A New Approach for Achieving Earlier and More Accurate Diagnosis of Connective Tissue Disease-Related Interstitial Lung Disease: TGFB and PDGFA as Novel Promising Biomarkers
Abstract
1. Introduction
2. Results
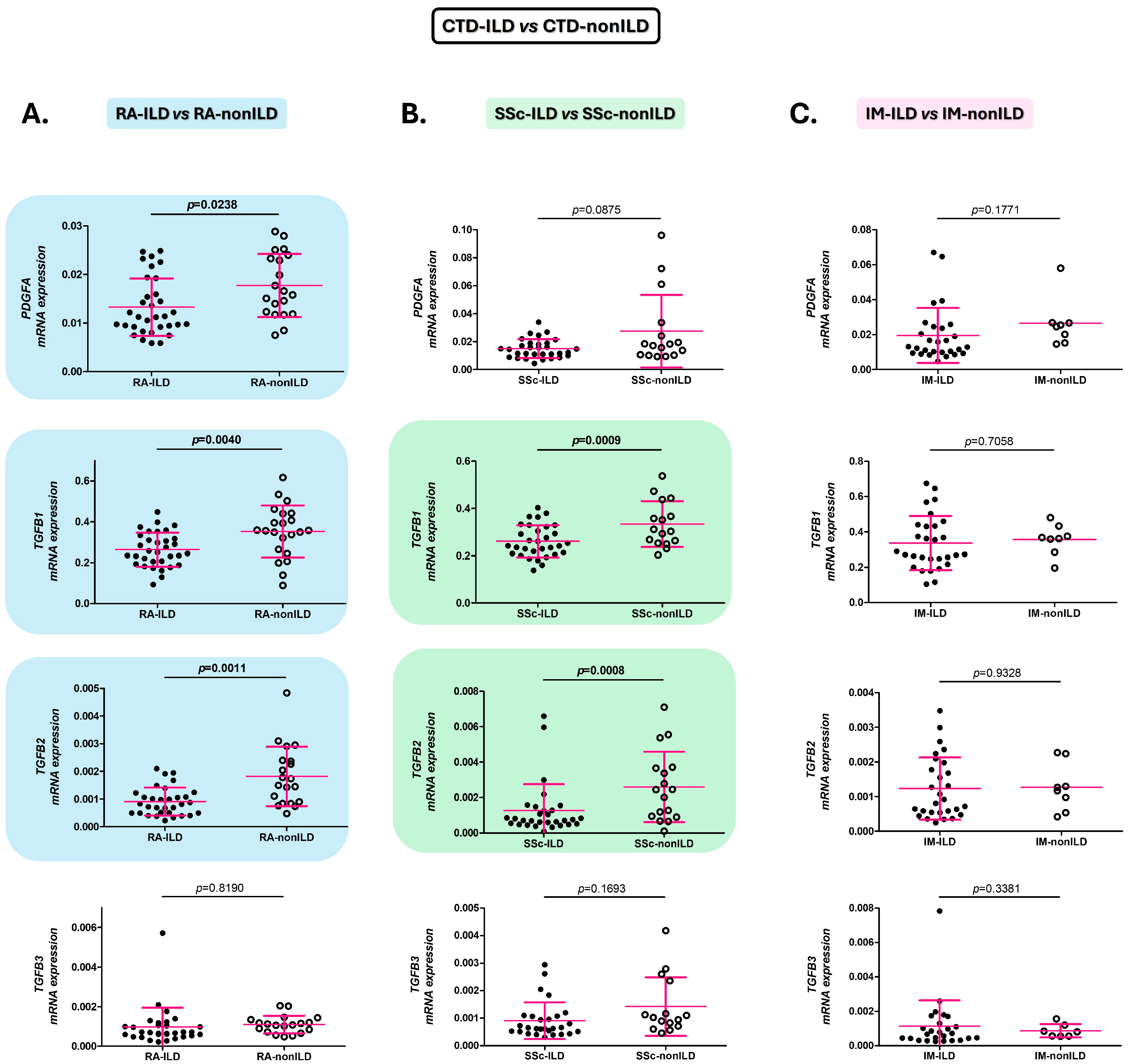
2.1. Alterations of the PDGFA, TGFB1, and TGFB2 Expression in the Blood Are Associated with the Presence of ILD in RA and SSc Patients
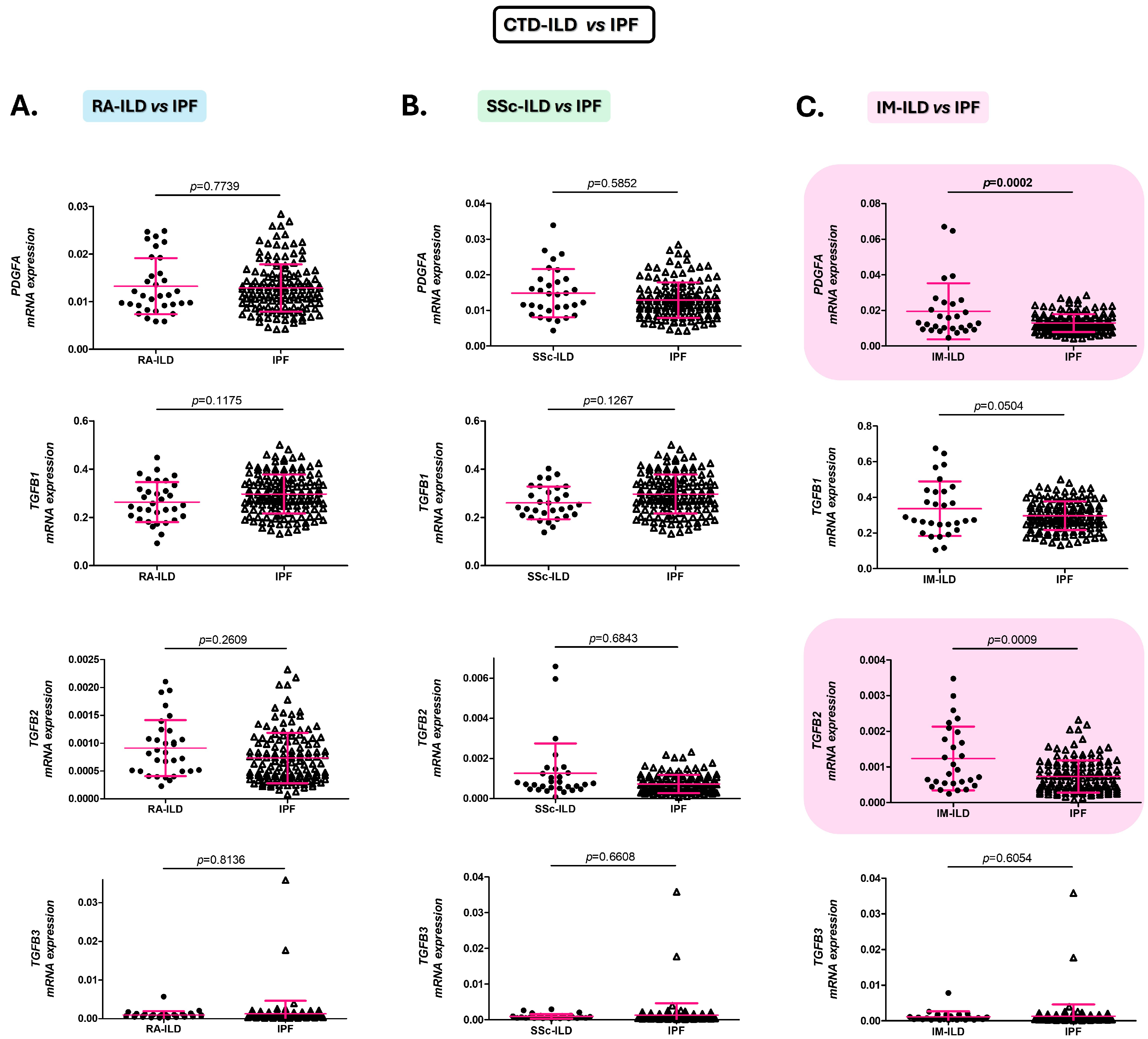
2.2. Alterations of the PDGFA and TGFB2 Expression in the Blood Distinguish Patients with IM-ILD from Those with IPF
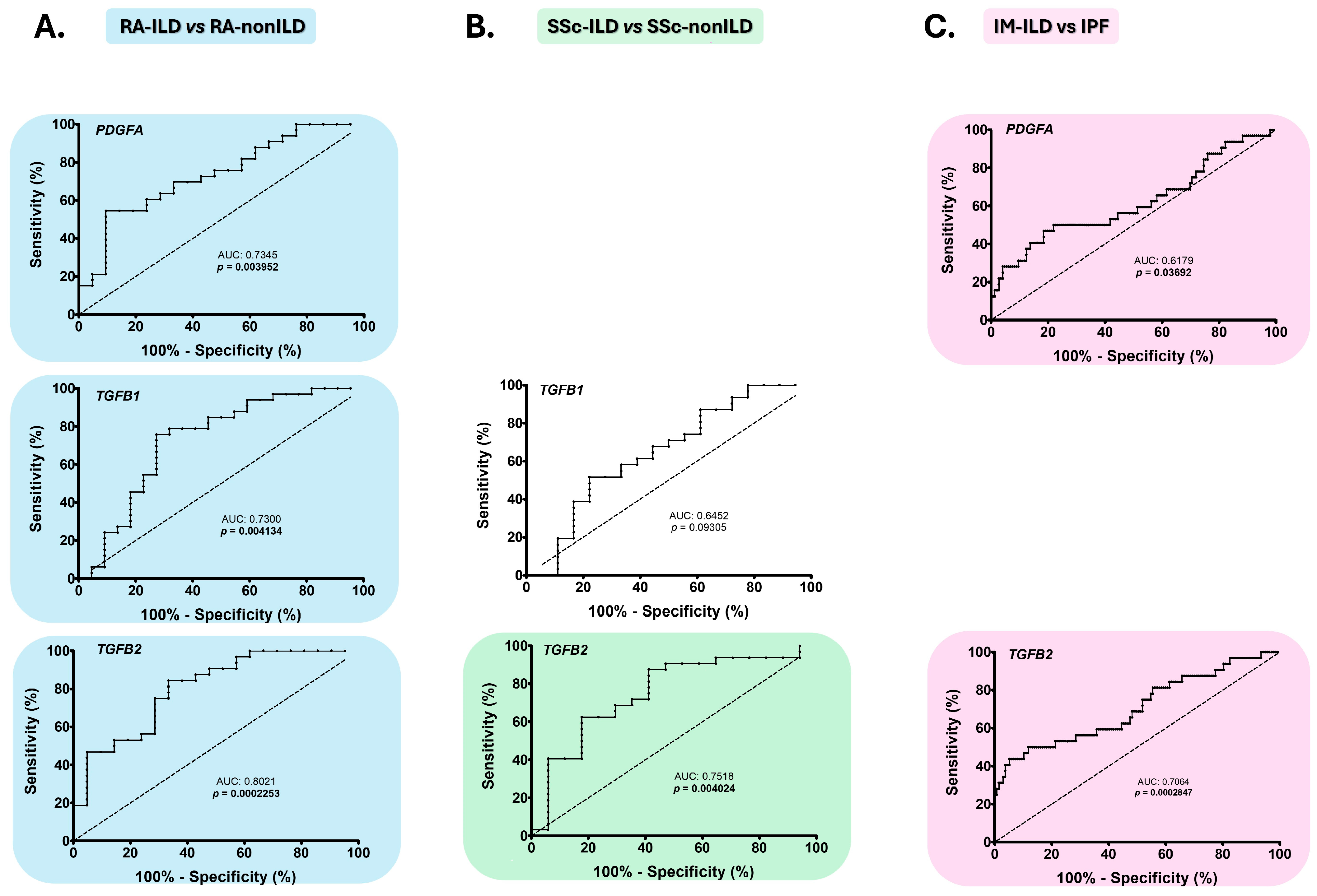
2.3. PDGFA, TGFB1, and TGFB2 as Early Diagnostic Biomarkers of the Presence of ILD in RA and SSc
2.4. PDGFA and TGFB2 as Biomarkers for the Differential Diagnosis of IM-ILD and IPF Patients
2.5. Changes in PDGFA, TGFB2, and TGFB3 Expression Are Associated with the Prognosis of RA-ILD and SSc-ILD Patients
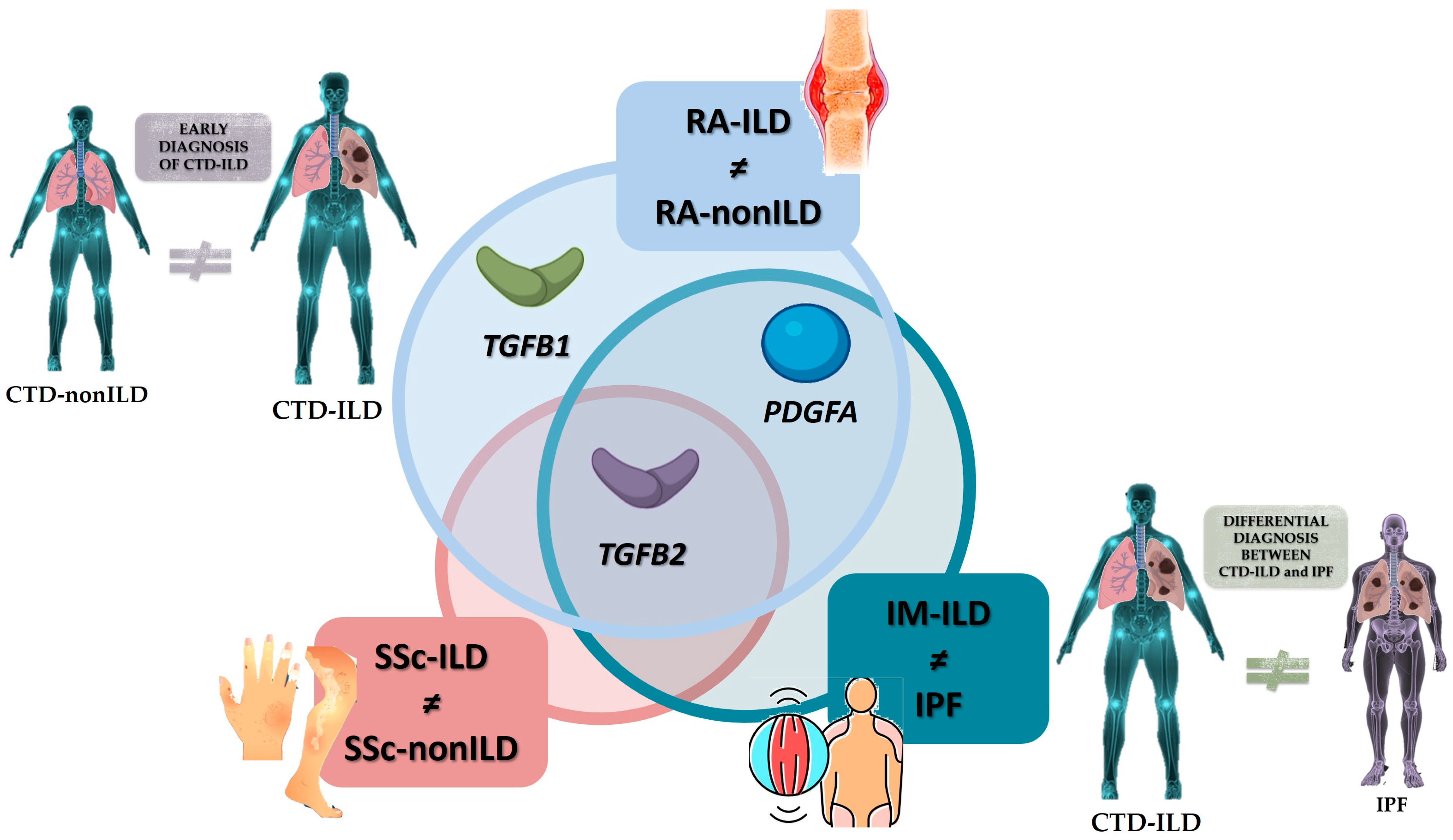
3. Discussion
4. Materials and Methods
4.1. Patient Populations
4.2. TGFB1, TGFB2, TGFB3, and PDGFA mRNA Expression Studies
4.3. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACA | anti-centromere antibodies. |
| ACPA | anti-cyclic citrullinated peptide antibodies. |
| ACR | American College of Rheumatology. |
| ANA | anti-nuclear antibodies. |
| Anti-Jo-1 | anti-histidyl tRNA synthetase antibodies. |
| Anti-PL-7 | anti-threonyl tRNA synthetase antibodies. |
| Anti-PL-12 | anti-alanyl tRNA synthetase antibodies. |
| ATA | anti-topoisomerase I antibodies. |
| ATS | American Thoracic Society. |
| ANOVA | analysis of variance. |
| AUC | area under the curve. |
| bDMARDs | biologic disease-modifying anti-rheumatic drugs. |
| CI | confident interval. |
| csDMARDs | conventional synthetic disease-modifying anti-rheumatic drugs. |
| CTDs | connective tissue diseases. |
| ERS | European Respiratory Society. |
| EULAR | European League Against Rheumatism. |
| FEV1 | forced expiratory volume in one second. |
| FVC | forced vital capacity. |
| HRCT | high-resolution computed tomography. |
| ILD | interstitial lung disease. |
| IM | inflammatory myopathies. |
| IPF | idiopathic pulmonary fibrosis. |
| PDGF | platelet-derived growth factor. |
| PFTs | pulmonary function tests. |
| RA | rheumatoid arthritis. |
| ROC | receiver operating characteristic. |
| SD | standard deviation. |
| SSc | systemic sclerosis. |
| TGF-β | transforming growth factor-β. |
| UIP | usual interstitial pneumonia.: |
References
- Spagnolo, P.; Distler, O.; Ryerson, C.J.; Tzouvelekis, A.; Lee, J.S.; Bonella, F.; Bouros, D.; Hoffmann-Vold, A.M.; Crestani, B.; Matteson, E.L. Mechanisms of Progressive Fibrosis in Connective Tissue Disease (CTD)-Associated Interstitial Lung Diseases (ILDs). Ann. Rheum. Dis. 2021, 80, 143–150. [Google Scholar] [CrossRef]
- Shi, X.; Young, C.D.; Zhou, H.; Wang, X. Transforming Growth Factor-β Signaling in Fibrotic Diseases and Cancer-Associated Fibroblasts. Biomolecules 2020, 10, 1666. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Transforming Growth Factor–ß in Tissue Fibrosis. J. Exp. Med. 2020, 217, e20190103. [Google Scholar] [CrossRef]
- Pardali, E.; Sanchez-Duffhues, G.; Gomez-Puerto, M.C.; Ten Dijke, P. TGF-β-Induced Endothelial-Mesenchymal Transition in Fibrotic Diseases. Int. J. Mol. Sci. 2017, 18, 2157. [Google Scholar] [CrossRef]
- Cerro Chiang, G.; Parimon, T. Understanding Interstitial Lung Diseases Associated with Connective Tissue Disease (CTD-ILD): Genetics, Cellular Pathophysiology, and Biologic Drivers. Int. J. Mol. Sci. 2023, 24, 2405. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Zou, J.; Hu, X.; Zheng, W.; Zhang, M.; Cheng, Z. Latent Transforming Growth Factor-β Binding Protein-2 Regulates Lung Fibroblast-to-Myofibroblast Differentiation in Pulmonary Fibrosis via NF-ΚB Signaling. Front. Pharmacol. 2021, 12, 788714. [Google Scholar] [CrossRef] [PubMed]
- Solinc, J.; Ribot, J.; Soubrier, F.; Pavoine, C.; Dierick, F.; Nadaud, S. The Platelet-Derived Growth Factor Pathway in Pulmonary Arterial Hypertension: Still an Interesting Target? Life 2022, 12, 658. [Google Scholar] [CrossRef]
- Atzeni, F.; Gerardi, M.C.; Barilaro, G.; Masala, I.F.; Benucci, M.; Sarzi-puttini, P. Interstitial Lung Disease in Systemic Autoimmune Rheumatic Diseases: A Comprehensive Review. Expert Rev. Clin. Immunol. 2018, 14, 69–82. [Google Scholar] [CrossRef]
- Cottin, V.I. Idiopathic Interstitial Pneumonias with Connective Tissue Diseases Features: A Review. Respirology 2016, 21, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Mathai, S.C.; Danoff, S.K. Management of Interstitial Lung Disease Associated with Connective Tissue Disease. BMJ 2016, 352, h6819. [Google Scholar] [CrossRef]
- Matson, S.M.; Demoruelle, M.K. Connective Tissue Disease Associated Interstitial Lung Disease. Rheum. Dis. Clin. N. Am. 2024, 50, 423–438. [Google Scholar] [CrossRef]
- Guiot, J.; Miedema, J.; Cordeiro, A.; De Vries-Bouwstra, J.K.; Dimitroulas, T.; Søndergaard, K.; Tzouvelekis, A.; Smith, V. Practical Guidance for the Early Recognition and Follow-up of Patients with Connective Tissue Disease-Related Interstitial Lung Disease. Autoimmun. Rev. 2024, 23, 103582. [Google Scholar] [CrossRef]
- Hariri, L.P.; Roden, A.C.; Chung, J.H.; Danoff, S.K.; Gomez Manjarres, D.C.; Hartwig, M.; Kheir, F.; King, C.; Kreider, M.; Lynch, D.A.; et al. The Role of Surgical Lung Biopsy in the Diagnosis of Fibrotic Interstitial Lung Disease: Perspective from the Pulmonary Fibrosis Foundation. Ann. Am. Thorac. Soc. 2021, 18, 1601–1609. [Google Scholar] [CrossRef]
- Enomoto, N. Relationship between Idiopathic Interstitial Pneumonias (IIPs) and Connective Tissue Disease-Related Interstitial Lung Disease (CTD-ILD): A Narrative Review. Respir. Investig. 2024, 62, 465–480. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, Z.; Wan, N.; Yang, L.; Jin, Z.; Jin, F.; Huang, Z.; Chen, M.; Wang, H.; Feng, J. Potential Biomarkers for Diagnosis and Disease Evaluation of Idiopathic Pulmonary Fibrosis. Chin. Med. J. 2023, 136, 1278–1290. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Liu, C.; Tan, C.; Zhang, J. Predictive Biomarkers of Disease Progression in Idiopathic Pulmonary Fibrosis. Heliyon 2023, 10, e23543. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Handa, R. Management of Connective Tissue Disease–Related Interstitial Lung Disease. Curr. Pulmonol. Rep. 2022, 11, 86–98. [Google Scholar] [CrossRef]
- Stainer, A.; Tonutti, A.; De Santis, M.; Amati, F.; Ceribelli, A.; Bongiovanni, G.; Torrisi, C.; Iacopino, A.; Mangiameli, G.; Aliberti, S.; et al. Unmet Needs and Perspectives in Rheumatoid Arthritis-Associated Interstitial Lung Disease: A Critical Review. Front. Med. 2023, 10, 1129939. [Google Scholar] [CrossRef]
- Hoffmann-Vold, A.M.; Fretheim, H.; Meier, C.; Maurer, B. Circulating Biomarkers of Systemic Sclerosis—Interstitial Lung Disease. J. Scleroderma Relat. Disord. 2020, 5, 41–47. [Google Scholar] [CrossRef]
- Pulito-Cueto, V.; Genre, F.; López-Mejías, R.; Mora-Cuesta, V.M.; Iturbe-Fernández, D.; Portilla, V.; Sebastián Mora-Gil, M.; Ocejo-Vinyals, J.G.; Gualillo, O.; Blanco, R.; et al. Endothelin-1 as a Biomarker of Idiopathic Pulmonary Fibrosis and Interstitial Lung Disease Associated with Autoimmune Diseases. Int. J. Mol. Sci. 2023, 24, 1275. [Google Scholar] [CrossRef]
- Pulito-Cueto, V.; Remuzgo-Martínez, S.; Genre, F.; Atienza-Mateo, B.; Mora-Cuesta, V.M.; Iturbe-Fernández, D.; Lera-Gómez, L.; Sebastián Mora-Gil, M.; Prieto-Peña, D.; Portilla, V.; et al. Elevated VCAM-1, MCP-1 and ADMA Serum Levels Related to Pulmonary Fibrosis of Interstitial Lung Disease Associated with Rheumatoid Arthritis. Front. Mol. Biosci. 2022, 9, 1056121. [Google Scholar] [CrossRef] [PubMed]
- Pulito-Cueto, V.; Remuzgo-Martínez, S.; Genre, F.; Atienza-Mateo, B.; Mora-Cuesta, V.M.; Iturbe-Fernández, D.; Lera-Gómez, L.; Mora-Gil, M.S.; Portilla, V.; Corrales, A.; et al. E-Selectin, ICAM-1, and ET-1 Biomarkers Address the Concern of the Challenging Diagnosis of Interstitial Lung Disease in Patients with Autoimmune Diseases. Int. J. Mol. Sci. 2023, 24, 12518. [Google Scholar] [CrossRef]
- Antoniou, K.M.; Margaritopoulos, G.; Economidou, F.; Siafakas, N.M. Pivotal Clinical Dilemmas in Collagen Vascular Diseases Associated with Interstitial Lung Involvement. Eur. Respir. J. 2009, 33, 882–896. [Google Scholar] [CrossRef]
- Ysamat Marfá, R.; Benito Ysamat, A.; Espejo Pérez, S.; Blanco Negredo, M.; Roldán Molina, R. Lung Disease Associated with Connective Tissue Disease. Radiologia 2013, 55, 107–117. [Google Scholar] [CrossRef]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and Mechano: Regulation of Connective Tissue Remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, L.; Wang, M.; Zhou, S.; Lu, Y.; Cui, H.; Racanelli, A.C.; Zhang, L.; Ye, T.; Ding, B.; et al. Targeting Fibrosis, Mechanisms and Clinical Trials. Signal Transduct. Target. Ther. 2022, 7, 206. [Google Scholar] [CrossRef]
- Biernacka, A.; Dobaczewski, M.; Frangogiannis, N.G. TGF-β Signaling in Fibrosis. Growth Factors 2011, 29, 196–202. [Google Scholar] [CrossRef]
- Van Kalsbeek, D.; Brooks, R.; Shaver, D.; Ebel, A.; Hershberger, D.; Schmidt, C.; Poole, J.A.; Ascherman, D.P.; Thiele, G.M.; Mikuls, T.R.; et al. Peripheral Blood Biomarkers for Rheumatoid Arthritis–Associated Interstitial Lung Disease: A Systematic Review. ACR Open Rheumatol. 2023, 5, 201–226. [Google Scholar] [CrossRef]
- Ji, Y.; Huang, J.; Zong, J.; Lü, D.; Xin, C.; He, J.; Chen, Y.; Chen, X.; Yu, S.; Li, J. The Serum Levels of Cytokines in Patients Withrheumatoid Arthritis Associated Interstitial Lungdisease and Their Clinical Significance. Zhonghua Jie He He Hu Xi Za Zhi 2008, 31, 264–267. [Google Scholar] [PubMed]
- Jian, X.; Guo, G.; Ruan, Y.; Zhao, B.; Wang, Y.; Ning, Q.; Zhang, Y. Clinical Observation of Rheumatoid Arthritis Associated Interstitial Lung Disease Patients and Changes of Serum Cytokines Thereof. Zhonghua Yi Xue Za Zhi 2008, 88, 1884–1887. [Google Scholar] [PubMed]
- Gochuico, B.R.; Avila, N.A.; Chow, C.K.; Novero, L.J.; Wu, H.P.; Ren, P.; MacDonald, S.D.; Travis, W.D.; Stylianou, M.P.; Rosas, I.O. Progressive Preclinical Interstitial Lung Disease in Rheumatoid Arthritis. Arch. Intern. Med. 2008, 168, 159–166. [Google Scholar] [CrossRef]
- Bonella, F.; Patuzzo, G.; Lunardi, C. Biomarker Discovery in Systemic Sclerosis: State of the Art. Curr. Biomark. Find. 2015, 5, 47–68. [Google Scholar] [CrossRef]
- Silver, R.M.; Wells, A.U. Histopathology and Bronchoalveolar Lavage. Rheumatology 2008, 47, v62–v64. [Google Scholar] [CrossRef] [PubMed]
- Sargent, J.L.; Milano, A.; Bhattacharyya, S.; Varga, J.; Connolly, M.K.; Chang, H.Y.; Whitfield, M.L. A TGFΒ-Responsive Gene Signature Is Associated with a Subset of Diffuse Scleroderma with Increased Disease Severity. J. Investig. Dermatol. 2010, 130, 694–705. [Google Scholar] [CrossRef]
- Muruganandam, M.; Ariza-Hutchinson, A.; Patel, R.A.; Sibbitt, W.L. Biomarkers in the Pathogenesis, Diagnosis, and Treatment of Systemic Sclerosis. J. Inflamm. Res. 2023, 16, 4633–4660. [Google Scholar] [CrossRef]
- Christmann, R.B.; Sampaio-Barros, P.; Stifano, G.; Borges, C.L.; De Carvalho, C.R.; Kairalla, R.; Parra, E.R.; Spira, A.; Simms, R.; Capellozzi, V.L.; et al. Association of Interferon- and Transforming Growth Factor β-Regulated Genes and Macrophage Activation with Systemic Sclerosis-Related Progressive Lung Fibrosis. Arthritis Rheumatol. 2014, 66, 714–725. [Google Scholar] [CrossRef]
- Desai, B.; Mattson, J.; Paintal, H.; Nathan, M.; Shen, F.; Beaumont, M.; Malinao, M.C.; Li, Y.; Canfield, J.; Basham, B.; et al. Differential Expression of Monocyte/Macrophage-Selective Markers in Human Idiopathic Pulmonary Fibrosis. Exp. Lung Res. 2011, 37, 227–238. [Google Scholar] [CrossRef]
- da Silva, T.C.P.; Silva, M.G.; Shinjo, S.K. Relevance of Serum Angiogenic Cytokines in Adult Patients with Dermatomyositis. Adv. Rheumatol. 2018, 58, 17. [Google Scholar] [CrossRef] [PubMed]
- Chanda, D.; Otoupalova, E.; Smith, S.R.; Volckaert, T.; De Langhe, S.P.; Thannickal, V.J. Developmental Pathways in the Pathogenesis of Lung Fibrosis. Mol. Aspects Med. 2019, 65, 56–69. [Google Scholar] [CrossRef]
- Inui, N.; Sakai, S.; Kitagawa, M. Molecular Pathogenesis of Pulmonary Fibrosis, with Focus on Pathways Related to Tgf-β and the Ubiquitin-Proteasome Pathway. Int. J. Mol. Sci. 2021, 22, 6107. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Hu, Y. TGF-Β1: Gentlemanly Orchestrator in Idiopathic Pulmonary Fibrosis (Review). Int. J. Mol. Med. 2021, 48, 132. [Google Scholar] [CrossRef] [PubMed]
- Burks, T.N.; Cohn, R.D. Role of TGF-β Signaling in Inherited and Acquired Myopathies. Skelet. Muscle 2011, 1, 19. [Google Scholar] [CrossRef] [PubMed]
- Shimojima, Y.; Ishii, W.; Matsuda, M.; Ikeda, S.I. Phenotypes of Peripheral Blood Lymphocytes and Cytokine Expression in Polymyositis and Dermatomyositis before Treatment and after Clinical Remission. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2012, 5, 77–87. [Google Scholar] [CrossRef]
- White, N.; Parsons, R.; Collins, G.; Barnett, A. Evidence of Questionable Research Practices in Clinical Prediction Models. BMC Med. 2023, 21, 339. [Google Scholar] [CrossRef]
- Akiyama, M.; Kaneko, Y. Pathogenesis, Clinical Features, and Treatment Strategy for Rheumatoid Arthritis-Associated Interstitial Lung Disease. Autoimmun. Rev. 2022, 21, 103056. [Google Scholar] [CrossRef]
- Jacquerie, P.; Henket, M.; André, B.; Moermans, C.; de Seny, D.; Gester, F.; Louis, R.; Malaise, M.; Guiot, J. Inflammatory Profile of Induced Sputum Composition in Systemic Sclerosis and Comparison with Healthy Volunteers. Sci. Rep. 2021, 11, 10679. [Google Scholar] [CrossRef]
- Sun, T.; Huang, Z.; Liang, W.-C.; Yin, J.; Lin, W.Y.; Wu, J.; Vernes, J.-M.; Lutman, J.; Caplazi, P.; Jeet, S.; et al. TGFb2 and TGFb3 Isoforms Drive Fibrotic Disease Pathogenesis. Sci. Translacional Med. 2021, 13, eabe0407. [Google Scholar] [CrossRef]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O.; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid Arthritis Classification Criteria: An American College of Rheumatology/European League Against Rheumatism Collaborative Initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef]
- Van Den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-cerinic, M.; Naden, R.P.; Medsger, T.A., Jr.; Carreira, P.E.; et al. Arthritis & Rheumatism 2013 Classification Criteria for Systemic Sclerosis. Arthritis Rheum. 2013, 65, 2737–2747. [Google Scholar] [CrossRef]
- Lundberg, I.E.; Tjärnlund, A.; Bottai, M.; Werth, V.P.; Pilkington, C.; de Visser, M.; Alfredsson, L.; Amato, A.A.; Barohn, R.J.; Liang, M.H.; et al. 2017 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Adult and Juvenile Idiopathic Inflammatory Myopathies and Their Major Subgroups. Arthritis Rheumatol. 2017, 69, 2271–2282. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Costabel, U.; Hansell, D.M.; King, T.E.; Lynch, D.A.; Nicholson, A.G.; Ryerson, C.J.; Ryu, J.H.; Selman, M.; Wells, A.U.; et al. An Official American Thoracic Society/European Respiratory Society Statement: Update of the International Multidisciplinary Classification of the Idiopathic Interstitial Pneumonias. Am. J. Respir. Crit. Care Med. 2013, 188, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Antoniou, K.M.; Bissell, B.D.; Bouros, D.; Buendia-Roldan, I.; Caro, F.; Crestani, B.; et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2022, 205, E18–E47. [Google Scholar] [CrossRef] [PubMed]
- Lynch, D.A.; Sverzellati, N.; Travis, W.D.; Brown, K.K.; Colby, T.V.; Galvin, J.R.; Goldin, J.G.; Hansell, D.M.; Inoue, Y.; Johkoh, T.; et al. Diagnostic Criteria for Idiopathic Pulmonary Fibrosis: A Fleischner Society White Paper. Lancet Respir. Med. 2018, 6, 138–153. [Google Scholar] [CrossRef]
- Cottin, V. Treatment of Progressive Fibrosing Interstitial Lung Diseases: A Milestone in the Management of Interstitial Lung Diseases. Eur. Respir. Rev. 2019, 28, 190109. [Google Scholar] [CrossRef]
- Van Den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A., Jr.; Carreira, P.E.; et al. 2013 Classification Criteria for Systemic Sclerosis: An American College of Rheumatology/European League against Rheumatism Collaborative Initiative. Ann. Rheum. Dis. 2013, 72, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
| PDGFA | TGFB1 | TGFB2 | TGFB3 | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | r | p | r | p | r | p | r | p |
| RA duration | 0.23 | 0.24 | 0.37 | 0.05 | −0.07 | 0.74 | 0.06 | 0.78 |
| ILD duration | −0.06 | 0.77 | 0.17 | 0.38 | −0.15 | 0.42 | −0.09 | 0.63 |
| FVC (% predicted) | −0.21 | 0.28 | −0.22 | 0.26 | −0.16 | 0.40 | 0.22 | 0.25 |
| FEV1 (% predicted) | −0.19 | 0.31 | −0.27 | 0.16 | −0.21 | 0.29 | 0.35 | 0.07 |
| DLCO (% predicted) | 0.04 | 0.88 | −0.36 | 0.13 | 0.17 | 0.48 | −0.06 | 0.82 |
| Category | MEAN ± SD | p | MEAN ± SD | p | MEAN ± SD | p | MEAN ± SD | p |
| RF− | 0.0162 ± 0.0067 | 0.43 | 0.3314 ± 0.0213 | 0.16 | 0.0011 ± 0.0006 | 0.97 | 0.0008 ± 0.0002 | 0.65 |
| RF+ | 0.0130 ± 0.0060 | 0.2531 ± 0.0833 | 0.0012 ± 0.0018 | 0.0077 ± 0.0353 | ||||
| ACPA− | 0.0131 ± 0.0073 | 0.89 | 0.2560 ± 0.0772 | 0.95 | 0.0032 ± 0.0048 | 0.01 | 0.0018 ± 0.0026 | 0.97 |
| ACPA+ | 0.0134 ± 0.0060 | 0.2611 ± 0.0847 | 0.0009 ± 0.0005 | 0.0078 ± 0.0360 | ||||
| UIP pattern, probable UIP pattern or Indeterminate for UIP pattern | 0.0120 ± 0.0058 | 0.04 | 0.2499 ± 0.0794 | 0.17 | 0.0013 ± 0.0020 | 0.72 | 0.0010 ± 0.0011 | 0.52 |
| NSIP pattern or Non-NSIP pattern | 0.0161 ± 0.0053 | 0.2939 ± 0.0867 | 0.0010 ± 0.0052 | 0.0196 ± 0.0592 | ||||
| Progressive pulmonary fibrosis− | 0.0137 ± 0.0057 | 0.33 | 0.2739 ± 0.0778 | 0.09 | 0.0012 ± 0.0018 | 0.80 | 0.0077 ± 0.0353 | 0.92 |
| Progressive pulmonary fibrosis+ | 0.0109 ± 0.0073 | 0.2032 ± 0.0938 | 0.0010 ± 0.0007 | 0.0008 ± 0.0004 | ||||
| Antifibrotic treatment− | 0.0134 ± 0.0059 | 0.37 | 0.2650 ± 0.0836 | 0.48 | 0.0012 ± 0.0017 | 0.57 | 0.0070 ± 0.0359 | 0.95 |
| Antifibrotic treatment+ | 0.0080 ± 0.0000 | 0.2052 ± 0.0000 | 0.0019 ± 0.0000 | 0.0014 ± 0.0000 | ||||
| sDMARDs− | 0.0118 ± 0.0040 | 0.31 | 0.2595 ± 0.0830 | 0.90 | 0.0009 ± 0.0005 | 0.29 | 0.0008 ± 0.0004 | 0.40 |
| sDMARDs+ | 0.0140 ± 0.0068 | 0.2649 ± 0.0876 | 0.0015 ± 0.0023 | 0.0121 ± 0.0453 | ||||
| bDMARDs− | 0.0125 ± 0.0048 | 0.58 | 0.2425 ± 0.0730 | 0.08 | 0.0009 ± 0.0005 | 0.28 | 0.0106 ± 0.0429 | 0.52 |
| bDMARDs+ | 0.0136 ± 0.0071 | 0.2954 ± 0.0938 | 0.0017 ± 0.0028 | 0.0012 ± 0.0015 | ||||
| PDGFA | TGFB1 | TGFB2 | TGFB3 | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | r | p | r | p | r | p | r | p |
| SSc duration | 0.04 | 0.84 | −0.15 | 0.45 | −0.13 | 0.53 | −0.02 | 0.91 |
| ILD duration | −0.12 | 0.54 | −0.15 | 0.46 | −0.22 | 0.27 | −0.06 | 0.78 |
| FVC (% predicted) | 0.24 | 0.22 | 0.07 | 0.74 | 0.35 | 0.08 | −0.21 | 0.33 |
| FEV1 (% predicted) | 0.30 | 0.13 | 0.16 | 0.42 | 0.40 | 0.04 | −0.18 | 0.39 |
| DLCO (% predicted) | 0.09 | 0.70 | −0.18 | 0.44 | 0.22 | 0.38 | −0.21 | 0.40 |
| Category | MEAN ± SD | p | MEAN ± SD | p | MEAN ± SD | p | MEAN ± SD | p |
| ANAs− | 0.0044 ± 0.0000 | 0.10 | 0.3220 ± 0.0000 | 0.71 | 0.0001 ± 0.0000 | 0.30 | 0.0004 ± 0.0000 | 0.63 |
| ANA+ | 0.0155 ± 0.0652 | 0.2604 ± 0.6789 | 0.0013 ± 0.0015 | 0.0028 ± 0.0096 | ||||
| ACA− | 0.0154 ± 0.0067 | 0.46 | 0.2624 ± 0.0688 | 0.94 | 0.0013 ± 0.0015 | 0.66 | 0.0028 ± 0.0096 | 0.97 |
| ACA+ | 0.0070 ± 0.0000 | 0.2639 ± 0.0000 | 0.0007 ± 0.0000 | 0.0007 ± 0.0000 | ||||
| ATA (anti-Scl-70)− | 0.0126 ± 0.0036 | 0.13 | 0.2609 ± 0.0335 | 0.72 | 0.0012 ± 0.0018 | 0.92 | 0.0011 ±0.0005 | 0.29 |
| ATA (anti-Scl-70)+ | 0.0172 ± 0.0076 | 0.2673 ± 0.0839 | 0.0013 ± 0.0015 | 0.0040 ± 0.0125 | ||||
| UIP pattern, probable UIP pattern or Indeterminate for UIP pattern | 0.0137 ± 0.0077 | 0.88 | 0.2580 ± 0.0859 | 0.70 | 0.0005 ± 0.0002 | 0.08 | 0.0069 ± 0.0177 | 0.02 |
| NSIP pattern or Non-NSIP pattern | 0.0153 ± 0.0067 | 0.2618 ± 0.0638 | 0.0016 ± 0.0017 | 0.0010 ± 0.0007 | ||||
| Progressive pulmonary fibrosis− | 0.0153 ± 0.0646 | 0.76 | 0.2613 ± 0.0607 | 0.48 | 0.0013 ± 0.0014 | 0.86 | 0.0009 ± 0.0006 | 0.02 |
| Progressive pulmonary fibrosis+ | 0.0135 ± 0.0078 | 0.2569 ± 0.0896 | 0.0013 ± 0.0019 | 0.0073 ± 0.0176 | ||||
| Antifibrotic treatment− | 0.0149 ± 0.0067 | 0.60 | 0.2587 ± 0.0687 | 0.86 | 0.0013 ± 0.0015 | 0.46 | 0.0028 ± 0.0096 | 0.89 |
| Antifibrotic treatment+ | 0.0146 ± 0.0095 | 0.2813 ± 0.0670 | 0.0006 ± 0.0002 | 0.0007 ± 0.0005 | ||||
| sDMARDs− | 0.0152 ± 0.0040 | 0.95 | 0.2505 ± 0.0676 | 0.30 | 0.0018 ± 0.0021 | 0.56 | 0.0011 ± 0.0005 | 0.77 |
| sDMARDs+ | 0.0151 ± 0.0076 | 0.2663 ± 0.0738 | 0.0012 ± 0.0014 | 0.0033 ± 0.0112 | ||||
| bDMARDs− | 0.0159 ± 0.0069 | 0.88 | 0.2459 ± 0.0619 | 0.14 | 0.0017 ± 0.0020 | 0.40 | 0.0047 ± 0.0138 | 0.65 |
| bDMARDs+ | 0.0156 ± 0.0076 | 0.2971 ± 0.0801 | 0.0011 ± 0.0009 | 0.0010 ± 0.0009 | ||||
| Vasodilators treatment− | 0.0156 ± 0.0678 | 0.78 | 0.2956 ± 0.0670 | 0.46 | 0.0012 ± 0.0009 | 0.42 | 0.0010 ± 0.0008 | 0.91 |
| Vasodilators treatment+ | 0.0159 ± 0.0074 | 0.2527 ± 0.0732 | 0.0016 ± 0.0019 | 0.0042 ± 0.1288 | ||||
| PDGFA | TGFB1 | TGFB2 | TGFB3 | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | r | p | r | p | r | p | r | p |
| IM duration | −0.20 | 0.32 | 0.02 | 0.93 | 0.01 | 0.96 | 0.17 | 0.43 |
| ILD duration | 0.14 | 0.53 | 0.02 | 0.92 | −0.16 | 0.48 | 0.14 | 0.53 |
| FVC (% predicted) | 0.05 | 0.80 | −0.03 | 0.88 | −0.16 | 0.48 | 0.01 | 0.96 |
| FEV1 (% predicted) | 0.08 | 0.73 | 0.01 | 0.97 | −0.07 | 0.74 | −0.04 | 0.85 |
| DLCO (% predicted) | −0.09 | 0.75 | −0.11 | 0.70 | −0.08 | 0.80 | 0.43 | 0.14 |
| Category | MEAN ± SD | p | MEAN ± SD | p | MEAN ± SD | p | MEAN ± SD | p |
| Anti-Jo1− | 0.0216 ± 0.0201 | 0.90 | 0.3334 ± 0.1490 | 0.81 | 0.0013 ± 0.0009 | 0.38 | 0.0073 ± 0.0284 | 0.59 |
| Anti-Jo1+ | 0.0236 ± 0.0234 | 0.3513 ± 0.1891 | 0.0009 ± 0.0007 | 0.0006 ± 0.0006 | ||||
| Anti-PL7− | 0.0226 ± 0.0198 | 0.79 | 0.3507 ± 0.1601 | 0.40 | 0.0012 ± 0.0008 | 0.50 | 0.0072 ± 0.0284 | 0.61 |
| Anti-PL7+ | 0.0195 ± 0.0236 | 0.2822 ± 0.1169 | 0.0016 ± 0.0011 | 0.0010 ± 0.0005 | ||||
| Anti-PL12− | 0.0222 ± 0.0212 | 0.82 | 0.3329 ± 0.1467 | 0.72 | 0.0012 ± 0.0009 | 0.83 | 0.0067 ± 0.0272 | 0.72 |
| Anti-PL12+ | 0.0193 ± 0.0094 | 0.3679 ± 0.2380 | 0.0011 ± 0.0006 | 0.0010 ± 0.0007 | ||||
| UIP pattern, probable UIP pattern or Indeterminate for UIP pattern | 0.0251 ± 0.0199 | 0.73 | 0.3454 ± 0.1684 | 0.89 | 0.0013 ± 0.0009 | 0.60 | 0.0011 ± 0.0007 | 0.11 |
| NSIP pattern or Non-NSIP pattern | 0.0201 ± 0.0249 | 0.3279 ± 0.1572 | 0.0011 ± 0.0010 | 0.0007 ± 0.0005 | ||||
| Progressive pulmonary fibrosis− | 0.0213 ± 0.0204 | 0.62 | 0.3346 ± 0.1444 | 0.81 | 0.0012 ± 0.0010 | 0.68 | 0.0070 ± 0.0285 | 0.57 |
| Progressive pulmonary fibrosis+ | 0.0287 ± 0.0312 | 0.3197 ± 0.3097 | 0.0014 ± 0.0008 | 0.0008 ± 0.0008 | ||||
| Antifibrotic treatment− | 0.0225 ± 0.0216 | 0.71 | 0.3498 ± 0.1542 | 0.11 | 0.0012 ± 0.0009 | 0.96 | 0.0067 ± 0.0278 | 0.99 |
| Antifibrotic treatment+ | 0.0107 ± 0.0030 | 0.1421 ± 0.0526 | 0.0011 ± 0.0009 | 0.0004 ± 0.0001 | ||||
| sDMARDs− | 0.0282 ± 0.0272 | 0.60 | 0.3761 ± 0.1588 | 0.29 | 0.0016 ± 0.0011 | 0.11 | 0.0010 ± 0.0006 | 0.09 |
| sDMARDs+ | 0.0173 ± 0.0107 | 0.2997 ± 0.1489 | 0.0009 ± 0.0007 | 0.0101 ± 0.0344 | ||||
| bDMARDs− | 0.0261 ± 0.0223 | 0.21 | 0.3537 ± 0.1721 | 0.42 | 0.0012 ± 0.0009 | 0.98 | 0.0075 ± 0.0291 | 0.84 |
| bDMARDs+ | 0.0111 ± 0.0060 | 0.2796 ± 0.0732 | 0.0012 ± 0.0010 | 0.0009 ± 0.0006 | ||||
| Study Objective Groups | Comparative Groups | ||||||
|---|---|---|---|---|---|---|---|
| RA-ILD n = 33 | SSc-ILD n = 31 | IM-ILD n = 29 | RA-nonILD n = 22 | SSc-nonILD n = 18 | IM-nonILD n = 8 | IPF n = 148 | |
| Sex (women), n (%) | 13 (39.39) | 19 (61.29) | 18 (62.07) | 14 (63.64) | 16 (88.89) | 6 (75.00) | 123 (83.11) |
| Age at study (years), mean ± SD | 70.73 ± 7.1 | 63.06 ± 10.2 | 62.45 ± 19.0 | 64.68 ± 11.29 | 57.94 ± 13.76 | 63.86 ± 19.17 | 70.60 ± 7.12 |
| Smoking ever, n (%) | 26 (78.79) | 16 (51.61) | 21 (72.41) | 13 (59.09) | 10 (55.56) | 3 (37.5) | 130 (87.84) |
| Antibody status | |||||||
| RF+, n (%) | 29 (87.87) | - | - | 12 (54.55) | - | - | - |
| ACPA+, n (%) | 28 (84.84) | - | - | 14 (63.64) | - | - | - |
| ANA+, n (%) | - | 29 (93.55) | - | - | 16 (88.89) | - | - |
| ACA+, n (%) | - | 1 (3.23) | - | - | 8 (44.44) | - | - |
| ATA (anti-Scl70)+, n (%) | - | 17 (54.84) | - | - | 3 (16.67) | - | - |
| Anti-Jo1+, n (%) | - | - | 5 (17.24) | - | - | 0 (0.00) | - |
| Anti-PL7+, n (%) | - | - | 6 (20.69) | - | - | 1 (12.5) | - |
| Anti-PL12+, n (%) | - | - | 3 (10.34) | - | - | 0 (0.00) | - |
| CTD duration (years), mean ± SD | 6.72 ± 7.13 | 7.01 ± 7.06 | 7.96 ± 21.29 | 9.70 ± 9.65 | 9.94 ± 7.92 | 5.27 ± 1.58 | - |
| ILD duration (years), mean ± SD | 3.88 ± 4.28 | 5.64 ± 6.95 | 3.88 ± 4.28 | - | - | - | 3.43 ± 3.49 |
| Pulmonary function tests | |||||||
| FVC (% predicted), mean ± SD | 85.46 ± 26.60 | 78.75 ± 24.16 | 79.58 ± 24.77 | 103.90 ± 17.51 | 107.20 ± 16.12 | 113.00 ± 0.00 | 75.73 ± 18.94 |
| FEV1 (% predicted), mean ± SD | 83.82 ± 24.52 | 77.11 ± 22.99 | 81.21 ± 25.57 | 101.10 ± 22.72 | 102.50 ±18.14 | 119.00 ± 0.00 | 77.57 ± 19.39 |
| DLCO (% predicted), mean ± SD | 44.34 ± 19.50 | 39.60 ± 17.71 | 52.18 ± 16.21 | 77.49 ± 17.85 | 70.68 ± 15.46 | 101.0 ± 0.00 | 36.47 ± 16.11 |
| HRCT | |||||||
| Pulmonary involvement in HRCT, n (%) | 33 (100.0) | 31 (100.0) | 29 (100.0) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 148 (100.00) |
| UIP pattern, n (%) | 17 (51.52) | 8 (25.80) | 7 (24.14) | - | - | - | 148 (100.00) |
| Probable UIP pattern, n (%) | 5 (15.15) | 1 (3.23) | 6 (20.69) | - | - | - | 0 (0.00) |
| Indeterminate for UIP pattern, n (%) | 1 (3.03) | 0 (0.00) | 0 (0.00) | - | - | - | 0 (0.00) |
| NSIP pattern, n (%) | 9 (27.27) | 20 (64.52) | 9 (31.03) | - | - | - | 0 (0.00) |
| Non-NSIP pattern, n (%) | 1 (3.03) | 2 (6.45) | 2 (6.90) | - | - | - | 0 (0.00) |
| Progressive pulmonary fibrosis, n (%) | 5 (15.15) | 8 (25.81) | 3 (10.34) | - | - | - | 148 (100.00) |
| Treatments | |||||||
| Antifibrotics, n (%) | 1 (3.03) | 2 (6.45) | 2 (6.90) | - | - | - | 98 (66.21) |
| sDMARDs, n (%) | 17 (52.52) | 22 (70.97) | 15 (51.72) | 19 (86.36) | 11 (61.11) | 6 (75.00) | - |
| bDMARDs, n (%) | 12 (36.36) | 10 (32.26) | 7 (24.14) | 4 (18.18) | 2 (36.00) | 3 (37.5) | - |
| Vasodilatators, n (%) | - | 17 (54.84) | - | - | 13 (72.22) | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pulito-Cueto, V.; Atienza-Mateo, B.; Batista-Liz, J.C.; Nieto-Nieto, R.; Vaquera-Illescas, C.; Sebastián Mora-Gil, M.; Iturbe-Fernández, D.; Mora-Cuesta, V.M.; Serrano-Combarro, A.; Izquierdo-Cuervo, S.; et al. A New Approach for Achieving Earlier and More Accurate Diagnosis of Connective Tissue Disease-Related Interstitial Lung Disease: TGFB and PDGFA as Novel Promising Biomarkers. Int. J. Mol. Sci. 2025, 26, 10722. https://doi.org/10.3390/ijms262110722
Pulito-Cueto V, Atienza-Mateo B, Batista-Liz JC, Nieto-Nieto R, Vaquera-Illescas C, Sebastián Mora-Gil M, Iturbe-Fernández D, Mora-Cuesta VM, Serrano-Combarro A, Izquierdo-Cuervo S, et al. A New Approach for Achieving Earlier and More Accurate Diagnosis of Connective Tissue Disease-Related Interstitial Lung Disease: TGFB and PDGFA as Novel Promising Biomarkers. International Journal of Molecular Sciences. 2025; 26(21):10722. https://doi.org/10.3390/ijms262110722
Chicago/Turabian StylePulito-Cueto, Verónica, Belén Atienza-Mateo, Joao C. Batista-Liz, Rebeca Nieto-Nieto, Clara Vaquera-Illescas, María Sebastián Mora-Gil, David Iturbe-Fernández, Víctor M. Mora-Cuesta, Ana Serrano-Combarro, Sheila Izquierdo-Cuervo, and et al. 2025. "A New Approach for Achieving Earlier and More Accurate Diagnosis of Connective Tissue Disease-Related Interstitial Lung Disease: TGFB and PDGFA as Novel Promising Biomarkers" International Journal of Molecular Sciences 26, no. 21: 10722. https://doi.org/10.3390/ijms262110722
APA StylePulito-Cueto, V., Atienza-Mateo, B., Batista-Liz, J. C., Nieto-Nieto, R., Vaquera-Illescas, C., Sebastián Mora-Gil, M., Iturbe-Fernández, D., Mora-Cuesta, V. M., Serrano-Combarro, A., Izquierdo-Cuervo, S., Aguirre Portilla, C., Cifrián, J. M., Blanco, R., & López-Mejías, R. (2025). A New Approach for Achieving Earlier and More Accurate Diagnosis of Connective Tissue Disease-Related Interstitial Lung Disease: TGFB and PDGFA as Novel Promising Biomarkers. International Journal of Molecular Sciences, 26(21), 10722. https://doi.org/10.3390/ijms262110722






