p38 Regulates FoxO3a-Mediated SOD2 Expression to Prevent Cd-Induced Oxidative Stress in Neuronal Cells
Abstract
1. Introduction
2. Results
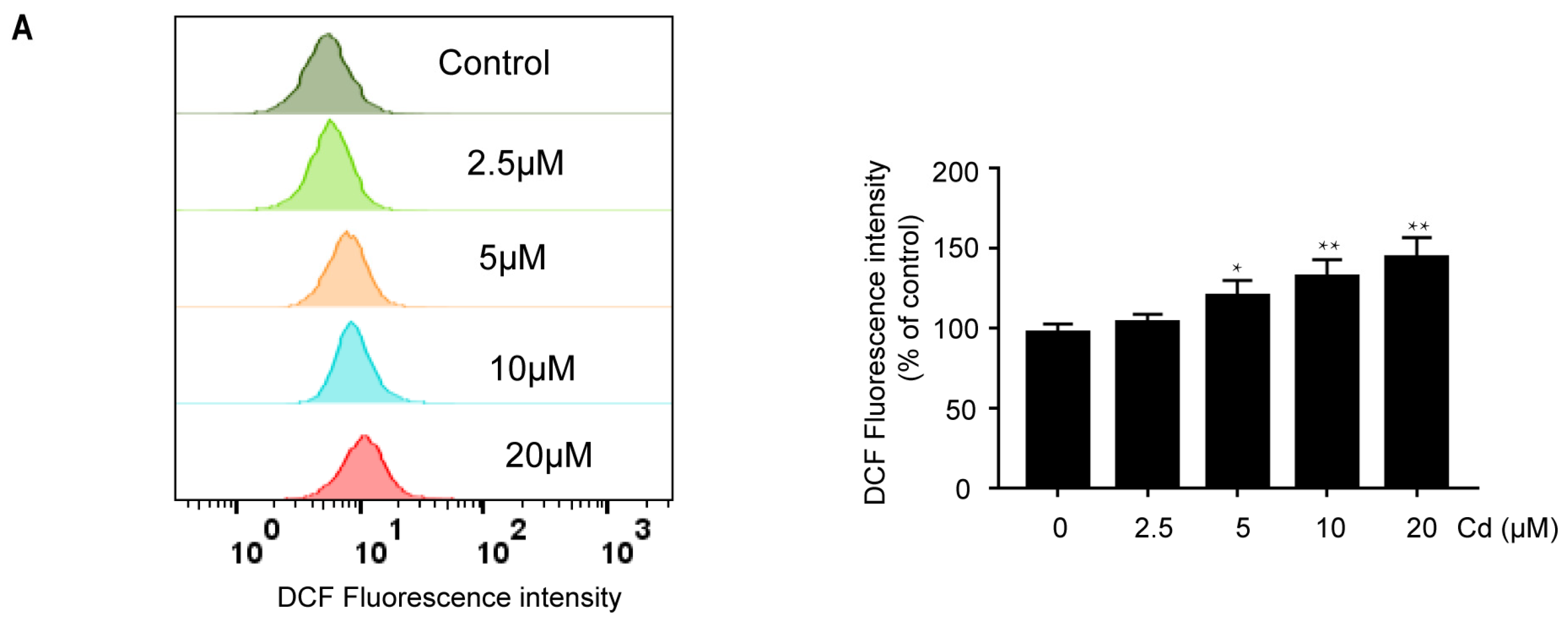
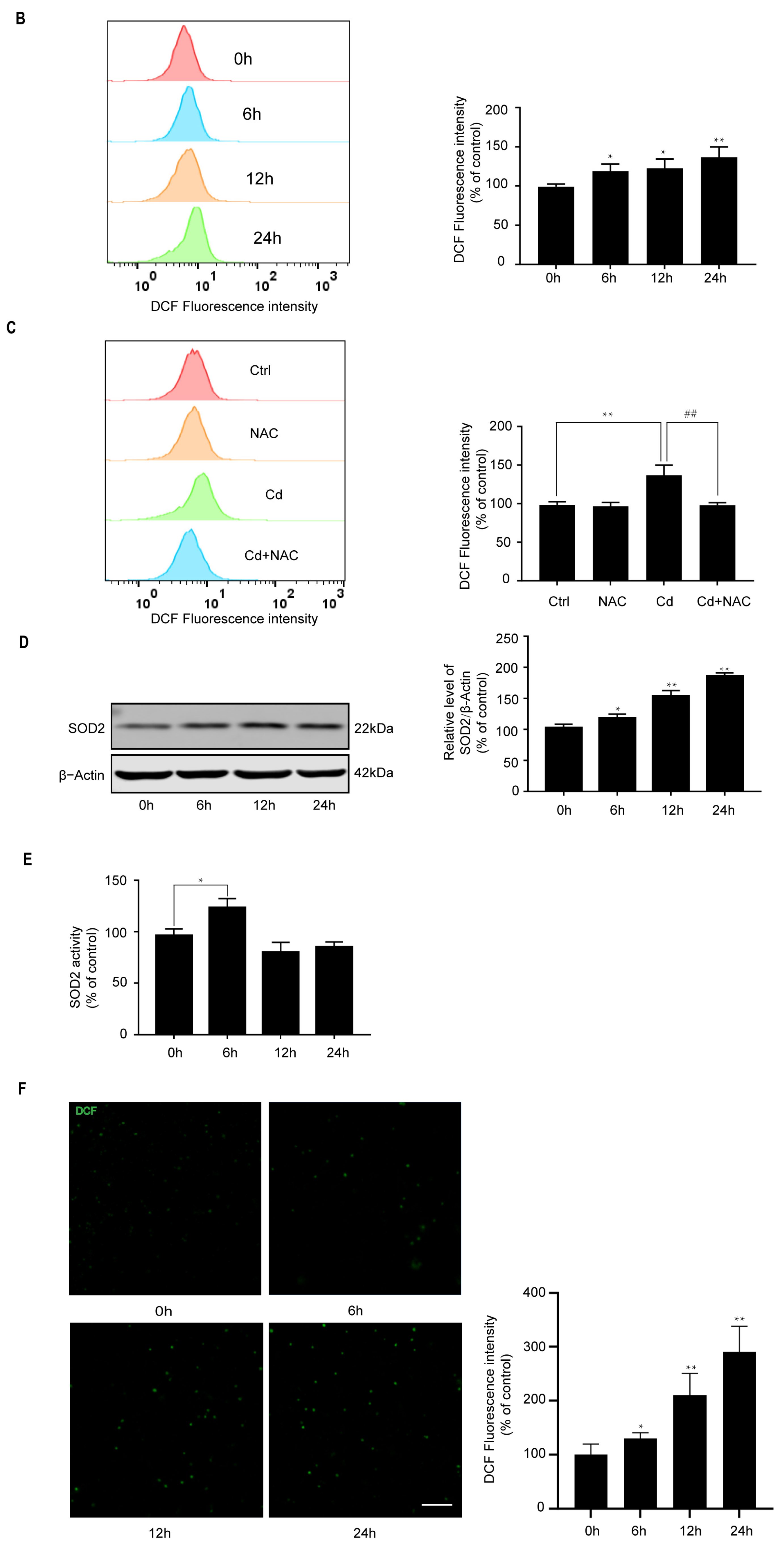
2.1. Cd Induces Oxidative Stress and Activates Antioxidant Defense Response in Neuronal Cells
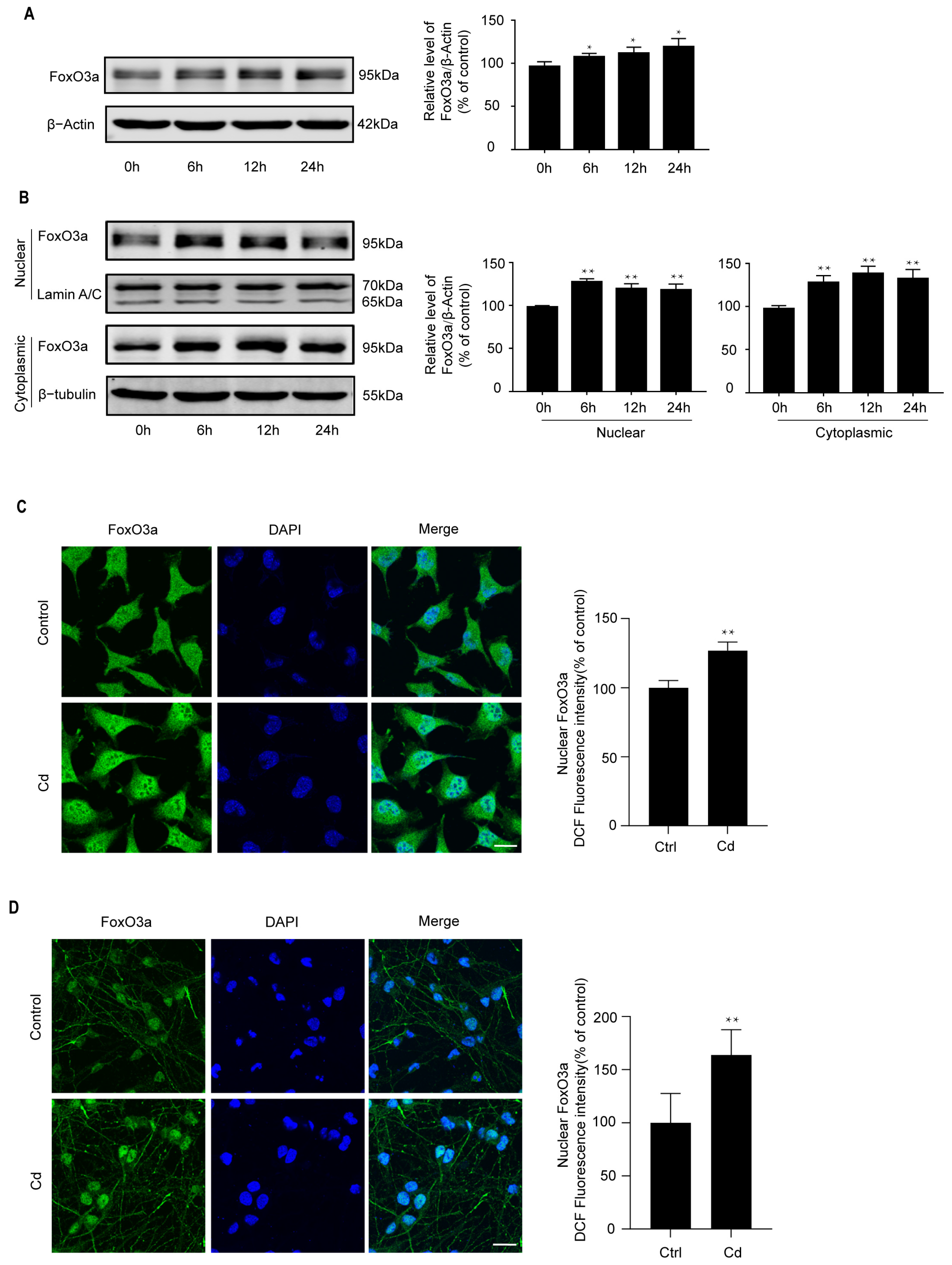
2.2. Cd Induces Nuclear Expression of FoxO3a in Neuronal Cells
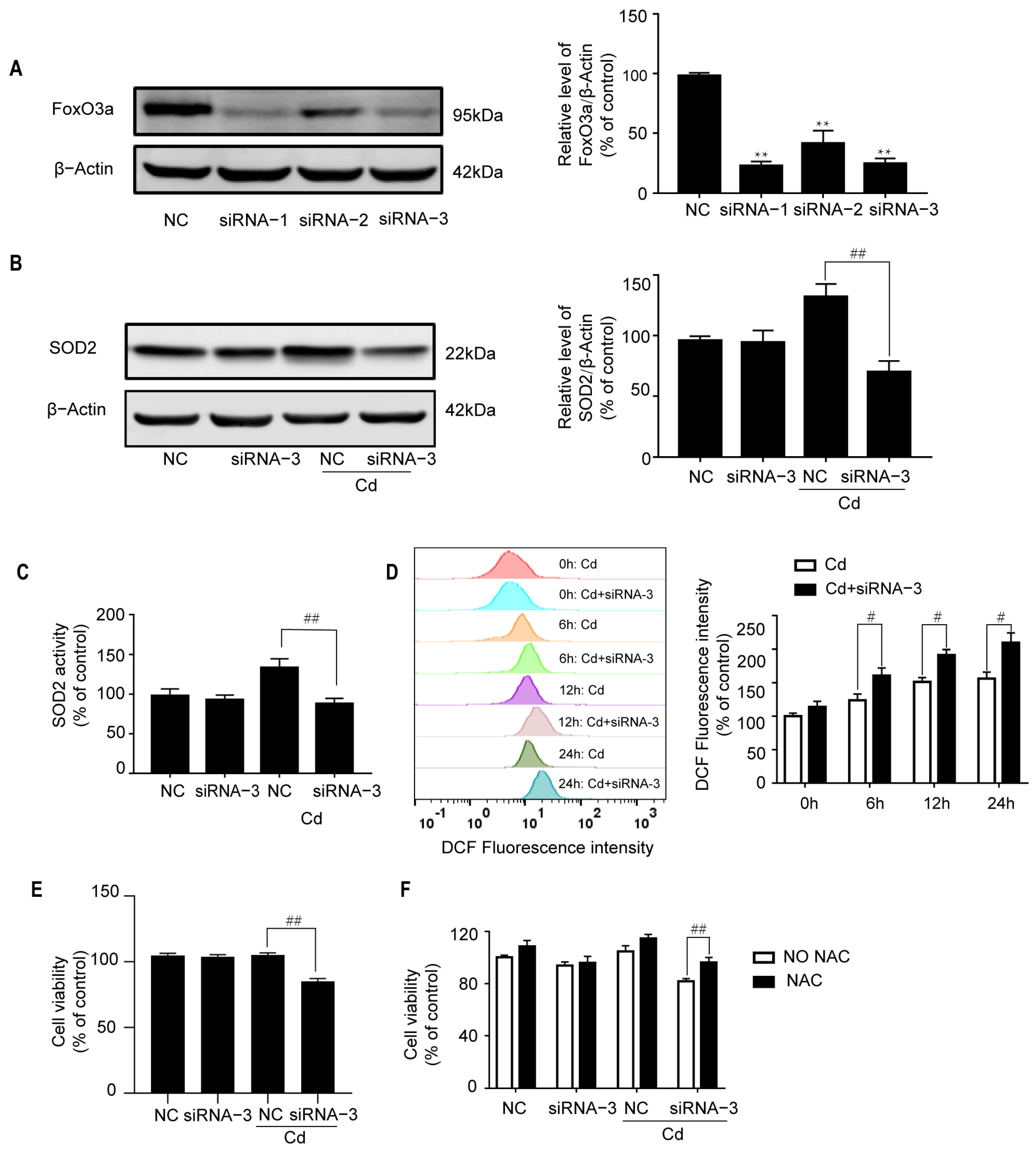
2.3. Knockdown of FoxO3a Increases Cd-Induced Oxidative Stress
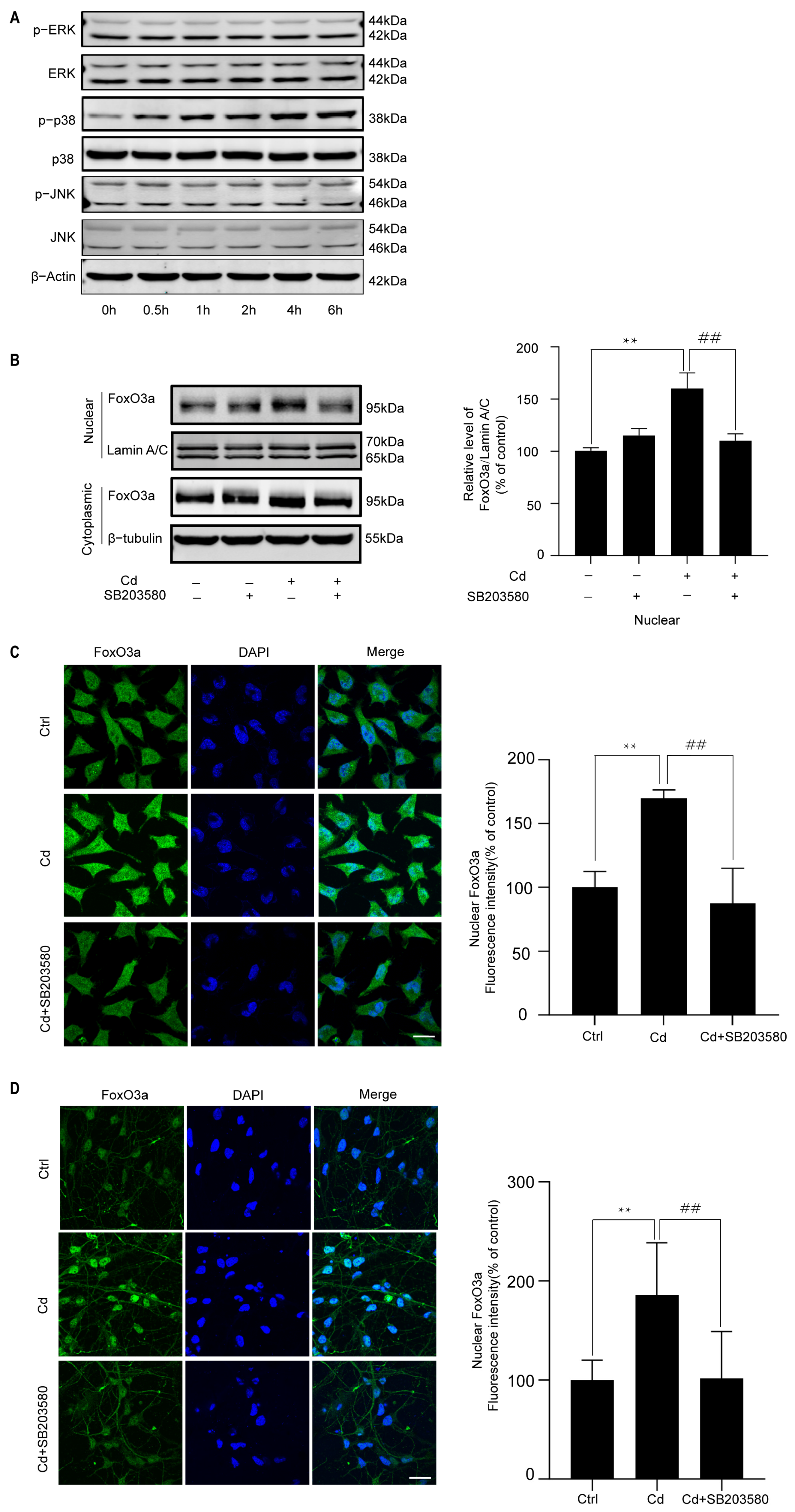
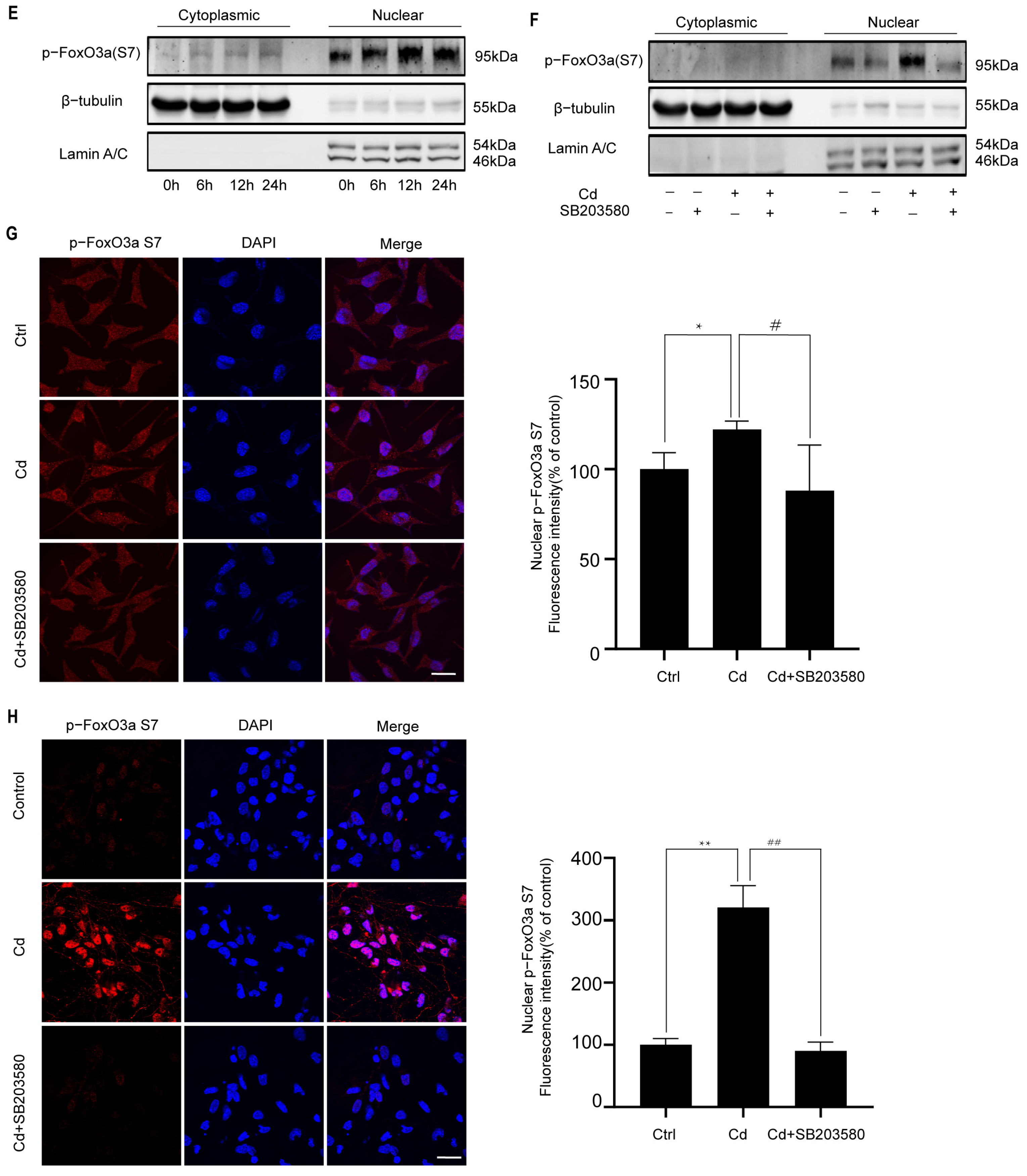
2.4. p38 Regulates the Cd-Induced Nuclear Expression of FoxO3a
2.5. The Effects of the p38/FoxO3a Pathway on the Cd-Induced Oxidative Stress
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Reagents
4.3. Cadmium Treatment
4.4. Cell Viability
4.5. Reactive Oxygen Species (ROS) Measurement
4.6. Knockdown of FoxO3a
4.7. Western Blot Analysis
4.8. Immunofluorescence
4.9. Measurement of SOD2 Enzyme Activity
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gaberšek, M.; Watts, M.J.; Gosar, M. Attic dust: An archive of historical air contamination of the urban environment and potential hazard to health? J. Hazard. Mater. 2022, 432, 128745. [Google Scholar] [CrossRef]
- Jannetto, P.J.; Cowl, C.T. Elementary Overview of Heavy Metals. Clin. Chem. 2023, 69, 336–349. [Google Scholar] [CrossRef]
- Zhao, D.; Lin, G.B.; Liu, C.; Juhasz, A.L.; Ma, L.Q. Health risk assessment of dietary cadmium exposure based on cadmium bioavailability in food: Opportunities and challenges. J. Hazard. Mater. 2025, 488, 137359. [Google Scholar] [CrossRef]
- Zhang, H.; Reynolds, M. Cadmium exposure in living organisms: A short review. Sci. Total Environ. 2019, 678, 761–767. [Google Scholar] [CrossRef]
- Charkiewicz, A.E.; Omeljaniuk, W.J.; Nowak, K.; Garley, M.; Nikliński, J. Cadmium Toxicity and Health Effects—A Brief Summary. Molecules 2023, 28, 6620. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, X.; Lu, S.; Chen, Q.; Wang, Z.; Lai, L.; Liu, Q.; Zhu, X.; Luo, L.; Li, J.; et al. Long-term exposure to cadmium disrupts neurodevelopment in mature cerebral organoids. Sci. Total Environ. 2024, 912, 168923. [Google Scholar] [CrossRef]
- Arruebarrena, M.A.; Hawe, C.T.; Lee, Y.M.; Branco, R.C. Mechanisms of Cadmium Neurotoxicity. Int. J. Mol. Sci. 2023, 24, 16558. [Google Scholar] [CrossRef]
- Branca, J.J.V.; Fiorillo, C.; Carrino, D.; Paternostro, F.; Taddei, N.; Gulisano, M.; Pacini, A.; Becatti, M. Cadmium-Induced Oxidative Stress: Focus on the Central Nervous System. Antioxidants 2020, 9, 492. [Google Scholar] [CrossRef] [PubMed]
- Cuypers, A.; Vanbuel, I.; Iven, V.; Kunnen, K.; Vandionant, S.; Huybrechts, M.; Hendrix, S. Cadmium-induced oxidative stress responses and acclimation in plants require fine-tuning of redox biology at subcellular level. Free Radic. Biol. Med. 2023, 199, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Su, Q.; Yue, C.; Zou, H.; Zhu, J.; Zhao, H.; Song, R.; Liu, Z. The Effect of Oxidative Stress-Induced Autophagy by Cadmium Exposure in Kidney, Liver, and Bone Damage, and Neurotoxicity. Int. J. Mol. Sci. 2022, 23, 13491. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.E.; Kim, G.S.; Chan, P.H. Neuroprotection by interleukin-6 is mediated by signal transducer and activator of transcription 3 and antioxidative signaling in ischemic stroke. Stroke 2011, 42, 3574–3579. [Google Scholar] [CrossRef]
- Nie, S.; Shi, Z.; Shi, M.; Li, H.; Qian, X.; Peng, C.; Ding, X.; Zhang, S.; Lv, Y.; Wang, L.; et al. PPARγ/SOD2 Protects Against Mitochondrial ROS-Dependent Apoptosis via Inhibiting ATG4D-Mediated Mitophagy to Promote Pancreatic Cancer Proliferation. Front. Cell Dev. Biol. 2022, 9, 745554. [Google Scholar] [CrossRef]
- Sarsour, E.H.; Kalen, A.L.; Goswami, P.C. Manganese superoxide dismutase regulates a redox cycle within the cell cycle. Antioxid. Redox Signal 2014, 20, 1618–1627. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.; Zhang, T.; Zhang, R.; Liu, R.; Chen, Y. Molecular mechanism on cadmium-induced activity changes of catalase and superoxide dismutase. Int. J. Biol. Macromol. 2015, 77, 59–67. [Google Scholar] [CrossRef]
- Liu, Y.; Ao, X.; Ding, W.; Ponnusamy, M.; Wu, W.; Hao, X.; Yu, W.; Wang, Y.; Li, P.; Wang, J. Critical role of FOXO3a in carcinogenesis. Mol. Cancer 2018, 17, 104. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, V.S.; Torres, F.F.; da Silva, D.G.H. FoxO3 and oxidative stress: A multifaceted role in cellular adaptation. J. Mol. Med. 2023, 101, 83–99. [Google Scholar] [CrossRef]
- Sanphui, P.; Biswas, S.C. FoxO3a is activated and executes neuron death via Bim in response to β-amyloid. Cell Death Dis. 2013, 4, e625. [Google Scholar] [CrossRef]
- Liu, C.; Tian, Q.; Li, Z.; Wang, G.; Han, W.; Jiang, S.; Sun, Z.; Xu, Q.; Wang, L.; Liao, J.; et al. FOXO3a-BAP1 axis regulates neuronal ferroptosis in early brain injury after subarachnoid hemorrhage. Redox Biol. 2025, 82, 103550. [Google Scholar] [CrossRef] [PubMed]
- Ferber, E.C.; Peck, B.; Delpuech, O.; Bell, G.P.; East, P.; Schulze, A. FOXO3a regulates reactive oxygen metabolism by inhibiting mitochondrial gene expression. Cell Death Differ. 2012, 19, 968–979. [Google Scholar] [CrossRef] [PubMed]
- Jacome Burbano, M.S.; Robin, J.D.; Bauwens, S.; Martin, M.; Donati, E.; Martínez, L.; Lin, P.; Sacconi, S.; Magdinier, F.; Gilson, E. Non-canonical telomere protection role of FOXO3a of human skeletal muscle cells regulated by the TRF2-redox axis. Commun. Biol. 2023, 6, 561. [Google Scholar] [CrossRef]
- Cui, Y.; Bai, M.; Gao, S.; Zhao, H.; Mei, X. Zinc ions facilitate metabolic bioenergetic recovery post spinal cord injury by activating microglial mitophagy through the STAT3-FOXO3a-SOD2 pathway. Free Radic. Biol. Med. 2025, 227, 64–79. [Google Scholar] [CrossRef]
- Liu, H.; Song, Y.; Qiu, H.; Liu, Y.; Luo, K.; Yi, Y.; Jiang, G.; Lu, M.; Zhang, Z.; Yin, J.; et al. Downregulation of FOXO3a by DNMT1 promotes breast cancer stem cell properties and tumorigenesis. Cell Death Differ. 2020, 27, 966–983. [Google Scholar] [CrossRef]
- Meng, L.; Feng, B.; Luan, L.; Fang, Z.; Zhao, G. MeCP2 inhibits ischemic neuronal injury by enhancing methylation of the FOXO3a promoter to repress the SPRY2-ZEB1 axis. Exp. Mol. Med. 2022, 54, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Malladi, N.; Lahamge, D.; Somwanshi, B.S.; Tiwari, V.; Deshmukh, K.; Balani, J.K.; Chakraborty, S.; Alam, M.J.; Banerjee, S.K. Paricalcitol attenuates oxidative stress and inflammatory response in the liver of NAFLD rats by regulating FOXO3a and NFκB acetylation. Cell. Signal. 2024, 121, 111299. [Google Scholar] [CrossRef] [PubMed]
- Tenbaum, S.P.; Ordóñez-Morán, P.; Puig, I.; Chicote, I.; Arqués, O.; Landolfi, S.; Fernández, Y.; Herance, J.R.; Gispert, J.D.; Mendizabal, L.; et al. β-catenin confers resistance to PI3K and AKT inhibitors and subverts FOXO3a to promote metastasis in colon cancer. Nat. Med. 2012, 18, 892–901. [Google Scholar] [CrossRef]
- Wang, X.; Chen, W.R.; Xing, D. A pathway from JNK through decreased ERK and Akt activities for FOXO3a nuclear translocation in response to UV irradiation. J. Cell. Physiol. 2012, 227, 1168–1178. [Google Scholar] [CrossRef]
- Yao, S.; Fan, L.Y.; Lam, E.W. The FOXO3-FOXM1 axis: A key cancer drug target and a modulator of cancer drug resistance. Semin. Cancer Biol. 2018, 50, 77–89. [Google Scholar] [CrossRef]
- Kops, G.J.; Dansen, T.B.; Polderman, P.E.; Saarloos, I.; Wirtz, K.W.; Coffer, P.J.; Huang, T.T.; Bos, J.L.; Medema, R.H.; Burgering, B.M. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature 2002, 419, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Ruankham, W.; Suwanjang, W.; Phopin, K.; Songtawee, N.; Prachayasittikul, V.; Prachayasittikul, S. Modulatory Effects of Alpha-Mangostin Mediated by SIRT1/3-FOXO3a Pathway in Oxidative Stress-Induced Neuronal Cells. Front. Nutr. 2021, 8, 714463. [Google Scholar] [CrossRef]
- Carew, R.M.; Browne, M.B.; Hickey, F.B.; Brazil, D.P. Insulin receptor substrate 2 and FoxO3a signalling are involved in E-cadherin expression and transforming growth factor-β1-induced repression in kidney epithelial cells. FEBS J. 2011, 278, 3370–3380. [Google Scholar] [CrossRef]
- Ciesielski, T.; Weuve, J.; Bellinger, D.C.; Schwartz, J.; Lanphear, B.; Wright, R.O. Cadmium exposure and neurodevelopmental outcomes in U.S. children. Environ. Health Perspect. 2012, 120, 758–763. [Google Scholar] [CrossRef]
- Gade, M.; Comfort, N.; Re, D.B. Sex-specific neurotoxic effects of heavy metal pollutants: Epidemiological, experimental evidence and candidate mechanisms. Environ. Res. 2021, 201, 111558. [Google Scholar] [CrossRef]
- Eaves, L.A.; Choi, G.; Hall, E.; Sillé, F.C.M.; Fry, R.C.; Buckley, J.P.; Keil, A.P. Prenatal Exposure to Toxic Metals and Neural Tube Defects: A Systematic Review of the Epidemiologic Evidence. Environ. Health Perspect. 2023, 131, 86002. [Google Scholar] [CrossRef]
- Yang, R.; Roshani, D.; Gao, B.; Li, P.; Shang, N. Metallothionein: A Comprehensive Review of Its Classification, Structure, Biological Functions, and Applications. Antioxidants 2024, 13, 825. [Google Scholar] [CrossRef] [PubMed]
- Dikalova, A.E.; Itani, H.A.; Nazarewicz, R.R.; McMaster, W.G.; Flynn, C.R.; Uzhachenko, R.; Fessel, J.P.; Gamboa, J.L.; Harrison, D.G.; Dikalov, S.I. Sirt3 Impairment and SOD2 Hyperacetylation in Vascular Oxidative Stress and Hypertension. Circ. Res. 2017, 121, 564–574. [Google Scholar] [CrossRef]
- Pi, H.; Xu, S.; Reiter, R.J.; Guo, P.; Zhang, L.; Li, Y.; Li, M.; Cao, Z.; Tian, L.; Xie, J.; et al. SIRT3-SOD2-mROS-dependent autophagy in cadmium-induced hepatotoxicity and salvage by melatonin. Autophagy 2015, 11, 1037–1051. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Kou, X.; Zhan, T.; Wei, G.; He, F.; Mao, X.; Yang, H. Apoptotic vesicles resist oxidative damage in noise-induced hearing loss through activation of FOXO3a-SOD2 pathway. Stem Cell Res. Ther. 2023, 14, 88. [Google Scholar] [CrossRef]
- Fu, B.; Zhao, J.; Peng, W.; Wu, H.; Zhang, Y. Resveratrol rescues cadmium-induced mitochondrial injury by enhancing transcriptional regulation of PGC-1α and SOD2 via the Sirt3/FoxO3a pathway in TCMK-1 cells. Biochem. Biophys. Res. Commun. 2017, 486, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Kyriazis, I.D.; Hoffman, M.; Gaignebet, L.; Lucchese, A.M.; Markopoulou, E.; Palioura, D.; Wang, C.; Bannister, T.D.; Christofidou-Solomidou, M.; Oka, S.I.; et al. KLF5 Is Induced by FOXO1 and Causes Oxidative Stress and Diabetic Cardiomyopathy. Circ. Res. 2021, 128, 335–357. [Google Scholar] [CrossRef]
- Yang, Y.; Song, L.; Yu, L.; Zhang, J.; Zhang, B. H4K12 lactylation potentiates mitochondrial oxidative stress via the Foxo1 pathway in diabetes-induced cognitive impairment. J. Adv. Res. 2025. [Google Scholar] [CrossRef]
- Sun, D.; Chen, S.; Li, S.; Wang, N.; Zhang, S.; Xu, L.; Zhu, S.; Li, H.; Gu, Q.; Xu, X.; et al. Enhancement of glycolysis-dependent DNA repair regulated by FOXO1 knockdown via PFKFB3 attenuates hyperglycemia-induced endothelial oxidative stress injury. Redox Biol. 2023, 59, 102589. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, J.; Zhu, Y.; Zhao, R.; Xie, Z.; Qu, X.; Duan, Y.; Li, N.; Tang, D.; Luo, X. BMP9 alleviates iron accumulation-induced osteoporosis via the USP10/FOXO1/GPX4 axis. J. Adv. Res. 2025. [Google Scholar] [CrossRef]
- Palma, F.R.; He, C.; Danes, J.M.; Paviani, V.; Coelho, D.R.; Gantner, B.N.; Bonini, M.G. Mitochondrial Superoxide Dismutase: What the Established, the Intriguing, and the Novel Reveal About a Key Cellular Redox Switch. Antioxid. Redox Signal. 2020, 32, 701–714. [Google Scholar] [CrossRef]
- Wang, B.; Xiao, J.L.; Ling, Y.H.; Meng, X.J.; Wu, B.; Yang, X.Y.; Zou, F. BNIP3 upregulation by ERK and JNK mediates cadmium-induced necrosis in neuronal cells. Toxicol. Sci. 2014, 140, 393–402. [Google Scholar] [CrossRef]
- Ho, K.K.; McGuire, V.A.; Koo, C.Y.; Muir, K.W.; de Olano, N.; Maifoshie, E.; Kelly, D.J.; McGovern, U.B.; Monteiro, L.J.; Gomes, A.R.; et al. Phosphorylation of FOXO3a on Ser-7 by p38 promotes its nuclear localization in response to doxorubicin. J. Biol. Chem. 2012, 287, 1545–1555. [Google Scholar] [CrossRef]
- Marzi, L.; Combes, E.; Vié, N.; Ayrolles-Torro, A.; Tosi, D.; Desigaud, D.; Perez-Gracia, E.; Larbouret, C.; Montagut, C.; Iglesias, M.; et al. FOXO3a and the MAPK p38 are activated by cetuximab to induce cell death and inhibit cell proliferation and their expression predicts cetuximab efficacy in colorectal cancer. Br. J. Cancer 2016, 115, 1223–1233. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, L.; Chen, L.; Gong, B.; Jia, D.; Sun, Q. Nuclear transport proteins: Structure, function, and disease relevance. Signal Transduct. Target. Ther. 2023, 8, 425. [Google Scholar] [CrossRef]
- Mis, M.; O’Brien, S.; Steinhart, Z.; Lin, S.; Hart, T.; Moffat, J.; Angers, S. IPO11 mediates βcatenin nuclear import in a subset of colorectal cancers. J. Cell Biol. 2020, 219, e201903017. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Su, Z.; Tavana, O.; Gu, W. Understanding the complexity of p53 in a new era of tumor suppression. Cancer Cell 2024, 42, 946–967. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zheng, J.; Zhao, L.; Wang, X.; Zhang, L.; Wang, J.; Zhang, Y.; Shi, R.; Han, J.; Han, W.; et al. E3 ligase RAD18 targets phosphorylated IRF3 to terminate IFNB1 transcription. Nat. Immunol. 2025, 26, 1581–1595. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, G.; Esposito, A.; Choi, H.; Matarese, M.; Benedetti, V.; Di Malta, C.; Monfregola, J.; Medina, D.L.; Lippincott-Schwartz, J.; Ballabio, A. mTOR-dependent phosphorylation controls TFEB nuclear export. Nat. Commun. 2018, 9, 3312. [Google Scholar] [CrossRef] [PubMed]
- Tormos, A.M.; Taléns-Visconti, R.; Nebreda, A.R.; Sastre, J. p38 MAPK: A dual role in hepatocyte proliferation through reactive oxygen species. Free Radic. Res. 2013, 47, 905–916. [Google Scholar] [CrossRef]
- Romero-Becerra, R.; Santamans, A.M.; Folgueira, C.; Sabio, G. p38 MAPK Pathway in the Heart: New Insights in Health and Disease. Int. J. Mol. Sci. 2020, 21, 7412. [Google Scholar] [CrossRef] [PubMed]
- McGill, M.M.; Richman, A.R.; Boyd, J.R.; Sabikunnahar, B.; Lahue, K.G.; Montgomery, T.L.; Caldwell, S.; Varnum, S.; Frietze, S.; Krementsov, D.N. p38 MAP Kinase Signaling in Microglia Plays a Sex-Specific Protective Role in CNS Autoimmunity and Regulates Microglial Transcriptional States. Front. Immunol. 2021, 12, 715311. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, T.; Ruan, S.; Liu, X.; Li, F.; Zhang, H.; Zou, F.; Wang, B. p38 Regulates FoxO3a-Mediated SOD2 Expression to Prevent Cd-Induced Oxidative Stress in Neuronal Cells. Int. J. Mol. Sci. 2025, 26, 10919. https://doi.org/10.3390/ijms262210919
Lin T, Ruan S, Liu X, Li F, Zhang H, Zou F, Wang B. p38 Regulates FoxO3a-Mediated SOD2 Expression to Prevent Cd-Induced Oxidative Stress in Neuronal Cells. International Journal of Molecular Sciences. 2025; 26(22):10919. https://doi.org/10.3390/ijms262210919
Chicago/Turabian StyleLin, Tianji, Shijuan Ruan, Xinyu Liu, Fangfei Li, Hangqian Zhang, Fei Zou, and Bin Wang. 2025. "p38 Regulates FoxO3a-Mediated SOD2 Expression to Prevent Cd-Induced Oxidative Stress in Neuronal Cells" International Journal of Molecular Sciences 26, no. 22: 10919. https://doi.org/10.3390/ijms262210919
APA StyleLin, T., Ruan, S., Liu, X., Li, F., Zhang, H., Zou, F., & Wang, B. (2025). p38 Regulates FoxO3a-Mediated SOD2 Expression to Prevent Cd-Induced Oxidative Stress in Neuronal Cells. International Journal of Molecular Sciences, 26(22), 10919. https://doi.org/10.3390/ijms262210919




