Elucidating Circular Ribonucleic Acid Mechanisms Associated with Splicing Factor 3 Inhibition in Cervical Cancer
Abstract
1. Introduction
2. Results
2.1. Theophylline Alters Cell Cycle Progression in CCa Cells
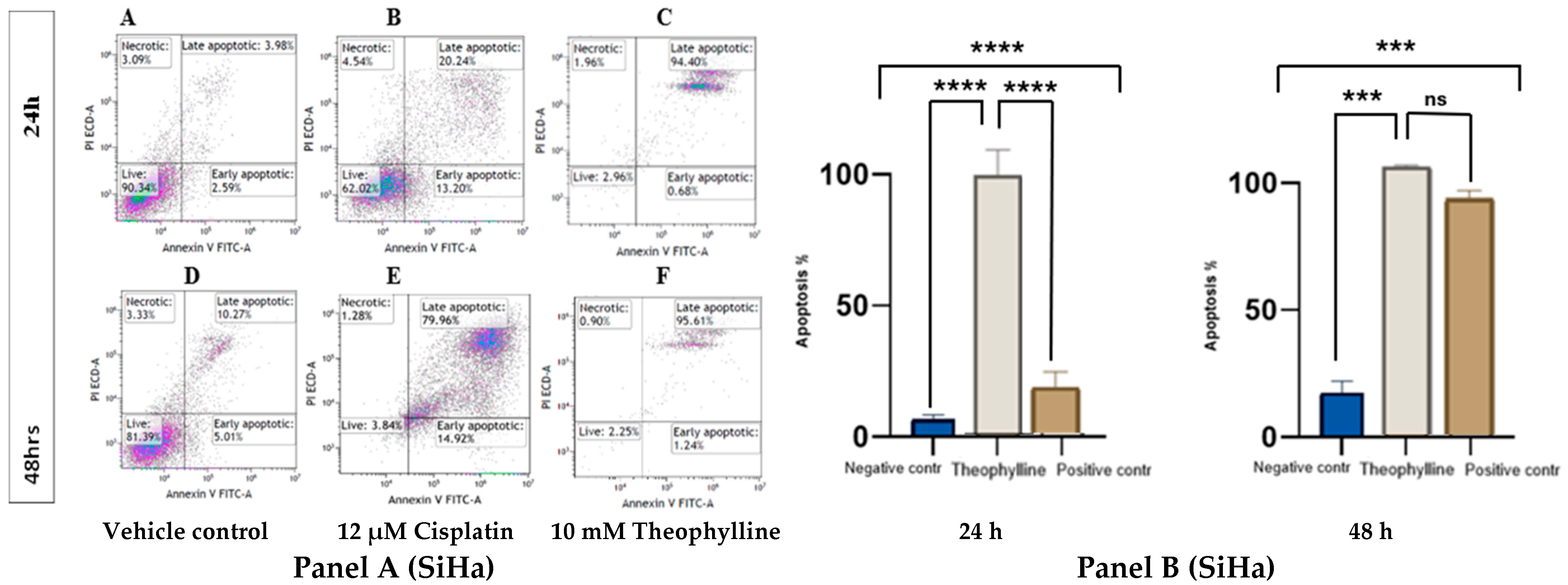
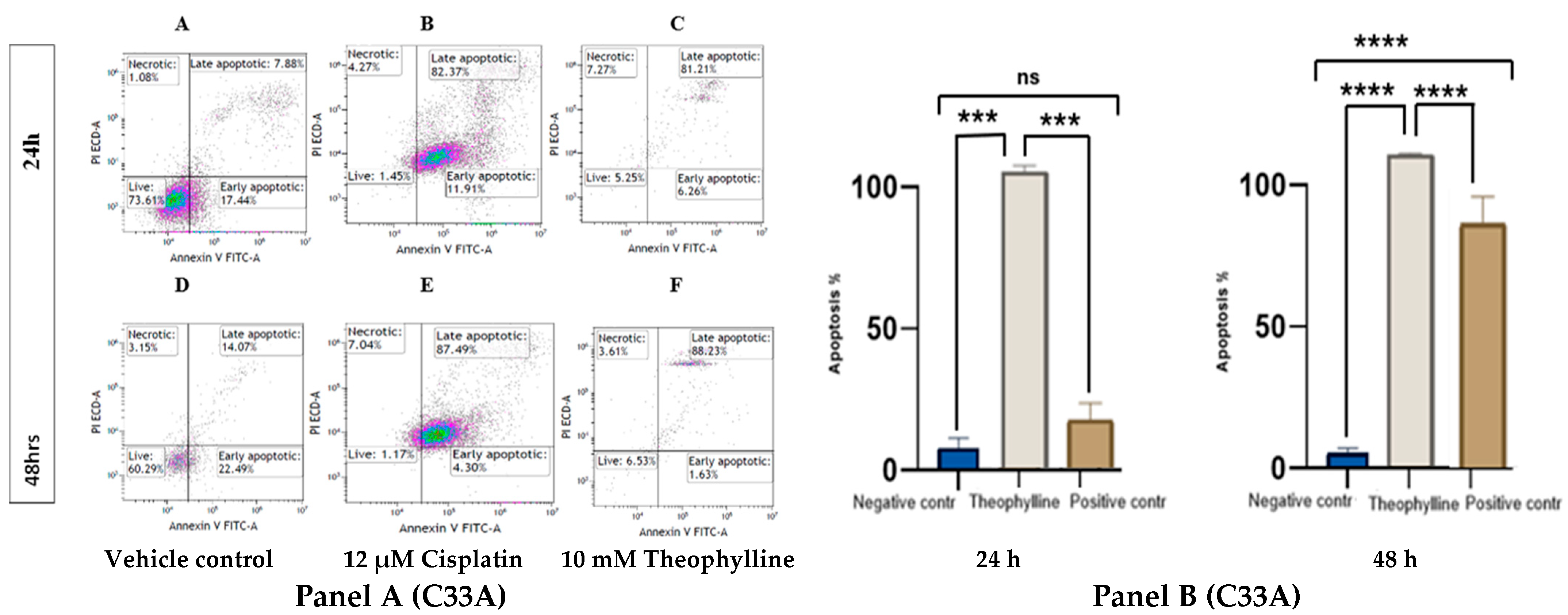
2.2. Theophylline Induces Apoptosis in CCa Cell Line SiHa and C33A
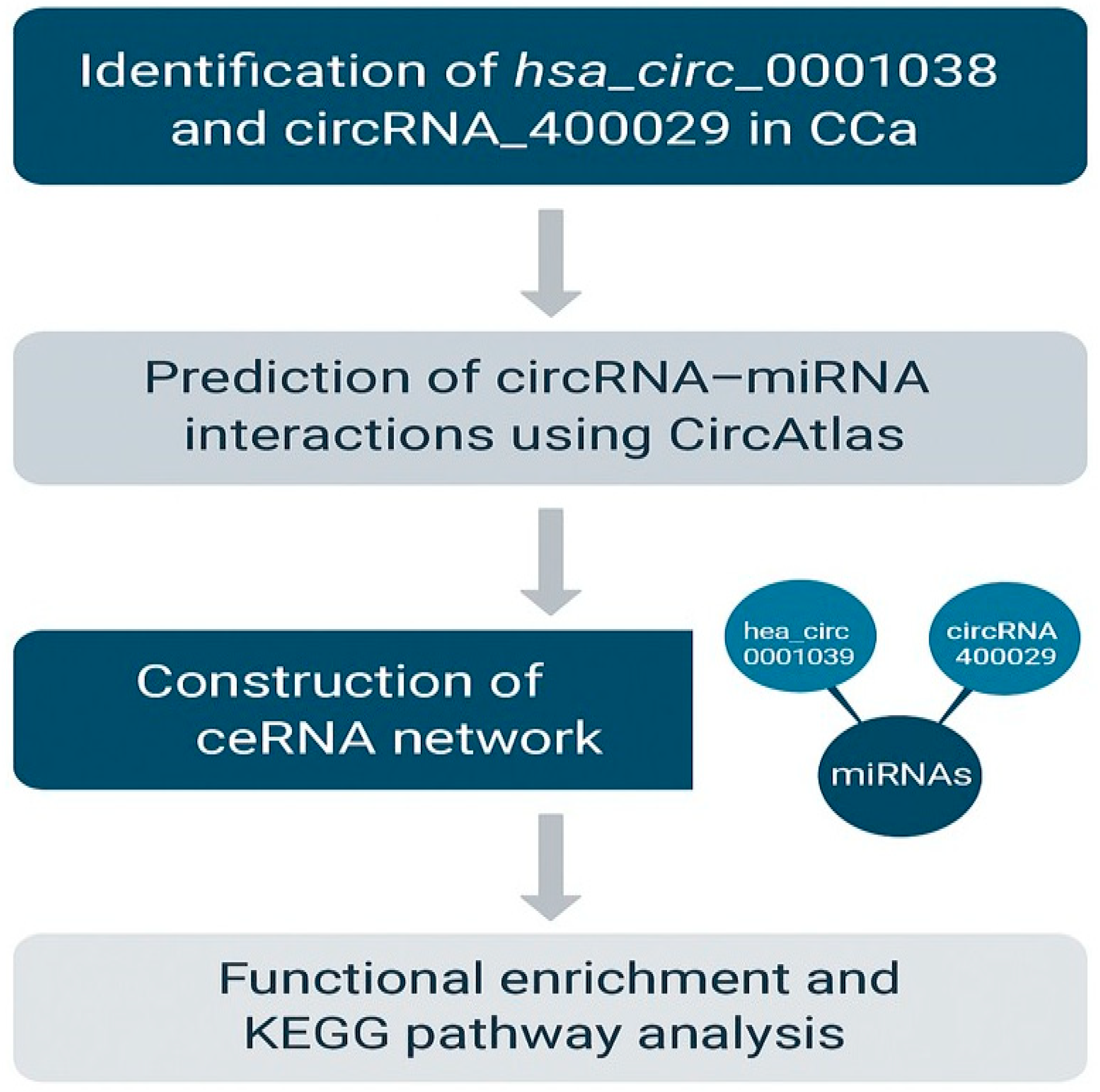
2.3. In Silico Bioinformatics Analysis of Differentially Expressed circRNAs hsa_circ_0001038 & circRNA_400029 in CCa
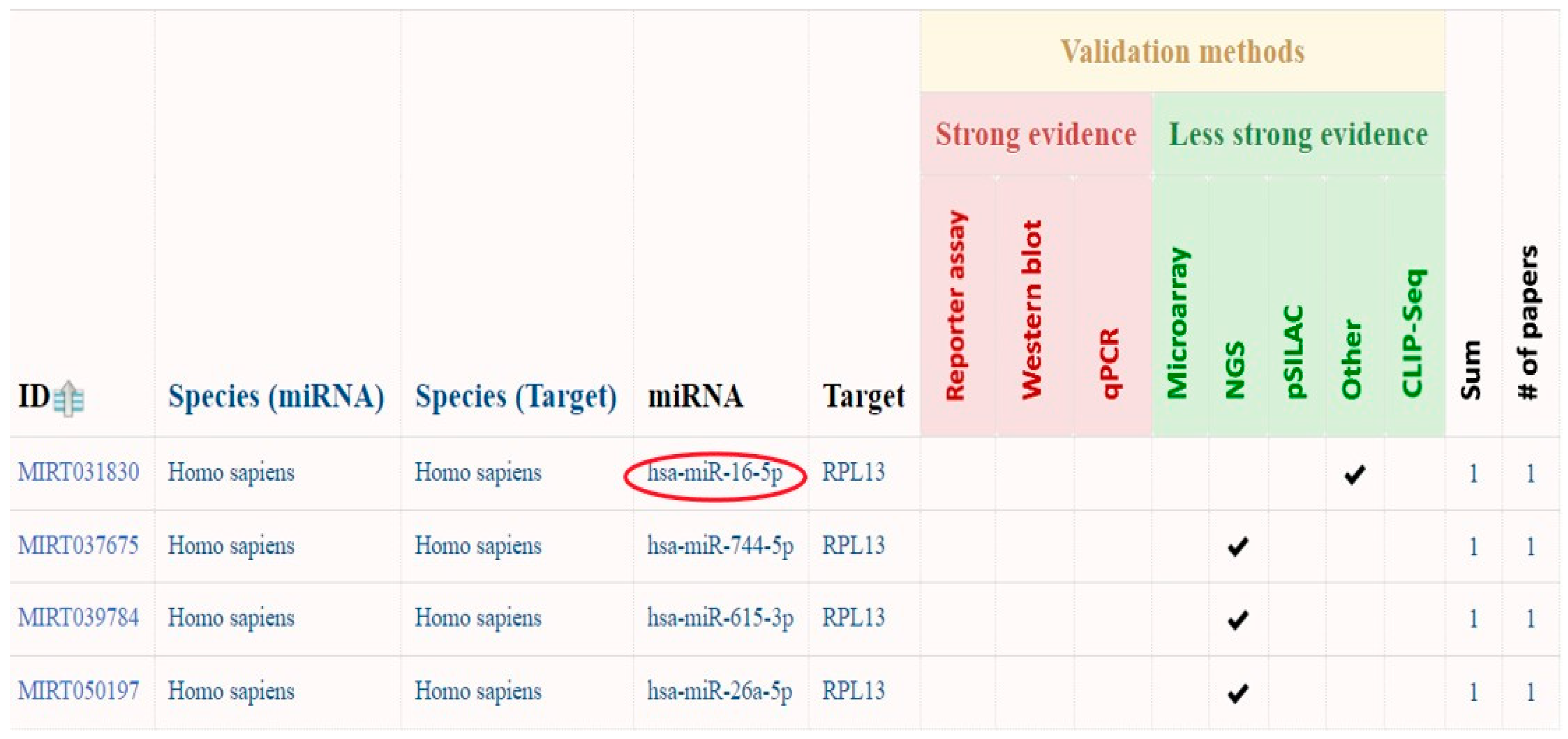
2.3.1. Prediction of circRNA_400029 miRNA Interactions Using miRTarBase
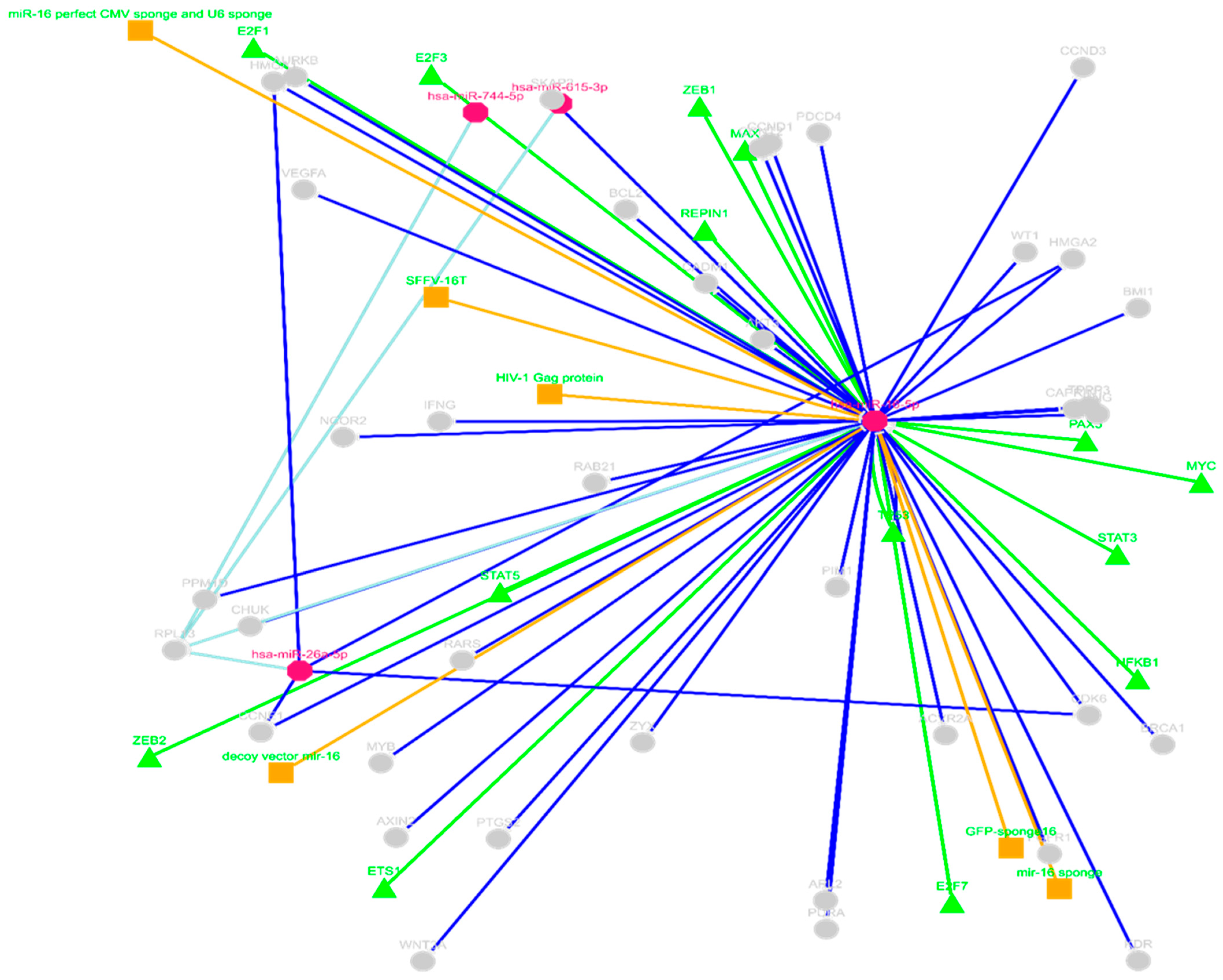
2.3.2. ceRNAs Network of circRNA_400029—miRNA Using miRTarBase
2.3.3. MiR-16-5p—Gene Target Pathway Analysis Using KEGG Pathway
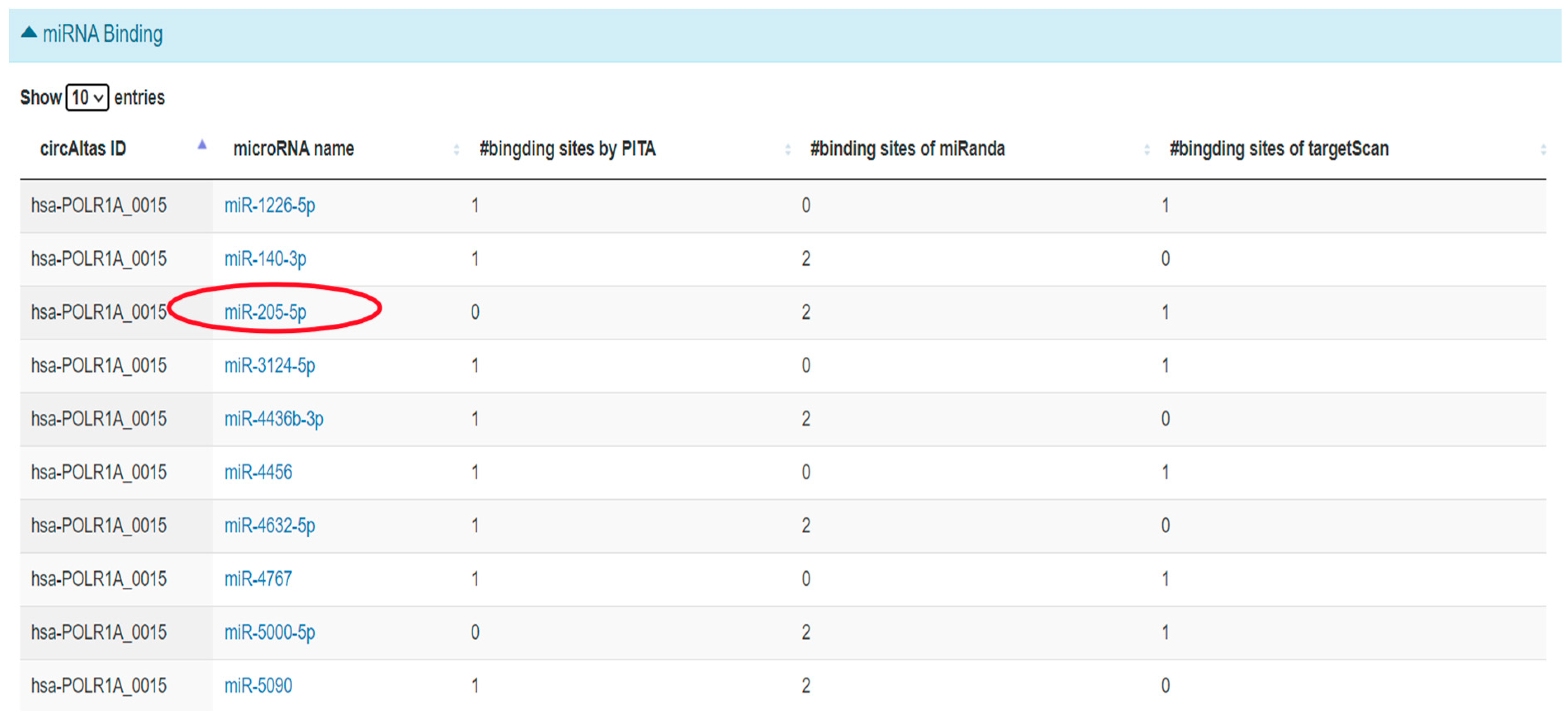
2.3.4. Prediction of hsa_circ_0001038—miRNA Interactions Using circAtlas
2.3.5. ceRNA Network of miR-205-5p Using miRTarBase
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Drug Treatment
4.2. Propidium Iodide (PI) Cell Cycle FACS Analysis
4.3. Apoptosis Assay by FACS
4.4. Bioinformatics Analysis
4.5. Statistical Analysis
5. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Castle, P.E.; Einstein, M.H.; Sahasrabuddhe, V.V. Cervical cancer prevention and control in women living with human immunodeficiency virus. CA Cancer J. Clin. 2021, 71, 505–526. [Google Scholar] [CrossRef]
- Zhang, J.; Qiu, H.; Qing, Y.; Hu, Y.; Deng, H. A commentary on ‘Local excision as a viable alternative to hysterectomy for early-stage cervical cancer in women of reproductive age: A population-based cohort study’. Int. J. Surg. 2024, 110, 3120–3121. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, H.; Zhang, L.; Qiao, Y. Cervical cancer: Epidemiology, risk factors and screening. Chin. J. Cancer Res. 2020, 32, 720. [Google Scholar] [CrossRef]
- Yousaf, S.; Shehzadi, A.; Ahmad, M.; Asrar, A.; Ahmed, I.; Iqbal, H.M.; Bule, M.H. Recent advances in HPV biotechnology: Understanding host-virus interactions and cancer progression—A review. Int. J. Surg. 2024, 110, 8025–8036. [Google Scholar] [CrossRef]
- Kumar, L.; Harish, P.; Malik, P.S.; Khurana, S. Chemotherapy and targeted therapy in the management of cervical cancer. Curr. Probl. Cancer 2018, 42, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Chargari, C.; Peignaux, K.; Escande, A.; Renard, S.; Lafond, C.; Petit, A.; Kee, D.L.C.; Durdux, C.; Haie-Méder, C. Radiotherapy of cervical cancer. Cancer Radiothérapie 2022, 26, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Hua, K. Cervical cancer: Emerging immune landscape and treatment. OncoTargets Ther. 2020, 13, 8037–8047. [Google Scholar] [CrossRef]
- Movahedpour, A.; Khatami, S.H.; Khorsand, M.; Salehi, M.; Savardashtaki, A.; Mirmajidi, S.H.; Negahdari, B.; Khanjani, N.; Naeli, P.; Vakili, O.; et al. Exosomal noncoding RNAs: Key players in glioblastoma drug resistance. Mol. Cell. Biochem. 2021, 476, 4081–4092. [Google Scholar] [CrossRef]
- Ali, S.A.; Peffers, M.J.; Ormseth, M.J.; Jurisica, I.; Kapoor, M. The non-coding RNA interactome in joint health and disease. Nat. Rev. Rheumatol. 2021, 17, 692–705. [Google Scholar] [CrossRef] [PubMed]
- Movahedpour, A.; Khatami, S.H.; Karami, N.; Vakili, O.; Naeli, P.; Jamali, Z.; Shabaninejad, Z.; Tazik, K.; Behrouj, H.; Ghasemi, H. Exosomal noncoding RNAs in prostate cancer. Clin. Chim. Acta 2022, 537, 127–132. [Google Scholar] [CrossRef]
- Dragomir, M.P.; Manyam, G.C.; Ott, L.F.; Berland, L.; Knutsen, E.; Ivan, C.; Lipovich, L.; Broom, B.M.; Calin, G.A. FuncPEP: A database of functional peptides encoded by non-coding RNAs. Non-Coding RNA 2020, 6, 41. [Google Scholar] [CrossRef]
- Dhanoa, J.K.; Sethi, R.S.; Verma, R.; Arora, J.S.; Mukhopadhyay, C.S. Long non-coding RNA: Its evolutionary relics and biological implications in mammals: A review. J. Anim. Sci. Technol. 2018, 60, 25. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Ren, H.; Guo, M.; Qian, J.; Yang, Y.; Gu, C. Review on circular RNAs and new insights into their roles in cancer. Comput. Struct. Biotechnol. J. 2021, 19, 910–928. [Google Scholar] [CrossRef]
- Xu, S.; Zhou, L.; Ponnusamy, M.; Zhang, L.; Dong, Y.; Zhang, Y.; Wang, Q.; Liu, J.; Wang, K. A comprehensive review of circRNA: From purification and identification to disease marker potential. PeerJ 2018, 6, e5503. [Google Scholar] [CrossRef]
- Yu, C.-Y.; Kuo, H.-C. The emerging roles and functions of circular RNAs and their generation. J. Biomed. Sci. 2019, 26, 29. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhu, Q. Mechanisms regulating abnormal circular RNA biogenesis in cancer. Cancers 2021, 13, 4185. [Google Scholar] [CrossRef]
- Bolha, L.; Ravnik-Glavač, M.; Glavač, D. Circular RNAs: Biogenesis, function, and a role as possible cancer biomarkers. Int. J. Genom. 2017, 2017, 6218353. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, A.S.; Asnani, M.; Black, K.L.; Hayer, K.E.; Taylor, D.; Thomas-Tikhonenko, A. The role of SRSF3 splicing factor in generating circular RNAs. bioRxiv 2019, preprint. [Google Scholar] [CrossRef]
- Zhou, Z.; Gong, Q.; Lin, Z.; Wang, Y.; Li, M.; Wang, L.; Ding, H.; Li, P. Emerging roles of SRSF3 as a therapeutic target for cancer. Front. Oncol. 2020, 10, 577636. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, J.; Yang, Z.; Chen, P.; Wang, D.B. CircRNA_400029 promotes the aggressive behaviors of cervical cancer by regulation of miR-1285-3p/TLN1 axis. J. Cancer 2022, 13, 541–553. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Wang, W.; Guo, X. Overexpression of circular RNA hsa_circ_0001038 promotes cervical cancer cell progression by acting as a ceRNA for miR-337-3p. Gene 2020, 733, 144273. [Google Scholar] [CrossRef]
- Song, T.; Xu, A.; Chen, X.; Gao, J.; Gao, F.; Kong, X. Circular RNA circRNA_101996 promoted cervical cancer development by regulating miR-1236-3p/TRIM37 axis. Kaohsiung J. Med. Sci. 2021, 37, 547–556. [Google Scholar] [CrossRef]
- Basera, A.; Hull, R.; Demetriou, D.; Bates, D.O.; Kaufmann, A.M.; Dlamini, Z.; Marima, R. Competing endogenous RNA (ceRNA) networks and splicing switches in cervical cancer: HPV oncogenesis, clinical significance and therapeutic opportunities. Microorganisms 2022, 10, 1852. [Google Scholar] [CrossRef]
- Chang, Y.-L.; Hsu, Y.-J.; Chen, Y.; Wang, Y.-W.; Huang, S.-M. Theophylline exhibits anti-cancer activity via suppressing SRSF3 in cervical and breast cancer cell lines. Oncotarget 2017, 8, 101461–101474. [Google Scholar] [CrossRef]
- Zhuang, L.; Huang, C.; Zhao, M.; Cheng, J.; Zhang, X. miR-205 targets ZEB1 and inhibits proliferation and invasion in cervical cancer cells. Oncol. Rep. 2015, 34, 2723–2732. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Zhang, J.; He, Y.; Shi, H.; Zhang, Z. miR-205-5p inhibits epithelial–mesenchymal transition and tumor progression in cervical cancer by targeting the PTEN/AKT pathway. J. Cell. Biochem. 2020, 121, 232–240. [Google Scholar] [CrossRef]
- Tang, Q.; Hann, S.S. Biological roles and mechanisms of circular RNA in human cancers. OncoTargets Ther. 2020, 2020, 2067–2092. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Liu, W.; Li, Y.; Liu, X.; Xu, Y. Circular RNA hsa_circ_0001038 promotes cervical cancer progression through miR-337-3p/STAT3 axis regulation. Biomed. Pharmacother. 2021, 139, 111553. [Google Scholar] [CrossRef]
- Chen, H.; Liu, X.; Wang, S.; Sun, Y.; Li, J. Circular RNA circRNA_400029 promotes cervical cancer cell proliferation and invasion by regulating the miR-1285-3p/TSPAN3 axis. Cancer Cell Int. 2021, 21, 175. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, Z.; Jiang, Y.; Shen, R.; Gu, M.; Xu, W.; Gu, X. Inhibition of splicing factor 3b subunit 1 (SF3B1) reduced cell proliferation, induced apoptosis and resulted in cell cycle arrest by regulating homeobox A10 (HOXA10) splicing in AGS and MKN28 human gastric cancer cells. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e919460-1. [Google Scholar] [CrossRef]
- Pérez-Pérez, D.; Reyes-Vidal, I.; Chávez-Cortez, E.G.; Sotelo, J.; Magaña-Maldonado, R. Methylxanthines: Potential therapeutic agents for glioblastoma. Pharmaceuticals 2019, 12, 130. [Google Scholar] [CrossRef] [PubMed]
- Petasny, M.; Bentata, M.; Pawellek, A.; Baker, M.; Kay, G.; Salton, M. Splicing to keep cycling: The importance of pre-mRNA splicing during the cell cycle. Trends Genet. 2021, 37, 266. [Google Scholar] [CrossRef] [PubMed]
- Bradley, R.K.; Anczuków, O. RNA splicing dysregulation and the hallmarks of cancer. Nat. Rev. Cancer 2023, 23, 135–155. [Google Scholar] [CrossRef] [PubMed]
- Che, Y.; Bai, M.; Lu, K.; Fu, L. Splicing factor SRSF3 promotes the progression of cervical cancer through regulating DDX5. Mol. Carcinog. 2023, 62, 210–223. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nyalambisa, A.; Alabi, B.A.; Dlamini, Z.; Marima, R. Elucidating Circular Ribonucleic Acid Mechanisms Associated with Splicing Factor 3 Inhibition in Cervical Cancer. Int. J. Mol. Sci. 2025, 26, 10883. https://doi.org/10.3390/ijms262210883
Nyalambisa A, Alabi BA, Dlamini Z, Marima R. Elucidating Circular Ribonucleic Acid Mechanisms Associated with Splicing Factor 3 Inhibition in Cervical Cancer. International Journal of Molecular Sciences. 2025; 26(22):10883. https://doi.org/10.3390/ijms262210883
Chicago/Turabian StyleNyalambisa, Amahle, Babatunde Adebola Alabi, Zodwa Dlamini, and Rahaba Marima. 2025. "Elucidating Circular Ribonucleic Acid Mechanisms Associated with Splicing Factor 3 Inhibition in Cervical Cancer" International Journal of Molecular Sciences 26, no. 22: 10883. https://doi.org/10.3390/ijms262210883
APA StyleNyalambisa, A., Alabi, B. A., Dlamini, Z., & Marima, R. (2025). Elucidating Circular Ribonucleic Acid Mechanisms Associated with Splicing Factor 3 Inhibition in Cervical Cancer. International Journal of Molecular Sciences, 26(22), 10883. https://doi.org/10.3390/ijms262210883





