Cage-Farming Causes Histopathological Alterations in the Renal Tissues of the Rainbow Trout Oncorhynchus mykiss (Walbaum, 1792)
Abstract
1. Introduction
2. Results
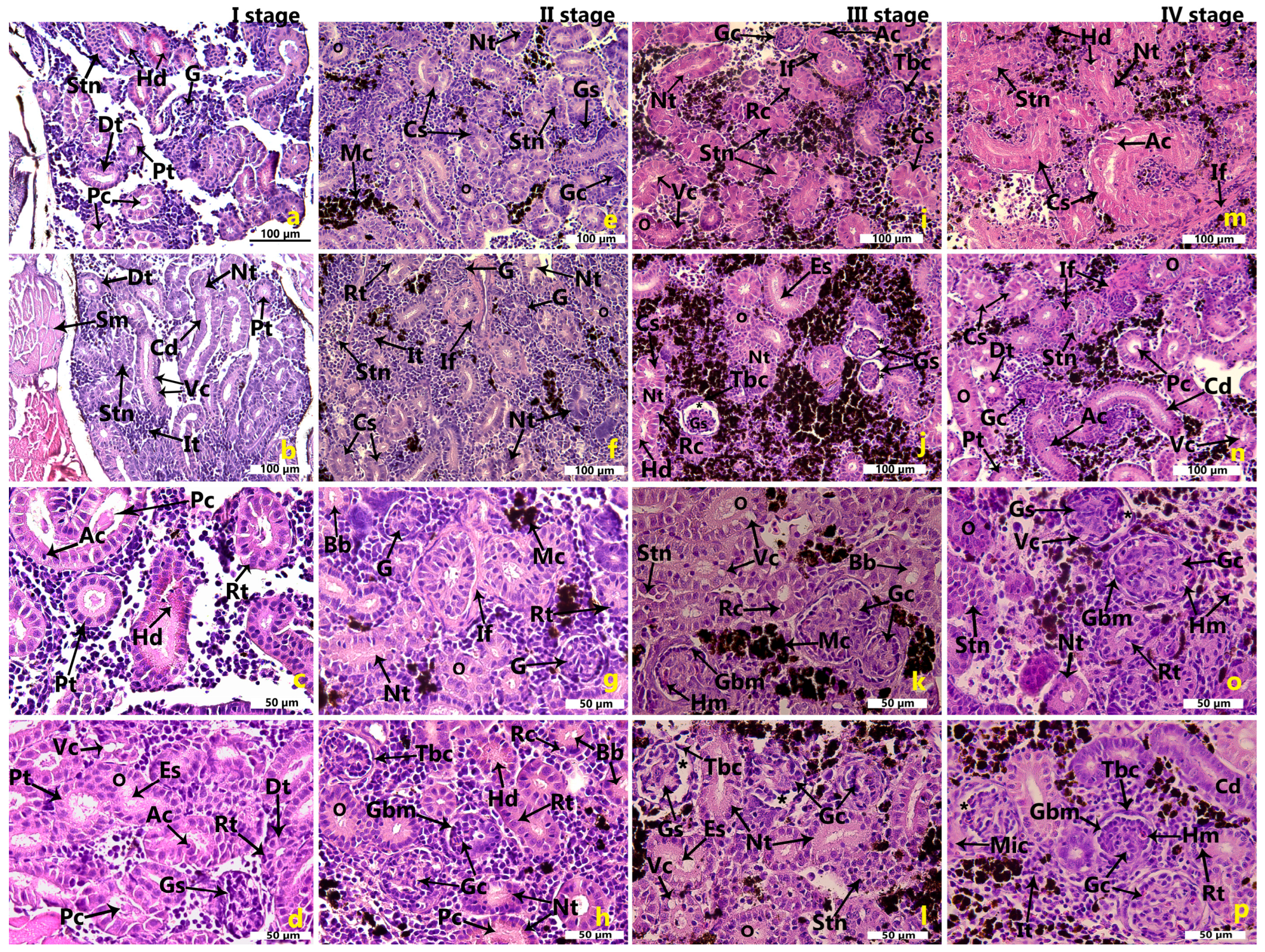
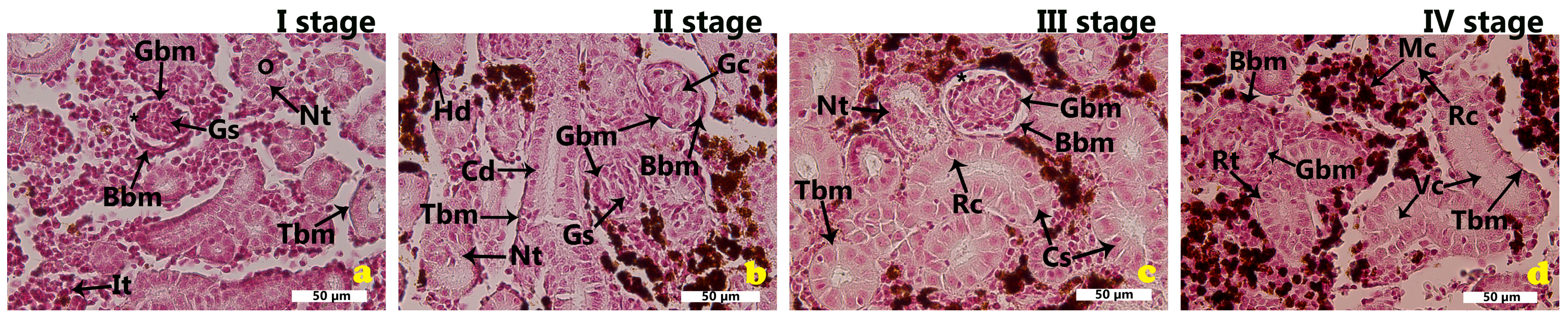
2.1. Morphological Features of the Renal Tissues in the Cage-Farmed Rainbow Trout During Different Growth Stages
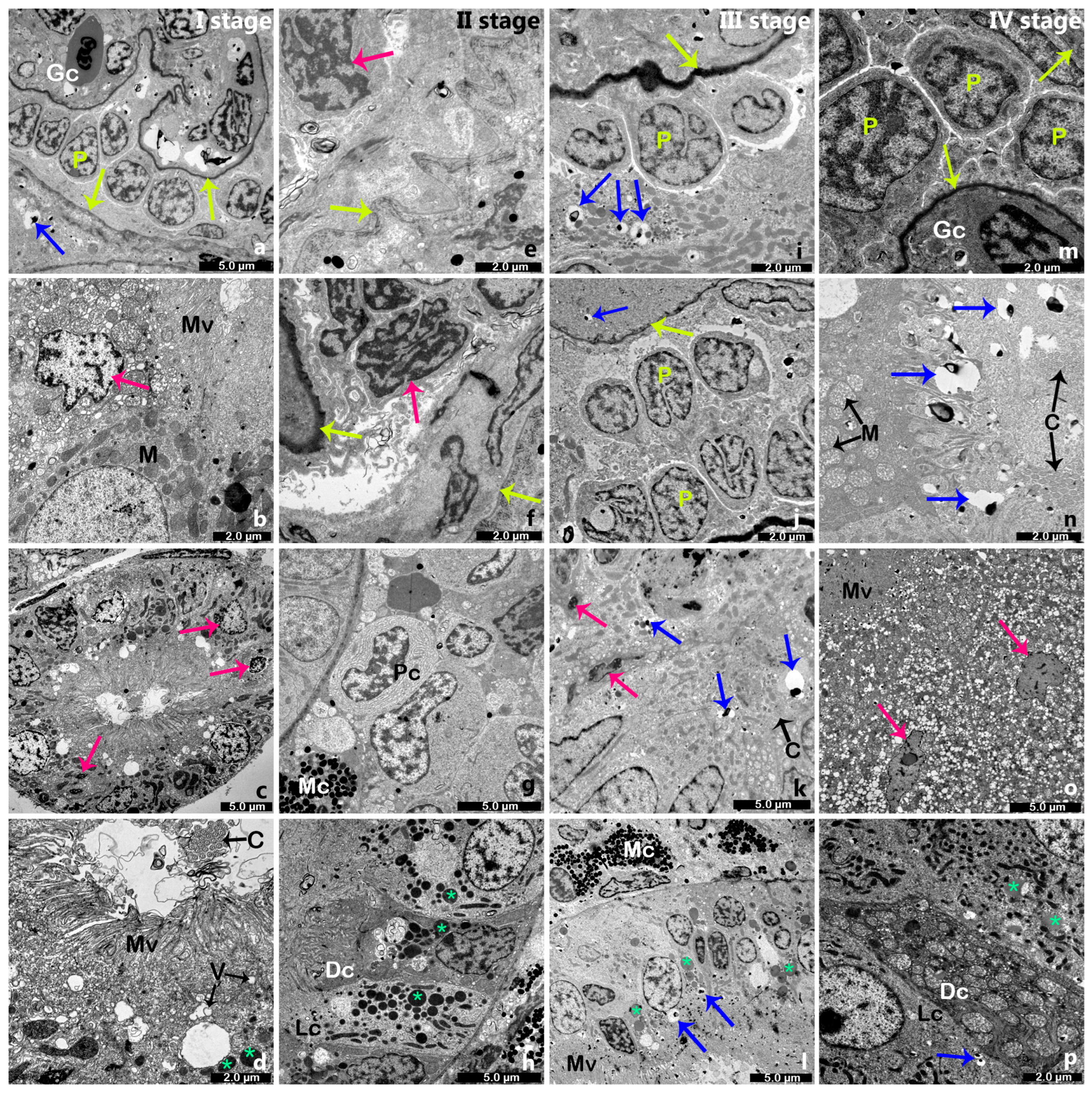
2.2. Electron Microscopy of the Renal Tissue in Cage-Farmed Rainbow Trout
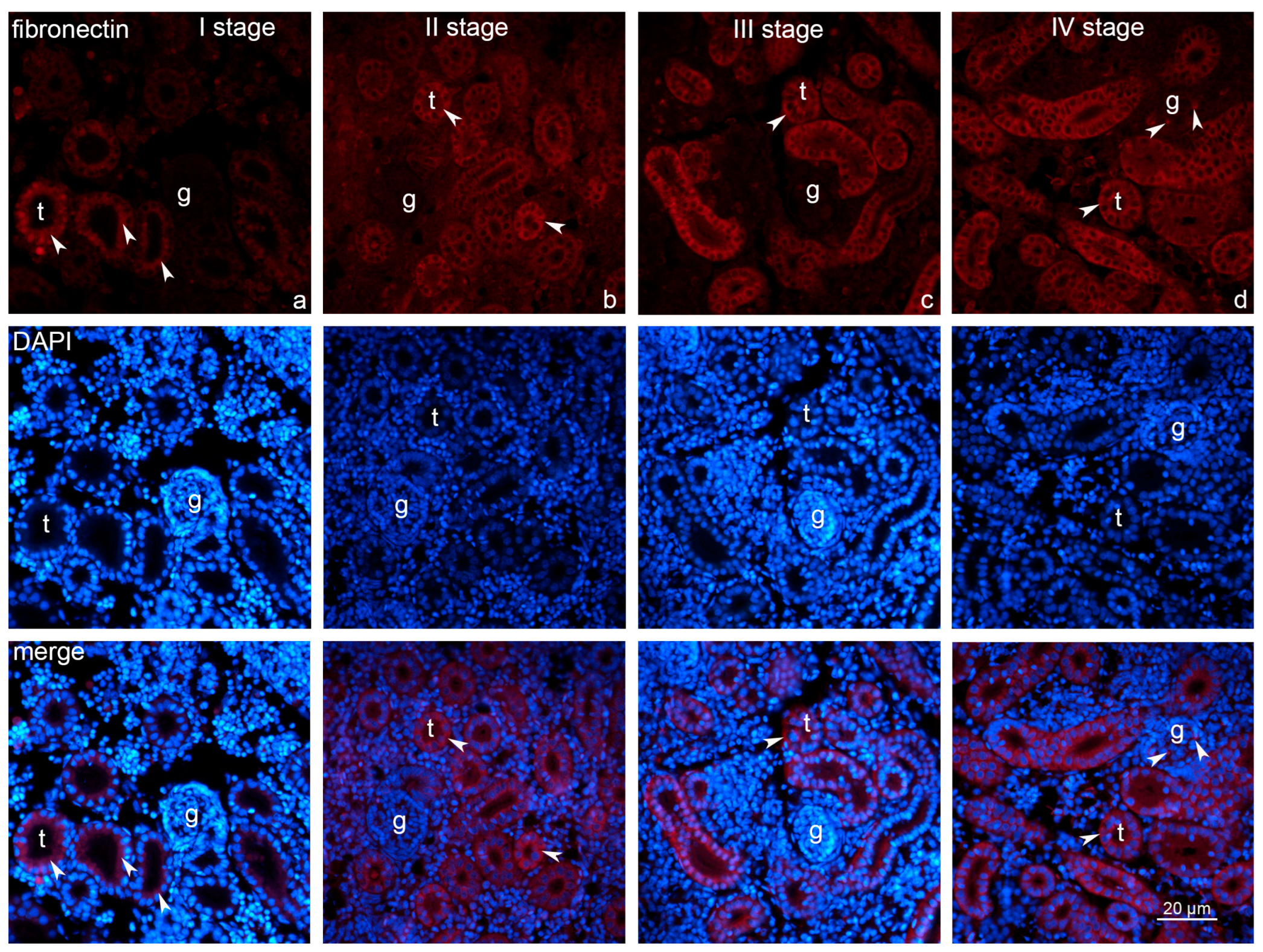
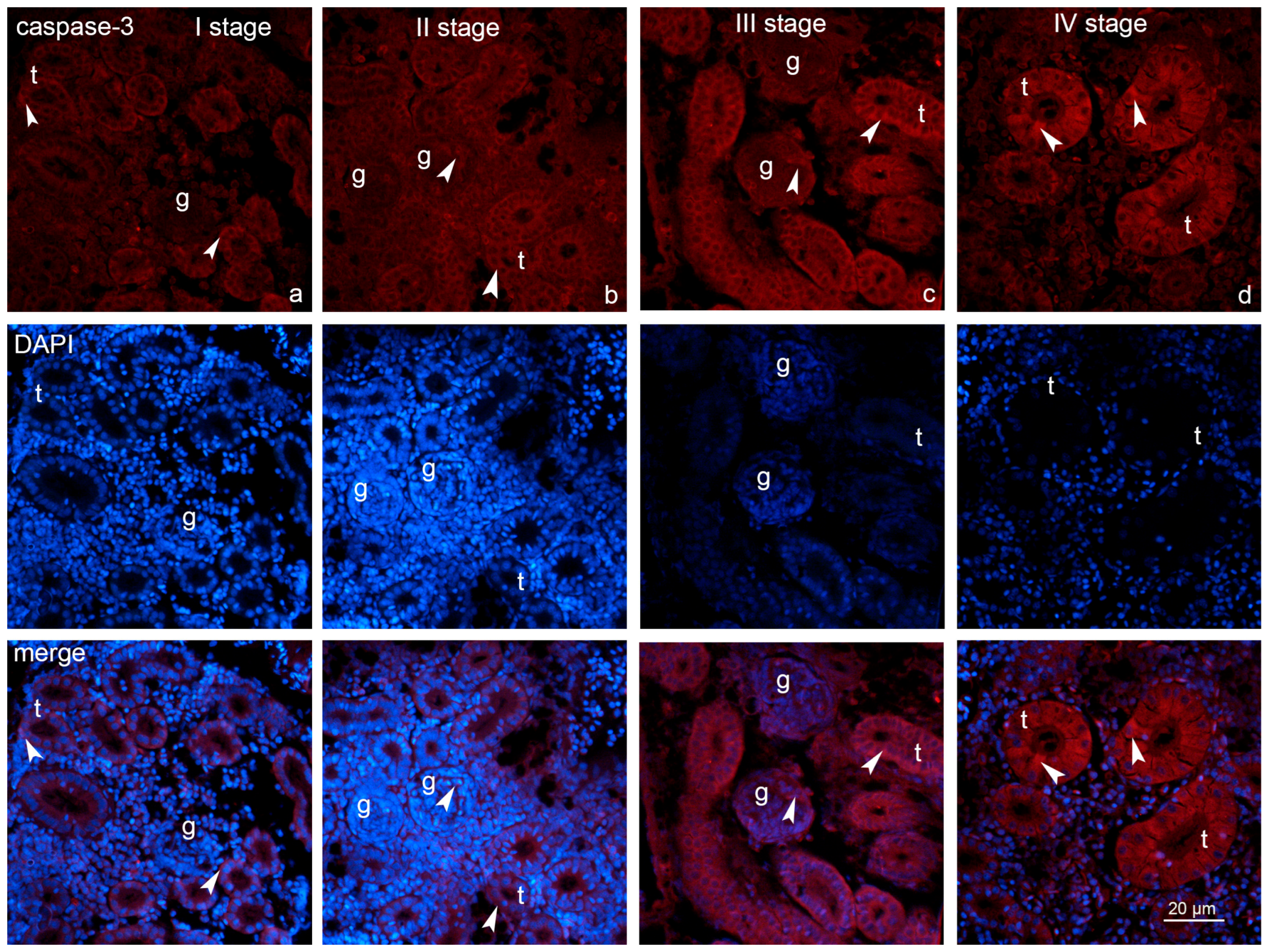
2.3. Expression of Fibronectin and Caspase-3
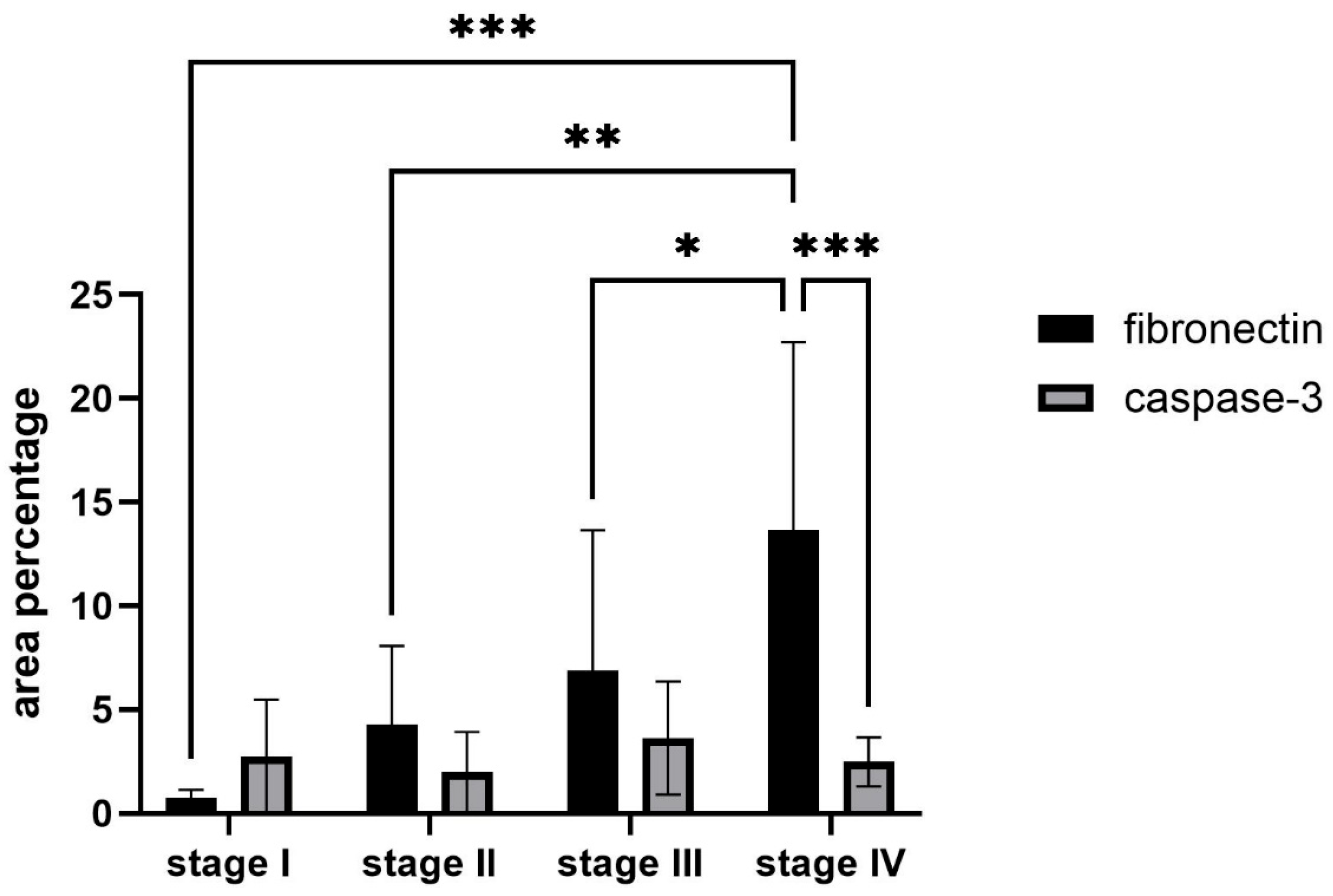
2.4. Statistical Analysis of the Fibronectin and Caspase-3 Measurements
2.5. Semi-Quantitative Analysis of the Morphological Outcomes of the Rainbow Trout Kidney
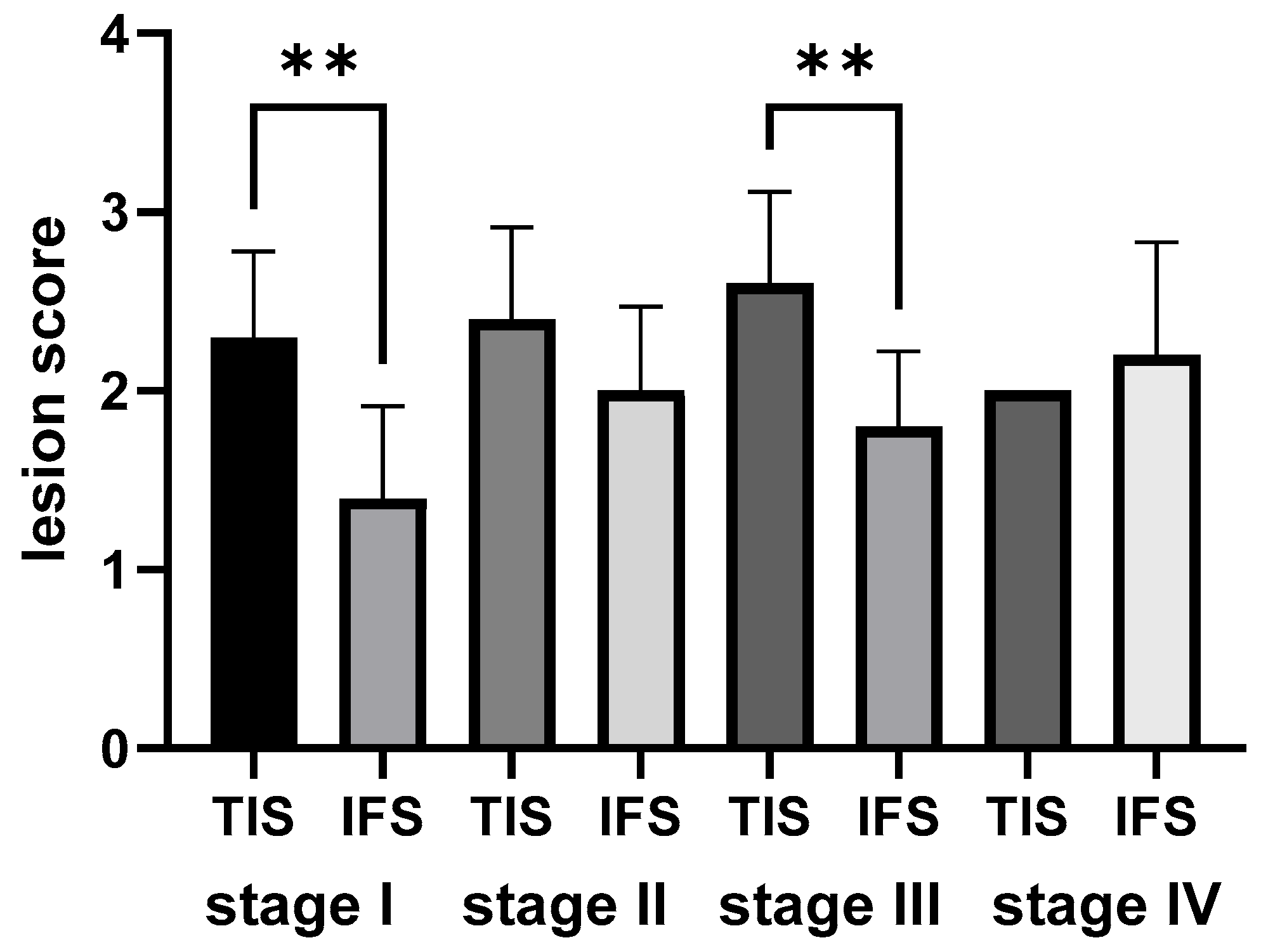
2.5.1. Semi-Quantitative Analysis of the Morphological Outcomes of the Rainbow Trout Kidney Within Each Growth Stage
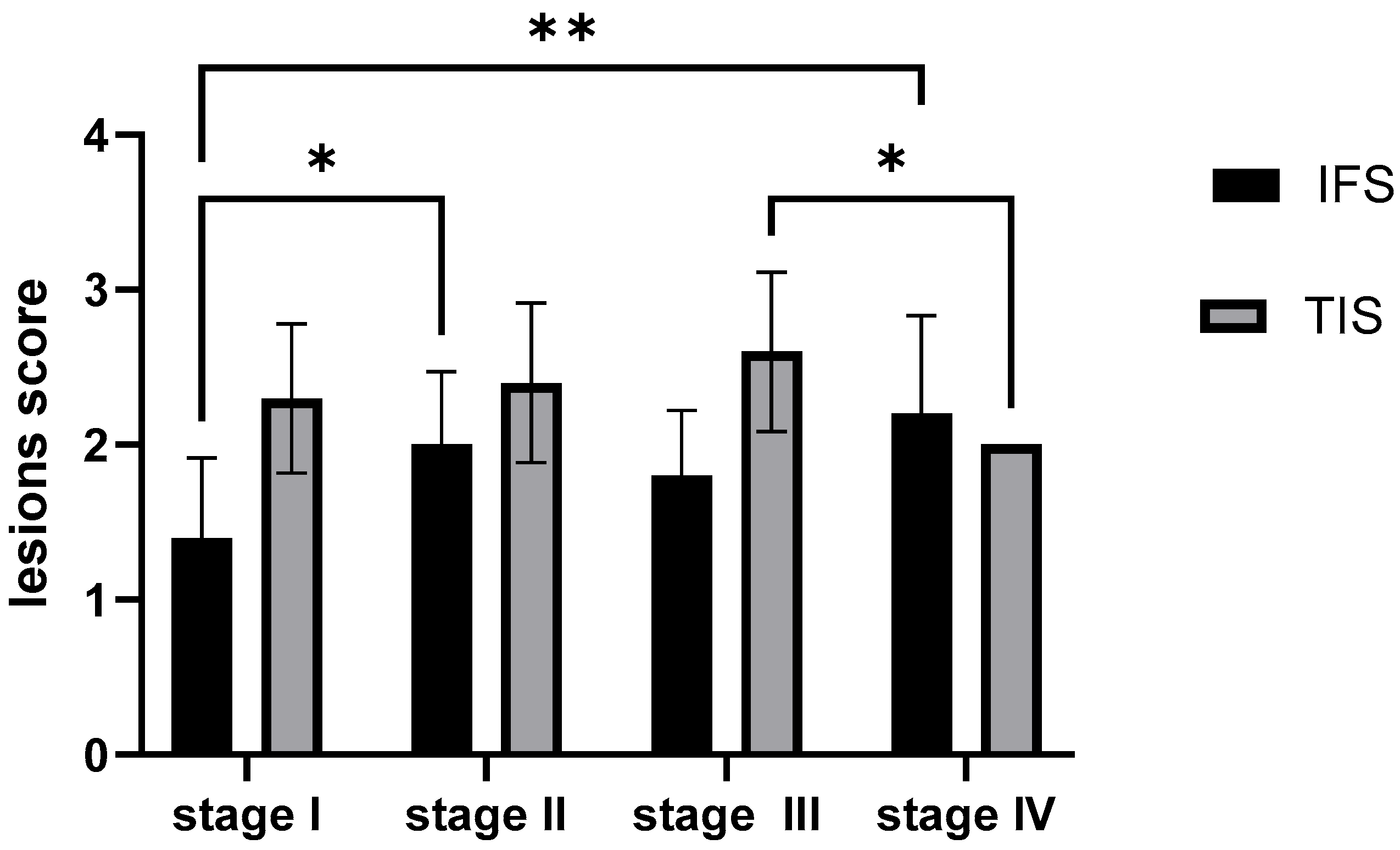
2.5.2. Semi-Quantitative Analysis of the Morphological Outcomes of the Rainbow Trout Kidney Across Four Growth Stages
3. Discussion
4. Materials and Methods
4.1. Collection of Samples
4.2. Renal Tissue Preparation
4.2.1. Hematoxylin–Eosin (H&E)
4.2.2. Periodic Acid Schiff—PAS
4.3. Immunofluorescence
4.4. Transmission Electron Microscopy
4.5. Statistical Analysis
4.5.1. Quantitative Analysis of the Fibronectin and Caspase-3 Expression
4.5.2. Semi-Quantitative Analysis of the Morphological Outcomes (Tubular Injury and Fibrosis)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2024—Blue Transformation in Action; FAO: Rome, Italy, 2024; p. 264. [Google Scholar]
- Boyd, C.E.; Tucker, C.S. Pond Aquaculture Water Quality Management; Springer: New York, NY, USA, 2011. [Google Scholar]
- Ashley, P.J. Fish welfare: Current issues in aquaculture. Appl. Anim. Behav. Sci. 2007, 104, 199–235. [Google Scholar] [CrossRef]
- Ellis, T.; Yildiz, H.Y.; López-Olmeda, J.; Spedicato, M.T.; Tort, L.; Øverli, Ø.; Martins, C.I. Cortisol and finfish welfare. Fish Physiol. Biochem. 2012, 38, 163–188. [Google Scholar] [CrossRef] [PubMed]
- Soto, D.; Aguilar-Manjarrez, J.; Hishamunda, N. Building an ecosystem approach to aquaculture. In Proceedings of the FAO/Universitat de les Illes Balears Expert Workshop, Palma de Mallorca, Spain, 7–11 May 2007; p. 221. [Google Scholar]
- Mwainge, V.; Caleb, O.; Aura, C.; Alice, M.; Veronica, O.; Nyaboke, H.; Ngoko, K.O.; Nyaundi, J. An overview of fish disease and parasite occurrence in the cage culture of Oreochromis niloticus: A case study in Lake Victoria, Kenya. Aquat. Ecosyst. Health Manag. 2021, 24, 43–55. [Google Scholar] [CrossRef]
- Öz, M.; Yavuz, O.; Bolukbas, F. Histopathology changes in the rainbow trout (Onchorhyncus mykiss) consuming boric acid supplemented fish fodder. J. Trace Elem. Med. Biol. 2020, 62, 126581. [Google Scholar] [CrossRef] [PubMed]
- Okwuosa, O.B.; Eyo, J.E.; Omovwohwovie, E.E. Role Of Fish as Bioindicators: A Review. IRE J. 2019, 2, 15. [Google Scholar]
- Camargo, M.; Martinez, C. Histopathology of gills, kidney and liver of a Neotropical fish caged in an urban stream. Neotrop. Ichthyol. 2007, 5, 327–336. [Google Scholar] [CrossRef]
- Shefat, S.; Karim, M. Nutritional Diseases of Fish in Aquaculture and Their Management: A Review. Acta Sci. Pharm. Sci. 2018, 2, 50–58. [Google Scholar]
- Zheng, Y.; Liu, J.; Xu, J.; Fan, H.; Wang, Y.; Zhuang, P.; Hu, M. Comparison of Artificial Feed and Natural Food by the Growth and Blood Biochemistry in Chinese Sturgeon Acipenser sinensis. Fishes 2023, 8, 45. [Google Scholar] [CrossRef]
- Idi-Ogede, A.; Adeshina, I.; Abdulraheem, I.; Abdulsalami, S. Histopathology of the gills, livers and kidney of Clarias gariepinus (Burchell, 1822) exposed to sniper 1000EC under laboratory conditions. Acta Biol. 2019, 26, 19–30. [Google Scholar] [CrossRef]
- Oğuz, A.R. A histological study of the kidney structure of Van fish (Alburnus tarichi) acclimated to highly alkaline water and freshwater. Mar. Freshw. Behav. Physiol. 2015, 48, 135–144. [Google Scholar] [CrossRef]
- Fiocchi, E.; Civettini, M.; Carbonara, P.; Zupa, W.; Lembo, G.; Manfrin, A. Development of molecular and histological methods to evaluate stress oxidative biomarkers in sea bass (Dicentrarchus labrax). Fish Physiol. Biochem. 2020, 46, 1577–1588. [Google Scholar] [CrossRef]
- Luft, F.C. Biomarkers and predicting acute kidney injury. Acta Physiol. 2021, 231, e13479. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wu, H. Chronic Kidney Disease in Diabetes: Biomarkers for Early Detection and Management. Int. J. Biol. Life Sci. 2024, 6, 23–27. [Google Scholar] [CrossRef]
- Cengiz, E.I. Gill and kidney histopathology in the freshwater fish Cyprinus carpio after acute exposure to deltamethrin. Environ. Toxicol. Pharmacol. 2006, 22, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Benli, A.C.; Köksal, G.; Ozkul, A. Sublethal ammonia exposure of Nile tilapia (Oreochromis niloticus L.): Effects on gill, liver and kidney histology. Chemosphere 2008, 72, 1355–1358. [Google Scholar] [CrossRef]
- Dey, B.; Abraham, T.J.; Singha, J.; Roy, A.; Karmakar, S.; Kumar Patil, P.; Roy, U. Histopathological changes and tissue residue concentrations of monosex Nile tilapia (Oreochromis niloticus, L) fries exposed to oxytetracycline. Aquac. Int. 2022, 30, 2113–2128. [Google Scholar] [CrossRef]
- Bardhan, A.; Abraham, T.J.; Singha, J.; Rajisha, R.; Krishna, E.K.N.; Panda, S.K.; Patil, P.K. Impacts of Oral Florfenicol Medication and Residues on the Kidney and Liver of Nile Tilapia Oreochromis niloticus (L.). Vet. Sci. 2023, 10, 36. [Google Scholar] [CrossRef]
- Velmurugan, B.; Selvanayagam, M.; Cengiz, E.I.; Unlu, E. The effects of monocrotophos to different tissues of freshwater fish Cirrhinus mrigala. Bull. Environ. Contam. Toxicol. 2007, 78, 450–454. [Google Scholar] [CrossRef]
- Dantzler, W. Comparative Physiology of the Vertebrate Kidney; Springer: New York, NY, USA, 2016; 292p. [Google Scholar]
- Fernandes, C.; Marcondes, S.; Galindo, G.; Franco-Belussi, L. Kidney anatomy, histology and histometric traits associated to renosomatic index in Gymnotus inaequilabiatus (Gymnotiformes: Gymnotidae). Neotrop. Ichthyol. 2019, 17, e190107. [Google Scholar] [CrossRef]
- Genten, F.; Terwinghe, E.; Danguy, A. Atlas of Fish Histology; CRC Press: Boca Raton, FL, USA, 2009; p. 215. [Google Scholar]
- Bjørgen, H.; Koppang, E.O. Anatomy of teleost fish immune structures and organs. Immunogenetics 2021, 73, 53–63. [Google Scholar] [CrossRef]
- Kamei, C.N.; Drummond, I.A. Zebrafish as a Model for Studying Kidney Regeneration. Curr. Pathobiol. Rep. 2014, 2, 53–59. [Google Scholar] [CrossRef][Green Version]
- Davidson, A.J. Uncharted waters: Nephrogenesis and renal regeneration in fish and mammals. Pediatr. Nephrol. 2011, 26, 1435–1443. [Google Scholar] [CrossRef]
- Davidson, A.J. Kidney regeneration in fish. Nephron Exp. Nephrol. 2014, 126, 45. [Google Scholar] [CrossRef]
- Hirashio, S.; Yamada, Y.; Mandai, K.; Hara, S.; Masaki, T. A Case of Fibronectin Glomerulopathy Caused by Missense Mutations in the Fibronectin 1 Gene. Kidney Int. Rep. 2017, 2, 969–972. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kalmár, T.; Jakab, D.; Maróti, Z.; Pásztor, G.; Turkevi-Nagy, S.; Kemény, É.; Hopfer, H.; Becker, J.U.; Bereczki, C.; Iványi, B. Fibronectin Glomerulopathy Without Typical Renal Biopsy Features in a 4-Year-Old Girl with Incidentally Discovered Proteinuria and a G417V FN1 Gene Mutation. Int. J. Mol. Sci. 2025, 26, 641. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, A.D. The Extracellular Matrix. In Encyclopedia of Cell Biology, 2nd ed.; Bradshaw, R.A., Hart, G.W., Stahl, P.D., Eds.; Academic Press: Oxford, UK, 2023; pp. 211–221. [Google Scholar]
- Skoczynski, K.; Kraus, A.; Daniel, C.; Büttner-Herold, M.; Amann, K.; Schiffer, M.; Hermann, K.; Herrnberger-Eimer, L.; Tamm, E.R.; Buchholz, B. The extracellular matrix protein fibronectin promotes metanephric kidney development. Pflug. Arch. 2024, 476, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-S.; Cheng, C.-C.; Chen, Y.-R.; Chen, F.-W.; Cheng, Y.-M.; Wang, J.-M. Epithelial CEBPD activates fibronectin and enhances macrophage adhesion in renal ischemia-reperfusion injury. Cell Death Discov. 2024, 10, 328. [Google Scholar] [CrossRef]
- Kaur, J.; Reinhardt, D. Extracellular Matrix (ECM) Molecules. In Stem Cell Biology and Tissue Engineering in Dental Sciences; Academic Press: Cambridge, MA, USA, 2015; pp. 25–43. [Google Scholar]
- Klemis, V.; Ghura, H.; Federico, G.; Würfel, C.; Bentmann, A.; Gretz, N.; Miyazaki, T.; Gröne, H.J.; Nakchbandi, I.A. Circulating fibronectin contributes to mesangial expansion in a murine model of type 1 diabetes. Kidney Int. 2017, 91, 1374–1385. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, M.; Zhang, Y. Identification of fibronectin 1 (FN1) and complement component 3 (C3) as immune infiltration-related biomarkers for diabetic nephropathy using integrated bioinformatic analysis. Bioengineered 2021, 12, 5386–5401. [Google Scholar] [CrossRef]
- Klair, N.; Mahmood, S.B.; El-Rifai, R.; Nast, C.C.; Bu, L.; Bregman, A. Fibronectin glomerulopathy in a kidney allograft biopsy. BMC Nephrol. 2023, 24, 359. [Google Scholar] [CrossRef]
- Humphreys, B.D. Mechanisms of Renal Fibrosis. Annu. Rev. Physiol. 2018, 80, 309–326. [Google Scholar] [CrossRef]
- Bowers, S.L.K.; Davis-Rodriguez, S.; Thomas, Z.M.; Rudomanova, V.; Bacon, W.C.; Beiersdorfer, A.; Ma, Q.; Devarajan, P.; Blaxall, B.C. Inhibition of fibronectin polymerization alleviates kidney injury due to ischemia-reperfusion. Am. J. Physiol. Ren. Physiol. 2019, 316, F1293–F1298. [Google Scholar] [CrossRef] [PubMed]
- Woroniecki, R.P.; Schnaper, H.W. Progression of glomerular and tubular disease in pediatrics. Semin. Nephrol. 2009, 29, 412–424. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tavares, M.B.; Chagas de Almeida Mda, C.; Martins, R.T.; de Sousa, A.C.; Martinelli, R.; dos-Santos, W.L. Acute tubular necrosis and renal failure in patients with glomerular disease. Ren. Fail. 2012, 34, 1252–1257. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chang, J.; Yao, B.; Niu, A.; Kelly, E.; Breeggemann, M.C.; Abboud Werner, S.L.; Harris, R.C.; Zhang, M.Z. Proximal tubule-derived colony stimulating factor-1 mediates polarization of renal macrophages and dendritic cells, and recovery in acute kidney injury. Kidney Int. 2015, 88, 1274–1282. [Google Scholar] [CrossRef]
- Erekat, N.S. Programmed Cell Death in Diabetic Nephropathy: A Review of Apoptosis, Autophagy, and Necroptosis. Med. Sci. Monit. 2022, 28, e937766. [Google Scholar] [CrossRef]
- Jing, Z.; Chen, Q.; Yan, C.; Zhang, C.; Xu, Z.; Huang, X.; Wu, J.; Li, Y.; Yang, S. Effects of Chronic Heat Stress on Kidney Damage, Apoptosis, Inflammation, and Heat Shock Proteins of Siberian Sturgeon (Acipenser baerii). Animals 2023, 13, 3733. [Google Scholar] [CrossRef]
- Miao, N.; Wang, B.; Xu, D.; Wang, Y.; Gan, X.; Zhou, L.; Xue, H.; Zhang, W.; Wang, X.; Lu, L. Caspase-11 promotes cisplatin-induced renal tubular apoptosis through a caspase-3-dependent pathway. Am. J. Physiol. Ren. Physiol. 2018, 314, F269–F279. [Google Scholar] [CrossRef]
- Hussar, P. Apoptosis Regulators Bcl-2 and Caspase-3. Encyclopedia 2022, 2, 1624–1636. [Google Scholar] [CrossRef]
- Martin-Sanchez, D.; Poveda, J.; Fontecha-Barriuso, M.; Ruiz-Andres, O.; Sanchez-Niño, M.D.; Ruiz-Ortega, M.; Ortiz, A.; Sanz, A.B. Targeting of regulated necrosis in kidney disease. Nefrologia 2018, 38, 125–135. [Google Scholar] [CrossRef]
- Gobe, G. Identification of apoptosis in kidney tissue sections. Methods Mol. Biol. 2009, 466, 175–192. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, S. From Apoptosis to Regulated Necrosis: An Evolving Understanding of Acute Kidney Injury. In Current Understanding of Apoptosis-Programmed Cell Death; Tutar, Y., Tutar, L., Eds.; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar]
- Liu, Q.; Lei, Z.; Guo, J.; Liu, A.; Lu, Q.; Fatima, Z.; Khaliq, H.; Shabbir, M.A.B.; Maan, M.K.; Wu, Q.; et al. Mequindox-Induced Kidney Toxicity Is Associated with Oxidative Stress and Apoptosis in the Mouse. Front. Pharmacol. 2018, 9, 436. [Google Scholar] [CrossRef]
- Wen, S.; Wang, Z.H.; Zhang, C.X.; Yang, Y.; Fan, Q.L. Caspase-3 Promotes Diabetic Kidney Disease Through Gasdermin E-Mediated Progression to Secondary Necrosis During Apoptosis. Diabetes Metab. Syndr. Obes. 2020, 13, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Lan, S.; Dieudé, M.; Sabo-Vatasescu, J.P.; Karakeussian-Rimbaud, A.; Turgeon, J.; Qi, S.; Gunaratnam, L.; Patey, N.; Hébert, M.J. Caspase-3 Is a Pivotal Regulator of Microvascular Rarefaction and Renal Fibrosis after Ischemia-Reperfusion Injury. J. Am. Soc. Nephrol. 2018, 29, 1900–1916. [Google Scholar] [CrossRef] [PubMed]
- Clementi, A.; Virzì, G.M.; Manani, S.M.; de Cal, M.; Battaglia, G.G.; Ronco, C.; Zanella, M. Plasma Cell-Free DNA and Caspase-3 Levels in Patients with Chronic Kidney Disease. J. Clin. Med. 2023, 12, 5616. [Google Scholar] [CrossRef]
- Brown, J.A.; Taylor, S.M.; Gray, C.J. Glomerular ultrastructure of the trout, Salmo gairdneri. Cell Tissue Res. 1983, 230, 205–218. [Google Scholar] [CrossRef]
- Anderson, B.G.; Loewen, R.D. Renal morphology of freshwater trout. Am. J. Anat. 1975, 143, 93–114. [Google Scholar] [CrossRef]
- Koc, N.D. The comparative research of kidneys of rainbow trout (Oncorhynchus mykiss, Walbaum 1792) and rat (Rattus norvegicus, Berkenhout 1769) in morphometric and histologic manner. Pak. J. Biol. Sci. 2006, 9, 445–447. [Google Scholar] [CrossRef][Green Version]
- Ferguson, H.W. Systemic Pathology of Fish: A Text and Atlas of Normal Tissues in Teleosts and Their Responses in Disease, 2nd ed.; Scotia Press: London, UK, 2006. [Google Scholar][Green Version]
- Hassan, M.; Melad, A.A.N.; Zakariah, M.I.; Yusoff, N.A.H. Histopathological Alterations in Gills, Liver and Kidney of African Catfish (Clarias gariepinus, Burchell 1822) Exposed to Melaleuca cajuputi Extract. Trop. Life Sci. Res. 2023, 34, 177–196. [Google Scholar] [CrossRef]
- Yancheva, V.; Georgieva, E.; Velcheva, I.; Iliev, I.; Stoyanova, S.; Vasileva, T.; Bivolarski, V.; Todorova-Bambaldokova, D.; Zulkipli, N.; Antal, L.; et al. Assessment of the exposure of two pesticides on common carp (Cyprinus carpio Linnaeus, 1758): Are the prolonged biomarker responses adaptive or destructive? Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 261, 109446. [Google Scholar] [CrossRef]
- Dorlikar, A.; Mohite, A. The effect of cypermethrin (25% ec) on histology of kidney of Ophiocephalus striatus. Int. J. Entomol. Res. 2019, 4, 31–35. [Google Scholar]
- Burlaka, I.; Nilsson, L.M.; Scott, L.; Holtbäck, U.; Eklöf, A.C.; Fogo, A.B.; Brismar, H.; Aperia, A. Prevention of apoptosis averts glomerular tubular disconnection and podocyte loss in proteinuric kidney disease. Kidney Int. 2016, 90, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Abraham, T.J.; Bora, M.; Bardhan, A.; Sen, A.; Das, R.; Nadella, R.K.; Patil, P.K. In-feed oxolinic acid induces oxidative stress and histopathological alterations in Nile tilapia Oreochromis niloticus. Toxicol. Rep. 2025, 14, 102020. [Google Scholar] [CrossRef] [PubMed]
- Bojarski, B.; Kot, B.; Witeska, M. Antibacterials in Aquatic Environment and Their Toxicity to Fish. Pharmaceuticals 2020, 13, 189. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, S.; Abdulmotalib, J.; Al-Rudainy, A.; Mohammad, S. Effect of environmental pollutants on fish health: An overview. Pharmaceuticals 2024, 13, 189. [Google Scholar] [CrossRef]
- Chevalier, R.L. The proximal tubule is the primary target of injury and progression of kidney disease: Role of the glomerulotubular junction. Am. J. Physiol. Ren. Physiol. 2016, 311, F145–F161. [Google Scholar] [CrossRef]
- Liu, B.C.; Tang, T.T.; Lv, L.L.; Lan, H.Y. Renal tubule injury: A driving force toward chronic kidney disease. Kidney Int. 2018, 93, 568–579. [Google Scholar] [CrossRef]
- Deane, J.A.; Ricardo, S.D. Emerging roles for renal primary cilia in epithelial repair. Int. Rev. Cell Mol. Biol. 2012, 293, 169–193. [Google Scholar] [CrossRef]
- Rabah, S.O. Acute Taxol nephrotoxicity: Histological and ultrastructural studies of mice kidney parenchyma. Saudi J. Biol. Sci. 2010, 17, 105–114. [Google Scholar] [CrossRef]
- McConnachie, D.J.; Stow, J.L.; Mallett, A.J. Ciliopathies and the Kidney: A Review. Am. J. Kidney Dis. 2021, 77, 410–419. [Google Scholar] [CrossRef]
- Dell, K.M. The role of cilia in the pathogenesis of cystic kidney disease. Curr. Opin. Pediatr. 2015, 27, 212–218. [Google Scholar] [CrossRef]
- Fitzsimons, L.A. An Emerging Role for Primary Cilia of the Renal Glomerulus: Implications and Considerations for Pathogenesis of Glomerular Diseases. In Cell Communication and Signaling in Health and Disease; Heinbockel, T., Ed.; IntechOpen: Rijeka, Croatia, 2024. [Google Scholar]
- Negron, J.F.; Lockshin, R.A. Activation of apoptosis and caspase-3 in zebrafish early gastrulae. Dev. Dyn. 2004, 231, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Sawant, P.B.; Bera, A.; Dasgupta, S.; Sawant, B.T.; Chadha, N.K.; Pal, A.K. p53 Dependent Apoptotic Cell Death Induces Embryonic Malformation in Carassius auratus under Chronic Hypoxia. PLoS ONE 2014, 9, e102650. [Google Scholar] [CrossRef] [PubMed]
- Trimarchi, H. Mechanisms of Podocyte Detachment, Podocyturia, and Risk of Progression of Glomerulopathies. Kidney Dis. 2020, 6, 324–329. [Google Scholar] [CrossRef]
- Sanders, P.W.; Booker, B.B.; Bishop, J.B.; Cheung, H.C. Mechanisms of intranephronal proteinaceous cast formation by low molecular weight proteins. J. Clin. Investig. 1990, 85, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Djudjaj, S.; Boor, P. Cellular and molecular mechanisms of kidney fibrosis. Mol. Asp. Med. 2019, 65, 16–36. [Google Scholar] [CrossRef]
- Panizo, S.; Martínez-Arias, L.; Alonso-Montes, C.; Cannata, P.; Martín-Carro, B.; Fernández-Martín, J.L.; Naves-Díaz, M.; Carrillo-López, N.; Cannata-Andía, J.B. Fibrosis in Chronic Kidney Disease: Pathogenesis and Consequences. Int. J. Mol. Sci. 2021, 22, 408. [Google Scholar] [CrossRef]
- Li, S.; Livingston, M.J.; Ma, Z.; Hu, X.; Wen, L.; Ding, H.F.; Zhou, D.; Dong, Z. Tubular cell senescence promotes maladaptive kidney repair and chronic kidney disease after cisplatin nephrotoxicity. JCI Insight 2023, 8, e166643. [Google Scholar] [CrossRef]
- Reimschuessel, R. A fish model of renal regeneration and development. ILAR J. 2001, 42, 285–291. [Google Scholar] [CrossRef]
- Gewin, L.S. Renal fibrosis: Primacy of the proximal tubule. Matrix Biol. 2018, 68–69, 248–262. [Google Scholar] [CrossRef]
- Guo, C.; Cui, Y.; Jiao, M.; Yao, J.; Zhao, J.; Tian, Y.; Dong, J.; Liao, L. Crosstalk between proximal tubular epithelial cells and other interstitial cells in tubulointerstitial fibrosis after renal injury. Front. Endocrinol. 2023, 14, 1256375. [Google Scholar] [CrossRef] [PubMed]
- Bülow, R.D.; Boor, P. Extracellular Matrix in Kidney Fibrosis: More Than Just a Scaffold. J. Histochem. Cytochem. 2019, 67, 643–661. [Google Scholar] [CrossRef] [PubMed]
- Tandon, A.; Singh, N.N.; Brave, V.R.; Sreedhar, G. Image analysis assisted study of mitotic figures in oral epithelial dysplasia and squamous cell carcinoma using differential stains. J. Oral Biol. Craniofac. Res. 2016, 6, S18–S23. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kim, H.S.; Moon, K.C. Primary renal well-differentiated neuroendocrine tumors: Report of six cases with an emphasis on the Ki-67 index and mitosis. Diagn. Pathol. 2019, 14, 12. [Google Scholar] [CrossRef]
- Suhasini, P.D.; Mulki, S.; Supriya, H. Apoptotic and mitotic indices in oral epithelial dysplasia and squamous cell carcinoma: A comparative study. J. Oral Maxillofac. Pathol. 2022, 26, 598. [Google Scholar] [CrossRef]
- Mantle, P.; Upadhyay, R.; Herman, D.; Batuman, V. Renal Apoptosis in Male Rats Induced by Extensive Dietary Exposure to Ochratoxins. Int. J. Mol. Sci. 2025, 26, 4553. [Google Scholar] [CrossRef]
- Kurosaka, K.; Takahashi, M.; Watanabe, N.; Kobayashi, Y. Silent cleanup of very early apoptotic cells by macrophages. J. Immunol. 2003, 171, 4672–4679. [Google Scholar] [CrossRef]
- Miao, X.; Wu, X.; You, W.; He, K.; Chen, C.; Pathak, J.L.; Zhang, Q. Tailoring of apoptotic bodies for diagnostic and therapeutic applications:advances, challenges, and prospects. J. Transl. Med. 2024, 22, 810. [Google Scholar] [CrossRef]
- Lan, S.; Yang, B.; Migneault, F.; Turgeon, J.; Bourgault, M.; Dieudé, M.; Cardinal, H.; Hickey, M.J.; Patey, N.; Hébert, M.J. Caspase-3-dependent peritubular capillary dysfunction is pivotal for the transition from acute to chronic kidney disease after acute ischemia-reperfusion injury. Am. J. Physiol. Ren. Physiol. 2021, 321, F335–F351. [Google Scholar] [CrossRef]
- Parrish, A.B.; Freel, C.D.; Kornbluth, S. Cellular mechanisms controlling caspase activation and function. Cold Spring Harb. Perspect. Biol. 2013, 5, a008672. [Google Scholar] [CrossRef]
- Baena-Lopez, L.A.; Wang, L.; Wendler, F. Cellular stress management by caspases. Curr. Opin. Cell Biol. 2024, 86, 102314. [Google Scholar] [CrossRef]
- Cheng, P.; Andersen, P.; Hassel, D.; Kaynak, B.L.; Limphong, P.; Juergensen, L.; Kwon, C.; Srivastava, D. Fibronectin mediates mesendodermal cell fate decisions. Development 2013, 140, 2587–2596. [Google Scholar] [CrossRef]
- Patten, J.; Wang, K. Fibronectin in development and wound healing. Adv. Drug Deliv. Rev. 2021, 170, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Fornaro, M.; Plescia, J.; Chheang, S.; Tallini, G.; Zhu, Y.M.; King, M.; Altieri, D.C.; Languino, L.R. Fibronectin protects prostate cancer cells from tumor necrosis factor-alpha-induced apoptosis via the AKT/survivin pathway. J. Biol. Chem. 2003, 278, 50402–50411. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.L.; Lin, H.T.; Chen, Y.W.; Chen, Y.C.; Tsai, F.T.; Chang, Y.L.; Chiou, S.H.; Sheu, D.C.; Ku, H.H. Fibronectin suppresses lipopolysaccharide-induced liver damage and promotes the cytoprotection abilities of hepatocyte-like cells derived from human bone marrow mesenchymal stem cells. Transplant. Proc. 2007, 39, 3444–3445. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Chen, L.; Zhuang, Y.; Han, Y.; Tang, W.; Xia, F. Fibronectin 1 inhibits the apoptosis of human trophoblasts by activating the PI3K/Akt signaling pathway. Int. J. Mol. Med. 2020, 46, 1908–1922. [Google Scholar] [CrossRef]
- Dhanani, K.C.H.; Samson, W.J.; Edkins, A.L. Fibronectin is a stress responsive gene regulated by HSF1 in response to geldanamycin. Sci. Rep. 2017, 7, 17617. [Google Scholar] [CrossRef]
- Kliewe, F.; Kaling, S.; Lötzsch, H.; Artelt, N.; Schindler, M.; Rogge, H.; Schröder, S.; Scharf, C.; Amann, K.; Daniel, C.; et al. Fibronectin is up-regulated in podocytes by mechanical stress. FASEB J. 2019, 33, 14450–14460. [Google Scholar] [CrossRef]
- Liu, X.Y.; Liu, R.X.; Hou, F.; Cui, L.J.; Li, C.Y.; Chi, C.; Yi, E.; Wen, Y.; Yin, C.H. Fibronectin expression is critical for liver fibrogenesis in vivo and in vitro. Mol. Med. Rep. 2016, 14, 3669–3675. [Google Scholar] [CrossRef]
- Eskandari, E.; Eaves, C.J. Paradoxical roles of caspase-3 in regulating cell survival, proliferation, and tumorigenesis. J. Cell Biol. 2022, 221, e202201159. [Google Scholar] [CrossRef]
- Mokhtar, D.M.; Abdelhafez, E.A. An overview of the structural and functional aspects of immune cells in teleosts. Histol. Histopathol. 2021, 36, 399–414. [Google Scholar] [CrossRef] [PubMed]
- Urbinati, E.; Zanuzzo, F.; Biller, J. Stress and immune system in fish. In Biology and Physiology of Freshwater Neotropical Fish; Academic Press: Cambridge, MA, USA, 2020; pp. 93–114. [Google Scholar]
- Salkova, E.; Gela, D.; Pecherkova, P.; Flajshans, M. Examination of white blood cell indicators for three different ploidy level sturgeon species reared in an indoor recirculation aquaculture system for one year. Vet. Med. 2022, 67, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Levy-Pereira, N.; Carriero, M.M.; Maganha, S.R.L.; Meira, C.M.; Lázaro, T.M.; Rocha, N.R.A.; Maia, A.A.M.; Wiegertjes, G.; Fernandes, A.M.; Sousa, R.L.M. Phagocytotic activity and gene expression of leukocytes isolated from Astyanax lacustris. Braz. J. Biol. 2023, 83, e264570. [Google Scholar] [CrossRef] [PubMed]
- Ghielli, M.; Verstrepen, W.; Nouwen, E.; De Broe, M.E. Regeneration processes in the kidney after acute injury: Role of infiltrating cells. Exp. Nephrol. 1998, 6, 502–507. [Google Scholar] [CrossRef]
- Cowled, P.; Fitridge, R. Pathophysiology of Reperfusion Injury. In Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists; Fitridge, R., Thompson, M., Eds.; University of Adelaide Press: Adelaide, Australia, 2011. [Google Scholar]
- Kaur, A.; Naveenkumar, B.; Sood, N.; Holeyappa, S. Blood cells morphology of fish as a diagnostic and species identification marker in aquaculture. J. Exp. Zool. India 2019, 22, 987–993. [Google Scholar]
- Pirarat, N.; Maita, M.; Endo, M.; Katagiri, T. Lymphoid apoptosis in Edwardsiella tarda septicemia in tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2007, 22, 608–616. [Google Scholar] [CrossRef]
- Dounousi, E.; Koliousi, E.; Papagianni, A.; Ioannou, K.; Zikou, X.; Katopodis, K.; Kelesidis, A.; Tsakiris, D.; Siamopoulos, K.C. Mononuclear leukocyte apoptosis and inflammatory markers in patients with chronic kidney disease. Am. J. Nephrol. 2012, 36, 531–536. [Google Scholar] [CrossRef]
- Breda, P.C.; Wiech, T.; Meyer-Schwesinger, C.; Grahammer, F.; Huber, T.; Panzer, U.; Tiegs, G.; Neumann, K. Renal proximal tubular epithelial cells exert immunomodulatory function by driving inflammatory CD4(+) T cell responses. Am. J. Physiol. Ren. Physiol. 2019, 317, F77–F89. [Google Scholar] [CrossRef]
- Cristofori, P.; Defazio, R.; Chiusolo, A.; Mongillo, M.; Bartolucci, G.B.; Chiara, F.; Trevisan, A. Hyaline droplet accumulation in kidney of rats treated with hexachloro-1:3-butadiene: Influence of age, dose and time-course. J. Appl. Toxicol. 2013, 33, 183–189. [Google Scholar] [CrossRef]
- Zhao, G.D.; Gao, R.; Hou, X.T.; Zhang, H.; Chen, X.T.; Luo, J.Q.; Yang, H.F.; Chen, T.; Shen, X.; Yang, S.C.; et al. Endoplasmic Reticulum Stress Mediates Renal Tubular Vacuolation in BK Polyomavirus-Associated Nephropathy. Front. Endocrinol. 2022, 13, 834187. [Google Scholar] [CrossRef]
- Han, X.Q.; Cui, Z.W.; Ma, Z.Y.; Wang, J.; Hu, Y.Z.; Li, J.; Ye, J.M.; Tafalla, C.; Zhang, Y.A.; Zhang, X.J. Phagocytic Plasma Cells in Teleost Fish Provide Insights into the Origin and Evolution of B Cells in Vertebrates. J. Immunol. 2024, 213, 730–742. [Google Scholar] [CrossRef]
- Manera, M. Rodlet Cell Morpho–Numerical Alterations as Key Biomarkers of Fish Responses to Toxicants and Environmental Stressors. Toxics 2024, 12, 832. [Google Scholar] [CrossRef]
- Reite, O.B. The rodlet cells of teleostean fish: Their potential role in host defence in relation to the role of mast cells/eosinophilic granule cells. Fish Shellfish Immunol. 2005, 19, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Bielek, E. Rodlet cells in teleosts: New ultrastructural observations on the distribution of the cores in trout (Oncorhynchus mykiss, Salmo trutta L.). J. Submicrosc. Cytol. Pathol. 2002, 34, 271–278. [Google Scholar]
- Bielek, E. Membrane transformations in degenerating rodlet cells in fishes of two teleostean families (Salmonidae, Cyprinidae). Anat. Rec. 2008, 291, 1693–1706. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.; Nowak, B. Effect of ranching time on melanomacrophage centres in anterior kidney and spleen of Southern bluefin tuna, Thunnus maccoyii. Fish Shellfish Immunol. 2016, 59, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Fogo, A.B.; Harris, R.C. Crosstalk between glomeruli and tubules. Nat. Rev. Nephrol. 2025, 21, 189–199. [Google Scholar] [CrossRef]
- Sugiyama, H.; Kashihara, N.; Makino, H.; Yamasaki, Y.; Ota, A. Apoptosis in glomerular sclerosis. Kidney Int. 1996, 49, 103–111. [Google Scholar] [CrossRef]
- Appel, D.; Kershaw, D.B.; Smeets, B.; Yuan, G.; Fuss, A.; Frye, B.; Elger, M.; Kriz, W.; Floege, J.; Moeller, M.J. Recruitment of podocytes from glomerular parietal epithelial cells. J. Am. Soc. Nephrol. 2009, 20, 333–343. [Google Scholar] [CrossRef]
- Liu, B.C.; Tang, T.T.; Lv, L.L. How Tubular Epithelial Cell Injury Contributes to Renal Fibrosis. Adv. Exp. Med. Biol. 2019, 1165, 233–252. [Google Scholar] [CrossRef]
- Cao, X.; Wang, J.; Zhang, T.; Liu, Z.; Liu, L.; Chen, Y.; Li, Z.; Zhao, Y.; Yu, Q.; Liu, T.; et al. Chromatin accessibility dynamics dictate renal tubular epithelial cell response to injury. Nat. Commun. 2022, 13, 7322. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.L.; Li, X.Y.; Zhou, Y.; Wang, B.; Lv, L.L.; Liu, B.C. Renal tubular epithelial cells response to injury in acute kidney injury. eBioMedicine 2024, 107, 105294. [Google Scholar] [CrossRef] [PubMed]
- Schnaper, H.W. The Tubulointerstitial Pathophysiology of Progressive Kidney Disease. Adv. Chronic Kidney Dis. 2017, 24, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Lozic, M.; Filipovic, N.; Juric, M.; Kosovic, I.; Benzon, B.; Solic, I.; Kelam, N.; Racetin, A.; Watanabe, K.; Katsuyama, Y.; et al. Alteration of Cx37, Cx40, Cx43, Cx45, Panx1, and Renin Expression Patterns in Postnatal Kidneys of Dab1-/- (yotari) Mice. Int. J. Mol. Sci. 2021, 22, 1284. [Google Scholar] [CrossRef]
- Restović, I.; Vučemilo, M.; Obad, M.; Kević, N.; Kelam, N.; Racetin, A.; Bočina, I. Expression of dendrin, neurofilament and glial fibrillary acidic protein in the brain of the dogfish Scyliorhinus canicula L. Period. Biol. 2023, 125, 43–55. [Google Scholar] [CrossRef]
- Clifton-Hadley, R.; Bucke, D.; Richards, R. A study of the sequential clinical and pathological changes during proliferative kidney disease in rainbow trout, Salmo gairdneri Richardson. J. Fish Dis. 2006, 10, 335–352. [Google Scholar] [CrossRef]
- Bettge, K.; Wahli, T.; Segner, H.; Schmidt-Posthaus, H. Proliferative kidney disease in rainbow trout: Time- and temperature-related renal pathology and parasite distribution. Dis. Aquat. Organ. 2009, 83, 67–76. [Google Scholar] [CrossRef]
- Meyerholz, D.K.; Beck, A.P. Fundamental Concepts for Semiquantitative Tissue Scoring in Translational Research. ILAR J. 2018, 59, 13–17. [Google Scholar] [CrossRef]
- Bailey, C.; Strepparava, N.; Ros, A.; Wahli, T.; Schmidt-Posthaus, H.; Segner, H.; Tafalla, C. It’s a hard knock life for some: Heterogeneity in infection life history of salmonids influences parasite disease outcomes. J. Anim. Ecol. 2021, 90, 2573–2593. [Google Scholar] [CrossRef]
| Stage I | Stage II | Stage III | Stage IV | |
|---|---|---|---|---|
| Age (months) | 1 | 11 | 13 | 18 |
| Avg. length (cm) | 3.885 | 15.33 | 21.68 | 27.78 |
| Avg. weight (g) | 0.468 | 34.45 | 102.09 | 226.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ugrin, M.; Fernandez Godoy, M.; Restović, I.; Hrabar, J.; Kević, N.; Bočina, I. Cage-Farming Causes Histopathological Alterations in the Renal Tissues of the Rainbow Trout Oncorhynchus mykiss (Walbaum, 1792). Int. J. Mol. Sci. 2025, 26, 10876. https://doi.org/10.3390/ijms262210876
Ugrin M, Fernandez Godoy M, Restović I, Hrabar J, Kević N, Bočina I. Cage-Farming Causes Histopathological Alterations in the Renal Tissues of the Rainbow Trout Oncorhynchus mykiss (Walbaum, 1792). International Journal of Molecular Sciences. 2025; 26(22):10876. https://doi.org/10.3390/ijms262210876
Chicago/Turabian StyleUgrin, Marina, María Fernandez Godoy, Ivana Restović, Jerko Hrabar, Nives Kević, and Ivana Bočina. 2025. "Cage-Farming Causes Histopathological Alterations in the Renal Tissues of the Rainbow Trout Oncorhynchus mykiss (Walbaum, 1792)" International Journal of Molecular Sciences 26, no. 22: 10876. https://doi.org/10.3390/ijms262210876
APA StyleUgrin, M., Fernandez Godoy, M., Restović, I., Hrabar, J., Kević, N., & Bočina, I. (2025). Cage-Farming Causes Histopathological Alterations in the Renal Tissues of the Rainbow Trout Oncorhynchus mykiss (Walbaum, 1792). International Journal of Molecular Sciences, 26(22), 10876. https://doi.org/10.3390/ijms262210876









