A Review on the Current and Future State of Urinary Tract Infection Diagnostics
Abstract
1. Introduction
1.1. Prevalence
1.2. Microbial Etiological Agents
1.3. Treatment
| Type of UTI | Treatment | Comment [Reference] |
|---|---|---|
| Uncomplicated | Nitrofurantoin | Treatment of uncomplicated lower UTIs; effective against most Gram-positive and Gram-negative microorganisms [51] |
| Sulfamethoxazole with trimethoprim | High rates of resistance preclude their use as empiric treatment of UTIs in several communities [52] | |
| Fosfomycin | High effectiveness against ESBL-producing strains [23] | |
| Furazidin | Higher antibacterial activity of furazidin compared to nitrofurantoin [53] | |
| Beta-lactams (amoxicillin with clavulanic acid, cefaclor) | It should be used for 7 days and its effectiveness is lower than that of fluoroquinolones [23] | |
| Fluoroquinolone | High effectiveness with 3-day treatment [23] | |
| Complicated | Piperacillin-tazobactam | Treatment of Enterococcus, Staphylococcus or Pseudomonas [22] |
| Piperacillin-tazobactam, fluoroquinolones, cefepime, or ceftazidime | Treatment of suspected Pseudomonas infections [23] | |
| Intravenous fosfomycin | Treatment of complicated UTIs, particularly ESBL-producing bacteria [23] | |
| Aminoglycosides | Reserved for patients in whom other antibiotics cannot be used due to resistance or allergy [19] | |
| Ceftazidime/avibactam | Considered a last-line antibiotic [54] | |
| Meropenem/vaborbactam | Effective against K. pneumoniae producing carbapenemases (KPC) [55] | |
| Plazomicin | Effective against the majority of E. coli and K. pneumoniae strains, including ESBL-producing strains [56] | |
| Tebipenem | Treatment of pyelonephritis and complicated UTIs [57] | |
| Cefiderocol | Synthetic siderophore cephalosporin; effective against many carbapenem-resistant bacteria [54] |
1.4. Aim of the Manuscript
2. Methodology for Selecting Scientific Literature
3. Common Diagnostic Techniques for UTIs
3.1. Culture and Disk Diffusion Tests
| Test | Disk Content | Type of Detected Antibiotic Resistance Mechanism |
|---|---|---|
| DDST | Disks with meropenem and EDTA [69] | Metallo-β-lactamases [69] |
| Temocillin disks | Temocillin [70] | TEM-1, SHV-1, OXA-1, OXA-48 [70] |
| CDT | Meropenem and meropenem combined with phenylboronic acid [71] | Carbapenems and AmpC β-lactamases [71] |
| CIM | Suspension onto an agar plate with E. coli ATCC 25922 [72] | Carbapenems [72] |
| MAST, Rosco, Liofilchem | One disk containing carbapenem and another an inhibitor compared with carbapenem alone [73] | KPC, MBL, and OXA-48 carbapenemases, ESBL and MRSA [73,77] |
3.2. Point-of-Care Tests
3.3. Automated Spectrophotometric Systems
4. Specialistic Diagnostic Methods for UTIs
4.1. Analysis of Selected Genes
4.2. Mass Spectrometry
4.3. Immunoenzymatic Assays
4.4. Flow Cytometry
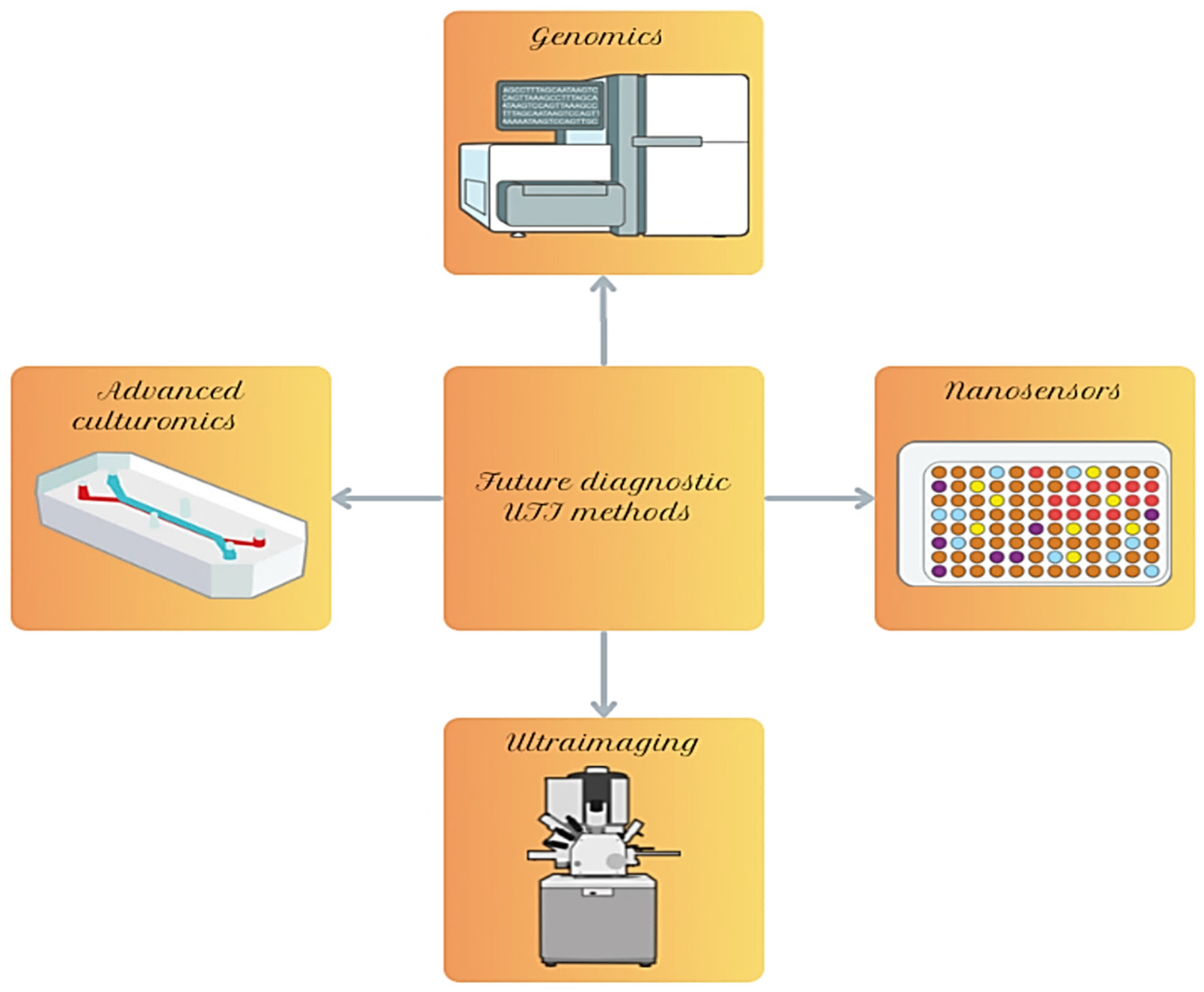
5. Modern Technologies and Future Directions in UTI Diagnostics
5.1. Advanced Methods of Genomics and Genetic Modifications
5.2. Nanosensors
5.3. Ultraimaging
5.4. Modern Methods of Culturing Microorganisms and Their Biofilms
6. Limitations and Future Perspectives of UTI Diagnostics
7. Conclusions
- -
- Culture methods remain the gold standard for UTI diagnosis;
- -
- Automatic analysis systems based on spectrophotometric measurements or microbial genetic/protein profiles are gaining importance, but are limited by price and staff training;
- -
- The newest methods, such as nanosensors or cultivation of biofilms in microfluidic systems, are very promising, although, for now, are poorly validated.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trześniewska-Ofiara, Z.; Mendrycka, M.; Woźniak-Kosek, A. Urinary Tract Infections: Epidemiological and Clinical Aspects. Med. Stud. 2024, 40, 208–215. [Google Scholar] [CrossRef]
- Mititelu, M.; Olteanu, G.; Neacșu, S.M.; Stoicescu, I.; Dumitrescu, D.-E.; Gheorghe, E.; Tarcea, M.; Busnatu, Ș.S.; Ioniță-Mîndrican, C.-B.; Tafuni, O.; et al. Incidence of Urinary Infections and Behavioral Risk Factors. Nutrients 2024, 16, 446. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhan, J.; Zhang, K.; Chen, H.; Cheng, S. Global, Regional, and National Burden of Urinary Tract Infections from 1990 to 2019: An Analysis of the Global Burden of Disease Study 2019. World J. Urol. 2022, 40, 755–763. [Google Scholar] [CrossRef]
- Schappert, S.M.; Rechtsteiner, E.A. Ambulatory Medical Care Utilization Estimates for 2007. Vital Health Stat. 2011, 169, 1–38. [Google Scholar]
- Simmering, J.E.; Tang, F.; Cavanaugh, J.E.; Polgreen, L.A.; Polgreen, P.M. The Increase in Hospitalizations for Urinary Tract Infections and the Associated Costs in the United States, 1998–2011. Open Forum Infect. Dis. 2017, 4, ofw281. [Google Scholar] [CrossRef] [PubMed]
- Foxman, B. The Epidemiology of Urinary Tract Infection. Nat. Rev. Urol. 2010, 7, 653–660. [Google Scholar] [CrossRef]
- de Lafforest, S.; Magnier, A.; Vallée, M.; Bey, E.; Le Goux, C.; Saint, F.; Therby, A.; Zahar, J.R.; Sotto, A.; Bruyere, F.; et al. FUrTIHF: French Urinary Tract Infections in Healthcare Facilities—Five-Year Historic Cohort (2014–2018). J. Hosp. Infect. 2021, 116, 29–36. [Google Scholar] [CrossRef]
- O’Brien, M.; Marijam, A.; Mitrani-Gold, F.S.; Terry, L.; Taylor-Stokes, G.; Joshi, A.V. Unmet Needs in Uncomplicated Urinary Tract Infection in the United States and Germany: A Physician Survey. BMC Infect. Dis. 2023, 23, 281. [Google Scholar] [CrossRef]
- Mwang’onde, B.J.; Mchami, J.I. The Aetiology and Prevalence of Urinary Tract Infections in Sub-Saharan Africa: A Systematic Review. J. Health Biol. Sci. 2022, 10, 1. [Google Scholar] [CrossRef]
- NHS England New Awareness Campaign to Help Reduce Hospital Admissions for Urinary Tract Infections. Available online: https://www.england.nhs.uk/2023/10/new-awareness-campaign-to-help-reduce-hospital-admissions-for-urinary-tract-infections/ (accessed on 1 October 2025).
- Haugom, L.E.A.; Ruths, S.; Emberland, K.E.; Eliassen, K.E.R.; Rortveit, G.; Wensaas, K.-A. Consultations and Antibiotic Treatment for Urinary Tract Infections in Norwegian Primary Care 2006–2015, a Registry-Based Study. BMC Fam. Pr. 2021, 22, 127. [Google Scholar] [CrossRef]
- Yuan, S.; Shi, Y.; Li, M.; Hu, X.; Bai, R. Trends in Incidence of Urinary Tract Infection in Mainland China from 1990 to 2019. Int. J. Gen. Med. 2021, 14, 1413–1420. [Google Scholar] [CrossRef]
- Franklin, M.; Emden, M.R.; Bauer, E.; Sacks, N.; Kawamatsu, S.; Kawano, Y.; Mitrani-Gold, F.S.; Joshi, A.V.; Ju, S.; Preib, M.T. 2840. Prevalence of Acute Uncomplicated Cystitis in Japan. Open Forum Infect. Dis. 2023, 10, ofad500.2450. [Google Scholar] [CrossRef]
- Hooton, T.M.; Scholes, D.; Hughes, J.P.; Winter, C.; Roberts, P.L.; Stapleton, A.E.; Stergachis, A.; Stamm, W.E. A Prospective Study of Risk Factors for Symptomatic Urinary Tract Infection in Young Women. N. Engl. J. Med. 1996, 335, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Schmiemann, G.; Kranz, J.; Mandraka, F.; Schubert, S.; Wagenlehner, F.; Gágyor, I. The Diagnosis, Treatment, and Prevention of Recurrent Urinary Tract Infection. Dtsch. Arztebl. Int. 2024, 121, 373–382. [Google Scholar] [CrossRef]
- Aggarwal, N.; Leslie, S.W. Recurrent Urinary Tract Infections; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Foxman, B.; Barlow, R.; D’Arcy, H.; Gillespie, B.; Sobel, J.D. Urinary Tract Infection. Ann. Epidemiol. 2000, 10, 509–515. [Google Scholar] [CrossRef]
- Harrington, R.D.; Hooton, T.M. Urinary Tract Infection Risk Factors and Gender. J. Gend. Specif. Med. 2000, 3, 27–34. [Google Scholar]
- Habak, P.J.; Carlson, K.; Griggs, R.P., Jr. Urinary Tract Infection in Pregnancy; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Urinary Tract Infections in Pregnant Individuals. Obstet. Gynecol. 2023, 142, 435–445. [CrossRef]
- Lipsky, B.A. Urinary Tract Infections in Men. Ann. Intern. Med. 1989, 110, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Sabih, A.; Leslie, S.W. Complicated Urinary Tract Infections; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Holecki, M.; Duława, J.; Hryniewicz, W.; Imiela, J.; Klinger, M.; Pawlik, K.; Wanke-Rytt, M. Rekomendacje Diagnostyki, Terapii i Profilaktyki Zakażeń Układu Moczowego u Dorosłych; Narodowy Instytut Leków: Warsaw, Poland, 2015. [Google Scholar]
- Choi, J.B.; Min, S.K. Complicated Urinary Tract Infection in Patients with Benign Prostatic Hyperplasia. J. Infect. Chemother. 2021, 27, 1284–1287. [Google Scholar] [CrossRef]
- Woodford, H.J.; George, J. Diagnosis and Management of Urinary Infections in Older People. Clin. Med. 2011, 11, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Dutta, C.; Pasha, K.; Paul, S.; Abbas, M.S.; Nassar, S.T.; Tasha, T.; Desai, A.; Bajgain, A.; Ali, A.; Mohammed, L. Urinary Tract Infection Induced Delirium in Elderly Patients: A Systematic Review. Cureus 2022, 14, e32321. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhao, J.; Wang, L.; Han, C.; Yan, R.; Zhu, P.; Qian, T.; Yu, S.; Zhu, X.; He, W. Epidemiological Trends and Predictions of Urinary Tract Infections in the Global Burden of Disease Study 2021. Sci. Rep. 2025, 15, 4702. [Google Scholar] [CrossRef] [PubMed]
- Bono, M.J.; Leslie, S.W. Uncomplicated Urinary Tract Infections; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Zhou, Y.; Zhou, Z.; Zheng, L.; Gong, Z.; Li, Y.; Jin, Y.; Huang, Y.; Chi, M. Urinary Tract Infections Caused by Uropathogenic Escherichia coli: Mechanisms of Infection and Treatment Options. Int. J. Mol. Sci. 2023, 24, 10537. [Google Scholar] [CrossRef]
- Ballén, V.; Cepas, V.; Ratia, C.; Gabasa, Y.; Soto, S.M. Clinical Escherichia coli: From Biofilm Formation to New Antibiofilm Strategies. Microorganisms 2022, 10, 1103. [Google Scholar] [CrossRef]
- Krzyżek, P. What Is a Biofilm? Lessons Learned from Interactions with Immune Cells. Int. J. Mol. Sci. 2024, 25, 11684. [Google Scholar] [CrossRef]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary Tract Infections: Epidemiology, Mechanisms of Infection and Treatment Options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef]
- Juszczak, K.; Dybowski, B.; Holecki, M.; Hryniewicz, W.; Klimek, H.; Kłoda, K.; Sieroszewski, P.; Drewa, T. Wytyczne Towarzystw Naukowych (PTU, PTGiP, PTMR) Dotyczące Diagnostyki, Terapii i Postępowania w Pozaszpitalnych Zakażeniach Dolnych Dróg Moczowych. Przegląd Urol. 2024, 142, 14–35. [Google Scholar]
- Jurałowicz, E.; Bartoszko-Tyczkowska, A.; Tyczkowska-Sieroń, E.; Kurnatowska, I. Etiology and Bacterial Susceptibility to Antibiotics in Patients with Recurrent Lower Urinary Tract Infections. Pol. Arch. Intern. Med. 2020, 130, 373–381. [Google Scholar] [CrossRef]
- Stefaniuk, E.; Suchocka, U.; Bosacka, K.; Hryniewicz, W. Etiology and Antibiotic Susceptibility of Bacterial Pathogens Responsible for Community-Acquired Urinary Tract Infections in Poland. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1363–1369. [Google Scholar] [CrossRef]
- Clarke, K.; Hall, C.L.; Wiley, Z.; Tejedor, S.C.; Kim, J.S.; Reif, L.; Witt, L.; Jacob, J.T. Catheter-Associated Urinary Tract Infections in Adults: Diagnosis, Treatment, and Prevention. J. Hosp. Med. 2020, 15, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Medina-Polo, J.; Naber, K.G.; Bjerklund Johansen, T.E. Healthcare-Associated Urinary Tract Infections in Urology. GMS Infect. Dis. 2021, 9, Doc05. [Google Scholar] [CrossRef]
- Bagińska, N.; Cieślik, M.; Górski, A.; Jończyk-Matysiak, E. The Role of Antibiotic Resistant A. baumannii in the Pathogenesis of Urinary Tract Infection and the Potential of Its Treatment with the Use of Bacteriophage Therapy. Antibiotics 2021, 10, 281. [Google Scholar] [CrossRef] [PubMed]
- Schuler, F.; Barth, P.J.; Niemann, S.; Schaumburg, F. A Narrative Review on the Role of Staphylococcus aureus Bacteriuria in S. aureus Bacteremia. Open Forum Infect. Dis. 2021, 8, ofab158. [Google Scholar] [CrossRef]
- Behzadi, P.; Behzadi, E.; Ranjbar, R. Urinary Tract Infections and Candida albicans. Cent. Eur. J. Urol. 2015, 68, 96–101. [Google Scholar] [CrossRef]
- Dias, V. Candida Species in the Urinary Tract: Is It a Fungal Infection or Not? Future Microbiol. 2020, 15, 81–83. [Google Scholar] [CrossRef]
- Gajdács, M.; Dóczi, I.; Ábrók, M.; Lázár, A.; Burián, K. Epidemiology of Candiduria and Candida Urinary Tract Infections in Inpatients and Outpatients: Results from a 10-Year Retrospective Survey. Cent. Eur. J. Urol. 2019, 72, 209–214. [Google Scholar] [CrossRef]
- Fazeli, A.; Kordbacheh, P.; Nazari, A.; Daie Ghazvini, R.; Mirhendi, H.; Safara, M.; Bakhshi, H.; Yaghoubi, R. Candiduria in Hospitalized Patients and Identification of Isolated Candida Species by Morphological and Molecular Methods in Ilam, Iran. Iran. J. Public Health 2019, 48, 156–161. [Google Scholar]
- Graninger, W. Pivmecillinam?Therapy of Choice for Lower Urinary Tract Infection. Int. J. Antimicrob. Agents 2003, 22, 73–78. [Google Scholar] [CrossRef]
- Thompson, D.; Xu, J.; Ischia, J.; Bolton, D. Fluoroquinolone Resistance in Urinary Tract Infections: Epidemiology, Mechanisms of Action and Management Strategies. BJUI Compass 2024, 5, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Hooton, T.M.; Stamm, W.E. Isolation of Fluoroquinolone-Resistant Rectal Escherichia coli after Treatment of Acute Uncomplicated Cystitis. J. Antimicrob. Chemother. 2005, 56, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Kaittanis, C.; Santra, S.; Perez, J.M. Emerging Nanotechnology-Based Strategies for the Identification of Microbial Pathogenesis. Adv. Drug Deliv. Rev. 2010, 62, 408–423. [Google Scholar] [CrossRef]
- Trautner, B.W. Management of Catheter-Associated Urinary Tract Infection. Curr. Opin. Infect. Dis. 2010, 23, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mireles, A.; Hreha, T.N.; Hunstad, D.A. Pathophysiology, Treatment, and Prevention of Catheter-Associated Urinary Tract Infection. Top. Spinal Cord Inj. Rehabil. 2019, 25, 228–240. [Google Scholar] [CrossRef] [PubMed]
- McAteer, J.; Lee, J.H.; Cosgrove, S.E.; Dzintars, K.; Fiawoo, S.; Heil, E.L.; Kendall, R.E.; Louie, T.; Malani, A.N.; Nori, P.; et al. Defining the Optimal Duration of Therapy for Hospitalized Patients with Complicated Urinary Tract Infections and Associated Bacteremia. Clin. Infect. Dis. 2023, 76, 1604–1612. [Google Scholar] [CrossRef]
- Squadrito, F.J.; del Portal, D. Nitrofurantoin; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Bader, M.S.; Loeb, M.; Leto, D.; Brooks, A.A. Treatment of Urinary Tract Infections in the Era of Antimicrobial Resistance and New Antimicrobial Agents. Postgrad. Med. 2020, 132, 234–250. [Google Scholar] [CrossRef]
- Bielec, F.; Brauncajs, M.; Pastuszak-Lewandoska, D. Nitrofuran Derivatives Cross-Resistance Evidence—Uropathogenic Escherichia coli Nitrofurantoin and Furazidin In Vitro Susceptibility Testing. J. Clin. Med. 2023, 12, 5166. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, D.; Wang, Y.; Ni, W. Cefiderocol for the Treatment of Multidrug-Resistant Gram-Negative Bacteria: A Systematic Review of Currently Available Evidence. Front. Pharmacol. 2022, 13, 896971, Erratum in Front. Pharmacol. 2022, 18, 976792. [Google Scholar] [CrossRef]
- Kaye, K.S.; Bhowmick, T.; Metallidis, S.; Bleasdale, S.C.; Sagan, O.S.; Stus, V.; Vazquez, J.; Zaitsev, V.; Bidair, M.; Chorvat, E.; et al. Effect of Meropenem-Vaborbactam vs Piperacillin-Tazobactam on Clinical Cure or Improvement and Microbial Eradication in Complicated Urinary Tract Infection. JAMA 2018, 319, 788. [Google Scholar] [CrossRef]
- Clark, J.A.; Burgess, D.S. Plazomicin: A New Aminoglycoside in the Fight against Antimicrobial Resistance. Ther. Adv. Infect. Dis. 2020, 7, 2049936120952604. [Google Scholar] [CrossRef]
- Eckburg, P.B.; Muir, L.; Critchley, I.A.; Walpole, S.; Kwak, H.; Phelan, A.-M.; Moore, G.; Jain, A.; Keutzer, T.; Dane, A.; et al. Oral Tebipenem Pivoxil Hydrobromide in Complicated Urinary Tract Infection. N. Engl. J. Med. 2022, 386, 1327–1338. [Google Scholar] [CrossRef]
- WHO. WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance; WHO: Geneva, Switzerland, 2024. [Google Scholar]
- Zdzisław, M.; Dorota, K.; Magdalena, P. Antybiotyki w Dobie Narastającej Antybiotykoopornosci. Antybiotyki w Dobie Narastającej Antybiotykoopornosci, 1st ed.; Wydawnictwo Naukowe PWN: Warsaw, Poland, 2021. [Google Scholar]
- Wardal, E.; Żabicka, D.; Hryniewicz, W.; Sadowy, E. VanA-Enterococcus faecalis in Poland: Hospital Population Clonal Structure and VanA Mobilome. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 1245–1261. [Google Scholar] [CrossRef]
- Bosacka, K.; Hryniewicz, W.; Mikołajczyk, A.; Stefaniuk, E. Właściwości i Zastosowanie Podłoży Bakteriologicznych. Postępy Mikrobiol. 2016, 55, 320–329. [Google Scholar]
- Marczewska, J.; Mysłowska, K. Nadzór Nad Materiałami Stosowanymi w Badaniach Mikrobiologicznych. Cz. 1: Podłoża Hodowlane. LAB Lab. Apar. Badania 2013, 18, 38–44. [Google Scholar]
- Perry, J.D. A Decade of Development ofChromogenic Culture Media for Clinical Microbiology in an Eraof Molecular Diagnostics. Clin. Microbiol. Rev. 2017, 30, 449–479, Erratum in Clin. Microbiol. Rev. 2017, 30. [Google Scholar] [CrossRef] [PubMed]
- Brennan-Kohn, T. Urine Good Hands: Diagnosing UTIs with Urine Cultures. Available online: https://asm.org/articles/2021/february/urine-good-hands-diagnosing-utis-with-urine-cultur (accessed on 1 October 2025).
- Hertz, M.A.; Johansen, I.S.; Rosenvinge, F.S.; Brasen, C.L.; Andersen, E.S.; Østergaard, C.; Skovsted, T.A.; Petersen, E.R.B.; Nielsen, S.L.; Mogensen, C.B.; et al. Urine Flow Cytometry and Dipstick Analysis in Diagnosing Bacteriuria and Urinary Tract Infections among Adults in the Emergency Department—A Diagnostic Accuracy Trial. Diagnostics 2024, 14, 412. [Google Scholar] [CrossRef]
- Elżbieta, B.; Teresa, N. Przegląd Wykrywanych Mechanizmów Oporności Bakteri. Lab. Med. 2019, 3, 20–27. [Google Scholar]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic Susceptibility Testing by a Standardized Single Disk Method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial disk susceptibility tests. Clin. Lab. Standars Inst. NCCLS 2012, 32, M02-A11. [Google Scholar]
- Picão, R.C.; Andrade, S.S.; Nicoletti, A.G.; Campana, E.H.; Moraes, G.C.; Mendes, R.E.; Gales, A.C. Metallo-β-Lactamase Detection: Comparative Evaluation of Double-Disk Synergy versus Combined Disk Tests for IMP-, GIM-, SIM-, SPM-, or VIM-Producing Isolates. J. Clin. Microbiol. 2008, 46, 2028–2037. [Google Scholar] [CrossRef]
- van Dijk, K.; Voets, G.M.; Scharringa, J.; Voskuil, S.; Fluit, A.C.; Rottier, W.C.; Leverstein-Van Hall, M.A.; Stuart, J.W.T.C. A Disc Diffusion Assay for Detection of Class A, B and OXA-48 Carbapenemases in Enterobacteriaceae Using Phenyl Boronic Acid, Dipicolinic Acid and Temocillin. Clin. Microbiol. Infect. 2014, 20, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Pournaras, S.; Zarkotou, O.; Poulou, A.; Kristo, I.; Vrioni, G.; Themeli-Digalaki, K.; Tsakris, A. A Combined Disk Test for Direct Differentiation of Carbapenemase-Producing Enterobacteriaceae in Surveillance Rectal Swabs. J. Clin. Microbiol. 2013, 51, 2986–2990. [Google Scholar] [CrossRef] [PubMed]
- van der Zwaluw, K.; de Haan, A.; Pluister, G.N.; Bootsma, H.J.; de Neeling, A.J.; Schouls, L.M. The Carbapenem Inactivation Method (CIM), a Simple and Low-Cost Alternative for the Carba NP Test to Assess Phenotypic Carbapenemase Activity in Gram-Negative Rods. PLoS ONE 2015, 10, e0123690. [Google Scholar] [CrossRef] [PubMed]
- Doyle, D.; Peirano, G.; Lascols, C.; Lloyd, T.; Church, D.L.; Pitout, J.D.D. Laboratory Detection of Enterobacteriaceae That Produce Carbapenemases. J. Clin. Microbiol. 2012, 50, 3877–3880. [Google Scholar] [CrossRef]
- Noster, J.; Thelen, P.; Hamprecht, A. Detection of Multidrug-Resistant Enterobacterales-From ESBLs to Carbapenemases. Antibiotics 2021, 10, 1140. [Google Scholar] [CrossRef] [PubMed]
- Al Mamari, A.M.K.; Al Jabri, Z.; Sami, H.; Rizvi, S.G.A.; Chan, M.F.; Al Siyabi, T.; Al Muharrmi, Z.; Rizvi, M. Evaluation of Six Commercial and In-House Phenotypic Tests for Detection of AmpC β-Lactamases: Is Routine Detection Possible? JAC Antimicrob. Resist. 2023, 5, dlad101. [Google Scholar] [CrossRef]
- Zakaria, N.D.; Hamzah, H.H.; Salih, I.L.; Balakrishnan, V.; Abdul Razak, K. A Review of Detection Methods for Vancomycin-Resistant Enterococci (VRE) Genes: From Conventional Approaches to Potentially Electrochemical DNA Biosensors. Biosensors 2023, 13, 294. [Google Scholar] [CrossRef]
- Cekin, Y.; Yazisiz, H.; Kuskucu, M.; Ongut, G.; Baysan, B.; Kilinckaya, H.; Ogunc, D.; Midilli, K.; Ozen, N.; Colak, D. Evaluation of the BD Phoenix System for Detection of Methicilin Resistance in Staphylococcus aureus Isolates in Comparison to BD GeneOhm MRSA Assay. Clin. Lab. 2014, 60, 863–867. [Google Scholar] [CrossRef]
- Mambatta, A.; Jayarajan, J.; Rashme, V.; Harini, S.; Menon, S.; Kuppusamy, J. Reliability of Dipstick Assay in Predicting Urinary Tract Infection. J. Fam. Med. Prim. Care 2015, 4, 265. [Google Scholar] [CrossRef]
- Hullegie, S.; Wootton, M.; Verheij, T.J.M.; Thomas-Jones, E.; Bates, J.; Hood, K.; Gal, M.; Francis, N.A.; Little, P.; Moore, M.; et al. Clinicians’ Interpretations of Point of Care Urine Culture versus Laboratory Culture Results: Analysis from the Four-Country POETIC Trial of Diagnosis of Uncomplicated Urinary Tract Infection in Primary Care. Fam. Pr. 2017, 34, 392–399. [Google Scholar] [CrossRef]
- Tomlinson, E.; Ward, M.; Cooper, C.; James, R.; Stokes, C.; Begum, S.; Watson, J.; Hay, A.D.; Jones, H.E.; Thom, H.; et al. Point-of-Care Tests for Urinary Tract Infections to Reduce Antimicrobial Resistance: A Systematic Review and Conceptual Economic Model. Health Technol. Assess. 2024, 28, 1–109. [Google Scholar] [CrossRef]
- Greeff, A.; Jeffery, B.; Pattinson, R.C. Uricult Trio as a Screening Test for Bacteriuria in Pregnancy. S. Afr. Med. J. 2002, 92, 306–309. [Google Scholar] [PubMed]
- Aspevall, O.; Kjerstadius, T.; Lindberg, L.; Hallander, H. Performance of Uricult Trio Assessed by a Comparison Method and External Control Panels in Primary Healthcare. Scand. J. Clin. Lab. Investig. 2000, 60, 381–386. [Google Scholar] [CrossRef]
- Scarparo, C.; Piccoli, P.; Ricordi, P.; Scagnelli, M. Evaluation of the DipStreak, a New Device with an Original Streaking Mechanism for Detection, Counting, and Presumptive Identification of Urinary Tract Pathogens. J. Clin. Microbiol. 2002, 40, 2169–2178. [Google Scholar] [CrossRef]
- Nakasone, I.; Kinjo, T.; Yamane, N.; Kisanuki, K.; Shiohira, C.M. Laboratory-Based Evaluation of the Colorimetric VITEK-2 Compact System for Species Identification and of the Advanced Expert System for Detection of Antimicrobial Resistances: VITEK-2 Compact System Identification and Antimicrobial Susceptibility Testing. Diagn. Microbiol. Infect. Dis. 2007, 58, 191–198. [Google Scholar] [CrossRef]
- Ozyurt, O.K.; Cetinkaya, O.; Ozhak, B.; Ongut, G.; Turhan, O.; Yazisiz, H.; Donmez, L.; Kuskucu, M.A.; Midilli, K.; Ogunc, D. Evaluation of the BD Phoenix CPO Detect Test for the Detection of Carbapenemase-Producing Enterobacterales. Future Microbiol. 2023, 18, 399–405. [Google Scholar] [CrossRef]
- Thomson, K.S.; Robledo, I.E.; Vázquez, G.J.; Moland, E.S. KPC Screening by Updated BD Phoenix and Vitek 2 Automated Systems. J. Clin. Microbiol. 2011, 49, 3386–3387. [Google Scholar] [CrossRef][Green Version]
- Aupaix, A.; Lamraoui, K.; Rodriguez-Villalobos, H.; Anantharajah, A.; Verroken, A. Comparison of Two Commercial Broth Microdilution Panels for Multidrug-Resistant Gram-Negative Bacteria: Thermo ScientificTM Sensititre DKMGN vs. Beckman Coulter MicroScan NMDRM1. Front. Microbiol. 2024, 15, 1480687. [Google Scholar] [CrossRef]
- Green, M.R.; Sambrook, J. Polymerase Chain Reaction. Cold Spring Harb. Protoc. 2019, 2019, pdb.top095109. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, R.; Munshi, A.; Panda, S.K. Polymerase Chain Reaction. Natl. Med. J. India 1992, 5, 115–119. [Google Scholar]
- Mackay, I.M. Real-Time PCR in Virology. Nucleic Acids Res. 2002, 30, 1292–1305. [Google Scholar] [CrossRef] [PubMed]
- Elnifro, E.M.; Ashshi, A.M.; Cooper, R.J.; Klapper, P.E. Multiplex PCR: Optimization and Application in Diagnostic Virology. Clin. Microbiol. Rev. 2000, 13, 559–570. [Google Scholar] [CrossRef]
- Voets, G.M.; Fluit, A.C.; Scharringa, J.; Cohen Stuart, J.; Leverstein-van Hall, M.A. A Set of Multiplex PCRs for Genotypic Detection of Extended-Spectrum β-Lactamases, Carbapenemases, Plasmid-Mediated AmpC β-Lactamases and OXA β-Lactamases. Int. J. Antimicrob. Agents 2011, 37, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Traczewski, M.M.; Carretto, E.; Canton, R.; Moore, N.M. Multicenter Evaluation of the Xpert Carba-R Assay for Detection of Carbapenemase Genes in Gram-Negative Isolates. J. Clin. Microbiol. 2018, 56, e00272-18. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Lee, J.Y.; Park, J.Y.; Jeon, M.J. Xpert Carba-R Assay for Detection of Carbapenemase-Producing Organisms in Patients Admitted to Emergency Rooms. Medicine 2020, 99, e23410. [Google Scholar] [CrossRef]
- Vergara, A.; Moreno-Morales, J.; Roca, I.; Pitart, C.; Kostyanev, T.; Rodriguez-Baño, J.; Goossens, H.; Marco, F.; Vila, J. A Comparative Study between Real-Time PCR and Loop-Mediated Isothermal Amplification to Detect Carbapenemase and/or ESBL Genes in Enterobacteriaceae Directly from Bronchoalveolar Lavage Fluid Samples. J. Antimicrob. Chemother. 2020, 75, 1453–1457. [Google Scholar] [CrossRef]
- Haldorsen, B.C.; Janice, J.; Samuelsen, Ø. Evaluation of the Amplex Eazyplex® SuperBug Acineto Test for Detection of Acquired OXA and NDM Carbapenemases in Acinetobacter spp. J. Glob. Antimicrob. Resist. 2021, 24, 340–341. [Google Scholar] [CrossRef]
- García-Fernández, S.; Morosini, M.-I.; Marco, F.; Gijón, D.; Vergara, A.; Vila, J.; Ruiz-Garbajosa, P.; Cantón, R. Evaluation of the Eazyplex® SuperBug CRE System for Rapid Detection of Carbapenemases and ESBLs in Clinical Enterobacteriaceae Isolates Recovered at Two Spanish Hospitals. J. Antimicrob. Chemother. 2015, 70, 1047–1050. [Google Scholar] [CrossRef] [PubMed]
- Sękowska, A.; Bogiel, T. The Evaluation of Eazyplex® SuperBug CRE Assay Usefulness for the Detection of ESBLs and Carbapenemases Genes Directly from Urine Samples and Positive Blood Cultures. Antibiotics 2022, 11, 138. [Google Scholar] [CrossRef]
- Zalas-Więcek, P.; Gospodarek-Komkowska, E.; Smalczewska, A. Rapid Detection of Genes Encoding Extended-Spectrum Beta-Lactamase and Carbapenemase in Clinical Escherichia coli Isolates with Eazyplex SuperBug CRE System. Microb. Drug Resist. 2020, 26, 1245–1249. [Google Scholar] [CrossRef]
- Probst, K.; Boutin, S.; Bandilla, M.; Heeg, K.; Dalpke, A.H. Fast and Automated Detection of Common Carbapenemase Genes Using Multiplex Real-Time PCR on the BD MAXTM System. J. Microbiol. Methods 2021, 185, 106224. [Google Scholar] [CrossRef]
- García-Fernández, S.; Simner, P.J.; Thomson, G.; Faron, M.; Bosboom, R.; van Griethuijsen, A.; García-Castillo, M.; Harris, R.; Ledeboer, N.A.; Cantón, R.; et al. Rapid Identification from Rectal Swabs of the Clinically Most Relevant Carbapenemase Genes from Gram-Negative Bacteria Using the BD MAX Check-Points CPO Assay. Diagn. Microbiol. Infect. Dis. 2022, 102, 115554. [Google Scholar] [CrossRef]
- Yoo, I.Y.; Shin, D.P.; Heo, W.; Ha, S.I.; Cha, Y.J.; Park, Y.-J. Comparison of BD MAX Check-Points CPO Assay with Cepheid Xpert Carba-R Assay for the Detection of Carbapenemase-Producing Enterobacteriaceae Directly from Rectal Swabs. Diagn. Microbiol. Infect. Dis. 2022, 103, 115716. [Google Scholar] [CrossRef]
- Yoo, I.Y.; Huh, K.; Shim, H.J.; Yun, S.A.; Chung, Y.N.; Kang, O.K.; Huh, H.J.; Lee, N.Y. Evaluation of the BioFire FilmArray Pneumonia Panel for Rapid Detection of Respiratory Bacterial Pathogens and Antibiotic Resistance Genes in Sputum and Endotracheal Aspirate Specimens. Int. J. Infect. Dis. 2020, 95, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Ruan, S.-Y.; Pan, S.-C.; Lee, T.-F.; Chien, J.-Y.; Hsueh, P.-R. Performance of a Multiplex PCR Pneumonia Panel for the Identification of Respiratory Pathogens and the Main Determinants of Resistance from the Lower Respiratory Tract Specimens of Adult Patients in Intensive Care Units. J. Microbiol. Immunol. Infect. 2019, 52, 920–928. [Google Scholar] [CrossRef]
- Berinson, B.; Both, A.; Berneking, L.; Christner, M.; Lütgehetmann, M.; Aepfelbacher, M.; Rohde, H. Usefulness of BioFire FilmArray BCID2 for Blood Culture Processing in Clinical Practice. J. Clin. Microbiol. 2021, 59, e0054321. [Google Scholar] [CrossRef]
- Peri, A.M.; Ling, W.; Furuya-Kanamori, L.; Harris, P.N.A.; Paterson, D.L. Performance of BioFire Blood Culture Identification 2 Panel (BCID2) for the Detection of Bloodstream Pathogens and Their Associated Resistance Markers: A Systematic Review and Meta-Analysis of Diagnostic Test Accuracy Studies. BMC Infect. Dis. 2022, 22, 794. [Google Scholar] [CrossRef]
- Dingle, T.C.; Butler-Wu, S.M. MALDI-TOF Mass Spectrometry for Microorganism Identification. Clin. Lab. Med. 2013, 33, 589–609. [Google Scholar] [CrossRef] [PubMed]
- Neonakis, I.K.; Spandidos, D.A. Detection of Carbapenemase Producers by Matrix-Assisted Laser Desorption-Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF MS). Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1795–1801. [Google Scholar] [CrossRef]
- Correa-Martínez, C.L.; Idelevich, E.A.; Sparbier, K.; Kostrzewa, M.; Becker, K. Rapid Detection of Extended-Spectrum β-Lactamases (ESBL) and AmpC β-Lactamases in Enterobacterales: Development of a Screening Panel Using the MALDI-TOF MS-Based Direct-on-Target Microdroplet Growth Assay. Front. Microbiol. 2019, 10, 13. [Google Scholar] [CrossRef]
- Dortet, L.; Bonnin, R.A.; Pennisi, I.; Gauthier, L.; Jousset, A.B.; Dabos, L.; Furniss, R.C.D.; Mavridou, D.A.I.; Bogaerts, P.; Glupczynski, Y.; et al. Rapid Detection and Discrimination of Chromosome- and MCR-Plasmid-Mediated Resistance to Polymyxins by MALDI-TOF MS in Escherichia coli: The MALDIxin Test. J. Antimicrob. Chemother. 2018, 73, 3359–3367. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Tien, N.; Liu, Y.-C.; Cho, D.-Y.; Chen, J.-W.; Tsai, Y.-T.; Huang, Y.-C.; Chao, H.-J.; Chen, C.-J. Rapid Identification of Methicillin-Resistant Staphylococcus aureus Using MALDI-TOF MS and Machine Learning from over 20,000 Clinical Isolates. Microbiol. Spectr. 2022, 10, e0048322. [Google Scholar] [CrossRef]
- Veenemans, J.; Welker, M.; van Belkum, A.; Saccomani, M.C.; Girard, V.; Pettersson, A.; Verhulst, C.; Kluytmans-Vandenbergh, M.; Kluytmans, J. Comparison of MALDI-TOF MS and AFLP for Strain Typing of ESBL-Producing Escherichia coli. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 829–838. [Google Scholar] [CrossRef]
- Gato, E.; Arroyo, M.J.; Méndez, G.; Candela, A.; Rodiño-Janeiro, B.K.; Fernández, J.; Rodríguez-Sánchez, B.; Mancera, L.; Arca-Suárez, J.; Beceiro, A.; et al. Direct Detection of Carbapenemase-Producing Klebsiella Pneumoniae by MALDI-TOF Analysis of Full Spectra Applying Machine Learning. J. Clin. Microbiol. 2023, 61, e0175122. [Google Scholar] [CrossRef]
- Gato, E.; Anantharajah, A.; Arroyo, M.J.; Artacho, M.J.; Caballero, J.d.D.; Candela, A.; Chudějová, K.; Constanso, I.P.; Elías, C.; Fernández, J.; et al. Multicenter Performance Evaluation of MALDI-TOF MS for Rapid Detection of Carbapenemase Activity in Enterobacterales: The Future of Networking Data Analysis with Online Software. Front. Microbiol. 2022, 12, 789731. [Google Scholar] [CrossRef] [PubMed]
- Hleba, L.; Hlebova, M.; Kovacikova, E.; Kovacik, A. MALDI-TOF MS Indirect Beta-Lactamase Detection in Ampicillin-Resistant Haemophilus Influenzae. Microorganisms 2023, 11, 1018. [Google Scholar] [CrossRef] [PubMed]
- Toprak, N.U.; Akgul, O.; Sóki, J.; Soyletir, G.; Nagy, E.; Leitner, E.; Wybo, I.; Tripkovic, V.; Justesen, U.S.; Jean-Pierre, H.; et al. Detection of Beta-Lactamase Production in Clinical Prevotella Species by MALDI-TOF MS Method. Anaerobe 2020, 65, 102240. [Google Scholar] [CrossRef]
- Tabatabaei, M.S.; Ahmed, M. Enzyme-Linked Immunosorbent Assay (ELISA); StatPearls: Treasure Island, FL, USA, 2022; pp. 115–134. [Google Scholar]
- Valencio, A.; da Silva, M.A.; Santos, F.F.; Polatto, J.M.; Machado, M.M.F.; Piazza, R.M.F.; Gales, A.C. Capture ELISA for KPC Detection in Gram-Negative Bacilli: Development and Standardisation. Microorganisms 2023, 11, 1052. [Google Scholar] [CrossRef]
- Hujer, A.M.; Page, M.G.P.; Helfand, M.S.; Yeiser, B.; Bonomo, R.A. Development of a Sensitive and Specific Enzyme-Linked Immunosorbent Assay for Detecting and Quantifying CMY-2 and SHV β-Lactamases. J. Clin. Microbiol. 2002, 40, 1947–1957. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, K.M. Flow Cytometry: An Overview. Curr. Protoc. Immunol. 2018, 120, 5.1.1–5.1.11. [Google Scholar] [CrossRef]
- Boonen, K.J.M.; Koldewijn, E.L.; Arents, N.L.A.; Raaymakers, P.A.M.; Scharnhorst, V. Urine Flow Cytometry as a Primary Screening Method to Exclude Urinary Tract Infections. World J. Urol. 2013, 31, 547–551. [Google Scholar] [CrossRef]
- Mejuto, P.; Luengo, M.; Díaz-Gigante, J. Automated Flow Cytometry: An Alternative to Urine Culture in a Routine Clinical Microbiology Laboratory? Int. J. Microbiol. 2017, 2017, 8532736. [Google Scholar] [CrossRef]
- Conkar, S.; Mir, S. Urine Flow Cytometry in the Diagnosis of Urinary Tract Infection. Indian J. Pediatr. 2018, 85, 995–999. [Google Scholar] [CrossRef] [PubMed]
- Broeren, M.; Nowacki, R.; Halbertsma, F.; Arents, N.; Zegers, S. Urine Flow Cytometry Is an Adequate Screening Tool for Urinary Tract Infections in Children. Eur. J. Pediatr. 2019, 178, 363–368. [Google Scholar] [CrossRef]
- Riesenfeld, C.S.; Schloss, P.D.; Handelsman, J. Metagenomics: Genomic Analysis of Microbial Communities. Annu. Rev. Genet. 2004, 38, 525–552. [Google Scholar] [CrossRef] [PubMed]
- Almas, S.; Carpenter, R.E.; Rowan, C.; Tamrakar, V.K.; Bishop, J.; Sharma, R. Advantage of Precision Metagenomics for Urinary Tract Infection Diagnostics. Front. Cell. Infect. Microbiol. 2023, 13, 1221289. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Yang, Y.; Zhao, J.; Yan, T.; Tian, X. Application of Metagenomic Next-Generation Sequencing in the Diagnosis and Treatment of Recurrent Urinary Tract Infection in Kidney Transplant Recipients. Front. Public Health 2022, 10, 901549. [Google Scholar] [CrossRef]
- Jeff, C.; Quarton, S.; Hatton, C.; Parekh, D.; Thickett, D.; McNally, A.; Sapey, E. Metagenomics in the Diagnosis and Treatment of Urinary Tract Infections: A Systematic Review and Meta-Analysis. Diagn. Microbiol. Infect. Dis. 2025, 113, 116995. [Google Scholar] [CrossRef]
- Ng, P.C.; Kirkness, E.F. Whole Genome Sequencing. Methods Mol. Biol. 2010, 628, 215–226. [Google Scholar]
- Ahmed, M.Y.; Gorish, B.M.T.; Alhaj, E.M.; Elrhim, M.A.E.A.; Siddig, S.S.; Altayb, H.N. Whole-Genome Sequencing of Multidrug-Resistant Escherichia coli Causing Urinary Tract Infection in an Immunocompromised Patient: A Case Report. J. Med. Case Rep. 2024, 18, 326. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Tomar, J. CRISPR/CAS 9 Mediated Treatment for UTIs. Int. J. Mod. Trends Sci. Technol. 2020, 6, 82–94. [Google Scholar] [CrossRef]
- Kang, S.; Kim, J.; Hur, J.K.; Lee, S.-S. CRISPR-Based Genome Editing of Clinically Important Escherichia coli SE15 Isolated from Indwelling Urinary Catheters of Patients. J. Med. Microbiol. 2017, 66, 18–25. [Google Scholar] [CrossRef]
- Lou, J.; Wang, B.; Li, J.; Ni, P.; Jin, Y.; Chen, S.; Xi, Y.; Zhang, R.; Duan, G. The CRISPR-Cas System as a Tool for Diagnosing and Treating Infectious Diseases. Mol. Biol. Rep. 2022, 49, 11301–11311. [Google Scholar] [CrossRef]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold Nanoparticles in Chemical and Biological Sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, B.; Yin, G.; Wang, J.; He, M.; Yang, Y.; Wang, T.; Tang, T.; Yu, X.-A.; Tian, J. Rapid Fluorescence Sensor Guided Detection of Urinary Tract Bacterial Infections. Int. J. Nanomed. 2022, 17, 3723–3733. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Xie, G.; Zhang, X.; Yuan, J.; Hou, Y.; Chen, H. Fast Detection of E. coli with a Novel Fluorescent Biosensor Based on a FRET System between UCNPs and GO@Fe3O4 in Urine Specimens. Anal. Methods 2021, 13, 2209–2214. [Google Scholar] [CrossRef] [PubMed]
- Crintea, A.; Carpa, R.; Mitre, A.-O.; Petho, R.I.; Chelaru, V.-F.; Nădășan, S.-M.; Neamti, L.; Dutu, A.G. Nanotechnology Involved in Treating Urinary Tract Infections: An Overview. Nanomaterials 2023, 13, 555. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, Y.; Hong, W.; Huang, M.; Wu, M.; Zhao, X. Applications of Genome Editing Technology in the Targeted Therapy of Human Diseases: Mechanisms, Advances and Prospects. Signal Transduct. Target. Ther. 2020, 5, 1. [Google Scholar] [CrossRef]
- Yacouba, A.; Bellali, S.; Haddad, G.; Mavros, N.; Fontanini, A.; Dubourg, G.; Lagier, J.; Raoult, D.; Bou Khalil, J. Use of Scanning Electron Microscopy and Energy Dispersive X-ray for Urine Analysis: A Preliminary Investigation. Microsc. Res. Tech. 2023, 86, 1249–1257. [Google Scholar] [CrossRef]
- Brooks, T.; Keevil, C.W. A Simple Artificial Urine for the Growth of Urinary Pathogens. Lett. Appl. Microbiol. 1997, 24, 203–206. [Google Scholar] [CrossRef]
- Rimbi, P.T.; O’Boyle, N.; Douce, G.R.; Pizza, M.; Rosini, R.; Roe, A.J. Enhancing a Multi-Purpose Artificial Urine for Culture and Gene Expression Studies of Uropathogenic Escherichia coli Strains. J. Appl. Microbiol. 2024, 135, lxae067. [Google Scholar] [CrossRef]
- Ipe, D.S.; Ulett, G.C. Evaluation of the in Vitro Growth of Urinary Tract Infection-Causing Gram-Negative and Gram-Positive Bacteria in a Proposed Synthetic Human Urine (SHU) Medium. J. Microbiol. Methods 2016, 127, 164–171. [Google Scholar] [CrossRef]
- Pinto, H.; Simões, M.; Borges, A. Prevalence and Impact of Biofilms on Bloodstream and Urinary Tract Infections: A Systematic Review and Meta-Analysis. Antibiotics 2021, 10, 825. [Google Scholar] [CrossRef] [PubMed]
- Buckner, E.; Buckingham-Meyer, K.; Miller, L.A.; Parker, A.E.; Jones, C.J.; Goeres, D.M. Coupon Position Does Not Affect Pseudomonas aeruginosa and Staphylococcus aureus Biofilm Densities in the CDC Biofilm Reactor. J. Microbiol. Methods 2024, 223, 106960. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, A.L.; Vu, H.; Martinez, T.; Peniata, L.; Kawaguchi, B.; Armbruster, D.A.; Ashton, N.N.; Williams, D.L. In Vitro Antibiofilm Efficacy of Ertapenem, Tobramycin, and Moxifloxacin against Biofilms Grown in a Glass Bead or CDC Biofilm Reactor®. PLoS ONE 2025, 20, e0318487. [Google Scholar] [CrossRef]
- Franco-Duarte, R.; Černáková, L.; Kadam, S.; Kaushik, K.S.; Salehi, B.; Bevilacqua, A.; Corbo, M.R.; Antolak, H.; Dybka-Stępień, K.; Leszczewicz, M.; et al. Advances in Chemical and Biological Methods to Identify Microorganisms—From Past to Present. Microorganisms 2019, 7, 130. [Google Scholar] [CrossRef]
- Assi, F.; Jabri, B.; Melalka, L.; Sekhsokh, Y.; Zouhdi, M. Direct MALDI-TOF MS-Based Method for Rapid Identification of Microorganisms and Antibiotic Susceptibility Testing in Urine Specimens. Iran. J. Microbiol. 2025, 17, 92–98. [Google Scholar] [CrossRef]
- Baimakhanova, B.; Sadanov, A.; Berezin, V.; Baimakhanova, G.; Trenozhnikova, L.; Orasymbet, S.; Seitimova, G.; Kalmakhanov, S.; Xetayeva, G.; Shynykul, Z.; et al. Emerging Technologies for the Diagnosis of Urinary Tract Infections: Advances in Molecular Detection and Resistance Profiling. Diagnostics 2025, 15, 2469. [Google Scholar] [CrossRef]
- Tran, A.; Alby, K.; Kerr, A.; Jones, M.; Gilligan, P.H. Cost Savings Realized by Implementation of Routine Microbiological Identification by Matrix-Assisted Laser Desorption Ionization–Time of Flight Mass Spectrometry. J. Clin. Microbiol. 2015, 53, 2473–2479. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.; Mariz, M.; Tiago, I.; Martins, J.; Alarico, S.; Ferreira, P. A Review on Urinary Tract Infections Diagnostic Methods: Laboratory-Based and Point-of-Care Approaches. J. Pharm. Biomed. Anal. 2022, 219, 114889. [Google Scholar] [CrossRef]
- Tang, M.; Yang, J.; Li, Y.; Zhang, L.; Peng, Y.; Chen, W.; Liu, J. Diagnostic Accuracy of MALDI-TOF Mass Spectrometry for the Direct Identification of Clinical Pathogens from Urine. Open Med. 2020, 15, 266–273. [Google Scholar] [CrossRef]
- Bermudez, T.; Schmitz, J.E.; Boswell, M.; Humphries, R. Novel Technologies for the Diagnosis of Urinary Tract Infections. J. Clin. Microbiol. 2025, 63, e0030624. [Google Scholar] [CrossRef] [PubMed]
- Bizzini, A.; Greub, G. Matrix-Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometry, a Revolution in Clinical Microbial Identification. Clin. Microbiol. Infect. 2010, 16, 1614–1619. [Google Scholar] [CrossRef] [PubMed]
- van Belkum, A.; Welker, M.; Pincus, D.; Charrier, J.-P.; Girard, V. Matrix-Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometry in Clinical Microbiology: What Are the Current Issues? Ann. Lab. Med. 2017, 37, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.; Deng, J.; Zhang, J.; Wu, H.; Wu, Y.; Bin, L.; Li, D.; Liu, J.; Yu, R.; Lin, H.; et al. Rapid and Accurate Diagnosis of Urinary Tract Infections Using Targeted Next-Generation Sequencing: A Multicenter Comparative Study with Metagenomic Sequencing and Traditional Culture Methods. J. Infect. 2025, 90, 106459. [Google Scholar] [CrossRef]
- Jennings, L.; Van Deerlin, V.M.; Gulley, M.L. Recommended Principles and Practices for Validating Clinical Molecular Pathology Tests. Arch. Pathol. Lab. Med. 2009, 133, 743–755. [Google Scholar] [CrossRef]
- Burd, E.M. Validation of Laboratory-Developed Molecular Assays for Infectious Diseases. Clin. Microbiol. Rev. 2010, 23, 550–576. [Google Scholar] [CrossRef]
- Szmulik, M.; Trześniewska-Ofiara, Z.; Mendrycka, M.; Woźniak-Kosek, A. A Novel Approach to Screening and Managing the Urinary Tract Infections Suspected Sample in the General Human Population. Front. Cell. Infect. Microbiol. 2022, 12, 915288. [Google Scholar] [CrossRef]
- Bil-Lula, I.; Ćwiklińska, A.; Kamińska, D.; Kamińska, J.; Kopczyński, Z.; Kozłowska, D.; Krzywonos-Zawadzka, A.; Mantur, M.; Mertas, A.; Pietruczuk, M.; et al. The Polish Society for Laboratory Diagnostics Guidelines on Urine Particles Analysis in a Medical Diagnostic Laboratory. J. Lab. Diagn. 2019, 55, 145–198. [Google Scholar] [CrossRef]
| Risk Factor | Examples |
|---|---|
| Anatomical abnormalities | Bladder diverticula, posterior urethral valve |
| Foreign body | Catheter, stent, nephrostomy |
| Obstruction | Enlarged prostate, stones, kidney cysts |
| Functional disorders | Vesicoureteral reflux, neurogenic bladder |
| Other | Pregnancy, diabetes, immunosuppression, resistant pathogens |
| Methods [Reference] | Time | Sensitivity | Resistance Detection | Costs | Bedside Test | Limitations | |
|---|---|---|---|---|---|---|---|
| Classical molecular methods | Genie expert [93,94] | <1 day | +++ | Detects only known and defined resistance mechanisms (mainly carbapenemases) | +++ | +/− | No distinction between colonization and infection Limited by its inability to detect all resistance determinants, particularly those outside the targeted panel |
| Genie II [95,96,97,98,99] | <15 min | Detects only known and defined resistance mechanisms (mainly ESBL and carbapenemases) | ++ | + | |||
| BD-MAX [100,101,102] | 2–2.5 h | ++ | − | ||||
| Bio-Fire [103,104,105,106] | >1 h | +++ | |||||
| MALDI-TOF MS [107,110,111,112,113,114,115,116] | >2 h | +++ | Detects cephalosporinases, carbapenemases, MCR, MRSA, VRE | + | − | No distinction between colonization and infection Differences with urine culture results | |
| ELISA [118,119] | 1.5–2.5 h | ++ | Enables direct prediction of beta-lactam resistance | + | − | Risk of cross-reactions Detects mechanism of resistance, but not a microbial strain | |
| Flow cytometry [120,122,124] | 3–10 min | + | − | ++ | − | Leukocyturia or biofilm may distort the result | |
| Advanced molecular methods | Metagenomics [126,127] | 24–48 h | ++++ | Can analyze hundreds of resistance genes | ++++ | − | Time-consuming No distinction between colonization and infection |
| Whole genome sequencing [129,130] | 24–72 h | Can analyze all resistance genes | − | Time-consuming No distinction between colonization and infection | |||
| CRISP [131,132,133] | 30–60 min | Can analyze specific resistance genes | ++ | +/− | Risk of false positive results Limited numbers of sets available on the market | ||
| Nanosensors [47,134,135,136] | 10–30 min | +++ | − | ++ | − | The need for additional tests Limited number of sets available on the market | |
| SEM [137] | <24 h | + | − | +++ | − | Limited to visualization of microorganisms, but unable to indicate a microbial strain and the mechanism of resistance | |
| Culture | Classical media [61,62,63] | >24 h | + | Detects cephalosporinases, carbapenemases, MCR, MRSA, VRE | + | − | Time-consuming and requiring a set of disposable materials to detect different resistance mechanisms |
| Artificial urine [141,142,144,145] | >24 h | Theoretically able to detect mechanisms of resistance (unvalidated) | + | Time-consuming and requiring a set of disposable materials to detect different resistance mechanisms Unvalidated | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dudzik, Ł.; Krzyżek, P.; Dworniczek, E. A Review on the Current and Future State of Urinary Tract Infection Diagnostics. Int. J. Mol. Sci. 2025, 26, 10847. https://doi.org/10.3390/ijms262210847
Dudzik Ł, Krzyżek P, Dworniczek E. A Review on the Current and Future State of Urinary Tract Infection Diagnostics. International Journal of Molecular Sciences. 2025; 26(22):10847. https://doi.org/10.3390/ijms262210847
Chicago/Turabian StyleDudzik, Łucja, Paweł Krzyżek, and Ewa Dworniczek. 2025. "A Review on the Current and Future State of Urinary Tract Infection Diagnostics" International Journal of Molecular Sciences 26, no. 22: 10847. https://doi.org/10.3390/ijms262210847
APA StyleDudzik, Ł., Krzyżek, P., & Dworniczek, E. (2025). A Review on the Current and Future State of Urinary Tract Infection Diagnostics. International Journal of Molecular Sciences, 26(22), 10847. https://doi.org/10.3390/ijms262210847








