Urine Extracellular Vesicle miRNA Changes Induced by Vicadrostat with/Without Empagliflozin in Patients with Chronic Kidney Disease
Abstract
1. Introduction
2. Results
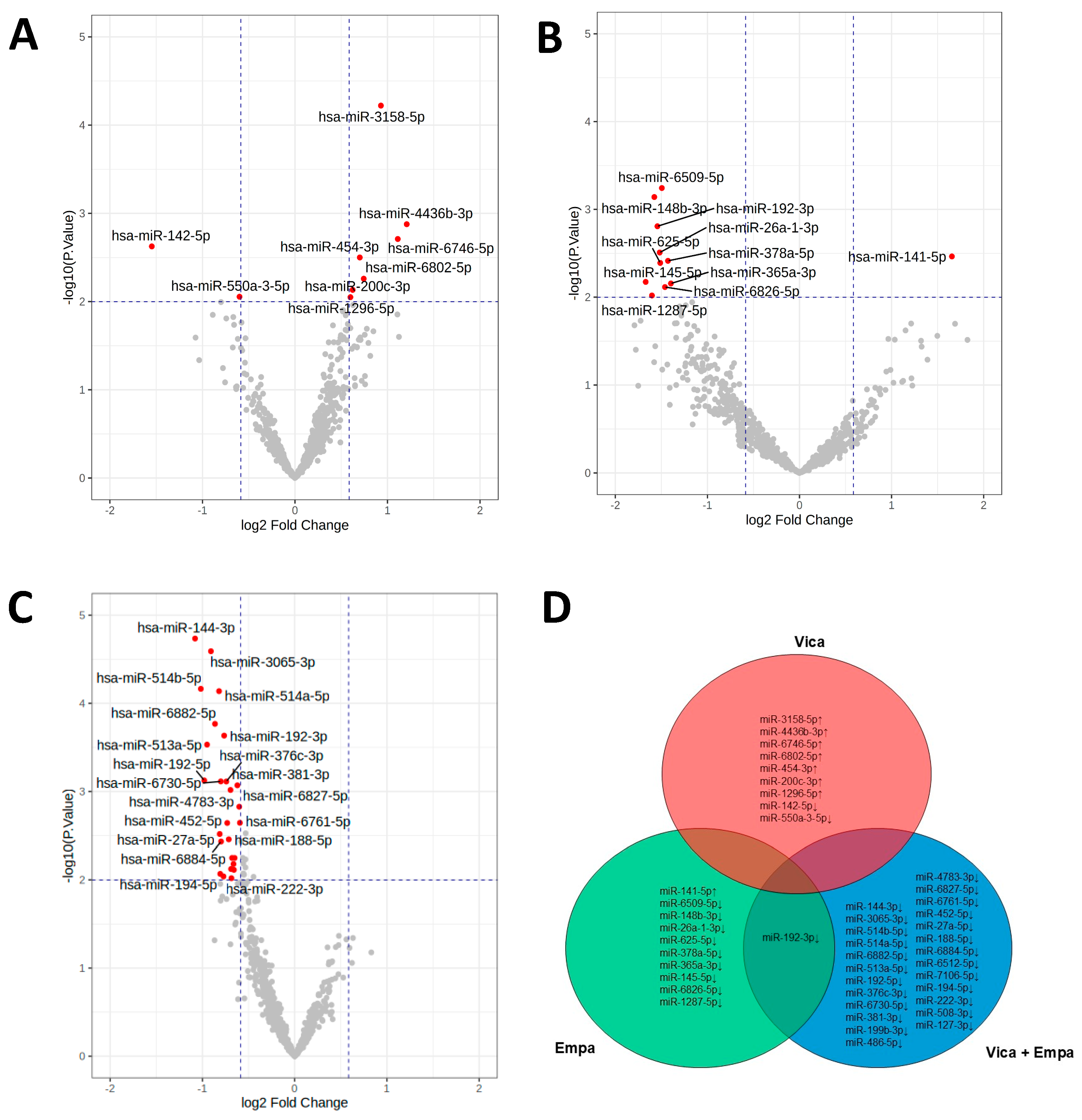
2.1. Effects of Vicadrostathigh, Empagliflozin, and Vicadrostathigh Plus Empagliflozin Resulted in Unique uEV miRNA Profiles
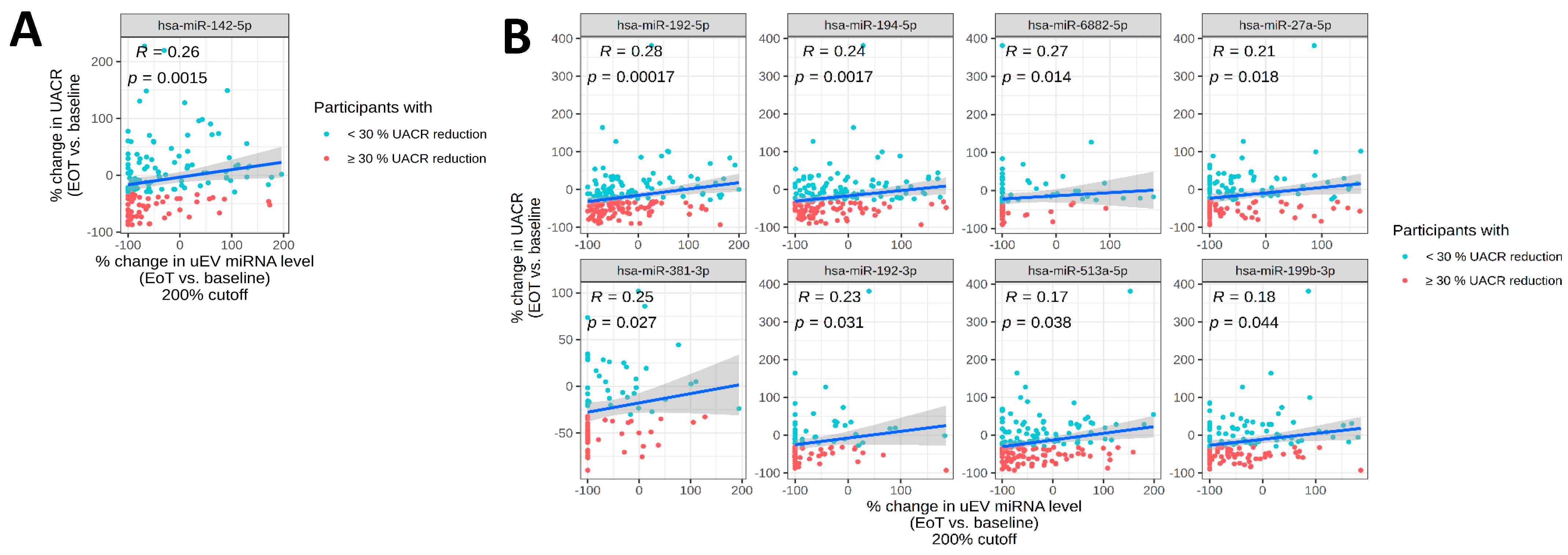
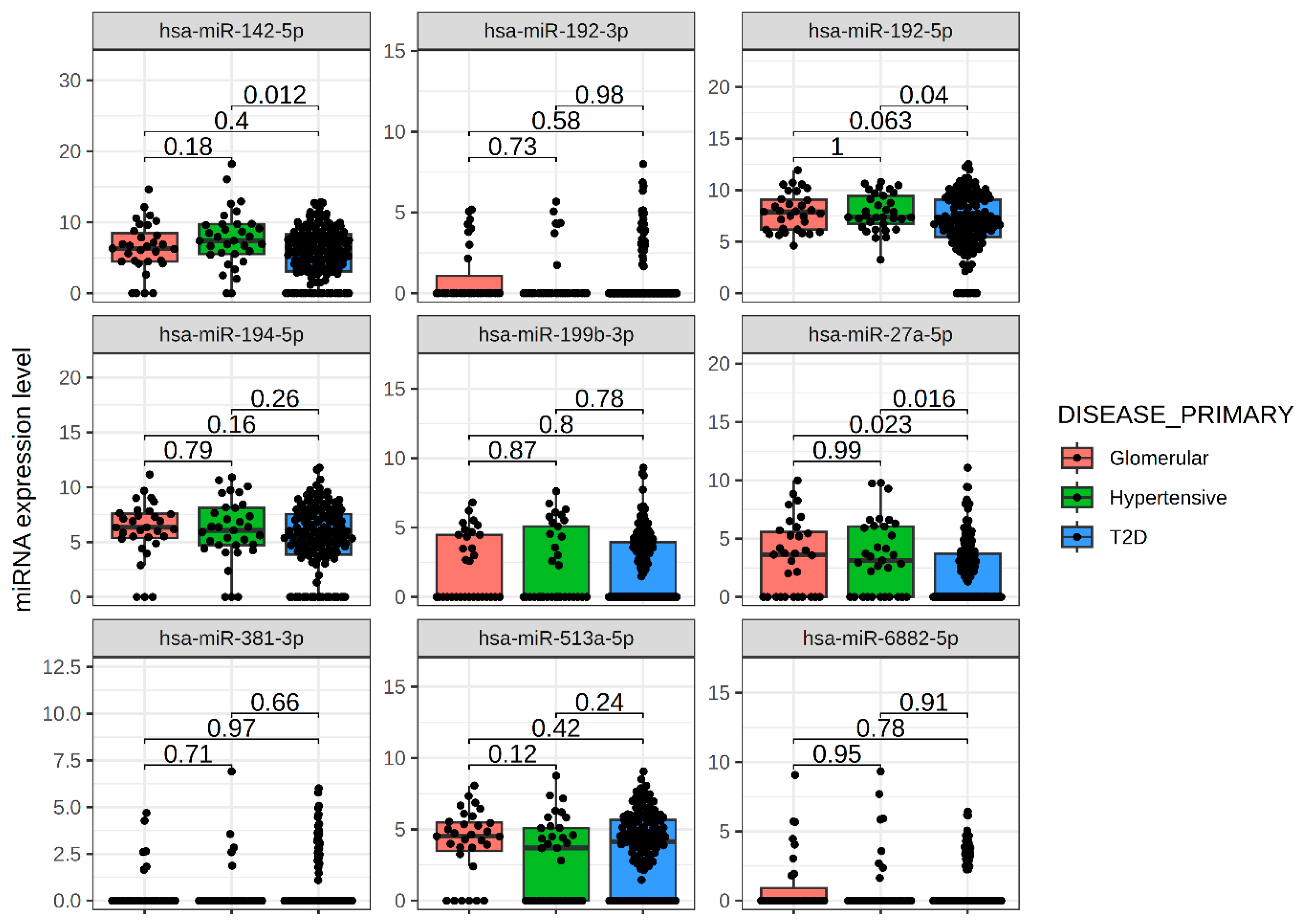
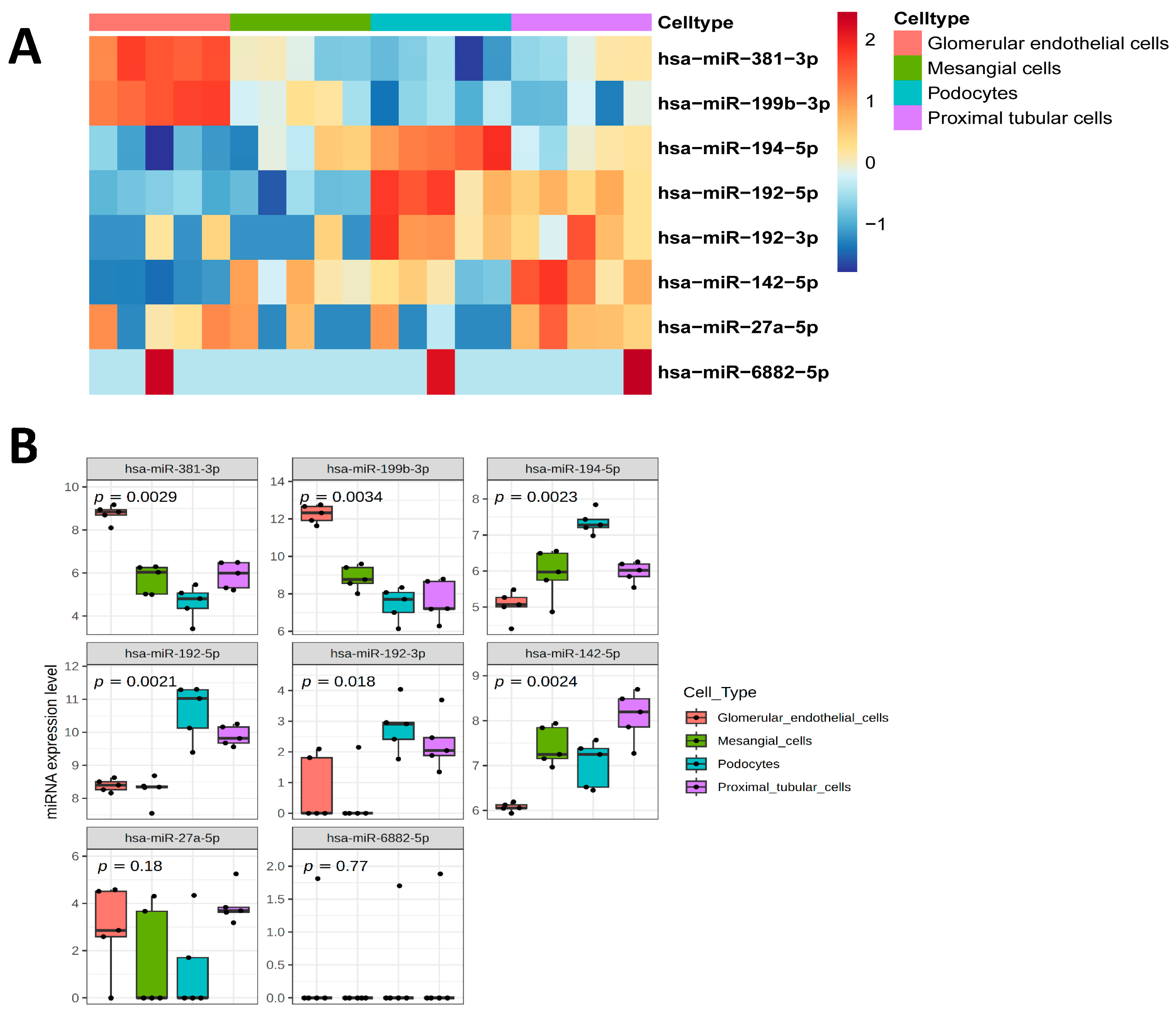
2.2. Changes in uEV miRNA Expression Profiles Associated with Albuminuria
2.3. Sustained Effects of Vicadrostat on uEV miRNAs Four Weeks Post-Treatment
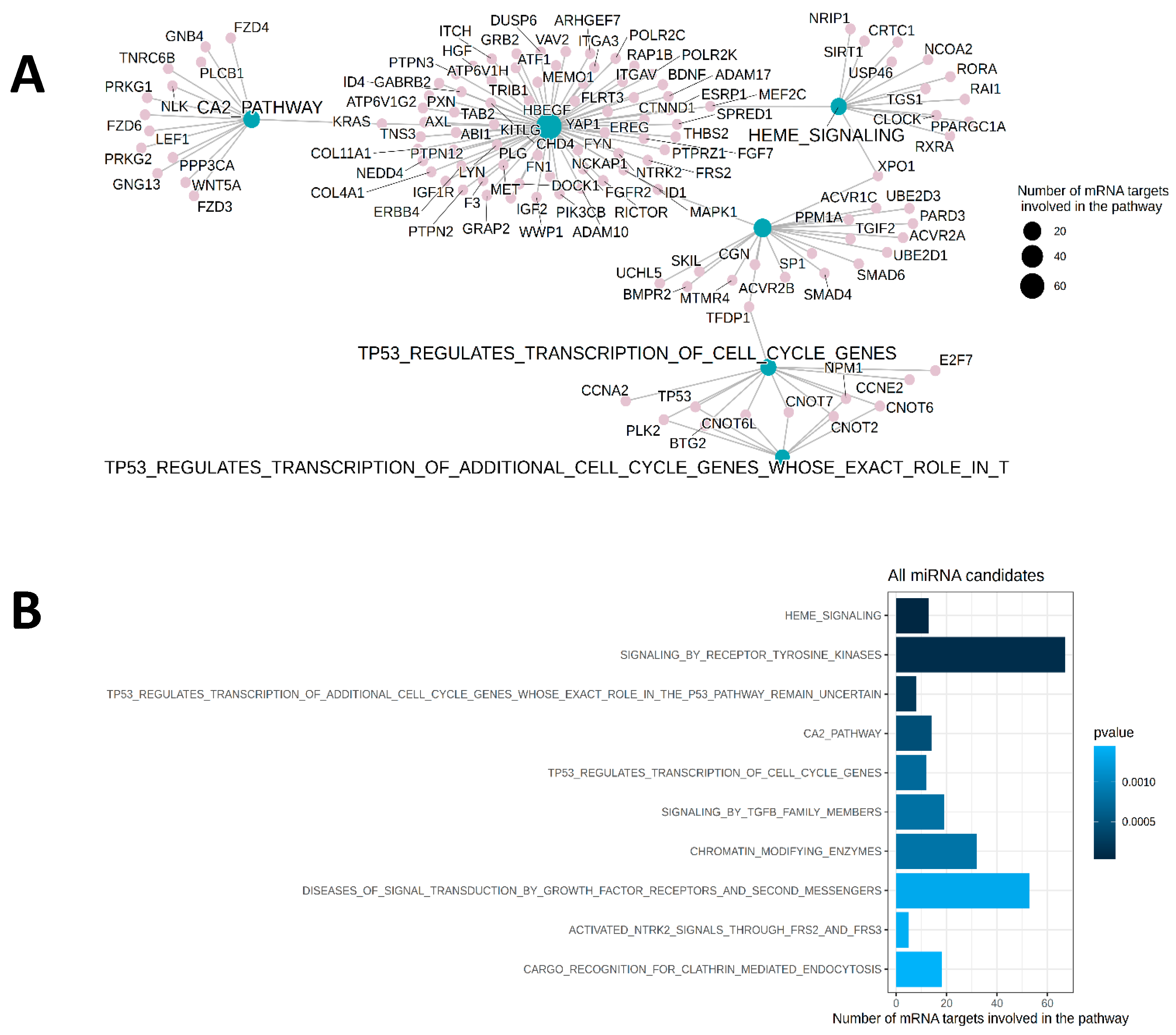
2.4. Functional Assessments of Treatment Effects
2.5. Correlations of uEV miRNA Expression with UACR and eGFR
3. Discussion
4. Materials and Methods
4.1. Clinical Trial
4.2. Urinary EV Small RNA Sequencing
4.3. Bioinfomatic Analysis
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xie, Y.; Bowe, B.; Mokdad, A.H.; Xian, H.; Yan, Y.; Li, T.; Maddukuri, G.; Tsai, C.Y.; Floyd, T.; Al-Aly, Z. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 2018, 94, 567–581. [Google Scholar] [CrossRef]
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Deng, L.; Guo, S.; Liu, Y.; Zhou, Y.; Liu, Y.; Zheng, X.; Yu, X.; Shuai, P. Global, regional, and national burden of chronic kidney disease and its underlying etiologies from 1990 to 2021: A systematic analysis for the Global Burden of Disease Study 2021. BMC Public Health 2025, 25, 636. [Google Scholar] [CrossRef]
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.W.; et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet 2018, 392, 2052–2090. [Google Scholar] [CrossRef] [PubMed]
- Francis, A.; Harhay, M.N.; Ong, A.C.M.; Tummalapalli, S.L.; Ortiz, A.; Fogo, A.B.; Fliser, D.; Roy-Chaudhury, P.; Fontana, M.; Nangaku, M.; et al. Chronic kidney disease and the global public health agenda: An international consensus. Nat. Rev. Nephrol. 2024, 20, 473–485. [Google Scholar] [CrossRef]
- Rossing, P.; Caramori, M.L.; Chan, J.C.N.; Heerspink, H.J.L.; Hurst, C.; Khunti, K.; Liew, A.; Michos, E.D.; Navaneethan, S.D.; Olowu, W.A.; et al. Executive summary of the KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease: An update based on rapidly emerging new evidence. Kidney Int. 2022, 102, 990–999. [Google Scholar] [CrossRef]
- Gilligan, S.; Raphael, K.L. Hyperkalemia and hypokalemia in CKD: Prevalence, risk factors, and clinical outcomes. Adv. Chronic Kidney Dis. 2017, 24, 315–318. [Google Scholar] [CrossRef]
- Rossignol, P.; Ruilope, L.M.; Cupisti, A.; Ketteler, M.; Wheeler, D.C.; Pignot, M.; Cornea, G.; Schulmann, T.; Lund, L.H. Recurrent hyperkalaemia management and use of renin-angiotensin-aldosterone system inhibitors: A European multi-national targeted chart review. Clin. Kidney J. 2020, 13, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Epstein, M.; Kovesdy, C.P.; Clase, C.M.; Sood, M.M.; Pecoits-Filho, R. Aldosterone, mineralocorticoid receptor activation, and CKD: A review of evolving treatment paradigms. Am. J. Kidney Dis. 2022, 80, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, K.R.; Hauske, S.J.; Canziani, M.E.; Caramori, M.L.; Cherney, D.; Cronin, L.; Heerspink, H.J.L.; Hugo, C.; Nangaku, M.; Rotter, R.C.; et al. Efficacy and safety of aldosterone synthase inhibition with and without empagliflozin for chronic kidney disease: A randomised, controlled, phase 2 trial. Lancet 2024, 403, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Agborbesong, E.; Bissler, J.; Li, X. Liquid Biopsy at the Frontier of Kidney Diseases: Application of Exosomes in Diagnostics and Therapeutics. Genes 2023, 14, 1367. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.S.; Taylor, J.; Valentine, H.R.; Irlam, J.J.; Eustace, A.; Hoskin, P.J.; Miller, C.J.; West, C.M. Enhanced stability of microRNA expression facilitates classification of FFPE tumour samples exhibiting near total mRNA degradation. Br. J. Cancer. 2012, 107, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Trionfini, P.; Benigni, A.; Remuzzi, G. MicroRNAs in kidney physiology and disease. Nat. Rev. Nephrol. 2015, 11, 23–33. [Google Scholar] [CrossRef]
- Liu, Z.; Fu, Y.; Yan, M.; Zhang, S.; Cai, J.; Chen, G.; Dong, Z. microRNAs in kidney diseases: Regulation, therapeutics, and biomarker potential. Pharmacol. Ther. 2024, 262, 108709. [Google Scholar] [CrossRef]
- Erdbrügger, U.; Le, T.H. Extracellular Vesicles in Renal Diseases: More than Novel Biomarkers? J. Am. Soc. Nephrol. 2016, 27, 12–26. [Google Scholar] [CrossRef]
- Ramezani, A.; Devaney, J.M.; Cohen, S.; Wing, M.R.; Scott, R.; Knoblach, S.; Singhal, R.; Howard, L.; Kopp, J.B.; Raj, D.S. Circulating and urinary microRNA profile in focal segmental glomerulosclerosis: A pilot study. Eur. J. Clin. Investig. 2015, 45, 394–404. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, S.; Ding, W.; Zhu, C.; Jiang, G.; Li, H. Recent Advances in miRNA Biomarkers for Diagnosis and Prognosis of Focal Segmental Glomerulosclerosis. Kidney Dis. 2025, 11, 283–291. [Google Scholar] [CrossRef]
- Barutta, F.; Tricarico, M.; Corbelli, A.; Annaratone, L.; Pinach, S.; Grimaldi, S.; Bruno, G.; Cimino, D.; Taverna, D.; Deregibus, M.C.; et al. Urinary exosomal micro-RNAs in incipient diabetic nephropathy. PLoS ONE 2013, 8, e73798. [Google Scholar] [CrossRef] [PubMed]
- Delić, D.; Eisele, C.; Schmid, R.; Baum, P.; Wiech, F.; Gerl, M.; Zimdahl, H.; Pullen, S.S.; Urquhart, R. Urinary Exosomal miRNA Signature in Type II Diabetic Nephropathy Patients. PLoS ONE 2016, 11, e0150154. [Google Scholar] [CrossRef]
- Lee, C.C.; Chen, C.C.; Hsu, C.K.; Chen, Y.T.; Chen, C.Y.; Yang, K.J.; Hung, M.J.; Wu, I.W. Urinary microRNA in Diabetic Kidney Disease: A Literature Review. Medicina 2023, 59, 354. [Google Scholar] [CrossRef]
- Barreiro, K.; Lay, A.C.; Leparc, G.; Tran, V.D.T.; Rosler, M.; Dayalan, L.; Burdet, F.; Ibberson, M.; Coward, R.J.M.; Huber, T.B.; et al. An in vitro approach to understand contribution of kidney cells to human urinary extracellular vesicles. J. Extracell. Vesicles 2023, 12, e12304. [Google Scholar] [CrossRef]
- Tuttle, K.R.; Rossing, P.; Hauske, S.J.; Cronin, L.; Hussain, J.; de Zeeuw, D.; Heerspink, H.J.L. Methods Article for a Study Protocol: Study Design and Baseline Characteristics for Aldosterone Synthase Inhibition in Chronic Kidney Disease. Am. J. Nephrol. 2024, 55, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.J.; Yao, Y.; Cai, X.Y.; Fang, G.Y. Emerging Role of MiR-192-5p in Human Diseases. Front. Pharmacol. 2021, 12, 614068. [Google Scholar] [CrossRef] [PubMed]
- Puppo, M.; Bucci, G.; Rossi, M.; Giovarelli, M.; Bordo, D.; Moshiri, A.; Gorlero, F.; Gherzi, R.; Briata, P. miRNA-Mediated KHSRP Silencing Rewires Distinct Post-transcriptional Programs during TGF-β-Induced Epithelial-to-Mesenchymal Transition. Cell Rep. 2016, 16, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Guan, M.; Zheng, Z.; Zhang, Q.; Tang, C.; Xu, W.; Xiao, Z.; Wang, L.; Xue, Y. miRNAs in Urine Extracellular Vesicles as Predictors of Early-Stage Diabetic Nephropathy. J. Diabetes Res. 2016, 2016, 7932765. [Google Scholar] [CrossRef]
- Jenkins, R.H.; Martin, J.; Phillips, A.O.; Bowen, T.; Fraser, D.J. Transforming growth factor β1 represses proximal tubular cell microRNA-192 expression through decreased hepatocyte nuclear factor DNA binding. Biochem. J. 2012, 443, 407–416. [Google Scholar] [CrossRef]
- Zhang, Y.; Qian, B.; Yang, Y.; Niu, F.; Lin, C.; Yuan, H.; Wang, J.; Wu, T.; Shao, Y.; Shao, S.; et al. Visceral Adipocyte-Derived Extracellular Vesicle miR-27a-5p Elicits Glucose Intolerance by Inhibiting Pancreatic β-Cell Insulin Secretion. Diabetes. 2024, 73, 1832–1847. [Google Scholar] [CrossRef]
- Romay, M.C.; Che, N.; Becker, S.N.; Pouldar, D.; Hagopian, R.; Xiao, X.; Lusis, A.J.; Berliner, J.A.; Civelek, M. Regulation of NF-κB signaling by oxidized glycerophospholipid and IL-1β induced miRs-21-3p and -27a-5p in human aortic endothelial cells. J. Lipid Res. 2015, 56, 38–50. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, Y.; Ran, Q.; Yao, Y.; Zhang, H.; Ju, J.; Yang, T.; Zhang, W.; Yu, X.; He, S. MicroRNA-381-3p Functions as a Dual Suppressor of Apoptosis and Necroptosis and Promotes Proliferation of Renal Cancer Cells. Front. Cell. Dev. Biol. 2020, 8, 290. [Google Scholar] [CrossRef]
- Zhou, G.; Zhang, X.; Wang, W.; Zhang, W.; Wang, H.; Xin, G. Both Peripheral Blood and Urinary miR-195-5p, miR-192-3p, miR-328-5p and Their Target Genes PPM1A, RAB1A and BRSK1 May Be Potential Biomarkers for Membranous Nephropathy. Med. Sci. Monit. 2019, 25, 1903–1916. [Google Scholar] [CrossRef]
- Ali, H.; Malik, M.Z.; Abu-Farha, M.; Abubaker, J.; Cherian, P.; Nizam, R.; Jacob, S.; Bahbahani, Y.; Naim, M.; Ahmad, S.; et al. Global analysis of urinary extracellular vesicle small RNAs in autosomal dominant polycystic kidney disease. J. Gene Med. 2024, 26, e3674. [Google Scholar] [CrossRef]
- Miao, X.; Tian, Y.; Wu, L.; Zhao, H.; Liu, J.; Gao, F.; Zhang, W.; Liu, Q.; Guo, H.; Yang, L.; et al. CircRTN4 aggravates mesangial cell dysfunction by activating the miR-513a-5p/FN axis in lupus nephritis. Lab. Investig. 2022, 102, 966–978. [Google Scholar] [CrossRef] [PubMed]
- Gholaminejad, A.; Abdul Tehrani, H.; Gholami Fesharaki, M. Identification of candidate microRNA biomarkers in renal fibrosis: A meta-analysis of profiling studies. Biomarkers 2018, 23, 713–724. [Google Scholar] [CrossRef]
- Su, S.; Zhao, Q.; He, C.; Huang, D.; Liu, J.; Chen, F.; Chen, J.; Liao, J.Y.; Cui, X.; Zeng, Y.; et al. miR-142-5p and miR-130a-3p are regulated by IL-4 and IL-13 and control profibrogenic macrophage program. Nat. Commun. 2015, 6, 8523. [Google Scholar] [CrossRef] [PubMed]
- Talebi, F.; Ghorbani, S.; Chan, W.F.; Boghozian, R.; Masoumi, F.; Ghasemi, S.; Vojgani, M.; Power, C.; Noorbakhsh, F. MicroRNA-142 regulates inflammation and T cell differentiation in an animal model of multiple sclerosis. J. Neuroinflammation 2017, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Mahtal, N.; Lenoir, O.; Tinel, C.; Anglicheau, D.; Tharaux, P.L. MicroRNAs in kidney injury and disease. Nat. Rev. Nephrol. 2022, 18, 643–662. [Google Scholar] [CrossRef]
- Garmaa, G.; Nagy, R.; Kói, T.; Uyen Nguyen, D.T.; Gergő, D.; Kleiner, D.; Csupor, D.; Hegyi, P.; Kökény, G. Panel miRNAs are potential diagnostic markers for chronic kidney diseases: A systematic review and meta-analysis. BMC Nephrol. 2024, 25, 261. [Google Scholar] [CrossRef]
- Deshpande, S.D.; Putta, S.; Wang, M.; Lai, J.Y.; Bitzer, M.; Nelson, R.G.; Lanting, L.L.; Kato, M.; Natarajan, R. Transforming growth factor-β-induced cross talk between p53 and a microRNA in the pathogenesis of diabetic nephropathy. Diabetes. 2013, 62, 3151–3162. [Google Scholar] [CrossRef]
- Lei, L.; Xiang, Y.X.; Luo, M.L.; Zhang, Z.Y.; Wu, H.W.; Tang, C.; Cui, T.J.; Zhang, X.M.; Wang, X.H.; Delic, D.; et al. Intercellular Communication Network of CellChat Uncovers Mechanisms of Kidney Fibrosis Based on Single-Cell RNA Sequencing. Kidney Blood Press. Res. 2025, 50, 276–299. [Google Scholar] [CrossRef]
- Chung, A.C.; Huang, X.R.; Meng, X.; Lan, H.Y. miR-192 mediates TGF-beta/Smad3-driven renal fibrosis. J. Am. Soc. Nephrol. 2010, 21, 1317–1325. [Google Scholar] [CrossRef]
- Putta, S.; Lanting, L.; Sun, G.; Lawson, G.; Kato, M.; Natarajan, R. Inhibiting microRNA-192 ameliorates renal fibrosis in diabetic nephropathy. J. Am. Soc. Nephrol. 2012, 23, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wang, C.; Yu, H.; Ding, M.; Zhang, C.; Lu, X.; Zhang, C.Y.; Zhang, C. Increased urinary exosomal microRNAs in children with idiopathic nephrotic syndrome. EBioMedicine 2019, 39, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lan, M.; Chen, Y. Minimal Information for Studies of Extracellular Vesicles (MISEV): Ten-Year Evolution (2014–2023). Pharmaceutics 2024, 16, 1394. [Google Scholar] [CrossRef]
- Enderle, D.; Spiel, A.; Coticchia, C.M.; Berghoff, E.; Mueller, R.; Schlumpberger, M.; Sprenger-Haussels, M.; Shaffer, J.M.; Lader, E.; Skog, J.; et al. Characterization of RNA from Exosomes and Other Extracellular Vesicles Isolated by a Novel Spin Column-Based Method. PLoS ONE 2015, 10, e0136133. [Google Scholar] [CrossRef]
- Buschmann, D.; Kirchner, B.; Hermann, S.; Märte, M.; Wurmser, C.; Brandes, F.; Kotschote, S.; Bonin, M.; Steinlein, O.K.; Pfaffl, M.W.; et al. Evaluation of serum extracellular vesicle isolation methods for profiling miRNAs by next-generation sequencing. J. Extracell. Vesicles 2018, 7, 1481321. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010, 11, R25. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, X. miRDB: An online database for prediction of functional microRNA targets. Nucleic Acids Res. 2020, 48(D1), D127–D131. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, X. Prediction of functional microRNA targets by integrative modeling of microRNA binding and target expression data. Genome Biol. 2019, 20, 18. [Google Scholar] [CrossRef]

| Vicahigh | Vicalow | Empa | Vicahigh + Empa | Vicalow + Empa | Placebo | |
|---|---|---|---|---|---|---|
| n = 99 | n = 59 | n = 54 | n = 101 | n = 62 | n = 62 | |
| Gender | ||||||
| Female | 35 (35%) | 15 (25%) | 23 (43%) | 32 (32%) | 21 (34%) | 17 (27%) |
| Male | 64 (65%) | 44 (75%) | 31 (57%) | 69 (68%) | 41 (66%) | 45 (73%) |
| Age (years) | 65 (10) | 65 (11) | 65 (10) | 61 (13) | 66 (11) | 62 (12) |
| Ethnicity/Race | ||||||
| Asian | 25 (25%) | 20 (34%) | 13 (24%) | 38 (38%) | 14 (23%) | 12 (19%) |
| Black or African American | 14 (14%) | 3 (5.1%) | 9 (17%) | 8 (7.9%) | 6 (9.7%) | 9 (15%) |
| White Other/Mixed | 56 (57%) 4 (4.0%) | 33 (56%) 3 (5.1%) | 31 (57%) 1 (1.9%) | 52 (51%) 3 (3.0%) | 41 (66%) 1 (1.6%) | 38 (61%) 3 (4.8%) |
| Diabetes | ||||||
| Yes | 64 (65%) | 41 (69%) | 38 (70%) | 65 (64%) | 54 (87%) | 42 (68%) |
| No | 35 (35%) | 18 (31%) | 16 (30%) | 36 (36%) | 8 (13%) | 20 (32%) |
| BMI (kg/m2) | 29.9 (4.7) | 30.0 (4.9) | 29.5 (5.5) | 29.4 (5.5) | 30.1 (5.7) | 30.5 (6.2) |
| eGFR (mL/min/1.73 m2) | 55 (17) | 53 (16) | 49 (18) | 52 (19) | 50 (17) | 56 (19) |
| UACR (mg/g) | 615 (593) | 848 (1,350) | 690 (810) | 759 (897) | 770 (843) | 715 (747) |
| SBP (mmHg) | 137 (16) | 135 (20) | 133 (13) | 134 (15) | 135 (16) | 134 (16) |
| DBP (mmHg) | 77 (9) | 78 (9) | 77 (9) | 77 (9) | 76 (9) | 81 (10) |
| Serum potassium (mmol/L) | 4.29 (0.51) | 4.24 (0.39) | 4.23 (0.41) | 4.28 (0.36) | 4.35 (0.40) | 4.29 (0.39) |
| Serum aldosterone (pmol/L) Participants with | 174 (236) | 195 (181) | 160 (132) | 148 (133) | 163 (125) | 175 (134) |
| <30% reduction | 49 (49%) | 40 (68%) | 40 (68%) | 38 (38%) | 40 (65%) | 53 (85%) |
| ≥30% reduction | 49 (49%) | 19 (32%) | 19 (32%) | 63 (62%) | 21 (34%) | 9 (15%) |
| Unknown | 1 (1.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1.6%) | 0 (0%) |
| miRNA | EoT/Baseline (Vicahigh/Empa) | p-Value | EoT/Baseline (Vicahigh) | p-Value | Correlation with UACR (p-Value) |
|---|---|---|---|---|---|
| miR-142-5p | −1.83 | 0.048 | −2.92 | 0.002 | 0.26 (0.001) |
| miR-192-5p | −1.97 | 0.001 | −1.03 | 0.906 | 0.28 (<0.001) |
| miR-194-5p | −1.71 | 0.009 | −1.34 | 0.246 | 0.24 (0.002) |
| miR-27a-5p | −1.76 | 0.003 | −1.37 | 0.134 | 0.21 (0.018) |
| miR-381-3p | −1.54 | 0.001 | 1.08 | 0.551 | 0.25 (0.027) |
| miR-192-3p | −1.70 | 0.000 | −1.07 | 0.647 | 0.23 (0.031) |
| miR-199b-3p | −1.58 | 0.008 | −1.39 | 0.076 | 0.18 (0.044) |
| miR-513a-5p | −1.93 | 0.000 | 1.25 | 0.283 | 0.17 (0.038) |
| miR-6882-5p | −1.82 | 0.000 | 1.09 | 0.619 | 0.27 (0.014) |
| miRNA | Functions | References |
|---|---|---|
| miR-192-5p | Key role in transforming growth factor beta (TGFβ) signaling pathway. Reduced expression can be linked to reduced extracellular matrix proliferation and attenuated epithelial–mesenchymal transformation. Increased expression in patients with albuminuria. | [23,24,25] |
| miR-194-5p | Involved in TGFβ signaling pathway; reduced expression in patients with albuminuria. | [26] |
| miR-27a-5p | Increased miR-27a-5p in the pancreatic islets of genetic and dietary mouse models of obesity is mainly derived from visceral adipocyte-secreted EVs and serves as a pathogenic factor driving β-cell insulin secretion injury; involved in regulation of nuclear factor kappa B (NF-kB) signaling. | [27,28] |
| miR-381-3p | Functions as a dual suppressor of apoptosis and necroptosis and promotes proliferation of kidney cancer cells. | [29] |
| miR-192-3p | Increased level in urinary sediment obtained from membranous nephropathy compared to healthy controls by participating in inflammation and apoptosis. | [30] |
| miR-199b-3p | Significantly upregulated in ADPKD (Autosomal dominant polycystic kidney disease) patient urine extracellular vesicles. | [31] |
| miR-513a-5p | CircRTN4 (circular RNA derived from exon 4 and 5 of the Reticulon 4 (RTN4) mRNA) exacerbates mesangial cell dysfunction by activating the miR-513a-5p/FN (fibronectin) axis in lupus nephritis. | [32] |
| miR-6882-5p | Unknown. | |
| miR-142-5p | Increased expression in kidney fibrosis (results obtained from a meta-analysis). miR-142-5p is regulated by IL-4 and IL-13 and controls profibrogenic macrophage program; miR-142a-5p overexpression in activated lymphocytes shifts the pattern of T cell differentiation towards Th1 cells. | [33,34,35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delic, D.; Gashaw, I.; Duran-Fernandez, I.; Cronin, L.; Hauske, S.J.; Rossing, P.; Tuttle, K.R. Urine Extracellular Vesicle miRNA Changes Induced by Vicadrostat with/Without Empagliflozin in Patients with Chronic Kidney Disease. Int. J. Mol. Sci. 2025, 26, 10810. https://doi.org/10.3390/ijms262210810
Delic D, Gashaw I, Duran-Fernandez I, Cronin L, Hauske SJ, Rossing P, Tuttle KR. Urine Extracellular Vesicle miRNA Changes Induced by Vicadrostat with/Without Empagliflozin in Patients with Chronic Kidney Disease. International Journal of Molecular Sciences. 2025; 26(22):10810. https://doi.org/10.3390/ijms262210810
Chicago/Turabian StyleDelic, Denis, Isabella Gashaw, Ileana Duran-Fernandez, Lisa Cronin, Sibylle J. Hauske, Peter Rossing, and Katherine R. Tuttle. 2025. "Urine Extracellular Vesicle miRNA Changes Induced by Vicadrostat with/Without Empagliflozin in Patients with Chronic Kidney Disease" International Journal of Molecular Sciences 26, no. 22: 10810. https://doi.org/10.3390/ijms262210810
APA StyleDelic, D., Gashaw, I., Duran-Fernandez, I., Cronin, L., Hauske, S. J., Rossing, P., & Tuttle, K. R. (2025). Urine Extracellular Vesicle miRNA Changes Induced by Vicadrostat with/Without Empagliflozin in Patients with Chronic Kidney Disease. International Journal of Molecular Sciences, 26(22), 10810. https://doi.org/10.3390/ijms262210810








