Comparative Analysis of Genetic Risk for Viral-Induced Axonal Loss in Genetically Diverse Mice
Abstract
1. Introduction
2. Results
2.1. Lesion Burden Varied by Strain and Region, with Axonal Degeneration Only Found in Two Strains
2.2. Genetic Predisposition to Neurological Disease Was Not Solely Based on the H2 Haplotype
2.3. QTL Associated with Lesion Locations Indicated That Genetic Background Was a Contributing Factor to Lesion Burden
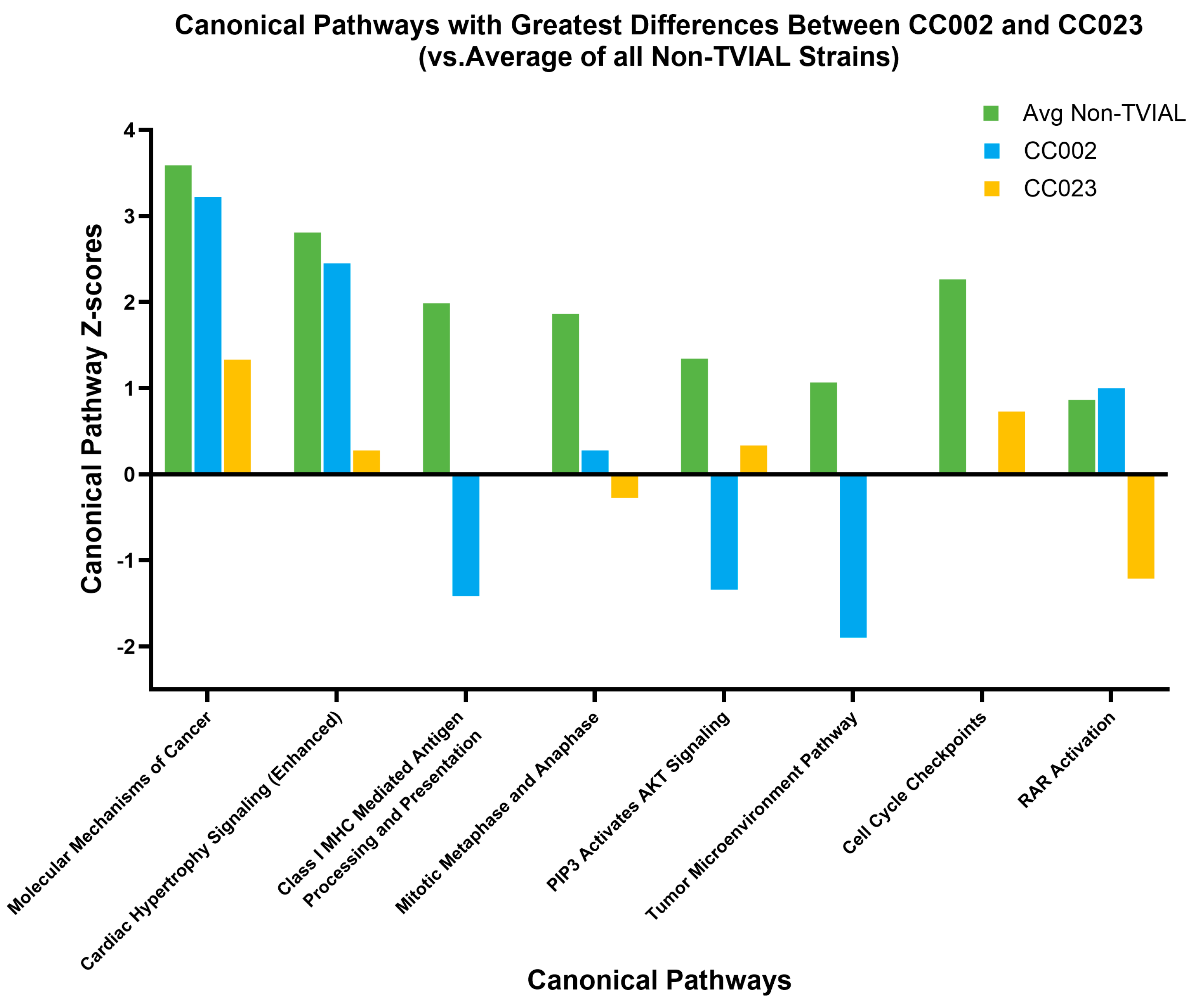
2.4. Axonal Degeneration in CC Strains Differed from TVID Typically Associated with Inbred Mice
| Lesion Location | SNP ID | Chr | Pos (Mb) | Pos (cM) | LOD | p-Value | % Var | Proximal (Mb) | Distal (Mb) |
|---|---|---|---|---|---|---|---|---|---|
| Level 3 | UNC17077443 | 9 | 106.56 | 50.69 | 10.52 | 2.90 × 10−8 | 93.23 | 103.85 | 107.63 |
| Cervical | UNC16238010 | 9 | 41.92 | 18.35 | 281.49 | 1.04 × 10−275 | 100.00 | 41.90 | 42.40 |
| Cervical | UNC17405188 | 10 | 9.94 | 0.77 | 293.27 | 1.91 × 10−287 | 100.00 | 9.91 | 16.47 |
| Cervical | UNC23765091 | 14 | 29.12 | 7.06 | 290.82 | 5.33 × 10−285 | 100.00 | 25.79 | 57.26 |
| Cervical | UNC26402456 | 16 | 16.96 | 6.57 | 277.77 | 5.25 × 10−272 | 100.00 | 12.84 | 17.34 |
| Lumbar | JAX00171047 | 9 | 49.57 | 23.55 | 10.58 | 2.57 × 10−8 | 93.33 | 44.03 | 49.63 |
| Lumbar | UNC29023821 | 18 | 34.05 | 12.68 | 10.98 | 1.13 × 10−8 | 93.97 | 33.99 | 34.05 |
2.5. Gene Expression Distinguished TVIAL-Affected Mouse Strains from Those Not Affected by TVIAL
2.6. Biomarkers and QTL-Linked Genes Revealed a Regulatory Architecture Underlying TVIAL Susceptibility
3. Discussion
3.1. Polygenic QTL Signatures Underlie Lesion Susceptibility and Include Loci Not Previously Associated with TMEV Pathology
3.2. Canonical Pathway Divergence Between CC002 and CC023 Reflected Distinct Biological Strategies Leading to Axonal Degeneration
3.3. Biomarker Integration Suggested Core and Strain-Specific Susceptibility Signatures
3.4. Different Genetic Signatures Underlying Axonal Loss Models Variation Seen in Human MS
3.5. Limitations and Future Directions
4. Materials and Methods
4.1. Mice
4.2. RNA Sequencing Dataset
4.3. Histological Evaluation
4.4. Association of Quantitative Trait Loci (QTL) with Lesion Burden
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bjornevik, K.; Cortese, M.; Healy, B.C.; Kuhle, J.; Mina, M.J.; Leng, Y.; Elledge, S.J.; Niebuhr, D.W.; Scher, A.I.; Munger, K.L.; et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 2022, 375, 296–301. [Google Scholar] [CrossRef]
- Soldan, S.S.; Berti, R.; Salem, N.; Secchiero, P.; Flamand, L.; Calabresi, P.A.; Brennan, M.B.; Maloni, H.W.; Mcfarland, H.F.; Lin, H.-C.; et al. Association of human herpes virus 6 (HHV-6) with multiple sclerosis: Increased IgM response to HHV-6 early antigen and detection of serum HHV-6 DNA. Nat. Med. 1997, 3, 1394–1397. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Sheu, J.J.; Kao, S.; Lin, H.C. Increased risk of multiple sclerosis following herpes zoster: A nationwide, population-based study. J. Infect. Dis. 2011, 204, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Küry, P.; Nath, A.; Créange, A.; Dolei, A.; Marche, P.; Gold, J.; Giovannoni, G.; Hartung, H.-P.; Perron, H. Human Endogenous Retroviruses in Neurological Diseases. Trends Mol. Med. 2018, 24, 379–394. [Google Scholar] [CrossRef]
- Meier, U.C.; Cipian, R.C.; Karimi, A.; Ramasamy, R.; Middeldorp, J.M. Cumulative Roles for Epstein-Barr Virus, Human Endogenous Retroviruses, and Human Herpes Virus-6 in Driving an Inflammatory Cascade Underlying MS Pathogenesis. Front. Immunol. 2021, 12, 757302. [Google Scholar] [CrossRef]
- Brahic, M.; Bureau, J.F. Genetics of susceptibility to Theiler’s virus infection. Bioessays 1998, 20, 627–633. [Google Scholar] [CrossRef]
- Butterfield, R.J.; Roper, R.J.; Rhein, D.M.; Melvold, R.W.; Haynes, L.; Ma, R.Z.; Doerge, R.W.; Teuscher, C. Sex-specific quantitative trait loci govern susceptibility to Theiler’s murine encephalomyelitis virus-induced demyelination. Genetics 2003, 163, 1041–1046. [Google Scholar] [CrossRef]
- Clatch, R.J.; Melvold, R.W.; Dal Canto, M.C.; Miller, S.D.; Lipton, H.L. The Theiler’s murine encephalomyelitis virus (TMEV) model for multiple sclerosis shows a strong influence of the murine equivalents of HLA-A, B, and C. J. Neuroimmunol. 1987, 15, 121–135. [Google Scholar] [CrossRef]
- Clatch, R.J.; Melvold, R.W.; Miller, S.D.; Lipton, H.L. Theiler’s murine encephalomyelitis virus (TMEV)-induced demyelinating disease in mice is influenced by the H-2D region: Correlation with TEMV-specific delayed-type hypersensitivity. J. Immunol. 1985, 135, 1408–1414. [Google Scholar] [CrossRef]
- Rodriguez, M.; David, C.S. Demyelination induced by Theiler’s virus: Influence of the H-2 haplotype. J. Immunol. 1985, 135, 2145–2148. [Google Scholar] [CrossRef]
- Rodriguez, M.; Leibowitz, J.; David, C.S. Susceptibility to Theiler’s virus-induced demyelination. Mapping of the gene within the H-2D region. J. Exp. Med. 1986, 163, 620–631. [Google Scholar] [CrossRef]
- Dal Canto, M.C.; Kim, B.S.; Miller, S.D.; Melvold, R.W. Theiler’s Murine Encephalomyelitis Virus (TMEV)-Induced Demyelination: A Model for Human Multiple Sclerosis. Methods 1996, 10, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Lipton, H.L. Theiler’s virus infection in mice: An unusual biphasic disease process leading to demyelination. Infect. Immun. 1975, 11, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Libbey, J.E.; Kirkman, N.J.; Smith, M.C.; Tanaka, T.; Wilcox, K.S.; White, H.S.; Fujinami, R.S. Seizures following picornavirus infection. Epilepsia 2008, 49, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.; Pavelko, K.; Coffman, R.L. Gamma interferon is critical for resistance to Theiler’s virus-induced demyelination. J. Virol. 1995, 69, 7286–7290. [Google Scholar] [CrossRef]
- DePaula-Silva, A.B.; Hanak, T.J.; Libbey, J.E.; Fujinami, R.S. Theiler’s murine encephalomyelitis virus infection of SJL/J and C57BL/6J mice: Models for multiple sclerosis and epilepsy. J. Neuroimmunol. 2017, 308, 30–42. [Google Scholar] [CrossRef]
- Afrasiabi, A.; Fewings, N.L.; Schibeci, S.D.; Keane, J.T.; Booth, D.R.; Parnell, G.P.; Swaminathan, S. The Interaction of Human and Epstein-Barr Virus miRNAs with Multiple Sclerosis Risk Loci. Int J Mol Sci 2021, 22, 2927. [Google Scholar] [CrossRef]
- Ascherio, A.; Munger, K. Epidemiology of multiple sclerosis: From risk factors to prevention. Semin. Neurol. 2008, 28, 17–28. [Google Scholar] [CrossRef]
- Hassani, A.; Khan, G. Epstein-Barr Virus and miRNAs: Partners in Crime. in the Pathogenesis of Multiple Sclerosis? Front. Immunol. 2019, 10, 695. [Google Scholar] [CrossRef]
- Mechelli, R.; Umeton, R.; Bellucci, G.; Bigi, R.; Rinaldi, V.; Angelini, D.F.; Guerrera, G.; Pignalosa, F.C.; Ilari, S.; Patrone, M.; et al. A disease-specific convergence of host and Epstein-Barr virus genetics in multiple sclerosis. Proc. Natl. Acad. Sci. USA 2025, 122, e2418783122. [Google Scholar] [CrossRef]
- Wahbeh, F.; Sabatino, J.J. Epstein-Barr Virus in Multiple Sclerosis: Past, Present, and Future. Neurol. Neuroimmunol. Neuroinflamm. 2025, 12, e200460. [Google Scholar] [CrossRef]
- Naito, S.; Namerow, N.; Mickey, M.R.; Terasaki, P.I. Multiple sclerosis: Association with HL-A3. Tissue Antigens 1972, 2, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Jersild, C.; Fog, T.; Hansen, G.S.; Thomsen, M.; Svejgaard, A.; Dupont, B. Histocompatibility determinants in multiple sclerosis, with special reference to clinical course. Lancet 1973, 2, 1221–1225. [Google Scholar] [CrossRef] [PubMed]
- Jersild, C.; Svejgaard, A.; Fog, T. HL-A antigens and multiple sclerosis. Lancet 1972, 1, 1240–1241. [Google Scholar] [CrossRef]
- Afrasiabi, A.; Ahlenstiel, C.; Swaminathan, S.; Parnell, G.P. The interaction between Epstein-Barr virus and multiple sclerosis genetic risk loci: Insights into disease pathogenesis and therapeutic opportunities. Clin. Transl. Immunol. 2023, 12, e1454. [Google Scholar] [CrossRef]
- Bar-Or, A.; Pender, M.P.; Khanna, R.; Steinman, L.; Hartung, H.-P.; Maniar, T.; Croze, E.; Aftab, B.T.; Giovannoni, G.; Joshi, M.A. Epstein-Barr Virus in Multiple Sclerosis: Theory and Emerging Immunotherapies. Trends Mol. Med. 2020, 26, 296–310. [Google Scholar] [CrossRef]
- Lawley, K.S.; Rech, R.R.; Perez Gomez, A.A.; Hopkins, L.; Han, G.; Amstalden, K.; Welsh, C.J.; Young, C.R.; Jones-Hall, Y.; Threadgill, D.W.; et al. Viral Clearance and Neuroinflammation in Acute TMEV Infection Vary by Host Genetic Background. Int. J. Mol. Sci. 2022, 23, 10482. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lawley, K.S.; Rech, R.R.; Elenwa, F.; Han, G.; Perez Gomez, A.A.; Amstalden, K.; Welsh, C.J.; Young, C.R.; Threadgill, D.W.; Brinkmeyer-Langford, C.L. Host genetic diversity drives variable central nervous system lesion distribution in chronic phase of Theiler's Murine Encephalomyelitis Virus (TMEV) infection. PLoS One 2021, 16, e0256370. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gomez, A.A.P.; Karmakar, M.; Carroll, R.J.; Lawley, K.S.; Amstalden, K.; Young, C.R.; Threadgill, D.W.; Welsh, C.J.; Brinkmeyer-Langford, C. Genetic and immunological contributors to virus-induced paralysis. Brain Behav. Immun. Health 2021, 18, 100395. [Google Scholar] [CrossRef]
- Gómez, A.A.P.; Karmakar, M.; Carroll, R.J.; Lawley, K.S.; Amstalden, K.; Young, C.R.; Threadgill, D.W.; Welsh, C.J.; Brinkmeyer-Langford, C. Serum Cytokines Predict Neurological Damage in Genetically Diverse Mouse Models. Cells 2022, 11, 2044. [Google Scholar] [CrossRef]
- Churchill, G.A.; Airey, D.C.; Allayee, H.; Angel, J.M.; Attie, A.D.; Beatty, J.; Beavis, W.D.; Belknap, J.K.; Bennett, B.; Berrettini, W.; et al. The Collaborative Cross, a community resource for the genetic analysis of complex traits. Nat. Genet. 2004, 36, 1133–1137. [Google Scholar] [CrossRef]
- Brinkmeyer-Langford, C.; Amstalden, K.; Konganti, K.; Hillhouse, A.; Lawley, K.; Perez-Gomez, A.; Young, C.R.; Welsh, C.J.; Threadgill, D.W. Resilience in Long-Term Viral Infection: Genetic Determinants and Interactions. Int. J. Mol. Sci. 2021, 22, 11379. [Google Scholar] [CrossRef]
- Brahic, M.; Bureau, J.F.; Michiels, T. The genetics of the persistent infection and demyelinating disease caused by Theiler’s virus. Annu. Rev. Microbiol. 2005, 59, 279–298. [Google Scholar] [CrossRef]
- Lipton, H.L.; Dal Canto, M.C. Susceptibility of inbred mice to chronic central nervous system infection by Theiler’s murine encephalomyelitis virus. Infect. Immun. 1979, 26, 369–374. [Google Scholar] [CrossRef]
- Gomez, J.A.; Wapinski, O.L.; Yang, Y.W.; Bureau, J.-F.; Gopinath, S.; Monack, D.M.; Chang, H.Y.; Brahic, M.; Kirkegaard, K. The NeST long ncRNA controls microbial susceptibility and epigenetic activation of the interferon-gamma locus. Cell 2013, 152, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Welsh, C.J.; Tonks, P.; Borrow, P.; Nash, A.A. Theiler’s virus: An experimental model of virus-induced demyelination. Autoimmunity 1990, 6, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Melvold, R.W.; Jokinen, D.M.; Knobler, R.L.; Lipton, H.L. Variations in genetic control of susceptibility to Theiler’s murine encephalomyelitis virus (TMEV)-induced demyelinating disease. I. Differences between susceptible SJL/J and resistant BALB/c strains map near the T cell beta-chain constant gene on chromosome 6. J. Immunol. 1987, 138, 1429–1433. [Google Scholar] [PubMed]
- Gerhauser, I.; Hansmann, F.; Ciurkiewicz, M.; Loscher, W.; Beineke, A. Facets of Theiler’s Murine Encephalomyelitis Virus-Induced Diseases: An Update. Int. J. Mol. Sci. 2019, 20, 448. [Google Scholar] [CrossRef]
- Oleszak, E.L.; Chang, J.R.; Friedman, H.; Katsetos, C.D.; Platsoucas, C.D. Theiler’s virus infection: A model for multiple sclerosis. Clin. Microbiol. Rev. 2004, 17, 174–207. [Google Scholar] [CrossRef]
- Kreutzer, M.; Seehusen, F.; Kreutzer, R.; Pringproa, K.; Kummerfeld, M.; Claus, P.; Deschl, U.; Kalkul, A.; Beineke, A.; Baumgärtner, W.; et al. Axonopathy is associated with complex axonal transport defects in a model of multiple sclerosis. Brain Pathol. 2012, 22, 454–471. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, I.; Fujinami, R.S. Inside-Out versus Outside-In models for virus induced demyelination: Axonal damage triggering demyelination. Springer Semin. Immunopathol. 2002, 24, 105–125. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, S.; Pellegrini, S. Experimental In Vivo Models of Multiple Sclerosis: State of the Art. In Multiple Sclerosis: Perspectives in Treatment and Pathogenesis; Zagon, I.S., McLaughlin, P.J., Eds.; Exon Publications: Brisbane, Australia, 2017. [Google Scholar]
- Turrin, N.P. Central nervous system Toll-like receptor expression in response to Theiler’s murine encephalomyelitis virus-induced demyelination disease in resistant and susceptible mouse strains. Virol. J. 2008, 5, 154. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Kang, H.S.; Kim, B.S. Th17 cells enhance viral persistence and inhibit T cell cytotoxicity in a model of chronic virus infection. J. Exp. Med. 2009, 206, 313–328. [Google Scholar] [CrossRef]
- Jin, Y.H.; Kang, H.S.; Hou, W.; Meng, L.; Kim, B.S. The level of viral infection of antigen-presenting cells correlates with the level of development of Theiler’s murine encephalomyelitis virus-induced demyelinating disease. J. Virol. 2015, 89, 1867–1878. [Google Scholar] [CrossRef]
- Cirillo, E.; Parnell, L.D.; Evelo, C.T. A Review of Pathway-Based Analysis Tools That Visualize Genetic Variants. Front. Genet. 2017, 8, 174. [Google Scholar] [CrossRef]
- Mears, H.V.; Sweeney, T.R. Mouse Ifit1b is a cap1-RNA-binding protein that inhibits mouse coronavirus translation and is regulated by complexing with Ifit1c. J. Biol. Chem. 2020, 295, 17781–17801. [Google Scholar] [CrossRef]
- Fensterl, V.; Sen, G.C. Interferon-induced Ifit proteins: Their role in viral pathogenesis. J. Virol. 2015, 89, 2462–2468. [Google Scholar] [CrossRef]
- Drenan, R.M.; Grady, S.R.; Whiteaker, P.; McClure-Begley, T.; McKinney, S.; Miwa, J.M.; Bupp, S.; Heintz, N.; McIntosh, J.M.; Bencherif, M.; et al. In vivo activation of midbrain dopamine neurons via sensitized, high-affinity alpha 6 nicotinic acetylcholine receptors. Neuron 2008, 60, 123–136. [Google Scholar] [CrossRef]
- Thorgeirsson, T.E.; Gudbjartsson, D.F.; Surakka, I.; Vink, J.M.; Amin, N.; Geller, F.; Sulem, P.; Rafnar, T.; Esko, T.; Walter, S.; et al. Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nat. Genet. 2010, 42, 448–453. [Google Scholar] [CrossRef]
- Grünblatt, E.; Zander, N.; Bartl, J.; Jie, L.; Monoranu, C.-M.; Arzberger, T.; Ravid, R.; Roggendorf, W.; Gerlach, M.; Riederer, P. Comparison analysis of gene expression patterns between sporadic Alzheimer’s and Parkinson’s disease. J. Alzheimer’s Dis. 2007, 12, 291–311. [Google Scholar] [CrossRef]
- Reith, M.E.A.; Kortagere, S.; Wiers, C.E.; Sun, H.; Kurian, M.A.; Galli, A.; Volkow, N.D.; Lin, Z. The dopamine transporter gene SLC6A3: Multidisease risks. Mol. Psychiatry 2022, 27, 1031–1046. [Google Scholar] [CrossRef]
- Krebs, C.J.; Robins, D.M. A pair of mouse KRAB zinc finger proteins modulates multiple indicators of female reproduction. Biol. Reprod. 2010, 82, 662–668. [Google Scholar] [CrossRef]
- Ferrer, I.; Perez, E.; Dalfo, E.; Barrachina, M. Abnormal levels of prohibitin and ATP synthase in the substantia nigra and frontal cortex in Parkinson’s disease. Neurosci. Lett. 2007, 415, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Ali, N.; Banerjee, E.; Singh, R.; Naskar, A.; Paidi, R.K.; Mohanakumar, K.P. Low Levels of Prohibitin in Substantia Nigra Makes Dopaminergic Neurons Vulnerable in Parkinson’s Disease. Mol. Neurobiol. 2018, 55, 804–821. [Google Scholar] [CrossRef] [PubMed]
- Wintachai, P.; Wikan, N.; Kuadkitkan, A.; Jaimipuk, T.; Ubol, S.; Pulmanausahakul, R.; Auewarakul, P.; Kasinrerk, W.; Weng, W.; Panyasrivanit, M.; et al. Identification of prohibitin as a Chikungunya virus receptor protein. J. Med. Virol. 2012, 84, 1757–1770. [Google Scholar] [CrossRef] [PubMed]
- Kuadkitkan, A.; Wikan, N.; Fongsaran, C.; Smith, D.R. Identification and characterization of prohibitin as a receptor protein mediating DENV-2 entry into insect cells. Virology 2010, 406, 149–161. [Google Scholar] [CrossRef]
- Sim, H.; Lee, J.-E.; Yoo, H.M.; Cho, S.; Lee, H.; Baek, A.; Kim, J.; Seo, H.; Kweon, M.-N.; Kim, H.G.; et al. Iroquois Homeobox Protein 2 Identified as a Potential Biomarker for Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 3455. [Google Scholar] [CrossRef]
- Mariani, E.; Frabetti, F.; Tarozzi, A.; Pelleri, M.C.; Pizzetti, F.; Casadei, R. Meta-Analysis of Parkinson’s Disease Transcriptome Data Using TRAM Software: Whole Substantia Nigra Tissue and Single Dopamine Neuron Differential Gene Expression. PLoS ONE 2016, 11, e0161567. [Google Scholar] [CrossRef]
- Lee, A.S.; Kranzusch, P.J.; Cate, J.H. eIF3 targets cell-proliferation messenger RNAs for translational activation or repression. Nature 2015, 522, 111–114. [Google Scholar] [CrossRef]
- Tang, R.; Langdon, W.Y.; Zhang, J. Negative regulation of receptor tyrosine kinases by ubiquitination: Key roles of the Cbl family of E3 ubiquitin ligases. Front. Endocrinol. 2022, 13, 971162. [Google Scholar] [CrossRef]
- Wu, J.; Song, L.; Lu, M.; Gao, Q.; Xu, S.; Zhou, P.; Ma, T. The multifaceted functions of DNA-PKcs: Implications for the therapy of human diseases. MedComm (2020) 2024, 5, e613. [Google Scholar] [CrossRef] [PubMed]
- Mollinari, C.; Cardinale, A.; Lupacchini, L.; Martire, A.; Chiodi, V.; Martinelli, A.; Rinaldi, A.M.; Fini, M.; Pazzaglia, S.; Domenici, M.R.; et al. The DNA repair protein DNA-PKcs modulates synaptic plasticity via PSD-95 phosphorylation and stability. EMBO Rep. 2024, 25, 3707–3737. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhong, L.; Yang, X.; Andersson, T.; Huang, M.; Tang, S.J. WNT5A signaling contributes to Abeta-induced neuroinflammation and neurotoxicity. PLoS ONE 2011, 6, e22920. [Google Scholar]
- Jiang, X.; Liu, J.; Guan, Y.; Zhao, Z.; Meng, F.; Wang, X.; Gao, X.; Zhou, F.; Chen, Y.; Wang, X. The mechanism of the WNT5A and FZD4 receptor mediated WNT/beta-catenin pathway in the degeneration of ALS spinal cord motor neurons. Biochem. Biophys. Res. Commun. 2022, 609, 23–30. [Google Scholar] [CrossRef]
- Casper, D.; Davies, P. Stimulation of choline acetyltransferase activity by retinoic acid and sodium butyrate in a cultured human neuroblastoma. Brain Res. 1989, 478, 74–84. [Google Scholar] [CrossRef]
- Koshy, A.M.; Mendoza-Parra, M.A. Retinoids: Mechanisms of Action in Neuronal Cell Fate Acquisition. Life 2023, 13, 2279. [Google Scholar] [CrossRef]
- Ugalde-Muñiz, P.; Hernández-Luna, M.G.; García-Velasco, S.; Lugo-Huitrón, R.; Murcia-Ramírez, J.; Martínez-Tapia, R.J.; Noriega-Navarro, R.; Navarro, L. Activation of dopamine D2 receptors attenuates neuroinflammation and ameliorates the memory impairment induced by rapid eye movement sleep deprivation in a murine model. Front. Neurosci. 2022, 16, 988167. [Google Scholar] [CrossRef]
- Zhu, J.; Hu, Z.; Han, X.; Wang, D.; Jiang, Q.; Ding, J.; Xiao, M.; Wang, C.; Lu, M.; Hu, G. Dopamine D2 receptor restricts astrocytic NLRP3 inflammasome activation via enhancing the interaction of beta-arrestin2 and NLRP3. Cell Death Differ. 2018, 25, 2037–2049. [Google Scholar] [CrossRef]
- Reyland, M.E.; Jones, D.N. Multifunctional roles of PKCdelta: Opportunities for targeted therapy in human disease. Pharmacol. Ther. 2016, 165, 1–13. [Google Scholar] [CrossRef]
- Gordon, R.; Singh, N.; Lawana, V.; Ghosh, A.; Harischandra, D.S.; Jin, H.; Hogan, C.; Sarkar, S.; Rokad, D.; Panicker, N.; et al. Protein kinase Cdelta upregulation in microglia drives neuroinflammatory responses and dopaminergic neurodegeneration in experimental models of Parkinson’s disease. Neurobiol. Dis. 2016, 93, 96–114. [Google Scholar] [CrossRef]
- Jin, H.; Kanthasamy, A.; Anantharam, V.; Rana, A.; Kanthasamy, A.G. Transcriptional regulation of pro-apoptotic protein kinase Cdelta: Implications for oxidative stress-induced neuronal cell death. J. Biol. Chem. 2011, 286, 19840–19859. [Google Scholar] [CrossRef] [PubMed]
- Kanthasamy, A.G.; Kitazawa, M.; Kanthasamy, A.; Anantharam, V. Role of proteolytic activation of protein kinase Cdelta in oxidative stress-induced apoptosis. Antioxid. Redox Signal 2003, 5, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Usui, N.; Berto, S.; Konishi, A.; Kondo, M.; Konopka, G.; Matsuzaki, H.; Shimada, S. Zbtb16 regulates social cognitive behaviors and neocortical development. Transl. Psychiatry 2021, 11, 242, Erratum in Transl. Psychiatry 2021, 11, 279. [Google Scholar] [CrossRef] [PubMed]
- Blazie, S.M.; Takayanagi-Kiya, S.; McCulloch, K.A.; Jin, Y. Eukaryotic initiation factor EIF-3.G augments mRNA translation efficiency to regulate neuronal activity. Elife 2021, 10, e68336. [Google Scholar] [CrossRef]
- Garcia-Esparcia, P.; Hernández-Ortega, K.; Koneti, A.; Gil, L.; Delgado-Morales, R.; Castaño, E.; Carmona, M.; Ferrer, I. Altered machinery of protein synthesis is region- and stage-dependent and is associated with alpha-synuclein oligomers in Parkinson’s disease. Acta Neuropathol. Commun. 2015, 3, 76. [Google Scholar] [CrossRef]
- Di Filippo, M.; Gaetani, L.; Centonze, D.; Hegen, H.; Kuhle, J.; Teunissen, C.E.; Tintoré, M.; Villar, L.M.; Willemse, E.A.; Zetterberg, H.; et al. Fluid biomarkers in multiple sclerosis: From current to future applications. Lancet Reg. Health Eur. 2024, 44, 101009. [Google Scholar] [CrossRef]
- Kingwell, E.; Marriott, J.J.; Jetté, N.; Pringsheim, T.; Makhani, N.; A Morrow, S.; Fisk, J.D.; Evans, C.; Béland, S.G.; Kulaga, S.; et al. Incidence and prevalence of multiple sclerosis in Europe: A systematic review. BMC Neurol. 2013, 13, 128. [Google Scholar] [CrossRef]
- Gross, C.C.; Schulte-Mecklenbeck, A.; Steinberg, O.V.; Wirth, T.; Lauks, S.; Bittner, S.; Schindler, P.; Baranzini, S.E.; Groppa, S.; Bellmann-Strobl, J.; et al. Multiple sclerosis endophenotypes identified by high-dimensional blood signatures are associated with distinct disease trajectories. Sci. Transl. Med. 2024, 16, eade8560. [Google Scholar] [CrossRef]
- Korsukewitz, C.; Wiendl, H. Emerging trends and challenges in multiple sclerosis in Europe: Rethinking classification and addressing COVID-19 impact. Lancet Reg. Health Eur. 2024, 44, 101017. [Google Scholar] [CrossRef]
- Nazish, S.; Shahid, R.; Zafar, A.; Alshamrani, F.; Al Sulaiman, A.; Alabdali, M.; Aljaafari, D.; Al Wabari, E.; A Alkhamis, F. Clinical Presentations and Phenotypic Spectrum of Multiple Sclerosis at a University Hospital in Saudi Arabia. J. Clin. Neurol. 2018, 14, 359–365. [Google Scholar] [CrossRef]
- Portaccio, E.; Magyari, M.; Havrdova, E.K.; Ruet, A.; Brochet, B.; Scalfari, A.; Di Filippo, M.; Tur, C.; Montalban, X.; Amato, M.P. Multiple sclerosis: Emerging epidemiological trends and redefining the clinical course. Lancet Reg. Health Eur. 2024, 44, 100977. [Google Scholar] [CrossRef] [PubMed]
- The Lancet Regional Health—Europe. Is the field of multiple sclerosis ready for a biologically driven continuum framework? Lancet Reg. Health Eur. 2024, 44, 101054. [Google Scholar] [CrossRef] [PubMed]
- Koh, D.R.; Fung-Leung, W.P.; Ho, A.; Gray, D.; Acha-Orbea, H.; Mak, T.W. Less mortality but more relapses in experimental allergic encephalomyelitis in CD8-/- mice. Science 1992, 256, 1210–1213. [Google Scholar] [CrossRef] [PubMed]
- Encinas, J.A.; Wicker, L.S.; Peterson, L.B.; Mukasa, A.; Teuscher, C.; Sobel, R.; Weiner, H.L.; Seidman, C.E.; Seidman, J.; Kuchroo, V.K. QTL influencing autoimmune diabetes and encephalomyelitis map to a 0.15-cM region containing Il2. Nat. Genet. 1999, 21, 158–160. [Google Scholar] [CrossRef]
- Zamvil, S.; Nelson, P.; Trotter, J.; Mitchell, D.; Knobler, R.; Fritz, R.; Steinman, L. T-cell clones specific for myelin basic protein induce chronic relapsing paralysis and demyelination. Nature 1985, 317, 355–358. [Google Scholar] [CrossRef]
- Matsushima, G.K.; Morell, P. The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol. 2001, 11, 107–116. [Google Scholar] [CrossRef]
- Jeffery, N.D.; Blakemore, W.F. Remyelination of mouse spinal cord axons demyelinated by local injection of lysolecithin. J. Neurocytol. 1995, 24, 775–781. [Google Scholar] [CrossRef]
- Sato, F.; Tanaka, H.; Hasanovic, F.; Tsunoda, I. Theiler’s virus infection: Pathophysiology of demyelination and neurodegeneration. Pathophysiology 2011, 18, 31–41. [Google Scholar] [CrossRef]
- Tsunoda, I.; Kuang, L.-Q.; Libbey, J.E.; Fujinami, R.S. Axonal injury heralds virus-induced demyelination. Am. J. Pathol. 2003, 162, 1259–1269. [Google Scholar] [CrossRef]
- Chesler, E.J. Out of the bottleneck: The Diversity Outcross and Collaborative Cross mouse populations in behavioral genetics research. Mamm. Genome 2014, 25, 3–11. [Google Scholar] [CrossRef]
- Collaborative Cross Consortium. The genome architecture of the Collaborative Cross mouse genetic reference population. Genetics 2012, 190, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Eldridge, R.; Osorio, D.; Amstalden, K.; Edwards, C.; Young, C.R.; Cai, J.J.; Konganti, K.; Hillhouse, A.; Threadgill, D.W.; Welsh, C.J.; et al. Antecedent presentation of neurological phenotypes in the Collaborative Cross reveals four classes with complex sex-dependencies. Sci. Rep. 2020, 10, 7918. [Google Scholar] [CrossRef]
- Threadgill, D.W.; Miller, D.R.; Churchill, G.A.; de Villena, F.P. The collaborative cross: A recombinant inbred mouse population for the systems genetic era. ILAR J. 2011, 52, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Clatch, R.J.; Lipton, H.L.; Miller, S.D. Characterization of Theiler’s murine encephalomyelitis virus (TMEV)-specific delayed-type hypersensitivity responses in TMEV-induced demyelinating disease: Correlation with clinical signs. J. Immunol. 1986, 136, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Clatch, R.J.; Lipton, H.L.; Miller, S.D. Class II-restricted T cell responses in Theiler’s murine encephalomyelitis virus (TMEV)-induced demyelinating disease. II. Survey of host immune responses and central nervous system virus titers in inbred mouse strains. Microb. Pathog. 1987, 3, 327–337. [Google Scholar] [CrossRef]
- Lipton, H.L.; Dal Canto, M.C. Chronic neurologic disease in Theiler’s virus infection of SJL/J mice. J. Neurol. Sci. 1976, 30, 201–207. [Google Scholar] [CrossRef]
- Lipton, H.L.; Melvold, R. Genetic analysis of susceptibility to Theiler’s virus-induced demyelinating disease in mice. J. Immunol. 1984, 132, 1821–1825. [Google Scholar] [CrossRef]
- Melvold, R.W.; Jokinen, D.M.; Miller, S.D.; Dal Canto, M.C.; Lipton, H.L. Identification of a locus on mouse chromosome 3 involved in differential susceptibility to Theiler’s murine encephalomyelitis virus-induced demyelinating disease. J. Virol. 1990, 64, 686–690. [Google Scholar] [CrossRef]
- Welsh, C.; Bustamante, L.; Nayak, M.; Welsh, T.; Dean, D.; Meagher, M. The effects of restraint stress on the neuropathogenesis of Theiler’s virus infection II: NK cell function and cytokine levels in acute disease. Brain Behav. Immun. 2004, 18, 166–174. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.; Green, J.; Pollard, J., Jr.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef]
- Calculating and Interpreting the p-Values for Functions, Pathways, and Lists in IPA Germantown, MD, USA: Qiagen; IPA 8.5. Available online: https://qiagen.my.salesforce-sites.com/KnowledgeBase/?id=kA41i000000L5pXCAS&categoryName=BioX (accessed on 31 October 2025).
- Rao, D.B.; Little, P.B.; Sills, R.C. Subsite awareness in neuropathology evaluation of National Toxicology Program (NTP) studies: A review of select neuroanatomical structures with their functional significance in rodents. Toxicol. Pathol. 2014, 42, 487–509. [Google Scholar] [CrossRef]
- Konganti, K.; Ehrlich, A.; Rusyn, I.; Threadgill, D.W. gQTL: A Web Application for QTL Analysis Using the Collaborative Cross Mouse Genetic Reference Population. G3 Genes|Genomes|Genet. 2018, 8, 2559–2562. [Google Scholar] [CrossRef]
- Kinsella, R.J.; Kähäri, A.; Haider, S.; Zamora, J.; Proctor, G.; Spudich, G.; Almeida-King, J.; Staines, D.; Derwent, P.; Kerhornou, A.; et al. Ensembl BioMarts: A hub for data retrieval across taxonomic space. Database 2011, 2011, bar030. [Google Scholar] [CrossRef]
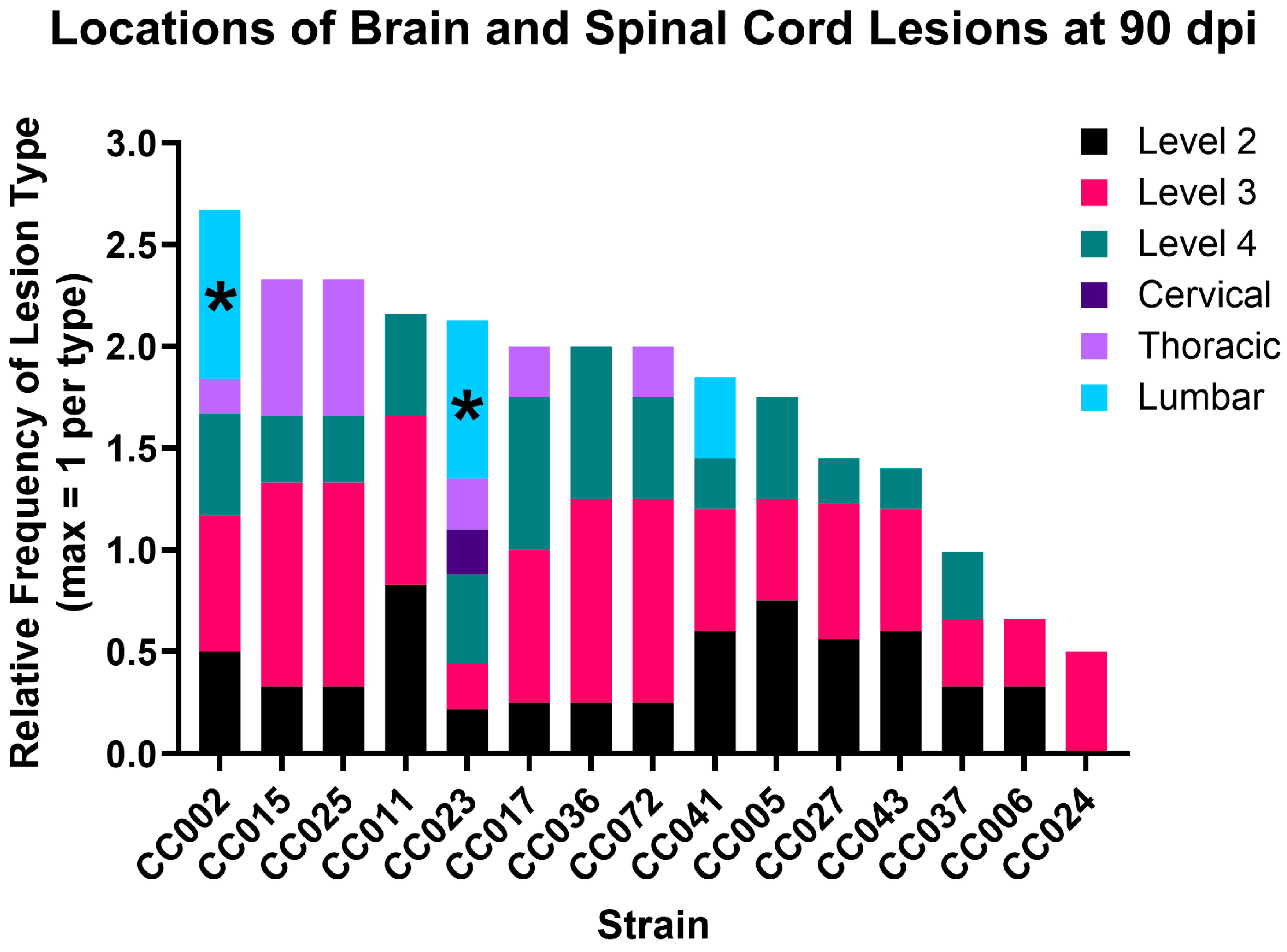
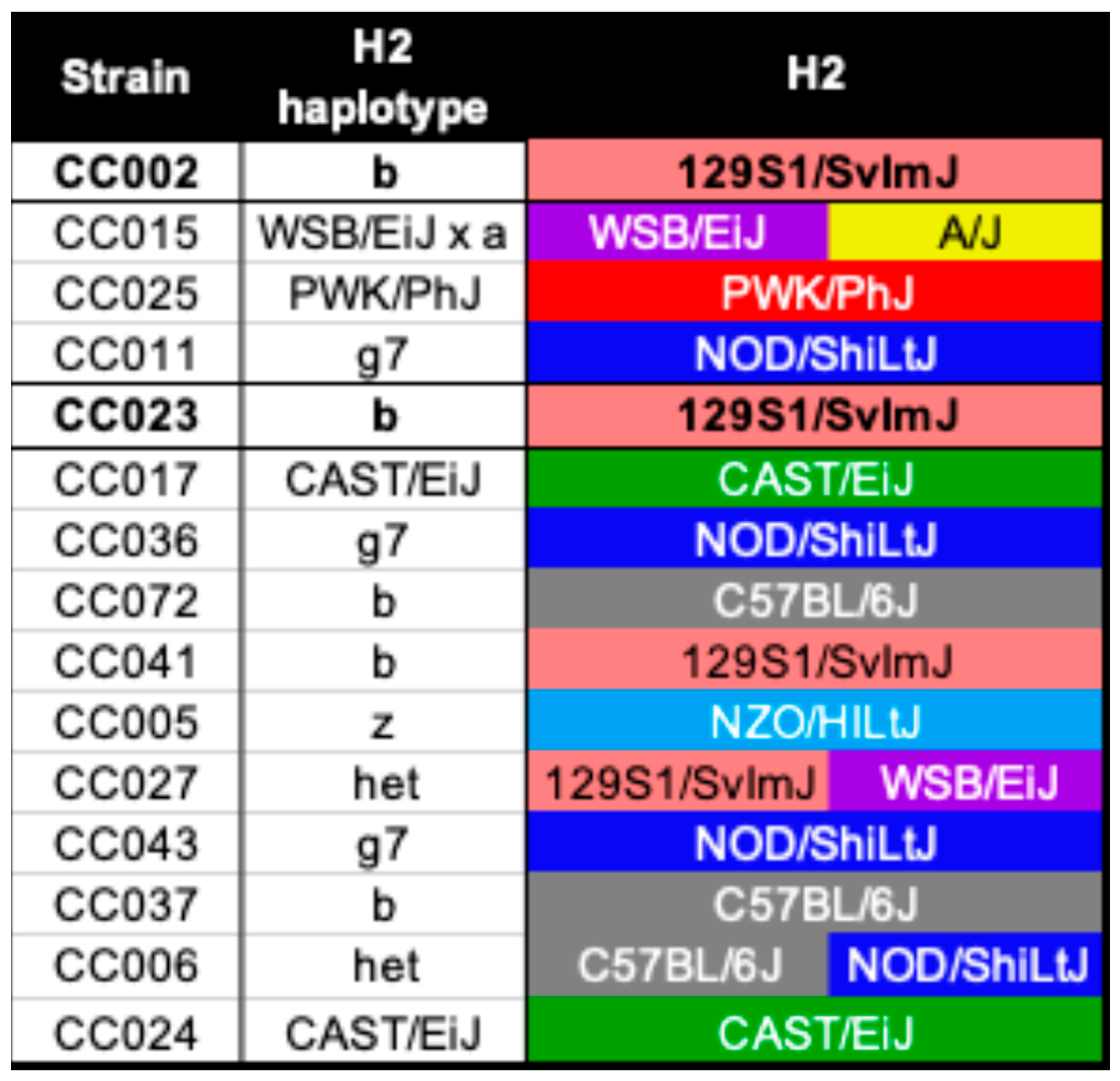
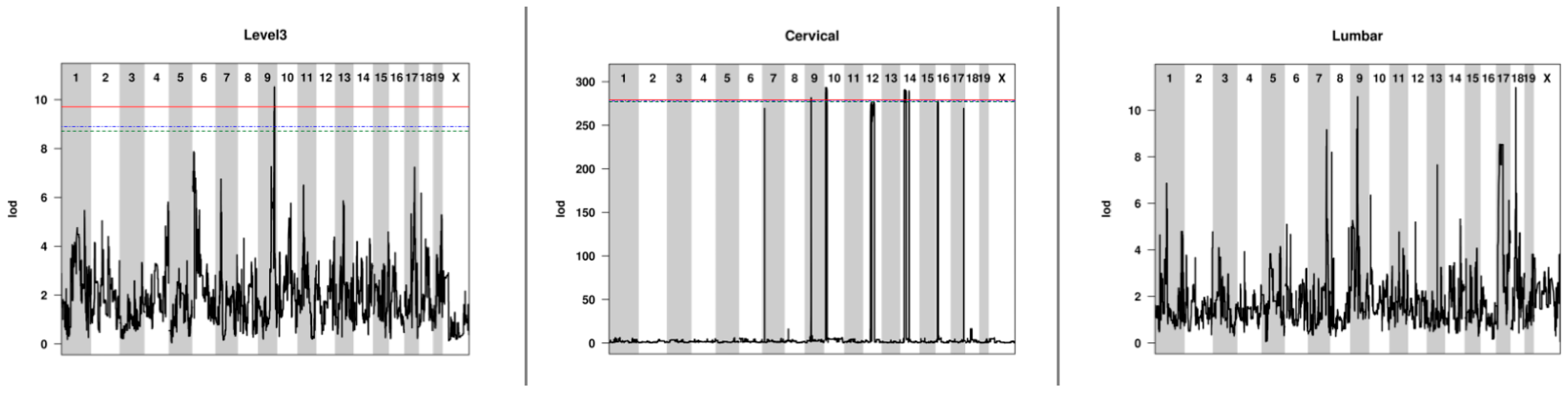
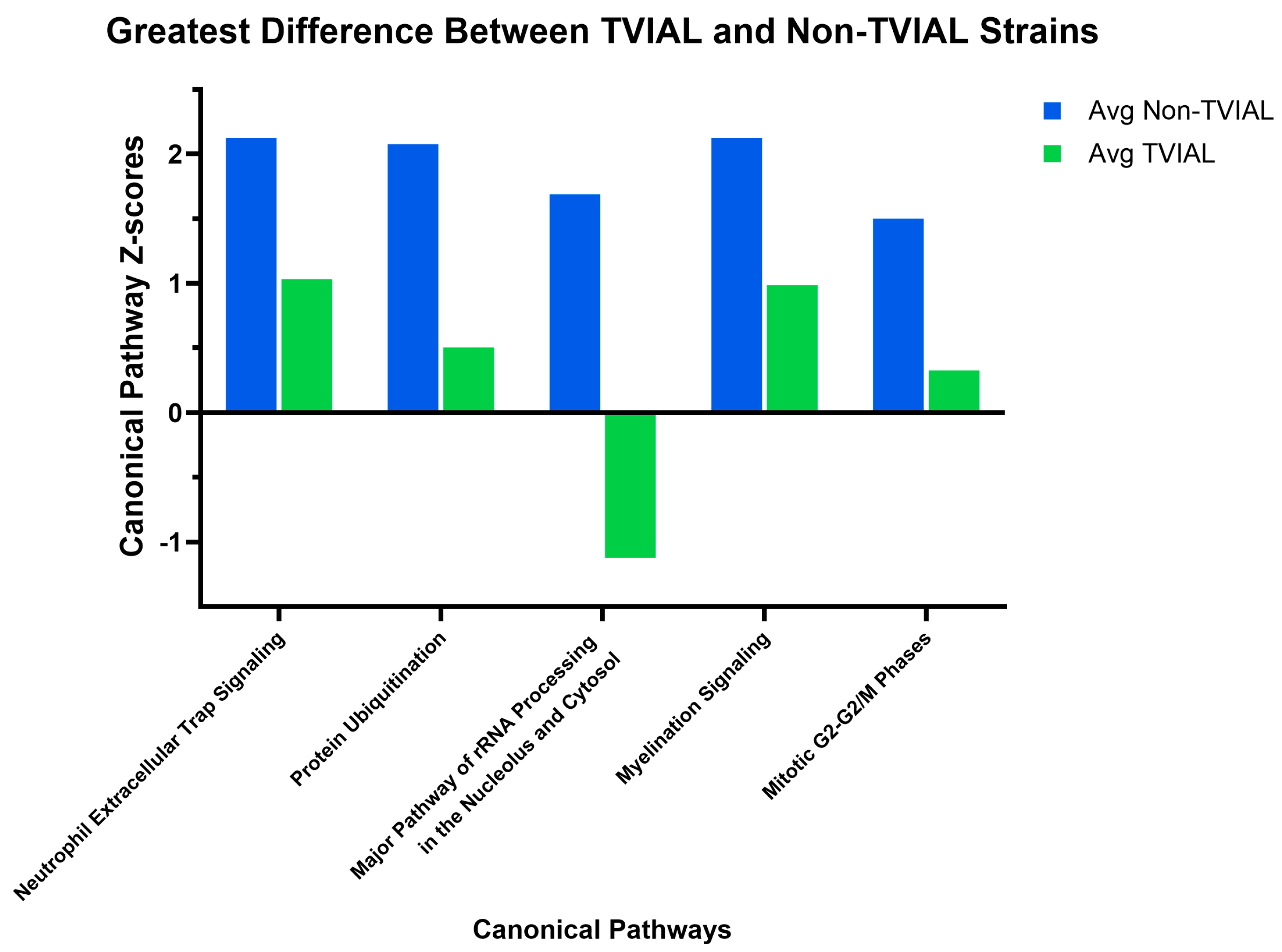
| Strain | Level 2 | Level 3 | Level 4 | Cervical | Thoracic | Lumbar |
|---|---|---|---|---|---|---|
| CC002 | 50% | 67% | 50% | 0% | 17% | 83% |
| CC005 | 75% | 50% | 50% | 0% | 0% | 0% |
| CC006 | 33% | 33% | 0% | 0% | 0% | 0% |
| CC011 | 83% | 83% | 50% | 0% | 0% | 0% |
| CC015 | 33% | 100% | 33% | 0% | 67% | 0% |
| CC017 | 25% | 75% | 75% | 0% | 25% | 0% |
| CC023 | 22% | 22% | 44% | 22% | 25% | 78% |
| CC024 | 0% | 50% | 0% | 0% | 0% | 0% |
| CC025 | 33% | 100% | 33% | 0% | 67% | 0% |
| CC027 | 56% | 67% | 22% | 0% | 0% | 0% |
| CC036 | 25% | 100% | 75% | 0% | 0% | 0% |
| CC037 | 33% | 33% | 33% | 0% | 0% | 0% |
| CC041 | 60% | 60% | 25% | 0% | 0% | 40% |
| CC043 | 60% | 60% | 20% | 0% | 0% | 0% |
| CC072 | 50% | 100% | 0% | 0% | 0% | 0% |
| Strain | TMEV-Infected F | TMEV-Infected M | Sham-Infected F | Sham-Infected M |
|---|---|---|---|---|
| CC002 | 4 (1) | 3 (2) | 3 (1) | 3 (1) |
| CC005 | 2 (2) | 2 (4) | 2 (3) | 2 (3) |
| CC006 | 2 (3) | 1 (2) | 1 (4) | 1 (2) |
| CC011 | 2 (3) | 4 (4) | 2 (3) | 2 (3) |
| CC015 | 1 (1) | 2 (2) | 2 (1) | 1 (2) |
| CC017 | 3 (1) | 1 (3) | 1 (2) | 2 (3) |
| CC023 | 5 (1) | 4 (1) | 4 (3) | 3 (2) |
| CC024 | 1 (1) | 1 (1) | 1 (1) | 1 (0) |
| CC025 | 3 (1) | 2 (1) | 1 (1) | 0 (0) |
| CC027 | 4 (3) | 5 (1) | 5 (2) | 5 (3) |
| CC036 | 1 (1) | 3 (6) | 1 (1) | 1 (2) |
| CC037 | 2 (3) | 1 (4) | 2 (2) | 2 (3) |
| CC041 | 0 (2) | 4 (2) | 0 (2) | 2 (0) |
| CC043 | 1 (0) | 2 (2) | 1 (1) | 1 (1) |
| CC072 | 0 (0) | 2 (2) | 0 (1) | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, T.W.; Perez-Gomez, A.; Lawley, K.; Young, C.R.; Welsh, C.J.; Brinkmeyer-Langford, C.L. Comparative Analysis of Genetic Risk for Viral-Induced Axonal Loss in Genetically Diverse Mice. Int. J. Mol. Sci. 2025, 26, 10727. https://doi.org/10.3390/ijms262110727
Kang TW, Perez-Gomez A, Lawley K, Young CR, Welsh CJ, Brinkmeyer-Langford CL. Comparative Analysis of Genetic Risk for Viral-Induced Axonal Loss in Genetically Diverse Mice. International Journal of Molecular Sciences. 2025; 26(21):10727. https://doi.org/10.3390/ijms262110727
Chicago/Turabian StyleKang, Tae Wook, Aracely Perez-Gomez, Koedi Lawley, Colin R. Young, C. Jane Welsh, and Candice L. Brinkmeyer-Langford. 2025. "Comparative Analysis of Genetic Risk for Viral-Induced Axonal Loss in Genetically Diverse Mice" International Journal of Molecular Sciences 26, no. 21: 10727. https://doi.org/10.3390/ijms262110727
APA StyleKang, T. W., Perez-Gomez, A., Lawley, K., Young, C. R., Welsh, C. J., & Brinkmeyer-Langford, C. L. (2025). Comparative Analysis of Genetic Risk for Viral-Induced Axonal Loss in Genetically Diverse Mice. International Journal of Molecular Sciences, 26(21), 10727. https://doi.org/10.3390/ijms262110727







