Cytoplasmic and Nuclear Effects on Agronomic Traits in Diploid Interspecific Potato Hybrids
Abstract
1. Introduction
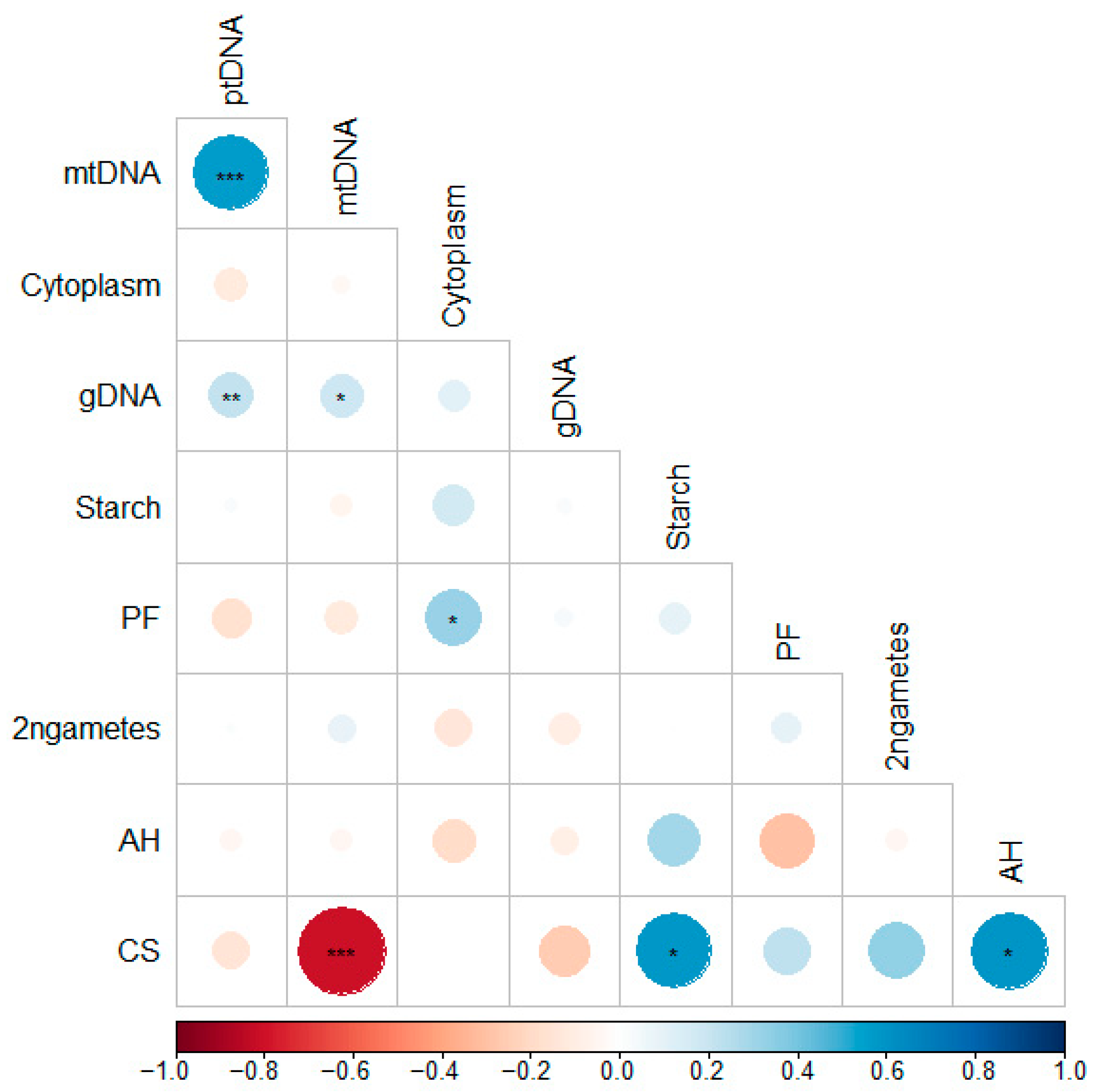
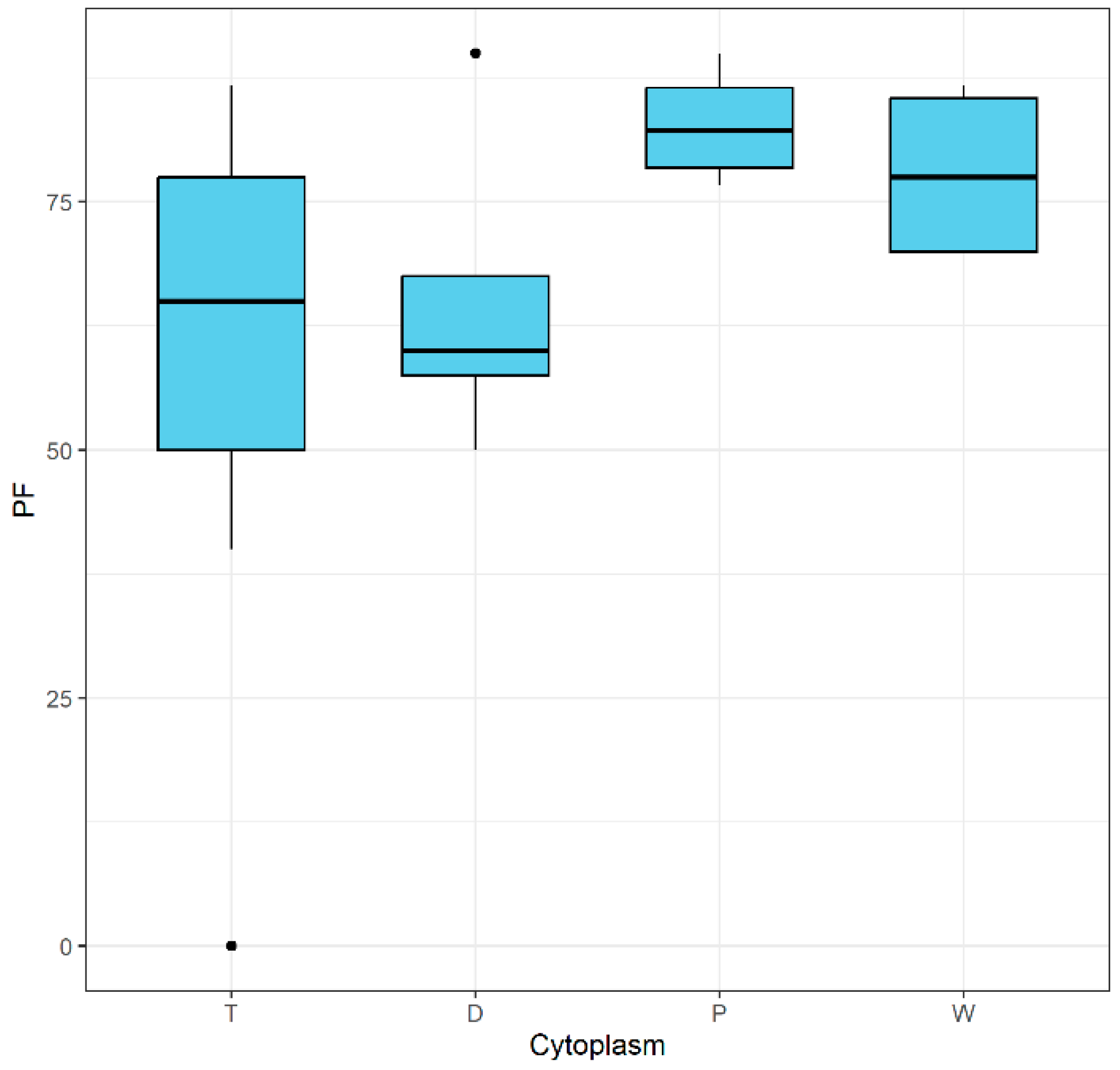
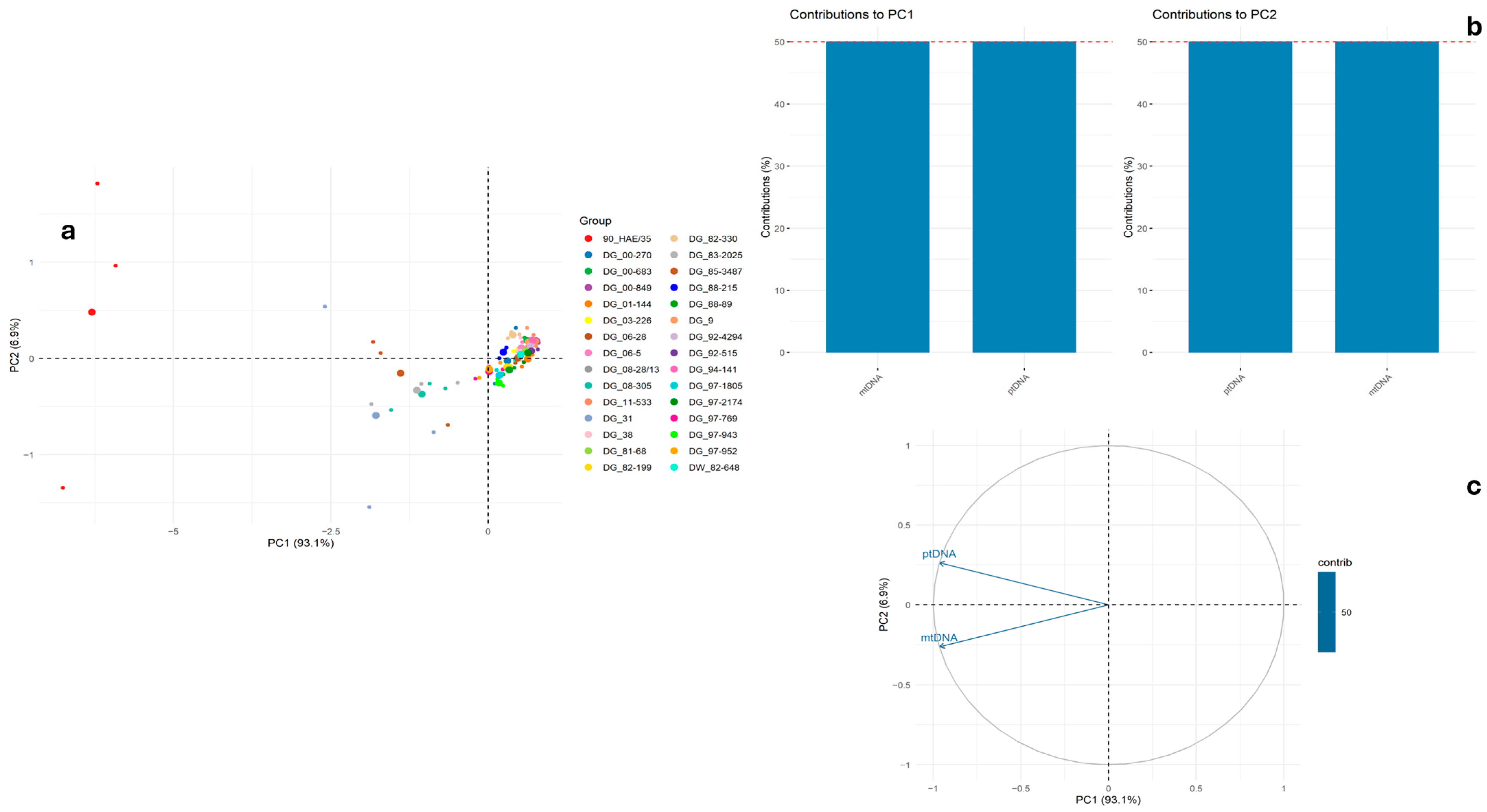
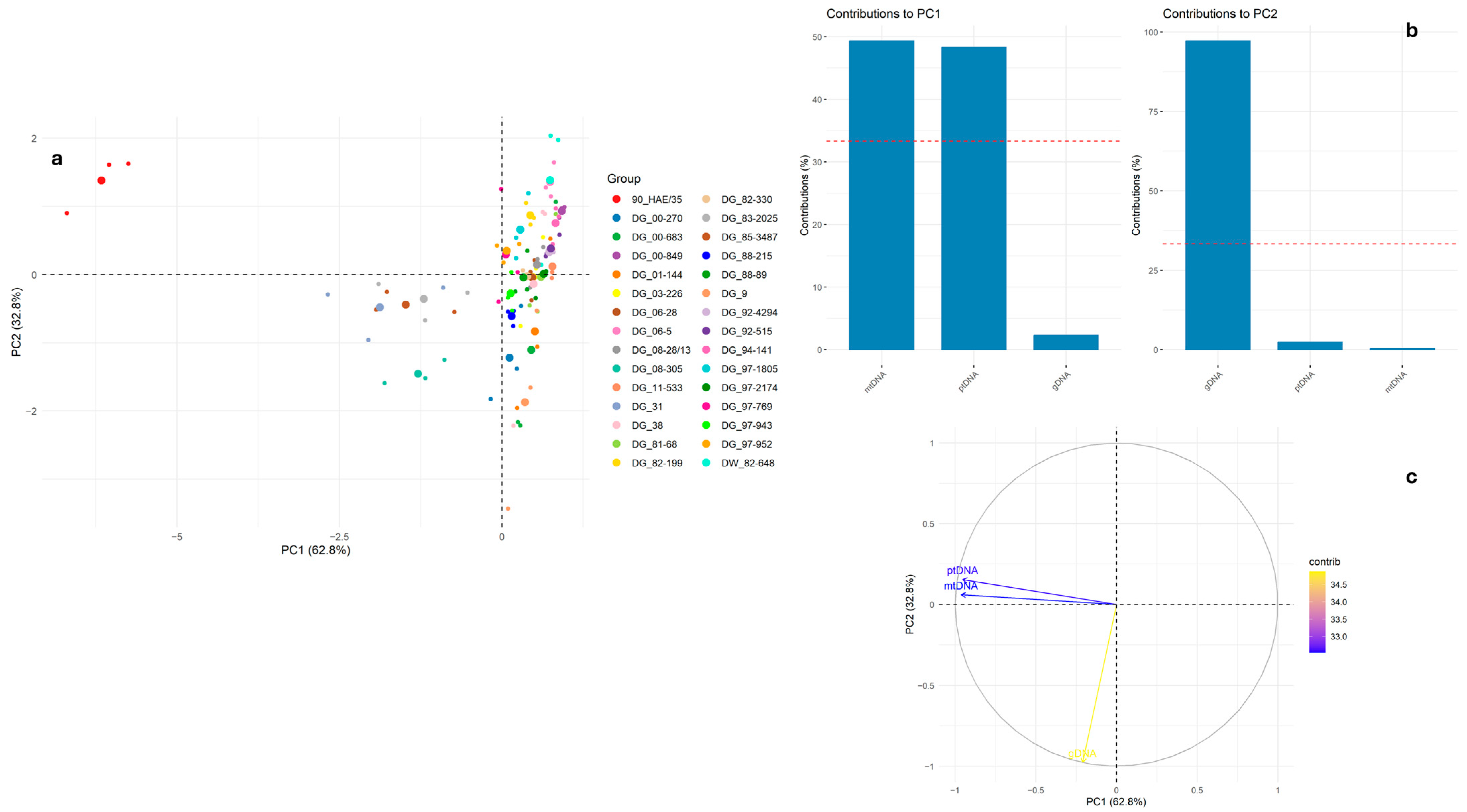
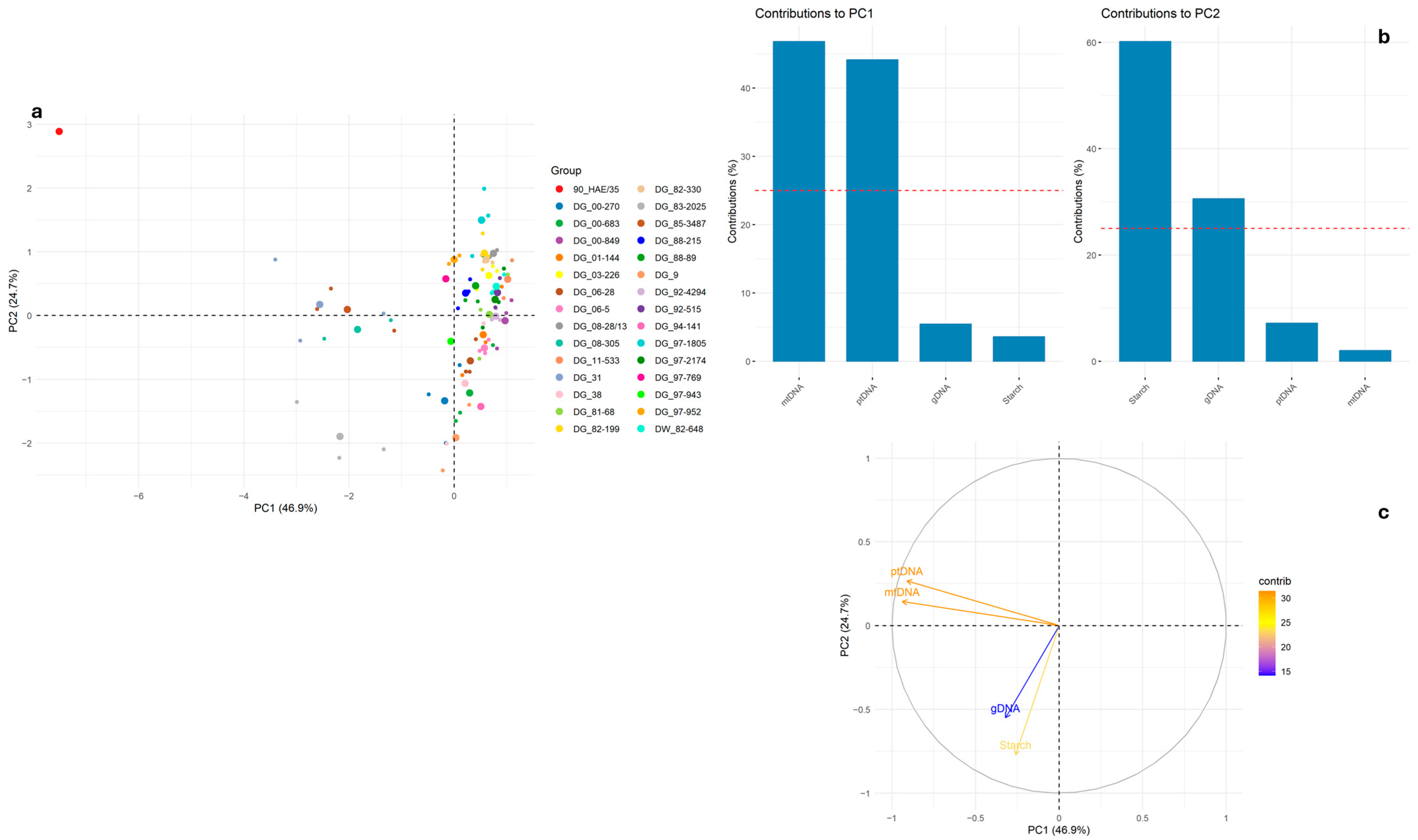
2. Results and Discussion
3. Materials and Methods
3.1. Plant Material
3.2. Sample Preparation for Flow Cytometry (FCM)
3.3. Genome Size Estimation
3.4. DNA Extraction
3.5. qPCR for Determination of Organelle DNA Content
3.6. Determination of Cytoplasm Type
3.7. Determination of Agronomic Traits
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Machida-Hirano, R. Diversity of potato genetic resources. Breed. Sci. 2015, 65, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Woodson, J.D.; Chory, J. Coordination of gene expression between organellar and nuclear genomes. Nat. Rev. Genet. 2008, 9, 383–395. [Google Scholar] [CrossRef]
- Strand, Å. Plastid-to-nucleus signalling. Curr. Opin. Plant Biol. 2004, 7, 621–625. [Google Scholar] [CrossRef]
- Nott, A.; Jung, H.S.; Koussevitzky, S.; Chory, J. Plastid-to-nucleus retrograde signaling. Annu. Rev. Plant Biol. 2006, 57, 739–759. [Google Scholar] [CrossRef]
- Loudya, N.; Barkan, A.; López-Juez, E. Plastid Retrograde Signaling: A Developmental Perspective. Plant Cell 2024, 36, 3903–3913. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, Z.; Qin, A.; Zhou, Y.; Sun, S.; Liu, Y.; Hu, M.; Yang, J.; Sun, X. Mitochondrial ATP Synthase beta-Subunit Affects Plastid Retrograde Signaling in Arabidopsis. Int. J. Mol. Sci. 2024, 25, 7829. [Google Scholar] [CrossRef]
- Marathe, S.; Grotewold, E.; Otegui, M.S. Should I Stay or Should I Go? Trafficking of Plant Extra-Nuclear Transcription Factors. Plant Cell 2024, 36, 1524–1539. [Google Scholar] [CrossRef]
- The Potato Genome Sequencing Consortium. Genome sequence and analysis of the tuber crop potato. Nature 2011, 475, 189–195. [Google Scholar] [CrossRef]
- Leisner, C.P.; Hamilton, J.P.; Crisovan, E.; Manrique-Carpintero, N.C.; Marand, A.P.; Newton, L.; Pham, G.M.; Jiang, J.; Douches, D.S.; Jansky, S.H.; et al. Genome sequence of M6, a diploid inbred clone of the high-glycoalkaloid-producing tuber-bearing potato species Solanum chacoense, reveals residual heterozygosity. Plant J. 2018, 94, 562–570, Erratum in Plant J. 2018, 96, 482. https://doi.org/10.1111/tpj.14075. [Google Scholar] [CrossRef]
- Zhou, Q.; Tang, D.; Huang, W.; Yang, Z.; Zhang, Y.; Hamilton, J.P.; Visser, R.G.F.; Bachem, C.W.B.; Robin Buell, C.; Zhang, Z.; et al. Haplotype-resolved genome analyses of a heterozygous diploid potato. Nat. Genet. 2020, 52, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- van Lieshout, N.; van der Burgt, A.; de Vries, M.E.; Ter Maat, M.; Eickholt, D.; Esselink, D.; van Kaauwen, M.P.W.; Kodde, L.P.; Visser, R.G.F.; Lindhout, P.; et al. Solyntus, the New Highly Contiguous Reference Genome for Potato (Solanum tuberosum). G3 Genes|Genomes|Genet. 2020, 10, 3489–3495. [Google Scholar] [CrossRef]
- Tang, D.; Jia, Y.; Zhang, J.; Li, H.; Cheng, L.; Wang, P.; Bao, Z.; Liu, Z.; Feng, S.; Zhu, X.; et al. Genome evolution and diversity of wild and cultivated potatoes. Nature 2022, 606, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Pellicer, J.; Hidalgo, O.; Dodsworth, S.; Leitch, I.J. Genome Size and Its Impact on the Evolution of Land Plants. Genes 2018, 9, 88. [Google Scholar] [CrossRef]
- Ortiz, R.; Mihovilovich, E. Genetics and Cytogenetics of the Potato. In The Potato Crop; Campos, H., Ortiz, O., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Birky, C.W., Jr. Uniparental inheritance of mitochondrial and chloroplast genes: Mechanisms and evolution. Proc. Natl. Acad. Sci. USA 1995, 92, 11331–11338. [Google Scholar] [CrossRef]
- Salvato, F.; Havelund, J.F.; Chen, M.; Rao, R.S.P.; Wrzesinska-Rogowska, A.; Jensen, O.N.; Gang, D.R.; Thelen, J.J.; Møller, I.M. The potato tuber mitochondrial proteome. Plant Physiol. 2014, 164, 637–653. [Google Scholar] [CrossRef]
- Lössl, A.; Adler, N.; Horn, R.; Frei, U.; Wenzel, G. Chondriome-type characterization of potato: Mt α, β, γ, δ, ε and novel plastid-mitochondrial configurations in somatic hybrids. Theor. Appl. Genet. 1999, 98, 1–10. [Google Scholar] [CrossRef]
- Hosaka, K.; Sanetomo, R. Development of rapid identification method for potato cytoplasm and its use for evaluating Japanese collections. Theor. Appl. Genet. 2012, 125, 1237–1251. [Google Scholar] [CrossRef] [PubMed]
- Sanetomo, R.; Gebhardt, C. Cytoplasmic genome types of European potatoes and their effects on complex agronomic traits. BMC Plant Biol. 2015, 15, 162. [Google Scholar] [CrossRef]
- Varré, J.S.; D’Agostino, N.; Touzet, P.; Gallina, S.; Tamburino, R.; Cantarella, C.; Ubrig, E.; Cardi, T.; Drouard, L.; Gualberto, J.M.; et al. Complete Sequence, Multichromosomal Architecture and Transcriptome Analysis of the Solanum tuberosum Mitochondrial Genome. Int. J. Mol. Sci. 2019, 20, 4788. [Google Scholar] [CrossRef]
- Achakkagari, S.R.; Tai, H.H.; Davidson, C.; De Jong, H.; Strömvik, M.V. The complete mitogenome assemblies of 10 diploid potato clones reveal recombination and overlapping variants. DNA Res. 2021, 28, dsab009. [Google Scholar] [CrossRef]
- Achakkagari, S.R.; Bozan, I.; Anglin, N.L.; Ellis, D.; Tai, H.H.; Strömvik, M.V. Complete mitogenome assemblies from a panel of 13 diverse potato taxa. Mitochondrial DNA B Resour. 2021, 6, 894–897. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, P.; Gómez, I.; Holuigue, L.; Araya, A.; Jordana, X. Transfer of rps14 from the mitochondrion to the nucleus in maize implied integration within a gene encoding the iron-sulphur subunit of succinate dehydrogenase and expression by alternative splicing. Plant J. 1999, 18, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.S.; Salvato, F.; Thal, B.; Eubel, H.; Thelen, J.J.; Møller, I.M. The proteome of higher plant mitochondria. Mitochondrion 2017, 33, 22–37. [Google Scholar] [CrossRef]
- Achakkagari, S.R.; Kyriakidou, M.; Tai, H.H.; Anglin, N.L.; Ellis, D.; Strömvik, M.V. Complete plastome assemblies from a panel of 13 diverse potato taxa. PLoS ONE 2020, 15, e0240124. [Google Scholar] [CrossRef]
- Fey, V.; Wagner, R.; Braütigam, K.; Wirtz, M.; Hell, R.; Dietzmann, A.; Leister, D.; Oelmüller, R.; Pfannschmidt, T. Retrograde plastid redox signals in the expression of nuclear genes for chloroplast proteins of Arabidopsis thaliana. J. Biol. Chem. 2005, 280, 5318–5328, Erratum in J. Biol. Chem. 2005, 16, 2020. https://doi.org/10.1016/S0021-9258(20)65941-5. [Google Scholar] [CrossRef]
- Maciejewska, U.; Skierski, J.S.; Szczerbakowa, A. Nuclear DNA content of Solanum species grown in vitro, as determined by flow cytometry. Acta Physiol. Plant. 1999, 21, 37–43. [Google Scholar] [CrossRef]
- Wang, D.; Zheng, Z.; Li, Y.; Hu, H.; Wang, Z.; Du, X.; Zhang, S.; Zhu, M.; Dong, L.; Ren, G.; et al. Which factors contribute most to genome size variation within angiosperms? Ecol. Evol. 2021, 11, 2660–2663. [Google Scholar] [CrossRef] [PubMed]
- Śliwka, J.; Wasilewicz-Flis, I.; Jakuczun, H.; Janiszewska, M.; Smyda-Dajmund, P.; McLean, K.; Zimnoch-Guzowska, E.; Bryan, G.J.; Sharma, S.K. Historical data provide new insights into inheritance of traits important for diploid potato breeding. Planta 2025, 261, 69. [Google Scholar] [CrossRef]
- Jansky, S.H.; Fajardo, D.A. Tuber starch amylose content is associated with cold-induced sweetening in potato. Food Sci. Nutr. 2014, 2, 628–633. [Google Scholar] [CrossRef]
- Sołtys-Kalina, D.; Szajko, K.; Wasilewicz-Flis, I.; Mańkowski, D.; Marczewski, W.; Śliwka, J. Quantitative trait loci for starch-corrected chip color after harvest, cold storage and after reconditioning mapped in diploid potato. Mol. Genet. Genom. 2020, 295, 209–219. [Google Scholar] [CrossRef]
- Szajko, K.; Sołtys-Kalina, D.; Heidorn-Czarna, M.; Smyda-Dajmund, P.; Wasilewicz-Flis, I.; Jańska, H.; Marczewski, W. Transcriptomic and proteomic data provide new insights into cold-treated potato tubers with T- and D-type cytoplasm. Planta 2022, 255, 97. [Google Scholar] [CrossRef]
- Michalovova, M.; Vyskot, B.; Kejnovsky, E. Analysis of plastid and mitochondrial DNA insertions in the nucleus (NUPTs and NUMTs) of six plant species: Size, relative age and chromosomal localization. Heredity 2013, 111, 314–320. [Google Scholar] [CrossRef]
- Watanabe, K. Potato genetics, genomics, and applications. Breed. Sci. 2015, 65, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Dionne, L.A. Cytoplasmic sterility in derivatives of Solanum demissum. Am. Potato J. 1961, 38, 117–120. [Google Scholar] [CrossRef]
- Santayana, M.; Aponte, M.; Kante, M.; Eyzaguirre, R.; Gastelo, M.; Lindqvist-Kreuze, H. Cytoplasmic Male Sterility Incidence in Potato Breeding Populations with Late Blight Resistance and Identification of Breeding Lines with a Potential Fertility Restorer Mechanism. Plants 2022, 11, 3093. [Google Scholar] [CrossRef]
- Anisimova, I.N.; Alpatieva, N.V.; Karabitsina, Y.I.; Gavrilenko, T.A. Nucleotide sequence polymorphism in the RFL-PPR genes of potato. J. Genet. 2019, 98, 87. [Google Scholar] [CrossRef]
- Gaborieau, L.; Brown, G.G.; Mireau, H. The Propensity of Pentatricopeptide Repeat Genes to Evolve into Restorers of Cytoplasmic Male Sterility. Front. Plant Sci. 2016, 7, 1816. [Google Scholar] [CrossRef] [PubMed]
- Xiao-Ming, Z.; Junrui, W.; Li, F.; Sha, L.; Hongbo, P.; Lan, Q.; Jing, L.; Yan, S.; Weihua, Q.; Lifang, Z.; et al. Inferring the evolutionary mechanism of the chloroplast genome size by comparing whole-chloroplast genome sequences in seed plants. Sci. Rep. 2017, 7, 1555. [Google Scholar] [CrossRef] [PubMed]
- Stensballe, A.; Hald, S.; Bauw, G.; Blennow, A.; Welinder, K.G. The amyloplast proteome of potato tuber. FEBS J. 2008, 275, 1723–1741. [Google Scholar] [CrossRef]
- Niu, S.; Zhang, G.; Li, X.; Haroon, M.; Si, H.; Fan, G.; Li, X.-Q. Organelle DNA contents and starch accumulation in potato tubers. Theor. Appl. Genet. 2019, 132, 205–216. [Google Scholar] [CrossRef]
- Doležel, J.; Bartos, J. Plant DNA flow cytometry and estimation of nuclear genome size. Ann. Bot. 2005, 95, 99–110. [Google Scholar] [CrossRef]
- Doležel, J.; Sgorbati, S.; Lucretti, S. Comparison of three DNA fluorochromes for flow cytometric estimation of nuclear DNA content in plants. Physiol. Plant. 1992, 85, 625–631. [Google Scholar] [CrossRef]
- Doležel, J.; Bartos, J.; Voglmayr, H.; Greilhuber, J. Nuclear DNA content and genome size of trout and human. Cytometry A 2003, 51, 127–128. [Google Scholar] [CrossRef] [PubMed]
- Smyda-Dajmund, P.; Śliwka, J.; Wasilewicz-Flis, I.; Jakuczun, H.; Zimnoch-Guzowska, E. Genetic composition of interspecific potato somatic hybrids and autofused 4x plants evaluated by DArT and cytoplasmic DNA markers. Plant Cell Rep. 2016, 35, 1345–1358. [Google Scholar] [CrossRef]
- Smyda-Dajmund, P.; Śliwka, J.; Janiszewska, M.; Zimnoch-Guzowska, E. Cytoplasmic diversity of potato relatives preserved at Plant Breeding and Acclimatization Institute in Poland. Mol. Biol. Rep. 2020, 47, 3929–3935. [Google Scholar] [CrossRef]
- Lunden, P.A. Underldokerd over forhold mellom popetens spesi-fikk av vekt og derestor vstoff og stivelsesinnhold Forhold. Fors. Landbruket 1956, 7, 81–107. [Google Scholar]
- Wasilewicz-Flis, I.; Jakuczun, H. Estimation of pollen fertility in potato. Monogr. I Rozpr. Nauk. IHAR Radzików Poland 2001, 10, 121–122. [Google Scholar]
- Jakuczun, H.; Zgórska, K.; Zimnoch-Guzowska, E. An investigation of the level of reducing sugars in diploid potatoes before and after cold storage. Potato Res. 1995, 38, 331–338. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 5 August 2025).
- Wei, T.; Simko, V. R Package ‘Corrplot’: Visualization of a Correlation Matrix (Version 0.95). Available online: https://github.com/taiyun/corrplot (accessed on 5 August 2025).
- Kassambara, A. _ggpubr: ‘ggplot2’ Based Publication Ready Plots_. R Package Version 0.6.0. 2023. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 5 August 2025).
- Kassambara, A.; Mundt, F. factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R package Version 1.0.7. 2020. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 4 November 2025).
| Potato Diploid Hybrid | Growth Conditions | 2C-Value 1 (pg) | ±SD | Peak CV 2 (%) Sample | Peak CV (%) Standard |
|---|---|---|---|---|---|
| DG 08-28/13 | in vitro | 1.739 ns | 0.024 | 3.12 | 3.42 |
| post-in vitro planting | 1.778 ns | 0.034 | 3.06 | 3.18 | |
| 1st tuber generation | 1.770 ns | 0.035 | 2.96 | 3.14 | |
| DG 00-683 | in vitro | 1.797 ns | 0.077 | 3.36 | 3.15 |
| post-in vitro planting | 1.747 ns | 0.039 | 3.69 | 3.25 | |
| 1st tuber generation | 1.751 ns | 0.011 | 4.12 | 3.65 | |
| DG 08-305 | in vitro | 1.746 ns | 0.059 | 2.97 | 3.05 |
| post-in vitro planting | 1.720 ns | 0.015 | 3.02 | 3.54 | |
| 1st tuber generation | 1.723 ns | 0.011 | 3.54 | 4.04 |
| Trait | W | p * |
|---|---|---|
| ptDNA | 0.42752 | 2.2 × 10−16 |
| mtDNA | 0.61772 | 5.844 × 10−14 |
| Cytoplasm | 0.67698 | 9.025 × 10−13 |
| gDNA | 0.96597 | 0.01852 |
| Starch | 0.96254 | 0.0258 |
| PF | 0.88719 | 0.00297 |
| 2n gametes | 0.60373 | 3.266 × 10−7 |
| AH | 0.66192 | 1.386 × 10−5 |
| CS | 0.88965 | 0.1377 |
| Cytoplasm | Diff | Lwr | Upr | p Adj |
|---|---|---|---|---|
| D-T | 4.25 | −23.6377 | 32.13765 | 0.975263 |
| P-T | 22 | −5.88765 | 49.88765 | 0.161192 |
| W-T | 17.16667 | −10.721 | 45.05432 | 0.352392 |
| P-D | 17.75 | −18.2528 | 53.75281 | 0.542416 |
| W-D | 12.91667 | −23.0861 | 48.91947 | 0.762085 |
| W-P | −4.83333 | −40.8361 | 31.16947 | 0.982821 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smyda-Dajmund, P.; Macko-Podgórni, A.; Sołtys-Kalina, D. Cytoplasmic and Nuclear Effects on Agronomic Traits in Diploid Interspecific Potato Hybrids. Int. J. Mol. Sci. 2025, 26, 10841. https://doi.org/10.3390/ijms262210841
Smyda-Dajmund P, Macko-Podgórni A, Sołtys-Kalina D. Cytoplasmic and Nuclear Effects on Agronomic Traits in Diploid Interspecific Potato Hybrids. International Journal of Molecular Sciences. 2025; 26(22):10841. https://doi.org/10.3390/ijms262210841
Chicago/Turabian StyleSmyda-Dajmund, Paulina, Alicja Macko-Podgórni, and Dorota Sołtys-Kalina. 2025. "Cytoplasmic and Nuclear Effects on Agronomic Traits in Diploid Interspecific Potato Hybrids" International Journal of Molecular Sciences 26, no. 22: 10841. https://doi.org/10.3390/ijms262210841
APA StyleSmyda-Dajmund, P., Macko-Podgórni, A., & Sołtys-Kalina, D. (2025). Cytoplasmic and Nuclear Effects on Agronomic Traits in Diploid Interspecific Potato Hybrids. International Journal of Molecular Sciences, 26(22), 10841. https://doi.org/10.3390/ijms262210841






