Dual Role of the Spinal Endocannabinoid System in Response to Noxious Stimuli: Antinociceptive Pathways and Neuropathic Pain Mechanisms
Abstract
1. Introduction
2. Spinal Cord Physiology and Central Sensitization in Neuropathic Pain
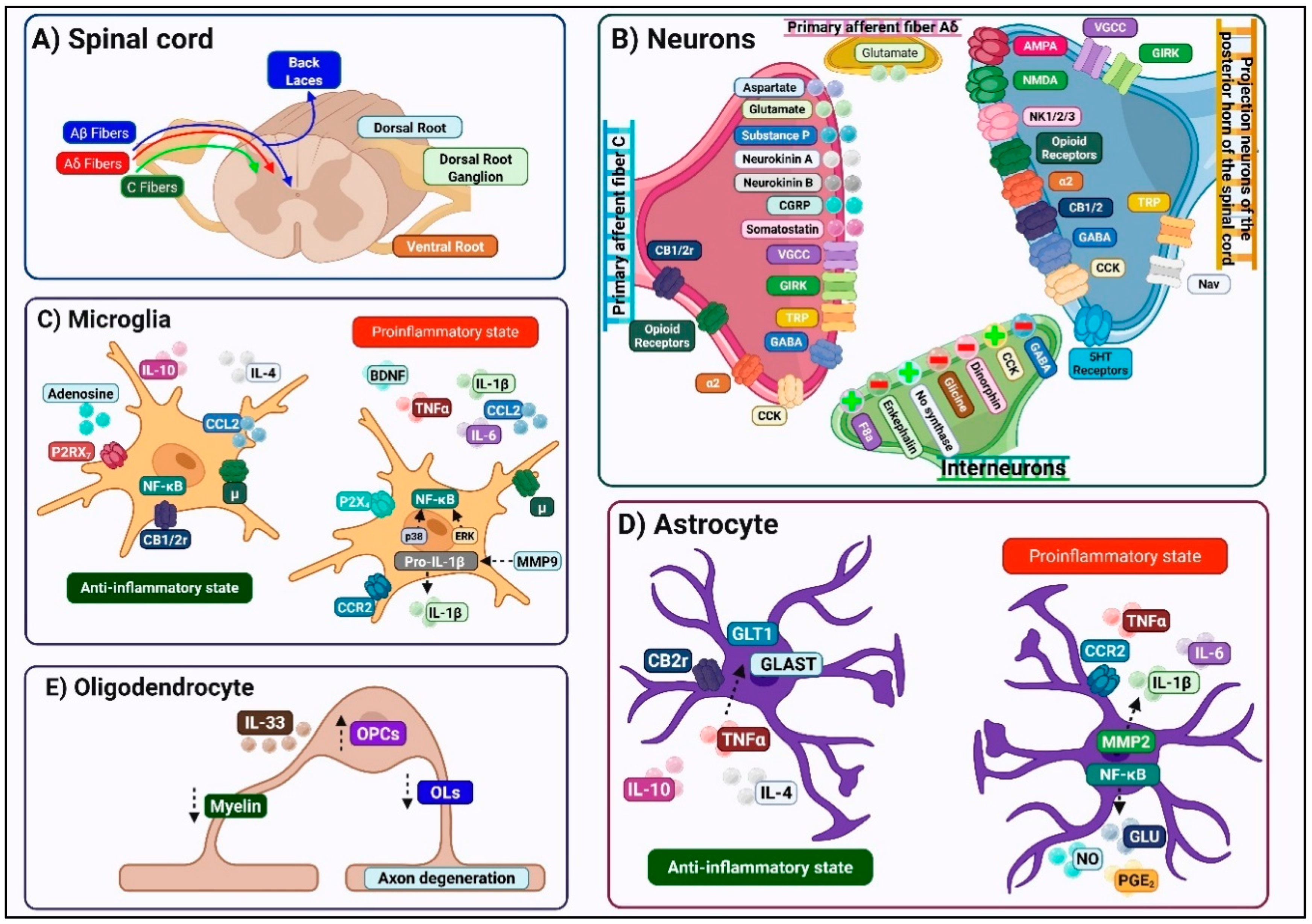
2.1. Structural and Functional Organization of the DH
2.2. Modulatory Systems Regulating Spinal Nociceptive Transmission
2.2.1. Descending Pain Modulatory Pathways
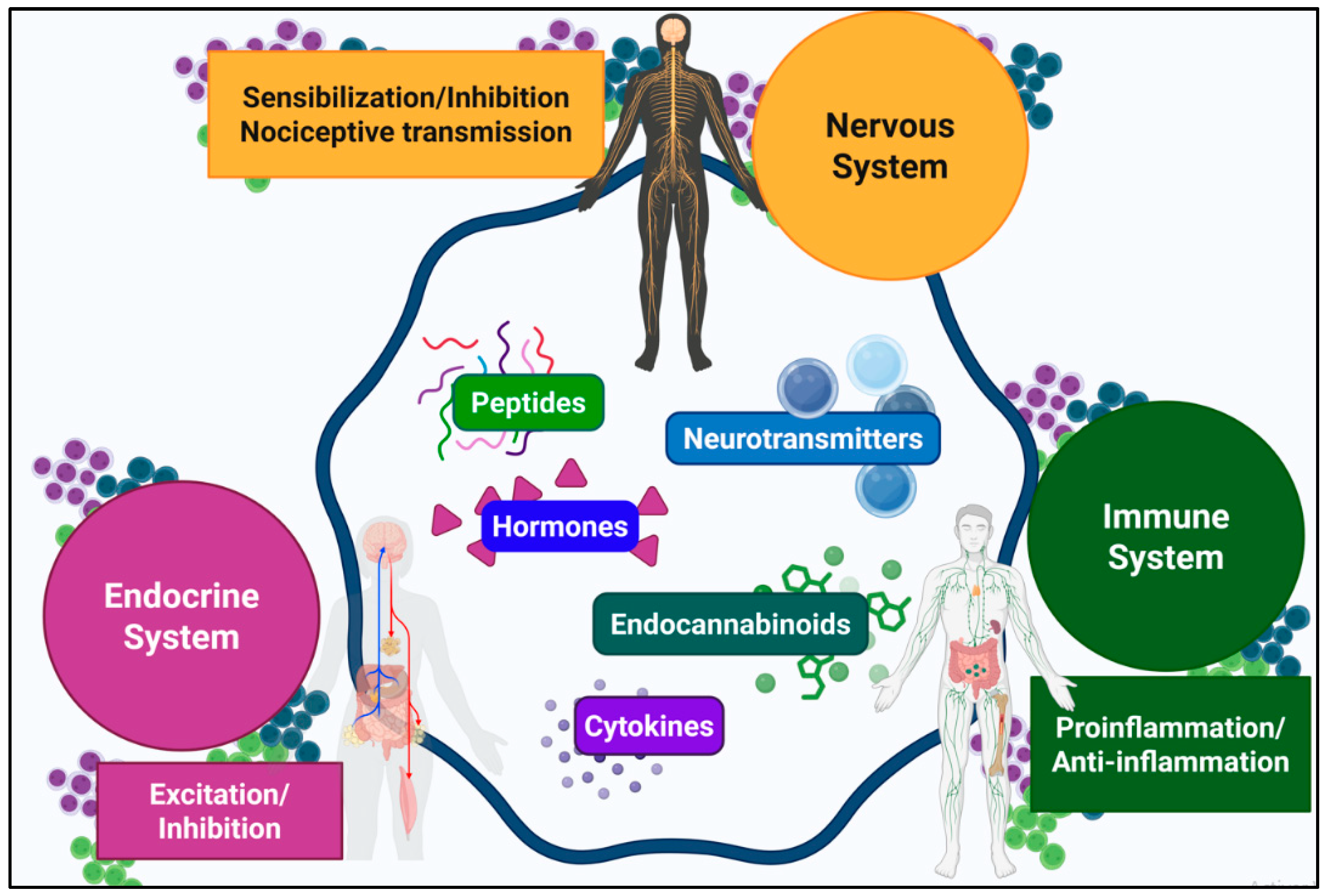
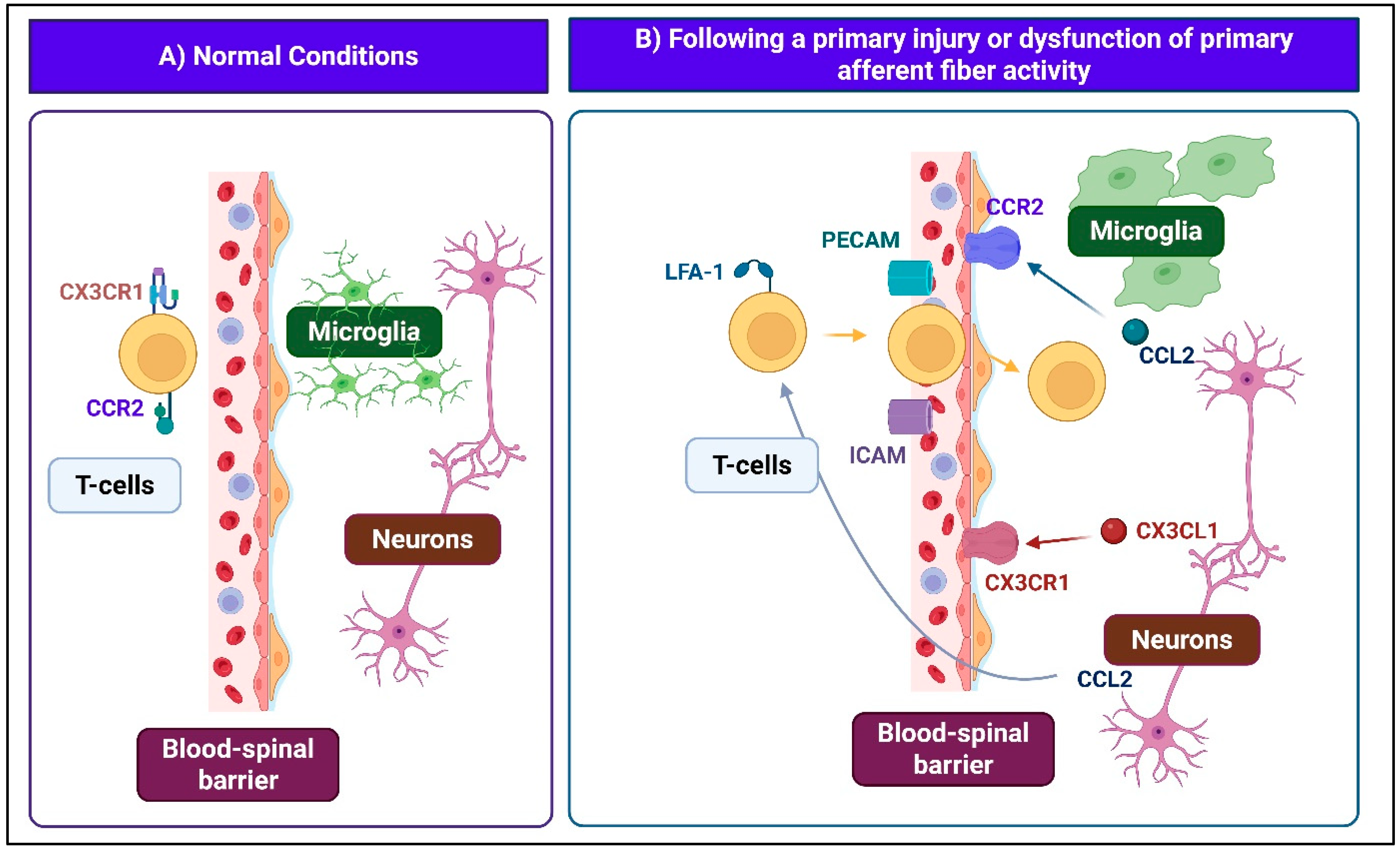
2.2.2. Spinal Neuroimmune Interactions
2.2.3. Neuroendocrine Modulation in the Spinal Cord
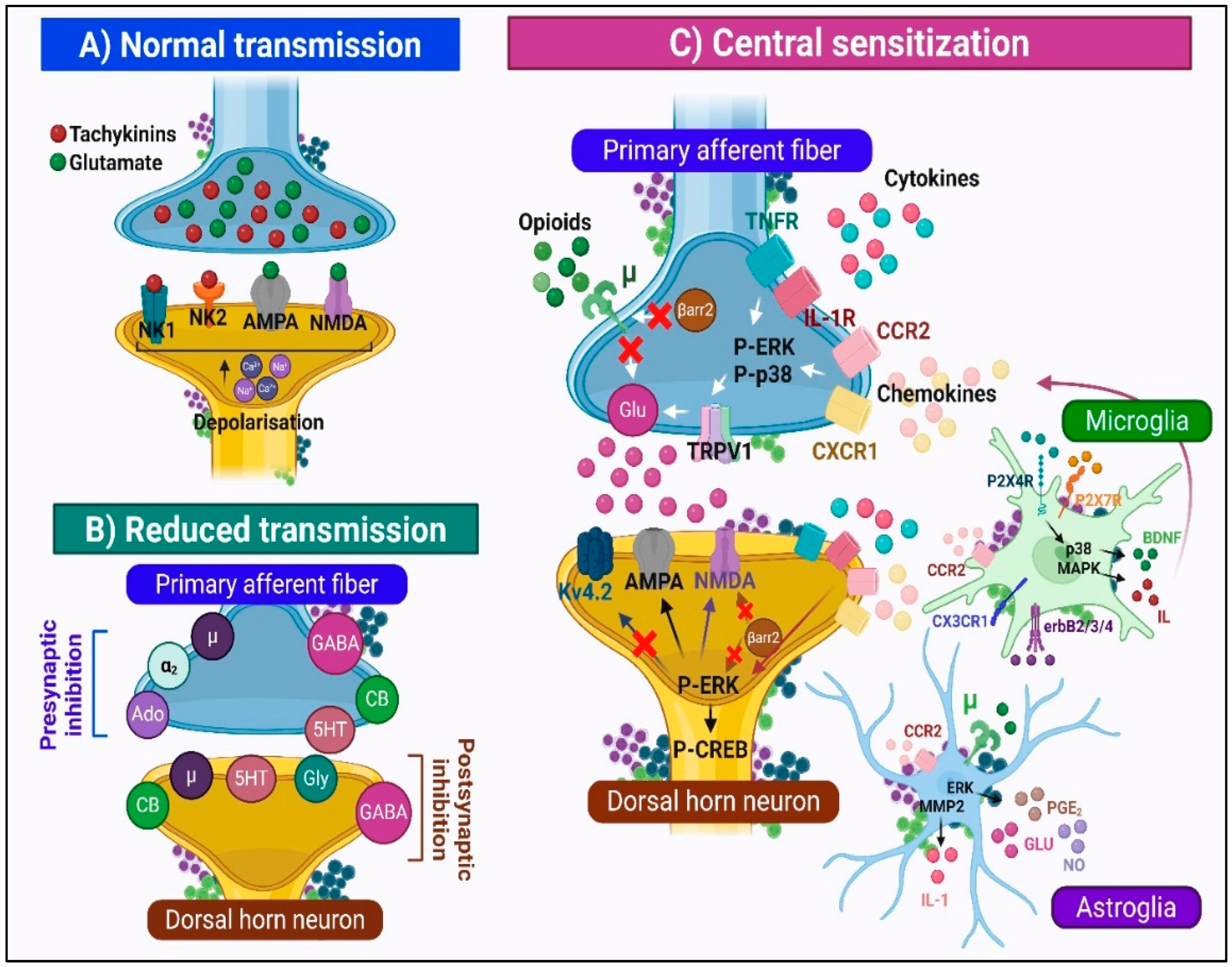
2.3. Synaptic Mechanisms of Nociceptive Transmission
2.3.1. Normal Physiological State
2.3.2. Central Sensitization: The Transition to Pathological Pain Processing
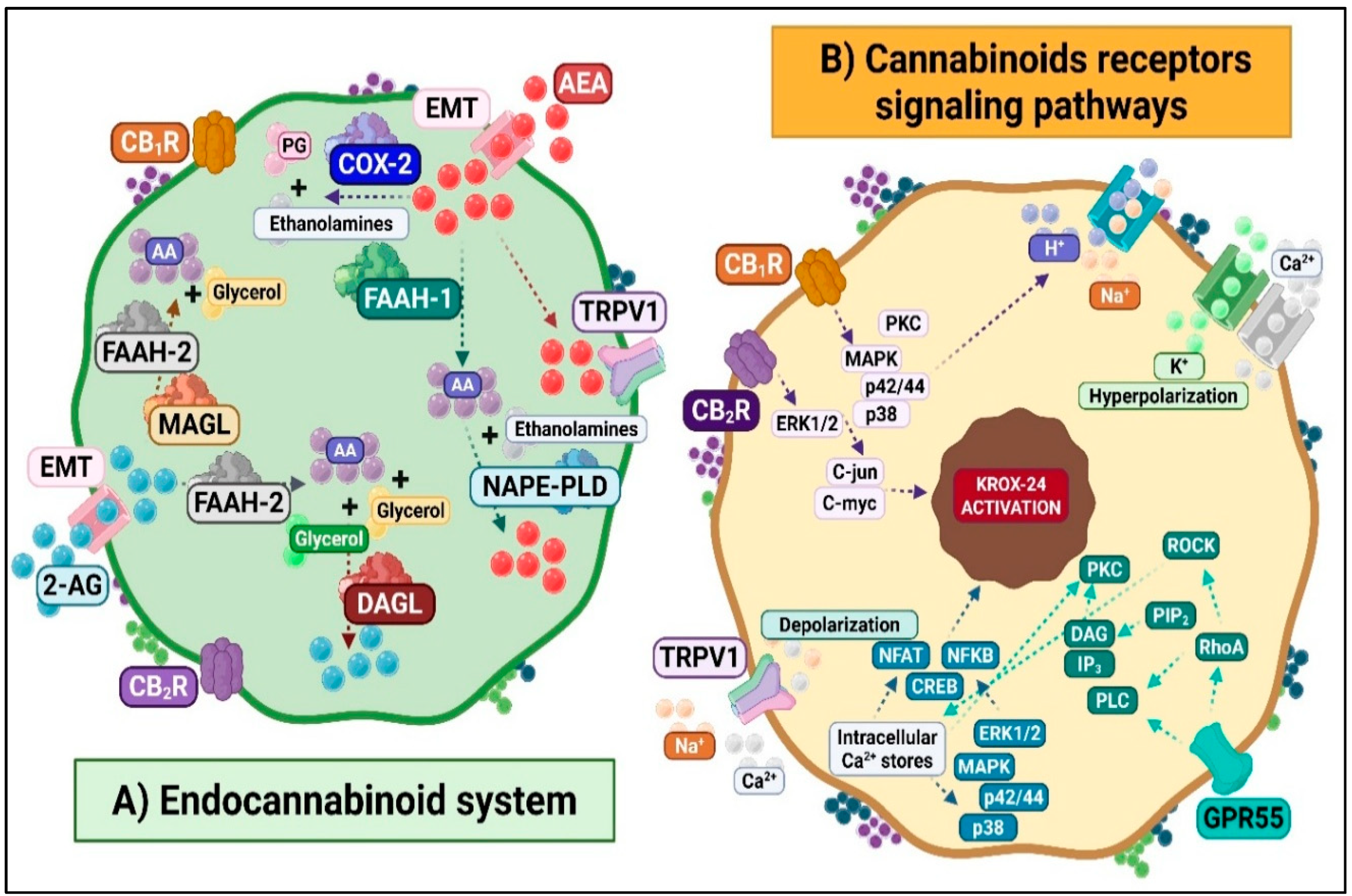
3. Spinal Endocannabinoid System: Implications in Neuropathic Pain
3.1. Molecular and Cellular Responses of the ECS to Noxious Stimuli
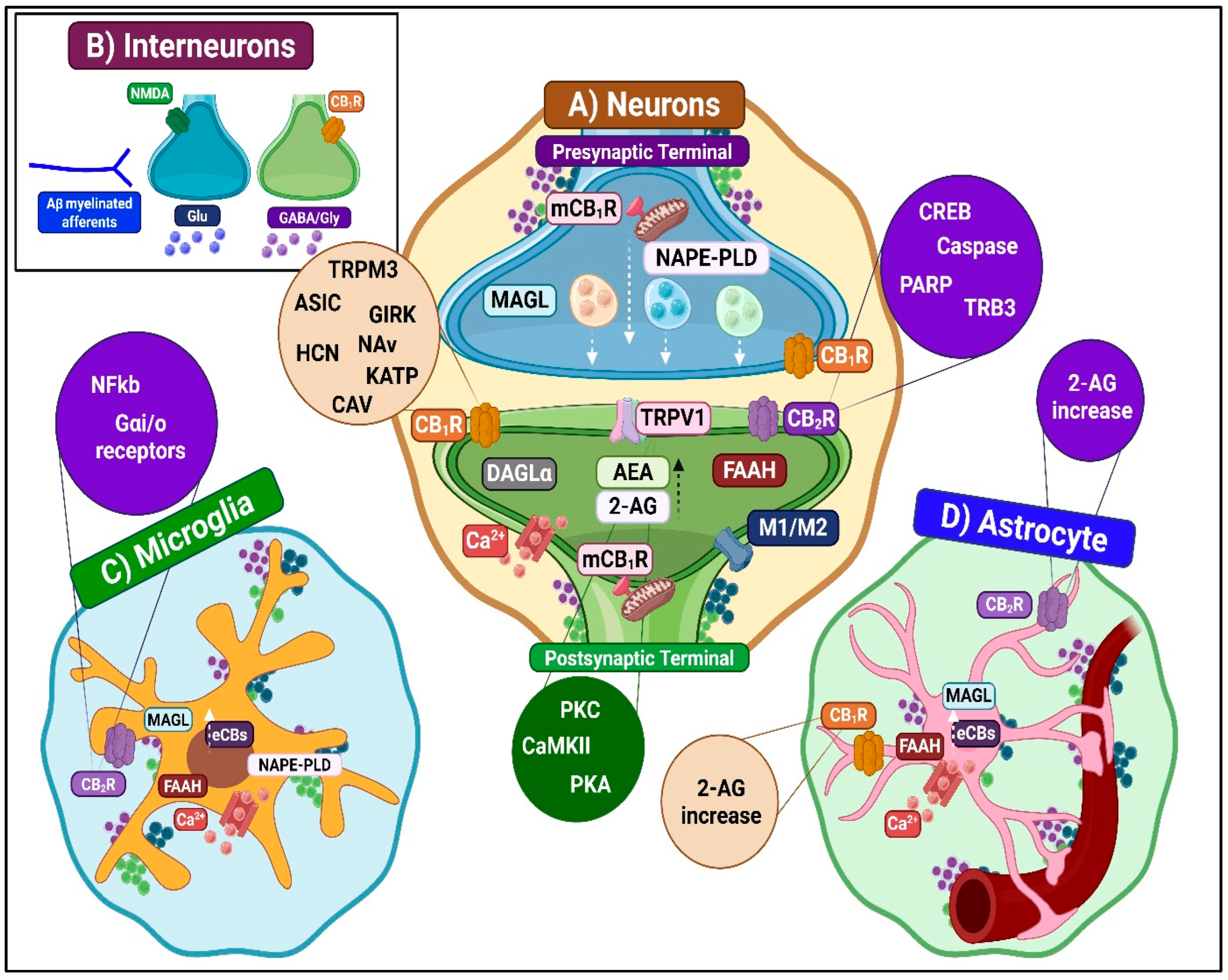
3.1.1. Spinal ECS and Antinociceptive Mechanisms
Inhibition of Ascending Nociceptive Transmission
- CB1R-mediated inhibition
- CB2R-mediated inhibition
- TRPV1-mediated inhibition
- Other receptor contributions
Enhancement of Descending Inhibitory Modulation
Neuroimmune Modulation
- Microglia-dependent antinociceptive mechanisms
- Astrocyte-dependent antinociceptive mechanisms
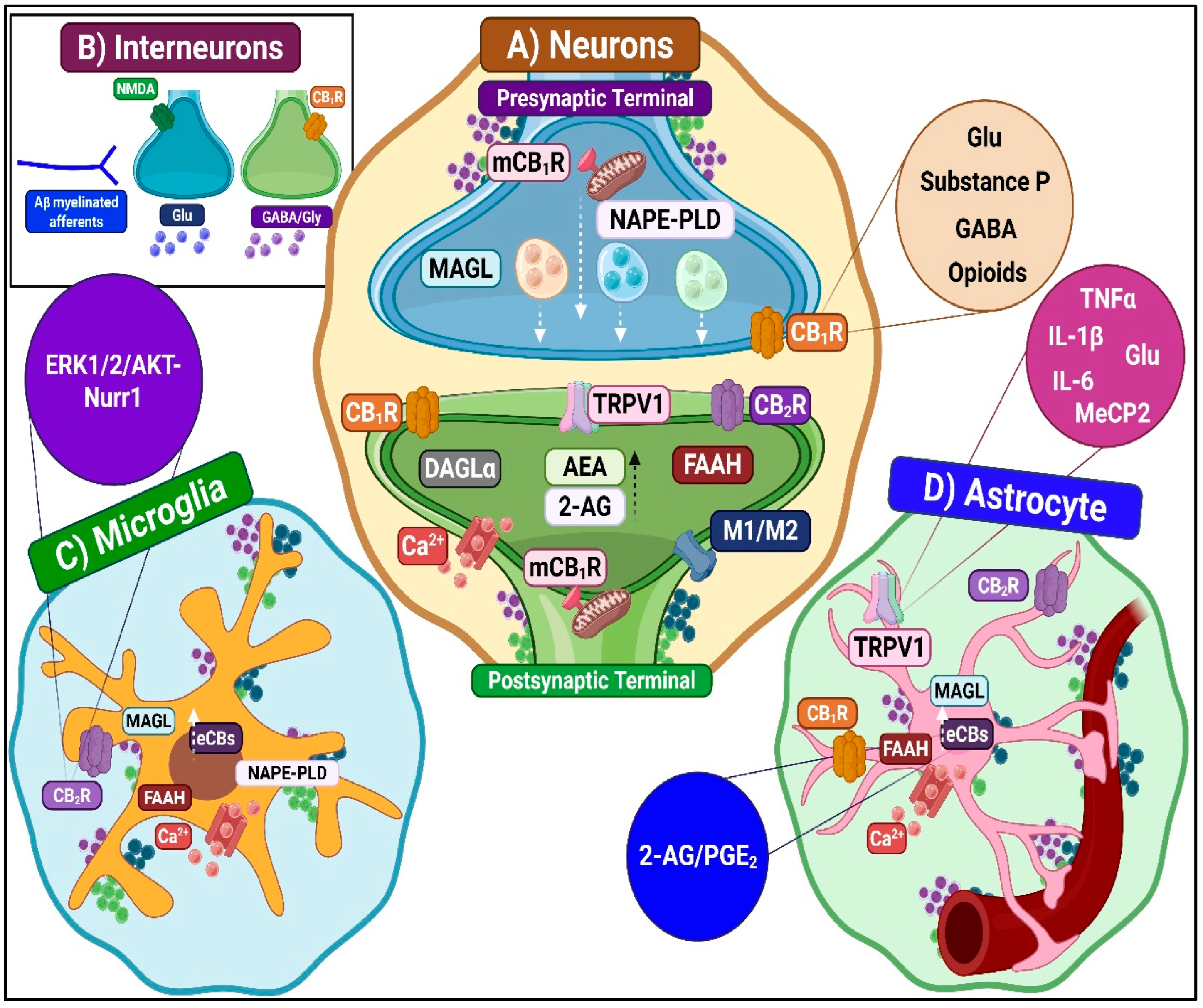
3.1.2. Spinal ECS and Pronociceptive Mechanisms
Potentiation of Ascending Nociceptive Transmission
- CB1R-mediated pronociception
- CB2R-mediated pronociception
- TRPV1-mediated pronociception
- Contributions from other receptors
Enhancement of Descending Excitatory Modulation
Neuroimmune Modulation
- Microglia-dependent pronociceptive mechanisms
- Astrocyte-dependent pronociceptive mechanisms
3.2. Determinants in Endocannabinoid System Plasticity
3.2.1. Diversity of the Ligand Concentration
3.2.2. Diverse Distributions of Activated Receptors
3.2.3. Diversity of Cannabinoid-Mediated Signaling Secondary to Altered ECS Receptor and Enzyme Expression
Signaling Related to GPCRs
Signaling Related to TRPV1 Channels
4. Therapeutic Implications
4.1. General Considerations
4.1.1. Use of the Intrathecal Route
4.1.2. Manipulation of Endocannabinoid Levels and Adaptive Responses to Injury
4.1.3. Therapies Targeting Glial Cells
4.1.4. Multimodal Analgesia
4.2. Specific Considerations
4.2.1. ECS-Related Strategies
Spinal CB1R-Mediated Modulation
Spinal CB2R-Mediated Modulation
Spinal Non-CB1R/CB2R Cannabinoid Receptors Modulation
Development of Agents Enhancing Endogenous Cannabinoid Signaling: Indirect Agonist Strategies
Development of Targeted Therapeutics for Selective Modulation of ECS Signaling Networks
- Allosteric modulators of cannabinoid receptors
- Drug design for cannabinoid receptor dimer
- Biased agonists in cannabinoid pharmacology
4.2.2. Other Strategies
Immune Response Modulation
- Selective inhibitors of microglial activation
- Modulators of pro-inflammatory signaling pathways
- Regulators of pathological astrocytic activity
- Pharmacological agents that interfere with aberrant intercellular glial communication.
Endocrine Response Modulation
Pharmacogenomics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Name | Category |
| 2-AG | 2-Arachidonoylglycerol | Endocannabinoid (Neurotransmitter) |
| 5-HT | 5-Hydroxytryptamine | Neurotransmitter |
| 5HT2A | 5-Hydroxytryptamine receptor 2A | Receptor (GPCR) |
| AA | Arachidonic Acid | Signal Molecule |
| AA-5-HT | N-Arachidonoyl Serotonin | Signal Molecule/Endocannabinoid |
| A-beta fibers | Large-diameter myelinated sensory fibers | Cell/Structure |
| A-delta fibers | Small-diameter thinly myelinated sensory fibers | Cell/Structure |
| AEA | Anandamide | Endocannabinoid (Neurotransmitter) |
| Ado | Adenosine | Neurotransmitter |
| AMPA | Alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid | Receptor (Ionotropic) |
| AMPAR | Alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (glutamate receptor) | Receptor (Ionotropic) |
| AP-1 | Activator Protein-1 | Transcription Factor |
| ASIC | Acid-Sensing Ion Channel | Ion Channel |
| BDNF | Brain-Derived Neurotrophic Factor | Neurotrophin/Signal Molecule |
| BB | Blood–Brain Barrier | Structure |
| BSCB | Blood–Spinal Cord Barrier | Structure |
| βarr2 | Beta-Arrestin 2 | Signal Molecule/Protein |
| Ca2+ | Calcium ion | Ion |
| CaM | Calmodulin | Signal Protein |
| CAV | Voltage-Gated Calcium Channel | Ion Channel |
| CB | Cannabinoid Receptor (generic) | Receptor (GPCR) |
| CB1R | Cannabinoid Receptor Type 1 | Receptor (GPCR) |
| CB2R | Cannabinoid Receptor Type 2 | Receptor (GPCR) |
| CB1/2 | Cannabinoid Receptor Types 1 and 2 | Receptor (GPCR) |
| CCL2 | CC Chemokine Ligand 2 | Chemokine (Signal) |
| CCR2 | CC Chemokine Receptor 2 | Receptor (GPCR) |
| cAMP | Cyclic Adenosine Monophosphate | Second Messenger |
| C-jun | Jun Proto-Oncogene | Transcription Factor |
| C-myc | Myc Proto-Oncogene | Transcription Factor |
| CGRP | Calcitonin Gene-Related Peptide | Neuropeptide/Signal Molecule |
| CNR1/CNR2 | CB1R/CB2R genes | Gene |
| CNS | Central Nervous System | Structure |
| COX/COX-2 | Cyclooxygenase/Cyclooxygenase-2 | Enzyme |
| CREB | cAMP Response Element-Binding Protein | Transcription Factor |
| CSF | Cerebrospinal Fluid | Structure/Fluid |
| CSF1R | Colony-Stimulating Factor 1 Receptor | Receptor (Tyrosine Kinase) |
| Cx43 | Connexin-43 | Gap Junction Protein |
| CX3CL1 | CX3C Chemokine Ligand 1 | Chemokine (Signal) |
| CX3CR1 | CX3C Chemokine Receptor 1 | Receptor (GPCR) |
| CYP2D6/CYP3A4 | Cytochrome P450 | Enzyme |
| DAG | Diacylglycerol | Signal/Lipid |
| DAGL/DAGLα | Diacylglycerol Lipase (α isoform) | Enzyme |
| DOR | Delta Opioid Receptor | Receptor (GPCR) |
| DRG | Dorsal Root Ganglion | Structure/Cell Group |
| eCB/eCBs/ECBs | Endocannabinoids | Neurotransmitter |
| ECS | Endocannabinoid System | Signaling System |
| eEPSCs | Evoked Excitatory Postsynaptic Currents | Electrophysiological Event |
| EMT/AMT | Endocannabinoid/Anandamide Membrane Transporter | Transporter |
| ERK/ERK1/2 | Extracellular Signal-Regulated Kinase(s) | Signal Protein/Enzyme |
| erbB2/3/4 | Erythroblastic Leukemia Viral Oncogene Homolog | Receptor (Tyrosine Kinase) |
| FAAH/FAAH-1/FAAH-2 | Fatty Acid Amide Hydrolase | Enzyme |
| GABA | Gamma-Aminobutyric Acid | Neurotransmitter |
| GABAR | GABA Receptor | Receptor (Ionotropic/Metabotropic) |
| Gαi/o | G Protein αi/o Subunit | Signal Molecule/Protein |
| GDR | Dorsal Root Ganglion | Structure/Cell Group |
| GFAP | Glial Fibrillary Acidic Protein | Glial Cell Marker |
| GIRK | G Protein-Coupled Inwardly Rectifying Potassium Channel | Ion Channel |
| Glu | Glutamate | Neurotransmitter |
| GLT-1/GLAST | Astrocytic Glutamate Transporters | Transporter |
| Gly | Glycine | Neurotransmitter |
| GlyR | Glycine Receptor | Receptor (Ionotropic) |
| GPR55 | G Protein-Coupled Receptor 55 | Receptor (GPCR) |
| GPR119 | G Protein-Coupled Receptor 119 | Receptor (GPCR) |
| HCN/HCN1/2 | Hyperpolarization-Activated Cyclic Nucleotide-Gated Channel | Ion Channel |
| ICAM | Intercellular Adhesion Molecule | Adhesion Molecule |
| IL | Interleukin | Cytokine/Signal |
| IL-1 beta | Interleukin 1 beta | Cytokine |
| IL-1R | Interleukin 1 receptor | Receptor (Cytokine) |
| IL-6 | Interleukin 6 | Cytokine |
| IL-10 | Interleukin 10 | Cytokine |
| IL-33 | Interleukin 33 | Cytokine |
| iNOS | Inducible Nitric Oxide Synthase | Enzyme |
| IP3 | Inositol Trisphosphate | Second Messenger |
| IT | Intrathecal | Route of Administration/Procedure |
| JAK/STAT | Janus Kinase/Signal Transducer and Activator of Transcription | Signal Protein/Pathway |
| JNK | c-Jun N-terminal Kinase | Signal Protein/Enzyme |
| K+ | Potassium Ion | Ion |
| KATP | ATP-Sensitive Potassium Channel | Ion Channel |
| KCC2 | Potassium-Chloride Cotransporter 2 | Transporter |
| Kv4.2 | Potassium Voltage-Gated Channel Subfamily D Member 2 | Ion Channel |
| KROX 24 | Transcription Factor KROX 24 | Transcription Factor |
| LFA-1 | Lymphocyte Function-Associated Antigen 1 | Adhesion Molecule |
| MAGL | Monoacylglycerol Lipase | Enzyme |
| MAPK | Mitogen-Activated Protein Kinase | Signal Protein/Enzyme |
| M1/M2 | Microglial Phenotypes | Cell/Phenotype |
| MMP-2/MMP-9 | Matrix Metalloproteinases | Enzyme |
| MeCP2 | Methyl CpG-Binding Protein 2 | Epigenetic Marker/Protein |
| mCB1R | Mitochondrial Cannabinoid Receptor 1 | Receptor (GPCR) |
| Na+ | Sodium Ion | Ion |
| NAPE-PLD | N-Acyl Phosphatidylethanolamine Phospholipase D | Enzyme |
| Nav1.7/SCN9A | Voltage-Gated Sodium Channel/Gene | Ion Channel/Gene |
| NFAT | Nuclear Factor of Activated T-Cells | Transcription Factor |
| NF-kappa-B | Nuclear Factor Kappa B | Transcription Factor |
| NGF | Nerve Growth Factor | Neurotrophin/Signal Molecule |
| NK1/NK2 | Neurokinin 1/2 Receptor | Receptor (GPCR) |
| NMDA/NMDAR | N-Methyl-D-Aspartate Receptor | Receptor (Ionotropic) |
| NO | Nitric Oxide | Signal Molecule |
| NSAIDs | Non-Steroidal Anti-Inflammatory Drugs | Drug Class |
| OPRM1 | Mu-Opioid Receptor Gene | Gene |
| OPCs | Oligodendrocyte Precursor Cells | Cell Type |
| OX1 | Orexin receptor type 1 | Receptor (GPCR) |
| p38/P-p38 | Stress-Activated MAP Kinases | Signal Protein/Enzyme |
| PARP | Poly(ADP-ribose) Polymerase | Enzyme |
| PBMC | Peripheral Blood Mononuclear Cell | Cell Type |
| P-CREB | Phosphorylated cAMP Response Element-Binding Protein | Transcription Factor (activated) |
| P-ERK | Phosphorylated ERK | Signal Protein (activated) |
| PECAM | Platelet Endothelial Cell Adhesion Molecule | Adhesion Molecule |
| PG/PGE2 | Prostaglandin/Prostaglandin E2 | Signal Molecule |
| PIP2 | Phosphatidylinositol 4,5-Bisphosphate | Second Messenger/Lipid |
| PKC | Protein Kinase C | Enzyme |
| PKA | Protein Kinase A | Enzyme |
| PLC | Phospholipase C | Enzyme |
| PPAR/PPARs | Peroxisome Proliferator-Activated Receptor(s) | Nuclear Receptor/Transcription Factor |
| PR | Progesterone Receptor | Nuclear Receptor |
| ROCK | Rho-Associated Protein Kinase | Enzyme |
| RhoA | Ras Homolog Family Member A | Signal Molecule/Protein |
| RE | Reticulum Endoplasmic | Organelle |
| SCN9A | Sodium Channel, Voltage-Gated, Type IX, Alpha Subunit Gene | Gene |
| siRNA | Small Interfering RNA | Genetic Tool |
| SNPs | Single Nucleotide Polymorphisms | Genetic Variation |
| STAT/STAT3 | Signal Transducer and Activator of Transcription | Signal Protein/Transcription Factor |
| TGF-beta | Transforming Growth Factor Beta | Cytokine/Signal |
| T-cells | T- lymphocytes | Cell Type |
| TNF | Tumor Necrosis Factor Alpha | Cytokine |
| TNFR | Tumor Necrosis Factor Receptor | Receptor |
| TRP | Transient Receptor Potential Channel Family | Ion Channel |
| TRPA1 | Transient Receptor Potential Ankyrin 1 | Ion Channel |
| TRPM3 | Transient Receptor Potential Melastatin 3 | Ion Channel |
| TRPV1 | Transient Receptor Potential Vanilloid 1 | Ion Channel |
| VGCC | Voltage-Gated Calcium Channel | Ion Channel |
References
- Cao, B.; Xu, Q.; Shi, Y.; Zhao, R.; Li, H.; Zheng, J.; Liu, F.; Wan, Y.; Wei, B. Pathology of pain and its implications for therapeutic interventions. Signal Transduct. Target. Ther. 2024, 9, 155. [Google Scholar] [CrossRef]
- Karcz, M.; Abd-Elsayed, A.; Chakravarthy, K.; Aman, M.M.; Strand, N.; Malinowski, M.N.; Latif, U.; Dickerson, D.; Suvar, T.; Lubenow, T.; et al. Pathophysiology of Pain and Mechanisms of Neuromodulation: A Narrative Review (A Neuron Project). J. Pain Res. 2024, 17, 3757–3790. [Google Scholar] [CrossRef] [PubMed]
- Comitato, A.; Bardoni, R. Presynaptic Inhibition of Pain and Touch in the Spinal Cord: From Receptors to Circuits. Int. J. Mol. Sci. 2021, 22, 414. [Google Scholar] [CrossRef]
- Yang, J.X.; Wang, H.F.; Chen, J.Z.; Li, H.Y.; Hu, J.C.; Yu, A.A.; Wen, J.J.; Chen, S.J.; Lai, W.D.; Wang, S.; et al. Potential Neuroimmune Interaction in Chronic Pain: A Review on Immune Cells in Peripheral and Central Sensitization. Front. Pain Res. 2022, 3, 946846. [Google Scholar] [CrossRef]
- Antal, M. Molecular Anatomy of Synaptic and Extrasynaptic Neurotransmission Between Nociceptive Primary Afferents and Spinal Dorsal Horn Neurons. Int. J. Mol. Sci. 2025, 26, 2356. [Google Scholar] [CrossRef]
- Abd-Elsayed, A.; Vardhan, S.; Aggarwal, A.; Vardhan, M.; Diwan, S.A. Mechanisms of Action of Dorsal Root Ganglion Stimulation. Int. J. Mol. Sci. 2024, 25, 3591. [Google Scholar] [CrossRef]
- Curatolo, M. Central Sensitization and Pain: Pathophysiologic and Clinical Insights. Curr. Neuropharmacol. 2024, 22, 15–22. [Google Scholar] [CrossRef]
- Attal, N.; Bouhassira, D.; Colvin, L. Advances and challenges in neuropathic pain: A narrative review and future directions. Br. J. Anaesth. 2023, 131, 79–92. [Google Scholar] [CrossRef]
- da Silva, M.D.V.; Martelossi-Cebinelli, G.; Yaekashi, K.M.; Carvalho, T.T.; Borghi, S.M.; Casagrande, R.; Verri, W.A. A Narrative Review of the Dorsal Root Ganglia and Spinal Cord Mechanisms of Action of Neuromodulation Therapies in Neuropathic Pain. Brain Sci. 2024, 14, 589. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Kuner, R.; Jensen, T.S. Neuropathic Pain: From Mechanisms to Treatment. Physiol. Rev. 2021, 101, 259–301. [Google Scholar] [CrossRef]
- Rexed, B. The cytoarchitectonic organization of the spinal cord in the cat. J. Comp. Neurol. 1952, 96, 414–495. [Google Scholar] [CrossRef]
- Gilbert, J.E.; Zhang, T.; Esteller, R.; Grill, W.M. Network model of nociceptive processing in the superficial spinal dorsal horn reveals mechanisms of hyperalgesia, allodynia, and spinal cord stimulation. J. Neurophysiol. 2023, 130, 1103–1117. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.A.; Hughes, D.I. Defining populations of dorsal horn interneurons. Pain 2020, 161, 2434–2436. [Google Scholar] [CrossRef]
- Donnelly, C.R.; Andriessen, A.S.; Chen, G.; Wang, K.; Jiang, C.; Maixner, W.; Ji, R.-R. Central Nervous System Targets: Glial Cell Mechanisms in Chronic Pain. Neurotherapeutics 2020, 17, 846–860. [Google Scholar] [CrossRef]
- Long, J.; Tian, G.; He, K.; Su, Y.; Wang, Z.; Huang, L.; Yao, Y.; Li, X.; Lin, Y. The role of microglia in neuropathic pain: A systematic review of animal experiments. Brain Res. Bull. 2025, 228, 111410. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, Y.; Bai, J. Spinal astrocytes involved in the pathogenesis and treatment of neuropathic pain. Front. Cell Neurosci. 2025, 19, 1547524. [Google Scholar] [CrossRef]
- Kim, W.; Angulo, M.C. Unraveling the role of oligodendrocytes and myelin in pain. J. Neurochem. 2025, 169, e16206. [Google Scholar] [CrossRef]
- Bannister, K.; Dickenson, A.H. Central Nervous System Targets: Supraspinal Mechanisms of Analgesia. Neurotherapeutics 2020, 17, 839–845. [Google Scholar] [CrossRef]
- Sirucek, L.; Ganley, R.P.; Zeilhofer, H.U.; Schweinhardt, P. Diffuse noxious inhibitory controls and conditioned pain modulation: A shared neurobiology within the descending pain inhibitory system? Pain 2023, 164, 463–468. [Google Scholar] [CrossRef]
- Malcangio, M. Role of the immune system in neuropathic pain. Scand. J. Pain 2019, 20, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.; Kuhn, J.A.; Braz, J.M.; Basbaum, A.I. Neuronal aromatase expression in pain processing regions of the medullary and spinal cord dorsal horn. J. Comp. Neurol. 2017, 525, 3414–3428. [Google Scholar] [CrossRef] [PubMed]
- Park, G.W.; Kim, H.; Won, S.H.; Kim, N.H.; Choi, S.R. Neurosteroids and neurological disorders. Korean J. Physiol. Pharmacol. 2025, 29, 157–164. [Google Scholar] [CrossRef]
- Diviccaro, S.; Cioffi, L.; Falvo, E.; Giatti, S.; Melcangi, R.C. Allopregnanolone: An overview on its synthesis and effects. J. Neuroendocrinol. 2022, 34, e12996. [Google Scholar] [CrossRef]
- Mensah-Nyagan, A.G.; Meyer, L.; Patte-Mensah, C. Modulatory role of neurosteroidogenesis in the spinal cord during peripheral nerve injury-induced chronic pain. Front. Neuroendocrinol. 2024, 72, 101116. [Google Scholar] [CrossRef]
- Coyoy-Salgado, A.; Segura-Uribe, J.; Salgado-Ceballos, H.; Castillo-Mendieta, T.; Sanchez-Torres, S.; Freyermuth-Trujillo, X.; Orozco-Barrios, C.; Orozco-Suarez, S.; Feria-Romero, I.; Pinto-Almazán, R.; et al. Evaluating Sex Steroid Hormone Neuroprotection in Spinal Cord Injury in Animal Models: Is It Promising in the Clinic? Biomedicines 2024, 12, 1478. [Google Scholar] [CrossRef]
- Dedek, A.; Hildebrand, M.E. Advances and Barriers in Understanding Presynaptic N-Methyl-D-Aspartate Receptors in Spinal Pain Processing. Front. Mol. Neurosci. 2022, 15, 864502. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Sun, X.; Wang, Q.; Zhang, J.; Wen, H.; Chen, W.J.; Zhu, S. Structural insights into the diverse actions of magnesium on NMDA receptors. Neuron 2025, 113, 1006–1018.e4. [Google Scholar] [CrossRef] [PubMed]
- De Preter, C.C.; Heinricher, M.M. The ‘in’s and out’s’ of descending pain modulation from the rostral ventromedial medulla. Trends Neurosci. 2024, 47, 447–460. [Google Scholar] [CrossRef]
- Song, Q.; E, S.; Zhang, Z.; Liang, Y. Neuroplasticity in the transition from acute to chronic pain. Neurotherapeutics 2024, 21, e00464. [Google Scholar] [CrossRef]
- Luongo, L.; Guida, F.; Maione, S.; Jacobson, K.A.; Salvemini, D. Adenosine Metabotropic Receptors in Chronic Pain Management. Front. Pharmacol. 2021, 12, 651038. [Google Scholar] [CrossRef]
- Hao, S.; Shi, W.; Liu, W.; Chen, Q.Y.; Zhuo, M. Multiple modulatory roles of serotonin in chronic pain and injury-related anxiety. Front. Synaptic Neurosci. 2023, 15, 1122381. [Google Scholar] [CrossRef]
- Huang, S.; Chen, Y.; Jia, Y.; Yang, T.; Su, W.; Zhu, Z.; Xue, P.; Feng, F.; Zhao, Y.; Chen, G. Delayed inhibition of ERK and p38 attenuates neuropathic pain without affecting motor function recovery after peripheral nerve injury. Neuropharmacology 2022, 202, 108835. [Google Scholar] [CrossRef]
- Xie, R.G.; Chu, W.G.; Liu, D.L.; Wang, X.; Ma, S.B.; Wang, F.; Wang, F.-D.; Lin, Z.; Wu, W.-B.; Lu, N.; et al. Presynaptic NMDARs on spinal nociceptor terminals state-dependently modulate synaptic transmission and pain. Nat. Commun. 2022, 13, 728. [Google Scholar] [CrossRef]
- Olivero, G.; Vergassola, M.; Cisani, F.; Roggeri, A.; Pittaluga, A. Presynaptic Release-regulating Metabotropic Glutamate Receptors: An Update. Curr. Neuropharmacol. 2020, 18, 655–672. [Google Scholar] [CrossRef] [PubMed]
- Koga, K.; Kobayashi, K.; Tsuda, M.; Kubota, K.; Kitano, Y.; Furue, H. Voltage-gated calcium channel subunit alpha(2)delta-1 in spinal dorsal horn neurons contributes to aberrant excitatory synaptic transmission and mechanical hypersensitivity after peripheral nerve injury. Front. Mol. Neurosci. 2023, 16, 1099925. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhang, J.; Xu, J.; Wang, Y.; Zheng, X.; Wang, W. Differential Neuronal Activation of Nociceptive Pathways in Neuropathic Pain After Spinal Cord Injury. Cell. Mol. Neurobiol. 2025, 45, 18. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Bair, M.; Descalzi, G. Reactive Astrocytes: Critical Players in the Development of Chronic Pain. Front. Psychiatry 2021, 12, 682056. [Google Scholar] [CrossRef]
- Rezende, B.; Alencar, A.K.N.; de Bem, G.F.; Fontes-Dantas, F.L.; Montes, G.C. Endocannabinoid System: Chemical Characteristics and Biological Activity. Pharmaceuticals 2023, 16, 148. [Google Scholar] [CrossRef]
- Maccarrone, M.; Di Marzo, V.; Gertsch, J.; Grether, U.; Howlett, A.C.; Hua, T.; Makriyannis, A.; Piomelli, D.; Ueda, N.; van der Stelt, M. Goods and Bads of the Endocannabinoid System as a Therapeutic Target: Lessons Learned after 30 Years. Pharmacol. Rev. 2023, 75, 885–958. [Google Scholar] [CrossRef]
- Kurosu, K.; Islam, A.; Sato, T.; Kahyo, T.; Banno, T.; Sato, N.; Matsuyama, Y.; Setou, M. Expression and Kinetics of Endogenous Cannabinoids in the Brain and Spinal Cord of a Spare Nerve Injury (SNI) Model of Neuropathic Pain. Cells 2022, 11, 4130. [Google Scholar] [CrossRef]
- Li, X.; Shen, L.; Hua, T.; Liu, Z.J. Structural and Functional Insights into Cannabinoid Receptors. Trends Pharmacol. Sci. 2020, 41, 665–677. [Google Scholar] [CrossRef]
- Iannotti, F.A.; Vitale, R.M. The Endocannabinoid System and PPARs: Focus on Their Signalling Crosstalk, Action and Transcriptional Regulation. Cells 2021, 10, 586. [Google Scholar] [CrossRef]
- Kibret, B.G.; Ishiguro, H.; Horiuchi, Y.; Onaivi, E.S. New Insights and Potential Therapeutic Targeting of CB2 Cannabinoid Receptors in CNS Disorders. Int. J. Mol. Sci. 2022, 23, 975. [Google Scholar] [CrossRef]
- Ouyang, Z.; Han, Z.; Xu, D.; Yan, B.; Wang, J.; Ye, S.; Wu, S.; Qiao, A. Structural insights into the activation of G protein-coupled receptor 119 by the agonist GSK1292263 and ligands selectivity among novel cannabinoid receptors. Int. J. Biol. Macromol. 2025, 319 Pt 1, 145476. [Google Scholar] [CrossRef]
- Chang, H.; Li, X.; Shen, L.; Ge, X.; Hao, S.; Wu, L.; Liu, S.; Liu, J.; Cherezov, V.; Hua, T. Structure basis of ligand recognition and activation of GPR55. Cell Res. 2025, 35, 80–83. [Google Scholar] [CrossRef]
- Vázquez-Carrera, M.; Wahli, W. PPARs as Key Mediators in the Regulation of Metabolism and Inflammation. Int. J. Mol. Sci. 2022, 23, 5025. [Google Scholar] [CrossRef]
- Pontearso, M.; Slepicka, J.; Bhattacharyya, A.; Spicarova, D.; Palecek, J. Dual effect of anandamide on spinal nociceptive transmission in control and inflammatory conditions. Biomed. Pharmacother. 2024, 173, 116369. [Google Scholar] [CrossRef]
- Martinez-Torres, S.; Mesquida-Veny, F.; Del Rio, J.A.; Hervera, A. Injury-induced activation of the endocannabinoid system promotes axon regeneration. iScience 2023, 26, 106814. [Google Scholar] [CrossRef] [PubMed]
- Finn, D.P.; Haroutounian, S.; Hohmann, A.G.; Krane, E.; Soliman, N.; Rice, A.S.C. Cannabinoids, the endocannabinoid system, and pain: A review of preclinical studies. Pain 2021, 162 (Suppl. 1), S5–S25. [Google Scholar] [CrossRef] [PubMed]
- Sagar, D.R.; Jhaveri, M.D.; Richardson, D.; Gray, R.A.; de Lago, E.; Fernandez-Ruiz, J.; Barrett, D.A.; Kendall, D.A.; Chapman, V. Endocannabinoid regulation of spinal nociceptive processing in a model of neuropathic pain. Eur. J. Neurosci. 2010, 31, 1414–1422. [Google Scholar] [CrossRef] [PubMed]
- Lutz, B. Neurobiology of cannabinoid receptor signaling. Dialogues Clin. Neurosci. 2020, 22, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Parnell, J.; Martin, N.; Dedek, A.; Rudyk, C.; Landrigan, J.; Bellavance, J.; VanDerLoo, S.; Tsai, E.C.; Hildebrand, M.E. Cannabinoid CB1 Receptor Expression and Localization in the Dorsal Horn of Male and Female Rat and Human Spinal Cord. Can. J. Pain 2023, 7, 2264895. [Google Scholar] [CrossRef]
- Yang, F.; Xu, Q.; Shu, B.; Tiwari, V.; He, S.Q.; Vera-Portocarrero, L.P.; Dong, X.; Linderoth, B.; Raja, S.N.; Wang, Y.; et al. Activation of cannabinoid CB1 receptor contributes to suppression of spinal nociceptive transmission and inhibition of mechanical hypersensitivity by Abeta-fiber stimulation. Pain 2016, 157, 2582–2593. [Google Scholar] [CrossRef] [PubMed]
- Hojo, M.; Sudo, Y.; Ando, Y.; Minami, K.; Takada, M.; Matsubara, T.; Kanaide, M.; Taniyama, K.; Sumikawa, K.; Uezono, Y. mu-Opioid receptor forms a functional heterodimer with cannabinoid CB1 receptor: Electrophysiological and FRET assay analysis. J. Pharmacol. Sci. 2008, 108, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Milligan, A.L.; Szabo-Pardi, T.A.; Burton, M.D. Cannabinoid Receptor Type 1 and Its Role as an Analgesic: An Opioid Alternative? J. Dual Diagn. 2020, 16, 106–119. [Google Scholar] [CrossRef]
- Guenther, K.G.; Hohmann, A.G. Cannabinoid CB(2) receptor-mediated analgesia: Mechanism-based insights and therapeutic potential. Br. J. Pharmacol. 2025, 182, 5090–5118. [Google Scholar] [CrossRef]
- Ghosh, K.; Zhang, G.F.; Chen, H.; Chen, S.R.; Pan, H.L. Cannabinoid CB2 receptors are upregulated via bivalent histone modifications and control primary afferent input to the spinal cord in neuropathic pain. J. Biol. Chem. 2022, 298, 101999. [Google Scholar] [CrossRef]
- Rakotoarivelo, V.; Mayer, T.Z.; Simard, M.; Flamand, N.; Di Marzo, V. The Impact of the CB(2) Cannabinoid Receptor in Inflammatory Diseases: An Update. Molecules 2024, 29, 3381. [Google Scholar] [CrossRef]
- Grabon, W.; Rheims, S.; Smith, J.; Bodennec, J.; Belmeguenai, A.; Bezin, L. CB2 receptor in the CNS: From immune and neuronal modulation to behavior. Neurosci. Biobehav. Rev. 2023, 150, 105226. [Google Scholar] [CrossRef]
- Rahman, M.M.; Jo, Y.Y.; Kim, Y.H.; Park, C.K. Current insights and therapeutic strategies for targeting TRPV1 in neuropathic pain management. Life Sci. 2024, 355, 122954. [Google Scholar] [CrossRef]
- Kang, S.Y.; Seo, S.Y.; Bang, S.K.; Cho, S.J.; Choi, K.H.; Ryu, Y. Inhibition of Spinal TRPV1 Reduces NMDA Receptor 2B Phosphorylation and Produces Anti-Nociceptive Effects in Mice with Inflammatory Pain. Int. J. Mol. Sci. 2021, 22, 11177. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, N.; Altier, C. TRPV1 and MOR working in tandem: Implications for pain and opioids use. Neuropsychopharmacology 2020, 45, 225–226. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Song, F.H.; Gao, S.J.; Wu, J.Y.; Li, D.Y.; Zhang, L.Q.; Zhou, Y.Q.; Liu, D.Q.; Mei, W. Peroxisome proliferator-activated receptor gamma: A promising therapeutic target for the treatment of chronic pain. Brain Res. 2025, 1850, 149366. [Google Scholar] [CrossRef] [PubMed]
- Zuniga-Romero, A.; Rivera-Plata, Q.; Arrieta, J.; Flores-Murrieta, F.J.; Rodriguez-Silverio, J.; Reyes-Garcia, J.G.; Huerta-Cruz, J.C.; Ramírez-Martínez, G.; Rocha-González, H.I. GPR55 and GPR119 Receptors Contribute to the Processing of Neuropathic Pain in Rats. Pharmaceuticals 2022, 15, 67. [Google Scholar] [CrossRef]
- Bouchet, C.A.; Ingram, S.L. Cannabinoids in the descending pain modulatory circuit: Role in inflammation. Pharmacol. Ther. 2020, 209, 107495. [Google Scholar] [CrossRef]
- Lee, M.T.; Mackie, K.; Chiou, L.C. Alternative pain management via endocannabinoids in the time of the opioid epidemic: Peripheral neuromodulation and pharmacological interventions. Br. J. Pharmacol. 2023, 180, 894–909. [Google Scholar] [CrossRef]
- Afridi, R.; Tsuda, M.; Ryu, H.; Suk, K. The Function of Glial Cells in the Neuroinflammatory and Neuroimmunological Responses. Cells 2022, 11, 659. [Google Scholar] [CrossRef]
- Ye, Y.; Su, X.; Tang, J.; Zhu, C. Neuropathic Pain Induced by Spinal Cord Injury from the Glia Perspective and Its Treatment. Cell. Mol. Neurobiol. 2024, 44, 81. [Google Scholar] [CrossRef]
- Karavis, M.Y.; Siafaka, I.; Vadalouca, A.; Georgoudis, G. Role of Microglia in Neuropathic Pain. Cureus 2023, 15, e43555. [Google Scholar] [CrossRef]
- Martinez Ramirez, C.E.; Ruiz-Perez, G.; Stollenwerk, T.M.; Behlke, C.; Doherty, A.; Hillard, C.J. Endocannabinoid signaling in the central nervous system. Glia 2023, 71, 5–35. [Google Scholar] [CrossRef]
- Li, J.; Wang, P.; Zhou, T.; Jiang, W.; Wu, H.; Zhang, S.; Deng, L.; Wang, H. Neuroprotective effects of interleukin 10 in spinal cord injury. Front. Mol. Neurosci. 2023, 16, 1214294. [Google Scholar] [CrossRef]
- Chen, O.; Luo, X.; Ji, R.R. Macrophages and microglia in inflammation and neuroinflammation underlying different pain states. Med. Rev. 2023, 3, 381–407. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, Y.; Zhai, X.; Xie, L.; Guo, Y.; Chen, C.; Li, Y.; Wang, F.; Zhu, Z.; Zheng, L.; et al. Microglial phagocytosis and regulatory mechanisms after stroke. J. Cereb. Blood Flow Metab. 2022, 42, 1579–1596. [Google Scholar] [CrossRef]
- McQuade, A.; Kang, Y.J.; Hasselmann, J.; Jairaman, A.; Sotelo, A.; Coburn, M.; Shabestari, S.K.; Chadarevian, J.P.; Fote, G.; Tu, C.H.; et al. Gene expression and functional deficits underlie TREM2-knockout microglia responses in human models of Alzheimer’s disease. Nat. Commun. 2020, 11, 5370, Correction in Nat. Commun. 2023, 14, 1194. [Google Scholar] [CrossRef]
- Illes, P.; Rubini, P.; Ulrich, H.; Zhao, Y.; Tang, Y. Regulation of Microglial Functions by Purinergic Mechanisms in the Healthy and Diseased CNS. Cells 2020, 9, 1108. [Google Scholar] [CrossRef]
- Franco, R.; Lillo, A.; Rivas-Santisteban, R.; Reyes-Resina, I.; Navarro, G. Microglial Adenosine Receptors: From Preconditioning to Modulating the M1/M2 Balance in Activated Cells. Cells 2021, 10, 1124. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Cheng, W.; Wang, Y.; Chen, X.; Zhang, L.; Li, Y.; Shen, F.; Yuan, D.; Hong, P.; Huang, W.; et al. The Dual Role of A2aR in Neuroinflammation: Modulating Microglial Polarization in White Matter Lesions. eNeuro 2025, 12, ENEURO.0579-24.2025. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Wu, Y.; Tian, Z.; Xu, Y.; Wu, C.; Wang, Z. Microglial Cannabinoid CB(2) Receptors in Pain Modulation. Int. J. Mol. Sci. 2023, 24, 2348. [Google Scholar] [CrossRef]
- Stella, N. Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia 2010, 58, 1017–1030. [Google Scholar] [CrossRef]
- van den Hoogen, N.J.; Harding, E.K.; Davidson, C.E.D.; Trang, T. Cannabinoids in Chronic Pain: Therapeutic Potential Through Microglia Modulation. Front. Neural Circuits 2021, 15, 816747. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhu, Y.; Ren, Y.; Zhao, L.; Zhao, J.; Yin, S.; Ni, H.; Zhu, R.; Cheng, L.; Xie, N. Targeting resident astrocytes attenuates neuropathic pain after spinal cord injury. eLife 2024, 13, RP95672. [Google Scholar] [CrossRef] [PubMed]
- Eraso-Pichot, A.; Pouvreau, S.; Olivera-Pinto, A.; Gomez-Sotres, P.; Skupio, U.; Marsicano, G. Endocannabinoid signaling in astrocytes. Glia 2023, 71, 44–59. [Google Scholar] [CrossRef]
- Kasatkina, L.A.; Rittchen, S.; Sturm, E.M. Neuroprotective and Immunomodulatory Action of the Endocannabinoid System under Neuroinflammation. Int. J. Mol. Sci. 2021, 22, 5431. [Google Scholar] [CrossRef] [PubMed]
- Quintero, J.M.; Diaz, L.E.; Galve-Roperh, I.; Bustos, R.H.; Leon, M.X.; Beltran, S.; Dodd, S. The endocannabinoid system as a therapeutic target in neuropathic pain: A review. Expert Opin. Ther. Targets 2024, 28, 739–755. [Google Scholar] [CrossRef]
- Spicarova, D.; Palecek, J. Anandamide-Mediated Modulation of Nociceptive Transmission at the Spinal Cord Level. Physiol. Res. 2024, 73 (Suppl. 1), S435–S448. [Google Scholar] [CrossRef]
- Hu, J.; Fan, W.; Xu, Y.; Li, X.; Zhang, H.; Li, S.; Xue, L. Maladaptive changes in the homeostasis of AEA-TRPV1/CB1R induces pain-related hyperactivity of nociceptors after spinal cord injury. Cell Biosci. 2025, 15, 2. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, W.; Lao, L.; Marvizon, J.C. Cannabinoid CB1 receptor facilitation of substance P release in the rat spinal cord, measured as neurokinin 1 receptor internalization. Eur. J. Neurosci. 2010, 31, 225–237. [Google Scholar] [CrossRef]
- Christie, M.J.; Mallet, C. Endocannabinoids can open the pain gate. Sci. Signal. 2009, 2, pe57. [Google Scholar] [CrossRef]
- Choi, S.I.; Lim, J.Y.; Yoo, S.; Kim, H.; Hwang, S.W. Emerging Role of Spinal Cord TRPV1 in Pain Exacerbation. Neural Plast. 2016, 2016, 5954890. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, Y.; Ye, X.; Zhang, N.; Pan, L.; Wang, B. TRP (transient receptor potential) ion channel family: Structures, biological functions and therapeutic interventions for diseases. Signal Transduct. Target. Ther. 2023, 8, 261. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sun, W.; Zhu, Y.; Zhao, F.; Deng, S.; Tian, M.; Wang, Y.; Gong, Y. TRPV1: The key bridge in neuroimmune interactions. J. Intensive Med. 2024, 4, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Back, S.K.; Davies, A.J.; Jeong, H.; Jo, H.J.; Chung, G.; Na, H.S.; Bae, Y.C.; Kim, S.J.; Kim, J.S.; et al. TRPV1 in GABAergic interneurons mediates neuropathic mechanical allodynia and disinhibition of the nociceptive circuitry in the spinal cord. Neuron 2012, 74, 640–647. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, T.; Meng, F.; Jiang, M.; Wu, S.; Xu, H. The endocannabinoid system in the brain undergoes long-lasting changes following neuropathic pain. iScience 2024, 27, 111409. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.H.; Hou, X.Y.; Liu, H.Z.; Zhou, Z.R.; Lv, S.S.; Yi, L.X.; Li, H.; Zhang, Y.Q. Descending projection neurons in the primary sensorimotor cortex regulate neuropathic pain and locomotion in mice. Nat. Commun. 2025, 16, 5918. [Google Scholar] [CrossRef]
- Muller, L.; Di Benedetto, S. Neuroimmune crosstalk in chronic neuroinflammation: Microglial interactions and immune modulation. Front. Cell Neurosci. 2025, 19, 1575022. [Google Scholar] [CrossRef]
- Han, Q.W.; Shao, Q.H.; Wang, X.T.; Ma, K.L.; Chen, N.H.; Yuan, Y.H. Author Correction: CB2 receptor activation inhibits the phagocytic function of microglia through activating ERK/AKT-Nurr1 signal pathways. Acta Pharmacol. Sin. 2022, 3, 2253–2266, Correction in: Acta Pharmacol. Sin. 2024, 45, 2228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jin, T.; Wang, H.; Han, S.; Liang, Y. Microglia in morphine tolerance: Cellular and molecular mechanisms and therapeutic potential. Front. Pharmacol. 2024, 15, 1499799. [Google Scholar] [CrossRef]
- Cheng, T.; Xu, Z.; Ma, X. The role of astrocytes in neuropathic pain. Front. Mol. Neurosci. 2022, 15, 1007889. [Google Scholar] [CrossRef]
- Wang, H.; Chen, C.; Lin, Y.; Tian, Z.; Yan, Z.; Zeng, X.; Yang, Y.; Lin, M.; Ai, Q.; Liu, X.; et al. Connexin43 and Its Regulation of Astrocyte Gap Junction Function: Influencing Depression Progression by Mediating Electrical and Chemical Signals. CNS Neurosci. Ther. 2025, 31, e70600. [Google Scholar] [CrossRef]
- Lu, H.J.; Gao, Y.J. Astrocytes in Chronic Pain: Cellular and Molecular Mechanisms. Neurosci. Bull. 2023, 39, 425–439. [Google Scholar] [CrossRef]
- Sanz-Galvez, R.; Falardeau, D.; Kolta, A.; Inglebert, Y. The role of astrocytes from synaptic to non-synaptic plasticity. Front. Cell. Neurosci. 2024, 18, 1477985. [Google Scholar] [CrossRef]
- Yang, X.L.; Wang, X.; Shao, L.; Jiang, G.T.; Min, J.W.; Mei, X.Y.; He, X.H.; Liu, W.H.; Huang, W.X.; Peng, B.W. TRPV1 mediates astrocyte activation and interleukin-1beta release induced by hypoxic ischemia (HI). J. Neuroinflamm. 2019, 16, 114. [Google Scholar] [CrossRef]
- Maldonado, R.; Banos, J.E.; Cabanero, D. The endocannabinoid system and neuropathic pain. Pain 2016, 157 (Suppl. S1), S23–S32. [Google Scholar] [CrossRef] [PubMed]
- Schiano Moriello, A.; Allara, M.; Iannotti, F.A.; Marcolongo, G.; Piscitelli, F.; Verde, R.; Di Marzo, V.; Petrosino, S. Deciphering the interaction between N-palmitoyl-D-glucosamine and the endocannabinoidome. Sci. Rep. 2025, 15, 21094. [Google Scholar] [CrossRef]
- Woodhams, S.G.; Sagar, D.R.; Burston, J.J.; Chapman, V. The role of the endocannabinoid system in pain. Handb. Exp. Pharmacol. 2015, 227, 119–143. [Google Scholar]
- Garcia-Ovejero, D.; Arevalo-Martin, A.; Petrosino, S.; Docagne, F.; Hagen, C.; Bisogno, T.; Watanabe, M.; Guaza, C.; Di Marzo, V.; Molina-Holgado, E. The endocannabinoid system is modulated in response to spinal cord injury in rats. Neurobiol. Dis. 2009, 33, 57–71. [Google Scholar] [CrossRef]
- Petrosino, S.; Palazzo, E.; de Novellis, V.; Bisogno, T.; Rossi, F.; Maione, S.; Di Marzo, V. Changes in spinal and supraspinal endocannabinoid levels in neuropathic rats. Neuropharmacology 2007, 52, 415–422. [Google Scholar] [CrossRef]
- Argueta, D.A.; Avalos, B.; Goel, Y.; Tran, H.; Fotio, Y.; Piomelli, D.; Di Patrizio, N.V.; Gupta, K. Neuroprotective, anti-inflammatory, and analgesic activity of palmitoylethanolamide in sickle cell mice. Blood Adv. 2025, 9, 3056–3068. [Google Scholar] [CrossRef]
- Carrier, E.J.; Kearn, C.S.; Barkmeier, A.J.; Breese, N.M.; Yang, W.; Nithipatikom, K.; Pfister, S.L.; Campbell, W.B.; Hillard, C.J. Cultured rat microglial cells synthesize the endocannabinoid 2-arachidonylglycerol, which increases proliferation via a CB2 receptor-dependent mechanism. Mol. Pharmacol. 2004, 65, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Witting, A.; Walter, L.; Wacker, J.; Moller, T.; Stella, N. P2X7 receptors control 2-arachidonoylglycerol production by microglial cells. Proc. Natl. Acad. Sci. USA 2004, 101, 3214–3219. [Google Scholar] [CrossRef] [PubMed]
- Yagami, T.; Koma, H.; Yamamoto, Y. Pathophysiological Roles of Cyclooxygenases and Prostaglandins in the Central Nervous System. Mol. Neurobiol. 2016, 53, 4754–4771. [Google Scholar] [CrossRef]
- Sagar, D.R.; Gaw, A.G.; Okine, B.N.; Woodhams, S.G.; Wong, A.; Kendall, D.A.; Chapman, V. Dynamic regulation of the endocannabinoid system: Implications for analgesia. Mol. Pain 2009, 5, 59. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.; Morales, P.; Reggio, P.H. Cannabinoid Ligands Targeting TRP Channels. Front. Mol. Neurosci. 2018, 11, 487. [Google Scholar] [CrossRef] [PubMed]
- Docs, K.; Balazs, A.; Papp, I.; Szucs, P.; Hegyi, Z. Reactive spinal glia convert 2-AG to prostaglandins to drive aberrant astroglial calcium signaling. Front. Cell Neurosci. 2024, 18, 1382465. [Google Scholar] [CrossRef]
- Ou, M.; Chen, Y.; Liu, J.; Zhang, D.; Yang, Y.; Shen, J.; Miao, C.; Tang, S.-J.; Liu, X.; Mulkey, D.K.; et al. Spinal astrocytic MeCP2 regulates Kir4.1 for the maintenance of chronic hyperalgesia in neuropathic pain. Prog. Neurobiol. 2023, 224, 102436. [Google Scholar] [CrossRef]
- Palazzo, E.; Luongo, L.; Novellis, V.; Rossi, F.; Maione, S. The Role of Cannabinoid Receptors in the Descending Modulation of Pain. Pharmaceuticals 2010, 3, 2661–2673. [Google Scholar] [CrossRef]
- Zhu, C.; Lan, X.; Wei, Z.; Yu, J.; Zhang, J. Allosteric modulation of G protein-coupled receptors as a novel therapeutic strategy in neuropathic pain. Acta Pharm. Sin. B 2024, 14, 67–86. [Google Scholar] [CrossRef] [PubMed]
- Bosier, B.; Muccioli, G.G.; Hermans, E.; Lambert, D.M. Functionally selective cannabinoid receptor signalling: Therapeutic implications and opportunities. Biochem. Pharmacol. 2010, 80, 1–12. [Google Scholar] [CrossRef]
- Marti-Solano, M. A multi-dimensional view of context-dependent G protein-coupled receptor function. Biochem. Soc. Trans. 2023, 51, 13–20. [Google Scholar] [CrossRef]
- Milligan, G. G protein-coupled receptor dimerisation: Molecular basis and relevance to function. Biochim. Biophys. Acta 2007, 1768, 825–835. [Google Scholar] [CrossRef]
- Rios, C.; Gomes, I.; Devi, L.A. mu opioid and CB1 cannabinoid receptor interactions: Reciprocal inhibition of receptor signaling and neuritogenesis. Br. J. Pharmacol. 2006, 148, 387–395. [Google Scholar] [CrossRef]
- Leo, L.M.; Abood, M.E. CB1 Cannabinoid Receptor Signaling and Biased Signaling. Molecules 2021, 26, 5413. [Google Scholar] [CrossRef] [PubMed]
- Ibsen, M.S.; Finlay, D.B.; Patel, M.; Javitch, J.A.; Glass, M.; Grimsey, N.L. Cannabinoid CB1 and CB2 Receptor-Mediated Arrestin Translocation: Species, Subtype, and Agonist-Dependence. Front. Pharmacol. 2019, 10, 350. [Google Scholar] [CrossRef]
- Starowicz, K.; Makuch, W.; Korostynski, M.; Malek, N.; Slezak, M.; Zychowska, M.; Petrosino, S.; De Petrocellis, L.; Cristino, L.; Przewlocka, B.; et al. Full inhibition of spinal FAAH leads to TRPV1-mediated analgesic effects in neuropathic rats and possible lipoxygenase-mediated remodeling of anandamide metabolism. PLoS ONE 2013, 8, e60040. [Google Scholar] [CrossRef]
- Yaksh, T.L.; Santos, G.G.D.; Borges Paes Lemes, J.; Malange, K. Neuraxial Drug Delivery in Pain Management: An Overview of Past, Present, and Future. Best Pract. Res. Clin. Anaesthesiol. 2023, 37, 243–265. [Google Scholar] [CrossRef]
- Deer, T.R.; Hayek, S.M.; Grider, J.S.; Pope, J.E.; Brogan, S.E.; Gulati, A.; Hagedorn, J.M.; Strand, N.; Hah, J.; Yaksh, T.L.; et al. The Polyanalgesic Consensus Conference (PACC)®: Updates on Clinical Pharmacology and Comorbidity Management in Intrathecal Drug Delivery for Cancer Pain. Neuromodulation 2025, 28, 1029–1053. [Google Scholar] [CrossRef] [PubMed]
- Deer, T.R.; Hayek, S.M.; Grider, J.S.; Hagedorn, J.M.; McDowell, G.C.; Kim, P.; Dupoiron, D.; Goel, V.; Duarte, R.; Pilitsis, J.G.; et al. The Polyanalgesic Consensus Conference (PACC)®: Intrathecal Drug Delivery Guidance on Safety and Therapy Optimization When Treating Chronic Noncancer Pain. Neuromodulation 2024, 27, 1107–1139. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, A.G.; Tsou, K.; Walker, J.M. Cannabinoid modulation of wide dynamic range neurons in the lumbar dorsal horn of the rat by spinally administered WIN55,212-2. Neurosci. Lett. 1998, 257, 119–122. [Google Scholar] [CrossRef]
- Johanek, L.M.; Simone, D.A. Cannabinoid agonist, CP 55,940, prevents capsaicin-induced sensitization of spinal cord dorsal horn neurons. J. Neurophysiol. 2005, 93, 989–997. [Google Scholar] [CrossRef]
- Kohno, K.; Tsuda, M. Neuron-microglia interactions modulating neuropathic pain. Int. Immunol. 2025, 37, 589–598. [Google Scholar] [CrossRef]
- Garcia-Dominguez, M. Neuroinflammation: Mechanisms, Dual Roles, and Therapeutic Strategies in Neurological Disorders. Curr. Issues Mol. Biol. 2025, 47, 417. [Google Scholar] [CrossRef]
- Shinu, P.; Morsy, M.A.; Nair, A.B.; Mouslem, A.K.A.; Venugopala, K.N.; Goyal, M.; Bansal, M.; Jacob, S.; Deb, P.K. Novel Therapies for the Treatment of Neuropathic Pain: Potential and Pitfalls. J. Clin. Med. 2022, 11, 3002. [Google Scholar] [CrossRef]
- Ji, R.R.; Suter, M.R. p38 MAPK, microglial signaling, and neuropathic pain. Mol. Pain. 2007, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.; Urits, I.; Orhurhu, V.; Peck, J.; Orhurhu, M.S.; Giacomazzi, S.; Smoots, D.; Piermarini, C.; Manchikanti, L.; Kaye, A.D.; et al. The Role of the Cannabinoid System in Pain Control: Basic and Clinical Implications. Curr. Pain Headache Rep. 2020, 24, 35. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Picco, L.; Murnion, B.; Winters, B.; Matheson, J.; Graham, M.; Campbell, G.; Parvaresh, L.; Khor, K.-E.; Betz-Stablein, B.; et al. Opioid-sparing effect of cannabinoids for analgesia: An updated systematic review and meta-analysis of preclinical and clinical studies. Neuropsychopharmacology 2022, 47, 1315–1330. [Google Scholar] [CrossRef]
- Subedi, A.; Etemad, A.; Tiwari, A.; Huang, Y.; Chatterjee, B.; McLeod, S.M.; Lu, Y.; Gonzalez, D.; Ghosh, K.; Sirito, M.; et al. Nerve injury inhibits Oprd1 and Cnr1 transcription through REST in primary sensory neurons. Sci. Rep. 2024, 14, 26612. [Google Scholar] [CrossRef]
- Liu, Y.; Jia, M.; Wu, C.; Zhang, H.; Chen, C.; Ge, W.; Wan, K.; Lan, Y.; Liu, S.; Li, Y.; et al. Transcriptomic Profiling in Mice With CB1 receptor Deletion in Primary Sensory Neurons Suggests New Analgesic Targets for Neuropathic Pain. Front. Pharmacol. 2021, 12, 781237. [Google Scholar] [CrossRef]
- Goncalves, M.R.; da Conceicao, M.S.; Jesus, C.H.A.; Gasparin, A.T.; Rosa, E.S.; da Cunha, J.M. Spinal cannabinoid CB1 or CB2 receptors activation attenuates mechanical allodynia in streptozotocin-induced diabetic rats. Behav. Pharmacol. 2022, 33, 158–164. [Google Scholar] [CrossRef]
- Lei, X.; Chen, M.; Liu, X.; Yan, Y.; Li, X.; Gong, F.; Yu, D.; Liu, X. Activation of cannabinoid CB1 receptors suppresses HCN channels function in dorsal root ganglion neurons of rats. Brain Res. Bull. 2025, 229, 111425. [Google Scholar] [CrossRef] [PubMed]
- Harding, E.K.; Zamponi, G.W. Central and peripheral contributions of T-type calcium channels in pain. Mol. Brain 2022, 15, 39. [Google Scholar] [CrossRef]
- Gadotti, V.M.; Antunes, F.T.T.; Zamponi, G.W. Analgesia by intrathecal delta-9-tetrahydrocannabinol is dependent on Cav3.2 calcium channels. Mol. Brain 2023, 16, 47. [Google Scholar] [CrossRef]
- Ferreira, M.V.; Jesus, C.H.A.; Bonfim da Costa, J.P.; Oliveira, G.; Liebl, B.; Verri, W., Jr.; Zanoveli, J.M.; da Cunha, J.M. Aspirin-triggered lipoxin A4 reduces neuropathic pain and anxiety-like behaviours in male diabetic rats: Antinociceptive enhancement by cannabinoid receptor agonists. Eur. J. Pharmacol. 2025, 989, 177254. [Google Scholar] [CrossRef]
- Guindon, J.; Hohmann, A.G. Cannabinoid CB2 receptors: A therapeutic target for the treatment of inflammatory and neuropathic pain. Br. J. Pharmacol. 2008, 153, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Brownjohn, P.W.; Ashton, J.C. Spinal cannabinoid CB2 receptors as a target for neuropathic pain: An investigation using chronic constriction injury. Neuroscience 2012, 203, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Racz, I.; Nadal, X.; Alferink, J.; Banos, J.E.; Rehnelt, J.; Martin, M.; Pintado, B.; Gutierrez-Adan, A.; Sanguino, E.; Bellora, N.; et al. Interferon-gamma is a critical modulator of CB(2) cannabinoid receptor signaling during neuropathic pain. J. Neurosci. 2008, 28, 12136–12145. [Google Scholar] [CrossRef] [PubMed]
- Wilkerson, J.L.; Alberti, L.B.; Thakur, G.A.; Makriyannis, A.; Milligan, E.D. Peripherally administered cannabinoid receptor 2 (CB(2)R) agonists lose anti-allodynic effects in TRPV1 knockout mice, while intrathecal administration leads to anti-allodynia and reduced GFAP, CCL2 and TRPV1 expression in the dorsal spinal cord and DRG. Brain Res. 2022, 1774, 147721. [Google Scholar] [CrossRef]
- Kamata, Y.; Kambe, T.; Chiba, T.; Yamamoto, K.; Kawakami, K.; Abe, K.; Taguchi, K. Paclitaxel Induces Upregulation of Transient Receptor Potential Vanilloid 1 Expression in the Rat Spinal Cord. Int. J. Mol. Sci. 2020, 21, 4341. [Google Scholar] [CrossRef]
- Son, D.B.; Choi, W.; Kim, M.; Go, E.J.; Jeong, D.; Park, C.K.; Kim, Y.H.; Lee, H.; Suh, J.W. Decursin Alleviates Mechanical Allodynia in a Paclitaxel-Induced Neuropathic Pain Mouse Model. Cells 2021, 10, 547. [Google Scholar] [CrossRef]
- Armin, S.; Muenster, S.; Abood, M.; Benamar, K. GPR55 in the brain and chronic neuropathic pain. Behav. Brain Res. 2021, 406, 113248. [Google Scholar] [CrossRef]
- Kohlberger, M.; Gadermaier, G. SELEX: Critical factors and optimization strategies for successful aptamer selection. Biotechnol. Appl. Biochem. 2022, 69, 1771–1792. [Google Scholar] [CrossRef]
- Jiang, W.; Yu, W.; Tan, Y. Activation of GPR55 alleviates neuropathic pain and chronic inflammation. Biotechnol. Appl. Biochem. 2025, 72, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, R.; Hossain, M.Z.; Takahashi, K.; Saito, I.; Kitagawa, J. Attenuation of allodynia and microglial reactivity by inhibiting the degradation of 2-arachidonoylglycerol following injury to the trigeminal nerve in mice. Heliyon 2022, 8, e10034. [Google Scholar] [CrossRef]
- Wilkerson, J.L.; Niphakis, M.J.; Grim, T.W.; Mustafa, M.A.; Abdullah, R.A.; Poklis, J.L.; Dewey, W.L.; Akbarali, H.; Banks, M.L.; Wise, L.E.; et al. The Selective Monoacylglycerol Lipase Inhibitor MJN110 Produces Opioid-Sparing Effects in a Mouse Neuropathic Pain Model. J. Pharmacol. Exp. Ther. 2016, 357, 145–156. [Google Scholar] [CrossRef]
- Crowe, M.S.; Wilson, C.D.; Leishman, E.; Prather, P.L.; Bradshaw, H.B.; Banks, M.L.; Kinsey, S.G. The monoacylglycerol lipase inhibitor KML29 with gabapentin synergistically produces analgesia in mice. Br. J. Pharmacol. 2017, 174, 4523–4539. [Google Scholar] [CrossRef]
- Woodhams, S.G.; Wong, A.; Barrett, D.A.; Bennett, A.J.; Chapman, V.; Alexander, S.P. Spinal administration of the monoacylglycerol lipase inhibitor JZL184 produces robust inhibitory effects on nociceptive processing and the development of central sensitization in the rat. Br. J. Pharmacol. 2012, 167, 1609–1619. [Google Scholar] [CrossRef]
- Vallee, M.; Vitiello, S.; Bellocchio, L.; Hebert-Chatelain, E.; Monlezun, S.; Martin-Garcia, E.; Kasanetz, F.; Baillie, G.L.; Panin, F.; Cathala, A.; et al. Pregnenolone can protect the brain from cannabis intoxication. Science 2014, 343, 94–98. [Google Scholar] [CrossRef]
- Lebovitz, E.E.; Keller, J.M.; Kominsky, H.; Kaszas, K.; Maric, D.; Iadarola, M.J. Positive allosteric modulation of TRPV1 as a novel analgesic mechanism. Mol. Pain 2012, 8, 70. [Google Scholar] [CrossRef]
- Wang, Y.; Zhuang, Y.; DiBerto, J.F.; Zhou, X.E.; Schmitz, G.P.; Yuan, Q.; Jain, M.K.; Liu, W.; Melcher, K.; Jiang, Y.; et al. Structures of the entire human opioid receptor family. Cell 2023, 186, 413–427.e17. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.J.; Pediani, J.D.; Milligan, G. Heteromultimerization of cannabinoid CB(1) receptor and orexin OX(1) receptor generates a unique complex in which both protomers are regulated by orexin A. J. Biol. Chem. 2011, 286, 37414–37428. [Google Scholar] [CrossRef] [PubMed]
- Bushlin, I.; Gupta, A.; Stockton, S.D.; Jr Miller, L.K.; Devi, L.A. Dimerization with cannabinoid receptors allosterically modulates delta opioid receptor activity during neuropathic pain. PLoS ONE 2012, 7, e49789. [Google Scholar] [CrossRef] [PubMed]
- Rozenfeld, R.; Devi, L.A. Exploring a role for heteromerization in GPCR signalling specificity. Biochem. J. 2011, 433, 11–18. [Google Scholar] [CrossRef]
- Nagi, K.; Onaran, H.O. Biased agonism at G protein-coupled receptors. Cell. Signal. 2021, 83, 109981. [Google Scholar] [CrossRef]
- Shen, S.; Wu, C.; Lin, G.; Yang, X.; Zhou, Y.; Zhao, C.; Miao, Z.; Tian, X.; Wang, K.; Yang, Z.; et al. Structure-based identification of a G protein-biased allosteric modulator of cannabinoid receptor CB1. Proc. Natl. Acad. Sci. USA 2024, 121, e2321532121. [Google Scholar] [CrossRef]
- Guenther, K.G.; Wirt, J.L.; Oliva, I.; Saberi, S.A.; Crystal, J.D.; Hohmann, A.G. The cannabinoid CB(2) agonist LY2828360 suppresses neuropathic pain behavior and attenuates morphine tolerance and conditioned place preference in rats. Neuropharmacology 2025, 265, 110257. [Google Scholar] [CrossRef] [PubMed]
- Magni, G.; Riboldi, B.; Ceruti, S. Human Glial Cells as Innovative Targets for the Therapy of Central Nervous System Pathologies. Cells 2024, 13, 606. [Google Scholar] [CrossRef] [PubMed]
- Du, E.R.; Fan, R.P.; Rong, L.L.; Xie, Z.; Xu, C.S. Regulatory mechanisms and therapeutic potential of microglial inhibitors in neuropathic pain and morphine tolerance. J. Zhejiang Univ. Sci. B 2020, 21, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.A.; Kim, T.U.; Chang, M.C. Minocycline for Controlling Neuropathic Pain: A Systematic Narrative Review of Studies in Humans. J. Pain Res. 2021, 14, 139–145. [Google Scholar] [CrossRef]
- Jin, S.X.; Zhuang, Z.Y.; Woolf, C.J.; Ji, R.R. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J. Neurosci. 2003, 23, 4017–4022. [Google Scholar] [CrossRef]
- Tsuda, M.; Mizokoshi, A.; Shigemoto-Mogami, Y.; Koizumi, S.; Inoue, K. Activation of p38 mitogen-activated protein kinase in spinal hyperactive microglia contributes to pain hypersensitivity following peripheral nerve injury. Glia 2004, 45, 89–95. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, W.; Chabot, J.G.; Quirion, R. Morphological evidence for the involvement of microglial p38 activation in CGRP-associated development of morphine antinociceptive tolerance. Peptides 2010, 31, 2179–2184. [Google Scholar] [CrossRef]
- Vincenzi, F.; Pasquini, S.; Contri, C.; Cappello, M.; Nigro, M.; Travagli, A.; Merighi, S.; Gessi, S.; Borea, P.A.; Varani, K. Pharmacology of Adenosine Receptors: Recent Advancements. Biomolecules 2023, 13, 1387. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Chen, S.; Xie, Y.; Cheng, Z.; Zhu, X.; Guo, Q. Role of M1/M2 macrophages in pain modulation. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2024, 49, 1155–1163. [Google Scholar]
- Starobova, H.; Nadar, E.I.; Vetter, I. The NLRP3 Inflammasome: Role and Therapeutic Potential in Pain Treatment. Front. Physiol. 2020, 11, 1016. [Google Scholar] [CrossRef]
- Chen, C.; Smith, M.T. The NLRP3 inflammasome: Role in the pathobiology of chronic pain. Inflammopharmacology 2023, 31, 1589–1603. [Google Scholar] [CrossRef]
- Gui, W.S.; Wei, X.; Mai, C.L.; Murugan, M.; Wu, L.J.; Xin, W.J.; Zhou, L.J.; Liu, X.G. Interleukin-1beta overproduction is a common cause for neuropathic pain, memory deficit, and depression following peripheral nerve injury in rodents. Mol. Pain 2016, 12, 1744806916646784. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.Y.; Liu, L.; Song, F.H.; Gao, S.J.; Wu, J.Y.; Li, D.Y.; Zhang, L.Q.; Liu, D.Q.; Zhou, Y.Q.; Mei, W. Targeting the JAK2/STAT3 signaling pathway for chronic pain. Aging Dis. 2024, 15, 186–200. [Google Scholar] [CrossRef]
- Rose, C.R.; Felix, L.; Zeug, A.; Dietrich, D.; Reiner, A.; Henneberger, C. Astroglial Glutamate Signaling and Uptake in the Hippocampus. Front. Mol. Neurosci. 2017, 10, 451. [Google Scholar] [CrossRef]
- Brambilla, R.; Bracchi-Ricard, V.; Hu, W.H.; Frydel, B.; Bramwell, A.; Karmally, S.; Green, E.J.; Bethea, J.R. Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J. Exp. Med. 2005, 202, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Alam, A.; Chen, Q.; Eusman, M.A.; Pal, A.; Eguchi, S.; Wu, L.; Ma, D. The role of microglia in the pathobiology of neuropathic pain development: What do we know? Br. J. Anaesth. 2017, 118, 504–516. [Google Scholar] [CrossRef]
- Chen, G.; Park, C.K.; Xie, R.G.; Berta, T.; Nedergaard, M.; Ji, R.R. Connexin-43 induces chemokine release from spinal cord astrocytes to maintain late-phase neuropathic pain in mice. Brain 2014, 137 Pt 8, 2193–2209. [Google Scholar] [CrossRef]
- Morioka, N.; Nakamura, Y.; Zhang, F.F.; Hisaoka-Nakashima, K.; Nakata, Y. Role of Connexins in Chronic Pain and Their Potential as Therapeutic Targets for Next-Generation Analgesics. Biol. Pharm. Bull. 2019, 42, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.; Malcangio, M. Fractalkine/CX(3)CR(1) Pathway in Neuropathic Pain: An Update. Front. Pain Res. 2021, 2, 684684. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Mattern, C.; Ghoumari, A.; Oudinet, J.P.; Liere, P.; Labombarda, F.; Sitruk-Ware, R.; De Nicola, A.; Guennoun, R. Revisiting the roles of progesterone and allopregnanolone in the nervous system: Resurgence of the progesterone receptors. Prog. Neurobiol. 2014, 113, 6–39. [Google Scholar] [CrossRef]
- Coronel, M.F.; Labombarda, F.; De Nicola, A.F.; Gonzalez, S.L. Progesterone reduces the expression of spinal cyclooxygenase-2 and inducible nitric oxide synthase and prevents allodynia in a rat model of central neuropathic pain. Eur. J. Pain 2014, 18, 348–359. [Google Scholar] [CrossRef]
- Labombarda, F.; Jure, I.; Gonzalez, S.; Lima, A.; Roig, P.; Guennoun, R.; Schumacher, M.; De Nicola, A.F. A functional progesterone receptor is required for immunomodulation, reduction of reactive gliosis and survival of oligodendrocyte precursors in the injured spinal cord. J. Steroid Biochem. Mol. Biol. 2015, 154, 274–284. [Google Scholar] [CrossRef]
- Petrovic, J.; Pesic, V.; Lauschke, V.M. Frequencies of clinically important CYP2C19 and CYP2D6 alleles are graded across Europe. Eur. J. Hum. Genet. 2020, 28, 88–94. [Google Scholar] [CrossRef]
- Babayeva, M.; Loewy, Z.G. Cannabis Pharmacogenomics: A Path to Personalized Medicine. Curr. Issues Mol. Biol. 2023, 45, 3479–3514. [Google Scholar] [CrossRef]
- Zorina-Lichtenwalter, K.; Parisien, M.; Diatchenko, L. Genetic studies of human neuropathic pain conditions: A review. Pain 2018, 159, 583–594. [Google Scholar] [CrossRef] [PubMed]
| Strategy | Mechanism/Target | Evidence | Examples/ Specific Agents | Advantages | Limitations/Notes |
|---|---|---|---|---|---|
| Intrathecal (IT) administration | Direct delivery into CSF, modulation at the dorsal horn and DRG | Robust preclinical/translational support | Cannabinoid agonists (levonantradol, WIN55,212-2, CP-55,940) FAAH inhibitors (URB597, AA-5-HT) MAGL inhibitors (URB602) | Lower doses, reduced systemic effects | Requires specialized technique, careful patient selection |
| Endocannabinoid (eCB) modulation | Modulation of endogenous eCB levels through regulation of synthesis/transport, selective activation of receptor subtypes, and intervention in supramolecular complexes | Enhances analgesia; context/time-dependent plasticity | Enzyme inhibitors (FAAH, MAGL), Selective CB2R agonists and Targeting of receptor heteromers | Potentiates endogenous signaling with fewer central effects Considers glial cell heterogeneity (M1/M2 microglia, A1/A2 astrocytes) | CB1R desensitization; variable responses depending on microenvironment Activation of CB1R on inhibitory interneurons in neuropathic pain can induce synaptic disinhibition by reducing the presynaptic release of inhibitory neurotransmitters |
| Glial-targeted therapies | Decrease microglial/astrocytic activation and neuroinflammation. | Strong preclinical support; mixed clinical results | See ‘Modulation of immune response’ in the specific strategies section. | Reduces central sensitization | Must preserve homeostatic glial functions |
| Multimodal analgesia | A combination of different analgesic therapies to simultaneously target multiple pain pathways | Synergistic, less tolerance | See Section 4.1.4 in the specific strategies section | Dose sparing and reduced adverse events | Requires rational design of combinations and timing |
| Intervention | Molecular Focus | Spinal Mechanism | Examples | Advantages | Limitations/Warnings | ||
|---|---|---|---|---|---|---|---|
| ECS-related strategies | Receptor-Based Intervention | CB1R agonism IT | Neuronal CB1R | Antinociception ↓ eEPSCs; ↓ HCN1/2 in DRG (↓ excitability) | ACEA, THC | Potent antihyperalgesic | CB1R desensitization/downregulation with sustained exposure |
| CB2R agonism IT | Neuronal/glial CB2R | Antinociception/anti-inflammatory; ↓ central sensitization; low tolerance | HU-308, L-759,632, AM1710, AM724, THC derivatives, Sch35966 | Safer profile (minimal CNS adverse effects) | Glia-context variability requires high selectivity | ||
| Non-CB1/CB2 receptors IT | Neuronal/glial TRPV1 channel, PPARs and GPR119 receptor | TRPV1 silencing/antagonism; PPARs regulate glial activity and directly contribute at the spinal level to decrease hyperexcitability and prevent excessive propagation of nociceptive signals; The GPR119 receptor modulates ascending pain transmission at the spinal level through distinct G-protein-coupling mechanisms | Selective antagonists, negative allosteric modulators, inhibition of phosphorylation cascades, promotion of TRPV1 internalization and reduction of glial neuroinflammation (example: TRPV1-targeting siRNA); Pioglitazone, a PPARγ agonist; AS1269574, a GPR119 agonist) | Targeted effects; potential synergy with ECS Some antinociceptive effects of PPARγ agonists (such as pioglitazone) in neuropathic pain models are extremely rapid (within 5 minutes after intrathecal administration), indicating a non-genomic mechanism in addition to the classical nuclear pathway | TRPV1: thermoregulation issues; PPAR agonist efficacy exhibits significant context-dependent variability Although AS1269574 suggests a role for GPR119 in spinal neuropathic pain, prior evidence lacks pharmacological specificity | ||
| Selective Modulation of Endocannabinoid System Signaling Networks | Metabolic enzymes of eCBs Endocannabinoid transport systems | Neuronal/glial degradation enzymes (FAAH, MAGL), membrane transporters (EMT/AMT) and synthesis enzymes (NAPE-PLD, DAGL) | ↑ AEA/2-AG; potentiates CB2R/CB1R | URB597 (FAAH), URB602 (MAGL), JZ | Reduced psychoactive liability compared to direct agonists, with sustained analgesic efficacy and diminished tolerance development, particularly when combined with glial modulators | Sometimes, weaker/transient effects, limited specificity | |
| Heteromers/dimers | Neuronal/glial CB1–OX1/CB1–DOR/CB1–5HT2A | Distinct pharmacology, bivalent ligands are highly selective | Bivalent ligands | Higher potency/selectivity | Early stage; limited clinical translation | ||
| Biased agonism | Neuronal/glial CB1R/CB2R | Favor G-protein vs β-arrestin pathways for analgesia | LY2828360 (G protein-biased CB2R agonist), CB-05 (G protein-biased CB2R agonist) | Optimizes efficacy/adverse effects | No clinical consensus yet; requires pathway biomarkers | ||
| Other Strategies | Glial and immune modulation (beyond ECS) | Microglial inhibitors | Microglia (M1 → M2; survival/activation) | ↓ p38 MAPK; ↓ cytokines; possible depletion/reprogramming | Minocycline; p38i (SB203580, FR167653); CSF1R i (PLX5622); pentoxifylline; A3 agonists; IL-10/TGF-β pathways | These compounds suppress microglial activation, attenuate proinflammatory cytokine signaling cascades and restore homeostatic inhibitory modulation within the spinal cord | Must preserve homeostatic functions; therapeutic window critical |
| Proinflammatory pathways | Inflammasome/IL-1β/COX | NLRP3 inhibition; anti-IL-1β; ↓ PGE2; ↑ PPAR-γ | MCC950; anakinra/anti-IL-1β; NSAIDs | Defined effects; combinable | Systemic immunosuppression risk (mitigated with local delivery) | ||
| Astrocytic regulation | Astrocyte JAK/STAT, p38-MAPK, NF-κB; GLT-1/GLAST | ↓ GFAP/cytokines; restores glutamate uptake; BSCB effects | AG490 (JAK2/STAT3); SB203580; IKKi (BMS-345541) | Reduces nociceptive amplification | Timing/dose critical to avoid homeostatic disruption | ||
| Neuro-glial communication | Connexin-43; fractalkine/CX3CR1; CGRP-p38-NF-κB | ↓ pathological ATP/glutamate release; ↓ microglial activation | Carbenoxolone; Cx43 peptides (Gap26/Gap27); anti-CX3CR1 | Interrupts sensitization loops | Specificity and local delivery are key | ||
| Endocrine response modulation | Neurosteroids | Neuronal/glial PR | Anti-inflammatory; NF-κB inhibition; cytokine regulation | Progesterone | Neuroprotective; reduces persistent neuroinflammation | PR-dependency; timing and dosing critical | |
| Pharmacogenomics | Detect Individual genetic variations in genes encoding metabolizing enzymes, transporters, and receptors to determine personalized drug responses | Genetic variations in drug-metabolizing enzymes—such as CYP2D6 and CYP3A4; Polymorphisms in nociceptive genes—such as SNPs in OPRM1 (μ-opioid receptor), CNR1/CNR2 genes (encoding CB1R/CB2R) and SCN9A (Nav1.7 sodium channel), IL1B and TNF haplotypes predisposing to heightened neuroinflammation and poor anti-inflammatory therapy outcomes | Optimize spinal analgesic interventions. | Personalized therapy; reduces trial-and-error | Requires validated panels and access to testing | ||
| Multimodal analgesia | A combination of different analgesic therapies to simultaneously target multiple pain pathways | Neuronal/glial | Synergistic antinociceptive effects of cannabinoid and other receptors in neuronal and glial cells → Crosstalk with complementary antinociceptive systems | p38 MAP kinase inhibitors + opioid agonists; Cannabinoids + opioids; FAAH and MAGL inhibitors + glial modulators; Minocycline + propentofylline (for microglial and astrocyte inhibition) | Co-expression targets in dorsal horn; synergy → Dose reduction Reduced opioid tolerance/adverse effects Break central sensitization | Timing and immune monitoring | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saldaña, R.; Carrascosa, A.J.; Torregrosa, A.B.; Navarrete, F.; García-Gutiérrez, M.S.; Manzanares, J. Dual Role of the Spinal Endocannabinoid System in Response to Noxious Stimuli: Antinociceptive Pathways and Neuropathic Pain Mechanisms. Int. J. Mol. Sci. 2025, 26, 10692. https://doi.org/10.3390/ijms262110692
Saldaña R, Carrascosa AJ, Torregrosa AB, Navarrete F, García-Gutiérrez MS, Manzanares J. Dual Role of the Spinal Endocannabinoid System in Response to Noxious Stimuli: Antinociceptive Pathways and Neuropathic Pain Mechanisms. International Journal of Molecular Sciences. 2025; 26(21):10692. https://doi.org/10.3390/ijms262110692
Chicago/Turabian StyleSaldaña, Raquel, Antonio J. Carrascosa, Abraham B. Torregrosa, Francisco Navarrete, María Salud García-Gutiérrez, and Jorge Manzanares. 2025. "Dual Role of the Spinal Endocannabinoid System in Response to Noxious Stimuli: Antinociceptive Pathways and Neuropathic Pain Mechanisms" International Journal of Molecular Sciences 26, no. 21: 10692. https://doi.org/10.3390/ijms262110692
APA StyleSaldaña, R., Carrascosa, A. J., Torregrosa, A. B., Navarrete, F., García-Gutiérrez, M. S., & Manzanares, J. (2025). Dual Role of the Spinal Endocannabinoid System in Response to Noxious Stimuli: Antinociceptive Pathways and Neuropathic Pain Mechanisms. International Journal of Molecular Sciences, 26(21), 10692. https://doi.org/10.3390/ijms262110692








