Membrane Lipids and Osmolytes Rearrangements Under Cell Wall Stress in Aspergillus niger
Abstract
1. Introduction
2. Results
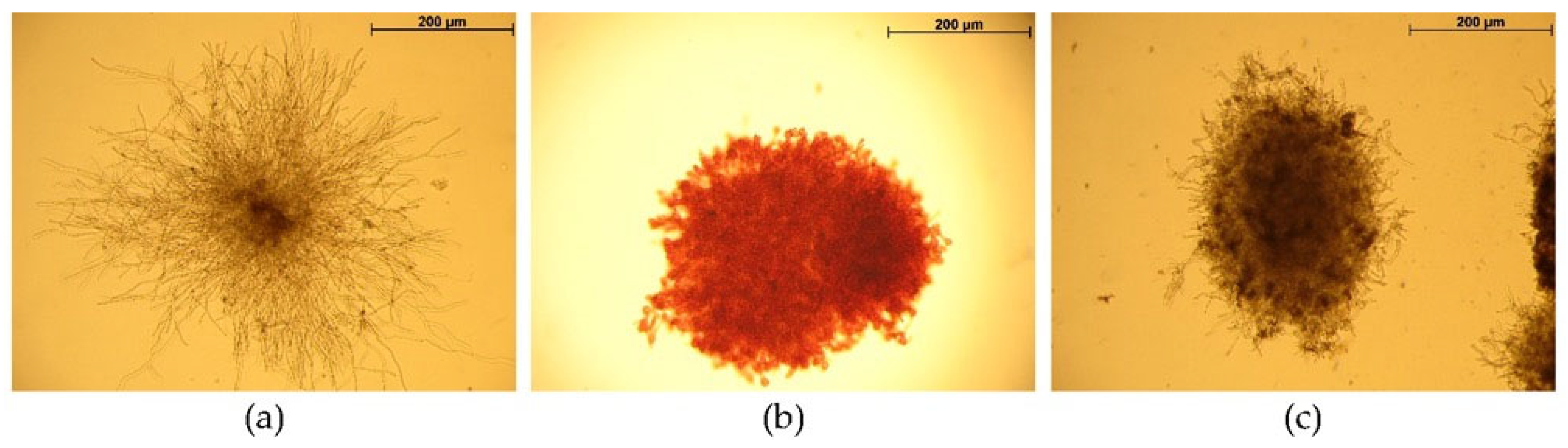
2.1. The Influence of Azo Dyes on A. niger Mycelium Morphology
2.2. The Influence of Azo Dyes on the Composition of the Main Polysaccharides in the A. niger Cell Wall
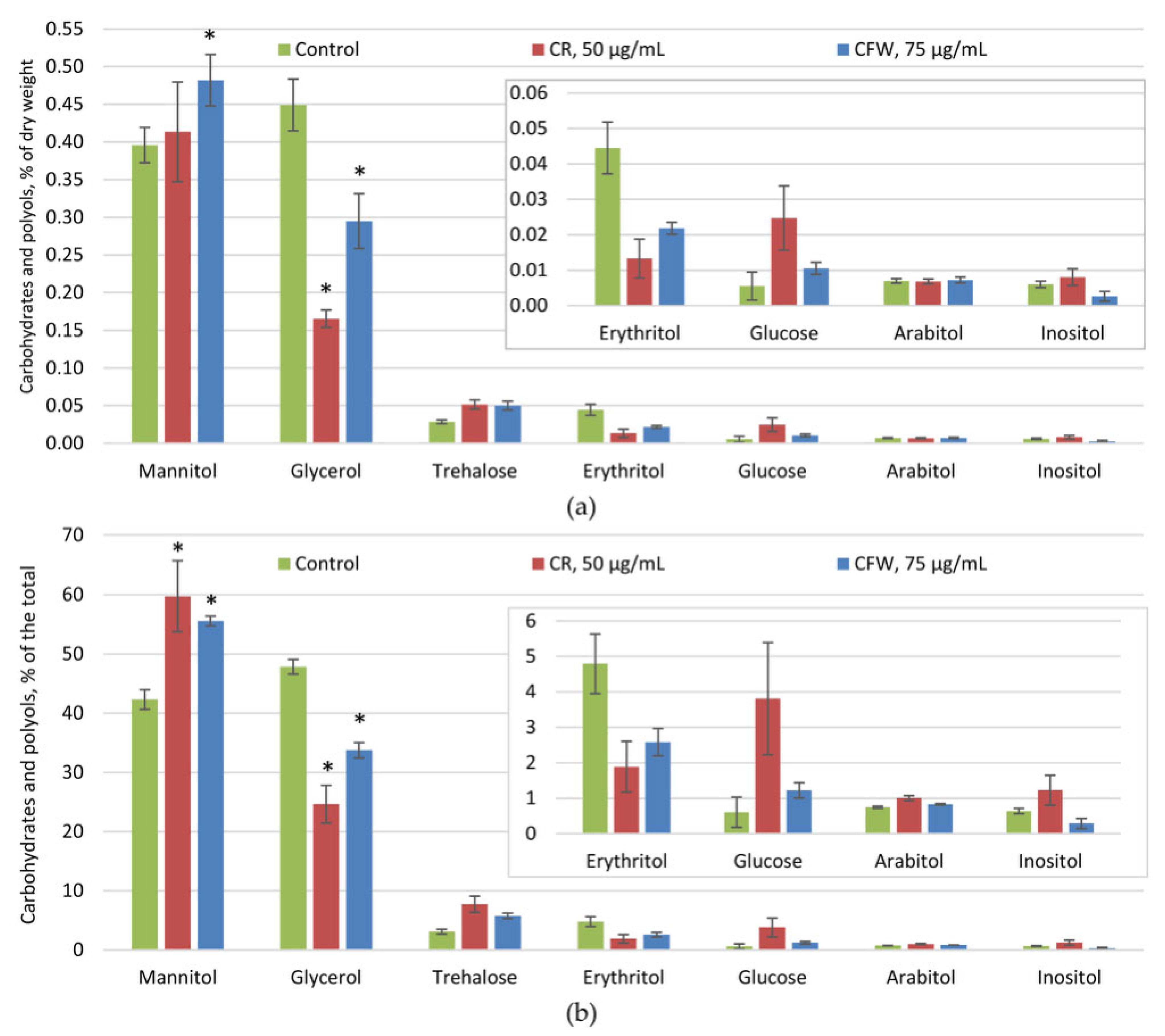
2.3. The Effect of Azo Dyes on the Composition of A. niger Osmolytes
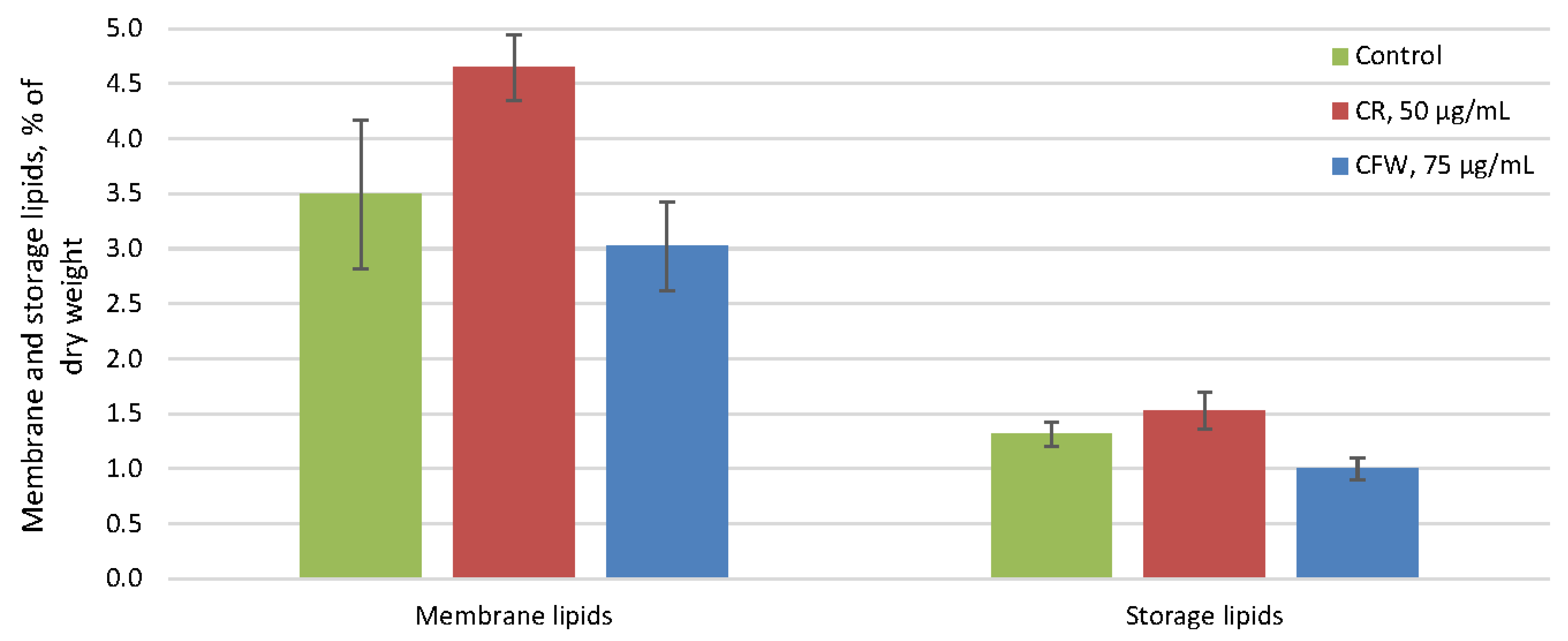
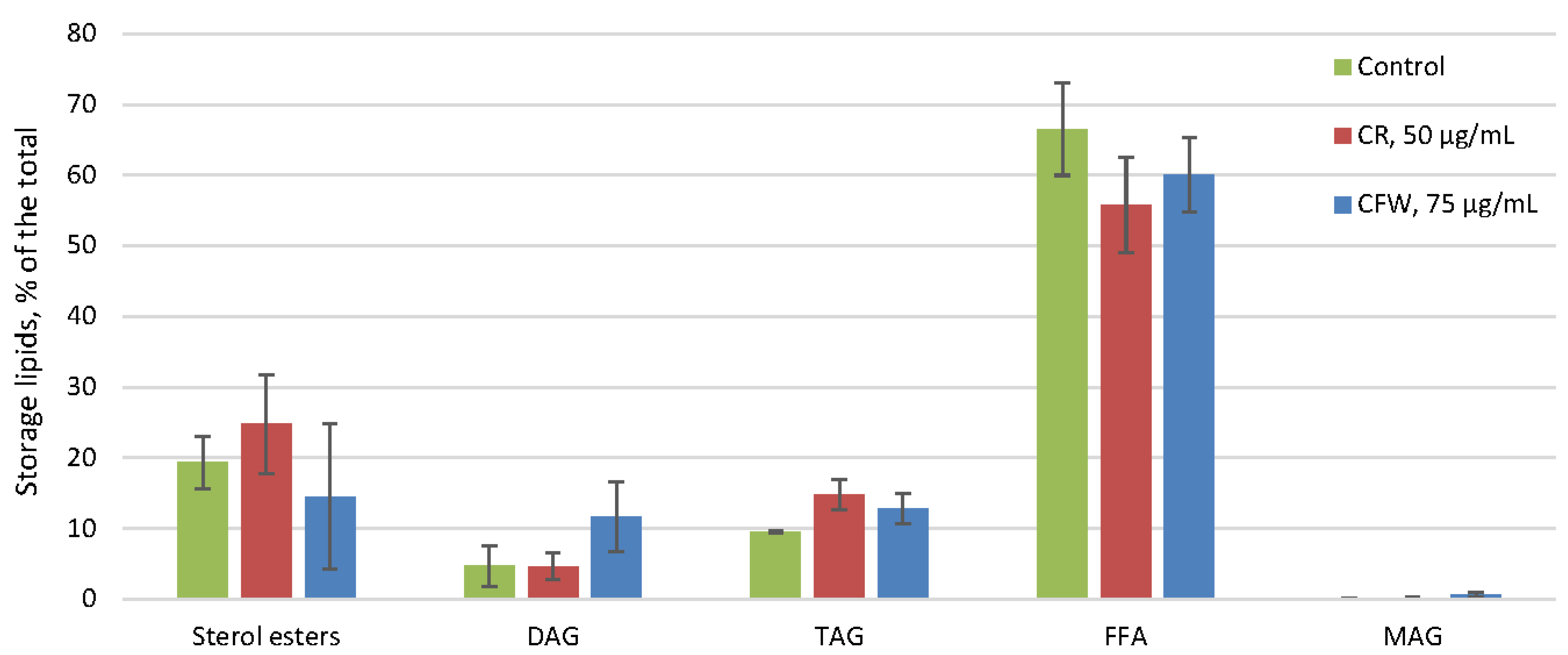
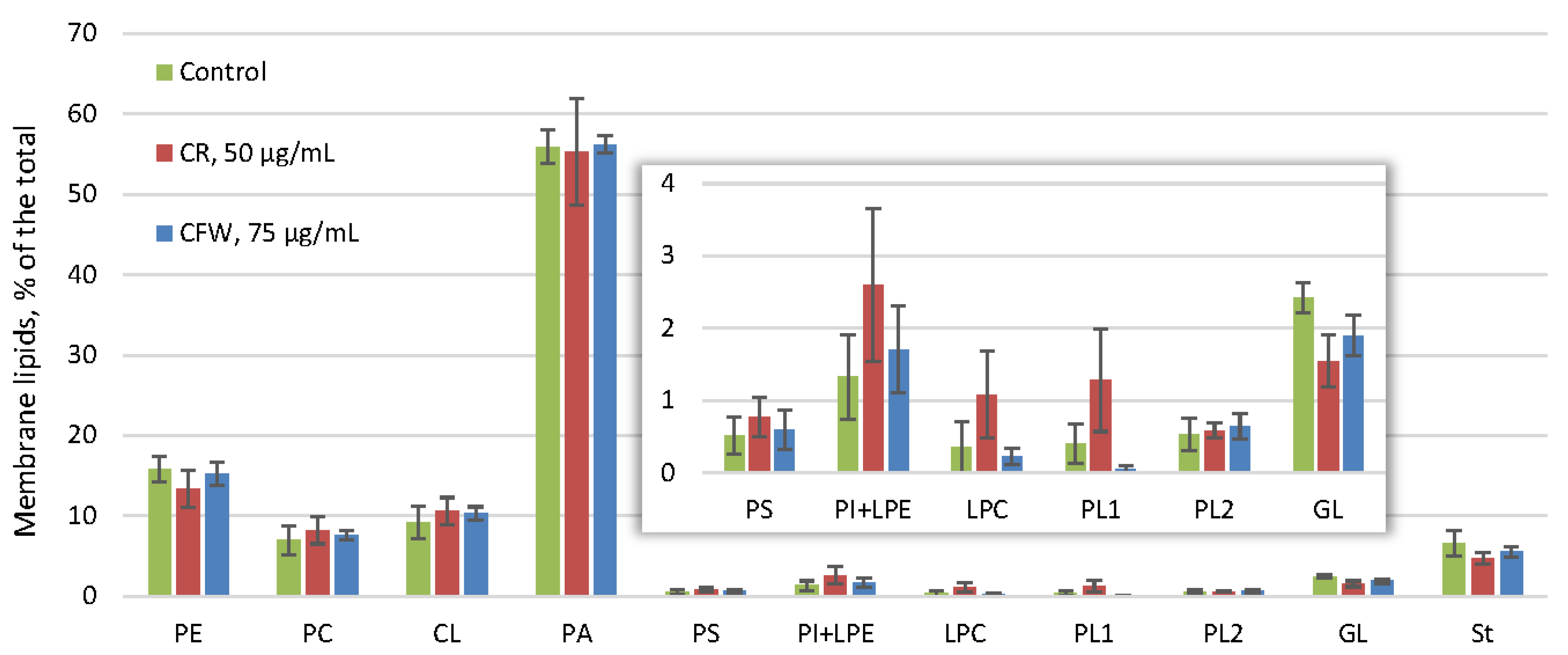
2.4. The Effect of Azo Dyes on the Composition of A. niger Storage and Membrane Lipids and Their Fatty Acids
3. Discussion
4. Materials and Methods
4.1. Objects of Study and Cultivation Protocol
4.2. Lipids, Carbohydrates, and Polyols Analysis
4.3. Cell Wall Isolation
4.4. Analysis of Chitin and Glucan
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stewart, G.G. The Structure and Function of the Yeast Cell Wall, Plasma Membrane and Periplasm. In Brewing and Distilling Yeasts; Springer International Publishing: Cham, Switzerland, 2017; pp. 55–75. [Google Scholar]
- Gow, N.A.R. Fungal Cell Wall Biogenesis: Structural Complexity, Regulation and Inhibition. Fungal Genet. Biol. 2025, 179, 103991. [Google Scholar] [CrossRef]
- Levin, D.E. Regulation of Cell Wall Biogenesis in Saccharomyces Cerevisiae: The Cell Wall Integrity Signaling Pathway. Genetics 2011, 189, 1145–1175. [Google Scholar] [CrossRef] [PubMed]
- Free, S.J. Fungal Cell Wall Organization and Biosynthesis. In Advances in Genetics; Academic Press Inc.: Cambridge, MA, USA, 2013; Volume 81, pp. 33–82. [Google Scholar]
- Gow, N.A.R.; Lenardon, M.D. Architecture of the Dynamic Fungal Cell Wall. Nat. Rev. Microbiol. 2023, 21, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Herrera, J.; Ortiz-Castellanos, L. Cell Wall Glucans of Fungi. A Review. Cell Surf. 2019, 5, 100022. [Google Scholar] [CrossRef]
- Kang, X.; Kirui, A.; Muszyński, A.; Widanage, M.C.D.; Chen, A.; Azadi, P.; Wang, P.; Mentink-Vigier, F.; Wang, T. Molecular Architecture of Fungal Cell Walls Revealed by Solid-State NMR. Nat. Commun. 2018, 9, 2747. [Google Scholar] [CrossRef]
- Garcia-Rubio, R.; de Oliveira, H.C.; Rivera, J.; Trevijano-Contador, N. The Fungal Cell Wall: Candida, Cryptococcus, and Aspergillus Species. Front. Microbiol. 2020, 10, 2993. [Google Scholar] [CrossRef]
- Ram, A.F.J. The Cell Wall Stress Response in Aspergillus niger Involves Increased Expression of the Glutamine: Fructose-6-Phosphate Amidotransferase-Encoding Gene (GfaA) and Increased Deposition of Chitin in the Cell Wall. Microbiology 2004, 150, 3315–3326. [Google Scholar] [CrossRef]
- Curto, M.Á.; Butassi, E.; Ribas, J.C.; Svetaz, L.A.; Cortés, J.C.G. Natural Products Targeting the Synthesis of β(1,3)-D-Glucan and Chitin of the Fungal Cell Wall. Existing Drugs and Recent Findings. Phytomedicine 2021, 88, 153556. [Google Scholar] [CrossRef]
- Ram, A.F.J.; Klis, F.M. Identification of Fungal Cell Wall Mutants Using Susceptibility Assays Based on Calcofluor White and Congo Red. Nat. Protoc. 2006, 1, 2253–2256. [Google Scholar] [CrossRef]
- Liu, Z.; Raj, S.; van Rhijn, N.; Fraczek, M.; Michel, J.-P.; Sismeiro, O.; Legendre, R.; Varet, H.; Fontaine, T.; Bromley, M.; et al. Functional Genomic and Biochemical Analysis Reveals Pleiotropic Effect of Congo Red on Aspergillus fumigatus. mBio 2021, 12, e00863-21. [Google Scholar] [CrossRef]
- Damveld, R.A.; Arentshorst, M.; VanKuyk, P.A.; Klis, F.M.; van den Hondel, C.A.M.J.J.; Ram, A.F.J. Characterisation of CwpA, a Putative Glycosylphosphatidylinositol-Anchored Cell Wall Mannoprotein in the Filamentous Fungus Aspergillus niger. Fungal Genet. Biol. 2005, 42, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Futagami, T.; Seto, K.; Kajiwara, Y.; Takashita, H.; Omori, T.; Takegawa, K.; Goto, M. The Putative Stress Sensor Protein MtlA Is Required for Conidia Formation, Cell Wall Stress Tolerance, and Cell Wall Integrity in Aspergillus nidulans. Biosci. Biotechnol. Biochem. 2014, 78, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, M.R.; Lorenz, A.; Nitsche, B.M.; van den Hondel, C.A.; Ram, A.F.; Meyer, V. The Capacity of Aspergillus niger to Sense and Respond to Cell Wall Stress Requires at Least Three Transcription Factors: RlmA, MsnA and CrzA. Fungal Biol. Biotechnol. 2014, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Martín, H.; Molina, M. Special Issue “The Fungal Cell Wall Integrity Pathway”. J. Fungi 2023, 9, 293. [Google Scholar] [CrossRef]
- de Oliveira Bruder Nascimento, A.C.M.; dos Reis, T.F.; de Castro, P.A.; Hori, J.I.; Bom, V.L.P.; de Assis, L.J.; Ramalho, L.N.Z.; Rocha, M.C.; Malavazi, I.; Brown, N.A.; et al. Mitogen Activated Protein Kinases SakA HOG1 and MpkC Collaborate for Aspergillus fumigatus Virulence. Mol. Microbiol. 2016, 100, 841–859. [Google Scholar] [CrossRef]
- Nikolaou, E.; Agrafioti, I.; Stumpf, M.; Quinn, J.; Stansfield, I.; Brown, A.J. Phylogenetic Diversity of Stress Signalling Pathways in Fungi. BMC Evol. Biol. 2009, 9, 44. [Google Scholar] [CrossRef]
- Manfiolli, A.O.; Mattos, E.C.; De Assis, L.J.; Silva, L.P.; Ulas, M.; Brown, N.A.; Silva-Rocha, R.; Bayram, Ö.; Goldman, G.H. Aspergillus fumigatus High Osmolarity Glycerol Mitogen Activated Protein Kinases Saka and Mpkc Physically Interact during Osmotic and Cell Wall Stresses. Front. Microbiol. 2019, 10, 918. [Google Scholar] [CrossRef]
- Ost, K.J.; Student, M.; Cord-Landwehr, S.; Moerschbacher, B.M.; Ram, A.F.J.; Dirks-Hofmeister, M.E. Cell Walls of Filamentous Fungi—Challenges and Opportunities for Biotechnology. Appl. Microbiol. Biotechnol. 2025, 109, 125. [Google Scholar] [CrossRef]
- Yoshimi, A.; Miyazawa, K.; Kawauchi, M.; Abe, K. Cell Wall Integrity and Its Industrial Applications in Filamentous Fungi. J. Fungi 2022, 8, 435. [Google Scholar] [CrossRef]
- Cortés, J.C.G.; Curto, M.-Á.; Carvalho, V.S.D.; Pérez, P.; Ribas, J.C. The Fungal Cell Wall as a Target for the Development of New Antifungal Therapies. Biotechnol. Adv. 2019, 37, 107352. [Google Scholar] [CrossRef]
- Lima, D.M.C.G.; Costa, T.P.C.; Emri, T.; Pócsi, I.; Pupin, B.; Rangel, D.E.N. Fungal Tolerance to Congo Red, a Cell Wall Integrity Stress, as a Promising Indicator of Ecological Niche. Fungal Biol. 2021, 125, 646–657. [Google Scholar] [CrossRef]
- Csillag, K.; Emri, T.; Rangel, D.E.N.; Pócsi, I. PH-Dependent Effect of Congo Red on the Growth of Aspergillus nidulans and Aspergillus niger. Fungal Biol. 2023, 127, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwe, T.M.; Gerritsen, A.; Arentshorst, M.; Punt, P.J.; Ram, A.F.J. Rab GDP-Dissociation Inhibitor GdiA Is an Essential Gene Required for Cell Wall Chitin Deposition in Aspergillus niger. Fungal Genet. Biol. 2020, 136, 103319. [Google Scholar] [CrossRef] [PubMed]
- Kovács, Z.; Szarka, M.; Kovács, S.; Boczonádi, I.; Emri, T.; Abe, K.; Pócsi, I.; Pusztahelyi, T. Effect of Cell Wall Integrity Stress and RlmA Transcription Factor on Asexual Development and Autolysis in Aspergillus nidulans. Fungal Genet. Biol. 2013, 54, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Feofilova, E.P.; Nemtsev, D.V.; Tereshina, V.M.; Memorskaya, A.S. Developmental Change of the Composition and Content of the Chitin-Glucan Complex in the Fungus Aspergillus niger. Appl. Biochem. Microbiol. 2006, 42, 545–549. [Google Scholar] [CrossRef]
- Jennings, D.H. Polyol Metabolism in Fungi. In Advances in Microbial Physiology; Rose, A.H., Tempest, D.W., Eds.; Academic Press: London, UK, 1985; Volume 25, pp. 149–193. ISBN 0-12027725-4. [Google Scholar]
- Yancey, P.H. Organic Osmolytes as Compatible, Metabolic and Counteracting Cytoprotectants in High Osmolarity and Other Stresses. J. Exp. Biol. 2005, 208, 2819–2830. [Google Scholar] [CrossRef]
- Yancey, P.H.; Siebenaller, J.F. Co-Evolution of Proteins and Solutions: Protein Adaptation versus Cytoprotective Micromolecules and Their Roles in Marine Organisms. J. Exp. Biol. 2015, 218, 1880–1896. [Google Scholar] [CrossRef]
- Ianutsevich, E.A.; Danilova, O.A.; Groza, N.V.; Kotlova, E.R.; Tereshina, V.M. Heat Shock Response of Thermophilic Fungi: Membrane Lipids and Soluble Carbohydrates under Elevated Temperatures. Microbiology 2016, 162, 989–999. [Google Scholar] [CrossRef]
- Bondarenko, S.A.; Ianutsevich, E.A.; Danilova, O.A.; Grum-Grzhimaylo, A.A.; Kotlova, E.R.; Kamzolkina, O.V.; Bilanenko, E.N.; Tereshina, V.M. Membrane Lipids and Soluble Sugars Dynamics of the Alkaliphilic Fungus Sodiomyces Tronii in Response to Ambient PH. Extremophiles 2017, 21, 743–754. [Google Scholar] [CrossRef]
- Ianutsevich, E.A.; Danilova, O.A.; Grum-Grzhimaylo, O.A.; Tereshina, V.M. The Role of Osmolytes and Membrane Lipids in the Adaptation of Acidophilic Fungi. Microorganisms 2023, 11, 1733. [Google Scholar] [CrossRef]
- Ianutsevich, E.A.; Danilova, O.A.; Grum-Grzhimaylo, O.A.; Tereshina, V.M. Membrane Lipids and Osmolytes in the Response of the Acidophilic Basidiomycete Phlebiopsis Gigantea to Heat, Cold, and Osmotic Shocks. Int. J. Mol. Sci. 2024, 25, 3380. [Google Scholar] [CrossRef]
- Danilova, O.A.; Ianutsevich, E.A.; Bondarenko, S.A.; Antropova, A.B.; Tereshina, V.M. Membrane Lipids and Osmolytes Composition of Xerohalophilic Fungus Aspergillus penicillioides during Growth on High NaCl and Glycerol Media. Microbiology 2022, 91, 503–513. [Google Scholar] [CrossRef]
- Danilova, O.A.; Ianutsevich, E.A.; Kochkina, G.A.; Tereshina, V.M. Adaptation of the Psychrophilic Mucor psychrophilus (Mucorales, Mucoromycota) to Lower Temperatures and under Conditions of Heat and Osmotic Shocks. Fungal Biol. 2025, 129, 101532. [Google Scholar] [CrossRef] [PubMed]
- Ianutsevich, E.A.; Danilova, O.A.; Groza, N.V.; Tereshina, V.M. Membrane Lipids and Cytosol Carbohydrates in Aspergillus niger under Osmotic, Oxidative, and Cold Impact. Microbiology 2016, 85, 302–310. [Google Scholar] [CrossRef]
- Morozova, E.V.; Kozlov, V.P.; Tereshina, V.M.; Memorskaya, A.S.; Feofilova, E.P. Changes in Lipid Composition and Carbohydrate Composition of Aspergillus niger Conidia during Germination. Appl. Biochem. Microbiol. 2002, 38, 129–133. [Google Scholar] [CrossRef]
- Davì, V.; Tanimoto, H.; Ershov, D.; Haupt, A.; De Belly, H.; Le Borgne, R.; Couturier, E.; Boudaoud, A.; Minc, N. Mechanosensation Dynamically Coordinates Polar Growth and Cell Wall Assembly to Promote Cell Survival. Dev. Cell 2018, 45, 170–182.e7. [Google Scholar] [CrossRef]
- Elbein, A.D.; Pan, Y.T.; Pastuszak, I.; Carroll, D. New Insights on Trehalose: A Multifunctional Molecule. Glycobiology 2003, 13, 17R–27R. [Google Scholar] [CrossRef]
- Iturriaga, G.; Suárez, R.; Nova-Franco, B. Trehalose Metabolism: From Osmoprotection to Signaling. Int. J. Mol. Sci. 2009, 10, 3793–3810. [Google Scholar] [CrossRef]
- Tapia, H.; Koshland, D.E. Trehalose Is a Versatile and Long-Lived Chaperone for Desiccation Tolerance. Curr. Biol. 2014, 24, 2758–2766. [Google Scholar] [CrossRef]
- Argüelles, J.-C.; Guirao-Abad, J.P.; Sánchez-Fresneda, R. Trehalose: A Crucial Molecule in the Physiology of Fungi. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–9. ISBN 978-0-12-809633-8. [Google Scholar]
- Kahraman, M.; Sevim, G.; Bor, M. The Role of Proline, Glycinebetaine, and Trehalose in Stress-Responsive Gene Expression. In Osmoprotectant-Mediated Abiotic Stress Tolerance in Plants; Hossain, M., Kumar, V., Burritt, D., Fujita, M., Mäkelä, P., Eds.; Springer International Publishing: Cham, Swizterland, 2019; pp. 241–256. ISBN 9783030274221. [Google Scholar]
- Kosar, F.; Akram, N.A.; Sadiq, M.; Al-Qurainy, F.; Ashraf, M. Trehalose: A Key Organic Osmolyte Effectively Involved in Plant Abiotic Stress Tolerance. J. Plant Growth Regul. 2019, 38, 606–618. [Google Scholar] [CrossRef]
- Pokotylo, I.; Kravets, V.; Martinec, J.; Ruelland, E. The Phosphatidic Acid Paradox: Too Many Actions for One Molecule Class? Lessons from Plants. Prog. Lipid Res. 2018, 71, 43–53. [Google Scholar] [CrossRef]
- Kooijman, E.E.; Chupin, V.; de Kruijff, B.; Burger, K.N.J. Modulation of Membrane Curvature by Phosphatidic Acid and Lysophosphatidic Acid. Traffic 2003, 4, 162–174. [Google Scholar] [CrossRef]
- McMahon, H.T.; Gallop, J.L. Membrane Curvature and Mechanisms of Dynamic Cell Membrane Remodelling. Nature 2005, 438, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Zhukovsky, M.A.; Filograna, A.; Luini, A.; Corda, D.; Valente, C. Phosphatidic Acid in Membrane Rearrangements. FEBS Lett. 2019, 593, 2428–2451. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Huo, Y.; Yang, N.; Wei, T. Phosphatidic Acid: From Biophysical Properties to Diverse Functions. FEBS J. 2024, 291, 1870–1885. [Google Scholar] [CrossRef] [PubMed]
- Salsaa, M.; Case, K.; Greenberg, M.L. Orchestrating Phospholipid Biosynthesis: Phosphatidic Acid Conducts and Opi1p Performs. J. Biol. Chem. 2017, 292, 18729–18730. [Google Scholar] [CrossRef]
- Tereshina, V.M.; Memorskaya, A.S.; Kotlova, E.R. The Effect of Different Heat Influences on Composition of Membrane Lipids and Cytosol Carbohydrates in Mycelial Fungi. Microbiology 2011, 80, 455–460. [Google Scholar] [CrossRef]
- Fabri, J.H.T.M.; Rocha, M.C.; Malavazi, I. Overview of the Interplay Between Cell Wall Integrity Signaling Pathways and Membrane Lipid Biosynthesis in Fungi: Perspectives for Aspergillus fumigatus. Curr. Protein Pept. Sci. 2020, 21, 265–283. [Google Scholar] [CrossRef]
- Inouye, M.; Phadtare, S. Cold-Shock Response and Adaptation to Near-Freezing Temperature in Cold-Adapted Yeasts. In Cold-adapted Yeasts: Biodiversity, Adaptation Strategies and Biotechnological Significance; Buzzini, P., Margesin, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 243–257. ISBN 978-3-642-39680-9. [Google Scholar]
- Gostinčar, C.; Gunde-Cimerman, N. Overview of Oxidative Stress Response Genes in Selected Halophilic Fungi. Genes 2018, 9, 143. [Google Scholar] [CrossRef]
- Tiwari, S.; Thakur, R.; Shankar, J. Role of Heat-Shock Proteins in Cellular Function and in the Biology of Fungi. Biotechnol. Res. Int. 2015, 2015, 132635. [Google Scholar] [CrossRef]
- Iwama, R. Phospholipid Dynamics in Aspergillus Species: Relations between Biological Membrane Composition and Cellular Morphology. Biosci. Biotechnol. Biochem. 2025, 89, 515–522. [Google Scholar] [CrossRef]
- Wilson, R.A.; Chang, P.-K.; Dobrzyn, A.; Ntambi, J.M.; Zarnowski, R.; Keller, N.P. Two Δ9-Stearic Acid Desaturases Are Required for Aspergillus nidulans Growth and Development. Fungal Genet. Biol. 2004, 41, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Calvo, A.M.; Gardner, H.W.; Keller, N.P. Genetic Connection between Fatty Acid Metabolism and Sporulation in Aspergillus nidulans. J. Biol. Chem. 2001, 276, 25766–25774. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, H.J.; Roseman, S. Quantitative Estimation of Chitin in Fungi. J. Bacteriol. 1957, 74, 222–224. [Google Scholar] [CrossRef] [PubMed]
- Nichols, B.W. Separation of the Lipids of Photosynthetic Tissues: Improvements in Analysis by Thin-Layer Chromatography. Biochim. Biophys. Acta—Spec. Sect. Lipids Relat. Subj. 1963, 70, 417–422. [Google Scholar] [CrossRef]
- Kotlova, E.R.; Senik, S.V.; Kücher, T.; Shavarda, A.L.; Kiyashko, A.A.; Psurtseva, N.V.; Sinyutina, N.F.; Zubarev, R.A. Alterations in the Composition of Membrane Glycero-and Sphingolipids in the Course of Flammulina Velutipes Surface Culture Development. Microbiology 2009, 78, 193–201. [Google Scholar] [CrossRef]
- Benning, C.; Huang, Z.H.; Gage, D.A. Accumulation of a Novel Glycolipid and a Betaine Lipid in Cells of Rhodobacter Sphaeroides Grown under Phosphate Limitation. Arch. Biochem. Biophys. 1995, 317, 103–111. [Google Scholar] [CrossRef]
- Kates, M. Techniques of Lipidology: Isolation, Analysis and Identification of Lipids. In Laboratory Techniques in Biochemistry and Molecular Biology; Work, T.S., Work, E., Eds.; North-Holland Publishing Company: Amsterdam, The Netherlands, 1972; Volume 3, pp. 267–610. ISBN 9780444533425. [Google Scholar]
- Weete, J.D. Introduction to Fungal Lipids. In Fungal Lipid Biochemistry; Kritchevsky, D., Ed.; Springer: Boston, MA, USA, 1974; Volume 1, pp. 3–36. ISBN 9781468428315. [Google Scholar]
- Somogyi, M. Determination of Blood Sugar. J. Biol. Chem. 1945, 160, 69–73. [Google Scholar] [CrossRef]
- Brobst, K.M. Gas–Liquid Chromatography of Trimethylsilyl Derivatives: Analysis of Corn Syrup. In General Carbohydrate Method; Whistler, R.L., BeMiller, J.N., Eds.; Academic Press: New York, NY, USA; London, UK, 1972; pp. 3–8. ISBN 9780127462066. [Google Scholar]
- Boas, N.F. Method for the Determination of Hexosamines in Tissues. J. Biol. Chem. 1953, 204, 553–563. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ianutsevich, E.A.; Danilova, O.A.; Saharova, S.A.; Tereshina, V.M. Membrane Lipids and Osmolytes Rearrangements Under Cell Wall Stress in Aspergillus niger. Int. J. Mol. Sci. 2025, 26, 10888. https://doi.org/10.3390/ijms262210888
Ianutsevich EA, Danilova OA, Saharova SA, Tereshina VM. Membrane Lipids and Osmolytes Rearrangements Under Cell Wall Stress in Aspergillus niger. International Journal of Molecular Sciences. 2025; 26(22):10888. https://doi.org/10.3390/ijms262210888
Chicago/Turabian StyleIanutsevich, Elena A., Olga A. Danilova, Sofiya A. Saharova, and Vera M. Tereshina. 2025. "Membrane Lipids and Osmolytes Rearrangements Under Cell Wall Stress in Aspergillus niger" International Journal of Molecular Sciences 26, no. 22: 10888. https://doi.org/10.3390/ijms262210888
APA StyleIanutsevich, E. A., Danilova, O. A., Saharova, S. A., & Tereshina, V. M. (2025). Membrane Lipids and Osmolytes Rearrangements Under Cell Wall Stress in Aspergillus niger. International Journal of Molecular Sciences, 26(22), 10888. https://doi.org/10.3390/ijms262210888





