Decreased Glucocorticoid Receptor Expression and Function in Cord Blood Immune Cells from Preterm Neonates with Morbidity
Abstract
1. Introduction
2. Results
2.1. Ex Vivo CBMC Glucocorticoid Sensitivity in Preterm Newborns
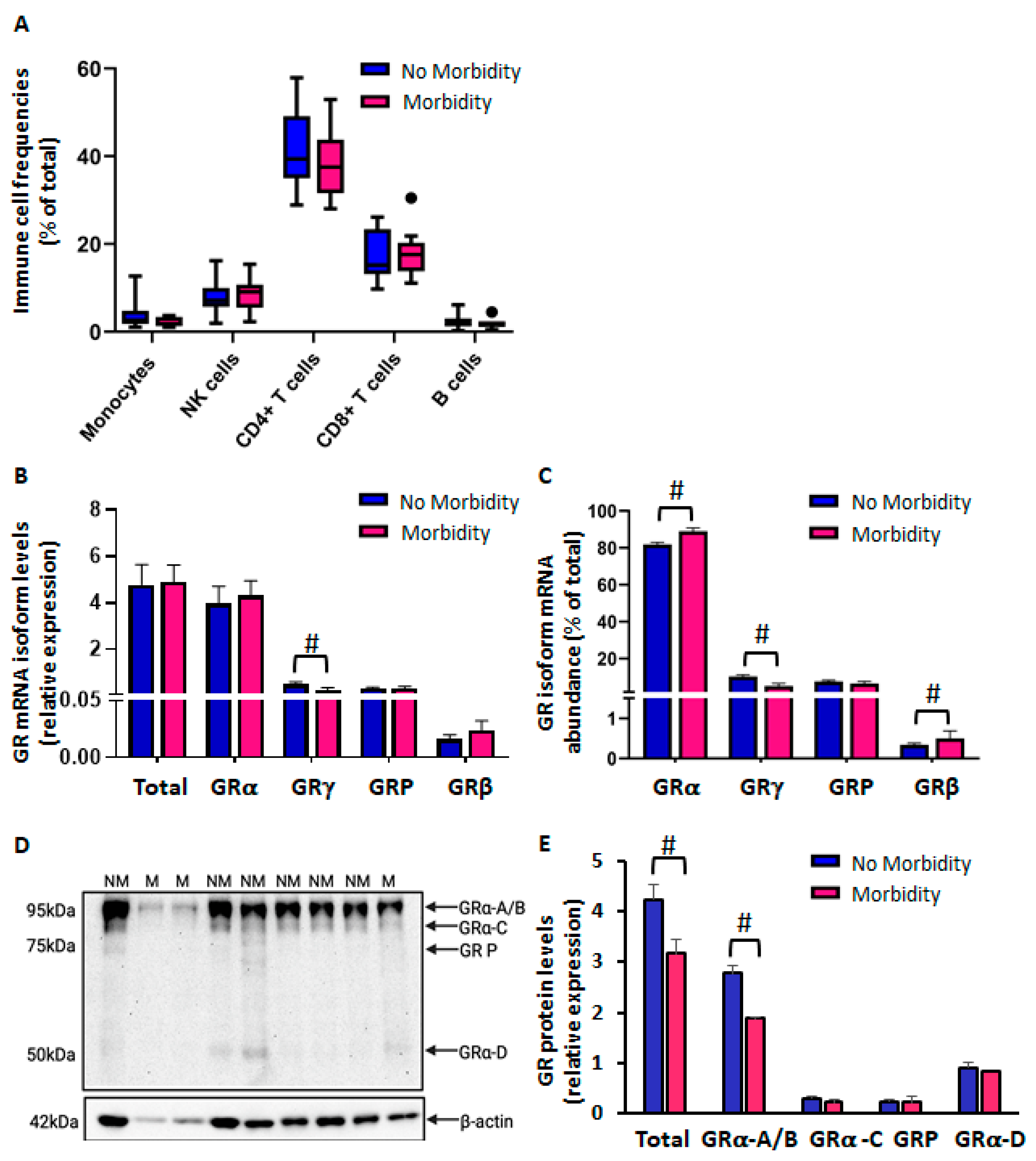
2.1.1. Reduced CBMC GR Protein Levels in Preterm Newborns in Association with Morbidity
2.1.2. Differential Glucocorticoid and Lipopolysaccharide-Mediated Regulation of CBMC-GR Expression According to Morbidity
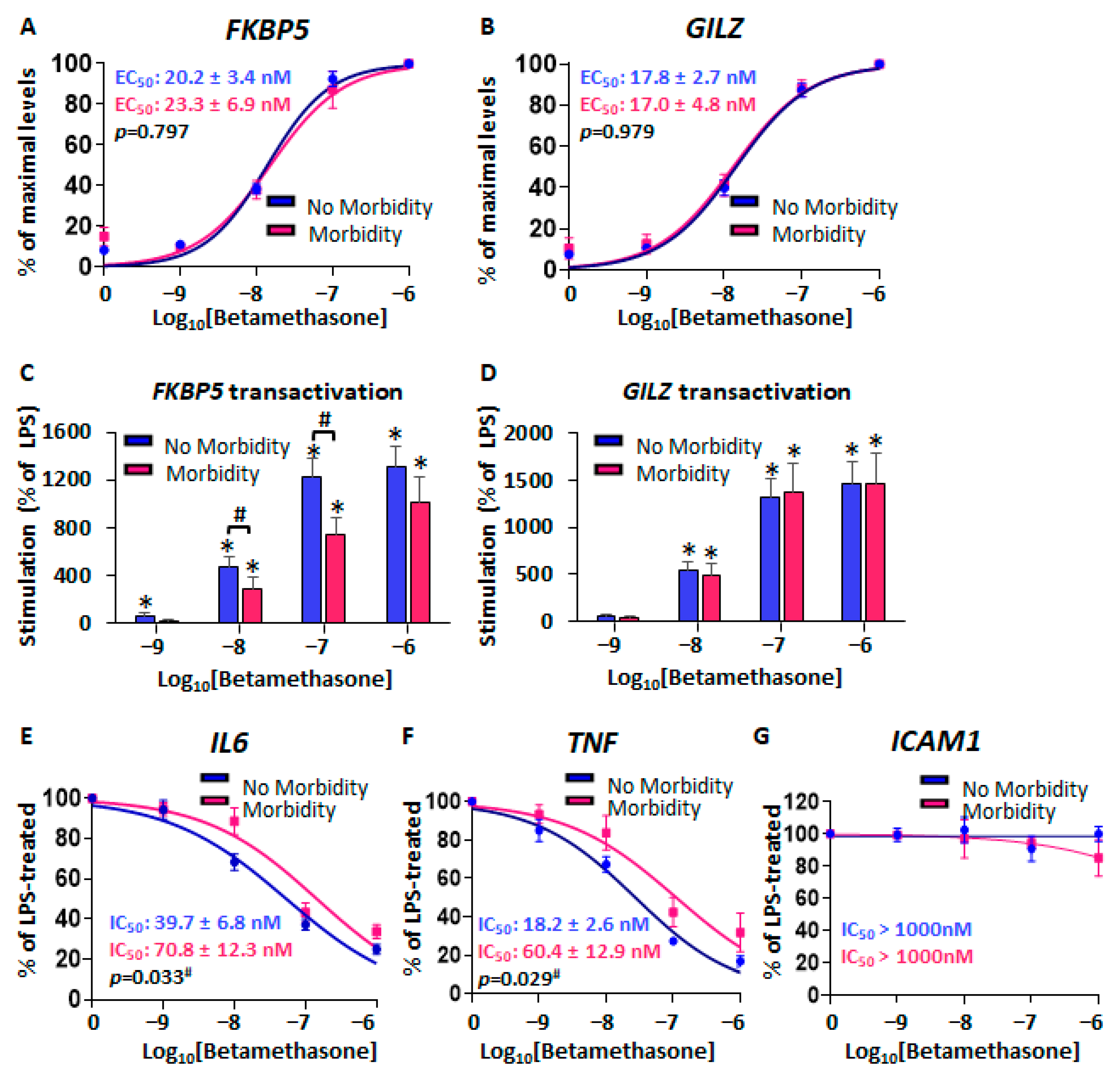
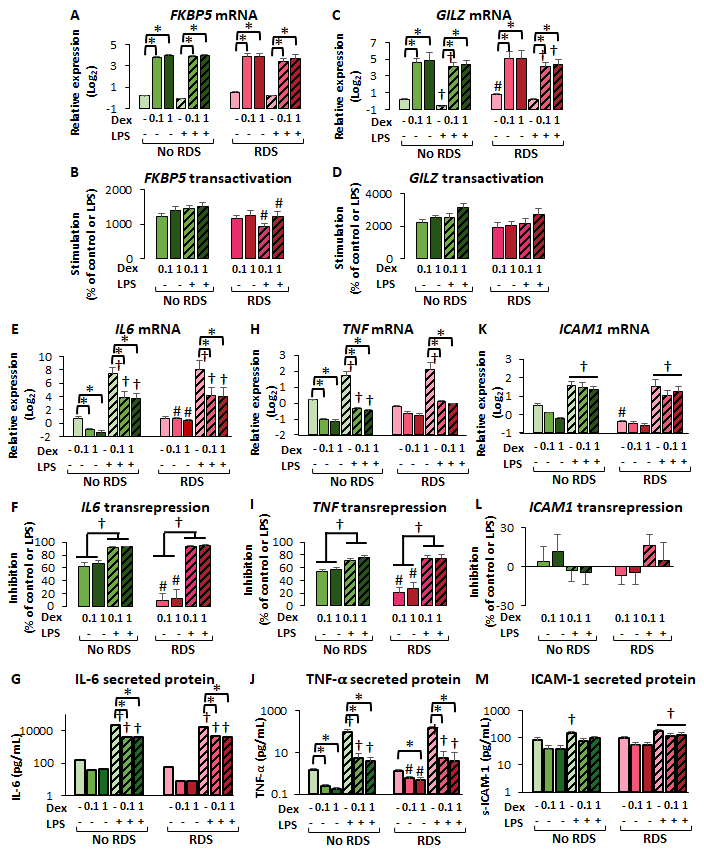
2.1.3. Reduced Glucocorticoid-Mediated Transrepression in CBMCs from Neonates with Morbidity
2.2. Ex Vivo CBWBC Glucocorticoid Sensitivity in Preterm Newborns
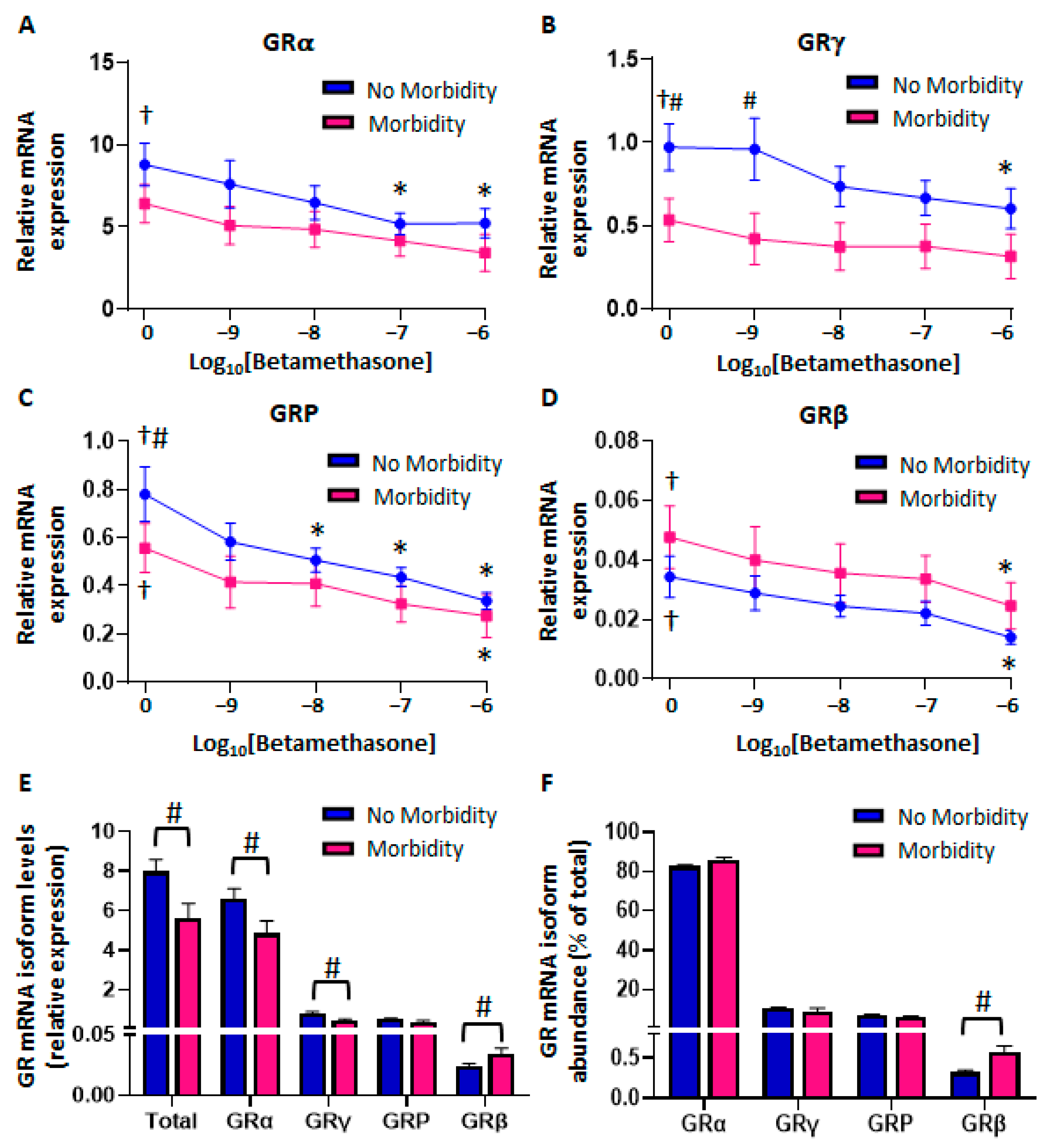
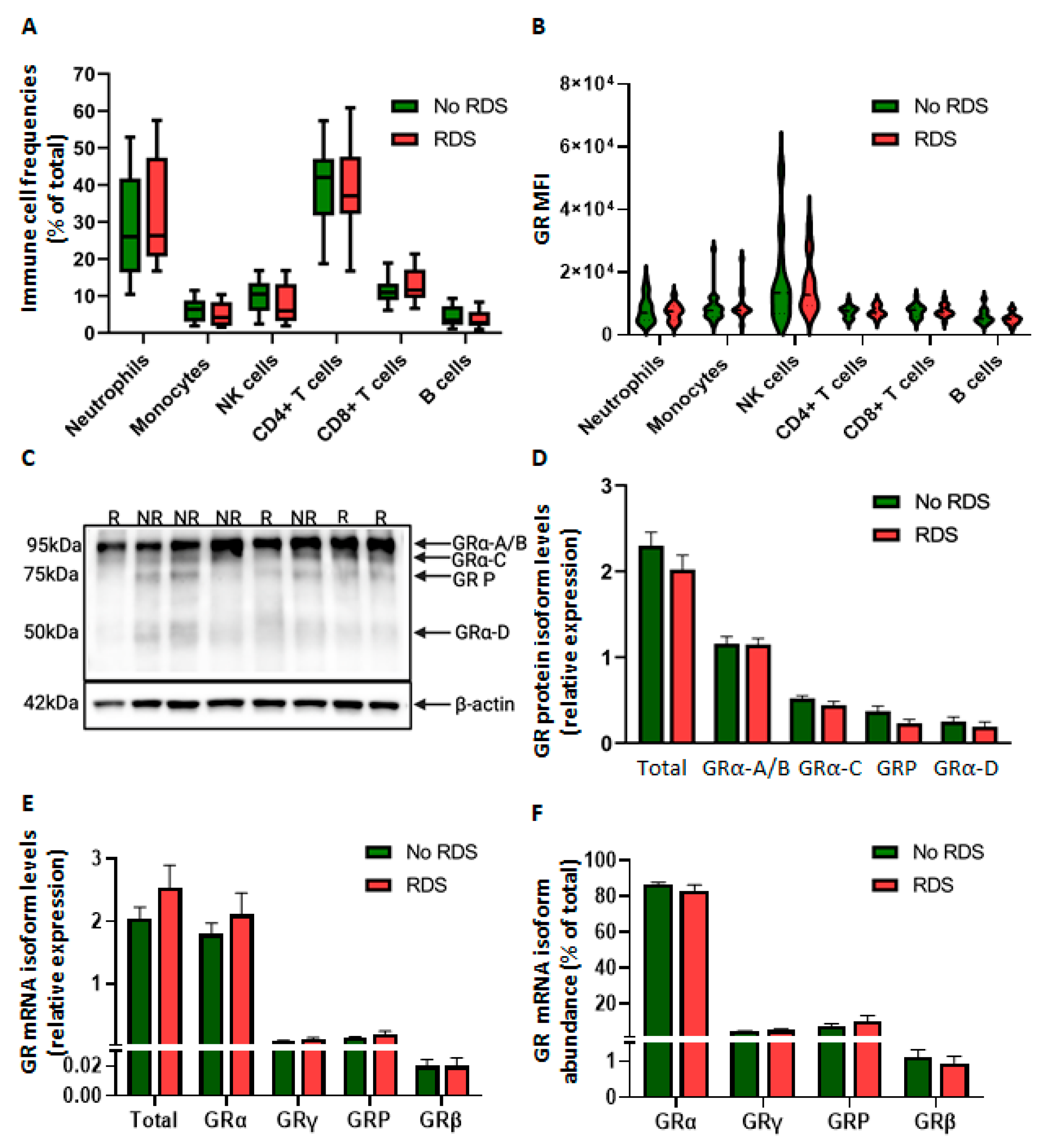
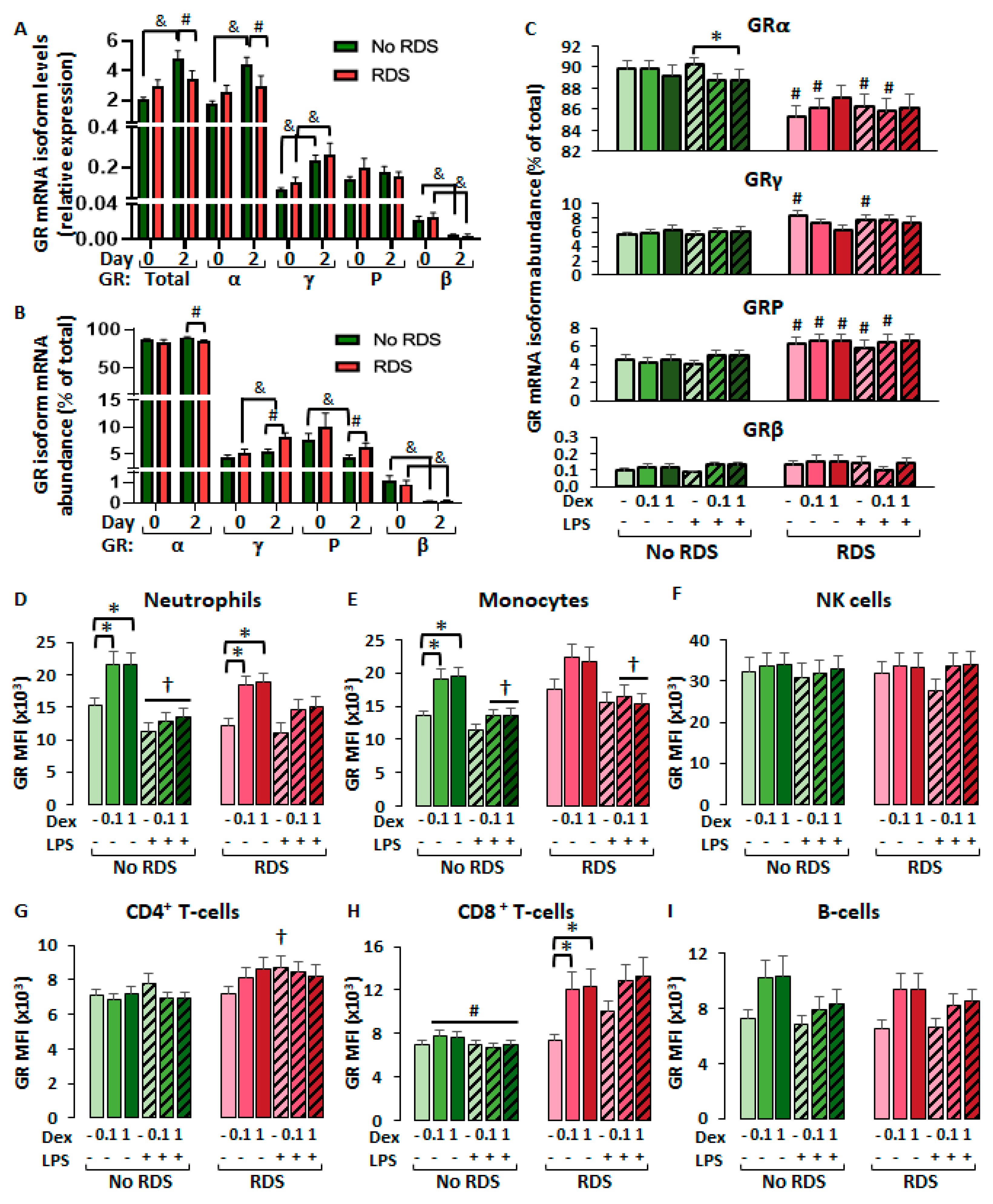
2.2.1. Similar GR Expression in Baseline CBWBCs According to Morbidity
2.2.2. Differential Glucocorticoid-Mediated Regulation of CBWBC-GR Expression According to Morbidity
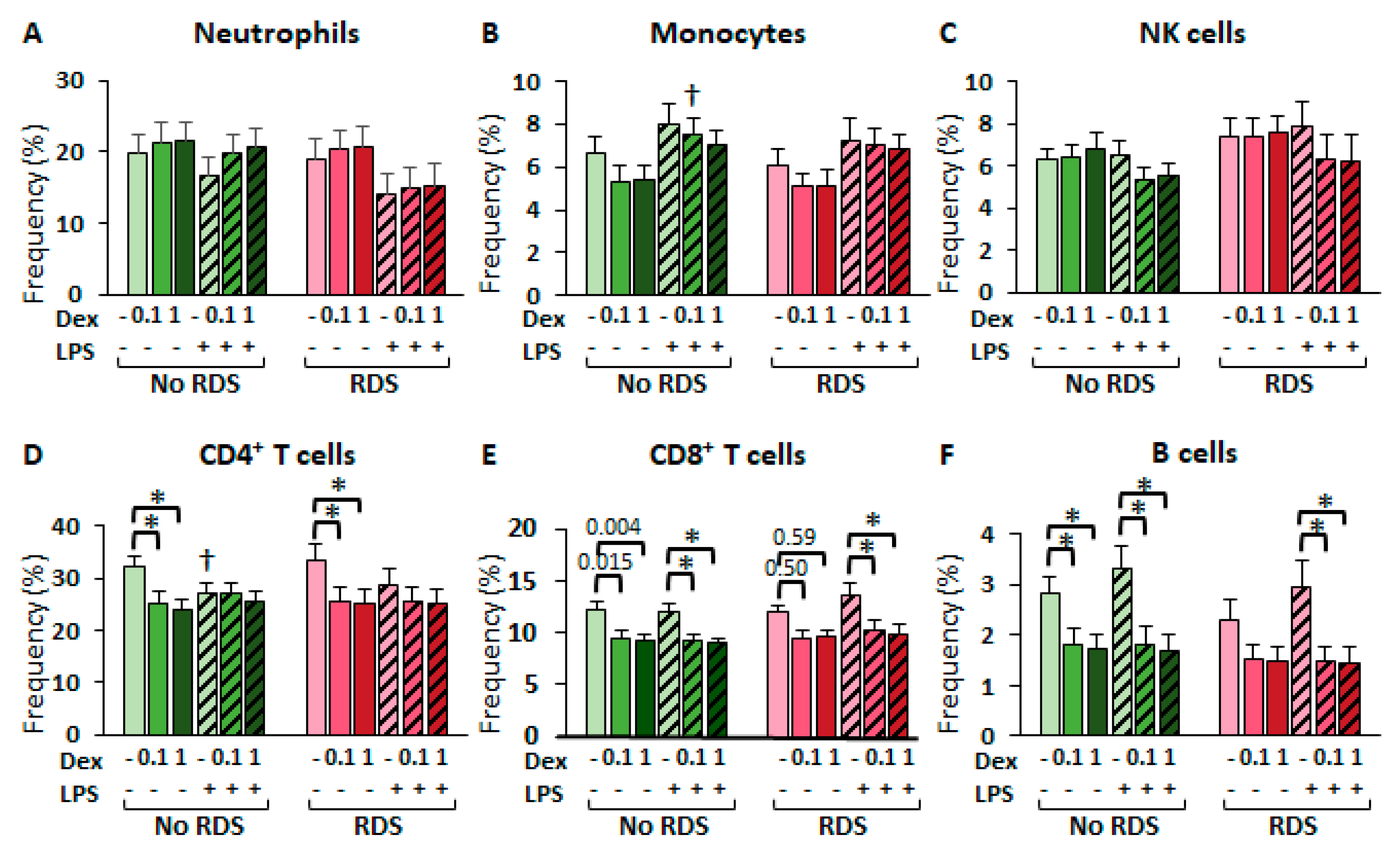
2.2.3. Glucocorticoid Effects in CBWBC Immune Cell Frequencies
2.2.4. Reduced GR-Mediated Transrepression in CBWBCs from Neonates with RDS
3. Discussion
Study Strengths and Limitations
4. Materials and Methods
4.1. Study Design and Human Subjects
4.1.1. Preterm Cohort 1: Frozen CBMCs
4.1.2. Preterm Cohort 2: Fresh CBWBCs
4.2. Immune Cell Characterization and Intracellular GR Levels by Flow Cytometry
4.3. Cell Culture
4.4. RNA Extraction and Real-Time PCR
4.5. Total Protein Isolation, SDS-PAGE, and Immunoblotting
4.6. ELISA Experiments
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACS | Antenatal Corticosteroid |
| BMI | Body Mass Index |
| BPD | Bronchopulmonary Dysplasia |
| CBMC | Cord Blood Mononuclear Cells |
| CBWBC | Cord blood White Blood Cells |
| EC50 | Half Maximal Effective Concentration |
| GR | Glucocorticoid Receptor |
| GRE | Glucocorticoid Response Element |
| IC50 | Half Maximal Inhibitory Concentration |
| IUGR | Intrauterine Growth Restriction |
| IVH | Intraventricular Hemorrhage |
| LPS | Lipopolysaccharides |
| NEC | Necrotizing Enterocolitis |
| NK | Natural Killer |
| PBMC | Peripheral Blood Mononuclear Cells |
| PPROM | Preterm Premature Rupture of Membranes |
| RDS | Respiratory Distress Syndrome |
References
- Anti, N.A.O.; Gheorghe, C.P.; Deming, D.D.; Adeoye, O.O.; Zhang, L.; Mata-Greenwood, E. Perinatal Glucocorticoid Sensitivity in the Preterm Newborn: Molecular Mechanisms, Endogenous Determinants, and Clinical Implications. Front. Endocrinol. 2025, 16, 1587891. [Google Scholar] [CrossRef]
- Busada, J.T.; Cidlowski, J.A. Mechanisms of Glucocorticoid Action During Development. Curr. Top. Dev. Biol. 2017, 125, 147–170. [Google Scholar] [CrossRef]
- McGoldrick, E.; Stewart, F.; Parker, R.; Dalziel, S.R. Antenatal Corticosteroids for Accelerating Fetal Lung Maturation for Women at Risk of Preterm Birth. Cochrane Database Syst. Rev. 2020, 2021, CD004454. [Google Scholar] [CrossRef]
- Takahashi, T.; Jobe, A.H.; Fee, E.L.; Newnham, J.P.; Schmidt, A.F.; Usuda, H.; Kemp, M.W. The Complex Challenge of Antenatal Steroid Therapy Nonresponsiveness. Am. J. Obstet. Gynecol. 2022, 227, 696–704. [Google Scholar] [CrossRef]
- Lockett, J.; Inder, W.J.; Clifton, V.L. The Glucocorticoid Receptor: Isoforms, Functions, and Contribution to Glucocorticoid Sensitivity. Endocr. Rev. 2024, 45, 593–624. [Google Scholar] [CrossRef] [PubMed]
- Vettorazzi, S.; Nalbantoglu, D.; Gebhardt, J.C.M.; Tuckermann, J. A Guide to Changing Paradigms of Glucocorticoid Receptor Function—A Model System for Genome Regulation and Physiology. FEBS J. 2022, 289, 5718–5743. [Google Scholar] [CrossRef] [PubMed]
- Vandevyver, S.; Dejager, L.; Libert, C. Comprehensive Overview of the Structure and Regulation of the Glucocorticoid Receptor. Endocr. Rev. 2014, 35, 671–693. [Google Scholar] [CrossRef]
- Hudson, W.H.; Vera, I.M.S.d.; Nwachukwu, J.C.; Weikum, E.R.; Herbst, A.G.; Yang, Q.; Bain, D.L.; Nettles, K.W.; Kojetin, D.J.; Ortlund, E.A. Cryptic Glucocorticoid Receptor-Binding Sites Pervade Genomic NF-κB Response Elements. Nat. Commun. 2018, 9, 1337. [Google Scholar] [CrossRef] [PubMed]
- Weikum, E.R.; de Vera, I.M.S.; Nwachukwu, J.C.; Hudson, W.H.; Nettles, K.W.; Kojetin, D.J.; Ortlund, E.A. Tethering Not Required: The Glucocorticoid Receptor Binds Directly to Activator Protein-1 Recognition Motifs to Repress Inflammatory Genes. Nucleic Acids Res. 2017, 45, 8596–8608. [Google Scholar] [CrossRef]
- Gerber, A.N.; Newton, R.; Sasse, S.K. Repression of Transcription by the Glucocorticoid Receptor: A Parsimonious Model for the Genomics Era. J. Biol. Chem. 2021, 296, 100687. [Google Scholar] [CrossRef]
- Quax, R.A.; Manenschijn, L.; Koper, J.W.; Hazes, J.M.; Lamberts, S.W.J.; van Rossum, E.F.C.; Feelders, R.A. Glucocorticoid Sensitivity in Health and Disease. Nat. Rev. Endocrinol. 2013, 9, 670–686. [Google Scholar] [CrossRef]
- Wilkinson, L.; Verhoog, N.J.D.; Louw, A. Disease- and Treatment-associated Acquired Glucocorticoid Resistance. Endocr. Connect. 2018, 7, R328–R349. [Google Scholar] [CrossRef]
- Ballard, P.L.; Ballard, R.A. Cytoplasmic Receptor for Glucocorticoids in Lung of the Human Fetus and Neonate. J. Clin. Investig. 1974, 53, 477–486. [Google Scholar] [CrossRef][Green Version]
- Kerepesi, T.; Arany, P. Low Levels of Glucocorticoid Binding Sites in Circulating Lymphocytes of Premature Infants Suffering from Hyaline Membrane Disease. J. Steroid. Biochem. 1985, 22, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Ataseven, F.; Salis, O.; Aygun, C.; Bedir, A.; Kucukoduk, S. Clinical Significance of Glucocorticoid Receptor Expression in Premature Infants with Respiratory Distress Syndrome. J. Matern. Fetal. Neonatal Med. 2017, 30, 430–433. [Google Scholar] [CrossRef]
- de Vries-van de Vlugt, B.C.; Lentjes, E.G.; Romijn, F.P.; Berger, H.M.; Massa, G.G. Glucocorticoid Receptors in Cord Blood Lymphocytes of Healthy Neonates and of Preterms Suffering from Respiratory Distress Syndrome. Acta Paediatr. 1997, 86, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Go, H.; Sato, M.; Hashimoto, K.; Imamura, T.; Kaneko, M.; Sato, T.; Goto, A.; Ishii, T.; Ishibashi, N.; Momoi, N.; et al. Glucocorticoid Receptor Expression in Whole Blood with Preterm Infants. J. Pediatr. Endocrinol. Metab. 2013, 26, 77–84. [Google Scholar] [CrossRef]
- Yamamoto, A.; Motokura, K.; Iwanaga, K.; Niwa, F.; Takita, J.; Kawai, M. Glucocorticoid Receptor Expression Pattern in Very Low-Birth Weight Infants Changes Drastically within the First Week of Life. Horm. Res. Paediatr. 2022, 96, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Bessler, H.; Mendel, C.; Straussberg, R.; Gurary, N.; Aloni, D.; Sirota, L. Effects of Dexamethasone on IL-1β, IL-6, and TNF-α Production by Mononuclear Cells of Newborns and Adults. Neonatology 1999, 75, 225–233. [Google Scholar] [CrossRef]
- Sahni, M.; Yeboah, B.; Das, P.; Shah, D.; Ponnalagu, D.; Singh, H.; Nelin, L.D.; Bhandari, V. Novel Biomarkers of Bronchopulmonary Dysplasia and Bronchopulmonary Dysplasia-Associated Pulmonary Hypertension. J. Perinatol. 2020, 40, 1634–1643. [Google Scholar] [CrossRef]
- Jonsson, B.; Tullus, K.; Brauner, A.; Lu, Y.; Noack, G. Early Increase of TNF alpha and IL-6 in Tracheobronchial Aspirate Fluid Indicator of Subsequent Chronic Lung Disease in Preterm Infants. Arch. Dis. Child.-Fetal Neonatal Ed. 1997, 77, F198–F201. [Google Scholar] [CrossRef]
- Ramsay, P.L.; O’Brian Smith, E.; Hegemier, S.; Welty, S.E. Early Clinical Markers for the Development of Bronchopulmonary Dysplasia: Soluble E-Selectin and ICAM-1. Pediatrics 1998, 102, 927–932. [Google Scholar] [CrossRef]
- Martin, C.B.; Oshiro, B.T.; Sands, L.D.; Kabir, S.; Thorpe, D.; Clark, T.C.; Yao, R.; Mata-Greenwood, E. Vitamin-D Dysregulation in Early- and Late-Onset Preeclampsia: A Gestational-Age Matched Study. J. Steroid Biochem. Mol. Biol. 2020, 203, 105729. [Google Scholar] [CrossRef]
- Amsterdam, A.; Sasson, R. The Anti-Inflammatory Action of Glucocorticoids Is Mediated by Cell Type Specific Regulation of Apoptosis. Mol. Cell. Endocrinol. 2002, 189, 1–9. [Google Scholar] [CrossRef]
- Bird, A.D.; Tan, K.H.; Olsson, P.F.; Zieba, M.; Flecknoe, S.J.; Liddicoat, D.R.; Mollard, R.; Hooper, S.B.; Cole, T.J. Identification of Glucocorticoid-Regulated Genes That Control Cell Proliferation during Murine Respiratory Development. J. Physiol. 2007, 585, 187–201. [Google Scholar] [CrossRef]
- Brewer, J.A.; Kanagawa, O.; Sleckman, B.P.; Muglia, L.J. Thymocyte Apoptosis Induced by T Cell Activation Is Mediated by Glucocorticoids In Vivo. J. Immunol. 2002, 169, 1837–1843. [Google Scholar] [CrossRef]
- Cole, T.J.; Blendy, J.A.; Monaghan, A.P.; Krieglstein, K.; Schmid, W.; Aguzzi, A.; Fantuzzi, G.; Hummler, E.; Unsicker, K.; Schütz, G. Targeted Disruption of the Glucocorticoid Receptor Gene Blocks Adrenergic Chromaffin Cell Development and Severely Retards Lung Maturation. Genes Dev. 1995, 9, 1608–1621. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.J.; Solomon, N.M.; Van Driel, R.; Monk, J.A.; Bird, D.; Richardson, S.J.; Dilley, R.J.; Hooper, S.B. Altered Epithelial Cell Proportions in the Fetal Lung of Glucocorticoid Receptor Null Mice. Am. J. Respir. Cell Mol. Biol. 2004, 30, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Kokkinopoulou, I.; Moutsatsou, P. Mitochondrial Glucocorticoid Receptors and Their Actions. Int. J. Mol. Sci. 2021, 22, 6054. [Google Scholar] [CrossRef] [PubMed]
- Marseglia, L.; D’Angelo, G.; Granese, R.; Falsaperla, R.; Reiter, R.J.; Corsello, G.; Gitto, E. Role of oxidative stress in neonatal respiratory distress syndrome. Free Rad. Biol. Med. 2019, 142, 132–137. [Google Scholar] [CrossRef]
- Reichardt, H.M.; Kaestner, K.H.; Tuckermann, J.; Kretz, O.; Wessely, O.; Bock, R.; Gass, P.; Schmid, W.; Herrlich, P.; Angel, P.; et al. DNA binding of the glucocorticoid receptor is not essential for survival. Cell 1998, 93, 531–541. [Google Scholar] [CrossRef]
- Cuevas Guaman, M.; Sbrana, E.; Shope, C.; Showalter, L.; Hu, M.; Meloche, S.; Aagaard, K. Administration of Antenatal Glucocorticoids and Postnatal Surfactant Ameliorates Respiratory Distress Syndrome–Associated Neonatal Lethality in Erk3−/− Mouse Pups. Pediatr. Res. 2014, 76, 24–32. [Google Scholar] [CrossRef]
- Habermehl, D.; Parkitna, J.R.; Kaden, S.; Brügger, B.; Wieland, F.; Gröne, H.-J.; Schütz, G. Glucocorticoid Activity during Lung Maturation Is Essential in Mesenchymal and Less in Alveolar Epithelial Cells. Mol. Endocrinol. 2011, 25, 1280–1288. [Google Scholar] [CrossRef]
- Li, A.; Hardy, R.; Stoner, S.; Tuckermann, J.; Seibel, M.; Zhou, H. Deletion of Mesenchymal Glucocorticoid Receptor Attenuates Embryonic Lung Development and Abdominal Wall Closure. PLoS ONE 2013, 8, e63578. [Google Scholar] [CrossRef]
- Schmidt, A.F.; Kannan, P.S.; Bridges, J.; Presicce, P.; Jackson, C.M.; Miller, L.A.; Kallapur, S.G.; Chougnet, C.A.; Jobe, A.H. Prenatal Inflammation Enhances Antenatal Corticosteroid–Induced Fetal Lung Maturation. JCI Insight 2020, 5, e139452. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Trakooljul, N.; Hadlich, F.; Ponsuksili, S.; Wimmers, K.; Murani, E. Transcriptome Analysis of Porcine PBMCs Reveals Lipopolysaccharide-Induced Immunomodulatory Responses and Crosstalk of Immune and Glucocorticoid Receptor Signaling. Virulence 2021, 12, 1808–1824. [Google Scholar] [CrossRef]
- Liden, J.; Rafter, I.; Truss, M.; Gustafsson, J.-Å.; Okret, S. Glucocorticoid Effects on NF-κB Binding in the Transcription of the ICAM-1 Gene. Biochem. Biophy. Res. Commun. 2000, 273, 1008–1014. [Google Scholar] [CrossRef]
- Amrani, Y.; Lazaar, A.L.; Panettieri, R.A. Up-Regulation of ICAM-1 by Cytokines in Human Tracheal Smooth Muscle Cells Involves an NF-kB- Dependent Signaling Pathway That Is Only Partially Sensitive to Dexamethasone. J. Immunol. 1999, 163, 2128–2134. [Google Scholar] [CrossRef] [PubMed]
- Ballabh, P.; Kumari, J.; Krauss, A.N.; Shin, J.J.; Jain, A.; Auld, P.A.M.; Lesser, M.L.; Cunningham-Rundles, S. Soluble E-Selectin, Soluble L-Selectin and Soluble ICAM-1 in Bronchopulmonary Dysplasia, and Changes with Dexamethasone. Pediatrics 2003, 111, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.K.; Hilton, S.V.; Merritt, T.A.; Hallman, M.; Mannino, F.; Boynton, B.R. Respiratory distress syndrome treated with human surfactant: Radiographic findings. Radiology 1985, 157, 329–334. [Google Scholar] [CrossRef]
- Mahoney, A.D.; Jain, L. Respiratory disorders in moderately preterm, late preterm, and early term infants. Clin. Perinatol. 2013, 40, 665–678. [Google Scholar] [CrossRef]
- Jensen, E.A.; Dysart, K.; Gantz, M.G.; McDonald, S.; Bamat, N.A.; Keszler, M.; Kirpalani, H.; Laughon, M.M.; Poindexter, B.B.; Duncan, A.F.; et al. The Diagnosis of Bronchopulmonary Dysplasia in Very Preterm Infants: An Evidence-Based Approach. Am. J. Respir. Crit. Care Med. 2019, 200, 751–759. [Google Scholar] [CrossRef]
- Papile, L.A.; Burstein, J.; Burstein, R.; Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1500 gm. J. Pediatr. 1978, 92, 529–534. [Google Scholar] [CrossRef]
- Bell, M.J.; Ternberg, J.L.; Feigin, R.D.; Keating, J.P.; Marshall, R.; Barton, L.; Brotherton, T. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann. Surg. 1978, 187, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.D.; Cooper, D.; Haddad, F.; Zaldivar, F.; Kraft, M.; Radom-Aizik, S. Glucocorticoid receptor expression on circulating leukocytes in healthy and asthmatic adolescents in response to exercise. Pediatr. Res. 2017, 82, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.D.; Radom-Aizik, S.; Haddad, F.; Zaldivar, F.; Kraft, M.; Cooper, D.M. Glucocorticoid receptor expression on circulating leukocytes differs between healthy male and female adults. J. Clin. Transl. Sci. 2017, 1, 108–114. [Google Scholar] [CrossRef]
- Chen, J.; Bruns, A.H.; Donnelly, H.K.; Wunderink, R.G. Comparative in vitro stimulation with lipopolysaccharide to study TNF alpha gene expression in fresh whole blood, fresh and frozen peripheral blood mononuclear cells. J. Immunol. Methods 2010, 357, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Vermeer, H.; Hendriks-Stegeman, B.I.; Verrijn Stuart, A.A.; van Buul-Offers, S.C.; Jansen, M. A comparison of in vitro bioassays to determine cellular glucocorticoid sensitivity. Eur. J. Endocrin. 2004, 150, 41–47. [Google Scholar] [CrossRef][Green Version]
- Mata-Greenwood, E.; Jackson, P.N.; Pearce, W.J.; Zhang, L. Endothelial glucocorticoid receptor promoter methylation according to dexamethasone sensitivity. J. Mol. Endocrin. 2015, 55, 133–146. [Google Scholar] [CrossRef]
- Mata-Greenwood, E.; Stewart, J.M.; Steinhorn, R.H.; Pearce, W.J. Role of BCL2-Associated Athanogene 1 in Differential Sensitivity of Human Endothelial Cells to Glucocorticoids. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1046–1055. [Google Scholar] [CrossRef]
| Total (n = 26) | No Morbidity (n = 15) | Morbidity (n = 11) | p-Value | |
|---|---|---|---|---|
| Maternal characteristics | ||||
| Maternal age (years) | 28.9 ± 5.7 | 29.8 ± 1.3 | 27.8 ± 1.9 | 0.511 |
| Pre-gravid BMI (kg/m2) | 26.4 ± 5.2 | 26.1 ± 1.8 | 26.9 ± 1.7 | 0.762 |
| Mode of delivery (C-section, n) | 20 | 11 | 9 | 0.674 |
| ACS treatment (n) | 21 | 10 | 11 | 0.046 |
| ACS-to-delivery interval (days) | 4.9 ± 1 | 7.0 ± 3.0 | 3.0 ± 1.1 | 0.113 |
| Pregnancy complications | ||||
| Preeclampsia (n) | 12 | 7 | 5 | 1.000 |
| PPROM (n) | 7 | 4 | 3 | 1.000 |
| Fetal/neonatal characteristics | ||||
| Gestational age (weeks) | 31.4 ± 6.2 | 33.3 ± 0.4 | 28.9 ± 0.9 | <0.001 |
| Fetal sex (male, n) | 16 | 7 | 9 | 0.109 |
| Birthweight (g) | 1708 ± 335 | 2008 ± 115 | 1297 ± 251 | 0.022 |
| Birthweight percentile (%) | 32.0 ± 6.3 | 34.9 ± 6.1 | 28.0 ± 10.2 | 0.546 |
| Cord blood cortisol levels (ng/mL) | 101.2 ± 19.9 | 97.4 ± 4.8 | 106.5 ± 5.4 | 0.223 |
| Neonatal complications (n) | ||||
| RDS | 9 | |||
| BPD | 4 | |||
| NEC | 4 | |||
| IVH | 2 | |||
| Neonatal demise | 3 |
| Total (n = 40) | No RDS (n = 25) | RDS (n = 15) | p | |
|---|---|---|---|---|
| Maternal characteristics | ||||
| Maternal age (years) | 28.9 ± 0.7 | 29.2 ± 0.7 | 28.5 ± 1.3 | 0.654 |
| Pre-gravid BMI (kg/m2) | 29.6 ± 1.5 | 29.2 ± 1.9 | 30.3 ± 2.2 | 0.743 |
| Mode of delivery (C-section, n) | 32 | 21 | 11 | 0.444 |
| ACS exposure (n) | 40 | 25 | 15 | 1.000 |
| ACS-to-delivery interval (days) | 8.1 ± 1.5 | 8.1 ± 1.9 | 8.0 ± 2.4 | 0.964 |
| Pregnancy complications (n) | ||||
| Maternal diabetes | 11 | 5 | 6 | 0.273 |
| PPROM | 4 | 4 | 0 | 0.278 |
| Preeclampsia | 18 | 10 | 8 | 0.517 |
| IUGR | 12 | 8 | 4 | 1.000 |
| Fetal/neonatal characteristics | ||||
| Multiple gestation (n) | 16 | 10 | 6 | 1.000 |
| Gestational age (weeks) | 32.5 ± 0.3 | 32.9 ± 0.3 | 31.8 ± 0.6 | 0.118 |
| Fetal sex (male, n) | 19 | 10 | 9 | 0.328 |
| Birthweight (g) | 1819 ± 81 | 1848 ± 95 | 1767 ± 153 | 0.624 |
| Birthweight percentile (%) | 27.8 ± 4.9 | 21.4 ± 23.1 | 33.7 ± 9.7 | 0.500 |
| Cord blood cortisol levels (ng/mL) | 63.1 ± 4.4 | 57.4 ± 2.5 | 72.7 ± 10.8 | 0.186 |
| Neonatal complications (n) | ||||
| BPD | 1 | 0 | 1 | 0.375 |
| NEC | 6 | 3 | 3 | 0.654 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anti, N.A.O.; Deming, D.D.; Gheorghe, C.P.; Tugung, A.; Gray-Hutto, N.; Zhang, L.; Mata-Greenwood, E. Decreased Glucocorticoid Receptor Expression and Function in Cord Blood Immune Cells from Preterm Neonates with Morbidity. Int. J. Mol. Sci. 2025, 26, 10686. https://doi.org/10.3390/ijms262110686
Anti NAO, Deming DD, Gheorghe CP, Tugung A, Gray-Hutto N, Zhang L, Mata-Greenwood E. Decreased Glucocorticoid Receptor Expression and Function in Cord Blood Immune Cells from Preterm Neonates with Morbidity. International Journal of Molecular Sciences. 2025; 26(21):10686. https://doi.org/10.3390/ijms262110686
Chicago/Turabian StyleAnti, Nana A. O., Douglas D. Deming, Ciprian P. Gheorghe, Ashra Tugung, Nikia Gray-Hutto, Lubo Zhang, and Eugenia Mata-Greenwood. 2025. "Decreased Glucocorticoid Receptor Expression and Function in Cord Blood Immune Cells from Preterm Neonates with Morbidity" International Journal of Molecular Sciences 26, no. 21: 10686. https://doi.org/10.3390/ijms262110686
APA StyleAnti, N. A. O., Deming, D. D., Gheorghe, C. P., Tugung, A., Gray-Hutto, N., Zhang, L., & Mata-Greenwood, E. (2025). Decreased Glucocorticoid Receptor Expression and Function in Cord Blood Immune Cells from Preterm Neonates with Morbidity. International Journal of Molecular Sciences, 26(21), 10686. https://doi.org/10.3390/ijms262110686








