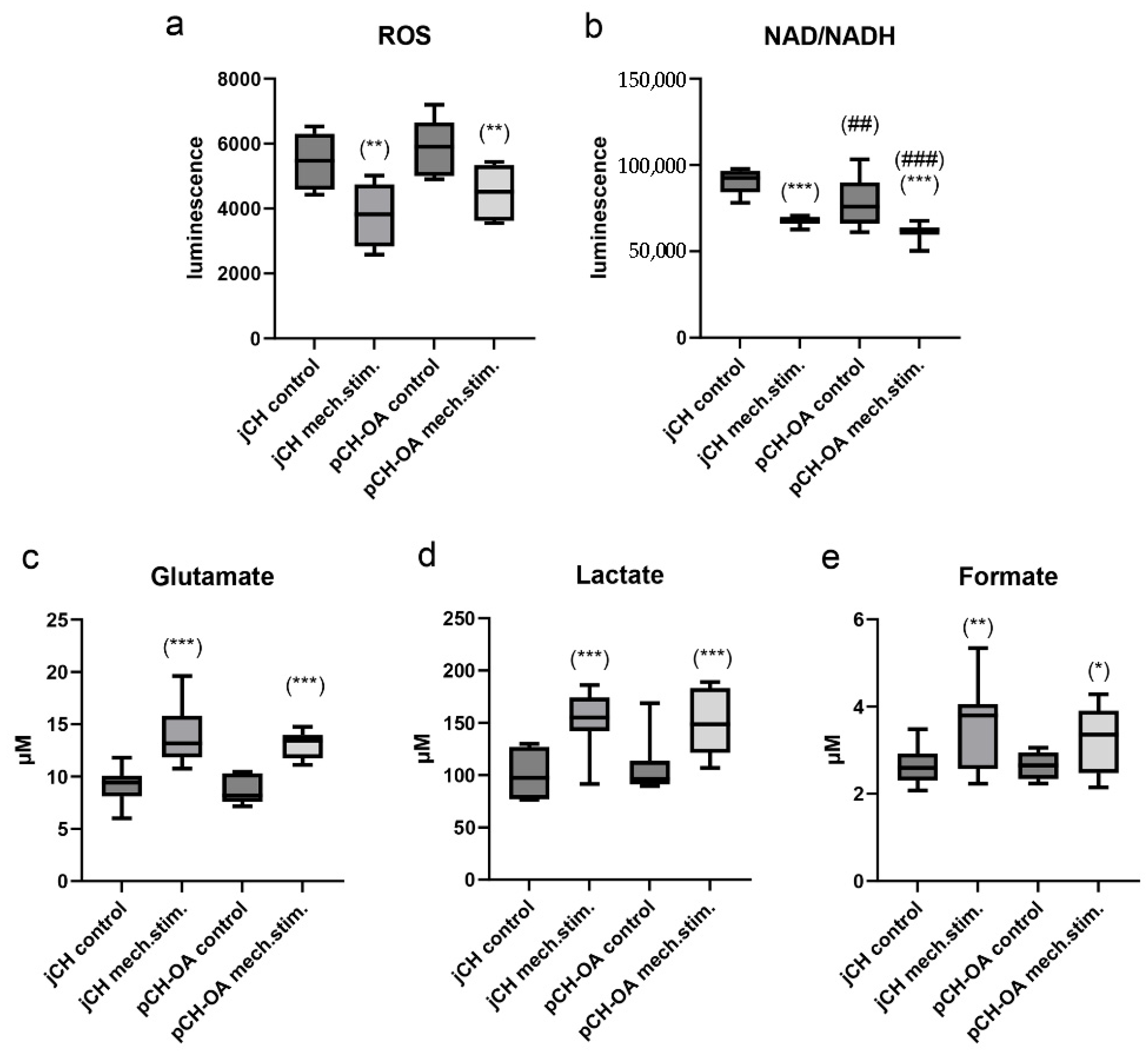
2.3. Impact of Cyclic Tensile Strain on Chondrocyte Metabolism
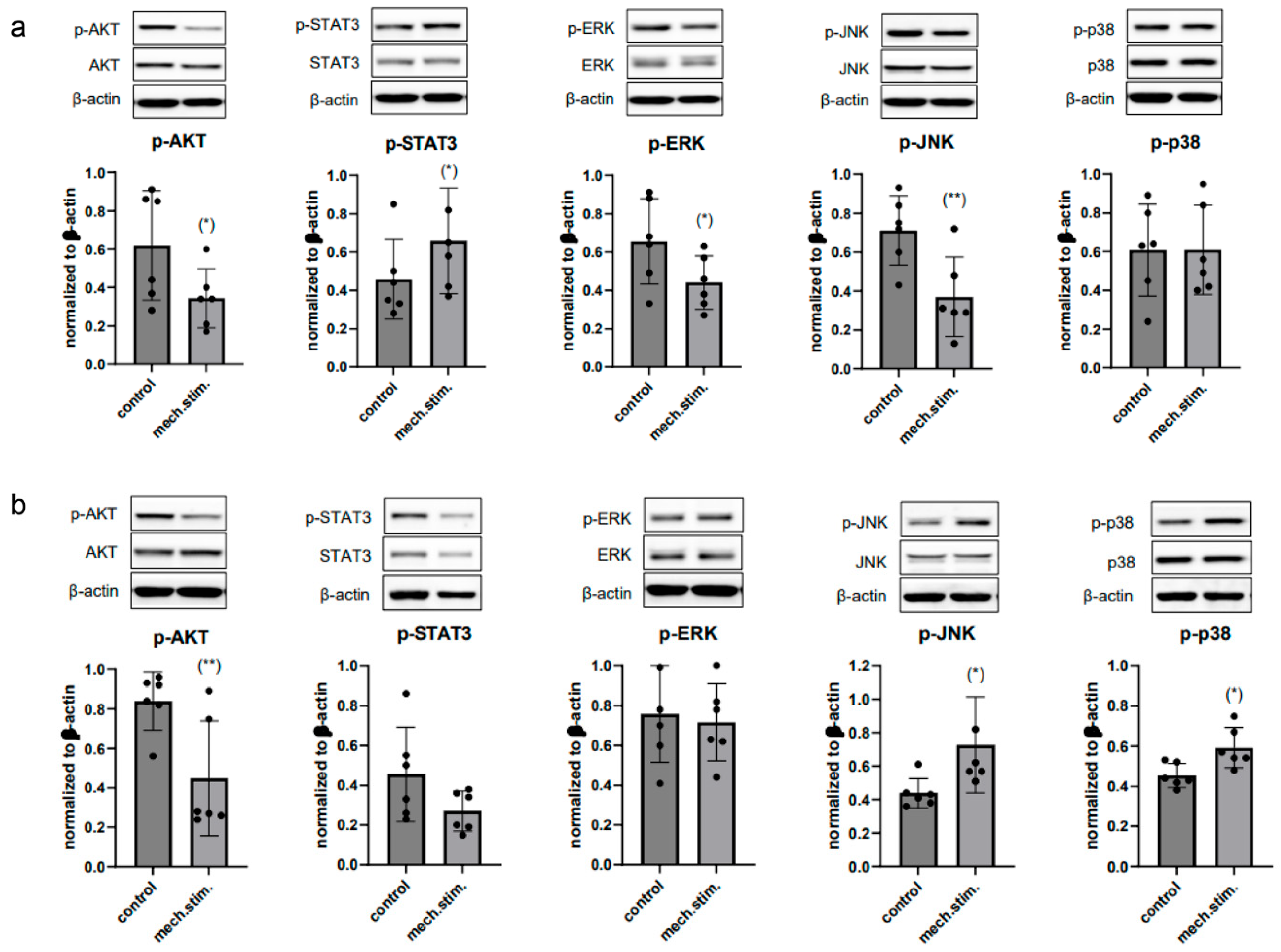
2.4. Cyclic Tensile Strain Alters Protein Phosphorylation
3. Discussion
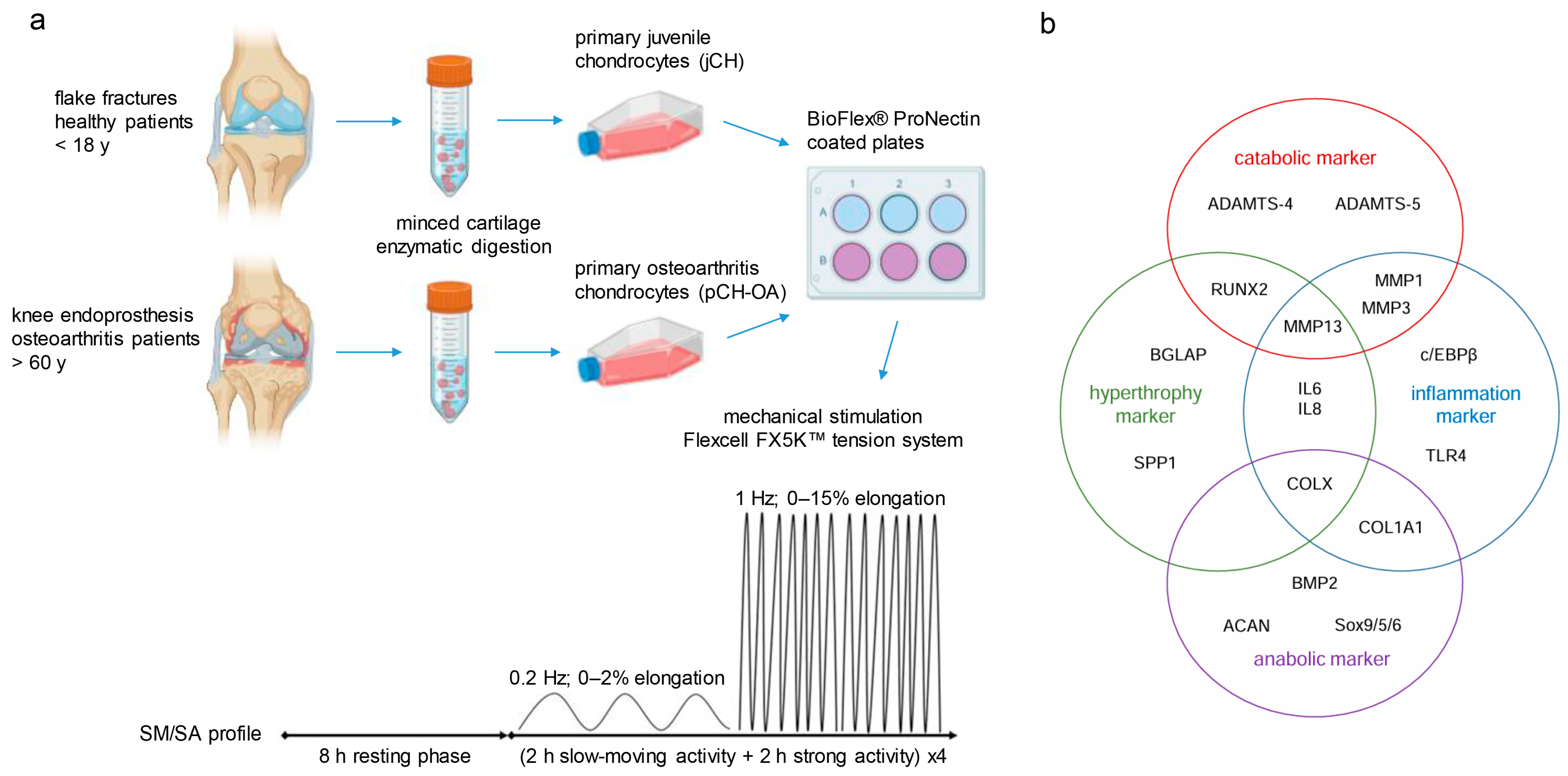
4. Materials and Methods
4.1. Cartilage Samples and Cell Culture of Primary Cells
4.2. Mechanical Stimulation of Chondrocytes
4.3. Reverse Transcription Polymerase Chain Reaction
4.4. Metabolic Assays
4.5. Protein Expression and Phosphorylation Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACAN | aggregan |
| BGLAP | osteocalcin |
| BIOMS | Biological Outcome Measurements |
| BMP2 | bone morphogenetic protein 2 |
| cEBPβ | CCAAT/enhancer binding proteins |
| COL | collagen |
| ECM | extracellular matrix |
| IL | interleukin |
| jCH | juvenile chondrocytes |
| MAPK | mitogen-activated protein kinases |
| MMPs | matrix metalloproteinases |
| NADH | nicotinamide adenine dinucleotide |
| OA | Osteoarthritis |
| PCA | principal component analysis |
| ROS | reactive oxygen species |
| RUNX2 | Runt-related transcription factor 2 |
| SM/SA | slow moving/strong activity profile |
| SPP1 | osteopontin |
| TLR4 | Toll-like-Receptor 4 |
| VAR | variance |
References
- Ramage, L.; Nuki, G.; Salter, D.M. Signalling cascades in mechanotransduction: Cell-matrix interactions and mechanical loading. Scand. J. Med. Sci. Sports 2009, 19, 457–469. [Google Scholar] [CrossRef]
- Wang, N.; Lu, Y.; Rothrauff, B.B.; Zheng, A.; Lamb, A.; Yan, Y.; Lipa, K.E.; Lei, G.; Lin, H. Mechanotransduction pathways in articular chondrocytes and the emerging role of estrogen receptor-alpha. Bone Res. 2023, 11, 13. [Google Scholar] [CrossRef]
- Riegger, J.; Brenner, R.E. Pathomechanisms of Posttraumatic Osteoarthritis: Chondrocyte Behavior and Fate in a Precarious Environment. Int. J. Mol. Sci. 2020, 21, 1560. [Google Scholar] [CrossRef]
- Chang, S.H.; Mori, D.; Kobayashi, H.; Mori, Y.; Nakamoto, H.; Okada, K.; Taniguchi, Y.; Sugita, S.; Yano, F.; Chung, U.-I.; et al. Excessive mechanical loading promotes osteoarthritis through the gremlin-1-NF-κB pathway. Nat. Commun. 2019, 10, 1442. [Google Scholar] [CrossRef]
- Hodgkinson, T.; Kelly, D.C.; Curtin, C.M.; O’Brien, F.J. Mechanosignalling in cartilage: An emerging target for the treatment of osteoarthritis. Nat. Rev. Rheumatol. 2022, 18, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Vincent, T.L. Targeting mechanotransduction pathways in osteoarthritis: A focus on the pericellular matrix. Curr. Opin. Pharmacol. 2013, 13, 449–454. [Google Scholar] [CrossRef]
- Vincent, T.L.; Wann, A.K.T. Mechanoadaptation: Articular cartilage through thick and thin. J. Physiol. 2019, 597, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Glasson, S.S.; Askew, R.; Sheppard, B.; Carito, B.; Blanchet, T.; Ma, H.L.; Flannery, C.R.; Peluso, D.; Kanki, K.; Yang, Z.; et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature 2005, 434, 644–648, Erratum in Nature 2007, 446, 102. [Google Scholar] [CrossRef]
- Clements, K.M.; Flannelly, J.K.; Tart, J.; Brockbank, S.M.V.; Wardale, J.; Freeth, J.; Parker, A.E.; Newham, P. Matrix metalloproteinase 17 is necessary for cartilage aggrecan degradation in an inflammatory environment. Ann. Rheum. Dis. 2011, 70, 683–689. [Google Scholar] [CrossRef]
- Troeberg, L.; Nagase, H. Proteases involved in cartilage matrix degradation in osteoarthritis. Biochim. Biophys. Acta 2012, 1824, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Mixon, A.; Bahar-Moni, A.S.; Faisal, T.R. Mechanical characterization of articular cartilage degraded combinedly with MMP-1 and MMP-9. J. Mech. Behav. Biomed. Mater. 2022, 129, 105131. [Google Scholar] [CrossRef]
- Ansari, M.Y.; Ahmad, N.; Haqqi, T.M. Oxidative stress and inflammation in osteoarthritis pathogenesis: Role of polyphenols. Biomed. Pharmacother. 2020, 129, 110452. [Google Scholar] [CrossRef]
- Wu, X.; Fan, X.; Crawford, R.; Xiao, Y.; Prasadam, I. The Metabolic Landscape in Osteoarthritis. Aging Dis. 2022, 13, 1166–1182. [Google Scholar] [CrossRef]
- van Doormaal, M.C.M.; Meerhoff, G.A.; Vlieland, T.P.M.; Peter, W.F. A clinical practice guideline for physical therapy in patients with hip or knee osteoarthritis. Musculoskelet. Care 2020, 18, 575–595. [Google Scholar] [CrossRef]
- Lawford, B.J.; Hall, M.; Hinman, R.S.; Van der Esch, M.; Harmer, A.R.; Spiers, L.; Kimp, A.; Dell, A.; Bennell, K.L. Exercise for osteoarthritis of the knee. Cochrane Database Syst. Rev. 2024, 12, CD004376. [Google Scholar] [CrossRef]
- Lohberger, B.; Kaltenegger, H.; Weigl, L.; Mann, A.; Kullich, W.; Stuendl, N.; Leithner, A.; Steinecker-Frohnwieser, B. Mechanical exposure and diacerein treatment modulate integrin-FAK-MAPKs mechanotransduction in human osteoarthritis chondrocytes. Cell Signal. 2019, 56, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Salter, D.M.; Millward-Sadler, S.J.; Nuki, G.; Wright, M.O. Differential responses of chondrocytes from normal and osteoarthritic human articular cartilage to mechanical stimulation. Biorheology 2002, 39, 97–108. [Google Scholar] [CrossRef] [PubMed]
- De Croos, J.N.; Dhaliwal, S.S.; Grynpas, M.D.; Pilliar, R.M.; Kandel, R.A. Cyclic compressive mechanical stimulation induces sequential catabolic and anabolic gene changes in chondrocytes resulting in increased extracellular matrix accumulation. Matrix Biol. 2006, 25, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shao, Y.; Yao, Z.; Liu, L.; Zhang, H.; Yin, J.; Xie, H.; Li, K.; Lai, P.; Zeng, H.; et al. Mechanical overloading promotes chondrocyte senescence and osteoarthritis development through downregulating FBXW7. Ann. Rheum. Dis. 2022, 81, 676–686. [Google Scholar] [CrossRef]
- Deng, X.; Xu, H.; Pan, C.; Hao, X.; Liu, J.; Shang, X.; Chi, R.; Hou, W.; Xu, T. Moderate mechanical strain and exercise reduce inflammation and excessive autophagy in osteoarthritis by downregulating mitofusin 2. Life Sci. 2023, 332, 122020. [Google Scholar] [CrossRef]
- Farnsworth, N.L.; Antunez, L.R.; Bryant, S.J. Dynamic compressive loading differentially regulates chondrocyte anabolic and catabolic activity with age. Biotechnol. Bioeng. 2013, 110, 2046–2057. [Google Scholar] [CrossRef]
- Mukherjee, A.; Das, B. The role of inflammatory mediators and matrix metalloproteinases (MMPs) in the progression of osteoarthritis. Biomater. Biosyst. 2024, 13, 100090. [Google Scholar] [CrossRef]
- Ikeda, T.; Kawaguchi, H.; Kamekura, S.; Ogata, N.; Mori, Y.; Nakamura, K.; Ikegawa, S.; Chung, U.-I. Distinct roles of Sox5, Sox6, and Sox9 in different stages of chondrogenic differentiation. J. Bone Miner. Metab. 2005, 23, 337–340. [Google Scholar] [CrossRef]
- Deng, Z.H.; Li, Y.S.; Gao, X.; Lei, G.H.; Huard, J. Bone morphogenetic proteins for articular cartilage regeneration. Osteoarthr. Cartil. 2018, 26, 1153–1161. [Google Scholar] [CrossRef]
- Whitty, C.; Pernstich, C.; Marris, C.; McCaskie, A.; Jones, M.; Henson, F. Sustained delivery of the bone morphogenetic proteins BMP-2 and BMP-7 for cartilage repair and regeneration in osteoarthritis. Osteoarthr. Cartil. Open. 2022, 4, 100240. [Google Scholar] [CrossRef]
- Rashid, H.; Smith, C.M.; Convers, V.; Clark, K.; Javed, A. Runx2 deletion in hypertrophic chondrocytes impairs osteoclast mediated bone resorption. Bone 2024, 181, 117014. [Google Scholar] [CrossRef] [PubMed]
- Bartels, Y.L.; van Lent, P.L.E.M.; van der Kraan, P.M.; Blom, A.B.; Bonger, K.M.; van den Bosch, M.H.J. Inhibition of TLR4 signalling to dampen joint inflammation in osteoarthritis. Rheumatology 2024, 63, 608–618. [Google Scholar] [CrossRef]
- Smeriglio, P.; Lai, J.H.; Dhulipala, L.; Behn, A.W.; Goodman, S.B.; Smith, R.L.; Maloney, W.J.; Yang, F.; Bhutani, N. Comparative potential of juvenile and adult human articular chondrocytes for cartilage tissue formation in three-dimensional biomimetic hydrogels. Tissue Eng. Part A 2015, 21, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Shu, B.; Zhang, M.; Xie, R.; Wang, M.; Jin, H.; Hou, W.; Tang, D.; Harris, S.E.; Mishina, Y.; O’Keefe, R.J.; et al. BMP2, but not BMP4, is crucial for chondrocyte proliferation and maturation during endochondral bone development. J. Cell Sci. 2011, 124, 3428–3440. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, R.; Yin, R.; Yin, W. Correlation of bone morphogenetic protein-2 levels in serum and synovial fluid with disease severity of knee osteoarthritis. Med. Sci. Monit. 2015, 21, 363–370. [Google Scholar] [CrossRef]
- Zhao, C.; Jiang, W.; Zhou, N.; Liao, J.; Yang, M.; Hu, N.; Liang, X.; Xu, W.; Chen, H.; Liu, W.; et al. Sox9 augments BMP2-induced chondrogenic differentiation by downregulating Smad7 in mesenchymal stem cells (MSCs). Genes Dis. 2017, 4, 229–239, Erratum in Genes Dis. 2023, 10, 624–626. https://doi.org/10.1016/j.gendis.2023.02.003. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Ohno, S.; Tanimoto, K.; Ijuin, C.; Tanaka, N.; Doi, T.; Kato, Y.; Tanne, K. The effects of high magnitude cyclic tensile load on cartilage matrix metabolism in cultured chondrocytes. Eur. J. Cell Biol. 2000, 79, 601–609. [Google Scholar] [CrossRef]
- Huang, J.; Ballou, L.R.; Hasty, K.A. Cyclic equibiaxial tensile strain induces both anabolic and catabolic responses in articular chondrocytes. Gene 2007, 404, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Bleuel, J.; Zaucke, F.; Bruggemann, G.P.; Niehoff, A. Effects of cyclic tensile strain on chondrocyte metabolism: A systematic review. PLoS ONE 2015, 10, e0119816. [Google Scholar] [CrossRef] [PubMed]
- Oka, Y.; Murata, K.; Kano, T.; Ozone, K.; Kanemura, N. Impact of controlling abnormal joint movement on the effectiveness of subsequent exercise intervention in mouse models of early knee osteoarthritis. Cartilage 2019, 13, 1947603519885007. [Google Scholar] [CrossRef]
- Zhang, H.; Ji, L.; Yang, Y.; Wie, Y.; Bai, L. The therapeutic effects of treadmill exercise on osteoarthritis in rats by inhibiting the HDAC3/NF-KappaB pathway in vivo and in vitro. Front. Physiol. 2019, 10, 1060. [Google Scholar] [CrossRef]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef]
- Jiang, W.; Liu, H.; Wan, R.; Wu, Y.; Shi, Z.; Huang, W. Mechanisms linking mitochondrial mechanotransduction and chondrocyte biology in the pathogenesis of osteoarthritis. Ageing Res. Rev. 2021, 67, 101315. [Google Scholar] [CrossRef]
- Segarra-Queralt, M.; Piella, G.; Noailly, J. Network-based modelling of mechano-inflammatory chondrocyte regulation in early osteoarthritis. Front. Bioeng. Biotechnol. 2023, 11, 1006066. [Google Scholar] [CrossRef]
- Henrotin, Y.E.; Bruckner, P. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthr. Cartil. 2003, 11, 747–755. [Google Scholar] [CrossRef]
- Bačenková, D.; Trebuňová, M.; Demeterová, J.; Živčák, J. Human Chondrocytes, Metabolism of Articular Cartilage, and Strategies for Application to Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 17096. [Google Scholar] [CrossRef]
- Bolduc, J.A.; Collins, J.A.; Loeser, R.F. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic. Biol. Med. 2019, 132, 73–82. [Google Scholar] [CrossRef]
- Li, D.; Ni, S.; Miao, K.S.; Zhuang, C. PI3K/Akt and caspase pathways mediate oxidative stress-induced chondrocyte apoptosis. Cell Stress Chaperones 2019, 24, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhang, Z.; Sheng, P.; Mobasheri, A. The role of metabolism in chondrocyte dysfunction and the progression of osteoarthritis. Ageing Res. Rev. 2021, 66, 101249. [Google Scholar] [CrossRef]
- Arra, M.; Abu-Amer, Y. Cross-talk of inflammation and chondrocyte intracellular metabolism in osteoarthritis. Osteoarthr. Cartil. 2023, 31, 1012–1021. [Google Scholar] [CrossRef]
- Loeser, R.F.; Erickson, E.A.; Long, D.L. Mitogen-activated protein kinases as therapeutic targets in osteoarthritis. Curr. Opin. Rheumatol. 2008, 20, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Ren, Q.; Jiao, L.; Huang, J.; Yi, J.; Chen, J.; Lai, J.; Ji, G.; Zheng, T. The potential roles of JAK/STAT signaling in the progression of osteoarthritis. Front. Endocrinol. 2022, 13, 1069057. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Ning, K.; Sun, M.L.; Zhang, X.A. Regulation and therapy, the role of JAK2/STAT3 signaling pathway in OA: A systematic review. Cell Commun. Signal. 2023, 21, 67. [Google Scholar] [CrossRef]
- Verhoeven, Y.; Tilborghs, S.; Jacobs, J.; De Waele, J.; Quatannens, D.; Deben, C.; Prenen, H.; Pauwels, P.; Trinh, X.B.; Wouters, A.; et al. The potential and controversy of targeting STAT family members in cancer. Semin. Cancer Biol. 2020, 60, 41–56. [Google Scholar] [CrossRef]


| BIOM | Group | n | Mean | SD | Levene Test [p] | t-Test [p] | Cohen’s d |
|---|---|---|---|---|---|---|---|
| COL1 | jCH | 9 | −1.84 | 0.48 | 0.93 | 0.021 * | 1.20 |
| pCH-OA | 9 | −1.30 | 0.43 | ||||
| MMP3 | jCH | 9 | 6.73 | 1.55 | 0.75 | 0.039 * | 1.06 |
| pCH-OA | 9 | 5.10 | 1.51 | ||||
| SOX5 | jCH | 9 | 9.03 | 1.98 | 0.00 ** | 0.024 * | 1.26 |
| pCH-OA | 9 | 7.18 | 0.64 | ||||
| ACAN | jCH | 9 | 8.44 | 1.11 | 0.15 | 0.058 (*) | 0.96 |
| pCH-OA | 9 | 7.59 | 0.57 | ||||
| RUNX2 | jCH | 9 | 4.81 | 0.69 | 0.24 | 0.079 (*) | 0.88 |
| pCH-OA | 9 | 5.36 | 0.54 |
| BIOM | Group | n | Mean Difference | SD | Levene Test [p] | t-Test [p] | Cohen’s d |
|---|---|---|---|---|---|---|---|
| IL6 | jCH | 9 | 0.91 | 0.25 | 0.077 | 0.097 (*) | 0.83 |
| pCH-OA | 9 | 0.76 | 0.16 | ||||
| IL8 | jCH | 9 | 1.17 | 0.69 | 0.084 | 0.046 * | 1.06 |
| pCH-OA | 9 | 0.59 | 0.39 | ||||
| TLR4 | jCH | 9 | 1.78 | 0.74 | 0.141 | 0.029 * | 1.17 |
| pCH-OA | 9 | 1.05 | 0.50 | ||||
| MMP1 | jCH | 9 | 1.81 | 0.53 | 0.245 | 0.022 * | 1.19 |
| pCH-OA | 9 | 1.27 | 0.35 | ||||
| BMP2 | jCH | 9 | 1.51 | 0.34 | 0.341 | 0.092 (*) | −0.85 |
| pCH-OA | 9 | 1.75 | 0.21 | ||||
| RUNX2 | jCH | 9 | 1.16 | 0.25 | 0.706 | 0.048 * | 1.01 |
| pCH-OA | 9 | 0.93 | 0.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lohberger, B.; Grote, V.; Kaltenegger, H.; Glänzer, D.; Sadoghi, P.; Kraus, T.; Steinecker-Frohnwieser, B. Juvenile and Osteoarthritic Human Chondrocytes Under Cyclic Tensile Strain: Transcriptional, Metabolic and Kinase Responses. Int. J. Mol. Sci. 2025, 26, 10934. https://doi.org/10.3390/ijms262210934
Lohberger B, Grote V, Kaltenegger H, Glänzer D, Sadoghi P, Kraus T, Steinecker-Frohnwieser B. Juvenile and Osteoarthritic Human Chondrocytes Under Cyclic Tensile Strain: Transcriptional, Metabolic and Kinase Responses. International Journal of Molecular Sciences. 2025; 26(22):10934. https://doi.org/10.3390/ijms262210934
Chicago/Turabian StyleLohberger, Birgit, Vincent Grote, Heike Kaltenegger, Dietmar Glänzer, Patrick Sadoghi, Tanja Kraus, and Bibiane Steinecker-Frohnwieser. 2025. "Juvenile and Osteoarthritic Human Chondrocytes Under Cyclic Tensile Strain: Transcriptional, Metabolic and Kinase Responses" International Journal of Molecular Sciences 26, no. 22: 10934. https://doi.org/10.3390/ijms262210934
APA StyleLohberger, B., Grote, V., Kaltenegger, H., Glänzer, D., Sadoghi, P., Kraus, T., & Steinecker-Frohnwieser, B. (2025). Juvenile and Osteoarthritic Human Chondrocytes Under Cyclic Tensile Strain: Transcriptional, Metabolic and Kinase Responses. International Journal of Molecular Sciences, 26(22), 10934. https://doi.org/10.3390/ijms262210934








