1. Introduction
Pancreatic cancer, a highly aggressive malignancy of the digestive system, claims nearly half a million lives annually worldwide and portends a profoundly poor prognosis. It ranks as the sixth leading cause of cancer-related mortality, accounting for approximately 5% of all global cancer deaths [
1]. The past 25 years have witnessed a more-than-twofold increase in the global incidence of pancreatic cancer, presenting a mounting public health burden. Well-documented risk factors for the disease comprise smoking, obesity, diabetes, and chronic alcohol use [
2]. The disease is characterized by a dismal median survival of just four months and a five-year survival rate of 13%, with projections indicating it will become the second-leading cause of cancer mortality within the next decade. Typically asymptomatic in its early stages, pancreatic cancer is frequently diagnosed at an advanced and inoperable stage [
3]. Consequently, while surgical resection remains the cornerstone of curative-intent treatment, only a small subset of patients qualify. Furthermore, cornerstone chemotherapeutic agents like gemcitabine offer constrained therapeutic benefits. These limitations collectively contribute to the persistently high rates of recurrence and metastasis observed with current treatment modalities [
4]. This dire clinical outlook underscores the urgent and unmet need for the development of novel, effective pharmacological therapies.
Natural products serve as a cornerstone of oncology therapeutics, prized for their favorable safety profiles and potent pharmacological activities. Among them, fungal secondary metabolites constitute a vast and largely untapped reservoir of therapeutic candidates. These compounds display unparalleled chemical diversity that eclipses the scope of synthetic chemistry and frequently operate through exquisitely structured bioactive molecules that mediate highly specific and novel mechanisms of action. Consequently, they represent an indispensable strategic asset in confronting future disease challenges, with demonstrated efficacy across antimicrobial, antiviral, and antitumor applications [
5].
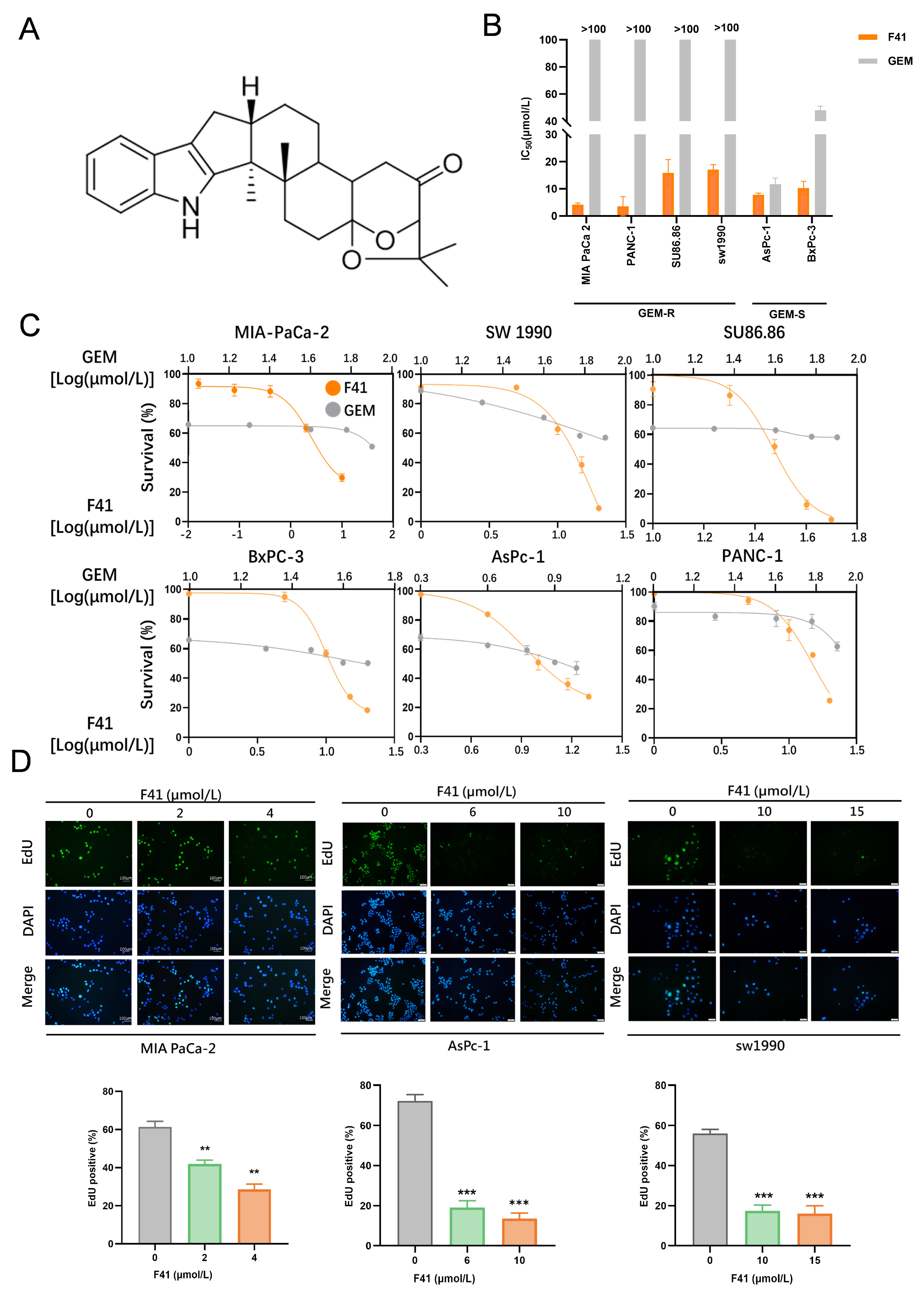
Compound F41 (C
27H
33NO
3) (
Figure 1A), an indole-diterpenoid secondary metabolite, was isolated from the entomopathogenic fungus Penicillium sp. found on the cereal cyst nematode [
6]. The molecular weight of F41 is 419.56 g/mol. Indole-diterpenoids, exemplified by F41, are a class of secondary metabolites sparsely distributed in fungi, primarily within the genera Claviceps, Aspergillus, Epichloë, and Penicillium [
7]. F41 shares the characteristic core scaffold common to all indole-diterpenoids, comprising a cyclic diterpenoid unit fused to an indole moiety. The diterpenoid component is partially derived from the mevalonic acid pathway, where four isoprene units assemble into a twenty-carbon skeleton. This hydrophobic framework dictates the molecule’s fundamental three-dimensional architecture [
8]. The indole ring, conversely, originates from the shikimic acid pathway and is derived from tryptophan. This electron-rich planar heterocycle is a critical pharmacophore, frequently engaging in π-π stacking and hydrogen-bonding interactions with biological targets [
9]. These two distinct structures are elegantly conjugated via catalysis by fungus-specific enzymes. The parent scaffold is often further decorated with various oxygen-containing functional groups, such as hydroxyl, ketone, and epoxide moieties. These groups not only modulate the molecule’s polarity but, more importantly, serve as key interaction sites for specific binding to biological targets, including ion channels and cellular organelles. Owing to their unique hybrid polycyclic systems and precise stereochemistry, indole-diterpenoids exhibit remarkable and diverse pharmacological activities. For instance, the indole-diterpenoids Paxilline and Nodulisporic Acid are potent modulators of high-conductance calcium-activated potassium (BK) channels and glutamate-gated chloride channels (GluCls), respectively. Their complex three-dimensional structures enable highly specific recognition of these ion channels, underpinning their therapeutic potential for treating neurological disorders such as essential tremor, epilepsy, and anxiety [
10]. Other indole-diterpenoids demonstrate additional pharmacologic effects, including antibacterial and antiviral activities, as well as inhibition of protein tyrosine phosphatase activity [
11]. Thus, indole-diterpenoids, represented by F41, constitute a highly promising family of natural products with significant medicinal value, positioning them as a crucial source for innovative drug discovery. However, the antitumor activity of F41, particularly against pancreatic cancer, had remained unreported. Therefore, we investigated the efficacy and underlying mechanisms of F41 against pancreatic cancer. Herein, we report for the first time the potent anti-pancreatic cancer activity of this fungus-derived indole-diterpenoid, F41.
3. Discussion
Pancreatic cancer is one of the most aggressive and lethal solid malignancies. Although surgical resection can be effective, its impact on overall survival remains minimal due to the high rates of recurrence and metastasis [
15]. While gemcitabine-based monotherapy or combination chemotherapy may improve patient prognosis to some extent [
16], the development of drug resistance often hinders long-term treatment efficacy [
17]. Thus, there is an urgent need for novel therapeutic agents against pancreatic cancer. Natural products derived from plants and microorganisms have attracted increasing attention for their unique pharmacological activities and their ability to induce tumor cell death through multiple pathways, including endoplasmic reticulum (ER) stress-mediated apoptosis, the extrinsic death receptor pathway, and the intrinsic mitochondrial pathway, demonstrating considerable anticancer potential [
18].
Compound F41 is an indole-diterpenoid derived from fungal secondary metabolites—a diverse group of natural products with various structures and significant antitumor activities. For instance, Plinabulin, from the marine fungus Aspergillus sp., targets tubulin and inhibits mitosis, offering a treatment strategy for non-small-cell lung cancer [
19]. Versicolactone B, isolated from a marine-derived strain of Aspergillus versicolor, exhibits potent cytotoxicity across multiple cancer cell lines. It induces S-phase arrest and promotes the formation of apoptotic bodies and nuclear condensation in PANC-1 pancreatic cancer cells, ultimately leading to apoptosis [
20]. These findings prompted us to investigate the anti-pancreatic cancer effects of F41.
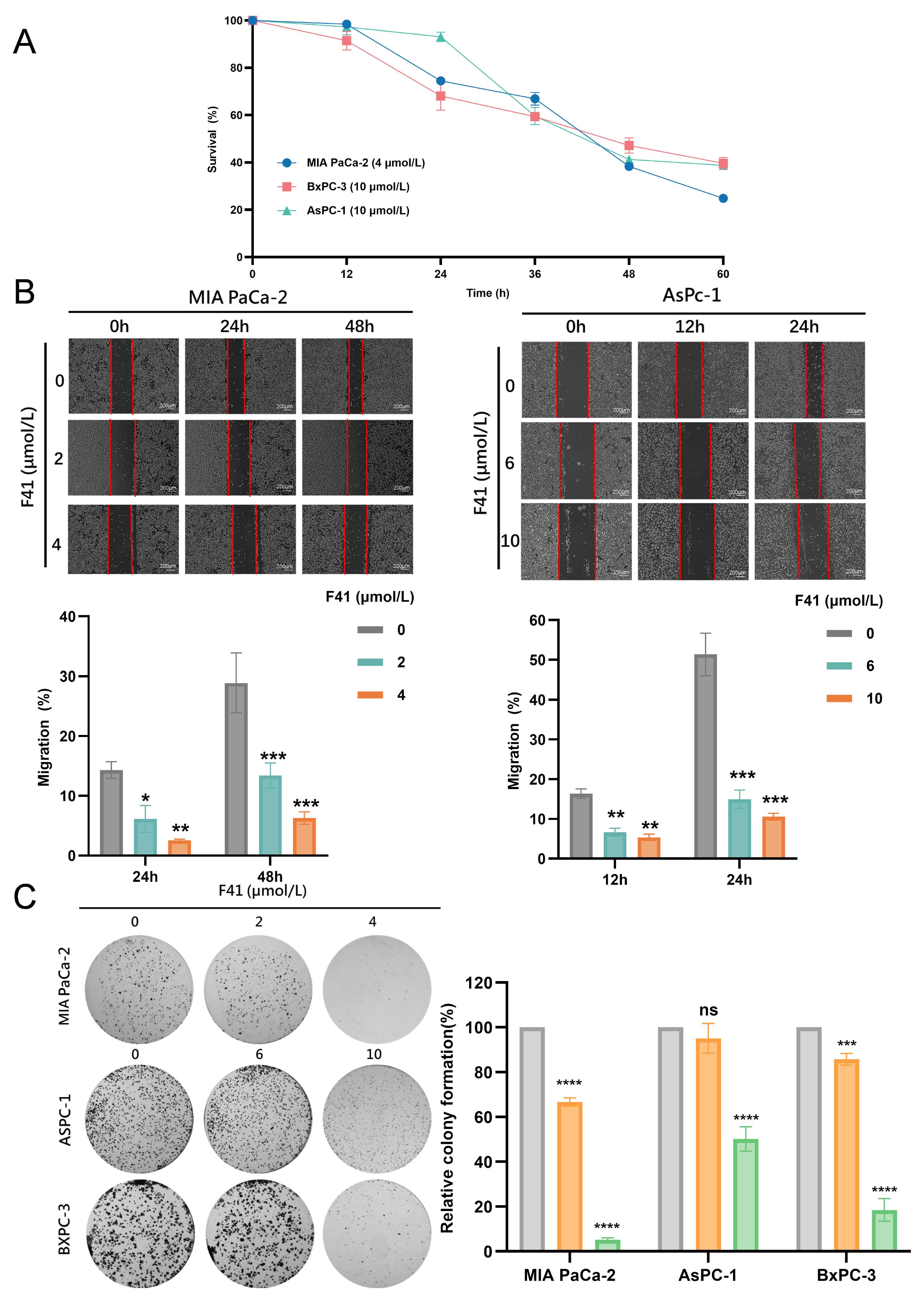
Our results demonstrate that F41 exhibits superior potency over gemcitabine, with significantly lower IC50 values against both gemcitabine-resistant (GEM-R; MIA PaCa-2, PANC-1, SW 1990, and SU86.86) and gemcitabine-sensitive (GEM-S; BxPC-3 and AsPC-1) cell lines. This positions F41 as a promising therapeutic candidate capable of potentially overcoming gemcitabine resistance in clinical settings. Furthermore, functional assays—including EdU incorporation, colony formation, and wound healing—consistently revealed that F41 concentration- and time-dependently suppresses DNA replication, clonogenic survival, and migratory capacity in pancreatic cancer cells, irrespective of their gemcitabine sensitivity.
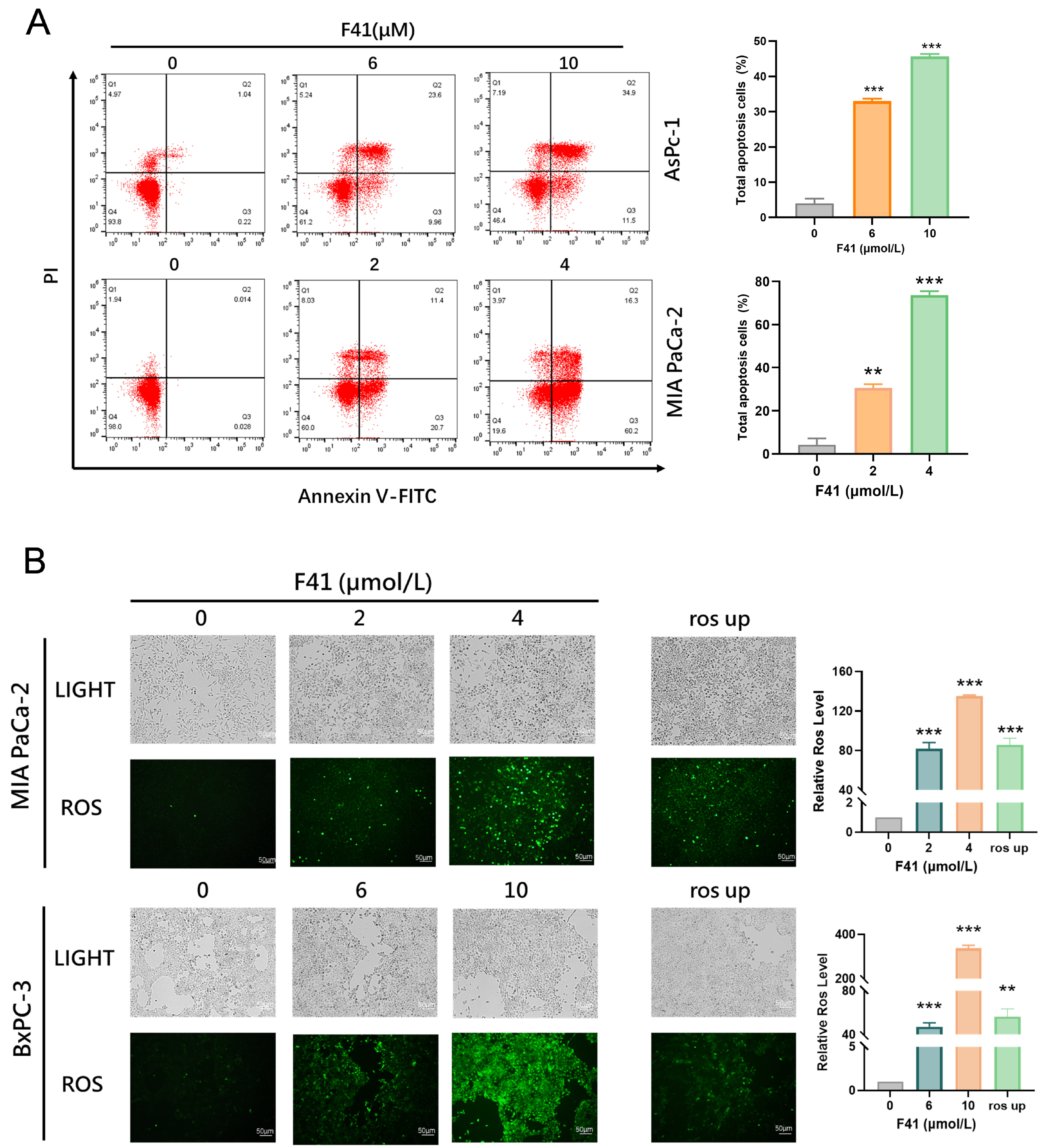
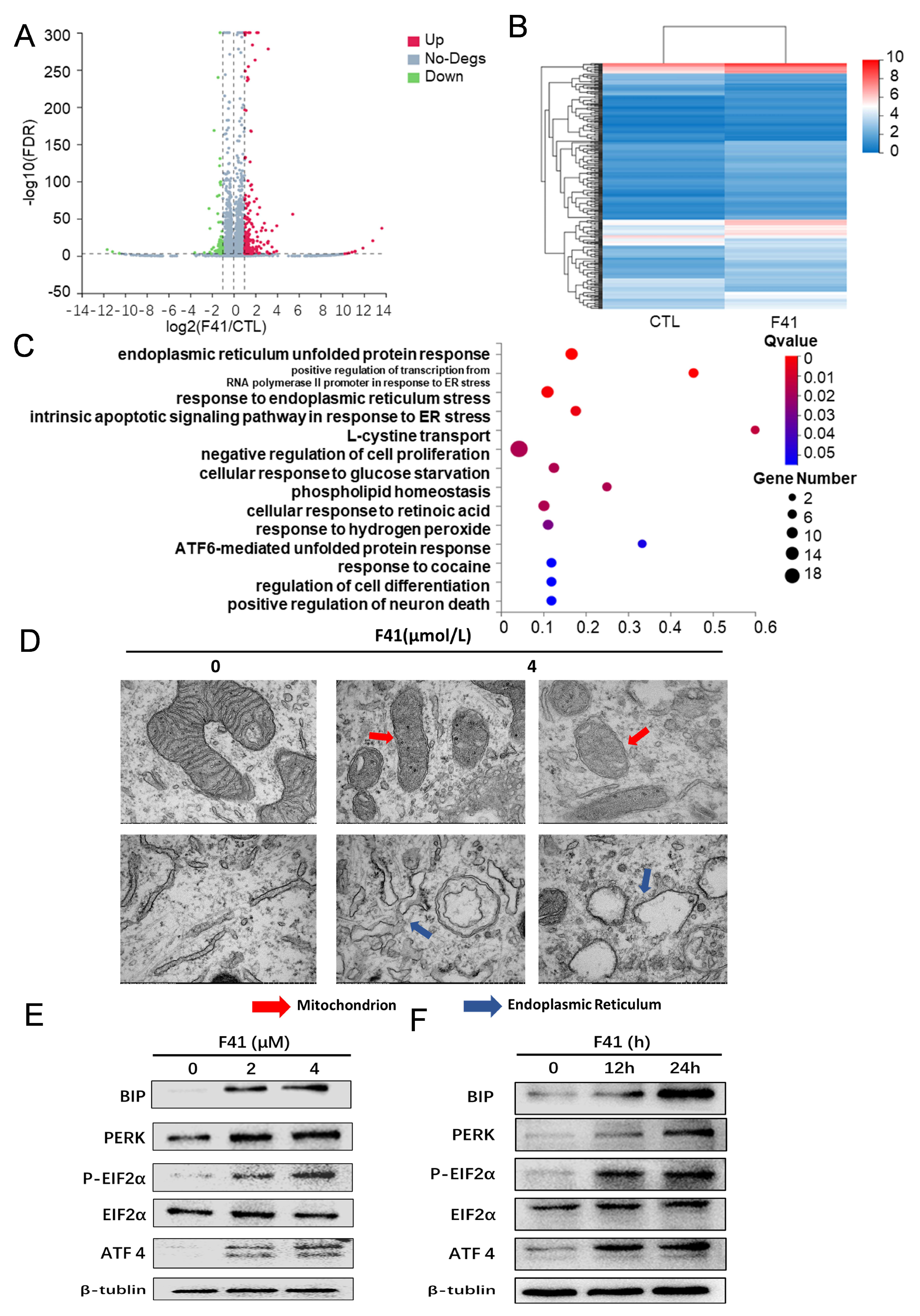
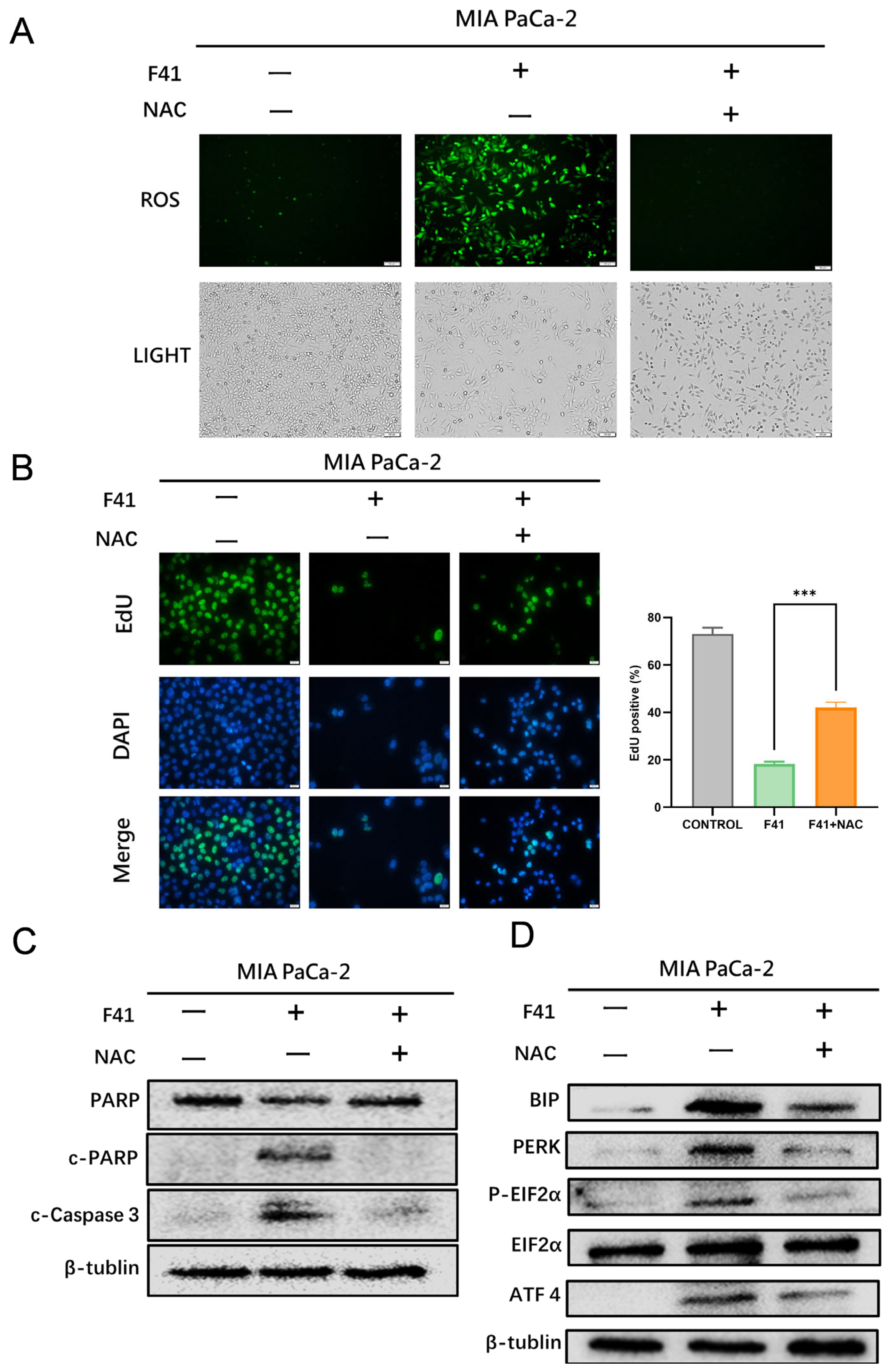
Having established the efficacy of F41, we next explored its underlying mechanism. Our data reveal that F41 induces apoptosis in pancreatic cancer cells by significantly elevating intracellular ROS levels. This observation is consistent with established knowledge that external stimuli can trigger mitochondrial ROS generation, leading to oxidative stress and subsequent apoptosis, thereby implicating this pathway in the mode of action of F41 [
21]. We therefore propose that F41 exerts its antitumor effects by inducing radical oxygen species production and apoptotic cell death. Transcriptome sequencing and transmission electron microscopy revealed that F41 induces ER stress and causes structural damage to both the ER and mitochondria. ER stress has been implicated in various diseases, including cancer, metabolic disorders, and chronic inflammation, and has emerged as a target for small molecules and gene therapies, highlighting its therapeutic relevance [
22].
Using the ROS scavenger NAC, we demonstrated that NAC attenuates F41-induced proliferation inhibition and reverses ROS accumulation, apoptosis, and ER stress activation. Previous studies have shown that natural compounds such as curcumin and schisandrin A can induce ROS-dependent ER stress, leading to tumor cell apoptosis [
23,
24]. Collectively, our findings support the conclusion that F41 induces apoptosis in pancreatic cancer cells through ROS-dependent ER stress activation, and that these effects can be partially reversed by NAC.
4. Materials and Methods
4.1. Cell Culture
The human pancreatic cancer cell lines employed in this research (AsPC-1, BxPC-3, MIA PaCa-2, Panc-1, SW1990, and SU86.86) were acquired from the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences (Beijing, China). These cells were grown in DMEM or RPMI-1640 medium containing 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin. All cell lines were kept in a humidified incubator at 37 °C with 5% CO2 for routine propagation.
4.2. Cell Viability Assay (MTT)
Cells in the logarithmic growth phase were plated in 96-well plates (NEST, Wuxi, China) at 7 × 103 cells per well in 200 μL of complete medium for a 24 h incubation at 37 °C with 5% CO2. Following this, we replaced the medium with fresh medium containing serially diluted test compounds. After a 48 h treatment period, we carefully removed the medium and added 150 μL of MTT solution (0.5 mg/mL in PBS; Sigma-Aldrich, St. Louis, MO, USA) to each well. The plates were then returned to the incubator for 4 h. Next, the MTT solution was aspirated, and we dissolved the resulting formazan crystals in 150 μL of dimethyl sulfoxide (DMSO). Absorbance was read at 490 nm using a BioTek ELx800 microplate reader (BioTek Instruments, Winooski, VT, USA), with all experiments including a minimum of three biological replicates.
4.3. Colony Formation Assay
Cells were plated in 6-well plates at a density of 1000 cells per well and given 24 h to adhere. Following this attachment period, the culture medium was replaced with a drug-containing medium. The cells were then cultured for 7 days. After this incubation, the cells were washed twice with phosphate-buffered saline (PBS; Zhongke Maichen, Beijing, China, ZM-001), fixed with 4% paraformaldehyde (Beyotime, Shanghai, China, P0099) for 15 min at room temperature, and stained using 0.1% crystal violet (Beyotime, Shanghai, China, C0121) for another 15 min. Excess stain was rinsed off with distilled water, and the plates were air-dried prior to being scanned. Colonies were assessed, and those containing more than 50 cells were counted.
4.4. Western Blot Analysis
Following a 24 h adherence period after seeding in 6-well plates at 2.5 × 105 cells/well, cells were treated as required. Subsequently, the cells were lysed on ice for 30 min using RIPA buffer (Beyotime, Shanghai, China, P0013B) containing protease and phosphatase inhibitors (Beyotime, Shanghai, China, P1081). The resulting lysates were then centrifuged at 12,000× g for 20 min at 4 °C to collect the supernatant. Protein concentration in the supernatants was quantified with a BCA assay kit (Beyotime, Shanghai, China, P0012). Equal protein aliquots (20 μg per lane) were resolved by 10% SDS-PAGE and electrophoretically transferred onto PVDF membranes (Millipore, Burlington, MA, USA, IPVH00010). The membranes were blocked with 5% non-fat milk for 1 h at room temperature before being probed with primary antibodies (diluted 1:1000 in TBST) overnight at 4 °C. After washing, the membranes were incubated with HRP-conjugated secondary antibodies (1:5000) for 2 h at room temperature. Finally, protein bands were detected using an ECL substrate (Millipore, Burlington, MA, USA, WBKLS0100).
4.5. Apoptosis Analysis by Flow Cytometry
The assessment of cell apoptosis was conducted using a commercial Annexin V-FITC/PI double-staining kit in accordance with the provided instructions. Post-treatment, cells were harvested, subjected to PBS washing, and resuspended to 1 × 106 cells/mL. A 100 μL sample of the suspension was then co-stained with 5 μL of Annexin V-FITC and 10 μL of propidium iodide (PI), followed by a 20 min incubation in darkness. Flow cytometric analysis was immediately performed after incubation, and the acquired data were analyzed with the FlowJo software (Version 10.8.1, Ashland, OR, USA).
4.6. Wound Healing Assay
A cell migration assay was performed using the wound healing method. Briefly, cells were seeded in 6-well plates at 4–5 × 105 cells per well and cultured to ~70% confluence. A linear wound was created in the monolayer with a sterile 200 μL pipette tip. Post-washing with PBS, the cells were cultured in a low-serum medium (3% FBS). Wound closure was monitored by imaging identical locations at 0, 12, 24, and 48 h with an inverted microscope. The acquired images were analyzed with the ImageJ (Version 1.54, Washington, DC, USA) software to calculate the percentage of wound closure.
4.7. Cell Proliferation Assay (EdU)
Cell proliferation was assessed with the EdU Apollo488 In Vitro Kit. Cells were plated on coverslips in 24-well plates at a density of 2–4 × 104 cells per well. Following a 24 h drug treatment, the cells were pulsed with 50 μM EdU working solution for 2 h. The cells then underwent a series of processing steps: fixation with 4% paraformaldehyde for 15 min, permeabilization with 0.25% Triton X-100 for 20 min, and finally fluorescent staining involving the Apollo reaction and DAPI nuclear counterstain for 30 min. Fluorescence microscope images were acquired from random fields, and the proportion of EdU-positive cells was quantified.
4.8. Transmission Electron Microscopy (TEM)
Ultrastructural alterations in F41-treated cells were investigated by transmission electron microscopy (TEM). MIA PaCa-2 cells were plated in 6-well plates at 2 × 105 cells per well and cultured for 24 h. After attachment, the cells were exposed to fresh medium containing F41, with untreated cells serving as negative controls. Following a 24 h incubation, the cells were harvested by trypsinization after PBS washing and pelleted by centrifugation at 800× g for 5 min. The cell pellets underwent primary fixation with 2.5% glutaraldehyde, followed by post-fixation in 1% osmium tetroxide containing 1.5% potassium ferrocyanide for 1 h. Subsequently, the samples were dehydrated through a graded ethanol series (50% to 100%), and en bloc staining was performed with 2% uranyl acetate in 70% ethanol at room temperature. After dehydration and staining, the samples were embedded in epoxy resin. Ultrathin sections (60 nm) were cut using an ultramicrotome, mounted on copper grids, and finally observed under a JEM-1400 Plus transmission electron microscope (JEOL, Tokyo, Japan) at an accelerating voltage of 120 kV.
4.9. Transcriptome Sequencing and Analysis
Total RNA was extracted from control and F41-treated cells using a TRIzol reagent (Carlsbad, CA, USA). After quality control, cDNA library construction and sequencing were performed on the BGISEQ-500RS platform by BGI (Shenzhen, China). Raw sequencing data were quality-controlled and aligned to the reference genome. Gene expression levels were quantified using the RSEM software (Version 1.2.12, University of Wisconsin-Madison, WI, USA). Differentially expressed genes (DEGs) were identified using thresholds of FDR < 0.001 and |log2(fold change)| > 1. Gene Ontology (GO) functional enrichment analysis was performed using the Phyper algorithm with Bonferroni correction (Q-value ≤ 0.05 considered significant).
4.10. Intracellular ROS Detection
Intracellular ROS production was assessed with the DCFH-DA probe. Briefly, after plating in 6-well plates at a density of 2.5 × 105 cells per well and reaching adherence, cells were subjected to a 24 h drug treatment. The cells were then loaded with 10 μM DCFH-DA by incubation for 20 min at 37 °C in the dark. Following three washes with serum-free medium to remove excess probe, fluorescence was detected and imaged using a fluorescence microscope, or quantified by flow cytometry. In this experiment, Rosup treatment was utilized to establish the positive control.
4.11. Reagents and Instruments
DMEM/RPMI-1640 medium and fetal bovine serum were purchased from Gibco (Grand Island, NY, USA). Penicillin–streptomycin, trypsin-EDTA, and PBS were products of Zhongke Maichen Technology (Beijing, China). The TRIzol was obtained from Invitrogen (Carlsbad, CA, USA). The EdU detection kit was obtained from RiboBio (Guangzhou, China). The ROS detection kit, Annexin V-FITC Apoptosis Detection Kit, RIPA lysis buffer, BCA protein assay kit, and others were purchased from Beyotime Biotechnology (Nantong, China). The primary antibodies against PERK (C33E10) and BIP (C50B12) were from Cell Signaling Technology (Danvers, MA, USA). The primary antibodies against EIF2α, p-EIF2α, ATF4, cleaved PARP, PARP, and β-tubulin were from Beyotime (Nantong, China). HRP-conjugated secondary antibodies were from Zhongshan Golden Bridge (Beijing, China). ECL chemiluminescence substrate was from Millipore (Burlington, Massachusetts, USA). MTT, DMSO, N-acetylcysteine (NAC), and other reagents were from Sigma-Aldrich (St. Louis, MO, USA). DAPI staining solution was from Invitrogen (Carlsbad, CA, USA). Major instruments used included an Olympus inverted microscope IX73 (Olympus Corporation, Tokyo, Japan), BD FACSCalibur flow cytometer (Becton, Dickinson and Company, Franklin Lakes, NJ, USA), BioTek ELx800 microplate reader (BioTek Instruments, Winooski, VT, USA), Thermo cell culture incubator (Thermo Fisher Scientific, Waltham, MA, USA), and SpectraMax chemiluminescence imaging system (Molecular Devices, San Jose, CA, USA).
4.12. Statistical Analysis
All experiments were independently repeated at least three times. Data are presented as mean ± standard deviation. Comparisons between groups were performed using the unpaired Student’s t-test or one-way analysis of variance (ANOVA), with appropriate post hoc tests. A p-value of less than 0.05 was considered statistically significant. All statistical analyses were conducted using the GraphPad Prism software (Version 10.0, La Jolla, CA, USA).