Advanced Spectroscopic Studies of the AIE-Enhanced ESIPT Effect in a Selected 1,3,4-Thiadiazole Derivative in Liposomal Systems with DPPC
Abstract
1. Introduction
2. Results
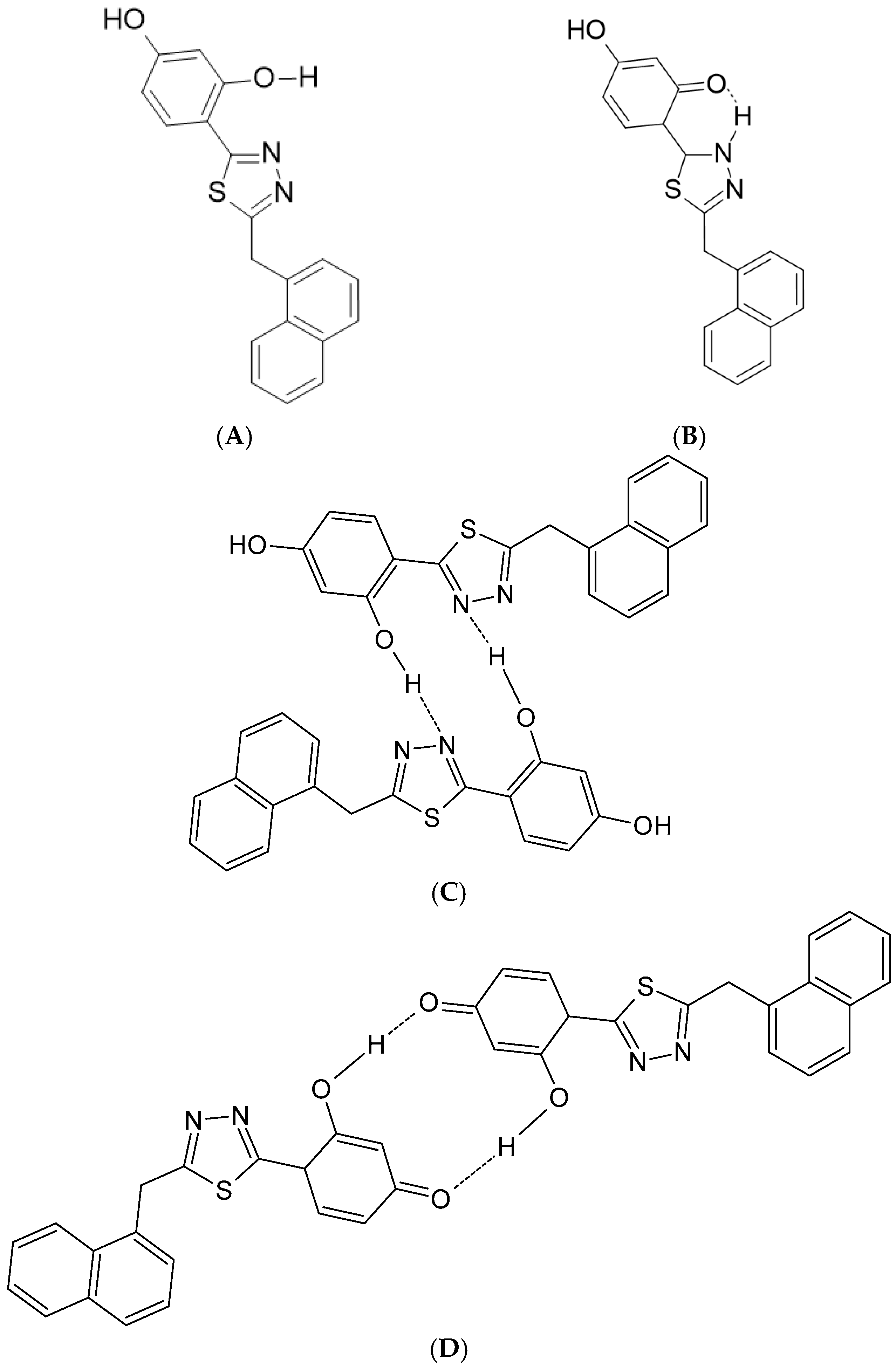
2.1. Structure and Stationary Spectroscopy
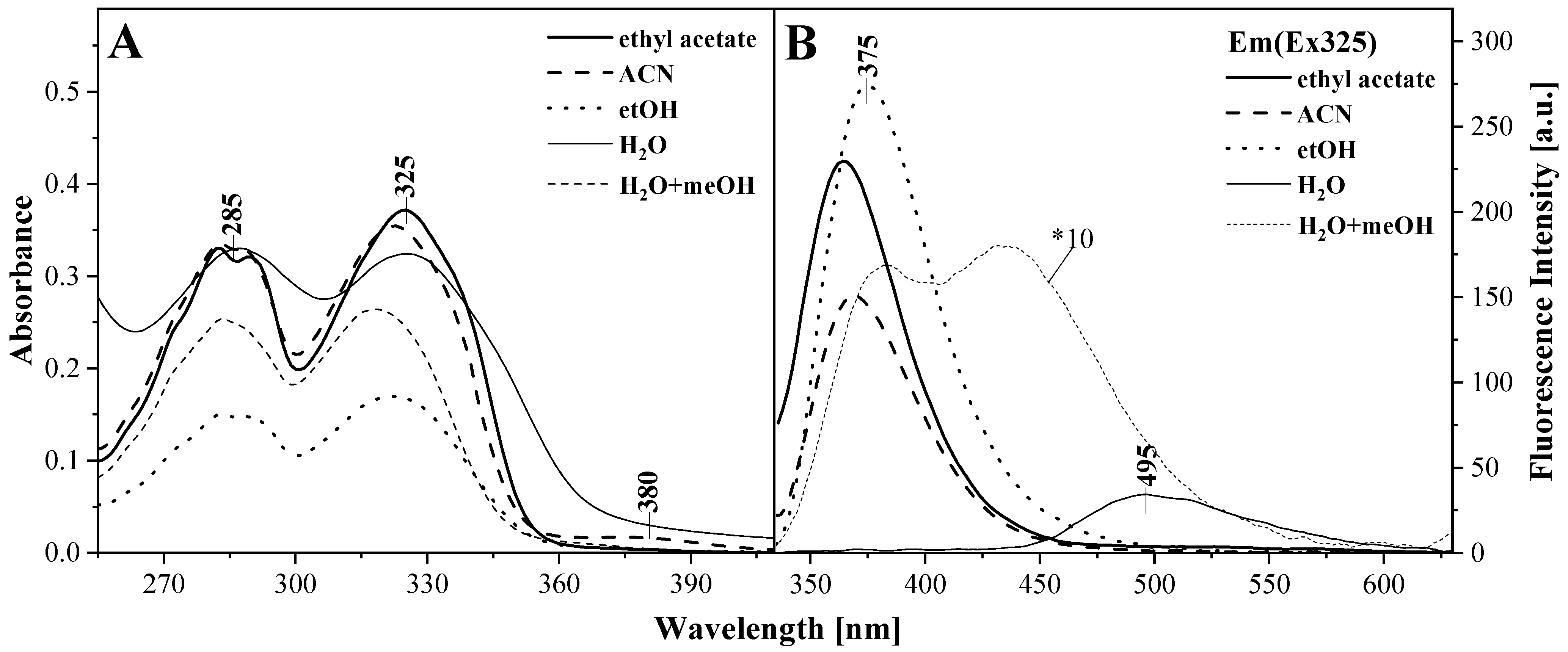
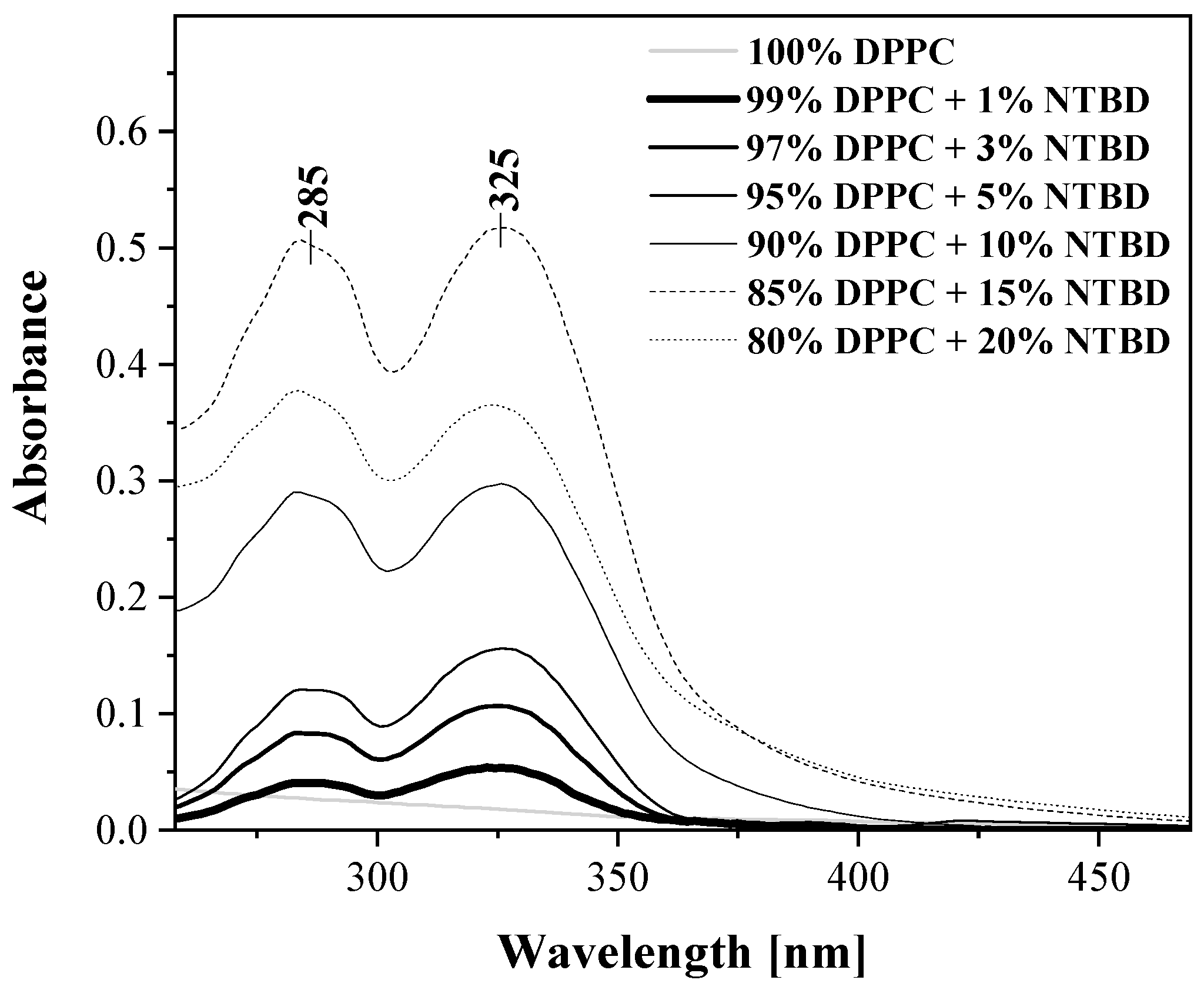
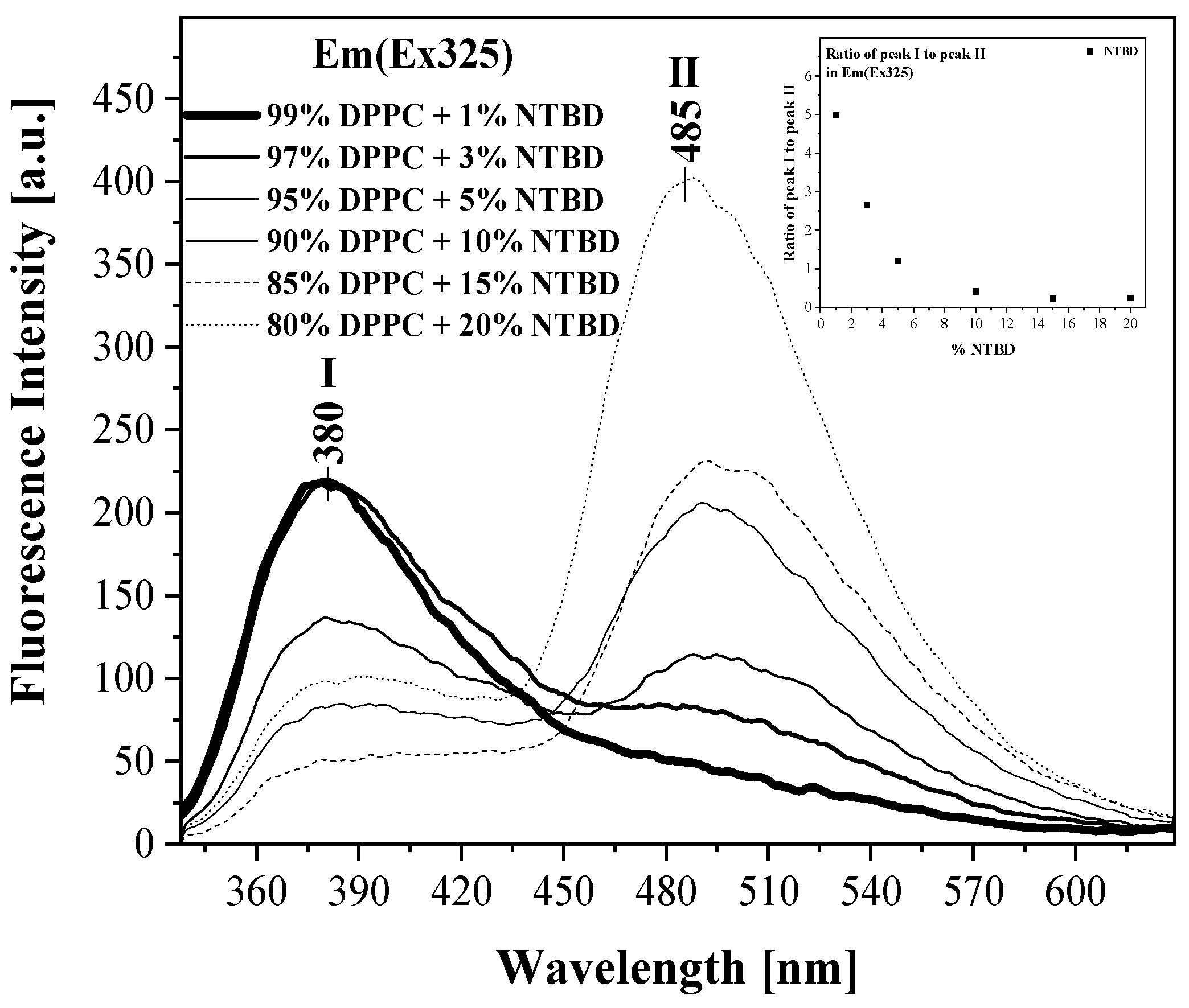
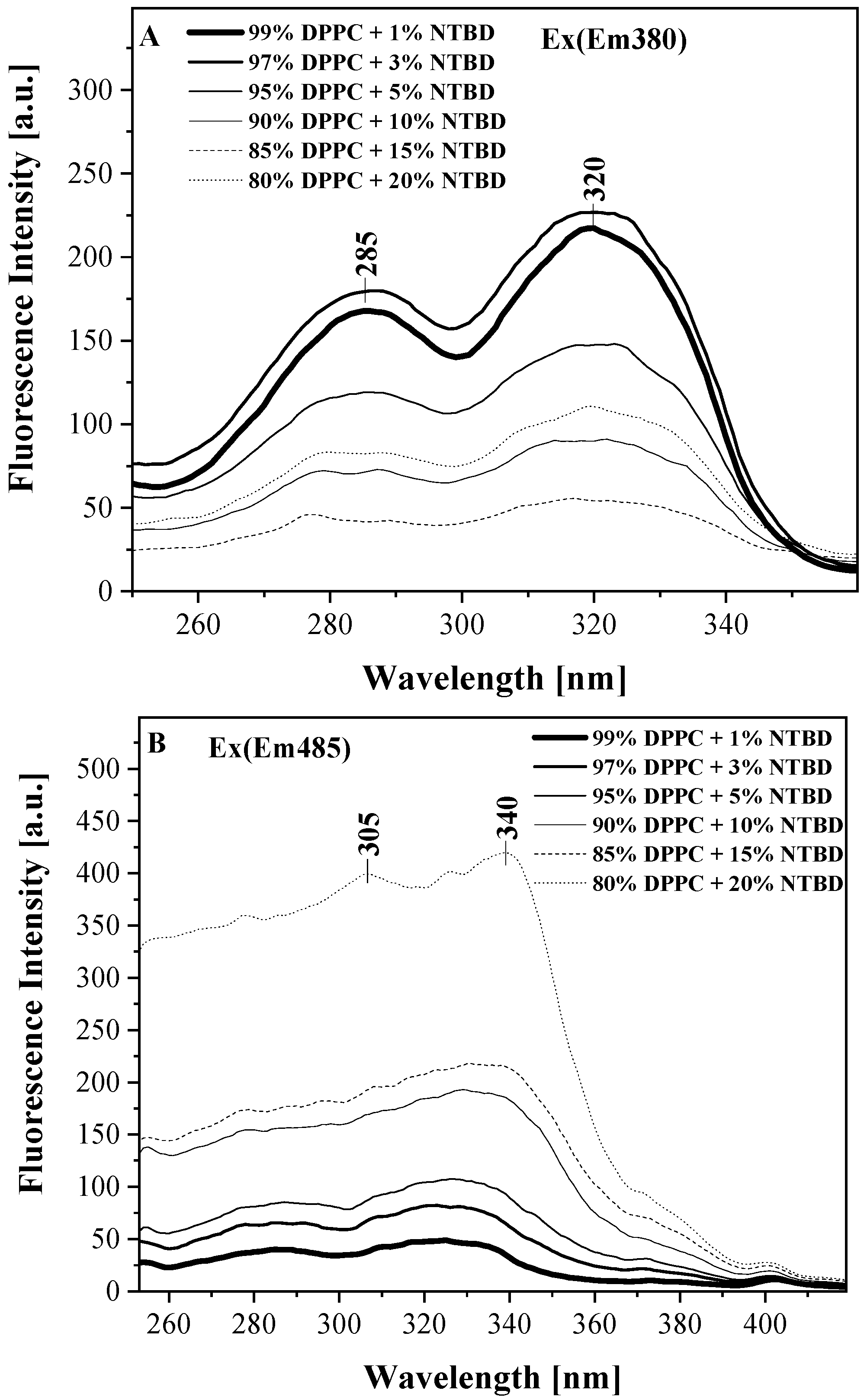
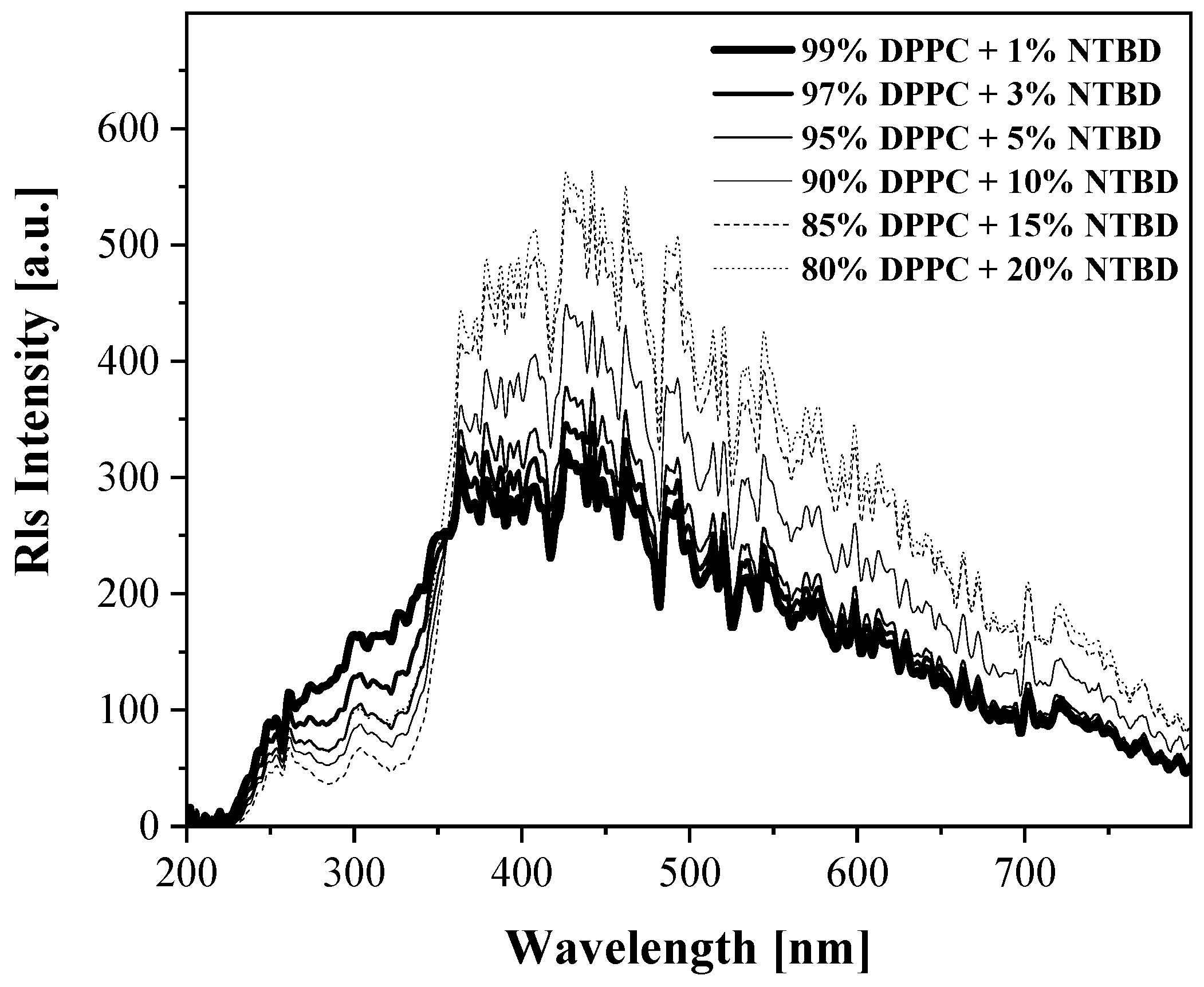
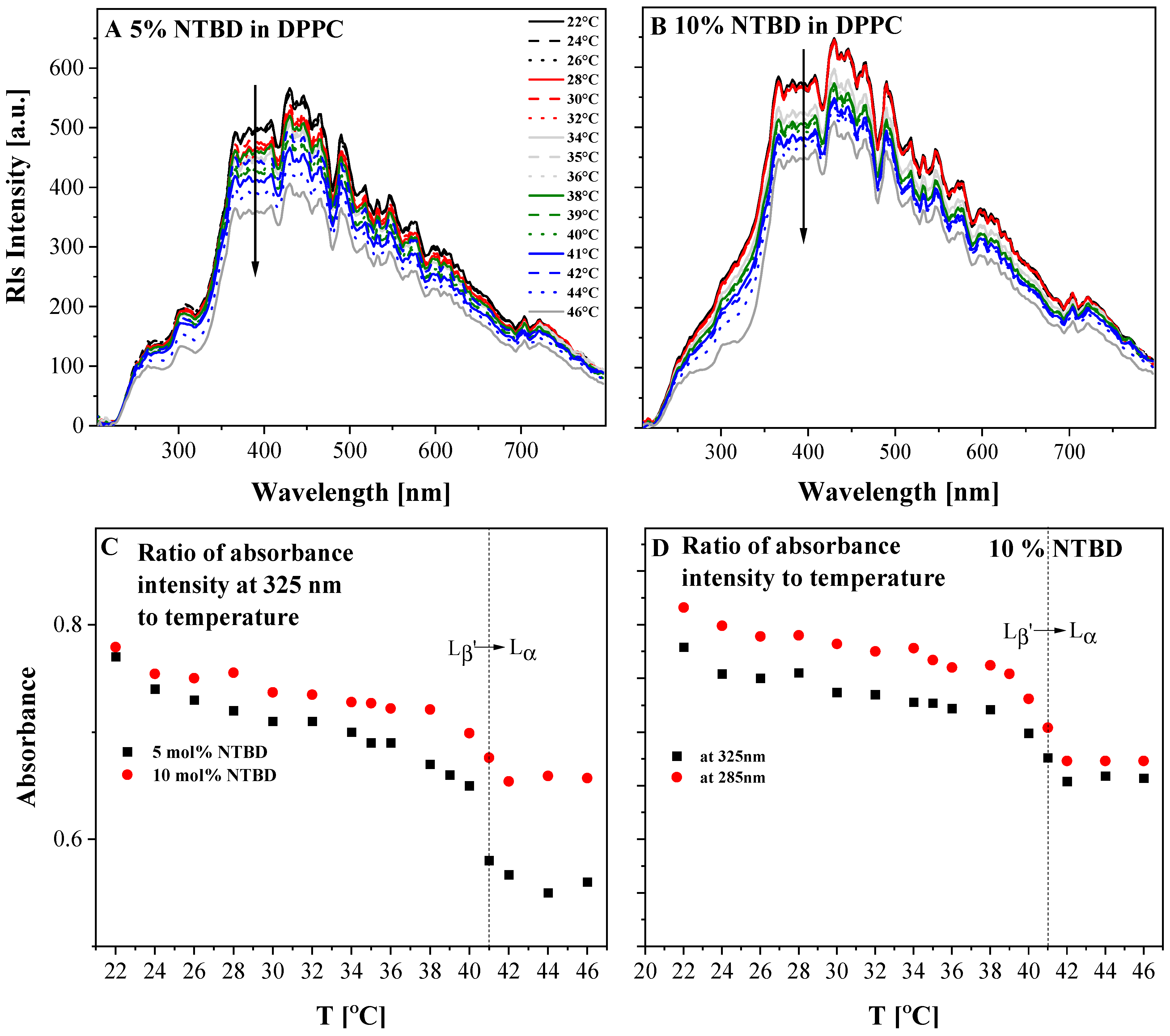
2.2. UV-Vis, Temperature-Dependent Fluorescence—Spectroscopic Measurements as a Function of Environmental Temperature Changes
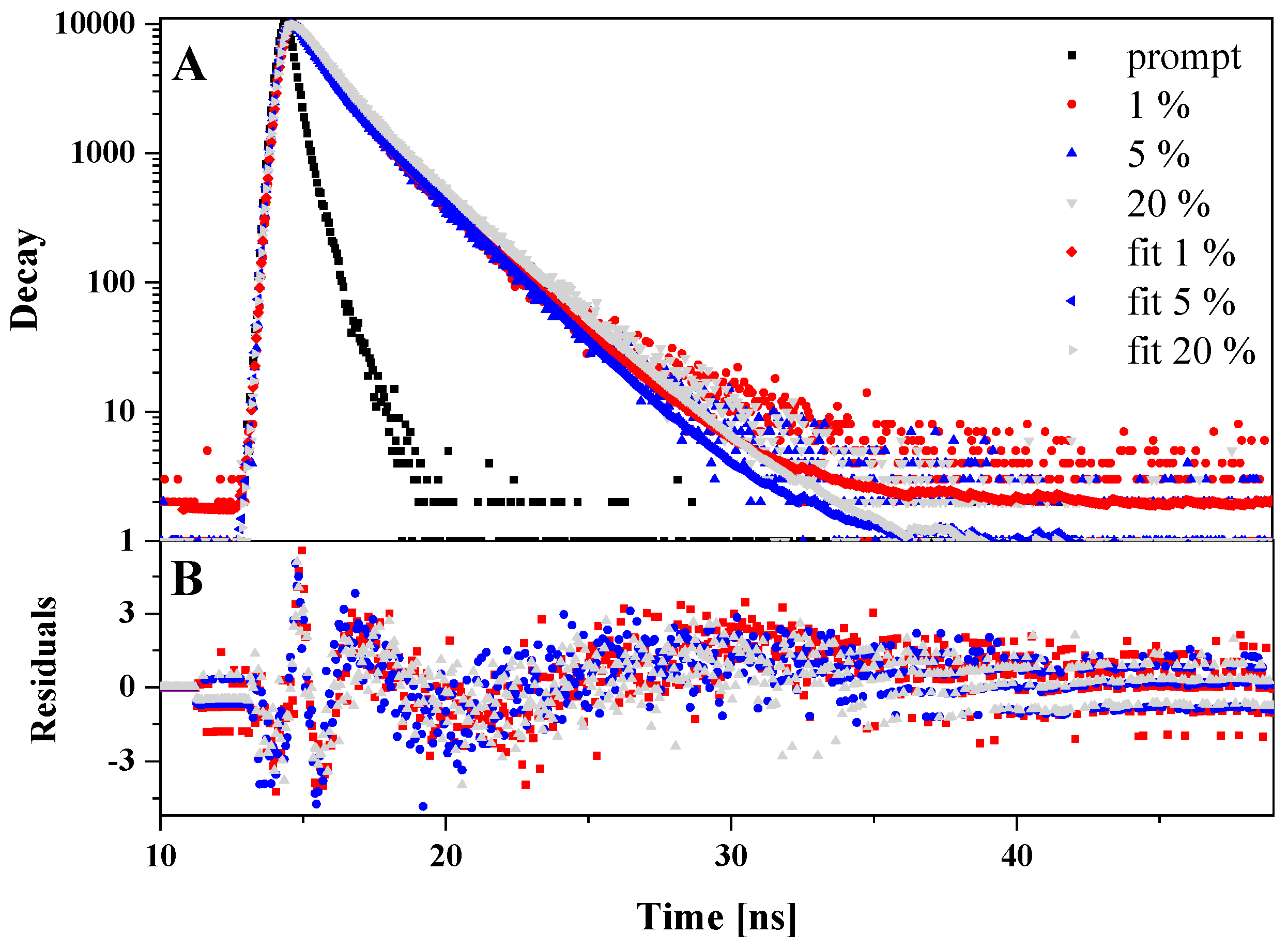
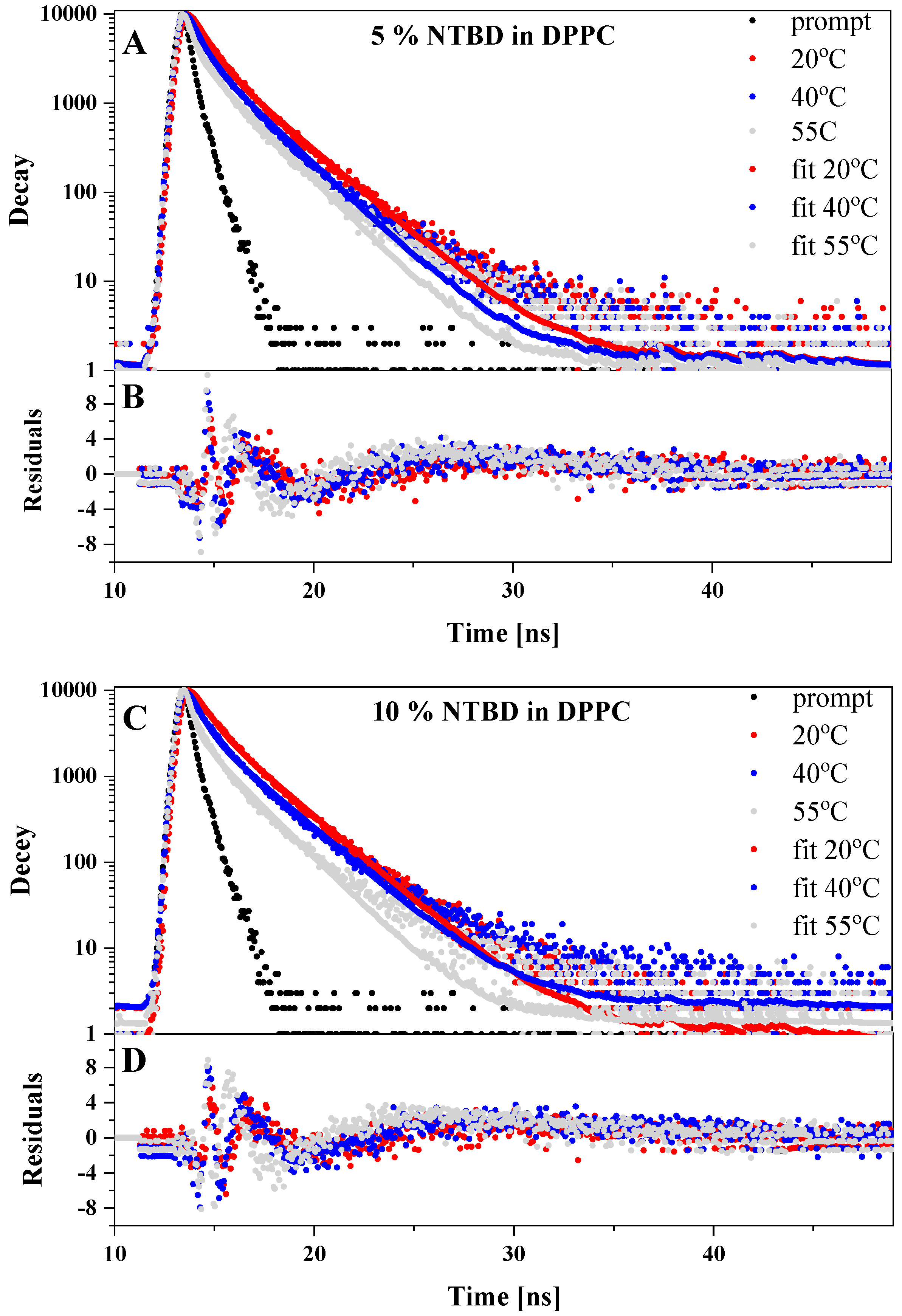
2.3. Temperature Dependence of Fluorescence Lifetimes
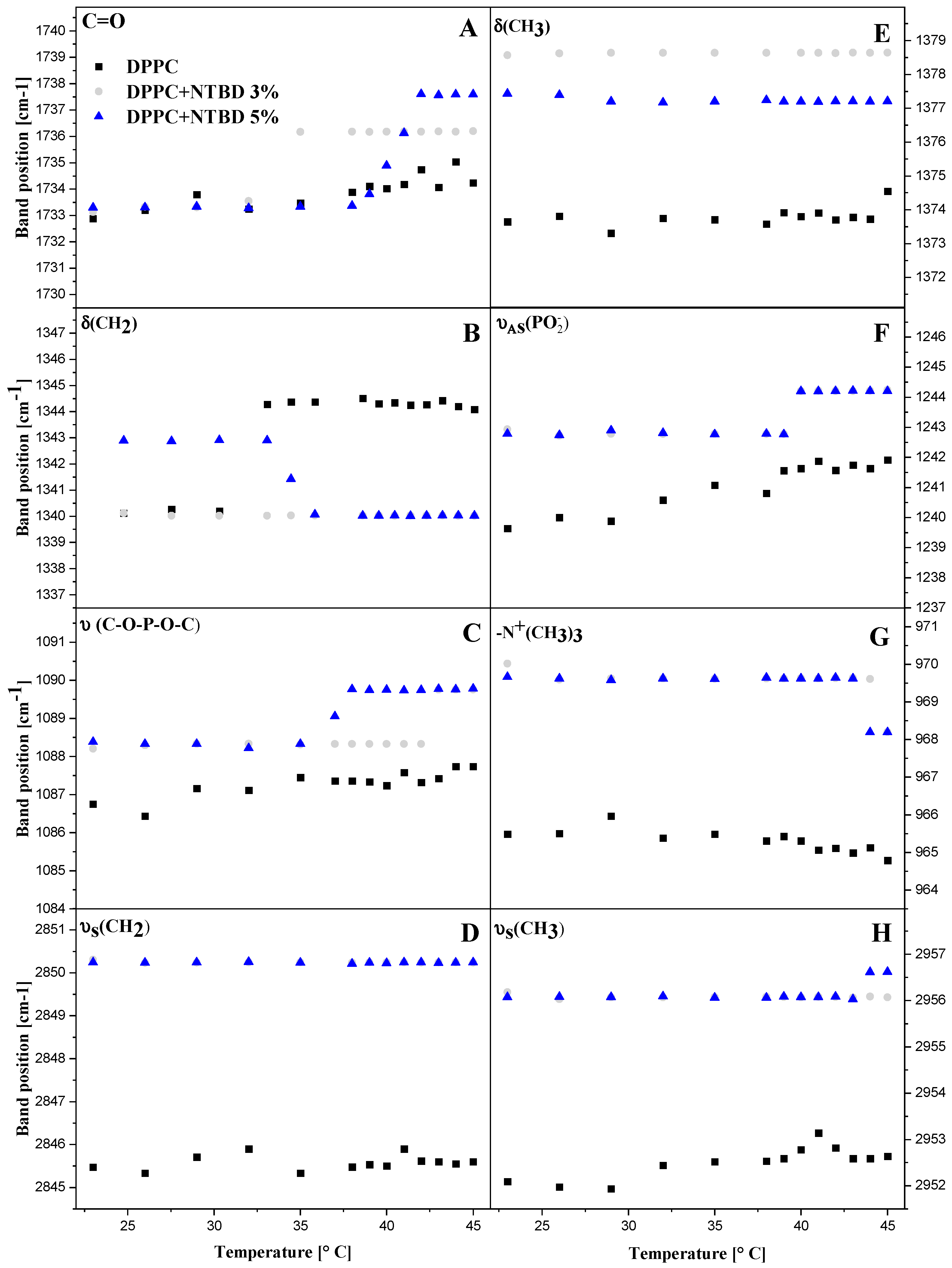
2.4. Infrared Spectroscopy—FTIR in the Analysis of Conformational Changes in the Lipid Membrane Induced by NTBD
2.4.1. Hydrophilic Region of the Membrane
2.4.2. Hydrophobic Region of the Membrane
2.5. XRD of Multilayer
2.6. Single Crystal X-Ray Diffraction
3. Materials and Methods
3.1. Methods
3.2. Measurements of Electronic Absorption and Fluorescence Spectra
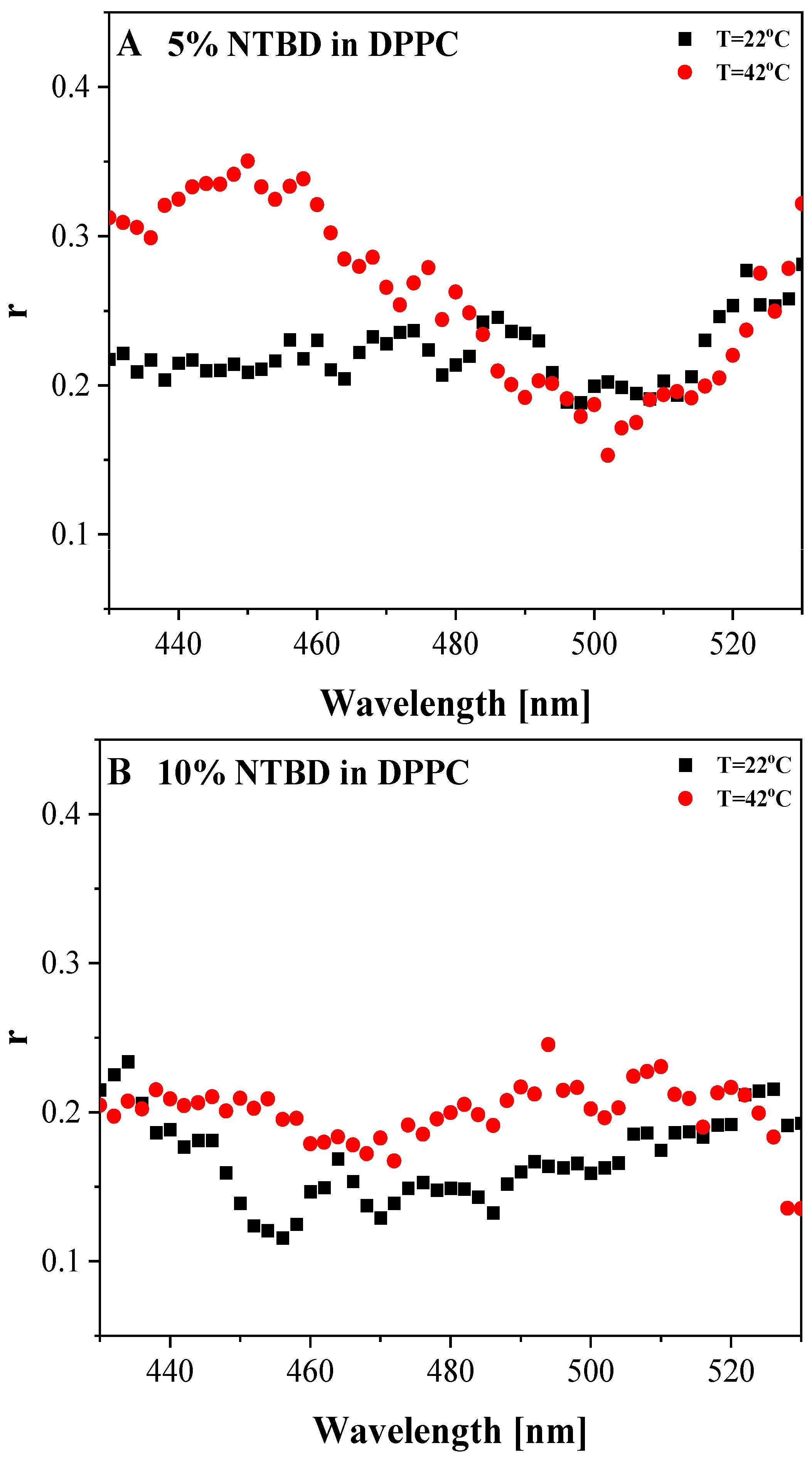
3.3. Anisotropy Measurements
3.4. Time-Correlated Single Photon Counting (TCSPC)
3.5. DLS Methods
3.6. FTIR Spectroscopy
3.7. XRD
3.8. Fluorescence Quantum Yields
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eugster, R.; Luciani, P. Liposomes: Bridging the Gap from Lab to Pharmaceuticals. Curr. Opin. Colloid Interface Sci. 2025, 75, 101875. [Google Scholar] [CrossRef]
- Basak, S.; Das, T.K. Liposome-Based Drug Delivery Systems: From Laboratory Research to Industrial Production—Instruments and Challenges. ChemEngineering 2025, 9, 56. [Google Scholar] [CrossRef]
- Mankan, E.; Karakas, C.Y.; Saroglu, O.; Mzoughi, M.; Sagdic, O.; Karadag, A. Food-Grade Liposome-Loaded Delivery Systems: Current Trends and Future Perspectives. Foods 2025, 14, 2978. [Google Scholar] [CrossRef]
- Luo, M.; Oomah, B.D.; Akoetey, W.; Zhang, Y.; Daneshfozoun, H.; Hosseinian, F. Liposomes as Sustainable Delivery Systems in Food, Cosmetic, and Pharmaceutical Applications. J. Am. Oil Chem. Soc. 2025, 102, 547–568. [Google Scholar] [CrossRef]
- Chelliah, R.; Rubab, M.; Vijayalakshmi, S.; Karuvelan, M.; Barathikannan, K.; Oh, D.-H. Liposomes for Drug Delivery: Classification, Therapeutic Applications, and Limitations. Next Nanotechnol. 2025, 8, 100209. [Google Scholar] [CrossRef]
- Kyrychenko, A.M.; Kovalenko, O.H. Prospects of Liposomes Application in Agriculture. Mikrobiolohichnyi Zhurnal 2025, 87, 72–82. [Google Scholar] [CrossRef]
- Pamunuwa, G.K.; Karunaratne, D.N. Liposomal Delivery of Plant Bioactives Enhances Potency in Food Systems: A Review. J. Food Qual. 2022, 2022, 5272592. [Google Scholar] [CrossRef]
- Jampílek, J.; Kráľová, K. Application of Nanotechnology In Agriculture And Food Industry, Its Prospects And Risks. Ecol. Chem. Eng. S 2015, 22, 321–361. [Google Scholar] [CrossRef]
- Singh, A.K.; Nair, A.V.; Shah, S.S.; Ray, S.; Singh, N.D.P. ESIPT-, AIE-, and AIE + ESIPT-Based Light-Activated Drug Delivery Systems and Bioactive Donors for Targeted Disease Treatment. J. Med. Chem. 2023, 66, 3732–3745. [Google Scholar] [CrossRef]
- David, M.; Budziak-Wieczorek, I.; Karcz, D.; Florescu, M.; Matwijczuk, A. Insight into Dual Fluorescence Effects Induced by Molecular Aggregation Occurring in Membrane Model Systems Containing 1,3,4-Thiadiazole Derivatives. Eur. Biophys. J. 2021, 50, 1083–1101. [Google Scholar] [CrossRef]
- Roohi, H.; Rouhi, M. Recent Advances in the ESIPT-Based Drug Delivery Systems for Targeted Diseases Treatment. Dye. Pigment. 2025, 240, 112863. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, H.; Yang, J.; Yao, Y.; Li, L. Solvents/Photo/Pillar[5]Arene Triple Responsive Morphology and Luminescence Transformation from an Amphiphilic Dicyanostilbene-Functionalized Thiophene. Chin. Chem. Lett. 2023, 34, 108452. [Google Scholar] [CrossRef]
- Chen, T.; Wang, J.; Tang, R.; Huang, Y.; Zhao, Q.; Yao, Y. An Amphiphilic [2]Biphenyl-Extended Pillar[6]Arene: Synthesis, Controllable Self-Assembly in Water and Application in Cell-Imaging. Chin. Chem. Lett. 2023, 34, 108088. [Google Scholar] [CrossRef]
- Matysiak, J. Biological and Pharmacological Activities of 1, 3, 4-Thiadiazole Based Compounds. Mini Rev. Med. Chem. 2015, 15, 762–775. [Google Scholar] [CrossRef]
- Hu, Y.; Li, C.-Y.; Wang, X.-M.; Yang, Y.-H.; Zhu, H.-L. 1,3,4-Thiadiazole: Synthesis, Reactions, and Applications in Medicinal, Agricultural, and Materials Chemistry. Chem. Rev. 2014, 114, 5572–5610. [Google Scholar] [CrossRef]
- Dawood, K.M.; Gomha, S.M. Synthesis and Anti-cancer Activity of 1,3,4-Thiadiazole and 1,3-Thiazole Derivatives Having 1,3,4-Oxadiazole Moiety. J. Heterocycl. Chem. 2015, 52, 1400–1405. [Google Scholar] [CrossRef]
- Szeliga, M. Thiadiazole Derivatives as Anticancer Agents. Pharmacol. Rep. 2020, 72, 1079–1100. [Google Scholar] [CrossRef]
- Szukalska, A.; Zajac, D.; Cyprych, K.; Mysliwiec, J. Ultra-Photostable Random Lasing Coming from the Benzothiadiazole Derivative Dye-Doped Organic System. J. Phys. Chem. C 2023, 127, 24618–24625. [Google Scholar] [CrossRef]
- Loto, R.T.; Loto, C.A.; Popoola, A.P.I. Corrosion Inhibition of Thiourea and Thiadiazole Derivatives: A Review. J. Mater. Environ. Sci. 2012, 3, 885–894. [Google Scholar]
- Wen, G.-L.; Wang, Y.-Y.; Liu, P.; Guo, C.-Y.; Zhang, W.-H.; Shi, Q.-Z. A Series of 1-D to 3-D Metal–Organic Coordination Architectures Assembled with V-Shaped Bis (Pyridyl) Thiadiazole under Co-Ligand Intervention. Inorganica Chim. Acta 2009, 362, 1730–1738. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, S.; Miao, L.; Lü, C.; An, Y. A New 1,3,4-Thiadiazole-Based ESIPT Probe for Detection of Hcy/Cys and GSH by Two-Channel Fluorescent Procedure and Its Potential Applications. Res. Chem. Intermed. 2023, 49, 2191–2208. [Google Scholar] [CrossRef]
- Budziak-Wieczorek, I.; Kaczmarczyk, D.; Rząd, K.; Gagoś, M.; Stepulak, A.; Myśliwa-Kurdziel, B.; Karcz, D.; Starzak, K.; Burdziński, G.; Srebro-Hooper, M. Cooperativity of ESPT and Aggregation-Induced Emission Effects—An Experimental and Theoretical Analysis of a 1, 3, 4-Thiadiazole Derivative. Int. J. Mol. Sci. 2024, 25, 3352. [Google Scholar] [CrossRef]
- Budziak-Wieczorek, I.; Ślusarczyk, L.; Myśliwa-Kurdziel, B.; Kurdziel, M.; Srebro-Hooper, M.; Korona-Glowniak, I.; Gagoś, M.; Gładyszewski, G.; Stepulak, A.; Kluczyk, D. Spectroscopic Characterization and Assessment of Microbiological Potential of 1, 3, 4-Thiadiazole Derivative Showing ESIPT Dual Fluorescence Enhanced by Aggregation Effects. Sci. Rep. 2022, 12, 22140. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, Y. A Theoretical Study on ESPT Mechanism of DALL-AcOH Complex. Commun. Comput. Chem. 2016, 4, 1–8. [Google Scholar]
- Li, Y.; Cao, B.; Zhou, Q.; Zhang, X.; Li, B.; Su, X.; Shi, Y. Enhancing Fluorescence of Benzimidazole Derivative via Solvent-Regulated ESIPT and TICT Process: A TDDFT Study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 258, 119862. [Google Scholar] [CrossRef]
- Brancato, G.; Signore, G.; Neyroz, P.; Polli, D.; Cerullo, G.; Abbandonato, G.; Nucara, L.; Barone, V.; Beltram, F.; Bizzarri, R. Dual Fluorescence through Kasha’s Rule Breaking: An Unconventional Photomechanism for Intracellular Probe Design. J. Phys. Chem. B 2015, 119, 6144–6154. [Google Scholar] [CrossRef]
- Nandi, A.; Manna, B.; Ghosh, R. Interplay of Exciton–Excimer Dynamics in 9, 10-Diphenylanthracene Nanoaggregates and Thin Films Revealed by Time-Resolved Spectroscopic Studies. Phys. Chem. Chem. Phys. 2019, 21, 11193–11202. [Google Scholar] [CrossRef]
- Manikandan, I.; Chang, C.-H.; Chen, C.-L.; Sathish, V.; Li, W.-S.; Malathi, M. Aggregation Induced Emission Enhancement (AIEE) Characteristics of Quinoline Based Compound—A Versatile Fluorescent Probe for pH, Fe (III) Ion, BSA Binding and Optical Cell Imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 182, 58–66. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, H.; Qin, L.; Hu, Y.; Lü, C.; An, Y. A 1, 3, 4-Thiadiazole Functionalized Schiff Base Based Fluorescence Enhancement and Colorimetric Probe for Detection of Cu (II) Ion and Its Potential Applications. Chem. Phys. 2023, 565, 111740. [Google Scholar] [CrossRef]
- Massue, J.; Pariat, T.; M. Vérité, P.; Jacquemin, D.; Durko, M.; Chtouki, T.; Sznitko, L.; Mysliwiec, J.; Ulrich, G. Natural Born Laser Dyes: Excited-State Intramolecular Proton Transfer (ESIPT) Emitters and Their Use in Random Lasing Studies. Nanomaterials 2019, 9, 1093. [Google Scholar] [CrossRef]
- Yi, S.-Z.; Li, B.-N.; Fu, P.-Y.; Pan, M.; Su, C.-Y. Interplay of Dual-Proton Transfer Relay to Achieve Full-Color Panel Luminescence in Excited-State Intramolecular Proton Transfer (ESIPT) Fluorophores. ACS Appl. Mater. Interfaces 2023, 15, 3172–3181. [Google Scholar] [CrossRef]
- Moraes, E.S.; Teixeira Alves Duarte, L.G.; Germino, J.C.; Atvars, T.D.Z. Near Attack Conformation as Strategy for ESIPT Modulation for White-Light Generation. J. Phys. Chem. C 2020, 124, 22406–22415. [Google Scholar] [CrossRef]
- Sedgwick, A.C.; Wu, L.; Han, H.-H.; Bull, S.D.; He, X.-P.; James, T.D.; Sessler, J.L.; Tang, B.Z.; Tian, H.; Yoon, J. Excited-State Intramolecular Proton-Transfer (ESIPT) Based Fluorescence Sensors and Imaging Agents. Chem. Soc. Rev. 2018, 47, 8842–8880. [Google Scholar] [CrossRef]
- Azcárate, J.C.; Díaz, S.A.; Fauerbach, J.A.; Gillanders, F.; Rubert, A.A.; Jares-Erijman, E.A.; Jovin, T.M.; Fonticelli, M.H. ESIPT and FRET Probes for Monitoring Nanoparticle Polymer Coating Stability. Nanoscale 2017, 9, 8647–8656. [Google Scholar] [CrossRef]
- Zhao, J.; Ji, S.; Chen, Y.; Guo, H.; Yang, P. Excited State Intramolecular Proton Transfer (ESIPT): From Principal Photophysics to the Development of New Chromophores and Applications in Fluorescent Molecular Probes and Luminescent Materials. Phys. Chem. Chem. Phys. 2012, 14, 8803–8817. [Google Scholar] [CrossRef]
- Sahu, S.; Das, M.; Bharti, A.K.; Krishnamoorthy, G. Proton Transfer Triggered Proton Transfer: A Self-Assisted Twin Excited State Intramolecular Proton Transfer. Phys. Chem. Chem. Phys. 2018, 20, 27131–27139. [Google Scholar] [CrossRef]
- Matwijczuk, A.; Górecki, A.; Kamiński, D.; Myśliwa-Kurdziel, B.; Fiedor, L.; Niewiadomy, A.; Karwasz, G.P.; Gagoś, M. Influence of Solvent Polarizability on the Keto-Enol Equilibrium in 4-[5-(Naphthalen-1-Ylmethyl)-1,3,4-Thiadiazol-2-Yl]Benzene-1,3-Diol. J. Fluoresc. 2015, 25, 1867–1874. [Google Scholar] [CrossRef]
- Shui, S.; Xu, J.; Yang, G.; Fu, H.; Wang, B. Supramolecular Aggregation Properties of Naphthalimide-Decorated Imino-Naphthol and Imino-Naphthalene Based on Fluorescent Materials. J. Mol. Struct. 2022, 1270, 133809. [Google Scholar] [CrossRef]
- Kasha, M. Molecular Excitons in Small Aggregates. In Spectroscopy of the Excited State; Bartolo, B., Pacheco, D., Goldberg, V., Eds.; Springer: Boston, MA, USA, 1976; pp. 337–363. ISBN 978-1-4684-2795-0. [Google Scholar]
- Chen, L.; Fu, P.; Wang, H.; Pan, M. Excited-State Intramolecular Proton Transfer (ESIPT) for Optical Sensing in Solid State. Adv. Opt. Mater. 2021, 9, 2001952. [Google Scholar] [CrossRef]
- Kwon, J.E.; Park, S.Y. Advanced Organic Optoelectronic Materials: Harnessing Excited-State Intramolecular Proton Transfer (ESIPT) Process. Adv. Mater. 2011, 23, 3615–3642. [Google Scholar] [CrossRef]
- Wu, Y.; Peng, C.; Zhan, Q.; Lou, X.; Liu, S.; Lin, X.; Han, Y.; Cao, P.; Cao, T. Towards Trans -Dual Deuterated Cyclopropanes via Photoredox Synergistic Deuteration with D 2 O. Chem. Sci. 2025, 16, 9535–9542. [Google Scholar] [CrossRef]
- Matsuki, H.; Miyazaki, E.; Sakano, F.; Tamai, N.; Kaneshina, S. Thermotropic and Barotropic Phase Transitions in Bilayer Membranes of Ether-Linked Phospholipids with Varying Alkyl Chain Lengths. Biochim. Biophys. Acta (BBA)—Biomembr. 2007, 1768, 479–489. [Google Scholar] [CrossRef]
- Neunert, G.; Tomaszewska-Gras, J.; Gauza-Włodarczyk, M.; Witkowski, S.; Polewski, K. Assessment of DPPC Liposome Disruption by Embedded Tocopheryl Malonate. Appl. Sci. 2023, 13, 6219. [Google Scholar] [CrossRef]
- Poojari, C.; Wilkosz, N.; Lira, R.B.; Dimova, R.; Jurkiewicz, P.; Petka, R.; Kepczynski, M.; Róg, T. Behavior of the DPH Fluorescence Probe in Membranes Perturbed by Drugs. Chem. Phys. Lipids 2019, 223, 104784. [Google Scholar] [CrossRef]
- Rauf, M.A.; Hisaindee, S.; Saleh, N. Spectroscopic Studies of Keto–Enol Tautomeric Equilibrium of Azo Dyes. RSC Adv. 2015, 5, 18097–18110. [Google Scholar] [CrossRef]
- Pandey, H.; Rani, R.; Agarwal, V. Liposome and Their Applications in Cancer Therapy. Braz. Arch. Biol. Technol. 2016, 59, e16150477. [Google Scholar] [CrossRef]
- Li, Y.; Dahal, D.; Pang, Y. Fluorescence Lifetimes of NIR-Emitting Molecules with Excited-State Intramolecular Proton Transfer. Molecules 2023, 28, 125. [Google Scholar] [CrossRef]
- Yun, I.; Cho, E.-S.; Jang, H.-O.; Kim, U.-K.; Choi, C.-H.; Chung, I.-K.; Kim, I.-S.; Wood, W.G. Amphiphilic Effects of Local Anesthetics on Rotational Mobility in Neuronal and Model Membranes. Biochim. Biophys. Acta (BBA)—Biomembr. 2002, 1564, 123–132. [Google Scholar] [CrossRef]
- Yang, H.; Jiang, P. Self-Cleaning Diffractive Macroporous Films by Doctor Blade Coating. Langmuir 2010, 26, 12598–12604. [Google Scholar] [CrossRef]
- Hoser, A.A.; Kamiński, D.M.; Matwijczuk, A.; Niewiadomy, A.; Gagoś, M.; Woźniak, K. On Polymorphism of 2-(4-Fluorophenylamino)-5-(2, 4-Dihydroxybenzeno)-1, 3, 4-Thiadiazole (FABT) DMSO Solvates. CrystEngComm 2013, 15, 1978–1988. [Google Scholar] [CrossRef]
- Hoser, A.A.; Kamiński, D.M.; Skrzypek, A.; Matwijczuk, A.; Niewiadomy, A.; Gagoś, M.; Woźniak, K. Interplay of Inter- and Intramolecular Interactions in Crystal Structures of 1,3,4-Thiadiazole Resorcinol Derivatives. Cryst. Growth Des. 2018, 18, 3851–3862. [Google Scholar] [CrossRef]
- Matysiak, J.; Nasulewicz, A.; Pełczyńska, M.; Świtalska, M.; Jaroszewicz, I.; Opolski, A. Synthesis and Antiproliferative Activity of Some 5-Substituted 2-(2,4-Dihydroxyphenyl)-1,3,4-Thiadiazoles. Eur. J. Med. Chem. 2006, 41, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Phase Annealing in SHELX-90: Direct Methods for Larger Structures. Acta Cryst. A 1990, 46, 467–473. [Google Scholar] [CrossRef]
- Oxford Diffraction, CrysAlis PRO, Oxford Diffraction Ltd., Yarnton, England, 2009.—References—Scientific Research Publishing. Available online: https://www.scirp.org/reference/referencespapers?referenceid=490878 (accessed on 16 August 2025).
- Jones, G.; Jackson, W.R.; Choi, C.Y.; Bergmark, W.R. Solvent Effects on Emission Yield and Lifetime for Coumarin Laser Dyes. Requirements for a Rotatory Decay Mechanism. J. Phys. Chem. 1985, 89, 294–300. [Google Scholar] [CrossRef]
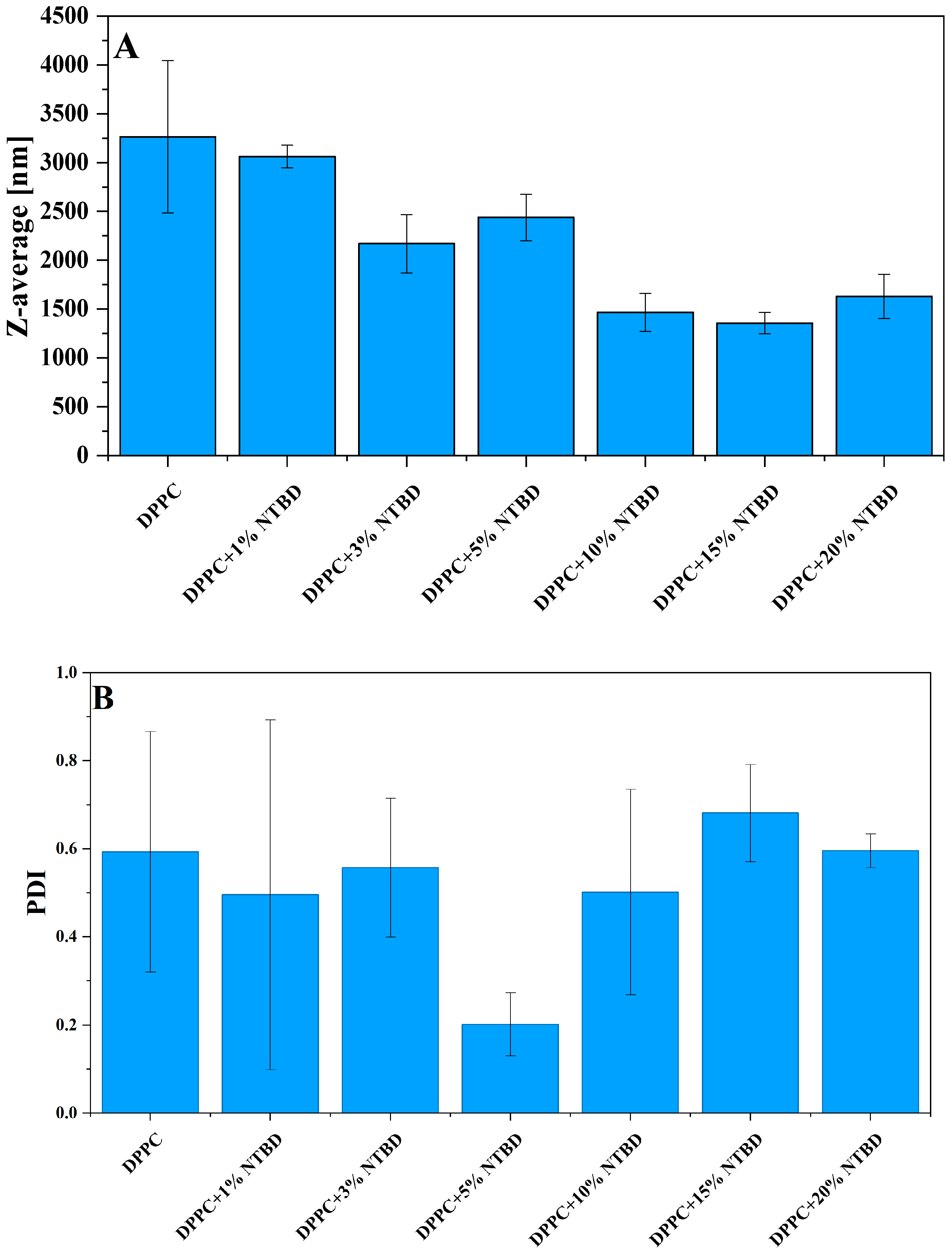
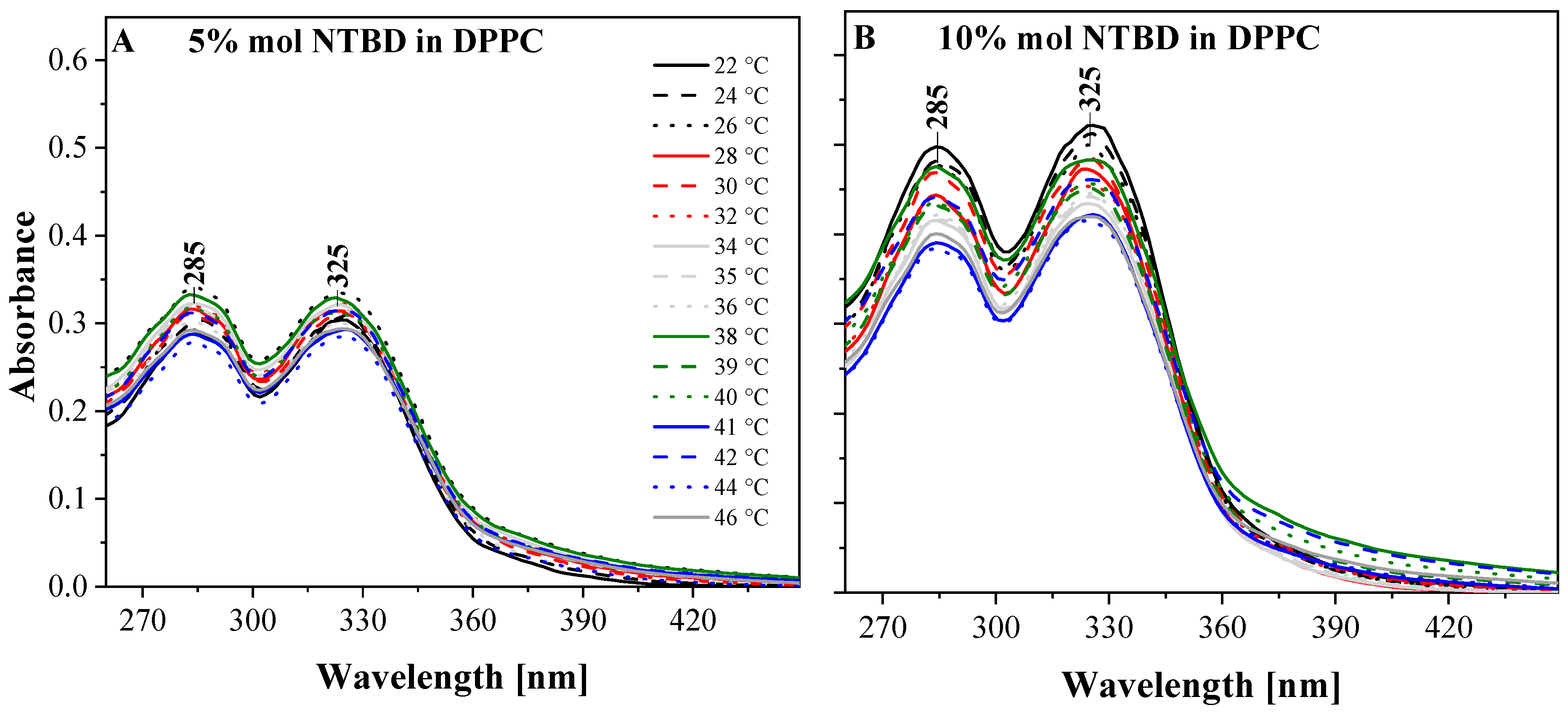
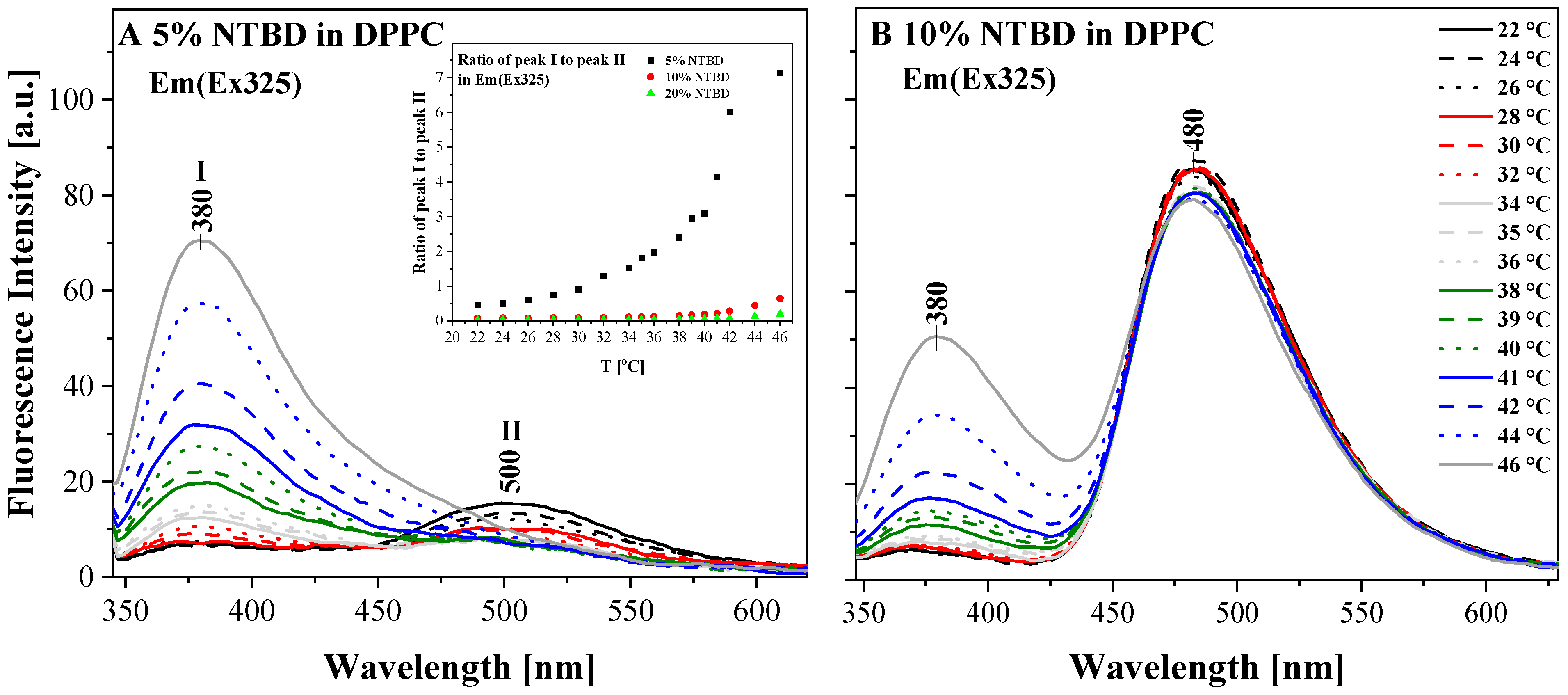
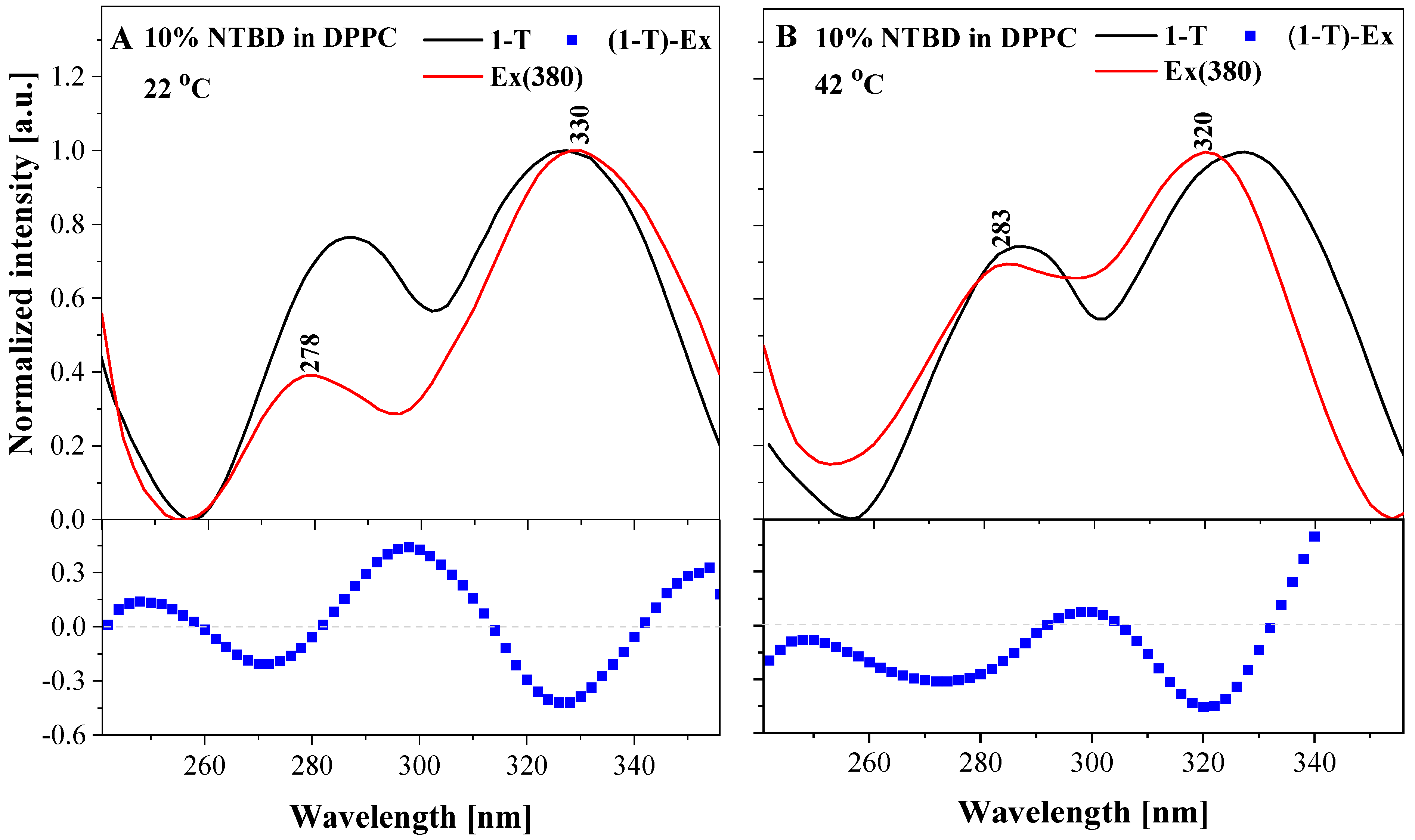
| NTBD % mol | I a | λex | ΦF |
|---|---|---|---|
| 5% | 5885 | 325 | 0.1942 |
| 10% | 8515 | 325 | 0.1730 |
| 15% | 12,253 | 325 | 0.2193 |
| 20% | 21,325 | 325 | 0.3098 |
| NTBD % mol | <τ> | τ1 | f1 | τ2 | f2 |
|---|---|---|---|---|---|
| 1% | 1.43 ± 0.02 | 0.66 ± 0.02 | 0.48 ± 0.01 | 2.14 ± 0.01 | 0.52 ± 0.01 |
| 3% | 1.38 ± 0.02 | 0.55 ± 0.02 | 0.42 ± 0.01 | 1.98 ± 0.01 | 0.58 ± 0.01 |
| 5% | 1.41 ± 0.02 | 0.49 ± 0.02 | 0.38 ± 0.01 | 1.98 ± 0.01 | 0.62 ± 0.01 |
| 10% | 1.55 ± 0.02 | 0.64 ± 0.02 | 0.38 ± 0.01 | 2.10 ± 0.01 | 0.62 ± 0.01 |
| 15% | 1.57 ± 0.02 | 0.60 ± 0.02 | 0.35 ± 0.01 | 2.08 ± 0.01 | 0.65 ± 0.01 |
| 20% | 1.61 ± 0.02 | 0.76 ± 0.02 | 0.40 ± 0.01 | 2.19 ± 0.01 | 0.60 ± 0.01 |
| Temp °C | <τ> | τ1 | f | τ2 | f |
|---|---|---|---|---|---|
| 5% NTBD | |||||
| 20 | 1.47 ± 0.02 | 0.54 ± 0.01 | 0.44 ± 0.01 | 2.20 ± 0.01 | 0.56 ± 0.01 |
| 25 | 1.42 ± 0.02 | 0.50 ± 0.01 | 0.44 ± 0.01 | 2.15 ± 0.01 | 0.56 ± 0.01 |
| 30 | 1.34 ± 0.02 | 0.42 ± 0.01 | 0.42 ± 0.01 | 2.02 ± 0.01 | 0.58 ± 0.01 |
| 35 | 1.30 ± 0.02 | 0.40 ± 0.01 | 0.45 ± 0.01 | 2.02 ± 0.01 | 0.55 ± 0.01 |
| 40 | 1.23 ± 0.02 | 0.37 ± 0.01 | 0.47 ± 0.01 | 1.99 ± 0.01 | 0.53 ± 0.01 |
| 45 | 1.15 ± 0.02 | 0.33 ± 0.01 | 0.51 ± 0.01 | 2.00 ± 0.02 | 0.49 ± 0.01 |
| 50 | 1.06 ± 0.03 | 0.22 ± 0.01 | 0.51 ± 0.02 | 1.94 ± 0.02 | 0.49 ± 0.02 |
| 55 | 0.93 ± 0.04 | 0.13 ± 0.01 | 0.54 ± 0.02 | 1.85 ± 0.02 | 0.46 ± 0.02 |
| 10% NTBD | |||||
| 20 | 1.53 ± 0.02 | 0.52 ± 0.01 | 0.38 ± 0.01 | 2.14 ± 0.01 | 0.62 ± 0.01 |
| 25 | 1.50 ± 0.02 | 0.55 ± 0.02 | 0.43 ± 0.01 | 2.21 ± 0.01 | 0.57 ± 0.01 |
| 30 | 1.25 ± 0.02 | 0.34 ± 0.01 | 0.45 ± 0.01 | 1.99 ± 0.01 | 0.55 ± 0.01 |
| 35 | 1.39 ± 0.02 | 0.42 ± 0.02 | 0.44 ± 0.01 | 2.15 ± 0.02 | 0.56 ± 0.01 |
| 40 | 1.33 ± 0.03 | 0.38 ± 0.02 | 0.45 ± 0.01 | 2.12 ± 0.02 | 0.55 ± 0.01 |
| 45 | 1.08 ± 0.03 | 0.32 ± 0.01 | 0.55 ± 0.01 | 2.02 ± 0.02 | 0.45 ± 0.01 |
| 50 | 0.93 ± 0.03 | 0.19 ± 0.01 | 0.57 ± 0.01 | 1.91 ± 0.02 | 0.43 ± 0.02 |
| 55 | 0.65 ± 0.10 | 0.06 ± 0.01 | 0.66 ± 0.06 | 1.81 ± 0.02 | 0.34 ± 0.06 |
| 20% NTBD | |||||
| 20 | 1.66 ± 0.02 | 0.61 ± 0.02 | 0.35 ± 0.01 | 2.24 ± 0.01 | 0.65 ± 0.01 |
| 25 | 1.65 ± 0.02 | 0.63 ± 0.02 | 0.38 ± 0.01 | 2.28 ± 0.01 | 0.62 ± 0.01 |
| 30 | 1.54 ± 0.02 | 0.51 ± 0.02 | 0.39 ± 0.01 | 2.19 ± 0.01 | 0.61 ± 0.01 |
| 35 | 1.48 ± 0.02 | 0.46 ± 0.01 | 0.41 ± 0.01 | 2.19 ± 0.01 | 0.59 ± 0.01 |
| 40 | 1.46 ± 0.03 | 0.41 ± 0.02 | 0.40 ± 0.01 | 2.17 ± 0.01 | 0.60 ± 0.01 |
| 45 | 1.26 ± 0.03 | 0.36 ± 0.02 | 0.51 ± 0.01 | 2.19 ± 0.02 | 0.49 ± 0.01 |
| 50 | 1.20 ± 0.03 | 0.24 ± 0.01 | 0.50 ± 0.01 | 2.17 ± 0.02 | 0.50 ± 0.01 |
| 55 | 1.25 ± 0.03 | 0.18 ± 0.01 | 0.46 ± 0.01 | 2.16 ± 0.02 | 0.54 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skrzypek, A.; Budziak-Wieczorek, I.; Ślusarczyk, L.; Górecki, A.; Kamiński, D.; Kwaśniewska, A.; Okoń, S.; Różyło, I.; Matwijczuk, A. Advanced Spectroscopic Studies of the AIE-Enhanced ESIPT Effect in a Selected 1,3,4-Thiadiazole Derivative in Liposomal Systems with DPPC. Int. J. Mol. Sci. 2025, 26, 10643. https://doi.org/10.3390/ijms262110643
Skrzypek A, Budziak-Wieczorek I, Ślusarczyk L, Górecki A, Kamiński D, Kwaśniewska A, Okoń S, Różyło I, Matwijczuk A. Advanced Spectroscopic Studies of the AIE-Enhanced ESIPT Effect in a Selected 1,3,4-Thiadiazole Derivative in Liposomal Systems with DPPC. International Journal of Molecular Sciences. 2025; 26(21):10643. https://doi.org/10.3390/ijms262110643
Chicago/Turabian StyleSkrzypek, Alicja, Iwona Budziak-Wieczorek, Lidia Ślusarczyk, Andrzej Górecki, Daniel Kamiński, Anita Kwaśniewska, Sylwia Okoń, Igor Różyło, and Arkadiusz Matwijczuk. 2025. "Advanced Spectroscopic Studies of the AIE-Enhanced ESIPT Effect in a Selected 1,3,4-Thiadiazole Derivative in Liposomal Systems with DPPC" International Journal of Molecular Sciences 26, no. 21: 10643. https://doi.org/10.3390/ijms262110643
APA StyleSkrzypek, A., Budziak-Wieczorek, I., Ślusarczyk, L., Górecki, A., Kamiński, D., Kwaśniewska, A., Okoń, S., Różyło, I., & Matwijczuk, A. (2025). Advanced Spectroscopic Studies of the AIE-Enhanced ESIPT Effect in a Selected 1,3,4-Thiadiazole Derivative in Liposomal Systems with DPPC. International Journal of Molecular Sciences, 26(21), 10643. https://doi.org/10.3390/ijms262110643










