IGF Pathway Components as Potential Biomarkers in Gastric Cancer
Abstract
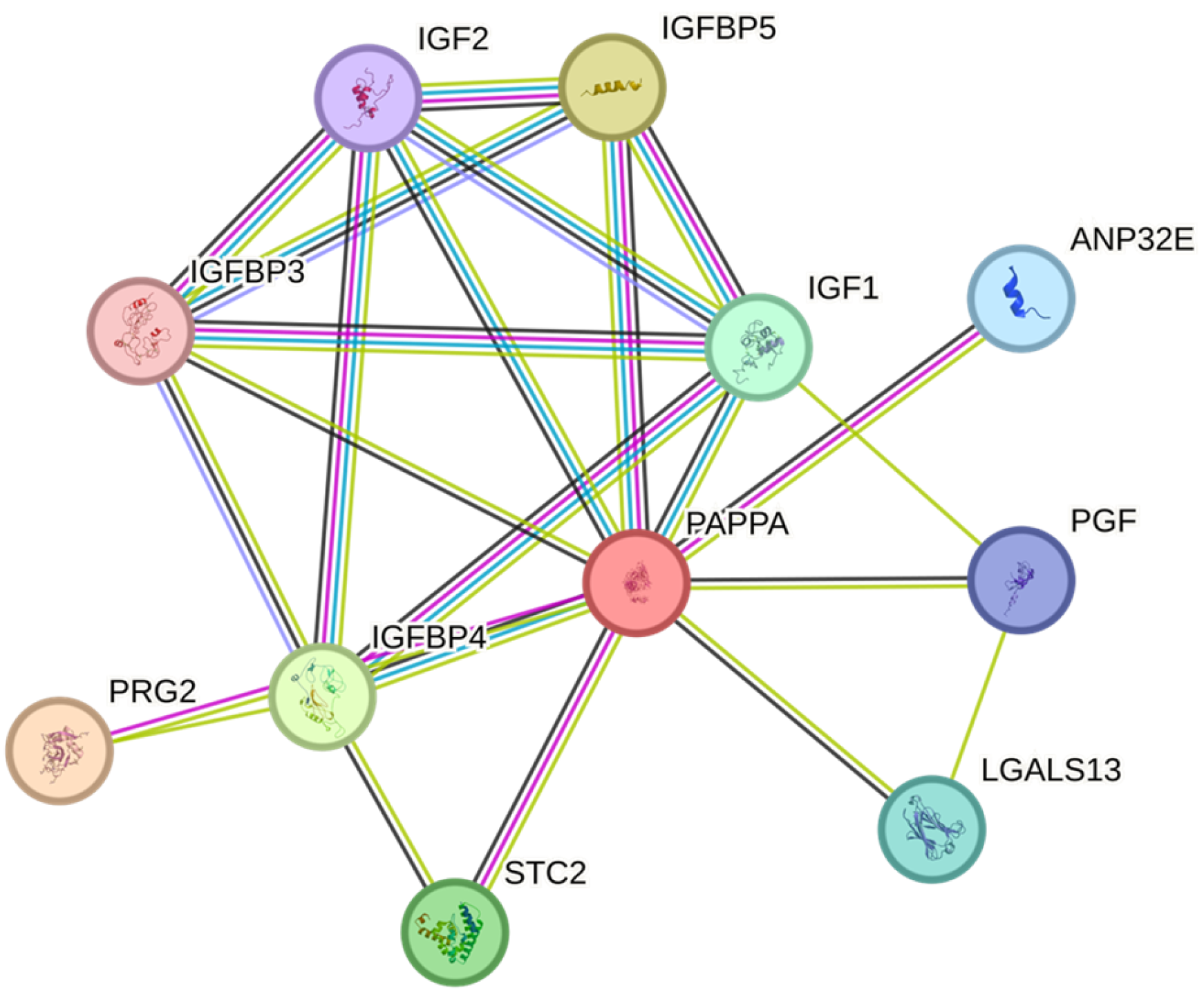
1. Introduction
2. Results
2.1. ELISA Results
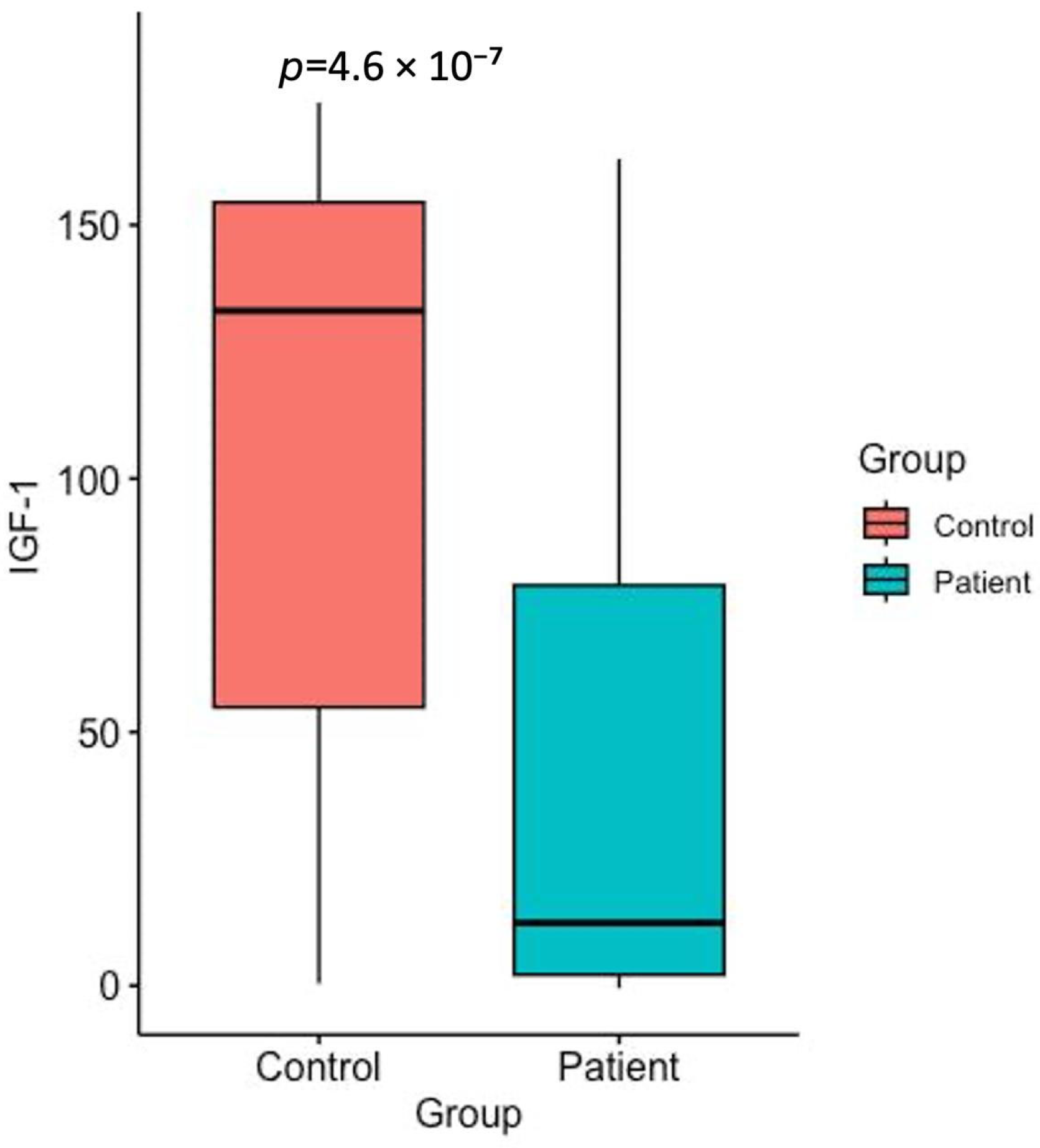
2.2. IGF-1 Levels
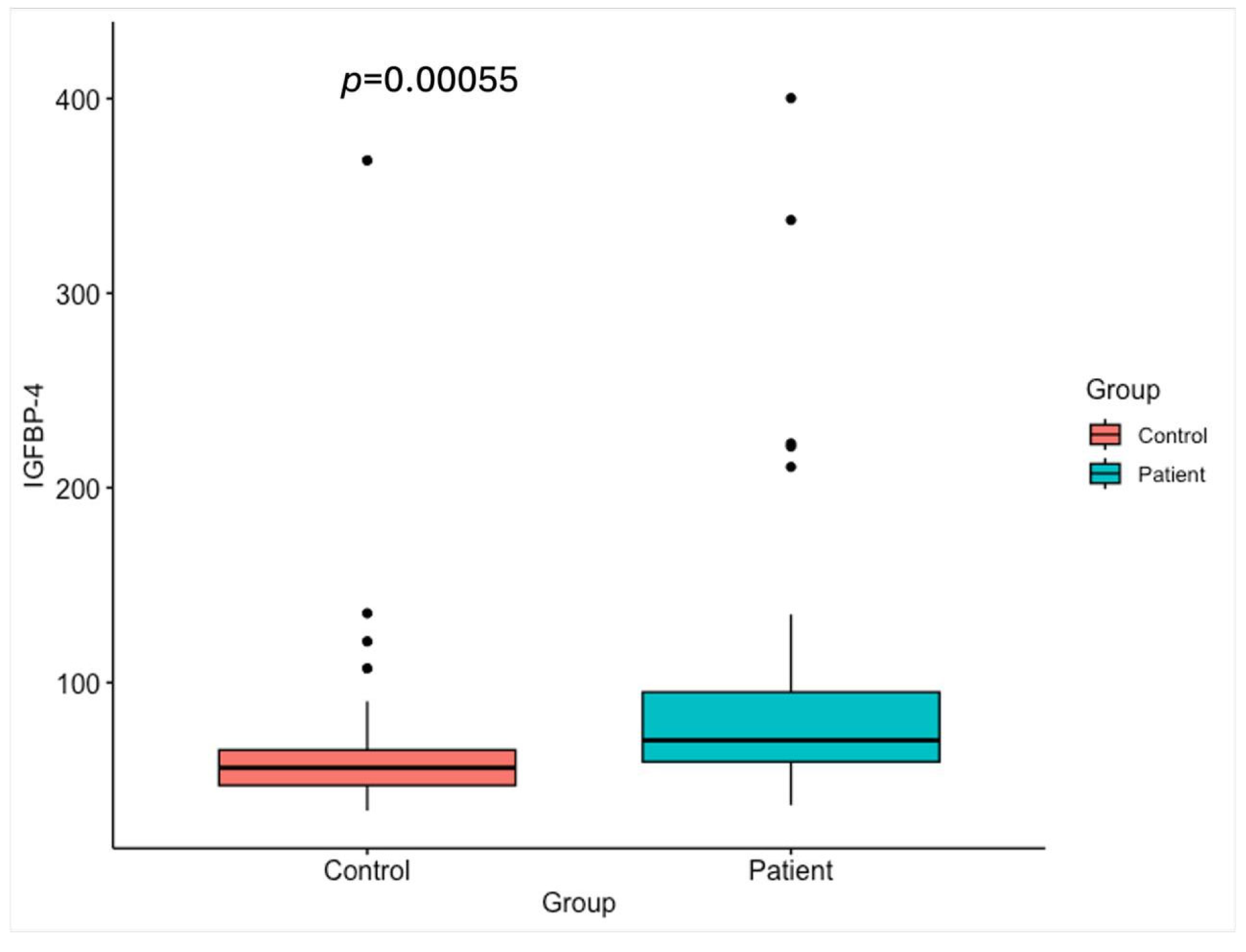
2.3. IGFBP-4 Levels
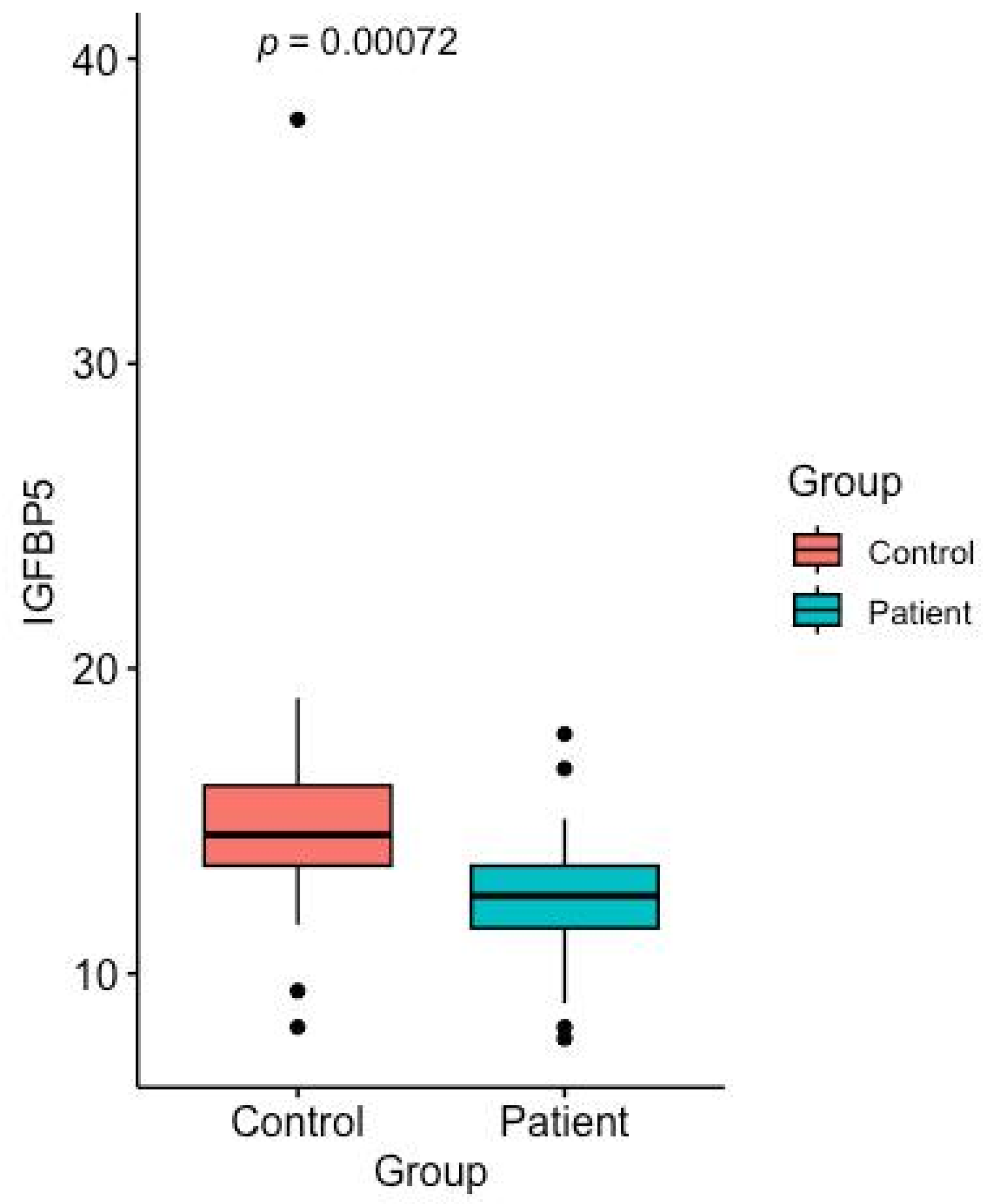
2.4. IGFBP-5 Levels
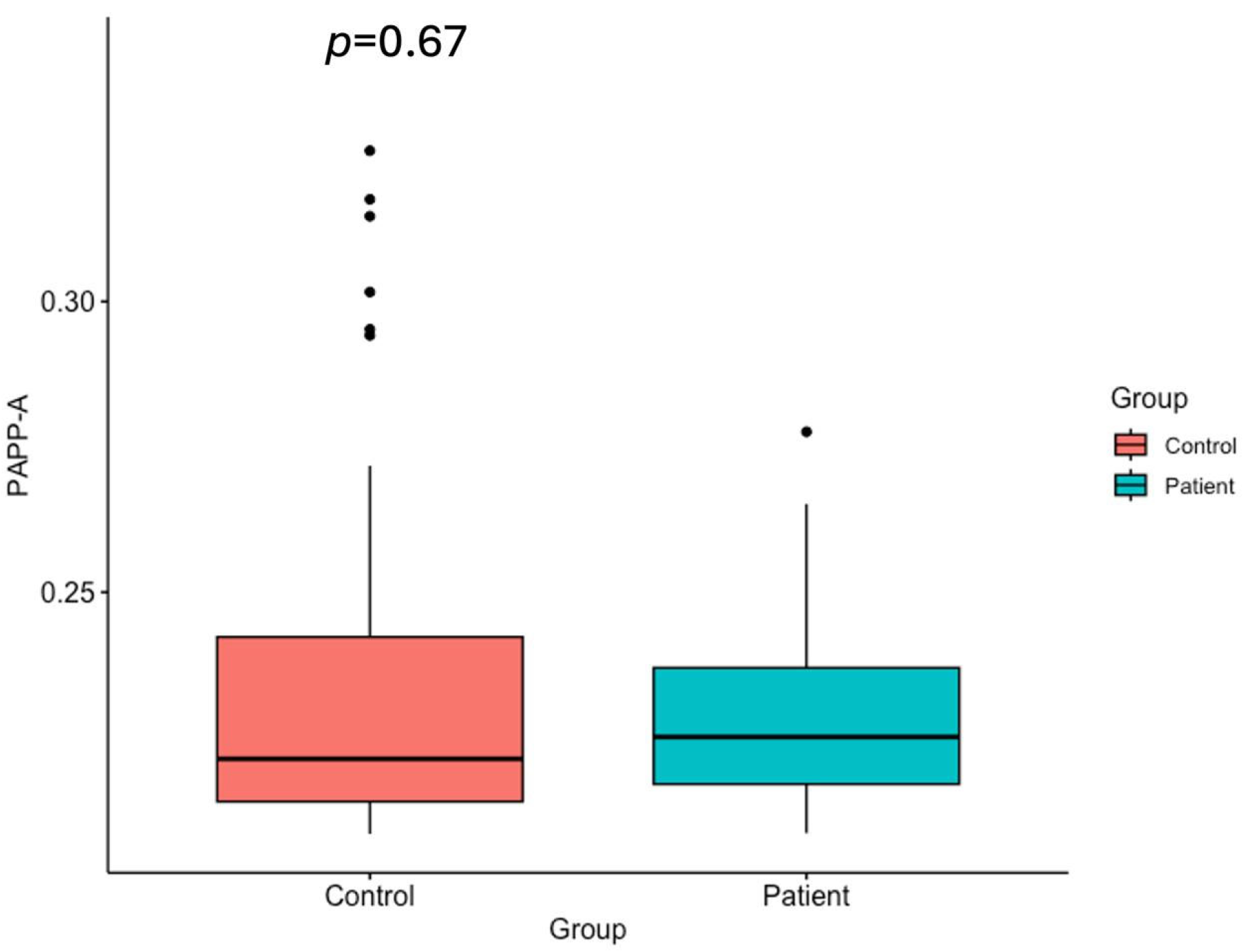
2.5. PAPP-A Levels
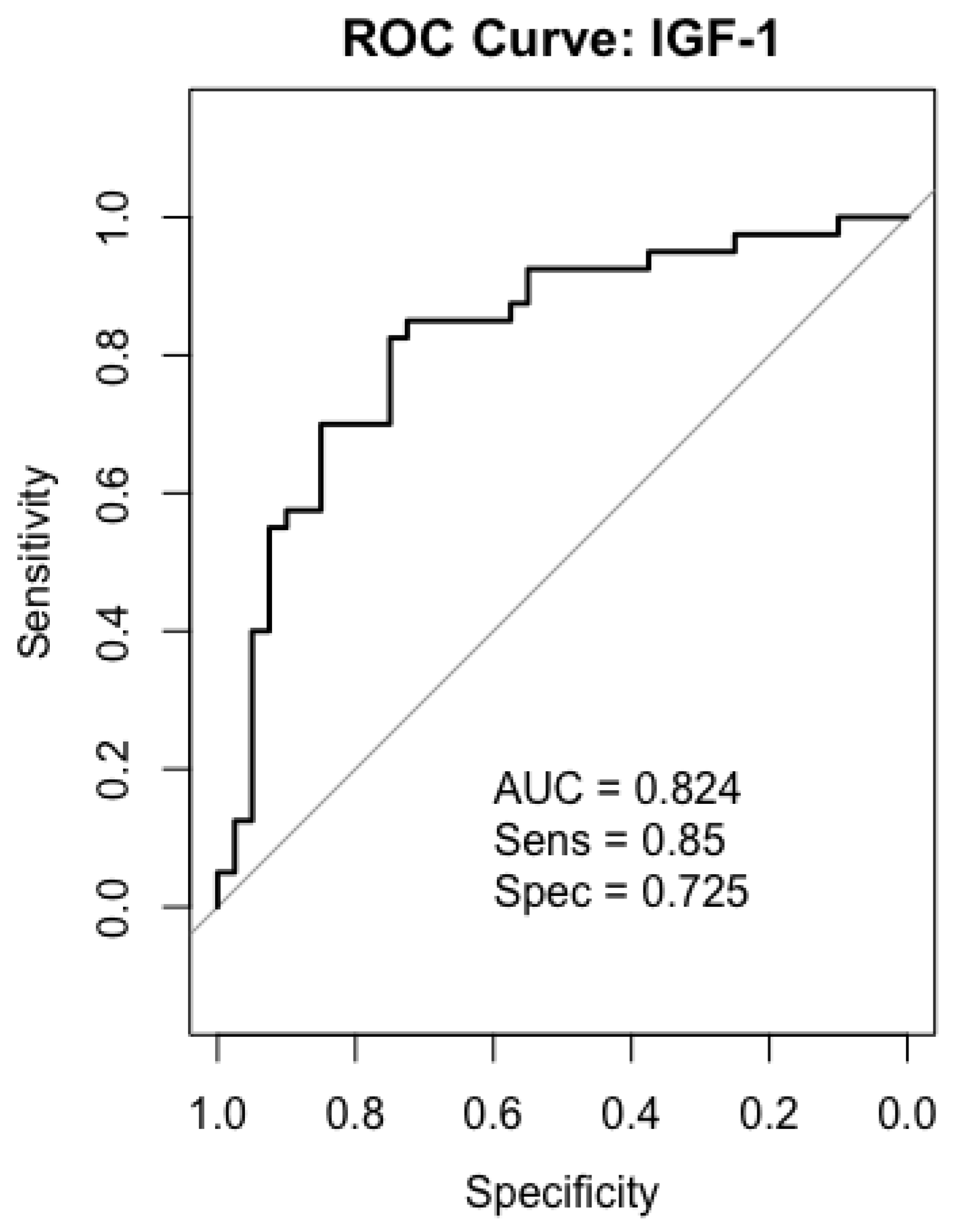
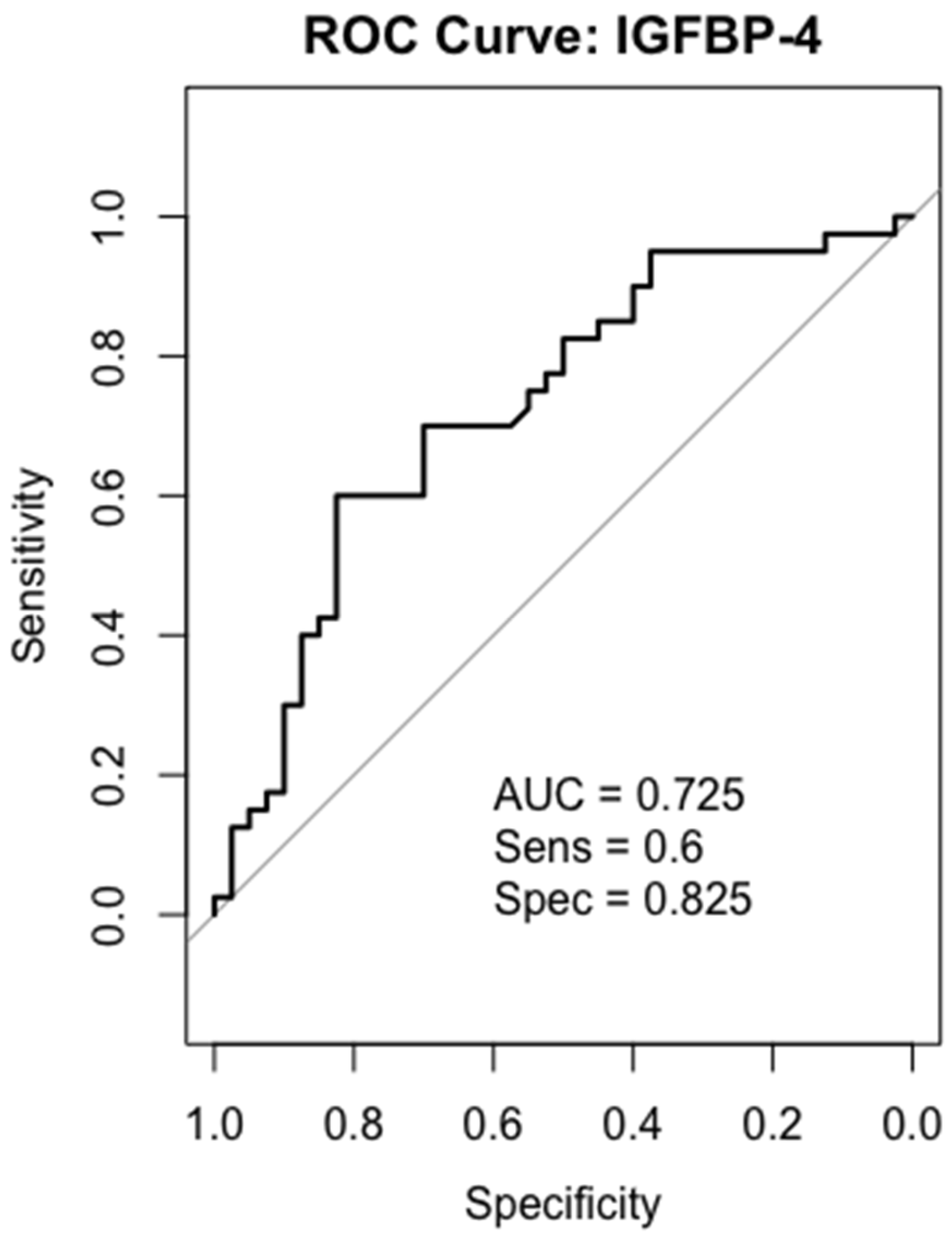
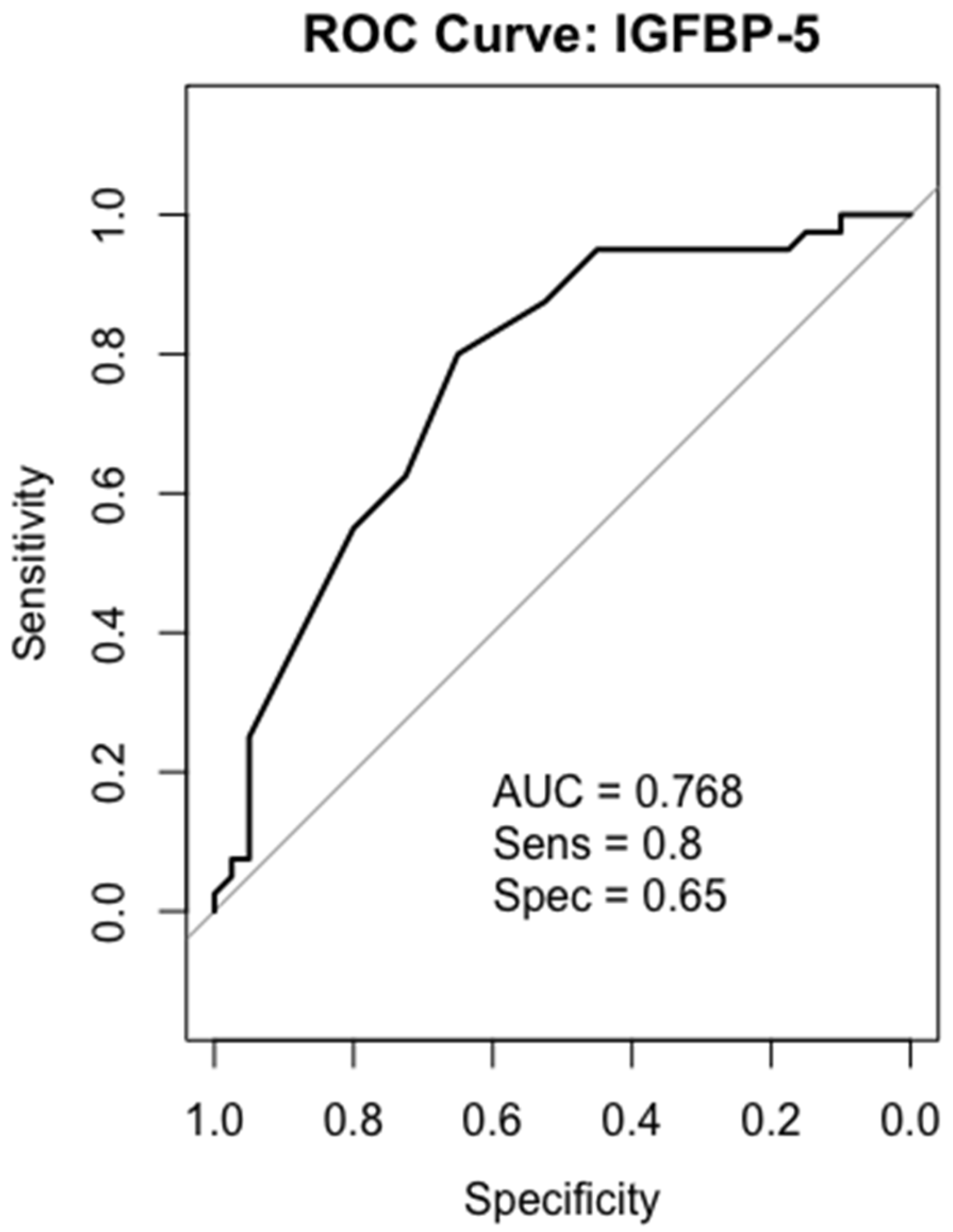
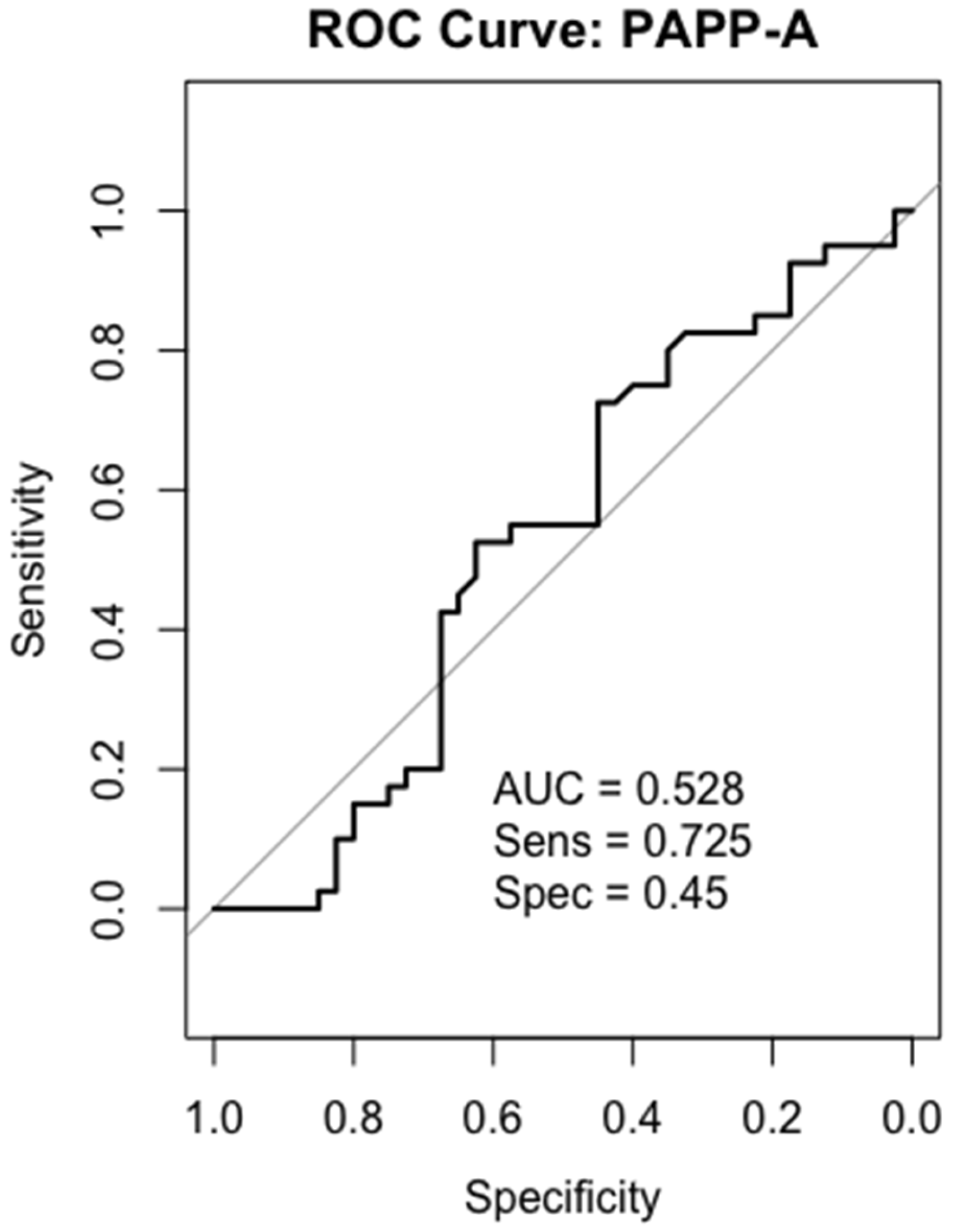
2.6. Receiver-Operating Characteristics (ROC) Analysis
2.7. IGF-1
2.8. IGFBP-4
2.9. IGFBP-5
2.10. PAPP-A
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Measurement of the IGF Components by Enzyme-Linked Immunoassay (ELISA)
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GC | Gastric cancer |
| IGF | Insulin-like growth factor |
| PAPP-A | Pregnancy-associated plasma protein A |
| IGFBP | Insulin-like growth factor binding protein |
| ELISA | Enzyme-linked immunoassays |
| CV | Coefficient of variation |
| LOD | Limit of detection |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Hu, B.; El Hajj, N.; Sittler, S.; Lammert, N.; Barnes, R.; Meloni-Ehrig, A. Gastric cancer: Classification, histology and application of molecular pathology. J. Gastrointest. Oncol. 2012, 3, 251–261. [Google Scholar] [CrossRef]
- Laurén, P. The Two Histological Main Types of Gastric Carcinoma: Diffuse and So-Called Intestinal-Type Carcinoma. An Attempt at a Histo-Clinical Classification. Acta Pathol. Microbiol. Scand. 1965, 64, 31–49. [Google Scholar] [CrossRef]
- Machlowska, J.; Baj, J.; Sitarz, M.; Maciejewski, R.; Sitarz, R. Gastric cancer: Epidemiology, risk factors, classification, genomic characteristics and treatment strategies. Int. J. Mol. Sci. 2020, 21, 4012. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z. Regulation of cell cycle progression by growth factor-induced cell signaling. Cells 2021, 10, 3327. [Google Scholar] [CrossRef]
- Weroha, S.J.; Haluska, P. IGF signaling in cancer. Endocrinol. Metab. Clin. N. Am. 2012, 41, 335–350. [Google Scholar] [CrossRef]
- Parveen, N.; Subhakumari, K.N.; Krishnan, S. Pregnancy Associated Plasma Protein-A (PAPP-A) Levels in Acute Coronary Syndrome: A Case Control Study in a Tertiary Care Centre. Indian J. Clin. Biochem. IJCB 2015, 30, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Kong, Q.; Yin, J.; Zhang, J.; Jiang, Y. Insulin-like growth factor receptor signaling in tumorigenesis and drug resistance: A challenge for cancer therapy. J. Hematol. Oncol. 2020, 13, 64. [Google Scholar] [CrossRef] [PubMed]
- Fruman, D.A.; Chiu, H.; Hopkins, B.D.; Bagrodia, S.; Cantley, L.C.; Abraham, R.T. The PI3K Pathway in Human Disease. Cell 2017, 170, 605–635. [Google Scholar] [CrossRef] [PubMed]
- Coşkun, U.; Günel, N.; Sancak, B.; Günel, U.; Onuk, E.; Bayram, O.; Yílmaz, E.; Candan, S.; Ozkan, S. Significance of serum vascular endothelial growth factor, insulin-like growth factor-I levels and nitric oxide activity in breast cancer patients. Breast 2003, 12, 104–110. [Google Scholar] [CrossRef]
- Gianuzzi, X.; Palma-Ardiles, G.; Hernandez-Fernandez, W.; Pasupuleti, V.; Hernandez, A.V.; Perez-Lopez, F.R. Insulin growth factor (IGF) 1, IGF-binding proteins and ovarian cancer risk: A systematic review and meta-analysis. Maturitas 2016, 94, 22–29. [Google Scholar] [CrossRef]
- El-Mesallamy, H.O.; Hamdy, N.M.; Zaghloul, A.S.; Sallam, A.M.M. Clinical value of circulating lipocalins and insulin-like growth factor axis in pancreatic cancer diagnosis. Pancreas 2013, 42, 149–154. [Google Scholar] [CrossRef]
- Altinkaynak, K.; Bïlïcï, M.; Bakan, N.; Akçay, F. Circulating levels of IGF-I and IGFBP-3 in gastric cancer. Turkish J. Med. Sci. 2012, 42 (Suppl. 2), 1458–1462. [Google Scholar] [CrossRef]
- Hjortebjerg, R. IGFBP-4 and PAPP-A in normal physiology and disease. Growth Horm. IGF Res. 2018, 41, 7–22. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhu, S.; Yin, W.; Liu, X.; Hu, Y. IGFBP-4 expression is adversely associated with lung cancer prognosis. Oncol. Lett. 2017, 14, 6876–6880. [Google Scholar] [CrossRef]
- Mosig, R.A.; Lobl, M.; Senturk, E.; Shah, H.; Cohen, S.; Chudin, E.; Fruscio, R.; Marchini, S.; D’INcalci, M.; Sachidanandam, R.; et al. IGFBP-4 tumor and serum levels are increased across all stages of epithelial ovarian cancer. J. Ovarian Res. 2012, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.J.; Ball, M.; Rukhlova, M.; Slinn, J.; L’Abbe, D.; Iqbal, U.; Monette, R.; Hagedorn, M.; O’Connor-McCourt, M.D.; Durocher, Y.; et al. IGFBP-4 anti-angiogenic and anti-tumorigenic effects are associated with anti-cathepsin B activity. Neoplasia 2013, 15, 554–567. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, J.; Zhang, X.; Zhang, M.; Fu, Y. Comprehensive Analysis of IGFBPs as Biomarkers in Gastric Cancer. Front. Oncol. 2021, 11, 723131. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.J.; Dickson, K.A.; McDougall, F.; Baxter, R.C. Insulin-like growth factor-binding protein-5 inhibits the growth of human breast cancer cells in vitro and in vivo. J. Biol. Chem. 2003, 278, 29676–29685. [Google Scholar] [CrossRef]
- Meadows, K.A.; Holly, J.M.; Stewart, C.E.H. Tumor necrosis factor-α-induced apoptosis is associated with suppression of insulin-like growth factor binding protein-5 secretion in differentiating murine skeletal myoblasts. J. Cell. Physiol. 2000, 183, 330–337. [Google Scholar] [CrossRef]
- Waters, J.A.; Urbano, I.; Robinson, M.; House, C.D. Insulin-like growth factor binding protein 5: Diverse roles in cancer. Front. Oncol. 2022, 12, 1052457. [Google Scholar] [CrossRef]
- Conover, C.A.; Oxvig, C. PAPP-A and cancer. J. Mol. Endocrinol. 2018, 61, T1–T10. [Google Scholar] [CrossRef]
- Boldt, H.B.; Conover, C.A. Pregnancy-associated plasma protein-A (PAPP-A): A local regulator of IGF bioavailability through cleavage of IGFBPs. Growth Horm. IGF Res. 2007, 17, 10–18. [Google Scholar] [CrossRef]
- Mansfield, A.S.; Visscher, D.W.; Hart, S.N.; Wang, C.; Goetz, M.P.; Oxvig, C.; Conover, C.A. Pregnancy-associated plasma protein-A expression in human breast cancer. Growth Horm. IGF Res. 2014, 24, 264–267. [Google Scholar] [CrossRef]
- Pan, H.; Hanada, S.; Zhao, J.; Mao, L.; Ma, M.Z.-Q. Protein Secretion Is Required for Pregnancy-Associated Plasma Protein-A to Promote Lung Cancer Growth In Vivo. PLoS ONE 2012, 7, e48799. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.A.; Haluska, P., Jr.; Bale, L.K.; Oxvig, C.; Conover, C.A. A Novel Neutralizing Antibody Targeting Pregnancy-Associated Plasma Protein-A Inhibits Ovarian Cancer Growth and Ascites Accumulation in Patient Mouse Tumorgrafts. Mayo Clin. 2015, 14, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Conover, C.A. The IGF-p53 connection in cancer. Growth Horm. IGF Res. 2018, 39, 25–28. [Google Scholar] [CrossRef]
- Yu, S.; Zheng, S.; Qiu, J.; Lin, H.; Yu, X.; Chen, H.; Wu, J.; Wu, W.; Chen, J.; Chen, Y.; et al. Overexpression of metalloproteinase PAPPA accelerates cancer progression and correlates with immune cell infiltration in gastric cancer: Insights from bioinformatics and in vitro investigations. Cancer Cell Int. 2025, 25, 38. [Google Scholar] [CrossRef]
- Brahmkhatri, V.P.; Prasanna, C.; Atreya, H.S. Insulin-like growth factor system in cancer: Novel targeted therapies. BioMed Res. Int. 2015, 2015, 538019. [Google Scholar] [CrossRef]
- Lee, D.Y.; Yang, D.H.; Kang, C.W.; Kim, S.J.; Joo, C.U.; Cho, S.C.; Kim, J.S. Serum insulin-like growth factors (IGFs) and IGF binding protein (IGFBP)-3 in patients with gastric cancer: IGFBP-3 protease activity induced by surgery. J. Korean Med Sci. 1997, 12, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Franciosi, C.M.; Piacentini, M.G.; Conti, M.; Romano, F.; Musco, F.; Caprotti, R.; Rovelli, F.; Uggeri, F. IGF-1 and IGF-1BP3 in gastric adenocarcinoma. Preliminary study. Hepatogastroenterology 2003, 50, 297–300. [Google Scholar]
- Liu, H.; Gu, H.; Kutbi, E.H.; Tan, S.C.; Low, T.Y.; Zhang, C. Association of IGF-1 and IGFBP-3 levels with gastric cancer: A systematic review and meta-analysis. Int. J. Clin. Pract. 2021, 75, e14764. [Google Scholar] [CrossRef]
- Oxvig, C. The role of PAPP-A in the IGF system: Location, location, location. J. Cell Commun. Signal. 2015, 9, 177–187. [Google Scholar] [CrossRef]
- Ng, E.-H.; Ji, C.-Y.; Tan, P.-H.; Lin, V.; Soo, K.-C.; Lee, K.-O. Altered serum levels of insulin-like growth-factor binding proteins in breast cancer patients. Ann. Surg. Oncol. 1998, 5, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Nur, S.I.; Ozturk, A.; Kavas, M.; Bulut, I.; Alparslan, S.; Aydogan, E.S.; Atinkaya, B.C.; Kolay, M.; Coskun, A. Igfbp-4: A promising biomarker for lung cancer. J. Med. Biochem. 2021, 40, 237–244. [Google Scholar] [CrossRef]
- Yi, H.; Hwang, P.; Yang, D.-H.; Kang, C.-W.; Lee, D.-Y. Expression of the insulin-like growth factors (IGFs) and the IGF-binding proteins (IGFBPs) in human gastric cancer cells. Eur. J. Cancer 2001, 37, 2257–2263. [Google Scholar] [CrossRef] [PubMed]
- Baxter, R.C. Signaling Pathways of the Insulin-like Growth Factor Binding Proteins. Endocr. Rev. 2023, 44, 753–778. [Google Scholar] [CrossRef]
- Johnson, S.K.; Dennis, R.A.; Barone, G.W.; Lamps, L.W.; Haun, R.S. Differential Expression of Insulin-like Growth Factor Binding Protein-5 in Pancreatic Adenocarcinomas: Identification Using DNA Microarray. Mol. Carcinog. 2006, 45, 814–827. [Google Scholar] [CrossRef]
- Wang, H.; Arun, B.K.; Wang, H.; Fuller, G.N.; Zhang, W.; Middleton, L.P.; Sahin, A.A. IGFBP2 and IGFBP5 overexpression correlates with the lymph node metastasis in T1 breast carcinomas. Breast J. 2008, 14, 261–267. [Google Scholar] [CrossRef]
- Wang, S.; Hong, Q.; Geng, X.; Chi, K.; Cai, G.; Wu, D. Insulin-Like Growth Factor Binding Protein 5—A Probable Target of Kidney Renal Papillary Renal Cell Carcinoma. BioMed Res. Int. 2019, 2019, 3210324. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, Z.; Li, D.; Xu, J.; Zeng, Z. Gene expression and prognosis of insulin-like growth factor-binding protein family members in non-small cell lung cancer. Oncol. Rep. 2019, 42, 1981–1995. [Google Scholar] [CrossRef]
- Wang, J.; Ding, N.; Li, Y.; Cheng, H.; Wang, D.; Yang, Q.; Deng, Y.; Yang, Y.; Li, Y.; Ruan, X.; et al. Insulin-like growth factor binding protein 5 (IGFBP5) functions as a tumor suppressor in human melanoma cells. Oncotarget 2015, 6, 20636–20649. [Google Scholar] [CrossRef]
- Akkiprik, M.; Feng, Y.; Wang, H.; Chen, K.; Hu, L.; Sahin, A.; Krishnamurthy, S.; Ozer, A.; Hao, X.; Zhang, W. Multifunctional roles of insulin-like growth factor binding protein 5 in breast cancer. Breast Cancer Res. 2008, 10, 212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, W.; Cao, L.; Xu, J.; Qian, Y.; Chen, H.; Zhang, Y.; Kang, W.; Gou, H.; Wong, C.C.; et al. PKNOX2 suppresses gastric cancer through the transcriptional activation of IGFBP5 and p53. Oncogene 2019, 38, 4590–4604. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shen, S.; Yan, Z.; Yan, L.; Ding, H.; Wang, A.; Xu, Q.; Sun, L.; Yuan, Y. Expression characteristics and their functional role of IGFBP gene family in pan-cancer. BMC Cancer 2023, 23, 371. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.; Grabellus, F.; Ferrera, L.; Galietta, L.; Schwindenhammer, B.; Mühlenberg, T.; Taeger, G.; Eilers, G.; Treckmann, J.; Breitenbuecher, F.; et al. DOG1 regulates growth and IGFBP-5 in gastrointestinal stromal tumors. Cancer Res. 2013, 73, 3661–3670. [Google Scholar] [CrossRef]
- Bulut, I.; Gulcan, E.; Coskun, A.; Ciftci, A.; Cetinkaya, E.; Altiay, G.; Caglar, T. Relationship Between Pregnancy-Associated Plasma Protein-A and Lung Cancer. Clin. Investig. 2009, 337, 241–244. [Google Scholar] [CrossRef]
- Hjortebjerg, R.; Høgdall, C.; Hansen, K.H.; Høgdall, E.; Frystyk, J. The IGF—PAPP-A—Stanniocalcin Axis in Serum and Ascites Associates with Prognosis in Patients with Ovarian Cancer. Int. J. Mol. Sci. 2024, 25, 2014. [Google Scholar] [CrossRef]
- Lu, Y.; Li, S.; Wang, T.; Liao, X.; Mao, L.; Li, Z. PAPP-A functions as a tumor suppressor and is downregulated in renal cell carcinoma. FEBS Open Bio 2021, 11, 1593–1606. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Review Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Lin, T.-M.; Halbert, S.P.; Spellacy, W.N. Measurement of pregnancy-associated plasma proteins during human gestation. J. Clin. Investig. 1974, 54, 576–582. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Control (n = 40) | Cancer (n = 40) | |
|---|---|---|
| Median (IQR) ± SD | Median (IQR) ± SD | |
| IGF-1 (ng/mL) | 133.1 ± 56.0 (54.9–154.5) | 12.37 ± 50.4 (2.1–78.9) |
| IGFBP-4 (ng/mL) | 56.2 ± 53.1 (47.2–65.4) | 70.3 ± 77.0 (59.3–95.0) |
| IGFBP-5 (ng/mL) | 14.56 ± 4.2 (13.54–16.16) | 12.56 ± 2.0 (11.50–13.54) |
| PAPP-A (ng/mL) | 0.221 ± 0.03 (0.214–0.242) | 0.225 ± 0.016 (0.217–0.237) |
| Cancerous | Healthy | |
|---|---|---|
| n | 40 | 40 |
| Age, m (max–min) | 58 (45–65) | 53 (45–65) |
| Male/Female | 29/11 | 32/8 |
| Variable | n | Percentage (%) |
|---|---|---|
| Histology | ||
| Adenocarcinoma | 21 | 26.0 |
| Intestinal type | 2 | 19.5 |
| Diffuse type | 15 | 2.7 |
| Neuroendocrine tumor | 1 | 2.4 |
| Other | 1 | 2.4 |
| Stages | ||
| Stage IV | 14 | 35 |
| Stage III | 14 | 35 |
| Stage II | 11 | 27.5 |
| Stage I | 1 | 2.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceylaner, B.; Sahin, F.; Yildiz, A.; Tastekin, D.; Gok, A.F.K.; Baykal, A.T. IGF Pathway Components as Potential Biomarkers in Gastric Cancer. Int. J. Mol. Sci. 2025, 26, 10880. https://doi.org/10.3390/ijms262210880
Ceylaner B, Sahin F, Yildiz A, Tastekin D, Gok AFK, Baykal AT. IGF Pathway Components as Potential Biomarkers in Gastric Cancer. International Journal of Molecular Sciences. 2025; 26(22):10880. https://doi.org/10.3390/ijms262210880
Chicago/Turabian StyleCeylaner, Betul, Furkan Sahin, Anil Yildiz, Didem Tastekin, Ali Fuat Kaan Gok, and Ahmet Tarik Baykal. 2025. "IGF Pathway Components as Potential Biomarkers in Gastric Cancer" International Journal of Molecular Sciences 26, no. 22: 10880. https://doi.org/10.3390/ijms262210880
APA StyleCeylaner, B., Sahin, F., Yildiz, A., Tastekin, D., Gok, A. F. K., & Baykal, A. T. (2025). IGF Pathway Components as Potential Biomarkers in Gastric Cancer. International Journal of Molecular Sciences, 26(22), 10880. https://doi.org/10.3390/ijms262210880






