GC-MS Profiling and Antimicrobial Activity of Eight Essential Oils Against Opportunistic Pathogens with Biofilm-Forming Potential
Abstract
1. Introduction
2. Results
2.1. GC-MS Analysis of EO
2.2. Antibacterial Effect
3. Discussion
3.1. Translational Aspects: Safety Thresholds and Delivery Considerations for the Topical Oral Use of Essential Oils
3.2. Limitations of the Study
4. Materials and Methods
4.1. Samples
4.2. GC-MS Analysis
4.3. Bacterial Strains
4.4. Antimicrobial Activity Evaluation
4.5. Biofilm Growth Assay
4.6. In Silico Toxicity Prediction
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ștefănescu, R.; Ősz, B.-E.; Pintea, A.; Laczkó-Zöld, E.; Tero-Vescan, A.; Vari, C.-E.; Fulop, E.; Blaș, I.; Vancea, S. Fennel Essential Oil as a Complementary Therapy in the Management of Diabetes. Pharmaceutics 2023, 15, 2657. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Schneider, R.; Singer, N.; Singer, T. Medical Aromatherapy Revisited—Basic Mechanisms, Critique, and a New Development. Hum. Psychopharmacol. Clin. Exp. 2019, 34, e2683. [Google Scholar] [CrossRef] [PubMed]
- Sadgrove, N.; Jones, G. A Contemporary Introduction to Essential Oils: Chemistry, Bioactivity and Prospects for Australian Agriculture. Agriculture 2015, 5, 48–102. [Google Scholar] [CrossRef]
- Lupia, C.; Castagna, F.; Bava, R.; Naturale, M.D.; Zicarelli, L.; Marrelli, M.; Statti, G.; Tilocca, B.; Roncada, P.; Britti, D.; et al. Use of Essential Oils to Counteract the Phenomena of Antimicrobial Resistance in Livestock Species. Antibiotics 2024, 13, 163. [Google Scholar] [CrossRef]
- Coșeriu, R.L.; Vintilă, C.; Pribac, M.; Mare, A.D.; Ciurea, C.N.; Togănel, R.O.; Cighir, A.; Simion, A.; Man, A. Antibacterial Effect of 16 Essential Oils and Modulation of Mex Efflux Pumps Gene Expression on Multidrug-Resistant Pseudomonas aeruginosa Clinical Isolates: Is Cinnamon a Good Fighter? Antibiotics 2023, 12, 163. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial Plant Compounds, Extracts and Essential Oils: An Updated Review on Their Effects and Putative Mechanisms of Action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef]
- ALrashidi, A.A.; Noumi, E.; Snoussi, M.; Feo, V.D. Chemical Composition, Antibacterial and Anti-Quorum Sensing Activities of Pimenta dioica L. Essential Oil and Its Major Compound (Eugenol) against Foodborne Pathogenic Bacteria. Plants 2022, 11, 540. [Google Scholar] [CrossRef] [PubMed]
- Maggio, F.; Rossi, C.; Serio, A.; Chaves-Lopez, C.; Casaccia, M.; Paparella, A. Anti-Biofilm Mechanisms of Action of Essential Oils by Targeting Genes Involved in Quorum Sensing, Motility, Adhesion, and Virulence: A Review. Int. J. Food Microbiol. 2025, 426, 110874. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Setzer, W.N. Chemical Composition and Biological Activities of Essential Oils of Curcuma Species. Nutrients 2018, 10, 1196. [Google Scholar] [CrossRef]
- Nostro, A.; Blanco, A.R.; Cannatelli, M.A.; Enea, V.; Flamini, G.; Morelli, I.; Sudano Roccaro, A.; Alonzo, V. Susceptibility of Methicillin-Resistant Staphylococci to Oregano Essential Oil, Carvacrol and Thymol. FEMS Microbiol. Lett. 2004, 230, 191–195. [Google Scholar] [CrossRef]
- Friedman, M.; Henika, P.R.; Mandrell, R.E. Bactericidal Activities of Plant Essential Oils and Some of Their Isolated Constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J. Food Prot. 2002, 65, 1545–1560. [Google Scholar] [CrossRef]
- Afkar, S. Assessment of Chemical Compositions and Antibacterial Activity of the Essential Oil of Mentha piperita in Response to Salicylic Acid. Nat. Prod. Res. 2024, 38, 3562–3573. [Google Scholar] [CrossRef]
- Ben Hsouna, A.; Ben Halima, N.; Smaoui, S.; Hamdi, N. Citrus Lemon Essential Oil: Chemical Composition, Antioxidant and Antimicrobial Activities with Its Preservative Effect against Listeria monocytogenes Inoculated in Minced Beef Meat. Lipids Health Dis. 2017, 16, 146. [Google Scholar] [CrossRef]
- Hoch, C.C.; Petry, J.; Griesbaum, L.; Weiser, T.; Werner, K.; Ploch, M.; Verschoor, A.; Multhoff, G.; Bashiri Dezfouli, A.; Wollenberg, B. 1,8-Cineole (Eucalyptol): A Versatile Phytochemical with Therapeutic Applications across Multiple Diseases. Biomed. Pharmacother. 2023, 167, 115467. [Google Scholar] [CrossRef]
- Setzer, W.N.; Duong, L.; Poudel, A.; Mentreddy, S.R. Variation in the Chemical Composition of Five Varieties of Curcuma longa Rhizome Essential Oils Cultivated in North Alabama. Foods 2021, 10, 212. [Google Scholar] [CrossRef]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef]
- Public Statement on the Use of Herbal Medicinal Products Containing Thujone. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/public-statement-use-herbal-medicinal-products-containing-thujone-revision-1_en.pdf (accessed on 12 March 2025).
- Baser, K.H.C.; Haskologlu, I.C.; Erdag, E. An Updated Review of Research into Carvacrol and Its Biological Activities. Rec. Nat. Prod. 2025, 19, 308–349. [Google Scholar] [CrossRef]
- Fuentes, C.; Fuentes, A.; Barat, J.M.; Ruiz, M.J. Relevant Essential Oil Components: A Minireview on Increasing Applications and Potential Toxicity. Toxicol. Mech. Methods 2021, 31, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel for Cosmetic Ingredient Safety. Safety Assessment of Melaleuca alternifolia (Tea Tree)-Derived Ingredients as Used in Cosmetics. Available online: https://www.cir-safety.org/sites/default/files/Tea%20Tree_0.pdf (accessed on 12 March 2025).
- Liu, H.Y.; Prentice, E.L.; Webber, M.A. Mechanisms of Antimicrobial Resistance in Biofilms. npj Antimicrob. Resist. 2024, 2, 27. [Google Scholar] [CrossRef]
- Ștefănescu, R.; Esianu, S. Uleiuri Volatile Utilizate în Practica Farmaceutică și în Parfumerie; University Press: Targu Mures, Romania, 2018. [Google Scholar]
- Angelini, E.; Dziuba, L. Essential Oils. In Handbook of Molecular Gastronomy; CRC Press: Boca Raton, FL, USA, 2021; ISBN 978-0-429-16870-3. [Google Scholar]
- European Pharmacopoeia—11th Edition; Council of Europe: Strasbourg, France, 2025.
- Kim, N.-C. Need for Pharmacopeial Quality Standards for Botanical Dietary Supplements and Herbal Medicines. Food Suppl. Biomater. Health 2021, 1, e10. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Landini, P.; Antoniani, D.; Burgess, J.G.; Nijland, R. Molecular Mechanisms of Compounds Affecting Bacterial Biofilm Formation and Dispersal. Appl. Microbiol. Biotechnol. 2010, 86, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-G.; Liu, T.; Hu, Q.-P.; Cao, X.-M. Chemical Composition, Antibacterial Properties and Mechanism of Action of Essential Oil from Clove Buds against Staphylococcus aureus. Molecules 2016, 21, 1194. [Google Scholar] [CrossRef]
- Bai, J.; Li, J.; Chen, Z.; Bai, X.; Yang, Z.; Wang, Z.; Yang, Y. Antibacterial Activity and Mechanism of Clove Essential Oil against Foodborne Pathogens. LWT 2023, 173, 114249. [Google Scholar] [CrossRef]
- Oniga, I.; Pușcaș, C.; Silaghi-Dumitrescu, R.; Olah, N.-K.; Sevastre, B.; Marica, R.; Marcus, I.; Sevastre-Berghian, A.C.; Benedec, D.; Pop, C.E.; et al. Origanum vulgare Ssp. Vulgare: Chemical Composition and Biological Studies. Molecules 2018, 23, 2077. [Google Scholar] [CrossRef]
- Han, F.; Ma, G.; Yang, M.; Yan, L.; Xiong, W.; Shu, J.; Zhao, Z.; Xu, H. Chemical Composition and Antioxidant Activities of Essential Oils from Different Parts of the Oregano. J. Zhejiang Univ. Sci. B 2017, 18, 79–84. [Google Scholar] [CrossRef]
- Ultee, A.; Bennik, M.H.J.; Moezelaar, R. The Phenolic Hydroxyl Group of Carvacrol Is Essential for Action against the Food-Borne Pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef]
- Man, A.; Santacroce, L.; Iacob, R.; Mare, A.; Man, L. Antimicrobial Activity of Six Essential Oils Against a Group of Human Pathogens: A Comparative Study. Pathogens 2019, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Catherine, A.A.; Deepika, H.; Negi, P.S. Antibacterial Activity of Eugenol and Peppermint Oil in Model Food Systems. J. Essent. Oil Res. 2012, 24, 481–486. [Google Scholar] [CrossRef]
- Kim, M.; Sowndhararajan, K.; Park, S.J.; Kim, S. Effect of Inhalation of Isomers, (+)-α-Pinene and (+)-β-Pinene on Human Electroencephalographic Activity According to Gender Difference. Eur. J. Integr. Med. 2018, 17, 33–39. [Google Scholar] [CrossRef]
- Yazgan, H. Investigation of Antimicrobial Properties of Sage Essential Oil and Its Nanoemulsion as Antimicrobial Agent. LWT 2020, 130, 109669. [Google Scholar] [CrossRef]
- Russo, A.; Formisano, C.; Rigano, D.; Senatore, F.; Delfine, S.; Cardile, V.; Rosselli, S.; Bruno, M. Chemical Composition and Anticancer Activity of Essential Oils of Mediterranean Sage (Salvia officinalis L.) Grown in Different Environmental Conditions. Food Chem. Toxicol. 2013, 55, 42–47. [Google Scholar] [CrossRef]
- Public Statement on Salvia officinalis L., Aetheroleum; European Medicines Agency: London, UK, 2016.
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (Tea Tree) Oil: A Review of Antimicrobial and Other Medicinal Properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial Activity of Terpenes and Terpenoids Present in Essential Oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef]
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Agents That Inhibit Bacterial Biofilm Formation. Future Med. Chem. 2015, 7, 647–671. [Google Scholar] [CrossRef] [PubMed]
- Miranda, S.W.; Asfahl, K.L.; Dandekar, A.A.; Greenberg, E.P. Pseudomonas aeruginosa Quorum Sensing. Adv. Exp. Med. Biol. 2022, 1386, 95–115. [Google Scholar] [CrossRef] [PubMed]
- Elcocks, E.R.; Spencer-Phillips, P.T.N.; Adukwu, E.C. Rapid Bactericidal Effect of Cinnamon Bark Essential Oil against Pseudomonas aeruginosa. J. Appl. Microbiol. 2020, 128, 1025–1037. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tang, C.; Zhang, R.; Ye, S.; Zhao, Z.; Huang, Y.; Xu, X.; Lan, W.; Yang, D. Metabolomics Analysis to Evaluate the Antibacterial Activity of the Essential Oil from the Leaves of Cinnamomum camphora (Linn.) Presl. J. Ethnopharmacol. 2020, 253, 112652. [Google Scholar] [CrossRef]
- Prabuseenivasan, S.; Jayakumar, M.; Ignacimuthu, S. In Vitro Antibacterial Activity of Some Plant Essential Oils. BMC Complement. Altern. Med. 2006, 6, 39. [Google Scholar] [CrossRef]
- Ranjitkar, S.; Zhang, D.; Sun, F.; Salman, S.; He, W.; Venkitanarayanan, K.; Tulman, E.R.; Tian, X. Cytotoxic Effects on Cancerous and Non-Cancerous Cells of Trans-Cinnamaldehyde, Carvacrol, and Eugenol. Sci. Rep. 2021, 11, 16281. [Google Scholar] [CrossRef]
- Gerosa, R.; Borin, M.; Menegazzi, G.; Puttini, M.; Cavalleri, G. In Vitro Evaluation of the Cytotoxicity of Pure Eugenol. J. Endod. 1996, 22, 532–534. [Google Scholar] [CrossRef]
- Abu-Darwish, M.S.; Cabral, C.; Ferreira, I.V.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Al-bdour, T.H.; Salgueiro, L. Essential Oil of Common Sage (Salvia officinalis L.) from Jordan: Assessment of Safety in Mammalian Cells and Its Antifungal and Anti-Inflammatory Potential. BioMed Res. Int. 2013, 2013, 538940. [Google Scholar] [CrossRef]
- Jünger, H.; Jaun-Ventrice, A.; Guldener, K.; Ramseier, C.A.; Reissmann, D.R.; Schimmel, M. Anti-Inflammatory Potential of an Essential Oil-Containing Mouthwash in Elderly Subjects Enrolled in Supportive Periodontal Therapy: A 6-Week Randomised Controlled Clinical Trial. Clin. Oral Investig. 2020, 24, 3203–3211. [Google Scholar] [CrossRef]
- Tisserand, R.; Young, R. Essential Oil Safety; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 978-0-443-06241-4. [Google Scholar]
- Janani, K.; Teja, K.; Ajitha, P. Cytotoxicity of Oregano Essential Oil and Calcium Hydroxide on L929 Fibroblast Cell: A Molecular Level Study. J. Conserv. Dent. 2021, 24, 457. [Google Scholar] [CrossRef]
- Breban-Schwarzkopf, D. Eugenol—A Natural Alternative in Dentistry: An in vitro and in ovo Biosafety Assessment. Farmacia 2024, 72, 1011–1018. [Google Scholar] [CrossRef]
- Silva, W.M.F.; Bona, N.P.; Pedra, N.S.; Cunha, K.F.D.; Fiorentini, A.M.; Stefanello, F.M.; Zavareze, E.R.; Dias, A.R.G. Risk Assessment of in Vitro Cytotoxicity, Antioxidant and Antimicrobial Activities of Mentha piperita L. Essential Oil. J. Toxicol. Environ. Health Part A 2022, 85, 230–242. [Google Scholar] [CrossRef]
- Ramage, G.; Milligan, S.; Lappin, D.F.; Sherry, L.; Sweeney, P.; Williams, C.; Bagg, J.; Culshaw, S. Antifungal, Cytotoxic, and Immunomodulatory Properties of Tea Tree Oil and Its Derivative Components: Potential Role in Management of Oral Candidosis in Cancer Patients. Front. Microbiol. 2012, 3, 220. [Google Scholar] [CrossRef] [PubMed]
- Eucast: MIC Determination. Available online: https://www.eucast.org/ast_of_bacteria/mic_determination (accessed on 14 March 2025).
- Mare, A.D.; Man, A.; Ciurea, C.N.; Toma, F.; Cighir, A.; Mareș, M.; Berța, L.; Tanase, C. Silver Nanoparticles Biosynthesized with Spruce Bark Extract—A Molecular Aggregate with Antifungal Activity against Candida Species. Antibiotics 2021, 10, 1261. [Google Scholar] [CrossRef] [PubMed]
- Kerekes, E.B.; Vidács, A.; Takó, M.; Petkovits, T.; Vágvölgyi, C.; Horváth, G.; Balázs, V.L.; Krisch, J. Anti-Biofilm Effect of Selected Essential Oils and Main Components on Mono- and Polymicrobic Bacterial Cultures. Microorganisms 2019, 7, 345. [Google Scholar] [CrossRef]
- Martínez, A.; Manrique-Moreno, M.; Klaiss-Luna, M.C.; Stashenko, E.; Zafra, G.; Ortiz, C. Effect of Essential Oils on Growth Inhibition, Biofilm Formation and Membrane Integrity of Escherichia coli and Staphylococcus aureus. Antibiotics 2021, 10, 1474. [Google Scholar] [CrossRef] [PubMed]
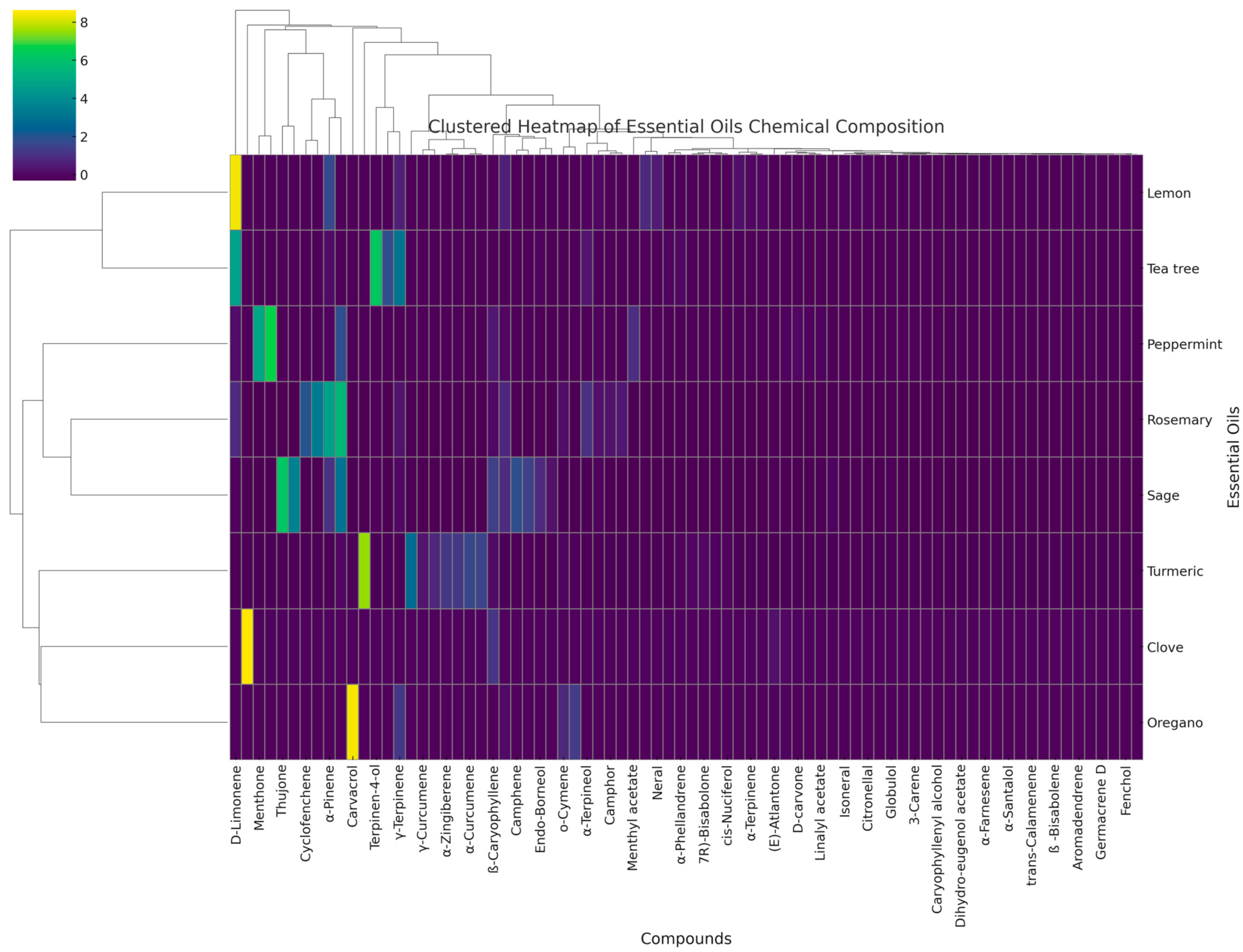
| Rt (min) | Compound | Molecular Mass | RI | RIL | Clove | Lemon | Oregano | Peppermint | Rosemary | Sage | Tea Tree | Turmeric |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peak Area (%) | ||||||||||||
| 4.107 | α-Pinene | C10H16 | 928 | 928 | - | 11.6 | - | 0.6 | 20.14 | 4.13 | 1.66 | - |
| 4.31 | Cyclofenchene | C10H16 | 943 | 946 | - | - | - | - | 9.24 | - | - | - |
| 4.313 | Camphene | C10H16 | 946 | 948 | - | - | - | - | - | 6.46 | - | - |
| 4.694 | Sabinene | C10H16 | 970 | 974 | - | - | - | - | - | - | 0.91 | - |
| 4.739 | β-Phellandrene | C10H20 | 974 | 1005 | - | 2.21 | - | 0.13 | - | - | 0.16 | - |
| 4.887 | β-Pinene | C10H16 | 984 | 974 | - | 4.68 | 1.91 | 1.33 | 4.22 | 3.61 | - | - |
| 5.114 | α-Phellandrene | C10H20 | 1001 | 1004 | - | - | - | - | 0.16 | - | 1.15 | 0.42 |
| 5.156 | 3-Carene | C10H16 | 1004 | 1008 | - | 0.14 | - | 0.18 | - | - | - | - |
| 5.252 | 2-Carene | C10H16 | 1011 | 1003 | - | - | - | - | - | - | 9.8 | - |
| 5.289 | α-Terpinene | C10H16 | 1014 | 1017 | - | 1.04 | - | - | - | - | - | - |
| 5.373 | o-Cymene | C10H14 | 1020 | 1026 | - | - | 7.29 | tr | 1.75 | 0.18 | - | 0.69 |
| 5.488 | D-Limonene | C10H16 | 1024 | 1029 | - | 52.91 | - | 2.11 | 4.41 | 0.64 | 23.9 | - |
| 5.509 | Eucalyptol | C10H18O | 1028 | 1031 | - | - | - | 11.45 | 23.11 | 10.27 | - | 0.8 |
| 5.87 | γ-Terpinene | C10H16 | 1055 | 1059 | - | 4.62 | 10.2 | - | 2.32 | 1.09 | 16.41 | tr |
| 6.314 | Terpinolene | C10H18 | 1088 | 1087 | - | 5.95 | - | - | - | - | - | - |
| 6.324 | Carvomenthene | C10H18 | 1089 | 1023 | - | - | - | - | - | 0.7 | - | - |
| 6.468 | Linalool | C10H18O | 1101 | 1099 | - | 0.98 | - | 0.57 | 2.11 | 0.56 | 0.15 | - |
| 6.653 | Thujone | C10H16O | 1112 | 1114 | - | - | - | - | - | 19.83 | - | - |
| 6.72 | Fenchol | C10H18O | 1117 | 1115 | - | 0.11 | - | - | - | - | tr | - |
| 7.236 | Camphor | C10H16O | 1154 | 1143 | - | - | - | - | 2.32 | - | - | - |
| 7.253 | Citronellal | C10H18O | 1155 | 1153 | - | 0.32 | - | - | - | - | - | - |
| 7.262 | (+)-2-Bornanone | C10H16O | 1155 | 1149 | - | - | - | - | - | 11.73 | - | - |
| 7.362 | Menthone | C10H18O | 1162 | 1150 | - | - | tr | 28.98 | - | - | - | - |
| 7.426 | Isoborneol | C10H18O | 1167 | 1158 | - | - | - | - | 14.49 | - | 0.11 | - |
| 7.429 | Isoneral | C10H16O | 1167 | 1165 | - | 0.68 | - | - | - | - | - | - |
| 7.528 | Endo-Borneol | C10H18O | 1174 | 1166 | - | - | - | - | 0.29 | 3.39 | 0.53 | - |
| 7.681 | Isogeranial | C10H16O | 1185 | 1185 | - | 1.23 | - | - | - | - | - | - |
| 7.728 | Levomenthol | C10H20O | 1188 | 1177 | - | - | - | 38.8 | - | - | - | - |
| 7.76 | Terpinen-4-ol | C10H18O | 1191 | 1177 | - | - | - | - | 0.59 | 0.51 | 31.56 | tr |
| 7.87 | α-Terpineol | C10H18O | 1198 | 1189 | - | 0.44 | - | - | 5.11 | 0.15 | 2.7 | - |
| 8.257 | cis-Carveol | C10H16O | 1226 | 1227 | 0.15 | |||||||
| 8.583 | Pulegone | C10H16O | 1252 | 1234 | - | - | - | 0.56 | - | - | - | - |
| 8.643 | D-carvone | C10H14O | 1256 | 1242 | - | 0.11 | tr | 1.24 | - | - | - | - |
| 8.701 | Geraniol | C10H18O | 1261 | 1252 | - | 0.22 | - | - | - | - | - | - |
| 8.703 | Linalyl acetate | C12H20O2 | 1261 | 1257 | - | - | - | 0.65 | - | - | - | - |
| 8.754 | Chavicol | C10H14O3 | 1265 | 1252 | 0.25 | - | - | - | - | - | - | - |
| 8.967 | Neral | C10H16O | 1280 | 1238 | - | 4.33 | - | - | - | - | - | - |
| 9.197 | Bornyl acetate | C12H20O2 | 1298 | 1283 | - | - | - | - | - | 1.77 | - | - |
| 9.227 | Isobornyl acetate | C12H20O2 | 1300 | 1284 | - | - | - | - | 2.84 | - | - | - |
| 9.254 | Thymol | C10H14O | 1302 | 1290 | - | - | 11.33 | - | - | - | - | 0.14 |
| 9.29 | Menthyl acetate | C12H22O2 | 1305 | 1295 | - | - | - | 6.5 | - | - | - | - |
| 9.528 | Carvacrol | C10H14O | 1322 | 1300 | - | - | 65.95 | - | - | - | - | - |
| 10.042 | α-Cubebene | C15H24 | 1359 | 1351 | - | - | - | 0.11 | - | tr | - | - |
| 10.344 | Eugenol | C10H12O | 1381 | 1357 | 79.75 | - | - | - | - | - | - | - |
| 10.418 | α-Copaene | C15H24 | 1387 | 1376 | - | - | - | 0.26 | tr | - | tr | - |
| 10.43 | Geranyl acetate | C12H20O2 | 1387 | 1379 | - | 0.43 | - | - | - | - | - | - |
| 10.599 | Germacrene D | C15H24 | 1400 | 1480 | - | tr | - | - | - | - | - | - |
| 10.745 | Sesquithujene | C15H24 | 1411 | 1410 | - | - | - | - | - | - | - | tr |
| 10.772 | Methyleugenol | C11H14O2 | 1414 | 1401 | 0.19 | - | - | - | - | - | - | - |
| 11.03 | β-Caryophyllene | C15H24 | 1436 | 1420 | 11.42 | 0.24 | - | 3.22 | 1.41 | 5.26 | tr | 1.22 |
| 11.128 | β-Copaene | C15H24 | 1442 | 1433 | - | tr | - | - | - | - | - | - |
| 11.269 | Aromadendrene | C15H24 | 1453 | 1440 | - | - | - | tr | - | tr | tr | - |
| 11.406 | trans-Isoeugenol | C10H12O2 | 1464 | 1454 | 0.12 | - | - | - | - | - | - | - |
| 11.475 | Humullene | C15H24 | 1469 | 1454 | 2.37 | - | - | 0.14 | - | 5.3 | - | 0.31 |
| 11.709 | γ-Curcumene | C15H22 | 1488 | 1480 | - | - | - | - | - | - | - | 1.99 |
| 11.768 | α-Curcumene | C15H22 | 1493 | 1482 | - | - | - | - | - | - | - | 6.4 |
| 11.879 | β-Humullene | C15H24 | 1502 | 1442 | - | - | - | - | - | tr | - | - |
| 11.909 | α-Zingiberene | C15H24 | 1504 | 1495 | - | - | - | - | - | - | - | 5.01 |
| 12.013 | α-Farnesene | C15H24 | 1513 | 1504 | 0.18 | - | - | - | - | - | - | - |
| 12.061 | β-Bisabolene | C15H24 | 1517 | 1508 | - | - | - | - | - | - | - | 2.03 |
| 12.1 | β-Curcumene | C15H22 | 1520 | 1512 | - | - | - | - | - | - | - | 0.41 |
| 12.268 | β-Sesquiphellandrene | C15H24 | 1534 | 1523 | - | - | - | - | - | - | - | 3.44 |
| 12.284 | Cadinene | C15H24 | 1535 | 1533 | tr | - | - | - | - | - | - | - |
| 12.297 | trans-Calamenene | C15H22 | 1536 | 1528 | - | - | - | - | - | tr | - | - |
| 12.332 | Dihydro-eugenol acetate | C12H16O | 1539 | 1536 | 0.24 | - | - | - | - | - | - | - |
| 12.462 | α-Bisabolene | C15H24 | 1550 | 1540 | - | tr | - | - | - | - | - | - |
| 12.930 | Caryophyllenyl alcohol | C15H26O | 1603 | 1580 | 0.28 | - | - | - | - | - | - | - |
| 12.973 | cis-Nuciferol | C15H22O | 1593 | 1744 | - | - | - | - | - | - | - | 0.6 |
| 13.09 | Caryophyllene oxide | C15H24O | 1603 | 1580 | 1.21 | - | 0.54 | 0.54 | tr | 0.21 | - | - |
| 13.182 | Globulol | C15H26O | 1611 | 1581 | - | - | - | - | - | 0.24 | - | - |
| 13.275 | α-Santalol | C15H24O | 1619 | 1683 | 0.14 | - | - | - | - | - | - | - |
| 13.92 | α-Tumerone | C15H22O | 1676 | 1668 | - | - | - | - | - | - | - | 10.41 |
| 14.051 | Ar-Turmerone | C15H20O | 1687 | 1668 | - | - | - | - | - | - | - | 26.52 |
| 14.284 | γ-Atlantone | C15H22O | 1708 | 1679 | - | - | - | - | - | - | - | 6.2 |
| 14.399 | β-Tumerone | C15H22O | 1718 | 1699 | - | - | - | - | - | - | - | 4.94 |
| 14.796 | (6R,7R)-Bisabolone | C15H24O | 1753 | 1742 | - | - | - | - | - | - | - | 0.79 |
| 15.165 | (E)-Atlantone | C15H22O | 1785 | 1775 | - | - | - | - | - | - | - | 0.93 |
| Total | 96.15 | 92.39 | 97.22 | 97.37 | 94.51 | 76.03 | 89.04 | 73.25 | ||||
| Monoterpene hydrocarbons | - | 83.15 | 19.4 | 4.35 | 42.24 | 16.81 | 53.99 | 1.11 | ||||
| Oxigenated monoterpenes | - | 9 | 77.28 | 88.75 | 50.86 | 48.21 | 35.05 | 0.94 | ||||
| Sesquiterpenes hydrocarbons | 13.97 | 0.24 | - | 3.73 | 1.41 | 10.56 | - | 21.08 | ||||
| Oxigenated sesquiterpenes | 1.63 | - | 0.54 | 0.54 | - | 0.45 | - | 50.39 | ||||
| Phenylpropanoid derivatives | 80.55 | - | - | - | - | - | - | - | ||||
| Essential Oil | MIC/MBC | ||||
|---|---|---|---|---|---|
| MSSA (ATCC 29213) | MRSA (ATCC 43300) | P. aeruginosa (ATCC 27853) | E. coli (ATCC 25922) | K. pneumoniae (ATCC 700603) | |
| Sage | 0.4%/0.4% | 3.125%/3.125% | 3.125%/3.125% | 3.125%/3.125% | - |
| Turmeric | 3.125%/- | 3.125%/- | - | - | - |
| Oregano | 0.4%/0.4% | 0.05%/3.125% | 3.125%/3.125% | 0.05%/0.05% | 3.125%/3.125% |
| Clove | 3.125%/3.125% | 0.4%/0.4% | 0.4%/0.4% | 0.4%/0.4% | 3.125%/3.125% |
| Lemon | 3.125%/- | 3.125%/3.125% | - | 3.125%/3.125% | 3.125% |
| Peppermint | 3.125%/- | - | - | 3.125% | - |
| Rosemary | 0.05%/0.4% | 0.05%/3.125% | 3.125%/3.125% | 0.4%/3.125% | 3.125%/3.125% |
| Tea tree | 0.05%/0.05% | 0.05%/0.05% | 0.4%/0.4% | 0.4%/0.4% | 0.4%/3.125% |
| Essential Oil | Concentration (%) | MSSA | MRSA | P. aeruginosa | E. coli | K. pneumoniae |
|---|---|---|---|---|---|---|
| BI | BI | BI | BI | BI | ||
| Sage | 50 | ++ | +++ | +++ | +/− | ++ |
| 25 | ++ | ++ | +++ | +/− | +/− | |
| 12.5 | +/− | +/− | ++ | ↑ | +/− | |
| 6.25 | ↑ | +/− | ↑ | ↑ | +/− | |
| Turmeric | 50 | ++ | ++ | ++ | ↑ | ↑ |
| 25 | ++ | ++ | ++ | ↑ | ↑ | |
| 12.5 | ++ | ++ | ++ | ↑ | ↑ | |
| 6.25 | +/− | ++ | ++ | ↑ | ↑ | |
| Oregano | 50 | +++ | ++ | +++ | +/− | ++ |
| 25 | ++ | +/− | +++ | +/− | +/− | |
| 12.5 | +/− | +/− | +++ | +/− | +/− | |
| 6.25 | +/− | +/− | ++ | +/− | +/− | |
| Clove | 50 | +++ | +++ | +++ | ++ | ++ |
| 25 | +++ | +++ | ++ | ++ | ++ | |
| 12.5 | +++ | +++ | +/− | ++ | ++ | |
| 6.25 | ++ | ++ | +/− | ++ | +++ | |
| Lemon | 50 | +++ | +++ | +++ | ++ | ++ |
| 25 | ++ | +++ | +++ | ++ | ++ | |
| 12.5 | ++ | +/− | +++ | ↑ | +/− | |
| 6.25 | +/− | +/− | +++ | ↑ | +/− | |
| Peppermint | 50 | +++ | +++ | +++ | ++ | +/− |
| 25 | +++ | +++ | +++ | +/− | +/− | |
| 12.5 | ++ | ++ | +++ | +/− | +/− | |
| 6.25 | +/− | +/− | +++ | ↑ | ↑ | |
| Rosemary | 50 | +++ | +++ | +++ | ++ | ++ |
| 25 | +++ | +++ | +++ | ++ | +/− | |
| 12.5 | ++ | ++ | ++ | +/− | +/− | |
| 6.25 | +/− | +/− | ++ | +/− | +/− | |
| Tea tree | 50 | ++ | ++ | ++ | ++ | +/− |
| 25 | +/− | +/− | +/− | +/− | ↑ | |
| 12.5 | +/− | ↑ | ↑ | +/− | ↑ | |
| 6.25 | ↑ | ↑ | ↑ | ↑ | ↑ |
| Essential Oil | MIC % (v/v) | Main Bioactive Compound | Predicted LD50 (mg/kg) | Toxicity Class | Toxicological Endpoints Target | Comments |
|---|---|---|---|---|---|---|
| Sage | 0.4% | Thujone | 500 | IV | Neurotoxicity, BBB | Use restricted in cosmetics and food products due to neurotoxicity; regulated under EU Regulation 1334/2008; TDI = 10 µg/kg body weight/day [18] |
| Turmeric | 3.125% | ar-Turmerone | 2000 | IV | BBB | Limited toxicity data |
| Oregano | 0.4% | Carvacrol | 810 | IV | BBB, Mitochondrial membrane potential, CYP2C9 | Potent antimicrobial activity; may irritate skin or mucosa at high concentrations [19] |
| Clove | 3.125% | Eugenol | 650 | IV | Neurotoxicity, BBB | Commonly used in dentistry as analgesic and antiseptic; recognized for safe usage at low concentrations [20] |
| Lemon | 3.125% | Limonene | 4400 | V | Cardiotoxicity, BBB, CYP2C9 | Widely used in cosmetics and fragrances; potential skin sensitizer; generally recognized as safe by FDA |
| Peppermint | 3.125% | Menthol | 940 | IV | BBB | Approved for oral and topical used; cooling agent in OTC products; can produce mucosal irritation in high doses |
| Rosemary | 0.05% | Eucalyptol | 2480 | V | BBB | Common in mouthwashes and cough syrups; approved for use in cosmetics and oral hygiene products |
| Tea tree | 0.05% | Terpinen-4-ol | 1016 | IV | BBB | Well-tolerated in topical formulations at <1%; cytotoxicity at higher concentrations [21] |
| Essential Oil/Major Compound | MIC % (v/v) | Non-Cytotoxic Level (%)/Cell Line # | Non-Irritant Dermal Concentrations (%) * | Suggested Delivery Formulation | Observations | References |
|---|---|---|---|---|---|---|
| Sage (thujone) | 0.4% | 0.03%/TR146 | recommended concentration: ≤0.4% | short contact rinse; synergistic formulation mouthwash | thujone is convulsant and neurotoxic; avoid internal use | [49,50,51] |
| Turmeric (ar-Turmerone) | 3.125% | n.d. | non-irritant concentrations: 4% | controlled delivery formulations; flavoring; recommended concentrations: ≤2% | limited data on oral safety | [51] |
| Oregano (carvacrol) | 0.4% | 0.005–0.008%/L969, HGF | non-irritant concentrations: 2% | diluted mouthwash; short exposure; recommended concentrations: <1% | highly irritant if used undiluted | [51,52] |
| Clove (eugenol) | 3.125% | Eugenol cytotoxicity: 0.01% after 24 h exposure | non-irritant concentrations: <3% | root canal use; diluted mouthwash; lozenges; synergistic with clorhexidine; recommended concentration: <1% | eugenol is frequently used in dental products in concentrations of 0.05–0.1% | [51,53] |
| Lemon (limonene) | 3.125% | n.d. | non-irritant concentrations: 2–5%; | diluted mouthwash; flavoring; recommended concentrations: ≤2% | oxidation products of limonene can cause skin sensitization | [51] |
| Peppermint (menthol) | 3.125% | 0.01%/HaCaT | non-irritant concentrations: <10%; low overall toxicity | mouthwash, hydrogels, lozenges, spray | has GRAS status; frequently used in dental products; pulegone content should be <1% | [51,54] |
| Rosemary (eucalyptol) | 0.05% | n.d. | non-irritant concentrations: <10% | mouthwash, hydrogels, low-dose lozenges, spray | generally well tolerated | [51] |
| Tea tree (terpinen-4-ol) | 0.05% | ≤0.03%/HaCaT, HGF | 10–20% | mouthwash, hydrogels, not lozenges; recommended concentration: 2.5% | tea tree oil is prone to oxidation | [51,55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ștefănescu, R.; Laczkó-Zöld, E.; Ciurea, C.; Tero-Vescan, A.; Ősz, B.; Vancea, S.; Sita, D.; Mare, A. GC-MS Profiling and Antimicrobial Activity of Eight Essential Oils Against Opportunistic Pathogens with Biofilm-Forming Potential. Int. J. Mol. Sci. 2025, 26, 10928. https://doi.org/10.3390/ijms262210928
Ștefănescu R, Laczkó-Zöld E, Ciurea C, Tero-Vescan A, Ősz B, Vancea S, Sita D, Mare A. GC-MS Profiling and Antimicrobial Activity of Eight Essential Oils Against Opportunistic Pathogens with Biofilm-Forming Potential. International Journal of Molecular Sciences. 2025; 26(22):10928. https://doi.org/10.3390/ijms262210928
Chicago/Turabian StyleȘtefănescu, Ruxandra, Eszter Laczkó-Zöld, Cristina Ciurea, Amelia Tero-Vescan, Bianca Ősz, Szende Vancea, Dragoș Sita, and Anca Mare. 2025. "GC-MS Profiling and Antimicrobial Activity of Eight Essential Oils Against Opportunistic Pathogens with Biofilm-Forming Potential" International Journal of Molecular Sciences 26, no. 22: 10928. https://doi.org/10.3390/ijms262210928
APA StyleȘtefănescu, R., Laczkó-Zöld, E., Ciurea, C., Tero-Vescan, A., Ősz, B., Vancea, S., Sita, D., & Mare, A. (2025). GC-MS Profiling and Antimicrobial Activity of Eight Essential Oils Against Opportunistic Pathogens with Biofilm-Forming Potential. International Journal of Molecular Sciences, 26(22), 10928. https://doi.org/10.3390/ijms262210928









