Levobupivacaine Administration Suppressed Cell Metabolism in Human Adenocarcinoma A549 Cells
Abstract
1. Introduction
2. Results
2.1. Levobupivacaine Inhibited the Cellular Activity of A549 Cells Through Its Interaction with ACE2
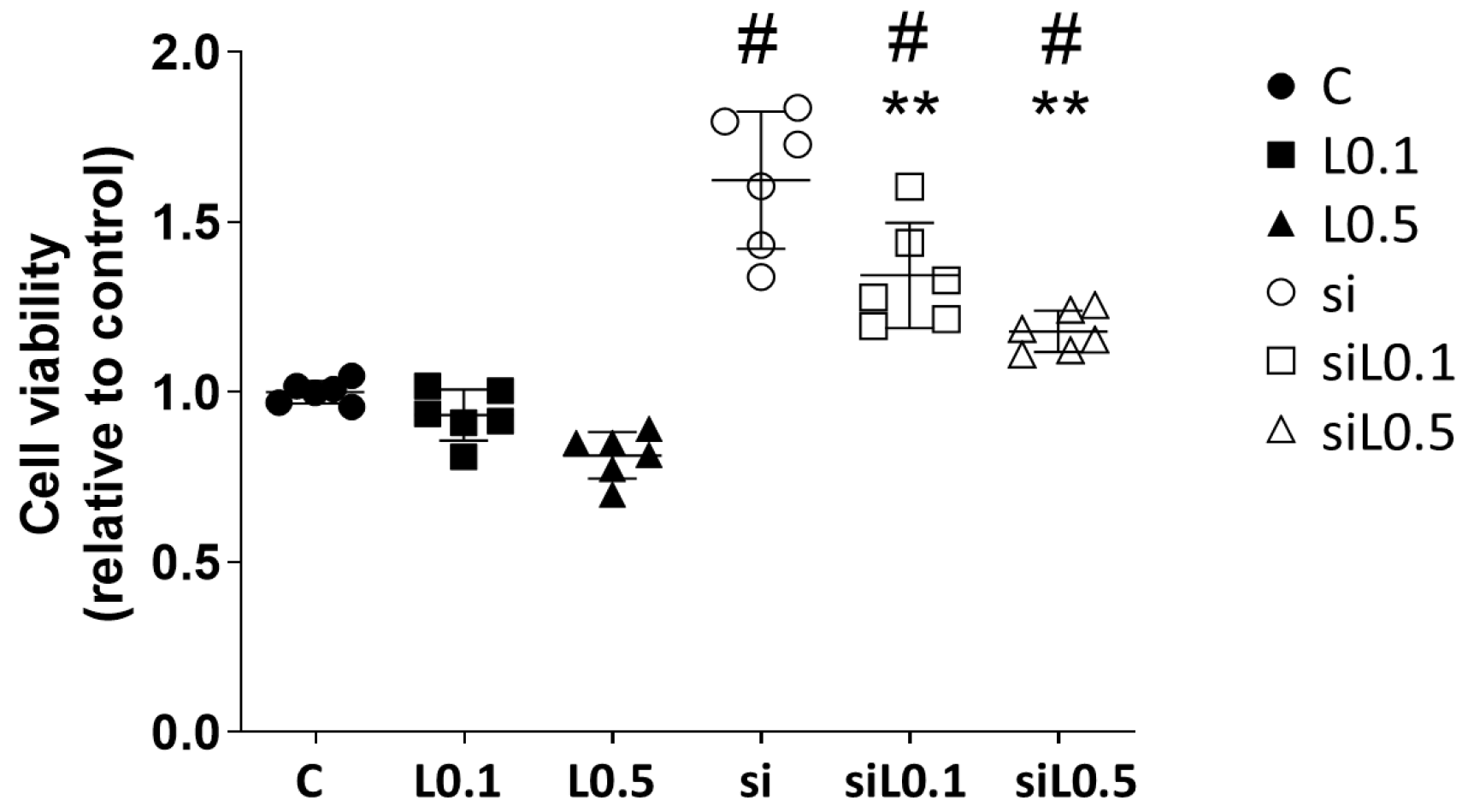
2.1.1. Levobupivacaine Treatment Did Not Alter the A549 Cells’ Proliferation, but ACE2 Knockdown Promoted the Cells’ Proliferation
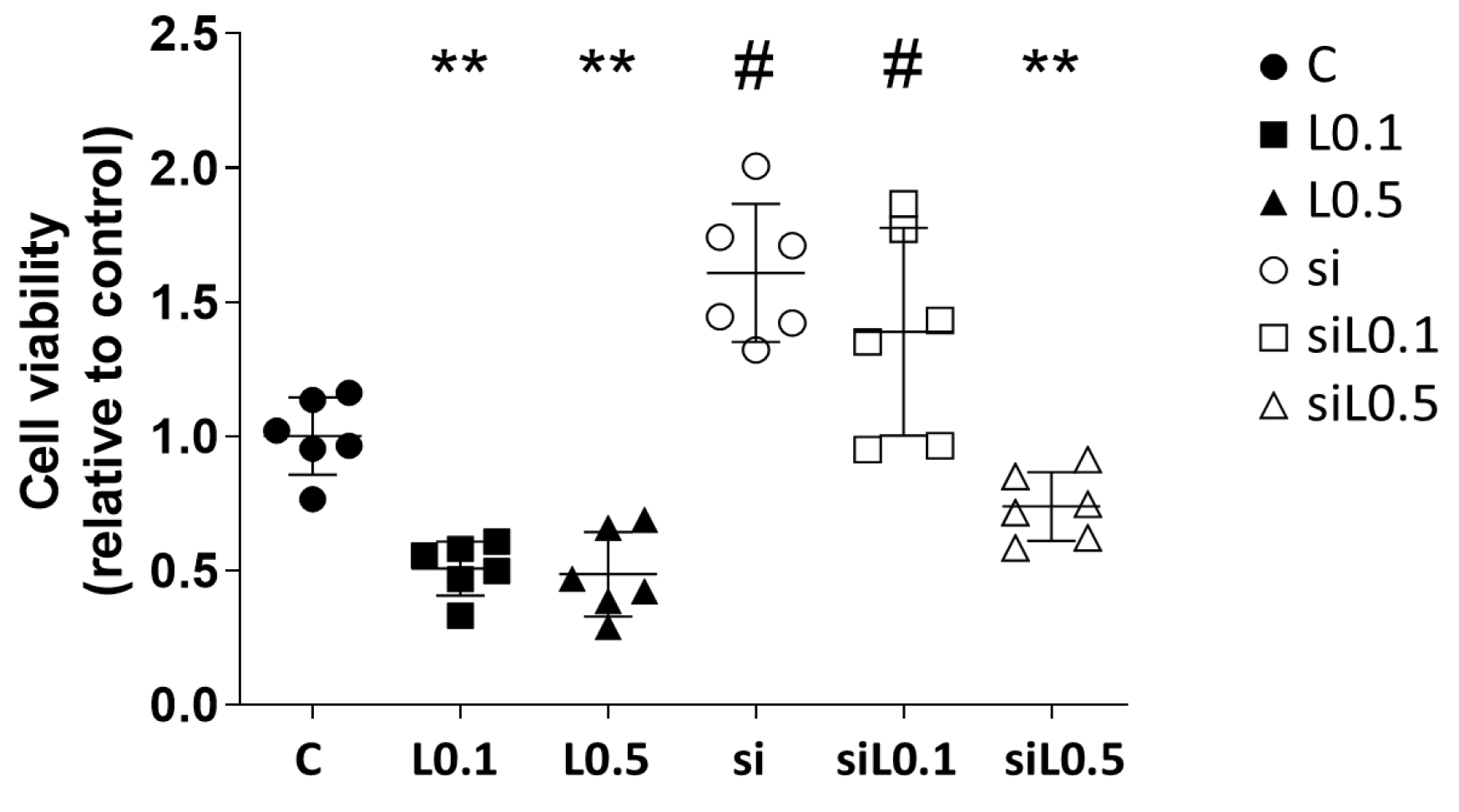
2.1.2. Levobupivacaine Treatment Resulted in the Suppression of ATP Synthesis, Which Was Reversed by the ACE2 Knockdown
2.2. mRNA Changes with Levobupivacaine and ACE2 siRNA Administration in A549 Cells
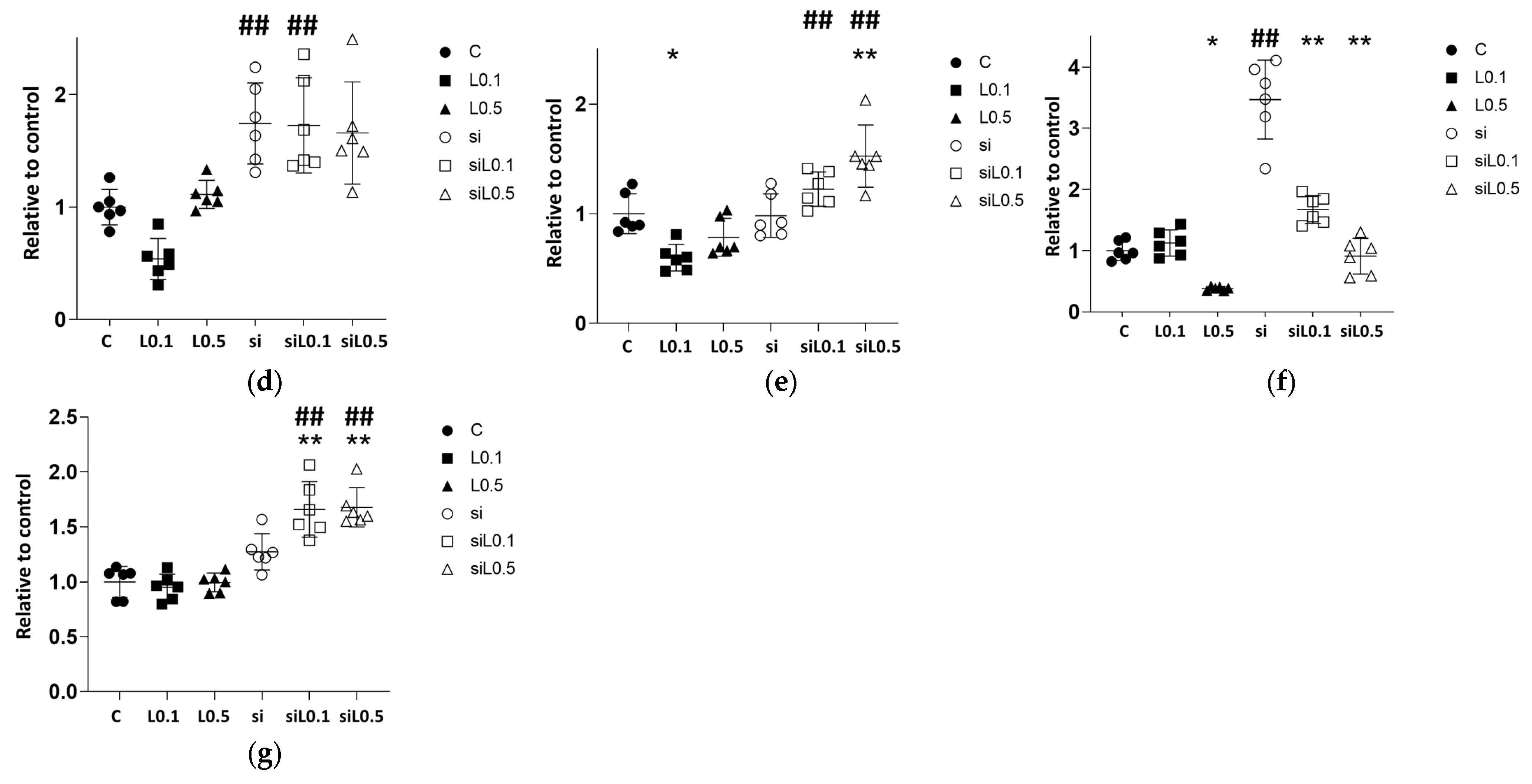
2.2.1. Levobupivacaine Treatment Led to an Increase in ACE2 mRNA Expression, Whereas the Administration of siRNA Significantly Reduced the Expression of ACE2
2.2.2. Levobupivacaine Suppressed the Expressions of Cancer Malignancy Markers, but This Suppressive Effect Was Reversed by the Combined Administration of ACE2 siRNA
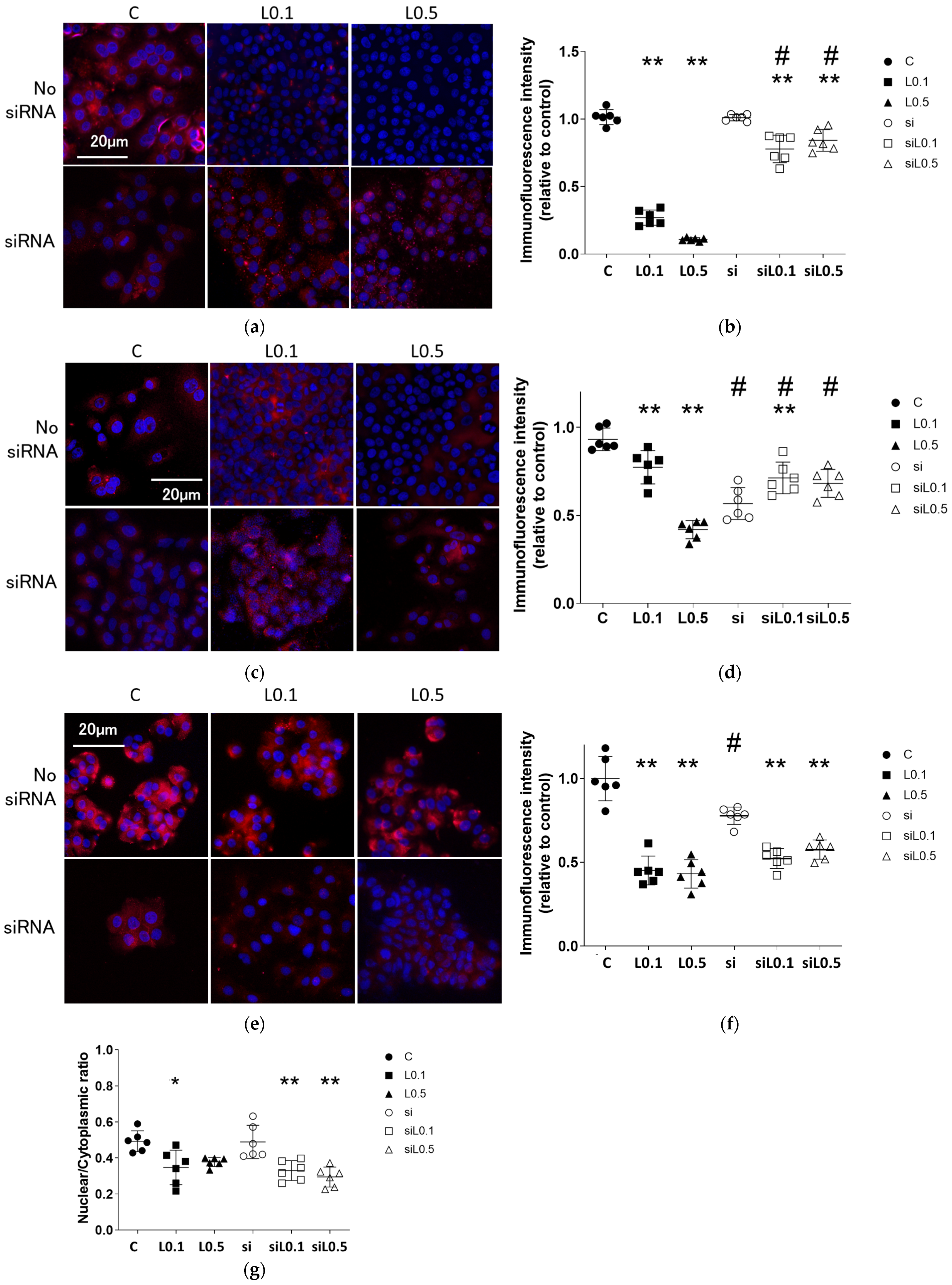
2.3. Immunofluorescent Analysis
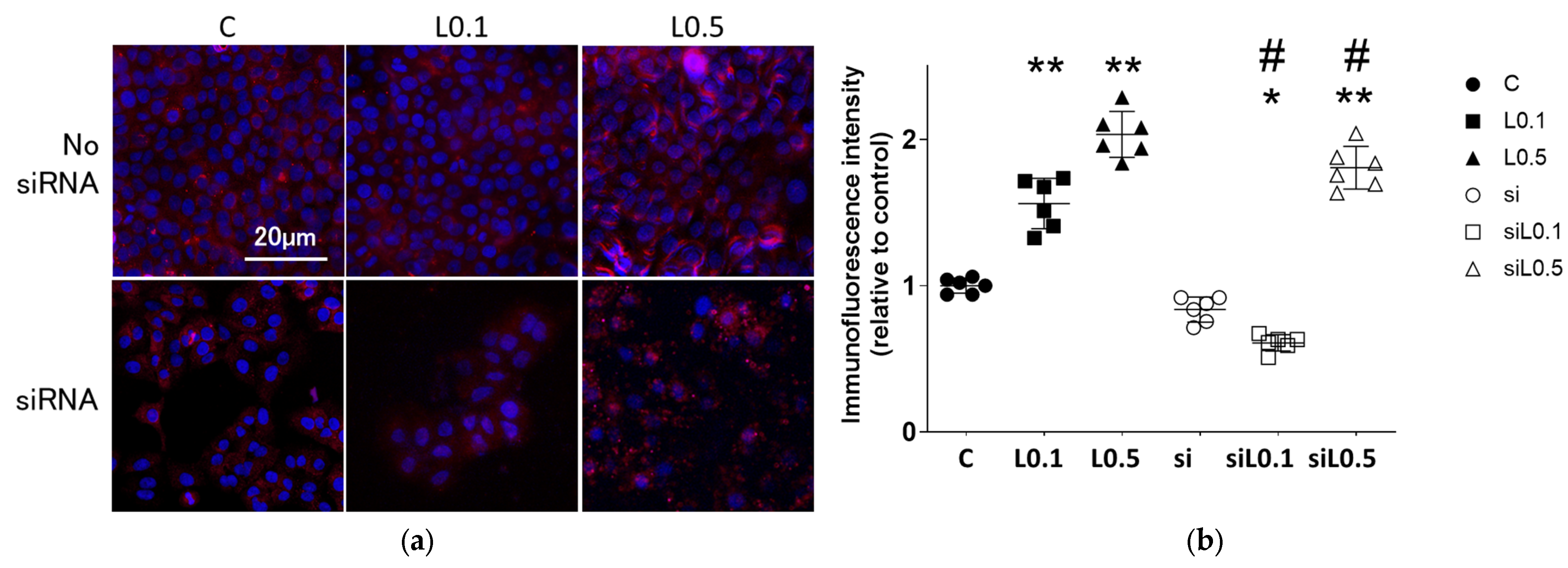
2.3.1. Levobupivacaine Treatment Enhanced the Expression of the Anticancer Marker ACE2
2.3.2. Levobupivacaine Suppressed the Expression of Cancer Malignancy Markers, but the Combined Administration of ACE2 siRNA Reversed This Suppressive Effect
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Anesthetic Administration
4.3. siRNA Transfection
4.4. Cell Proliferation Test (Cell Counting Kit-8, CCK-8 Assay)
4.5. ATP Analysis
4.6. RNA Extraction
4.7. qRT-PCR
4.8. Immunofluorescence Study
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Agency for Research on Cancer; World Health Organization. Cancer Factsheets. 2022. Available online: https://gco.iarc.fr/today/fact-sheets-cancer/fact-sheets-cancers (accessed on 26 September 2025).
- Luo, G.; Zhang, Y.; Rumgay, H.; Morgan, E.; Langselius, O.; Vignat, J.; Colombet, M.; Bray, F. Estimated worldwide variation and trends in incidence of lung cancer by histological subtype in 2022 and over time: A population-based study. Lancet Respir. Med. 2025, 13, 348–363. [Google Scholar] [CrossRef]
- Huh, J.; Hwang, W. The Role of Anesthetic Management in Lung Cancer Recurrence and Metastasis: A Comprehensive Review. J. Clin. Med. 2024, 13, 6681. [Google Scholar] [CrossRef]
- Leone, S.; Di Cianni, S.; Casati, A.; Fanelli, G. Pharmacology, toxicology, and clinical use of new long acting local anesthetics, ropivacaine and levobupivacaine. Acta Biomed. 2008, 79, 92–105. [Google Scholar]
- Casati, A.; Borghi, B.; Fanelli, G.; Cerchierini, E.; Santorsola, R.; Sassoli, V.; Grispigni, C.; Torri, G. A double-blinded, randomized comparison of either 0.5% levobupivacaine or 0.5% ropivacaine for sciatic nerve block. Anesth. Analg. 2002, 94, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Yamamoto, M.; Tomihari, M.; Ishikawa, M. Ropivacaine Administration Suppressed A549 Lung Adenocarcinoma Cell Proliferation and Migration via ACE2 Upregulation and Inhibition of the Wnt1 Pathway. Int. J. Mol. Sci. 2024, 25, 9334. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Wei, X.; Pang, L.; Wu, Y.; Hu, B.; Ruan, Y.; Liu, Z.; Liu, J.; Wang, T. Prognostic and Immunological Value of Angiotensin-Converting Enzyme 2 in Pan-Cancer. Front. Mol. Biosci. 2020, 7, 189. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, M.; Yu, H.; Sun, X.; Li, Q.; Yang, M.; Qiu, X.; Su, H.; Gong, A.; Du, F. Immunoinducible Carbon Dot-Incorporated Hydrogels as a Photothermal-Derived Antigen Depot to Trigger a Robust Antitumor Immune Response. ACS Appl. Mater. Interfaces 2023, 15, 7700–7712. [Google Scholar] [CrossRef]
- Bao, W.; Xiao, Z.; Wang, Z.; Liu, D.; Tan, P.; Huang, M. Comprehensive analysis of the long non-coding RNA expression profile and functional roles in a contrast-induced acute kidney injury rat model. Exp. Ther. Med. 2021, 22, 739. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.M.; Lin, B.F.; Wong, C.S.; Chuang, W.T.; Chou, Y.T.; Wu, Z.F. Levobuipivacaine-Induced Dissemination of A549 Lung Cancer Cells. Sci. Rep. 2017, 7, 8646. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, R.; Cheng, Y.; Wu, X.; Xi, S.; Sun, Y.; Jiang, H. Lidocaine inhibits the proliferation of lung cancer by regulating the expression of GOLT1A. Cell Prolif. 2017, 50, e12316. [Google Scholar] [CrossRef]
- Hsieh, W.H.; Liao, S.W.; Chan, S.M.; Hou, J.D.; Wu, S.Y.; Ho, B.Y.; Chen, K.Y.; Tai, Y.T.; Fang, H.W.; Fang, C.Y.; et al. Lidocaine induces epithelial-mesenchymal transition and aggravates cancer behaviors in non-small cell lung cancer A549 cells. Oncol. Lett. 2023, 26, 346. [Google Scholar] [CrossRef]
- Ma, L.; Cong, W.; Zhang, H.; Zhang, W.; Zhan, Y.; Liu, Y.; Zhang, J.; Wang, Z.; Gao, Y.; Han, B.; et al. Lidocaine Inhibits the Proliferation of Non-Small Cell Lung Cancer and Exerts Anti-Inflammatory Effects Through the TLR-9/MyD88/NF-kappaB Pathway. DNA Cell Biol. 2025, 44, 161–171. [Google Scholar] [CrossRef]
- Wang, H.W.; Wang, L.Y.; Jiang, L.; Tian, S.M.; Zhong, T.D.; Fang, X.M. Amide-linked local anesthetics induce apoptosis in human non-small cell lung cancer. J. Thorac. Dis. 2016, 8, 2748–2757. [Google Scholar] [CrossRef]
- Zuo, X.; Ren, S.; Zhang, H.; Tian, J.; Tian, R.; Han, B.; Liu, H.; Dong, Q.; Wang, Z.; Cui, Y.; et al. Chemotherapy induces ACE2 expression in breast cancer via the ROS-AKT-HIF-1alpha signaling pathway: A potential prognostic marker for breast cancer patients receiving chemotherapy. J. Transl. Med. 2022, 20, 509. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Li, C.; Hu, X.; Wang, J.; Liu, L.; Zhang, X.; Tong, Y. Association Between ACE2 and Lung Diseases. Infect. Drug Resist. 2024, 17, 1771–1780. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Song, L.; Bai, Y.; Liu, P.; Liu, Y.; Xie, F.; Xie, L. Decipering the Molecular Mechanism of ACE2 Regulating A549 Cells. Front. Genet. 2021, 12, 653725. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Ni, L.; Wan, H.; Fan, L.; Fei, X.; Ma, Q.; Gao, B.; Xiang, Y.; Che, J.; Li, Q. Overexpression of ACE2 produces antitumor effects via inhibition of angiogenesis and tumor cell invasion in vivo and in vitro. Oncol. Rep. 2011, 26, 1157–1164. [Google Scholar] [CrossRef]
- Kim, S.J.; Rabbani, Z.N.; Dewhirst, M.W.; Vujaskovic, Z.; Vollmer, R.T.; Schreiber, E.G.; Oosterwijk, E.; Kelley, M.J. Expression of HIF-1alpha, CA IX, VEGF, and MMP-9 in surgically resected non-small cell lung cancer. Lung Cancer 2005, 49, 325–335. [Google Scholar] [CrossRef]
- Choi, J.Y.; Jang, Y.S.; Min, S.Y.; Song, J.Y. Overexpression of MMP-9 and HIF-1alpha in Breast Cancer Cells under Hypoxic Conditions. J. Breast Cancer 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, P.; Fukuda, R.; Kumar, G.; Krishnamachary, B.; Zeller, K.I.; Dang, C.V.; Semenza, G.L. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell 2007, 11, 407–420. [Google Scholar] [CrossRef]
- Wicks, E.E.; Semenza, G.L. Hypoxia-inducible factors: Cancer progression and clinical translation. J. Clin. Investig. 2022, 132, e159839. [Google Scholar] [CrossRef]
- Chellini, F.; Tani, A.; Parigi, M.; Palmieri, F.; Garella, R.; Zecchi-Orlandini, S.; Squecco, R.; Sassoli, C. HIF-1alpha/MMP-9 Axis Is Required in the Early Phases of Skeletal Myoblast Differentiation under Normoxia Condition In Vitro. Cells 2023, 12, 2851. [Google Scholar] [CrossRef]
- Liu, H.L.; Liu, D.; Ding, G.R.; Liao, P.F.; Zhang, J.W. Hypoxia-inducible factor-1alpha and Wnt/beta-catenin signaling pathways promote the invasion of hypoxic gastric cancer cells. Mol. Med. Rep. 2015, 12, 3365–3373. [Google Scholar] [CrossRef]
- Wan, R.; Mo, Y.; Chien, S.; Li, Y.; Li, Y.; Tollerud, D.J.; Zhang, Q. The role of hypoxia inducible factor-1alpha in the increased MMP-2 and MMP-9 production by human monocytes exposed to nickel nanoparticles. Nanotoxicology 2011, 5, 568–582. [Google Scholar] [CrossRef]
- Wu, B.; Crampton, S.P.; Hughes, C.C. Wnt signaling induces matrix metalloproteinase expression and regulates T cell transmigration. Immunity 2007, 26, 227–239. [Google Scholar] [CrossRef]
- Hong, C.F.; Chen, W.Y.; Wu, C.W. Upregulation of Wnt signaling under hypoxia promotes lung cancer progression. Oncol. Rep. 2017, 38, 1706–1714. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. HIF-1: Upstream and downstream of cancer metabolism. Curr. Opin. Genet. Dev. 2010, 20, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L.; Roth, P.H.; Fang, H.M.; Wang, G.L. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J. Biol. Chem. 1994, 269, 23757–23763. [Google Scholar] [CrossRef] [PubMed]
- Kaidi, A.; Williams, A.C.; Paraskeva, C. Interaction between beta-catenin and HIF-1 promotes cellular adaptation to hypoxia. Nat. Cell Biol. 2007, 9, 210–217. [Google Scholar] [CrossRef]
- Jiang, N.; Zou, C.; Zhu, Y.; Luo, Y.; Chen, L.; Lei, Y.; Tang, K.; Sun, Y.; Zhang, W.; Li, S.; et al. HIF-1a-regulated miR-1275 maintains stem cell-like phenotypes and promotes the progression of LUAD by simultaneously activating Wnt/beta-catenin and Notch signaling. Theranostics 2020, 10, 2553–2570. [Google Scholar] [CrossRef]
- Dong, S.; Liang, S.; Cheng, Z.; Zhang, X.; Luo, L.; Li, L.; Zhang, W.; Li, S.; Xu, Q.; Zhong, M.; et al. ROS/PI3K/Akt and Wnt/beta-catenin signalings activate HIF-1alpha-induced metabolic reprogramming to impart 5-fluorouracil resistance in colorectal cancer. J. Exp. Clin. Cancer Res. 2022, 41, 15. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, S.; Sng, N.; Carretero, J.; Welner, R.; Hayashi, Y.; Yamamoto, M.; Tan, A.J.; Yamaguchi, N.; Yasuda, H.; Li, D.; et al. beta-catenin contributes to lung tumor development induced by EGFR mutations. Cancer Res. 2014, 74, 5891–5902. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Lee, J.H.; Jiang, H.; Wang, C.; Wang, S.; Zheng, Z.; Shao, F.; Xu, D.; Xia, Y.; Li, J.; et al. beta-Catenin induces transcriptional expression of PD-L1 to promote glioblastoma immune evasion. J. Exp. Med. 2020, 217, e20200684. [Google Scholar] [CrossRef]
- Vallee, A.; Lecarpentier, Y.; Vallee, J.N. The Key Role of the WNT/beta-Catenin Pathway in Metabolic Reprogramming in Cancers under Normoxic Conditions. Cancers 2021, 13, 5557. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwasaki, M.; Yamamoto, M.; Tomihari, M.; Fujii, K.; Ishikawa, M. Levobupivacaine Administration Suppressed Cell Metabolism in Human Adenocarcinoma A549 Cells. Int. J. Mol. Sci. 2025, 26, 10833. https://doi.org/10.3390/ijms262210833
Iwasaki M, Yamamoto M, Tomihari M, Fujii K, Ishikawa M. Levobupivacaine Administration Suppressed Cell Metabolism in Human Adenocarcinoma A549 Cells. International Journal of Molecular Sciences. 2025; 26(22):10833. https://doi.org/10.3390/ijms262210833
Chicago/Turabian StyleIwasaki, Masae, Makiko Yamamoto, Masahiro Tomihari, Kaori Fujii, and Masashi Ishikawa. 2025. "Levobupivacaine Administration Suppressed Cell Metabolism in Human Adenocarcinoma A549 Cells" International Journal of Molecular Sciences 26, no. 22: 10833. https://doi.org/10.3390/ijms262210833
APA StyleIwasaki, M., Yamamoto, M., Tomihari, M., Fujii, K., & Ishikawa, M. (2025). Levobupivacaine Administration Suppressed Cell Metabolism in Human Adenocarcinoma A549 Cells. International Journal of Molecular Sciences, 26(22), 10833. https://doi.org/10.3390/ijms262210833





