Instant Cascara Beverage as a Neuroimmune Modulator of the Brain–Gut Axis: Sex-Dependent Effects in Healthy Rats
Abstract
1. Introduction
2. Results
2.1. General Health and Behavioral Studies
2.1.1. General Health Parameters
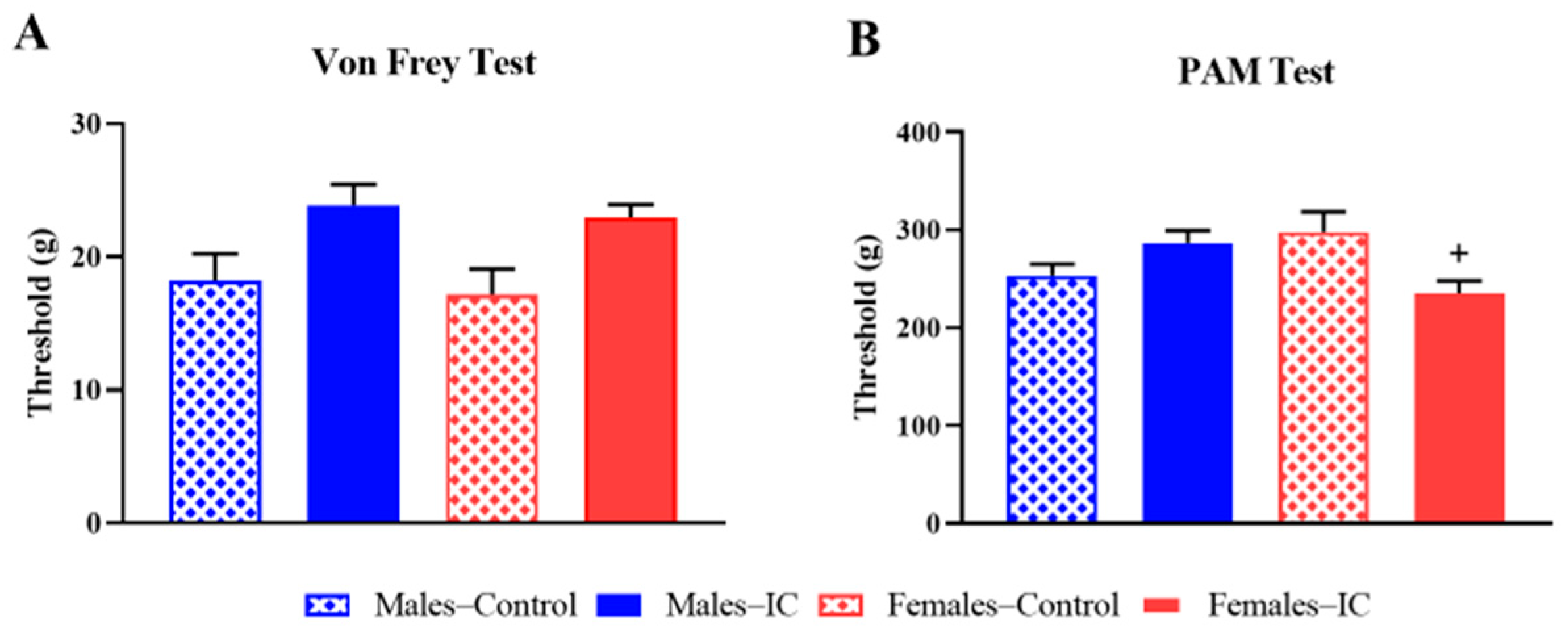
2.1.2. Locomotor Activity and Somatic Sensitivity
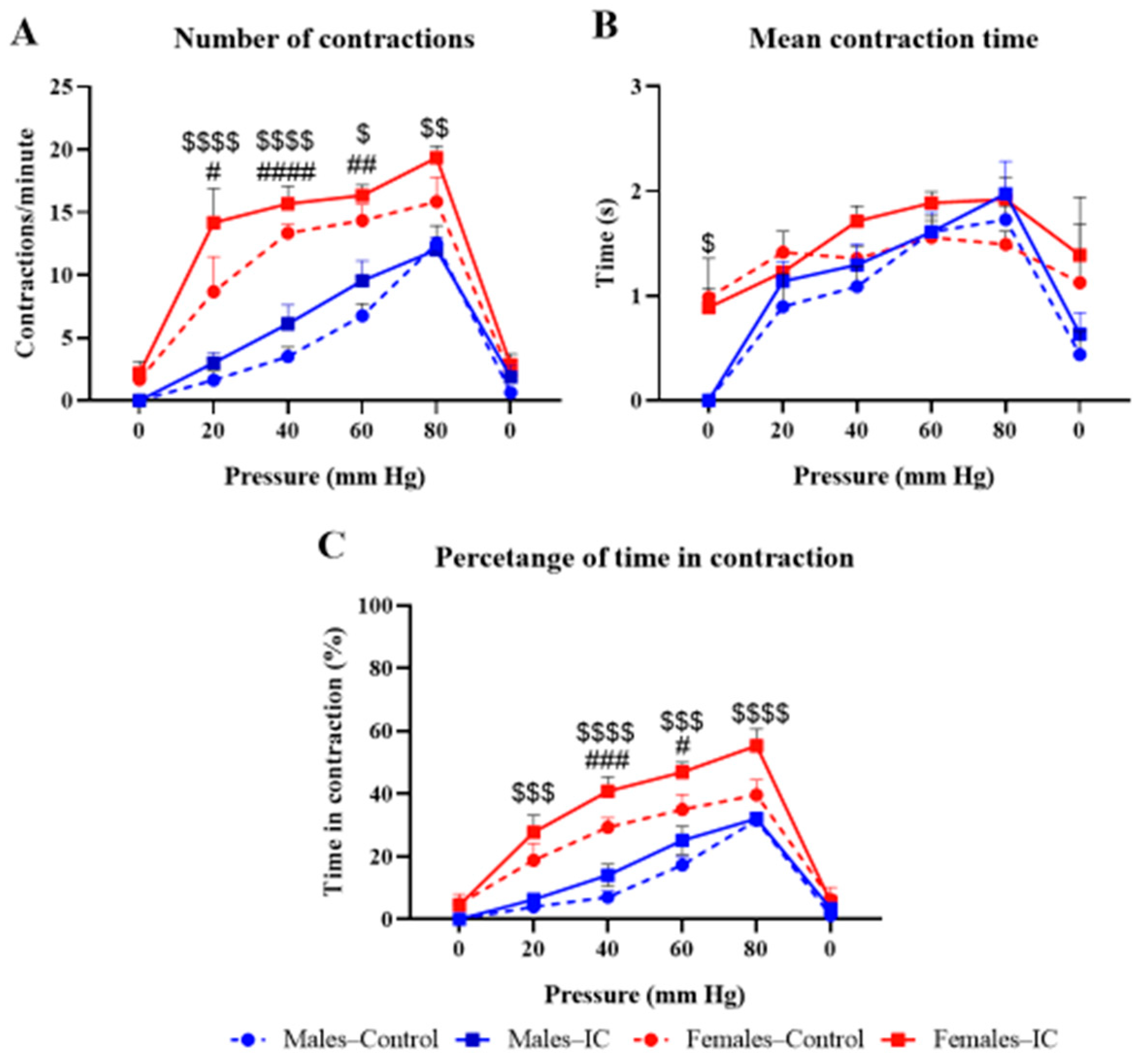
2.1.3. Visceral Sensitivity
2.2. Histological and Immunohistochemical Colonic Studies
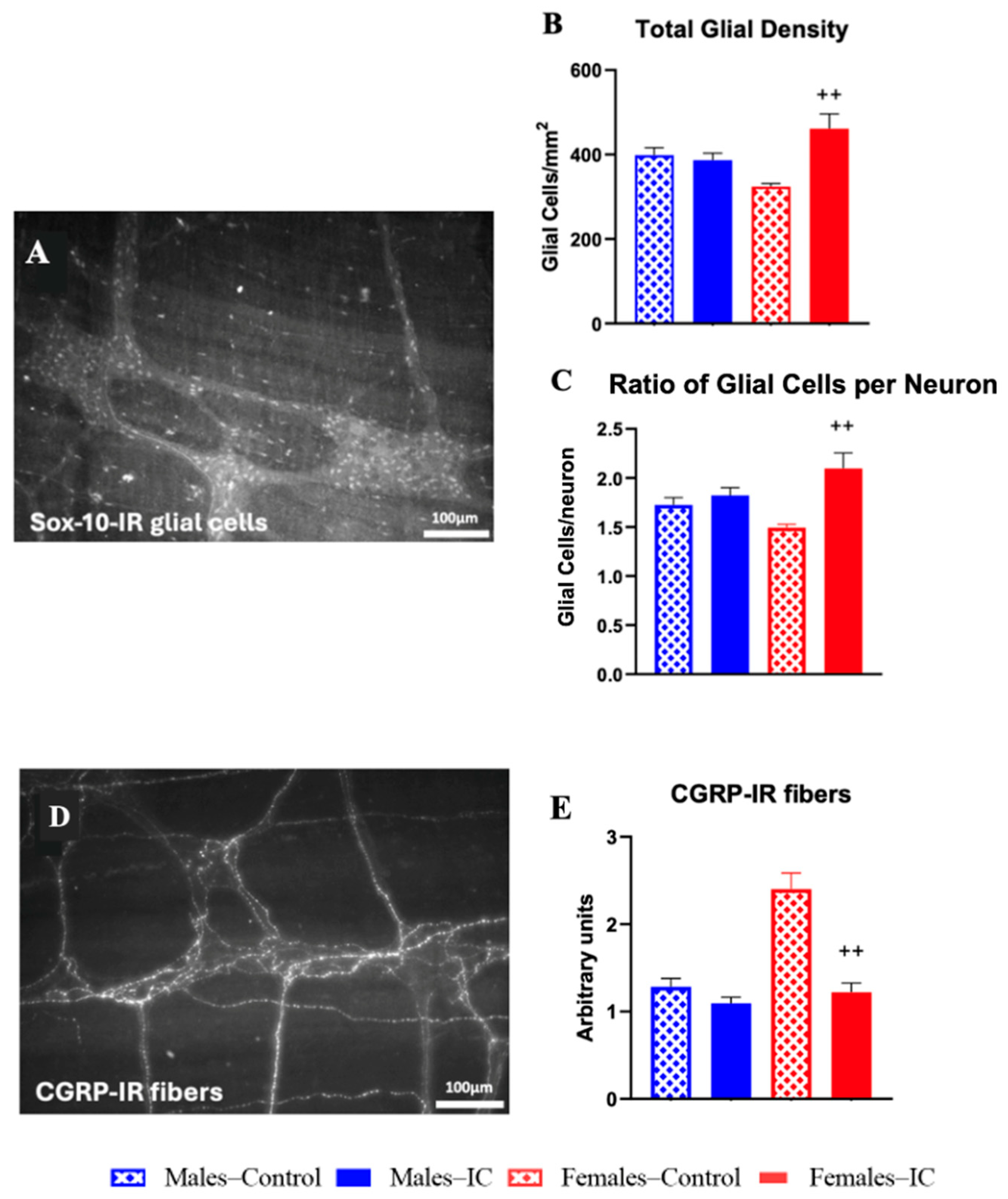
2.2.1. Colonic Myenteric Plexus
2.2.2. Colonic Wall Immunocytes
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Animals and Experimental Groups
4.3. Instant Cascara Beverage
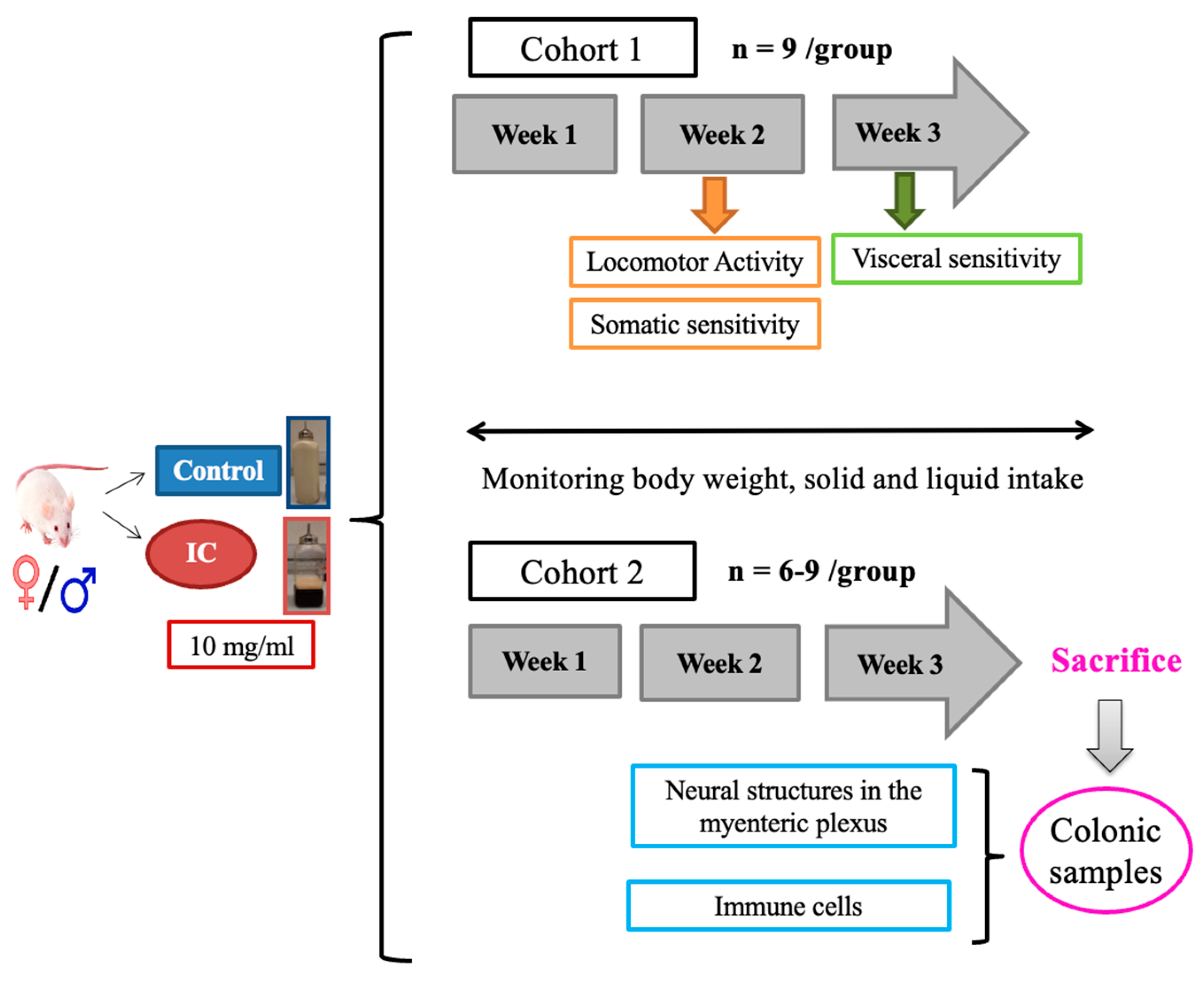
4.4. Experimental Protocol
4.5. Body Weight, Solid and Liquid Intake
4.6. Vaginal Cytology Smear in Female Rats
4.7. Locomotor Activity Analysis
4.8. Somatic Sensitivity
4.8.1. Von Frey Test
4.8.2. Pressure Administration Device Test
4.9. Visceral Sensitivity
4.10. Enteric Glial Cells and CGRP-IR Varicose Fibers in the Distal Colon Myenteric Plexus
4.11. Immune Cells in the Distal Colon Wall
4.11.1. Mast Cell Quantification
4.11.2. Eosinophils
4.11.3. Macrophages and Neutrophils
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Asp | Aspartic acid |
| ATP | Adenosine Triphosphate |
| BGA | Brain–Gut Axis |
| CGA | Chlorogenic acid |
| CGRP | Calcitonin Gene-Related Peptide |
| CGRP-IR | Calcitonin Gene-related Peptide |
| CNS | Central Nervous System |
| ENS | Enteric Nervous System |
| GABA | Gamma-Aminobutyric Acid |
| Glu | Glutamic acid |
| H&E | Hematoxilina y Eosina |
| IC | Instant Cascara |
| IR | Immunoreactive |
| IL-6 | Interleukin-6 |
| PAM | Pressure administration |
| PBS | Phosphate-Buffered Saline |
| RyR | Ryanodine receptors |
| SCFAs | Short-Chain Fatty Acids |
| TNF-α | Tumor Necrosis Factor alpha |
References
- Iriondo-Dehond, A.; Iriondo-Dehond, M.; Del Castillo, M.D. Applications of Compounds from Coffee Processing By-Products. Biomolecules 2020, 10, 1219. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Technical Report on the Notification of Cherry Pulp from Coffea Arabica L. and Coffea Canephora Pierre Ex A. Froehner as a Traditional Food from a Third Country Following Article 14 of Regulation (EU) 2015/2283. EFSA Support. Publ. 2021, 18, 6657E. [Google Scholar] [CrossRef]
- López-Parra, M.B.; Gómez-Domínguez, I.; Iriondo-DeHond, M.; Villamediana Merino, E.; Sánchez-Martín, V.; Mendiola, J.A.; Iriondo-DeHond, A.; del Castillo, M.D. The Impact of the Drying Process on the Antioxidant and Anti-Inflammatory Potential of Dried Ripe Coffee Cherry Pulp Soluble Powder. Foods 2024, 13, 1114. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Elizondo, A.S.; Iriondo-DeHond, M.; Ríos, M.B.; Mufari, R.; Mendiola, J.A.; Ibañez, E.; del Castillo, M.D. Assessment of Healthy and Harmful Maillard Reaction Products in a Novel Coffee Cascara Beverage: Melanoidins and Acrylamide. Foods 2020, 9, 620. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, R.P.; Lima, F.D.; Carvalho, N.R.; Bresciani, G.; Royes, L.F. Caffeine Effects on Systemic Metabolism, Oxidative-Inflammatory Pathways, and Exercise Performance. Nutr. Res. 2020, 80, 1–17. [Google Scholar] [CrossRef]
- Cui, W.Q.; Wang, S.T.; Pan, D.; Chang, B.; Sang, L.X. Caffeine and Its Main Targets of Colorectal Cancer. World J. Gastrointest. Oncol. 2020, 12, 149–172. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Bagdas, D.; Gul, Z.; Meade, J.A.; Cam, B.; Cinkilic, N.; Gurun, M.S. Pharmacologic Overview of Chlorogenic Acid and Its Metabolites in Chronic Pain and Inflammation. Curr. Neuropharmacol. 2019, 18, 216–228. [Google Scholar] [CrossRef]
- Bié, J.; Sepodes, B.; Fernandes, P.C.B.; Ribeiro, M.H.L. Polyphenols in Health and Disease: Gut Microbiota, Bioaccessibility, and Bioavailability. Compounds 2023, 3, 40–72. [Google Scholar] [CrossRef]
- De La Cruz, S.T.; Iriondo-DeHond, A.; Herrera, T.; Lopez-Tofiño, Y.; Galvez-Robleño, C.; Prodanov, M.; Velazquez-Escobar, F.; Abalo, R.; Del Castillo, M.D. An Assessment of the Bioactivity of Coffee Silverskin Melanoidins. Foods 2019, 8, 68. [Google Scholar] [CrossRef]
- Hyland, N.P.; Cryan, J.F. A Gut Feeling about GABA: Focus on GABAB Receptors. Front. Pharmacol. 2010, 1, 124. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.; Saint-Paul, M.C.; Dadone, B.; Patouraux, S.; Vivinus, M.H.; Ouvrier, D.; Michiels, J.F.; Piche, T.; Tulic, M.K. Inflammatory Cell Distribution in Colon Mucosa as a New Tool for Diagnosis of Irritable Bowel Syndrome: A Promising Pilot Study. Neurogastroenterol. Motil. 2018, 30, e13223. [Google Scholar] [CrossRef]
- Lu, S.; Zhao, Q.; Guan, Y.; Sun, Z.; Li, W.; Guo, S.; Zhang, A. The Communication Mechanism of the Gut-Brain Axis and Its Effect on Central Nervous System Diseases: A Systematic Review. Biomed. Pharmacother. 2024, 178, 117207. [Google Scholar] [CrossRef]
- Smolilo, D.J.; Hibberd, T.J.; Costa, M.; Wattchow, D.A.; De Fontgalland, D.; Spencer, N.J. Intrinsic Sensory Neurons Provide Direct Input to Motor Neurons and Interneurons in Mouse Distal Colon via Varicose Baskets. J. Comp. Neurol. 2020, 528, 2033–2043. [Google Scholar] [CrossRef]
- Yang, D.; Almanzar, N.; Chiu, I.M. The Role of Cellular and Molecular Neuroimmune Crosstalk in Gut Immunity. Cell. Mol. Immunol. 2023, 20, 1259–1269. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, T.; Oshima, T.; Huang, X.; Tomita, T.; Fukui, H.; Miwa, H. Microinflammation in the Intestinal Mucosa and Symptoms of Irritable Bowel Syndrome. J. Gastroenterol. 2022, 57, 62–69. [Google Scholar] [CrossRef]
- Farmer, A.D.; Aziz, Q. Mechanisms of Visceral Pain in Health and Functional Gastrointestinal Disorders. Scand. J. Pain 2014, 5, 51–60. [Google Scholar] [CrossRef]
- Kim, N.; Yun, M.; Oh, Y.J.; Choi, H.J. Mind-Altering with the Gut: Modulation of the Gut-Brain Axis with Probiotics. J. Microbiol. 2018, 56, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.M.; Makai, G.E.H. Fibromyalgia and Irritable Bowel Syndrome in Female Pelvic Pain. Semin. Reprod. Med. 2018, 36, 136–142. [Google Scholar] [CrossRef]
- Erdrich, S.; Hawrelak, J.A.; Myers, S.P.; Harnett, J.E. A Systematic Review of the Association between Fibromyalgia and Functional Gastrointestinal Disorders. Therap. Adv. Gastroenterol. 2020, 13, 1756284820977402. [Google Scholar] [CrossRef]
- Larauche, M.; Mulak, A.; Taché, Y. Stress and Visceral Pain: From Animal Models to Clinical Therapies. Exp. Neurol. 2012, 233, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, B. Extraintestinal Manifestations in Irritable Bowel Syndrome: A Systematic Review. Therap. Adv. Gastroenterol. 2022, 15, 17562848221114558. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Barceló, P.; Bagues, A.; Benítez-Álvarez, D.; López-Tofiño, Y.; Gálvez-Robleño, C.; López-Gómez, L.; del Castillo, M.D.; Abalo, R. Evaluation of the Effects of Instant Cascara Beverage on the Brain-Gut Axis of Healthy Male and Female Rats. Nutrients 2023, 16, 65. [Google Scholar] [CrossRef]
- Gallego-Barceló, P.; Benítez-Álvarez, D.; Bagues, A.; Silván-Ros, B.; Montalbán-Rodríguez, A.; López-Gómez, L.; Vera, G.; del Castillo, M.D.; Uranga, J.A.; Abalo, R. Ex Vivo Study of Colon Health, Contractility and Innervation in Male and Female Rats after Regular Exposure to Instant Cascara Beverage. Foods 2024, 13, 2474. [Google Scholar] [CrossRef] [PubMed]
- Jacenik, D.; Bagüés, A.; López-Gómez, L.; López-Tofiño, Y.; Iriondo-Dehond, A.; Serra, C.; Banovcanová, L.; Gálvez-Robleño, C.; Fichna, J.; Del Castillo, M.D.; et al. Changes in Fatty Acid Dietary Profile Affect the Brain–Gut Axis Functions of Healthy Young Adult Rats in a Sex-Dependent Manner. Nutrients 2021, 13, 1864. [Google Scholar] [CrossRef]
- López-Gómez, L.; López-Tofiño, Y.; Abalo, R. Dependency on Sex and Stimulus Quality of Nociceptive Behavior in a Conscious Visceral Pain Rat Model. Neurosci. Lett. 2021, 746, 135667. [Google Scholar] [CrossRef]
- Huang, Z.; Liao, L.; Wang, Z.; Lu, Y.; Yan, W.; Cao, H.; Tan, B. An Efficient Approach for Wholemount Preparation of the Myenteric Plexus of Rat Colon. J. Neurosci. Methods 2021, 348, 109012. [Google Scholar] [CrossRef]
- Kunke, M.; Kaehler, M.; Boni, S.; Schröder, K.; Weier, A.; Chunder, R.; Kuerten, S.; Böttner, M.; Cascorbi, I.; Neunlist, M.; et al. SOX10-Mediated Regulation of Enteric Glial Phenotype in Vitro and Its Relevance for Neuroinflammatory Disorders. J. Mol. Neurosci. 2025, 75, 26. [Google Scholar] [CrossRef]
- Schou, W.S.; Ashina, S.; Amin, F.M.; Goadsby, P.J.; Ashina, M. Calcitonin Gene-Related Peptide and Pain: A Systematic Review. J. Headache Pain 2017, 18, 34. [Google Scholar] [CrossRef]
- Bernardini, N.; Segnani, C.; Ippolito, C.; De Giorgio, R.; Colucci, R.; Faussone-Pellegrini, M.S.; Chiarugi, M.; Campani, D.; Castagna, M.; Mattii, L.; et al. Immunohistochemical Analysis of Myenteric Ganglia and Interstitial Cells of Cajal in Ulcerative Colitis. J. Cell. Mol. Med. 2012, 16, 318. [Google Scholar] [CrossRef]
- Kmeťová, K.; Marônek, M.; Borbélyová, V.; Hodosy, J.; Celec, P. Acute Effect of Cola and Caffeine on Locomotor Activity in Drosophila and Rat. Physiol. Res. 2021, 70, 287–292. [Google Scholar] [CrossRef]
- Zhou, Q.; Verne, G.N. Molecular Mechanisms and Pathways in Visceral Pain. Cells 2025, 14, 1146. [Google Scholar] [CrossRef] [PubMed]
- Yip, J.L.K.; Balasuriya, G.K.; Spencer, S.J.; Hill-Yardin, E.L. The Role of Intestinal Macrophages in Gastrointestinal Homeostasis: Heterogeneity and Implications in Disease. Cell Mol. Gastroenterol. Hepatol. 2021, 12, 1701–1718. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Bättig, K.; Holmén, J.; Nehlig, A.; Zvartau, E.E. Actions of Caffeine in the Brain with Special Reference to Factors That Contribute to Its Widespread Use. Pharmacol. Rev. 1999, 51, 83–133. [Google Scholar] [CrossRef]
- Marin, M.T.; Zancheta, R.; Paro, A.H.; Possi, A.P.M.; Cruz, F.C.; Planeta, C.S. Comparison of Caffeine-Induced Locomotor Activity between Adolescent and Adult Rats. Eur. J. Pharmacol. 2011, 660, 363–367. [Google Scholar] [CrossRef]
- Haddad, M.; Cherchi, F.; Alsalem, M.; Al-saraireh, Y.M.; Madae’en, S. Adenosine Receptors as Potential Therapeutic Analgesic Targets. Int. J. Mol. Sci. 2023, 24, 13160. [Google Scholar] [CrossRef] [PubMed]
- Okumura, T.; Nozu, T.; Ishioh, M.; Igarashi, S.; Kumei, S.; Ohhira, M. Adenosine A1 Receptor Agonist Induces Visceral Antinociception via 5-HT1A, 5-HT2A, Dopamine D1 or Cannabinoid CB1 Receptors, and the Opioid System in the Central Nervous System. Physiol. Behav. 2020, 220, 112881. [Google Scholar] [CrossRef]
- Christofi, F.L.; Zhang, H.; Yu, J.G.; Guzman, J.; Xue, J.; Kim, M.; Wang, Y.Z.; Cooke, H.J. Differential Gene Expression of Adenosine A1, A2a, A2b, and A3 Receptors in the Human Enteric Nervous System. J. Comp. Neurol. 2001, 439, 46–64. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.; Ferreirinha, F.; Silva, I.; Duarte-Araújo, M.; Correia-De-Sá, P. Localization and Function of Adenosine Receptor Subtypes at the Longitudinal Muscle-Myenteric Plexus of the Rat Ileum. Neurochem. Int. 2011, 59, 1043–1055. [Google Scholar] [CrossRef]
- Peachey, J.A.; Hourani, S.M.O.; Kitchen, I. Ontogeny of Adenosine Receptors in the Longitudinal Muscle and Muscularis Mucosae of the Rat Distal Colon. Naunyn Schmiedebergs Arch. Pharmacol. 1999, 359, 140–146. [Google Scholar] [CrossRef]
- Ren, J.; Bertrand, P.P. Purinergic Receptors and Synaptic Transmission in Enteric Neurons. Purinergic Signal. 2007, 4, 255. [Google Scholar] [CrossRef]
- Tammpere, A.; Brusberg, M.; Axenborg, J.; Hirsch, I.; Larsson, H.; Lindström, E. Evaluation of Pseudo-Affective Responses to Noxious Colorectal Distension in Rats by Manometric Recordings. Pain 2005, 116, 220–226. [Google Scholar] [CrossRef]
- Temple, J.L.; Bernard, C.; Lipshultz, S.E.; Czachor, J.D.; Westphal, J.A.; Mestre, M.A. The Safety of Ingested Caffeine: A Comprehensive Review. Front. Psychiatry 2017, 8, 80. [Google Scholar] [CrossRef]
- Arvidsson, S.; Larsson, M.; Larsson, H.; Lindström, E.; Martinez, V. Assessment of Visceral Pain-Related Pseudo-Affective Responses to Colorectal Distension in Mice by Intracolonic Manometric Recordings. J. Pain 2006, 7, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Machado, N.J.; Ardais, A.P.; Nunes, A.; Szabó, E.C.; Silveirinha, V.; Silva, H.B.; Kaster, M.P.; Cunha, R.A. Impact of Coffee Intake on Measures of Wellbeing in Mice. Nutrients 2024, 16, 2920. [Google Scholar] [CrossRef]
- Domaszewski, P.; Konieczny, M.; Pakosz, P.; Matuska, J.; Skorupska, E.; Santafé, M.M. Obesity as an Influencing Factor for the Occurrence of Caffeine-Induced Effects in Women. Nutr. Metab. Cardiovasc. Dis. 2025, 35, 103836. [Google Scholar] [CrossRef]
- Becker, J.B.; Prendergast, B.J.; Liang, J.W. Female Rats Are Not More Variable than Male Rats: A Meta-Analysis of Neuroscience Studies. Biol. Sex Differ. 2016, 7, 34. [Google Scholar] [CrossRef]
- Ferrari, L.F.; Khomula, E.V.; Araldi, D.; Levine, J.D. Marked Sexual Dimorphism in the Role of the Ryanodine Receptor in a Model of Pain Chronification in the Rat. Sci. Rep. 2016, 6, 31221. [Google Scholar] [CrossRef]
- Bradshaw, H.; Miller, J.; Ling, Q.; Malsnee, K.; Ruda, M.A. Sex Differences and Phases of the Estrous Cycle Alter the Response of Spinal Cord Dynorphin Neurons to Peripheral Inflammation and Hyperalgesia. Pain 2000, 85, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Nicotra, L.; Tuke, J.; Grace, P.M.; Rolan, P.E.; Hutchinson, M.R. Sex Differences in Mechanical Allodynia: How Can It Be Preclinically Quantified and Analyzed? Front. Behav. Neurosci. 2014, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Prusator, D.K.; Greenwood-Van Meerveld, B. Sex-Related Differences in Pain Behaviors Following Three Early Life Stress Paradigms. Biol. Sex Differ. 2016, 7, 29. [Google Scholar] [CrossRef]
- Michna, L.; Lu, Y.P.; Lou, Y.R.; Wagner, G.C.; Conney, A.H. Stimulatory Effect of Oral Administration of Green Tea and Caffeine on Locomotor Activity in SKH-1 Mice. Life Sci. 2003, 73, 1383–1392. [Google Scholar] [CrossRef]
- Iriondo-Dehond, A.; Uranga, J.A.; Del Castillo, M.D.; Abalo, R. Effects of Coffee and Its Components on the Gastrointestinal Tract and the Brain–Gut Axis. Nutrients 2021, 13, 88. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Zhao, X.; Yu, L.; Yin, Y.; Zhang, X.D.; Wang, L.; Li, J.X.; Zhu, Q.; Luo, J.L. Current Status of GABA Receptor Subtypes in Analgesia. Biomed. Pharmacother. 2023, 168, 115800. [Google Scholar] [CrossRef]
- Auteri, M.; Zizzo, M.G.; Serio, R. GABA and GABA Receptors in the Gastrointestinal Tract: From Motility to Inflammation. Pharmacol. Res. 2015, 93, 11–21. [Google Scholar] [CrossRef]
- Alfadda, A.A.; Storr, M.A.; Shaffer, E.A. Eosinophilic Colitis: Epidemiology, Clinical Features, and Current Management. Therap. Adv. Gastroenterol. 2011, 4, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, E.; de Kleijn, R.; Colzato, L.S.; Alkemade, A.; Forstmann, B.U.; Nieuwenhuis, S. Neurotransmitters as Food Supplements: The Effects of GABA on Brain and Behavior. Front. Psychol. 2015, 6, 1520. [Google Scholar] [CrossRef]
- Mesías, M.; Delgado-Andrade, C. Melanoidins as a Potential Functional Food Ingredient. Curr. Opin. Food Sci. 2017, 14, 37–42. [Google Scholar] [CrossRef]
- Pokusaeva, K.; Johnson, C.; Luk, B.; Uribe, G.; Fu, Y.; Oezguen, N.; Matsunami, R.K.; Lugo, M.; Major, A.; Mori-Akiyama, Y.; et al. GABA-producing Bifidobacterium Dentium Modulates Visceral Sensitivity in the Intestine. Neurogastroenterol. Motil. 2016, 29, e12904. [Google Scholar] [CrossRef] [PubMed]
- O’Riordan, K.J.; Collins, M.K.; Moloney, G.M.; Knox, E.G.; Aburto, M.R.; Fülling, C.; Morley, S.J.; Clarke, G.; Schellekens, H.; Cryan, J.F. Short Chain Fatty Acids: Microbial Metabolites for Gut-Brain Axis Signalling. Mol. Cell. Endocrinol. 2022, 546, 111572. [Google Scholar] [CrossRef]
- Machado, F.; Gómez-Domínguez, I.; Hurtado-Ribeira, R.; Martin, D.; Coimbra, M.A.; del Castillo, M.D.; Coreta-Gomes, F. In Vitro Human Colonic Fermentation of Coffee Arabinogalactan and Melanoidin-Rich Fractions. Int. J. Biol. Macromol. 2024, 275, 133740. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.; Taine, E.G.; Meng, D.; Cui, T.; Tan, W. Chlorogenic Acid: A Systematic Review on the Biological Functions, Mechanistic Actions, and Therapeutic Potentials. Nutrients 2024, 16, 924. [Google Scholar] [CrossRef] [PubMed]
- Kabała, K.; Janicka, M. Relationship between the GABA Pathway and Signaling of Other Regulatory Molecules. Int. J. Mol. Sci. 2024, 25, 10749. [Google Scholar] [CrossRef] [PubMed]
- Del Castillo, M.D.; Ibáñez, E.; Amigo-Benavent, M.; Herrero, M.; Plaza, M.; Ullate, M. Application of Products of Coffee Silverskin in Anti-Ageing Cosmetics and Functional Food. WIPO Patent WO2013/004873, 10 January 2013. [Google Scholar]
- Cora, M.C.; Kooistra, L.; Travlos, G. Vaginal Cytology of the Laboratory Rat and Mouse: Review and Criteria for the Staging of the Estrous Cycle Using Stained Vaginal Smears. Toxicol. Pathol. 2015, 43, 776–793. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, J.K.; Greenwood-Van Meerveld, B. Amygdala Activation by Corticosterone Alters Visceral and Somatic Pain in Cycling Female Rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G1080–G1085. [Google Scholar] [CrossRef]
- Bagüés, A.; Martín-Fontelles, M.I.; Esteban-Hernández, J.; Sánchez-Robles, E.M. Characterization of the Nociceptive Effect of Carrageenan: Masseter versus Gastrocnemius. Muscle Nerve 2017, 56, 804–813. [Google Scholar] [CrossRef]
- Vera, G.; Castillo, M.; Cabezos, P.A.; Chiarlone, A.; Martín, M.I.; Gori, A.; Pasquinelli, G.; Barbara, G.; Stanghellini, V.; Corinaldesi, R.; et al. Enteric Neuropathy Evoked by Repeated Cisplatin in the Rat. Neurogastroenterol. Motil. 2011, 23, 370. [Google Scholar] [CrossRef]
- Castro, M.; Valero, M.S.; López-Tofiño, Y.; López-Gómez, L.; Girón, R.; Martín-Fontelles, M.I.; Uranga, J.A.; Abalo, R. Radiographic and Histopathological Study of Gastrointestinal Dysmotility in Lipopolysaccharide-Induced Sepsis in the Rat. Neurogastroenterol. Motil. 2023, 35, e14639. [Google Scholar] [CrossRef]

| Period of Time | Males–Control | Males–IC | Females–Control | Females–IC |
|---|---|---|---|---|
| 0–10 min | 629 ± 54 | 694 ± 59 | 598 ± 91 | 874 ± 64 + |
| 11–20 min | 308 ± 51 & | 387 ± 31 &&&& | 365 ± 55 & | 495 ± 55 &&& |
| 21–30 min | 192 ± 72 && | 135 ± 41 &&&& | 174 ± 46 &&& | 178 ± 62 &&&& |
| Parameters | Changes by Sex | Changes by Beverage | |
|---|---|---|---|
| General Health (body weight, solid and liquid intakes) | ♀ < ♂ | N.S. | |
| Locomotion | N.S. | ♀(IC > W) | |
| Sensitivity | Tactile | N.S. | N.S. |
| Skeletal muscle | N.S. | ♀(IC > W) | |
| Visceral | ♀ > ♂ | N.S. | |
| Colonic neuroimmune modulation | Glial cells | N.S. | ♀(IC > W) |
| CGRP varicose fibers | N.S. | ♀(IC < W) | |
| M2 Macrophages | ♀ > ♂ (only in IC) | ♂ (IC < W) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallego-Barceló, P.; López-Tofiño, Y.; López-Gómez, L.; Vera, G.; Bagues, A.; Esteban-Hernández, J.; Castillo, M.D.d.; Uranga, J.A.; Abalo, R. Instant Cascara Beverage as a Neuroimmune Modulator of the Brain–Gut Axis: Sex-Dependent Effects in Healthy Rats. Int. J. Mol. Sci. 2025, 26, 10691. https://doi.org/10.3390/ijms262110691
Gallego-Barceló P, López-Tofiño Y, López-Gómez L, Vera G, Bagues A, Esteban-Hernández J, Castillo MDd, Uranga JA, Abalo R. Instant Cascara Beverage as a Neuroimmune Modulator of the Brain–Gut Axis: Sex-Dependent Effects in Healthy Rats. International Journal of Molecular Sciences. 2025; 26(21):10691. https://doi.org/10.3390/ijms262110691
Chicago/Turabian StyleGallego-Barceló, Paula, Yolanda López-Tofiño, Laura López-Gómez, Gema Vera, Ana Bagues, Jesús Esteban-Hernández, María Dolores del Castillo, José Antonio Uranga, and Raquel Abalo. 2025. "Instant Cascara Beverage as a Neuroimmune Modulator of the Brain–Gut Axis: Sex-Dependent Effects in Healthy Rats" International Journal of Molecular Sciences 26, no. 21: 10691. https://doi.org/10.3390/ijms262110691
APA StyleGallego-Barceló, P., López-Tofiño, Y., López-Gómez, L., Vera, G., Bagues, A., Esteban-Hernández, J., Castillo, M. D. d., Uranga, J. A., & Abalo, R. (2025). Instant Cascara Beverage as a Neuroimmune Modulator of the Brain–Gut Axis: Sex-Dependent Effects in Healthy Rats. International Journal of Molecular Sciences, 26(21), 10691. https://doi.org/10.3390/ijms262110691










