Sub-15 nm Nanoparticles for Drug Delivery: Emerging Frontiers and Therapeutic Potential
Abstract
1. Introduction
2. Types of Sub-15 nm Nanoparticles
2.1. Sub-15 nm Polymeric Nanoparticles and Dendrimers for Cancer Drug Delivery
2.1.1. Polymeric Nanoparticles
2.1.2. Dendrimers
2.1.3. Comparative Considerations
2.2. Lipid-Based Nanoparticles Below 15 nm in Size for Cancer Therapy
2.2.1. Lipid Nanoparticles (LNPs)
2.2.2. Liposomes
2.2.3. Nanoemulsions
2.2.4. Solid Lipid Nanoparticles (SLNs)
2.2.5. Comparison of Lipid-Based Nanoparticles
2.3. Metallic Nanoparticles Below 15 nm in Size for Cancer Therapy
2.3.1. Gold Nanoparticles (AuNPs)
- Enhanced diffusion through tumor interstitium;
- Efficient renal clearance for ultrasmall AuNPs (<5–8 nm);
- High surface-area-to-volume ratio for functionalization with targeting ligands, drugs, and imaging agents.
- Drug carriers, via surface conjugation or thiol-linker attachment of doxorubicin, paclitaxel, etc.;
- Photothermal agents, where NIR laser exposure induces localized hyperthermia for tumor ablation;
- Radiosensitizers, enhancing the effect of radiation therapy by increasing local dose deposition [58].
2.3.2. Silver Nanoparticles (AgNPs)
- Induction of reactive oxygen species (ROS)
- DNA damage and mitochondrial disruption
2.3.3. Iron Oxide Nanoparticles
- Magnetic resonance imaging (MRI) contrast agents, especially T1-weighted imaging, for early tumor detection;
- Hyperthermia therapy, using alternating magnetic fields to generate localized heat;
- Targeted drug delivery, when functionalized with anticancer drugs, peptides, or antibodies [80].
- Benefit from an optimal size that supports effective tumor targeting and in situ distribution.
- Maintain a favorable balance between releasing therapeutic agents (Fe2+ for triggering •OH generation) and achieving accumulation within the tumor.
- Exhibit a therapeutic profile that, at least in the case of the 10 nm particles, leads to the best antitumor effect in vivo compared to smaller (e.g., <~5 nm) or larger nanoparticles.
- The nanoparticles display a superparamagnetic nature which imparts a high relaxivity of ~225 mM−1·s−1 for T2-weighted magnetic resonance imaging.
- They are also amenable as contrast agents in photoacoustic and NIR imaging, enhancing their utility as theranostic agents.
2.3.4. Comparative Considerations Between Metallic Nanoparticles
2.4. Quantum Dots and Carbon-Based Nanoparticles Below 15 nm in Size for Cancer Therapy
- (a)
- High Surface-Area-to-Volume Ratio:
- (b)
- Multifunctionality for Imaging and Therapeutics:
- (c)
- Applications in Molecular Imaging and Targeted Drug Delivery:
2.4.1. Graphene Quantum Dots (GQDs)
- Strong and tunable photoluminescence (PL);
- High photostability and low photobleaching;
- Intrinsic biocompatibility and low toxicity;
- Surface functional groups (–OH, –COOH, –NH2) for conjugation.
- Fluorescence imaging: GQDs offer bright and stable emission for cellular and in vivo tumor imaging, often in the near-infrared (NIR) range for deep tissue penetration. Challenges (GQDs)—Although metal-free and generally biocompatible, GQDs can still generate ROS under photoexcitation; batch-to-batch control surface groups and size dispersion < 10 nm should be verified with orthogonal methods to ensure consistent brightness and safety.
- Drug delivery: GQDs can be loaded or conjugated with chemotherapeutic agents (e.g., doxorubicin, paclitaxel) via π-π stacking and hydrogen bonding.
- Photodynamic and photothermal therapy (PDT/PTT): GQDs generate reactive oxygen species (ROS) upon light excitation, inducing apoptosis in tumor cells. Their photothermal conversion efficiency also allows heat-induced cancer cell ablation [95]. Challenges (QGDs—PDT/PTT)—Phototoxicity windows, heat generation thresholds, and tumor-to-normal selectivity require careful dose and light-dose control; CQAs should include size distribution and surface chemistry that modulate ROS and heat generation.
2.4.2. Carbon Nanotubes (CNTs)
- High mechanical strength and thermal conductivity;
- Exceptional near-infrared (NIR) absorbance;
- Large surface area for drug or gene attachment;
- Intrinsic fluorescence and Raman scattering for imaging.
- Drug and gene delivery: CNTs are excellent vehicles for loading chemotherapeutic agents (e.g., cisplatin, doxorubicin), siRNA, or DNA through covalent or non-covalent interactions. Despite strong loading and NIR absorption, sub-15 nm CNTs face biopersistence-linked inflammation risks and typically require robust surface functionalization to improve dispersibility and biocompatibility; these constraints should be considered alongside the efficacy data. Functionalization with PEG, folic acid, or peptides improves dispersibility and targeting [108].
- Photothermal therapy (PTT): SWCNTs efficiently convert NIR light into heat, achieving localized tumor ablation.
- Biopersistence and slow clearance;
- Inflammatory responses and cytotoxicity;
- Need for extensive surface functionalization to enhance solubility and biocompatibility.
2.4.3. Comparative Insight and Outlook Between GQDs and CNTs
2.4.4. Synthesis of Sub-15 nm Nanoparticles
2.5. Magnetic Nanomaterials and Metal-Organic Frameworks (MOFs) for Quantum Technologies
2.5.1. Why Size Matters in Nanoscale Confinement
2.5.2. Room-Temperature Entanglement in a MOF
2.5.3. Relevance to Sub-15 nm Bionanotechnology
3. Unique Physicochemical Properties of Sub-15 nm Nanoparticles
3.1. Size-Dependent Surface Area and Reactivity
3.1.1. Surface-Area-to-Volume Ratio: A Key Driver of Nano-Bio Interactions
- Greater surface functionalization (e.g., with targeting ligands, PEG chains, drugs, fluorophores)
- Enhanced dispersion in aqueous media due to higher surface energy
- More rapid interactions with the cellular membrane and proteins
- Drug delivery (higher loading per unit mass of carrier)
- Catalytic cancer therapy (e.g., Fenton-like reactions in chemodynamic therapy)
- Diagnostic signal amplification (e.g., in fluorescence or photoacoustic imaging)
3.1.2. Surface Reactivity and Chemical Functionality
- Higher chemical reactivity: The atoms on the surface are less coordinated than those in the bulk, leading to more available sites for reactions or interactions with biomolecules.
- Enhanced redox activity: For metal or metal oxide nanoparticles, this translates to increased ROS generation, which is useful in therapies like photodynamic or chemodynamic treatment of tumors.
- Increased adsorption capacity: Smaller nanoparticles can adsorb a higher quantity of drugs or proteins due to their large surface area and increased surface free energy.
- Gold nanoparticles (AuNPs) < 10 nm show dramatically higher binding affinities for thiolated ligands than larger AuNPs, enabling denser and more stable functional coatings [145].
- Graphene quantum dots (GQDs) < 5 nm possess abundant edge sites and oxygen-containing functional groups, offering superior reactivity for drug conjugation and ROS generation under light irradiation [95].
3.1.3. Quantum Effects and Size-Dependent Optical Properties
- Optical absorbance and fluorescence: Smaller particles exhibit blue-shifted emission and tunable photoluminescence, enabling size-controlled imaging probes.
- Photothermal and photodynamic conversion efficiency: Higher surface reactivity improves energy transfer for cancer cell ablation.
3.1.4. Implications for Drug Delivery and Cancer Therapy [59]
- Efficient cellular uptake: Their small size and reactive surfaces allow better interaction with cell membranes, promoting endocytosis.
- Improved tumor penetration: Smaller nanoparticles navigate through the dense extracellular matrix more effectively than larger systems, achieving more uniform drug distribution within tumors.
- Targeted delivery: The abundance of surface sites allows for multivalent conjugation of targeting moieties, enhancing specificity to tumor cells or receptors (e.g., folate, RGD peptides).
- Stimuli-responsiveness: Reactive surfaces can be engineered to respond to tumor-specific cues such as pH, redox, or enzymatic activity.
- Potential toxicity or oxidative stress
- Instability due to agglomeration
- Non-specific protein adsorption (opsonization)
3.2. Enhanced Cellular Uptake and Biodistribution
3.2.1. Size as a Determinant of Cellular Uptake Pathways
- Enter cells more rapidly than larger particles
- Preferentially undergo caveolae- or clathrin-mediated endocytosis, which allows escape from lysosomal degradation
- Access subcellular compartments such as the nucleus, mitochondria, or endoplasmic reticulum more readily [158]
3.2.2. Tumor Penetration and Interstitial Diffusion
- Superior interstitial diffusion in 3D tumor spheroids and dense tumor stroma
- Ability to overcome size-exclusion effects posed by collagen networks and ECM pores (~20–50 nm)
- Enhanced paracellular transport and transcytosis across endothelial barriers [136]
3.2.3. Biodistribution and Pharmacokinetics
- Circulation time: Depending on surface properties (e.g., PEGylation), sub-15 nm particles may circulate long enough to exploit the enhanced permeability and retention (EPR) effect, though ultrasmall particles (<5 nm) may undergo rapid renal clearance.
- Renal clearance: Nanoparticles below ~6 nm are rapidly filtered by the kidneys and excreted in urine, which may reduce systemic toxicity and off-target accumulation.
- RES evasion: Sub-15 nm particles can partially evade RES capture by combining hydration-layer stealth (PEG, polysarcosine, poly(2-oxazoline), zwitterions) with corona-quality control (dysopsonin-favoring interfaces), which together extend circulation and improve tumor exposure.
- Liver and spleen accumulation: Although liver uptake is common for many nanoparticles, the reduced opsonization of smaller particles improves their biodistribution to peripheral and tumor tissues.
3.2.4. Enhanced Endosomal Escape and Intracellular Targeting
- Can disrupt endosomal membranes more effectively due to high local surface energy;
- Have been observed to traffic into the nucleus (especially for particles < 10 nm) without requiring nuclear localization signals;
- Are suitable for mitochondrial or lysosomal targeting when functionalized appropriately.
3.3. Stability, Aggregation, and Surface Charge Considerations of Sub-15 nm Nanoparticles
3.3.1. Colloidal Stability and Thermodynamic Considerations
- Van der Waals attractions that dominate at the nanoscale;
- Electrolyte-induced screening of surface charges in saline solutions;
- Protein corona formation, leading to bridging and aggregation.
- Electrostatic stabilization involves generating a high surface charge (zeta potential > |±30| mV) to repel nearby particles.
- Steric stabilization relies on hydrophilic polymers (e.g., PEG, Pluronic, polysaccharides) that form a hydration shell to prevent particle-particle contact.
3.3.2. Aggregation Behavior and Its Implications
- Loss of size-specific advantages, such as enhanced tumor penetration and renal clearance
- Inaccurate biodistribution, as aggregated particles behave like larger ones (>100 nm)
- Reduced targeting efficacy, due to shielding or loss of surface ligands
- Increased immunogenicity and toxicity, as aggregates can be rapidly recognized by the reticuloendothelial system (RES)
3.3.3. Surface Charge and Zeta Potential
- Highly positive zeta potentials (>+30 mV) may enhance cellular uptake via electrostatic attraction to negatively charged cell membranes but also increase serum protein adsorption and cytotoxicity.
- Highly negative zeta potentials (<–30 mV) contribute to colloidal stability but may limit membrane interaction and uptake.
- Near-neutral zeta potentials (~±10 mV) often indicate poor colloidal stability and increased risk of aggregation unless sterically stabilized.
3.3.4. Strategies to Improve Stability of Sub-15 nm Nanoparticles
- (i)
- Surface functionalization:
- ○
- PEGylation for steric repulsion and protein resistance;
- ○
- Ligand grafting (e.g., citrate, polypeptides, poloxamers) to introduce charge or hydrophilicity;
- ○
- Zwitterionic coatings to achieve charge neutrality and reduce opsonization.
- (ii)
- Core–shell architectures:
- ○
- Encapsulation within liposomes, micelles, or polymeric shells to isolate core particles and enhance dispersion;
- ○
- Examples: Lipid-coated quantum dots, micelle-encapsulated AuNPs.
- (iii)
- Buffer optimization:
- ○
- Use of low-ionic-strength buffers during storage and formulation;
- ○
- Addition of stabilizers like trehalose, sucrose, or PVP during freeze-drying.
- (iv)
- Protein corona control:
- (v)
- Stealth by corona design:
- ○
- Pre-adsorb benign proteins or use of biomimetic interfaces (e.g., albumin, HDL-mimetics) to favor dysopsonin coronas that reduce RES uptake without sacrificing targeting [171].
3.4. Drug Delivery and Controlled Release from Sub-15 nm Nanoparticles
3.4.1. Passive Targeting via the EPR Effect
- Enhanced tumor penetration: Their small size enables deep diffusion into the tumor interstitium, reaching hypoxic and poorly vascularized regions that are typically inaccessible to larger carriers (>50 nm) [176].While EPR remains a central concept, extensive literature shows that its magnitude is heterogeneous across tumor types, sites, and models, and median tumor delivery can be very low in preclinical meta-analyses, which helps explain variable clinical translation. Readers are referred to detailed reviews/meta-analyses for the current state of the field and strategies to enhance delivery (vascular normalization, flow restoration, microenvironment modulation, and active transport). These syntheses collectively emphasize that passive extravasation alone rarely suffices, and that active endothelial processes (transcytosis) likely contribute substantially to tumor entry for many nanocarriers [177,178,179,180].
- Reduced off-target accumulation: Compared to larger particles that accumulate in the liver and spleen, ultrasmall particles can achieve more favorable biodistribution profiles with proper surface functionalization (e.g., PEGylation).
- Prolonged circulation: When coated with stealth polymers or zwitterionic/PEG-alternative brushes, sub-15 nm nanoparticles reduce opsonization and RES clearance, improving effective EPR capture.Liposomes and polymeric micelles (~10–20 nm) have demonstrated deeper tumor penetration and enhanced therapeutic efficacy in breast and pancreatic cancer models [8].
3.4.2. Active Targeting via Surface Ligand Functionalization
- Small molecules (e.g., folic acid);
- Peptides (e.g., RGD, TAT);
- Antibodies or antibody fragments;
- Aptamers or nucleic acids.
- Selective accumulation in tumor cells overexpressing specific receptors (e.g., folate receptor, integrins, EGFR);
- Receptor-mediated endocytosis, improving cellular uptake and intracellular drug delivery;
- Minimized off-target toxicity through selective targeting;
- Transcytosis-enabled targeting (endothelial crossing): Beyond receptor-mediated cellular uptake, receptor-mediated transcytosis across endothelial cells is increasingly recognized as a dominant entry route for nanoparticles into tumors. In mouse and human samples, quantitative imaging and modeling indicate that the vast majority (often > 90%) of nanoparticles enter tumors via active trans-endothelial transport, rather than through static inter-endothelial gaps alone. For sub-15 nm carriers, size, curvature, and ligand display can favor caveolae-/vesicle-mediated uptake and vesiculo-vacuolar organelle (VVO) transport, aligning with classic observations of VEGF-induced hyperpermeability. Design levers include ligands to receptors involved in endothelial trafficking (e.g., transferrin receptor, ICAM-1, albumin/GP60 pathways, integrins), zwitterionic/stealth coronas that reduce nonspecific adhesion yet preserve receptor binding, and compact particle sizes that fit vesicular pathways [183,184].
- 10–15 nm AuNPs functionalized with RGD peptides showed increased uptake in integrin-positive tumors and improved photothermal therapy outcomes [185]. The challenges associated with targeting ligands are the target expression heterogeneity, off-target binding, and immunogenicity of larger ligands. These necessitate quantitative control of surface ligand density and size distribution as CQAs, confirmed with orthogonal analytics.
3.4.3. Controlled Drug Release Mechanisms
- Protected during circulation
- Released specifically at the tumor site or within cancer cells
- Responsive to internal or external stimuli
- pH-responsive systems: Exploit the acidic tumor microenvironment or endosomal compartments to trigger release (e.g., acid-labile bonds, protonation of polymer backbones).
- Redox-responsive systems: Use disulfide linkages that are cleaved in the presence of elevated intracellular glutathione.
- Enzyme-sensitive carriers: Release payload in response to matrix metalloproteinases (MMPs) or cathepsins overexpressed in tumors.
- Photothermal and photodynamic triggers: Light-responsive nanoparticles (e.g., AuNPs, GQDs) release drugs upon NIR irradiation [191].
- Iron oxide nanoparticles < 15 nm functionalized with cathepsin-cleavable linkers for enzyme-triggered doxorubicin release [192].
- The prodrug is prepared by conjugating 6-mercaptopurine (6-MP) to 50 kDa hyaluronic acid via a carbonyl vinyl sulfide linker, resulting in a drug conjugate with a 6-MP content of 6.9 wt%.
- The HA-GS-MP nanoparticles exhibit excellent water solubility with a hydrodynamic size of ca. 15 nm, which places them at the upper end of the specified 5–15 nm range.
3.4.4. Combination Therapies and Theranostics
- Sub-10 nm carbon dots delivering both paclitaxel and siRNA while enabling fluorescence imaging
- Gold nanoshells < 15 nm used for combining photothermal therapy and drug delivery under image guidance
- In vivo studies in mice bearing subcutaneously implanted HeLa cells showed significant tumor inhibition. For example, an approximately 99% tumor inhibition ratio was shown by the combination of MNPs and H2O2 after treatment for 17 days. This highlights how nanoparticles in this size range can be optimized for effective cancer therapy.
- These nanoparticles also serve as efficient magnetic resonance (MR) imaging contrast agents. In vitro and in vivo studies indicated that 13 nm MNPs could be used as highly sensitive T2-weighted MR imaging agents. Their relaxivity was determined to be r2 = 104 s−1·mM−1, making them suitable for tracking and targeting tumors via MR imaging. The study also reports that the MR signal was much more negative, and the intensity was significantly diminished with the increase in the concentration of 13 nm MNPs in vitro.
- In vivo, tumors were clearly visualized with a 3-fold decrease in MR signal intensity at the tumor site after 24 h following treatment, indicating successful targeting and accumulation at the desired location.
3.5. Sub-15 nm Nanoparticles for Blood–Brain Barrier Penetration
3.5.1. The Blood–Brain Barrier: A Key Challenge in Neuropharmacology
- Tight junctions between endothelial cells
- Efflux pumps (e.g., P-glycoprotein)
- Astrocytic endfeet and pericytes
3.5.2. Size-Dependent BBB Penetration
- Size within or below endothelial pore limits (~20 nm) enables paracellular or transcytotic transport.
- Low steric hindrance supports uptake via adsorptive- or receptor-mediated transcytosis.
- Increased diffusivity compared to larger particles enhances passage through dense brain parenchyma after BBB crossing.
- The engineered nanoparticles are designed for targeted drug delivery. The surface modification with Angiopep-2, in particular, improved the ability of the nanoparticles to cross the blood–brain barrier (BBB) and target the inflammatory microenvironment of glioma. The study reported a higher uptake by activated neutrophils (uptake efficiency increased from 24.9% for the uncoated system to 31.1% for ANG-2 EM@PPC), which is important for hitchhiking on these cells to deliver drugs effectively (Zhao et al., 2024) [204].
- Furthermore, the nanoparticles influenced cellular processes by altering the death pathway of neutrophils from neutrophil extracellular traps-osis (NETosis) to apoptosis. This modification was confirmed by both Western blot and flow cytometry (with apoptotic body production reaching as high as 77.7%), suggesting that nanoparticles in this size range can be finely tuned to affect cellular responses.
- In animal models of in situ glioma, all formulations of the engineered nanoparticles (including those with ANG-2 modification) demonstrated effective distribution to brain tissue with higher affinity and internalization by neutrophils at the tumor site, compared to the control (DiR group) (Zhao et al., 2024) [204].
3.5.3. Strategies for Enhancing BBB Crossing
- (i)
- Passive mechanisms:
- Nanoparticles with hydrophobic coatings, zwitterionic surfaces, or low protein adsorption may exploit transient BBB permeability or adsorptive transcytosis.
- (ii)
- Active targeting mechanisms:
- Receptor-mediated transcytosis (RMT):
- ▪
- Transferrin receptor (TfR): Used by functionalizing NPs with transferrin, lactoferrin, or TfR-binding antibodies;
- ▪
- Low-density lipoprotein receptor (LDLR): Targeted using apolipoprotein E (ApoE) or mimetic peptides;
- ▪
- Insulin receptor targeting brain tumor and neurodegenerative drug delivery.
- 10–15 nm PEGylated AuNPs conjugated with transferrin showed efficient accumulation in glioma tissue in vivo and enabled imaging and drug delivery [205].
- (iii)
- Carrier-mediated transport (CMT):
- Exploits glucose, amino acid, or peptide transporters to carry drug-loaded ultrasmall NPs across the BBB.
3.5.4. Imaging and Diagnostic Applications
- Iron oxide nanoparticles (<15 nm) act as MRI contrast agents (T1 or T2) for early detection of brain tumors or lesions.
- Graphene quantum dots and carbon dots (<10 nm) provide fluorescent and photoacoustic signals, enabling real-time imaging of BBB passage and brain accumulation.
- Radiolabeled gold nanoparticles have been explored for PET imaging in neuro-oncology.
3.5.5. Therapeutic Applications in Neurological Diseases
- (i)
- Brain tumors (e.g., glioblastoma):
- Sub-15 nm NPs loaded with doxorubicin, temozolomide, or siRNA have shown efficient accumulation in intracranial tumors, especially when actively targeted.
- (ii)
- Neurodegenerative diseases:
- NPs delivering siRNA, antioxidants, or neuroprotective peptides (e.g., nerve growth factor) can halt or slow the progression of Alzheimer’s or Parkinson’s disease.
- Graphene quantum dots have shown ROS scavenging ability and Aβ aggregation inhibition, offering therapeutic benefit in Alzheimer’s models [207].
- (iii)
- Stroke and neuroinflammation:
- Anti-inflammatory drugs and neuroprotective agents delivered via sub-15 nm carriers reduce BBB breakdown and oxidative damage post-stroke.
- Nanoparticles of this size have unique advantages for drug delivery, particularly when targeting central nervous system tumors. In the referenced study, investigators used polyamidoamine dendrimers— nanoparticles whose sizes are tightly controlled due to their multigenerational structure (with sizes increasing by only 1 to 2 nm per successive generation)—to explore how size affects transvascular delivery into malignant glioma cells (Sarin et al., 2008) [208]. Although the study specifically focused on the ability of these nanoparticles to traverse the blood-brain tumor barrier, the findings are informative for the broader 5–15 nm range.
- The study demonstrated that nanoparticles must be below a certain critical size to effectively permeate the pore structures of the blood-brain tumor barrier. According to the authors, the intravenously administered functionalized dendrimers, less than approximately 11.7 to 11.9 nm in diameter, were able to traverse the pores of the blood-brain tumor barrier of RG-2 malignant gliomas, while larger ones could not.
- Given that the 5–15 nm range spans both sub-threshold (for example, those around 5–11 nm) and suprathreshold (above 11.9 nm) sizes, nanoparticles at the lower end would be more likely to cross the barrier.
- Furthermore, within the subset of permeable nanoparticles (those below roughly 11.7–11.9 nm), having long blood half-lives was crucial for effective accumulation within glioma cells. This means that even if a nanoparticle is small enough to cross the barrier, its ability to remain in circulation for sufficient periods is essential for it to localize within target tumor cells. In practical terms, nanoparticles in the 5–15 nm range that are engineered to have both an optimal size (ideally below the 11.7–11.9 nm threshold) and extended circulation times are more promising as vehicles for targeted drug delivery
3.5.6. Safety and Clearance Considerations
- Renal clearance, reducing the risk of long-term accumulation in the CNS;
- Minimized immune activation, especially with stealth coatings (e.g., PEG, zwitterionic ligands);
- Lower cytotoxicity, especially for carbon-based or polymeric systems.
3.6. Applications of Sub-15 nm Nanoparticles in Vaccines and Immunotherapy
- Cellular Uptake: The authors noted a size-dependent increase in cellular uptake by dendritic cells (DCs) and subsequent T-cell cross-priming and activation. This indicates that even within the smaller range, the nanoparticles are actively taken up by immune cells.
- Lymph Node Delivery: Upon injection into a mouse footpad, it was observed that both 22 and 33 nm OVAGNPs showed much higher delivery efficiency to draining LNs than did 10 nm OVA-GNPs. Thus, although the 10 nm particles (derived from 7 nm GNPs) are capable of local cell uptake, their efficiency in reaching lymph nodes (and hence in orchestrating systemic immune responses) appears limited compared to somewhat larger sizes.
- Immune Response Threshold: The study concludes that the size threshold for induction of potent cellular responses and T-cell poly-functionality by GNPs lies between 10 nm and 22 nm. This suggests that while nanoparticles in the 5–15 nm range (with the example particle having an effective size of ~10 nm) can induce immune responses, they may fall below the optimal threshold for inducing maximum CD8+ T-cell activation. Indeed, an ex vivo restimulation assay revealed that frequencies of OVA-specific CD8+ T cells were higher in mice immunized with 22 and 333 nm OVA-GNPs than in those immunized with 10 nm OVA-GNPs.
3.6.1. Rational for Sub-15 nm Nanoparticles in Immunomodulation
- Efficient lymph node targeting: Nanoparticles below 20–30 nm drain rapidly through lymphatic capillaries and accumulate in lymph nodes, where antigen-presenting cells (APCs) reside [214].
- Improved uptake by dendritic cells: Smaller particles are preferentially internalized by DCs via clathrin- and caveolae-mediated endocytosis, facilitating antigen processing and presentation.
- Surface engineering flexibility: High surface area allows for co-loading of antigens, adjuvants (e.g., CpG, MPLA), and targeting ligands (e.g., mannose) on the same particle.
- Enhanced antigen stability and cross-presentation: Sub-15 nm particles protect protein or peptide antigens from degradation and promote MHC class I cross-presentation for cytotoxic T cell activation.
3.6.2. Vaccine Delivery Applications
- Antigen-loaded polymeric micelles, dendrimers, liposomes, or LNPs
- Surface-conjugated peptides, proteins, or mRNA
- Immunostimulatory adjuvants, such as toll-like receptor (TLR) agonists
- Rapid lymph node trafficking, which enhances immune priming
- Controlled release of antigen, mimicking natural pathogen exposure
- Reduced systemic toxicity compared to soluble adjuvants
- LNPs ~15 nm in size were used in mRNA COVID-19 vaccines, demonstrating high efficiency in delivering nucleic acid vaccines and triggering robust humoral and cellular immunity [39].
3.6.3. Cancer Immunotherapy
- Deliver tumor-associated antigens (TAAs) or neo-antigens to APCs;
- Stimulate cytotoxic CD8+ T-cell responses;
- Reprogramming the tumor microenvironment (TME) by delivering immunomodulators;
- Co-deliver checkpoint inhibitors or siRNA targeting immune suppressive genes (e.g., PD-L1, IDO).
- (i)
- Tumor antigen delivery:
- Dendritic cell-targeted nanoparticles < 15 nm carrying TAAs (e.g., gp100, TRP2 peptides) induced tumor-specific T-cell responses and delayed tumor progression in melanoma models [220].
- (ii)
- mRNA-based cancer vaccines:
- Sub-15 nm LNPs encapsulating mRNA coding for tumor neoantigens have shown promise in personalized cancer vaccines, enabling endogenous antigen expression and potent immune activation.
- (iii)
- Immunogenic cell death (ICD):
- Small nanoparticles (~10 nm) loaded with doxorubicin or oxaliplatin can induce ICD in tumor cells, releasing danger-associated molecular patterns (DAMPs) and enhancing antigen presentation [221].
- (iv)
- Immunomodulator delivery:
- Sub-15 nm dendrimers delivering IL-2, TGF-β inhibitors, or checkpoint-blocking peptides locally within tumors modulate the TME and restore T-cell function.
3.6.4. Challenges and Considerations
- Lower antigen loading capacity due to limited volume;
- Stability issues, requiring robust surface coating (e.g., PEG, zwitterionic polymers);
- Risk of rapid renal clearance if below renal filtration threshold (~5–6 nm);
- Potential immune tolerance if poorly immunogenic antigens are presented without sufficient adjuvanticity.
3.7. Sub-15 nm Nanoparticles in Bioimaging and Theranostics
3.7.1. Advantages of Using Sub-15 nm Nanoparticles in Bioimaging
- Efficient tumor penetration and distribution, particularly in solid tumors with dense stroma or poor vascularization;
- Improved renal clearance, reducing background signal and systemic toxicity for diagnostic agents;
- High surface area for conjugation of targeting ligands and imaging probes (e.g., fluorophores, radioisotopes, contrast agents);
- Size-dependent quantum effects, enabling tunable emission for fluorescence imaging.
3.7.2. Fluorescence and Optical Imaging
- Size-tunable photoluminescence due to quantum confinement (emission ranges from UV to NIR);
- High quantum yield and photostability;
- Surface functionalization for targeting tumors, specific receptors, or organelles.
- <10 nm graphene quantum dots functionalized with folate exhibited NIR fluorescence imaging and targeted detection of ovarian tumors in vivo [95].
- They are tailored for targeting specific molecular markers, as each batch is functionalized with distinct melanoma targeting ligands.
- Their ultrasmall size offers enhanced tissue penetration and rapid clearance, reducing non–specific background.
- Their near–infrared fluorescence facilitates deep tissue imaging with high contrast.
- When combined with PET imaging, they enable precise, image–guided, and multiplexed interrogation of cancer metastases.
- The assembled nanoparticle (solid immersion lens) SIL is used for achieving wide-field and real-time super-resolution optical imaging. This means that devices based on such nanoparticles are capable of breaking the optical diffraction limit, enabling visualization of nano-scale details that are not normally resolvable with standard optical microscopes.
- The technology also proves versatile, being applicable to the observation of nanomaterials, cancer cells, and living cells or bacteria. This broad applicability underscores how the unique properties of nanoparticles in this size range (here exemplified by 15 nm TiO2) can be harnessed for diverse biological and material science applications.
- The technique is highlighted as offering a fast, wide-field, real-time, non-destructive, and low-cost solution for improving the quality of optical microscopic observation, which is a significant benefit in both research and diagnostic settings.
3.7.3. Magnetic Resonance Imaging (MRI)
- (a)
- Increases blood half-life and tumor uptake;
- (b)
- Enhances T1-weighted contrast at low concentrations;
- (c)
- Reduces liver/spleen accumulation compared to larger SPIONs.
- Sub-5 nm Mn-doped iron oxide nanoparticles offered dual T1/T2 imaging of brain tumors with minimal off-target retention [232].
3.7.4. Computed Tomography (CT) and Photoacoustic Imaging
- CT contrast agents: Sub-15 nm AuNPs accumulate in tumors and lymph nodes, enabling high-resolution, real-time anatomical imaging.
- Photoacoustic imaging: Small AuNPs and semiconducting polymer dots enable deep tissue visualization with ultrasound-coupled optical contrast [233].
- The synthesis method can produce ultrapure, size-tunable nanoparticles, with reported sizes even below 7 nm, fitting well within the 5–15 nm range.
- These nanoparticles exhibit a strong and broad plasmonic peak in the 640–700 nm region, with an extended tail over 800 nm, favoring their use in biomedical applications that require tissue-penetrating optical properties.
- Biological testing revealed that these nanoparticles exhibit low cytotoxicity and excellent cell uptake.
- They have been successfully used in photothermal therapy, signifying their potential to advance modalities such as contrast imaging, photoacoustic imaging, and surface-enhanced Raman scattering (SERS).
3.7.5. Nuclear Imaging (PET/SPECT)
- Rapid clearance reduces radiation dose.
- Small size enables receptor-mediated targeting.
- Useful for whole-body imaging of cancer metastasis and therapeutic response
- 64Cu-labeled dendrimers (~5–8 nm) conjugated with RGD peptides demonstrated PET imaging of integrin-expressing tumors with an excellent signal-to-noise ratio [235].
3.7.6. Therapeutic Applications: Integrated Diagnosis and Therapy
- Co-delivery of therapeutic agents (e.g., drugs, siRNA) and imaging labels.
- Real-time monitoring of biodistribution, tumor accumulation, and therapeutic response.
- Stimuli-responsive release triggered by pH, redox, enzymes, or light.
- Gold nanoshells (~15 nm) for photoacoustic imaging and NIR-triggered photothermal therapy.
- GQDs delivering doxorubicin and enabling fluorescence imaging-guided chemotherapy.
- Iron oxide nanoparticles releasing immune checkpoint inhibitors with MRI tracking.
4. Engineering Sub-15 nm Nanoparticles
4.1. Material Selection
- Lipid-based systems: Lipid micelles and small unilamellar liposomes (≤15 nm) have been engineered using high-pressure extrusion or microfluidics [238].
- Biomolecular nanostructures: Protein-based and DNA-origami-based nanoparticles are emerging tools for engineering precise and uniform structures in the sub-15 nm range [241].
4.2. Synthesis and Size Control Techniques
- Self-assembly: For polymeric micelles and dendrimers, critical micelle concentration (CMC), solvent polarity, temperature, and molecular weight ratios are optimized to yield sub-15 nm sizes [242].
- Microfluidics: Continuous-flow microfluidic systems allow fine control over mixing and nucleation kinetics, producing monodisperse sub-15 nm particles with high reproducibility [243].
- Reverse microemulsion: This technique enables synthesis of ultrasmall inorganic nanoparticles by confining nucleation and growth within nanoscopic water droplets in an oil phase [244].
- Ultrasonication and extrusion: Lipid-based systems are downsized using high-energy mechanical processes, often combined with surfactants to maintain structural integrity at nanoscale dimensions [238].
4.3. Surface Functionalization and Stabilization
- Zwitterionic and hydrophilic coatings: Zwitterionic polymers and hydrophilic moieties like polysaccharides offer alternatives to PEG for stealth behavior [247].
- Ligand conjugation: Antibodies, peptides, aptamers, and small molecules (e.g., folic acid, RGD peptides) can be attached to the nanoparticle surface for receptor-mediated targeting [248].
- Charge tuning: Slightly negative or near-neutral surface charges are generally favored to reduce nonspecific interactions with serum proteins and avoid rapid clearance [249].
4.4. Encapsulation and Drug Loading Strategies
- Core-loading: Hydrophobic drugs can be solubilized in the core of micelles or lipid-based carriers. However, the core volume is limited in sub-15 nm particles, often leading to lower drug loading [250].
- Surface adsorption or conjugation: Small molecule drugs or nucleic acids can be electrostatically bound or covalently conjugated to nanoparticle surfaces using cleavable linkers (e.g., pH-sensitive, redox-responsive) [251].
- Matrix entrapment: Inorganic particles may encapsulate drugs within porous frameworks or through coordination chemistry, especially in metal–organic hybrids or mesoporous silica [252].
4.5. Considerations for Scalability and Reproducibility
- Process scalability: Techniques like microfluidics, flash nanoprecipitation, and high-shear homogenization are being optimized for GMP-compatible production [253].
- Batch consistency: Real-time monitoring tools such as dynamic DLS, TEM, and field-flow fractionation (FFF) are employed to ensure narrow size distributions and reproducibility [254].
- Stability optimization: Lyophilization with cryoprotectants or formulation in aqueous buffers with stabilizers (e.g., trehalose, surfactants) is often necessary for long-term storage [255].
Sub-15 nm Nanoparticles in Industry
5. Regulatory and Quality Considerations
5.1. Regulatory Classification and Product Definition
5.2. Quality by Design (QbD) and Critical Quality Attributes (CQAs)
5.3. Analytical Challenges and Method Standardization
5.4. Toxicological Considerations and Non-Clinical Evaluation
- Cross biological barriers more readily (e.g., blood–brain barrier, placental barrier);
- Evade immune surveillance or, conversely, provoke unexpected immune responses;
- Exhibit nonlinear dose–response relationships.
- Tailored nonclinical safety assessments, including immunotoxicity, genotoxicity, and reproductive toxicity;
- Evaluation of accumulation and clearance in organs such as the kidney, liver, and spleen;
- Long-term studies for chronic exposure when applicable.
5.5. Sterility, Endotoxins, and Impurities
5.6. Regulatory Submissions and Clinical Translation
- Raw material characterization and specifications.
- Manufacturing process flow and in-process controls.
- Validation of analytical methods.
- Stability data under ICH conditions.
- Non-clinical safety data relevant to nanoparticle-specific behavior.
6. The Future Prospects of Sub-15 nm Nanoparticles
6.1. Smart Nanoparticles and Precision Engineering
6.2. Crossing Biological Barriers and Enhancing Penetration
6.3. Clinical Translation and Regulatory Science
Clinical Landscape of Sub-15 nm Nanoparticles
6.4. Biodegradable and Clearance Optimized Nanomaterials
6.5. Personalized Nanomedicine and Immune Modulation
7. Conclusions
- (a)
- There is a need to establish quantitative design rules for ultrasmall formats (size–corona–charge windows that maximize target engagement) while preserving stability/clearance using standardized in vitro–in vivo correlation (IVIVC) panels and barrier models relevant to tumors and CNS.
- (b)
- Barrier-aware engineering systematically tests how softness, aspect ratio, and ligand topology govern penetration across the ECM, mucosa, and nuclear envelopes; we need to couple these studies with responsive (smart) chemistries that activate cargo release only after barrier transit.
- (c)
- There is also a need to build assay batteries for the toxicology of sub-15 nm nanoparticles that capture ultrasmall-specific hazards (immune modulation, nonlinear dose–response, off-target barrier crossing) and agree on minimal datasets for repeat-dose studies and long-term clearance.
- (d)
- Lastly, we need to develop metrology and reference materials for validated methods (size, polydispersity, protein corona, ligand density) and community reference particles in the 5–15 nm regime to ensure cross-lab reproducibility and support regulatory review.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AF4/FFF | Asymmetric flow field-flow fractionation/field-flow fractionation |
| AuNP/AgNP | Gold/silver nanoparticle |
| BBB | Blood–brain barrier |
| CAGR | Compound annual growth rate |
| CMC | Critical micellar concentration |
| CNT | Carbon nanotube |
| COVID-19 | Coronavirus disease 2019 |
| CQA | Critical quality attributes |
| DHLA | Dihydrolipoic acid |
| DLS | Dynamic light scattering |
| CVD | Chemical vapor deposition |
| DOX | Doxorubicin |
| EPR | Enhanced permeability and retention |
| GBM | Glioblastoma |
| GQD | Graphene quantum dot |
| LNP | Lipid nanoparticle |
| LDL | Low-density lipoprotein |
| LSPR | Localized surface plasmon resonance |
| MOF | Metal–organic frameworks |
| MUA | 11-mercaptoundecanoic acid |
| MWCNT | Multi-walled carbon nanotube |
| NIR | Near infra-red |
| NLP | Nanolipoprotein particle |
| NTA | Nanoparticle tracking analysis |
| PAMAM | Poly(amidoamine) |
| PEG | Polyethylene glycol |
| PD-L1 | Programmed death-ligand 1 |
| PTX | Paclitaxel |
| RGD | Arg-Gly-Asp peptide |
| SWCNT | Single-walled carbon nanotube |
| 5TT | Quintet multiexciton state |
| TEM/SEM | Transmission/scanning electron microscopy |
| PTT/PDT | Photothermal/photodynamic therapy |
| SLN | Solid lipid nanoparticle |
| SPR | Surface plasmon resonance |
| SPION | superparamagnetic iron oxide nanoparticle |
References
- Fortune Business Insights Pvt. Ltd.: Pune, Maharashtra, India. US: +1 833 909 2966. Available online: www.fortunebusinessinsights.com/nanomedicine-market-110983 (accessed on 23 October 2025).
- Yang, S.; Meel, V.D.R.; Chen, X.; Lammers, T. The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics 2020, 10, 7921–7924. [Google Scholar] [CrossRef]
- Wu, J. The enhanced permeability and retention (EPR) effect: The significance of the concept and methods to enhance its application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef]
- Ikeda-Imafuku, M.; Wang, L.L.W.; Rodrigues, D.; Shaha, S.; Zhao, Z.; Mitragotri, S. Strategies to improve the EPR effect: A mechanistic perspective and clinical translation. J. Control. Release 2022, 345, 512–536. [Google Scholar] [CrossRef]
- Choi, H.S.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.; Ipe, B.; Bawendi, M.G.; Frangioni, J.H. Renal clearance of quantum dots. Nat. Biotechnol. 2007, 25, 1165–1170. [Google Scholar] [CrossRef]
- Liu, J.; Yu, M.; Zhou, C.; Yang, S.; Ning, X.; Zheng, J. Passive tumor targeting of renal-clearable luminescent gold nanoparticles: Long tumor retention and fast normal tissue clearance. J. Am. Chem. Soc. 2013, 135, 4978–4981. [Google Scholar] [CrossRef]
- Longmire, M.; Choyke, P.L.; Kobayashi, H. Clearance properties of nano-sized particles and molecules as imaging agents: Considerations and caveats. Nanomedicine 2008, 3, 703–717. [Google Scholar] [CrossRef]
- Cabral, H.; Matsumoto, Y.; Mizuno, K.; Chen, Q.; Murakami, M.; Kimura, M.; Terada, Y.; Kano, M.R.; Miyazono, K.; Uesaka, M.; et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumors depends on size. Nat. Nanotechnol. 2011, 6, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Koo, H.; Sun, I.C.; Yuk, S.H.; Choi, K.; Kim, K.; Kwon, I.C. Tumor-targeting multi-functional nanoparticles for theragnosis: New paradigm for cancer therapy. Adv. Drug Deliv. Rev. 2012, 64, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Fadeel, B.; Farcal, L.; Hardy, B.; Vazquez-Campos, S.; Hristozov, D.; Marcomini, A.; Lynch, I.; Valsami-Jones, E.; Alenius, H.; Savolainen, K. Advanced tools for the safety assessment of nanomaterials. Nat. Nanotechnol. 2018, 13, 537–543. [Google Scholar] [CrossRef]
- Austria, E., Jr.; Bilek, M.; Varamini, P.; Akhavan, B. Breaking biological barriers: Engineering polymeric nanoparticles for cancer therapy. Nano Today 2025, 60, 102552. [Google Scholar] [CrossRef]
- Vo, Y.; Raveendran, R.; Cao, C.; Tian, L.; Lai, R.Y.; Stenzel, M.H. Tadpole-like cationic single-chain nanoparticles display high cellular uptake. J. Mater. Chem. B 2024, 12, 12627–12640. [Google Scholar] [CrossRef]
- Kröger, A.P.P.; Paulusse, J.M.J. Single-chain polymer nanoparticles in controlled drug delivery and targeted imaging. J. Control. Release 2018, 286, 326–347. [Google Scholar] [CrossRef]
- Feng, G.; Liu, J.; Liu, R.; Mao, D.; Tomczak, N.; Liu, B. Ultrasmall conjugated polymer nanoparticles with high specificity for targeted cancer cell imaging. Adv. Sci. 2017, 4, 1600407. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, A.S.; Chauhan, P.N.; Noolvi, M.N.; Chaturvedi, K.; Ganguly, K.; Shukla, S.S.; Nadagouda, M.N.; Aminabhavi, T.M. Polymeric micelles: Basic research to clinical practice. Int. J. Pharm. 2017, 532, 249–268. [Google Scholar] [CrossRef]
- Yadav, H.K.S.; Almokdad, A.A.; Shaluf, S.I.M.; Debe, M.S. Polymer-Based Nanomaterials for Drug-Delivery Carriers; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128140338. [Google Scholar]
- Alsehli, M. Polymeric nanocarriers as stimuli-responsive systems for targeted tumor (cancer) therapy: Recent advances in drug delivery. Saudi Pharm. J. 2020, 28, 255–265. [Google Scholar] [CrossRef]
- Luxenhofer, R.; Han, Y.; Schulz, A.; Tong, J.; He, Z.; Kabanovv, A.; Jordan, R. Poly(2-oxazoline)s as polymer therapeutics. Macromol. Rapid Commun. 2012, 32, 1613–1631. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Rho, S.; Stiles, W.R.; Hu, S.; Baek, Y.; Hwang, D.W.; Kashiwagi, S. Size-dependent EPR effect of polymeric nanoparticles on tumor targeting. Adv. Healthc. Mater. 2020, 9, 1901223. [Google Scholar] [CrossRef]
- Lima, T.; Bernfur, K.; Vilanova, M.; Cedervall, T. Understanding the lipid and protein corona formation on different sized polymeric nanoparticles. Sci. Rep. 2020, 10, 1129. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, M.; Stigler, J.; Elia, G.; Lynch, I.; Cedervall, T.; Dawson, K.A. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl. Acad. Sci. USA 2008, 105, 14265–14270. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.-X. Aggregation-induced emission (AIE) polymeric micelles for imaging-guided photodynamic therapy. Nanomaterials 2018, 8, 921. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, R.; Chen, C. The Nano-Bio interactions of nanomedicines: Understanding the biochemical driving forces and redox reactions. Acc. Chem. Res. 2019, 52, 1507–1519. [Google Scholar] [CrossRef]
- Huo, M.; Yuan, J.; Tao, L.; Wei, Y. Redox-responsive polymers for drug delivery: From molecular design to applications. Polym. Chem. 2014, 5, 1519–1528. [Google Scholar] [CrossRef]
- Meher, N.; Ashley, G.W.; Bidkar, A.P.; Dhrona, S.; Fong, C.; Fontaine, D.; Vera, D.R.B.; Wilson, D.M.; Seo, Y.; Santi, D.V.; et al. Prostate-specific membrane antigen targeted deep tumor penetration of polymer nanocarriers. ACS Appl. Mater. Interfaces 2022, 14, 50569–50582. [Google Scholar] [CrossRef]
- Zhang, R.; Fan, X.; Meng, Z.; Lin, H.; Jin, Q.; Gong, F.; Dong, Z.; Li, Y.; Chen, Q.; Liu, Z.; et al. Renal Clearable Ru-based Coordination Polymer Nanodots for Photoacoustic Imaging Guided Cancer Therapy. Theranostics 2019, 9, 8266–8276. [Google Scholar] [CrossRef]
- Emam, M.H.; Elezaby, R.S.; Swidan, S.A.; Loutfy, S.A.; Hathout, R.M. Cerium Oxide Nanoparticles/Polyacrylonitrile Nanofibers as Impervious Barrier against Viral Infections. Pharmaceutics 2023, 15, 1494. [Google Scholar] [CrossRef] [PubMed]
- Mignani, S.; Rodrigues, J.; Tomas, H.; Zablocka, M.; Shi, X.; Caminade, A.; Majoral, J. Dendrimers in combination with natural products and analogues as anti-cancer agents. Chem. Soc. Rev. 2018, 47, 514–532. [Google Scholar] [CrossRef]
- Mittal, P.; Saharan, A.; Verma, R.; Altalbawy, F.M.A.; Alfaidi, M.A.; Batiha, G.E.S.; Akter, W.; Gautam, R.K.; Uddin, M.S.; Rahman, M.S. Dendrimers: A new Race of Pharmaceutical Nanocarriers. BioMed Res. Int. 2021, 2021, 8844030. [Google Scholar] [CrossRef]
- Greish, K.; Mathur, A.; Bakhiet, M.; Taurin, S. Nanomedicine: Is it lost in translation? Ther. Deliv. 2018, 9, 269–285. [Google Scholar] [CrossRef]
- Singh, P.; Gupta, U.; Asthna, A.; Jain, N.K. Folate and Folate-PEG-PAMAM Dendrimers: Synthesis, characterization, and targeted anticancer drug delivery potential in tumor bearing mice. Bioconjug. Chem. 2008, 19, 2239–2252. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Guo, H.; Ouyang, Z.; Fan, Y.; Cao, X.; Xia, J.; Shi, X.; Guo, R. Multifunctional Core-Shell Tecto Dendrimers Incorporated with Gold Nanoparticles for Targeted Dual Mode CT/MR Imaging of Tumors. ACS Appl. Bio Mater. 2021, 4, 1803–1812, Correction in ACS Appl. Bio Mater. 2021, 4, 4652. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, Y.; Zhao, Q.; Wang, Z.; Xu, Z.; Jia, X. An Enzyme-Responsive Nanogel Carrier Based on PAMAM Dendrimers for Drug Delivery. ACS Appl. Mater. Interfaces 2016, 8, 19899–19906. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Xie, L.; Banerjee, S.; Mao, G.; Padhye, S.; Sarkar, F.H.; Iyer, A.K. Hyaluronic acid conjugated polyamidoamine dendrimers for targeted delivery of 3,4-difluorobenzylidene curcumin to CD44 overexpressing pancreatic cancer cells. Colloids Surf. B Biointerfaces 2015, 136, 413–423. [Google Scholar] [CrossRef]
- Yang, J.; Wang, K.; Zheng, Y.; Piao, Y.; Wang, J.; Tang, J.; Shen, Y.; Zhou, Z. Molecularly Precise, Bright, Photostable, and Biocompatible Cyanine Nanodots as Alternatives to Quantum Dots for Biomedical Applications. Angew. Chem. Int. Ed. 2022, 61, e202202128. [Google Scholar] [CrossRef] [PubMed]
- Tomalia, D.A.; Reyna, L.A.; Svenson, S. Dendrimers as multi-purpose nanodevices for oncology drug delivery and diagnostic imaging. Biochem. Soc. Trans. 2007, 35 Pt 1, 61–67. [Google Scholar] [CrossRef]
- Twibanire, J.A.; Grindley, T.B. Polyester dendrimers: Smart carriers for drug delivery. Ploymers 2014, 6, 179–213. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Jayaraman, M.; Ansell, S.M.; Mui, B.L.; Tam, Y.K.; Chen, J.; Du, X.; Butler, D.; Eltepu, L.; Matsuda, S.; Narayanannair, J.K.; et al. Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angew. Chem. Int. Ed. 2012, 51, 8529–8533. [Google Scholar] [CrossRef]
- Mui, B.L.; Tam, Y.K.; Jayaraman, M.; Ansell, S.M.; Du, X.; Tam, Y.Y.C.; Lin, P.J.; Chen, S.; Narayanannair, J.K.; Rajeev, K.G.; et al. Influence of polyethylene glycol lipid desorption rates on pharmacokinetics and pharmacodynamics of siRNA lipid nanoparticles. Mol. Ther. Nucleic Acids 2013, 2, e139. [Google Scholar] [CrossRef]
- Scharadin, T.M.; He, W.; Yiannakou, Y.; Tomilov, A.A.; Saldana, M.; Cortopassi, G.A.; Carraway, K.L., 3rd; Coleman, M.A.; Henderson, P.T. Synthesis and biochemical characterization of EGF receptor in a water-soluble membrane model system. PLoS ONE 2017, 12, e0177761. [Google Scholar] [CrossRef]
- Onyüksel, H.; Jeon, E.; Rubinstein, I. Nanomicellar paclitaxel increases cytotoxicity of multidrug resistant breast cancer cells. Cancer Lett. 2009, 274, 327–330. [Google Scholar] [CrossRef]
- Nikanjam, M.; Blakely, E.A.; Bjornstad, K.A.; Shu, X.; Budinger, T.F.; Forte, T.M. Synthetic nano-low density lipoprotein as targeted drug delivery vehicle for glioblastoma multiforme. Int. J. Pharm. 2007, 328, 86–94. [Google Scholar] [CrossRef]
- Choi, H.; Liu, T.; Qiao, H.; Chacko, A.M.; Hu, S.H.; Chen, S.Y.; Zhou, R.; Chen, I.W. Biomimetic nanosurfactant stabilizes sub-50 nanometer phospholipid particles enabling high paclitaxel payload and deep tumor penetration. Biomaterials 2018, 181, 240–251. [Google Scholar] [CrossRef]
- Senior, J.; Gregoriadis, G. Stability of small unilamellar liposomes in serum and clearance from the circulation: The effect of the phospholipid and cholesterol components. Biochim. Biophys. Acta 1982, 712, 398–402. [Google Scholar] [CrossRef]
- Immordino, M.L.; Dosio, F.; Cattel, L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 2006, 1, 297–315. [Google Scholar]
- Solans, C.; Solé, I. Nano-emulsions: Formation by low-energy methods. Curr. Opin. Colloid Interface Sci. 2012, 17, 246–254. [Google Scholar] [CrossRef]
- Shaker, D.S.; Ishak, R.A.; Ghoneim, A.; Elhuoni, M.A. Nanoemulsion: A review on mechanisms for the transdermal delivery of hydrophobic and hydrophilic drugs. Sci. Pharm. 2019, 87, 17. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Z.; Xiong, R.; Luan, M.; Qian, Z.; Zhang, Q.; Wang, S. A multi-component paclitaxel-loaded β-elemene nanoemulsion by transferrin modification enhances anti-non-small-cell lung cancer treatment. Int. J. Pharm. 2024, 663, 124570. [Google Scholar] [CrossRef] [PubMed]
- Hoseny, S.S.; Soliman, A.M.; Fahmy, S.R.; Sadek, S.A. Development of a novel pomegranate polysaccharide nanoemulsion formulation with anti-inflammatory, antioxidant, and antitumor properties. Curr. Drug Deliv. 2023, 20, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Azadi, S.; Osanloo, M.; Zarenezhad, E.; Farjam, M.; Jalali, A.; Ghanbariasad, A. Nano-scaled emulsion and nanogel containing Mentha pulegium essential oil: Cytotoxicity on human melanoma cells and effects on apoptosis regulator genes. BMC Complement. Med. Ther. 2023, 23, 6. [Google Scholar] [CrossRef]
- Lin, Y.H.; Wang, C.C.; Lin, Y.H.; Chen, B.H. Preparation of catechin nanoemulsion from oolong tea leaf waste and its inhibition of prostate cancer cells DU-145 and tumors in mice. Molecules 2021, 26, 3260. [Google Scholar] [CrossRef]
- 54Inbaraj, B.S.; Hua, L.H.; Chen, B.H. Comparative study on inhibition of pancreatic cancer cells by resveratrol gold nanoparticles and a resveratrol nanoemulsion prepared from grape skin. Pharmaceutics 2021, 13, 1871. [Google Scholar] [CrossRef]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef] [PubMed]
- Pardeike, J.; Hommoss, A.; Müller, R.H. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int. J. Pharm. 2009, 366, 170–184. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Gold nanoparticles: Interesting optical properties and recent applications in cancer diagnostics and therapy. Nanomedicine 2006, 2, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Albanese, A.; Tang, P.S.; Chan, W.C. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Zhang, Q.; Xu, H.; Yan, Z. Effects of nanoparticle size on antitumor activity of 10-hydroxycamptothecin-conjugated gold nanoparticles: In vitro and in vivo studies. Int. J. Nanomed. 2016, 11, 929–940. [Google Scholar] [CrossRef]
- Steckiewicz, K.P.; Barcinska, E.; Malankowska, A.; Zauszkiewicz-Pawlak, A.; Nowaczyk, G.; Zaleska-Medynska, A.; Inkielewicz-Stepniak, I. Impact of gold nanoparticles shape on their cytotoxicity against human osteoblast and osteosarcoma in in vitro model. Evaluation of the safety of use and anti-cancer potential. J. Mater. Sci. Mater. Med. 2019, 30, 22. [Google Scholar] [CrossRef]
- Selim, A.A.; Sakr, T.M.; Essa, B.M.; Sayed, G.H.; Anwer, K.E. (99m)Tc-labeled benzenesulfonamide derivative-entrapped gold citrate nanoparticles as an auspicious tumour targeting. Sci. Rep. 2025, 15, 4687. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Laksee, S.; Puthong, S.; Kongkavitoon, P.; Palaga, T.; Muangsin, N. Facile and green synthesis of pullulan derivative-stabilized Au nanoparticles as drug carriers for enhancing anticancer activity. Carbohydr. Polym. 2018, 198, 495–508. [Google Scholar] [CrossRef]
- Venkatpurwar, V.; Shiras, A.; Pokharkar, V. Porphyran capped gold nanoparticles as a novel carrier for delivery of anticancer drug: In vitro cytotoxicity study. Int. J. Pharm. 2011, 409, 314–320. [Google Scholar] [CrossRef]
- Fratoddi, I.; Venditti, I.; Battocchio, C.; Polzonetti, G.; Cametti, C.; Russo, M.V. Core shell hybrids based on noble metal nanoparticles and conjugated polymers: Synthesis and characterization. Nanoscale Res. Lett. 2011, 6, 98. [Google Scholar] [CrossRef]
- Jana, B.; Kim, D.; Choi, H.; Kim, M.; Kim, K.; Kim, S.; Jin, S.; Park, M.H.; Lee, K.H.; Yoon, C.; et al. Drug resistance-free cytotoxic nanodrugs in composites for cancer therapy. J. Mater. Chem. B 2021, 9, 3143–3152. [Google Scholar] [CrossRef]
- Janic, B.; Brown, S.L.; Neff, R.; Liu, F.; Mao, G.; Chen, Y.; Jackson, L.; Chetty, I.J.; Movsas, B.; Wen, N. Therapeutic enhancement of radiation and immunomodulation by gold nanoparticles in triple negative breast cancer. Cancer Biol. Ther. 2021, 22, 124–135. [Google Scholar] [CrossRef]
- Setyawati, M.I.; Tay, C.Y.; Bay, B.H.; Leong, D.T. Gold nanoparticles induced endothelial leakiness depends on particle size and endothelial cell origin. ACS Nano 2017, 11, 5020–5030. [Google Scholar] [CrossRef] [PubMed]
- Mardina, V.; Fadlly, T.A.; Harmawan, T.; Sufriadi, E.; Iqramullah, M.; Umar, H.; Ilyas, S. Green synthesis of gold nanoparticles from the aqueous extracts of Sphagneticola trilobata (L.) J.F. Pruski as anti-breast cancer agents. J. Adv. Pharm. Technol. Res. 2024, 15, 75–80. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gurunathan, S.; Park, J.H.; Han, J.W.; Kim, J.H. Comparative assessment of the apoptotic potential of silver nanoparticles synthesized by Bacillus tequilensis and Calocybe indica in MDA-MB-231 human breast cancer cells: Targeting p53 for anticancer therapy. Int. J. Nanomed. 2015, 10, 4203–4222, Correction in Int. J. Nanomed. 2022, 17, 5207–5208. [Google Scholar] [CrossRef] [PubMed]
- Satapathy, S.R.; Mohapatra, P.; Preet, R.; Das, D.; Sarkar, B.; Choudhuri, T.; Wyatt, M.D.; Kundu, C.N. Silver-based nanoparticles induce apoptosis in human colon cancer cells mediated through p53. Nanomedicine 2013, 8, 1307–1322. [Google Scholar] [CrossRef]
- Ahmed, S.; Saifullah; Ahmad, M.; Swami, B.L.; Ikram, S. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J. Radiat. Res. Appl. Sci. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Tripathi, D.; Modi, A.; Narayan, G.; Rai, S.P. Green and cost-effective synthesis of silver nanoparticles from endangered medicinal plant Withania coagulans and their potential biomedical properties. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 100, 152–164. [Google Scholar] [CrossRef]
- Xia, Q.H.; Ma, Y.J.; Wang, J.W. Biosynthesis of silver nanoparticles using Taxus yunnanensis callus and their antibacterial activity and cytotoxicity in human cancer cells. Nanomaterials 2016, 6, 160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Majeed, S.; Danish, M.; Zakariya, N.A.; Hashim, R.; Ansari, M.T.; Alkahtani, S.; Hasnain, M.S. In vitro evaluation of antibacterial, antioxidant, and antidiabetic activities and glucose uptake through 2-NBDG by Hep-2 liver cancer cells treated with green synthesized silver nanoparticles. Oxid. Med. Cell. Longev. 2022, 2022, 1646687. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Z.; Zhai, S.; Cheng, Y.; Liu, J.; Liu, B. In vitro cytotoxicity of silver nanoparticles in primary rat hepatic stellate cells. Mol. Med. Rep. 2013, 8, 1365–1372. [Google Scholar] [CrossRef]
- Lotfy, W.A.; Alkersh, B.M.; Sabry, S.A.; Ghozlan, H.A. Biosynthesis of silver nanoparticles by Aspergillus terreus: Characterization, optimization, and biological activities. Front. Bioeng. Biotechnol. 2021, 9, 633468. [Google Scholar] [CrossRef]
- Lee, N.; Choi, S.H.; Hyeon, T. Iron oxide based nanoparticles for multimodal imaging and magnetoresponsive therapy. Chem. Rev. 2011, 115, 10637–10689. [Google Scholar] [CrossRef] [PubMed]
- Moiseeva, E.O.; Kolosnjaj-Tabi, J.; Clément, O.; Wilhelm, C. Ultrasmall maghemite nanoparticles as MRI contrast agent: Unique combination of aggregation stability, low toxicity, and tumor visualization. Nanomedicine 2025, 65, 102811. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Sant, S.; Wang, B.; Laurent, S.; Sen, T. Superparamagnetic iron oxide nanoparticles: Promises for diagnosis and treatment of cancer. Chem. Rev. 2011, 111, 253–280. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Tian, X.; Lu, Y.; Xing, H.; Li, Z.; Chen, Y.; Zhang, Y.; Zhu, D.; Yang, Y.; Ma, L. Appropriate size of Fe3O4 nanoparticles for cancer therapy by ferroptosis. ACS Appl. Bio Mater. 2022, 5, 1692–1699. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Rathi, B.S.; Chauhan, D.S.; Balhara, M.; Hooda, A. Biodegradable protein-stabilized inorganic nanoassemblies for photothermal radiotherapy of hepatoma cells. ACS Omega 2022, 7, 8928–8937. [Google Scholar] [CrossRef]
- Khaniabadi, P.M.; Shahbazi-Gahrouei, D.; Aziz, A.A.; Dheyab, M.A.; Khaniabadi, B.M.; Mehrdel, B.; Jameel, M.S. Trastuzumab conjugated porphyrin-superparamagnetic iron oxide nanoparticle: A potential PTTMRI bimodal agent for herceptin positive breast cancer. Photodiagnosis. Photodyn. Ther. 2020, 31, 101896. [Google Scholar] [CrossRef]
- Song, H.; Quan, F.; Yu, Z.; Zheng, M.; Ma, Y.; Xiao, H.; Ding, F. Carboplatin prodrug conjugated Fe3O4 nanoparticles for magnetically targeted drug delivery in ovarian cancer cells. J. Mater. Chem. B 2019, 7, 433–442. [Google Scholar] [CrossRef]
- Seggio, M.; Laneri, F.; Graziano, A.; Natile, M.; Fraix, A.; Sortino, S. Green Synthesis of Near-Infrared Plasmonic Gold Nanostructures by Pomegranate Extract and Their Supramolecular Assembling with Chemo- and Photo-Therapeutics. Nanomaterials 2022, 12, 4476. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. Quantum dot-A10 RNA aptamer-doxorubicin conjugate. In Molecular Imaging and Contrast Agent Database (MICAD); 2004–2013; National Center for Biotechnology Information: Bethesda, MD, USA, 25 August 2008. Available online: https://www.ncbi.nlm.nih.gov/books/NBK23117/ (accessed on 17 October 2025).
- Zhao, C.; Bai, Z.; Liu, X.; Zhang, Y.; Zou, B.; Zhong, H. Small GSH-capped CuInS2 quantum dots: MPA-assisted aqueous phase transfer and bioimaging applications. ACS Appl. Mater. Interfaces 2015, 7, 17623–17629. [Google Scholar] [CrossRef] [PubMed]
- Rodzik, Ł.; Lewandowska-Łańcucka, J.; Szuwarzyński, M.; Szczubiałka, K.; Nowakowska, M. Novel fluorescent CdTe quantum dot-thymine conjugate: Synthesis, properties and possible application. Nanotechnology 2017, 28, 045701. [Google Scholar] [CrossRef] [PubMed]
- Egloff, S.; Melnychuk, N.; Cruz Da Silva, E.; Reisch, A.; Martin, S.; Klymchenko, A.S. Amplified fluorescence in situ hybridization by small and bright dye-loaded polymeric nanoparticles. ACS Nano 2022, 16, 1381–1394. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, N.; Qin, Y.; Wu, F.; Xu, Z.; Lan, T.; Cheng, Z.; Zhao, P.; Liu, H. Affibody-functionalized Ag2S quantum dots for photoacoustic imaging of epidermal growth factor receptor overexpressed tumors. Nanoscale 2018, 10, 16581–16590. [Google Scholar] [CrossRef]
- Ahmad, J.; Wahab, R.; Siddiqui, M.A.; Saquib, Q.; Al-Khedhairy, A.A. Cytotoxicity and cell death induced by engineered nanostructures (quantum dots and nanoparticles) in human cell lines. J. Biol. Inorg. Chem. 2020, 25, 325–338. [Google Scholar] [CrossRef]
- Stavitskaya, A.; Sitmukhanova, E.; Sayfutdinova, A.; Khusnetdenova, E.; Mazurova, K.; Cherednichenko, K.; Naumenko, E.; Fakhrullin, R. Photoinduced antibacterial activity and cytotoxicity of CdS stabilized on mesoporous aluminosilicates and silicates. Pharmaceutics 2022, 14, 1309. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, Z.; Welsher, K.; Robinson, J.T.; Goodwin, A.; Zaric, S.; Dai, H. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 2014, 7, 548–557. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef]
- Hai, X.; Feng, J.; Chen, X.; Wang, J. Tuning the optical properties of graphene quantum dots for biosensing and bioimaging. J. Mater. Chem. B 2018, 6, 3219–3234. [Google Scholar] [CrossRef] [PubMed]
- Mazaheri Tehrani, M.; Erfani, M.; Amiri, M.; Goudarzi, M. Technetium-99m radiolabeling of graphene quantum dots (GQDs) as a new probe for glioblastoma tumor imaging. Int. J. Radiat. Biol. 2025, 101, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, W.; Wang, Y.; Tao, W.; Hou, T.; Cai, D.; Liu, L.; Liu, C.; Jiang, K.; Lin, J.; et al. The graphene quantum dots gated nanoplatform for photothermal-enhanced synergetic tumor therapy. Molecules 2024, 29, 615. [Google Scholar] [CrossRef]
- Vahedi, N.; Tabandeh, F.; Mahmoudifard, M. Hyaluronic acid-graphene quantum dot nanocomposite: Potential target drug delivery and cancer cell imaging. Biotechnol. Appl. Biochem. 2022, 69, 1068–1079. [Google Scholar] [CrossRef]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic nanoparticles and their targeted delivery applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, L.H.; Ramström, O.; Yan, M. Engineering nanomaterial surfaces for biomedical applications. Exp. Biol. Med. 2009, 234, 1128–1139. [Google Scholar] [CrossRef]
- Dias, A.P.; da Silva Santos, S.; da Silva, J.V.; Parise-Filho, R.; Ferreira, E.I.; El Seoud, O.; Giarolla, J. Dendrimers in the context of nanomedicine. Int. J. Pharm. 2020, 573, 118814. [Google Scholar] [CrossRef]
- Tegos, G.P.; Demidova, T.N.; Arcila-Lopez, D.; Lee, H.; Wharton, T.; Gali, H.; Hamblin, M.R. Cationic fullerenes are effective and selective antimicrobial photosensitizers. Chem. Biol. 2011, 12, 1127–1135. [Google Scholar] [CrossRef]
- Ji, H.; Yang, Z.; Jiang, W.; Geng, C.; Gong, M.; Xiao, H.; Wang, Z.; Cheng, L. Antiviral activity of nano carbon fullerene lipidosome against influenza virus in vitro. J. Huazhong Univ. Sci. Technol. Med. Sci. 2008, 28, 243–246. [Google Scholar] [CrossRef]
- Eatemadi, A.; Daraee, H.; Karimkhanloo, H.; Kouhi, M.; Zarghami, N.; Akbarzadeh, A.; Abasi, M.; Hanifehpour, Y.; Joo, S.W. Carbon nanotubes: Properties, synthesis, purification, and medical applications. Nanoscale Res. Lett. 2014, 9, 393. [Google Scholar] [CrossRef]
- Anzar, N.; Hasan, R.; Tyagi, M.; Yadav, N.; Narang, J. Carbon nanotube—A review on synthesis, properties and plethora of applications in the field of biomedical science. Sens. Int. 2020, 1, 100003. [Google Scholar] [CrossRef]
- Tangboriboon, N. Carbon and carbon nanotube drug delivery and its characterization, properties, and applications. Nanocarriers Drug Deliv. 2019, 2019, 451–467. [Google Scholar] [CrossRef]
- Liu, Z.; Davis, C.; Cai, W.; He, L.; Chen, X.; Dai, H. Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Res. 2007, 68, 6652–6660. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Antaris, A.L.; Dai, H. Multifunctional in vivo vascular imaging using near-infrared II fluorescent carbon nanotubes. Nat. Med. 2014, 20, 902–908. [Google Scholar] [CrossRef]
- Gamiño-Barocio, I.; Vázquez-Vázquez, E.F.; Hernández-Rodríguez, Y.M.; Cigarroa-Mayorga, O.E. Tuning the charge transfer in MWCNTs via the incorporation of ZnONPs and AgNPs: The role of carbon binding with ZnO/Ag heterostructures in reactive species formation. Nanomaterials 2024, 14, 1517. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.; Patharkar, A.; Kuche, K.; Maheshwari, R.; Deb, P.K.; Kalia, K.; Tekade, R.K. Functionalized carbon nanotubes as emerging delivery system for the treatment of cancer. Int. J. Pharm. 2018, 548, 540–558. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Xu, T.; Liao, H.; Yao, C.; Liu, Y.; Li, Z.; Chen, Z.; Pan, D.; Sun, L.; et al. Gram-scale synthesis of single-crystalline graphene quantum dots with superior optical properties. Nat. Commun. 2014, 5, 5357. [Google Scholar] [CrossRef]
- Shin, Y.; Lee, J.; Yang, J.; Park, J.; Lee, K.; Kim, S.; Park, Y.; Lee, H. Mass production of graphene quantum dots by one-pot synthesis directly from graphite in high yield. Small 2014, 10, 866–870. [Google Scholar] [CrossRef]
- Hoang, T.T.; Pham, H.; Tran, Q. A Facile Microwave-Assisted Hydrothermal Synthesis of Graphene Quantum Dots for Organic Solar Cell Efficiency Improvement. J. Nanomater. 2020, 8, 3207909. [Google Scholar] [CrossRef]
- Russo, P.; Liang, R.; Jabari, E.; Marzbanrad, E.; Toyserkani, E.; Zhou, Y.N. Single-step synthesis of graphene quantum dots by femtosecond laser ablation of graphene oxide dispersions. Nanoscale 2016, 8, 8863–8877. [Google Scholar] [CrossRef]
- Abbas, A.; Tabish, T.A.; Bull, S.J.; Lim, T.M.; Phan, A.N. High yield synthesis of graphene quantum dots from renewable biomass via microwave treatment. Sci. Rep. 2020, 10, 21262. [Google Scholar] [CrossRef]
- Price, B.; Lomeda, J.; Tour, J. Aggressively Oxidized Ultra-Short Single-Walled Carbon Nanotubes Having Oxidized Sidewalls. Chem. Mater. 2009, 21, 3917–3923. [Google Scholar] [CrossRef][Green Version]
- Furtado, C.; Kim, U.; Gutierrez, H.; Pan, L.; Dickey, E.; Eklund, P. Debundling and Dissolution of Single-Walled Carbon Nanotubes in Amide Solvents. J. Am. Chem. Soc. 2004, 126, 6095–6105. [Google Scholar] [CrossRef]
- Liu, L.; Xie, J.; Li, T.; Wu, H. Fabrication of nanopores with ultrashort single-walled carbon nanotubes inserted in a lipid bilayer. Nat. Protoc. 2015, 10, 1670–1678. [Google Scholar] [CrossRef]
- Bacha, K.; Chemotti, C.; Mbakidi, J.; Deleu, M.; Bouquillon, S. Dendrimers: Synthesis, Encapsulation Applications and Specific Interaction with the Stratum Corneum—A Review. Macromol 2023, 3, 343–370. [Google Scholar] [CrossRef]
- Mehta, M.; Bui, T.A.; Yang, X.; Aksoy, Y.; Goldys, E.M.; Deng, W. Lipid-Based Nanoparticles for Drug/Gene Delivery. ACS Mater. Au 2023, 3, 600–619. [Google Scholar] [CrossRef]
- Otsuka, H.; Nagasaki, Y.; Kataoka, K. PEGylated nanoparticles for biological and pharmaceutical applications K. Adv. Drug Deliv. Rev. 2003, 55, 403–419. [Google Scholar] [CrossRef]
- Cho, H.; Lai, T.; Tomoda, K.; Kwon, G. Polymeric Micelles for Multi-Drug Delivery in Cancer. Adv. Drug Deliv. Rev. 2014, 65, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Atkins, W.; McClary, W. Preparation of Lipid Nanodiscs with Lipid Mixtures. Curr Protoc. Protein Sci. 2019, 98, e100. [Google Scholar] [CrossRef]
- Bouchemal, K.; Briançon, S.; Perrier, E.; Fessiet, H. Nano-emulsion formulation using spontaneous emulsification: Solvent, oil and surfactant optimization. Int. J. Pharm. 2004, 280, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Solè, I.; Solans, C.; Maestro, A.; González, C.; Gutiérrez, J. Study of nano-emulsion formation by dilution of microemulsions. J. Colloid Interface Sci. 2012, 376, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Perala, S.; Kumar, S. On the Mechanism of Metal Nanoparticle Synthesis in the Brust–Schiffrin Method. Langmuir 2013, 29, 9863–9873. [Google Scholar] [CrossRef]
- Cliffel, D.; Zamborini, F.; Gross, S.; Murray, R. Mercaptoammonium-Monolayer-Protected, Water-Soluble Gold, Silver, and Palladium Clusters. Langmuir 2000, 16, 9699–9702. [Google Scholar] [CrossRef]
- Yu, W.; Falkner, J.C.; Yavuz, C.T.; Calvin, V.L. Synthesis of monodisperse iron oxidenanocrystals by thermal decomposition of iron carboxylate salts. Chem. Commun. 2004, 20, 2306–2307. [Google Scholar] [CrossRef]
- Lassenberger, A.; Grünewald, T.A.; van Oostrum, P.D.J.; Rennhofer, H.; Amenitsch, H.; Zirbs, R.; Lichtenegger, H.C.; Reimhult, E. Monodisperse Iron Oxide Nanoparticles by Thermal Decomposition: Elucidating Particle Formation by Second-Resolved in Situ Small-Angle X-ray Scattering. Chem. Mater. 2017, 29, 4511–4522. [Google Scholar] [CrossRef]
- Xie, J.; Peng, S.; Brower, N. One-pot synthesis of monodisperse iron oxidenanoparticles for potential biomedical application. Pure Appl. Chem. 2006, 78, 1003–1014. [Google Scholar] [CrossRef]
- Reiss, P.; Protière, M.; Li, L. Core/Shell semiconductor nanocrystals. Small 2009, 5, 154–168. [Google Scholar] [CrossRef]
- Winkler, R.; Ciria, M.; Ahmad, M.; Plank, H.; Marcuello, C. A Review of the Current State of Magnetic Force Microscopy to Unravel the Magnetic Properties of Nanomaterials Applied in Biological Systems and Future Directions for Quantum Technologies. Nanomaterials 2023, 13, 2585. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, A.; Tanaka, K.; Fuki, M.; Fujiwara, S.; Kimizuka, N.; Ryu, T.; Saigo, M.; Onda, K.; Kusumoto, R.; Ueno, N.; et al. Room-temperature quantum coherence of entangled multiexcitons in a metal-organic framework. Sci. Adv. 2024, 10, eadi3147. [Google Scholar] [CrossRef] [PubMed]
- Perrault, S.D.; Walkey, C.; Jennings, T.; Fischer, H.C.; Chan, W.C.W. Mediating tumor targeting efficiency of nanoparticles through design. Nano Lett. 2009, 9, 1909–1915. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Kim, B.Y.S.; Rutka, J.T.; Chan, W.C.W. Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotechnol. 2008, 3, 145–150. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Luehmann, H.; Xia, X.; Brown, P.; Jarreau, C.; Welch, M.; Xia, Y. Evaluating the pharmacokinetics and in vivo cancer targeting capability of Au nanocages by positron emission tomography imaging. ACS Nano 2012, 6, 5880–5888. [Google Scholar] [CrossRef]
- Hamada, T.; Morita, M.; Miyakawa, M.; Sugimoto, R.; Hatanaka, A.; Vestergaard, M.C.; Takagi, M. Size-dependent partitioning of nano/microparticles mediated by membrane lateral heterogeneity. J. Am. Chem. Soc. 2012, 134, 13990–13996. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.; Ma, H.; Huang, K.; Liu, J.; Wei, T.; Jin, S.; Zhang, J.; He, S.; Liang, X. Superior penetration and retention behavior of 50 nm gold nanoparticles in tumors. Cancer Res. 2013, 73, 319–330. [Google Scholar] [CrossRef]
- Chauhan, V.P.; Stylianopoulos, T.; Martin, J.D.; Popovic, Z.; Chen, O.; Kamoun, W.S.; Bawendi, M.G.; Fukumura, D.; Jain, R.K. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat. Nanotechnol. 2012, 7, 383–388. [Google Scholar] [CrossRef]
- Napierska, D.; Quarck, R.; Thomassen, L.C.J.; Lison, D.; Martens, J.A.; Delcroix, M.; Nemery, B.; Hoet, P.H. Amorphous silica nanoparticles promote monocyte adhesion to human endothelial cells: Size-dependent effect. Small 2012, 9, 430–438. [Google Scholar] [CrossRef]
- Arvizo, R.R.; Saha, S.; Wang, E.; Robertson, J.D.; Bhattacharya, R.; Mukherjee, P. Inhibition of tumor growth and metastasis by a self-therapeutic nanoparticle. Proc. Natl. Acad. Sci. USA 2013, 110, 6700–6705. [Google Scholar] [CrossRef]
- Ferreira, A.; Fernandez, J.; Federica, A.; Morelli, G.; Russo, L.; Violatto, M.; Cognet, V.; Barrientos, A.; Soliman, M.; Dobricic, M.; et al. Tuning of Ultrasmall Gold Nanoparticles Surface Properties Affect Their Biological Fate. Part. Part. Syst. Charact. 2024, 41, 2300168. [Google Scholar] [CrossRef]
- Andrian, T.; Pujals, S.; Albertazzi, L. Quantifying the effect of PEG architecture on nanoparticle ligand availability using DNA-PAINT. Nanoscale Adv. 2021, 3, 6876–6881. [Google Scholar] [CrossRef]
- Sperling, R.A.; Rivera Gil, P.; Zhang, F.; Zanella, M.; Parak, W.J. Biological applications of gold nanoparticles. Chem. Soc. Rev. 2008, 37, 1896–1908. [Google Scholar] [CrossRef]
- Hamida, R.S.; Ali, M.A.; Sharif, F.T.; Sonbol, H.; Bin-Meferij, M.M. Biofabrication of silver nanoparticles using Nostoc muscorum Lukesova 2/91: Optimization, characterization, and biological applications. Int. J. Nanomed. 2023, 18, 5625–5649. [Google Scholar] [CrossRef]
- Sumathi, P.; Renuka, N.; Subramanian, R.; Periyasami, G.; Rahaman, M.; Karthikeyan, P. Prospective in vitro A431 cell line anticancer efficacy of zirconia nanoflakes derived from Enicostemma littorale aqueous extract. Cell Biochem. Funct. 2023, 41, 676–686. [Google Scholar] [CrossRef]
- Abdelhalim, M.A. Uptake of gold nanoparticles in several rat organs after intraperitoneal administration in vivo: A fluorescence study. BioMed Res. Int. 2013, 2013, 353695. [Google Scholar] [CrossRef] [PubMed]
- Kimta, N.; Chauhan, A.; Puri, S.; Kumari, A.; Sharma, R.; Kumar, A.; Kapoor, D. Phytomediated copper oxide nanoparticles derived from the fronds of Adiantum venustum D.Don: Evaluation of their biomedical potential. Appl. Biochem. Biotechnol. 2025, 197, 398–426. [Google Scholar] [CrossRef]
- Huang, R.H.; Sobol, N.B.; Younes, A.; Mamun, T.; Lewis, J.S.; Ulijn, R.V.; O’Brien, S. Comparison of methods for surface modification of barium titanate nanoparticles for aqueous dispersibility: Toward biomedical utilization of perovskite oxides. ACS Appl. Mater. Interfaces 2020, 12, 51135–51147. [Google Scholar] [CrossRef] [PubMed]
- Hassanien, R.; Husein, D.Z.; Al-Hakkani, M.F. Biosynthesis of copper nanoparticles using aqueous Tilia extract: Antimicrobial and anticancer activities. Heliyon 2018, 4, e01077. [Google Scholar] [CrossRef] [PubMed]
- Almalki, M.A.; Khalifa, A.Y.Z. Silver nanoparticles synthesis from Bacillus sp KFU36 and its anticancer effect in breast cancer MCF-7 cells via induction of apoptotic mechanism. J. Photochem. Photobiol. B 2020, 204, 111786. [Google Scholar] [CrossRef]
- Wan, X.; Liu, M.; Ma, M.; Chen, D.; Wu, N.; Li, L.; Li, Z.; Lin, G.; Wang, X.; Xu, G. The ultrasmall biocompatible CuS@BSA nanoparticle and its photothermal effects. Front. Pharmacol. 2019, 10, 141. [Google Scholar] [CrossRef]
- Li, C.; Guan, H.; Li, Z.; Wang, F.; Wu, J.; Zhang, B. Study on different particle sizes of DOX-loaded mixed micelles for cancer therapy. Colloids Surf. B Biointerfaces 2020, 196, 111303. [Google Scholar] [CrossRef] [PubMed]
- Yücel, O.; Şengelen, A.; Emik, S.; Önay-Uçar, E.; Arda, N.; Gürdağ, G. Folic acid-modified methotrexate conjugated gold nanoparticles as nano-sized trojans for drug delivery to folate receptor-positive cancer cells. Nanotechnology 2020, 31, 355101. [Google Scholar] [CrossRef]
- Bahadori, F.; Topçu, G.; Eroğlu, M.S.; Onyüksel, H. A new lipid-based nano formulation of vinorelbine. AAPS PharmSciTech 2014, 15, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Dziawer, Ł.; Majkowska-Pilip, A.; Gaweł, D.; Godlewska, M.; Pruszyński, M.; Jastrzębski, J.; Wąs, B.; Bilewicz, A. Trastuzumab-modified gold nanoparticles labeled with 211At as a prospective tool for local treatment of HER2-positive breast cancer. Nanomaterials 2019, 9, 632. [Google Scholar] [CrossRef]
- Rejman, J.; Oberle, V.; Zuhorn, I.S.; Hoekstra, D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem. J. 2004, 377 Pt 1, 159–169. [Google Scholar] [CrossRef]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef]
- Silva, M.O.D.; Carneiro, M.L.B.; Siqueira, J.L.N.; Báo, S.N.; Souza, A.R. Development of a promising antitumor compound based on rhodium (II) succinate associated with iron oxide nanoparticles coated with lauric acid/albumin hybrid: Synthesis, colloidal stability and cytotoxic effect in breast carcinoma cells. J. Nanosci. Nanotechnol. 2018, 18, 3832–3843. [Google Scholar] [CrossRef]
- Rennick, J.J.; Johnston, A.P.R.; Parton, R.G. Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat. Nanotechnol. 2021, 16, 266–276. [Google Scholar] [CrossRef]
- Bugno, J.; Poellmann, M.J.; Sokolowski, K.; Hsu, H.J.; Kim, D.H.; Hong, S. Tumor penetration of Sub10 nm nanoparticles: Effect of dendrimer properties on their penetration in multicellular tumor spheroids. Nanomedicine 2019, 21, 102059. [Google Scholar] [CrossRef]
- Wong, C.; Stylianopoulos, T.; Cui, J.; Martin, J.; Chauhan, V.P.; Jiang, W.; Popovic, Z.; Jain, R.K.; Bawendi, M.G.; Fukumura, D. Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc. Natl. Acad. Sci. USA 2011, 108, 2426–2431. [Google Scholar] [CrossRef] [PubMed]
- Edge, D.; Shortt, C.M.; Gobbo, O.L.; Teughels, S.; Prina-Mello, A.; Volkov, Y.; MacEneaney, P.; Radomski, M.W.; Markos, F. Pharmacokinetics and bio-distribution of novel super paramagnetic iron oxide nanoparticles (SPIONs) in the anaesthetized pig. Clin. Exp. Pharmacol. Physiol. 2016, 43, 319–326. [Google Scholar] [CrossRef]
- Guerrero-Florez, V.; Mendez-Sanchez, S.C.; Patrón-Soberano, O.A.; Rodríguez-González, V.; Blach, D.; Martínez, O.F. Gold nanoparticle-mediated generation of reactive oxygen species during plasmonic photothermal therapy: A comparative study for different particle sizes, shapes, and surface conjugations. J. Mater. Chem. B 2020, 8, 2862–2875. [Google Scholar] [CrossRef]
- Huang, K.; Ma, H.; Liu, J.; Huo, S.; Kumar, A.; Wei, T.; Zhang, X.; Jin, S.; Gan, Y.; Wang, P.C.; et al. Size-dependent localization and penetration of ultrasmall gold nanoparticles in cancer cells, multicellular spheroids, and tumors in vivo. ACS Nano 2012, 6, 4483–4493. [Google Scholar] [CrossRef]
- Sierpe, R.; Donoso-González, O.; Lang, E.; Noyong, M.; Simon, U.; Kogan, M.J.; Yutronic, N. Solid-state formation of a potential melphalan delivery nanosystem based on β-cyclodextrin and silver nanoparticles. Int. J. Mol. Sci. 2023, 24, 3990. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.J.B.; Bispo, D.; Vilela, C.; Botas, A.M.P.; Ferreira, R.A.S.; Menezes, A.C.; Campos, F.; Oliveira, H.; Abreu, M.H.; Santos, S.A.O.; et al. One-minute synthesis of size-controlled fucoidan-gold nanosystems: Antitumoral activity and dark field imaging. Materials 2020, 13, 1076. [Google Scholar] [CrossRef]
- Koutsiouki, K.; Angelopoulou, A.; Ioannou, E.; Voulgari, E.; Sergides, A.; Magoulas, G.E.; Bakandritsos, A.; Avgoustakis, K. TAT peptide-conjugated magnetic PLA–PEG nanocapsules for the targeted delivery of paclitaxel: In vitro and cell studies. AAPS PharmSciTech 2017, 18, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Lévy, R.; Thanh, N.T.K.; Doty, R.C.; Hussain, I.; Nichols, R.J.; Schiffrin, D.J.; Brust, M.; Fernig, D.G. Rational and combinatorial design of peptide capping ligands for gold nanoparticles. J. Am. Chem. Soc. 2004, 126, 10076–10084. [Google Scholar] [CrossRef]
- Wen, P.; Ke, W.; Dirisala, A.; Toh, K.; Tanaka, M.; Li, J. Stealth and pseudo-stealth nanocarriers. Adv. Drug Deliv. Rev. 2023, 198, 114895. [Google Scholar] [CrossRef] [PubMed]
- Cedervall, T.; Lynch, I.; Lindman, S.; Berggard, T.; Thulin, E.; Nilsson, H.; Dawson, K.A.; Linse, S. Understanding the nanoparticle–protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc. Natl. Acad. Sci. USA 2007, 104, 2050–2055. [Google Scholar] [CrossRef]
- Walkey, C.D.; Chan, W.C. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem. Soc. Rev. 2012, 41, 2780–2799. [Google Scholar] [CrossRef]
- Chen, B.W.; He, Y.C.; Sung, S.Y.; Le, T.T.H.; Hsieh, C.L.; Chen, J.Y.; Wei, Z.H.; Yao, D.J. Synthesis and characterization of magnetic nanoparticles coated with polystyrene sulfonic acid for biomedical applications. Sci. Technol. Adv. Mater. 2020, 21, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhao, J.; Ma, J.; Yang, H.; Zhang, X.; Cao, Y.; Liu, P. GMT8 aptamer conjugated PEGylated Ag@Au core-shell nanoparticles as a novel radiosensitizer for targeted radiotherapy of glioma. Colloids Surf. B Biointerfaces 2022, 211, 112330. [Google Scholar] [CrossRef]
- Jain, R.K.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef]
- Cheng, Y.; He, C.; Riviere, J.; Monteiro-Riviere, N.; Lin, Z. Meta-Analysis of Nanoparticle Delivery to Tumors Using a Physiologically Based Pharmacokinetic Modeling and Simulation Approach. ACS Nano 2020, 14, 3075–3095. [Google Scholar] [CrossRef]
- Belyaev, I.; Griaznova, O.; Yaremenko, A.; Deyev, S.; Zelepukin, I. Beyond the EPR effect: Intravital microscopy analysis of nanoparticle drug delivery to tumors. Adv. Drug Deliv. Rev. 2025, 219, 115550. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huo, Y.; Yao, L.; Xu, Y.; Meng, F.; Li, H.; Sun, K.; Zhou, G.; Kohane, D.; Tao, K. Transcytosis of Nanomedicine for Tumor Penetration. Nano Lett. 2019, 19, 8010–8020. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, Y.; Zhang, X.; Shi, X.; Parak, W.; Pich, A. Transvascular transport of nanocarriers for tumor delivery. Nat. Commun. 2024, 15, 8172. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, R.; Chi, Y.; Sun, B.; Wang, C.; Guo, J.; Wang, C.; Zhao, Y. Graphene quantum dot nanocarriers with high drug loading for efficient delivery and therapy. Adv. Healthc. Mater. 2015, 4, 1630–1638. [Google Scholar] [CrossRef]
- Beckford Vera, D.R.; Fontaine, S.D.; VanBrocklin, H.F.; Hearn, B.R.; Reid, R.; Ashley, G.W.; Santi, D.V. PET imaging of the EPR effect in tumor xenografts using small 15 nm diameter polyethylene glycols labeled with zirconium-89. Mol. Cancer Ther. 2020, 19, 673–679. [Google Scholar] [CrossRef]
- Sindhwani, S.; Syed, A.M.; Ngai, J.; Kingston, B.R.; Maiorino, L.; Rothschild, J.; Macmillan, P.; Zhang, Y.; Rajesh, N.U.; Hoang, T.; et al. The entry of nanoparticles into solid tumours. Nat. Mater. 2020, 19, 566–575. [Google Scholar] [CrossRef]
- Li, J.; Kataoka, K. Chemo-physical Strategies to Advance the in Vivo Functionality of Targeted Nanomedicine: The Next Generation. J. Am. Chem. Soc. 2021, 143, 538–559. [Google Scholar] [CrossRef]
- Choi, C.H.J.; Alabi, C.A.; Webster, P.; Davis, M.E. Mechanism of active targeting in solid tumors with antibody functionalized nanoparticles. Proc. Natl. Acad. Sci. USA 2010, 107, 1235–1240. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.D.; Shin, D.M.; Simons, J.W.; Nie, S. Nanotechnology for targeted cancer therapy. Expert Rev. Anticancer Ther. 2007, 7, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Mullen, D.G.; McNerny, D.Q.; Desai, A.; Shloush, J.; Karnas, E.; Widin, J.; Daly, W.; Hong, S.; Baker, J.R., Jr.; Orr, B.G.; et al. Design and synthesis of a dendrimer-based modular drug delivery platform. Bioconjug. Chem. 2011, 22, 679–689. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, Y.J.; Park, Y. Folic acid and chitosan-functionalized gold nanorods and triangular silver nanoplates for the delivery of anticancer agents. Int. J. Nanomed. 2022, 17, 1881–1902. [Google Scholar] [CrossRef]
- Wu, T.; Ding, X.; Su, B.; Soodeen-Lalloo, A.K.; Zhang, L.; Shi, J.-Y. Magnetic resonance imaging of tumor angiogenesis using dual-targeting RGD10–NGR9 ultrasmall superparamagnetic iron oxide nanoparticles. Clin. Transl. Oncol. 2018, 20, 599–606. [Google Scholar] [CrossRef]
- Wu, F.; Chen, P.M.; Gardinier, T.C.; Turker, M.Z.; Venkatesan, A.M.; Patel, V.; Khor, T.; Bradbury, M.S.; Wiesner, U.B.; Adams, G.P.; et al. Dual-targeted ultrasmall silica nanoparticles for accurate cancer imaging and therapy. ACS Nano 2022, 16, 20021–20033. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S.; Cheng, G.; Yu, P.; Chang, J. Light-Responsive Nanomaterials for Cancer Therapy. Engineering 2022, 13, 18–30. [Google Scholar] [CrossRef]
- Kuang, T.; Liu, Y.; Gong, T.; Peng, X.; Hu, X.; Yu, Z. Enzyme-Responsive Nanoparticles for Anti-Cancer Drug Delivery. Curr. Nanosci. 2015, 11, 1. [Google Scholar] [CrossRef]
- Catarata, R.; Azim, N.; Bhattacharya, S.; Zhai, L. Controlled drug release from polyelectrolyte-drug conjugate nanoparticles. J. Mater. Chem. B 2020, 8, 2887–2894. [Google Scholar] [CrossRef]
- Huang, X.; Cao, J.; Zhang, Y.; Liu, T.; Yan, H. Polyethylenimine modified with 2,3-dimethylmaleic anhydride potentiates the antitumor efficacy of conventional chemotherapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 558–568. [Google Scholar] [CrossRef]
- Qiu, J.; Cheng, R.; Zhang, J.; Sun, H.; Deng, C.; Meng, F.; Zhong, Z. Glutathione-sensitive hyaluronic acid-mercaptopurine prodrug linked via carbonyl vinyl sulfide: A robust and CD44-targeted nanomedicine for leukemia. Biomacromolecules 2017, 18, 3207–3214. [Google Scholar] [CrossRef]
- Wang, X.; Li, F.; Yan, X.; Ma, Y.; Miao, Z.H.; Dong, L.; Chen, H.; Lu, Y.; Zha, Z. Ambient aqueous synthesis of ultrasmall Ni(0.85)Se nanoparticles for noninvasive photoacoustic imaging and combined photothermal-chemotherapy of cancer. ACS Appl. Mater. Interfaces 2017, 9, 41782–41793. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, Y.X.; Gao, Y.J.; Gao, F.P.; Fan, Y.S.; Li, X.J.; Duan, Z.Y.; Wang, H. Anti-bacterial and in vivo tumor treatment by reactive oxygen species generated by magnetic nanoparticles. J. Mater. Chem. B 2013, 1, 5100–5107. [Google Scholar] [CrossRef]
- Hao, R.; Yu, J.; Ge, Z.; Zhao, L.; Sheng, F.; Xu, L.; Li, G.; Hou, Y. Developing Fe3O4 nanoparticles into an efficient multimodality imaging and therapeutic probe. Nanoscale 2013, 5, 11954–11963. [Google Scholar] [CrossRef] [PubMed]
- Starha, P.; Stavárek, M.; Tuček, J.; Trávníček, Z. 4-aminobenzoic acid-coated maghemite nanoparticles as potential anticancer drug magnetic carriers: A case study on highly cytotoxic Cisplatin-like complexes involving 7-azaindoles. Molecules 2014, 19, 1622–1634. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, K.S.; Husen, A.; Sohrab, S.S.; Yassin, M.O. Recent status of nanomaterial fabrication and their potential applications in neurological disease management. Nanoscale Res. Lett. 2018, 13, 231. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood–brain barrier to treat neurodegenerative diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Mohammadinejad, R.; Kailasa, S.K.; Ahmadi, Z.; Afshar, E.G.; Pardakhty, A. Carbon dots as versatile nanoarchitectures for the treatment of neurological disorders and their theranostic applications: A review. Adv. Colloid Interface Sci. 2020, 278, 102123. [Google Scholar] [CrossRef]
- Shen, Z.; Liu, T.; Yang, Z.; Zhou, Z.; Tang, W.; Fan, W.; Liu, Y.; Mu, J.; Li, L.; Bregadze, V.I.; et al. Small-sized gadolinium oxide-based nanoparticles for high-efficiency theranostics of orthotopic glioblastoma. Biomaterials 2020, 235, 119783. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Li, J.; Ni, M.; Wang, J.; Wang, X.; Cheng, L.; Niu, W.; Zhang, Y.; Wang, Y. Establishment of an engineered bacterial membrane biomimetic nanodrug delivery system and its role in the treatment of glioma. Sichuan Da Xue Xue Bao Yi Xue Ban 2024, 55, 861–871. [Google Scholar] [CrossRef]
- Dixit, S.; Novak, T.; Miller, K.; Zhu, Y.; Kenney, M.E.; Broome, A.-M. Transferrin receptor–targeted theranostic gold nanoparticles for photosensitizer delivery in brain tumors. Nanoscale 2015, 7, 1782–1790. [Google Scholar] [CrossRef]
- McNelles, S.A.; Knight, S.D.; Janzen, N.; Valliant, J.F.; Adronov, A. Synthesis, radiolabeling, and in vivo imaging of PEGylated high-generation polyester dendrimers. Biomacromolecules 2015, 16, 3033–3041. [Google Scholar] [CrossRef]
- Hou, T.; Yang, Q.; Ding, M.; Wang, X.; Mei, K.; Guan, P.; Wang, C.; Hu, X. Blood-brain barrier permeable carbon nano-assemblies for amyloid-β clearance and neurotoxic attenuation. Colloids Surf. B Interfaces 2024, 244, 114182. [Google Scholar] [CrossRef]
- Sarin, H.; Kanevsky, A.S.; Wu, H.; Brimacombe, K.R.; Fung, S.H.; Sousa, A.A.; Auh, S.; Wilson, C.M.; Sharma, K.; Aronova, M.A.; et al. Effective transvascular delivery of nanoparticles across the blood-brain tumor barrier into malignant glioma cells. J. Transl. Med. 2008, 6, 80. [Google Scholar] [CrossRef]
- Hsu, J.C.; Barragan, D.; Tward, A.E.; Hajfathalian, M.; Amirshaghaghi, A.; Mossburg, K.J.; Rosario-Berríos, D.N.; Bouché, M.; Andrianov, A.K.; Delikatny, E.J.; et al. A biodegradable ‘one-for-all’ nanoparticle for multimodality imaging and enhanced photothermal treatment of breast cancer. Adv. Healthc. Mater. 2024, 13, e2303018. [Google Scholar] [CrossRef]
- Kim, Y.J.; Rahman, M.M.; Lee, S.M.; Kim, J.M.; Park, K.; Kang, J.H.; Seo, Y.R. Assessment of in vivo genotoxicity of citrate coated silver nanoparticles via transcriptomic analysis of rabbit liver tissue. Int. J. Nanomed. 2019, 14, 393–405. [Google Scholar] [CrossRef]
- Zhu, M.; Du, L.; Zhao, R.; Wang, H.Y.; Zhao, Y.; Nie, G.; Wang, R.F. Cell-penetrating nanoparticles activate the inflammasome to enhance antibody production by targeting microtubule-associated protein 1-light chain 3 for degradation. ACS Nano 2020, 14, 3703–3717. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Li, S.; Shi, Z.; Su, K.; Ding, Y.; Zhang, L.; Tang, Q.; Han, J.; Zhao, H.; Wang, F. Recombinant ferritin-based nanoparticles as neoantigen carriers significantly inhibit tumor growth and metastasis. J. Nanobiotechnol. 2024, 22, 562. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Ahn, S.; Lee, J.; Kim, J.Y.; Choi, M.; Gujrati, V.; Kim, H.; Kim, J.; Shin, E.C.; Jon, S. Effects of gold nanoparticle-based vaccine size on lymph node delivery and cytotoxic T-lymphocyte responses. J. Control. Release 2017, 256, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.T.; Viles, A.J.V.D.; Simeoni, E.; Angeli, V.; Randolph, G.J.; Neil, C.P.; Lee, L.K.; Swartz, M.A.; Hubbell, J.A. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat. Biotechnol. 2007, 25, 1159–1164. [Google Scholar] [CrossRef]
- Tam, H.H.; Melo, M.B.; Kang, M.; Pelet, J.M.; Ruda, V.M.; Foley, M.H.; Hu, J.K.; Kumari, S.; Crampton, J.E.; Baldeon, A.D.; et al. Sustained antigen availability during germinal center initiation enhances antibody responses to vaccination. Proc. Natl. Acad. Sci. USA 2016, 113, E6639–E6648. [Google Scholar] [CrossRef]
- Demento, S.L.; Cui, W.; Criscione, J.M.; Stern, E.; Tulipan, J.; Kaech, S.M.; Fahmy, T.M. Role of sustained antigen release from nanoparticle vaccines in shaping the T cell memory phenotype. Biomaterials 2012, 33, 4957–4964. [Google Scholar] [CrossRef]
- Bachmann, M.F.; Jennings, G.T. Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010, 10, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Manolova, V.; Flace, A.; Bauer, M.; Schwarz, K.; Saudan, P.; Bachmann, M.F. Nanoparticles target distinct dendritic cell populations according to their size. Eur. J. Immunol. 2008, 38, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.H.; Cheong, T.C.; Min, J.H.; Wu, J.H.; Lee, S.J.; Kim, D.; Yang, J.S.; Kim, S.; Kim, Y.K.; Seong, S.Y. A multifunctional core–shell nanoparticle for dendritic cell-based cancer immunotherapy. Nat. Nanotechnol. 2011, 6, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Lynn, G.M.; Sedlik, C.; Baharom, F.; Zhu, Y.; Ramirez-Valdez, R.A.; Coble, V.L.; Tobin, K.; Nichols, S.R.; Itzkowitz, Y.; Zaidi, N.B.; et al. Peptide–TLR-7/8a conjugate vaccines chemically programmed for nanoparticle self-assembly enhance CD8⁺ T-cell immunity to tumor antigens. Nat. Biotechnol. 2020, 38, 320–332. [Google Scholar] [CrossRef]
- Kuai, R.; Yuan, W.; Son, S.; Nam, J.; Xu, Y.; Fan, Y.; Schwendeman, A.; Moon, J.J. Elimination of established tumors with nanodisc-based combination chemoimmunotherapy. Sci. Adv. 2018, 4, eaao1736. [Google Scholar] [CrossRef]
- Sargsian, A.; Koutsoumpou, X.; Girmatsion, H.; Egil, C.; Buttiens, K.; Luci, C.R.; Soenen, S.J.; Manshian, B.B. Silver nanoparticle induced immunogenic cell death can improve immunotherapy. J. Nanobiotechnol. 2024, 22, 691. [Google Scholar] [CrossRef]
- Ryvolova, M.; Chomoucka, J.; Drbohlavova, J.; Kopel, P.; Babula, P.; Hynek, D.; Adam, V.; Eckschlager, T.; Hubalek, J.; Stiborova, M.; et al. Sensor applications of engineered nanoparticles in biomedicine: State of the art. Sensors 2012, 12, 14792–14820. [Google Scholar] [CrossRef]
- Naseri, N.; Ajorlou, E.; Asghari, F.; Pilehvar-Soltanahmadi, Y. Artificial cells nanomedicine and biotechnology. Artif. Cells Nanomed. Biotechnol. 2017, 46, 1111–1121. [Google Scholar] [CrossRef]
- Segets, D.; Lucas, J.M.; Klupp Taylor, R.N.; Scheele, M.; Zheng, H.; Alivisatos, A.P.; Peukert, W. Determination of the Quantum Dot Band Gap Dependence on Particle Size from Optical Absorbance and Transmission Electron Microscopy Measurements. ACS Nano 2012, 6, 9021–9032. [Google Scholar] [CrossRef] [PubMed]
- Thiruppathi, R.; Mishra, S.; Ganapathy, M.; Padmanabhan, P.; Gulyas, B. Nanoparticle Functionalization and Its Potentials for Molecular Imaging. Adv. Sci. 2017, 4, 1600279. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; van Rooy, I.; Hak, S.; Fay, F.; Tang, J.; Davies, C.d.L.; Skobe, M.; Fisher, E.A.; Radu, A.; Fayad, Z.A.; et al. Near-Infrared Fluorescence Energy Transfer Imaging of Nanoparticle Accumulation and Dissociation Kinetics in Tumor-Bearing Mice. ACS Nano 2013, 7, 10362–10370. [Google Scholar] [CrossRef][Green Version]
- Santra, S.; Dutta, D.; Walter, G.A.; Moudgil, B.M. Fluorescent Nanoparticle Probes for Cancer Imaging. Technol. Cancer Res. Treat. 2005, 4, 593–602. [Google Scholar] [CrossRef]
- Gil, H.M.; Price, T.W.; Chelani, K.; Bouillard, J.S.G.; Galaminus, S.D.J.; Stasiuk, G.J. NIR-quantum dots in biomedical imaging and their future. iScience 2021, 24, 102189. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Madajewski, B.; Ma, K.; Karassawa Zanoni, D.; Stambuk, H.; Turker, M.Z.; Monette, S.; Zhang, L.; Yoo, B.; Chen, P.; et al. Molecular phenotyping and image–guided surgical treatment of melanoma using spectrally distinct ultrasmall core–shell silica nanoparticles. Sci. Adv. 2019, 5, eaax5208. [Google Scholar] [CrossRef]
- Wang, W.; Yan, B.; Wang, H.; Chen, Y.; Nie, X.; Yi, C.; Wang, Z.; Xu, Z.; Zeng, J.; Fan, W. Wide-field and real-time super-resolution optical imaging by titanium dioxide nanoparticle-assembled solid immersion lens. Small 2023, 19, e2207596. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Lee, N.; Kim, H.; Kim, J.; Choi, S.H.; Kim, J.H.; Kim, T.; Song, I.C.; Park, S.P.; Moon, W.K.; et al. Uniform mesoporous dye-doped silica nanoparticles decorated with multiple nanocrystals for simultaneous enhanced magnetic resonance imaging, fluorescence and drug delivery. J. Am. Chem. Soc. 2010, 132, 552–557. [Google Scholar] [CrossRef]
- Wu, Y.; Huang, K.; Chen, G.; Lin, L. Advances in photoacoustic imaging of breast cancer. Sensors 2025, 25, 4812. [Google Scholar] [CrossRef]
- Popov, A.A.; Tselikov, G.; Dumas, N.; Berard, C.; Metwally, K.; Jones, N.; Al-Kattan, A.; Larrat, B.; Braguer, D.; Mensah, S.; et al. Laser-synthesized TiN nanoparticles as promising plasmonic alternative for biomedical applications. Sci. Rep. 2019, 9, 1194. [Google Scholar] [CrossRef]
- Cai, W.; Shin, Q.W.; Chen, K.; Gheysens, O.; Wang, S.X.; Gambhir, S.S.; Chen, X. Peptide-labeled near-infrared quantum dots for imaging tumor vasculature in living subjects. Nano Lett. 2006, 6, 669–676. [Google Scholar] [CrossRef]
- Kwon, G.S.; Kataoka, K. Block copolymer micelles as long-circulating drug vehicles. Adv. Drug Deliv. Rev. 1995, 16, 295–309. [Google Scholar] [CrossRef]
- Svenson, S.; Tomalia, D.A. Dendrimers in biomedical applications—Reflections on the field. Adv. Drug Deliv. Rev. 2005, 57, 2106–2129. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, M.R. Liposomes: An overview of manufacturing techniques. Cell Mol. Biol. Lett. 2005, 10, 711–719. [Google Scholar]
- Derfus, A.M.; Chan, W.C.W.; Bhatia, S.N. Probing the cytotoxicity of semiconductor quantum dots. Nano Lett. 2004, 4, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Shi, S.; Goel, S. Ultrasmall silica nanoparticles in translational biomedical research: Overview and outlook. Adv. Drug Deliv. Rev. 2023, 192, 114638. [Google Scholar] [CrossRef] [PubMed]
- Seeman, N.C.; Sleiman, H.F. DNA nanotechnology. Nat. Rev. Mater. 2018, 3, 17068. [Google Scholar] [CrossRef]
- Bae, Y.; Kataoka, K. Intelligent polymeric micelles from functional poly(ethylene glycol)-poly(amino acid) block copolymers. Adv. Drug Deliv. Rev. 2009, 61, 768–784. [Google Scholar] [CrossRef]
- Valencia, P.M.; Basto, P.A.; Zhang, L.; Rhee, M.; Langer, R.; Farokhzad, O.C.; Karnik, R. Single-step assembly of homogenous lipid–polymer nanoparticles for delivery of siRNA. ACS Nano 2010, 4, 1671–1679. [Google Scholar] [CrossRef]
- Pileni, M.P. Reverse micelles as microreactors. J. Phys. Chem. 1993, 97, 6961–6973. [Google Scholar] [CrossRef]
- Owens, D.E.; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, F.; Liu, S.; Jiang, S. Anti-PEG antibodies in the clinic: Current issues and beyond PEGylation. J. Control. Release 2016, 244, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Gao, H. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm. Sin. B 2016, 6, 268–286. [Google Scholar] [CrossRef]
- Tenzer, S.; Docter, D.; Rosfa, S.; Wlodarski, A.; Kuharev, J.; Rekik, A.; Knauer, S.K.; Bantz, C.; Nawroth, T.; Bier, C.; et al. Nanoparticle size is a critical physicochemical determinant of the human blood plasma corona: A comprehensive quantitative proteomic analysis. ACS Nano 2011, 5, 7155–7167. [Google Scholar] [CrossRef]
- Dong, Y.; Feng, S.S. Poly(d,l-lactide-co-glycolide)/montmorillonite nanoparticles for oral delivery of anticancer drugs. Biomaterials 2005, 26, 6068–6076. [Google Scholar] [CrossRef]
- Wang, Y.; Kohane, D.S. External triggering and triggered targeting strategies for drug delivery. Nat. Rev. Mater. 2017, 2, 17020. [Google Scholar] [CrossRef]
- Rosenholm, J.M.; Sahlgren, C.; Lindén, M. Towards multifunctional, targeted drug delivery systems using mesoporous silica nanoparticles–opportunities & challenges. Nanoscale 2010, 2, 1870–1883. [Google Scholar] [CrossRef] [PubMed]
- Hood, R.R.; DeVoe, D.L. High-throughput continuous flow production of nanoscale liposomes by microfluidic vertical flow focusing. Small 2015, 11, 5790–5799. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Maa, Y.F.; Prestrelski, S.J. Biopharmaceutical powders: Particle formation and formulation considerations. Curr. Pharm. Biotechnol. 2000, 1, 283–302. [Google Scholar] [CrossRef] [PubMed]
- NNCrystal US Corporation. Available online: https://nn-labs.com (accessed on 17 October 2025).
- Thermo Fisher Scientific. Available online: https://www.thermofisher.com/us/en/home/life-science/cell-analysis/cellular-imaging/fluorescence-microscopy-and-immunofluorescence-if.html (accessed on 17 October 2025).
- Ocean NanoTech. Available online: https://oceannanotech.com/web/ (accessed on 17 October 2025).
- Nanosys Shoei Chemical Inc. Available online: https://nanosys.com/ (accessed on 17 October 2025).
- Nanoco Technologies. Available online: https://www.nanocotechnologies.com/ (accessed on 17 October 2025).
- BOC Sciences. Available online: https://www.bocsci.com/superparamagnetic-iron-oxide-nano.html (accessed on 17 October 2025).
- CD Bioparticles. Available online: https://www.cd-bioparticles.com/product/carbon-dots-list-220.html (accessed on 17 October 2025).
- Yuan, F.; Yuan, T.; Sui, L.; Wang, Z.; Xi, Z.; Li, Y.; Li, X.; Fan, L.; Tan, Z.; Chen, A.; et al. Engineering triangular carbon quantum dots with unprecedented narrow bandwidth emission for multicolored LEDs. Nat. Commun. 2018, 9, 2249. [Google Scholar] [CrossRef]
- International Council for Harmonization (ICH). ICH Q8(R2): Pharmaceutical Development; ICH: Geneva, Switzerland, 2009. [Google Scholar]
- U.S. Food and Drug Administration. Considering Whether an FDA-Regulated Product Involves the Application of Nanotechnology; Guidance for Industry; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2014.
- European Medicines Agency (EMA). Reflection Paper on Nanotechnology-Based Medicinal Products for Human Use; EMA/CHMP/806058/2009 Rev. 2; European Medicines Agency: London, UK, 2019. [Google Scholar]
- Tong, F.; Wang, Y.; Gao, H. Progress and challenges in the translation of cancer nanomedicines. Curr. Opin. Biotechnol. 2024, 85, 103045. [Google Scholar] [CrossRef]
- Desai, N. Nanoparticle drug delivery system design considerations for oncology applications. Adv. Drug Deliv. Rev. 2012, 64, 1539–1549. [Google Scholar] [CrossRef]
- Park, K. Facing the truth about nanotechnology in drug delivery. ACS Nano 2013, 7, 7442–7447. [Google Scholar] [CrossRef]
- Sapsford, K.E.; Algar, W.R.; Berti, L.; Gemmill, K.B.; Casey, B.J.; Oh, E.; Stewart, M.H.; Medintz, L. Functionalizing nanoparticles with biological molecules: Developing chemistries that facilitate nanotechnology. Chem. Rev. 2013, 113, 1904–2074. [Google Scholar] [CrossRef]
- Bremer-Hoffmann, S.; Halamoda-Kenzaoui, B.; Borgos, S.E. Identification of regulatory needs for nanomedicine. J. Interdiscip. Nanomed. 2018, 3, 4–15. [Google Scholar] [CrossRef]
- Nanotechnology Characterization Lab (NCL). NCL Method Protocols: Stability Testing of Nanomaterials; Frederick National Lab: Frederick, MD, USA, 2020.
- Dobrovolskaia, M.A.; Clogston, J.D.; Neun, B.W.; Hall, J.B.; Patri, A.K.; McNeil, S.E. Assessment of hemolytic properties of nanoparticles. Nanomedicine 2013, 9, 725–733. [Google Scholar] [CrossRef]
- FDA Center for Drug Evaluation and Research (CDER). Drug Development and Drug Interactions: Table of Substrates, Inhibitors and Inducers; FDA: Silver Spring, MD, USA, 2017.
- Zhang, L.; Gu, F.X.; Chan, J.M.; Wang, A.Z.; Langer, R.S.; Farokhzad, O.C. Nanoparticles in medicine: Therapeutic applications and developments. Clin. Pharmacol. Ther. 2008, 83, 761–769. [Google Scholar] [CrossRef]
- Chang, D.; Ma, Y.; Xu, X.; Xie, J.; Ju, S. Stimuli-responsive polymeric nanoplatforms for cancer therapy. Front. Bioeng. Biotechnol. 2021, 9, 707319. [Google Scholar] [CrossRef]
- Urakova, A.; Baksheev, A.; Pryadko, A.; Grubova, I.; Surmeneva, M.; Chernozem, P.; Mukhortova, Y.; Wagner, D.; Gerasimov, E.; Kazantsev, S.; et al. Sub-20-nm magnetite-based core-shell nanoparticles with strong magnetic, magnetoelectric, and nanocatalytic properties. Ceram. Int. 2025, 51, 21702–21713. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Z.; Zhang, C.; Zhu, S.; Li, L.; Gu, Z.; Zhao, Y. Ultrasmall BiOI Quantum Dots with Efficient Renal Clearance for Enhanced Radiotherapy of Cancer. Adv. Sci. 2020, 7, 1902561. [Google Scholar] [CrossRef]
- Hare, J.I.; Lammers, T.; Ashford, M.B.; Puri, S.; Storm, G.; Barry, S.T. Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Adv. Drug Deliv. Rev. 2017, 108, 25–38. [Google Scholar] [CrossRef]
- Phillips, E.; Penate-Medina, O.; Zanzonico, P.B.; Carvajal, R.; Mohan, P.; Ye, Y.; Humm, J.; Gönen, M.; Kalaigian, H.; Schöder, H.; et al. Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe. Sci. Transl. Med. 2014, 29, 260ra149. [Google Scholar] [CrossRef] [PubMed]
- Kumthekar, P.; Ko, C.H.; Paunesku, T.; Dixit, K.; Sonabend, A.M.; Bloch, O.; Tate, M.; Schwartz, M.; Zuckerman, L.; Lezon, R.; et al. A first-in-human phase 0 clinical study of RNA interference-based spherical nucleic acids in patients with recurrent glioblastoma. Sci. Transl. Med. 2021, 13, eabb3945. [Google Scholar] [CrossRef] [PubMed]
- Jebari-Benslaiman, S.; Uribe, K.; Benito-Vicente, A.; Galicia-Garcia, U.; Larrea-Sebal, A.; Alloza, I.; Vandenbroeck, K.; Ostolaza, H.; Martín, C. Cholesterol Efflux Efficiency of Reconstituted HDL Is Affected by Nanoparticle Lipid Composition. Biomedicines 2020, 8, 373. [Google Scholar] [CrossRef] [PubMed]
- Cabral, H.; Nishiyama, N.; Kataoka, K. Supramolecular nanodevices: From design validation to theranostic nanomedicine. Acc. Chem. Res. 2011, 44, 999–1008. [Google Scholar] [CrossRef]
- Zhu, G.-H. Controlling nanoparticle clearance for translational success. Trends Pharmacol. Sci. 2022, 43, 611–622. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Simberg, D. Translational gaps in nanomedicine: Barriers to bench-to-bedside transition. Trends Pharmacol. Sci. 2018, 39, 927–937. [Google Scholar] [CrossRef]
- Fang, R.H.; Kroll, A.V.; Gao, W.; Zhang, L. Cell membrane coating nanotechnology. Adv. Mater. 2018, 30, e1706759. [Google Scholar] [CrossRef]
- Yoo, J.-W.; Doshi, N.; Mitragotri, S. Adaptive micro and nanoparticles: Temporal control over carrier properties to facilitate drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 1247–1256. [Google Scholar] [CrossRef]
- Ortiz-Perez, A.; van Tilborg, D.; van der Meel, R.; Grisoni, F.; Albertazzi, L. Machine learning–guided high-throughput nanoparticle design. Digit. Discov. 2024, 3, 1280–1291. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Monopoli, M.P.; Aberg, C.; Salvati, A.; Dawson, K.A. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 2012, 7, 779–786. [Google Scholar] [CrossRef]
- Fronya, A.A.; Antonenko, S.V.; Karpov, N.V.; Pokryshkin, N.S.; Eremina, A.S.; Yakunin, V.G.; Kharin, A.Y.; Syuy, A.V.; Volkov, V.S.; Dombrovska, Y.; et al. Germanium Nanoparticles Prepared by Laser Ablation in Low Pressure Helium and Nitrogen Atmosphere for Biophotonic Applications. Materials 2022, 15, 5308. [Google Scholar] [CrossRef]
- Grande-Tovar, C.D.; Castro, J.I.; Valencia Llano, C.H.; Tenorio, D.L.; Saavedra, M.; Zapata, P.A.; Chaur, M.N. Polycaprolactone (PCL)-polylactic acid (PLA)-glycerol (Gly) composites incorporated with zinc oxide nanoparticles (ZnO-NPs) and tea tree essential oil (TTEO) for tissue engineering applications. Pharmaceutics 2022, 15, 43. [Google Scholar] [CrossRef]
- Ashokkumar, T.; Prabhu, D.; Geetha, R.; Govindaraju, K.; Manikandan, R.; Arulvasu, C.; Singaravelu, G. Apoptosis in liver cancer (HepG2) cells induced by functionalized gold nanoparticles. Colloids Surf. B Biointerfaces 2014, 123, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Baharara, J.; Namvar, F.; Ramezani, T.; Hosseini, N.; Mohamad, R. Green synthesis of silver nanoparticles using Achillea biebersteinii flower extract and its anti-angiogenic properties in the rat aortic ring model. Molecules 2014, 19, 4624–4634. [Google Scholar] [CrossRef]
- Darwesh, O.M.; Marzoog, A.; Matter, I.A.; Okla, M.K.; El-Tayeb, M.A.; Aufy, M.; Dawoud, T.M.; Abdel-Maksoud, M.A. Natural dyes developed by microbial-nanosilver to produce antimicrobial and anticancer textiles. Microb. Cell Factories 2024, 23, 189. [Google Scholar] [CrossRef]
- Nam, J.; Won, N.; Jin, H.; Chung, H.; Kim, S. pH-Induced aggregation of gold nanoparticles for photothermal cancer therapy. J. Am. Chem. Soc. 2009, 131, 13639–13645. [Google Scholar] [CrossRef]
- Mabrouk, M.; Kenawy, S.H.; El-Bassyouni, G.E.; Ibrahim Soliman, A.A.E.; Hamzawy, E.M. Cancer Cells Treated by Clusters of Copper Oxide Doped Calcium Silicate. Adv. Pharm. Bull. 2019, 9, 102–109. [Google Scholar] [CrossRef]
- Salama, H.E.; Saad, G.R.; Sabaa, M.W. Synthesis, characterization, and biological activity of cross-linked chitosan biguanidine loaded with silver nanoparticles. J. Biomater. Sci. Polym. Ed. 2016, 27, 1880–1898. [Google Scholar] [CrossRef]
- Tang, S.; Li, Y.; Fang, Y.; Tu, M.; Wu, S.; Cen, Y.; Xu, J. Simultaneously delivery of functional gallium ions and hydrogen sulfide to endow potentiated treatment efficacy in chemo- and PARPi-resistant ovarian cancer. J. Nanobiotechnol. 2025, 23, 73. [Google Scholar] [CrossRef]
- Kaba, S.I.; Egorova, E.M. In vitro studies of the toxic effects of silver nanoparticles on HeLa and U937 cells. Nanotechnol. Sci. Appl. 2015, 8, 19–29. [Google Scholar] [CrossRef]
- Kang, S.H.; Hong, S.P.; Kang, B.S. Targeting chemo-proton therapy on C6 cell line using superparamagnetic iron oxide nanoparticles conjugated with folate and paclitaxel. Int. J. Radiat. Biol. 2018, 94, 1006–1016. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, Z.; Zhao, T.; Liu, X.; Jian, L. Mixed Micelles of Doxorubicin Overcome Multidrug Resistance by Inhibiting the Expression of P-Glycoprotein. J. Biomed. Nanotechnol. 2015, 11, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Punnoose, A.; Dodge, K.; Rasmussen, J.W.; Chess, J.; Wingett, D.; Anders, C. Cytototoxicity of ZnO nanoparticles can be tailored by modifying their surface structure: A green chemistry approach for safer nanomaterials. ACS Sustain. Chem. Eng. 2014, 2, 1666–1673. [Google Scholar] [CrossRef] [PubMed]
- Kossatz, S.; Ludwig, R.; Dähring, H.; Ettelt, V.; Rimkus, G.; Marciello, M.; Salas, G.; Patel, V.; Teran, F.J.; Hilger, I. High therapeutic efficiency of magnetic hyperthermia in xenograft models achieved with moderate temperature dosages in the tumor area. Pharm. Res. 2014, 31, 3274–3288. [Google Scholar] [CrossRef]
- Kasparis, G.; Sangnier, A.P.; Wang, L.; Efstathiou, C.; LaGrow, A.P.; Sergides, A.; Wilhelm, C.; Thanh, N.T.K. Zn-doped iron oxide nanoparticles with high magnetization and photothermal efficiency for cancer treatment. J. Mater. Chem. B 2023, 11, 787–801. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Choi, J.Y.; Na, H.B.; Seo, T.S. Quantitation of Oxidative Stress Gene Expression in Human Cell Lines Treated with Water-Dispersible MnO Nanoparticles. J. Nanosci. Nanotechnol. 2015, 15, 4126–4135. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Guo, D.; Choi, H.-S.; Kim, J.-H.; Lee, J.; Kim, Y.-S.; Kim, J. Block Copolymer-Encapsulated CaWO4 Nanocrystals: A Facile Route to Efficient Radiation Sensitizers. ACS Appl. Mater. Interfaces 2016, 8, 8608–8619. [Google Scholar] [CrossRef] [PubMed]
- Rozalen, M.; Sanchez-Polo, M.; Fernandez-Perales, M.; Widmann, T.J.; Rivera-Utrilla, J. Methotrexate-conjugated silver nanoparticles: Synthesis, characterization, and biological evaluation. RSC Adv. 2020, 10, 9540–9552. [Google Scholar] [CrossRef]
- Lévy, M.; Wilhelm, C.; Siaugue, J.-M.; Horner, O.; Bacri, J.-C.; Gazeau, F. Magnetically induced hyperthermia: Size-dependent heating power of γ-Fe2O3 nanoparticles. J. Phys. Condens. Matter 2008, 20, 204133. [Google Scholar] [CrossRef]
- Davaran, S.; Rasoulianboroujeni, M.; Hashemi, M.; Shiralizadeh Dezfuli, A.; Karimzadeh, L.; Azami, R.; Samani, S.M. Synthesis and characterization of thermally and pH-sensitive magnetic nanocomposite for controlled delivery of doxorubicin. Asian Pac. J. Cancer Prev. 2014, 15, 49–54. [Google Scholar] [CrossRef]
- Hameed, M.K.; Gul, M.T.; Khan, A.A.; Kanu, G.A.; AbuOdeh, R.O.; Kim, S.; Han, C.; Mohamed, A.A. Enhanced delivery of doxorubicin via transferrin-coated arylated gold nanoparticles. Int. J. Pharm. 2025, 673, 125418. [Google Scholar] [CrossRef]
- Dechsri, K.; Suwanchawalit, C.; Chitropas, P.; Ngawhirunpat, T.; Rojanarata, T.; Opanasopit, P.; Pengnam, S. Rapid microwave-assisted synthesis of pH-sensitive carbon-based nanoparticles for the controlled release of doxorubicin to cancer cells. AAPS PharmSciTech 2023, 24, 135. [Google Scholar] [CrossRef]
- Ahamed, M. Silica nanoparticles-induced cytotoxicity, oxidative stress and apoptosis in cultured A431 and A549 cells. Hum. Exp. Toxicol. 2013, 32, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Kumar, N.; Prasad, P.; Shirbhate, S.; Sehrawat, S.; Lochab, B. Stable dispersions of covalently tethered polymer improved graphene oxide nanoconjugates as an effective vector for siRNA delivery. ACS Appl. Mater. Interfaces 2018, 10, 14577–14593. [Google Scholar] [CrossRef]
- Cheng, Y.; Dai, Q.; Morshed, R.A.; Fan, X.; Wegscheid, M.L.; Wainwright, D.A.; Han, Y.; Zhang, L.; Auffinger, B.; Tobias, A.L.; et al. Blood–brain barrier permeable gold nanoparticles: An efficient delivery platform for enhanced malignant glioma therapy and imaging. Small 2014, 10, 5137–5150. [Google Scholar] [CrossRef]
- Gawali, S.L.; Barick, K.C.; Shetake, N.G.; Rajan, V.; Pandey, B.N.; Kumar, N.N.; Priyadarsini, K.I.; Hassan, P.A. pH-labile magnetic nanocarriers for intracellular drug delivery to tumor cells. ACS Omega 2019, 4, 11728–11736. [Google Scholar] [CrossRef]
- Kandil, E.I.; El-Sonbaty, S.M.; Moawed, F.S.; Khedr, O.M. Anticancer redox activity of gallium nanoparticles accompanied with low dose of gamma radiation in female mice. Tumour Biol. 2018, 40, 1010428317749676. [Google Scholar] [CrossRef]
- Netala, V.R.; Bukke, S.; Domdi, L.; Soneya, S.G.; Reddy, S.; Bethu, M.S.; Kotakdi, V.S.; Saritha, K.V.; Tartte, V. Biogenesis of silver nanoparticles using leaf extract of Indigofera hirsuta L. and their potential biomedical applications (3-in-1 system). Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 1), 1138–1148. [Google Scholar] [CrossRef]
- Yuan, Y.G.; Gurunathan, S. Combination of graphene oxide–silver nanoparticle nanocomposites and cisplatin enhances apoptosis and autophagy in human cervical cancer cells. Int. J. Nanomed. 2017, 12, 6537–6558. [Google Scholar] [CrossRef]
- Rio, I.S.R.; Rodrigues, A.R.O.; Rodrigues, J.M.; Queiroz, M.R.P.; Calhelha, R.C.; Ferreira, I.C.F.R.; Almeida, B.G.; Pires, A.; Pereira, A.M.; Araújo, J.P.; et al. Magnetoliposomes based on magnetic/plasmonic nanoparticles loaded with tricyclic lactones for combined cancer therapy. Pharmaceutics 2021, 13, 1905. [Google Scholar] [CrossRef]
- Dobrucka, R.; Dlugaszewska, J.; Kaczmarek, M. Cytotoxic and antimicrobial effects of biosynthesized ZnO nanoparticles using Chelidonium majus extract. Biomed. Microdevices 2018, 20, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, F.; Mao, C.; Ming, X. Multiarm nanoconjugates for cancer cell-targeted delivery of photosensitizers. Mol. Pharm. 2018, 15, 2559–2569. [Google Scholar] [CrossRef]
- Laksee, S.; Sansanaphongpricha, K.; Puthong, S.; Sangphech, N.; Palaga, T.; Muangsin, N. New organic/inorganic nanohybrids of targeted pullulan derivative/gold nanoparticles for effective drug delivery systems. Int. J. Biol. Macromol. 2020, 162, 561–577. [Google Scholar] [CrossRef] [PubMed]
- Abdelhalim, M. Alterations in gold nanoparticle levels are size dependent, with the smaller ones inducing the most toxic effects and related to the time of exposure of the gold nanoparticles. West Indian Med. J. 2015, 65, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Mahmood, S.; Aziz, T.; Azeem, A.; Rajpoot, Z.; Rehman, S.U.; Al-Asmari, F.; Alahmari, A.S.; Saleh, O.; Sameeh, M.Y.; et al. Green-synthesis of silver nanoparticles AgNPs from Podocarpus macrophyllus for targeting GBM and LGG brain cancers via NOTCH2 gene interactions. Sci. Rep. 2024, 14, 25489. [Google Scholar] [CrossRef]
- Vodnik, V.V.; Mojić, M.; Stamenović, U.; Otoničar, M.; Ajdžanović, V.; Maksimović-Ivanić, D.; Mijatović, S.; Marković, M.M.; Barudžija, T.; Filipović, B.; et al. Development of genistein-loaded gold nanoparticles and their antitumor potential against prostate cancer cell lines. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 124, 112078. [Google Scholar] [CrossRef]
- Bobyk, L.; Edouard, M.; Deman, P.; Vautrin, M.; Pernet-Gallay, K.; Delaroche, J.; Adam, J.F.; Estève, F.; Ravanat, J.L.; Elleaume, H. Photoactivation of gold nanoparticles for glioma treatment. Nanomedicine 2013, 9, 1089–1097. [Google Scholar] [CrossRef]
- Isikawa, M.; Guidelli, E. Microfluidic synthesis of theranostic nanoparticles with near-infrared scintillation: Toward next-generation dosimetry in X-ray-induced photodynamic therapy. ACS Appl. Mater. Interfaces 2022, 14, 324–336. [Google Scholar] [CrossRef]
- Mohammadi-Samani, S.; Miri, R.; Salmanpour, M.; Khalighian, N.; Sotoudeh, S.; Erfani, N. Preparation and assessment of chitosan-coated superparamagnetic Fe3O4 nanoparticles for controlled delivery of methotrexate. Res. Pharm. Sci. 2013, 8, 25–33. [Google Scholar]
- Vladimirov, G.K.; Remenshchikov, V.E.; Nesterova, A.M.; Volkov, V.V.; Vladimirov, Y.A. Comparison of the size and properties of the cytochrome c/cardiolipin nanoparticles in a sediment and non-polar medium. Biochemistry 2019, 84, 923–930. [Google Scholar] [CrossRef]
- Miyano, T.; Wijagkanalan, W.; Kawakami, S.; Yamashita, F.; Hashida, M. Anionic amino acid dendrimer-trastuzumab conjugates for specific internalization in HER2-positive cancer cells. Mol. Pharm. 2010, 7, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Logaranjan, K.; Raiza, A.J.; Gopinath, S.C.; Chen, Y.; Pandian, K. Shape- and Size-Controlled Synthesis of Silver Nanoparticles Using Aloe vera Plant Extract and Their Antimicrobial Activity. Nanoscale Res. Lett. 2016, 11, 520. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, H.; Wang, Y.; Yu, X.; Zhang, S.; Zhang, Q.; Cheng, Y. A Facile Strategy to Prepare Dendrimer-stabilized Gold Nanorods with Sub-10-nm Size for Efficient Photothermal Cancer Therapy. Sci. Rep. 2016, 6, 22764. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zhou, X.; Xu, J.; Sun, R.; Xia, H.; Ding, J.; Chin, Y.E.; Chai, Z.; Shi, H.; Gao, M. Furin enzyme and pH synergistically triggered aggregation of gold nanoparticles for activated photoacoustic imaging and photothermal therapy of tumors. Anal. Chem. 2021, 93, 9277–9285. [Google Scholar] [CrossRef]
- Ekkapongpisit, M.; Giovia, A.; Follo, C.; Caputo, G.; Isidoro, C. Biocompatibility, endocytosis, and intracellular trafficking of mesoporous silica and polystyrene nanoparticles in ovarian cancer cells: Effects of size and surface charge. Colloids Surf. B Biointerfaces 2012, 94, 251–259. [Google Scholar] [CrossRef]
- Kc, B.; Paudel, S.N.; Rayamajhi, S.; Karna, D.; Adhikari, S.; Shrestha, B.G.; Bisht, G. Enhanced preferential cytotoxicity through surface modification: Synthesis, characterization and comparative in vitro evaluation of Triton-X-100 modified and unmodified zinc oxide nanoparticles in human breast cancer cell (MDA-MB-231). Chem. Cent. J. 2016, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, D.P.; Hirsch, L.R.; Halas, N.J.; Payne, J.D.; West, J.L. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett. 2004, 209, 171–176. [Google Scholar] [CrossRef]
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv. Drug Deliv. Rev. 2001, 47, 113–131. [Google Scholar] [CrossRef] [PubMed]
- Tüncel, Ö.; Kahraman, E.; Bağci, G.; Atabey, N.; Özçelik, S. Engineered silica nanoparticles are biologically safe vehicles to deliver drugs or genes to liver cells. Mater. Sci. Eng. C 2021, 119, 111585. [Google Scholar] [CrossRef]
- Etame, A.B.; Smith, C.A.; Chan, W.C.; Rutka, J.T. Design and potential application of PEGylated gold nanoparticles with size-dependent permeation through brain microvasculature. Nanomedicine 2011, 7, 992–1000. [Google Scholar] [CrossRef]
- Tabujew, I.; Willig, M.; Leber, N.; Freidel, C.; Negwer, I.; Koynov, K.; Helm, M.; Landfester, K.; Zentel, R.; Peneva, K.; et al. Overcoming the barrier of CD8(+)T cells: Two types of nano-sized carriers for siRNA transport. Acta Biomater. 2019, 100, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Bejko, M.; Yaman, Y.A.; Keyes, A.; Bagur, A.; Rosa, P.; Gayot, M.; Weill, F.; Mornet, S.; Sandre, O. Structure-function relationship of iron oxide nanoflowers: Optimal sizes for magnetic hyperthermia depending on alternating magnetic field conditions. ChemPhysChem 2024, 25, e202400023. [Google Scholar] [CrossRef]
- Mezher, A.H.; Salehpour, M.; Saadati, Z. Folic acid-functionalized and acetyl-terminated dendrimers as nanovectors for co-delivery of sorafenib and 5-fluorouracil. Arch. Biochem. Biophys. 2024, 762, 110176. [Google Scholar] [CrossRef]
- Brinker, C.J.; Liu, K.; Zhu, W. Dual-Stage Irradiation of Size-Switchable Albumin Nanocluster for Cascaded Tumor Enhanced Penetration and Photothermal Therapy. ACS Nano 2022, 16, 13919–13932. [Google Scholar] [CrossRef]
- Govindaraju, K.; Krishnamoorthy, K.; Alsagaby, S.A.; Singaravelu, G.; Premanathan, M. Green synthesis of silver nanoparticles for selective toxicity towards cancer cells. IET Nanobiotechnol. 2015, 9, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Amina, M.; Al Musayeib, N.M.; Al-Hamoud, G.A.; Al-Dbass, A.; El-Ansary, A.; Ali, M.A. Prospective of biosynthesized L.satiVum oil/PEG/Ag-MgO bionanocomposite film for its antibacterial and anticancer potential. Saudi J. Biol. Sci. 2021, 28, 5971–5985. [Google Scholar] [CrossRef]
- Liu, J.; Guo, X.; Luo, Z.; Zhang, J.; Li, M.; Cai, K. Hierarchically stimuli-responsive nanovectors for improved tumor penetration and programed tumor therapy. Nanoscale 2018, 10, 13737–13750. [Google Scholar] [CrossRef]
- Alam, A.; Tanveer, F.; Khalil, A.T.; Zohra, T.; Khamlich, S.; Alam, M.M.; Salman, M.; Ali, M.; Ikram, A.; Shinwari, Z.K.; et al. Silver nanoparticles biosynthesized from secondary metabolite producing marine actinobacteria and evaluation of their biomedical potential. Antonie Van Leeuwenhoek 2021, 114, 1497–1516. [Google Scholar] [CrossRef]
- Fazilati, M. Folate decorated magnetite nanoparticles: Synthesis and targeted therapy against ovarian cancer. Cell Biol. Int. 2014, 38, 154–163. [Google Scholar] [CrossRef]
- Mohandoss, S.; Palanisamy, S.; You, S.; Shim, J.J.; Lee, Y.R. Supramolecular nanogels based on gelatin-cyclodextrin-stabilized silver nanocomposites with antibacterial and anticancer properties. J. Biomater. Sci. Polym. Ed. 2022, 33, 689–704. [Google Scholar] [CrossRef]
- Bishoyi, A.K.; Mandhata, C.P.; Sahoo, C.R.; Samal, P.; Dubey, D.; Jali, B.R.; Alamri, A.M.; Khan, M.S.; Padhy, R.N. Biogenic Synthesis and Characterization of Silver Nanoparticles with Cyanobacterium Oscillatoria salina Using Against MDR Pathogenic Bacteria and Their Antiproliferative and Toxicity Study. Cell Biochem. Funct. 2025, 43, e70043. [Google Scholar] [CrossRef]
- Vasilakaki, M.; Ntallis, N.; Yaacoub, N.; Muscas, G.; Peddis, D.; Trohidou, K.N. Optimising the magnetic performance of Co ferrite nanoparticles via organic ligand capping. Nanoscale 2018, 10, 21244–21253. [Google Scholar] [CrossRef]
- Abdelrahman, S.E.S.A.H.; El Hawary, S.; Mohsen, E.; El Raey, M.A.; Selim, H.M.R.M.; Hamdan, A.M.E.; Ghareeb, M.A.; Hamed, A.A. Bio-fabricated zinc oxide nanoparticles mediated by endophytic fungus Aspergillus sp. SA17 with antimicrobial and anticancer activities: In vitro supported by in silico studies. Front. Microbiol. 2024, 15, 1366614. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, N.E.; Hussein, M.H.; El-Sawah, A.A. Bio-fabrication of silver nanoparticles by phycocyanin, characterization, in vitro anticancer activity against breast cancer cell line and in vivo cytotxicity. Sci. Rep. 2017, 7, 10844. [Google Scholar] [CrossRef]
- Whitener, R.; Mosley, R.J.; Wower, J.; Byrne, M.E. Nucleic acid biohybrid nanocarriers with high therapeutic payload and controllable extended release of daunomycin for cancer therapy. J. Biomed. Mater. Res. A 2021, 109, 1256–1265. [Google Scholar] [CrossRef] [PubMed]
- Maiti, D.; Yu, H.; Mochida, Y.; Won, S.; Yamashita, S.; Naito, M.; Miyata, K.; Kim, H.J. Terbium–Rose Bengal coordination nanocrystals-induced ROS production under low-dose X-rays in cultured cancer cells for photodynamic therapy. ACS Appl. Bio Mater. 2023, 6, 2505–2513. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.J.; Chen, B.H.; Inbaraj, B.S.; Chien, J.T. Preparation of allyl isothiocyanate nanoparticles, their anti-inflammatory activity towards RAW 264.7 macrophage cells and anti-proliferative effect on HT1376 bladder cancer cells. J. Sci. Food Agric. 2019, 99, 3106–3116. [Google Scholar] [CrossRef]
- Kudłacik-Kramarczyk, S.; Drabczyk, A.; Głąb, M.; Gajda, P.; Czopek, A.; Zagorska, A.; Jaromin, A.; Gebernator, J.; Makara, A.; Tyliszczak, B. The development of the innovative synthesis methodology of albumin nanoparticles supported by their physicochemical, cytotoxic and hemolytic evaluation. Materials 2021, 14, 4386. [Google Scholar] [CrossRef]
- Han, M.; Huang-Fu, M.Y.; Guo, W.W.; Guo, N.N.; Chen, J.; Liu, H.N.; Xie, Z.Q.; Lin, M.T.; Wei, Q.C.; Gao, J.Q. MMP-2-sensitive HA end-conjugated poly(amidoamine) dendrimers via click reaction to enhance drug penetration into solid tumor. ACS Appl. Mater. Interfaces 2017, 9, 42459–42470. [Google Scholar] [CrossRef]
- Zhang, N.; Xin, X.; Feng, N.; Wu, D.; Zhang, J.; Yu, T.; Jiang, Q.; Gao, M.; Yang, H.; Zhao, S.; et al. Combining fruquintinib and doxorubicin in size-converted nano-drug carriers for tumor therapy. ACS Biomater. Sci. Eng. 2022, 8, 1907–1920. [Google Scholar] [CrossRef]
- Liu, T.; Chao, Y.; Gao, M.; Liang, C.; Chen, Q.; Song, G.; Cheng, L.; Liu, Z. Ultra-small MoS2 nanorods with rapid body clearance for photothermal cancer therapy. Nano Res. 2016, 9, 3003–3017. [Google Scholar] [CrossRef]
- Mou, J.; Li, P.; Liu, C.; Xu, H.; Song, L.; Wang, J.; Zhang, K.; Chen, Y.; Shi, J.; Chen, H. Ultrasmall Cu2-x S Nanodots for Highly Efficient Photoacoustic Imaging-Guided Photothermal Therapy. Small 2015, 11, 2275–2283. [Google Scholar] [CrossRef]
- Mao, F.; Wen, L.; Sun, C.; Zhang, S.; Wang, G.; Zeng, J.; Wang, Y.; Ma, J.; Gao, M.; Li, Z. Ultrasmall biocompatible Bi2Se3 nanodots for multimodal imaging-guided synergistic radiophotothermal therapy against cancer. ACS Nano 2016, 10, 11145–11155. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Chen, M.; Zheng, N. Sub-10-nm Pd nanosheets with renal clearance for efficient near-infrared photothermal cancer therapy. Small 2014, 10, 3139–3144. [Google Scholar] [CrossRef] [PubMed]
- Caizer, I.S.; Caizer, C. Superparamagnetic hyperthermia study with cobalt ferrite nanoparticles covered with γ-cyclodextrins by computer simulation for application in alternative cancer therapy. Int. J. Mol. Sci. 2022, 23, 4350. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; Zhang, L.; Chen, X.; Liu, L. MoO3 nanodots with pH-responsive degradation for chemo–chemodynamic therapy. Adv. Funct. Mater. 2018, 28, 1802157. [Google Scholar] [CrossRef]
- Liao, F.H.; Yu, C.C.; Hsu, C.H.; Lee, Y.H.; Chen, C.W.; Chang, Y.C.; Chou, P.T. Ultra-small platinum nanoparticle-enabled catalysis and hypoxia reversal for cancer therapy. ACS Appl. Bio Mater. 2021, 4, 6776–6785. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, Y.; Xiong, T.; Han, S.; Li, C.; He, Y.; He, Y.; Zhao, G.; Wang, T.; Wang, L.; et al. Clustered cobalt nanorods initiate ferroptosis up upregulating heme oxygenase 1 for radiotherapy sensitization. Small 2023, 19, e2206415. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Parveen, S.; Panda, J.J. The present and future of nanotechnology in human health care. Nanomedicine 2007, 3, 20–31. [Google Scholar] [CrossRef]
- Lu, J.; Liong, M.; Zink, J.I.; Tamanoi, F. Mesoporous silica nanoparticles as a delivery system for hydrophobic anticancer drugs. Small 2007, 3, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, L.R.; Stafford, R.J.; Bankson, J.A.; Sershen, S.R.; Rivera, B.; Price, R.E.; Hazle, J.D.; Halas, N.J.; West, J.L. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl. Acad. Sci. USA 2003, 100, 13549–13554. [Google Scholar] [CrossRef] [PubMed]
- Sarmento, B.; Ribeiro, A.; Veiga, F.; Sampaio, P.; Neufeld, R.; Ferreira, D. Alginate/chitosan nanoparticles are effective for oral insulin delivery. Pharm. Res. 2007, 24, 2198–2206. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Balas, F.; Arcos, D. Mesoporous materials for drug delivery. Angew. Chem. Int. Ed. 2001, 40, 1538–1540. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, V.; Epple, M. Inorganic nanoparticles as carriers of nucleic acids into cells. Angew. Chem. Int. Ed. 2010, 49, 9576–9597. [Google Scholar] [CrossRef]
- Hans, M.L.; Lowman, A.M. Biodegradable nanoparticles for drug delivery and targeting. Curr. Opin. Solid State Mater. Sci. 2002, 6, 319–327. [Google Scholar] [CrossRef]
- Douglas, T.; Young, M. Viruses: Making friends with old foes. Science 2006, 312, 873–875. [Google Scholar] [CrossRef]
- Duncan, R.; Izzo, L. Dendrimer biocompatibility and toxicity. Adv. Drug Deliv. Rev. 2005, 57, 2215–2237. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, D.; Xi, J.; Au, L.; Siekkinen, A.; Warsen, A.; Li, Z.Y.; Zhang, H.; Xia, Y.; Li, X. Gold nanocages: Bioconjugation and their potential use as optical imaging contrast agents. Nano Lett. 2005, 5, 473–477. [Google Scholar] [CrossRef]
- Jensen, A.W.; Wilson, S.R.; Schuster, D.I. Biological applications of fullerenes. Bioorg. Med. Chem. 1996, 4, 767–779. [Google Scholar] [CrossRef]
- Vinogradov, S.V.; Bronich, T.K.; Kabanov, A.V. Nanosized cationic hydrogels for drug delivery: Preparation, properties and interactions with cells. Adv. Drug Deliv. Rev. 2002, 54, 135–147. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef]
- Harris, J.M.; Chess, R.B. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2003, 2, 214–221. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Sinha, V.R.; Bansal, K.; Kaushik, R.; Kumria, R.; Trehan, A. Poly-ε-caprolactone microspheres and nanospheres: An overview. Int. J. Pharm. 2004, 278, 1–23. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, J.; Zhao, Q.; Liu, L.; Zhang, Z. Functional graphene oxide as a nanocarrier for controlled loading and targeted delivery of anticancer drugs. Small 2010, 6, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Lynn, D.M.; Langer, R. Degradable poly(β-amino esters): Synthesis, characterization, and self-assembly with plasmid DNA. J. Am. Chem. Soc. 2000, 122, 10761–10768. [Google Scholar] [CrossRef]
- Calderón, M.; Quadir, M.A.; Sharma, S.K.; Haag, R. Dendritic polyglycerols for biomedical applications. Adv. Mater. 2010, 22, 190–218. [Google Scholar] [CrossRef]
- Davis, M.E.; Zuckerman, J.E.; Choi, C.H.J.; Seligson, D.; Tolcher, A.; Alabi, C.A.; Yen, Y.; Heidel, J.D.; Ribas, A. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature 2010, 464, 1067–1070. [Google Scholar] [CrossRef]
- Fan, Y.; Miao, W.; Yan, R.; Li, C.; Wang, X. Tannylated lipid nanoparticles for prolonged circulation and PET imaging-guided cancer therapy. Biomater. Adv. 2025, 175, 214325. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Yan, X.; Zhang, L. Comparison of short-term toxicity between elemental nano-Se and selenite in mice. Life Sci. 2005, 76, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Pham, B.T.T.; Colvin, E.K.; Pham, N.T.H.; Kim, B.J.; Fuller, E.S.; Moon, E.A.; Barbey, R.; Yuen, S.; Rickman, B.H.; Bryce, N.S.; et al. Biodistribution and clearance of stable superparamagnetic maghemite iron oxide nanoparticles in mice following intraperitoneal administration. Int. J. Mol. Sci. 2018, 19, 205. [Google Scholar] [CrossRef] [PubMed]
- Torres Martin de Rosales, R.; Tavaré, R.; Glaria, A.; Varma, G.; Protti, A.; Blower, P.J. (99m)Tc-bisphosphonate-iron oxide nanoparticle conjugates for dual-modality biomedical imaging. Bioconjug. Chem. 2011, 22, 455–465. [Google Scholar] [CrossRef]
- Kumar, C.G.; Poornachandra, Y.; Mamidyala, S.K. Green synthesis of.bacterial gold nanoparticles conjugated to resveratrol as delivery vehicles. Colloids Surf. B Biointerfaces 2014, 123, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Riccò, R.; Nizzero, S.; Penna, E.; Meneghello, A.; Cretaio, E.; Enrichi, F. Ultra-small dye-doped silica nanoparticles via modified sol-gel technique. J. Nanopart. Res. 2018, 20, 117. [Google Scholar] [CrossRef]
- Pourali, P.; Svoboda, M.; Neuhöferová, E.; Dzmitruk, V.; Benson, V. Accumulation and toxicity of biologically produced gold nanoparticles in different types of specialized mammalian cells. Biotechnol. Appl. Biochem. 2024, 71, 766–778. [Google Scholar] [CrossRef]
- Li, X.; Qiu, L.; Zhu, P.; Tao, X.; Imanaka, T.; Zhao, J.; Huang, Y.; Tu, Y.; Cao, X. Epidermal growth factor-ferritin H-chain protein nanoparticles for tumor active targeting. Small 2012, 8, 2505–2514. [Google Scholar] [CrossRef]
- Le, P.; Vaidya, R.; Smith, L.D.; Han, Z.; Zahid, M.U.; Winter, J.; Sarkar, S.; Chung, H.J.; Perez-Pinera, P.; Selvin, P.R.; et al. Optimizing quantum dot probe size for single-receptor imaging. ACS Nano 2020, 14, 8343–8358. [Google Scholar] [CrossRef]
- Suh, M.; Park, J.Y.; Ko, G.B.; Kim, J.Y.; Hwang, D.W.; Rees, L.; E Conway, G.; Doak, S.H.; Kang, H.; Lee, N.; et al. Optimization of micelle-encapsulated extremely small sized iron oxide nanoparticles as a T1 contrast imaging agent: Biodistribution and safety profile. J. Nanobiotechnol. 2024, 22, 419. [Google Scholar] [CrossRef]
- Reimer, P.; Weissleder, R.; Lee, A.S.; Wittenberg, J.; Brady, T.J. Receptor imaging: Application to MR imaging of liver cancer. Radiology 1990, 177, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Rhyner, M.N.; Smith, A.M.; Gao, X.; Mao, H.; Yang, L.; Nie, S. Quantum dots and multifunctional nanoparticles: New contrast agents for tumor imaging. Nanomedicine 2006, 1, 209–217. [Google Scholar] [CrossRef]
- Quan, B.; Lee, C.; Yoo, J.S.; Piao, Y. Facile scalable synthesis of highly monodisperse small silica nanoparticles using alkaline buffer solution and their application for efficient sentinel lymph node mapping. J. Mater. Chem. B 2017, 5, 586–594. [Google Scholar] [CrossRef]
- Alzahrani, H.; Alkaltham, M.S.; Alsulami, T.; Alzahrani, A.; Althawab, S.A. Synthesis of magnetic N-doped carbon dots as pH-responsive targeted molecule cargo and its antioxidant and antibacterial behaviour. Acta Pharm. 2025, 75, 383–406. [Google Scholar] [CrossRef]
- Tufani, A.; Qureshi, A.; Niazi, J.H. Iron oxide nanoparticles based magnetic luminescent quantum dots (MQDs) synthesis and biomedical/biological applications: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111545. [Google Scholar] [CrossRef]
- Thurn, K.T.; Arora, H.; Paunesku, T.; Wu, A.; Brown, E.M.; Doty, C.; Kremer, J.; Woloschak, G. Endocytosis of titanium dioxide nanoparticles in prostate cancer PC-3M cells. Nanomedicine 2011, 7, 123–130. [Google Scholar] [CrossRef]
- Tolstik, E.; Osminkina, L.A.; Akimov, D.; Gongalsky, M.B.; Kudryavtsev, A.A.; Timoshenko, V.Y.; Heintzmann, R.; Sivakov, V.; Popp, J. Linear and Non-Linear Optical Imaging of Cancer Cells with Silicon Nanoparticles. Int. J. Mol. Sci. 2016, 17, 1536. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, H.J.; Luo, Y.L.; Xu, C.F.; Du, X.J.; Du, J.Z.; Wang, J. Enhanced primary tumor penetration facilitates nanoparticle draining into lymph nodes after systemic injection for tumor metastasis inhibition. ACS Nano 2019, 13, 8648–8658. [Google Scholar] [CrossRef] [PubMed]
- Cheyne, R.W.; Smith, T.A.; Trembleau, L.; McLaughlin, A.C. Synthesis and characterisation of biologically compatible TiO2 nanoparticles. Nanoscale Res. Lett. 2011, 6, 423. [Google Scholar] [CrossRef]
- Ali, M.R.; Snyder, B.; El-Sayed, M.A. Synthesis and optical properties of small Au nanorods using a seedless growth technique. Langmuir 2012, 28, 9807–9815. [Google Scholar] [CrossRef]
- Ahn, K.Y.; Ko, H.K.; Lee, B.R.; Lee, E.J.; Lee, J.H.; Byun, Y.; Kwon, I.C.; Kim, K.; Lee, J. Engineered protein nanoparticles for in vivo tumor detection. Biomaterials 2014, 35, 6422–6429. [Google Scholar] [CrossRef]
- Liu, K.; Yan, X.; Xu, Y.J.; Dong, L.; Hao, L.N.; Song, Y.H.; Li, F.; Su, Y.; Wu, Y.D.; Qian, H.S.; et al. Sequential growth of CaF2:Yb,Er@CaF2:Gd nanoparticles for efficient magnetic resonance angiography and tumor diagnosis. Biomater. Sci. 2017, 5, 2403–2415. [Google Scholar] [CrossRef]
- Zou, P.; Yu, Y.; Wang, Y.A.; Zhong, Y.; Welton, A.; Galbán, C.; Wang, S.; Sun, D. Superparamagnetic iron oxide nanotheranostics for targeted cancer cell imaging and pH-dependent intracellular drug release. Mol. Pharm. 2010, 7, 1974–1984. [Google Scholar] [CrossRef]
- Reynders, H.; Van Zundert, I.; Silva, R.; Carlier, B.; Deschaume, O.; Bartic, C.; Rocha, S.; Basov, S.; Van Bael, M.J.; Himmelreich, U.; et al. Label-free iron oxide nanoparticles as multimodal contrast agents in cells using multi-photon and magnetic resonance imaging. Int. J. Nanomed. 2021, 16, 8375–8389. [Google Scholar] [CrossRef]
- Eriksson, P.; Truong, A.H.T.; Brommesson, C.; du Rietz, A.; Kokil, G.R.; Boyd, R.D.; Hu, Z.; Dang, T.T.; Persson, P.O.A.; Uvdal, K. Cerium oxide nanoparticles with entrapped gadolinium for high T1 relaxivity and ROS-scavenging functionality. ACS Omega 2022, 7, 21337–21345. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.M.; Lim, S.T.; Sohn, M.H.; Jeong, H.J. Size control of (99m)Tc-tin colloid using PVP and buffer solution for sentinel lymph node detection. J. Korean Med. Sci. 2015, 30, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Li, Z.; Chen, K.; Hsu, A.R.; Xu, C.; Xie, J.; Sun, S.; Chen, X. PET/MRI dual-modality tumor imaging using arginine-glycine-aspartic (RGD)-conjugated radiolabeled iron oxide nanoparticles. J. Nucl. Med. 2008, 49, 1371–1379. [Google Scholar] [CrossRef]
- da Paz, M.C.; Santos, M.d.F.; Santos, C.M.; da Silva, S.W.; de Souza, L.B.; Lima, E.C.; Silva, R.C.; Lucci, C.M.; Morais, P.C.; Azevedo, R.B.; et al. Anti-CEA loaded maghemite nanoparticles as a theragnostic device for colorectal cancer. Int. J. Nanomed. 2012, 7, 5271–5282. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, J.; He, Y.; Sun, W.; Luo, Y.; Cai, H.; Pan, Y.; Shen, M.; Xia, J.; Shi, X. Hyaluronic acid-modified hydrothermally synthesized iron oxide nanoparticles for targeted tumor MR imaging. Biomaterials 2014, 35, 3666–3677. [Google Scholar] [CrossRef]
- Olvera-Aripez, J.; Camacho-López, S.; Flores-Castañeda, M.; Belman-Rodríguez, C.; Vilchis-Nestor, A.R.; Castro-Longoria, E. Biosynthesis of gold nanoparticles by fungi and its potential in SERS. Bioprocess Biosyst. Eng. 2024, 47, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Mulens-Arias, V.; Balfourier, A.; Nicolás-Boluda, A.; Carn, F.; Gazeau, F. Disturbance of adhesomes by gold nanoparticles reveals a size- and cell type-bias. Biomater. Sci. 2018, 7, 389–408. [Google Scholar] [CrossRef]
- Tang, R.; Xue, J.; Xu, B.; Shen, D.; Sudlow, G.P.; Achilefu, S. Tunable ultrasmall visible-to-extended near-infrared emitting silver sulfide quantum dots for integrin-targeted cancer imaging. ACS Nano 2015, 9, 220–230. [Google Scholar] [CrossRef]
- Li, D.; Wen, S.; Kong, M.; Liu, Y.; Hu, W.; Shi, B.; Shi, X.; Jin, D. Highly Doped Upconversion Nanoparticles for In Vivo Applications Under Mild Excitation Power. Anal. Chem. 2020, 92, 10913–10919. [Google Scholar] [CrossRef]
- Sabuncu, A.C.; Grubbs, J.; Qian, S.; Abdel-Fattah, T.M.; Stacey, M.W.; Beskok, A. Probing nanoparticle interactions in cell culture media. Colloids Surf. B Biointerfaces 2012, 95, 96–102. [Google Scholar] [CrossRef]
- Abdelhalim, M.A. Exposure to gold nanoparticles produces cardiac tissue damage that depends on the size and duration of exposure. Lipids Health Dis. 2011, 10, 205. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Peng, C.; Sun, W.; Yu, Z.; Zhou, B.; Li, D.; Luo, Y.; Ding, L.; Shen, M.; Shi, X. Formation of iron oxide nanoparticle-loaded γ-polyglutamic acid nanogels for MR imaging of tumors. J. Mater. Chem. B 2015, 3, 8684–8693. [Google Scholar] [CrossRef]
- Gowda, A.; Suman, T.C.; Anil, V.S.; Raghavan, S. Phytosynthesis of silver nanoparticles using aqueous sandalwood (Santalum album L.) leaf extract: Divergent effects of SW-AgNPs on proliferating plant and cancer cells. PLoS ONE 2024, 19, e0300115. [Google Scholar] [CrossRef]
- Arunkumar, P.; Thanalakshmi, M.; Kumar, P.; Premkumar, K. Micrococcus luteus mediated dual mode synthesis of gold nanoparticles: Involvement of extracellular α-amylase and cell wall teichuronic acid. Colloids Surf. B Biointerfaces 2013, 103, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Retnakumari, A.; Jayasimhan, J.; Chandran, P.; Menon, D.; Nair, S.; Mony, U.; Koyakutty, M. Cd33 monoclonal antibody conjugated Au cluster nano-bioprobe for targeted flow-cytometric detection of acute myeloid leukaemia. Nanotechnology 2011, 22, 285102. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Nguyen, V.P.; Jaiswal, S.; Kang, X.; Lee, M.; Paulus, Y.M.; Wang, T.D. Thin Layer-Protected Gold Nanoparticles for Targeted Multimodal Imaging with Photoacoustic and CT. Pharmaceuticals 2021, 14, 1075. [Google Scholar] [CrossRef]
- Tang, B.; Zhang, N.; Chen, Z.; Xu, K.; Zhuo, L.; An, L.; Yang, G. Probing hydroxyl radicals and their imaging in living cells by use of fam-DNA-Au nanoparticles. Chemistry 2008, 14, 522–528. [Google Scholar] [CrossRef]
- Le, P.; Chitoor, S.; Tu, C.; Lim, S.J.; Smith, A.M. Compact quantum dots for quantitative cytology. Methods Mol. Biol. 2020, 2064, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sharma, J.G.; Maji, S.; Malhotra, B.D. Nanostructured zirconia decorated reduced graphene oxide based efficient biosensing platform for non-invasive oral cancer detection. Biosens. Bioelectron. 2016, 78, 497504. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Ma, K.; Zhang, L.; Madajewski, B.; Zanzonico, P.; Sequeira, S.; Gonen, M.; Wiesner, U.; Bradbury, M.S. Target-or-Clear Zirconium-89 Labeled Silica Nanoparticles for Enhanced Cancer-Directed Uptake in Melanoma: A Comparison of Radiolabeling Strategies. Chem. Mater. 2017, 29, 8269–8281. [Google Scholar] [CrossRef]
- Ma, X.H.; Chen, K.; Wang, S.; Liu, S.Y.; Li, D.F.; Mi, Y.T.; Wu, Z.Y.; Qu, C.F.; Zhao, X.M. Bi-specific T1 positive-contrastenhanced magnetic resonance imaging molecular probe for hepatocellular carcinoma in an orthotopic mouse model. World J. Gastrointest. Oncol. 2022, 14, 858–871. [Google Scholar] [CrossRef]
- Wang, J.; Lu, Y.; Peng, F.; Zhong, Y.; Zhou, Y.; Jiang, X.; Su, Y.; He, Y. Photostable water-dispersible NIRemitting CdTe/CdS/ZnS core-shell-shell quantum dots for high-resolution tumor targeting. Biomaterials 2013, 34, 9509–9518. [Google Scholar] [CrossRef]
- Li, Y.; Bai, H.; Zhang, J.; Tang, J.; Li, Y.; Zhang, W.; Zhao, Z.; Xiao, Y.; Lü, Y. Fluorescent property of carbon dots extracted from cigarette smoke and the application in bio-imaging. Opt. Express 2022, 30, 47026–47037. [Google Scholar] [CrossRef]
- Mohammadi, M.; Khademi, S.; Choghazrdi, Y.; Irajirad, R.; Keshtkar, M.; Montazerabadi, A. Modified bismuth nanoparticles: A new targeted nanoprobe for computed tomography imaging of cancer. Cell J. 2022, 24, 515–521. [Google Scholar] [CrossRef]
- Yue, H.; Park, J.Y.; Chang, Y.; Lee, G.H. Ultrasmall Europium, Gadolinium, and Dysprosium Oxide Nanoparticles: Polyol Synthesis, Properties, and Biomedical Imaging Applications. Mini Rev. Med. Chem. 2020, 20, 1767–1780. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Guo, X.; Wang, H. A mitochondria-targeted ratiometric fluorescent sensor based on naphthalimide derivative-functionalized silica-based nanodots for imaging formaldehyde in living cells and zebrafish. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 15, 123970. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, B.; Sun, S. Dumbbell-like Au–Fe3O4 nanoparticles for dual-modality imaging. J. Am. Chem. Soc. 2015, 137, 1514–1517. [Google Scholar] [CrossRef]
- An, Y.; Ren, Y.; Bick, M.; Dudek, A.; Waworuntu, E.H.W.; Tang, J.; Chen, J.; Chang, B. Highly fluorescent copper nanoclusters for sensing and bioimaging. Biosens. Bioelectron. 2020, 154, 112078, Correction in Biosens. Bioelectron. 2020, 156, 112127. [Google Scholar] [CrossRef]
- Yuan, X.; Luo, Z.; Yu, Y.; Yao, Q.; Xie, J. Luminescent gold nanoclusters for bioimaging applications. Beilstein J. Nanotechnol. 2020, 11, 533–546. [Google Scholar] [CrossRef]
- Geng, P.; Yu, N.; Liu, X.; Zhu, Q.; Wen, M.; Ren, Q.; Qiu, P.; Zhang, H.; Li, M.; Chen, Z. Sub 5 nm Gd3+ -Hemoporfin Framework Nanodots for Augmented Sonodynamic Theranostics and Fast Renal Clearance. Adv. Healthc. Mater. 2021, 10, e2100703. [Google Scholar] [CrossRef]
- Hong, G.; Robinson, J.T.; Zhang, Y.; Diao, S.; Antaris, A.L.; Wang, Q.; Dai, H. In vivo fluorescence imaging with Ag2S short-wave infrared nanocrystals in the second near-infrared region. Nat. Commun. 2012, 3, 9818–9821. [Google Scholar] [CrossRef]
- Na, H.B.; Lee, J.H.; An, K.; Park, Y.I.; Park, M.; Lee, I.S.; Nam, D.H.; Kim, S.T.; Kim, S.H.; Kim, S.W.; et al. Development of a T1 contrast agent for magnetic resonance imaging using MnO nanoparticles. Angew. Chem. Int. Ed. 2007, 46, 5397–5401. [Google Scholar] [CrossRef]
- Erogbogbo, F.; Yong, K.T.; Roy, I.; Hu, R.; Law, W.C.; Zhao, W.; Ding, H.; Wu, F.; Kumar, R.; Swihart, M.T.; et al. In vivo targeted cancer imaging, sentinel lymph node mapping and multichannel imaging with biocompatible silicon nanocrystals. ACS Nano 2011, 5, 413–423. [Google Scholar] [CrossRef]
- Xie, J.; Zheng, Y.; Ying, J.Y. Protein-directed synthesis of highly fluorescent gold nanoclusters. J. Am. Chem. Soc. 2009, 131, 888–889. [Google Scholar] [CrossRef]
- Ma, J.J.; Yu, M.X.; Zhang, Z.; Cai, W.G.; Zhang, Z.L.; Zhu, H.L.; Cheng, Q.Y.; Tian, Z.Q.; Pang, D.W. Gd-DTPA-coupled Ag2Se quantum dots for dual-modality magnetic resonance imaging and fluorescence imaging in the second near-infrared window. Nanoscale 2018, 10, 10699–10704. [Google Scholar] [CrossRef]
- Stan, C.S.; Coroaba, A.; Simioescu, N.; Uritu, C.M.; Bejan, D.; Ursu, L.E.; Dascalu, A.I.; Doroftei, F.; Dobromir, M.; Albu, C.; et al. Mn-doped carbon dots as contrast agents for magnetic resonance and fluorescence imaging. Int. J. Mol. Sci. 2025, 26, 6293. [Google Scholar] [CrossRef]
- Jain, S.; Nehra, M.; Dilbaghi, N.; Chaudhary, G.R.; Kumar, S. Detection of Hg2+ Using a Dual-Mode Biosensing Probe Constructed Using Ratiometric Fluorescent Copper Nanoclusters@Zirconia Metal-Organic Framework/N-Methyl Mesoporphyrin IX and Colorimetry G-Quadruplex/Hemin Peroxidase-Mimicking G-Quadruplex DNAzyme. BME Front. 2024, 5, 0078. [Google Scholar] [CrossRef]
- Benezra, M.; Penate-Medina, O.; Zanzonico, P.B.; Schaer, D.; Ow, H.; Burns, A.; DeStanchina, E.; Longo, V.; Herz, E.; Iyer, S.; et al. Multimodal silica nanoparticles are effective cancer-targeted probes in a model of human melanoma. J. Clin. Investig. 2011, 121, 2768–2780. [Google Scholar] [CrossRef]
- Michalet, X.; Pinaud, F.F.; Bentolila, L.A.; Tsay, J.M.; Doose, S.; Li, J.J.; Sundaresan, G.; Wu, A.M.; Gambhir, S.S.; Weiss, S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 2005, 307, 538–544. [Google Scholar] [CrossRef]
- Jun, Y.W.; Lee, J.H.; Cheon, J. Chemical design of nanoparticle probes for high-performance magnetic resonance imaging. Angew. Chem. Int. Ed. 2008, 47, 5122–5135. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Cui, Y.; Levenson, R.M.; Chung, L.W.; Nie, S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotechnol. 2004, 22, 969–976. [Google Scholar] [CrossRef]
- Weissleder, R.; Elizondo, G.; Wittenberg, J.; Rabito, C.A.; Bengele, H.H.; Josephson, L. Ultrasmall superparamagnetic iron oxide: Characterization of a new class of contrast agents for MR imaging. Radiology 1995, 175, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Bulte, J.W.; Kraitchman, D.L. Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed. 2004, 17, 484–499. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Patil, S.; Bhargava, N.; Kang, J.F.; Riedel, L.M.; Seal, S.; Hickman, J.J. Auto-catalytic ceria nanoparticles offer neuroprotection to adult rat spinal cord neurons. Biomaterials 2007, 28, 1918–1925. [Google Scholar] [CrossRef]
- Karamipour, S.; Sadjadi, M.S.; Farhadyar, N. Fabrication and spectroscopic studies of folic acid-conjugated Fe3O4@Au core-shell for targeted drug delivery application. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 148, 146–155. [Google Scholar] [CrossRef]
- Song, X.; Li, C.L.; Qiu, N.; Lv, Q.Y.; Wu, X.; Cui, H.F. pH-sensitive biomimetic nanosystem based on large-pore mesoporous silica nanoparticles with high hyaluronidase loading for tumor deep penetration. ACS Appl. Mater. Interfaces 2023, 15, 38294–38308. [Google Scholar] [CrossRef] [PubMed]
- Ovejero, J.G.; Mayoral, A.; Cañete, M.; García, M.; Hernando, A.; Herrasti, P. Electrochemical synthesis and magnetic properties of MFe2O4 (M = Fe, Mn, Co, Ni) nanoparticles for potential biomedical applications. J. Nanosci. Nanotechnol. 2019, 19, 2008–2015. [Google Scholar] [CrossRef] [PubMed]
- Rajan, A.; Sharma, M.; Sahu, N.K. Assessing magnetic and inductive thermal properties of various surfactants functionalised Fe3O4 nanoparticles for hyperthermia. Sci. Rep. 2020, 10, 15045. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.J.; Lee, J.H.; Hong, S.C.; Lee, J.; Lee, J.; Han, D.W. Difference between toxicities of iron oxide magnetic nanoparticles with various surface-functional groups against human normal fibroblasts and fibrosarcoma cells. Materials 2013, 6, 4689–4706. [Google Scholar] [CrossRef]
- Wang, H.; Bi, J.; Zhu, B.W.; Tan, M. Multicolorful Carbon Dots for Tumor Theranostics. Curr. Med. Chem. 2018, 25, 2894–2909. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, S. Green Fabrication of Bioactive Silver Nanoparticles Using Mentha pulegium Extract under Alkaline: An Enhanced Anticancer Activity. ACS Omega 2021, 7, 1494–1504. [Google Scholar] [CrossRef]
- Cui, M.; Tang, Z.; Ahmad, Z.; Pan, C.; Lu, Y.; Ali, K.; Huang, S.; Lin, X.; Wahab, A.; Iqbal, M.Z.; et al. Facile synthesis of manganese-hafnium nanocomposites for multimodal MRI/CT imaging and in vitro photodynamic therapy of colon cancer. Colloids Surf. B Biointerfaces 2024, 237, 113834. [Google Scholar] [CrossRef]
- Namvar, F.; Azizi, S.; Rahman, H.S.; Mohamad, R.; Rasedee, A.; Soltani, M.; Rahim, R.A. Green synthesis, characterization, and anticancer activity of hyaluronan/zinc oxide nanocomposite. OncoTargets Ther. 2016, 9, 4549–4559. [Google Scholar] [CrossRef]
- AlSalhi, M.S.; Elangovan, K.; Ranjitsingh, A.J.A.; Murali, P.; Devanesan, S. Synthesis of silver nanoparticles using plant-derived 4-N-methyl benzoic acid and evaluation of antimicrobial, antioxidant and antitumor activity. Saudi J. Biol. Sci. 2019, 26, 970–978. [Google Scholar] [CrossRef]
- Rezaeian, A.; Amini, S.M.; Najafabadi, M.R.H.; Farsangi, Z.J.; Samadian, H. Plasmonic hyperthermia or radiofrequency electric field hyperthermia of cancerous cells through green-synthesized curcumin-coated gold nanoparticles. Lasers Med. Sci. 2021, 37, 1333–1341. [Google Scholar] [CrossRef]
- Matijević, M.; Žakula, J.; Korićanac, L.; Radoičić, M.; Liang, X.; Mi, L.; Tričković, J.F.; Šobot, A.V.; Stanković, M.N.; Nakarada, Đ.; et al. Controlled killing of human cervical cancer cells by combined action of blue light and C-doped TiO2 nanoparticles. Photochem. Photobiol. Sci. 2021, 20, 1087–1098. [Google Scholar] [CrossRef]
- Attar, M.M.; Amanpour, S.; Haghpanahi, M.; Haddadi, M.; Rezaei, G.; Muhammadnejad, S.; HajiAkhoundzadeh, M.; Barati, T.; Sadeghi, F.; Javadi, S. Thermal analysis of magnetic nanoparticle in alternating magnetic field on human HCT-116 colon cancer cell line. Int. J. Hyperth. 2016, 32, 858–867. [Google Scholar] [CrossRef]
- Feng, Q.; Liu, Y.; Huang, J.; Chen, K.; Huang, J.; Xiao, K. Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings. Sci. Rep. 2018, 8, 2082. [Google Scholar] [CrossRef]
- Cho, J.; Wang, M.; Gonzalez-Lepera, C.; Mawlawi, O.; Cho, S.H. Development of bimetallic (Zn@Au) nanoparticles as potential PET-imageable radiosensitizers. Med. Phys. 2016, 43, 4775. [Google Scholar] [CrossRef] [PubMed]
- Seemann, K.M.; Kovács, A.; Schmid, T.E.; Ilicic, K.; Multhoff, G.; Dunin-Borkowski, R.E.; Michelagnoli, C.; Cieplicka-Oryńczak, N.; Jana, S.; Colombi, G.; et al. Fe-Pt-Yb2O3 core–shell nanoparticles with switchable magnetic properties and β-emitting shell after activation. iScience 2023, 26, 107683. [Google Scholar] [CrossRef]
- Laxman, K.; Reddy, B.P.K.; Mishra, S.K.; Gopal, M.B.; Robinson, A.; De, A.; Srivastava, R.; Ravikanth, M. BF2-oxasmaragdyrin nanoparticles: A non-toxic, photostable, enhanced non-radiative decay-assisted efficient photothermal cancer theragnostic agent. ACS Appl. Mater. Interfaces 2020, 12, 52329–52342. [Google Scholar] [CrossRef]
- Gal, N.; Lassenberger, A.; Herrero-Nogareda, L.; Scheberl, A.; Charwat, V.; Kasper, C.; Reimhult, E. Interaction of size-tailored PEGylated iron oxide nanoparticles with lipid membranes and cells. ACS Biomater. Sci. Eng. 2017, 3, 249–259. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Othman, S.F.; Curtis, E.T.; Bauer, N.A.; Chauhan, N.; Kumar, D.; Jaggi, M.; Chauhan, S.C. Curcumin-loaded magnetic nanoparticles for breast cancer therapeutics and imaging applications. Int. J. Nanomed. 2012, 7, 1761–1779. [Google Scholar] [CrossRef] [PubMed]
- Hiremath, C.G.; Heggnnavar, G.B.; Kariduraganavar, M.Y.; Hiremath, M.B. Co-delivery of paclitaxel and curcumin to foliate positive cancer cells using Pluronic-coated iron oxide nanoparticles. Prog. Biomater. 2019, 8, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Grillo, R.; Gallo, J.; Stroppa, D.G.; Carbó-Argibay, E.; Lima, R.; Fraceto, L.F.; Bañobre-López, M. Sub-micrometer magnetic nanocomposites: Insights into the effect of magnetic nanoparticles interactions on the optimization of SAR and MRI performance. ACS Appl. Mater. Interfaces 2016, 8, 29293–29304. [Google Scholar] [CrossRef] [PubMed]
- Mulens-Arias, V.; Balfourier, A.; Nicolás-Boluda, A.; Carn, F.; Gazeau, F. Endocytosis-driven gold nanoparticle fractal rearrangement in cells and its influence on photothermal conversion. Nanoscale 2020, 12, 21832–21849. [Google Scholar] [CrossRef]
- Lan, H.; Jamil, M.; Ke, G.; Dong, N. The role of nanoparticles and nanomaterials in cancer diagnosis and treatment: A comprehensive review. Am. J. Cancer Res. 2023, 13, 5751–5784. [Google Scholar]
- Javid, A.; Ahmadian, S.; Saboury, A.A.; Kalantar, S.M.; Rezaei-Zarchi, S.; Shahzad, S. Biocompatible APTES-PEG modified magnetite nanoparticles: Effective carriers of antineoplastic agents to ovarian cancer. Appl. Biochem. Biotechnol. 2014, 173, 36–54. [Google Scholar] [CrossRef]
- Chen, Y.; Xiang, M.; Wang, Z. Application of Novel Hollow Carbon Nanosphere Drug-Loading System in Chemotherapy of Esophageal Squamous Cell Carcinoma. J. Nanosci. Nanotechnol. 2021, 21, 814–823. [Google Scholar] [CrossRef]
- Chen, H.; Qiu, Y.; Ding, D.; Lin, H.; Sun, W.; Wang, G.D.; Huang, W.; Zhang, W.; Lee, D.; Liu, G.; et al. Gadolinium-Encapsulated Graphene Carbon Nanotheranostics for Imaging-Guided Photodynamic Therapy. Adv. Mater. 2018, 30, e1802748. [Google Scholar] [CrossRef]
- Islam, S.N.; Naqvi, S.M.A.; Gorain, M.; Roy, G.; Kundu, G.C.; Ahmad, A. Sustainable Synthesis of Nitrogen-Embedded Cu2S Quantum Dots for In Vitro and In Vivo Breast Cancer Management. ACS Appl. Biomater. 2025, 8, 410–419. [Google Scholar] [CrossRef]
- Kharat, P.B.; Somvanshi, S.B.; Khirade, P.P.; Jadhav, K.M. Induction Heating Analysis of Surface-Functionalized Nanoscale CoFe2O4 for Magnetic Fluid Hyperthermia toward Noninvasive Cancer Treatment. ACS Omega 2020, 5, 23378–23384. [Google Scholar] [CrossRef]
- Chahardoli, A.; Karimi, N.; Sadeghi, F.; Fattahi, A. Green synthesis of gold nanoparticles using Nigella arvensis leaf extract and evaluation of their antibacterial, antioxidant, cytotoxicity, and catalytic properties. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 2), 579–588. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.; Yang, P.; Dai, Y.; Gai, S.; He, F.; Lin, J. Lutecium fluoride hollow mesoporous spheres with enhanced up-conversion luminescent bioimaging and light-triggered drug release by gold nanocrystals. ACS Appl. Mater. Interfaces 2014, 6, 15550–15563. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Huang, B.B.; Lai, C.M.; Lu, Y.S.; Shao, J.W. Advancements in the synthesis of carbon dots and their application in biomedicine. J. Photochem. Photobiol. B Biol. 2024, 255, 112920. [Google Scholar] [CrossRef]
- Moetasam Zorab, M.; Mohammadjani, N.; Ashengroph, M.; Alavi, M. Biosynthesis of quantum dots and their therapeutic applications in the diagnosis and treatment of cancer and SARS-CoV-2. Adv. Pharm. Bull. 2023, 13, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Fritsch-Decker, S.; An, Z.; Yan, J.; Hansjosten, I.; Al-Rawi, M.; Peravali, R.; Diabaté, S.; Weiss, C. Silica nanoparticles provoke cell death independent of p53 and BAX in human colon cancer cells. Nanomaterials 2019, 9, 1172. [Google Scholar] [CrossRef]
- Jakic, K.; Selc, M.; Macova, R.; Kurillova, A.; Kvitek, L.; Panacek, A.; Babelova, A. Effects of different-sized silver nanoparticles on morphological and functional alterations in lung cancer and non-cancer lung cells. Neoplasma 2023, 70, 390–401. [Google Scholar] [CrossRef]
- Dorniani, D.; Hussein, M.Z.; Kura, A.U.; Fakurazi, S.; Shaari, A.H.; Ahmad, Z. Preparation and characterization of 6-mercaptopurine-coated magnetite nanoparticles as a drug delivery system. Drug Des. Devel. Ther. 2013, 7, 1015–1026. [Google Scholar] [CrossRef]
- Prasad, A.I.; Parchur, A.K.; Juluri, R.R.; Jadhav, N.; Pandey, B.N.; Ningthoujam, R.S.; Vatsa, R.K. Bi-functional properties of Fe3O4@YPO4:Eu hybrid nanoparticles: Hyperthermia application. Dalton Trans. 2013, 42, 4885–4896. [Google Scholar] [CrossRef] [PubMed]
- Licciardi, M.; Scialabba, C.; Cavallaro, G.; Sangregorio, C.; Fantechi, E.; Giammona, G. Cell uptake enhancement of folate targeted polymer coated magnetic nanoparticles. J. Biomed. Nanotechnol. 2013, 9, 949–964. [Google Scholar] [CrossRef] [PubMed]
- Saratale, R.G.; Benelli, G.; Kumar, G.; Kim, D.S.; Saratale, G.D. Bio-fabrication of silver nanoparticles using the leaf extract of an ancient herbal medicine, dandelion (Taraxacum officinale), evaluation of their antioxidant, anticancer potential, and antimicrobial activity against phytopathogens. Environ. Sci. Pollut. Res. Int. 2018, 25, 10392–10406. [Google Scholar] [CrossRef]
- Liang, S.; Wang, Y.; Yu, J.; Zhang, C.; Xia, J.; Yin, D. Surface modified superparamagnetic iron oxide nanoparticles: As a new carrier for bio-magnetically targeted therapy. J. Mater. Sci. Mater. Med. 2007, 18, 2297–2302. [Google Scholar] [CrossRef]
- Van Hao, N.; Tung, D.H.; Hung, N.P.; Hoa, V.X.; Ha, N.T.; Khanh Van, N.T.; Tan, P.T.; Van Trinh, P. Green, facile and fast synthesis of silver nanoparticles by using solution plasma techniques and their antibacterial and anticancer activities. RSC Adv. 2023, 13, 21838–21849. [Google Scholar] [CrossRef]
- Raghubir, R.; Khan, S.; Arora, I.; Gupta, U.D.; Sharma, A.K. Shape-dependent riluzole delivery from iron oxide nanoparticles: Implications in osteosarcoma therapy. Colloids Surf. B Biointerfaces 2020, 193, 111080. [Google Scholar] [CrossRef]
- García-Cuellar, C.M.; Cabral-Romero, C.; Hernández-Delgadillo, R.; Solis-Soto, J.M.; Meester, I.; Sánchez-Pérez, Y.; Nakagoshi-Cepeda, S.E.; Pineda-Aguilar, N.; Sánchez-Nájera, R.I.; Nakagoshi-Cepeda, M.A.A.; et al. Bismuth Lipophilic Nanoparticles (BisBAL NP) Inhibit the Growth of Tumor Cells in a Mouse Melanoma Model. Anticancer Agents Med. Chem. 2022, 22, 2548–2557. [Google Scholar] [CrossRef]
- Singh, I.; Arora, R.; Dhiman, H.; Pahwa, R. Carbon quantum dots: Synthesis, characterization and biomedical application. Turk. J. Pharm. Sci. 2018, 15, 219–230. [Google Scholar] [CrossRef]
- Ramalingam, V.; Raja, S.; Harshavardhan, M. In situ one-step synthesis of polymer-functionalized palladium nanoparticles: An efficient anticancer agent against breast cancer. Dalton Trans. 2020, 49, 3510–3518. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, A.; Malankowska, A.; Nowaczyk, G.; Grześkowiak, B.F.; Tuśnio, K.; Słomski, R.; Zaleska-Medynska, A.; Jurga, S. Size and shape-dependent cytotoxicity profile of gold nanoparticles for biomedical applications. J. Mater. Sci. Mater. Med. 2017, 28, 92. [Google Scholar] [CrossRef] [PubMed]
- Jannathul Firdhouse, M.; Lalitha, P. Cytotoxicity of spherical gold nanoparticles synthesised using aqueous extracts of aerial roots of Rhaphidophora aurea intertwined over Lawsonia inermis and Areca catechu on MCF-7 cell line. IET Nanobiotechnol. 2017, 11, 2–11. [Google Scholar] [CrossRef]
- Li, H.; Kang, Z.; Liu, Y.; Lee, S.T. Carbon nanodots: Synthesis, properties and applications. J. Mater. Chem. 2012, 22, 24230–24253. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- Slowing, I.I.; Trewyn, B.G.; Giri, S.; Lin, V.S.Y. Mesoporous silica nanoparticles for drug delivery and biosensing applications. Adv. Funct. Mater. 2008, 18, 37–46. [Google Scholar] [CrossRef]
- Patri, A.K.; Majoros, I.J.; Baker, J.R., Jr. Dendritic polymer macromolecular carriers for drug delivery. Curr. Opin. Chem. Biol. 2002, 6, 466–471. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, L.; Liu, Z. Drug delivery with upconversion nanoparticles for multi-functional targeted cancer cell imaging and therapy. Biomaterials 2011, 32, 1110–11120. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Guo, H.; Liu, L.; Liu, Y.; Xie, L. Size-dependent cellular uptake and localization profiles of silver nanoparticles. Int. J. Nanomed. 2019, 14, 4247–4259. [Google Scholar] [CrossRef]
- Hayashi, K.; Nakamura, M.; Sakamoto, W.; Yogo, T.; Miki, H.; Ozaki, S.; Abe, M.; Matsumoto, T.; Ishimura, K. Superparamagnetic nanoparticle clusters for cancer theranostics combining magnetic resonance imaging and hyperthermia treatment. Theranostics 2013, 3, 366–376. [Google Scholar] [CrossRef]
- Witte, A.B.; Leistra, A.N.; Wong, P.T.; Bharathi, S.; Refior, K.; Smith, P.; Kaso, O.; Sinniah, K.; Choi, S.K. Atomic force microscopy probing of receptor–nanoparticle interactions for riboflavin receptor targeted gold–dendrimer nanocomposites. J. Phys. Chem. B 2014, 118, 2872–2882. [Google Scholar] [CrossRef]
- Ghandehari, S.; Tabrizi, M.H.; Ardalan, P.; Neamati, A.; Shali, R. Green synthesis of silver nanoparticles using Rubia tinctorum extract and evaluation of the anti-cancer properties in vitro. IET Nanobiotechnol. 2019, 13, 269–274. [Google Scholar] [CrossRef]
- Mistry, H.; Thakor, R.; Bariya, H. Biogenesis and characterization of proficient silver nanoparticles employing marine procured fungi Hamigera pallida and assessment of their antioxidative, antimicrobial and anticancer potency. Biotechnol. Lett. 2022, 44, 1097–1107. [Google Scholar] [CrossRef]
- Khan, M.S.; Alomari, A.; Tabrez, S.; Hassan, I.; Wahab, R.; Bhat, S.A.; Alafaleq, N.O.; Altwaijry, N.; Shaik, G.M.; Zaidi, S.K.; et al. Anticancer Potential of Biogenic Silver Nanoparticles: A Mechanistic Study. Pharmaceutics 2021, 13, 707. [Google Scholar] [CrossRef]
- Kajani, A.A.; Bordbar, A.-K.; Mehrgardi, M.A.; Zarkesh-Esfahani, S.H.; Motaghi, H.; Kardi, M.; Khosropour, A.R.; Ozdemir, J.; Benamara, M.; Beyzavi, H.; et al. Green and Facile Synthesis of Highly Photoluminescent Multicolor Carbon Nanocrystals for Cancer Therapy and Imaging. ACS Appl. Biomater. 2018, 1, 1458–1467. [Google Scholar] [CrossRef]
- Akerman, M.E.; Chan, W.C.; Laakkonen, P.; Bhatia, S.N.; Ruoslahti, E. Nanocrystal targeting in vivo. Proc. Natl. Acad. Sci. USA 2002, 99, 12617–12621. [Google Scholar] [CrossRef] [PubMed]
- Bures, Z.; Mamo, T.; Vlcek, M.; Lu, L.; Yaszemski, M.J. Signal protein-functionalized gold nanoparticles for nuclear targeting into osteosarcoma cells for use in radiosensitization experiments. Neoplasma 2020, 67, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Rizwana, H.; Bokahri, N.A.S.; Alkhattaf, F.; Albasher, G.A.; Aldehaish, H. Antifungal, Antibacterial, and Cytotoxic Activities of Silver Nanoparticles Synthesized from Aqueous Extracts of Mace-Arils of Myristica fragrans. Molecules 2021, 26, 7709. [Google Scholar] [CrossRef]
- Xu, J.; Yıldıztekin, M.; Han, D.; Keskin, C.; Baran, A.; Baran, M.F.; Eftekhari, A.; Ava, C.A.; Kandemir, S.İ.; Cebe, D.B.; et al. Biosynthesis, characterization, and investigation of antimicrobial and cytotoxic activities of silver nanoparticles using solanum tuberosum peel aqueous extract. Heliyon 2023, 9, e19061. [Google Scholar] [CrossRef]
- Hamida, R.S.; Ali, M.A.; Mugren, N.; Al-Zaban, M.I.; Bin-Meferij, M.M.; Redhwan, A. Planophila laetevirens-mediated synthesis of silver nanoparticles: Optimization, characterization, and anticancer and antibacterial potentials. ACS Omega 2023, 8, 29169–29188. [Google Scholar] [CrossRef]
- Adebayo-Tayo, B.C.; Ogunleye, G.E.; Ogbole, O. Biomedical application of greenly synthesized silver nanoparticles using the filtrate of trichoderma viride: Anticancer and immunomodulatory potentials. Polim. Med. 2019, 49, 57–62. [Google Scholar] [CrossRef]
- Balkrishna, A.; Sharma, V.K.; Das, S.; Mishra, N.; Bisht, L.; Joshi, A.; Sharma, N. Characterization and anti-cancerous effect of putranjiva roxburghii seed extract mediated silver nanoparticles on human colon (HCT-116), pancreatic (panc-1) and breast (mda-mb 231) cancer cell lines: A comparative study. Int. J. Nanomed. 2020, 15, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Alabssawy, A.N.; Abu-Elghait, M.; Azab, A.M.; Khalaf-Allah, H.M.M.; Ashry, A.S.; Ali, A.O.M.; Sabra, A.A.A.; Salem, S.S. Hindering the biofilm of microbial pathogens and cancer cell lines development using silver nanoparticles synthesized by epidermal mucus proteins from clarias gariepinus. BMC Biotechnol. 2024, 24, 28. [Google Scholar] [CrossRef]
- El-Naggar, N.E.; Hussein, M.H.; El-Sawah, A.A. Phycobiliprotein-mediated synthesis of biogenic silver nanoparticles, characterization, in vitro and in vivo assessment of anticancer activities. Sci. Rep. 2018, 8, 8925. [Google Scholar] [CrossRef] [PubMed]
- Oves, M.; Rauf, M.A.; Qari, H.A. Therapeutic applications of biogenic silver nanomaterial synthesized from the paper flower of bougainvillea glabra (miami, pink). Nanomaterials 2023, 13, 615. [Google Scholar] [CrossRef]
- Essawy, M.M.; Rafik, S.T.; Awaad, A.K.; Mourad, G.M.; El Achy, S.N. Photo-excitable zinc sulfide nanoparticles: A theranostic nanotool for cancer management. Oral Dis. 2023, 29, 3243–3258. [Google Scholar] [CrossRef]
- Zahraei, M.; Marciello, M.; Lazaro-Carrillo, A.; Villanueva, A.; Herranz, F.; Talelli, M.; Costo, R.; Monshi, A.; Shahbazi-Gahrouei, D.; Amirnasr, M.; et al. Versatile theranostics agents designed by coating ferrite nanoparticles with biocompatible polymers. Nanotechnology 2016, 27, 255702–255714. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; He, J.; Zhang, F.; Yu, J.; Zhang, W.; Gao, L.; Li, Y.; Zhou, M. Multistage-responsive clustered nanosystem to improve tumor accumulation and penetration for photothermal/enhanced radiation synergistic therapy. Biomaterials 2021, 268, 120590. [Google Scholar] [CrossRef]
- Quiñonero, G.; Gallo, J.; Carrasco, A.; Samitier, J.; Villasante, A. Engineering biomimetic nanoparticles through extracellular vesicle coating in cancer tissue models. Nanomaterials 2023, 13, 3097. [Google Scholar] [CrossRef]
- Arvapalli, D.M.; Sheardy, A.T.; Bang, J.J.; Wei, J. Antiproliferative and ROS regulation activity of photoluminescent curcumin-derived nanodots. ACS Appl. Bio Mater. 2021, 4, 8477–8486. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.G.; Hamed, A.A.; Elmetwalli, A.; Abdel-Monem, M.O.; El-Shora, H.M.; Alsallami, W.M. Assessment of myco-fabricated al2o3 NPs toxicity on cancer cells and pathogenic microbes by suppression of bacterial metabolic key enzymes. Int. J. Biol. Macromol. 2024, 283 Pt 3, 137073. [Google Scholar] [CrossRef]
- Heneweer, C.; Gendy, S.E.; Peñate-Medina, O. Liposomes and inorganic nanoparticles for drug delivery and cancer imaging. Ther. Deliv. 2012, 3, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Sinha, N.; Cifter, G.; Sajo, E.; Kumar, R.; Sridhar, S.; Nguyen, P.L.; Cormack, R.A.; Makrigiorgos, G.M.; Ngwa, W. Brachytherapy application with in situ dose painting administered by gold nanoparticle eluters. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Asadi, S.; Madrakian, T.; Ahmadi, M.; Aguirre, M.Á.; Afkhami, A.; Uroomiye, S.S.; Ghaffari, F.; Ranjbar, A. Aerosol assisted synthesis of a pH responsive curcumin anticancer drug nanocarrier using chitosan and alginate natural polymers. Sci. Rep. 2023, 13, 19389. [Google Scholar] [CrossRef]
- Kumar, A.; Ramamoorthy, S.; Sundaramurthy, A. Synthesis of Ag nanoparticles for selective dual detection of glutathione and dopamine using N, N-dimethyl-p-phenylenediamine mediated colorimetric probe. Chemosphere 2023, 342, 140124. [Google Scholar] [CrossRef]
- Khademi, S.; Sarkar, S.; Shakeri-Zadeh, A.; Attaran, N.; Kharrazi, S.; Solgi, R.; Reza Ay, M.; Azimian, H.; Ghadiri, H. Dual-energy CT imaging of nasopharyngeal cancer cells using multifunctional gold nanoparticles. IET Nanobiotechnol. 2019, 13, 957–961. [Google Scholar] [CrossRef]
- Affatigato, L.; Licciardi, M.; Bonamore, A.; Martorana, A.; Incocciati, A.; Boffi, A.; Militello, V. FerritinCoated SPIONs as New Cancer Cell Targeted Magnetic Nanocarrier. Molecules 2023, 28, 1163. [Google Scholar] [CrossRef]
- Feng, M.; Li, M.; Dai, R.; Xiao, S.; Tang, J.; Zhang, X.; Chen, B.; Liu, J. Multifunctional FeS2@SRF@BSA nanoplatform for chemo-combined photothermal enhanced photodynamic/chemodynamic combination therapy. Biomater. Sci 2021, 10, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Dong, L.; Zhang, B.; Liang, B.; Wang, L.; Ma, G.; Wang, L. Lentinan stabilized bimetallic PdPt3 dendritic nanoparticles with enhanced oxidase-like property for L-cysteine detection. Int. J. Biol. Macromol. 2022, 216, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Kirkby, C.; Ghasroddashti, E. Targeting mitochondria in cancer cells using gold nanoparticleenhanced radiotherapy: A Monte Carlo study. Med. Phys. 2015, 42, 1119–1128. [Google Scholar] [CrossRef]
- Liu, S.; Piao, J.; Liu, Y.; Tang, J.; Liu, P.; Yang, D.; Zhang, L.; Ge, N.; Jin, Z.; Jiang, Q.; et al. Radiosensitizing effects of different size bovine serum albumin-templated gold nanoparticles on H22 hepatoma-bearing mice. Nanomedicine 2018, 13, 1371–1383. [Google Scholar] [CrossRef]
- Shi, C.; Qi, H.; Ma, R.; Sun, Z.; Xiao, L.; Wei, G.; Huang, Z.; Liu, S.; Li, J.; Dong, M.; et al. N,S-selfdoped carbon quantum dots from fungus fibers for sensing tetracyclines and for bioimaging cancer cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110132. [Google Scholar] [CrossRef] [PubMed]
- Nallathamby, P.D.; Lee, K.J.; Desai, T.; Xu, X.H. Study of the multidrug membrane transporter of single living Pseudomonas aeruginosa cells using size-dependent plasmonic nanoparticle optical probes. Biochemistry 2010, 49, 5942–5953. [Google Scholar] [CrossRef]
- Mittal, A.K.; Tripathy, D.; Choudhary, A.; Aili, P.K.; Chatterjee, A.; Singh, I.P.; Banerjee, U.C. Biosynthesis of silver nanoparticles using Potentilla fulgens Wall. ex Hook. and its therapeutic evaluation as anticancer and antimicrobial agent. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 53, 120–127. [Google Scholar] [CrossRef]
- El-Sonbaty, S.M. Fungus-mediated synthesis of silver nanoparticles and evaluation of antitumor activity. Cancer Nanotechnol. 2013, 4, 73–79. [Google Scholar] [CrossRef]
- Nakhaeepour, Z.; Mashreghi, M.; Matin, M.M.; NakhaeiPour, A.; Housaindokht, M.R. Multifunctional CuO nanoparticles with cytotoxic effects on KYSE30 esophageal cancer cells, antimicrobial and heavy metal sensing activities. Life Sci. 2019, 234, 116758. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chang, W.H.; Wang, S.J.; Hsieh, W.Y. Fluorescent magnetic nanoparticles with specific targeting functions for combinded targeting, optical imaging and magnetic resonance imaging. J. Biomater. Sci. Polym. Ed. 2012, 23, 1903–1922. [Google Scholar] [CrossRef]
- Alves, M.N.; Paschoal, A.C.C.; Klimeck, T.D.F.; Kulogovski, C.; Marcon, B.H.; Aguir, A.M. Biological Synthesis of Low Cytotoxicity Silver Nanoparticles (AgNPs) by the Fungus Chaetomium thermophilum Sustainable Nanotechnology. J. Fungi 2022, 8, 605. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.V.; Prakash, T. Kinetics of cisplatin release by in-vitro using poly(D, L-lactide) coated Fe3O4 nanocarriers. IEEE Trans. Nanobiosci. 2013, 12, 60–63. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Chelliah, R.; MubarakAli, D.; Oh, D.H.; Kathiresan, K.; Wang, M.H. Unveiling the potentials of biocompatible silver nanoparticles on human lung carcinoma A549 cells and Helicobacter pylori. Sci. Rep. 2019, 9, 5787. [Google Scholar] [CrossRef]
- Song, G.; Kenney, M.; Chen, Y.S.; Zheng, X.; Deng, Y.; Chen, Z.; Wang, S.X.; Gambhir, S.S.; Dai, H.; Rao, J. Carboncoated FeCo nanoparticles as sensitive magnetic-particle-imaging tracers with photothermal and magnetothermal properties. Nat. Biomed. Eng. 2020, 4, 325–334. [Google Scholar] [CrossRef]
- Castillo, P.M.; de la Mata, M.; Casula, M.F.; Sánchez-Alcázar, J.A.; Zaderenko, A.P. PEGylated versus non-PEGylated magnetic nanoparticles as camptothecin delivery system. Beilstein J. Nanotechnol. 2014, 5, 1312–1319. [Google Scholar] [CrossRef]
- Majeed, S.; Aripin, F.H.B.; Shoeb, N.S.B.; Danish, M.; Ibrahim, M.N.M.; Hashim, R. Bioengineered silver nanoparticles capped with bovine serum albumin and its anticancer and apoptotic activity against breast, bone and intestinal colon cancer cell lines. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 254–263. [Google Scholar] [CrossRef]
- Zhan, M.; Wang, D.; Zhao, L.; Chen, L.; Ouyang, Z.; Mignani, S.; Majoral, J.P.; Zhao, J.; Zhang, G.; Shi, X.; et al. Phosphorus core-shell tecto dendrimers for enhanced tumor imaging: The rigidity of the backbone matters. Biomater. Sci. 2023, 11, 7387–7396. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, L.; Lin, R.; Wang, A.Y.; Yang, L.; Kuang, M.; Qian, W.; Mao, H. Casein-coated iron oxide nanoparticles for high MRI contrast enhancement and efficient cell targeting. ACS Appl. Mater. Interfaces 2013, 5, 4632–4639. [Google Scholar] [CrossRef]
- Fantechi, E.; Innocenti, C.; Zanardelli, M.; Fittipaldi, M.; Falvo, E.; Carbo, M.; Shullani, V.; Di Cesare Mannelli, L.; Ghelardini, C.; Ferretti, A.M.; et al. A smart platform for hyperthermia application in cancer treatment: Cobalt-doped ferrite nanoparticles mineralized in human ferritin cages. ACS Nano 2014, 8, 4705–4719. [Google Scholar] [CrossRef]
- Tok, K.; Barlas, F.B.; Bayır, E.; Şenışık, A.M.; Zihnioglu, F.; Timur, S. One step synthesis of tryptophanisatin carbon nano dots and bio-applications as multifunctional nanoplatforms. Colloids Surf. B Biointerfaces 2025, 249, 114533. [Google Scholar] [CrossRef] [PubMed]
- Alphandéry, E.; Idbaih, A.; Adam, C.; Delattre, J.Y.; Schmitt, C.; Gazeau, F.; Guyot, F.; Chebbi, I. Biodegraded magnetosomes with reduced size and heating power maintain a persistent activity against intracranial U87-Luc mouse GBM tumors. J. Nanobiotechnol. 2019, 17, 126. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, S.; Doyle, H.; Blasco, J.; Redmond, G.; Sheehan, D. Oxidative stress and toxicity of gold nanoparticles in Mytilus edulis. Aquat. Toxicol. 2010, 100, 178–186. [Google Scholar] [CrossRef]
- Vyas, S.P.; Goswami, R. Size-dependent cellular uptake and TLR4 attenuation by gold nanoparticles in lung adenocarcinoma cells. Nanomedicine 2019, 14, 229–253. [Google Scholar] [CrossRef]
- Lee, Y.J.; Song, K.; Cha, S.H.; Cho, S.; Kim, Y.S.; Park, Y. Sesquiterpenoids from Tussilago farfara Flower Bud Extract for the Eco-Friendly Synthesis of Silver and Gold Nanoparticles Possessing Antibacterial and Anticancer Activities. Nanomaterials 2019, 9, 819. [Google Scholar] [CrossRef]
- Engelberg, S.; Netzer, E.; Assaraf, Y.G.; Livney, Y.D. Selective eradication of human non-small cell lung cancer cells using aptamer-decorated nanoparticles harboring a cytotoxic drug cargo. Cell Death Dis. 2019, 10, 702. [Google Scholar] [CrossRef]
- Lazaro-Carrillo, A.; Filice, M.; Guillén, M.J.; Amaro, R.; Viñambres, M.; Tabero, A.; Paredes, K.O.; Villanueva, A.; Calvo, P.; Del Puerto Morales, M.; et al. Tailor-made PEG coated iron oxide nanoparticles as contrast agents for long lasting magnetic resonance molecular imaging of solid cancers. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110262. [Google Scholar] [CrossRef]
- Poderys, V.; Jarockyte, G.; Bagdonas, S.; Karabanovas, V.; Rotomskis, R. Protein-stabilized gold nanoclusters for PDT: ROS and singlet oxygen generation. J. Photochem. Photobiol. B Biol. 2020, 204, 111802. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Karmakar, S.; Yang, J.; Zhang, H.; Yang, Y.; Thorn, P.; Yu, C. Facile synthesis of ultra-small hybrid silica spheres for enhanced penetration in 3D glioma spheroids. Chem. Commun. 2014, 50, 1527–1529. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; He, J.; Gao, B.; Cao, J.; Li, D.; Li, Y.; Huang, G.; Zhou, X. Targeted Therapy of Non-Small Cell Lung Cancer and Liver Cancer: Functional Nanocarriers for the Delivery of Cisplatin and Tissue Factor Pathway Inhibitor-2. Chemotherapy 2023, 68, 73–86. [Google Scholar] [CrossRef]
- Glickson, J.D.; Lund-Katz, S.; Zhou, R.; Choi, H.; Chen, I.W.; Li, H.; Zheng, G. Lipoprotein nanoplatform for targeted delivery of diagnostic and therapeutic agents. Mol. Imaging 2008, 7, 101–110. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Pan, J.; Zhao, F.; Kan, D.; Cheng, R.; Zhang, X.; Sun, S.K. Rhenium Sulfide Nanoparticles as a Biosafe Spectral CT Contrast Agent for Gastrointestinal Tract Imaging and Tumor Theranostics in Vivo. ACS Appl. Mater. Interfaces 2019, 11, 33650–33658. [Google Scholar] [CrossRef]
- Hameed, S.; Bhattarai, P.; Gong, Z.; Liang, X.; Yue, X.; Dai, Z. Ultrasmall porphyrin-silica core-shell dots for enhanced fluorescence imaging-guided cancer photodynamic therapy. Nanoscale Adv. 2022, 5, 277–289. [Google Scholar] [CrossRef]
- Song, L.; Chen, Y.; Ding, J.; Wu, H.; Zhang, W.; Ma, M.; Zang, F.; Wang, Z.; Gu, N.; Zhang, Y. Rituximab conjugated iron oxide nanoparticles for targeted imaging and enhanced treatment against CD20-positive lymphoma. J. Mater. Chem. B 2020, 8, 895–907. [Google Scholar] [CrossRef]
- Wang, C.; Hsu, C.H.; Li, Z.; Hwang, L.P.; Lin, Y.C.; Chou, P.T.; Lin, Y.Y. Effective heating of magnetic nanoparticle aggregates for in vivo nano-theranostic hyperthermia. Int. J. Nanomed. 2017, 12, 6273–6287. [Google Scholar] [CrossRef]
- Xing, S.; Tan, L.H.; Yang, M.; Pan, M.; Lv, Y.; Tang, Q.; Yang, Y.; Chen, H. Highly controlled core/shell structures: Tunable conductive polymer shells on gold nanoparticles and nanochains. J. Mater. Chem. 2009, 19, 3286–3291. [Google Scholar] [CrossRef]
- Mishra, S.K.; Kannan, S. Microwave Synthesis of Chitosan Capped Silver-Dysprosium Bimetallic Nanoparticles: A Potential Nanotheranosis Device. Langmuir 2016, 32, 13687–13696. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Im, P.W.; Piao, Y. A Facile Route for the Preparation of Monodisperse Iron Nitride at Silica Core/shell Nanostructures. Front. Bioeng. Biotechnol. 2021, 9, 735727. [Google Scholar] [CrossRef] [PubMed]
- Abdelhalim, M.A.; Jarrar, B.M. Renal tissue alterations were size-dependent with smaller ones induced more effects and related with time exposure of gold nanoparticles. Lipids Health Dis. 2011, 10, 163. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, Y.; Guo, Q. Sub-10 nm Cu(5)FeS(4) cube for magnetic resonance imaging-guided photothermal therapy of cancer. Int. J. Nanomed. 2018, 13, 7987–7996. [Google Scholar] [CrossRef]
- El-Fiqi, A.; Allam, R.; Kim, H.W. Antioxidant cerium ions-containing mesoporous bioactive glass ultrasmall nanoparticles: Structural, physico-chemical, catalase-mimic and biological properties. Colloids Surf. B Biointerfaces 2021, 206, 111932. [Google Scholar] [CrossRef]
- Múzquiz-Ramos, E.M.; Cortés-Hernández, D.A.; Escobedo-Bocardo, J.C.; Zugasti-Cruz, A.; Ramírez-Gómez, X.S.; Osuna-Alarcón, J.G. In vitro and in vivo biocompatibility of apatite-coated magnetite nanoparticles for cancer therapy. J. Mater. Sci. Mater. Med. 2013, 24, 1035–1041. [Google Scholar] [CrossRef]
- Moharil, P.; Wan, Z.; Pardeshi, A.; Li, J.; Huang, H.; Luo, Z.; Rathod, S.; Zhang, Z.; Chen, Y.; Zhang, B.; et al. Engineering a folic acid-decorated ultrasmall gemcitabine nanocarrier for breast cancer therapy: Dual targeting of tumor cells and tumor-associated macrophages. Acta Pharm. Sin. B 2022, 12, 1148–1162. [Google Scholar] [CrossRef]
- Nian, D.; Shi, P.; Sun, J.; Ren, L.; Hao, X.; Han, J. Application of luteinizing hormone-releasing hormone ferrosoferric oxide nanoparticles in targeted imaging of breast tumors. J. Int. Med. Res. 2019, 47, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Lee, Y.J.; Kim, Y.J.; Park, Y. Synthesis of Au–Ag bimetallic nanoparticles using Korean red ginseng (Panax ginseng Meyer) root extract for chemo-photothermal anticancer therapy. Arch. Pharm. Res. 2023, 46, 659–678. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.Z.; Prasad, P.; Cai, P.; He, C.; Foltz, W.D.; Amini, M.A.; Gordijo, C.R.; Rauth, A.M.; Wu, X.Y. Manganese oxide and docetaxel co-loaded fluorescent polymer nanoparticles for dual modal imaging and chemotherapy of breast cancer. J. Control. Release 2015, 209, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Sommer, A.; Distel, L.V.; Hazemann, J.L.; Kröner, W.; Neuhuber, W.; Müller, P.; Proux, O.; Kryschi, C. Superparamagnetic iron oxide nanoparticles as novel X-ray enhancer for low-dose radiation therapy. J. Phys. Chem. B 2014, 118, 6159–6166. [Google Scholar] [CrossRef]
- Miri, A.; Sarani, M.; Khatami, M. Nickel-doped cerium oxide nanoparticles: Biosynthesis, cytotoxicity and UV protection studies. RSC Adv. 2020, 10, 3967–3977, Correction in RSC Adv. 2020, 10, 6735–6735. [Google Scholar] [CrossRef]
- Wu, D.; Ji, W.; Xu, S.; Li, Y.; Ji, Y.; Fu, K.; Yang, G. Near-infrared light-triggered size-shrinkable theranostic nanomicelles for effective tumor targeting and regression. Int. J. Pharm. 2024, 658, 124203. [Google Scholar] [CrossRef]
- Bogusz, K.; Tehei, M.; Cardillo, D.; Lerch, M.; Rosenfeld, A.; Dou, S.X.; Liu, H.K.; Konstantinov, K. High toxicity of Bi(OH)3 and α-Bi2O3 nanoparticles towards malignant 9L and MCF-7 cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 93, 958–967. [Google Scholar] [CrossRef]
- van Tilborg, G.A.; Mulder, W.J.; Deckers, N.; Storm, G.; Reutelingsperger, C.P.; Strijkers, G.J.; Nicolay, K. Annexin A5-functionalized bimodal lipid-based contrast agents for the detection of apoptosis. Bioconjug. Chem. 2006, 17, 741–749. [Google Scholar] [CrossRef]
- Huo, Y.; Singh, P.; Kim, Y.J.; Soshnikova, V.; Kang, J.; Markus, J.; Ahn, S.; Castro-Aceituno, V.; Mathiyalagan, R.; Chokkalingam, M.; et al. Biological synthesis of gold and silver chloride nanoparticles by Glycyrrhiza uralensis and in vitro applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 303–312. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, H.; Yang, Y.; Zhang, C.; Dong, X.; Du, J.; Yan, L.; Zhang, G.; Gu, Z.; Zhao, Y. Tumor microenvironment-manipulated radiocatalytic sensitizer based on bismuth heteropolytungstate for radiotherapy enhancement. Biomaterials 2019, 189, 11–22. [Google Scholar] [CrossRef]
- Thangudu, S.; Kalluru, P.; Vankayala, R. Preparation, cytotoxicity, and in vitro bioimaging of water soluble and highly fluorescent palladium nanoclusters. Bioengineering 2020, 7, 20. [Google Scholar] [CrossRef]
- Prateeksha; Singh, B.R.; Gupta, V.K.; Deeba, F.; Bajpai, R.; Pandey, V.; Naqvi, A.H.; Upreti, D.K.; Gathergood, N.; Jiang, Y.; et al. Non-Toxic and Ultra-Small Biosilver Nanoclusters Trigger Apoptotic Cell Death in Fluconazole-Resistant Candida albicans via Ras Signaling. Biomolecules 2019, 9, 47. [Google Scholar] [CrossRef]
- Pei, M.; Liu, K.; Qu, X.; Wang, K.; Chen, Q.; Zhang, Y.; Wang, X.; Wang, Z.; Li, X.; Chen, F.; et al. Enzyme-catalyzed synthesis of selenium-doped manganese phosphate for synergistic therapy of drug-resistant colorectal cancer. J. Nanobiotechnol. 2023, 21, 72. [Google Scholar] [CrossRef]
- Wenderich, K.; Zhu, K.; Bu, Y.; Tichelaar, F.D.; Mul, G.; Huijser, A. Photophysical characterization of Ru nanoclusters on nanostructured TiO2 by time-resolved photoluminescence spectroscopy. J. Phys. Chem. C Nanomater. Interfaces 2023, 127, 14353–14362. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Y.; Zhang, Y.; Liu, G.; Chen, C. Iridium nanoclusters for near-infrared photothermal cancer therapy. Biomaterials 2020, 255, 120200. [Google Scholar] [CrossRef]
- Li, J.; Dai, S.; Qin, R.; Shi, C.; Ming, J.; Zeng, X.; Wen, X.; Zhuang, R.; Chen, X.; Guo, Z.; et al. Ligand engineering of Titanium-Oxo Nanoclusters for Cerenkov Radiation-Reinforced Photo/Chemodynamic Tumor Therapy. ACS Appl. Mater. Interfaces. 2021, 13, 54727–54738. [Google Scholar] [CrossRef] [PubMed]
- Hola, K.; Zhang, Y.; Wang, Y.; Giannelis, E.P.; Zboril, R.; Rogach, A.L. Carbon dots—Emerging fluorescent nanomaterials for bioimaging, cancer therapy and optoelectronics. Nano Today 2014, 9, 590–603. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.J.; Zhang, D.; Yang, D.C. Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yu, M.; Zhou, C.; Zheng, J. Renal clearable inorganic nanoparticles: A new frontier of bionanotechnology. Mater. Today 2013, 16, 477–486. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, P.; Du, K.; Zhang, M.; Wen, D.; Lu, Y.; Feng, J.; Zhang, H. Near-infrared-light-responsive copper oxide nanoparticles as efficient theranostic nanoagents for photothermal tumor ablation. ACS Appl. Bio Mater. 2021, 4, 5266–5275. [Google Scholar] [CrossRef] [PubMed]
- Patri, A.K.; Kukowska-Latallo, J.F.; Baker, J.R. Targeted drug delivery with dendrimers: Comparison of the release kinetics of covalently conjugated drug and noncovalent drug inclusion complex. Adv. Drug Deliv. Rev. 2009, 61, 429–437. [Google Scholar] [CrossRef]
- Sun, Y.P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef] [PubMed]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramirez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef]
- Kratz, F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J. Control. Release 2008, 132, 171–183. [Google Scholar] [CrossRef]
- Jordan, A.; Scholz, R.; Wust, P.; Fähling, H.; Roland, F. Magnetic fluid hyperthermia (MFH): Cancer treatment with AC magnetic field induced excitation of biocompatible superparamagnetic nanoparticles. J. Magn. Magn. Mater. 1999, 201, 413–419. [Google Scholar] [CrossRef]
- Erogbogbo, F.; Yong, K.T.; Roy, I.; Xu, G.; Prasad, P.N.; Swihart, M.T. Biocompatible luminescent silicon quantum dots for imaging of cancer cells. ACS Nano 2008, 2, 873–878. [Google Scholar] [CrossRef]
- Chatterji, A.; Ochoa, W.F.; Paine, M.; Ratna, B.R.; Johnson, J.E.; Lin, T. New addresses on an addressable virus nanoblock: Uniquely reactive Lys residues on cowpea mosaic virus. Chem. Biol. 2004, 11, 855–863. [Google Scholar] [CrossRef]
- Murphy, C.J.; Gole, A.M.; Hunyadi, S.E.; Stone, J.W.; Sisco, P.N.; Alkilany, A.; Kinard, B.E.; Hankins, P. Gold nanoparticles in biology: Beyond toxicity to cellular imaging. Acc. Chem. Res. 2008, 41, 1721–1730. [Google Scholar] [CrossRef]
- Loo, C.; Lin, A.; Hirsch, L.; Lee, M.H.; Barton, J.; Halas, N.; West, J.; Drezek, R. Nanoshell-enabled photonics-based imaging and therapy of cancer. Technol. Cancer Res. Treat. 2004, 3, 33–40. [Google Scholar] [CrossRef]
- Rothemund, P.W. Folding DNA to create nanoscale shapes and patterns. Nature 2006, 440, 297–302. [Google Scholar] [CrossRef]
- Zhang, L.; Chan, J.M.; Gu, F.X.; Rhee, J.W.; Wang, A.Z.; Radovic-Moreno, A.F.; Alexis, F.; Langer, R.; Farokhzad, O.C. Self-assembled lipid–polymer hybrid nanoparticles: A robust drug delivery platform. ACS Nano 2008, 2, 1696–1702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, Y.; Ding, Y.; Povey, M.; York, D. Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids). J. Nanopart. Res. 2007, 9, 479–489. [Google Scholar] [CrossRef]
- Bianco, A.; Kostarelos, K.; Prato, M. Applications of carbon nanotubes in drug delivery. Curr. Opin. Chem. Biol. 2005, 9, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Coester, C.J.; Langer, K.; von Briesen, H.; Kreuter, J. Gelatin nanoparticles by two step desolvation—A new preparation method, surface modifications and cell uptake. J. Microencapsul. 2000, 17, 187–193. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Slatkin, D.N.; Smilowitz, H.M. The use of gold nanoparticles to enhance radiotherapy in mice. Phys. Med. Biol. 2004, 49, N309–N315. [Google Scholar] [CrossRef]
- Sun, S.; Murray, C.B.; Weller, D.; Folks, L.; Moser, A. Monodisperse FePt nanoparticles and ferromagnetic FePt nanocrystal superlattices. Science 2000, 287, 1989–1992. [Google Scholar] [CrossRef]
- Rhule, J.T.; Hill, C.L.; Judd, D.A.; Schinazi, R.F. Polyoxometalates in medicine. Chem. Rev. 1998, 98, 327–358. [Google Scholar] [CrossRef]
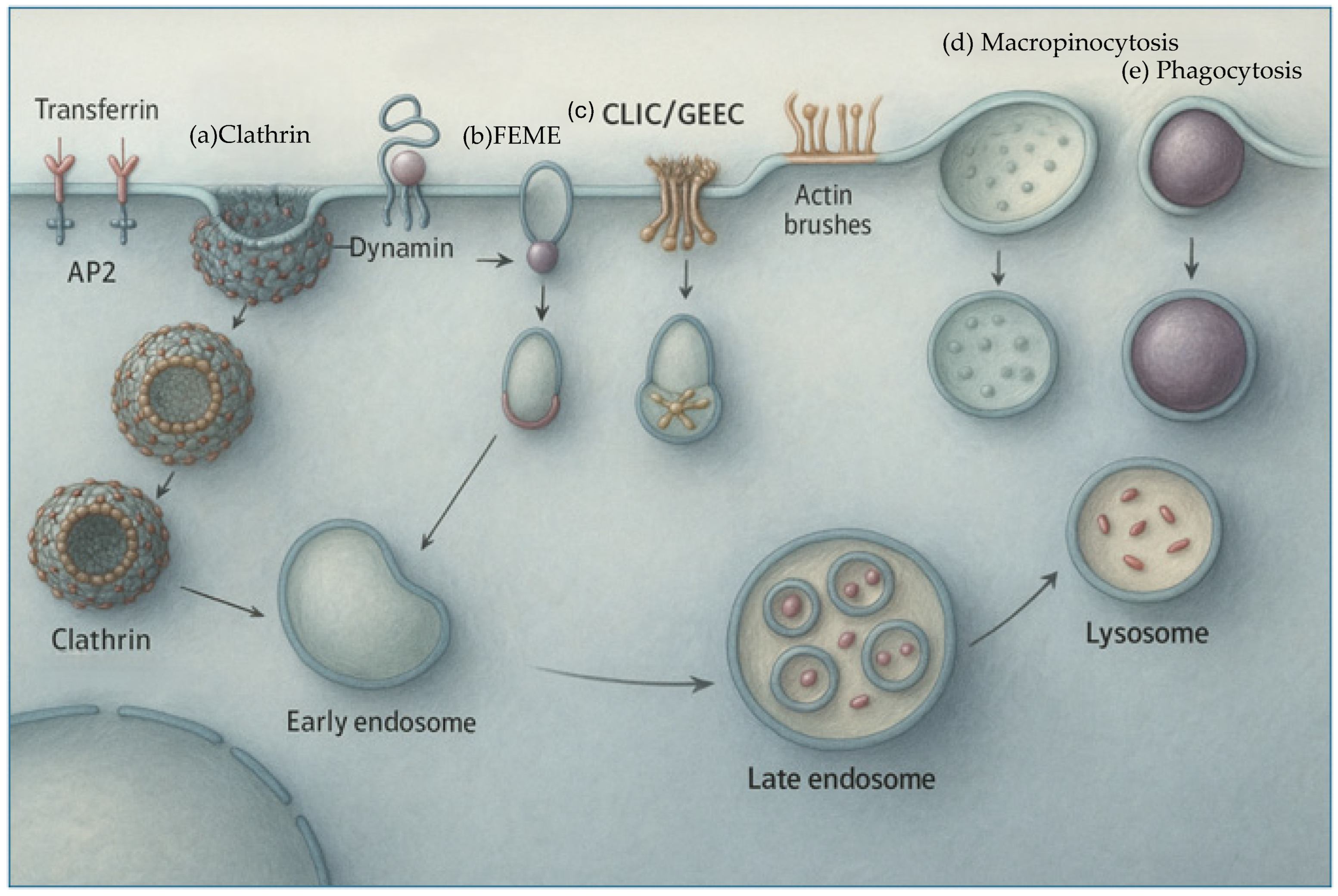
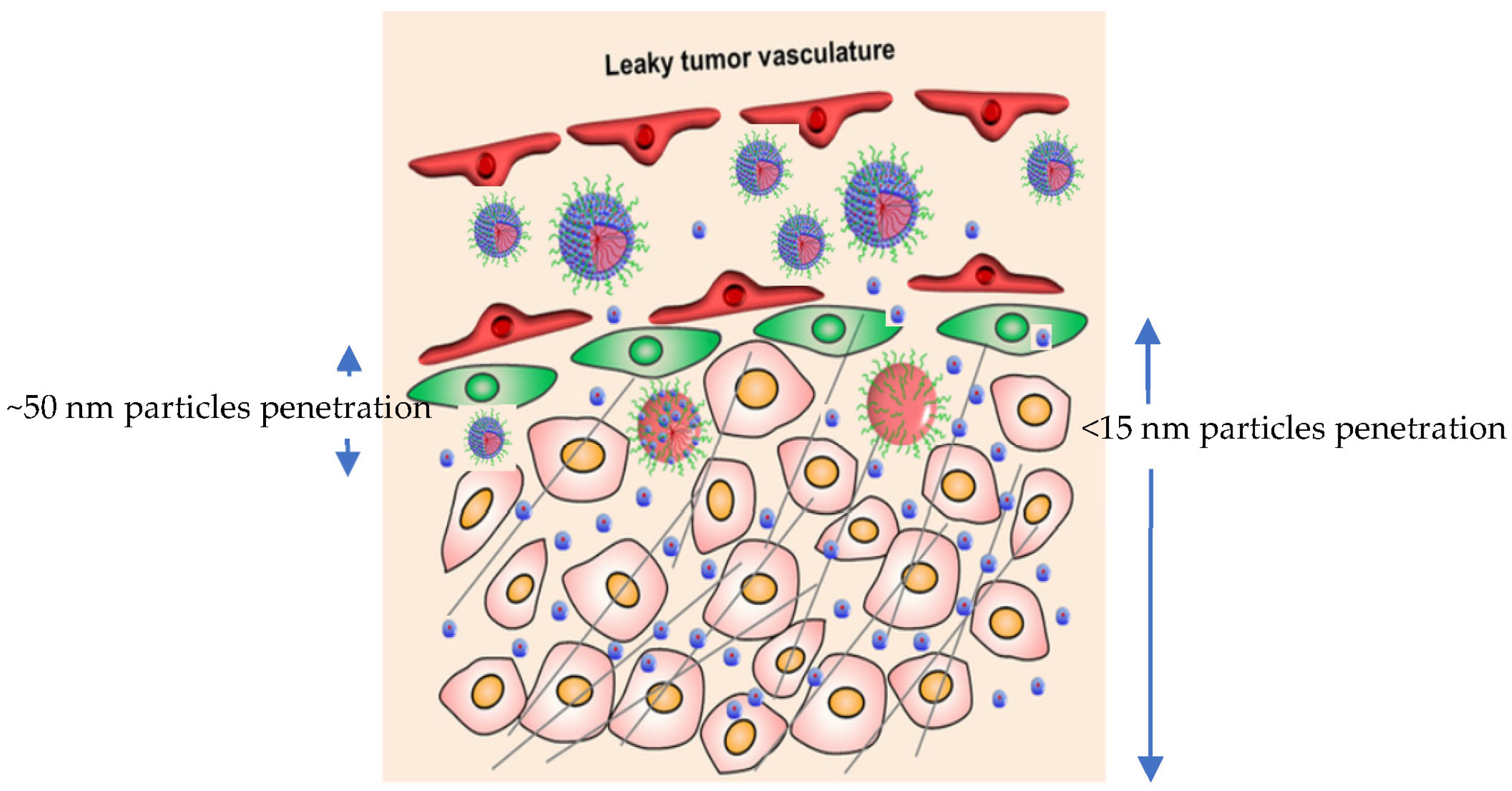
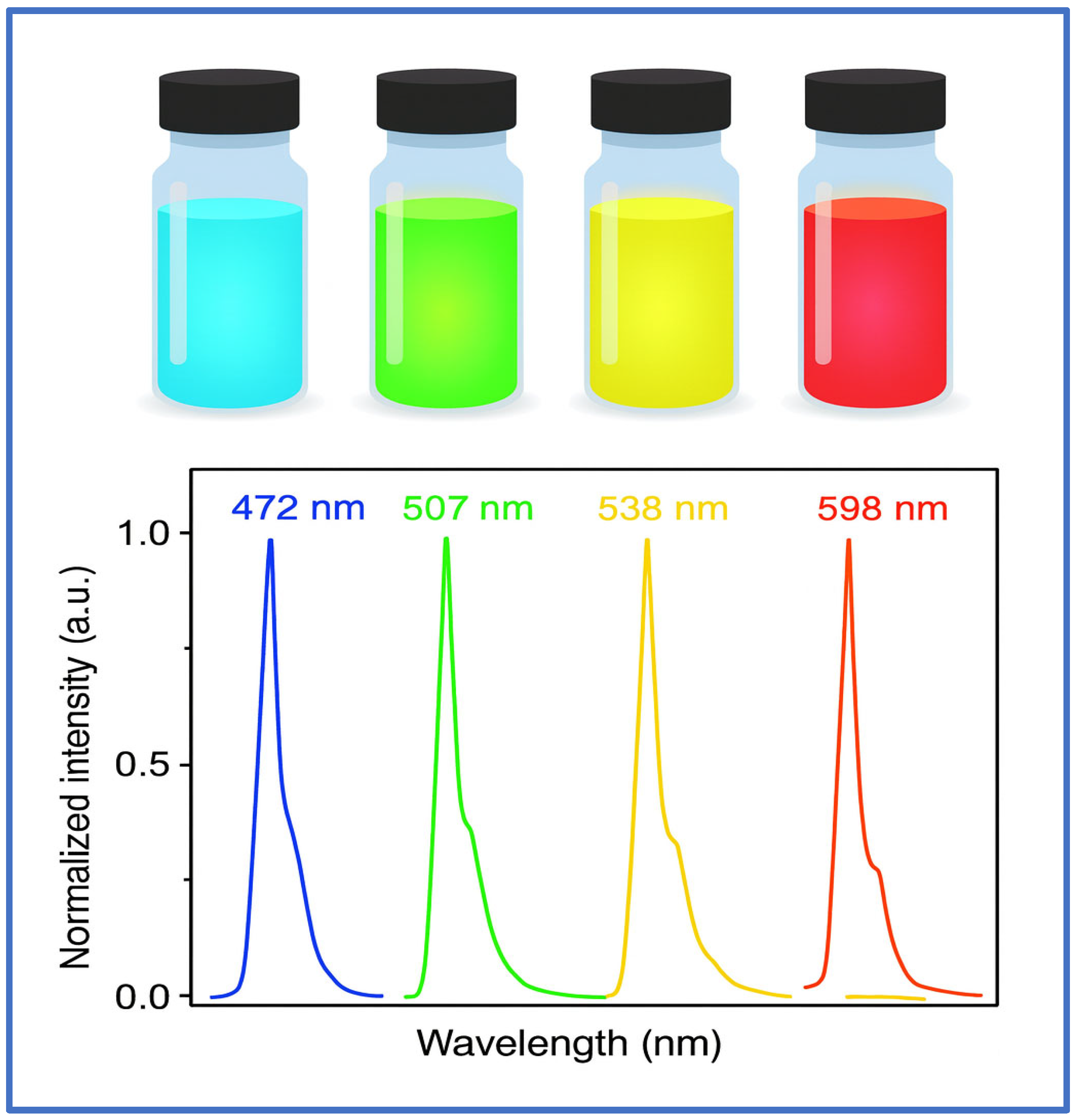
| Images | Type | Typical Size Range (nm) | Development Status | Unique Properties | Example Drugs/Applications | Key References |
|---|---|---|---|---|---|---|
 | Nanocapsules (a) | 5–20 (can be <15) | Preclinical, Some clinical | Reservoir structure with drug in core and polymer shell; tunable release kinetics | Curcumin, Dexamethasone, Insulin | [2,3] |
 | Nanospheres (b) | 10–50 (some <15 possible) | Preclinical | Matrix system where drug is uniformly dispersed in polymer matrix | Paclitaxel, 5-FU, Doxorubicin | [4,5] |
 | Micelles (c) | 5–15 | Clinical and preclinical | Amphiphilic self-assembly; core–shell; good for hydrophobic drugs; often PEGylated | Paclitaxel (Genexol®-PM), Doxorubicin, siRNA | [6,7,8] |
 | Dendrimers (d) | 1–10 | Clinical (few), preclinical | Hyperbranched, monodisperse, multivalent surface; ideal for targeted drug delivery | Cisplatin, siRNA, Methotrexate, NSAIDs | [9,10] |
 | Polymersomes (e) | 10–50 (rarely <15) | Emerging/preclinical | Bilayer vesicles; stable; carry both hydrophilic and hydrophobic drugs | Proteins, siRNA, Anticancer agents | [11,12] |
 | Polyplexes (f) | 5–15 | Preclinical, some clinical | Complex of cationic polymer with DNA/RNA; gene therapy focus | DNA/RNA vaccines, CRISPR-Cas9 delivery | [13,14] |
| Parameter | LNPs | Liposomes | Nanoemulsions | SLNs |
|---|---|---|---|---|
| Structure | Amorphous lipid core | Lipid bilayer | Oil droplets | Solid lipid matrix |
| Drug types | RNA, small molecules | Hydrophilic and hydrophobic | Hydrophobic | Mostly hydrophobic |
| Typical size (<15 nm achievable or not) | Yes | Yes | Yes | Yes (rarely) |
| Stability | Moderate to high | Moderate | High (kinetic) | High (physical) |
| Challenges | PEG-shedding, stability | Low loading, rapid clearance | Surfactant toxicity | Low drug loading at ultrasmall sizes |
| Property | Gold NPs | Silver NPs | Iron Oxide NPs |
|---|---|---|---|
| Typical size range (sub-15 nm) | 1–15 nm | 2–15 nm | 5–15 nm |
| Key applications | Photothermal, drug delivery, imaging | Cytotoxicity, antimicrobial, chemo | MRI, hyperthermia, CDT |
| Surface modification | Thiols, PEG, ligands | Polymers, bioreduction agents | Dextran, PEG, citrate |
| Advantages | Biocompatible, stable | Potent cytotoxicity | Magnetic targeting, imaging |
| Limitations | Expensive, limited biodegradation | Potential toxicity | Agglomeration, synthesis complexity |
| Parameter | Sub-15 nm Nanoparticles | 15–30 nm Nanoparticles | Implications/Trade-Off |
|---|---|---|---|
| Typical size regime | 5–15 nm (often near 10 nm) | 15–30 nm (EPR-optimized range) | Defines distinct physicochemical and biological behaviors |
| Tumor/tissue penetration | Excellent diffusion and deep stromal penetration; cross BBB and dense ECM | Moderate; relies mainly on EPR effect and leaky vasculature | Smaller systems favored for desmoplastic or poorly vascularized tumors |
| Drug/payload capacity | Limited core volume; lower loading; suited for potent small molecules or conjugates | Larger internal volume; higher encapsulation of macromolecules (siRNA, mRNA, proteins) | Larger systems preferred when payload size dominates |
| Colloidal and storage stability | Higher surface energy; prone to aggregation; requires PEGylation, zwitterionic, or lyophilized stabilization | Generally stable under physiological ionic strength; less surface-energy stress | Sub-15 nm systems need careful formulation control |
| Circulation and clearance | Rapid renal elimination (<5.5 nm full clearance); minimal RES uptake; short systemic half-life | Slower renal filtration; longer circulation; possible hepatic/splenic accumulation | Balance between safety (fast clearance) and retention (long exposure) |
| Biodistribution pattern | Diffuse distribution; low organ retention; favorable for repeat dosing | Prolonged tumor residence; potential off-target organ accumulation | Depends on therapy duration and dosing frequency |
| Biological interactions | High surface-area-to-volume ratio; quantum and charge-dependent behavior | Classical colloidal interactions; surface-driven uptake via endocytosis | Unique quantum/charge effects emerge only below ~15 nm |
| Formulation complexity | Demanding size control; requires advanced synthetic precision (SCNPs, dendrimers, ultrafine emulsions) | Easier scale-up using standard micelle/liposome/nanoemulsion techniques | Manufacturability favors mid-size range |
| Therapeutic applications | Imaging agents, photothermal/photodynamic nanodots, small-molecule chemotherapeutics, renal-clearable probes | High-payload formulations for nucleic acids, proteins, vaccines, long-acting depots | Use sub-15 nm when diffusion and clearance dominate; 15–30 nm when payload and persistence dominate |
| Regulatory/translational outlook | Emerging class; limited guidelines; requires new PK/Tox paradigms | Better-defined; more precedent from approved nanomedicines | Sub-15 nm systems are frontier candidates for next-generation precision nanomedicine |
| Feature | Graphene Quantum Dots (GQDs) | Carbon Nanotubes (CNTs) |
|---|---|---|
| Typical size | <10 nm | Diameter: 0.8–20 nm |
| Primary strengths | Bright/stable PL; metal-free; easy bioconjugation; PDT | Very high loading; strong PTT; PA imaging |
| Best-fit applications | Fluorescence imaging, PDT, light-guided chemo | PTT, gene/drug delivery at high dose density, PA/sensing |
| Drug loading (qualitative) | Moderate (π-π, H-bonding, conjugation) | High (adsorption, covalent, endohedral) |
| Renal clearance potential | Higher (≤10 nm, sheet-like) | Lower (needs oxidation for clearance) |
| Inflammation/bioperpersistence risk | Low-moderate | Moderate-high unless heavily functionalized |
| Typical functionalization | Carboxyl/amine/PEG/targeting ligands | Oxidation/PEGylation/amide/ester; targeting ligands |
| Safety focus | Oxidative stress at high dose | Lung/RES persistence |
| Intended Use | Preferential Platforms | Why Synthesis Fit | Watch-Outs for Production |
|---|---|---|---|
| Deep tumor imaging/PDT/PTT | GQDs, QDs (Cd-free), Au (<15 nm) | High PL or photothermal efficiency; straightforward top-down/bottom-up or Brust/seed routes | PL drop on aqueous transfer; aggregation in salts (add stealth) |
| Ligand-dense drug conjugates | Dendrimers | Precise size (G3–G5), controlled multivalency | Iterative steps, ligand-density QC, avoid inter-particle crosslinks |
| Solubilizing hydrophobes; fast penetration | Micelles; nanoemulsions (≤15 nm) | Self-assembly/emulsification is scalable; small hydrodynamic size | Dilution stability, Ostwald ripening; surfactant biocompatibility |
| Membrane protein display/receptor uptake | NLPs/HDL mimetics (~10 nm) | Belt-stabilized disks via cholate dialysis/microfluidics | Protein quality; batch reproducibility; stoichiometry control |
| PTT/hybrid ROS | CNTs (ultrashort) ± Ag/Au | CVD → shortening; metal decoration for ROS/SERS | Catalyst removal; biopersistence; robust dispersion/stealth needed |
| Parameter | Sub-15 nm Nanoparticles | Larger Nanoparticles (>50 nm) |
|---|---|---|
| Lymph node access | Efficient via lymphatic drainage | Limited, often require active transport |
| Cellular uptake by APCs | Rapid and efficient | Slower, may remain extracellular |
| Tumor penetration | High | Limited to perivascular regions |
| Immune activation | Favorable for T cell priming | May lead to immune evasion or sequestration |
| P (Size) | Modality | Indication(s) | Stage/ID | Key Human Findings |
|---|---|---|---|---|
| C’ dots (silica ~5–7 nm) | Optical-PET imaging | Melanoma, SLN mapping | First-in-human (2014) | Well tolerated; renal clearance; targeted tumor uptake [280]. |
| ELU001 (CDC) (~6–7 nm) | Drug-conjugate (exatecan), FRα-targeted | FRα+ solid tumors | Phase 1/2, NCT05001282 | Early safety reported; clinical activity under evaluation. |
| AGuIX (~3 nm) | MRI contrast + radiosensitizer | Brain metastases, GBM | Phase II, e.g., NCT04899908; Fast Track (2024) | MRI-visible tumor uptake; randomized studies in progress. |
| NU-0129 (SNA) (Au core ~13 nm; HD may be > 15 nm) | siRNA gene regulation | Recurrent GBM | Phase 0, NCT03020017 | Intratumoral delivery/target engagement after IV dosing [281]. |
| rHDL/CER-001 (~9–12 nm) | Imaging/CV therapy; exploratory oncology imaging | Atherosclerosis; pilot oncology imaging | Multiple clinical studies (CV); pilot 89Zr-HDL PET in cancer | Human-scale safety; size compatible with tumor imaging/drug delivery concepts [282]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De, T.; Trieu, V.; Myers, S.; Qazi, S.; Saund, S.; Lee, C. Sub-15 nm Nanoparticles for Drug Delivery: Emerging Frontiers and Therapeutic Potential. Int. J. Mol. Sci. 2025, 26, 10842. https://doi.org/10.3390/ijms262210842
De T, Trieu V, Myers S, Qazi S, Saund S, Lee C. Sub-15 nm Nanoparticles for Drug Delivery: Emerging Frontiers and Therapeutic Potential. International Journal of Molecular Sciences. 2025; 26(22):10842. https://doi.org/10.3390/ijms262210842
Chicago/Turabian StyleDe, Tapas, Vuong Trieu, Scott Myers, Sanjive Qazi, Saran Saund, and Cynthia Lee. 2025. "Sub-15 nm Nanoparticles for Drug Delivery: Emerging Frontiers and Therapeutic Potential" International Journal of Molecular Sciences 26, no. 22: 10842. https://doi.org/10.3390/ijms262210842
APA StyleDe, T., Trieu, V., Myers, S., Qazi, S., Saund, S., & Lee, C. (2025). Sub-15 nm Nanoparticles for Drug Delivery: Emerging Frontiers and Therapeutic Potential. International Journal of Molecular Sciences, 26(22), 10842. https://doi.org/10.3390/ijms262210842




