Gestational Diabetes Mellitus Alters Cytokine Profiles and Macrophage Polarization in Human Placenta
Abstract
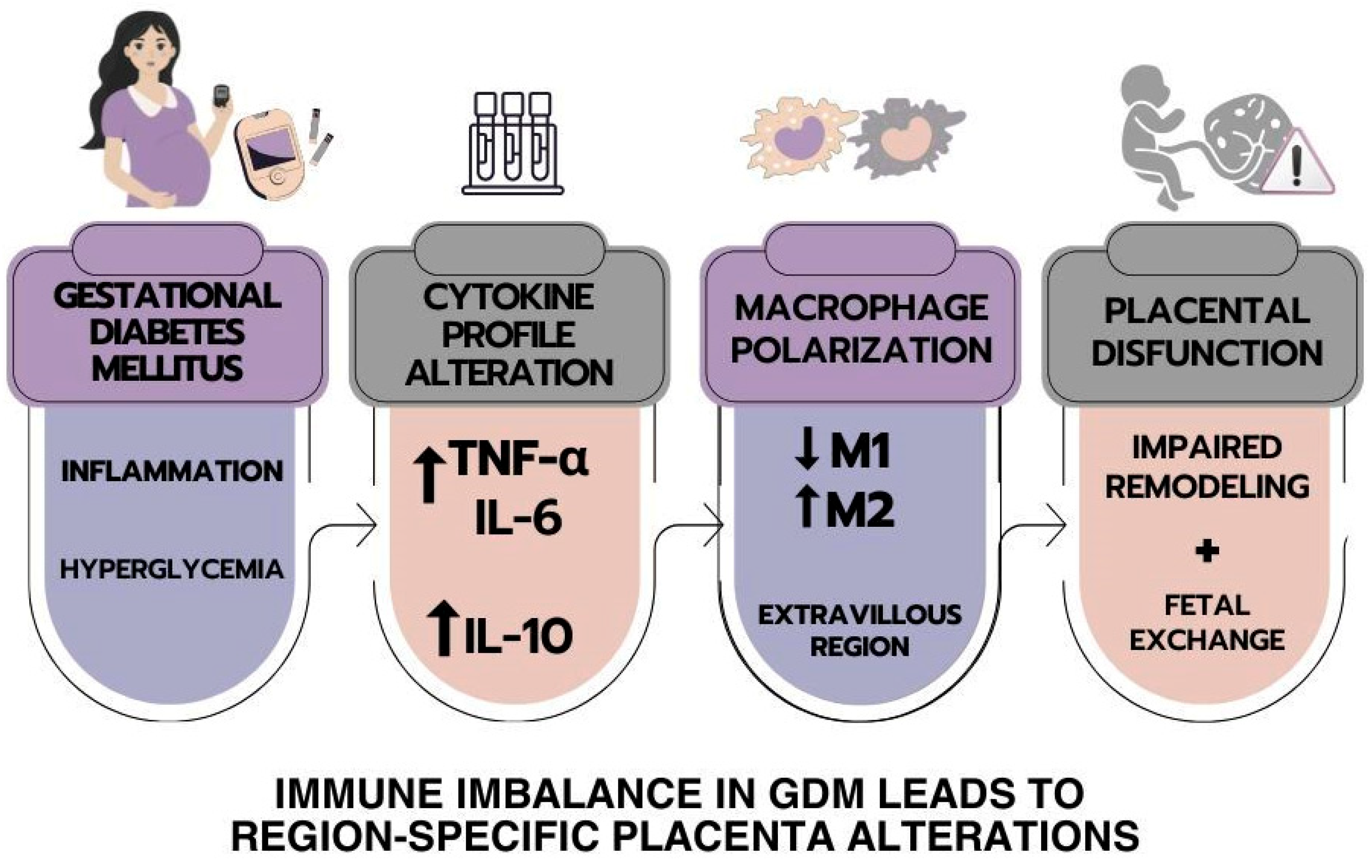
1. Introduction
2. Results
2.1. Clinical Characteristics of Pregnant Women and Newborns
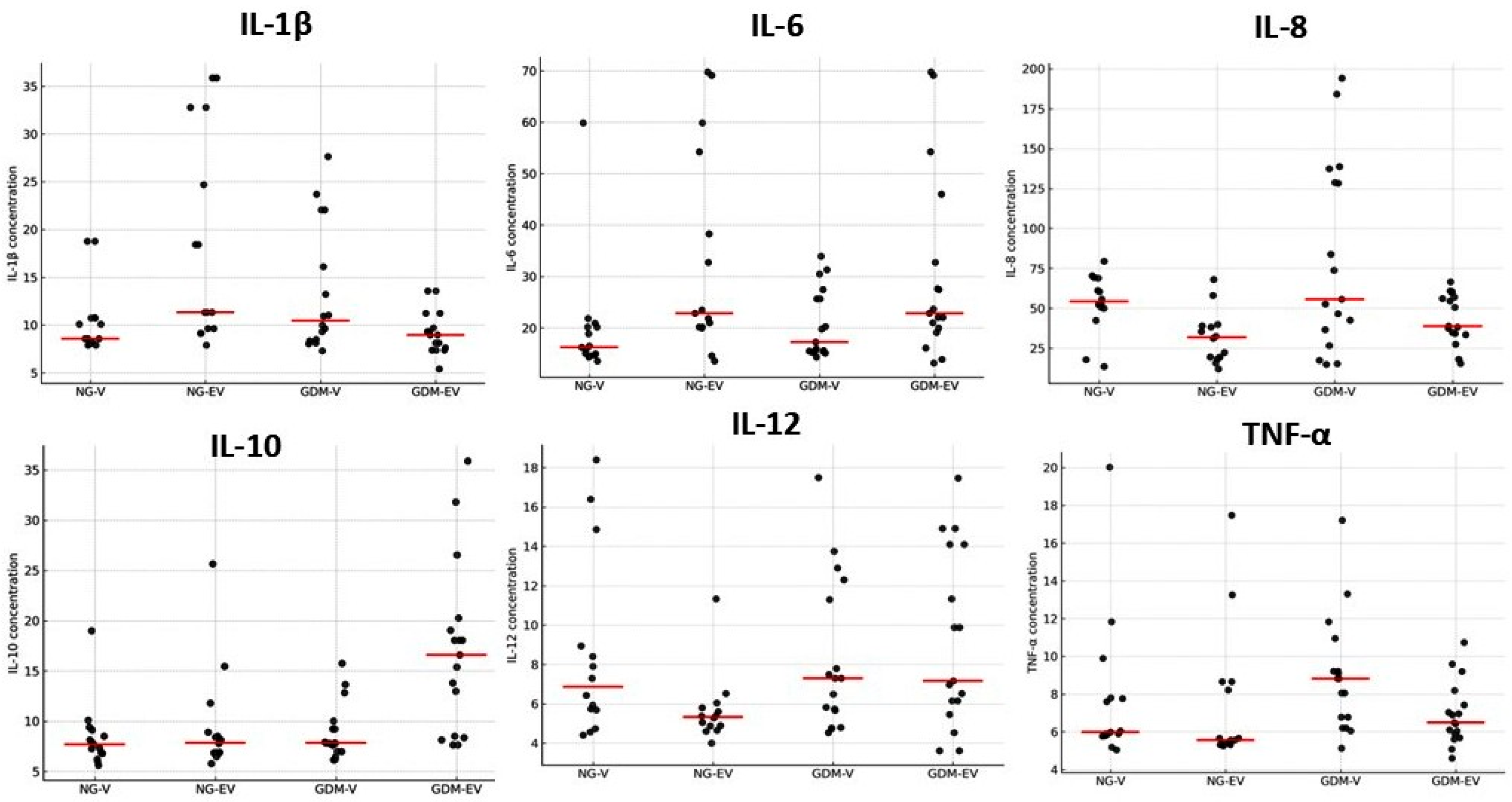
2.2. Placental Cytokines
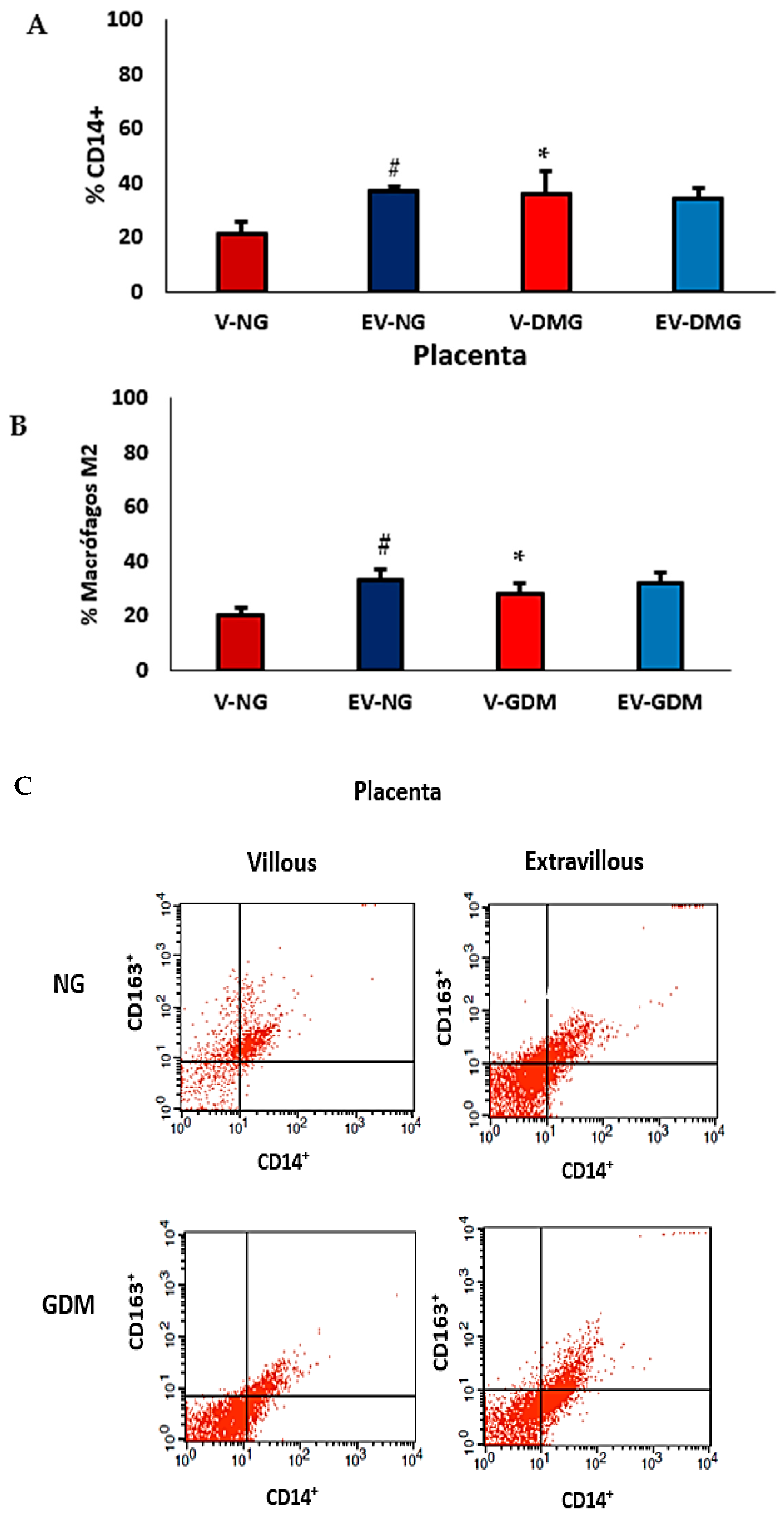
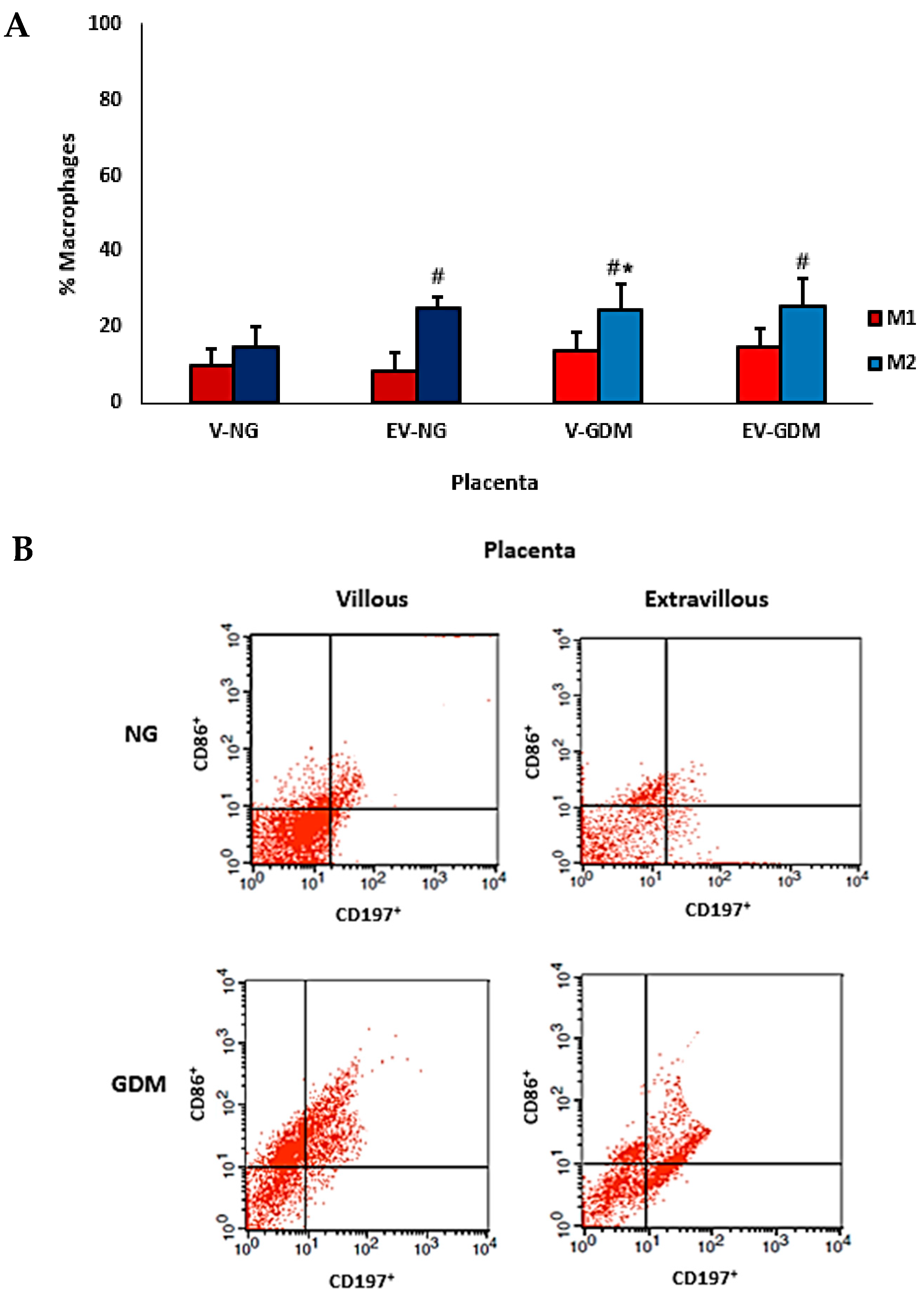
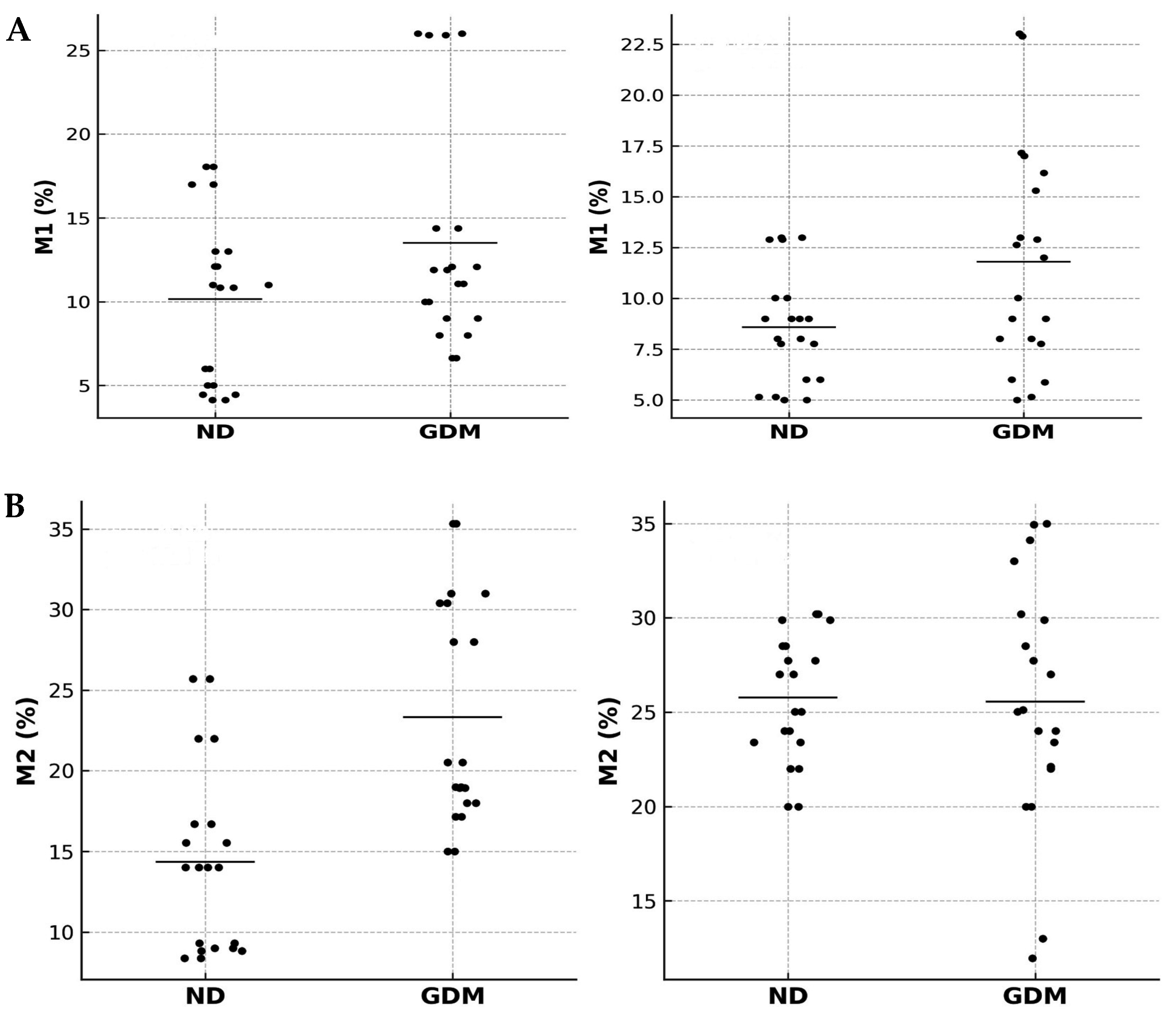
2.3. Immunophenotyping and Placental Macrophage Identification and Polarization
3. Discussion
4. Materials and Methods
4.1. Study and Subjects
4.2. Subject Follow-Up and Characterization
4.3. Placenta Sampling and Preparation of Macrophages
4.4. Quantification of Cytokines
4.5. Immunophenotyping Macrophage Identification and Polarization
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stetting, A.; Wong, J.; Murphy, H.R.; Ross, G.P. A clinical update on gestational diabetes mellitus. Endocr. Rev. 2022, 43, 763–793. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: A global perspective. Curr. Diabetes Rep. 2016, 16, 7. [Google Scholar] [CrossRef]
- Mitanchez, D.; Yzydorczyk, C.; Siddeek, B.; Boubred, F.; Benahmed, M.; Simeoni, U. Perinatal complications associated with pregestational and gestational diabetes. Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 213–224. [Google Scholar] [CrossRef]
- Hara, C.C.P.; França, E.L.; Fagundes, D.L.G.; Queiroz, A.A.; Rudge, M.V.C.; Honorio-França, A.C.; Paranhos Calderon, I.M.P. Characterization of natural killer cells and cytokines in maternal placenta and fetus of diabetic mothers. J. Immunol. Res. 2016, 2016, 7154524. [Google Scholar] [CrossRef]
- Fagundes, D.L.G.; França, E.L.; Gonzatti, M.B.; Rudge, M.V.C.; Calderon, I.M.; Honorio-França, A.C. The modulatory role of cytokines IL-4 and IL-17 in the functional activity of phagocytes in diabetic pregnant women. APMIS 2018, 126, 56–64. [Google Scholar] [CrossRef]
- Tauber, Z.; Burianova, A.; Koubova, K.; Mrstik, M.; Jirkovska, M.; Cizkova, K. The interplay of inflammation and placenta in maternal diabetes: Insights into Hofbauer cell expression patterns. Front. Immunol. 2024, 15, 1386528. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Jiang, W.; Yin, Z.; Li, N.; Tong, C.; Qi, H. Mechanistic study of preeclampsia and macrophage-associated molecular networks: Bioinformatics insights from multiple datasets. Front. Genet. 2024, 15, 1376971. [Google Scholar] [CrossRef]
- Bezemer, R.E.; Faas, M.; van Goor, H.; Gordijn, S.J.; Prins, J. Decidual macrophages and Hofbauer cells in fetal growth restriction. Front. Immunol. 2024, 15, 1379537. [Google Scholar] [CrossRef]
- Szukiewicz, D. Insights into reproductive immunology and placental lipid–cytokine interplay in macrosomia: A narrative review. Int. J. Mol. Sci. 2024, 25, 12135. [Google Scholar] [CrossRef]
- Holder, B.S.; Tower, C.L.; Forbes, K.; Mulla, M.J.; Aplin, J.D.; Abrahams, V.M. Macrophage function in normal and diabetic pregnancies. J. Reprod. Immunol. 2019, 132, 21–29. [Google Scholar]
- Cobo, T.; Kacerovsky, M.; Jacobsson, B. Placental histopathological findings in gestational diabetes mellitus. Placenta 2020, 98, 13–20. [Google Scholar] [CrossRef]
- Fierro, J.J.; Velásquez, M.; Cadavid, A.P.; de Leeuw, K. Effects of anti-beta 2-glycoprotein 1 antibodies and its association with pregnancy-related morbidity in antiphospholipid syndrome. Am. J. Reprod. Immunol. 2022, 87, e13509. [Google Scholar] [CrossRef]
- Knöfler, M.; Haider, S.; Saleh, L.; Pollheimer, J.; Gamage, T.K.J.B.; James, J. Human placenta and trophoblast development: Key molecular mechanisms and model systems. Cell Mol. Life Sci. 2019, 76, 3479–3496. [Google Scholar] [CrossRef]
- Olmos-Ortiz, A.; Flores-Espinosa, P.; Díaz, L.; Velázquez, P.; Ramírez-Isarraraz, C.; Zaga-Clavellina, V. Immunoendocrine Dysregulation during Gestational Diabetes Mellitus: The Central Role of the Placenta. Int. J. Mol. Sci. 2021, 22, 8087. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, F.; Peng, Y.; Chen, R.; Zhou, W.; Wang, H.; OuYang, J.; Yu, B.; Xu, Z. Transcriptomic profiling of human placenta in gestational diabetes mellitus at the single-cell level. Front. Endocrinol. 2021, 12, 679582. [Google Scholar] [CrossRef]
- Cui, S.S.; Zhang, P.; Sun, L.; Yuan, Y.L.L.; Wang, J.; Zhang, F.X.; Li, R. Mucin1 induced trophoblast dysfunction in gestational diabetes mellitus via Wnt/β-catenin signaling pathway. Biol. Res. 2023, 56, 48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.N.; Wu, Q.; Deng, Y.H. Phenotypic characterisation of regulatory T cells in patients with gestational diabetes mellitus. Sci. Rep. 2024, 14, 4881. [Google Scholar] [CrossRef]
- Zgutka, K.; Tkacz, M.; Tomasiak, P.; Piotrowska, K.; Ustianowski, P.; Pawlik, A.; Tarnowski, M. Gestational diabetes mellitus-induced inflammation in the placenta via IL-1β and Toll-like receptor pathways. Int. J. Mol. Sci. 2024, 25, 11409. [Google Scholar] [CrossRef]
- Schliefsteiner, C.; Peinhaupt, M.; Kopp, S.; Lögl, J.; Lang-Olip, I.; Hiden, U.; Heinemann, A.; Desoye, G.; Wadsack, C. Human placental Hofbauer cells maintain an anti-inflammatory M2 phenotype despite the presence of gestational diabetes mellitus. Front. Immunol. 2017, 8, 888. [Google Scholar] [CrossRef]
- França, D.C.H.; Honorio-França, A.C.; Silva, K.M.R.; Alves, F.C.B.; Bueno, G.; Costa, S.M.B.; Cotrim, A.C.D.M.; Barbosa, A.M.P.; França, E.L.; Rudge, M.V.C.; et al. Serotonin and interleukin 10 can influence the blood and urine viscosity in gestational diabetes mellitus and pregnancy-specific urinary incontinence. Int. J. Mol. Sci. 2023, 24, 17125. [Google Scholar] [CrossRef]
- França, D.C.H.; França, E.L.; Sobrevia, L.; Barbosa, A.M.P.; Ho, A.C. Integration of nutrigenomics, melatonin, serotonin and inflammatory cytokines in the pathophysiology of pregnancy-specific urinary incontinence in women with gestational diabetes mellitus. Front. Med. 2023, 10, 787230. [Google Scholar] [CrossRef]
- Zhang, Y.H.; He, M.; Wang, Y.; Liao, A.H. Modulators of the balance between M1 and M2 macrophages during pregnancy. Front. Immunol. 2017, 8, 120. [Google Scholar] [CrossRef]
- Sharma, S.; Banerjee, S.; Krueger, P.M.; Blois, S.M. Immunobiology of gestational diabetes mellitus in post-Medawar era. Front. Immunol. 2021, 12, 710095. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cui, D.; Yang, H. The distributional characteristics of M2 macrophages in the placental chorionic villi are altered among term pregnant women with uncontrolled type 2 diabetes mellitus. Placenta 2022, 120, 56–63. [Google Scholar] [CrossRef]
- Gosain, R.; Verma, N.; Jain, A.; Dixit, A. CD68 expression in the placenta of gestational diabetic mothers: A case-control study. Indian J. Pathol. Microbiol. 2023, 66, 727–731. [Google Scholar] [CrossRef]
- Silva, K.M.R.; França, D.C.H.; de Queiroz, A.A.; Fagundes-Triches, D.L.G.; de Marchi, P.G.F.; Morais, T.C.; Honorio-França, A.C.; França, E.L. Polarization of melatonin-modulated colostrum macrophages in the presence of breast tumor cell lines. Int. J. Mol. Sci. 2023, 24, 12400. [Google Scholar] [CrossRef]
- American Diabetes Association. Standards of Care in Diabetes. Diabetes Care 2025, 48 (Suppl. S1), S1–S352. [Google Scholar] [CrossRef]
- Soares, M.J.; Iqbal, K.; Kozai, K. Hypoxia and placental development. Birth Defects Res. 2017, 109, 1309–1329. [Google Scholar] [CrossRef]
- Hauguel-de Mouzon, S.; Guerre-Millo, M. The placenta cytokine network and inflammatory signals. Placenta 2006, 27, 794–798. [Google Scholar] [CrossRef]
- Moreli, J.B.; Corrêa-Silva, S.; Damasceno, D.C.; Sinzato, Y.K.; Lorenzon-Ojea, A.R.; Borbely, A.U.; Rudge, M.V.; Bevilacqua, E.; Calderon, I.M. Changes in the TNF-alpha/IL-10 ratio in hyperglycemia-associated pregnancies. Diabetes Res. Clin. Pract. 2015, 107, 362–369. [Google Scholar] [CrossRef]
- Mandalà, M. Oxidative Stress and Inflammation in Uterine–Vascular Adaptation During Pregnancy. Antioxidants 2025, 14, 1051. [Google Scholar] [CrossRef]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The pathophysiology of gestational diabetes mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef]
- Radaelli, T.; Varastehpour, A.; Catalano, P.; Hauguel-de Mouzon, S. Gestational diabetes induces placental genes for chronic stress and inflammatory pathways. Diabetes 2003, 52, 2951–2958. [Google Scholar] [CrossRef]
- Burton, G.J.; Fowden, A.L.; Thornburg, K.L. Placental origins of chronic disease. Physiol. Rev. 2016, 96, 1509–1565. [Google Scholar] [CrossRef] [PubMed]
- Colombini, A.; Libonati, F.; Cangelosi, D.; Lopa, S.; De Luca, P.; Coviello, D.A.; Moretti, M.; de Girolamo, L. Inflammatory priming with IL-1β promotes the immunomodulatory behavior of adipose derived stem cells. Front. Bioeng. Biotechnol. 2022, 10, 1000879. [Google Scholar] [CrossRef] [PubMed]
- Mallardo, M.; Ferraro, S.; Daniele, A.; Nigro, E. GDM-complicated pregnancies: Focus on adipokines. Mol. Biol. Rep. 2021, 48, 8171–8180. [Google Scholar] [CrossRef]
- Yockey, L.J.; Iwasaki, A. Interferons and proinflammatory cytokines in pregnancy and fetal development. Immunity 2018, 49, 397–412. [Google Scholar] [CrossRef]
- Wu, R.Y.; Xiao, K.; Hotte, N.; Tandon, P.; Elloumi, Y.; Ambrosio, L.; Dunsmore, G.; Elahi, S.; Kroeker, K.I.; Dieleman, L.A.; et al. Elevated IL-6 and IL-22 in Early Pregnancy Are Associated with Worse Disease Course in Women with Inflammatory Bowel Disease. Int. J. Mol. Sci. 2022, 23, 10281. [Google Scholar] [CrossRef]
- Vilotić, A.; Nacka-Aleksić, M.; Pirković, A.; Bojić-Trbojević, Ž.; Dekanski, D.; Jovanović Krivokuća, M. IL-6 and IL-8: An Overview of Their Roles in Healthy and Pathological Pregnancies. Int. J. Mol. Sci. 2022, 23, 14574. [Google Scholar] [CrossRef]
- Murthi, P.; Pinar, A.A.; Dimitriadis, E.; Samuel, C.S. Inflammasomes—A Molecular Link for Altered Immunoregulation and Inflammation Mediated Vascular Dysfunction in Preeclampsia. Int. J. Mol. Sci. 2020, 21, 1406. [Google Scholar] [CrossRef]
- Renaud, S.J.; Graham, C.H. The role of macrophages in utero-placental interactions during normal and pathological pregnancy. Immunol. Investig. 2008, 37, 535–564. [Google Scholar] [CrossRef]
- Abu-Raya, B.; Michalski, C.; Sadarangani, M.; Lavoie, P.M. Maternal immunological adaptation during normal pregnancy. Front. Immunol. 2020, 11, 575197. [Google Scholar] [CrossRef]
- Kang, J.; Lee, C.-N.; Li, H.-Y.; Hsu, K.-H.; Wang, S.-H.; Lin, S.-Y. Association of interleukin-10 methylation levels with gestational diabetes in a Taiwanese population. Front. Genet. 2018, 9, 222. [Google Scholar] [CrossRef]
- França, E.L.; Calderon, I.M.P.; Vieira, E.L.; Morceli, G.; Honorio-França, A.C. Transfer of maternal immunity to newborns of diabetic mothers. Clin. Dev. Immunol. 2012, 2012, 928187. [Google Scholar] [CrossRef]
- Fagundes, D.L.G.; França, E.L.; Morceli, G.; Rudge, M.V.C.; Calderon, I.M.P.; Honorio-França, A.C. The role of cytokines in the functional activity of phagocytes in blood and colostrum of diabetic mothers. Clin. Dev. Immunol. 2013, 2013, 590190. [Google Scholar] [CrossRef]
- Fagundes, D.L.G.; França, E.L.; da Silva Fernandes, R.T.; Hara, C.C.P.; Morceli, G.; Honorio-França, A.C.; Calderon, I.M.P. Changes in T-cell phenotype and cytokines profile in maternal blood, cord blood and colostrum of diabetic mothers. J. Matern. Fetal Neonatal Med. 2016, 29, 998–1004. [Google Scholar] [CrossRef]
- Paparini, D.E.; Grasso, E.; Aguilera, F.; Arslanian, M.A.; Lella, V.; Lara, B.; Schafir, A.; Gori, S.; Merech, F.; Hauk, V.; et al. Sex-specific phenotypical, functional and metabolic profiles of human term placenta macrophages. Biol. Sex Differ. 2024, 15, 80. [Google Scholar] [CrossRef]
- Yang, S.W.; Hwang, H.S.; Kang, Y.S. The role of placenta Hofbauer cells during pregnancy and pregnancy complications. Obstet. Gynecol. Sci. 2025, 68, 9–17. [Google Scholar] [CrossRef]
- Souza, E.G.; Hara, C.C.P.; Fagundes, D.L.G.; de Queiroz, A.A.; Morceli, G.; Calderon, I.M.P.; França, E.L.; Honorio-França, A.C. Maternal-foetal diabetes modifies neonatal Fc receptor expression on human leucocytes. Scand. J. Immunol. 2016, 84, 237–244. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, X.H.; Jin, L. Macrophage polarization in physiological and pathological pregnancy. Front. Immunol. 2019, 10, 792. [Google Scholar] [CrossRef]
- Mor, G.; Cardenas, I.; Abrahams, V.; Guller, S. Inflammation and pregnancy: The role of the immune system at the implantation site. Ann. N. Y. Acad. Sci. 2011, 1221, 80–87. [Google Scholar] [CrossRef]
- Svensson-Arvelund, J.; Ernerudh, J. The role of macrophages in promoting and maintaining homeostasis at the fetal-maternal interface. Am. J. Reprod. Immunol. 2015, 74, 100–109. [Google Scholar] [CrossRef]
- Ross, E.A.; Devitt, A.; Johnson, J.R. Macrophages: The good, the bad, and the gluttony. Front. Immunol. 2021, 12, 708186. [Google Scholar] [CrossRef] [PubMed]
- Milan, K.L.; Shree, R.A.; Nandana, N.; Leela, R.; Ramkumar, K.M. Role of macrophage reprogramming in the pathogenesis of gestational diabetes mellitus. Cytokine 2025, 196, 157041. [Google Scholar] [CrossRef]
- Wei, Y.; Lin, Z.; Yang, J. Macrophage plasticity and placental remodeling in pregnancy disorders. Cell. Mol. Life Sci. 2025, 82, 178. [Google Scholar]
- Mittal, R.; Goyal, S.; Singh, D. Unveiling gestational diabetes: An overview of pathophysiology and clinical consequences. Front. Physiol. 2025, 16, 1509352. [Google Scholar]
- Milan, K.L.; Fang, R.; Zhao, T. Hyperglycemia-induced placental macrophage dysregulation in gestational diabetes mellitus. Front. Immunol. 2025, 16, 1537845. [Google Scholar]
- Szarka, A.; Rigó, J., Jr.; Lázár, L.; Beko, G.; Molvarec, A. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol. 2010, 11, 59. [Google Scholar] [CrossRef]
- Luo, M.; Zhao, F.; Cheng, H.; Su, M.; Wang, Y. Macrophage polarization: An important role in inflammatory diseases. Front. Immunol. 2024, 15, 1352946. [Google Scholar] [CrossRef]
- Cotechini, T.; Graham, C.H. Aberrant maternal inflammation as a cause of pregnancy complications: A potential therapeutic target? Placenta 2015, 36, 960–966. [Google Scholar] [CrossRef]
- Barke, T.L.; Goldstein, J.A.; Sundermann, A.C.; Reddy, A.P.; Linder, J.E.; Correa, H.; Velez-Edwards, D.R.; Aronoff, D.M. Gestational diabetes mellitus is associated with increased CD163 expression and iron storage in the placenta. Am. J. Reprod. Immunol. 2018, 80, e13020. [Google Scholar] [CrossRef]
- Louis, M.B.P.; França, D.C.H.; Queiroz, A.A.; Calderon, I.M.P.; França, E.L.; Honorio-França, A.C. Melatonin hormone acts on cells of maternal blood and placenta from diabetic mothers. Front. Physiol. 2022, 12, 765928. [Google Scholar] [CrossRef]
- Rudge, M.V.C.; Calderon, I.M.P.; Ramos, M.D.; Abbade, J.F.; Rujolo, L.M. Perinatal outcome of pregnancies complicated by diabetes and by maternal daily hyperglycemia not related to diabetes: A retrospective 10-year analysis. Gynecol. Obstet. Investig. 2000, 50, 108–112. [Google Scholar] [CrossRef]
- Calderon, I.M.P.; Damasceno, D.C.; Amorin, R.L.; Costa, R.A.; Brasil, M.A.; Rudge, M.V.C. Morphometric study of placental villi and vessels in women with mild hyperglycemia or gestational or overt diabetes. Diabetes Res. Clin. Pract. 2007, 78, 65–71. [Google Scholar] [CrossRef]
- Vincent, Z.L.; Mitchell, M.D.; Ponnampalam, A.P. Regulation of MT1-MMP/MMP-2/TIMP-2 axis in human placenta. J. Inflamm. Res. 2015, 8, 193–200. [Google Scholar] [CrossRef]
- Fujimori, M.; França, E.L.; Fiorin, V.; Morais, T.C.; Honorio-França, A.C.; de Abreu, L.C. Changes in the biochemical and immunological components of serum and colostrum of overweight and obese mothers. BMC Pregnancy Childbirth 2015, 15, 166. [Google Scholar] [CrossRef] [PubMed]
- Borbely, A.U.; Sandri, S.; Fernandes, I.R.; Prado, K.M.; Cardoso, E.C.; Correa-Silva, S.; Albuquerque, R.; Knöfler, M.; Beltrão-Braga, P.; Campa, A.; et al. The term basal plate of the human placenta as a source of functional extravillous trophoblast cells. Reprod. Biol. Endocrinol. 2014, 12, 7. [Google Scholar] [CrossRef]
- Skytthe, M.K.; Graversen, J.H.; Moestrup, S.K. Targeting of CD163+ macrophages in inflammatory and malignant diseases. Int. J. Mol. Sci. 2020, 21, 1–32. [Google Scholar] [CrossRef]

| Parameters | ND | GDM | p-Value |
|---|---|---|---|
| Maternal | |||
| Age (years) | 26.4 ± 4.1 | 28.6 ± 6.7 | 0.4421 |
| Gestational age (weeks) | 39.2 ± 0.5 | 38.3 ± 0.9 | 0.0882 |
| Mean glycemia (mg/dL) | 81.9 ± 6.7 | 100.1 ± 11.6 * | 0.0341 |
| HbA1c (%) | 4.9 ± 0.5 | 5.8 ± 0.7 * | 0.0287 |
| BMI (1st trimester) | 26.9 ± 6.2 | 28.5 ± 7.8 | 0.7846 |
| BMI (3rd trimester) | 31.1 ± 7.7 | 32.4 ± 6.4 | 0.8237 |
| Newborn | |||
| Glucose | 77.6 ± 7.9 | 66.5 ± 14.1 | 0.1778 |
| Birth weight (%) | |||
| SGA | 15% (3) | 5% (1) | |
| AGA | 75% (15) | 70% (14) | |
| LGA | 10% (2) | 25% (5) | |
| Placental weight (g) | 609.3 ± 87.4 | 706.7 ± 92.7 | 0.7025 |
| Placental weight/fetal weight ratio | 0.167 ± 0.032 | 0.180 ± 0.037 * | 0.0032 |
| Placental Cytokines | Villous Layer/Extravillous Layer Ratio | p-Value | |
|---|---|---|---|
| ND | GDM | ||
| IL-1 β | 0.73 ± 0.2 | 1.08 ± 0.21 * | 0.0402 |
| IL-6 | 0.77 ± 0.25 | 0.79 ± 0.23 | 0.2663 |
| IL-8 | 2.0 ± 0.27 | 1.41 ± 0.81 * | 0.0473 |
| IL-10 | 0.87 ± 0.24 | 0.71 ± 0.33 | 0.1237 |
| IL-12 | 1.45 ± 0.61 | 1.08 ± 0.59 | 0.1283 |
| TNF-α | 0.97 ± 0.17 | 1.55 ± 0.61 * | 0.0325 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, M.R.; Marchi, G.F.d.; Silva, K.M.R.; França, D.C.H.; Silva, M.A.B.d.; Ribeiro Barbosa, J.; Melo, L.V.L.; França, E.L.; Honorio-França, A.C. Gestational Diabetes Mellitus Alters Cytokine Profiles and Macrophage Polarization in Human Placenta. Int. J. Mol. Sci. 2025, 26, 10867. https://doi.org/10.3390/ijms262210867
Barbosa MR, Marchi GFd, Silva KMR, França DCH, Silva MABd, Ribeiro Barbosa J, Melo LVL, França EL, Honorio-França AC. Gestational Diabetes Mellitus Alters Cytokine Profiles and Macrophage Polarization in Human Placenta. International Journal of Molecular Sciences. 2025; 26(22):10867. https://doi.org/10.3390/ijms262210867
Chicago/Turabian StyleBarbosa, Martalice Ribeiro, Gabriela Feres de Marchi, Kênia Maria Rezende Silva, Danielle Cristina Honorio França, Marcondes Alves Barbosa da Silva, Jakeline Ribeiro Barbosa, Laura Valdiane Luz Melo, Eduardo Luzía França, and Adenilda Cristina Honorio-França. 2025. "Gestational Diabetes Mellitus Alters Cytokine Profiles and Macrophage Polarization in Human Placenta" International Journal of Molecular Sciences 26, no. 22: 10867. https://doi.org/10.3390/ijms262210867
APA StyleBarbosa, M. R., Marchi, G. F. d., Silva, K. M. R., França, D. C. H., Silva, M. A. B. d., Ribeiro Barbosa, J., Melo, L. V. L., França, E. L., & Honorio-França, A. C. (2025). Gestational Diabetes Mellitus Alters Cytokine Profiles and Macrophage Polarization in Human Placenta. International Journal of Molecular Sciences, 26(22), 10867. https://doi.org/10.3390/ijms262210867








