Moon Jellyfish Mucin and Collagen Attenuate Catabolic Activity in Chondrocytes but Show Limited Efficacy in an Osteoarthritis Rat Model
Abstract
1. Introduction
2. Results
2.1. Measurement of Endotoxin Concentration
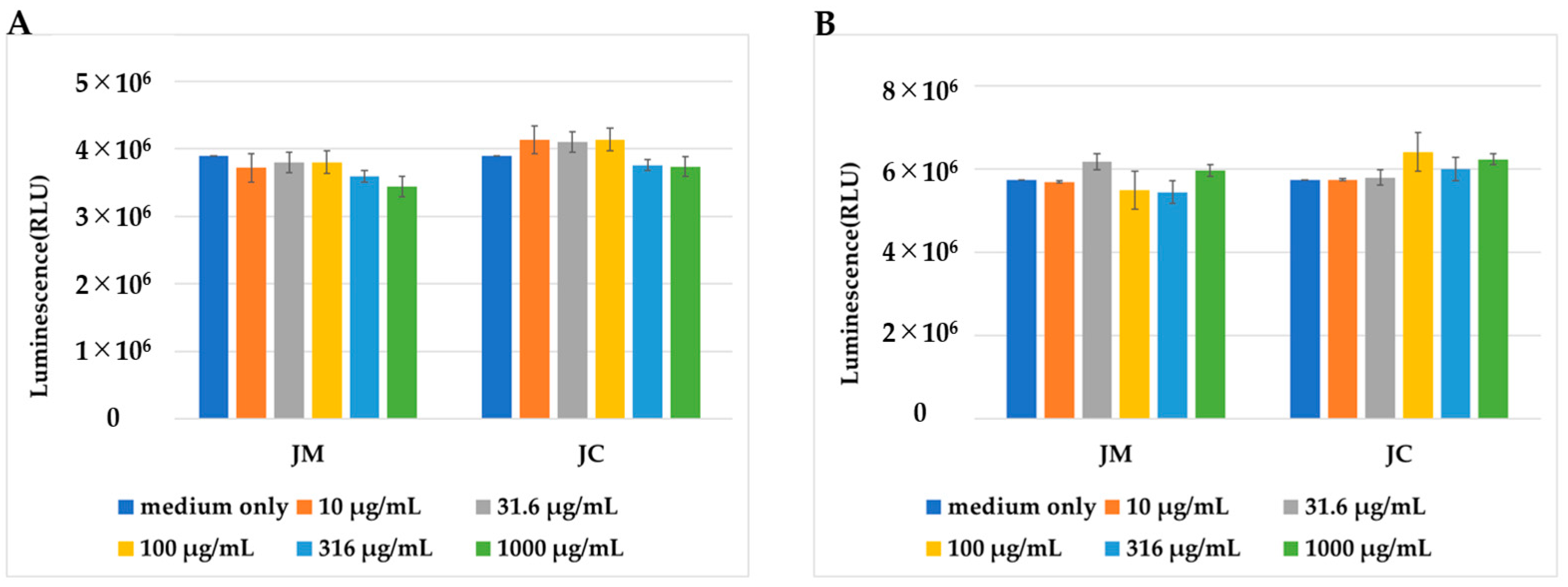
2.2. Effects of JM and JC on Cell Proliferation
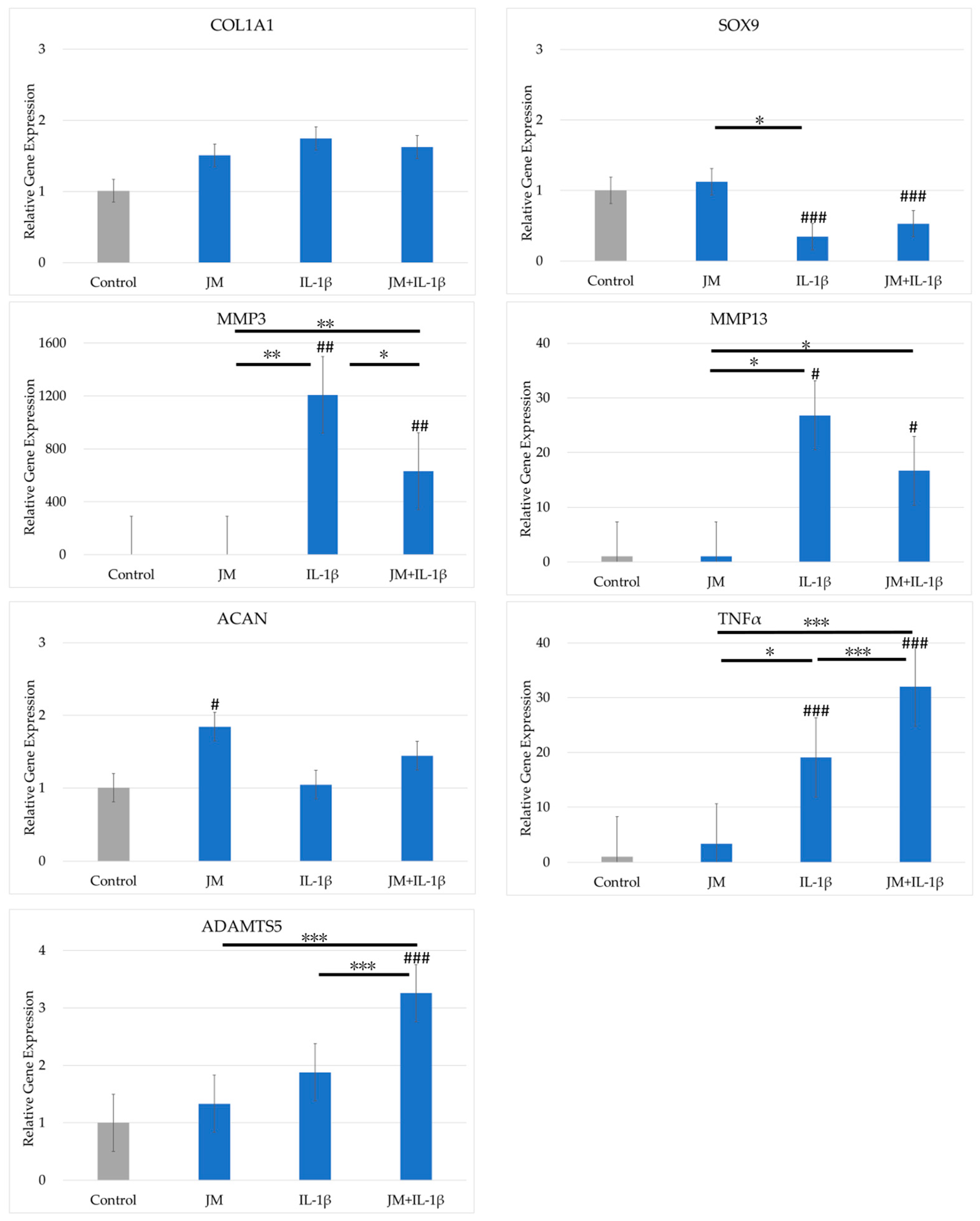
2.3. Effects of JM on Gene Expression in PDs
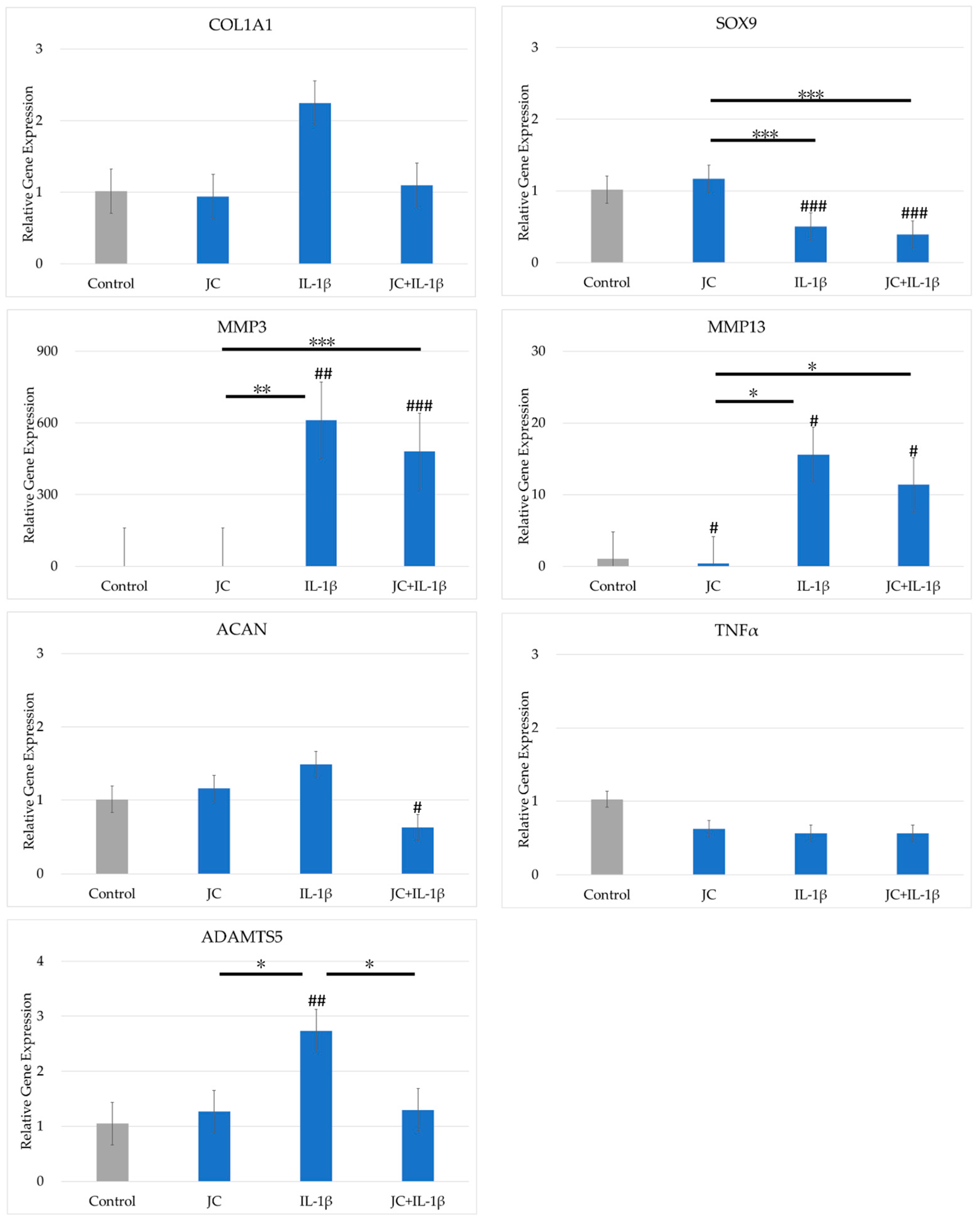
2.4. Effects of JC on Gene Expression in PDs
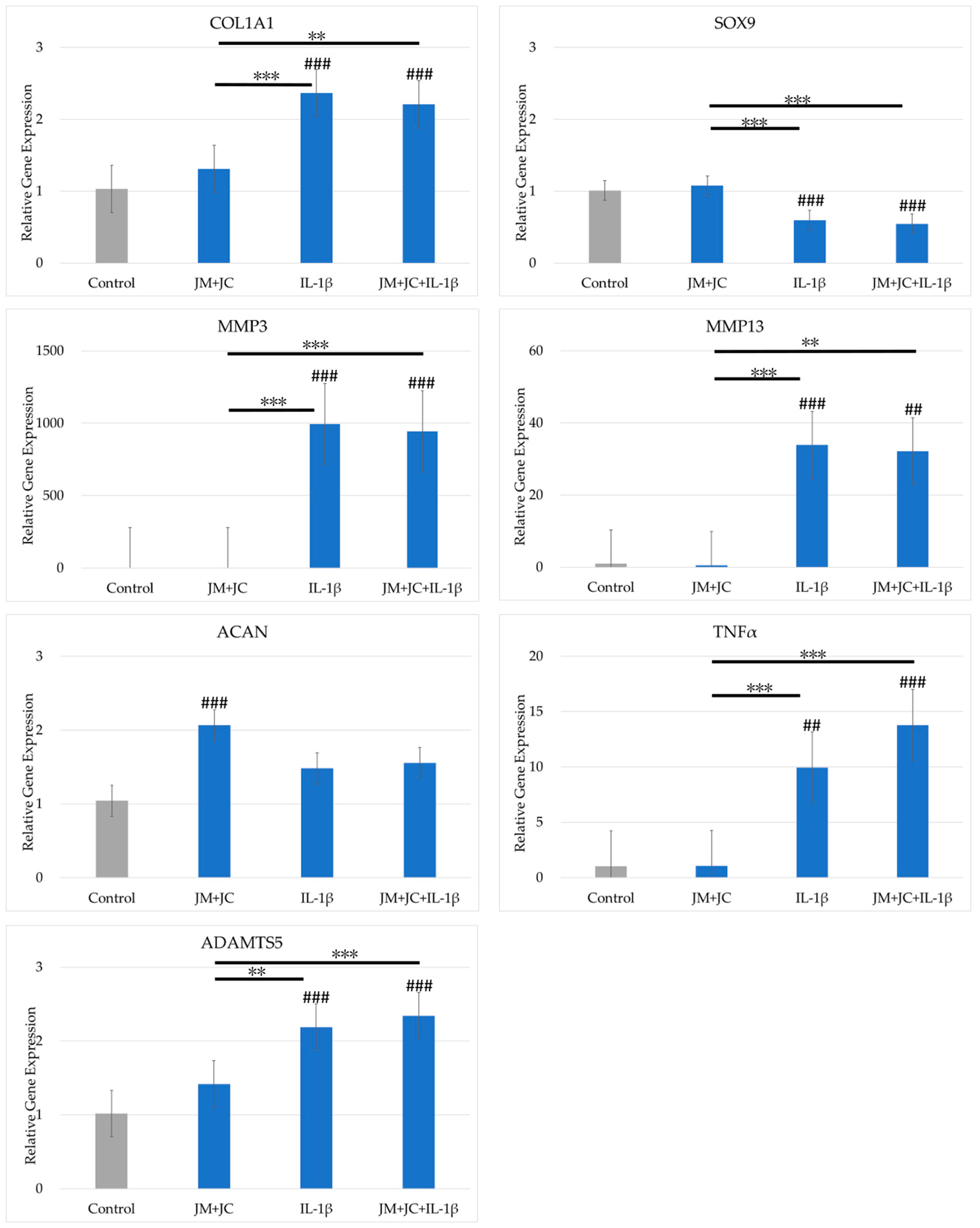
2.5. Effects of the Combination of JM and JC on Gene Expression in PDs
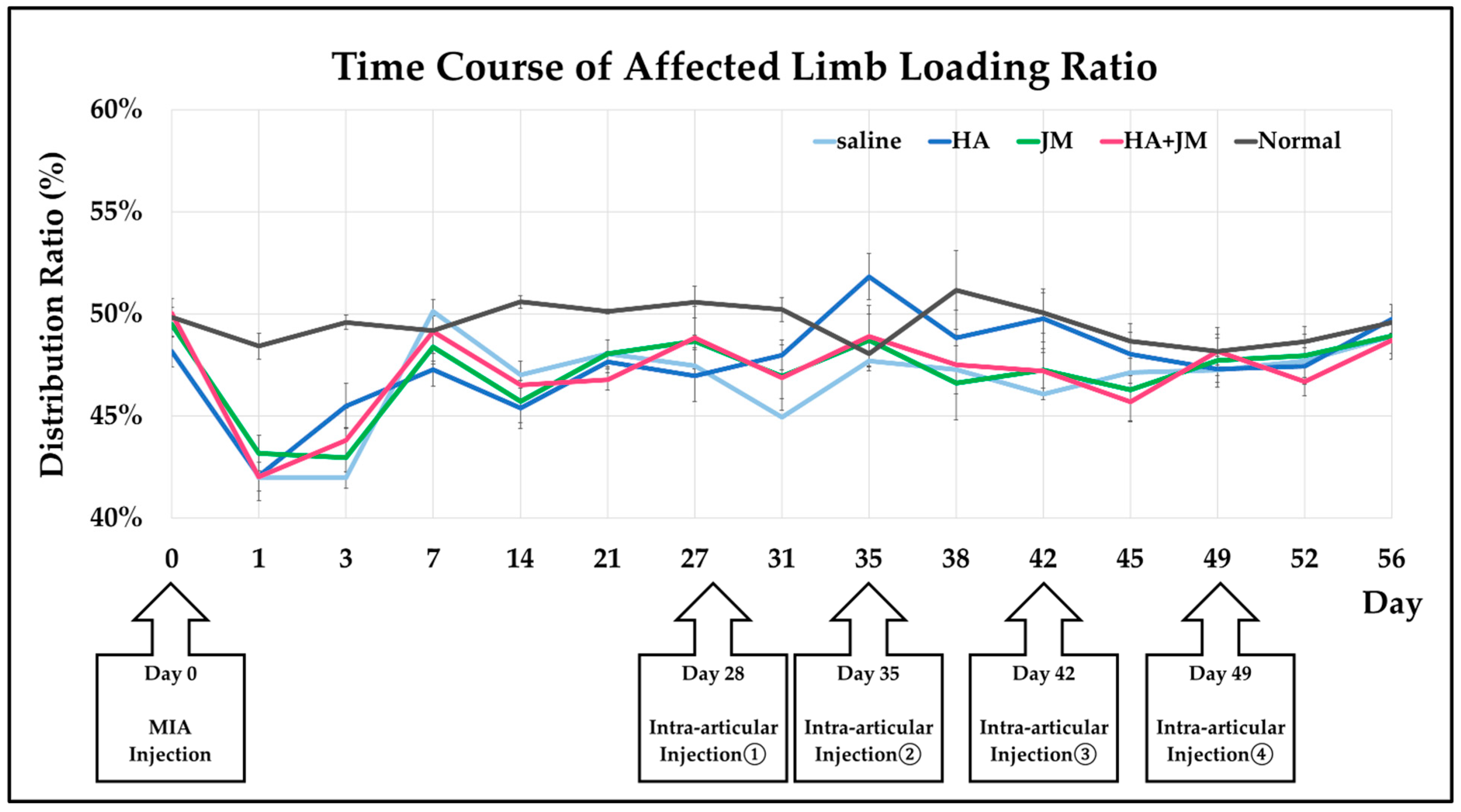
2.6. Changes in Weight-Bearing Ratio Following Intra-Articular Injections in MIA Model Rats
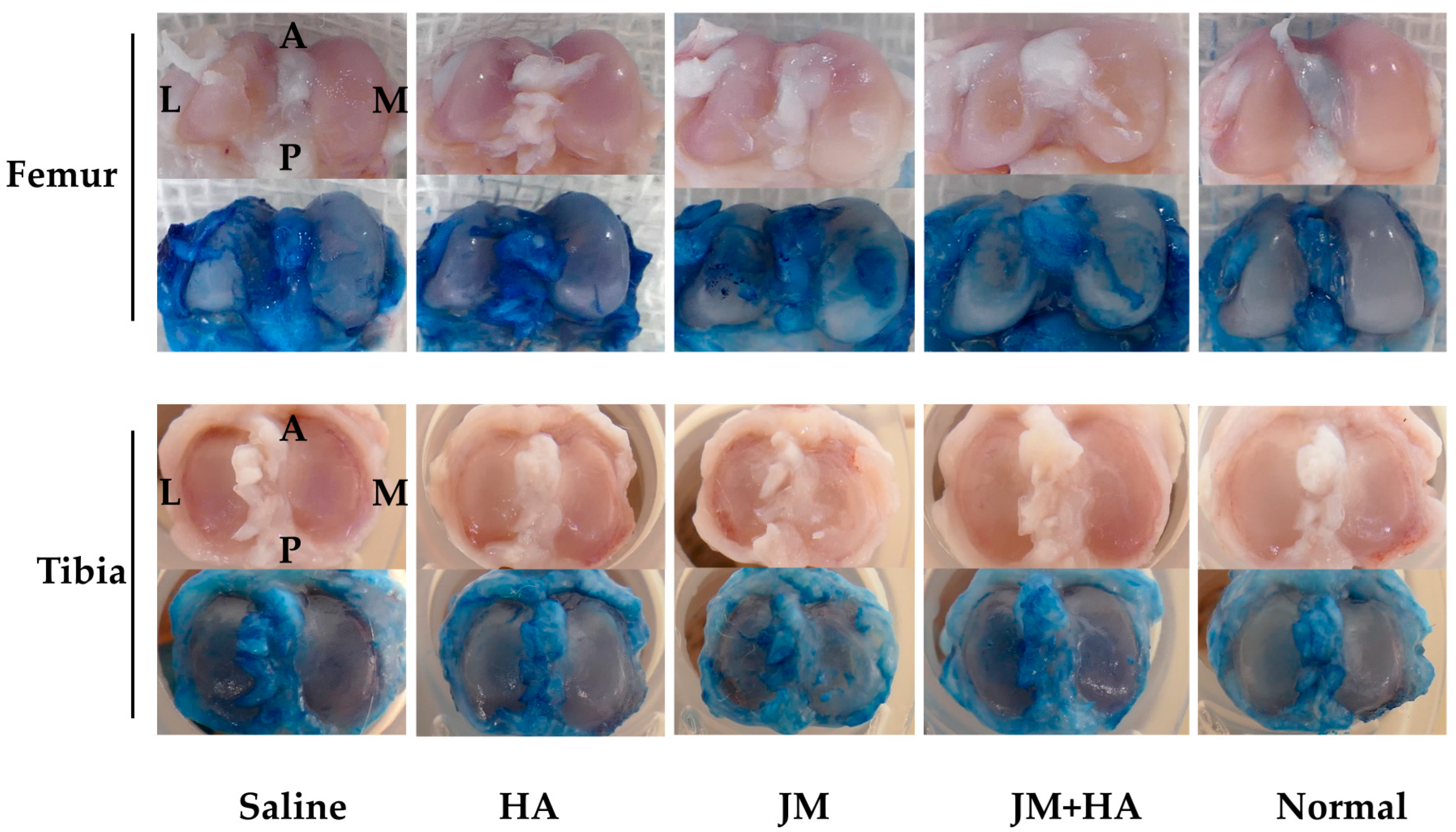
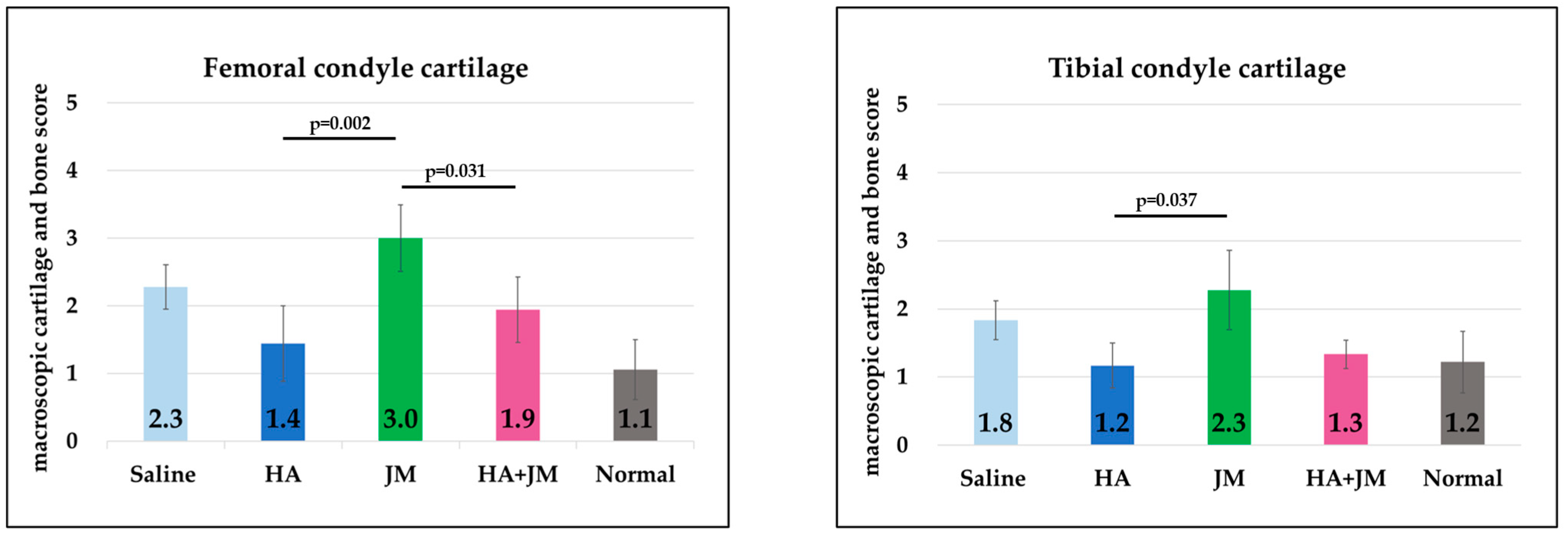
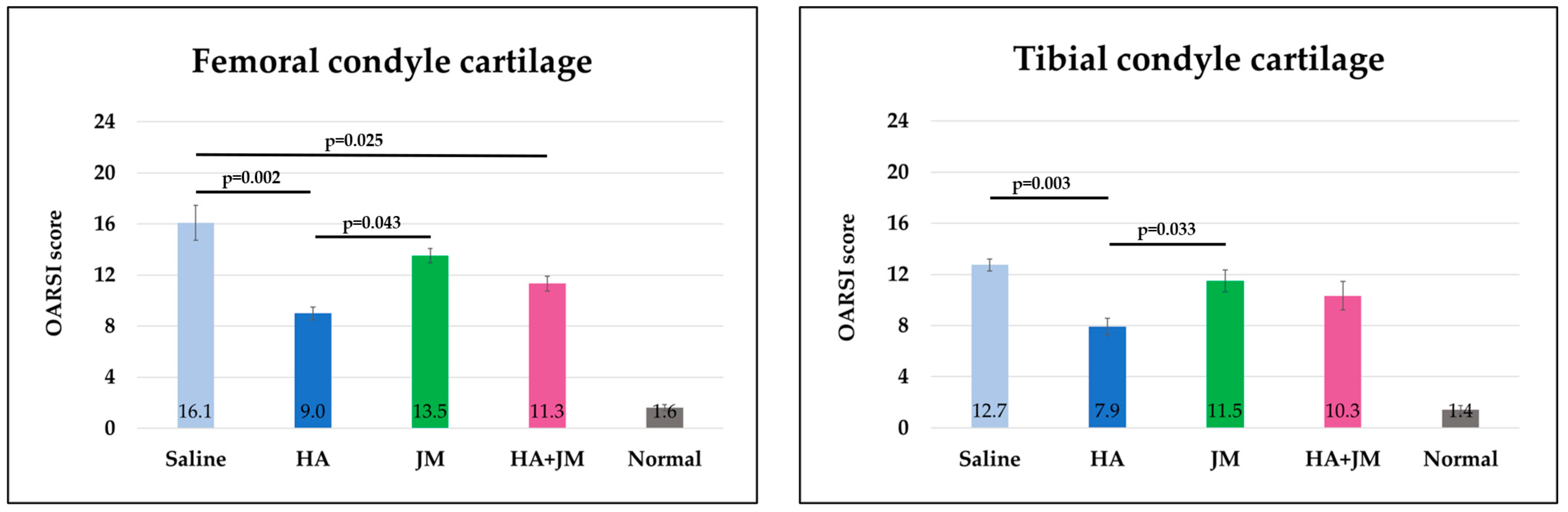
2.7. Comparison of Macroscopic Scores of Articular Cartilage
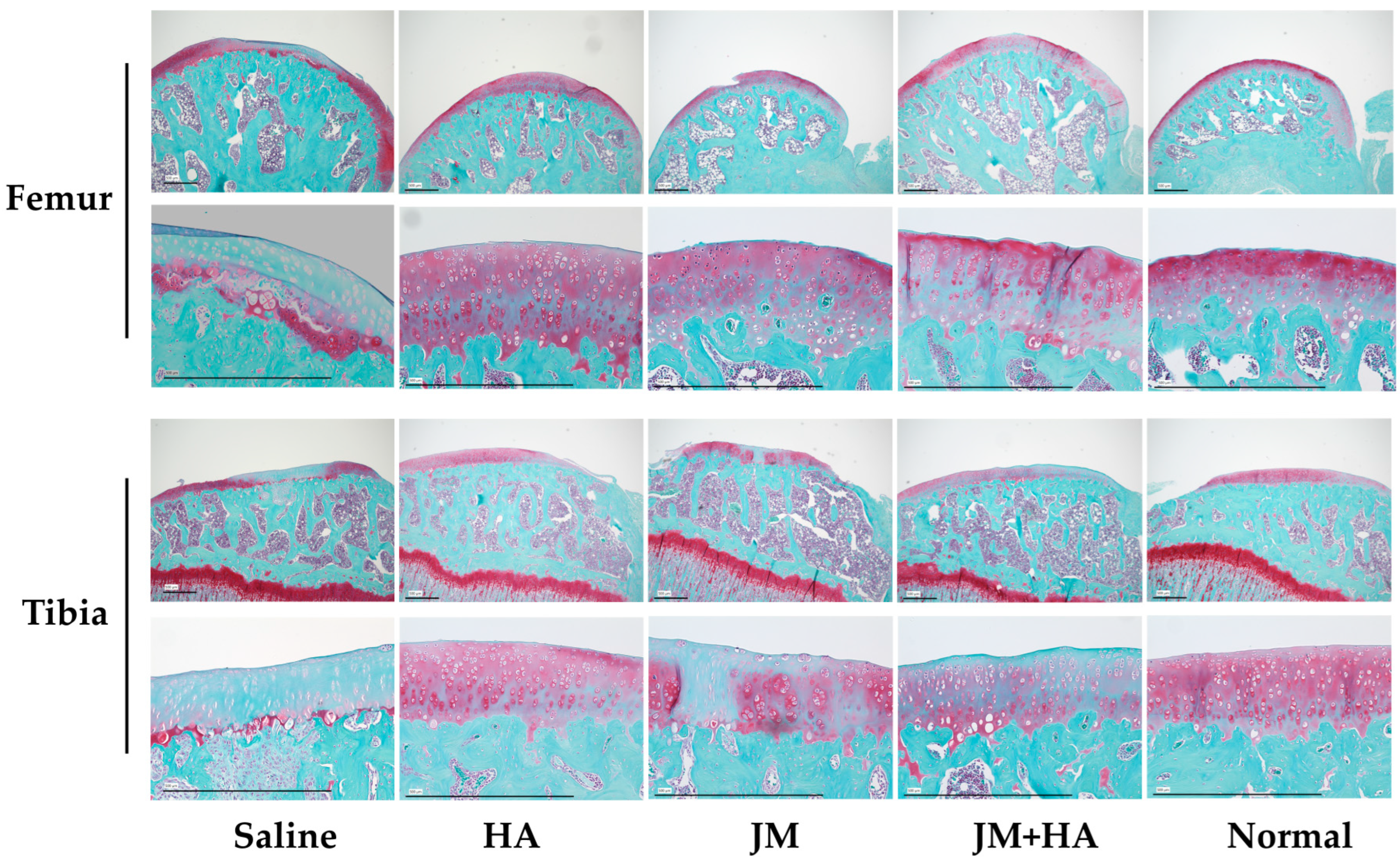
2.8. Comparison of Histological Scores of Articular Cartilage
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Isolation of PDs
4.1.2. Extraction and Purification of JM
4.1.3. Extraction and Purification of JC
4.2. Examination of the Cell Growth-Inhibitory Effects of JM and JC
4.3. Cell Culture
4.4. RNA Extraction, cDNA Synthesis, and RT-qPCR
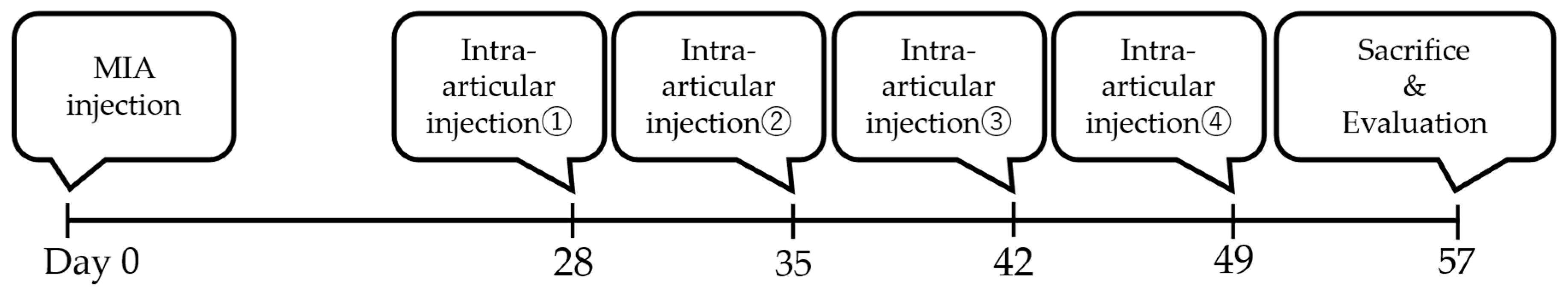
4.5. Establishment of MIA Model Rats and Intra-Articular Injections
4.5.1. Animals
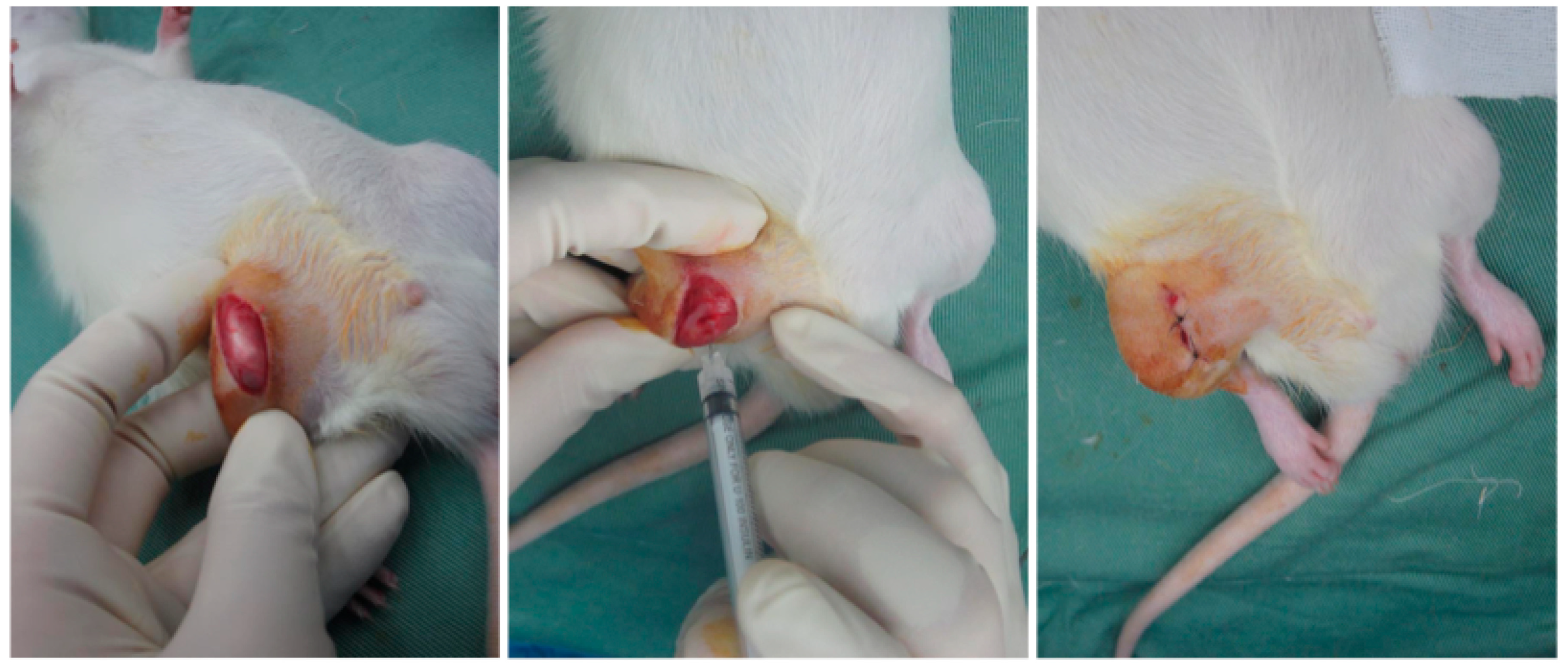
4.5.2. Induction of OA by MIA
4.5.3. Intra-Articular Injection
4.5.4. Pain Evaluation
4.5.5. Evaluation
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | Ascorbic acid |
| AB | Antibiotic antimycotic solution |
| ACAN | Aggrecan |
| ADAMTS5 | A Disintegrin and Metalloproteinase with Thrombospondin motifs 5 |
| ASC | Adipose derived stem cell |
| COL1 | Type I collagen |
| COL2 | Type II collagen |
| Ct | Cycle threshold |
| DMEM/F12 | Dulbecco’s Modified Eagle’s Medium–nutrient mixture F-12 |
| FBS | Fetal bovine serum |
| GAPDH | Glyceraldehyde-3-phosphate Dehydrogenase |
| HA | Hyaluronic acid |
| IL-1β | Interleukin-1β |
| JC | Jellyfish collagen |
| JM | Jellyfish mucin |
| MMP3 | Matrix Metalloproteinase-3 |
| MMP13 | Matrix Metalloproteinase-13 |
| MUC5AC | Mucin 5ac |
| OA | Osteoarthritis |
| PBS | Phosphate-buffered saline |
| PRP | Platelet-rich plasma |
| RT-qPCR | Quantitative reverse-transcription polymerase chain reaction |
| TGFβ | Transforming growth factor-β |
| TNFα | Tumor necrosis factor-α |
References
- Trouvin, A.-P.; Perrot, S. Pain in Osteoarthritis. Implications for Optimal Management. Jt. Bone Spine 2018, 85, 429–434. [Google Scholar] [CrossRef]
- Kulkarni, K.; Karssiens, T.; Kumar, V.; Pandit, H. Obesity and Osteoarthritis. Maturitas 2016, 89, 22–28. [Google Scholar] [CrossRef]
- Allen, P.R.; Denham, R.A.; Swan, A.V. Late Degenerative Changes after Meniscectomy. Factors Affecting the Knee after Operation. J. Bone Jt. Surg. Br. 1984, 66, 666–671. [Google Scholar] [CrossRef]
- Dulay, G.S.; Cooper, C.; Dennison, E.M. Knee Pain, Knee Injury, Knee Osteoarthritis & Work. Best Pract. Res. Clin. Rheumatol. 2015, 29, 454–461. [Google Scholar] [CrossRef]
- Song, J.; Baek, I.-J.; Chun, C.-H.; Jin, E.-J. Dysregulation of the NUDT7-PGAM1 Axis Is Responsible for Chondrocyte Death during Osteoarthritis Pathogenesis. Nat. Commun. 2018, 9, 3427. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Mobasheri, A.; Mozafari, M. Inflammatory Mediators in Osteoarthritis: A Critical Review of the State-of-the-Art, Current Prospects, and Future Challenges. Bone 2016, 85, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.-P.; Fahmi, H. Role of Proinflammatory Cytokines in the Pathophysiology of Osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef]
- Frischholz, S.; Berberich, O.; Böck, T.; Meffert, R.H.; Blunk, T. Resveratrol Counteracts IL-1β-Mediated Impairment of Extracellular Matrix Deposition in 3D Articular Chondrocyte Constructs. J. Tissue Eng. Regen. Med. 2020, 14, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Vincent, T.L. IL-1 in Osteoarthritis: Time for a Critical Review of the Literature. F1000Research 2019, 8, 934. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Izumi, M.; Aso, K.; Sugimura, N.; Kato, T.; Tani, T. Effects of Intra-Articular Hyaluronic Acid Injection on Immunohistochemical Characterization of Joint Afferents in a Rat Model of Knee Osteoarthritis. Eur. J. Pain Lond. Engl. 2015, 19, 334–340. [Google Scholar] [CrossRef]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic Acid: Molecular Mechanisms and Therapeutic Trajectory. Front. Vet. Sci. 2019, 6, 192. [Google Scholar] [CrossRef]
- Altman, R.; Hackel, J.; Niazi, F.; Shaw, P.; Nicholls, M. Efficacy and Safety of Repeated Courses of Hyaluronic Acid Injections for Knee Osteoarthritis: A Systematic Review. Semin. Arthritis Rheum. 2018, 48, 168–175. [Google Scholar] [CrossRef]
- Cook, C.S.; Smith, P.A. Clinical Update: Why PRP Should Be Your First Choice for Injection Therapy in Treating Osteoarthritis of the Knee. Curr. Rev. Musculoskelet. Med. 2018, 11, 583–592. [Google Scholar] [CrossRef]
- Pak, J.; Lee, J.H.; Pak, N.; Pak, Y.; Park, K.S.; Jeon, J.H.; Jeong, B.C.; Lee, S.H. Cartilage Regeneration in Humans with Adipose Tissue-Derived Stem Cells and Adipose Stromal Vascular Fraction Cells: Updated Status. Int. J. Mol. Sci. 2018, 19, 2146. [Google Scholar] [CrossRef]
- Wasai, S.; Sato, M.; Maehara, M.; Toyoda, E.; Uchiyama, R.; Takahashi, T.; Okada, E.; Iwasaki, Y.; Suzuki, S.; Watanabe, M. Characteristics of Autologous Protein Solution and Leucocyte-Poor Platelet-Rich Plasma for the Treatment of Osteoarthritis of the Knee. Sci. Rep. 2020, 10, 10572. [Google Scholar] [CrossRef] [PubMed]
- Ohta, N.; Sato, M.; Ushida, K.; Kokubo, M.; Baba, T.; Taniguchi, K.; Urai, M.; Kihira, K.; Mochida, J. Jellyfish Mucin May Have Potential Disease-Modifying Effects on Osteoarthritis. BMC Biotechnol. 2009, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Urai, M.; Nakamura, T.; Uzawa, J.; Baba, T.; Taniguchi, K.; Seki, H.; Ushida, K. Structural Analysis of O-Glycans of Mucin from Jellyfish (Aurelia aurita) Containing 2-Aminoethylphosphonate. Carbohydr. Res. 2009, 344, 2182–2187. [Google Scholar] [CrossRef] [PubMed]
- Pustlauk, W.; Paul, B.; Brueggemeier, S.; Gelinsky, M.; Bernhardt, A. Modulation of Chondrogenic Differentiation of Human Mesenchymal Stem Cells in Jellyfish Collagen Scaffolds by Cell Density and Culture Medium. J. Tissue Eng. Regen. Med. 2017, 11, 1710–1722. [Google Scholar] [CrossRef]
- Sumiyoshi, H.; Nakao, S.; Endo, H.; Yanagawa, T.; Nakano, Y.; Okamura, Y.; Kawaguchi, A.T.; Inagaki, Y. A Novel Composite Biomaterial Made of Jellyfish and Porcine Collagens Accelerates Dermal Wound Healing by Enhancing Reepithelization and Granulation Tissue Formation in Mice. Adv. Wound Care 2020, 9, 295–311. [Google Scholar] [CrossRef]
- Song, E.; Yeon Kim, S.; Chun, T.; Byun, H.-J.; Lee, Y.M. Collagen Scaffolds Derived from a Marine Source and Their Biocompatibility. Biomaterials 2006, 27, 2951–2961. [Google Scholar] [CrossRef]
- Widdowson, J.P.; Picton, A.J.; Vince, V.; Wright, C.J.; Mearns-Spragg, A. In Vivo Comparison of Jellyfish and Bovine Collagen Sponges as Prototype Medical Devices. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 1524–1533. [Google Scholar] [CrossRef]
- Bennett, E.P.; Mandel, U.; Clausen, H.; Gerken, T.A.; Fritz, T.A.; Tabak, L.A. Control of Mucin-Type O-Glycosylation: A Classification of the Polypeptide GalNAc-Transferase Gene Family. Glycobiology 2012, 22, 736–756. [Google Scholar] [CrossRef] [PubMed]
- Sewing, J.; Klinger, M.; Notbohm, H. Jellyfish Collagen Matrices Conserve the Chondrogenic Phenotype in Two- and Three-Dimensional Collagen Matrices. J. Tissue Eng. Regen. Med. 2017, 11, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Udo, M.; Muneta, T.; Tsuji, K.; Ozeki, N.; Nakagawa, Y.; Ohara, T.; Saito, R.; Yanagisawa, K.; Koga, H.; Sekiya, I. Monoiodoacetic Acid Induces Arthritis and Synovitis in Rats in a Dose- and Time-Dependent Manner: Proposed Model-Specific Scoring Systems. Osteoarthr. Cartil. 2016, 24, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Pritzker, K.P.H.; Gay, S.; Jimenez, S.A.; Ostergaard, K.; Pelletier, J.-P.; Revell, P.A.; Salter, D.; van den Berg, W.B. Osteoarthritis Cartilage Histopathology: Grading and Staging. Osteoarthr. Cartil. 2006, 14, 13–29. [Google Scholar] [CrossRef]
- Maehara, M.; Sato, M.; Toyoda, E.; Takahashi, T.; Okada, E.; Kotoku, T.; Watanabe, M. Characterization of Polydactyly-Derived Chondrocyte Sheets versus Adult Chondrocyte Sheets for Articular Cartilage Repair. Inflamm. Regen. 2017, 37, 22. [Google Scholar] [CrossRef]
- Ito, S.; Sato, M.; Yamato, M.; Mitani, G.; Kutsuna, T.; Nagai, T.; Ukai, T.; Kobayashi, M.; Kokubo, M.; Okano, T.; et al. Repair of Articular Cartilage Defect with Layered Chondrocyte Sheets and Cultured Synovial Cells. Biomaterials 2012, 33, 5278–5286. [Google Scholar] [CrossRef]
- Mihara, M.; Higo, S.; Uchiyama, Y.; Tanabe, K.; Saito, K. Different Effects of High Molecular Weight Sodium Hyaluronate and NSAID on the Progression of the Cartilage Degeneration in Rabbit OA Model. Osteoarthr. Cartil. 2007, 15, 543–549. [Google Scholar] [CrossRef]
- Tani, Y.; Sato, M.; Maehara, M.; Nagashima, H.; Yokoyama, M.; Yokoyama, M.; Yamato, M.; Okano, T.; Mochida, J. The Effects of Using Vitrified Chondrocyte Sheets on Pain Alleviation and Articular Cartilage Repair. J. Tissue Eng. Regen. Med. 2017, 11, 3437–3444. [Google Scholar] [CrossRef]
- Takatori, N.; Sato, M.; Toyoda, E.; Takahashi, T.; Okada, E.; Maehara, M.; Watanabe, M. Cartilage Repair and Inhibition of the Progression of Cartilage Degeneration after Transplantation of Allogeneic Chondrocyte Sheets in a Nontraumatic Early Arthritis Model. Regen. Ther. 2018, 9, 24–31. [Google Scholar] [CrossRef]
- Nomura, M.; Sakitani, N.; Iwasawa, H.; Kohara, Y.; Takano, S.; Wakimoto, Y.; Kuroki, H.; Moriyama, H. Thinning of Articular Cartilage after Joint Unloading or Immobilization. An Experimental Investigation of the Pathogenesis in Mice. Osteoarthr. Cartil. 2017, 25, 727–736. [Google Scholar] [CrossRef] [PubMed]
- García-Coronado, J.M.; Martínez-Olvera, L.; Elizondo-Omaña, R.E.; Acosta-Olivo, C.A.; Vilchez-Cavazos, F.; Simental-Mendía, L.E.; Simental-Mendía, M. Effect of Collagen Supplementation on Osteoarthritis Symptoms: A Meta-Analysis of Randomized Placebo-Controlled Trials. Int. Orthop. 2019, 43, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.E.; Oesser, S. Collagen Hydrolysate for the Treatment of Osteoarthritis and Other Joint Disorders: A Review of the Literature. Curr. Med. Res. Opin. 2006, 22, 2221–2232. [Google Scholar] [CrossRef] [PubMed]
- Bersellini Farinotti, A.; Wigerblad, G.; Nascimento, D.; Bas, D.B.; Morado Urbina, C.; Nandakumar, K.S.; Sandor, K.; Xu, B.; Abdelmoaty, S.; Hunt, M.A.; et al. Cartilage-Binding Antibodies Induce Pain through Immune Complex-Mediated Activation of Neurons. J. Exp. Med. 2019, 216, 1904–1924. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kuriyama, T.; Nakagawara, R.; Aihara, M.; Hamada-Sato, N. Allergy to Fish Collagen: Thermostability of Collagen and IgE Reactivity of Patients’ Sera with Extracts of 11 Species of Bony and Cartilaginous Fish. Allergol. Int. Off. J. Jpn. Soc. Allergol. 2016, 65, 450–458. [Google Scholar] [CrossRef]
| Primer | ID |
|---|---|
| COL1A1 | Hs00164004_m1 |
| COL2A1 | Hs00264051_m1 |
| SOX9 | Hs01001343_g1 |
| MMP3 | Hs00968305_m1 |
| MMP13 | Hs00233992_m1 |
| ACAN | Hs00153936_m1 |
| TGFβ | Hs00998133_m1 |
| TNFα | Hs00174128_m1 |
| ADAMTS5 | Hs00199841_m1 |
| GAPDH | Hs02758991_g1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omura, H.; Toyoda, E.; Baba, T.; Uchiyama, R.; Watanabe, M.; Sato, M. Moon Jellyfish Mucin and Collagen Attenuate Catabolic Activity in Chondrocytes but Show Limited Efficacy in an Osteoarthritis Rat Model. Int. J. Mol. Sci. 2025, 26, 10920. https://doi.org/10.3390/ijms262210920
Omura H, Toyoda E, Baba T, Uchiyama R, Watanabe M, Sato M. Moon Jellyfish Mucin and Collagen Attenuate Catabolic Activity in Chondrocytes but Show Limited Efficacy in an Osteoarthritis Rat Model. International Journal of Molecular Sciences. 2025; 26(22):10920. https://doi.org/10.3390/ijms262210920
Chicago/Turabian StyleOmura, Haruka, Eriko Toyoda, Takayuki Baba, Ryoka Uchiyama, Masahiko Watanabe, and Masato Sato. 2025. "Moon Jellyfish Mucin and Collagen Attenuate Catabolic Activity in Chondrocytes but Show Limited Efficacy in an Osteoarthritis Rat Model" International Journal of Molecular Sciences 26, no. 22: 10920. https://doi.org/10.3390/ijms262210920
APA StyleOmura, H., Toyoda, E., Baba, T., Uchiyama, R., Watanabe, M., & Sato, M. (2025). Moon Jellyfish Mucin and Collagen Attenuate Catabolic Activity in Chondrocytes but Show Limited Efficacy in an Osteoarthritis Rat Model. International Journal of Molecular Sciences, 26(22), 10920. https://doi.org/10.3390/ijms262210920






