Biochemical Effects of Natural and Nanoparticle Fish and Algal Oils in Gilt Pregnancy Diets on Base Excision Repair Enzymes in Newborn Piglets—Socioeconomic Implications for Regional Pig Farming—Preliminary Results
Abstract
1. Introduction
2. Results
2.1. Modulation of Cleaved Abasic Sites in the Liver of Newborn Piglets (First Day of Life) Following Maternal Oil Supplementation During Pregnancy
2.2. Modulation of Gene Expression in the Liver of Newborn Piglets (First Day of Life) Following Maternal Oil Supplementation During Pregnancy
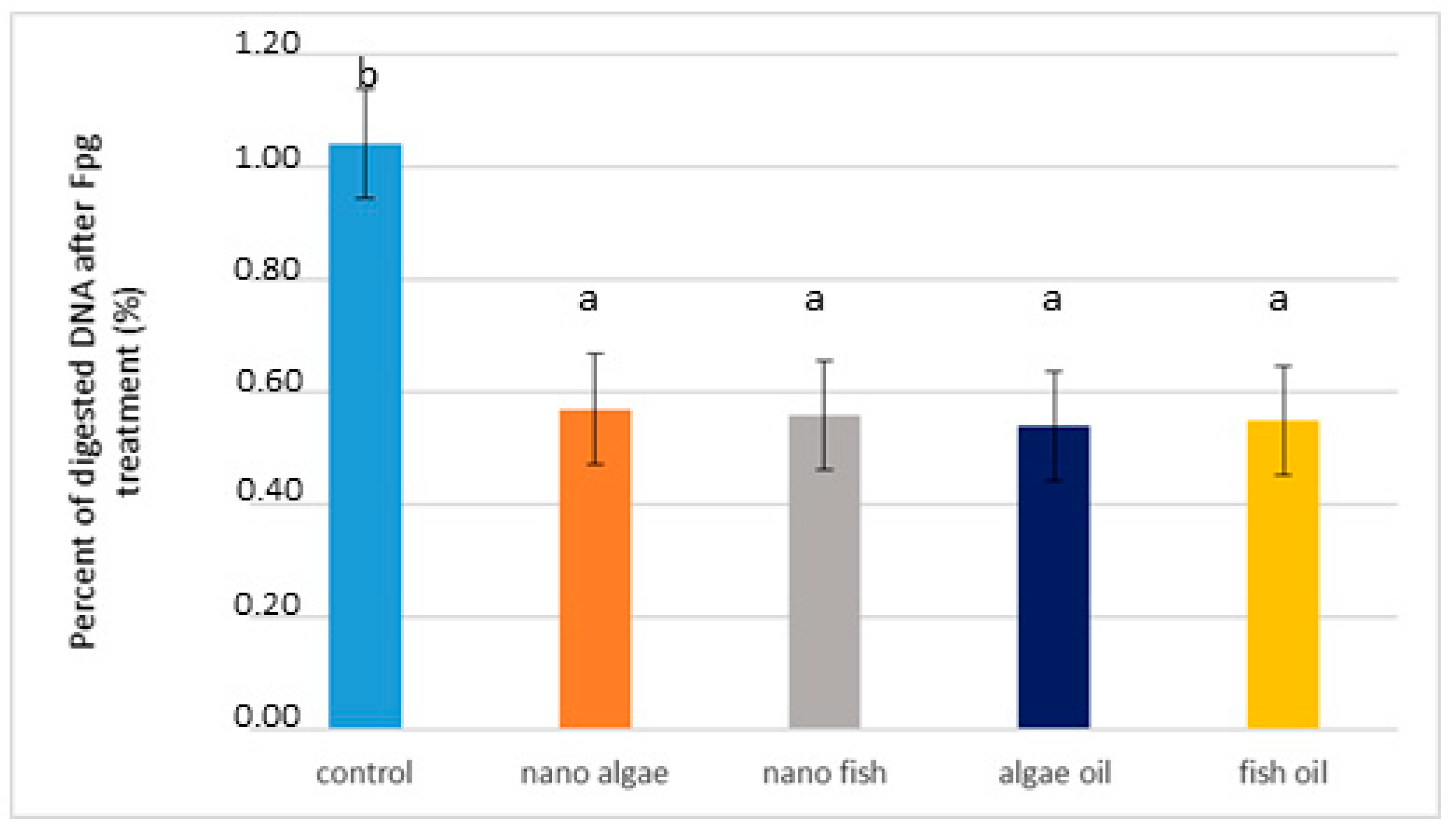
2.3. Modulation of FPG Protein Activity in the Liver of Newborn Piglets (First Day of Life) Following Maternal Oil Supplementation During Pregnancy
3. Discussion
Limitations of the Study
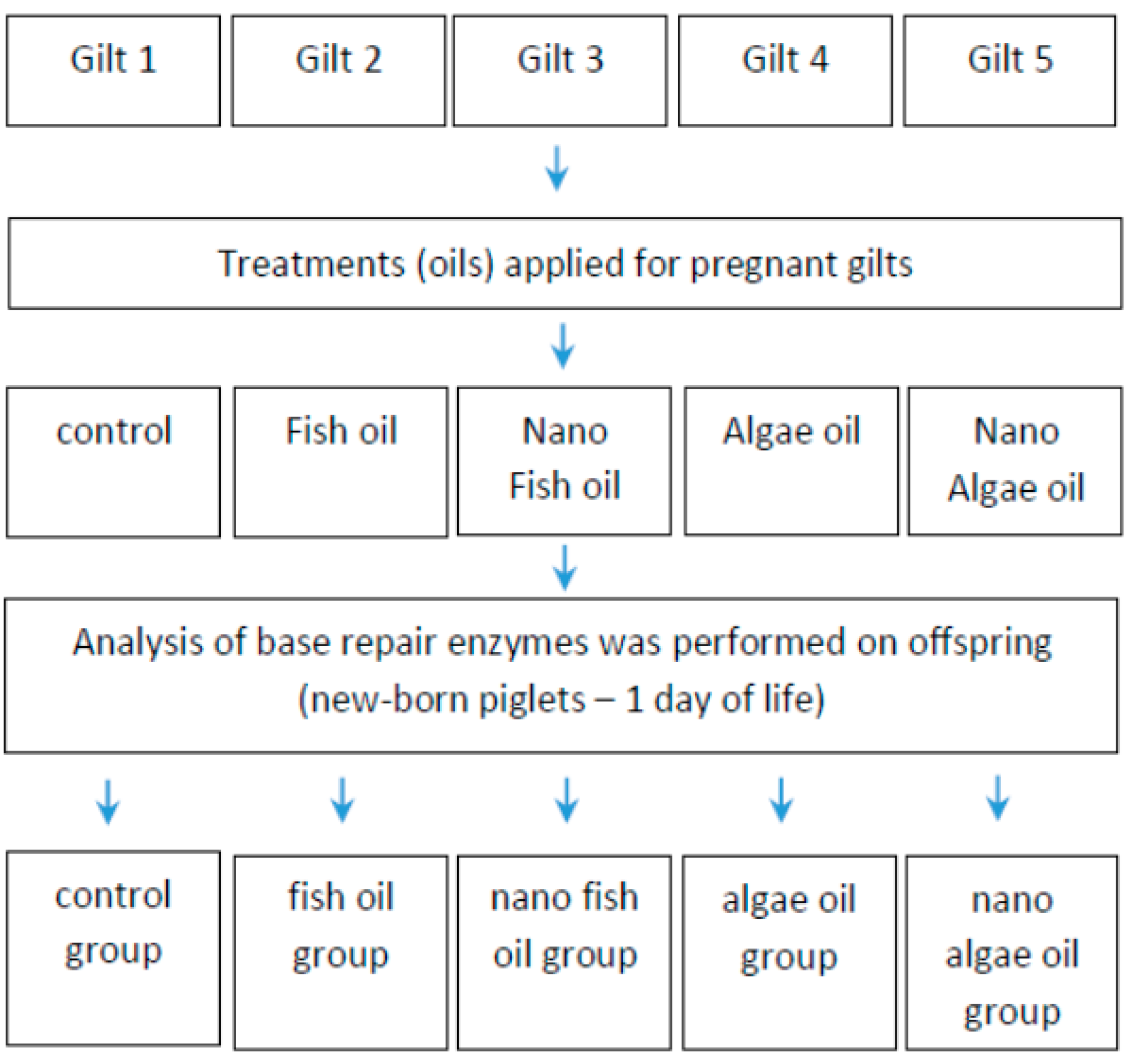
4. Materials and Methods
4.1. Animals Housing and Feeding
4.2. Characteristics of the Nanoparticles and Their Systems
4.3. DNA Repair Activity Assay
4.4. Real-Time Quantitative Reverse Transcription-Polymerase Chain Reaction (qPCR) Analysis of mRNA Abundance
4.5. Estimation of Genomic DNA Oxidative Damage from the Liver of Neborn Piglets
4.6. Statistical Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed]
- Allameh, A.; Niayesh-Mehr, R.; Aliarab, A.; Sebastiani, G.; Pantopoulos, K. Oxidative Stress in Liver Pathophysiology and Disease. Antioxidants 2023, 12, 1653. [Google Scholar] [CrossRef]
- Repetto, M.G.; Ossani, G.; Monserrat, A.J.; Boveris, A. Oxidative damage: The biochemical mechanism of cellular injury and necrosis in choline deficiency. Exp. Mol. Pathol. 2010, 88, 143–149. [Google Scholar] [CrossRef]
- Ames, B.N. Endogenous oxidative DNA damage, aging, and cancer. Free Radic. Res. Commun. 1989, 7, 121–128. [Google Scholar]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef]
- Oliński, R.; Gackowski, D.; Rozalski, R.; Foksinski, M.; Bialkowki, K. Oxidative DNA damage in cancer patients: A cause or a consequence of the disease development? Mutat. Res. 2003, 531, 177–190. [Google Scholar] [CrossRef]
- Mc Cord, J.M. The evolution of free radicals and oxidative stress. Am. J. Med. 2003, 108, 652–659. [Google Scholar] [CrossRef]
- Bartsch, H.; Nair, J. Oxidative stress and lipid peroxidation derived DNA-lesions in inflammation driven carcinogenesis. Cancer Detect. Prev. 2004, 28, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Lipiński, P.; Starzynski, R.R.; Drapier, J.C.; Bouton, C.; Bartlomiejczyk, T.; Sochanowicz, B.; Smuda, E.; Gajkowska, A.; Kruszewski, M. Induction of iron regulatory protein 1 RNA binding activity by nitric oxide is associated with a concomitant increase in the labile iron pool: Implications for DNA damage. Biochem. Biophys. Res. Commun. 2005, 327, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Obtułowicz, T.; Wilczura, A.; Speina, E.; Swoboda, M.; Janik, J.; Janowska, B.; Cieśla, J.M.; Kowalczyk, P.; Jawień, A.; Gackowski, D.; et al. Aberrant repair of etheno–DNA adducts in leukocytes and colon tissue of colon cancer patients. Free Radic. Biol. Med. 2010, 49, 1064–1071. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Jaworek, J.; Kot, M.; Sokolowska, B.; Bielen, A.; Janowska, B.; Ciesla, J.M.; Szparecki, G.; Sados, B.; Tudek, B. Inflammation increases oxidative DNA damage repair and stimulates preneoplastic changes in colons of newborn rats. J. Physiol. Pharmacol. 2016, 67, 277–286. [Google Scholar] [PubMed]
- Khokhlova, E.V.; Fesenko, Z.S.; Sopova, J.V.; Leonova, E.I. Features of DNA Repair in the Early Stages of Mammalian Embryonic Development. Genes 2020, 11, 1138. [Google Scholar] [CrossRef]
- Ba, X.; Boldogh, I. 8-Oxoguanine DNA glycosylase 1: Beyond repair of the oxidatively modified base lesions. Redox Biol. 2018, 14, 669–678. [Google Scholar] [CrossRef]
- Hang, B.; Medina, M.; Fraenkel-Conrat, H.; Singer, B. A 55-kDa protein isolated from human cells shows DNA glycosylase activity toward 3,N4-ethenocytosine and the GyT mismatch. Proc. Natl. Acad. Sci. USA 1998, 95, 13561–13566. [Google Scholar] [CrossRef]
- Eder, E.; Wacker, M.; Lutz, U.; Nair, J.; Fang, X.; Bartsch, H.; Beland, F.A.; Schlatter, J.; Lutz, W.K. Oxidative stress related DNA adducts in the liver of female rats fed with sunflower-, rapeseed-, olive- or coconut oil supplemented diets. Chem.-Biol. Interact. 2006, 159, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Langie, S.A.S.; Kowalczyk, P.; Tomaszewski, B.; Vasilaki, A.; Maas, L.M.; Moonen, E.J.; Palagani, A.; Godschalk, R.W.L.; Tudek, B.; van Schooten, F.J.; et al. Redox and epigenetic regulation of the APE1 gene in the hippocampus of piglets: The effect of early life exposures. DNA Repair 2014, 18, 52–62. [Google Scholar] [CrossRef]
- Velazquez, M.R.; Batistel, F.; Rodriguez, J.M.P.; Relling, A.E. Effects of maternal dietary omega-3 polyunsaturated fatty acids and methionine during late gestation on fetal growth, DNA methylation, and mRNA relative expression of genes associated with the inflammatory response, lipid metabolism and DNA, methylation in placenta and offspring’s liver in sheep. J. Anim. Sci. Biotechnol. 2020, 11, 111. [Google Scholar]
- Chiorcea-Paquim, A.M. 8-oxoguanine and 8-oxodeoxyguanosine Biomarkers of Oxidative DNA Damage: A Review on HPLC–ECD Determination. Molecules 2022, 27, 1620. [Google Scholar] [CrossRef]
- Prorok, P.; Saint-Pierre, C.; Gasparutto, D.; Fedorova, O.S.; Ishchenko, A.A.; Leh, H.; Buckle, M.; Tudek, B.; Saparbaev, M. Highly Mutagenic Exocyclic DNA Adducts Are Substrates for the Human Nucleotide Incision Repair Pathway. PLoS ONE 2012, 7, e51776, Erratum in: PLoS One 2013, 8.. [Google Scholar] [CrossRef]
- Laval, F.; Wink, D.A. Inhibition by nitric oxide of the repair protein, O6-methylguanine-DNA-methyltransferase. Carcin-Ogenesis 1994, 15, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Reed, A.J.; Zahurancik, W.J.; Daskalova, S.M.; Sidney, M.; Suo, H.Z. Interlocking activities of DNA polymerase β in the base excision repair pathway. Proc. Natl. Acad. Sci. USA 2022, 119, e2118940119. [Google Scholar] [CrossRef]
- Kay, J.; Thadhani, E.; Samson, L.; Engelward, B. Inflammation-Induced DNA kappa, Mutations and Cancer. DNA Repair (Amst) 2019, 83, 102673. [Google Scholar] [CrossRef]
- Bagga, D.; Wang, L.; Farias-Eisner, R.; Glaspy, J.A.; Reddy, S.T. Differential effects of prostaglandin derived from omega-6 and omega-3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Proc. Natl. Acad. Sci. USA 2003, 100, 1751–1756. [Google Scholar] [CrossRef]
- Fong, L.; Muhlhausler, B.S.; Gibson, R.A.; Xian, Z.C.J. Perinatal maternal dietary supplementation of Ω 3-fatty acids transiently affects bone marrow microenvironment, osteoblast and osteoclast formation, and bone mass in male offspring. Endocrinology 2012, 153, 2455–2465. [Google Scholar] [CrossRef]
- dos Santos Silva, P.; Kra, G.; Butenko, Y.; Daddam, J.R.; Levin, Y.; Zachut, M. Maternal supplementation with n-3 fatty acids affects placental lipid metabolism, inflammation, oxidative stress, the endocannabinoid system, and the neonate cytokine concentrations in dairy cows. J. Anim. Sci. Biotechnol. 2024, 15, 74. [Google Scholar] [CrossRef] [PubMed]
- Mathias, P.C.F.; Elmhiri, G.; de Oliveira, J.C.; Delayre-Orthez, C.; Barella, L.F.; Tόfolo, L.P.; Fabricio, G.S.; Chango, A.; Abdennebi-Najar, L. Maternal diet, bioactive molecules, and exercising as reprogramming tools of metabolic programming. Eur. J. Nutr. 2014, 53, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Richter, G.W. Studies of iron overload. Lysosomal proteolysis of rat liver ferritin. Pathol. Res. Pract. 1986, 181, 159–167. [Google Scholar] [CrossRef]
- Moghadam, F.V.; Pourahmad, R.; Mortazavi, A.; Davoodi, D.; Azizinezhad, R. Use of Fish Oil Nanoencapsulated with Gum Arabic Carrier in Low Fat Probiotic Fermented Milk. Food Sci. Anim. Resour. 2019, 39, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Babbs, C.F. Oxygen radicals in ulcerative colitis. Free Radic. Biol. Med. 1992, 13, 169–181. [Google Scholar] [CrossRef]
- Siscovick, D.S.; Barringer, T.A.; Fretts, A.M.; Wu, J.H.; Lichtenstein, A.H.; Costello, R.B.; Kris-Etherton, P.M.; Jacobson, T.A.; Engler, M.B.; Alger, H.M. Omega-3 polyunsaturated fatty acid (fish oil) supplementation and the prevention of clinical cardiovascular disease: A science advisory from the American Heart Association. Circulation 2017, 135, e867–e884. [Google Scholar] [CrossRef]
- Smolińska, K.; Szopa, A.; Sobczyński, J.; Serefko, A.; Dobrowolski, P. Nutritional Quality Implications: Exploring the Impact of a Fatty Acid-Rich Diet on Central Nervous System Development. Nutrients 2024, 16, 1093. [Google Scholar] [CrossRef] [PubMed]
- Mongan, D.; Healy, C.; Jones, H.J.; Zammit, S.; Cannon, M.; Cotter, D.R. Plasma polyunsaturated fatty acids and mental disorders in adolescence and early adulthood: Cross-sectional and longitudinal associations in a general population cohort. Transl. Psychiatry 2021, 11, 321. [Google Scholar] [CrossRef]
- Amador-Licona, N.; Diaz-Murillo, T.A.; Gabriel-Ortiz, G.; Pacheco-Moises, F.P.; Pereyra-Nobara, T.A.; Guizar-Mendoza, J.M.; Barbosa-Sabanero, G.; Orozco-Avina, G.; Moreno-Martinez, S.C.; Luna-Montalban, R.; et al. Omega 3 fatty acids supplementation and oxidative stress in HIV-seropositive patients. A Clinical Trial. PLoS ONE 2016, 11, e0151637. [Google Scholar] [CrossRef]
- Heshmati, J.; Morvaridzadeh, M.; Maroufizadeh, S.; Akbari, A.; Yavari, M.; Amirinejad, A.; Maleki-Hajiagha, A.; Sepidarkish, M. Omega-3 fatty acids supplementation and oxidative stress parameters: A systematic review and meta-analysis of clinical trials. Pharmacol. Res. 2019, 149, 104462. [Google Scholar] [CrossRef]
- Kosek, V.; Heczkova, M.; Novak, F.; Meisnerova, E.; Novákova, O.; Zelenka, J.; Bechynska, K.; Vrzacova, N.; Suttnar, J. The ω-3 Polyunsaturated Fatty Acids and Oxidative Stress in Long-Term Parenteral Nutrition Dependent Adult Patients: Functional Lipidomics Approach. Nutrients 2020, 12, 2351. [Google Scholar] [CrossRef]
- Leghi, G.E.; Muhlhausler, B.S. The effect of n-3 LCPUFA supplementation on oxidative stress and inflammation in the placenta and maternal plasma during pregnancy. Prostaglandins Leukot. Essent. Fat. Acids 2016, 113, 33–39. [Google Scholar] [CrossRef]
- Opgenorth, J.; Sordillo, L.M.; van de Haar, M.J. Colostrum supplementation with n-3 fatty acids and α-tocopherol alters plasma polyunsaturated fatty acid profile and decreases an indicator of oxidative stress in newborn calves. J. Dairy. Sci. 2020, 103, 3545–3553. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef] [PubMed]
- Maehre, H.K.; Jensen, I.J.; Elvevoll, E.O.; Eilertsen, K.E. Omega-3 Fatty Acids and Cardiovascular Diseases: Effects, Mechanisms and Dietary Relevance. Int. J. Mol. Sci. 2015, 16, 22636–22661. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.M.; Ma, D.W.L. Are all n-3 polyunsaturated fatty acids created equal? Lipids Health Dis. 2009, 8, 33. [Google Scholar] [CrossRef]
- Mahan, D.C.; Shields, R.G., Jr. Macro- and micromineral composition of pigs from birth to 145 kilograms of body weight. J. Anim. Sci. 1998, 76, 506–512. [Google Scholar] [CrossRef]
- Sobrino, A.; Walker, M.E.; Colas, R.A.; Dalli, J. Protective activities of distinct omega-3 enriched oils are linked to their ability to upregulate specialized pro-resolving mediators. PLoS ONE 2020, 15, e0242543. [Google Scholar] [CrossRef] [PubMed]
- Kemse, N.G.; Kale, A.A.; Joshi, S.R. Supplementation of maternal omega-3 fatty acids to pregnancy induced hypertension Wistar rats improves IL10 and VEGF levels. Prostaglandins Leukot. Essent. Fat. Acids 2016, 104, 25–32. [Google Scholar] [CrossRef]
- Jones, M.L.; Mark, P.J.; Waddell, B.J. Maternal dietary omega-3 fatty acids and placental function. Reproduction 2014, 147, R143–R152. [Google Scholar] [CrossRef] [PubMed]
- Keelan, J.A.; Mas, E.; D’Vaz, N.; Dunstan, J.A.; Li, S.; Barden, A.E.; Mark, P.J.; Waddell, B.J.; Prescott, S.L.; Mori, T.A. Effects of maternal n-3 fatty acid supplementation on placental cytokines, pro-resolving lipid mediators and their precursors. Reproduction 2015, 149, 171–178. [Google Scholar] [CrossRef]
- Ornoy, A. Prenatal origin of obesity and their complications: Gestational diabetes, maternal overweight and the paradoxical effects of fetal growth restriction and macrosomia. Reprod. Toxicol. 2011, 32, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Langie, S.A.S.; Kowalczyk, P.; Tudek, B.; Zabielski, R.; Dziaman, T.; Oliński, R.; van Schooten Frederik, J.; Godschalk, R.W.L. The effect of oxidative stress on nucleotide-excision repair in colon tissue of newborn piglets. Mutat. Res. 2010, 695, 75–80. [Google Scholar] [CrossRef]
- Zabielski, R.; Gajewski, Z.; Valverde Piedra, J.L.; Laubitz, D.; Wilczak, J.; Bałasinska, B.; Kulasek, G. The perinatal development of the gastrointestinal tract in piglets can be modified by supplementation of sow diet with bioactive substances. Livest. Sci. 2007, 109, 34–37. [Google Scholar] [CrossRef]
- Puzio, I.; Kapica, M.; Bienko, M.; Valverde Piedra, J.L.; Gajewski, Z.; Wilczak, J.; Kulasek, G.; Zabielski, R. Dietary bioactive substances influenced perinatal bone development in piglets. Livest. Sci. 2007, 108, 72–75. [Google Scholar] [CrossRef]
- Li, E.; Li, C.; Horn, N.; Ajuwon, K.M. PPARγ activation inhibits endocytosis of claudin-4 and protects against deoxynivalenol-induced intestinal barrier dysfunction in IPEC-J2 cells and weaned piglets. Toxicol. Lett. 2023, 375, 8–20. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Sobol, M.; Makulska, J.; Weglarz, A.; Kurylczyk, A.; Skiba, G. Administration of Natural Fish and Algal Oils in Nanoparticle Form to Pregnant Gilts and Newborn Piglets: Biochemical Effects and Spatial–Socio-Economic Implications for Regional Food Systems. Int. J. Mol. Sci. 2025, 26, 9158. [Google Scholar] [CrossRef]
- Tanghe, S.; Missotten, J.; Raes, K.; De Smet, S. The effect of different concentrations of linseed oil or fish oil in the maternal diet on the fatty acid composition and oxidative status of sows and piglets. J. Anim. Physiol. Anim. Nutr. 2015, 99, 938–949. [Google Scholar] [CrossRef]
- Delker, D.; Hatch, G.; Allen, J.; Crissman, B.; George, M.; Geter, D.; Kilburn, S.; Moore, T.; Nelson, G.; Roop, B.; et al. Molecular biomarkers of oxidative stress associated with bromate carcinogenicity. Toxicology 2006, 221, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Crispi, F.; Miranda, J.; Gratacós, E. Long-term cardiovascular consequences of fetal growth restriction: Biology, clinical implications, and opportunities for prevention of adult disease. Am. J. Obstet. Gynecol. 2018, 218, S869–S879. [Google Scholar] [CrossRef]
- Ghorbanzade, T.; Jafari, S.M.; Akhavan, S.; Hadavi, R. Nanoencapsulation of fish oil in nano-liposomes and its application in fortification of yogurt. Food Chem. 2017, 216, 146–152. [Google Scholar] [CrossRef]
- Zhu, Y.; Mierau, J.O.; Riphagen, I.J.; Heiner-Fokkema, M.R.; Dekker, L.H.; Navis, G.J.; Bakker, S.J.L. Types of fish consumption differ across socioeconomic strata and impact plasma omega-3 levels. Eur. J. Nutr. 2024, 63, 435–443. [Google Scholar] [CrossRef]
- Marco, A.; Kisliouk, T.; Tabachnik, T.; Meiri, N.; Weller, A. Overweight and CpG methylation of the Pomc promoter in offspring of high-fat-diet-fed dams are not. FASEB J. 2014, 28, 4148–4157. [Google Scholar] [CrossRef] [PubMed]
- Saffery, R.; Novakovic, B. Epigenetics as the mediator of fetal programming of adult onset disease: What is the evidence? Acta Obstet. Gynecol. Scand. 2014, 93, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.J.; Kim, Y.J. What is fetal programming?: A lifetime health is under the control of in utero health. Obstet. Gynecol. Sci. 2017, 60, 506–519. [Google Scholar] [CrossRef]
- Clonan, A.; Roberts, K.E.; Holdsworth, M. Socioeconomic and demographic drivers of red and processed meat consumption: Implications for health and environmental sustainability. Proc. Nutr. Soc. 2016, 75, 367–373. [Google Scholar] [CrossRef]
- Willits-Smith, A.; Brenna, J.T.; Moubarac, J.C.; Herforth, A. Demographic and Socioeconomic Correlates of Disproportionate Beef Consumption. Nutrients 2023, 15, 3795. [Google Scholar] [CrossRef] [PubMed]
- Ostro, M.J.; Cullis, P.R. Use of liposomes as injectable-drug delivery systems. Am. J. Health-Syst. Pharm. 1989, 46, 1576–1588. [Google Scholar] [CrossRef]
- WHO. Healthy Diet—Fact Sheet N°394; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/news-room/fact-sheets/detail/healthy-diet (accessed on 29 July 2025).
- Tavakoli, H.; Hosseini, O.; Jafari, S.M.; Katouzian, I. Evaluation of physicochemical and antioxidant properties of yogurt CRITICAL REVIEWS IN FOOD SCIENCE AND NUTRITION 21 enriched by olive leaf phenolics within nanoliposomes. J. Agric. Food Chem. 2018, 66, 9231–9240. [Google Scholar] [CrossRef]
- Landaeta-Díaz, L.; Salas, D.; Gutiérrez, V. Mapping Nutritional Inequality: A Primary Socio-Spatial Analysis of Food Deserts in Santiago de Chile. Urban Sci. 2024, 8, 129. [Google Scholar] [CrossRef]
- Tonnac, A.; de Mourot, J. Effect of dietary sources of n-3 fatty acids on pig performance and technological, nutritional and sensory qualities of pork. Animal 2018, 12, 1527–1535. [Google Scholar] [CrossRef]
- Afzal, S.; Manap, A.S.A.; Attiq, A.; Albokhadaim, I.; Kandeel, M.; Alhojaily, S.M. From imbalance to impairment: The central role of reactive oxygen species in oxidative stress-induced disorders and therapeutic exploration. Front. Pharmacol. Sec. Exp. Pharmacol. Drug Discov. 2023, 14, 1269581. [Google Scholar] [CrossRef] [PubMed]
- Andersson, L.; Haley, C.S.; Ellegren, H.; Knott, S.A.; Johansson, M.; Andersson, K.; Andersson-Eklund, L.; Edfors-Lilja, I.; Fredholm, M.; Hansson, I.; et al. Genetic mapping of quantitative trait loci for growth and fatness in pigs. Science 1994, 263, 1771–1774. [Google Scholar] [CrossRef] [PubMed]
- Koag, M.C.; Jung, H.; Lee, S. Mutagenesis mechanism of the major oxidative adenine lesion 7,8-dihydro-8-oxoadenine. Nucleic Acids Res. 2020, 48, 5119–5134. [Google Scholar] [CrossRef]
- Cai, F.; Sorg, O.; Granci, V.; Lecumberri, E.; Miralbell, R.; Dupertuis, Y.M.; Pichard, C. Interaction of ω-3 polyunsaturated fatty acids with radiation therapy in two different colorectal cancer cell lines. Clin. Nutr. 2014, 33, 164–170. [Google Scholar] [CrossRef]
- Feng, Z.; Hu, W.; Tang, M.S. Trans-4-hydroxy-2-nonenal inhibits nucleotide excision repair in human cells: A possible mechanism for lipid peroxidation induced carcinogenesis. Proc. Natl. Acad. Sci. USA 2004, 101, 8598–8602. [Google Scholar] [CrossRef]
- Feng, Z.; Hu, W.; Marnett, L.J.; Tang, M.S. Malondialdehyde, a major endogenous lipid peroxidation product, sensitizes human cells to UV- and BPDE-induced killing and mutagenesis through inhibition of nucleotide excision repair. Mutat. Res. 2006, 601, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Wypych, A.; Ożgo, M.; Bernaciak, M.; Herosimczyk, A.; Barszcz, M.; Gawin, K.; Ciechanowicz, A.K.; Kucia, M.; Pierzchała, M.; Poławska, E.; et al. Effect of feeding high fat diets differing in fatty acid composition on oxidative stress markers and protein expression profiles in mouse kidney. J. Anim. Feed Sci. 2024, 33, 170–184. [Google Scholar] [CrossRef]
- Hussein, N.; Ah-Sing, E.; Wilkinson, P.; Leach, C.; Griffin, B.A.; Millward, D.J. Long-chain conversion of linoleic acid and alpha-linolenic acid in response to marked changes in their dietary intake in men. J. Lipid Res. 2005, 46, 269–280. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Kothapalli, K.S.D.; Brenna, J.T. Desaturase and elongase limiting endogenous long chain polyunsaturated fatty acid biosynthesis. Curr. Opin. Clin. Nutr. Metab. Care 2016, 9, 103–110. [Google Scholar] [CrossRef]
- El Ghissassi, F.; Barbin, A.; Nair, J.; Bartsch, H. Formation of 1,N6-ethenoadenine and 3,N4-ethenocytosineby lipid peroxidation products and nucleic acid bases. Chem. Res. Toxicol. 1995, 8, 278–283. [Google Scholar] [CrossRef]
- da Silva, E.P., Jr.; Nachbar, R.T.; Levada-Pires, A.C.; Hirabara, S.M.; Lambertucci, R.H. Omega-3 fatty acids differentially modulate enzymatic anti-oxidant systems in skeletal muscle cells. Cell Stress Chaperones 2016, 21, 87–95. [Google Scholar] [CrossRef]
- Ferenc, K.; Marcinkowski, M.; Olszewski, J.; Kowalczyk, P.; Pilžys, T.; Garbicz, D.; Dib, N.; Świderska, B.; Matyba, P.; Gajewski, Z.; et al. The proteomic profile is altered but not repaired after bariatric surgery in type 2 diabetes pigs. Sci. Rep. 2024, 14, 10235. [Google Scholar] [CrossRef]
- Li, E.; Horn, N.; Ajuwon, K.M. EPA and DHA inhibit endocytosis of claudin-4 and protect against deoxynivalenol-induced intestinal barrier dysfunction through PPARγ dependent and independent pathways in jejunal IPEC-J2 cells. Food Res. Int. 2022, 157, 111420. [Google Scholar] [CrossRef]
- Richard, D.; Kefi, K.; Barbe, U.; Bausero, P.; Visioli, F. Polyunsaturated fatty acids as antioxidants. Pharmacol. Res. 2008, 57, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Chung, F.L. Formation of etheno adducts in reactions of enals autooxidation. Chem. Res. Toxicol. 1994, 7, 857–860. [Google Scholar] [CrossRef] [PubMed]
- McCowen, K.C.; Bistrian, B.R. Essential fatty acids and their derivatives. Curr. Opin. Gastroenterol. 2005, 21, 207–215. [Google Scholar] [CrossRef]
- Williams-Bey, Y.; Boularan, C.; Vural, A.; Huang, N.N.; Hwang, I.Y.; Shan-Shi, C.; Kehrl, J.H. Omega-3 free fatty acids suppress macrophage inflammasome activation by inhibiting NF-κB activation and enhancing autophagy. PLoS ONE 2014, 9, e97957. [Google Scholar] [CrossRef]
- Mas, E.; Woodman, R.J.; Burke, V.; Puddey, I.B.; Beilin, L.J.; Durand, T.; Mori, T.A. The omega-3 fatty acids EPA and DHA decrease plasma F(2)-isoprostanes: Results from two placebo-controlled interventions. Free Radic. Res. 2010, 44, 983–990. [Google Scholar] [CrossRef]
- Zhang, W. Nanoparticle Aggregation: Principles and Modeling. Adv. Exp. Med. Biol. 2014, 811, 19–43. [Google Scholar] [PubMed]
- Kulvietis, V.; Zalgeviciene, V.; Dzidziapetrience, J.; Rotomskis, R. Transport of nanoparticles through the placental barier. Tohoku J. Exp. Med. 2011, 225, 225–234. [Google Scholar] [CrossRef]
- Herd, H.; Daum, N.; Jones, A.T.; Huwer, H.; Ghandehari, H.; Leh, C.M. Nanoparticle geometry and surface orientation influences mode of cellular uptake. ACS Nano 2013, 7, 1961–1973. [Google Scholar] [CrossRef]
- Yudkina, A.V.; Bulgakov, N.A.; Kim, D.V.; Baranova, S.V.; Ishchenko, A.A.; Saparbaev, M.K.; Koval, V.V.; Zharkov, D.O. Abasic site–peptide cross-links are blocking lesions repaired by AP endonucleases. Nucleic Acids Res. 2023, 51, 6321–6336. [Google Scholar] [CrossRef] [PubMed]
- Bosona, T.; Gebresenbet, G. Cluster building and logistics network integration of local food supply chain. Biosyst. Eng. 2011, 108, 293–302. [Google Scholar] [CrossRef]
- Gollini, I.; Lu, B.; Charlton, M.; Brunsdon, C.; Harris, P. GWmodel: An R Package for Exploring Spatial Heterogeneity Using Geographically Weighted Models. arXiv 2013, arXiv:1306.0413. [Google Scholar] [CrossRef]
- Rogers, E.M. Diffusion of Innovations, 5th ed.; Free Press: New York, NY, USA, 2003. [Google Scholar]
- Simpson, D.A.C.; Feeney, S.; Boyle, C.; Stitt, A.W. Retinal VEGF mRNA measured by SYBR green I fluorescence: A versatile ap-proach to quantitative PCR. Mol. Vis. 2000, 6, 178–183. [Google Scholar]
- Luu-The, V.; Paquet, N.; Calvo, E.; Cumps, J. Improved real-time RT-PCR method for high-throughput measurements using second derivative calculation and double correction. Biotechniques 2005, 38, 287–293. [Google Scholar] [CrossRef]
- Kolendowski, B.; Hassan, H.; Krstic, M.; Isovic, M.; Thillainadesan, G.; Chambers, A.F.; Tuck, A.B.; Torchia, J. Genome-wide analysis reveals a role for TDG in estrogen receptor-mediated enhancer RNA tran-scription and 3-dimensional reorganization. Epigenet. Chromatin. 2018, 11, 5. [Google Scholar] [CrossRef]
- Han, P.; Du, Z.; Liu, X.; You, J.; Shi, X.E.; Sun, S.; Yang, G.; Li, X. Effects of maternal supplementation of fish oil during late gestation and lactation on growth performance, fecal microbiota structure and post-weaning diarrhoea of offspring piglets. Br. J. Nutr. 2023, 130, 966–977. [Google Scholar] [CrossRef]
- Llauradó-Calero, E.; García-Gudiño, J.; Hernández-García, F.I.; Izquierdo, M.; Torrallardona, D.; Esteve-García, E.; Tous, N. Effect of fish oil in Iberian sow diets on fatty acid, oxylipins and immune traits of colostrum and milk, and suckling piglets’ growth performance. Animal 2025, 19, 101430. [Google Scholar] [CrossRef]
- Roszkos, R.; Tóth, T.; Bazar, G.; Fébel, H.; Mézes, M. Effect of omega-3 polyunsaturated fatty acid supplementation on oxi dative stress parameters and sex hormone levels of modern genotype sows. Vet. Med. Sci. 2023, 9, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Dube, E. Nanoparticle-Enhanced Fish Feed: Benefits and Challenges. Fishes 2024, 9, 322. [Google Scholar] [CrossRef]
- Islas-Fabila, P.; Roldán-Santiago, P.; de la Cruz-Cruz, L.A.; Limón-Morales, O.; Dutro-Aceves, A.; Orozco-Gregorio, H.; Bonilla-Jaime, H. Importance of Selected Nutrients and Additives in the Feed of Pregnant Sows for the Survival of Newborn Piglets. Animals 2024, 14, 418. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, E.K.; Jensen, S.K.; Nguyen, D.N.; Xie, Z.; Bruun, T.S.; Strathe, A.V. Supplementing sow diets with docosahexaenoic acid alters fatty acid composition of sow blood and milk. Conference contribution in journal. Anim. Feed Sci. Technol. 2025, 324, 116334. [Google Scholar] [CrossRef]
- Ge, Z.; An, Y.; Lan, W.; Li, X. Effects of Dietary Supplementation of Omega-3 PUFA Enriched Fish Oil During Late-Pregnancy and Lactation on Reproductive Performance, Immune Activity and Fecal Microbiota Composition in Postpartum Sows. Vet. Sci. 2025, 12, 139. [Google Scholar] [CrossRef]
- Gelaye, Y. Application of nanotechnology in animal nutrition: Bibliographic review. Cogent Food Agric. 2024, 10, 2290308. [Google Scholar] [CrossRef]
- Fontana, B.L.; Henn, G.S.; Dos Santos, C.H.; Specht, L.; Schmitz, S.; Volken de Souza, C.F.; Lehn, D.N. Encapsulation of Zootechnical Additives for Poultry and Swine Feeding: A Systematic Review. ACS Omega 2025, 10, 6294–6305. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Rosa, A.; Almeida, A.; Martins, R.; Ribeiro, T.; Pintado, M.; Gonçalves, R.F.S.; Pinheiro, A.C.; Fonseca, A.J.M.; Maia, M.R.G.; et al. Omega-3 fatty acids from fish by-products: Innovative extraction and application in food and feed. Food Bioprod. Process. 2024, 145, 32–41. [Google Scholar] [CrossRef]
- Kazemi, M. Revolutionizing Veterinary Medicine: The Role of Nanoparticles in Advancing Animal Health, Nutrition and Disease Management. Vet. Med. Sci. Sep. 2025, 11, e70528. [Google Scholar] [CrossRef]
- Paslawski, R.; Kowalczyk, P.; Paslawska, U.; Wiśniewski, J.; Dzięgiel, P.; Janiszewski, A.; Kiczak, L.; Zacharski, M.; Gawdzik, B.; Kramkowski, K.; et al. Analysis of the Model of Atherosclerosis Formation in Pig Hearts as a Result of Impaired Activity of DNA Repair Enzymes. Int. J. Mol. Sci. 2024, 25, 2282. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion related to the Tolerable Upper Intake Level of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and docosapentaenoic acid (DPA). Eur. Food Saf. Auth. J. 2012, 10, 2815. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Guidance for Industry. Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers; Pharmacology and Toxicology; U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER): Rockville, MD, USA, 2005.
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyan te–phenol–chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Nolan, T.; Hands, R.E.; Bustin, A. Quantification of mRNA using real-time RT-PCR. Nat Protoc. 2006, 1, 1559–1582. [Google Scholar] [CrossRef]



| Supplementation | ||||||
|---|---|---|---|---|---|---|
| Item | Control | Nano-Algae | Nano-Fish | Algae Oil | Fish Oil | S.E. |
| Cleaved abasic sites [fmol ethenoadducts/μg protein/h] | ||||||
| εA | 1.205 b | 1.192 b | 0.637 a | 0.735 ab | 0.550 a | 0.126 |
| εC | 1.438 b | 1.045 ab | 0.812 a | 0.713 a | 0.675 a | 0.146 |
| 8oxoG | 1.352 b | 1.000 ab | 0.820 ab | 0.702 a | 0.642 a | 0.143 |
| Relative genes expression [A.U] | ||||||
| TDG | 1.297 b | 1.090 b | 0.473 a | 0.368 a | 0.527 a | 0.099 |
| MPG | 1.993 d | 1.142 c | 0.608 ab | 0.282 a | 0.563 ab | 0.176 |
| OGG1 | 2.050 b | 1.748 b | 0.840 a | 0.602 a | 0.747 a | 0.164 |
| 18S | 1.537 b | 1.297 b | 0.732 a | 0.528 a | 0.848 a | 0.106 |
| FPG treatment [percentage of genomic DNA recognised by FPG enzyme] | ||||||
| FPG | 0.960 b | 0.545 a | 0.542 a | 0.507 a | 0.532 a | 0.016 |
| Item | Day of Pregnancy | |
|---|---|---|
| Till 90 | 90–114 | |
| Nutritional value (per kg diet) | ||
| Crude protein, g | 145 | 175 |
| Lysine, g | 8.0 | 10.0 |
| Methionine | 3.0 | 3.0 |
| Methionine + Cystine | 6.0 | 7.0 |
| Threonine, g | 6.0 | 6.5 |
| Tryptophan, g | 2.0 | 2.0 |
| Crude fibre | 50.0 | 36.0 |
| Calcium, g | 8.0 | 9.0 |
| Total phosphorus | 6.0 | 6.5 |
| Digestible phosphorus, g | 2.0 | 3.0 |
| Vitamin A, IU | 13,000 | 12,500 |
| Vitamin E, mg | 100 | 100 |
| Vitamin D, IU | 2000 | 2000 |
| Metabolisable energy, MJ | 12.0 | 13.0 |
| Ingredient | Norsan Omega-3 Arktis (Per 10 mL) | Norsan Omega-3 Vegan (Per 10 mL) |
|---|---|---|
| Components | ||
| Sunflower oil | - | - |
| Fish oil | 8.8 g | - |
| Algae oil | - | 7.0 g |
| Olive oil | - | 2.2 g |
| Fatty acid composition | ||
| SFA | 1.8 g | - |
| MUFA | 3.9 g | - |
| PUFA | 2.3 g | 4.4 g |
| Omega-3 | 2.0 g | 4.0 g |
| EPA | 750 mg | 1218 mg |
| DPA | 95 mg | 314 mg |
| DHA | 975 mg | 2316 mg |
| Gene Specific Primer | Sequence (5′ to 3′) |
|---|---|
| MPG MpgF | GTCCTATCCGGCGACTTCC |
| MPG MpgR | CTTGTCTGGCAGGCCCTTTG C |
| Ogg1F | CTCAGAAATTCCAAGGTGTTC |
| Ogg1R | CCGCTCCACATGCCAGTG |
| 18SpfF | ATCCTTCGATGTCGGCTCTT |
| 18SpfR | ACTAACCTGTCTCAGACGGTC |
| TDGpeF | TAATGGGCAGTGATGACCC |
| TDGpeR | TGCAGCATTTAGCAGAGCTGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalczyk, P.; Sobol, M.; Makulska, J.; Węglarz, A.; Kurylczyk, A.; Schabikowski, M.; Skiba, G. Biochemical Effects of Natural and Nanoparticle Fish and Algal Oils in Gilt Pregnancy Diets on Base Excision Repair Enzymes in Newborn Piglets—Socioeconomic Implications for Regional Pig Farming—Preliminary Results. Int. J. Mol. Sci. 2025, 26, 10676. https://doi.org/10.3390/ijms262110676
Kowalczyk P, Sobol M, Makulska J, Węglarz A, Kurylczyk A, Schabikowski M, Skiba G. Biochemical Effects of Natural and Nanoparticle Fish and Algal Oils in Gilt Pregnancy Diets on Base Excision Repair Enzymes in Newborn Piglets—Socioeconomic Implications for Regional Pig Farming—Preliminary Results. International Journal of Molecular Sciences. 2025; 26(21):10676. https://doi.org/10.3390/ijms262110676
Chicago/Turabian StyleKowalczyk, Paweł, Monika Sobol, Joanna Makulska, Andrzej Węglarz, Apoloniusz Kurylczyk, Mateusz Schabikowski, and Grzegorz Skiba. 2025. "Biochemical Effects of Natural and Nanoparticle Fish and Algal Oils in Gilt Pregnancy Diets on Base Excision Repair Enzymes in Newborn Piglets—Socioeconomic Implications for Regional Pig Farming—Preliminary Results" International Journal of Molecular Sciences 26, no. 21: 10676. https://doi.org/10.3390/ijms262110676
APA StyleKowalczyk, P., Sobol, M., Makulska, J., Węglarz, A., Kurylczyk, A., Schabikowski, M., & Skiba, G. (2025). Biochemical Effects of Natural and Nanoparticle Fish and Algal Oils in Gilt Pregnancy Diets on Base Excision Repair Enzymes in Newborn Piglets—Socioeconomic Implications for Regional Pig Farming—Preliminary Results. International Journal of Molecular Sciences, 26(21), 10676. https://doi.org/10.3390/ijms262110676






