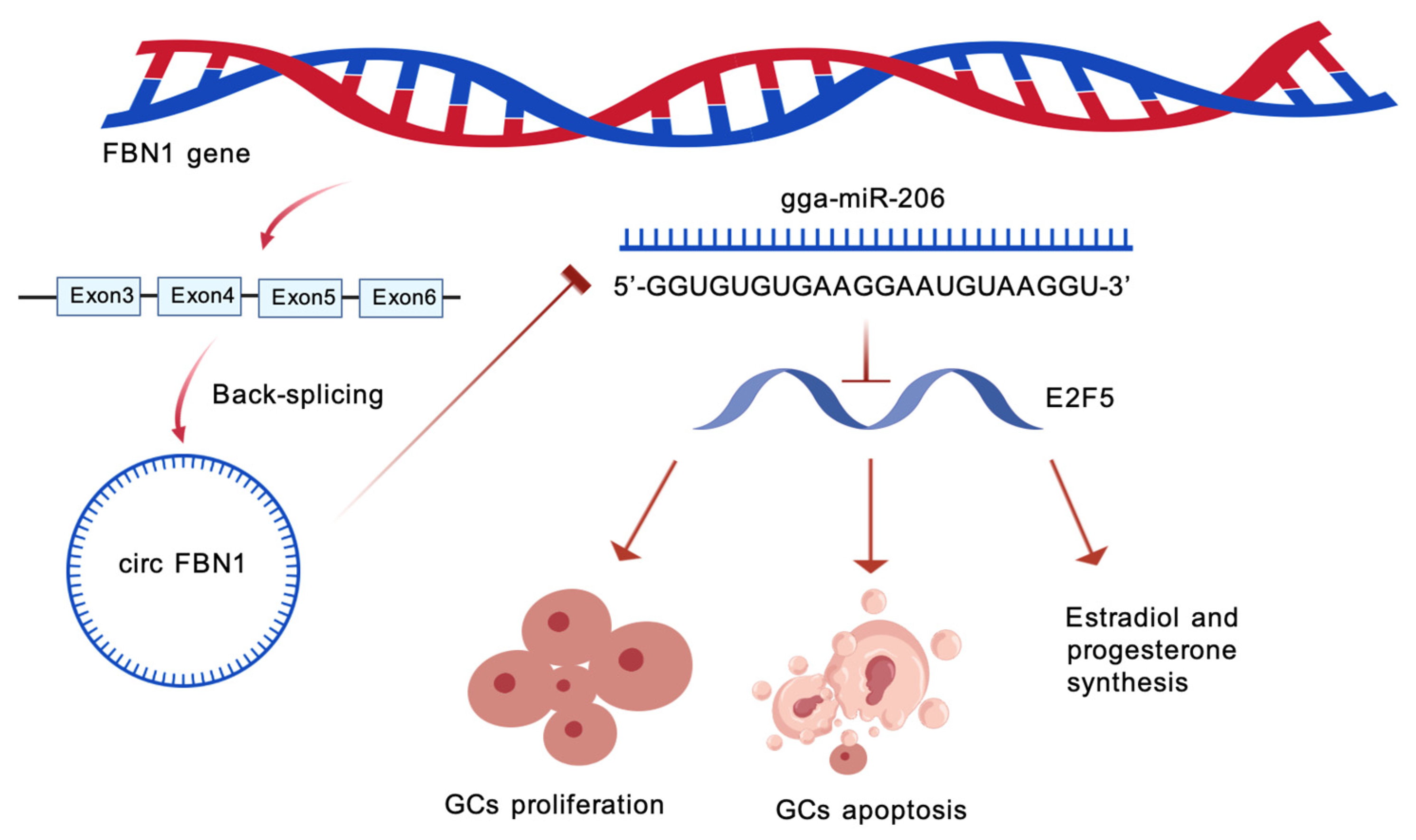
circ_0000132 Regulates Chicken Granulosa Cell Proliferation Apoptosis and E2/P4 Synthesis via miR-206 E2F5 Signaling
Abstract
1. Introduction
2. Results
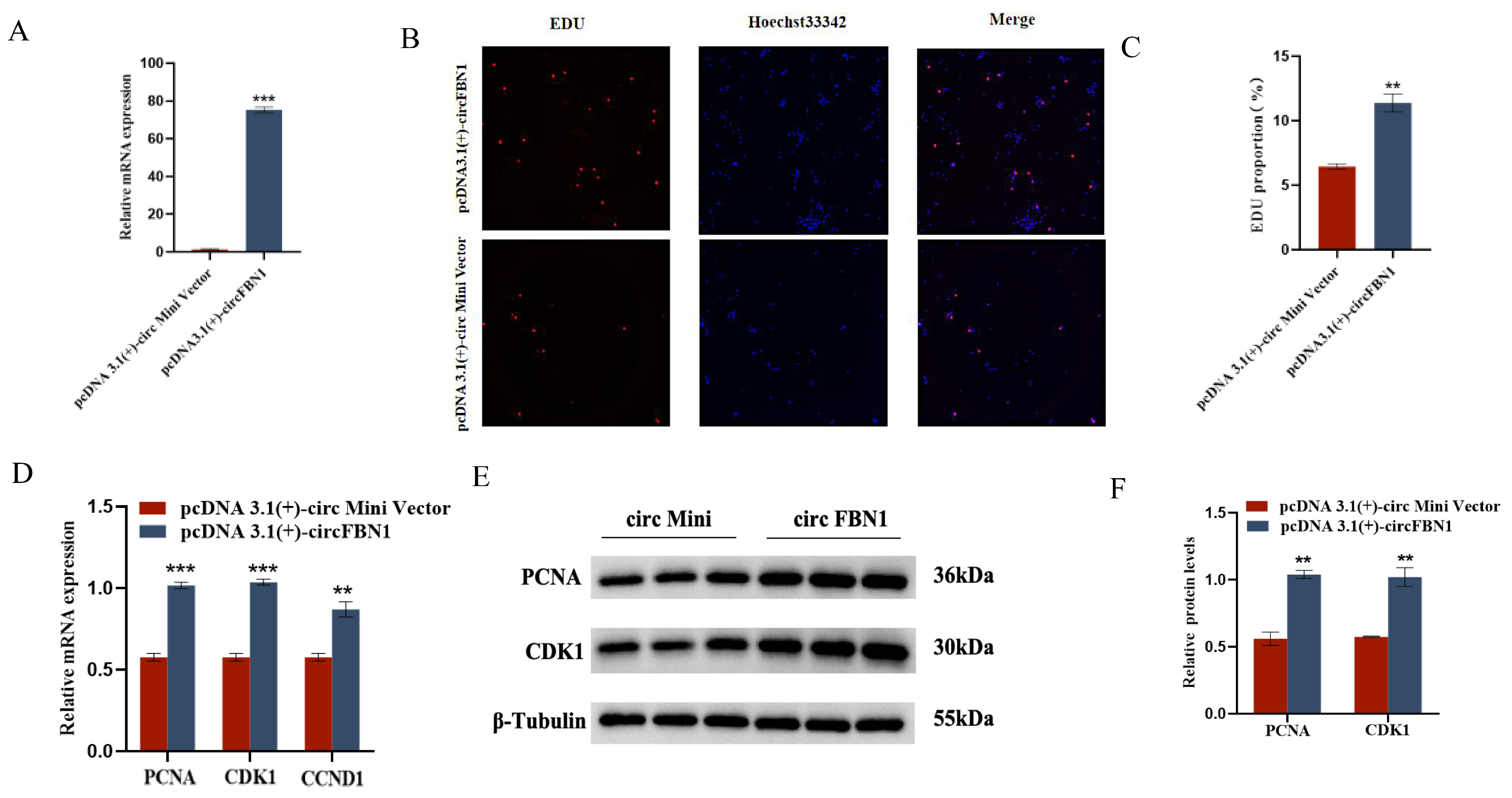
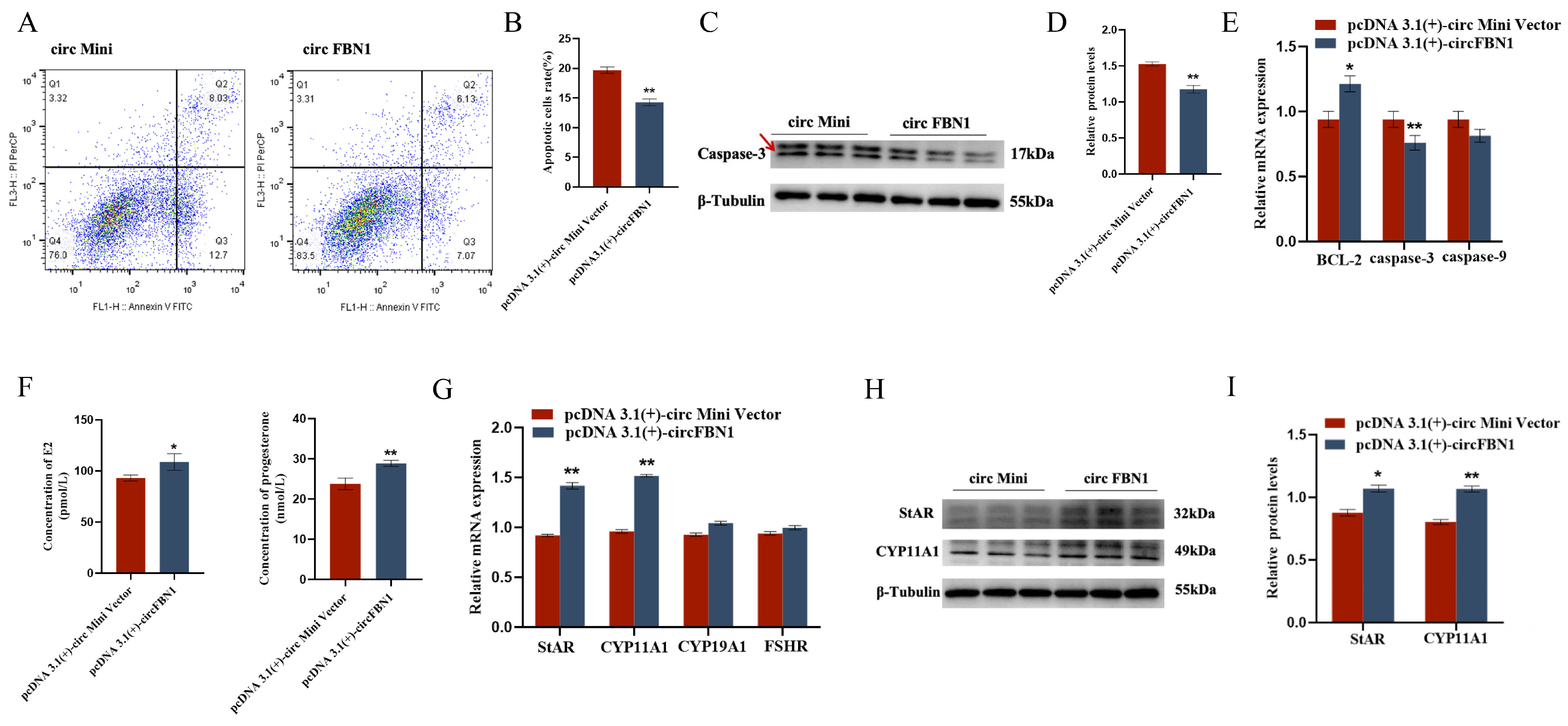
2.1. circ_0000132 Affects GC Proliferation, Apoptosis, and Steroid Synthesis and Secretion
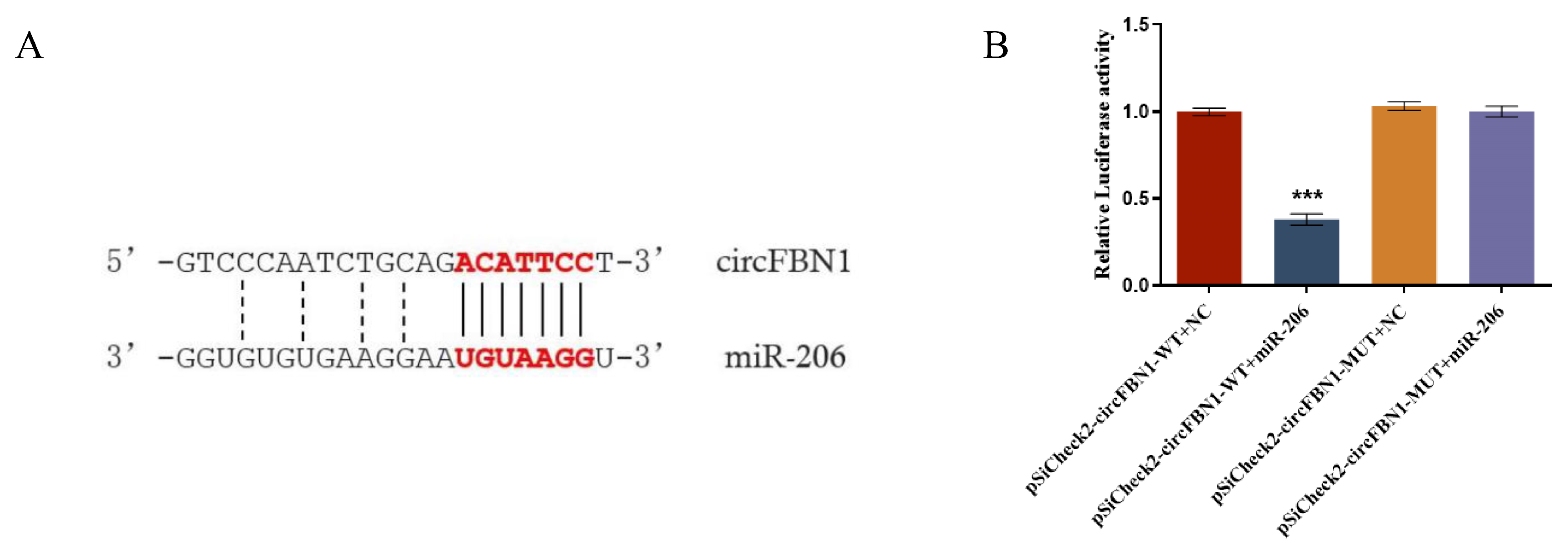
2.2. circ_0000132 Acted as a Sponge of gga-miR-206
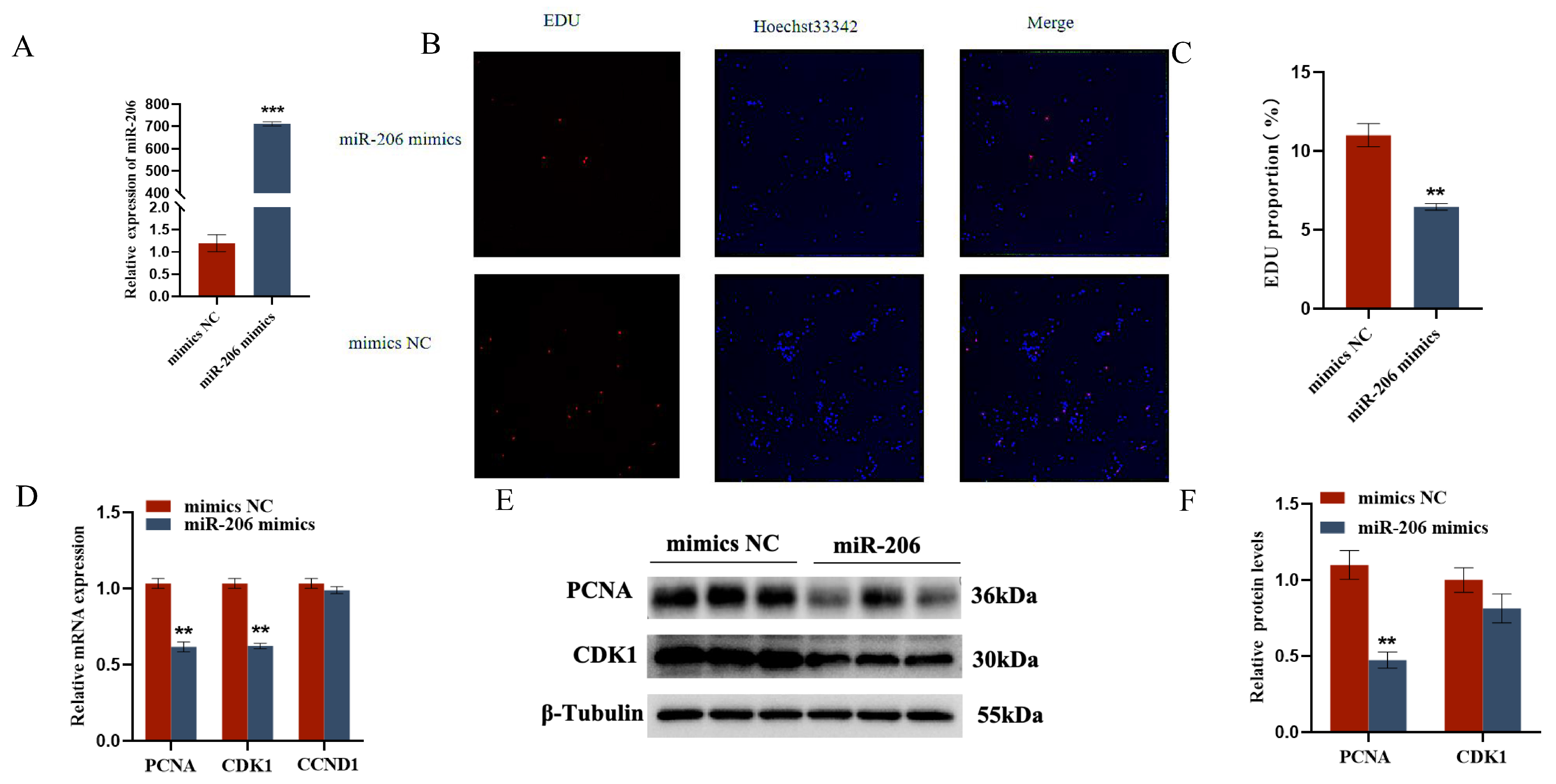
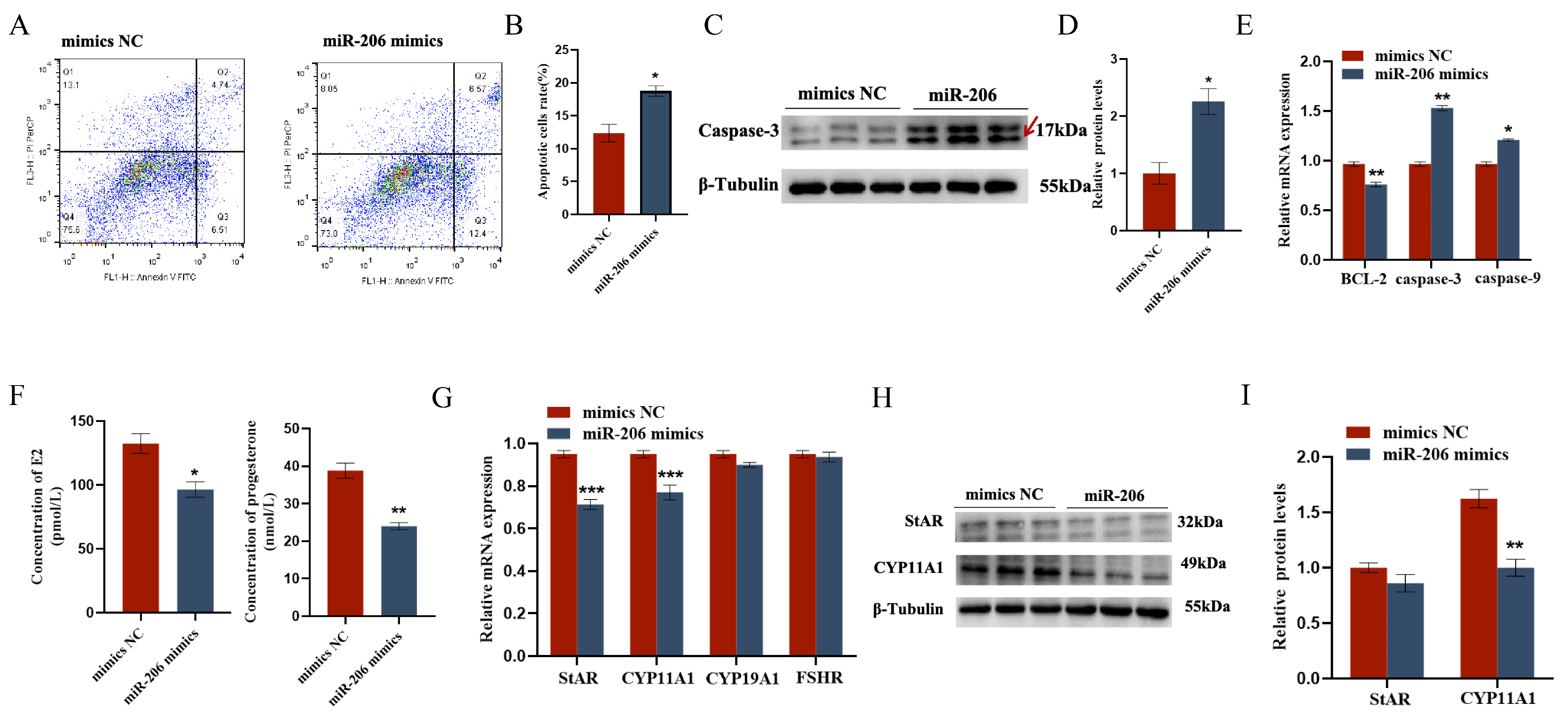
2.3. gga-miR-206 Affects GC Proliferation, Apoptosis, and Steroid Synthesis and Secretion
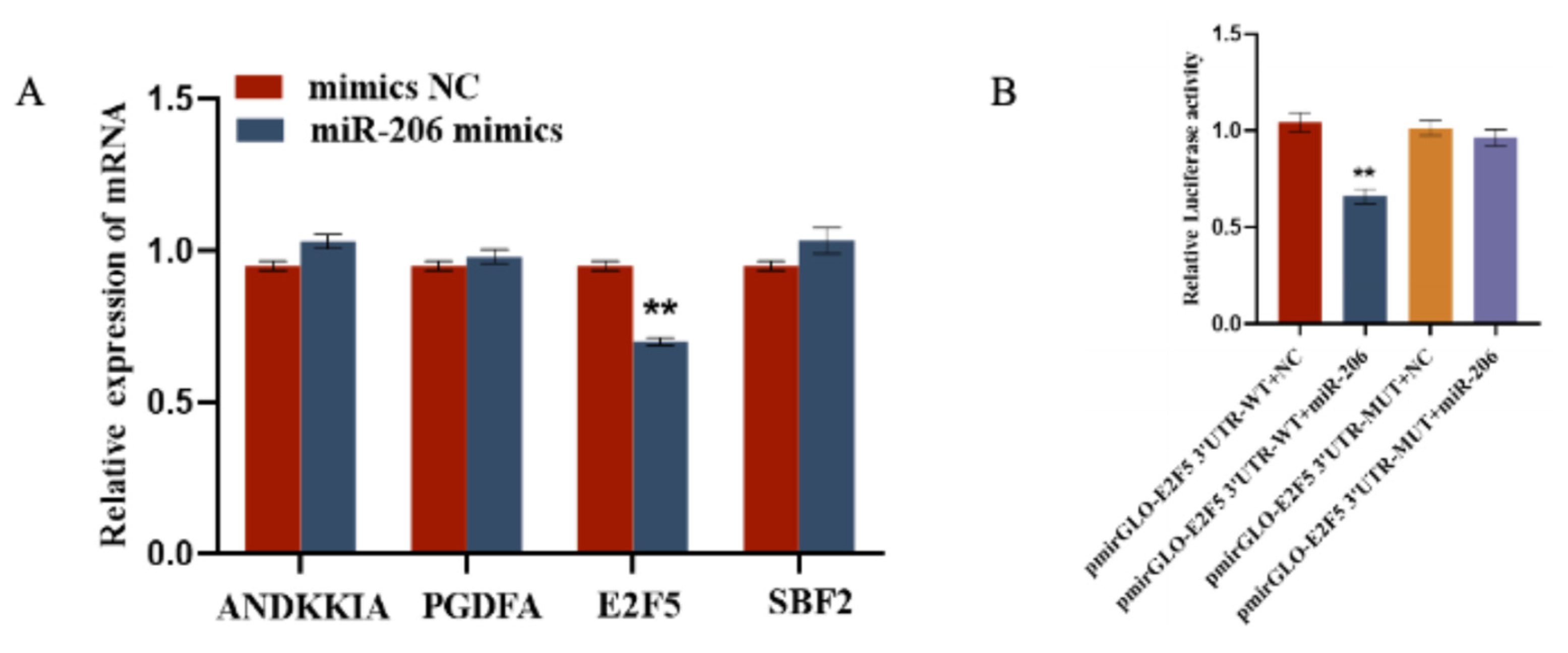
2.4. gga-miR-206 Targets the E2F5 Gene
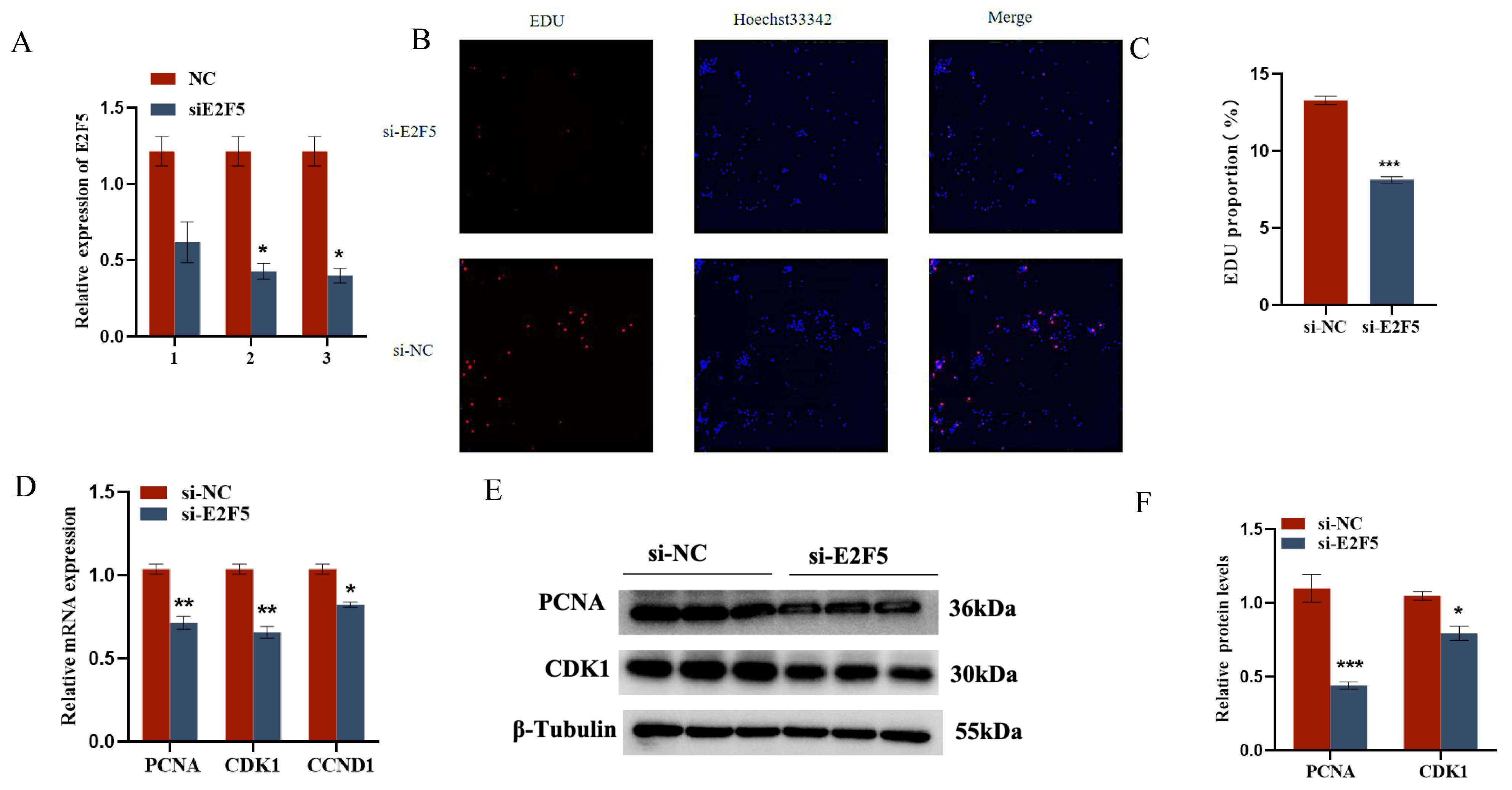
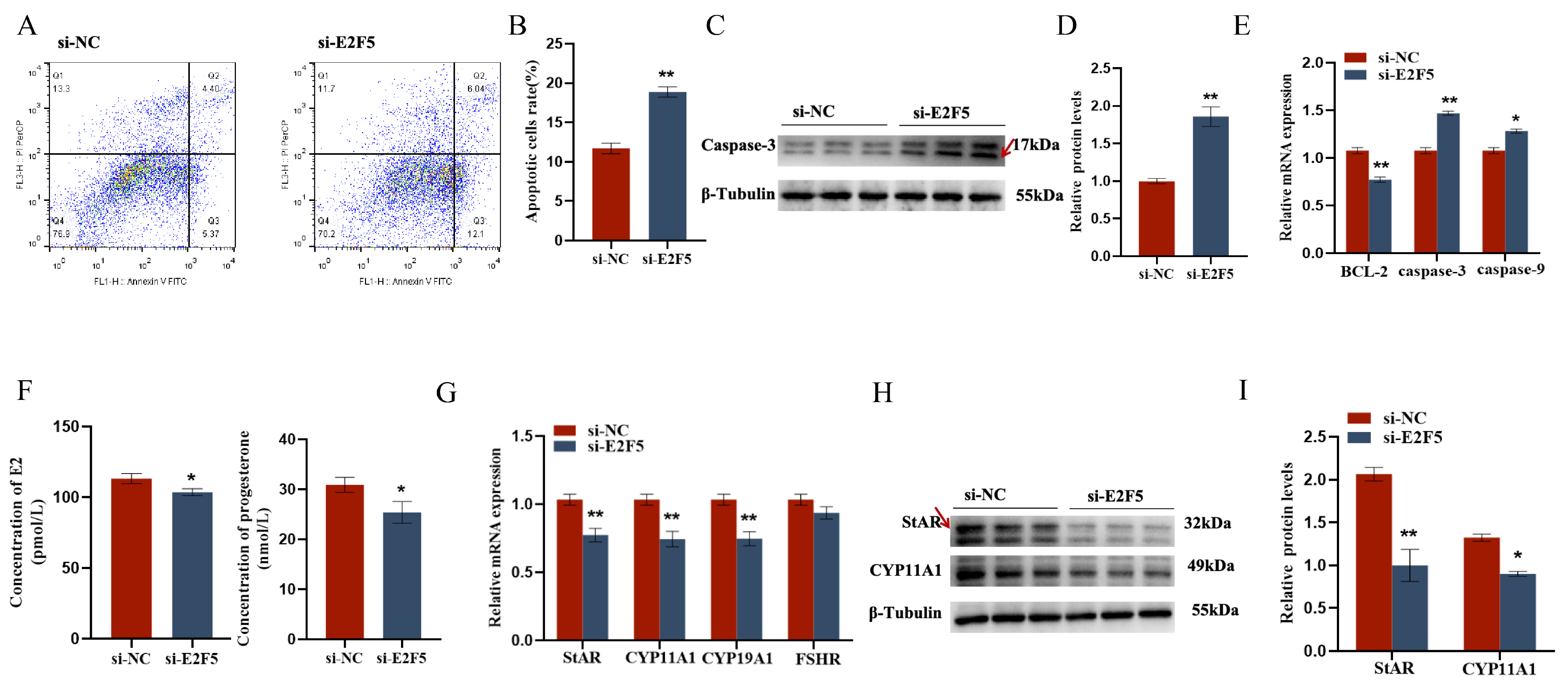
2.5. E2F5 Affects GC Proliferation, Apoptosis, and Steroid Synthesis and Secretion
3. Discussion
4. Materials and Methods
4.1. Chicken Follicle Harvesting
4.2. CircFBN1 Validation and qRT-PCR
4.3. Plasmid Construction and Dual-Luciferase Reporter Assay
4.4. GC Isolation and Culture
4.5. Cell Transfection
4.6. Cell Proliferation Assay
4.7. Cell Apoptosis Assay
4.8. Western Blot
4.9. ELISA Assay for Steroid Hormones
4.10. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sechman, A. The role of thyroid hormones in regulation of chicken ovarian steroidogenesis. Gen. Comp. Endocrinol. 2013, 190, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Flaws, J.A.; Abbud, R.; Mann, R.J.; Nilson, J.H.; Hirshfield, A.N. Chronically elevated luteinizing hormone depletes primordial follicles in the mouse ovary. Biol. Reprod. 1997, 57, 1233–1237. [Google Scholar] [CrossRef]
- Glister, C.; Groome, N.P.; Knight, P.G. Oocyte-mediated suppression of follicle-stimulating hormone- and insulin-like growth factor-induced secretion of steroids and inhibin-related proteins by bovine granulosa cells in vitro: Possible role of transforming growth factor alpha. Biol. Reprod. 2003, 68, 758–765. [Google Scholar] [CrossRef]
- Haughian, J.M.; Ginther, O.J.; Diaz, F.J.; Wiltbank, M.C. Gonadotropin-releasing hormone, estradiol, and inhibin regulation of follicle-stimulating hormone and luteinizing hormone surges: Implications for follicle emergence and selection in heifers. Biol. Reprod. 2013, 88, 165. [Google Scholar] [CrossRef]
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA circles function as efficient microRNA sponges. Nature 2013, 495, 384–388. [Google Scholar] [CrossRef]
- Jiang, X.; Peng, M.; Liu, Q.; Peng, Q.; Oyang, L.; Li, S.; Xu, X.; Shen, M.; Wang, J.; Li, H.; et al. Circular RNA hsa_circ_0000467 promotes colorectal cancer progression by promoting eIF4A3-mediated c-Myc translation. Mol. Cancer 2024, 23, 151. [Google Scholar] [CrossRef]
- Lu, T.; Cui, L.; Zhou, Y.; Zhu, C.; Fan, D.; Gong, H.; Zhao, Q.; Zhou, C.; Zhao, Y.; Lu, D.; et al. Transcriptome-wide investigation of circular RNAs in rice. RNA 2015, 21, 2076–2087. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Cai, G.; Wang, Y.; Zhuang, Q.; Cai, Z.; Li, Y.; Gao, S.; Li, F.; Zhang, C.; Zhao, B.; et al. Circular RNA-based neoantigen vaccine for hepatocellular carcinoma immunotherapy. MedComm 2024, 5, e667. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Zhou, S.; Dain, L.; Mei, L.; Zhu, G. Circular RNA: An emerging frontier in RNA therapeutic targets, RNA therapeutics, and mRNA vaccines. J. Control. Release 2022, 348, 84–94. [Google Scholar] [CrossRef]
- Yu, H.; Wen, Y.; Yu, W.; Lu, L.; Yang, Y.; Liu, C.; Hu, Z.; Fang, Z.; Huang, S. Optimized circular RNA vaccines for superior cancer immunotherapy. Theranostics 2025, 15, 1420–1438. [Google Scholar] [CrossRef]
- Gebremedhn, S.; Salilew-Wondim, D.; Ahmad, I.; Sahadevan, S.; Hossain, M.M.; Hoelker, M.; Rings, F.; Neuhoff, C.; Tholen, E.; Looft, C.; et al. MicroRNA Expression Profile in Bovine Granulosa Cells of Preovulatory Dominant and Subordinate Follicles during the Late Follicular Phase of the Estrous Cycle. PLoS ONE 2015, 10, e0125912. [Google Scholar] [CrossRef] [PubMed]
- Kehl, T.; Backes, C.; Kern, F.; Fehlmann, T.; Ludwig, N.; Meese, E.; Lenhof, H.P.; Keller, A. About miRNAs, miRNA seeds, target genes and target pathways. Oncotarget 2017, 8, 107167–107175. [Google Scholar] [CrossRef] [PubMed]
- Maalouf, S.W.; Liu, W.S.; Pate, J.L. MicroRNA in ovarian function. Cell Tissue Res. 2016, 363, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Stark, A.; Brennecke, J.; Bushati, N.; Russell, R.B.; Cohen, S.M. Animal MicroRNAs confer robustness to gene expression and have a significant impact on 3’UTR evolution. Cell 2005, 123, 1133–1146. [Google Scholar] [CrossRef]
- Wang, W.; Wu, K.; Jia, M.; Sun, S.; Kang, L.; Zhang, Q.; Tang, H. Dynamic Changes in the Global MicroRNAome and Transcriptome Identify Key Nodes Associated With Ovarian Development in Chickens. Front. Genet. 2018, 9, 491. [Google Scholar] [CrossRef]
- Wu, N.; Gaur, U.; Zhu, Q.; Chen, B.; Xu, Z.; Zhao, X.; Yang, M.; Li, D. Expressed microRNA associated with high rate of egg production in chicken ovarian follicles. Anim. Genet. 2017, 48, 205–216. [Google Scholar] [CrossRef]
- Ghanem, K.; Johnson, A.L. Follicle dynamics and granulosa cell differentiation in the turkey hen ovary. Poult. Sci. 2018, 97, 3755–3761. [Google Scholar] [CrossRef]
- Gomez-León, V.E.; Ginther, O.J.; Domingues, R.R.; Guimarães, J.D.; Wiltbank, M.C. Necessity for LH in selection and continued growth of the bovine dominant follicle. Reproduction 2020, 159, 559–569. [Google Scholar] [CrossRef]
- Guo, X.; Wang, Y.; Chen, Q.; Yuan, Z.; Chen, Y.; Guo, M.; Kang, L.; Sun, Y.; Jiang, Y. The Role of PTHLH in Ovarian Follicle Selection, Its Transcriptional Regulation and Genetic Effects on Egg Laying Traits in Hens. Front. Genet. 2019, 10, 430. [Google Scholar] [CrossRef]
- Hou, Y.; Hu, J.; Li, J.; Li, H.; Lu, Y.; Liu, X. MFN2 regulates progesterone biosynthesis and proliferation of granulosa cells during follicle selection in hens. J. Cell. Physiol. 2024, 239, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.L.; Bridgham, J.T.; Witty, J.P.; Tilly, J.L. Susceptibility of avian ovarian granulosa cells to apoptosis is dependent upon stage of follicle development and is related to endogenous levels of bcl-xlong gene expression. Endocrinology 1996, 137, 2059–2066. [Google Scholar] [CrossRef][Green Version]
- Li, J.; Bao, R.; Peng, S.; Zhang, C. The molecular mechanism of ovarian granulosa cell tumors. J. Ovarian Res. 2018, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Nowak, M.; Grzesiak, M.; Saito, N.; Kwaśniewska, M.; Sechman, A.; Hrabia, A. Expression of aquaporin 4 in the chicken ovary in relation to follicle development. Reprod. Domest. Anim. 2017, 52, 857–864. [Google Scholar] [CrossRef]
- Kim, D.; Ocón-Grove, O.; Johnson, A.L. Bone morphogenetic protein 4 supports the initial differentiation of hen (Gallus gallus) granulosa cells. Biol. Reprod. 2013, 88, 161. [Google Scholar] [CrossRef] [PubMed]
- Tajima, K.; Orisaka, M.; Yata, H.; Goto, K.; Hosokawa, K.; Kotsuji, F. Role of granulosa and theca cell interactions in ovarian follicular maturation. Microsc. Res. Technol. 2006, 69, 450–458. [Google Scholar] [CrossRef] [PubMed]
- McGee, E.A.; Hsueh, A.J. Initial and cyclic recruitment of ovarian follicles. Endocr. Rev. 2000, 21, 200–214. [Google Scholar]
- Cheng, J.; Wei, Y.; Zhao, Z.; Xing, Q.; Gao, Z.; Cheng, J.; Yu, C.; Pan, Y.; Yang, Y.; Shi, D.; et al. MiR-29c-5p regulates the function of buffalo granulosa cells to induce follicular atresia by targeting INHBA. Theriogenology 2023, 205, 50–62. [Google Scholar] [CrossRef]
- Cheng, J.; Xing, Q.; Pan, Y.; Yang, Y.; Zhang, R.; Shi, D.; Deng, Y. CircTEC Inhibits the Follicular Atresia in Buffalo (Bubalus bubalis) via Targeting miR-144-5p/FZD3 Signaling Axis. Int. J. Mol. Sci. 2024, 26, 153. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, L.; Zhang, Y.; Madaniyati, M.; Shi, S.; Huang, L.; Song, X.; Pang, W.; Chu, G.; Yang, G. miR-10a-5p inhibits steroid hormone synthesis in porcine granulosa cells by targeting CREB1 and inhibiting cholesterol metabolism. Theriogenology 2023, 212, 19–29. [Google Scholar] [CrossRef]
- Radecki, S.V.; Capdevielle, M.C.; Buonomo, F.C.; Scanes, C.G. Ontogeny of insulin-like growth factors (IGF-I and IGF-II) and IGF-binding proteins in the chicken following hatching. Gen. Comp. Endocrinol. 1997, 107, 109–117. [Google Scholar] [CrossRef]
- Saneyasu, T.; Nakamura, T.; Honda, K.; Kamisoyama, H. IGF-1 knockdown inhibits phosphorylation of Akt and ERK in chicken embryonic myotubes. Growth Horm. IGF Res. 2022, 65, 101478. [Google Scholar] [CrossRef]
- Zhou, S.; Zhao, D.; Liu, S.; Zeng, W.; Zhang, C. TGF-β1 sustains germ cell cyst reservoir via restraining follicle formation in the chicken. Cell Biol. Int. 2020, 44, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xu, J.; Wu, Y.; Liang, B.; Yan, M.; Sun, C.; Wang, D.; Hu, X.; Liu, L.; Hu, W.; et al. The potential role and mechanism of circRNA/miRNA axis in cholesterol synthesis. Int. J. Biol. Sci. 2023, 19, 2879–2896. [Google Scholar] [CrossRef]
- Mohanapriya, R.; Akshaya, R.L.; Selvamurugan, N. A regulatory role of circRNA-miRNA-mRNA network in osteoblast differentiation. Biochimie 2022, 193, 137–147. [Google Scholar] [CrossRef]
- You, G.; Long, H.; Shen, X.; Yin, H.; Zhang, S. Emerging roles of circular RNAs on the regulation of production traits in chicken. Poult. Sci. 2025, 104, 104612. [Google Scholar] [CrossRef]
- Li, J.; Si, S.J.; Wu, X.; Zhang, Z.H.; Li, C.; Tao, Y.Q.; Yang, P.K.; Li, D.H.; Li, Z.J.; Li, G.X.; et al. CircEML1 facilitates the steroid synthesis in follicular granulosa cells of chicken through sponging gga-miR-449a to release IGF2BP3 expression. Genomics 2023, 115, 110540. [Google Scholar] [CrossRef]
- Pan, Z.; Yang, C.; Zhao, R.; Jiang, X.; Yu, C.; Li, Z. Characterization of lncRNA/circRNA-miRNA-mRNA network to reveal potential functional ceRNAs in the skeletal muscle of chicken. Front. Physiol. 2022, 13, 969854. [Google Scholar] [CrossRef]
- Qing, Z.; Dongliu, L.; Xuedie, G.; Khoso, P.A.; Xiaodan, H.; Shu, L. MiR-144-3p targets STC1 to activate PI3K/AKT pathway to induce cell apoptosis and cell cycle arrest in selenium deficiency broilers. J. Inorg. Biochem. 2022, 226, 111665. [Google Scholar] [CrossRef]
- Ru, M.; Liang, H.; Ruan, J.; Haji, R.A.; Cui, Y.; Yin, C.; Wei, Q.; Huang, J. Chicken ovarian follicular atresia: Interaction network at organic, cellular, and molecular levels. Poult. Sci. 2024, 103, 103893. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.Y.; Edna, O.; Wang, J.; Zhang, H.J.; Zhou, J.M.; Qiu, K.; Wu, S.G. Histological and molecular difference in albumen quality between post-adolescent hens and aged hens. Poult. Sci. 2024, 103, 103618. [Google Scholar] [CrossRef] [PubMed]
- Navara, K.J. The role of steroid hormones in the adjustment of primary sex ratio in birds: Compiling the pieces of the puzzle. Integr. Comp. Biol. 2013, 53, 923–937. [Google Scholar] [CrossRef] [PubMed]
- Acosta, T.J.; Miyamoto, A. Vascular control of ovarian function: Ovulation, corpus luteum formation and regression. Anim. Reprod. Sci. 2004, 82–83, 127–140. [Google Scholar] [CrossRef]
- Meng, S.; Zhou, H.; Feng, Z.; Xu, Z.; Tang, Y.; Li, P.; Wu, M. CircRNA: Functions and properties of a novel potential biomarker for cancer. Mol. Cancer 2017, 16, 94. [Google Scholar] [CrossRef]
- Xu, S.; Zhou, L.; Ponnusamy, M.; Zhang, L.; Dong, Y.; Zhang, Y.; Wang, Q.; Liu, J.; Wang, K. A comprehensive review of circRNA: From purification and identification to disease marker potential. PeerJ 2018, 6, e5503. [Google Scholar] [CrossRef]
- Obi, P.; Chen, Y.G. The design and synthesis of circular RNAs. Methods 2021, 196, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Gilbert, A.B.; Evans, A.J.; Perry, M.M.; Davidson, M.H. A method for separating the granulosa cells, the basal lamina and the theca of the preovulatory ovarian follicle of the domestic fowl (Galus domesticus). J. Reprod. Fertil. 1977, 50, 179–181. [Google Scholar] [CrossRef] [PubMed]
| Primer | Sequence | Annealing Temp (°C) | Product Size (bp) |
|---|---|---|---|
| circFBN1 | F: GAACGCATTGTGGACAGCCT R: GGCTATCTGACCATTTGGGC | 60 | 274 |
| CYP19A1 | F: GGCCTCCAGCAGGTTGAAAG R: ATAGGCACTGTGGCAACTGG | 60 | 214 |
| E2F5 | F: GCCTTCCAGACTCAGTGTTG R: GGCTCCTCCATCTTTGCTAT | 60 | 148 |
| CDK1 | F: TGGCCTTGAACCACCCATAC R: AGGCAGGCAGGCAAAGATAA | 60 | 298 |
| CCND1 | F: ATAGTCGCCACTTGGATGCT R: AACCGGCTTTTCTTGAGGGG | 60 | 122 |
| CYP11A1 | F: GTGGACACGACTTCCATGACT R: GAGAGTCTCCTTGATGGCGG | 60 | 174 |
| PCNA | F: AACACTCAGAGCAGAAGAC R: GCACAGGAGATGACAACA | 60 | 225 |
| Caspase-9 | F: GCTTGTCCATCCCAGTCCAA R: CAGTCTGTGGTCGCTCTTGT | 60 | 95 |
| Caspase-3 | F: TGGCCCTCTTGAACTGAAAG R: TCCACTGTCTGCTTCAATACC | 60 | 139 |
| BCL2 | F: ATCGTCGCCTTCTTCGAGTT R: ATCCCATCCTCCGTTGTTCT | 60 | 150 |
| StAR | F: AACCTGCTTCACTCTGTATC R: CTCATTAACTTCCTCTTGTCTC | 60 | 151 |
| β-actin | F: GTCCACCGCAAATGCTTCTAA R: TGCGCATTTATGGGTTTTGTT | 60 | 78 |
| FSHR | F: AACCTGCTTCACTCTGTATC R: CTCATTAACTTCCTCTTGTCTC | 60 | 184 |
| PGDFA | F: GATGAGCGCAACGTGAGAAC R: CACCACTGATCCGGACAACA | 60 | 106 |
| SBF2 | F: GATGAGCGCAACGTGAGAAC R: CACCACTGATCCGGACAACA | 60 | 154 |
| ANDKKIA | F: AGAAGGTGGTTATGCTGTG R:TAAGAAGGAATGCGAGGAAT | 60 | 235 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Li, W.; Fu, G.; Liu, S.; Ma, T. circ_0000132 Regulates Chicken Granulosa Cell Proliferation Apoptosis and E2/P4 Synthesis via miR-206 E2F5 Signaling. Int. J. Mol. Sci. 2025, 26, 10779. https://doi.org/10.3390/ijms262110779
Yang H, Li W, Fu G, Liu S, Ma T. circ_0000132 Regulates Chicken Granulosa Cell Proliferation Apoptosis and E2/P4 Synthesis via miR-206 E2F5 Signaling. International Journal of Molecular Sciences. 2025; 26(21):10779. https://doi.org/10.3390/ijms262110779
Chicago/Turabian StyleYang, Huanqi, Wei Li, Guanhua Fu, Sihan Liu, and Tenghe Ma. 2025. "circ_0000132 Regulates Chicken Granulosa Cell Proliferation Apoptosis and E2/P4 Synthesis via miR-206 E2F5 Signaling" International Journal of Molecular Sciences 26, no. 21: 10779. https://doi.org/10.3390/ijms262110779
APA StyleYang, H., Li, W., Fu, G., Liu, S., & Ma, T. (2025). circ_0000132 Regulates Chicken Granulosa Cell Proliferation Apoptosis and E2/P4 Synthesis via miR-206 E2F5 Signaling. International Journal of Molecular Sciences, 26(21), 10779. https://doi.org/10.3390/ijms262110779





