Assessment of AOPP, TBARS, and Inflammatory Status in Diabetic Nephropathy and Hemodialyzed Patients
Abstract
1. Introduction
- I.
- To compare AOPP and TBARS values between the two patient groups.
- II.
- To analyze correlations between AOPP, TBARS, and selected clinical and biochemical indices.
- III.
- To assess the potential role of these oxidative stress markers as indicators of disease severity and progression.
2. Results
2.1. Demographic and Clinical Characteristics
2.2. Levels of AOPP and TBARS and Inflammatory Status
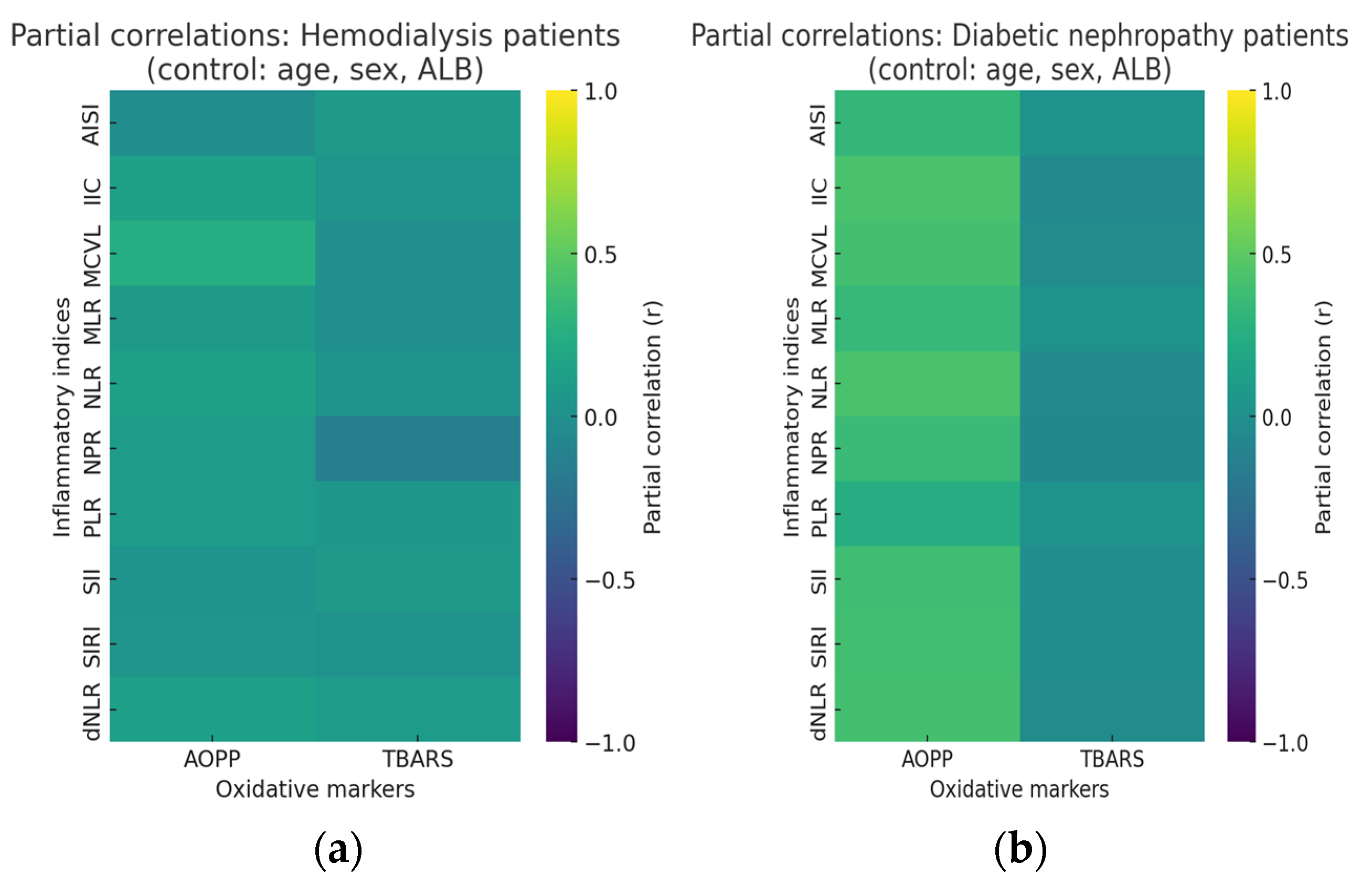
2.3. Partial Correlations of AOPP and TBARS and Inflammatory Status
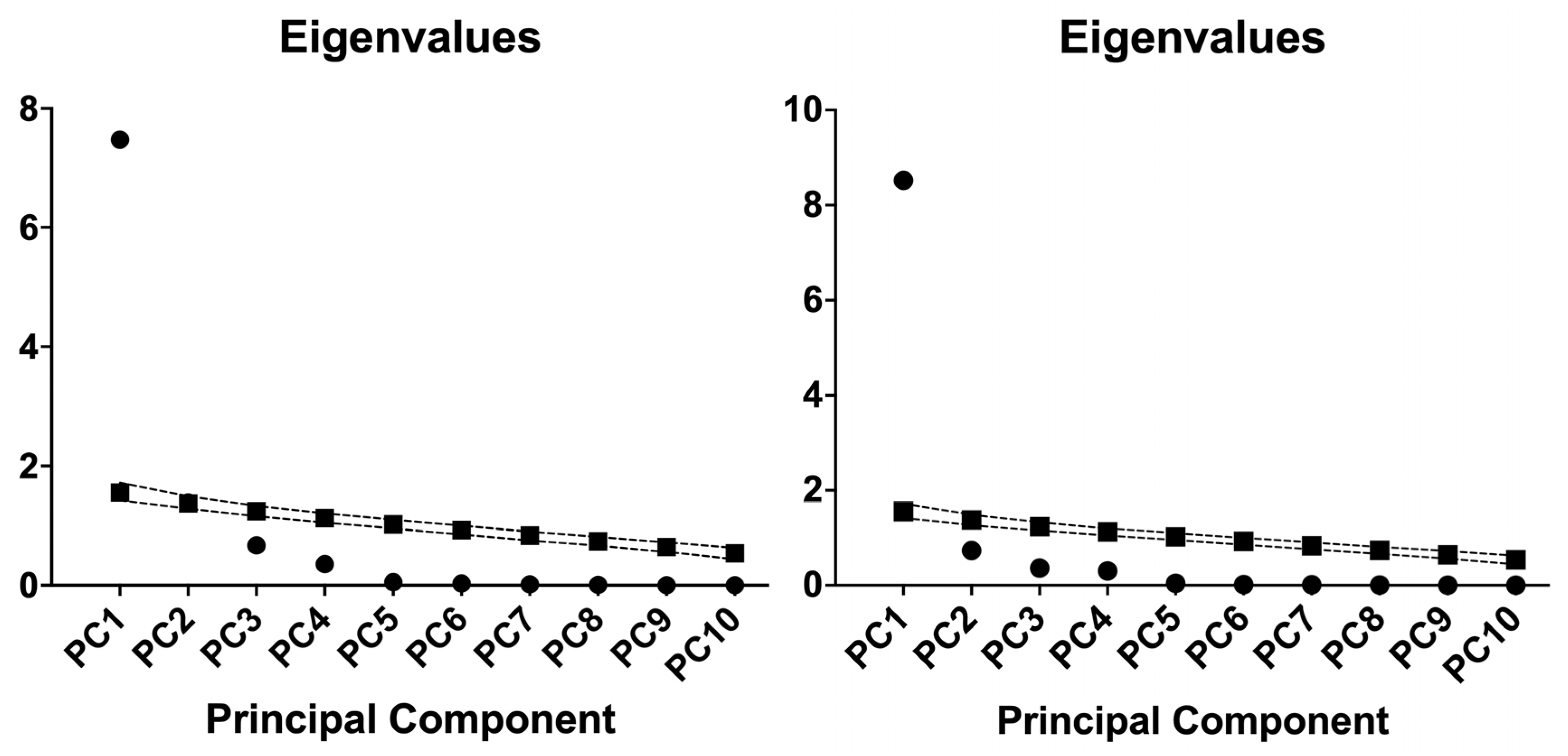
2.4. Principal Component Analysis of Inflammatory Indices in Hemodialysis and Diabetic Nephropathy Cohorts
2.5. Multiple Linear Regressions
2.5.1. Multiple Linear Regressions of HD Cohort
Multiple Linear Regressions of HD Cohort for AOPP
Multiple Linear Regressions of HD Cohort for TBARS
2.5.2. Multiple Linear Regressions of T2DM-DN Cohort
Multiple Linear Regressions of T2DM-DN Cohort for AOPP
Multiple Linear Regressions of T2DM-DN Cohort for TBARS
3. Discussion
3.1. Strengths and Limitations
3.2. Future Directions
4. Materials and Methods
4.1. Study Design and Setting
4.2. Ethical Considerations
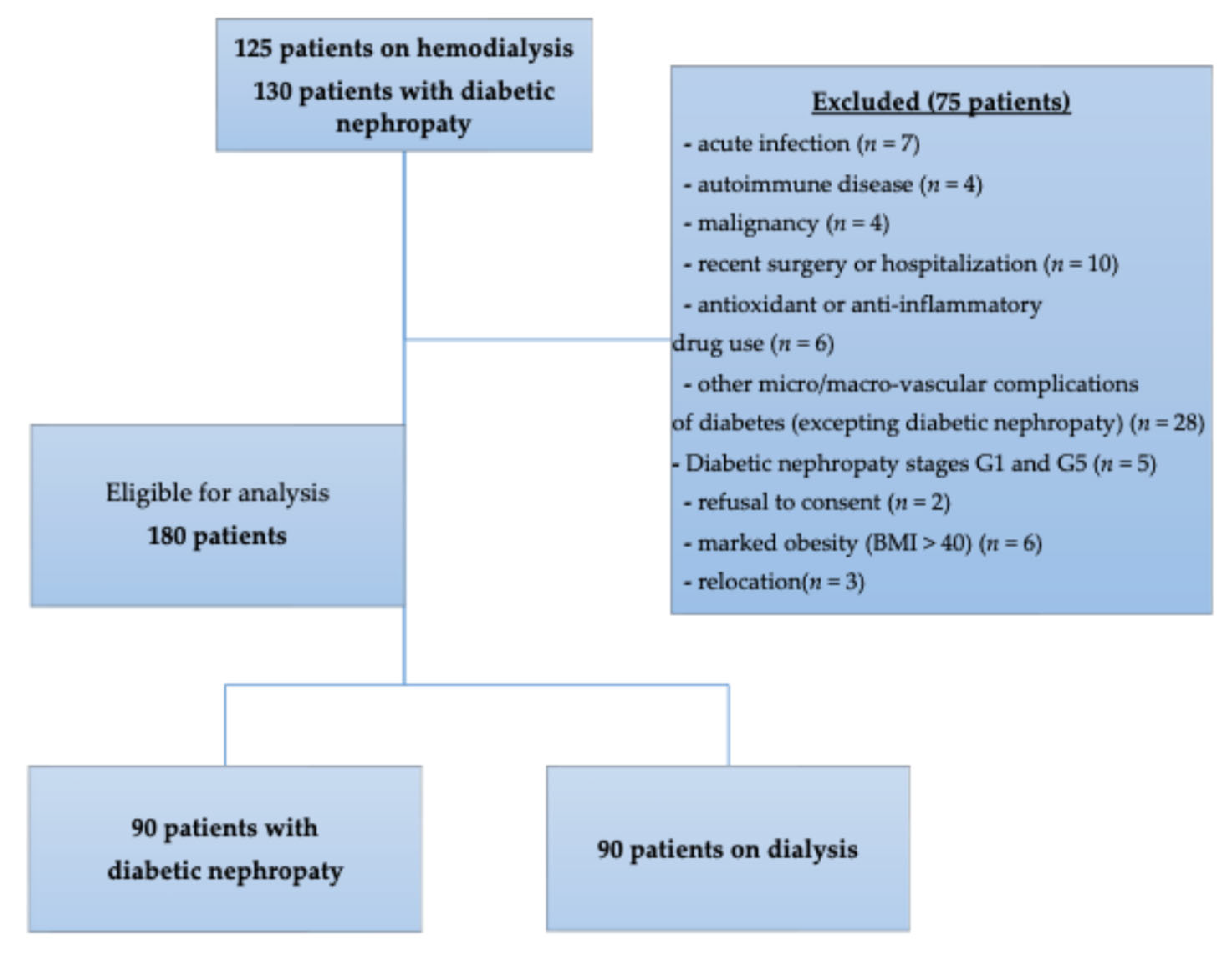
4.3. Participants
4.4. Evaluation of Inflammatory Status
4.5. Sampling Procedure
4.6. Laboratory Assays
4.7. Enzyme-Linked Immunosorbent Assay of AOPP and TBARS
4.8. Statistical Assessment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gyurászová, M.; Gurecká, R.; Bábíčková, J.; Tóthová, Ľ. Oxidative Stress in the Pathophysiology of Kidney Disease: Implications for Noninvasive Monitoring and Identification of Biomarkers. Oxid. Med. Cell. Longev. 2020, 2020, 5478708. [Google Scholar] [CrossRef]
- Jin, Q.; Liu, T.; Qiao, Y.; Liu, D.; Yang, L.; Mao, H.; Ma, F.; Wang, Y.; Peng, L.; Zhan, Y. Oxidative stress and inflammation in diabetic nephropathy: Role of polyphenols. Front. Immunol. 2023, 14, 1185317. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, C. Oxidative Stress: A Culprit in the Progression of Diabetic Kidney Disease. Antioxidants 2024, 13, 455. [Google Scholar] [CrossRef]
- Sagoo, M.K.; Gnudi, L. Diabetic nephropathy: Is there a role for oxidative stress? Free Radic. Biol. Med. 2018, 116, 50–63. [Google Scholar] [CrossRef]
- Joumaa, J.P.; Raffoul, A.; Sarkis, C.; Chatrieh, E.; Zaidan, S.; Attieh, P.; Harb, F.; Azar, S.; Ghadieh, H.E. Mechanisms, Biomarkers, and Treatment Approaches for Diabetic Kidney Disease: Current Insights and Future Perspectives. J. Clin. Med. 2025, 14, 727. [Google Scholar] [CrossRef] [PubMed]
- Liakopoulos, V.; Roumeliotis, S.; Gorny, X.; Dounousi, E.; Mertens, P.R. Oxidative Stress in Hemodialysis Patients: A Review of the Literature. Oxid. Med. Cell. Longev. 2017, 2017, 3081856. [Google Scholar] [CrossRef]
- Ling, X.C.; Kuo, K.-L. Oxidative stress in chronic kidney disease. Ren. Replace. Ther. 2018, 4, 53. [Google Scholar] [CrossRef]
- Arimura, N.; Watanabe, H.; Kato, H.; Imafuku, T.; Nakano, T.; Sueyoshi, M.; Chikamatsu, M.; Tokumaru, K.; Nagasaki, T.; Maeda, H.; et al. Advanced Oxidation Protein Products Contribute to Chronic-Kidney-Disease-Induced Adipose Inflammation through Macrophage Activation. Toxins 2023, 15, 179. [Google Scholar] [CrossRef]
- Vodošek Hojs, N.; Bevc, S.; Ekart, R.; Hojs, R. Oxidative Stress Markers in Chronic Kidney Disease with Emphasis on Diabetic Nephropathy. Antioxidants 2020, 9, 925. [Google Scholar] [CrossRef]
- Xue, K.; Wang, Y.; Wang, Y.; Fang, H. Advanced Oxidation Protein Product Promotes Oxidative Accentuation in Renal Epithelial Cells via the Soluble (Pro)renin Receptor-Mediated Intrarenal Renin-Angiotensin System and Nox4-H2O2 Signaling. Oxid. Med. Cell. Longev. 2021, 2021, 5710440. [Google Scholar] [CrossRef] [PubMed]
- Vinereanu, I.V.; Peride, I.; Niculae, A.; Tiron, A.T.; Caragheorgheopol, A.; Manda, D.; Checherita, I.A. The Relationship between Advanced Oxidation Protein Products, Vascular Calcifications and Arterial Stiffness in Predialysis Chronic Kidney Disease Patients. Medicina 2021, 57, 452. [Google Scholar] [CrossRef]
- Pasterk, L.; Lemesch, S.; Leber, B.; Trieb, M.; Curcic, S.; Stadlbauer, V.; Schuligoi, R.; Schicho, R.; Heinemann, A.; Marsche, G. Oxidized plasma albumin promotes platelet-endothelial crosstalk and endothelial tissue factor expression. Sci. Rep. 2016, 6, 22104. [Google Scholar] [CrossRef]
- Aguilar Diaz De Leon, J.; Borges, C.R. Evaluation of Oxidative Stress in Biological Samples Using the Thiobarbituric Acid Reactive Substances Assay. J. Vis. Exp. 2020, 159. [Google Scholar] [CrossRef]
- Trevisan, M.; Browne, R.; Ram, M.; Muti, P.; Freudenheim, J.; Carosella, A.M.; Armstrong, D. Correlates of Markers of Oxidative Status in the General Population. Am. J. Epidemiol. 2001, 154, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Ma, J.; Leng, T.; Yuan, Z.; Hu, T.; Liu, Q.; Shen, T. Advances in oxidative stress in pathogenesis of diabetic kidney disease and efficacy of TCM intervention. Ren. Fail. 2023, 45, 2146512. [Google Scholar] [CrossRef]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and Oxidative Stress in Chronic Kidney Disease-Potential Therapeutic Role of Minerals, Vitamins and Plant-Derived Metabolites. Int. J. Mol. Sci. 2019, 21, 263. [Google Scholar] [CrossRef] [PubMed]
- Tanase, D.M.; Gosav, E.M.; Anton, M.I.; Floria, M.; Seritean Isac, P.N.; Hurjui, L.L.; Tarniceriu, C.C.; Costea, C.F.; Ciocoiu, M.; Rezus, C. Oxidative Stress and NRF2/KEAP1/ARE Pathway in Diabetic Kidney Disease (DKD): New Perspectives. Biomolecules 2022, 12, 1227. [Google Scholar] [CrossRef]
- Darenskaya, M.; Kolesnikov, S.; Semenova, N.; Kolesnikova, L. Diabetic Nephropathy: Significance of Determining Oxidative Stress and Opportunities for Antioxidant Therapies. Int. J. Mol. Sci. 2023, 24, 12378. [Google Scholar] [CrossRef]
- Winiarska, A.; Knysak, M.; Nabrdalik, K.; Gumprecht, J.; Stompór, T. Inflammation and Oxidative Stress in Diabetic Kidney Disease: The Targets for SGLT2 Inhibitors and GLP-1 Receptor Agonists. Int. J. Mol. Sci. 2021, 22, 10822. [Google Scholar] [CrossRef]
- Conti, G.; Caccamo, D.; Siligato, R.; Gembillo, G.; Satta, E.; Pazzano, D.; Carucci, N.; Carella, A.; Del Campo, G.; Salvo, A.; et al. Association of Higher Advanced Oxidation Protein Products (AOPPs) Levels in Patients with Diabetic and Hypertensive Nephropathy. Medicina 2019, 55, 675. [Google Scholar] [CrossRef]
- Ito, F.; Sono, Y.; Ito, T. Measurement and Clinical Significance of Lipid Peroxidation as a Biomarker of Oxidative Stress: Oxidative Stress in Diabetes, Atherosclerosis, and Chronic Inflammation. Antioxidants 2019, 8, 72. [Google Scholar] [CrossRef]
- Sung, C.-C.; Hsu, Y.-C.; Chen, C.-C.; Lin, Y.-F.; Wu, C.-C. Oxidative Stress and Nucleic Acid Oxidation in Patients with Chronic Kidney Disease. Oxidative Med. Cell. Longev. 2013, 2013, 301982. [Google Scholar] [CrossRef]
- Varan, H.I.; Dursun, B.; Dursun, E.; Ozben, T.; Suleymanlar, G. Acute effects of hemodialysis on oxidative stress parameters in chronic uremic patients: Comparison of two dialysis membranes. Int. J. Nephrol. Renov. Dis. 2010, 3, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Dounousi, E.; Papavasiliou, E.; Makedou, A.; Ioannou, K.; Katopodis, K.P.; Tselepis, A.; Siamopoulos, K.C.; Tsakiris, D. Oxidative Stress Is Progressively Enhanced with Advancing Stages of CKD. Am. J. Kidney Dis. 2006, 48, 752–760. [Google Scholar] [CrossRef]
- Podkowińska, A.; Formanowicz, D. Chronic Kidney Disease as Oxidative Stress- and Inflammatory-Mediated Cardiovascular Disease. Antioxidants 2020, 9, 752. [Google Scholar] [CrossRef] [PubMed]
- Mancinelli, M.; Moscucci, F.; Cofini, V.; De Nino, A.L.; Bocale, R.; Savoia, C.; Baratta, F.; Desideri, G. Metabolic Determinants of Systemic Inflammation Dynamics During Hemodialysis: Insights from the Systemic Immune–Inflammation Index in a Single-Center Observational Study. Metabolites 2025, 15, 651. [Google Scholar] [CrossRef]
- Burlacu, A.; Namolovan, C.A.; Brinza, C.; Covic, A.; Floria, M.; Voroneanu, L.; Covic, A. Neutrophil-to-Lymphocyte Ratio (NLR)—Independent Prognostic Marker of Renal Function Decline in Chronic Kidney Disease: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 6822. [Google Scholar] [CrossRef]
- Mureșan, A.V.; Russu, E.; Arbănași, E.M.; Kaller, R.; Hosu, I.; Arbănași, E.M.; Voidăzan, S.T. The Predictive Value of NLR, MLR, and PLR in the Outcome of End-Stage Kidney Disease Patients. Biomedicines 2022, 10, 1272. [Google Scholar] [CrossRef]
- Kawalec, A.; Stojanowski, J.; Mazurkiewicz, P.; Choma, A.; Gaik, M.; Pluta, M.; Szymański, M.; Bruciak, A.; Gołębiowski, T.; Musiał, K. Systemic Immune Inflammation Index as a Key Predictor of Dialysis in Pediatric Chronic Kidney Disease with the Use of Random Forest Classifier. J. Clin. Med. 2023, 12, 6911. [Google Scholar] [CrossRef] [PubMed]
- Dopierała, M.; Nitz, N.; Król, O.; Wasicka-Przewoźna, K.; Schwermer, K.; Pawlaczyk, K. New and Emerging Biomarkers in Chronic Kidney Disease. Biomedicines 2025, 13, 1423. [Google Scholar] [CrossRef]
- Rysz, J.; Franczyk, B.; Ławiński, J.; Gluba-Brzózka, A. Oxidative Stress in ESRD Patients on Dialysis and the Risk of Cardiovascular Diseases. Antioxidants 2020, 9, 1079. [Google Scholar] [CrossRef]
- Villalpando-Sánchez, D.C.; Barajas-Medina, C.A.; Alvarez-Aguilar, C.; López-Ortiz, G.; Romero-Henríquez, L.F.; Gómez-García, A. Advanced Oxidative Protein Products Had a Diagnostic Accuracy for Identifying Chronic Kidney Disease in Adult Population. Metabolites 2024, 14, 37. [Google Scholar] [CrossRef]
- Deus, L.A.; Corrêa, H.d.L.; Neves, R.V.P.; Reis, A.L.; Honorato, F.S.; Silva, V.L.; Souza, M.K.; de Araújo, T.B.; de Gusmão Alves, L.S.; Sousa, C.V.; et al. Are Resistance Training-Induced BDNF in Hemodialysis Patients Associated with Depressive Symptoms, Quality of Life, Antioxidant Capacity, and Muscle Strength? An Insight for the Muscle–Brain–Renal Axis. Int. J. Environ. Res. Public Health 2021, 18, 11299. [Google Scholar] [CrossRef]
- Kozlowska, L.; Jagiello, K.; Ciura, K.; Sosnowska, A.; Zwiech, R.; Zbrog, Z.; Wasowicz, W.; Gromadzinska, J. The Effects of Two Kinds of Dietary Interventions on Serum Metabolic Profiles in Haemodialysis Patients. Biomolecules 2023, 13, 854. [Google Scholar] [CrossRef] [PubMed]
- Santos, I.S.R.; Martin-Pastor, M.; Tavares Júnior, A.G.; Queiroz, K.A.; da Silva Sólon, L.G.; Sousa, F.F.O.d. Metabolomic Profile and Its Correlation with the Plasmatic Levels of Losartan, EXP3174 and Blood Pressure Control in Hypertensive and Chronic Kidney Disease Patients. Int. J. Mol. Sci. 2023, 24, 9832. [Google Scholar] [CrossRef]
- Chaghouri, P.; Maalouf, N.; Peters, S.L.; Nowak, P.J.; Peczek, K.; Zasowska-Nowak, A.; Nowicki, M. Two Faces of Vitamin C in Hemodialysis Patients: Relation to Oxidative Stress and Inflammation. Nutrients 2021, 13, 791. [Google Scholar] [CrossRef] [PubMed]
- Navarro-García, J.A.; Rodríguez-Sánchez, E.; Aceves-Ripoll, J.; Abarca-Zabalía, J.; Susmozas-Sánchez, A.; González Lafuente, L.; Bada-Bosch, T.; Hernández, E.; Mérida-Herrero, E.; Praga, M.; et al. Oxidative Status before and after Renal Replacement Therapy: Differences Between Conventional High Flux Hemodialysis and on-Line Hemodiafiltration. Nutrients 2019, 11, 2809. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Ohtake, T. The Characteristics of Dialysis Membranes: Benefits of the AN69 Membrane in Hemodialysis Patients. J. Clin. Med. 2023, 12, 1123. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2024. Diabetes Care 2023, 47, S20–S42. [Google Scholar] [CrossRef]
- Stevens, P.E.; Ahmed, S.B.; Carrero, J.J.; Foster, B.; Francis, A.; Hall, R.K.; Herrington, W.G.; Hill, G.; Inker, L.A.; Kazancıoğlu, R.; et al. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- Xie, H.; Ruan, G.; Wei, L.; Deng, L.; Zhang, Q.; Ge, Y.; Song, M.; Zhang, X.; Lin, S.; Liu, X.; et al. The inflammatory burden index is a superior systemic inflammation biomarker for the prognosis of non-small cell lung cancer. J. Cachexia Sarcopenia Muscle 2023, 14, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Șerban, R.E.; Popescu, D.M.; Boldeanu, M.V.; Florescu, D.N.; Șerbănescu, M.S.; Șandru, V.; Panaitescu-Damian, A.; Forțofoiu, D.; Șerban, R.C.; Gherghina, F.L.; et al. The Diagnostic and Prognostic Role of Inflammatory Markers, Including the New Cumulative Inflammatory Index (IIC) and Mean Corpuscular Volume/Lymphocyte (MCVL), in Colorectal Adenocarcinoma. Cancers 2025, 17, 990. [Google Scholar] [CrossRef] [PubMed]
- Poenariu, I.S.; Boldeanu, L.; Ungureanu, B.S.; Caragea, D.C.; Cristea, O.M.; Pădureanu, V.; Siloși, I.; Ungureanu, A.M.; Statie, R.-C.; Ciobanu, A.E.; et al. Interrelation of Hypoxia-Inducible Factor-1 Alpha (HIF-1 α) and the Ratio between the Mean Corpuscular Volume/Lymphocytes (MCVL) and the Cumulative Inflammatory Index (IIC) in Ulcerative Colitis. Biomedicines 2023, 11, 3137. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Bai, Z.; Wu, Q.; Chu, G.; Zhang, Y.; Guo, X.; Qi, X. Inflammatory Indexes for Assessing the Severity and Disease Progression of Ulcerative Colitis: A Single-Center Retrospective Study. Front. Public Health 2022, 10, 851295. [Google Scholar] [CrossRef]
- CKD-EPI Equations for Glomerular Filtration Rate (GFR). Available online: https://www.mdcalc.com/calc/3939/ckd-epi-equations-glomerular-filtration-rate-gfr#evidence (accessed on 25 September 2025).
- AOPP Elisa Kit :: Human Advanced Oxidation Protein Products ELISA Kit. Available online: https://www.mybiosource.com/aopp-human-elisa-kits/advanced-oxidation-protein-products/722252 (accessed on 25 September 2025).
- TBARS Elisa Kit :: Human Thiobarbituric Acid Reactive Substance (TBARS) ELISA Kit. Available online: https://www.mybiosource.com/human-elisa-kits/thiobarbituric-acid-reactive-substance-tbars/166987 (accessed on 25 September 2025).
| Parameter | HD 90 Patients | T2DM-DN 90 Patients | p-Value |
|---|---|---|---|
| Age (years) [median (range)] | 61 (23–89) | 67 (40–83) | 0.03 * |
| Sex (n) Male/Female | 43/47 | 48/42 | 0.454 |
| Residence (n) Urban/Rural | 24/66 | 60/30 | <0.0001 * |
| Weight (kg) [median (range)] | 74.25 (39–103) | 84.50 (45–137) | <0.0001 * |
| Height (m) [median (range)] | 1.66 (1.19–1.98) | 1.68 (1.47–1.89) | 0.281 |
| BMI (kg/m2) [median (range)] | 26.40 (17.33–35.43) | 31.11 (20.82–45.55) | <0.0001 * |
| TG (mg/dL) [median (range)] | 133 (49–401) | 145 (57–397) | 0.668 |
| TC (mg/dL) [median (range)] | 171.50 (92–291) | 162 (84.00–364) | 0.777 |
| WBC (×103/μL) [median (range)] | 6.77 (2.72–10.30) | 8.42 (4.90–25.36) | <0.0001 * |
| NEU (×103/μL) [median (range)] | 4.20 (1.12–7.20) | 5.23 (2.68–23.67) | <0.0001 * |
| LYM (×103/μL) [median (range)] | 1.59 (0.20–3.22) | 1.92 (0.28–6.05) | 0.0005 * |
| BAS (×103/μL) [median (range)] | 0.05 (0.00–0.17) | 0.04 (0.01–0.40) | 0.0006 * |
| EOS (×103/μL) [median (range)] | 0.20 (0.00–0.82) | 0.16 (0.01–0.76) | 0.012 * |
| MON (×103/μL) [median (range)] | 0.60 (0.31–1.77) | 0.58 (0.20–1.19) | 0.211 |
| RBC (×103/μL) (Mean ± SD) | 3.60 ± 0.54 | 4.21 ± 0.67 | <0.0001 * |
| HCT (%) (Mean ± SD) | 34.21 ± 4.57 | 37.42 ± 6.71 | 0.0003 * |
| HGB (g/dL) [median (range)] | 10.90 (7.90–14.20) | 12.65 (8.10–17.30) | <0.0001 * |
| MCV (fL) [median (range)] | 96.60 (64.60–125.00) | 89.35 (69.00–106.30) | <0.0001 * |
| PLT (×103/μL) [median (range)] | 192.00 (57.20–438.00) | 240.50 (116.00–573.00) | <0.0001 * |
| Creatinine (mg/dL) [median (range)] | 8.81 (3.25–16.00) | 1.09 (0.59–2.83) | <0.0001 * |
| Urea (mg/dL) [median (range)] | 135.90 (36.38–387.30) | 52.00 (24.00–159.00) | <0.0001 * |
| eGFR (mL/min/1.73 m2) CKD-EPI [median (range)] | 5.51 (2.14–20.81) | 57.39 (16.55–89.96) | <0.0001 * |
| Na (Mean ± SD) | 138.6 ± 2.90 | 137.90 ± 4.04 | 0.198 |
| K [median (range)] | 4.80 (2.50–7.80) | 4.55 (3.10–7) | 0.006 * |
| Cl [median (range)] | 105.50 (99.00–122.00) | 102 (90.00–110) | <0.0001 * |
| ALT (mg/dL) [median (range)] | 16 (6.00–150) | 22 (7.00–136) | 0.001 * |
| AST (mg/dL) [median (range)] | 16 (4.00–186) | 21.39 (9.85–65.43) | <0.0001 * |
| Hemodialysis Vintage (months) | 48 ± 22 | - | - |
| Membrane type (Synthetic high-flux/Synthetic low-flux) (n/%) | - | 77/13 85.56%/14.44% | - |
| Years of diagnosis (years) | - | 12.06 ± 8.23 | - |
| HbA1c (%) | - | 9.57 ± 2.19 | - |
| Parameter | HD | T2DM-DN | p-Value | Parameter | HD | T2DM-DN | p-Value |
|---|---|---|---|---|---|---|---|
| CRP (mg/dL) [median (range)] | 4.50 (0.30–49.40) | 1.08 (0.02–30.42) | <0.0001 * | AISI [median (range)] | 317.30 (24.98–6087) | 355.80 (69.36–4707) | 0.09 |
| ALB (g/dL) [median (range)] | 4.10 (3.20–4.80) | 4.40 (3.50–5.30) | <0.0001 * | SIRI [median (range)] | 1.67 (0.30–21.97) | 1.43 (0.32–39.47) | 0.339 |
| IIC [median (range)] | 3.72 (1.44–34.66) | 3.00 (1.19–59.68) | 0.02 * | SII [median (range)] | 505.20 (77.83–6745) | 603.30 (192.70–6272) | 0.002 * |
| NPR [median (range)] | 22.25 (9.59–72.99) | 21.75 (8.67–130.50) | 0.775 | NLR [median (range)] | 2.57 (0.95–24.35) | 2.56 (1.00–54.07) | 0.06 |
| MCVL [median (range)] | 61.42 (23.99–434) | 46.28 (13.60–315.40) | <0.0001 * | MLR [median (range)] | 0.39 (0.11–4.51) | 0.28 (0.10–2.61) | <0.0001 * |
| AOPP [median (range)] | 25.80 (2.48–50) | 5.06 (2.29–25.79) | <0.0001 * | PLR [median (range)] | 128.40 (45.32–1385) | 124.70 (44.96–521.90) | 0.485 |
| TBARS [median (range)] | 8.49 (0.53–29.94) | 1.89 (0.31–15.78) | <0.0001 * | dNLR [median (range)] | 1.66 (0.70–4.25) | 1.81 (0.86–14.56) | 0.03 * |
| Parameter | Number | r-Value | p-Value | q-Value | Parameter | Number | r-Value | p-Value | q-Value | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HD | |||||||||||
| AOPP | AISI | 90 | −0.018 | 0.864 | 0.911 | TBARS | AISI | 90 | 0.083 | 0.439 | 0.798 |
| IIC | 90 | 0.148 | 0.167 | 0.798 | IIC | 90 | 0.041 | 0.704 | 0.911 | ||
| SII | 90 | 0.036 | 0.741 | 0.911 | SII | 90 | 0.085 | 0.431 | 0.798 | ||
| SIRI | 90 | 0.043 | 0.691 | 0.911 | SIRI | 90 | 0.019 | 0.863 | 0.911 | ||
| MCVL | 90 | 0.238 | 0.024 * | 0.489 | MCVL | 90 | −0.015 | 0.886 | 0.911 | ||
| NLR | 90 | 0.147 | 0.170 | 0.798 | NLR | 90 | 0.019 | 0.862 | 0.911 | ||
| MLR | 90 | 0.085 | 0.428 | 0.798 | MLR | 90 | −0.012 | 0.912 | 0.911 | ||
| PLR | 90 | 0.089 | 0.407 | 0.798 | PLR | 90 | 0.067 | 0.530 | 0.882 | ||
| dNLR | 90 | 0.131 | 0.219 | 0.798 | dNLR | 90 | 0.092 | 0.389 | 0.798 | ||
| NPR | 90 | 0.086 | 0.423 | 0.798 | NPR | 90 | −0.141 | 0.187 | 0.798 | ||
| T2DM-DN | |||||||||||
| AOPP | AISI | 90 | 0.325 | 0.001 * | 0.004 | TBARS | AISI | 90 | 0.018 | 0.864 | 0.882 |
| IIC | 90 | 0.428 | <0.0001 * | 0.0002 | IIC | 90 | −0.060 | 0.574 | 0.882 | ||
| SII | 90 | 0.384 | 0.0002 * | 0.0006 | SII | 90 | −0.021 | 0.847 | 0.882 | ||
| SIRI | 90 | 0.391 | 0.0001 * | 0.0005 | SIRI | 90 | −0.028 | 0.797 | 0.882 | ||
| MCVL | 90 | 0.401 | 0.0001 * | 0.0004 | MCVL | 90 | −0.033 | 0.760 | 0.882 | ||
| NLR | 90 | 0.429 | <0.0001 * | 0.0002 | NLR | 90 | −0.055 | 0.607 | 0.882 | ||
| MLR | 90 | 0.342 | 0.001 * | 0.002 | MLR | 90 | 0.016 | 0.882 | 0.882 | ||
| PLR | 90 | 0.248 | 0.018 * | 0.03 | PLR | 90 | 0.018 | 0.868 | 0.882 | ||
| dNLR | 90 | 0.404 | 0.0001 * | 0.0004 | dNLR | 90 | −0.039 | 0.719 | 0.882 | ||
| NPR | 90 | 0.353 | 0.0007 * | 0.001 | NPR | 90 | −0.075 | 0.482 | 0.876 | ||
| Component | Eigenvalue | Variance Explained (%) | Cumulative Proportion of Variance (%) | Component | Eigenvalue | Variance Explained (%) | Cumulative Proportion of Variance (%) |
|---|---|---|---|---|---|---|---|
| HD | T2DM-DN | ||||||
| PC1 | 7.473 | 74.73% | 74.73% | PC1 | 8.52 | 85.20% | 85.20% |
| PC2 | 1.386 | 13.86% | 88.60% | PC2 | 0.7327 | 7.33% | 92.52% |
| PC3 | 0.6726 | 6.73% | 95.32% | PC3 | 0.3636 | 3.64% | 96.16% |
| PC4 | 0.3574 | 3.57% | 98.90% | PC4 | 0.3053 | 3.05% | 99.21% |
| PC5 | 0.053 | 0.53% | 99.43% | PC5 | 0.0428 | 0.43% | 99.64% |
| PC6 | 0.0297 | 0.30% | 99.72% | PC6 | 0.0161 | 0.16% | 99.80% |
| PC7 | 0.0169 | 0.17% | 99.89% | PC7 | 0.0126 | 0.13% | 99.93% |
| PC8 | 0.0076 | 0.08% | 99.97% | PC8 | 0.0056 | 0.06% | 99.98% |
| PC9 | 0.0019 | 0.02% | 99.99% | PC9 | 0.0011 | 0.01% | 99.99% |
| PC10 | 0.0012 | 0.01% | 100.00% | PC10 | 0.0007 | 0.01% | 100.00% |
| Variable | AISI | IIC | SII | SIRI | MCVL | NLR | MLR | PLR | dNLR | NPR |
|---|---|---|---|---|---|---|---|---|---|---|
| PC1 loading-HD | −0.9415 | −0.9728 | −0.9769 | −0.9674 | −0.8821 | −0.9819 | −0.9558 | −0.9731 | −0.3762 | 0.0798 |
| PC1 loading-T2DM-DN | −0.9149 | −0.9791 | −0.9647 | −0.9653 | −0.9095 | −0.9783 | −0.924 | −0.7565 | −0.9409 | −0.8751 |
| Analysis of Variance | SS | DF | MS | F (DFn, DFd) | p Value | |
|---|---|---|---|---|---|---|
| Model 1 | Regression | 1743 | 5 | 348.7 | F (5, 84) = 1.298 | 0.2728 |
| Sex-HD | 383.4 | 1 | 383.4 | F (1, 84) = 1.426 | 0.2357 | |
| HD vintage | 248.3 | 1 | 248.3 | F (1, 84) = 0.9240 | 0.3392 | |
| Membrane-HD | 602.8 | 1 | 602.8 | F (1, 84) = 2.243 | 0.1380 | |
| Age-HD | 555.3 | 1 | 555.3 | F (1, 84) = 2.066 | 0.1543 | |
| BMI-HD | 2.34 | 1 | 2.34 | F (1, 84) = 0.008706 | 0.9259 | |
| Model 2 | Regression | 1958 | 5 | 391.7 | F (5, 84) = 1.472 | 0.2078 |
| Creatinine-HD | 1366 | 1 | 1366 | F (1, 84) = 5.131 | 0.0261 | |
| ALB-HD | 114.1 | 1 | 114.1 | F (1, 84) = 0.4286 | 0.5145 | |
| PC-inflammation | 282.3 | 1 | 282.3 | F (1, 84) = 1.061 | 0.3060 | |
| CRP-HD | 7.803 | 1 | 7.803 | F (1, 84) = 0.02932 | 0.8645 | |
| eGFR-HD | 816.1 | 1 | 816.1 | F (1, 84) = 3.066 | 0.0836 |
| Variable | Estimate | 95% CI (Profile Likelihood) | |t| | p Value | ||
|---|---|---|---|---|---|---|
| Model 1 | β0 | Intercept | 40.55 | 18.11 to 62.98 | 3.594 | 0.0005 |
| β1 | Sex-HD | 4.256 | −2.830 to 11.34 | 1.194 | 0.2357 | |
| β2 | HD vintage | 0.2286 | −0.2443 to 0.7014 | 0.9613 | 0.3392 | |
| β3 | Membrane-HD | −6.305 | −14.68 to 2.066 | 1.498 | 0.138 | |
| β4 | Age-HD | −0.3607 | −0.8598 to 0.1383 | 1.438 | 0.1543 | |
| β5 | BMI-HD | 0.03156 | −0.6411 to 0.7042 | 0.09331 | 0.9259 | |
| Model 2 | β0 | Intercept | 10.15 | −40.08 to 60.38 | 0.4017 | 0.6889 |
| β1 | Creatinine-HD | 2.467 | 0.3011 to 4.632 | 2.265 | 0.0261 | |
| β2 | ALB-HD | −3.055 | −12.34 to 6.226 | 0.6547 | 0.5145 | |
| β3 | PC-inflammation | 0.7471 | −0.6956 to 2.190 | 1.03 | 0.306 | |
| β4 | CRP-HD | 0.03098 | −0.3288 to 0.3908 | 0.1712 | 0.8645 | |
| β5 | eGFR-HD | 1.708 | −0.2318 to 3.648 | 1.751 | 0.0836 |
| Analysis of Variance | SS | DF | MS | F (DFn, DFd) | p Value | |
|---|---|---|---|---|---|---|
| Model 1 | Regression | 361.8 | 5 | 72.37 | F (5, 84) = 1.587 | 0.1727 |
| Sex-HD | 25.99 | 1 | 25.99 | F (1, 84) = 0.5699 | 0.4524 | |
| HD vintage | 252.6 | 1 | 252.6 | F (1, 84) = 5.538 | 0.0209 | |
| Membrane-HD | 0.09155 | 1 | 0.09155 | F (1, 84) = 0.002007 | 0.9644 | |
| Age-HD | 118.8 | 1 | 118.8 | F (1, 84) = 2.604 | 0.1103 | |
| BMI-HD | 1.297 | 1 | 1.297 | F (1, 84) = 0.02844 | 0.8665 | |
| Model 2 | Regression | 237 | 5 | 47.41 | F (5, 84) = 1.007 | 0.4190 |
| Creatinine-HD | 0.06341 | 1 | 0.06341 | F (1, 84) = 0.001346 | 0.9708 | |
| ALB-HD | 106.2 | 1 | 106.2 | F (1, 84) = 2.256 | 0.1369 | |
| PC-inflammation | 5.306 | 1 | 5.306 | F (1, 84) = 0.1127 | 0.7380 | |
| CRP-HD | 75.69 | 1 | 75.69 | F (1, 84) = 1.607 | 0.2084 | |
| eGFR-HD | 21.49 | 1 | 21.49 | F (1, 84) = 0.4564 | 0.5012 |
| Variable | Estimate | 95% CI (Profile Likelihood) | |t| | p Value | ||
|---|---|---|---|---|---|---|
| Model 1 | β0 | Intercept | 10.87 | 1.627 to 20.11 | 2.339 | 0.0217 |
| β1 | Sex-HD | 1.108 | −1.811 to 4.028 | 0.7549 | 0.4524 | |
| β2 | HD vintage | −0.2305 | −0.4253 to −0.03572 | 2.353 | 0.0209 | |
| β3 | Membrane-HD | 0.0777 | −3.371 to 3.526 | 0.0448 | 0.9644 | |
| β4 | Age-HD | 0.1668 | −0.03876 to 0.3724 | 1.614 | 0.1103 | |
| β5 | BMI-HD | 0.0235 | −0.2536 to 0.3006 | 0.1686 | 0.8665 | |
| Model 2 | β0 | Intercept | −2.857 | −23.99 to 18.27 | 0.2689 | 0.7887 |
| β1 | Creatinine-HD | 0.01681 | −0.8940 to 0.9276 | 0.03669 | 0.9708 | |
| β2 | ALB-HD | 2.948 | −0.9556 to 6.852 | 1.502 | 0.1369 | |
| β3 | PC-inflammation | 0.1024 | −0.5044 to 0.7093 | 0.3357 | 0.738 | |
| β4 | CRP-HD | −0.0965 | −0.2478 to 0.05486 | 1.268 | 0.2084 | |
| β5 | eGFR-HD | 0.2772 | −0.5388 to 1.093 | 0.6756 | 0.5012 |
| Analysis of Variance | SS | DF | MS | F (DFn, DFd) | p Value | |
|---|---|---|---|---|---|---|
| Model 1 | Regression | 198.3 | 4 | 49.57 | F (4, 85) = 1.250 | 0.2960 |
| Sex-DN | 2.672 | 1 | 2.672 | F (1, 85) = 0.06739 | 0.7958 | |
| Years of diagnosis-DN | 20.49 | 1 | 20.49 | F (1, 85) = 0.5167 | 0.4742 | |
| Age-DN | 87.12 | 1 | 87.12 | F (1, 85) = 2.197 | 0.1419 | |
| BMI-DN | 92.49 | 1 | 92.49 | F (1, 85) = 2.333 | 0.1304 | |
| Model 2 | Regression | 764.9 | 5 | 153 | F (5, 84) = 4.584 | 0.0010 |
| ALB-DN | 29.13 | 1 | 29.13 | F (1, 84) = 0.8727 | 0.3529 | |
| HbA1c-DN | 59.15 | 1 | 59.15 | F (1, 84) = 1.772 | 0.1867 | |
| PC-inflammation | 470.5 | 1 | 470.5 | F (1, 84) = 14.10 | 0.0003 | |
| CRP-DN | 0.04513 | 1 | 0.04513 | F (1, 84) = 0.001352 | 0.9708 | |
| eGFR-DN | 0.6729 | 1 | 0.6729 | F (1, 84) = 0.02016 | 0.8874 |
| Variable | Estimate | 95% CI (Profile Likelihood) | |t| | p Value | ||
|---|---|---|---|---|---|---|
| Model 1 | β0 | Intercept | 8.702 | −4.840 to 22.24 | 1.278 | 0.2049 |
| β1 | Sex-DN | −0.3596 | −3.114 to 2.394 | 0.2596 | 0.7958 | |
| β2 | Years of diagnosis-DN | 0.06061 | −0.1070 to 0.2282 | 0.7188 | 0.4742 | |
| β3 | Age-DN | −0.118 | −0.2764 to 0.04029 | 1.482 | 0.1419 | |
| β4 | BMI-DN | 0.2035 | −0.06140 to 0.4683 | 1.527 | 0.1304 | |
| Model 2 | β0 | Intercept | 3.975 | −10.43 to 18.38 | 0.5487 | 0.5847 |
| β1 | ALB-DN | 1.342 | −1.514 to 4.197 | 0.9342 | 0.3529 | |
| β2 | HbA1c-DN | −0.3768 | −0.9396 to 0.1860 | 1.331 | 0.1867 | |
| β3 | PC-inflammation | 1.134 | 0.5333 to 1.734 | 3.755 | 0.0003 | |
| β4 | CRP-DN | 0.0035 | −0.1856 to 0.1926 | 0.03677 | 0.9708 | |
| β5 | eGFR-DN | −0.0042 | −0.06343 to 0.05498 | 0.142 | 0.8874 |
| Analysis of Variance | SS | DF | MS | F (DFn, DFd) | p Value | |
|---|---|---|---|---|---|---|
| Model 1 | Regression | 18.9 | 4 | 4.725 | F (4, 85) = 0.4639 | 0.7620 |
| Sex-DN | 1.102 | 1 | 1.102 | F (1, 85) = 0.1082 | 0.7431 | |
| Years of diagnosis-DN | 3.779 | 1 | 3.779 | F (1, 85) = 0.3710 | 0.5441 | |
| Age-DN | 1.106 | 1 | 1.106 | F (1, 85) = 0.1085 | 0.7426 | |
| BMI-DN | 6.9 | 1 | 6.9 | F (1, 85) = 0.6774 | 0.4128 | |
| Model 2 | Regression | 26.14 | 5 | 5.227 | F (5, 84) = 0.5114 | 0.7669 |
| ALB-DN | 0.2306 | 1 | 0.2306 | F (1, 84) = 0.02256 | 0.8810 | |
| HbA1c-DN | 4.367 | 1 | 4.367 | F (1, 84) = 0.4273 | 0.5151 | |
| PC-inflammation | 6.003 | 1 | 6.003 | F (1, 84) = 0.5873 | 0.4456 | |
| CRP-DN | 2.234 | 1 | 2.234 | F (1, 84) = 0.2186 | 0.6413 | |
| eGFR-DN | 14.37 | 1 | 14.37 | F (1, 84) = 1.406 | 0.2391 |
| Variable | Estimate | 95% CI (Profile Likelihood) | |t| | p Value | ||
|---|---|---|---|---|---|---|
| Model 1 | β0 | Intercept | 3.67 | −3.193 to 10.53 | 1.063 | 0.2907 |
| β1 | Sex-DN | −0.2309 | −1.627 to 1.165 | 0.3289 | 0.7431 | |
| β2 | Years of diagnosis-DN | 0.02603 | −0.05894 to 0.1110 | 0.6091 | 0.5441 | |
| β3 | Age-DN | 0.0133 | −0.06696 to 0.09355 | 0.3295 | 0.7426 | |
| β4 | BMI-DN | −0.0556 | −0.1898 to 0.07867 | 0.823 | 0.4128 | |
| Model 2 | β0 | Intercept | 2.338 | −5.635 to 10.31 | 0.5831 | 0.5614 |
| β1 | ALB-DN | 0.1194 | −1.461 to 1.700 | 0.1502 | 0.881 | |
| β2 | HbA1c-DN | −0.1024 | −0.4139 to 0.2091 | 0.6537 | 0.5151 | |
| β3 | PC-inflammation | −0.1281 | −0.4604 to 0.2042 | 0.7663 | 0.4456 | |
| β4 | CRP-DN | 0.0246 | −0.08005 to 0.1293 | 0.4675 | 0.6413 | |
| β5 | eGFR-DN | 0.01953 | −0.01323 to 0.05230 | 1.186 | 0.2391 |
| Index | Formula |
|---|---|
| AISI | ((neutrophils × monocytes × platelets)/lymphocytes) |
| IIC | ((mean corpuscular volume × width of erythrocyte distribution × neutrophils)/(lymphocytes × 1000)) |
| SII | (neutrophils × platelets)/lymphocytes |
| SIRI | ((neutrophil × monocytes)/lymphocytes) |
| MCVL | mean corpuscular volume/lymphocytes |
| NLR | neutrophil-to-lymphocyte ratio |
| MLR | monocyte-to-lymphocyte ratio |
| PLR | platelet-to-lymphocyte ratio |
| dNLR | neutrophils/(leucocytes − neutrophils) |
| NPR | (neutrophil counts × 1000)/platelet counts |
| AOPP | TBARS | |
|---|---|---|
| Catalog Number | MBS722252 | MBS166987 |
| Sensitivity | 0.1 ng/mL | 0.022 nmol/mL |
| Detection range | 2.5–50 ng/mL | 0.05–30 nmol/mL |
| Cross reactivity | No significant cross-reactivity or interference between AOPP and analogs was observed. | - |
| Intra-/Inter-assay CV (%) | CV < 10%; CV < 12% | CV < 8%; CV < 10% |
| ISO Certification | Manufactured in an ISO 13485:2016 Certified Laboratory. | Manufactured in an ISO 9001:2015 Certified Laboratory. |
| Assay Type | Competitive | Quantitative Sandwich |
| Spike Recovery | 92–101% | - |
| Sample required/well | 40 microL | 40 microL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caragea, D.C.; Boldeanu, L.; Assani, M.-Z.; Caragea, M.-E.; Stroe-Ionescu, A.-Ș.; Popa, R.; Maria, D.-T.; Pădureanu, V.; Vere, C.C.; Boldeanu, M.V. Assessment of AOPP, TBARS, and Inflammatory Status in Diabetic Nephropathy and Hemodialyzed Patients. Int. J. Mol. Sci. 2025, 26, 10670. https://doi.org/10.3390/ijms262110670
Caragea DC, Boldeanu L, Assani M-Z, Caragea M-E, Stroe-Ionescu A-Ș, Popa R, Maria D-T, Pădureanu V, Vere CC, Boldeanu MV. Assessment of AOPP, TBARS, and Inflammatory Status in Diabetic Nephropathy and Hemodialyzed Patients. International Journal of Molecular Sciences. 2025; 26(21):10670. https://doi.org/10.3390/ijms262110670
Chicago/Turabian StyleCaragea, Daniel Cosmin, Lidia Boldeanu, Mohamed-Zakaria Assani, Mariana-Emilia Caragea, Alexandra-Ștefania Stroe-Ionescu, Romeo Popa, Daniela-Teodora Maria, Vlad Pădureanu, Cristin Constantin Vere, and Mihail Virgil Boldeanu. 2025. "Assessment of AOPP, TBARS, and Inflammatory Status in Diabetic Nephropathy and Hemodialyzed Patients" International Journal of Molecular Sciences 26, no. 21: 10670. https://doi.org/10.3390/ijms262110670
APA StyleCaragea, D. C., Boldeanu, L., Assani, M.-Z., Caragea, M.-E., Stroe-Ionescu, A.-Ș., Popa, R., Maria, D.-T., Pădureanu, V., Vere, C. C., & Boldeanu, M. V. (2025). Assessment of AOPP, TBARS, and Inflammatory Status in Diabetic Nephropathy and Hemodialyzed Patients. International Journal of Molecular Sciences, 26(21), 10670. https://doi.org/10.3390/ijms262110670









