Structure and Function of the Extracellular Matrix in Normal and Pathological Conditions: Looking at the Bicuspid Aortic Valve
Abstract
1. Introduction
1.1. General Principles on Structure and Function of the Extracellular Matrix
1.2. The Structure and Function of Basement Membranes and Connective Tissue
1.3. The Role of Macromolecules in the Composition of ECM
1.3.1. The Function of Proteoglycans: Key Components in the Organisation of the ECM and Cell Processes
1.3.2. The Role of Glycosaminoglycans to Modulate the ECM
2. The Collagen in the Organisation of the ECM
2.1. Fibrillar Collagens
2.2. Network-Forming Collagens
2.3. Stick-like Collagen-Forming Fibrils
3. Elastic Fibres and Elastin Function to Preserve Tissue Elasticity at the ECM Level
4. Laminins Fulfil the Role of Adhesion Proteins Within the ECM Network
5. Integrins: Mediators of Adhesion and Signalling Between Cells and ECM
5.1. Integrins, ECM, and Ligands: A Comprehensive Exploration
5.2. The Process of Integrin Activation, Its Functions in Physiological Contexts and Its Impact on the Disease
5.3. Fibrillin Dysregulation in Bicuspid Aortic Valve Disease
6. Remodelling of the ECM and Its Dynamic Nature
6.1. ADAMTSs, ADAMs and MMPs
6.2. Insight on Physiological and Pathological Functions of Matrix Metalloproteinases
6.3. ADAMTSs and ADAMs
Physiological and Pathological Functions
6.4. Heparanases
Physiological and Pathological Functions
7. Future Direction
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADAM | a disintegrin and metalloproteinase |
| ADAMTS | a disintegrin and metalloproteinase with thrombospondin motifs |
| AML | acute myeloid leukaemia |
| BAV | bicuspid aortic valve |
| BM | basement membrane |
| CAF | cancer-associated fibroblast; |
| CD44 | cluster of differentiation 44 |
| CNS | central nervous system |
| CR-CSCs | colorectal cancer stem cells |
| CS | chondroitin sulphate |
| DDRs | discoidin domain receptors |
| DES | desmosine |
| DS | dermatan sulphate |
| EBP | elastin-binding protein |
| ECM | extracellular matrix |
| EDPs | elastin-derived peptides |
| EGFR | epidermal growth factor receptor |
| EMILINs | elastin microfibril interfacers |
| EMT | epithelial-to-mesenchymal transition |
| ERC | elastin receptor complex |
| ERM | ezrin–radixin–moesin |
| FACITs | fibril-associated collagens with interrupted triple helices |
| FGFR | fibroblast growth factor receptor |
| GAG | glycosaminoglycan |
| GFs | growth factors |
| GPC | glypican |
| GPI | glycosylphosphatidylinositol |
| HA | hyaluronan; |
| HAS | hyaluronan synthase |
| HB-EGF | heparin-binding EGF |
| Hep | heparin |
| Hh | Hedgehog |
| HPSE | heparanase |
| HS | heparan sulphate |
| Hyal | hyaluronidase |
| IGFIR | insulin-like growth factor receptor I |
| LacCer | lactosylceramide |
| LOX | lysyl oxidase |
| LRP | lipoprotein receptor-related protein |
| MACITs | membrane-associated collagens with interrupted triple helices |
| MET | mesenchymal-to-epithelial transition |
| MMPs | matrix metalloproteinases |
| Neu-1 | neuraminidase-1 |
| OPN | osteopontin |
| PEGF | pigment epithelium-derived factor |
| PG | proteoglycan |
| RHAMM | receptor for hyaluronan-mediated motility |
| RIP | regulated intramembrane proteolysis |
| ROS | reactive oxygen species |
| RTK | receptor tyrosine kinase |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| SLRPs | small leucine-rich proteoglycans |
| SRGN | serglycin |
| TE | tropoelastin |
| TIMP | tissue inhibitor of metalloproteinases |
| TLR | toll-like receptor |
| TMEM | transmembrane protein |
| TN | tenascin |
| TSP | thrombospondin |
| VEGF | vascular endothelial growth factor |
| vWF | vonWillebrand factor. |
References
- Fedak, P.W.; de Sa, M.P.; Verma, S.; Nili, N.; Kazemian, P.; Butany, J.; Strauss, B.H.; Weisel, R.D.; David, T.E. Vascular matrix remodeling in patients with bicuspid aortic valve malformations: Implications for aortic dilatation. J. Thorac. Cardiovasc. Surg. 2003, 126, 797–806. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Gialeli, C.; Bouris, P.; Giannopoulou, E.; Skandalis, S.S.; Aletras, A.J.; Iozzo, R.V.; Karamanos, N.K. Cell-matrix interactions: Focus on proteoglycan-proteinase interplay and pharmacological targeting in cancer. FEBS J. 2014, 281, 5023–5042. [Google Scholar] [CrossRef] [PubMed]
- Fedak, P.W.; Verma, S.; David, T.E.; Leask, R.L.; Weisel, R.D.; Butany, J. Clinical and pathophysiological implications of a bicuspid aortic valve. Circulation 2002, 106, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.A.; Meng, L.; Zaia, J. Deep Sequencing of Complex Proteoglycans: A Novel Strategy for High Coverage and Site-specific Identification of Glycosaminoglycan-linked Peptides. Mol. Cell. Proteomics. 2018, 17, 1578–1590. [Google Scholar] [CrossRef] [PubMed]
- Mavrogonatou, E.; Pratsinis, H.; Papadopoulou, A.; Karamanos, N.K.; Kletsas, D. Extracellular matrix alterations in senescent cells and their significance in tissue homeostasis. Matrix Biol. 2019, 75–76, 27–42. [Google Scholar] [CrossRef]
- Niwa, K.; Perloff, J.K.; Bhuta, S.M.; Laks, H.; Drinkwater, D.C.; Child, J.S.; Miner, P.D. Structural abnormalities of great arterial walls in congenital heart disease: Light and electron microscopic analyses. Circulation 2001, 103, 393–400. [Google Scholar] [CrossRef]
- Bunton, T.E.; Biery, N.J.; Myers, L.; Gayraud, B.; Ramirez, F.; Dietz, H.C. Phenotypic alteration of vascular smooth muscle cells precedes elastolysis in a mouse model of Marfan syndrome. Circ. Res. 2001, 88, 37–43. [Google Scholar] [CrossRef]
- Urbanczyk, M.; Layland, S.L.; Schenke-Layland, K. The role of extracellular matrix in biomechanics and its impact on bioengineering of cells and 3D tissues. Matrix Biol. 2020, 85–86, 1–14. [Google Scholar] [CrossRef]
- Epstein, J.A.; Buck, C.A. Transcriptional regulation of cardiac development: Implications for congenital heart disease and DiGeorge syndrome. Pediatr. Res. 2000, 48, 717–724. [Google Scholar] [CrossRef]
- Karamanos, N.K. Extracellular matrix: Key structural and functional meshwork in health and disease. FEBS J. 2019, 286, 2826–2829. [Google Scholar] [CrossRef] [PubMed]
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E.H.; et al. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Manou, D.; Karamanos, N.K. The extracellular matrix as a multitasking player in disease. FEBS J. 2019, 286, 2830–2869. [Google Scholar] [CrossRef]
- Iozzo, R.V.; Theocharis, A.D.; Neill, T.; Karamanos, N.K. Complexity of matrix phenotypes. Matrix Biol. Plus 2020, 6–7, 100038. [Google Scholar] [CrossRef]
- Eckersley, A.; Ozols, M.; O’Cualain, R.; Keevill, E.J.; Foster, A.; Pilkington, S.; Knight, D.; Griffiths, C.E.M.; Watson, R.E.B.; Sherratt, M.J. Proteomic fingerprints of damage in extracellular matrix assemblies. Matrix Biol. Plus 2020, 5, 100027. [Google Scholar] [CrossRef]
- Csapo, R.; Gumpenberger, M.; Wessner, B. Skeletal Muscle Extracellular Matrix—What Do We Know About Its Composition, Regulation, and Physiological Roles? A Narrative Review. Front. Physiol. 2020, 11, 253. [Google Scholar] [CrossRef]
- Poole, J.J.A.; Mostaço-Guidolin, L.B. Optical Microscopy and the Extracellular Matrix Structure: A Review. Cells 2021, 10, 1760. [Google Scholar] [CrossRef]
- Karamanos, N.K.; Theocharis, A.D.; Neill, T.; Iozzo, R.V. Matrix modeling and remodeling: A biological interplay regulating tissue homeostasis and diseases. Matrix Biol. 2019, 75–76, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Karamanos, N.K.; Piperigkou, Z.; Theocharis, A.D.; Watanabe, H.; Franchi, M.; Baud, S.; Brézillon, S.; Götte, M.; Passi, A.; Vigetti, D.; et al. Proteoglycan Chemical Diversity Drives Multifunctional Cell Regulation and Therapeutics. Chem. Rev. 2018, 118, 9152–9232. [Google Scholar] [CrossRef]
- Lepedda, A.J.; Nieddu, G.; Formato, M.; Baker, M.B.; Fernández-Pérez, J.; Moroni, L. Glycosaminoglycans: From Vascular Physiology to Tissue Engineering Applications. Front. Chem. 2021, 9, 680836. [Google Scholar] [CrossRef] [PubMed]
- Rehan, I.F.; Elnagar, A.; Zigo, F.; Sayed-Ahmed, A.; Yamada, S. Biomimetic strategies for the deputization of proteoglycan functions. Front. Cell Dev. Biol. 2024, 12, 1391769. [Google Scholar] [CrossRef]
- Iozzo, R.V.; Schaefer, L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015, 42, 11–55. [Google Scholar] [CrossRef]
- Chan, C.K.; Rolle, M.W.; Potter-Perigo, S.; Braun, K.R.; Van Biber, B.P.; Laflamme, M.A.; Murry, C.E.; Wight, T.N. Differentiation of cardiomyocytes from human embryonic stem cells is accompanied by changes in the extracellular matrix production of versican and hyaluronan. J. Cell. Biochem. 2010, 111, 585–596. [Google Scholar] [CrossRef]
- Melrose, J. Perlecan, a modular instructive proteoglycan with diverse functional properties. Int. J. Biochem. Cell Biol. 2020, 128, 105849. [Google Scholar] [CrossRef]
- Kapoor, A.; Chen, C.G.; Iozzo, R.V. Endorepellin evokes an angiostatic stress signaling cascade in endothelial cells. Biol. Chem. 2020, 295, 6344–6356. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.G.; Iozzo, R.V. Angiostatic cues from the matrix: Endothelial cell autophagy meets hyaluronan biology. J. Biol. Chem. 2020, 295, 16797–16812. [Google Scholar] [CrossRef] [PubMed]
- Neill, T.; Buraschi, S.; Kapoor, A.; Iozzo, R.V. Proteoglycan-driven Autophagy: A Nutrient-independent Mechanism to Control Intracellular Catabolism. J. Histochem. Cytochem. 2020, 68, 733–746. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.J.; Farrugia, B.L.; Biose, I.J.; Bix, G.J.; Melrose, J. Perlecan, A Multi-Functional, Cell-Instructive, Matrix-Stabilizing Proteoglycan With Roles in Tissue Development Has Relevance to Connective Tissue Repair and Regeneration. Front. Cell Dev. Biol. 2022, 10, 856261. [Google Scholar] [CrossRef]
- Siegel, G.; Malmsten, M.; Ermilov, E. Anionic biopolyelectrolytes of the syndecan/perlecan superfamily: Physicochemical properties and medical significance. Adv. Colloid. Interface Sci. 2014, 205, 275–318. [Google Scholar] [CrossRef]
- Poluzzi, C.; Iozzo, R.V.; Schaefer, L. Endostatin and endorepellin: A common route of action for similar angiostatic cancer avengers. Adv. Drug Deliv. Rev. 2016, 97, 156–173. [Google Scholar] [CrossRef]
- Nemcova, A.; Jirkovska, A.; Dubsky, M.; Kolesar, L.; Bem, R.; Fejfarova, V.; Pysna, A.; Woskova, V.; Skibova, J.; Jude, E.B. Difference in Serum Endostatin Levels in Diabetic Patients with Critical Limb Ischemia Treated by Autologous Cell Therapy or Percutaneous Transluminal Angioplasty. Cell Transplant. 2018, 27, 1368–1374. [Google Scholar] [CrossRef]
- Xian, X.; Gopal, S.; Couchman, J.R. Syndecans as receptors and organizers of the extracellular matrix. Cell Tissue Res. 2010, 339, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Ricard-Blum, S.; Couchman, J.R. Conformations, interactions and functions of intrinsically disordered syndecans. Biochem. Soc. Trans. 2023, 51, 1083–1096. [Google Scholar] [CrossRef]
- Gondelaud, F.; Bouakil, M.; Le Fèvre, A.; Miele, A.E.; Chirot, F.; Duclos, B.; Liwo, A.; Ricard-Blum, S. Extended disorder at the cell surface: The conformational landscape of the ectodomains of syndecans. Matrix Biol. Plus 2021, 12, 100081. [Google Scholar] [CrossRef]
- Corti, F.; Wang, Y.; Rhodes, J.M.; Atri, D.; Archer-Hartmann, S.; Zhang, J.; Zhuang, Z.W.; Chen, D.; Wang, T.; Wang, Z.; et al. N-terminal syndecan-2 domain selectively enhances 6-O heparan sulfate chains sulfation and promotes VEGFA165-dependent neovascularization. Nat. Commun. 2019, 10, 1562. [Google Scholar] [CrossRef]
- Gondelaud, F.; Ricard-Blum, S. Structures and interactions of syndecans. FEBS J. 2019, 286, 2994–3007. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Filmus, J. Glypican-6 and Glypican-4 stimulate embryonic stomach growth by regulating Hedgehog and noncanonical Wnt signaling. Dev. Dyn. 2022, 251, 2015–2028. [Google Scholar] [CrossRef] [PubMed]
- Kolset, S.O.; Tveit, H. Serglycin--structure and biology. Cell. Mol. Life Sci. 2008, 65, 1073–1085. [Google Scholar] [CrossRef]
- Kolset, S.O.; Pejler, G. Serglycin: A structural and functional chameleon with wide impact on immune cells. J. Immunol. 2011, 187, 4927–4933. [Google Scholar] [CrossRef]
- Manou, D.; Karamanos, N.K.; Theocharis, A.D. Tumorigenic functions of serglycin: Regulatory roles in epithelial to mesenchymal transition and oncogenic signaling. Semin. Cancer Biol. 2020, 62, 108–115. [Google Scholar] [CrossRef]
- Simpson, M.A. Impacts of Hyaluronan on Extracellular Vesicle Production and Signaling. Cells 2025, 14, 139. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Chanmee, T.; Itano, N. Hyaluronan: Metabolism and Function. Biomolecules 2020, 10, 1525. [Google Scholar] [CrossRef]
- Sugita, S.; Naito, Y.; Zhou, L.; He, H.; Hao, Q.; Sakamoto, A.; Lee, J.W. Hyaluronic acid restored protein permeability across injured human lung microvascular endothelial cells. FASEB Bioadv. 2022, 4, 619–631. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, Y.; Zhang, S.; Wang, X.; Dou, H.; Yu, X.; Zhang, Z.; Yang, S.; Xiao, M. Extracellular matrix remodeling in tumor progression and immune escape: From mechanisms to treatments. Mol. Cancer 2023, 22, 48. [Google Scholar] [CrossRef]
- Tavianatou, A.G.; Caon, I.; Franchi, M.; Piperigkou, Z.; Galesso, D.; Karamanos, N.K. Hyaluronan: Molecular size-dependent signaling and biological functions in inflammation and cancer. FEBS J. 2019, 286, 2883–2908. [Google Scholar] [CrossRef]
- Taylor, K.R.; Yamasaki, K.; Radek, K.A.; Nardo, A.D.; Goodarzi, H.; Golenbock, D.; Beutler, B.; Gallo, R.L. Recognition of hyaluronan released in sterile injury involves a unique receptor complex dependent on Toll-like receptor 4, CD44, and MD-2. J. Biol. Chem. 2007, 282, 18265–18275. [Google Scholar] [CrossRef]
- Leng, Y.; Abdullah, A.; Wendt, M.K.; Calve, S. Hyaluronic acid, CD44 and RHAMM regulate myoblast behavior during embryogenesis. Matrix Biol. 2019, 79, 236–254. [Google Scholar] [CrossRef]
- Ishizuka, S.; Tsuchiya, S.; Ohashi, Y.; Terabe, K.; Askew, E.B.; Ishizuka, N.; Knudson, C.B.; Knudson, W. Hyaluronan synthase 2 (HAS2) overexpression diminishes the procatabolic activity of chondrocytes by a mechanism independent of extracellular hyaluronan. J. Biol. Chem. 2019, 294, 13562–13579. [Google Scholar] [CrossRef] [PubMed]
- Knudson, W.; Ishizuka, S.; Terabe, K.; Askew, E.B.; Knudson, C.B. The pericellular hyaluronan of articular chondrocytes. Matrix Biol. 2019, 78–79, 32–46. [Google Scholar] [CrossRef]
- Koide, T. Designed triple-helical peptides as tools for collagen biochemistry and matrix engineering. Philos. Trans. R Soc. Lond. B Biol Sci. 2007, 362, 1281–1291. [Google Scholar] [CrossRef] [PubMed]
- Varma, S.; Orgel, J.P.; Schieber, J.D. Nanomechanics of Type I Collagen. Biophys. J. 2016, 111, 50–56. [Google Scholar] [CrossRef]
- Kirkness, M.W.; Lehmann, K.; Forde, N.R. Mechanics and structural stability of the collagen triple helix. Curr. Opin. Chem. Biol. 2019, 53, 98–105. [Google Scholar] [CrossRef]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, e1801651. [Google Scholar] [CrossRef]
- Hu, J.; Li, J.; Jiang, J.; Wang, L.; Roth, J.; McGuinness, K.N.; Baum, J.; Dai, W.; Sun, Y.; Nanda, V.; et al. Design of synthetic collagens that assemble into supramolecular banded fibers as a functional biomaterial testbed. Nat. Commun. 2022, 13, 6761. [Google Scholar] [CrossRef] [PubMed]
- Kozel, B.A.; Mecham, R.P. Elastic fiber ultrastructure and assembly. Matrix Biol. 2019, 84, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Reichheld, S.E.; Muiznieks, L.D.; Lu, R.; Sharpe, S.; Keeley, F.W. Sequence variants of human tropoelastin affecting assembly, structural characteristics and functional properties of polymeric elastin in health and disease. Matrix Biol. 2019, 84, 68–80. [Google Scholar] [CrossRef]
- Halper, J.; Kjaer, M. Basic components of connective tissues and extracellular matrix: Elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins. Adv. Exp. Med. Biol. 2014, 802, 31–47. [Google Scholar] [CrossRef]
- Thomson, J.; Singh, M.; Eckersley, A.; Cain, S.A.; Sherratt, M.J.; Baldock, C. Fibrillin microfibrils and elastic fibre proteins: Functional interactions and extracellular regulation of growth factors. Semin. Cell Dev. Biol. 2019, 89, 109–117. [Google Scholar] [CrossRef]
- Wang, M.; McGraw, K.R.; Monticone, R.E.; Pintus, G. Unraveling Elastic Fiber-Derived Signaling in Arterial Aging and Related Arterial Diseases. Biomolecules 2025, 15, 153. [Google Scholar] [CrossRef]
- Kawecki, C.; Hézard, N.; Bocquet, O.; Poitevin, G.; Rabenoelina, F.; Kauskot, A.; Duca, L.; Blaise, S.; Romier, B.; Martiny, L.; et al. Elastin-derived peptides are new regulators of thrombosis. Arter. Thromb. Vasc. Biol. 2014, 34, 2570–2578. [Google Scholar] [CrossRef] [PubMed]
- Le Page, A.; Khalil, A.; Vermette, P.; Frost, E.H.; Larbi, A.; Witkowski, J.M.; Fulop, T. The role of elastin-derived peptides in human physiology and diseases. Matrix Biol. 2019, 84, 81–96. [Google Scholar] [CrossRef]
- Heinz, A. Elastases and elastokines: Elastin degradation and its significance in health and disease. Crit. Rev. Biochem. Mol. Biol. 2020, 55, 252–273. [Google Scholar] [CrossRef]
- Rodríguez, C.; Martínez-González, J. The Role of Lysyl Oxidase Enzymes in Cardiac Function and Remodeling. Cells 2019, 8, 1483. [Google Scholar] [CrossRef]
- Al-U’datt, D.; Allen, B.G.; Nattel, S. Role of the lysyl oxidase enzyme family in cardiac function and disease. Cardiovasc. Res. 2019, 115, 1820–1837. [Google Scholar] [CrossRef]
- Vallet, S.D.; Ricard-Blum, S. Lysyl oxidases: From enzyme activity to extracellular matrix cross-links. Essays Biochem. 2019, 63, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Vallet, S.D.; Miele, A.E.; Uciechowska-Kaczmarzyk, U.; Liwo, A.; Duclos, B.; Samsonov, S.A.; Ricard-Blum, S. Insights into the structure and dynamics of lysyl oxidase propeptide, a flexible protein with numerous partners. Sci. Rep. 2018, 8, 11768. [Google Scholar] [CrossRef] [PubMed]
- Lazar, T.; Martínez-Pérez, E.; Quaglia, F.; Hatos, A.; Chemes, L.B.; Iserte, J.A.; Méndez, N.A.; Garrone, N.A.; Saldaño, T.E.; Marchetti, J.; et al. PED in 2021: A major update of the protein ensemble database for intrinsically disordered proteins. Nucleic Acids Res. 2021, 49, D404–D411. [Google Scholar] [CrossRef]
- Vallet, S.D.; Guéroult, M.; Belloy, N.; Dauchez, M.; Ricard-Blum, S. A Three-Dimensional Model of Human Lysyl Oxidase, a Cross-Linking Enzyme. ACS Omega 2019, 4, 8495–8505, Erratum in ACS Omega 2020, 5, 14202. [Google Scholar] [CrossRef]
- Calabro, N.E.; Kristofik, N.J.; Kyriakides, T.R. Thrombospondin-2 and extracellular matrix assembly. Biochim. Biophys. Acta. 2014, 1840, 2396–2402. [Google Scholar] [CrossRef] [PubMed]
- Rosini, S.; Pugh, N.; Bonna, A.M.; Hulmes, D.J.S.; Farndale, R.W.; Adams, J.C. Thrombospondin-1 promotes matrix homeostasis by interacting with collagen and lysyl oxidase precursors and collagen cross-linking sites. Sci. Signal. 2018, 11, eaar2566. [Google Scholar] [CrossRef]
- Calabro, N.E.; Barrett, A.; Chamorro-Jorganes, A.; Tam, S.; Kristofik, N.J.; Xing, H.; Loye, A.M.; Sessa, W.C.; Hansen, K.; Kyriakides, T.R. Thrombospondin-2 regulates extracellular matrix production, LOX levels, and cross-linking via downregulation of miR-29. Matrix Biol. 2019, 82, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Chester, D.; Brown, A.C. The role of biophysical properties of provisional matrix proteins in wound repair. Matrix Biol. 2017, 60–61, 124–140. [Google Scholar] [CrossRef]
- Arnoldini, S.; Moscaroli, A.; Chabria, M.; Hilbert, M.; Hertig, S.; Schibli, R.; Béhé, M.; Vogel, V. Novel peptide probes to assess the tensional state of fibronectin fibers in cancer. Nat. Commun. 2017, 8, 1793. [Google Scholar] [CrossRef]
- Miéville, A.; Fonta, C.M.; Leo, C.; Christe, L.; Goldhahn, J.; Singer, G.; Vogel, V. Fibronectin Fibers Progressively Lose Their Tension in Invasive Human Breast Carcinoma while Being Tensed in DCIS and Healthy Breast Tissue. Adv. Sci. 2025, 12, e04351. [Google Scholar] [CrossRef]
- Di Russo, J.; Hannocks, M.J.; Luik, A.L.; Song, J.; Zhang, X.; Yousif, L.; Aspite, G.; Hallmann, R.; Sorokin, L. Vascular laminins in physiology and pathology. Matrix Biol. 2017, 57–58, 140–148. [Google Scholar] [CrossRef]
- Takahashi, K.; Aritomi, S.; Honkawa, F.; Asari, S.; Hirose, K.; Konishi, A. Efficient and cost-effective differentiation of induced neural crest cells from induced pluripotent stem cells using laminin 211. Regen. Ther. 2024, 26, 749–759. [Google Scholar] [CrossRef]
- Mohassel, P.; Foley, A.R.; Bönnemann, C.G. Extracellular matrix-driven congenital muscular dystrophies. Matrix Biol. 2018, 71–72, 188–204. [Google Scholar] [CrossRef] [PubMed]
- Matic Jelic, I.; Stokovic, N.; Ivanjko, N.; Pecina, M.; Kufner, V.; Bordukalo Niksic, T.; Vukicevic, S. Systemic inhibition of bone morphogenetic protein 1.3 as a possible treatment for laminin-related congenital muscular dystrophy. Int. Orthop. 2025, 49, 45–52. [Google Scholar] [CrossRef]
- Allamand, V.; Guicheney, P. Merosin-deficient congenital muscular dystrophy, autosomal recessive (MDC1A, MIM#156225, LAMA2 gene coding for alpha2 chain of laminin). Eur. J. Hum. Genet. 2002, 10, 91–94. [Google Scholar]
- Abedsaeidi, M.; Hojjati, F.; Tavassoli, A.; Sahebkar, A. Biology of Tenascin C and its Role in Physiology and Pathology. Curr. Med. Chem. 2024, 31, 2706–2731. [Google Scholar] [CrossRef] [PubMed]
- Loustau, T.; Abou-Faycal, C.; Erne, W.; Zur Wiesch, P.A.; Ksouri, A.; Imhof, T.; Mörgelin, M.; Li, C.; Mathieu, M.; Salomé, N.; et al. Modulating tenascin-C functions by targeting the MAtrix REgulating MOtif, “MAREMO”. Matrix Biol. 2022, 108, 20–38. [Google Scholar] [CrossRef]
- Imanaka-Yoshida, K.; Tawara, I.; Yoshida, T. Tenascin-C in cardiac disease: A sophisticated controller of inflammation, repair, and fibrosis. Am. J. Physiol. Cell. Physiol. 2020, 319, C781–C796. [Google Scholar] [CrossRef]
- Nagel, F.; Schaefer, A.K.; Gonçalves, I.F.; Acar, E.; Oszwald, A.; Kaiser, P.; Kain, R.; Trescher, K.; Eilenberg, W.H.; Brostjan, C.; et al. The expression and role of tenascin C in abdominal aortic aneurysm formation and progression. Interact. Cardiovasc. Thorac. Surg. 2022, 34, 841–848. [Google Scholar] [CrossRef]
- Jana, S.; Hu, M.; Shen, M.; Kassiri, Z. Extracellular matrix, regional heterogeneity of the aorta, and aortic aneurysm. Exp. Mol. Med. 2019, 51, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Meloty-Kapella, C.V.; Degen, M.; Chiquet-Ehrismann, R.; Tucker, R.P. Effects of tenascin-W on osteoblasts in vitro. Cell Tissue Res. 2008, 334, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Degen, M.; Scherberich, A.; Tucker, R.P. Tenascin-W: Discovery, Evolution, and Future Prospects. Front. Immunol. 2021, 11, 623305. [Google Scholar] [CrossRef] [PubMed]
- Tucker, R.P.; Degen, M. Revisiting the Tenascins: Exploitable as Cancer Targets? Front. Oncol. 2022, 12, 908247. [Google Scholar] [CrossRef]
- Hargus, G.; Cui, Y.; Schmid, J.S.; Xu, J.; Glatzel, M.; Schachner, M.; Bernreuther, C. Tenascin-R promotes neuronal differentiation of embryonic stem cells and recruitment of host-derived neural precursor cells after excitotoxic lesion of the mouse striatum. Stem Cells 2008, 26, 1973–1984. [Google Scholar] [CrossRef]
- Rathjen, F.G.; Hodge, R. Early Days of Tenascin-R Research: Two Approaches Discovered and Shed Light on Tenascin-R. Front. Immunol. 2021, 11, 612482. [Google Scholar] [CrossRef]
- Fernandez, M.C.; Duran, A.C.; Real, R.; López, D.; Fernández, B.; de Andrés, A.V.; Arqué, J.M.; Gallego, A.; Sans-Coma, V. Coronary artery anomalies and aortic valve morphology in the Syrian hamster. Lab. Anim. 2000, 34, 145–154. [Google Scholar] [CrossRef]
- Eisenberg, L.M.; Markwald, R.R. Molecular regulation of atrioventricular valvuloseptal morphogenesis. Circ. Res. 1995, 77, 1–6. [Google Scholar] [CrossRef]
- Towbin, J.A.; Belmont, J. Molecular determinants of left and right outflow tract obstruction. J. Am. J. Med. Genet. 2000, 97, 297–303. [Google Scholar] [CrossRef]
- Lee, T.C.; Zhao, Y.D.; Courtman, D.W.; Stewart, D.J. Abnormal aortic valve development in mice lacking endothelial nitric oxide synthase. Circulation 2000, 101, 2345–2348. [Google Scholar] [CrossRef]
- Loscalzo, M.L.; Goh, D.L.; Loeys, B.; Kent, K.C.; Spevak, P.J.; Dietz, H.C. Familial thoracic aortic dilation and bicommissural aortic valve: A prospective analysis of natural history and inheritance. Am. J. Med. Genet. A 2007, 143A, 1960–1967. [Google Scholar] [CrossRef] [PubMed]
- Cripe, L.; Andelfinger, G.; Martin, L.J.; Shooner, K.; Benson, D.W. Bicuspid aortic valve is heritable. J. Am. Coll. Cardiol. 2004, 44, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Gianakas, C.A.; Keeley, D.P.; Ramos-Lewis, W.; Park, K.; Jayadev, R.; Kenny, I.W.; Chi, Q.; Sherwood, D.R. Hemicentin-mediated type IV collagen assembly strengthens juxtaposed basement membrane linkage. J. Cell. Biol. 2023, 222, e202112096. [Google Scholar] [CrossRef] [PubMed]
- Keeley, D.P.; Sherwood, D.R. Tissue linkage through adjoining basement membranes: The long and the short term of it. Matrix Biol. 2019, 75–76, 58–71. [Google Scholar] [CrossRef]
- Yao, Y. Laminin: Loss-of-function studies. Cell. Mol. Life Sci. 2017, 74, 1095–1115. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, M.; Qian, Y.; Yang, Y.; Sun, Y.; Liu, B.; Wang, L.; Dong, M. Identification of a likely pathogenic structural variation in the LAMA1 gene by Bionano optical mapping. NPJ Genom. Med. 2020, 5, 31. [Google Scholar] [CrossRef]
- Kolasangiani, R.; Bidone, T.C.; Schwartz, M.A. Integrin Conformational Dynamics and Mechanotransduction. Cells 2022, 11, 3584. [Google Scholar] [CrossRef]
- Moreno-Layseca, P.; Icha, J.; Hamidi, H.; Ivaska, J. Integrin trafficking in cells and tissues. Nat. Cell Biol. 2019, 21, 122–132. [Google Scholar] [CrossRef]
- Kadry, Y.A.; Calderwood, D.A. Chapter 22: Structural and signaling functions of integrins. Biochim. et Biophys. Acta (BBA) Biomembr. 2020, 1862, 183206. [Google Scholar] [CrossRef]
- Yazlovitskaya, E.M.; Plosa, E.; Bock, F.; Viquez, O.M.; Mernaugh, G.; Gewin, L.S.; De Arcangelis, A.; Georges-Labouesse, E.; Sonnenberg, A.; Blackwell, T.S.; et al. The laminin-binding integrins regulate nuclear factor κB-dependent epithelial cell polarity and inflammation. J. Cell Sci. 2021, 134, jcs259161. [Google Scholar] [CrossRef]
- Halper, J. Proteoglycans and diseases of softs tissus. Adv. Exp. Med. Biol. 2014, 802, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Gubbiotti, M.A.; Buraschi, S.; Kapoor, A.; Iozzo, R.V. Proteoglycan signaling in tumor angiogenesis and endothelial cell autophagy. Semin. Cancer Biol. 2020, 62, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Frey, H.; Schroeder, N.; Manon-Jensen, T.; Iozzo, R.V.; Schaefer, L. Biological interplay between proteoglycans and their innate immune receptors in inflammation. FEBS J. 2013, 280, 2165–2179. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, U. A personal voyage through the proteoglycan field. Matrix Biol. 2014, 35, 3–7. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Karamanos, N.K. Proteoglycans remodeling in cancer: Underlying molecular mechanisms. Matrix Biol. 2019, 75–76, 220–259. [Google Scholar] [CrossRef]
- Neill, T.; Kapoor, A.; Xie, C.; Buraschi, S.; Iozzo, R.V. A functional outside-in signaling network of proteoglycans and matrix molecules regulating autophagy. Matrix Biol. 2021, 100–101, 118–149. [Google Scholar] [CrossRef]
- Couchman, J.R. Transmembrane signaling proteoglycans. Annu. Rev. Cell Dev. Biol. 2010, 26, 89–114. [Google Scholar] [CrossRef]
- Gopal, S.; Søgaard, P.; Multhaupt, H.A.; Pataki, C.; Okina, E.; Xian, X.; Pedersen, M.E.; Stevens, T.; Griesbeck, O.; Park, P.W.; et al. Transmembrane proteoglycans control stretch-activated channels to set cytosolic calcium levels. J. Cell Biol. 2015, 210, 1199–1211. [Google Scholar] [CrossRef] [PubMed]
- Moreth, K.; Iozzo, R.V.; Schaefer, L. Small leucine-rich proteoglycans orchestrate receptor crosstalk during inflammation. Cell Cycle 2012, 11, 2084–2091. [Google Scholar] [CrossRef]
- Dituri, F.; Gigante, G.; Scialpi, R.; Mancarella, S.; Fabregat, I.; Giannelli, G. Proteoglycans in Cancer: Friends or Enemies? A Special Focus on Hepatocellular Carcinoma. Cancers 2022, 14, 1902. [Google Scholar] [CrossRef] [PubMed]
- Piperigkou, Z.; Mohr, B.; Karamanos, N.; Götte, M. Shed proteoglycans in tumor stroma. Cell Tissue Res. 2016, 635, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Piperigkou, Z.; Karamanos, N.K. Evaluating the Effects of MicroRNAs on Proteoglycans and Matrix Constituents’ Expression and Functional Properties. Methods Mol. Biol. 2023, 2619, 257–271. [Google Scholar] [CrossRef]
- Human Protein Atlas. Available online: http://www.proteinatlas.org (accessed on 1 October 2025).
- Melo, F.R.; Vita, F.; Berent-Maoz, B.; Levi-Schaffer, F.; Zabucchi, G.; Pejler, G. Proteolytic histone modification by mast cell tryptase, a serglycin proteoglycan-dependent secretory granule protease. J. Biol Chem. 2014, 289, 7682–7690. [Google Scholar] [CrossRef]
- Bouris, P.; Manou, D.; Sopaki-Valalaki, A.; Kolokotroni, A.; Moustakas, A.; Kapoor, A.; Iozzo, R.V.; Karamanos, N.K.; Theocharis, A.D. Serglycin promotes breast cancer cell aggressiveness: Induction of epithelial to mesenchymal transition, proteolytic activity and IL-8 signaling. Matrix Biol. 2018, 74, 35–51. [Google Scholar] [CrossRef]
- Korpetinou, A.; Papachristou, D.J.; Lampropoulou, A.; Bouris, P.; Labropoulou, V.T.; Noulas, A.; Karamanos, N.K.; Theocharis, A.D. Increased Expression of Serglycin in Specific Carcinomas and Aggressive Cancer Cell Lines. Biomed. Res. Int. 2015, 2015, 690721. [Google Scholar] [CrossRef]
- Korpetinou, A.; Skandalis, S.; Labropoulou, V.; Smirlaki, G.; Noulas, A.; Karamanos, N.; Theocharis, A.D. Serglycin: At the crossroad of inflammation and malignancy. Front. Oncol. 2014, 3, 327. [Google Scholar] [CrossRef]
- Colwill, K.; Renewable Protein Binder Working Group; Gräslund, S. A roadmap to generate renewable protein binders to the human proteome. Nat. Methods 2011, 8, 551–558. [Google Scholar] [CrossRef]
- Kemberi, M.; Salmasi, Y.; Santamaria, S. The Role of ADAMTS Proteoglycanases in Thoracic Aortic Disease. Int. J. Mol. Sci. 2023, 24, 12135. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Hughes, M.; Krishnamoorthy, S.; Zou, S.; Zhang, L.; Wu, D.; Zhang, C.; Curci, J.A.; Coselli, J.S.; Milewicz, D.M.; et al. Critical Role of ADAMTS-4 in the Development of Sporadic Aortic Aneurysm and Dissection in Mice. Sci. Rep. 2017, 7, 12351. [Google Scholar] [CrossRef]
- Cikach, F.S.; Koch, C.D.; Mead, T.J.; Galatioto, J.; Willard, B.B.; Emerton, K.B.; Eagleton, M.J.; Blackstone, E.H.; Ramirez, F.; Roselli, E.E.; et al. Massive aggrecan and versican accumulation in thoracic aortic aneurysm and dissection. JCI Insight 2018, 3, e97167. [Google Scholar] [CrossRef]
- Goyal, A.; Neill, T.; Owens, R.T.; Schaefer, L.; Iozzo, R.V. Decorin activates AMPK, an energy sensor kinase, to induce autophagy in endothelial cells. Matrix Biol. 2014, 34, 46–54. [Google Scholar] [CrossRef]
- Karamanou, K.; Franchi, M.; Onisto, M.; Passi, A.; Vynios, D.H.; Brézillon, S. Evaluation of lumican effects on morphology of invading breast cancer cells, expression of integrins and downstream signaling. FEBS J. 2020, 287, 4862–4880. [Google Scholar] [CrossRef]
- Neill, T.; Iozzo, R.V. The Role of Decorin Proteoglycan in Mitophagy. Cancers 2022, 14, 804. [Google Scholar] [CrossRef]
- Lopez, S.G.; Moura, H.R.; Chow, E.; Kuo, J.C.; Paszek, M.J.; Bonassar, L.J. Recombinant Small Leucine-Rich Proteoglycans Modulate Fiber Structure and Mechanical Properties of Collagen Gels. ACS Biomater. Sci. Eng. 2025, 11, 4101–4115. [Google Scholar] [CrossRef]
- Sun, L.R.; Li, S.Y.; Guo, Q.S.; Zhou, W.; Zhang, H.M. SPOCK1 Involvement in Epithelial-to-Mesenchymal Transition: A New Target in Cancer Therapy? Cancer Manag. Res. 2020, 12, 3561–3569. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Rai, A.; Dohare, R.; Arora, S.; Ali, S.; Parveen, S.; Syed, M.A. Network-based identification of signature genes KLF6 and SPOCK1 associated with oral submucous fibrosis. Mol. Clin. Oncol. 2020, 12, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, A.; Yurchenco, P.D.; Iozzo, R.V. The nature and biology of basement membranes. Matrix Biol. 2017, 57–58, 1–11. [Google Scholar] [CrossRef]
- Willis, C.D.; Poluzzi, C.; Mongiat, M.; Iozzo, R.V. Endorepellin laminin-like globular 1/2 domains bind Ig3-5 of vascular endothelial growth factor (VEGF) receptor 2 and block pro-angiogenic signaling by VEGFA in endothelial cells. FEBS J. 2013, 280, 2271–2284. [Google Scholar] [CrossRef]
- Hakami, H.; Dinesh, N.E.H.; Nelea, V.; Lamarche-Vane, N.; Ricard-Blum, S.; Reinhardt, D.P. Fibulin-4 and latent-transforming growth factor beta-binding protein-4 interactions with syndecan-2 and syndecan-3 are required for elastogenesis. FASEB J. 2025, 39, e70505. [Google Scholar] [CrossRef] [PubMed]
- Lambaerts, K.; Wilcox-Adelman, S.A.; Zimmermann, P. The signaling mechanisms of syndecan heparan sulfate proteoglycans. Curr. Opin. Cell Biol. 2009, 21, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.; Kim, A.; Hwang, J.; Song, H.K.; Kim, Y.; Oh, E.S. Emerging Role of Syndecans in Extracellular Matrix Remodeling in Cancer. J. Histochem. Cytochem. 2020, 68, 863–870. [Google Scholar] [CrossRef]
- Multhaupt, H.A.; Yoneda, A.; Whiteford, J.R.; Oh, E.S.; Lee, W.; Couchman, J.R. Syndecan signaling: When, where and why? J Physiol Pharmacol. 2009, 60 (Suppl 4), 31–38. [Google Scholar] [PubMed]
- Whiteford, J.R.; Behrends, V.; Kirby, H.; Kusche-Gullberg, M.; Muramatsu, T.; Couchman, J.R. Syndecans promote integrin-mediated adhesion of mesenchymal cells in two distinct pathways. Exp. Cell Res. 2007, 313, 3902–3913. [Google Scholar] [CrossRef]
- Gopal, S.; Arokiasamy, S.; Pataki, C.; Whiteford, J.R.; Couchman, J.R. Syndecan receptors: Pericellular regulators in development and inflammatory disease. Open Biol. 2021, 11, 200377. [Google Scholar] [CrossRef]
- Li, N.; Spetz, M.R.; Ho, M. The Role of Glypicans in Cancer Progression and Therapy. J. Histochem. Cytochem. 2020, 68, 841–862. [Google Scholar] [CrossRef]
- Filmus, J.; Capurro, M. The role of glypicans in Hedgehog signaling. Matrix Biol. 2014, 35, 248–252. [Google Scholar] [CrossRef]
- Matsuda, K.; Maruyama, H.; Guo, F.; Kleeff, J.; Itakura, J.; Matsumoto, Y.; Lander, A.D.; Korc, M. Glypican-1 is overexpressed in human breast cancer and modulates the mitogenic effects of multiple heparin-binding growth factors in breast cancer cells. Cancer Res. 2001, 61, 5562–5569. [Google Scholar]
- Lu, H.; Niu, F.; Liu, F.; Gao, J.; Sun, Y.; Zhao, X. Elevated glypican-1 expression is associated with an unfavorable prognosis in pancreatic ductal adenocarcinoma. Cancer Med. 2017, 6, 1181–1191. [Google Scholar] [CrossRef]
- Campbell, D.H.; Lund, M.E.; Nocon, A.L.; Cozzi, P.J.; Frydenberg, M.; De Souza, P.; Schiller, B.; Beebe-Dimmer, J.L.; Ruterbusch, J.J.; Walsh, B.J. Detection of glypican-1 (GPC-1) expression in urine cell sediments in prostate cancer. PLoS ONE. 2018, 13, e0196017. [Google Scholar] [CrossRef]
- Melo, S.A.; Luecke, L.B.; Kahlert, C.; Fernandez, A.F.; Gammon, S.T.; Kaye, J.; LeBleu, V.S.; Mittendorf, E.A.; Weitz, J.; Rahbari, N.; et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015, 523, 177–182, Erratum in Nature 2022, 610, E15–E17. [Google Scholar] [CrossRef]
- Harada, E.; Serada, S.; Fujimoto, M.; Takahashi, Y.; Takahashi, T.; Hara, H.; Nakatsuka, R.; Sugase, T.; Nishigaki, T.; Saito, Y.; et al. Glypican-1 targeted antibody-based therapy induces preclinical antitumor activity against esophageal squamous cell carcinoma. Oncotarget 2017, 8, 24741–24752. [Google Scholar] [CrossRef] [PubMed]
- Raman, S.; Buongervino, S.N.; Lane, M.V.; Zhelev, D.V.; Zhu, Z.; Cui, H.; Martinez, B.; Martinez, D.; Wang, Y.; Upton, K.; et al. A GPC2 antibody-drug conjugate is efficacious against neuroblastoma and small-cell lung cancer via binding a conformational epitope. Cell Rep. Med. 2021, 2, 100344. [Google Scholar] [CrossRef]
- Li, N.; Torres, M.B.; Spetz, M.R.; Wang, R.; Peng, L.; Tian, M.; Dower, C.M.; Nguyen, R.; Sun, M.; Tai, C.H.; et al. CAR T cells targeting tumor-associated exons of glypican 2 regress neuroblastoma in mice. Cell Rep. Med. 2021, 2, 100297. [Google Scholar] [CrossRef] [PubMed]
- Capurro, M.I.; Xiang, Y.Y.; Lobe, C.; Filmus, J. Glypican-3 promotes the growth of hepatocellular carcinoma by stimulating canonical Wnt signaling. Cancer Res. 2005, 65, 6245–6254. [Google Scholar] [CrossRef] [PubMed]
- Capurro, M.; Martin, T.; Shi, W.; Filmus, J. Glypican-3 binds to Frizzled and plays a direct role in the stimulation of canonical Wnt signaling. Cell Sci. 2014, 127 Pt 7, 1565–1575. [Google Scholar] [CrossRef]
- De Cat, B.; Muyldermans, S.Y.; Coomans, C.; Degeest, G.; Vanderschueren, B.; Creemers, J.; Biemar, F.; Peers, B.; David, G. Processing by proprotein convertases is required for glypican-3 modulation of cell survival, Wnt signaling, and gastrulation movements. J. Cell Biol. 2003, 163, 625–635. [Google Scholar] [CrossRef]
- Gao, W.; Ho, M. The role of glypican-3 in regulating Wnt in hepatocellular carcinomas. Cancer Rep. 2011, 1, 14–19. [Google Scholar]
- Castillo, L.F.; Tascón, R.; Lago Huvelle, M.R.; Novack, G.; Llorens, M.C.; Dos Santos, A.F.; Shortrede, J.; Cabanillas, A.M.; Bal de Kier Joffé, E.; Labriola, L.; et al. Glypican-3 induces a mesenchymal to epithelial transition in human breast cancer cells. Oncotarget. 2016, 7, 60133–60154. [Google Scholar] [CrossRef]
- Umezu, T.; Shibata, K.; Kajiyama, H.; Yamamoto, E.; Nawa, A.; Kikkawa, F. Glypican-3 expression predicts poor clinical outcome of patients with early-stage clear cell carcinoma of the ovary. J. Clin. Pathol. 2010, 63, 962–966. [Google Scholar] [CrossRef] [PubMed]
- Stallcup, W.B.; You, W.K.; Kucharova, K.; Cejudo-Martin, P.; Yotsumoto, F. NG2 Proteoglycan-Dependent Contributions of Pericytes and Macrophages to Brain Tumor Vascularization and Progression. Microcirculation 2016, 23, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Mellai, M.; Annovazzi, L.; Bisogno, I.; Corona, C.; Crociara, P.; Iulini, B.; Cassoni, P.; Casalone, C.; Boldorini, R.; Schiffer, D. Chondroitin Sulphate Proteoglycan 4 (NG2/CSPG4) Localization in Low- and High-Grade Gliomas. Cells 2020, 9, 1538. [Google Scholar] [CrossRef]
- Ampofo, E.; Schmitt, B.M.; Menger, M.D.; Laschke, M.W. The regulatory mechanisms of NG2/CSPG4 expression. Cell. Mol. Biol. Lett. 2017, 22, 4. [Google Scholar] [CrossRef]
- Kurokawa, T.; Imai, K. Chondroitin sulfate proteoglycan 4: An attractive target for antibody-based immunotherapy. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2024, 100, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Diestel, U.; Resch, M.; Meinhardt, K.; Weiler, S.; Hellmann, T.V.; Mueller, T.D.; Nickel, J.; Eichler, J.; Muller, Y.A. Identification of a Novel TGF-β-Binding Site in the Zona Pellucida C-terminal (ZP-C) Domain of TGF-β-Receptor-3 (TGFR-3). PLoS ONE 2013, 8, e67214. [Google Scholar] [CrossRef]
- Kim, S.K.; Henen, M.A.; Hinck, A.P. Structural biology of betaglycan and endoglin, membrane-bound co-receptors of the TGF-beta family. Exp. Biol. Med. 2019, 244, 1547–1558. [Google Scholar] [CrossRef]
- Faissner, A.; Reinhard, J. The extracellular matrix compartment of neural stem and glial progenitor cells. Glia 2015, 63, 1330–1349. [Google Scholar] [CrossRef]
- Reinhard, J.; Brösicke, N.; Theocharidis, U.; Faissner, A. The extracellular matrix niche microenvironment of neural and cancer stem cells in the brain. Int. J. Biochem. Cell Biol. 2016, 81 Pt A, 174–183. [Google Scholar] [CrossRef]
- Maeda, N. Proteoglycans and neuronal migration in the cerebral cortex during development and disease. Front. Neurosci. 2015, 9, 98. [Google Scholar] [CrossRef]
- McKee, T.J.; Perlman, G.; Morris, M.; Komarova, S.V. Extracellular matrix composition of connective tissues: A systematic review and meta-analysis. Sci. Rep. 2019, 9, 10542. [Google Scholar] [CrossRef]
- Shivatare, S.S.; Shivatare, V.S.; Wong, C.H. Glycoconjugates: Synthesis, Functional Studies, and Therapeutic Developments. Chem. Rev. 2022, 122, 15603–15671, Erratum in Chem. Rev. 2024, 124, 6693–6696. [Google Scholar] [CrossRef]
- Soares da Costa, D.; Reis, R.L.; Pashkuleva, I. Sulfation of Glycosaminoglycans and Its Implications in Human Health and Disorders. Annu. Rev. Biomed. Eng. 2017, 19, 1–26. [Google Scholar] [CrossRef]
- Bartolini, B.; Caravà, E.; Caon, I.; Parnigoni, A.; Moretto, P.; Passi, A.; Vigetti, D.; Viola, M.; Karousou, E. Heparan Sulfate in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1245, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, M.; Doherty, G.G.; See, N.W.; Gandhi, N.S.; Ferro, V. From Cancer to COVID-19: A Perspective on Targeting Heparan Sulfate-Protein Interactions. Chem, Rec. 2021, 21, 3087–3101. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Pedersen, L.C.; Xu, D. Targeting heparan sulfate-protein interactions with oligosaccharides and monoclonal antibodies. Front. Mol. Biosci. 2023, 10, 1194293. [Google Scholar] [CrossRef] [PubMed]
- Purushothaman, A.; Sanderson, R.D. Heparanase: A Dynamic Promoter of Myeloma Progression. Adv. Exp. Med. Biol. 2020, 1221, 331–349. [Google Scholar] [CrossRef]
- Mitsou, I.; Multhaupt, H.A.B.; Couchman, J.R. Proteoglycans, ion channels and cell-matrix adhesion. Biochem. J. 2017, 474, 1965–1979. [Google Scholar] [CrossRef]
- Perrimon, N.; Bernfield, M. Specificities of heparan sulphate proteoglycans in developmental processes. Nature 2000, 404, 725–728. [Google Scholar] [CrossRef]
- Vlodavsky, I.; Kayal, Y.; Hilwi, M.; Soboh, S.; Sanderson, R.D.; Ilan, N. Heparanase-A single protein with multiple enzymatic and nonenzymatic functions. Proteoglycan. Res. 2023, 1, e6. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.Y.; Wang, B.; Ma, M.G.; Zhu, J.F. Cellulose-based Nanocarriers as Platforms for Cancer Therapy. Curr. Pharm. Des. 2017, 23, 5292–5300. [Google Scholar] [CrossRef]
- Silva, C.F.S.; Motta, J.M.; Teixeira, F.C.O.B.; Gomes, A.M.; Vilanova, E.; Kozlowski, E.O.; Borsig, L.; Pavão, M.S.G. Non-Anticoagulant Heparan Sulfate from the Ascidian Phallusia nigra Prevents Colon Carcinoma Metastasis in Mice by Disrupting Platelet-Tumor Cell Interaction. Cancers 2020, 12, 1353. [Google Scholar] [CrossRef]
- Piperigkou, Z.; Karamanou, K.; Afratis, N.A.; Bouris, P.; Gialeli, C.; Belmiro, C.L.; Pavão, M.S.; Vynios, D.H.; Tsatsakis, A.M. Biochemical and toxicological evaluation of nano-heparins in cell functional properties, proteasome activation and expression of key matrix molecules. Toxicol. Lett. 2016, 240, 32–42. [Google Scholar] [CrossRef]
- Floer, M.; Götte, M.; Wild, M.K.; Heidemann, J.; Gassar, E.S.; Domschke, W.; Kiesel, L.; Luegering, A.; Kucharzik, T. Enoxaparin improves the course of dextran sodium sulfate-induced colitis in syndecan-1-deficient mice. Am. J. Pathol. 2010, 176, 146–157. [Google Scholar] [CrossRef]
- Clausen, T.M.; Sandoval, D.R.; Spliid, C.B.; Pihl, J.; Perrett, H.R.; Painter, C.D.; Narayanan, A.; Majowicz, S.A.; Kwong, E.M.; McVicar, R.N.; et al. SARS-CoV-2 Infection Depends on Cellular Heparan Sulfate and ACE2. Cell 2020, 183, 1043–1057.e15. [Google Scholar] [CrossRef]
- Ho, L.T.; Harris, A.M.; Tanioka, H.; Yagi, N.; Kinoshita, S.; Caterson, B.; Quantock, A.J.; Young, R.D.; Meek, K.M. A comparison of glycosaminoglycan distributions, keratan sulphate sulphation patterns and collagen fibril architecture from central to peripheral regions of the bovine cornea. Matrix Biol. 2014, 38, 59–68. [Google Scholar] [CrossRef]
- Littlechild, S.L.; Young, R.D.; Caterson, B.; Yoshida, H.; Yamazaki, M.; Sakimura, K.; Quantock, A.J.; Akama, T.O. Keratan Sulfate Phenotype in the β-1,3-N-Acetylglucosaminyltransferase-7-Null Mouse Cornea. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1641–1651. [Google Scholar] [CrossRef]
- Melrose, J. Keratan sulfate (KS)-proteoglycans and neuronal regulation in health and disease: The importance of KS-glycodynamics and interactive capability with neuroregulatory ligands. J. Neurochem. 2019, 149, 170–194. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, B.; Thiagarajan, G.; Madhan, B.; Kar, K. Triple-helical peptides: An approach to collagen conformation, stability, and self-association. Biopolymers 2008, 89, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Heljasvaara, R.; Aikio, M.; Ruotsalainen, H.; Pihlajaniemi, T. Collagen XVIII in tissue homeostasis and dysregulation—Lessons learned from model organisms and human patients. Matrix Biol. 2017, 57–58, 55–75. [Google Scholar] [CrossRef]
- Oudart, J.B.; Monboisse, J.C.; Maquart, F.X.; Brassart, B.; Brassart-Pasco, S.; Ramont, L. Type XIX collagen: A new partner in the interactions between tumor cells and their microenvironment. Matrix Biol. 2017, 57–58, 169–177. [Google Scholar] [CrossRef]
- Bretaud, S.; Guillon, E.; Karppinen, S.M.; Pihlajaniemi, T.; Ruggiero, F. Collagen XV, a multifaceted multiplexin present across tissues and species. Matrix Biol. Plus 2020, 6–7, 100023. [Google Scholar] [CrossRef]
- El Hajj, E.C.; El Hajj, M.C.; Ninh, V.K.; Gardner, J.D. Inhibitor of lysyl oxidase improves cardiac function and the collagen/MMP profile in response to volume overload. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H463–H473. [Google Scholar] [CrossRef]
- Brancato, V.; Garziano, A.; Gioiella, F.; Urciuolo, F.; Imparato, G.; Panzetta, V.; Fusco, S.; Netti, P.A. 3D is not enough: Building up a cell instructive microenvironment for tumoral stroma microtissues. Acta Biomater. 2017, 47, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Werb, Z.; Lu, P. The Role of Stroma in Tumor Development. Cancer J. 2015, 21, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.S.; Rustgi, A.K. Matricellular proteins: Priming the tumour microenvironment for cancer development and metastasis. Br. J. Cancer 2013, 108, 755–761. [Google Scholar] [CrossRef]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, J.; Zhang, H.; Wang, J.; Hua, H.; Jiang, Y. The role of network-forming collagens in cancer progression. Int. J. Cancer 2022, 151, 833–842. [Google Scholar] [CrossRef]
- Bourgot, I.; Primac, I.; Louis, T.; Noël, A.; Maquoi, E. Reciprocal Interplay Between Fibrillar Collagens and Collagen-Binding Integrins: Implications in Cancer Progression and Metastasis. Front. Oncol. 2020, 10, 1488. [Google Scholar] [CrossRef]
- Zhan, H.X.; Zhou, B.; Cheng, Y.G.; Xu, J.W.; Wang, L.; Zhang, G.Y.; Hu, S.Y. Crosstalk between stromal cells and cancer cells in pancreatic cancer: New insights into stromal biology. Cancer Lett. 2017, 392, 83–93. [Google Scholar] [CrossRef]
- Birk, D.E. Type V collagen: Heterotypic type I/V collagen interactions in the regulation of fibril assembly. Micron 2001, 32, 223–237. [Google Scholar] [CrossRef]
- Velosa, A.P.P.; Brito, L.; de Jesus Queiroz, Z.A.; Carrasco, S.; Tomaz de Miranda, J.; Farhat, C.; Goldenstein-Schainberg, C.; Parra, E.R.; de Andrade, D.C.O.; Silva, P.L.; et al. Identification of Autoimmunity to Peptides of Collagen V α1 Chain as Newly Biomarkers of Early Stage of Systemic Sclerosis. Front. Immunol. 2021, 11, 604602. [Google Scholar] [CrossRef]
- Ricard-Blum, S.; Baffet, G.; Théret, N. Molecular and tissue alterations of collagens in fibrosis. Matrix Biol. 2018, 68–69, 122–149. [Google Scholar] [CrossRef]
- López, B.; González, A.; Lindner, D.; Westermann, D.; Ravassa, S.; Beaumont, J.; Gallego, I.; Zudaire, A.; Brugnolaro, C.; Querejeta, R.; et al. Osteopontin-mediated myocardial fibrosis in heart failure: A role for lysyl oxidase? Cardiovasc. Res. 2013, 99, 111–120. [Google Scholar] [CrossRef]
- Díez, J.; González, A.; Kovacic, J.C. Myocardial Interstitial Fibrosis in Nonischemic Heart Disease, Part 3/4: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 2204–2218. [Google Scholar] [CrossRef]
- Knudsen, L.; Ruppert, C.; Ochs, M. Tissue remodelling in pulmonary fibrosis. Cell Tissue Res. 2017, 367, 607–626. [Google Scholar] [CrossRef]
- Reungoat, E.; Grigorov, B.; Zoulim, F.; Pécheur, E.I. Molecular Crosstalk between the Hepatitis C Virus and the Extracellular Matrix in Liver Fibrogenesis and Early Carcinogenesis. Cancers 2021, 13, 2270. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Fertala, A.; Abboud, J.; Wang, M.; Rivlin, M.; Beredjiklian, P.K. The Molecular Basis of Genetic Collagen Disorders and Its Clinical Relevance. J. Bone Joint. Surg. Am. 2018, 100, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Uitto, J.; Has, C.; Vahidnezhad, H.; Youssefian, L.; Bruckner-Tuderman, L. Molecular pathology of the basement membrane zone in heritable blistering diseases: The paradigm of epidermolysis bullosa. Matrix Biol. 2017, 57–58, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Amendola, P.G.; Reuten, R.; Erler, J.T. Interplay Between LOX Enzymes and Integrins in the Tumor Microenvironment. Cancers 2019, 11, 729. [Google Scholar] [CrossRef]
- Mienaltowski, M.J.; Gonzales, N.L.; Beall, J.M.; Pechanec, M.Y. Basic Structure, Physiology, and Biochemistry of Connective Tissues and Extracellular Matrix Collagens. Adv. Exp. Med. Biol. 2021, 1348, 5–43. [Google Scholar] [CrossRef]
- Bella, J.; Hulmes, D.J. Fibrillar Collagens. In Fibrous Proteins: Structures and Mechanisms. Subcellular Biochemistry; Springer: Cham, Switzerland, 2017; Volume 82, pp. 457–490. [Google Scholar] [CrossRef]
- Väisänen, M.R.; Väisänen, T.; Tu, H.; Pirilä, P.; Sormunen, R.; Pihlajaniemi, T. The shed ectodomain of type XIII collagen associates with the fibrillar fibronectin matrix and may interfere with its assembly in vitro. Biochem. J. 2006, 393 Pt 1, 43–50. [Google Scholar] [CrossRef]
- Grässel, S.; Bauer, R.J. Collagen XVI in health and disease. Matrix Biol. 2013, 32, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Nagata, K. Roles of the endoplasmic reticulum-resident, collagen-specific molecular chaperone Hsp47 in vertebrate cells and human disease. J. Biol. Chem. 2019, 294, 2133–2141. [Google Scholar] [CrossRef]
- Sato, K.; Yomogida, K.; Wada, T.; Yorihuzi, T.; Nishimune, Y.; Hosokawa, N.; Nagata, K. Type XXVI collagen, a new member of the collagen family, is specifically expressed in the testis and ovary. J. Biol. Chem. 2002, 277, 37678–37684. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Sinkeviciute, D.; He, Y.; Karsdal, M.; Henrotin, Y.; Mobasheri, A.; Önnerfjord, P.; Bay-Jensen, A. The minor collagens in articular cartilage. Protein Cell 2017, 8, 560–572. [Google Scholar] [CrossRef]
- Ye, Y.; Shetye, S.S.; Birk, D.E.; Soslowsky, L.J. Regulatory Role of Collagen XI in the Establishment of Mechanical Properties of Tendons and Ligaments in Mice Is Tissue Dependent. J. Biomech. Eng. 2025, 147, 011003. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, N.; Tanaka, S.; Yoshioka, H.; Koch, M.; Gordon, M.K.; Ramirez, F. Collagen XXIV (Col24a1) gene expression is a specific marker of osteoblast differentiation and bone formation. Connect. Tissue Res. 2008, 49, 68–75. [Google Scholar] [CrossRef]
- Wang, W.; Olson, D.; Liang, G.; Franceschi, R.T.; Li, C.; Wang, B.; Wang, S.S.; Yang, S. Collagen XXIV (Col24α1) promotes osteoblastic differentiation and mineralization through TGF-β/Smads signaling pathway. Int. J. Biol. Sci. 2012, 8, 1310–1322. [Google Scholar] [CrossRef]
- Wang, W.; Li, F.; Wang, K.; Cheng, B.; Guo, X. PAPSS2 promotes alkaline phosphates activity and mineralization of osteoblastic MC3T3-E1 cells by crosstalk and Smads signal pathways. PLoS ONE 2012, 7, e43475. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, L.; Kong, D.; Hu, G.; Wei, B. Construction of Novel DNA Methylation-Based Prognostic Model to Predict Survival in Glioblastoma. J. Comput. Biol. 2020, 27, 718–728. [Google Scholar] [CrossRef]
- Bielajew, B.J.; Hu, J.C.; Athanasiou, K.A. Collagen: Quantification, biomechanics, and role of minor subtypes in cartilage. Nat. Rev. Mater. 2020, 5, 730–747. [Google Scholar] [CrossRef]
- Plumb, D.A.; Ferrara, L.; Torbica, T.; Knowles, L.; Mironov AJr Kadler, K.E.; Briggs, M.D.; Boot-Handford, R.P. Collagen XXVII organises the pericellular matrix in the growth plate. PLoS ONE 2011, 6, e29422. [Google Scholar] [CrossRef] [PubMed]
- Gonzaga-Jauregui, C.; Yesil, G.; Nistala, H.; Gezdirici, A.; Bayram, Y.; Nannuru, K.C.; Pehlivan, D.; Yuan, B.; Jimenez, J.; Sahin, Y.; et al. Functional biology of the Steel syndrome founder allele and evidence for clan genomics derivation of COL27A1 pathogenic alleles worldwide. Eur. J. Hum. Genet. 2020, 28, 1243–1264. [Google Scholar] [CrossRef] [PubMed]
- Kuivaniemi, H.; Tromp, G. Type III collagen (COL3A1): Gene and protein structure, tissue distribution, and associated diseases. Gene 2019, 707, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.A.; Handford, P.A. New insights into the structure, assembly and biological roles of 10-12 nm connective tissue microfibrils from fibrillin-1 studies. Biochem. J. 2016, 473, 827–838. [Google Scholar] [CrossRef]
- Wang, J.; Guo, F.; Chen, G.; Sun, J.; Tang, Q.; Chen, L. Spatial-Temporal Patterns and Inflammatory Factors of Bone Matrix Remodeling. Stem Cells Int. 2021, 2021, 4307961. [Google Scholar] [CrossRef]
- Yuan, Z.; Lin, B.; Wang, C.; Yan, Z.; Yang, F.; Su, H. Collagen remodeling-mediated signaling pathways and their impact on tumor therapy. J. Biol. Chem. 2025, 301, 108330. [Google Scholar] [CrossRef]
- Bella, J.; Liu, J.; Kramer, R.; Brodsky, B.; Berman, H.M. Conformational effects of Gly-X-Gly interruptions in the collagen triple helix. J. Mol. Biol. 2006, 362, 298–311. [Google Scholar] [CrossRef]
- Gjaltema, R.A.; Bank, R.A. Molecular insights into prolyl and lysyl hydroxylation of fibrillar collagens in health and disease. Crit. Rev. Biochem. Mol. Biol. 2017, 52, 74–95. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Taga, Y.; Zientek, K.; Mizuno, N.; Salo, A.M.; Semenova, O.; Tufa, S.F.; Keene, D.R.; Holden, P.; Mizuno, K.; et al. Type I and type V procollagen triple helix uses different subsets of the molecular ensemble for lysine posttranslational modifications in the rER. J. Biol. Chem. 2021, 296, 100453. [Google Scholar] [CrossRef]
- Ito, S.; Nagata, K. Biology of Hsp47 (Serpin H1), a collagen-specific molecular chaperone. Semin. Cell Dev. Biol. 2017, 62, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Doan, N.D.; DiChiara, A.S.; Del Rosario, A.M.; Schiavoni, R.P.; Shoulders, M.D. Mass Spectrometry-Based Proteomics to Define Intracellular Collagen Interactomes. Methods Mol. Biol. 2019, 1944, 95–114. [Google Scholar] [CrossRef]
- Kirchner, M.; Deng, H.; Xu, Y. Heterogeneity in proline hydroxylation of fibrillar collagens observed by mass spectrometry. PLoS ONE 2021, 16, e0250544. [Google Scholar] [CrossRef]
- Malfait, F.; Castori, M.; Francomano, C.A.; Giunta, C.; Kosho, T.; Byers, P.H. The Ehlers-Danlos syndromes. Nat. Rev. Dis. Primers. 2020, 6, 64. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Peng, Y.; Li, C.; Xie, B.; Wei, X.; Gui, B.; Meng, J.; Chen, S.; Fan, X. Genetic skeletal disorders: Phenotypic-genotypic characteristics and RhGH therapy responses of a pediatric cohort. Sci. Rep. 2025, 15, 20717. [Google Scholar] [CrossRef]
- López-Jiménez, A.J.; Basak, T.; Vanacore, R.M. Proteolytic processing of lysyl oxidase-like-2 in the extracellular matrix is required for crosslinking of basement membrane collagen IV. J. Biol. Chem. 2017, 292, 16970–16982. [Google Scholar] [CrossRef]
- Roy, A.; Gauld, J.W. Sulfilimine bond formation in collagen IV. Chem. Commun. 2024, 60, 646–657. [Google Scholar] [CrossRef]
- Bhave, G.; Colon, S.; Ferrell, N. The sulfilimine cross-link of collagen IV contributes to kidney tubular basement membrane stiffness. Am. J. Physiol. Renal Physiol. 2017, 313, F596–F602. [Google Scholar] [CrossRef]
- Summers, J.A.; Yarbrough, M.; Liu, M.; McDonald, W.H.; Hudson, B.G.; Pastor-Pareja, J.C.; Boudko, S.P. Collagen IV of basement membranes: IV. Adaptive mechanism of collagen IV scaffold assembly in Drosophila. J. Biol. Chem. 2023, 299, 105394. [Google Scholar] [CrossRef] [PubMed]
- Bleyer, A.J.; Westemeyer, M.; Xie, J.; Bloom, M.S.; Brossart, K.; Eckel, J.J.; Jones, F.; Molnar, M.Z.; Kotzker, W.; Anand, P.; et al. Genetic Etiologies for Chronic Kidney Disease Revealed through Next-Generation Renal Gene Panel. Am. J. Nephrol. 2022, 53, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Guiraud, S.; Migeon, T.; Ferry, A.; Chen, Z.; Ouchelouche, S.; Verpont, M.C.; Sado, Y.; Allamand, V.; Ronco, P.; Plaisier, E. HANAC Col4a1 Mutation in Mice Leads to Skeletal Muscle Alterations due to a Primary Vascular Defect. Am. J. Pathol. 2017, 187, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.S.; Ma, D.J.; Choi, J.; Shin, Y.J. COL8A2 Regulates the Fate of Corneal Endothelial Cells. Investig. Ophthalmol. Vis. Sci. 2020, 61, 26. [Google Scholar] [CrossRef]
- Lopes, J.; Adiguzel, E.; Gu, S.; Liu, S.L.; Hou, G.; Heximer, S.; Assoian, R.K.; Bendeck, M.P. Type VIII collagen mediates vessel wall remodeling after arterial injury and fibrous cap formation in atherosclerosis. Am. J. Pathol. 2013, 182, 2241–2253. [Google Scholar] [CrossRef]
- Schimmel, K.; Ichimura, K.; Reddy, S.; Haddad, F.; Spiekerkoetter, E. Cardiac Fibrosis in the Pressure Overloaded Left and Right Ventricle as a Therapeutic Target. Front. Cardiovasc. Med. 2022, 9, 886553. [Google Scholar] [CrossRef]
- Shen, G. The role of type X collagen in facilitating and regulating endochondral ossification of articular cartilage. Orthod. Craniofacial Res. 2005, 8, 11–17. [Google Scholar] [CrossRef]
- Sweeney, E.; Roberts, D.; Corbo, T.; Jacenko, O. Congenic mice confirm that collagen X is required for proper hematopoietic development. PLoS ONE 2010, 5, e9518. [Google Scholar] [CrossRef]
- Kahlert, U.D.; Shi, W.; Strecker, M.; Scherpinski, L.A.; Wartmann, T.; Dölling, M.; Perrakis, A.; Relja, B.; Mengoni, M.; Braun, A.; et al. COL10A1 allows stratification of invasiveness of colon cancer and associates to extracellular matrix and immune cell enrichment in the tumor parenchyma. Front. Oncol. 2022, 12, 1007514. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Lu, Q.; Tian, H.; Wang, K.; Liu, Z.; Xiong, Y.; Li, Y.; Ma, N.; Tian, H.; et al. The p.W651fsX666 mutation on COL10A1 results in impaired trimerization of normal collagen X to induce Schmid type Metaphyseal chondrodysplasia. Hum. Mol. Genet. 2025, 21, ddaf071. [Google Scholar] [CrossRef]
- Conradt, G.; Hausser, I.; Nyström, A. Epidermal or Dermal Collagen VII Is Sufficient for Skin Integrity: Insights to Anchoring Fibril Homeostasis. J. Investig. Dermatol. 2024, 144, 1301–1310.e7. [Google Scholar] [CrossRef] [PubMed]
- Nyström, A.; Velati, D.; Mittapalli, V.R.; Fritsch, A.; Kern, J.S.; Bruckner-Tuderman, L. Collagen VII plays a dual role in wound healing. J. Clin. Investig. 2013, 123, 3498–3509. [Google Scholar] [CrossRef] [PubMed]
- Hurskainen, T.; Moilanen, J.; Sormunen, R.; Franzke, C.W.; Soininen, R.; Loeffek, S.; Huilaja, L.; Nuutinen, M.; Bruckner-Tuderman, L.; Autio-Harmainen, H.; et al. Transmembrane collagen XVII is a novel component of the glomerular filtration barrier. Cell Tissue Res. 2012, 348, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Tabor, A.; LeQuang, J.A.K.; Pergolizzi, J., Jr. Epidermolysis Bullosa: Practical Clinical Tips From the Field. Cureus 2024, 16, e53774. [Google Scholar] [CrossRef]
- Koga, H.; Teye, K.; Yamashita, K.; Ishii, N.; Tsuruta, D.; Nakama, T. Detection of anti-type VII collagen IgE antibodies in epidermolysis bullosa acquisita. Br. J. Dermatol. 2019, 180, 1107–1113. [Google Scholar] [CrossRef]
- Cescon, M.; Gattazzo, F.; Chen, P.; Bonaldo, P. Collagen VI at a glance. J. Cell Sci. 2015, 128, 3525–3531. [Google Scholar] [CrossRef]
- Lamandé, S.R.; Bateman, J.F. Collagen VI disorders: Insights on form and function in the extracellular matrix and beyond. Matrix Biol. 2018, 71–72, 348–367. [Google Scholar] [CrossRef]
- Cescon, M.; Gregorio, I.; Eiber, N.; Borgia, D.; Fusto, A.; Sabatelli, P.; Scorzeto, M.; Megighian, A.; Pegoraro, E.; Hashemolhosseini, S.; et al. Collagen VI is required for the structural and functional integrity of the neuromuscular junction. Acta Neuropathol. 2018, 136, 483–499. [Google Scholar] [CrossRef]
- Veit, G.; Kobbe, B.; Keene, D.R.; Paulsson, M.; Koch, M.; Wagener, R. Collagen XXVIII, a novel von Willebrand factor A domain-containing protein with many imperfections in the collagenous domain. J. Biol. Chem. 2006, 281, 3494–3504. [Google Scholar] [CrossRef]
- Chen, P.; Cescon, M.; Bonaldo, P. The Role of Collagens in Peripheral Nerve Myelination and Function. Mol. Neurobiol. 2015, 52, 216–225. [Google Scholar] [CrossRef]
- Gebauer, J.M.; Kobbe, B.; Paulsson, M.; Wagener, R. Structure, evolution and expression of collagen XXVIII: Lessons from the zebrafish. Matrix Biol. 2016, 49, 106–119. [Google Scholar] [CrossRef]
- Yang, H.; Jin, L.; Sun, X. A thirteen-gene set efficiently predicts the prognosis of glioblastoma. Mol. Med. Rep. 2019, 19, 1613–1621. [Google Scholar] [CrossRef]
- Marneros, A.G.; Keene, D.R.; Hansen, U.; Fukai, N.; Moulton, K.; Goletz, P.L.; Moiseyev, G.; Pawlyk, B.S.; Halfter, W.; Dong, S.; et al. Collagen XVIII/endostatin is essential for vision and retinal pigment epithelial function. EMBO J. 2004, 23, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Seppinen, L.; Pihlajaniemi, T. The multiple functions of collagen XVIII in development and disease. Matrix Biol. 2011, 30, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Marneros, A.G.; Olsen, B.R. Physiological role of collagen XVIII and endostatin. FASEB J. 2005, 19, 716–728. [Google Scholar] [CrossRef]
- Amenta, P.S.; Scivoletti, N.A.; Newman, M.D.; Sciancalepore, J.P.; Li, D.; Myers, J.C. Proteoglycan-collagen XV in human tissues is seen linking banded collagen fibers subjacent to the basement membrane. J. Histochem. Cytochem. 2005, 53, 165–176. [Google Scholar] [CrossRef]
- Mutolo, M.J.; Morris, K.J.; Leir, S.H.; Caffrey, T.C.; Lewandowska, M.A.; Hollingsworth, M.A.; Harris, A. Tumor suppression by collagen XV is independent of the restin domain. Matrix Biol. 2012, 31, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Rasi, K.; Piuhola, J.; Czabanka, M.; Sormunen, R.; Ilves, M.; Leskinen, H.; Rysä, J.; Kerkelä, R.; Janmey, P.; Heljasvaara, R.; et al. Collagen XV is necessary for modeling of the extracellular matrix and its deficiency predisposes to cardiomyopathy. Circ. Res. 2010, 107, 1241–1252. [Google Scholar] [CrossRef]
- Dhungana, H.; Huuskonen, M.T.; Pihlajaniemi, T.; Heljasvaara, R.; Vivien, D.; Kanninen, K.M.; Malm, T.; Koistinaho, J.; Lemarchant, S. Lack of collagen XV is protective after ischemic stroke in mice. Cell Death Dis. 2017, 8, e2541. [Google Scholar] [CrossRef]
- Ricard-Blum, S.; Ballut, L. Matricryptins derived from collagens and proteoglycans. Front. Biosci. 2011, 16, 674–697. [Google Scholar] [CrossRef]
- de Castro Brás, L.E.; Frangogiannis, N.G. Extracellular matrix-derived peptides in tissue remodeling and fibrosis. Matrix Biol. 2020, 91–92, 176–187. [Google Scholar] [CrossRef]
- Li, L.; Huang, J.; Liu, Y. The extracellular matrix glycoprotein fibrillin-1 in health and disease. Front. Cell Dev. Biol. 2024, 11, 1302285. [Google Scholar] [CrossRef]
- Baldwin, A.K.; Simpson, A.; Steer, R.; Cain, S.A.; Kielty, C.M. Elastic fibres in health and disease. Expert. Rev. Mol. Med. 2013, 15, e8. [Google Scholar] [CrossRef] [PubMed]
- Craft, C.S.; Broekelmann, T.J.; Mecham, R.P. Microfibril-associated glycoproteins MAGP-1 and MAGP-2 in disease. Matrix Biol. 2018, 71–72, 100–111. [Google Scholar] [CrossRef]
- Doliana, R.; Mongiat, M.; Bucciotti, F.; Giacomello, E.; Deutzmann, R.; Volpin, D.; Bressan, G.M.; Colombatti, A. EMILIN, a component of the elastic fiber and a new member of the C1q/tumor necrosis factor superfamily of proteins. J. Biol. Chem. 1999, 274, 16773–16781. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.C.; Riley, G.P. ADAMTS proteinases: A multi-domain, multi-functional family with roles in extracellular matrix turnover and arthritis. Arthritis Res. Ther. 2005, 7, 160–169. [Google Scholar] [CrossRef]
- Kelwick, R.; Desanlis, I.; Wheeler, G.N.; Edwards, D.R. The ADAMTS (A Disintegrin and Metalloproteinase with Thrombospondin motifs) family. Genome Biol. 2015, 16, 113. [Google Scholar] [CrossRef]
- Ono, R.N.; Sengle, G.; Charbonneau, N.L.; Carlberg, V.; Bächinger, H.P.; Sasaki, T.; Lee-Arteaga, S.; Zilberberg, L.; Rifkin, D.B.; Ramirez, F.; et al. Latent transforming growth factor beta-binding proteins and fibulins compete for fibrillin-1 and exhibit exquisite specificities in binding sites. J. Biol. Chem. 2009, 284, 16872–16881. [Google Scholar] [CrossRef]
- Hedtke, T.; Schräder, C.U.; Heinz, A.; Hoehenwarter, W.; Brinckmann, J.; Groth, T.; Schmelzer, C.E.H. A comprehensive map of human elastin cross-linking during elastogenesis. FEBS J. 2019, 286, 3594–3610. [Google Scholar] [CrossRef] [PubMed]
- Schmelzer, C.E.H.; Duca, L. Elastic fibers: Formation, function, and fate during aging and disease. FEBS J. 2022, 289, 3704–3730. [Google Scholar] [CrossRef]
- Schmelzer, C.E.H.; Heinz, A.; Troilo, H.; Lockhart-Cairns, M.P.; Jowitt, T.A.; Marchand, M.F.; Bidault, L.; Bignon, M.; Hedtke, T.; Barret, A.; et al. Lysyl oxidase-like 2 (LOXL2)-mediated cross-linking of tropoelastin. FASEB J. 2019, 33, 5468–5481. [Google Scholar] [CrossRef]
- Schräder, C.U.; Heinz, A.; Majovsky, P.; Karaman Mayack, B.; Brinckmann, J.; Sippl, W.; Schmelzer, C.E.H. Elastin is heterogeneously cross-linked. J. Biol. Chem. 2018, 293, 15107–15119. [Google Scholar] [CrossRef]
- Sarohi, V.; Chakraborty, S.; Basak, T. Exploring the cardiac ECM during fibrosis: A new era with next-gen proteomics. Front. Mol. Biosci. 2022, 9, 1030226. [Google Scholar] [CrossRef]
- Bruce, M.C.; Lo, P.Y. A morphometric quantitation of developmental changes in elastic fibers in rat lung parenchyma: Variability with lung region and postnatal age. J. Lab. Clin. Med. 1991, 117, 226–233. [Google Scholar]
- Shapiro, S.D.; Endicott, S.K.; Province, M.A.; Pierce, J.A.; Campbell, E.J. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J. Clin. Investig. 1991, 1828–1834. [Google Scholar] [CrossRef]
- Li, B.; Daggett, V. Molecular basis for the extensibility of elastin. J. Muscle Res. Cell Motil. 2002, 23, 561–573. [Google Scholar] [CrossRef]
- Li, B.; Alonso, D.O.; Daggett, V. The molecular basis for the inverse temperature transition of elastin. J. Mol. Biol. 2001, 305, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Kielty, C.M.; Baldock, C.; Lee, D.; Rock, M.J.; Ashworth, J.L.; Shuttleworth, C.A. Fibrillin: From microfibril assembly to biomechanical function. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2002, 357, 207–217. [Google Scholar] [CrossRef]
- Reinhardt, D.P.; Gambee, J.E.; Ono, R.N.; Bächinger, H.P.; Sakai, L.Y. Initial steps in assembly of microfibrils. Formation of disulfide-cross-linked multimers containing fibrillin-1. J. Biol. Chem. 2000, 275, 2205–2210. [Google Scholar] [CrossRef] [PubMed]
- Carta, L.; Pereira, L.; Arteaga-Solis, E.; Lee-Arteaga, S.Y.; Lenart, B.; Starcher, B.; Merkel, C.A.; Sukoyan, M.; Kerkis, A.; Hazeki, N.; et al. Fibrillins 1 and 2 perform partially overlapping functions during aortic development. J. Biol. Chem. 2006, 281, 8016–8023. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, D.P.; Keene, D.R.; Corson, G.M.; Pöschl, E.; Bächinger, H.P.; Gambee, J.E.; Sakai, L.Y. Fibrillin-1: Organization in microfibrils and structural properties. J. Mol. Biol. 1996, 258, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Qian, R.Q.; Glanville, R.W. Alignment of fibrillin molecules in elastic microfibrils is defined by transglutaminase-derived cross-links. Biochemistry 1997, 36, 15841–15847. [Google Scholar] [CrossRef]
- Sabatier, L.; Djokic, J.; Fagotto-Kaufmann, C.; Chen, M.; Annis, D.S.; Mosher, D.F.; Reinhardt, D.P. Complex contributions of fibronectin to initiation and maturation of microfibrils. Biochem. J. 2013, 456, 283–295. [Google Scholar] [CrossRef]
- Muiznieks, L.D.; Sharpe, S.; Pomès, R.; Keeley, F.W. Role of Liquid-Liquid Phase Separation in Assembly of Elastin and Other Extracellular Matrix Proteins. J. Mol. Biol. 2018, 430, 4741–4753. [Google Scholar] [CrossRef]
- Broekelmann, T.J.; Kozel, B.A.; Ishibashi, H.; Werneck, C.C.; Keeley, F.W.; Zhang, L.; Mecham, R.P. Tropoelastin interacts with cell-surface glycosaminoglycans via its COOH-terminal domain. J. Biol. Chem. 2005, 280, 40939–40947. [Google Scholar] [CrossRef] [PubMed]
- Czirok, A.; Zach, J.; Kozel, B.A.; Mecham, R.P.; Davis, E.C.; Rongish, B.J. Elastic fiber macro-assembly is a hierarchical, cell motion-mediated process. J. Cell Physiol. 2006, 207, 97–106. [Google Scholar] [CrossRef]
- Kozel, B.A.; Ciliberto, C.H.; Mecham, R.P. Deposition of tropoelastin into the extracellular matrix requires a competent elastic fiber scaffold but not live cells. Matrix Biol. 2004, 23, 23–34. [Google Scholar] [CrossRef]
- Nakamura, T. Roles of short fibulins, a family of matricellular proteins, in lung matrix assembly and disease. Matrix Biol. 2018, 73, 21–33. [Google Scholar] [CrossRef]
- Gillery, P.; Jaisson, S. Post-translational modification derived products (PTMDPs): Toxins in chronic diseases? Clin. Chem. Lab. Med. 2014, 52, 33–38. [Google Scholar] [CrossRef]
- Dobberstein, R.C.; Tung, S.M.; Ritz-Timme, S. Aspartic acid racemisation in purified elastin from arteries as basis for age estimation. Int. J. Legal Med. 2010, 124, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Jaisson, S.; Desmons, A.; Doué, M.; Gorisse, L.; Pietrement, C.; Gillery, P. Measurement of Homocitrulline, A Carbamylation-derived Product, in Serum and Tissues by LC-MS/MS. Curr. Protoc. 2023, 3, e762. [Google Scholar] [CrossRef]
- Konova, E.; Baydanoff, S.; Atanasova, M.; Velkova, A. Age-related changes in the glycation of human aortic elastin. Exp. Gerontol. 2004, 39, 249–254. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Sakata, N.; Meng, J.; Sakamoto, M.; Noma, A.; Maeda, I.; Okamoto, K.; Takebayashi, S. Possible involvement of increased glycoxidation and lipid peroxidation of elastin in atherogenesis in haemodialysis patients. Nephrol. Dial. Transplant. 2002, 17, 630–636. [Google Scholar] [CrossRef]
- Sakata, N.; Noma, A.; Yamamoto, Y.; Okamoto, K.; Meng, J.; Takebayashi, S.; Nagai, R.; Horiuchi, S. Modification of elastin by pentosidine is associated with the calcification of aortic media in patients with end-stage renal disease. Nephrol. Dial. Transplant. 2003, 18, 1601–1609. [Google Scholar] [CrossRef]
- Meng, J.; Sakata, N.; Takebayashi, S. Increased glycoxidation and lipoperoxidation in the collagen of the myocardium in hemodialysis patients. Cardiovasc. Res. 2000, 47, 306–313. [Google Scholar] [CrossRef]
- Wang, Y.; Zeinali-Davarani, S.; Davis, E.C.; Zhang, Y. Effect of glucose on the biomechanical function of arterial elastin. J. Mech. Behav. Biomed. Mater. 2015, 49, 244–254. [Google Scholar] [CrossRef]
- Cocciolone, A.J.; Hawes, J.Z.; Staiculescu, M.C.; Johnson, E.O.; Murshed, M.; Wagenseil, J.E. Elastin, arterial mechanics, and cardiovascular disease. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H189–H205. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, M.F.; Hashimoto, J. Mechanical factors in arterial aging: A clinical perspective. J. Am. Coll. Cardiol. 2007, 50, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mandalà, M.; Pedatella, A.L.; Morales Palomares, S.; Cipolla, M.J.; Osol, G. Maturation is associated with changes in rat cerebral artery structure, biomechanical properties and tone. Acta Physiol. 2012, 205, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Hodis, S.; Zamir, M. Mechanical events within the arterial wall under the forces of pulsatile flow: A review. J. Mech. Behav. Biomed. Mater. 2011, 4, 1595–1602. [Google Scholar] [CrossRef]
- Lombard, C.; Arzel, L.; Bouchu, D.; Wallach, J.; Saulnier, J. Human leukocyte elastase hydrolysis of peptides derived from human elastin exon 24. Biochimie 2006, 88, 1915–1921. [Google Scholar] [CrossRef]
- Heinz, A.; Jung, M.C.; Jahreis, G.; Rusciani, A.; Duca, L.; Debelle, L.; Weiss, A.S.; Neubert, R.H.; Schmelzer, C.E. The action of neutrophil serine proteases on elastin and its precursor. Biochimie 2012, 94, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Schmelzer, C.E.; Jung, M.C.; Wohlrab, J.; Neubert, R.H.; Heinz, A. Does human leukocyte elastase degrade intact skin elastin? FEBS J. 2012, 279, 4191–4200. [Google Scholar] [CrossRef] [PubMed]
- Taddese, S.; Weiss, A.S.; Jahreis, G.; Neubert, R.H.; Schmelzer, C.E. In vitro degradation of human tropoelastin by MMP-12 and the generation of matrikines from domain 24. Matrix Biol. 2009, 28, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Heinz, A.; Taddese, S.; Sippl, W.; Neubert, R.H.; Schmelzer, C.E. Insights into the degradation of human elastin by matrilysin-1. Biochimie 2011, 93, 187–194. [Google Scholar] [CrossRef]
- Bossard, M.J.; Tomaszek, T.A.; Thompson, S.K.; Amegadzie, B.Y.; Hanning, C.R.; Jones, C.; Kurdyla, J.T.; McNulty, D.E.; Drake, F.H.; Gowen, M.; et al. Proteolytic activity of human osteoclast cathepsin K. Expression, purification, activation, and substrate identification. J. Biol. Chem. 1996, 271, 12517–12524. [Google Scholar] [CrossRef]
- Brömme, D.; Okamoto, K.; Wang, B.B.; Biroc, S. Human cathepsin O2, a matrix protein-degrading cysteine protease expressed in osteoclasts. Functional expression of human cathepsin O2 in Spodoptera frugiperda and characterization of the enzyme. J. Biol. Chem. 1996, 271, 2126–2132. [Google Scholar] [CrossRef]
- Panwar, P.; Hedtke, T.; Heinz, A.; Andrault, P.-M.; Hoehenwarter, W.; Granville, D.J.; Schmelzer, C.E.H.; Brömme, D. Expression of elastolytic cathepsins in human skin and their involvement in age-dependent elastin degradation. Biochim. et Biophys. Acta (BBA) Gen. Subj. 2020, 1864, 129544. [Google Scholar] [CrossRef]
- Maquart, F.-X.; Pasco, S.; Ramont, L.; Hornebeck, W.; Monboisse, J.-C. An introduction to matrikines: Extracellular matrix-derived peptides which regulate cell activity. Implication in tumor invasion. Crit. Rev. Oncol. Hematol. 2004, 49, 199–202. [Google Scholar] [CrossRef]
- Maurer, E.; Tang, C.; Schaff, M.; Bourdon, C.; Receveur, N.; Ravanat, C.; Eckly, A.; Hechler, B.; Gachet, C.; Lanza, F.; et al. Targeting platelet GPIbβ reduces platelet adhesion, GPIb signaling and thrombin generation and prevents arterial thrombosis. Arterioscler Thromb Vasc Biol. 2013, 33, 1221–1229. [Google Scholar] [CrossRef]
- Maurice, P.; Blaise, S.; Gayral, S.; Debelle, L.; Laffargue, M.; Hornebeck, W.; Duca, L. Elastin fragmentation and atherosclerosis progression: The elastokine concept. Trends Cardiovasc. Med. 2013, 23, 211–221. [Google Scholar] [CrossRef]
- Hornebeck, W.; Robinet, A.; Duca, L.; Antonicelli, F.; Wallach, J.; Bellon, G. The elastin connection and melanoma progression. Anticancer Res. 2005, 25, 2617–2625. [Google Scholar]
- Antonicelli, F.; Bellon, G.; Debelle, L.; Hornebeck, W. Elastin-elastases and inflamm-aging. Curr. Top. Dev. Biol. 2007, 79, 99–155. [Google Scholar] [CrossRef]
- Mora Huertas, A.C.; Schmelzer, C.E.; Hoehenwarter, W.; Heyroth, F.; Heinz, A. Molecular-level insights into aging processes of skin elastin. Biochimie 2016, 128–129, 163–173. [Google Scholar] [CrossRef]
- Scandolera, A.; Odoul, L.; Salesse, S.; Guillot, A.; Blaise, S.; Kawecki, C.; Maurice, P.; El Btaouri, H.; Romier-Crouzet, B.; Martiny, L.; et al. The Elastin Receptor Complex: A Unique Matricellular Receptor with High Anti-tumoral Potential. Front. Pharmacol. 2016, 7, 32. [Google Scholar] [CrossRef]
- Pierre, A.; Lemaire, F.; Meghraoui-Kheddar, A.; Audonnet, S.; Héry-Huynh, S.; Le Naour, R. Impact of aging on inflammatory and immune responses during elastin peptide-induced murine emphysema. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 316, L608–L620. [Google Scholar] [CrossRef]
- Wahart, A.; Hocine, T.; Albrecht, C.; Henry, A.; Sarazin, T.; Martiny, L.; El Btaouri, H.; Maurice, P.; Bennasroune, A.; Romier-Crouzet, B.; et al. Role of elastin peptides and elastin receptor complex in metabolic and cardiovascular diseases. FEBS J. 2019, 286, 2980–2993. [Google Scholar] [CrossRef] [PubMed]
- Lapis, K.; TímÁr, J. Role of elastin–matrix interactions in tumor progression. Semin. Cancer Biol. 2002, 12, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Hinek, A.; Bodnaruk, T.D.; Bunda, S.; Wang, Y.; Liu, K. Neuraminidase-1, a subunit of the cell surface elastin receptor, desialylates and functionally inactivates adjacent receptors interacting with the mitogenic growth factors PDGF-BB and IGF-2. Am. J. Pathol. 2008, 173, 1042–1056. [Google Scholar] [CrossRef] [PubMed]
- Privitera, S.; Prody, C.A.; Callahan, J.W.; Hinek, A. The 67-kDa Enzymatically inactive alternatively spliced variant of beta-galactosidase is identical to the elastin/laminin-binding protein. J. Biol. Chem. 1998, 273, 6319–6326. [Google Scholar] [CrossRef]
- Skeie, J.M.; Hernandez, J.; Hinek, A.; Mullins, R.F. Molecular responses of choroidal endothelial cells to elastin derived peptides through the elastin-binding protein (GLB1). Matrix Biol. 2012, 31, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Bennasroune, A.; Romier-Crouzet, B.; Blaise, S.; Laffargue, M.; Efremov, R.G.; Martiny, L.; Maurice, P.; Duca, L. Elastic fibers and elastin receptor complex: Neuraminidase-1 takes the center stage. Matrix Biol. 2019, 84, 57–67. [Google Scholar] [CrossRef]
- Rusciani, A.; Duca, L.; Sartelet, H.; Chatron-Colliet, A.; Bobichon, H.; Ploton, D.; Le Naour, R.; Blaise, S.; Martiny, L.; Debelle, L. Elastin peptides signaling relies on neuraminidase-1-dependent lactosylceramide generation. PLoS ONE 2010, 5, e14010. [Google Scholar] [CrossRef]
- Duca, L.; Lambert, E.; Debret, R.; Rothhut, B.; Blanchevoye, C.; Delacoux, F.; Hornebeck, W.; Martiny, L.; Debelle, L. Elastin peptides activate extracellular signal-regulated kinase 1/2 via a Ras-independent mechanism requiring both p110gamma/Raf-1 and protein kinase A/B-Raf signaling in human skin fibroblasts. Mol. Pharmacol. 2005, 67, 1315–1324. [Google Scholar] [CrossRef]
- Sun, X.; Gao, X.; Zhou, L.; Sun, L.; Lu, C. PDGF-BB-induced MT1-MMP expression regulates proliferation and invasion of mesenchymal stem cells in 3-dimensional collagen via MEK/ERK1/2 and PI3K/AKT signaling. Cell. Signal. 2013, 25, 1279–1287. [Google Scholar] [CrossRef]
- Govindarajan, G.; Eble, D.M.; Lucchesi, P.A.; Samarel, A.M. Focal adhesion kinase is involved in angiotensin II-mediated protein synthesis in cultured vascular smooth muscle cells. Circ. Res. 2000, 87, 710–716. [Google Scholar] [CrossRef]
- Albrecht, C.; Kuznetsov, A.S.; Appert-Collin, A.; Dhaideh, Z.; Callewaert, M.; Bershatsky, Y.V.; Urban, A.S.; Bocharov, E.V.; Bagnard, D.; Baud, S.; et al. Transmembrane Peptides as a New Strategy to Inhibit Neuraminidase-1 Activation. Front. Cell Dev. Biol. 2020, 8, 611121. [Google Scholar] [CrossRef] [PubMed]
- Szychowski, K.A.; Skóra, B.; Tobiasz, J.; Gmiński, J. Elastin-derived peptide VGVAPG decreases differentiation of mouse embryo fibroblast (3T3-L1) cells into adipocytes. Adipocyte 2020, 9, 234–245. [Google Scholar] [CrossRef]
- Tembely, D.; Henry, A.; Vanalderwiert, L.; Toussaint, K.; Bennasroune, A.; Blaise, S.; Sartelet, H.; Jaisson, S.; Galés, C.; Martiny, L.; et al. The Elastin Receptor Complex: An Emerging Therapeutic Target Against Age-Related Vascular Diseases. Front. Endocrinol. 2022, 13, 815356. [Google Scholar] [CrossRef]
- Bocquet, O.; Tembely, D.; Rioult, D.; Terryn, C.; Romier, B.; Bennasroune, A.; Blaise, S.; Sartelet, H.; Martiny, L.; Duca, L.; et al. Characterization of novel interactions with membrane NEU1 highlights new regulatory functions for the Elastin Receptor Complex in monocyte interaction with endothelial cells. Cell Biosci. 2021, 11, 206. [Google Scholar] [CrossRef] [PubMed]
- Maurice, P.; Baud, S.; Bocharova, O.V.; Bocharov, E.V.; Kuznetsov, A.S.; Kawecki, C.; Bocquet, O.; Romier, B.; Gorisse, L.; Ghirardi, M.; et al. New Insights into Molecular Organization of Human Neuraminidase-1: Transmembrane Topology and Dimerization Ability. Sci. Rep. 2016, 6, 38363. [Google Scholar] [CrossRef]
- Hyun, S.W.; Liu, A.; Liu, Z.; Cross, A.S.; Verceles, A.C.; Magesh, S.; Kommagalla, Y.; Kona, C.; Ando, H.; Luzina, I.G.; et al. The NEU1-selective sialidase inhibitor, C9-butyl-amide-DANA, blocks sialidase activity and NEU1-mediated bioactivities in human lung in vitro and murine lung in vivo. Glycobiology 2016, 26, 834–849. [Google Scholar] [CrossRef] [PubMed]
- Guillot, A.; Toussaint, K.; Ebersold, L.; ElBtaouri, H.; Thiebault, E.; Issad, T.; Peiretti, F.; Maurice, P.; Sartelet, H.; Bennasroune, A.; et al. Sialic acids cleavage induced by elastin-derived peptides impairs the interaction between insulin and its receptor in adipocytes 3T3-L1. J. Physiol. Biochem. 2024, 80, 363–379. [Google Scholar] [CrossRef]
- Romier, B.; Ivaldi, C.; Sartelet, H.; Heinz, A.; Schmelzer, C.E.H.; Garnotel, R.; Guillot, A.; Jonquet, J.; Bertin, E.; Guéant, J.-L.; et al. Production of Elastin-Derived Peptides Contributes to the Development of Nonalcoholic Steatohepatitis. Diabetes 2018, 67, 1604–1615. [Google Scholar] [CrossRef] [PubMed]
- Kiyozumi, D.; Nakano, I.; Sato-Nishiuchi, R.; Tanaka, S.; Sekiguchi, K. Laminin is the ECM niche for trophoblast stem cells. Life Sci. Alliance 2020, 3, e201900515. [Google Scholar] [CrossRef]
- Fujiwara, H.; Gu, J.; Sekiguchi, K. Rac regulates integrin-mediated endothelial cell adhesion and migration on laminin-8. Exp. Cell Res. 2004, 292, 67–77. [Google Scholar] [CrossRef]
- Durbeej, M. Laminins. Cell Tissue Res. 2010, 339, 259–268. [Google Scholar] [CrossRef]
- Hallmann, R.; Hannocks, M.-J.; Song, J.; Zhang, X.; Di Russo, J.; Luik, A.-L.; Burmeister, M.; Gerwien, H.; Sorokin, L. The role of basement membrane laminins in vascular function. Int. J. Biochem. Cell Biol. 2020, 127, 105823. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhang, X.; Buscher, K.; Wang, Y.; Wang, H.; Di Russo, J.; Li, L.; Lütke-Enking, S.; Zarbock, A.; Stadtmann, A.; et al. Endothelial Basement Membrane Laminin 511 Contributes to Endothelial Junctional Tightness and Thereby Inhibits Leukocyte Transmigration. Cell Rep. 2017, 18, 1256–1269. [Google Scholar] [CrossRef]
- Tunggal, P.; Smyth, N.; Paulsson, M.; Ott, M.-C. Laminins: Structure and genetic regulation. Microsc. Res. Tech. 2000, 51, 214–227. [Google Scholar] [CrossRef]
- Akter, L.; Flechsig, H.; Marchesi, A.; Franz, C.M. Observing Dynamic Conformational Changes within the Coiled-Coil Domain of Different Laminin Isoforms Using High-Speed Atomic Force Microscopy. Int. J. Mol. Sci. 2024, 25, 1951. [Google Scholar] [CrossRef]
- Yousif, L.F.; Di Russo, J.; Sorokin, L. Laminin isoforms in endothelial and perivascular basement membranes. Cell Adhes. Migr. 2013, 7, 101–110. [Google Scholar] [CrossRef]
- Rousselle, P.; Beck, K. Laminin 332 processing impacts cellular behavior. Cell Adhes. Migr. 2013, 7, 122–134. [Google Scholar] [CrossRef]
- Spenlé, C.; Simon-Assmann, P.; Orend, G.; Miner, J.H. Laminin α5 guides tissue patterning and organogenesis. Cell Adhes. Migr. 2013, 7, 90–100. [Google Scholar] [CrossRef]
- Simon-Assmann, P.; Orend, G.; Mammadova-Bach, E.; Spenle, C.; Lefebvre, O. Role of laminins in physiological and pathological angiogenesis. Int. J. Dev. Biol. 2011, 55, 455–465. [Google Scholar] [CrossRef]
- Kikkawa, Y.; Matsunuma, M.; Kan, R.; Yamada, Y.; Hamada, K.; Nomizu, M.; Negishi, Y.; Nagamori, S.; Toda, T.; Tanaka, M.; et al. Laminin α5_CD239_Spectrin is a candidate association that compensates the linkage between the basement membrane and cytoskeleton in skeletal muscle fibers. Matrix Biol. Plus 2022, 15, 100118. [Google Scholar] [CrossRef]
- Gawlik, K.I.; Holmberg, J.; Svensson, M.; Einerborg, M.; Oliveira, B.M.S.; Deierborg, T.; Durbeej, M. Potent pro-inflammatory and pro-fibrotic molecules, osteopontin and galectin-3, are not major disease modulators of laminin α2 chain-deficient muscular dystrophy. Sci. Rep. 2017, 7, 44059. [Google Scholar] [CrossRef] [PubMed]
- Yurchenco, P.D.; McKee, K.K. Linker Protein Repair of LAMA2 Dystrophic Neuromuscular Basement Membranes. Front. Mol. Neurosci. 2019, 12, 305. [Google Scholar] [CrossRef] [PubMed]
- Funk, S.D.; Bayer, R.H.; Malone, A.F.; McKee, K.K.; Yurchenco, P.D.; Miner, J.H. Pathogenicity of a Human Laminin β2 Mutation Revealed in Models of Alport Syndrome. J. Am. Soc. Nephrol. 2018, 29, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Accorsi, A.; Cramer, M.L.; Girgenrath, M. Fibrogenesis in LAMA2-Related Muscular Dystrophy Is a Central Tenet of Disease Etiology. Front. Mol. Neurosci. 2020, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Vilboux, T.; Malicdan, M.C.V.; Chang, Y.M.; Guo, J.; Zerfas, P.M.; Stephen, J.; Cullinane, A.R.; Bryant, J.; Fischer, R.; Brooks, B.P.; et al. Cystic cerebellar dysplasia and biallelic LAMA1 mutations: A lamininopathy associated with tics, obsessive compulsive traits and myopia due to cell adhesion and migration defects. J. Med. Genet. 2016, 53, 318–329. [Google Scholar] [CrossRef]
- Fabian, L.; Dowling, J.J. Zebrafish Models of LAMA2-Related Congenital Muscular Dystrophy (MDC1A). Front. Mol. Neurosci. 2020, 13, 122. [Google Scholar] [CrossRef] [PubMed]
- Gawlik, K.I.; Durbeej, M. A Family of Laminin α2 Chain-Deficient Mouse Mutants: Advancing the Research on LAMA2-CMD. Front. Mol. Neurosci. 2020, 13, 59. [Google Scholar] [CrossRef] [PubMed]
- Toutouna, L.; Beck-Woedl, S.; Feige, U.; Glaeser, B.; Komlosi, K.; Eckenweiler, M.; Luetzen, N.; Haack, T.B.; Fischer, J.; Bader, I.; et al. Novel homozygous LAMB1 in-frame deletion in a pediatric patient with brain anomalies and cerebrovascular event. Am. J. Med. Genet. Part A 2023, 191, 2656–2663. [Google Scholar] [CrossRef]
- Dietvorst, S.; Devriendt, K.; Lambert, J.; Boogaerts, A.; Bogaert, K.V.D.; Buyse, G.; Van Calenbergh, F. NID1-related autosomal dominant Dandy-Walker malformation with occipital cephalocele in three generations. Eur. J. Med Genet. 2023, 66, 104713. [Google Scholar] [CrossRef]
- Saarela, A.; Timonen, O.; Kirjavainen, J.; Liu, Y.; Silvennoinen, K.; Mervaala, E.; Kälviäinen, R. Novel LAMC3 pathogenic variant enriched in Finnish population causes malformations of cortical development and severe epilepsy. Epileptic Disord. 2024, 26, 498–509. [Google Scholar] [CrossRef]
- Hannigan, G.E.; Coles, J.G.; Dedhar, S. Integrin-linked kinase at the heart of cardiac contractility, repair, and disease. Circ. Res. 2007, 100, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Knöll, R.; Postel, R.; Wang, J.; Krätzner, R.; Hennecke, G.; Vacaru, A.M.; Vakeel, P.; Schubert, C.; Murthy, K.; Rana, B.K.; et al. Laminin-alpha4 and integrin-linked kinase mutations cause human cardiomyopathy via simultaneous defects in cardiomyo-cytes and endothelial cells. Circulation 2007, 116, 515–525. [Google Scholar] [CrossRef]
- Li, J.; Tzu, J.; Chen, Y.; Zhang, Y.; Nguyen, N.T.; Gao, J.; Bradley, M.; Keene, D.R.; Oro, A.E.; Miner, J.H.; et al. Laminin-10 is crucial for hair morphogenesis. EMBO J. 2003, 22, 2400–2410. [Google Scholar] [CrossRef]
- Sampaolo, S.; Napolitano, F.; Tirozzi, A.; Reccia, M.G.; Lombardi, L.; Farina, O.; Barra, A.; Cirillo, F.; Melone, M.A.B.; Gianfrancesco, F.; et al. Identification of the first dominant mutation of LAMA5 gene causing a complex multisystem syndrome due to dysfunction of the extracellular matrix. J. Med. Genet. 2017, 54, 710–720. [Google Scholar] [CrossRef]
- Maselli, R.A.; Arredondo, J.; Vázquez, J.; Chong, J.X.; Bamshad, M.J.; Nickerson, D.A.; Lara, M.; Ng, F.; Lo, V.L.; Pytel, P.; et al. A presynaptic congenital myasthenic syndrome attributed to a homozygous sequence variant in LAMA5. Ann. N. Y. Acad. Sci. 2018, 1413, 119–125. [Google Scholar] [CrossRef]
- Rebustini, I.T.; Patel, V.N.; Stewart, J.S.; Layvey, A.; Georges-Labouesse, E.; Miner, J.H.; Hoffman, M.P. Laminin α5 is necessary for submandibular gland epithelial morphogenesis and influences FGFR expression through β1 integrin signaling. Dev. Biol. 2007, 308, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Jiang, Z.; Yang, G. Laminins in osteogenic differentiation and pluripotency maintenance. Differentiation 2020, 114, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Barad, M.; Csukasi, F.; Bosakova, M.; Martin, J.H.; Zhang, W.; Taylor, S.P.; Lachman, R.S.; Zieba, J.; Bamshad, M.; Nickerson, D.; et al. Biallelic mutations in LAMA5 disrupts a skeletal noncanonical focal adhesion pathway and produces a distinct bent bone dysplasia. EBioMedicine 2020, 62, 103075. [Google Scholar] [CrossRef]
- Yap, L.; Tay, H.G.; Nguyen, M.T.X.; Tjin, M.S.; Tryggvason, K. Laminins in Cellular Differentiation. Trends Cell Biol. 2019, 29, 987–1000. [Google Scholar] [CrossRef]
- Yap, L.; Wang, J.-W.; Moreno-Moral, A.; Chong, L.Y.; Sun, Y.; Harmston, N.; Wang, X.; Chong, S.Y.; Vanezis, K.; Öhman, M.K.; et al. In Vivo Generation of Post-infarct Human Cardiac Muscle by Laminin-Promoted Cardiovascular Progenitors. Cell Rep. 2019, 26, 3231–3245.e9. [Google Scholar] [CrossRef]
- Kechagia, J.Z.; Ivaska, J.; Roca-Cusachs, P. Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol. 2019, 20, 457–473. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Ballestrem, C.; Streuli, C.H. The C terminus of talin links integrins to cell cycle progression. J. Cell Biol. 2011, 195, 499–513. [Google Scholar] [CrossRef]
- Lad, Y.; Harburger, D.S.; Calderwood, D.A. Integrin cytoskeletal interactions. Methods Enzymol. 2007, 426, 69–84. [Google Scholar] [CrossRef]
- Harburger, D.S.; Calderwood, D.A. Integrin signalling at a glance. J. Cell Sci. 2009, 122 Pt 2, 159–163. [Google Scholar] [CrossRef]
- Xiong, J.-P.; Stehle, T.; Diefenbach, B.; Zhang, R.; Dunker, R.; Scott, D.L.; Joachimiak, A.; Goodman, S.L.; Arnaout, M.A. Crystal structure of the extracellular segment of integrin alpha Vbeta3. Science 2001, 294, 339–345. [Google Scholar] [CrossRef]
- Salvati, M.; Cordero, F.M.; Pisaneschi, F.; Melani, F.; Gratteri, P.; Cini, N.; Bottoncetti, A.; Brandi, A. Synthesis, SAR and in vitro evaluation of new cyclic Arg-Gly-Asp pseudopentapeptides containing a s-cis peptide bond as integrin αvβ3 and αvβ5 ligands. Bioorganic Med. Chem. 2008, 16, 4262–4271. [Google Scholar] [CrossRef]
- Wu, Y.; Cao, Z.; Liu, W.; Cahoon, J.G.; Wang, K.; Wang, P.; Hu, L.; Chen, Y.; Moser, M.; Vella, A.T.; et al. Nanoscopy reveals integrin clustering reliant on kindlin-3 but not talin-1. Cell Commun Signal. 2025, 23, 12. [Google Scholar] [CrossRef]
- Ito, K.; Kon, S.; Nakayama, Y.; Kurotaki, D.; Saito, Y.; Kanayama, M.; Kimura, C.; Diao, H.; Morimoto, J.; Matsui, Y.; et al. The differential amino acid requirement within osteopontin in α4 and α9 integrin-mediated cell binding and migration. Matrix Biol. 2009, 28, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Lund, S.A.; Wilson, C.L.; Raines, E.W.; Tang, J.; Giachelli, C.M.; Scatena, M. Osteopontin mediates macrophage chemotaxis via α4 and α9 integrins and survival via the α4 integrin. J. Cell Biochem. 2013, 114, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Hui, T.; Sørensen, E.S.; Rittling, S.R. Osteopontin binding to the alpha 4 integrin requires highest affinity integrin conformation, but is independent of post-translational modifications of osteopontin. Matrix Biol. 2015, 41, 19–25. [Google Scholar] [CrossRef]
- Mohamed, K.E.-T. Role of osteopontin in cellular signaling and metastatic phenotype. Front. Biosci. 2008, 13, 4276–4284. [Google Scholar] [CrossRef]
- Sun, J.; Feng, A.; Chen, S.; Zhang, Y.; Xie, Q.; Yang, M.; Shao, Q.; Liu, J.; Yang, Q.; Kong, B.; et al. Osteopontin splice variants expressed by breast tumors regulate monocyte activation via MCP-1 and TGF-β1. Cell. Mol. Immunol. 2013, 10, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Tadokoro, S.; Shattil, S.J.; Eto, K.; Tai, V.; Liddington, R.C.; de Pereda, J.M.; Ginsberg, M.H.; Calderwood, D.A. Talin binding to integrin beta tails: A final common step in integrin activation. Science 2003, 302, 103–106. [Google Scholar] [CrossRef]
- Hamaia, S.; Farndale, R.W. Integrin recognition motifs in the human collagens. Adv. Exp. Med. Biol. 2014, 819, 127–142. [Google Scholar] [CrossRef]
- Reyes, C.D.; García, A.J. Engineering integrin-specific surfaces with a triple-helical collagen-mimetic peptide. J. Biomed. Mater. Res. Part A 2003, 65A, 511–523. [Google Scholar] [CrossRef]
- Miranti, C.K.; Brugge, J.S. Sensing the environment: A historical perspective on integrin signal transduction. Nat. Cell Biol. 2002, 4, E83–E90. [Google Scholar] [CrossRef]
- Playford, M.P.; Schaller, M.D. The interplay between Src and integrins in normal and tumor biology. Oncogene 2004, 23, 7928–7946. [Google Scholar] [CrossRef] [PubMed]
- Sen, M.; Koksal, A.C.; Yuki, K.; Wang, J.; Springer, T.A. Ligand- and cation-induced structural alterations of the leukocyte integrin LFA-1. J. Biol. Chem. 2018, 293, 6565–6577. [Google Scholar] [CrossRef] [PubMed]
- Calderwood, D.A.; Campbell, I.D.; Critchley, D.R. Talins and kindlins: Partners in integrin-mediated adhesion. Nat. Rev. Mol. Cell Biol. 2013, 14, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Calderwood, D.A. Integrin activation. J. Cell Sci. 2004, 117 Pt 5, 657–666. [Google Scholar] [CrossRef]
- Calderwood, D. Talin controls integrin activation. Biochem. Soc. Trans. 2004, 32 Pt 3, 434–437. [Google Scholar] [CrossRef]
- Böttcher, R.T.; Veelders, M.; Rombaut, P.; Faix, J.; Theodosiou, M.; Stradal, T.E.; Rottner, K.; Zent, R.; Herzog, F.; Fässler, R. Kindlin-2 recruits paxillin and Arp2/3 to promote membrane protrusions during initial cell spreading. J. Cell Biol. 2017, 216, 3785–3798. [Google Scholar] [CrossRef]
- Georgiadou, M.; Ivaska, J. Tensins: Bridging AMP-Activated Protein Kinase with Integrin Activation. Trends Cell Biol. 2017, 27, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Yan, L.; Zou, C.; Wang, K.; Chen, M.; Xu, B.; Zhou, Z.; Zhang, D. Integrins regulate stemness in solid tumor: An emerging therapeutic target. J. Hematol. Oncol. 2021, 14, 177. [Google Scholar] [CrossRef]
- Guo, W.; Giancotti, F.G. Integrin signalling during tumour progression. Nat. Rev. Mol. Cell Biol. 2004, 5, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Karamanou, K.; Franchi, M.; Proult, I.; Rivet, R.; Vynios, D.; Brézillon, S. Lumican Inhibits In Vivo Melanoma Metastasis by Altering Matrix-Effectors and Invadopodia Markers. Cells 2021, 10, 841. [Google Scholar] [CrossRef]
- Horton, E.R.; Humphries, J.D.; James, J.; Jones, M.C.; Askari, J.A.; Humphries, M.J. The integrin adhesome network at a glance. J. Cell Sci. 2016, 129, 4159–4163. [Google Scholar] [CrossRef]
- Totaro, A.; Panciera, T.; Piccolo, S. YAP/TAZ upstream signals and downstream responses. Nat. Cell Biol. 2018, 20, 888–899. [Google Scholar] [CrossRef]
- Alonso-Matilla, R.; Provenzano, P.P.; Odde, D.J. Optimal cell traction forces in a generalized motor-clutch model. Biophys. J. 2023, 122, 3369–3385. [Google Scholar] [CrossRef]
- Chernousov, M.; Faerman, A.; Frid, M.; Printseva, O.; Koteliansky, V. Monoclonal antibody to fibronectin which inhibits extracellular matrix assembly. FEBS Lett. 1987, 217, 124–128. [Google Scholar] [CrossRef]
- Mui, K.L.; Chen, C.S.; Assoian, R.K. The mechanical regulation of integrin–cadherin crosstalk organizes cells, signaling and forces. J. Cell Sci. 2016, 129, 1093–1100. [Google Scholar] [CrossRef]
- Dzobo, K.; Dandara, C. Architecture of Cancer-Associated Fibroblasts in Tumor Microenvironment: Mapping Their Origins, Heterogeneity, and Role in Cancer Therapy Resistance. OMICS 2020, 24, 314–339. [Google Scholar] [CrossRef] [PubMed]
- Humphries, J.D.; Byron, A.; Humphries, M.J. Integrin ligands at a glance. J. Cell Sci. 2006, 119 Pt 19, 3901–3903. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, B.; Ao, M.; White, L.M.; Means, A.L.; Brewer, B.M.; Yang, L.; Washington, M.K.; Shi, C.; Franco, O.E.; Weaver, A.M.; et al. Cancer-associated fibroblasts promote directional cancer cell migration by aligning fibronectin. J. Cell Biol. 2017, 216, 3799–3816. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Massagué, J. Epithelial-mesenchymal transitions: Twist in development and metastasis. Cell 2004, 118, 277–279. [Google Scholar] [CrossRef]
- Rapraeger, A.C.; Ell, B.J.; Roy, M.; Li, X.; Morrison, O.R.; Thomas, G.M.; Beauvais, D.M. Vascular endothelial-cadherin stimulates syndecan-1-coupled insulin-like growth factor-1 receptor and cross-talk between αVβ3 integrin and vascular endothelial growth factor receptor 2 at the onset of endothelial cell dissemination during angiogenesis. FEBS J. 2013, 280, 2194–2206. [Google Scholar] [CrossRef] [PubMed]
- Afratis, N.A.; Bouris, P.; Skandalis, S.S.; Multhaupt, H.A.; Couchman, J.R.; Theocharis, A.D.; Karamanos, N.K. IGF-IR cooperates with ERα to inhibit breast cancer cell aggressiveness by regulating the expression and localisation of ECM molecules. Sci. Rep. 2017, 7, 40138. [Google Scholar] [CrossRef] [PubMed]
- Afratis, N.A.; Nikitovic, D.; Multhaupt, H.A.; Theocharis, A.D.; Couchman, J.R.; Karamanos, N.K. Syndecans—Key regulators of cell signaling and biological functions. FEBS J. 2017, 284, 27–41. [Google Scholar] [CrossRef]
- Person, A.D.; Klewer, S.E.; Runyan, R.B. Cell biology of cardiac cushion development. Int. Rev. Cytol. 2005, 243, 287–335. [Google Scholar] [PubMed]
- Scansen, B.A. Coronary Artery Anomalies in Animals. Vet Sci. 2017, 4, 20. [Google Scholar] [CrossRef]
- Ivan den Hoff, M.J.; Moorman, A.F. Cardiac neural crest: The holy grail of cardiac abnormalities? Cardiovasc Res. 2000, 47, 212–226. [Google Scholar] [CrossRef]
- Dupuis, L.E.; Osinska, H.; Weinstein, M.B.; Hinton, R.B.; Kern, C.B. Insufficient versican cleavage and Smad2 phosphorylation results in bicuspid aortic and pulmonary valves. J Mol Cell Cardiol. 2013, 60, 50–59. [Google Scholar] [CrossRef]
- Braverman, A.C. Bicuspid aortic valve and associated aortic wall abnormalities. Curr. Opin. Cardiol. 1996, 11, 501–503. [Google Scholar] [CrossRef]
- de Sa, M.; Moshkovitz, Y.; Butany, J.; David, T.E. Histologic abnormalities of the ascending aorta and pulmonary trunk in patients with bicuspid aortic valve disease: Clinical relevance to the ross procedure. J. Thorac. Cardiovasc. Surg. 1999, 118, 588–596. [Google Scholar] [CrossRef]
- Spadaccio, C.; Montagnani, S.; Acar, C.; Nappi, F. Introducing bioresorbable scaffolds into the show. A potential adjunct to resuscitate Ross procedure. Int. J. Cardiol. 2015, 190, 50–52. [Google Scholar] [CrossRef]
- Nappi, F.; Spadaccio, C.; Al-Attar, N.; Acar, C. The Ross procedure at the crossroads: Lessons from biology: Is Dr Ross’s dream concluded? Int. J. Cardiol. 2015, 178, 37–39. [Google Scholar] [CrossRef]
- Parai, J.L.; Masters, R.G.; Walley, V.M.; Stinson, W.A.; Veinot, J.P. Aortic medial changes associated with bicuspid aortic valve: Myth or reality? Can. J. Cardiol. 1999, 15, 1233–1238. [Google Scholar]
- Veinot, J.P. Congenitally bicuspid aortic valve and associated aortic medical disease. Ann. Thorac. Surg. 2001, 71, 1067–1068. [Google Scholar] [CrossRef]
- Chung, A.W.; Au Yeung, K.; Sandor, G.G.; Judge, D.P.; Dietz, H.C.; van Breemen, C. Loss of elastic fiber integrity and reduction of vascular smooth muscle contraction resulting from the upregulated activities of matrix metalloproteinase-2 and -9 in the thoracic aortic aneurysm in Marfan syndrome. Circ Res. 2007, 101, 512–522. [Google Scholar] [CrossRef]
- Pereira, L.; Lee, S.Y.; Gayraud, B.; Andrikopoulos, K.; Shapiro, S.D.; Bunton, T.; Biery, N.J.; Dietz, H.C.; Sakai, L.Y.; Ramirez, F. Pathogenetic sequence for aneurysm revealed in mice underexpressing fibrillin-1. Proc. Natl. Acad. Sci. USA 1999, 96, 3819–3823. [Google Scholar] [CrossRef]
- Tan, J.L.; Davlouros, P.A.; McCarthy, K.P.; Gatzoulis, M.A.; Ho, S.Y. Intrinsic histological abnormalities of aortic root and ascending aorta in tetralogy of Fallot: Evidence of causative mechanism for aortic dilatation and aortopathy. Circulation 2005, 112, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.W.; Parks, W.C. Role of matrix metalloproteinases inabdominal aortic aneurysms. Ann. N. Y. Acad. Sci. 1996, 800, 157–174. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, K.J.; Nousari, H.C.; Anhalt, G.J.; Stone, C.D.; Laschinger, J.C. Immunohistochemical abnormalities of fibrillin in cardiovascular tissues in Marfan’s syndrome. Ann. Thorac. Surg. 1997, 63, 1012–1017. [Google Scholar] [CrossRef]
- Nappi, F.; Spadaccio, C.; Fraldi, M.; Montagnani, S.; Fouret, P.; Chachques, J.C.; Acar, C. A composite semiresorbable armoured scaffold stabilizes pulmonary autograft after the Ross operation: Mr Ross’s dream fulfilled. J. Thorac. Cardiovasc. Surg. 2016, 151, 155–164.e1. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Spadaccio, C.; Fouret, P.; Hammoudi, N.; Chachques, J.C.; Chello, M.; Acar, C. An experimental model of the Ross operation: Development of resorbable reinforcements for pulmonary autografts. J. Thorac. Cardiovasc. Surg. 2015, 149, 1134–1142. [Google Scholar] [CrossRef]
- Fu, K.; Bai, Z.; Chen, L.; Ye, W.; Wang, M.; Hu, J.; Liu, C.; Zhou, W. Antitumor activity and structure-activity relationship of heparanase inhibitors: Recent advances. Eur. J. Med. Chem. 2020, 193, 112221. [Google Scholar] [CrossRef]
- Sanderson, R.D.; Elkin, M.; Rapraeger, A.C.; Ilan, N.; Vlodavsky, I. Heparanase regulation of cancer, autophagy and inflammation: New mechanisms and targets for therapy. FEBS J. 2017, 284, 42–55. [Google Scholar] [CrossRef]
- Masola, V.; Bellin, G.; Gambaro, G.; Onisto, M. Heparanase: A Multitasking Protein Involved in Extracellular Matrix (ECM) Remodeling and Intracellular Events. Cells 2018, 7, 236. [Google Scholar] [CrossRef]
- Bacchetti, R.; Yuan, S.; Rainero, E. ADAMTS Proteases: Their Multifaceted Role in the Regulation of Cancer Metastasis. Dis. Res. 2024, 4, 40–52. [Google Scholar] [CrossRef]
- Zhong, S.; Khalil, R.A. A Disintegrin and Metalloproteinase (ADAM) and ADAM with thrombospondin motifs (ADAMTS) family in vascular biology and disease. Biochem. Pharmacol. 2019, 164, 188–204. [Google Scholar] [CrossRef]
- de Mendoza, A.; Sebé-Pedrós, A. Origin and evolution of eukaryotic transcription factors. Curr. Opin. Genet. Dev. 2019, 58–59, 25–32. [Google Scholar] [CrossRef]
- Souza, J.S.M.; Lisboa, A.B.P.; Santos, T.M.; Andrade, M.V.S.; Neves, V.B.S.; Teles-Souza, J.; Jesus, H.N.R.; Bezerra, T.G.; Falcão, V.G.O.; Oliveira, R.C.; et al. The evolution of ADAM gene family in eukaryotes. Genomics 2020, 112, 3108–3116. [Google Scholar] [CrossRef] [PubMed]
- Amar, S.; Smith, L.; Fields, G.B. Matrix metalloproteinase collagenolysis in health and disease. Biochim. et Biophys. Acta (BBA) Mol. Cell Res. 2017, 1864, 1940–1951. [Google Scholar] [CrossRef] [PubMed]
- Turunen, S.P.; Tatti-Bugaeva, O.; Lehti, K. Membrane-type matrix metalloproteases as diverse effectors of cancer progression. Biochim. et Biophys. Acta (BBA) Mol. Cell Res. 2017, 1864 Pt A, 1974–1988. [Google Scholar] [CrossRef] [PubMed]
- Tallant, C.; Marrero, A.; Gomis-Rüth, F. Matrix metalloproteinases: Fold and function of their catalytic domains. Biochim. et Biophys. Acta (BBA) Mol. Cell Res. 2010, 1803, 20–28. [Google Scholar] [CrossRef]
- Piperigkou, Z.; Manou, D.; Karamanou, K.; Theocharis, A.D. Strategies to Target Matrix Metalloproteinases as Therapeutic Approach in Cancer. Methods Mol. Biol. 2018, 1731, 325–348. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, Z.; Chen, D.; Li, Y.; Zhang, Q.; Su, J. Bone Regeneration Using MMP-Cleavable Peptides-Based Hydrogels. Gels 2021, 7, 199. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, L.; Wang, P.; Chen, D.; Wu, Z.; Tang, C. Periostin promotes migration and osteogenic differentiation of human periodontal ligament mesenchymal stem cells via the Jun amino-terminal kinases (JNK) pathway under inflammatory conditions. Cell Prolif. 2017, 50, e12369. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef]
- Zitka, O.; Kukacka, J.; Krizkov, S.; Huska, D.; Adam, V.; Masarik, M.; Prusa, R.; Kizek, R. Matrix metalloproteinases. Curr. Med. Chem. 2010, 17, 3751–3768. [Google Scholar] [CrossRef]
- Abilleira, S.; Bevan, S.; Markus, H.S. The role of genetic variants of matrix metalloproteinases in coronary and carotid atherosclerosis. J. Med. Genet. 2006, 43, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Moll, M.; Hobbs, B.D.; Pratte, K.A.; Zhang, C.; Ghosh, A.J.; Bowler, R.P.; Lomas, D.A.; Silverman, E.K.; DeMeo, D.L. Assessing inflammatory protein biomarkers in COPD subjects with and without alpha-1 antitrypsin deficiency. Respir. Res. 2025, 26, 247. [Google Scholar] [CrossRef] [PubMed]
- Tokura, T.; Matsushita, T.; Nishida, K.; Nagai, K.; Kanzaki, N.; Hoshino, Y.; Matsumoto, T.; Kuroda, R. SIRT1 antisense long noncoding RNA attenuates interleukin-1β-induced osteoarthritic gene expression in human chondrocytes through its mRNA interaction. Sci. Rep. 2025, 15, 23338. [Google Scholar] [CrossRef]
- Martin, M.D.; Matrisian, L.M. The other side of MMPs: Protective roles in tumor progression. Cancer Metastasis Rev. 2007, 26, 717–724. [Google Scholar] [CrossRef]
- Memtsas, V.; Zarros, A.; Theocharis, S. Matrix metalloproteinases in the pathophysiology and progression of gynecological malignancies: Could their inhibition be an effective therapeutic approach? Expert Opin. Ther. Targets 2009, 13, 1105–1120. [Google Scholar] [CrossRef]
- Ramnath, N.; Creaven, P.J. Matrix metalloproteinase inhibitors. Curr. Oncol. Rep. 2004, 6, 96–102. [Google Scholar] [CrossRef]
- Overall, C.M.; Kleifeld, O. Tumour microenvironment—Opinion: Validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat. Rev. Cancer 2006, 6, 227–239. [Google Scholar] [CrossRef]
- Jobin, P.G.; Butler, G.S.; Overall, C.M. New intracellular activities of matrix metalloproteinases shine in the moonlight. Biochim. et Biophys. Acta (BBA) Mol. Cell Res. 2017, 1864 Pt A, 2043–2055. [Google Scholar] [CrossRef]
- Kim, I.-S.; Yang, W.-S.; Kim, C.-H. Physiological Properties, Functions, and Trends in the Matrix Metalloproteinase Inhibitors in Inflammation-Mediated Human Diseases. Curr. Med. Chem. 2023, 30, 2075–2112. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K.; Senthebane, D.A.; Ganz, C.; Thomford, N.E.; Wonkam, A.; Dandara, C. Advances in Therapeutic Targeting of Cancer Stem Cells within the Tumor Microenvironment: An Updated Review. Cells 2020, 9, 1896. [Google Scholar] [CrossRef]
- Kanak, S.; Krzemińska, B. The Active compounds in plants of the genus Alchemilla with proven skin care and therapeutic formulations in the treatment of skin diseases. Prospect. Pharm. Sci. 2025, 22, 188–198. [Google Scholar] [CrossRef]
- Apte, S.S. (Ed.) ADAMTS Proteins: Concepts, Challenges, and Prospects. In ADAMTS Proteases. Methods in Molecular Biology; Humana: New York, NY, USA, 2020; Volume 2043, pp. 1–12. [Google Scholar]
- Mead, T.J.; Apte, S.S. ADAMTS proteins in human disorders. Matrix Biol. 2018, 71–72, 225–239. [Google Scholar] [CrossRef]
- Lambert, J.; Edwards, D.R. Analysis of ADAMTS effects on cell adhesion and migration. In ADAMTS Proteases: Methods and Protocols; Apte, S.S., Ed.; Springer: New York, NY, USA, 2020; pp. 179–193. [Google Scholar]
- Bekhouche, M.; Colige, A. The procollagen N-proteinases ADAMTS2, 3 and 14 in pathophysiology. Matrix Biol. 2015, 44–46, 46–53. [Google Scholar] [CrossRef]
- Bekhouche, M.; Leduc, C.; Dupont, L.; Janssen, L.; Delolme, F.; Goff, S.V.; Smargiasso, N.; Baiwir, D.; Mazzucchelli, G.; Zanella-Cleon, I.; et al. Determination of the substrate repertoire of ADAMTS2, 3, and 14 significantly broadens their functions and identifies extracellular matrix organization and TGF-β signaling as primary targets. FASEB J. 2016, 30, 1741–1756. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-J.; Kong, W.; Ilalov, K.; Yu, S.; Xu, K.K.; Prazak, L.; Fajardo, M.; Sehgal, B.; Di Cesare, P.E. ADAMTS-7: A metalloproteinase that directly binds to and degrades cartilage oligomeric matrix protein. FASEB J. 2006, 20, 988–990. [Google Scholar] [CrossRef]
- Colige, A.; Monseur, C.; Crawley, J.T.B.; Santamaria, S.; de Groot, R. Proteomic discovery of substrates of the cardiovascular protease ADAMTS7. J. Biol. Chem. 2019, 294, 8037–8045. [Google Scholar] [CrossRef] [PubMed]
- Kremer Hovinga, J.A.; Coppo, P.; Lämmle, B.; Moake, J.L.; Miyata, T.; Vanhoorelbeke, K. Thrombotic thrombocytopenic purpura. Nat. Rev. Dis. Primers 2017, 3, 17020. [Google Scholar] [CrossRef] [PubMed]
- South, K.; Lane, D.A. ADAMTS-13 and von Willebrand factor: A dynamic duo. J. Thromb. Haemost. 2018, 16, 6–18. [Google Scholar] [CrossRef]
- Jackson, H.W.; Defamie, V.; Waterhouse, P.; Khokha, R. TIMPs: Versatile extracellular regulators in cancer. Nat. Rev. Cancer 2017, 17, 38–53. [Google Scholar] [CrossRef]
- Shteper, P.J.; Zcharia, E.; Ashhab, Y.; Peretz, T.; Vlodavsky, I.; Ben-Yehuda, D. Role of promoter methylation in regulation of the mammalian heparanase gene. Oncogene 2003, 22, 7737–7749. [Google Scholar] [CrossRef]
- Baraz, L.; Haupt, Y.; Elkin, M.; Peretz, T.; Vlodavsky, I. Tumor suppressor p53 regulates heparanase gene expression. Oncogene 2006, 25, 3939–3947. [Google Scholar] [CrossRef]
- Vlodavsky, I.; Eldor, A.; Haimovitz-Friedman, A.; Matzner, Y.; Ishai-Michaeli, R.; Lider, O.; Naparstek, Y.; Cohen, I.R.; Fuks, Z. Expression of heparanase by platelets and circulating cells of the immune system: Possible involvement in diapedesis and extravasation. Invasion Metastasis 1992, 12, 112–127. [Google Scholar] [PubMed]
- D’SOuza, S.S.; Daikoku, T.; Farach-Carson, M.C.; Carson, D.D. Heparanase expression and function during early pregnancy in mice1. Biol. Reprod. 2007, 77, 433–441. [Google Scholar] [CrossRef]
- Zcharia, E.; Zilka, R.; Yaar, A.; Yacoby-Zeevi, O.; Zetser, A.; Metzger, S.; Sarid, R.; Naggi, A.; Casu, B.; Ilan, N.; et al. Heparanase accelerates wound angiogenesis and wound healing in mouse and rat models. FASEB J. 2005, 19, 211–221. [Google Scholar] [CrossRef]
- Ilan, N.; Elkin, M.; Vlodavsky, I. Regulation, function and clinical significance of heparanase in cancer metastasis and angiogenesis. Int. J. Biochem. Cell Biol. 2006, 38, 2018–2039. [Google Scholar] [CrossRef]
- Arvatz, G.; Shafat, I.; Levy-Adam, F.; Ilan, N.; Vlodavsky, I. The heparanase system and tumor metastasis: Is heparanase the seed and soil? Cancer Metastasis Rev. 2011, 30, 253–268. [Google Scholar] [CrossRef]
- Ramani, V.C.; Zhan, F.; He, J.; Barbieri, P.; Noseda, A.; Tricot, G.; Sanderson, R.D. Targeting heparanase overcomes chemoresistance and diminishes relapse in myeloma. Oncotarget 2016, 7, 1598–1607. [Google Scholar] [CrossRef]
- Beta Blockers and Angiotensin Receptor Blockers in Bicuspid Aortic Valve Disease Aortopathy (BAV Study) (BAV). Available online: https://clinicaltrials.gov/study/NCT01202721?tab=results (accessed on 25 October 2025).
- Biner, S.; Rafique, A.M.; Ray, I.; Cuk, O.; Siegel, R.J.; Tolstrup, K. Aortopathy is prevalent in relatives of bicuspid aortic valve patients. JACC 2009, 53, 2288–2295. [Google Scholar] [CrossRef] [PubMed]
- Tadros, T.M.; Klein, M.D.; Shapira, O.M. Ascending aortic dilatation associated with bicuspid aortic valve: Pathophysiology, molecular biology, and clinical implications. Circulation 2009, 119, 880–890. [Google Scholar] [CrossRef] [PubMed]
- Siu, S.C.; Silversides, C.K. Bicuspid aortic valve disease. J. Am. Coll. Cardiol. 2010, 55, 2789–2800. [Google Scholar] [CrossRef] [PubMed]
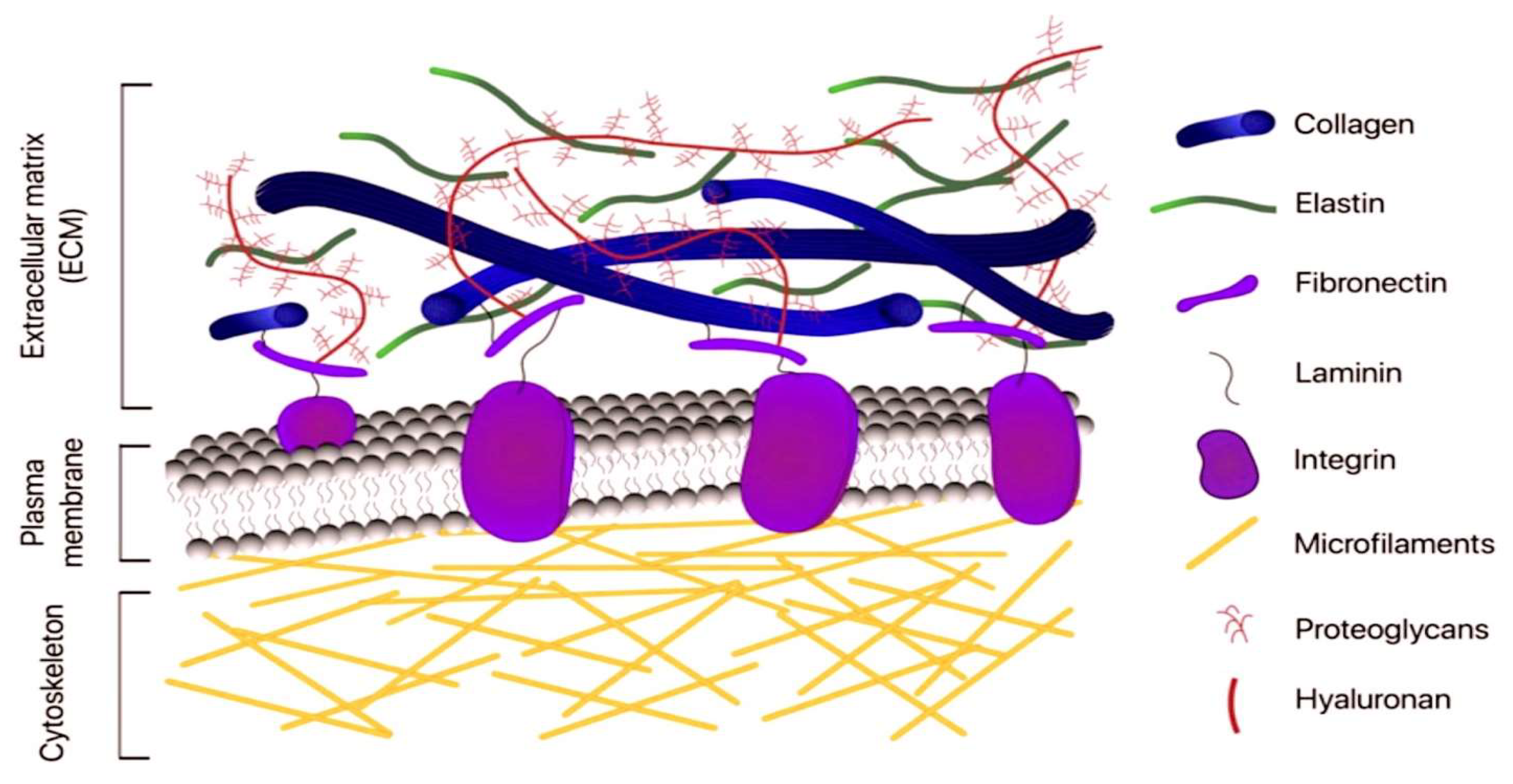
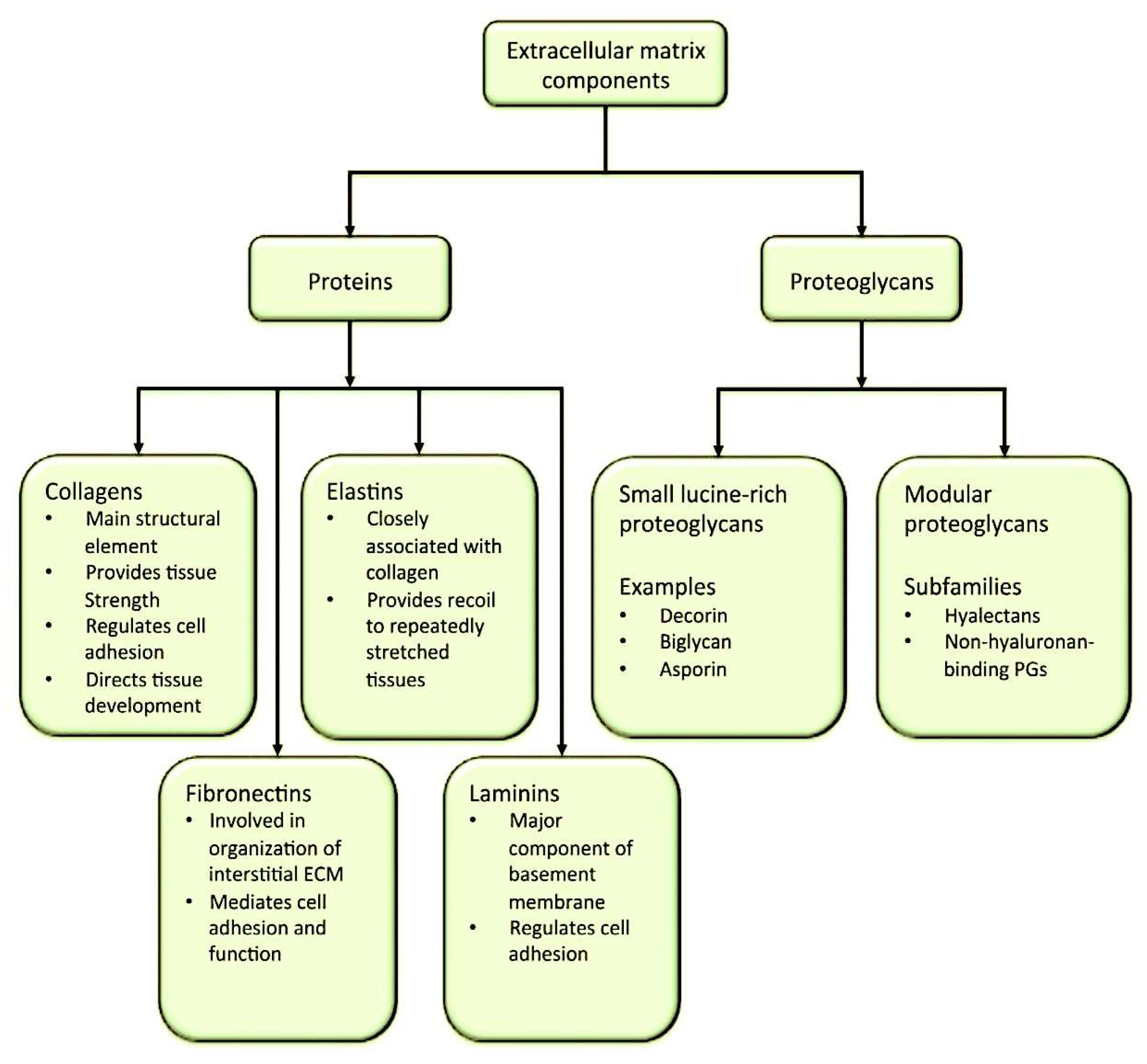

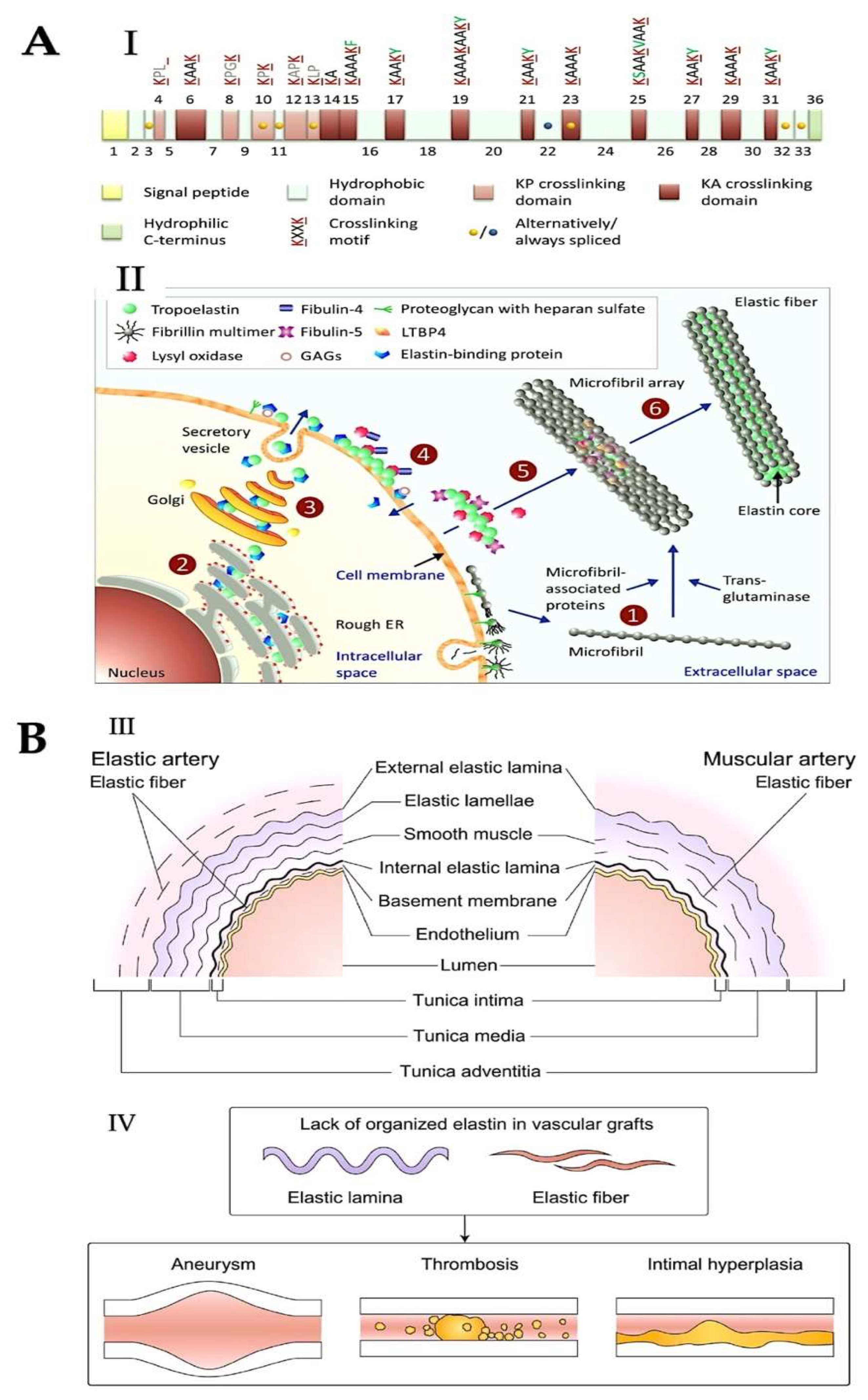
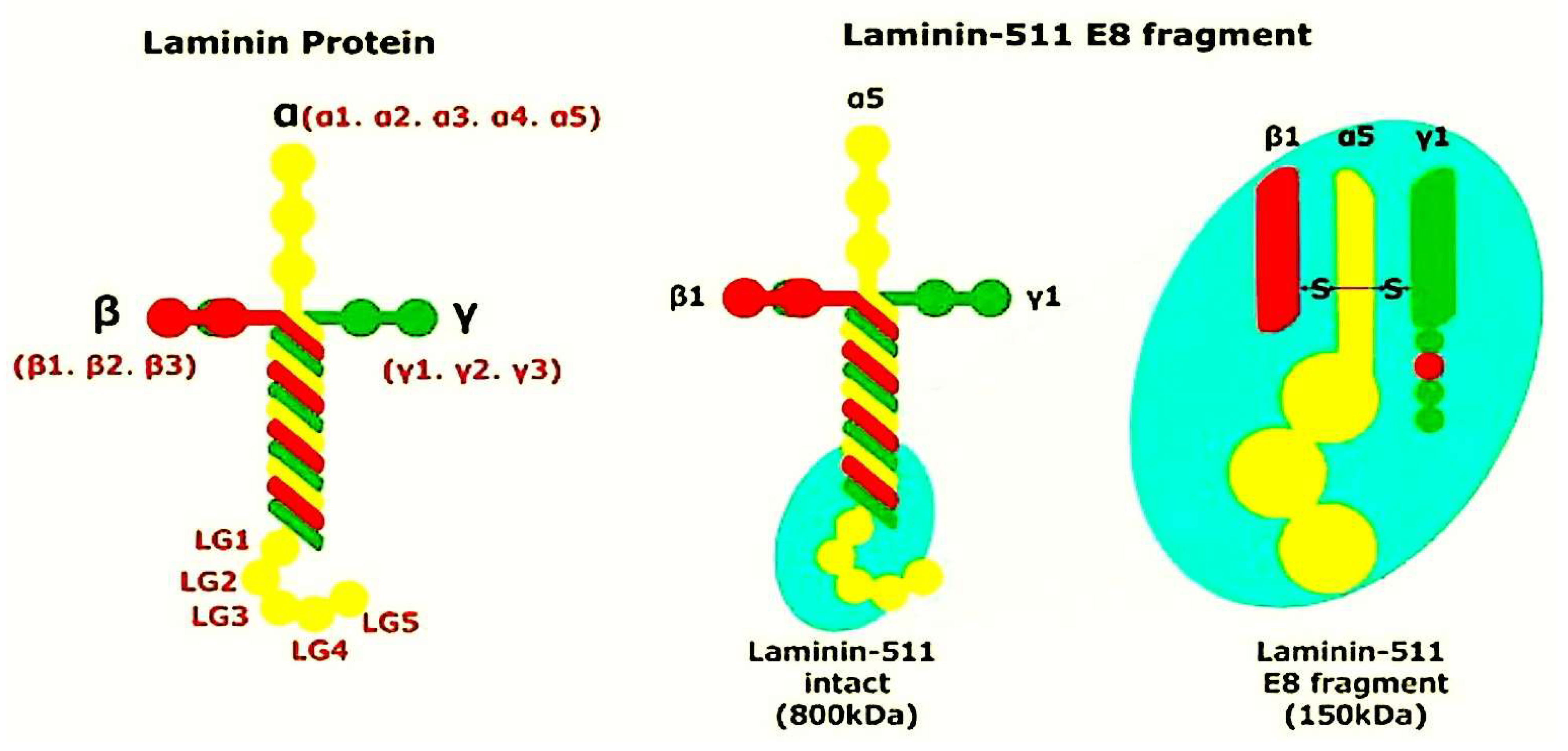
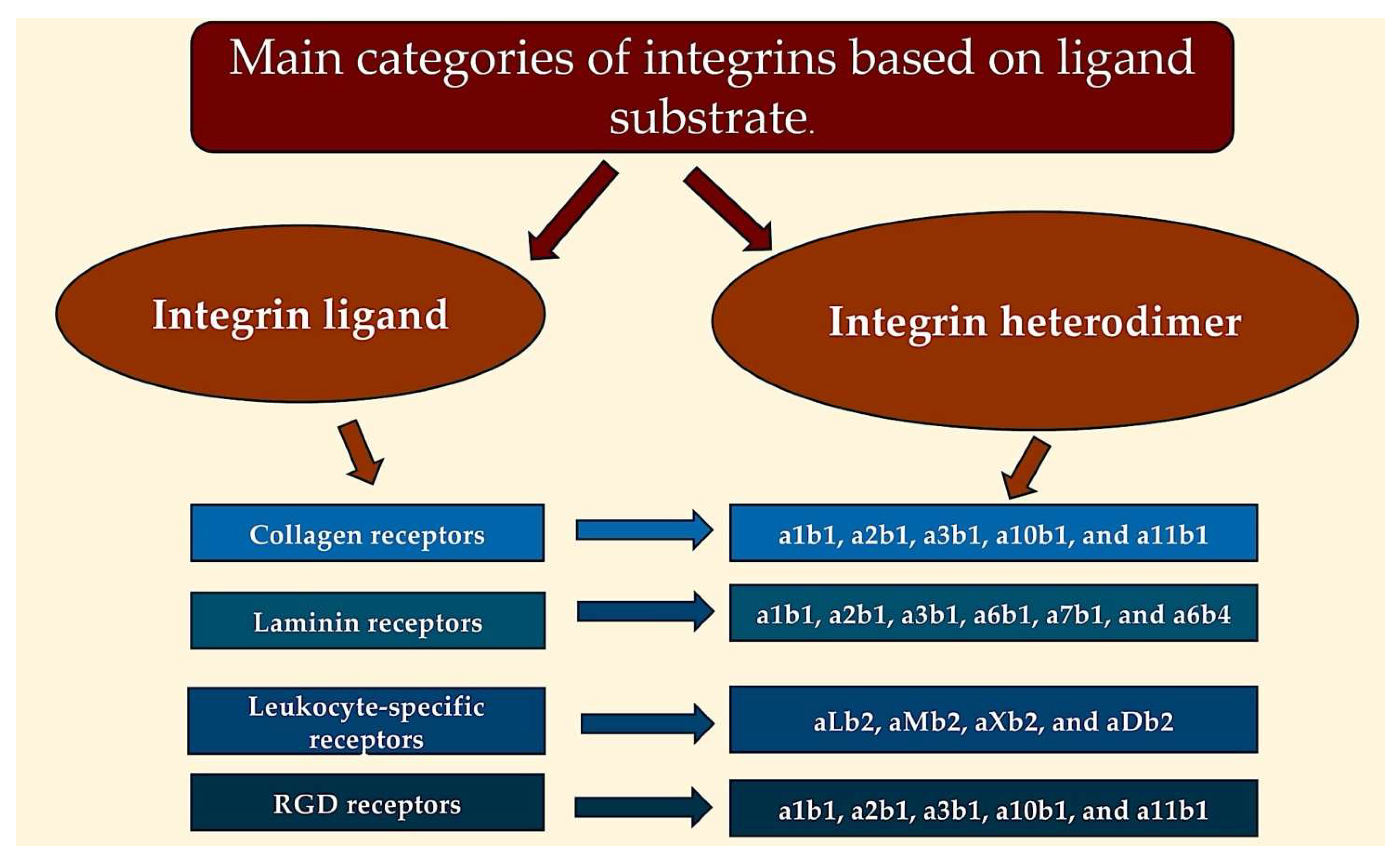

|
| Laminin Isoform | 111 | 211 | 121 | 221 | 332 | 311 | 321 | 411 | 421 | 511 | 521 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| α | α1 | α2 | α1 | α2 | α3 | α3 | α3 | α4 | α4 | α5 | α5 |
| β | β1 | β1 | β2 | β2 | β3 | β1 | β2 | β1 | β2 | β1 | β2 |
| γ | γ1 | γ1 | γ1 | γ1 | γ2 | γ1 | γ1 | γ1 | γ1 | γ1 | γ1 |
| Binding Protein | Integrin Heterodimer |
|---|---|
| Fibronectin | α v β 3, α v β 6, aIIb β 3, α v β 1, α 5 β 1, α 8 β 1, α 4 β 1, α 4 β 7 |
| vWF | α v β 3, α IIb β 3 |
| Vitronectin | α β 3, α v β 5, α 8 β 1, α IIb β 3 |
| Fibrillin | α v β 3 |
| Fibrinogen | α v β 3, α IIb β 3, α X β 2, α M β 2 |
| Factor X | α M β 2 |
| Developmental endothelial locus-1 | α β 3, α v β 5 |
| Inactivated complement component C3b | α X β 2, α M β 2 |
| TN | α v β 3, α 8 β 1, α 9 β 1 |
| Platelet endothelial cell adhesion molecule 1 | α v β 3 |
| E-cadherin | α E β 7 |
| Latency associated peptide transforming GF | α v β 1, α v β 8, α v β 6, α v β 3 |
| Mucosal addressin cell adhesion molecule 1 | α 4 β 7, α 4 β 1 |
| Bone sialoprotein | α v β 3, α v β 5 |
| Thrombospondin | α 3 β 1, α 2 β 1, α 4 β 1, α v β 3, α 3 β 1, α IIb β 3 |
| Laminin | α 3 β 1, α 6 β 1, α 6 β 4, α 7 β 1, α 1 β 1, α 2 β 1, α 10 β 1 |
| Collagen | α 1 β 1, α 5 β 1, α 10 β 1, α 11 β 1, α X β 2 |
| OPN | α 4 β 7, α 4 β 1, α 9 β 1, α 8 β 1, α 5 β 1, α v β 1, α v β 6, α v β 3, α v β 75 |
Pathological condition
HPSE inhibits apoptosis viaHS-mediated signaling Autophagy regulation Inducing angiogenesis Activating invasion, metastasis, EMT and EC degradation Involving in exosome formation
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nappi, F. Structure and Function of the Extracellular Matrix in Normal and Pathological Conditions: Looking at the Bicuspid Aortic Valve. Int. J. Mol. Sci. 2025, 26, 10825. https://doi.org/10.3390/ijms262210825
Nappi F. Structure and Function of the Extracellular Matrix in Normal and Pathological Conditions: Looking at the Bicuspid Aortic Valve. International Journal of Molecular Sciences. 2025; 26(22):10825. https://doi.org/10.3390/ijms262210825
Chicago/Turabian StyleNappi, Francesco. 2025. "Structure and Function of the Extracellular Matrix in Normal and Pathological Conditions: Looking at the Bicuspid Aortic Valve" International Journal of Molecular Sciences 26, no. 22: 10825. https://doi.org/10.3390/ijms262210825
APA StyleNappi, F. (2025). Structure and Function of the Extracellular Matrix in Normal and Pathological Conditions: Looking at the Bicuspid Aortic Valve. International Journal of Molecular Sciences, 26(22), 10825. https://doi.org/10.3390/ijms262210825







